- 1Department of Pharmacy, Tianjin First Central Hospital, Nankai University, Tianjin, China
- 2College of Chemistry, Nankai University, Tianjin, China
The administered infusion solution is a sterile preparation that can be used directly for intravenous infusion in patients by mixing one or more intravenous drugs using aseptic operation technology. The pharmacy intravenous admixture service (PIVAS) center is a professional technical service department in hospitals, where the majority of inpatient-administered infusion solutions are prepared. During the processes of dissolution, dilution, preparation, storage, and use of intravenous drugs, the quality control of the administered infusion solution can be affected by various factors. At present, there are no relevant standards or guidance documents for the quality control of administered infusion solutions. Cytotoxic drugs are still the main treatment option for cancer patients and are mainly prepared in PIVAS centers in most hospitals. In this study, we mainly focused on the quality control of cytotoxic drug-administered infusion solutions and explored associated factors (diluent, container, concentration, temperature, and light), physical stability (visual appearance, pH, osmolality, and particulate matter), chemical stability (content), and biological stability (sterility). Most of the studies reviewed in this paper have insufficient data on the related factors and physicochemical stability of the administered infusion solutions. Research on the sterility of administered infusion solutions is particularly limited, with only one article addressing this aspect. Ensuring the quality of cytotoxic drug-administered infusion solutions is vital for the safe administration of drugs to cancer patients, so it is very important to enhance associated research. This article summarized the relevant literature on the quality control of cytotoxic drug-administered infusion solutions and provided a reference for safer and more efficient use of these drugs in clinical practice.
1 Introduction
Intravenous infusion is one of the most important treatments and is commonly used in clinical practice, particularly for hospitalized patients. In 2020, the intravenous infusion rate of inpatients in secondary and higher-level hospitals was 86.10% in China (1). The utilization rate of venous infusion in inpatients is relatively high. According to the Annual Report of National Adverse Drug Reaction Monitoring (2022) in China, adverse drug reactions (ADRs) related to intravenous administration accounted for 57.8% (2). Oral administration exhibits highly variable pharmacokinetics. In order to increase bioavailability, intravenous administration is required in clinical practice. The pharmacy intravenous admixture service (PIVAS) center is a professional technical service department in hospitals that provides high-quality intravenous infusion to ensure safety and effectiveness. PIVAS centers are equipped with clean dispensing environments, advanced dispensing technologies, and professional medication order review systems, where pharmacists participate in the whole process. The PIVAS workflow is complex and mainly involves the following steps: the clinician prescribes intravenous therapy→ order information transfer→ receipt of PIVAS workstation→ the pharmacist reviews the order→ print label→ dispensing→ dispensing check→ mixing→ administered infusion solution check→ administered infusion solution packaging→ place in closed containers and lock→ sent to the ward. In order to avoid medication errors and decrease ADRs related to infusion, the PIVAS center incorporates two important steps: dispensing check and administered infusion solution check.
An administered infusion solution is a mixture of one or more types of intravenous drugs prepared using aseptic operation technology (to prevent bacteria from entering the human body or other objects), which can be directly used for intravenous infusion in patients in clinical settings. The administered infusion solution check is the last step in the PIVAS workflow, where the solution is verified according to specific standards for different drugs. This check includes examining the appearance of the infusion bag or bottle for cleanliness or leaks; assessing whether the administered infusion solution shows signs of discoloration, turbidity, precipitation, or crystallization; confirming whether the basic infusion and empty bottle match the drug name, specification, and dosage indicated on the label; and checking the rationality of drug compatibility and the suitability of the drug dosage. The visual appearance of the administered infusion solution is mainly considered. However, the time and conditions of storage will directly affect the quality of the administered infusion solution. In practice, more detailed information is often required about the administered infusion solution; for example, fluorouracil may need to be administered for several days to maintain steady plasma concentrations (3). Furthermore, some drugs such as monoclonal antibodies are very expensive, and in some special cases, their administered infusion solution cannot be used in a timely manner. However, the lack of stability data leads to drug waste. Thus, there is a compelling need for more comprehensive data on the practical use of administered infusion solutions (4).
Cancer remains a major public health concern in China, according to the latest report by the National Cancer Center (NCC) of China (5). Although targeted and monoclonal antibody drugs have been used in the treatment of tumors in recent years, cytotoxic drug-mediated chemotherapy is still the mainstream approach for tumor treatment. Cytotoxic agents not only target tumor cells but also damage healthy cells; for pharmacists and nurses, long-term exposure to these cytotoxic agents will cause certain damage to the normal body (6). To avoid occupational exposure to cytotoxic drugs, their reconstitution and preparation take place in PIVAS centers under strict aseptic conditions and were finally sent to the ward. Considering the pharmacokinetic/pharmacodynamic (PK/PD) variability and therapeutic index of cytotoxic drugs, they are used in a variety of ways to meet different clinical needs, such as outpatient transportations, implantable devices, venous pumping, intravenous infusion, intravenous injections, and ambulatory home chemotherapy (7, 8). The duration of the administered infusion solutions is often prolonged, even extending to several days; for example, ifosfamide may be administered as a continuous home-based infusion over 14 days for the treatment of soft tissue sarcoma (7). The physicochemical stability and sterility of cytotoxic drug-administered infusion solutions, which are crucial for ensuring their safety and effectiveness in clinical use, have been questioned (9).
