- 1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
- 2Department of Surgery and Cancer, Imperial College London, Hamlyn Centre, 3rd floor Surgical Innovation Centre, St Mary’s Hospital, London, United Kingdom
Current platforms for cancer surgery are inherently imprecise and this is manifest in high rates of incomplete excision and reoperative intervention. A prominent example is breast conserving surgery where intra-operative determination of margin involvement is challenging leading to high national average rates of positive resection margins needing revisional procedures. To meet these demands of improved precision it is valuable to image the resected tissue in real time in such a way that enables tissue characterization. A plethora of imaging methods have been proposed, with X-ray micro-CT appearing as one of the most promising due to its ability to scan the entire resection in 3D, as opposed to 2D imaging methods and/or approaches that only allow sampling the tissue at specific locations with limited field-of-view. A key, well-known limitation is the limited soft tissue sensitivity of X-rays, which has recently been overcome through the advent of X-ray phase contrast imaging (XPCI). The introduction of XPCI methods working with conventional sources (as opposed to specialized facilities such as synchrotrons) has spawn a series of exciting studies aiming at translating XPCI into clinical applications, which have recently extended into the realm of intra-operative imaging for breast conserving surgery and other areas. This article briefly introduces the XPCI technology, then reviews its existing applications in intra-operative imaging.
1 Intra-operative imaging
Most surgically resected specimens are examined via conventional histopathology, the results of which become available in a few weeks after surgery. While this is acceptable in some areas, there are others where immediate, real-time knowledge of e.g., margin status and tumor stage would be highly beneficial (Twengström et al., 2022; Partridge et al., 2024), and some where this knowledge is essential. A typical example of the latter is breast conserving surgery, as positive margin involvement detected days later at histopathology assessment commonly leads to a second, and sometimes even a third re-operation. Re-operations are a significant burden first and foremost to patients, to whom they cause discomfort, stress and worse cosmetic outcomes and breast-related quality of life, plus they are associated with a significant increase in healthcare costs (Grant et al., 2019). As can be expected, the incidence of re-operations varies globally and among centers, with median and peak approaching 20% and 40% respectively (Tang et al., 2017).
The ability to determine margin involvement in real time during the operation itself and provide immediate feedback to the surgical team would allow the latter to resect more tissue where necessary, thereby reducing the incidence of re-operations. Current practice in the UK involves imaging the resected tissue (“wide local excision”, WLE) with a specimen radiography system (St John et al., 2017a) which, however, only provides a partial solution due to a) the limited soft-tissue sensitivity of conventional X-rays (Pisano et al., 2000) and b) its 2D nature, which means margins orthogonal to the imaging axis are occluded in the projection, making their assessment extremely difficult (Streeter et al., 2022).
Several imaging techniques have been proposed to overcome the limitations of specimen radiography. Discussing them in detail lies beyond the scope of the present article, however a partial list would include Raman spectroscopy (Kong et al., 2014), optical coherence tomography (Nguyen et al., 2009) and methods based on radiofrequency (Dixon et al., 2015) and optical sectioning microscopy (Yoshitake et al., 2016; Tao et al., 2014). All these techniques probe specimens only at specific locations, which can be a barrier to effective and rapid implementation. The limited field of view can also lead to complex image mosaicking, and tissue interface artifacts can affect optical methods (Chang et al., 2015). Optical Coherence Tomography shows promise and has been used to illuminate the entire specimen surface, however the effective imaging depth is limited to approximately 2 mm (Maloney et al., 2018), and reported sensitivity values vary significantly, recently reported at 69% (Kennedy et al., 2020) vs. more optimistic estimates previously reported by Nguyen et al. (2009). Methods based on radiofrequency and bioimpedance spectroscopy have shown low specificity (Schnabel et al., 2014), with a recent UK trial failing to show any difference in reoperative intervention resulting from their use Bundred et al. (2022). Recent approaches also include detection of Cherenkov radiation directly from the tumor bed (Grootendorst et al., 2017), which requires administering radionuclides to the patients, and mass spectrometry coupled to a surgical scalpel (St John et al., 2017b), which is still under assessment. The latter method shows promise and is one of the key achievements in the area, but cannot detect close margins and requires collecting molecular signatures from all lesions.
Finally, it is important to mention cytopathological approaches such as frozen section and imprint cytology, sometimes referred to as intraoperative consultation (Sabel et al., 2012); these are highly accurate techniques, but they are also time consuming, costly and labour intensive; for this reason, no institutions in the UK currently uses them (St John et al., 2017a).
In this complex and evolving scenario, X-ray micro-CT was shown to possess several ideal characteristics, such as tissue penetration, spatial resolution, and especially full volumetric visualization of the entire specimen (Ritman, 2013) which, where contrast is appropriate, allows following strands protruding from the main tumor mass to ascertain whether they reach the specimens’ margins. Indeed, some studies have demonstrated accuracy comparable and possibly superior to other proposed methods (DiCorpo et al., 2020). However, the main limitation of conventional micro-CT is the already mentioned limited soft tissue sensitivity, which possibly explains why sensitivity values of ∼50% or just above are often reported (McClatchy et al., 2018; Qiu et al., 2018). X-ray phase contrast imaging (XPCI) was repeatedly demonstrated to overcome the soft-tissue sensitivity limitations of conventional X-rays while maintaining resolution, penetration power and full 3D visualization; in a proper implementation, it can therefore provide a valuable solution in intra-operative breast imaging and other surgical applications.
For completeness, we mention that x-ray diffraction has also been proposed for intra-operative use, due to its potential to increase specificity (Stryker et al., 2021). It has to be noted, however, that experimental implementations so far have been characterized by excessively long acquisition times (e.g., 5–7 h for a two-dimensional image in Moss et al., 2017). Although improvements in source technology and more efficient geometries such as the fan beam approach proposed by Stryker et al. could ameliorate this, it is unlikely the technique could reach the 10–15′ scan times required in intra-operative imaging in breast conserving surgery, especially in a (preferrable) three-dimensional imaging implementation.
