- School of Cancer and Pharmaceutical Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, United Kingdom
Introduction: Achieving negative margins during breast-conserving surgery (BCS) for breast cancer is critical to reduce re-excision rates and minimise local recurrence. Intraoperative imaging techniques using radiotracers such as 18F-fluorodeoxyglucose (18F-FDG) offer a promising solution. When administered intravenously, 18F-FDG accumulates preferentially in malignant tissues due to their elevated glycolytic activity, enabling molecular imaging of tumour margins. Technologies such as Cerenkov Luminescence Imaging (CLI), Flexible Auto-Radiography (FAR), and intraoperative PET/CT systems have emerged as tools to visualise radiotracer distribution in excised breast tissue, offering real-time insight into margin status.
Materials and methodology: CLI operates on the principle of detecting visible light photons generated by positrons from 18F-FDG travelling faster than light in tissue. FAR captures beta particles via a scintillating film to yield high-resolution surface maps of tracer activity. These modalities were evaluated both independently and in combination (CLI-FAR) using the LightPath® system, while the XEOS AURA 10 system was utilised for intraoperative PET/CT imaging. A series of feasibility studies and interventional trials assessed their diagnostic performance in real-time margin assessment during BCS.
Results: Grootendorst et al. (J. Nucl. Med., 2017, 58(6), 891–898) demonstrated that CLI achieved 89% sensitivity and 95% specificity in identifying positive margins in a cohort of 12 patients. Jurrius et al. (EJNMMI Res., 2021, 11(1)) reported 81.7% sensitivity and 46.2% specificity with FAR in 66 patients. The CLI-FAR technique, by Sinha et al. (Radiol. Adv., 2024, 1(2)), yielded 76.9% sensitivity and 97.8% specificity, reducing re-excision rates by 69%. PET/CT-based intraoperative imaging using the AURA 10 device, as evaluated by Crem et al. (ESMO Open, 2024, 9), achieved 91% sensitivity and 94% specificity, while Göker et al. (Acta Chir. Belg., 2020, 120(5), 366–374) reported 79% sensitivity and 72% specificity using micro-PET/CT. Radiation exposure to surgical staff across studies remained low (15–38 µSv), and imaging added minimal time to operative workflows.
Conclusion: Radionuclide-based intraoperative specimen imaging offers a viable, real-time solution for margin assessment in BCS. Techniques such as CLI, FAR, and intraoperative PET/CT demonstrate a strong correlation with histopathology, with the potential to significantly reduce re-excision rates. Challenges remain in imaging larger specimens and tumours with low metabolic activity. However, integrating these technologies into surgical practice presents a transformative opportunity for precision-guided oncologic surgery.
Introduction
Principles of nuclear medicine/radioactivity in intra-operative specimen imaging
The application of radiotracers has gained significant prominence in medicine, particularly as a well-established modality in the clinical management of breast cancer. This technique plays a crucial role primarily in staging, and monitoring therapeutic responses (Zhang-Yin, 2023). In the realm of molecular imaging, the use of the widely accessible radiotracer 18F-Fluorodeoxyglucose (18F-FDG), a glucose analogue employed in positron emission tomography (PET), presents a viable option for enhancing diagnostic accuracy and understanding metabolic processes (Robertson et al., 2009).
18F-fluorodeoxyglucose (18F-FDG) is a radiolabelled glucose analogue with a similar initial uptake mechanism to glucose. Following intravenous administration, 18F-FDG is internalised by cells through glucose transporters, predominantly Glucose Transporter Type 1 (GLUT1), and is subsequently phosphorylated by hexokinase to form 18F-FDG-6-phosphate (Vanhove et al., 2019). Unlike physiological glucose, 18F-FDG-6-phosphate is unable to progress through glycolysis due to the absence of the 2-hydroxyl moiety, leading to its metabolic entrapment within the cellular milieu. This phenomenon, referred to as “metabolic trapping,” results in a preferential accumulation of 18F-FDG in tissues characterised by elevated rates of glycolysis (Vanhove et al., 2019). Notably, breast cancer cells frequently demonstrate the Warburg effect, favouring aerobic glycolysis, which translates to significantly augmented glucose uptake compared to non-malignant tissues (Vanhove et al., 2019). Consequently, malignant breast tumours exhibit a concentration of 18F-FDG that is commensurate with their metabolic activity. Crucially, the uptake of 18F-FDG is associated with tumour aggressiveness and viability; more aggressive neoplasms (e.g., high-grade or triple negative subtypes) typically exhibit enhanced 18F-FDG uptake, whereas less aggressive tumours (such as lobular carcinoma) and very small lesions may manifest reduced uptake (Zhang-Yin, 2023). This underscores the biochemical rationale for employing 18F-FDG as a surrogate marker of tissue metabolism in oncologic imaging.