The administered infusion solution check is a vital step in the PIVAS workflow and serves as a guarantee for drug safety. However, there is no associated report emphasizing and summarizing the aspects of quality control. A European conference consensus addressed the practical stability of post-dilution or post-reconstitution but did not define the technical term “administered infusion solution,” which has been used for several years in China (9, 10). There are many factors that affect the quality of administered infusion solutions, such as diluent, concentration, container, temperature, and duration of storage. Quality control mainly includes the assessment of physical stability (visual appearance, pH, osmolality, and particle formation), chemical stability (content), and sterility (microbiology and endotoxin). In this context, we collected the data related to cytotoxic drug-administered infusion solutions. This paper mainly evaluates the stability and sterility of cytotoxic drugs in administered infusion solutions. The findings provide an important reference to evaluate the quality control of administered infusion solutions.
2 Factors
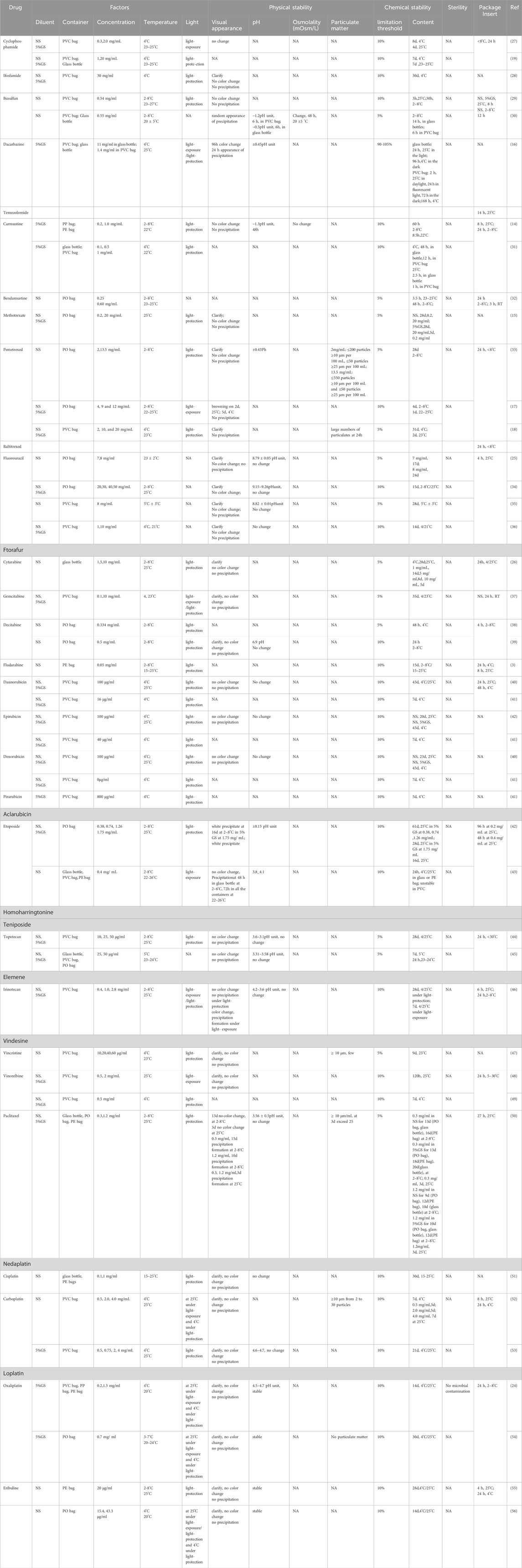
Cytotoxic drugs commonly used in clinical practice were selected from the medical insurance catalog in China. Intravenous administration includes intravenous injection and intravenous infusion. This paper mainly focuses on cytotoxic drugs administered by intravenous infusion, and injectable drugs such as Bleomycin and azacitidine are excluded. The stability of a single drug in a single solvent was screened, while the stability of multi-drug mixtures was not within the scope of this study. The quality control of administered infusion solutions includes the physical stability, chemical stability, and biological stability. As there is no specific standard to investigate the quality of administered infusion solutions, we mainly refer to the criteria of injection stability in the 2020 Chinese Pharmacopoeia. These criteria mainly include assessments of visual appearance, particulate matter, pH, content, and sterility. All results are presented in Table 1.