2 X-ray phase contrast imaging (XPCI)
Following pioneering experiments in the mid-60s (Bonse and Hart, 1965) XPCI found large diffusion in the mid-90s, mostly thanks to the advent of third generation synchrotron sources; its huge potential in medical imaging was rapidly recognized (Lewis, 2004). One of the earliest medical applications to be targeted was mammography, as various XPCI methods immediately showed image quality largely outperforming clinical practice (Arfelli et al., 1998; Pisano et al., 2000); indeed, mammography was also the area where the first clinical study on human patients was performed, still at a synchrotron (Castelli et al., 2011; Olivo and Castelli, 2014). This study was based on the simplest XPCI approach, propagation-based (PB) XPCI, which does not require the use of any optical elements (Figure 1), but requires a “spatially coherent” (simplifying, small and distant) focal spot, as is readily available at synchrotrons.
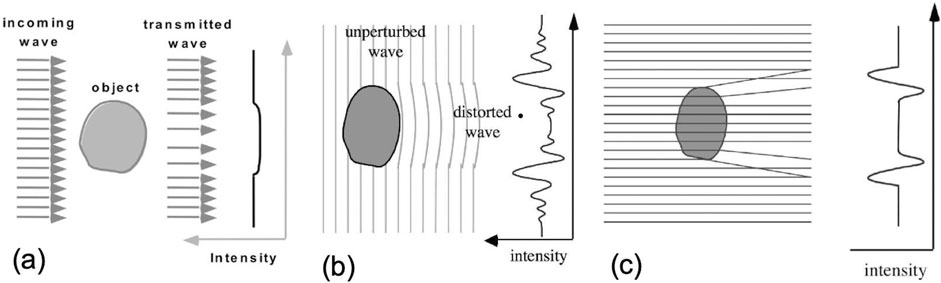
Figure 1. Propagation-based (PB) XPCI (b,c) compared to conventional X-ray imaging (a). In the latter, only the attenuation of X-rays is considered: an object becomes visible if it attenuates more (or less) than the surrounding background, which creates a “shadow” in the detected image (a). This is the main limitation of conventional X-rays, as the attenuation of soft-tissue tumors is very similar to that of the surrounding healthy soft tissue, making their detection difficult. In XPCI, the fact that X-rays are waves is exploited instead: waves travel with different speeds through different materials (b), leading to parts of the wavefront being phase-shifted with respect to others upon traversing an object. Allowed a sufficient distance to propagate, these phase shifts create interference patterns, the peaks and troughs of which are typically much more intense than signals created by attenuation. However, the detection of these patterns requires “spatially coherent” (small and distant) sources and detectors with high resolution. An approximated description relies on X-ray refraction, as refraction angles are proportional to the first derivative of the phase shifts, highest at details’ edges. As can be seen in (c), this approximated description cannot reproduce the fine fringes of the interference pattern in (b), but it does reproduce the pattern’s main minima and maxima. This was used as inspiration for the development of methods working with incoherent sources such as edge illumination.
At that stage, the necessity to rely on large, expensive and highly specialized facilities such as synchrotrons (only approximately 50 of which exist in the world) severely limited the diffusion of XPCI, and de facto prevented medical uses apart from specific studies like the above. However, in the mid-00s, techniques emerged that enabled XPCI to be implemented with conventional sources (Figure 2), such as Talbot-Lau interferometry (TLI, Pfeiffer et al., 2006) and Edge Illumination (EI, Olivo and Speller, 2007).

Figure 2. Talbot-Lau interferometry (TLI, (a)) and double- (b) and single- (c) mask versions of edge illumination (EI). In TLI, a phase grating with a small period (a few microns) is coherently illuminated so as to form a “Talbot self-image” which is analyzed at the detector by a second (absorption) grating. The regular structure of the grating allows “sectioning” an extended (i.e., incoherent) source with a third (“source”) grating creating sufficiently coherent “sourcelets”, which must be harmonically matched to the other two gratings. EI dispenses with coherence by using an amplitude modulator that directly “sections” the beam into beamlets, which are deflected by refraction (i.e., phase changes) induced by the sample. These deflections can be detected either via a second mask on the detector (b), or with a detector with sufficiently small pixels so as to resolve the beamlets directly (c). (c) Is a simpler implementation but it may restrict the available field-of-view, since 4-5 pixels per beamlet are required instead of just one (as is the case in (b)).
Later, the advent of novel source technology allowing higher fluxes from small focal spots (and therefore featuring higher spatial coherence than conventional X-ray tubes) such as those based on Liquid Metal Jet (LMJ) anodes (Larsson et al., 2011) allowed pre-existing methods such as PB XPCI to be implemented in more compact setups (Twengström et al., 2022). Concurrently, they also allowed additional techniques born at synchrotrons that require spatial coherence such as speckle-based imaging (Morgan et al., 2012), characterized by a “random” beam modulator, to be implemented on a laboratory scale (Zanette et al., 2014). More recent research making use of absorbing (as opposed to phase-shifting) random modulators, effectively a “hybrid” between EI (it its “single mask” embodiment, see e.g., Vittoria et al., 2017) and speckle-based imaging, may allow relaxing the spatial coherence requirements, ultimately leading to implementations with non-micro-focal X-ray sources (Magnin et al., 2023).
The development of these novel approaches allowed imaging tissue specimens outside synchrotrons and in standard labs, prospectively opening the way to digital histology and, more recently, intra-operative imaging.
3 Applications of XPCI to intra-operative imaging
Interest in the use of XPCI for tissue specimen imaging (for e.g., “digital histology”) arose already with synchrotrons which, due to their “ideal X-ray source” nature, allow superb image quality, including of breast tissue: exquisite examples can be seen in e.g., Baran et al. (2018) and Donato et al. (2024). Successful attempts were made to obtain at least comparable image quality with TLI (Hellerhoff et al., 2019; Polikarpov et al., 2023); example images are reported in Figure 3.