18F-FDG possesses a physical half-life of approximately 109.7 min (around 1.8 h) (Cunha, 2022). It undergoes decay via positron emission, resulting in the generation of two
Advancements in technology have facilitated the intraoperative use of 18F-FDG to enhance surgical decision-making, particularly within the context of breast-conserving surgery (BCS). BCS, also known as lumpectomy or wide local excision (WLE), necessitates the thorough excision of malignant breast tumours with clear surgical margins. Nonetheless, the attainment of negative margins presents a considerable challenge; historically, it has been documented that 20%–25% of patients undergoing BCS require re-excision due to positive margins (St John et al., 2017; Talsma et al., 2011; Jeevan et al., 2012; Pleijhuis et al., 2009). To address this issue, novel intraoperative imaging modalities have been developed to leverage the uptake of FDG within neoplastic tissue, allowing for real-time assessment of excised specimens. This enables surgeons to identify and resect any additional tissue that may be necessary. Among the innovative techniques emerging for intraoperative specimen imaging are Cerenkov Luminescence Imaging (CLI) (Grootendorst et al., 2016), Flexible Auto-Radiography (FAR) (Jurrius et al., 2021), and high-resolution intraoperative Positron Emission Tomography (PET). These methodologies allow surgeons to visualise residual FDG-avid disease within the excised tissue specimen during the surgical procedure, thereby significantly increasing the probability of achieving complete tumour resection in a single operative intervention.
Principles of cerenkov luminescence imaging (CLI)
Cerenkov radiation is the light emitted when a charged particle (such as a beta particle) travels through a dielectric medium (like water or tissue) at a speed greater than the speed of light in that medium (Robertson et al., 2009). In this situation, the particle polarises the surrounding molecules; once the particle passes, those polarised molecules relax back and release energy in the form of optical photons–the Cerenkov light (Grootendorst et al., 2016). The Russian scientist Pavel Cerenkov first documented this phenomenon in 1934, when he observed the emission of blue light emanating from a water vessel during radioactive decay (Das et al., 2014). Cerenkov Luminescence Imaging (CLI) is a molecular imaging modality that leverages this optical emission to visualise radiotracers in biological subjects. In CLI, no external light source is needed; instead, the patient or sample is injected with a beta-emitting radiotracer such as 18F-FDG, and the intrinsic Cerenkov light produced by the tracer’s decay is captured using sensitive optical cameras. This real-time imaging technique is employed within the imaging modality, incorporating the benefits of white light optical and PET imaging.
In a pioneering investigation, Grootendorst et al. (2016) first evaluated the application of CLI in BCS (Grootendorst et al., 2016). This study aimed to assess the efficacy of CLI in detecting margins positive for malignancy within lumpectomy specimens excised in women with breast cancer. Participants received an injection of 18F-FDG. The investigators analysed the capacity of CLI to identify FDG-avid tumour cells located at or in close proximity to the excised tissue margins, thereby facilitating immediate intraoperative re-excision when deemed necessary.
Grootendorst et al. initially investigated and substantiated the principle of utilising CLI on ten excised specimens by observing radioactivity within tumour cells. This was followed by an assessment of fifteen margins in twelve patients (Grootendorst et al., 2017). Prior to surgery, the patients received an intravenous injection of 18F-FDG at a dosage of approximately 150–230 MBq, administered 45–60 min before the procedure. The acquired images post-resection were systematically compared with histopathological assessments of margin status.
Principles of Flexible Auto-Radiography (FAR)
Flexible Auto-Radiography (FAR) is an innovative molecular imaging technique designed to visualise the distribution of radiopharmaceuticals, especially beta-emitting tracers such as 18F-FDG, on excised tissue specimens, with high spatial resolution and sensitivity (Jurrius et al., 2021). FAR indirectly detects scintillations generated from charged particles, such as positrons, interacting with a thin scintillating film. This innovative approach allows for a more precise localisation of radiopharmaceutical uptake, thereby improving the efficacy of diagnostic imaging and patient assessment in clinical settings. It is particularly well-suited for intraoperative surgical margin assessment in cancers such as breast cancer, where determining whether the tumour extends to the edge of the resected specimen is critical.
FAR was evaluated for intraoperative assessment of margins in patients undergoing WLE for invasive breast cancer in a pivotal first-in-human feasibility study by Jurrius et al. (2021). The Flexible Auto-Radiography system utilised a thin scintillating film detector in direct contact with the excised tissue, capturing beta particles emitted by 18F-FDG-labelled cancer cells. This proximity-based technique offers millimetre to sub-millimetre spatial resolution, far exceeding standard gamma-based approaches.
The prospective study included 66 patients, each receiving a preoperative injection of 18F-FDG (∼3 MBq/kg), with FAR performed intraoperatively on freshly excised lumpectomy specimens. Imaging was completed within 10 min of excision. FAR imaging was subsequently compared with corresponding histopathological margin status.
Cerenkov luminescence imaging combined with flexible auto-radiography (CLI-FAR)
Cerenkov Luminescence Imaging combined with Flexible Auto-Radiography (CLI-FAR) represents an innovative dual-modality imaging approach that synergistically integrates Cerenkov Luminescence Imaging (CLI) and Flexible Auto-Radiography (FAR). Both modalities have been evaluated for their efficacy in assessing the margins of BCS individually and combined.