2.1 Diluent
For continuous venous infusion, the drug is diluted in a suitable injection solution. The pH value and electrolyte composition of the diluent are important considerations. Therefore, under normal circumstances, diluents with a pH similar to that of the drugs are usually selected for dissolution and dilution. The diluent listed in the drug instructions should be used, and non-electrolyte solvents should be selected for drugs sensitive to electrolytes (11). The commonly used diluents for infusion solutions include 5% and 10% dextrose (5% and 10% GS), 5% glucose sodium chloride, and 0.9% sodium chloride (NS). Most studies included in our analysis chose NS and 5% GS as solvents to evaluate the stability and quality of administered infusion solutions.
2.2 Container
The containers for infusion solutions are often made of glass or plastic, such as bottles, cassettes, syringes, or bags. Plastic containers offer several advantages, such as small size, lightweight, ease of storage and shipping, and being unbreakable. These advantages have led to plastic gradually replacing glass bottles as the main material for infusion solution containers. At present, the plastic bags used in the pharmaceutical industry, at home and abroad, are made of polyvinyl chloride (PVC), polyethylene (PE), polypropylene (PP), polyolefin (PO), and ethylene–vinyl acetate (EVA). PVC bags contain the plasticizer di-(2-ethylhexyl) phthalate (DEHP) to make them soft and flexible, but leaching of harmful substances like DEHP into the solutions has been reported (12). Carmustine has been reported to adsorb onto PVC and EVA surfaces but not onto PE or glass containers, making it suitable only for PVC-free containers like PE or PP bags (13, 14). So, new types of infusion bags made from materials such as PP and PE supersede the PVC as the main material. In our paper, we list the containers used for the quality control of administered infusion solutions. Many articles were published years ago on the use of PVC bags, so we include some recent studies in our research. However, the available literature in this area remains relatively scarce.
2.3 Concentration
Concentration is crucial for cytotoxic drugs as the clinically relevant dose intervals range from tens of milligrams to a several grams, and therefore, diluted solutions are prepared in a wide range of concentrations. Some cytotoxic drugs have different indications at different doses; for example, high doses of methotrexate are used for breast cancer and head and neck cancer, while low doses are used for autoimmune diseases. Different concentrations also affect the stability of the administered infusion solution. For example, high concentrations of methotrexate (20 mg/mL) can be stored at room temperature for 28 days, whereas low concentrations of methotrexate (0.2 mg/mL) can only be stored for 3 days under the same conditions (15). We list concentration as the one of the factors influencing the quality of cytotoxic drug-administered infusion solutions.
2.4 Temperature
Temperature is one of the main factors affecting the stability of administered infusion solutions. In clinical practice, two temperature conditions, namely, room temperature (RT, 22°C–25°C, 25°C ± 2°C, and 25°C) and refrigeration (2°C–8°C, 4°C ± 3°C, and 4°C), were used for storing ready-to-use solutions.
2.5 Light
Light is the most important factor influencing the stability of the drug. Sunlight can have a dramatic effect on the stability of diluted solutions in polypropylene containers. Light protection is important for many cytotoxic drugs; for example, methotrexate and dacarbazine should be protected from direct sunlight during storage (16). We considered the effect of light on the physicochemical stability of administered infusion solutions, and most articles reviewed in our research studied this aspect.
3 Cytotoxic drug-administered infusion solution stability
The stability of injection includes physical stability, chemical stability, and biological stability. There is no relevant standard for the stability of administered infusion solutions, so we chose the standard of injection stability from the 2020 Chinese Pharmacopoeia as a reference to evaluate their quality.
3.1 Physical stability
Physical stability generally refers to changes in the physical properties, mainly including visual appearance, pH, osmolality, and particulate matter (Table 1).
3.1.1 Visual appearance
Visual appearance includes solution color, turbidity, and precipitation, and it is the first step in the quality control of administered infusion solutions. Pharmacists and nurses can directly observe the changes to ensure drug safety. The administered infusion solution shall be visually inspected under natural light, and there are no foreign bodies such as glass chips, fibers with length >2 mm, lumps with maximum particle size >2 mm, and unshaken particles. Although the chemical content is within limits, its color has changed, or some precipitation has formed in the long-term storage (17, 18). Therefore, visual appearance is important for the quality control of cytotoxic drug-administered infusion solutions (Table 1).