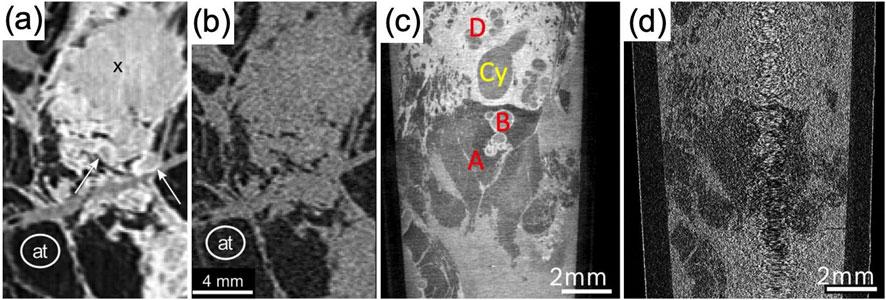
Figure 3. XPCI micro-CT images of breast tissue with TLI and laboratory sources. (a) Shows images of a tumor mass (indicated by X) surrounded by DCIS (white arrows), clearly differentiated thanks to XPCI; the same structures are hardly distinguishable in the conventional attenuation image in (b), although adipose tissue (indicated with at) is distinguishable in both. (c) Shows another example where TLI-based XPCI enables distinguishing milk ducts with columnar metaplasia (A), dilated ducts (B), cysts (Cy) and fibrosis (D); the same features ate not distinguishable in the attenuation image in (d). Panels (a,b) are adapted under the terms of the Creative Commons Attribute License, CC-BY 4.0 license from Hellerhoff et al. (2019), https://doi.org/10.1371/journal.pone.0210291. Panels (c,d) are adapted under the terms of the Creative Commons Attribute License, CC-BY 4.0 license from Polikarpov et al. (2023), https://doi.org/10.1038/s41598-023-35854-6.
The main issue with the results reported in Figure 3 is acquisition times, which range from ∼7 (Hellerhoff et al., 2019) to ∼90 h (Polikarpov et al., 2023), making application to intra-operative imaging impossible. TLI requires a procedure called phase stepping, whereby one of the gratings must be stepped laterally by sub-period increments at each CT angular projection (e.g., Hellerhoff et al. used 11 steps and Polikarpov et al. used 5). On top of extending acquisition times in proportion to the number of used steps, phase stepping a) adds dead times to the scans, associated with motor movements and return to starting position, and most of all b) imposes the use of a step-and-shoot acquisition procedure, as opposed to a “flyscan” one where the sample is continuously rotated, therefore eliminating any source of dead time. “Interlaced” approaches where stepping of the grating and sample rotation are combined were proposed at synchrotrons (Zanette et al., 2011) but, to the best of our knowledge, not translated to extensive studies of biological tissues. Options to avoid phase stepping, pioneered by Momose’s group (Takeda et al., 2007), require the use of very small detector pixels to directly resolve the Talbot self-image created by the phase grating (e.g., 1 micron in the cited paper). Since detectors typically have a few thousand pixels, this restricts the field-of-view to a few mm, making applicability to full WLEs practically impossible.
A breakthrough in the ability to achieve acquisition speeds compatible with intra-operative imaging in XPCI came from Diemoz et al. (2017) adaptation of Paganin et al.’s “single shot” phase retrieval method (Paganin et al., 2002) to EI. The Paganin et al. method enables retrieving the phase from a single image in PB XPCI based on a proportionality assumption between phase and attenuation in homogeneous objects. We note this is a reasonable assumption in breast tissue which, calcifications aside, is indeed a near-ideal sample for Paganin’s method; as can be seen from Figure 4, calcifications are still clearly visible, although their retrieval is not quantitatively exact. It should be noted, however, that the possible presence of clips or other metallic inserts would also cause problems–not only to Paganin retrieval but also to general CT reconstruction methods (De Man et al., 1999). As such, they should be handled following established strategies (e.g., Zhang et al., 2011), ideally before application of the Paganin algorithm. Some artefact reduction strategies have been developed specifically for phase-based X-ray methods (Kumschier et al., 2024).

Figure 4. XPCI micro-CT images of breast tissue with EI and laboratory sources. (a) Shows a phase CT slice where arrows 1 and 2 show an involved margin and tumor-induced inflammation, respectively; these are confirmed by H&E histopathology (b), the appearance of which is remarkably similar to the EI phase micro-CT image. Tumor inhomogeneity is also detected (red arrow), similarly to results shown in Figure 3a but with much faster scans. For comparison, (c) shows a conventional specimen radiography image of the same sample, highlighting the extreme difficulty in extracting the same information. (d) Uses a different sample to demonstrate the method’s ability to visualize DCIS (bottom half of the image), again with matching H&E results (top half). Adapted under the terms of the Creative Commons Attribute License, CC-BY 4.0 license from Massimi et al. (2021), https://doi.org/10.1038/s41598-021-83330-w.
Unlike TLI and speckle methods that are characterized by uneven image backgrounds, the relatively large mask periods in EI allow the acquisition of a perfectly flat field in the absence of a sample (see Figure 2b: despite the relative misalignment between the two masks, all detector pixels are illuminated in the same way). This allows a straightforward translation of Paganin’s method, enabling effective phase retrieval (for homogeneous samples) from single projections, which in turn allows flyscan acquisitions with continuous sample rotation. Based on this principle, Diemoz et al. demonstrated unprecedented phase-retrieved micro-CT scan times of 3 min for biological samples using conventional rotating anode X-ray sources. This innovation prompted a study on breast tissue specimens aimed at intra-operative imaging (Massimi et al., 2021), some examples from which are reported in Figure 4.