Sinha et al. conducted a prospective, single-arm, interventional feasibility study that spanned 13 months at a single centre in the United Kingdom (Guy’s Hospital), aiming to evaluate the combined diagnostic accuracy of CLI-FAR using 18F-FDG to characterise tumour margins intraoperatively in women undergoing breast-conserving surgery (BCS) (Sinha et al., 2024). In the study patients received a dose of 250 MBq (±10%) of 18F-FDG intravenously approximately 145 min before the anticipated intraoperative imaging. After inducing anaesthesia, tumour excision was performed using either a scalpel or low-energy diathermy. The orientation of the excised specimens was standardised through the application of sutures and clips. All specimens underwent initial imaging via three-dimensional intraoperative X-ray. CLI-FAR imaging was deployed to margins suspected of involvement; in the absence of suspicion, imaging was performed on the two margins deemed closest to the tumour. All intraoperative findings derived from CLI-FAR imaging were reviewed on each occasion by one of seven trained consultant surgeons and acted upon contemporaneously to excise further tissue if suspected to be positive with CLI-FAR.
Histopathologists who were blinded to the results of intraoperative optical imaging evaluated the excised specimens and reported margin status, tumour type and grade, receptor status, the presence or absence of lympho-vascular invasion, nodal status, and, where relevant, the response to neoadjuvant chemotherapy. The primary aim was to assess the diagnostic performance of this technique by correlating intraoperative imaging findings with standard histopathological analysis.
The LightPath® system
Both imaging techniques CLI and FAR are acquired utilising the LightPath® system (Lightpoint Medical Ltd., United Kingdom), an in vitro diagnostic apparatus that identifies the location and distribution of positron-emitting radionuclides within excised surgical specimens (see Figure 1). This system is a device featuring an ultrasensitive camera capable of detecting emitted activity within the 550–850 nm wavelength range. A PET imaging agent, which is preferentially absorbed by malignant cells compared to healthy tissues, is required for imaging purposes, such as 18F-FDG. As a result, the tumour emits beta particles at a greater rate and intensity, allowing differentiation from the adjacent healthy breast tissue. The LightPath® Imaging System can detect these beta particles directly by detecting Cerenkov luminescence or by assessing scintillations activated by charged particles exciting a scintillator using FAR.
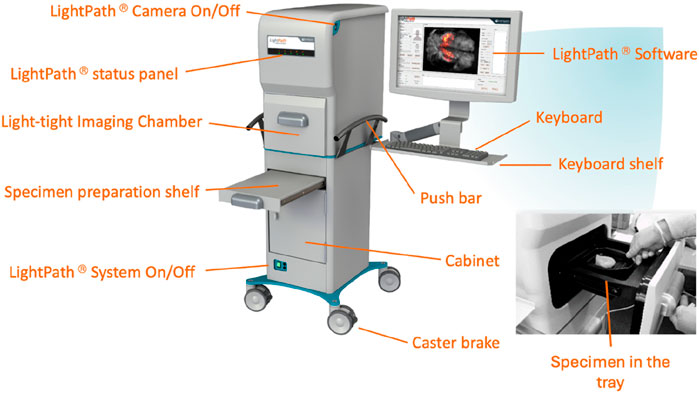
Figure 1. The LightPath® System with labels to identify parts of the system. The Light-tight Image Chamber is where the specimen is placed in a tray, which is demonstrated on the lower right side of the diagram above. The system is controlled by the keyboard and the images are visible on the LightPath Software on the screen.
The LightPath® Imaging System comprises a fully light-tight specimen chamber. It is equipped with an electron-multiplying charge-coupled device (EMCCD) camera (Andor iXon Ultra 897, Andor Technology Plc), a camera designated for white light imaging, a sample stage, and a white light source. The specimen may be covered with a 12-µm scintillator and a Mylar sheet for Flexible Auto-Radiography (FAR) or uncovered for Cerenkov Luminescence Imaging (CLI). Once the specimen is appropriately positioned within the light-tight enclosure, it is illuminated using the white light source. Subsequently, the white light source is disabled to allow the EMCCD camera to commence the detection of the LightPath® Image.
The electron-multiplying charge-coupled device (EMCCD) camera, along with the reference (white light) camera, constitutes the core of the LightPath® system. The EMCCD camera serves the purpose of detecting optical Cerenkov photons or scintillation photons generated by the radiopharmaceutical, thereby effectively facilitating the LightPath® in discerning the radiopharmaceutical distribution within excised tissue specimens. Both imaging modalities possess an acquisition performed over 300 s using 8 × 8-pixel binning.
The CLI images were acquired using a technique that employs non-invasive imaging to visualise tissues labelled with the 18F-FDG radiotracer, as illustrated in Figure 2. The ultra-sensitive camera of the LightPath® system detects emitted light and generates an image of the tissue. During the execution of FAR, a scintillator measuring 12 μm in thickness was affixed to the WLE. Additionally, a 3-μm mylar sheet was interposed between the specimen and the scintillator to prevent contamination of the scintillator, as illustrated in Figure 2.