3.1.2 pH
pH is a numerical indicator that describes the degree of pondus hydrogenii of a solution. pH is an important parameter for the stability of injections according to the 2020 Chinese Pharmacopoeia as changes in pH can affect the solubility of the drug and cause precipitation. The normal physiological pH of the human body is 7.35–7.45. Injections with extreme pH levels are more likely to cause vascular irritation, inflammatory reactions, or pain. Extreme pH (pH > 10 and <2) can increase the degradation rate of drugs such as cyclophosphamide (19). In order to increase drug availability and decrease the risk of local irritation, the pH limits are within 3.5–9 (20). The latest expert consensus in 2023 recommends that pH value variations less than 10% indicate that the administered infusion solution is stable (10, 21). We found that most articles did not provide enough detail about pH, such as terms like stable or unchanged, and some articles did not mention this parameter at all. We have listed these data according to the original data in the literature (Table 1).
3.1.3 Osmolality
Osmolality means the extra pressure applied on the surface of the solution just enough to prevent osmosis from occurring. Osmolality is another important parameter that must be considered regarding the quality control of administered infusion solutions. The osmolality of the blood is 285–310 mOsmol/kg, and the average physiological osmolality is approximately 297.5 mOsmol/kg. Either hypertonic solutions with an osmolality >600 mOsmol/kg or hypotonic solutions with an osmolality approximately <150 mOsmol/kg have been reported to possibly cause crenation as an adverse reaction to intravenous infusion (22). Each drug has a different osmolarity; compound such as 0.9% NaCl and 5% dextrose, as isotonic solutions, are used as diluents for intravenous administration in clinical practice. The latest expert consensus in 2023 recommends that the osmolality changes of 10% indicate that the solution is stable and physically compatible compared with the initial condition (0 h) (10). Most research studies selected in this paper do not list exact osmolality or do not explore this factor (Table 1).
3.1.4 Particulate matter
Particulate matter refers to small insoluble substances that are generally less than 50 μm and invisible to the naked eye. Infusion particles are those that enter the human body during the intravenous infusion process and cannot be removed from the body. The 2020 Chinese pharmacopoeia stipulates that particles ≥ 10 μm/mL should not exceed 25, particles ≥ 25 μm/mL should not exceed 3, and particles ≥50 μm should not be detected in more than 100 mL of an intravenous solution. The particles ≥ 10 μm should not exceed 6000, and particles ≥25 μm should not exceed 600 in each test sample for less than 100 mL of the intravenous solution. During infusion, the particles will adhere to the blood vessel wall and aggregate into larger particles, which may block microvessels, resulting in local blood vessel obstruction and insufficient blood supply. In addition, these particles will stimulate the body to produce allergic reaction and form granulomas. It is important to systematically evaluate the particulate matter. However, most studies focusing on the stability of ready-to-use infusion solutions do not list the data on particulate matter, highlighting the lack of standards and data on the administered infusion solutions (Table 1).
3.2 Chemical stability
Chemical stability refers to the content change due to hydrolysis or oxidation chemical degradation reactions (Table 1). The content of a drug will change over time, depending on the duration of storage and temperature. In clinical practice, given the general rule that drugs remain (i.e., at the recommended dilution) at up to 90% of their initial content, this 10% degradation has been widely used as the stability limit in published studies. Each drug has different pharmacodynamics, which results in different levels of stability. For anticancer drugs, which have a narrow therapeutic range for effective treatment, the classical 10% degradation limitation may not be appropriate. In such cases, the stability limitation is set at 5% or in compliance with the relevant standards in the 2020 Chinese Pharmacopoeia. However, most of the reference studies reviewed in our text chose 10% limitation, with only a few studies employing 5% or 95%–105% as the stability limit.
3.3 Biological stability
Biological stability generally refers to the deterioration and corruption due to microbial exposure. Sterility is another important factor in evaluating the stability of injections according to the 2020 Chinese Pharmacopoeia (Table 1). Administered infusion solutions are post-dilution or post-reconstitution products in PIVAS centers; however, there is no sterility data on administered infusion solutions. Most pharmaceutical industries frequently limit the use to 24 h after post-dilution or post-reconstitution only for bacteriological reasons, regardless of the true clinical practice, which, in many cases, could be longer. In practice, some cytotoxic drugs, such as 5-FU, may require continuous infusions for several days, so it is vital to test sterility (23). In this paper, we list sterility as an important part of administered infusion solution quality control. Only one article deals with microbial contamination of administered infusion solutions in our research (24). It demonstrated that the current attention to the quality control of administered infusion is not enough.
4 Discussion
The quality control of administered infusion solutions is a vital step from PIVAS to the ward and is indispensable to ensure the proper use of drugs. The quality control of cytotoxic drug-administered infusion solutions is important for patient safety in clinical practice and should be strengthened. In the paper, we summarized and listed the cytotoxic drug-administered infusion solution-associated data, mainly including the physical and chemical stability and sterility. However, there are some limitations to this analysis.
Many drugs selected are not commercially available products but are standard products for laboratory use only and, therefore, do not reflect clinical practice. This also means that pharmaceutical excipients in the commercial product cannot be analyzed, which prevents an accurate reflection of the drug’s true content in the administered infusion solution. In some cases, the admixtures were prepared in a laboratory to mimic a real-world environment in PIVAS centers; therefore, this information should be carefully applied for real-world practices.