A first part of the Massimi et al. study scanned >100 in vitro formalin-fixed breast tissue specimens, approximately half of which contained cancer. As well as with EI XPCI micro-CT, all specimens were also imaged with conventional specimen radiography and underwent histopathology evaluation. The study’s radiologist blindly scored both XPCI and conventional radiography images for cancer presence at margins, and histopathology was used as the ground truth against which both imaging methods were benchmarked. This showed comparable specificity (83%, 95% CI 70%–92% vs. 86%, 95% CI 73–93 for XPCI and specimen radiography, respectively), but a remarkable 260% improvement in sensitivity for the former (83%, 95% CI 60%–92% vs. 32%, 95% CI 20–49). Considering that several studies indicated that sensitivity was the last hurdle micro-CT needed to overcome to become the method of choice in intra-operative imaging (see §1), this is an extremely promising result.
For this study, the radiologist was trained ahead of undertaking the blind scoring of all XPCI images: they were given short presentations by the physicists group supported by the pathologist, during which they were able to see XPCI CT slices side-by-side with matching histopathology images. Following training, they looked at all XPCI images scoring them for cancer presence (yes/no) and cancer presence at margins (yes/no). They did the same with the conventional specimen radiography images, for which of course they needed no training. There was discussion as to whether it would be possible to train the surgical team on image interpretation so that a radiologist would not be needed, with or without the support of appropriately trained AI algorithms. These remain interesting and active areas of current and future research.
The second part of the Massimi et al. study demonstrated that the method could be seamlessly integrated into the clinical workflow, i.e., that it could scan entire, fresh WLEs in times compatible with those required by clinical workflows. An enhanced version of the scanner with a field-of-view of 9 × 9 cm2 (which 1 year of observations at the Barts hospital in London, UK indicated was sufficient to cover >90% of the cases) was deployed in a reasonable vicinity of the operating theatre. Through discussions with the surgical team, it was agreed that a maximum time of 15 min could be made available; 15 min scans were then performed, but post-scan analysis in which half the CT projections were discarded revealed that 7.5 min scans would have resulted in comparable image quality (Figure 5). It has to be specified though that these times refer only to the specimen scan and do not include the CT reconstruction time. Since the latter is a parallelizable process that can be implemented on GPUs, it was assumed the added time related to reconstruction could be considered negligible compared to the scan time in a prospective clinical system.

Figure 5. XPCI micro-CT images of full-size (container diameter 5 cm) fresh WLEs obtained with a dedicated intra-operative EI XPCI scanner and 15 (a) and 7.5 min (b) scan time. Adapted under the terms of the Creative Commons Attribute License, CC-BY 4.0 license from Massimi et al. (2021), https://doi.org/10.1038/s41598-021-83330-w.
Another option to speed up acquisitions is to apply Paganin et al.’s retrieval algorithm to PB XPCI, for which it was originally intended. However, unlike EI, PB XPCI requires a spatially coherent source which, from a lab deployment perspective, means a micro-focal source. The issue with micro-focal sources is their low power, which translates into low X-ray flux and therefore longer acquisitions. As an example, a widely used micro-focal source by Hamamatsu (model L12161-07) has 10 W of power in “small focus” mode, which is 120 times lower than that of the Rigaku Micro-Max 007 source used in the Massimi et al. study. Power directly translates into X-ray flux and therefore scan time.
A great opportunity in this space was created by the development of a new source technology (the LMJ) that can provide higher power from small focal spots. Indeed, to the best of our knowledge, the Twengström et al. (2022) study, using PB XPCI and the LMJ source, is the only other example where the intra-operative imaging problem was targeted using laboratory-scale sources and PB XPCI. This study, which only considered fixed tissue, showed excellent margin delineation in neuroendocrine tumors in pancreas, and less sharp (but still visible) margins in intrahepatic cholangiocarcinoma in liver (Twengström et al., 2022). Despite a source power of 100 W, which becomes competitive with the 1.2 kW of the Rigaku source used by Massimi et al. (2021) once the 68% absorption in the first mask and additional 50% absorption in the second mask are taken into account (leaving approximately 190 W of source power available for imaging), scans lasted between 1.5 and 3 h. This can have several explanations. The smaller pixel size (22 vs. 50 micron) requires a ∼5-fold increase in statistics to achieve the same pixel-to-pixel signal-to-noise ratio, which is possibly exacerbated by small pixel detectors often being less efficient than larger pixel ones; however, small pixels are necessary in PB XPCI. Additional factors could be the slightly longer source-to-detector distance (approximately 1 m vs. 70 cm in the Massimi et al. case, leading to a 2-fold reduction in flux), and the lack of an emission line at 17.5 keV that can boost soft tissue contrast. However, the Twengström et al. images had higher resolution than the Massimi et al. ones, which can lead to improved detection of fine details. Twengström et al. mention the option to reduce scan times by using higher power LMJ sources, which have indeed become available; however, higher power is often achieved by increasing the kVp, leading to significantly “harder” spectra that negatively affect image contrast.
For completeness, we mention two follow-up studies with EI XPCI. In the first one (Massimi et al., 2022), it was observed that, since only a fraction of the sample corresponding to the aperture in the pre-sample mask is illuminated (see Figures 2b,c), a resolution equal to the aperture size can ultimately be obtained in the images. This allowed the acquisition of images with a resolution of about 12 micron (comparable to the Twengström study). This enabled the visualization of tumor strands as thin as 30 micron (Figure 6); furthermore, contrast gets also boosted by the increased resolution, which enabled the detection of features classically considered X-ray invisible (e.g., tissue response to chemotherapy). However, this came at the cost of lengthening scan times to a few hours: reaching aperture-limited resolution requires stepping the sample (or the mask) at every CT projection in increments equal to an aperture, until a complete mask period is covered. Similarly to phase-stepping in TLI, this increases the scan time proportionally to the number of steps (given by mask period divided by mask aperture) and, most of all, imposes a step-and-shoot acquisition modality that adds dead times. However, it is important to note a key difference in the reason why stepping is applied: in TLI, it is a necessity to perform phase retrieval; in EI, it offers an option to increase spatial resolution, while retrieval is still performed on single frames. Without stepping, retrieved images with a resolution determined by the pixel size are obtained (as in Massimi et al., 2021). Importantly, this is achieved by implementing a different acquisition scheme on the same machine: e.g., the scanner used for intra-operative imaging could be used for more detailed tissue studies (virtual histology) during surgery downtimes.