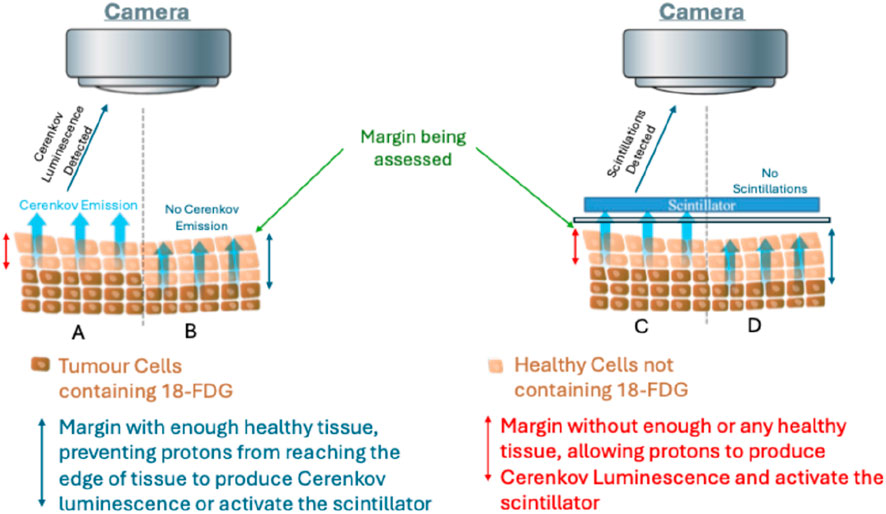
Figure 2. Schematic representation of proton-induced Cerenkov luminescence Imaging (CLI) and scintillation for Flexible Auto-Radiography (FAR) for intraoperative margin assessment. Panels A and B illustrate Cerenkov luminescence detection, where tumour cells containing 18-FDG are shown adjacent to healthy tissue. In Panel (A) (positive margin), insufficient or absent healthy tissue allows protons to reach the specimen surface, producing detectable Cerenkov luminescence. In Panel (B) (negative margin), the presence of more than 1 mm of healthy tissue prevents protons from reaching the edge, thereby blocking Cerenkov emission. Panels (C,D) depict scintillation detection, in which an external scintillator is placed over the tissue. In Panel (C) (positive margin), inadequate healthy tissue allows protons to activate the scintillator, producing detectable scintillations. In Panel (D) (negative margin), sufficient healthy tissue (>1 mm) prevents protons from reaching and activating the scintillator, resulting in no detectable scintillations. The cell distribution in this schematic is not drawn to scale and does not represent the true number or arrangement of tumour versus healthy cells, but is provided for illustrative purposes only. Additionally, only a portion of the tissue specimen is represented to demonstrate the principle of margin assessment.
Each margin must be imaged individually, given that the camera can solely capture a two-dimensional image at any given instance. The specimen must be positioned in the tray so that the margin is oriented towards the camera (image taken in cranio-caudal orientation with the margin of interest facing upwards).
The application of a scintillator for FAR provides the notable advantage of ensuring that only charged particles are capable of generating scintillations, thereby eliminating any diathermy artifacts associated with FAR. To ascertain activity within a designated wavelength spectrum of 550 nm ±10%, a band path filter is employed; this is necessary as the scintillator film generates scintillations within a restricted wavelength range.
Specimen 3D PET-CT imaging
The XEOS AURA 10 is the first and currently the only mobile PET/CT specimen imager designed for use in the operating room for intra-operative specimen imaging. It combines a high-sensitivity PET detector with a built-in CT to provide 3D tomographic images of the removed tissue, with sub-millimetre spatial resolution. Within minutes of excision, the specimen (e.g., a lumpectomy sample) is placed into the device’s receptacle, and a rapid PET/CT scan is performed. As a result of the small field of view and dedicated design, the system achieves roughly a five-fold higher spatial resolution than conventional whole-body PET. In practice, this means even sub-centimetre foci of residual FDG-avid tumour at the specimen margins can be detected in three dimensions. The co-registered CT scan provides an anatomical reference, allowing precise localisation of hot spots within the tissue. AURA 10 produces a detailed 3D image in under 10 min after specimen removal, giving near-real-time feedback to the surgeon. Early studies have shown that such intraoperative PET imaging is feasible and improves margin assessment (Rovera et al., 2023). For instance, Göker et al. (2020) demonstrated the use of an FDG “micro-PET/CT” on lumpectomy specimens, successfully identifying involved margins that warranted immediate additional resection (Göker et al., 2020).
The XEOS AURA 10 system has been utilised by Labert et al. to demonstrate the feasibility of employing this advanced imaging system intraoperatively during multi-organ resections. These procedures encompass a range of malignancies, including breast cancer, thyroid cancer, transitional cell carcinoma, renal cell carcinoma, prostate cancer, and skin cancer (Lambert et al., 2025).