The same drug may have multiple manufacturers, and the pharmaceutical excipients used by each manufacturer are different. Is it necessary to compare each product in the market? Can the analysis of only one product be used to represent all the other products in the market? In our research, we mainly listed one product for each drug. We also found that many drugs lack information regarding administered infusion solutions, especially some original products such as elemene and ifosfamide. Some frequently used cytotoxic drugs such as loplatin and nedaplatin also lack associated data on administered infusion solutions.
When reviewing package inserts, information regarding post-dilution or post-reconstitution is frequently limited to 24 h to meet licensing requirements. When medicines are being licensed, little attention is given to drug administration in the clinic. Therefore, pharmacists and medical staff need to pay more attention to the preservation and use of administered infusion solutions to ensure patient safety. It is vital to fill the gap between the package insert and clinical needs.
Most tertiary hospitals in China are equipped with PIVAS centers, where intravenous solutions are admixed. Although the environment in PIVAS centers is clean, it is necessary to check the sterility of administered infusion solutions after prolonged storage. Any microbial contamination can lead to serious effects in patients. There has been limited research on the sterility of administered infusion solutions. In addition to potential time-dependent physical and chemical changes, microorganism contamination poses a risk during post-dilution storage. The monitoring of heat sources and endotoxins after long-term storage of administered infusions is also crucial. Although most administered infusion solutions are used within 12 h of admixture, sterility should be the first consideration under some special conditions, such as continuous pumping for a long time, home infusion, and changing infusion time due to disease progression. Almost all tertiary hospitals can carry out microbiological examination, so pharmacists or nurses should take sterility into consideration to ensure drug safety for patients and avoid unnecessary waste.
With the continuous development of the tiered diagnosis and treatment model in China, daytime chemotherapy and home chemotherapy will become new models for the diagnosis and treatment of cancer patients. However, due to a lack of data on administered infusion solutions, the widespread application of these models to all chemotherapy regimens is limited. Improving these data will have huge impact on patients, the pharmaceutical industry, nursing staff, and economic aspects.
Another limitation to our research is the lack of associated data on degradation products, which is essential for the quality control of administered infusion solutions, especially for cytotoxic drugs. For example, high doses of 5-FU could increase cardiotoxicity because of small quantities of degradation products (fluoromalonaldehyde and fluoroacetaldehyde) produced during storage in administered infusion solutions (25). The limitations vary for each drug, and the content of by-products should be strictly detected, following the ChP criterion. Some drugs may retain more than 95% of their initial content; however, when degradation product levels exceed ChP limitations, it could compromise the chemical stability of infusion solutions due to the degradation product (26).
5 Conclusion
In order to standardize quality inspection, improve the quality control of the cytotoxic drug-administered infusion solutions, and ensure the safety and effectiveness of intravenous drug use, this paper established a quality control paradigm based on practical clinical needs including visual appearance (color, turbidity, and visual foreign body), pH, osmolality, particulate matter, drug content, and sterility. These indexes are analyzed comprehensively to assess the quality and stability of the administered infusion solution. At present, there is limited research on cytotoxic drug-administered infusion solutions, and there is a lack of relevant standards and technical guidance. With the development of regionalized PIVAS centers, the clinical demand for research in this area is increasing, which may drive further investigation. The administered infusion solution quality control is vital for patients, pharmacists, and nurses in clinical practice and has huge economical potential, so it is important to enhance relevant research in this field.