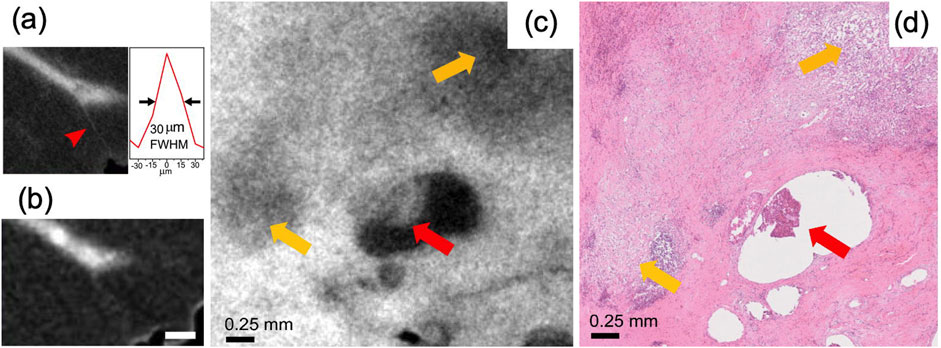
Figure 6. High resolution micro-CT images of breast tissue with EI and laboratory sources. (a) Shows how the “high resolution” acquisition mode allows the detection of ∼30 micron thick tumor strands (see profile in the inset), invisible in the faster, lower-resolution mode (b). Yellow arrows in (c) show the tissue’s response to chemotherapy, confirmed by the matching H&E image in (d). The red arrow indicates a residual infiltrating ductal carcinoma. Adapted with permission from IEEE Transactions on Medical Imaging (License Number 5987140428005) from Massimi et al. (2022), https://doi.org/10.1109/TMI.2021.3137964.
Finally, Partridge et al. (2024) applied EI XPCI micro-CT to the intra-operative imaging of full human esophagi resulting from esophagectomy procedures. An additional complication was encountered in this case: because esophagi consist almost entirely of muscle, contrast was hindered, also in XPCI, by muscle and water having practically the same X-ray refractive index. By partially displacing water via ethanol immersion, Partridge et al. (2024) obtained exquisite delineation of esophageal layers which enabled T-staging the tumors from the X-ray images alone. However, this required leaving the specimen immersed in ethanol for times too long to be compatible with intra-operative practice, even considering the longer duration of esophagectomy procedures compared to breast conserving surgery. Faster specimen preparation procedures are therefore needed to translate EI XPCI micro-CT into intra-operative imaging for esophagectomies.
4 Conclusions and perspectives
Various implementations of XPCI micro-CT with conventional sources have shown huge potential in digital histology, with EI and PB XPCI with LMJ sources showing promise also in intra-operative imaging, offering potentially the major achievements in the reviewed field–at least as far as x-ray based techniques are concerned.
In particular, Massimi et al. (2021) showed that (at-pixel resolution) EI XPCI micro-CT boosts the sensitivity for cancer detection at margins by a factor 2.6 compared to specimen radiography (the current standard practice in the United Kingdom), and that it is suitable to image full size, fresh WLEs in timeframes compatible with clinical workflows. For clinical translation, a key gap in research is the need for further clinical trials on fresh WLEs to ascertain sensitivity and specificity vs. different cancer types, ideally followed by a multi-center prospective randomized study to confirm the clinical and cost effectiveness of EI XPCI micro-CT for breast conserving surgery. From a technical perspective, a challenge is the need to maintain the two (pre- and post-sample) masks used in EI to achieve phase sensitivity aligned to within approximately a micron, in noisy and vibration-prone environments like hospitals. Methods to mitigate this based on automated alignment exist (Millard et al., 2013), and the technique has been repeatedly used in rugged environments such as factory floors (e.g., Astolfo et al., 2017). Options to use simplified approaches based on a single mask are also discussed in the literature (Vittoria et al., 2017).
PB XPCI with LMJ sources provides excellent image quality with higher resolution; a remaining gap is the need to speed up acquisitions to become compatible with intra-operative practice. This could be achieved by using higher power LMJ sources that do not compromise image contrast via excessively high average X-ray energy, better detector technology, or both.
If sufficient miniaturization is achieved, laser-based sources (Gambari et al., 2020) could also become an option in the future, especially considering their rapid progress (Doherty et al., 2023).
Options to significantly increase resolution in EI XPCI micro-CT were demonstrated by Massimi et al. (2022); these, however, came at the cost of lengthier step-and-shoot acquisitions. A method termed “cycloidal CT” was developed where the sample is roto-translated in the “structured” X-ray beam (i.e., the multiple beamlets created in EI by the pre-sample mask), which allows accessing the higher, “at aperture” resolution available in EI with flyscans instead of step-and-shoot acquisition (Hagen et al., 2020). A first, proof-of-concept assessment of the viability of cycloidal CT in intra operative specimen imaging has been conducted (Roche i Morgo et al., 2021) which, if further developed, could lead to a “best of both world” situation where very high resolution and scan times compatible with intra operative practice may become simultaneously available.
Finally, cost would also be an essential element of any health economics assessment looking at the adoption of the above x-ray solutions in clinical practice. EI requires masks that currently have a cost of approximately $10–20k; it should be noted, however, that most of these are “set-up” costs related to the fact that masks are being built as “one offs” to specific, custom designs; if mass-produced to a fixed design, it is reasonable to expect that their cost would reduce significantly. Therefore, they would add relatively little to the overall cost of a micro-CT machine: if the cost of the latter is considered acceptable for intra-operative use, then it is reasonable to assume that an EI version would be too. The current cost of a LMJ source is considerably higher than that of a standard x-ray source (including rotating anode); however, this too could reduce as the technology progresses.