Notably, Göker et al. initiated a proof-of-concept study involving twenty patients with early-stage breast cancer who underwent breast-conserving surgery (BCS). Participants received an intravenous injection of 4 MBq/kg of 18F-FDG approximately 30 min before the operation. Post-surgical excision, resected specimens were intraoperatively imaged using high-resolution micro-PET (β-CUBE) and micro-CT (X-CUBE) scanners provided by MOLECUBES. The PET/CT images were reconstructed and analysed utilising a supervised automated algorithm to segment regions of elevated 18F-FDG uptake, thus assessing margin status based on the spatial relationship between tumour signals and specimen boundaries. Surgeons, blinded to histopathological outcomes, independently interpreted the PET/CT images to evaluate margin status, with comparative results aligned against histopathological findings, which served as the gold standard.
Between June 2017 and June 2022, De Crem et al., alongside Göker, prospectively enrolled forty-one patients with early-stage breast cancer undergoing BCS to assess the feasibility of intraoperative micro-PET/CT imaging for margin evaluation (Göker et al., 2020; Crem et al., 2024). Following surgical excision, resected breast specimens were imaged using the high-resolution β-CUBE (micro-PET) and X-CUBE (micro-CT) systems, which were subsequently integrated into the AURA 10 device for intraoperative application. These imaging modalities facilitated three-dimensional visualisation of metabolic activity and anatomical details in real-time to ascertain margin status. The primary objective was to evaluate the diagnostic performance of this technique by contrasting intraoperative imaging findings with standard histopathological analysis.
Lambert et al. conducted margin assessment solely with the XEOS AURA 10, evaluating seven breast specimens intraoperatively (Lambert et al., 2025). Their primary aim was to assess the diagnostic performance of this technique by evaluating intraoperative imaging findings with standard histopathological analysis.
Materials and methods
This comprehensive review delineates an extensive overview of imaging methodologies employed across a broad spectrum of research designs, encompassing proof-of-concept observational studies, feasibility assessments, and interventional clinical trials.
This review primarily examines studies on patients undergoing breast-conserving surgery (BCS). The selection criteria for the included studies that highlight the use of advanced imaging techniques such as CLI, FAR, combined CLI-FAR and three-dimensional (3D) PET specimen imaging modalities. Studied using the LightPoint system or the XEOS AURA 10 system for 3D imaging were included.
A systematic literature search was conducted across multiple reputable databases, including PubMed and Cochrane Library, employing a comprehensive search strategy tailored to identify pertinent studies. The search focused on publications that investigated the application of CLI, FAR, and CLI-FAR using the LightPoint system or the XEOS AURA 10 system within the context of breast-conserving surgery. The search terms incorporated keywords such as “breast conserving surgery,” “CLI,” “FAR,” “CLI-FAR,” “XEOS AURA 10,” and “3D imaging,” among others. The identification process yielded a total of five studies that met the inclusion criteria.
In terms of study selection, three peer-reviewed articles were identified for their investigation into CLI, FAR, and the combined CLI-FAR imaging modalities. These studies were conducted by Grootendorst et al., Jurrius et al., and Sinha et al., each contributing valuable insights into the clinical applicability and performance of these imaging techniques in BCS. Additionally, two studies focusing on the application of the XEOS AURA 10 system for 3D imaging in breast tissue samples and intraoperative settings were included. These were conducted by De Crem et al. and Lambert et al., and provided critical evaluation of the system’s efficacy in surgical scenarios.
Results
Several key studies have investigated the use of intraoperative radiotracer-based specimen imaging across various devices and methodologies. The results of these are shown in Table 1.
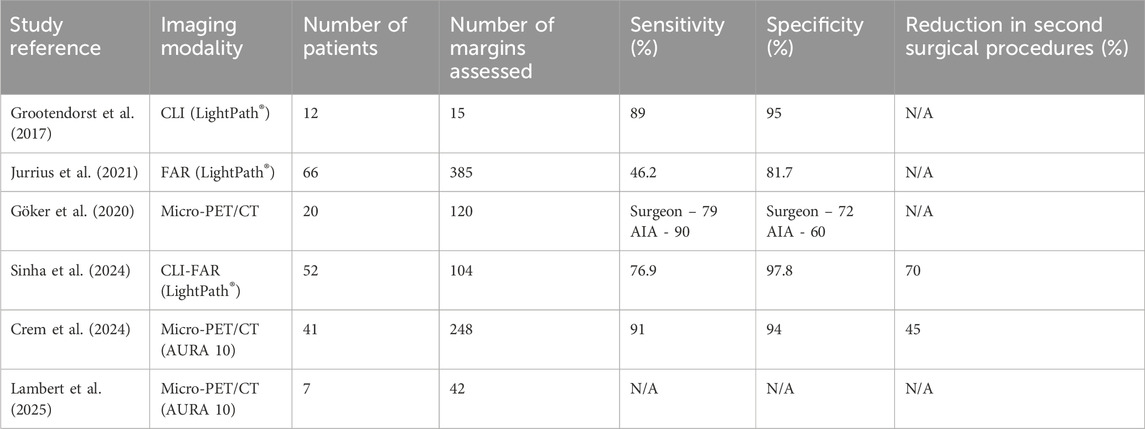
Table 1. Key Studies using intraoperative radiotracer-based specimen imaging. (AIA–Automated Image Analysis).