Author contributions
SW: writing–original draft and writing–review and editing. F-YZ: data curation, investigation, and writing–review and editing. XD: supervision and writing–review and editing. X-LP: investigation and writing–review and editing. CS: investigation and writing–review and editing. J-LT: project administration and writing–review and editing. D-PM: resources and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Program of Science Foundation of Tianjin (China) (20JCQNJC01390) and the China Medicine Education Association (2016-164-8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zuo, W, Zhou, L, Gao, D, Chen, X, Zhang, Y, Mei, D, et al. Investigation on status of intravenous infusion among in patientsin secondary and above general hospitalsin China. Chin Health Qual Management (2023) 30(4):30–3. doi:10.13912/j.cnki.chqm.2023.30.4.09
2. Annual report of national adverse drug reaction monitoring. Zhong guo yao wu jing jie (2022) 20(6):712–9. doi:10.19803/j.1672-8629.20230168
3. Szałek, E, Kamińska, A, Grześkowiak, E, Hanna, U, Monika, B, and Murawa, D. The stability of fludarabine phosphate in concentrate and diluted with sodium chloride 0.9. WSPOLCZESNA ONKOLOGIA-CONTEMPORARY ONCOLOGY (2011) 15(3):142–6. doi:10.5114/wo.2011.23003
4. Vigneron, J, Astier, A, Trittler, R, Hecq, JD, Daouphars, M, Larsson, I, et al. SFPO and ESOP recommendations for the practical stability of anticancer drugs: an update. Ann Pharmaceutiques Françaises (2013) 71(6):376–89. doi:10.1016/j.pharma.2013.06.002
5. Han, B, Zheng, R, Zeng, H, Wang, S, Sun, K, Chen, R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent (2024) 4(1):47–53. doi:10.1016/j.jncc.2024.01.006
6. Kaur, R, Bhardwaj, A, and Gupta, S. Cancer treatment therapies: traditional to modern approaches to combat cancers. Mol Biol Rep (2023) 50(11):9663–76. doi:10.1007/s11033-023-08809-3
7. Salman, D, Swinden, J, Barton, S, Peron, J-MR, and Nabhani-Gebara, S. Evaluation of the stability profile of anticancer drugs: a review of Ifosfamide and Mesna regimen for the treatment of metastatic soft tissue sarcoma. J Oncol Pharm Pract (2014) 22(1):86–91. doi:10.1177/1078155214549490
8. Zhang, Y, Kawedia, JD, Myers, AL, McIntyre, CM, Anderson, PM, Kramer, MA, et al. Physical and chemical stability of high-dose ifosfamide and mesna for prolonged 14-day continuous infusion. J Oncol Pharm Pract (2013) 20(1):51–7. doi:10.1177/1078155213478284
9. Bardin, C, Astier, A, Vulto, A, Sewell, G, Vigneron, J, Trittler, R, et al. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Ann Pharmaceutiques Françaises (2011) 69(4):221–31. doi:10.1016/j.pharma.2011.07.002
10. Tong, T, Haiwen, D, Wu, J, Gu, F, Liu, S, and Linqin, T, Expert consensus on physical stability and compatibility inspection indicators for ready to administer infusion solutions Herald of medicine 2023, 16 (18).
11. Association, GP. Expert recommendations for pharmacists and nurses on the safe administration of intravenous medication. Pharm Today (2023) 33(10):721–32. doi:10.12048/j.issn.1674⁃229X.2023.10.001
12. Dine, T, Kahlfi, F, Duban, M, Gressier, B, Luyckx, M, Brunet, C, et al. Effects of PVC bags sterilization process on the 5-fluorouracil stability. Biomaterials (1999) 20(7):655–61. doi:10.1016/s0142-9612(98)00221-x
13. Favier, M, De Cazanove, F, Coste, A, Cherti, N, and Bressolle, F. Stability of carmustine in polyvinyl chloride bags and polyethylene-lined trilayer plastic containers. Am J Health-System Pharm (2001) 58(3):238–41. doi:10.1093/ajhp/58.3.238
14. Knoll, L, Kraemer, I, and Thiesen, J. Physicochemical stability of carmustine-containing medicinal products after reconstitution and after dilution to ready-to-administer infusion solutions stored refrigerated or at room temperature. Eur J Hosp Pharm (2023) 30(1):11–6. doi:10.1136/ejhpharm-2020-002597
15. Nissen, KB, Jorgensen, LB, Berg, DL, and Andersen, G. Stability study of methotrexate in 0.9% sodium chloride injection and 5% dextrose injection with limit tests for impurities. Am J Health-System Pharm (2017) 74(9):e211–e223. doi:10.2146/ajhp150818
16. El Aatmani, M, Poujol, S, Astre, C, Malosse, F, and Pinguet, F. Stability of dacarbazine in amber glass vials and polyvinyl chloride bags. Am J Health-System Pharm (2002) 59(14):1351–6. doi:10.1093/ajhp/59.14.1351
17. Vidal, F, Cotteret, C, Negbane, A, Sebti, M, Hinterlang, M, Cisternino, S, et al. Stability of pemetrexed diarginine concentrates for solution in vials and diluted in 0.9% sodium chloride and dextrose 5% polyolefin infusion bags. Eur J Hosp Pharm (2022) 29(6):353–8. doi:10.1136/ejhpharm-2020-002620
18. Zhang, Y, and Trissel, LA. Physical and chemical stability of pemetrexed in infusion solutions. Ann Pharmacother (2006) 40(6):1082–5. doi:10.1345/aph.1g715
19. Beijnen, JH, van Gijn, R, Challa, EE, Kaijser, GP, and Underberg, WJ. Chemical stability of two sterile, parenteral formulations of cyclophosphamide (Endoxan) after reconstitution and dilution in commonly used infusion fluids. J Parenter Sci Technol : a Publ Parenter Drug Assoc (1992) 46(4):111–6.