Author contributions
AO: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing. DL: Conceptualization, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AO is supported by the Royal Academy of Engineering under their “Chairs in Emerging Technologies” scheme (grant CiET1819/2/78). DRL acknowledges the infrastructure support of the NIHR Imperial BRC.
Conflict of interest
AO is a named inventor on patents owned by UCL that protect the edge illumination technology, discussed among other in the paper.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arfelli, F., Assante, M., Bonvicini, V., Bravin, A., Cantatore, G., Castelli, E., et al. (1998). Low-dose phase contrast x-ray medical imaging. Phys. Med. Biol. 43, 2845–2852. doi:10.1088/0031-9155/43/10/013
Astolfo, A., Endrizzi, M., Vittoria, F. A., Diemoz, P. C., Price, B., Haig, I., et al. (2017). Large field of view, fast and low dose multimodal phase-contrast imaging at high x-ray energy. Sci. Rep. 7, 2187. doi:10.1038/s41598-017-02412-w
Baran, P., Mayo, S., McCormack, M., Pacile, S., Tromba, G., Dullin, C., et al. (2018). High-resolution x-ray phase contrast 3-D imaging of breast tissue specimens as a possible adjunct to histopathology. IEEE Trans. Med. Imaging 37, 2642–2650. doi:10.1109/TMI.2018.2845905
Bonse, U., and Hart, M. (1965). An X-ray interferometer. Appl. Phys. Lett. 6, 155–156. doi:10.1063/1.1754212
Bundred, N. J., Dixon, M., Achuthan, R., Barrett, E., Benson, J., Courtney, C. A., et al. (2022). Does the use of an intraoperative device to assess margins reduce need for re-excison after breast conserving surgery: multicentre randomised controlled trial. Eur. J. Surg. Oncol. 48, e221. doi:10.1016/j.ejso.2022.03.143
Castelli, E., Tonutti, M., Arfelli, F., Longo, R., Quaia, E., Rigon, L., et al. (2011). Mammography with synchrotron radiation: first clinical experience with phase-detection technique. Radiology 259, 684–694. doi:10.1148/radiol.11100745
Chang, T. P., Leff, D. R., Shousha, S., Hadjiminas, D. J., Ramakrishnan, R., Hughes, M. R., et al. (2015). Imaging breast cancer morphology using probe-based confocal laser endomicroscopy: towards a real-time intraoperative imaging tool for cavity scanning. Breast Cancer Res. Treat. 153, 299–310. doi:10.1007/s10549-015-3543-8
De Man, B., Nuyts, J., Dupont, P., Marchal, G., and Suetens, P. (1999). Metal streak artifacts in X-ray computed tomography: a simulation study. IEEE Trans. Nucl. Sci. 46, 691–696. doi:10.1109/23.775600
DiCorpo, D., Tiwari, A., Tang, R., Griffin, M., Aftreth, O., Bautista, P., et al. (2020). The role of Micro-CT in imaging breast cancer specimens. Breast Cancer Res. Treat. 180, 343–357. doi:10.1007/s10549-020-05547-z
Diemoz, P., Hagen, C., Endrizzi, M., Minuti, M., Bellazzini, R., Urbani, L., et al. (2017). Single-shot x-ray phase-contrast computed tomography with nonmicrofocal laboratory sources. Phys. Rev. Appl. 7, 044029. doi:10.1103/PhysRevApplied.7.044029
Dixon, J. M., Renshaw, L., Young, O., Kulkarni, D., Saleem, T., Sarfaty, M., et al. (2016). Intra-operative assessment of excised breast tumour margins using ClearEdge imaging device. Eur. J. Surg. Oncol. (EJSO) 42, 1834–1840. doi:10.1016/j.ejso.2016.07.141
Doherty, A., Fourmaux, S., Astolfo, A., Ziesche, R., Wood, J., Finlay, O., et al. (2023). Femtosecond multimodal imaging with a laser-driven X-ray source. Commun. Phys. 6, 288. doi:10.1038/s42005-023-01412-9
Donato, S., Arana Pena, L. M., Arfelli, F., Brombal, L., Colmo, L., Longo, R., et al. (2024). Integrating X-ray phase-contrast imaging and histology for comparative evaluation of breast tissue malignancies in virtual histology analysis. Sci. Rep. 14, 5831. doi:10.1038/s41598-024-56341-6
Gambari, M., Clady, R., Stolidi, A., Uteza, O., Sentis, M., and Ferré, A. (2020). Exploring phase contrast imaging with a laser-based Kα x-ray source up to relativistic laser intensity. Sci. Rep. 10, 6766. doi:10.1038/s41598-020-63614-3
Grant, Y., Al-Khudairi, R., St John, E., Barschkett, M., Cunningham, D., Al-Mufti, R., et al. (2019). Patient-level costs in margin re-excision for breast-conserving surgery. J. Br. Surg. 106, 384–394. doi:10.1002/bjs.11050
Grootendorst, M. R., Cariati, M., Pinder, S. E., Kothari, A., Douek, M., Kovacs, T., et al. (2017). Intraoperative assessment of tumour resection margins in breast-conserving surgery using 18F-FDG Cerenkov luminescence imaging: a first-in-human feasibility study. J. Nucl. Med. 58, 891–898. doi:10.2967/jnumed.116.181032
Hagen, C. K., Vittoria, F. A., Morgó, O. R. i., Endrizzi, M., and Olivo, A. (2020). Cycloidal computed tomography. Phys. Rev. Appl. 14, 014069. doi:10.1103/PhysRevApplied.14.014069
Hellerhoff, K., Birnbacher, L., Sztrokay-Gaul, A., Grandl, S., Auweter, S., Willner, M., et al. (2019). Assessment of intraductal carcinoma in situ (DCIS) using grating-based X-ray phase contrast CT at conventional X-Ray sources: an experimental ex-vivo study. Plos One 14, e0210291. doi:10.1371/journal.pone.0210291