Grootendorst et al. (2017) conducted a study involving an initial cohort of 10 patients aimed at optimising the imaging protocol (Jurrius et al., 2021). Subsequently, an additional 12 patients were incorporated into the analytical dataset. The imaging modality, CLI, successfully identified elevated tumour radiance in 10 out of these 12 patients. The quantitative analysis yielded a mean radiance measurement of 560 ± 160 photons/s/cm2/sr, accompanied by a tumour-to-background ratio of 2.41 ± 0.54. Furthermore, all 15 evaluable surgical margins were confirmed to be clear by both CLI and histopathological assessment. Notably, the concordance between margin distances appraised by CLI and those ascertained through histopathology demonstrated a robust agreement, as evidenced by a kappa statistic of 0.81. Additionally, the interrater reliability among surgeons interpreting CLI images was found to be high, achieving a kappa value of 0.912.
Göker et al. (2020) evaluated the MOLECUBES b-CUBE/X-CUBE micro-PET/CT system in a cohort of patients who underwent breast-conserving surgery (Göker et al., 2020). Their findings revealed a high sensitivity of 90% but moderate specificity of 60% using automated image analysis, with an area under the receiver operating characteristic curve (AUC) of 0.86. Surgeon interpretation yielded slightly lower but still robust sensitivity of 79% and specificity of 72%. FDG uptake was visualised clearly in all excised tumour specimens. Jurrius et al. (2021) reported on the use of Flexible Auto-Radiography (FAR) alone. This multicenter study showed an overall diagnostic accuracy of 80.5%, with a sensitivity of 46.2% and specificity of 81.7%. Notably, in a high-activity subgroup, FAR achieved a sensitivity of 71.4% and a negative predictive value of 98.4%. The mean radiation dose to staff during these procedures was approximately 38 µSv, which was within safe limits.
Sinha et al. (2024) investigated the dual-modality technique of Cerenkov Luminescence Imaging and Flexible Auto-Radiography (CLI-FAR) (Sinha et al., 2024). The study achieved a sensitivity of 76.9% and a specificity of 97.8% in detecting positive surgical margins, significantly reducing the reoperation rate by 69%. The average time for image interpretation was 6 min, demonstrating its feasibility for use during live surgical procedures without delaying operative workflow.
The BIMAP study (2024), conducted in Belgium using the XEOS AURA 10 PET/CT system, demonstrated a strong correlation between intraoperative imaging results and final histopathological margin assessment in cases of invasive ductal carcinoma (IDC) (Crem et al., 2024). This study reported that the estimated number needed to treat (NNT) to prevent one reoperation was four, supporting both the diagnostic accuracy and clinical utility of the technique in a general hospital setting.
Finally, Lambert et al. (2025) reviewed the practical implementation of the AURA 10 system in a general hospital setting without on-site PET/CT facilities (Lambert et al., 2025). Of the 32 surgical procedures, including seven for breast carcinoma, all PET/CT scans were completed intraoperatively with a median staff radiation dose of approximately 15 µSv per procedure. The imaging protocol used low levels of radiotracer activity and yielded high-quality, diagnostically useful images.
Discussion
Intra-operative assessment of surgical margins in BCS remains a critical determinant in mitigating reoperation rates. Multiple studies utilising 18F-FDG imaging modalities, such as CLI, FAR, and PET/CT systems - including Lightpath, XEOS AURA 10, β-CUBE, and X-CUBE - have accrued substantial evidence supporting their clinical efficacy (Zhang-Yin, 2023; Sinha et al., 2024; Grootendorst et al., 2016; Jurrius et al., 2021; Göker et al., 2020; Lambert et al., 2025; Crem et al., 2024).
Data from a multicentre investigation into FAR utilising 18F-FDG exhibited an overall accuracy of 80.5%, with a sensitivity of 46.2% and a specificity of 81.7% in detecting invasive carcinoma and ductal carcinoma in situ (DCIS) (Jurrius et al., 2021). Notably, integrating FAR with CLI for CLI-FAR imaging enhanced sensitivity to 76.9% and specificity to 97.8%, significantly decreasing reoperation rates by 69%.
Further proof-of-concept and first-in-human studies corroborated the diagnostic potential of utilising 18F-FDG imaging modalities. Goker et al. reported an automated image analysis area under the curve (AUC) of 0.86, while surgeon-led assessments achieved sensitivities of up to 79% and specificities of 72%. The modality was recognised for its rapid interpretation (approximately 1 min) and its seamless integration into the surgical workflow, minimising delays (Göker et al., 2020).
The XEOS AURA 10 device, which combines micro-PET and micro-CT for intra-operative evaluation of resection margins, demonstrated high diagnostic performance in a cohort of 41 patients, particularly in cases of invasive ductal carcinoma (IDC), with sensitivities and specificities of 91% and 94%, respectively (Crem et al., 2024). This capability to identify positive margins enabled four patients to avoid additional surgical interventions.
A significant advantage of assessing margins via mobile radiotracers lies in their capacity to detect metabolic activity, rather than relying solely on anatomical landmarks. This molecular perspective is pivotal in recognising microscopic residual disease that may evade detection through physical palpation or conventional imaging techniques. In contrast to traditional methods such as specimen radiography or frozen section analysis, intraoperative PET/CT provides a three-dimensional, volumetric assessment of excised tissue, alongside quantifiable radiotracer uptake values.