20. Roethlisberger, D, Mahler, H-C, Altenburger, U, and Pappenberger, A. If euhydric and isotonic do not work, what are acceptable pH and osmolality for parenteral drug dosage forms? J Pharm Sci (2017) 106(2):446–56. doi:10.1016/j.xphs.2016.09.034
21. Koller, AK, Krebs, S, and Dörje, F. Medication safety in intravenous therapy: a compatibility study of clonidine with drugs frequently used in intensive care. Pharmaceutics (2020) 13(1):21. doi:10.3390/pharmaceutics13010021
22. Stawny, M, Gostyńska, A, Nadolna, M, and Jelińska, A. Safe practice of Y-site drug administration: the case of colistin and parenteral nutrition. Pharmaceutics (2020) 12(3):292. doi:10.3390/pharmaceutics12030292
23. Raphaëlle, P, Elodie, P, Nathalie, J, Odile, A, Paul, S, Isabelle, M, et al. Safe disconnection of 5-fluorouracil elastomeric pumps: the benefit of a closed-system-transfer device designed for cytotoxic drug administration/perfusion. The J Vasc Access (2021) 24(4):653–9. doi:10.1177/11297298211044017
24. Eiden, C, Philibert, L, Bekhtari, K, Poujol, S, Malosse, F, and Pinguet, F. Physicochemical stability of oxaliplatin in 5% dextrose injection stored in polyvinyl chloride, polyethylene, and polypropylene infusion bags. Am J Health-System Pharm (2009) 66(21):1929–33. doi:10.2146/ajhp090039
25. Closset, M, Onorati, S, Colsoul, M-L, Goderniaux, N, Bihin, B, Jamart, J, et al. Long-term physicochemical stability of 5-fluorouracil at selected standardised rounded doses in polyolefin bags. J Oncol Pharm Pract (2023) 29(8):1878–83. doi:10.1177/10781552231152618
26. Ayed, WB, Drira, C, Soussi, MA, Ouesleti, H, Hamdene, B, Khrouf, M, et al. Physical and chemical stability of cytarabine in polypropylene syringes. J Oncol Pharm Pract (2020) 27(4):827–33. doi:10.1177/1078155220937405
27. Fleming, RA, Olsen, DJ, Savage, PD, and Fox, JL. Stability of ondansetron hydrochloride and cyclophosphamide in injectable solutions. Am J Health-System Pharm (1995) 52(5):514–6. doi:10.1093/ajhp/52.5.514
28. Dine, T, Lebegue, S, Benaji, B, Gressier, B, Segard, V, and Goudaliez, F. Stability and compatibility studies of four cytostatic agents (fluorouracil, dacarbazine, cyclophosphamide and ifosfamide) with PVC infusion bags. Pharm Sci Com (1994) 4:97–101.
29. Guichard, N, Bonnabry, P, Rudaz, S, and Fleury-Souverain, S. Stability of busulfan solutions in polypropylene syringes and infusion bags as determined with an original assay. Am J Health-System Pharm (2017) 74(22):1887–94. doi:10.2146/ajhp160516
30. Houot, M, Poinsignon, V, Mercier, L, Valade, C, Desmaris, R, Lemare, F, et al. Physico-chemical stability of busulfan in injectable solutions in various administration packages. Drugs in R&D (2013) 13(1):87–94. doi:10.1007/s40268-013-0003-y
31. Favier, M, De Cazanove, F, Coste, A, Cherti, N, and Bressolle, F. Stability of carmustine in polyvinyl chloride bags and polyethylene-lined trilayer plastic containers. Am J Health-System Pharm (2001) 58(3):238–41. doi:10.1093/ajhp/58.3.238
32. Vigneron, J, D’Huart, E, and Demoré, B. Stability of bendamustine solutions: influence of sodium chloride concentration, temperature and container. Pharm Technology Hosp Pharm (2018) 3(1):13–21. doi:10.1515/pthp-2017-0033
33. Nelson, L, Dwyer, P, Corris, M, Santillo, M, Davies, L, Milligan, K, et al. Stability of pemetrexed disodium in sodium chloride 0.9% w/v intravenous Viaflo infusion bags. Eur J Hosp Pharm (2023) 30(e1):e2–e9. doi:10.1136/ejhpharm-2021-002823
34. Komm, C, Rochani, A, Fox, T, and Kaushal, G. Stability of extemporaneously compounded 5-fluorouracil utilizing high performance liquid chromatography. Drug discoveries and Ther (2022) 16(1):1–7. doi:10.5582/ddt.2022.01011
35. Galanti, L, Lebitasy, MP, Hecq, JD, Cadrobbi, J, Vanbeckbergen, D, and Jamart, J. Long-term stability of 5-Fluorouracil in 0.9% sodium chloride after freezing, microwave thawing, and refrigeration. Can J Hosp Pharm (2009) 62(1):34–8. doi:10.4212/cjhp.v62i1.115