Kennedy, K. M., Zilkens, R., Allen, W. M., Foo, K. Y., Fang, Q., Chin, L., et al. (2020). Diagnostic accuracy of quantitative micro-elastography for margin assessment in breast conserving surgery. Cancer Res. 80, 1773–1783. doi:10.1158/0008-5472.CAN-19-1240
Kong, K., Zaabar, F., Rakha, E., Ellis, I., Koloydenko, A., and Notingher, I. (2014). Towards intra-operative diagnosis of tumours during breast conserving surgery by selective-sampling Raman micro-spectroscopy. Phys. Med. Biol. 59, 6141–6152. doi:10.1088/0031-9155/59/20/6141
Kumschier, T., Thalhammer, J., Schmid, C., Haeusele, J., Koehler, T., Pfeiffer, F., et al. (2024). Streak artefact removal in x-ray dark-field computed tomography using a convolutional neural network. Med. Phys. 51, 7404–7414. doi:10.1002/mp.17305
Larsson, D. H., Takman, P. A. C., Lundström, U., Burvall, A., and Hertz, H. M. (2011). A 24 keV liquid-metal-jet x-ray source for biomedical applications. Rev. Sci. Instrum. 82, 123701. doi:10.1063/1.3664870
Lewis, R. A. (2004). Medical phase contrast x-ray imaging: current status and future prospects. Phys. Med. Biol. 49, 3573–3583. doi:10.1088/0031-9155/49/16/005
Magnin, C., Quenot, L., Bohic, S., Mihai Cenda, D., Fernández Martínez, M., Lantz, B., et al. (2023). Dark-field and directional dark-field on low-coherence x ray sources with random mask modulations: validation with SAXS anisotropy measurements. Opt. Lett. 48, 5839–5842. doi:10.1364/OL.501716
Maloney, B. W., McClatchy, D. M., Pogue, B. W., Paulsen, K. D., Wells, W. A., and Barth, R. J. (2018). Review of methods for intraoperative margin detection for breast conserving surgery. J. Biomed. Opt. 23, 1–19. doi:10.1117/1.JBO.23.10.100901
Massimi, L., Suaris, T., Hagen, C. K., Endrizzi, M., Munro, P. R. T., Havariyoun, G., et al. (2021). Detection of involved margins in breast specimens with x-ray phase-contrast computed tomography. Sci. Rep. 11, 3663. doi:10.1038/s41598-021-83330-w
Massimi, L., Suaris, T., Hagen, C. K., Endrizzi, M., Munro, P. R. T., Havariyoun, G., et al. (2022). Volumetric high-resolution X-ray phase contrast virtual histology of breast specimens with a compact laboratory system. IEEE Trans. Med. Imaging 41, 1188–1195. doi:10.1109/TMI.2021.3137964
McClatchy, D. M., Zuurbier, R. A., Wells, W. A., Paulsen, K. D., and Pogue, B. W. (2018). Micro-computed tomography enables rapid surgical margin assessment during breast conserving surgery (BCS): correlation of whole BCS micro-CT readings to final histopathology. Breast Cancer Res. Treat. 172, 587–595. doi:10.1007/s10549-018-4951-3
Millard, T. P., Endrizzi, M., Ignatyev, K., Hagen, C. K., Munro, P. R. T., Speller, R. D., et al. (2013). Method for automatization of the alignment of a laboratory based x-ray phase contrast edge illumination system. Rev. Sci. Instrum. 84, 083702. doi:10.1063/1.4816827
Morgan, K. S., Paganin, D. M., and Siu, K. K. W. (2012). X-ray phase imaging with a paper analyzer. Appl. Phys. Lett. 100, 124102. doi:10.1063/1.3694918
Moss, R. M., Amin, A. S., Crews, C., Purdie, C. A., Jordan, L. B., Iacoviello, F., et al. (2017). Correlation of X-ray diffraction signatures of breast tissue and their histopathological classification. Sci. Rep. 7, 12998. doi:10.1038/s41598-017-13399-9
Nguyen, F. T., Zysk, A. M., Chaney, E. J., Kotynek, J. G., Oliphant, U. J., Bellafiore, F. J., et al. (2009). Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 69, 8790–8796. doi:10.1158/0008-5472.CAN-08-4340
Olivo, A., and Castelli, E. (2014). X-ray phase contrast imaging: from synchrotrons to conventional sources. Riv. Nuovo Cimento 37, 467–508. doi:10.1393/ncr/i2014-10104-8
Olivo, A., and Speller, R. (2007). A coded-aperture technique allowing x-ray phase contrast imaging with conventional sources. Appl. Phys. Lett. 91, 074106. doi:10.1063/1.2772193
Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R., and Wilkins, S. W. (2002). Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40. doi:10.1046/j.1365-2818.2002.01010.x
Partridge, T., Wolfson, P., Jiang, J., Massimi, L., Astolfo, A., Djurabekova, N., et al. (2024). T-staging oesophageal tumours with x-rays. Optica 4, 569–576. doi:10.1364/OPTICA.501948
Pfeiffer, F., Weitkamp, T., Bunk, O., and David, C. (2006). Phase retrieval and differential phase-contrast imaging with low-brilliance X-ray sources. Nat. Phys. 2, 258–261. doi:10.1038/nphys265
Pisano, E. D., Johnston, R. E., Chapman, D., Geradts, J., Iacocca, M. V., Livasy, C. A., et al. (2000). Human breast cancer specimens: diffraction-enhanced imaging with histologic correlation—improved conspicuity of lesion detail compared with digital radiography. Radiology 214, 895–901. doi:10.1148/radiology.214.3.r00mr26895
Polikarpov, M., Vila-Comamala, J., Wang, Z., Pereira, A., van Gogh, S., Gasser, C., et al. (2023). Towards virtual histology with x-ray grating interferometry. Sci. Rep. 13, 9049. doi:10.1038/s41598-023-35854-6