These approaches have been successfully applied in clinical settings without the requirement of adapting theatres with nuclear medicine infrastructure, emphasising the technology’s portability and practicality. Studies conducted by Jurrius et al. and Grootendorst et al. indicated relatively low radiation exposure levels for theatre personnel and the administration of small tracer doses, further supported the safety profile of these techniques (Grootendorst et al., 2016; Jurrius et al., 2021).
Nonetheless, certain limitations persist. CLI and FAR methodologies inherently face challenges in assessing the presence of positive margins. Specifically, chemiluminescence presents considerable constraints in CLI, necessitating substantial training and expertise to effectively differentiate it from radioactivity. Sinha et al. propose the utilisation of a standardised scintillator for FAR imaging, capable of accommodating specimens up to a 4 cm maximum size (Sinha et al., 2024). However, with the growing frequency of oncoplastic surgical procedures, specimens frequently exceed 4 cm in diameter. In response to this trend, larger scintillators have become increasingly available.
Additionally, FDG-PET demonstrates diminished efficacy in tumours characterised by low intrinsic glucose metabolism, such as invasive lobular carcinoma and in situ lesions, which may result in false-negative findings (Lambert et al., 2025). Although the spatial resolution of PET is considered high by typical standards, it may still constrain the ability to accurately delineate margins in very small or multifocal tumours. Furthermore, there exists a challenge in consistently synchronising the timing of injection and imaging procedures to ensure they occur within an optimal temporal window. Grootendorst et al. (2017) reported that patients received approximately 5 MBq/kg of 18F-FDG 45–60 min before surgical excision, with a mean interval of 86 ± 26 min between injection and excision. Specimens were imaged immediately after excision. Similarly, Jurrius et al. (2021) indicated that intraoperative Flexible Auto-Radiography (FAR) imaging was performed within 60–180 min post-injection. Subgroup analyses using an injection-to-imaging threshold of about 158 min and stratification based on decay-corrected 18F-FDG activity (∼97 MBq) did not show significant differences in imaging performance. A target interval of 60–120 min is generally recommended; however, acceptable results can be achieved up to approximately 180 min post-injection, depending on residual activity levels and logistical factors. Göker et al. and DeCrem et al. do not specify the interval between injection and imaging. Lambert et al. indicate that the injection was administered 60–90 min prior to tumour resection and imaging. Sinha et al. standardised image intensity across patients by administering a uniform dose of 250 MBq (±10%) of 18F-FDG, approximately 145 min before intraoperative imaging, to normalise imaging intensity.
Moreover, the interpretation of PET/CT imagery necessitates a learning curve, emphasising the importance of multidisciplinary collaboration between nuclear medicine, surgical teams, and pathology for optimal integration and patient outcomes. An emerging advancement in this field would be to assess whether diagnostic accuracy can be enhanced by involving radiologists or nuclear medicine physicians in image interpretation. Although this approach may require additional coordination and potentially introduce delays, it represents a promising direction for future research.
Conclusion
Intra-operative specimen imaging utilising radiotracers, particularly with devices such as the LightPoint machine employing CLI and FAR or the XEOS AURA 10, presents a rapid, viable, and efficacious methodology for margin assessment during BCS. This technique yields imaging results that exhibit a robust correlation with histopathological standards and has the potential to significantly diminish re-excision rates when employed within real-time intra-operative decision-making protocols.
Nonetheless, challenges remain in assessing tumours characterised by low metabolic activity and larger dimensions. Rigorous training, refinement of interpretative algorithms, and the development of tailored imaging protocols may enhance diagnostic reliability.
In summary, these findings provide compelling evidence for further integrating intra-operative specimen imaging utilising radiotracers into breast cancer surgical workflows, highlighting its value as a beneficial tool for improving oncological outcomes and mitigating patient burden.
Author contributions
AS: Data curation, Conceptualization, Writing – review and editing, Formal Analysis, Writing – original draft. AP: Supervision, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Guy’s and St Thomas Charity and the ECMC (CRUK and NIHR). The UK sites receive support from Cancer Research UK and Department of Health as Experimental Cancer Medicine Centres. Cancer Research UK Award ECMCQQR-2022/10005 new award for ECMC since March 2023 to March 2028.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Crem, A. S. D., Tummers, P., Depypere, H., Braems, G., Salihi, R., Vergauwen, G., et al. (2024). 89P BIMAP: breast cancer intra-operative margin assessment using PET-CT. ESMO Open 9, 103161. doi:10.1016/j.esmoop.2024.103161
Cunha, J. P. (2022). Fludeoxyglucose F 18 injection (FDG): side effects, uses, dosage, interactions, warnings. Available online at: https://www.rxlist.com/fludeoxyglucose-drug.htm (Accessed April 19, 2025).