Qiu, S.-Q., Dorrius, M. D., de Jongh, S. J., Jansen, L., de Vries, J., Schröder, C. P., et al. (2018). Micro-computed tomography (micro-CT) for intra-operative surgical margin assessment of breast cancer: a feasibility study in breast conserving surgery. Eur. J. Surg. Oncol. 44, 1708–1713. doi:10.1016/j.ejso.2018.06.022
Ritman, E. L. (2011). Current status of developments and applications of micro-CT. Annu. Rev. Biomed. Eng. 13, 531–552. doi:10.1146/annurev-bioeng-071910-124717
Roche i Morgo, O., Massimi, L., Suaris, T., Endrizzi, M., Munro, P. R. T., Savvidis, S., et al. (2021). Exploring the potential of cycloidal computed tomography for advancing intraoperative specimen imaging. Dev. X-Ray Tomogr. XIII 11840, 42. doi:10.1117/12.2594547
Sabel, M. S., Jorns, J. M., Wu, A., Myers, J., Newman, L. A., and Breslin, T. M. (2012). Development of an intraoperative pathology consultation service at a free-standing ambulatory surgical center: clinical and economic impact for patients undergoing breast cancer surgery. Am. J. Surg. 204, 66–77. doi:10.1016/j.amjsurg.2011.07.016
Schnabel, F., Boolbol, S. K., Gittleman, M., Karni, T., Tafra, L., Feldman, S., et al. (2014). A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann. Surg. Oncol. 21, 1589–1595. doi:10.1245/s10434-014-3602-0
St John, E. R., Al-Khudairi, R., Ashrafian, H., Athanasiou, T., Takats, Z., Hadjiminas, D. J., et al. (2017a). Diagnostic accuracy of intra-operative techniques for margin assessment in breast cancer surgery: a meta-analysis. Ann. Surg. 265, 300–310. doi:10.1097/SLA.0000000000001897
St John, E. R., Balog, J., McKenzie, J. S., Rossi, M., Covington, A., Muirhead, L., et al. (2017b). Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery. Breast. Cancer Res. 19, 59. doi:10.1186/s13058-017-0845-2
Streeter, S. S., Hunt, B., Paulsen, K. D., and Pogue, B. W. (2022). Emerging and future use of intra-surgical volumetric X-ray imaging and adjuvant tools for decision support in breast-conserving surgery. Curr. Opin. Biomed. Eng. 22, 100382. doi:10.1016/j.cobme.2022.100382
Stryker, S., Kapadia, A. J., and Greenberg, J. A. (2021). Simulation based evaluation of a fan beam coded aperture x-ray diffraction imaging system for biospecimen analysis. Phys. Med. Biol. 66, 065022. doi:10.1088/1361-6560/abe779
Takeda, Y., Yashiro, W., Suzuki, Y., Aoki, S., Hattori, T., and Momose, A. (2007). X-ray phase imaging with single phase grating. Jpn. J. Appl. Phys. 46, L89–L91. doi:10.1143/JJAP.46.L89
Tang, S. S.-K., Kaptanis, S., Haddow, J. B., Mondani, G., Elsberger, B., Tasoulis, M. K., et al. (2017). Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur. J. Cancer 84, 315–324. doi:10.1016/j.ejca.2017.07.032
Tao, Y. K., Shen, D., Sheikine, Y., Ahsen, O. O., Wang, H. H., Schmolze, D. B., et al. (2014). Assessment of breast pathologies using nonlinear microscopy. Proc. Natl. Acad. Sci. U. S. A. 111, 15304–15309. doi:10.1073/pnas.1416955111
Twengström, W., Moro, C. F., Romell, J., Larsson, J. C., Sparrelid, E., Björnstedt, M., et al. (2022). Can laboratory x-ray virtual histology provide intraoperative 3D tumor resection margin assessment? J. Med. Imaging 9, 031503. doi:10.1117/1.JMI.9.3.031503
Vittoria, F. A., Endrizzi, M., Kallon, G. K., Hagen, C. K., Iacoviello, F., De Coppi, P., et al. (2017). Multimodal phase-based x-ray microtomography with nonmicrofocal laboratory sources. Phys. Rev. Appl. 8, 064009. doi:10.1103/PhysRevApplied.8.064009
Yoshitake, T., Giacomelli, M. G., Cahill, L. C., Schmolze, D. B., Vardeh, H., Faulkner-Jones, B. E., et al. (2016). Direct comparison between confocal and multiphoton microscopy for rapid histopathological evaluation of unfixed human breast tissue. J. Biomed. Opt. 21, 126021. doi:10.1117/1.JBO.21.12.126021
Zanette, I., Bech, M., Pfeiffer, F., and Weitkamp, T. (2011). Interlaced phase stepping in phase-contrast x-ray tomography. Appl. Phys. Lett. 98, 094101. doi:10.1063/1.3559849
Zanette, I., Zhou, T., Burvall, A., Lundström, U., Larsson, D., Zdora, M., et al. (2014). Speckle-based x-ray phase contrast and dark-field imaging with a laboratory source. Phys. Rev. Lett. 112, 253903. doi:10.1103/PhysRevLett.112.253903
Keywords: X-ray phase contrast, advanced X-ray imaging, intra-operative imaging, breast conserving surgery, wide local excisions
Citation: Olivo A and Leff DR (2025) X-ray phase contrast for intra-operative specimen imaging in breast conserving surgery and other areas. Front. Med. Eng. 3:1608247. doi: 10.3389/fmede.2025.1608247
Received: 08 April 2025; Accepted: 04 September 2025;
Published: 17 September 2025.
Edited by:
Lorenzo Vannozzi, Institute of BioRobotics, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Viorel Nastasa, Extreme Light Infrastructure Nuclear Physics, RomaniaAndrew Leong, Los Alamos National Laboratory (DOE), United States
Copyright © 2025 Olivo and Leff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Olivo, YS5vbGl2b0B1Y2wuYWMudWs=
 Alessandro Olivo
Alessandro Olivo Daniel R. Leff
Daniel R. Leff