Das, S., Thorek, D. L. J., and Grimm, J. (2014). Cerenkov imaging. Adv. Cancer Res. 124, 213–234. doi:10.1016/B978-0-12-411638-2.00006-9
Göker, M., Marcinkowski, R., Van Bockstal, M., Keereman, V., Van Holen, R., Van Dorpe, J., et al. (2020). 18F-FDG micro-PET/CT for intra-operative margin assessment during breast-conserving surgery. Acta Chir. Belg. 120 (5), 366–374. doi:10.1080/00015458.2020.1774163
Grootendorst, M. R., Cariati, M., Kothari, A., Tuch, D. S., and Purushotham, A. (2016). Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin. Transl. Imaging 4, 353–366. doi:10.1007/s40336-016-0183-x
Grootendorst, M. R., Cariati, M., Pinder, S. E., Kothari, A., Douek, M., Kovacs, T., et al. (2017). Intraoperative assessment of tumor resection margins in breast-conserving surgery using 18F-FDG cerenkov luminescence imaging: a first-in-human feasibility study. J. Nucl. Med. 58 (6), 891–898. doi:10.2967/jnumed.116.181032
Jeevan, R., Cromwell, D. A., Trivella, M., Lawrence, G., Kearins, O., Pereira, J., et al. (2012). Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ (Online) 345 (7869), e4505. doi:10.1136/bmj.e4505
Jurrius, P. A. G. T., Grootendorst, M. R., Krotewicz, M., Cariati, M., Kothari, A., Patani, N., et al. (2021). Intraoperative [18F]FDG flexible autoradiography for tumour margin assessment in breast-conserving surgery: a first-in-human multicentre feasibility study. EJNMMI Res. 11 (1), 28. doi:10.1186/s13550-021-00759-w
Lambert, B., Vergucht, V., Dekeyser, S., De Craene, A., Ameye, F., Van Den Bossche, B., et al. (2025). Feasibility study on the implementation of a mobile high-resolution PET/CT scanner for surgical specimens: exploring clinical applications and practical considerations. Eur. J. Nucl. Med. Mol. Imaging 52, 2979–2994. doi:10.1007/s00259-025-07143-z
Pleijhuis, R. G., Graafland, M., De Vries, J., Bart, J., De Jong, J. S., and Van Dam, G. M. (2009). Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann. Surg. Oncol. 16 (10), 2717–2730. doi:10.1245/s10434-009-0609-z
Robertson, R., Germanos, M. S., Li, C., Mitchell, G. S., Cherry, S. R., and Silva, M. D. (2009). Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 54 (16), N355–N365. doi:10.1088/0031-9155/54/16/n01
Rovera, G., Grimaldi, S., Oderda, M., Finessi, M., Giannini, V., Passera, R., et al. (2023). Machine learning CT-based automatic nodal segmentation and PET semi-quantification of intraoperative 68Ga-PSMA-11 PET/CT images in high-risk prostate cancer: a pilot study. Diagnostics 13 (18), 3013. doi:10.3390/diagnostics13183013
Sinha, A., Peterson, Z., Shifa, B., Jeffery, H., Jurrius, P., Allen, S., et al. (2024). Cerenkov luminescence imaging and flexible autoradiography for specimen margin assessment during breast-conserving cancer surgery. Radiol. Adv. 1 (2), umae015. doi:10.1093/radadv/umae015
St John, E. R., Al-Khudairi, R., Ashrafian, H., Athanasiou, T., Takats, Z., Hadjiminas, D. J., et al. (2017). Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery. Ann. Surg. 265 (2), 300–310. doi:10.1097/sla.0000000000001897
Talsma, A. K., Reedijk, A. M. J., Damhuis, R. A. M., Westenend, P. J., and Vles, W. J. (2011). Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch hospitals. Eur. J. Surg. Oncol. 37 (4), 357–363. doi:10.1016/j.ejso.2011.01.008
Vanhove, K., Thomeer, M., Derveaux, E., Shkedy, Z., Owokotomo, O. E., Adriaensens, P., et al. (2019). Correlations between the metabolic profile and 18F-FDG-Positron Emission Tomography-Computed Tomography parameters reveal the complexity of the metabolic reprogramming within lung cancer patients. Sci. Rep. 9 (1), 16212–11. doi:10.1038/s41598-019-52667-8
Keywords: breast cancer, breast concerving therapy, oncology, re-excision, radionucleotides, breast surgeries
Citation: Sinha A and Purushotham A (2025) A review on the use of radionuclide imaging techniques to detect margin positivity in intraoperative specimens during breast-conserving surgery. Front. Med. Eng. 3:1628589. doi: 10.3389/fmede.2025.1628589
Received: 14 May 2025; Accepted: 25 September 2025;
Published: 14 October 2025.
Edited by:
James Michaelson, Harvard University, United StatesReviewed by:
Michael A. Lynes, University of Connecticut, United StatesJames Weaver, Massachusetts Institute of Technology, United States
Copyright © 2025 Sinha and Purushotham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnie Purushotham, YXJuaWUucHVydXNob3RoYW1Aa2NsLmFjLnVr
 Aaditya Sinha
Aaditya Sinha Arnie Purushotham*
Arnie Purushotham*