36. Martel, P, Petit, J, Pinguet, F, Poujol, S, Astre, C, and Fabbro, M. Long-term stability of 5-fluorouracil stored in PVC bags and in ambulatory pump reservoirs. J Pharm Biomed Anal (1996) 14(4):395–9. doi:10.1016/0731-7085(95)01635-x
37. Xu, Q, Zhang, V, and Trissel, LA. Physical and chemical stability of gemcitabine hydrochloride solutions. J Am Pharm Assoc (1999) 39(4):509–13. doi:10.1016/s1086-5802(16)30470-3
38. Laforgia, M, Amodio, L, Colangiulo, S, Ungaro, V, Gatti, L, Lucarelli, G, et al. LC-MS/MS analysis on infusion bags and filled syringes of decitabine: new data on physicochemical stability of an unstable molecule. ACS omega (2022) 7(29):25239–43. doi:10.1021/acsomega.2c02144
39. Kim, SH, Heeb, RM, and Krämer, I. Physicochemical stability of reconstituted decitabine (Dacogen®) solutions and ready-to-administer infusion bags when stored refrigerated or frozen. Pharm Technology Hosp Pharm (2017) 2(4):145–57. doi:10.1515/pthp-2017-0025
40. Wood, MJ, Irwin, WJ, and Scott, DK. Stability of doxorubicin, daunorubicin and epirubicin in plastic syringes and minibags. J Clin Pharm Ther (1990) 15(4):279–89. doi:10.1111/j.1365-2710.1990.tb00386.x
41. Dine, T, Cazin, JC, Gressier, B, Luyckx, M, Brunet, C, Cazin, M, et al. Stability and compatibility of four anthracyclines: doxorubicin, epirubicin, daunorubicin and pirarubicin with PVC infusion bags. Pharmaceutisch Weekblad Scientific Edition (1992) 14(6):365–9. doi:10.1007/bf01970174
42. D’Huart, E, Vigneron, J, Lider, P, and Demoré, B. Physicochemical stability of etoposide diluted at range concentrations between 0.38 and 1.75 mg/mL in polyolefin bags. Eur J Hosp Pharm (2020) 27(1):43–8. doi:10.1136/ejhpharm-2018-001571
43. Barthes, DM, Rochard, EB, Pouliquen, IJ, Rabouan, SM, and Courtois, PY. Stability and compatibility of etoposide in 0.9% sodium chloride injection in three containers. Am J Health-System Pharm (1994) 51(21):2706–9. doi:10.1093/ajhp/51.21.2706
44. Krämer, I, and Thiesen, J. Stability of topotecan infusion solutions in polyvinylchloride bags and elastomeric portable infusion devices. J Oncol Pharm Pract (1999) 5(2):75–82. doi:10.1177/107815529900500203
45. Craig, SB, Bhatt, UH, and Patel, K. Stability and compatibility of topotecan hydrochloride for injection with common infusion solutions and containers. J Pharm Biomed Anal (1997) 16(2):199–205. doi:10.1016/s0731-7085(97)00022-8
46. Thiesen, J, and Krämer, I. Physicochemical stability of irinotecan injection concentrate and diluted infusion solutions in PVC bags. J Oncol Pharm Pract (2000) 6(3):115–21. doi:10.1177/107815520000600305
47. Trissel, LA, Zhang, Y, and Cohen, MR. The stability of diluted vincristine sulfate used as a deterrent to inadvertent intrathecal injection. Hosp Pharm (2001) 36(7):740–5. doi:10.1177/001857870103600707
48. Lieu, CL, Chin, A, and Gill, MA. Five-day stability of vinorelbine in 5% dextrose injection and in 0.9% sodium chloride injection at room temperature. Int J Pharm compounding (1999) 3(1):67–8.
49. Dine, T, Luyckx, M, Cazin, JC, Brunet, C, Cazin, M, Goudaliez, F, et al. Stability and compatibility studies of vinblastine, vincristine, vindesine and vinorelbine with PVC infusion bags. Int J Pharmaceutics (1991) 77(2):279–85. doi:10.1016/0378-5173(91)90328-l
50. Donyai, P, and Sewell, GJ. Physical and chemical stability of paclitaxel infusions in different container types. J Oncol Pharm Pract (2006) 12(4):211–22. doi:10.1177/1078155206073589
51. Karbownik, A, Szałek, E, Urjasz, H, Głęboka, A, Mierzwa, E, and Grześkowiak, E. The physical and chemical stability of cisplatin (Teva) in concentrate and diluted in sodium chloride 0.9. Contemp Oncol (Poznan, Poland) (2012) 16(5):435–9. doi:10.5114/wo.2012.31775
52. Myers, AL, Zhang, YP, Kawedia, JD, Trinh, VA, Tran, H, Smith, JA, et al. Stability study of carboplatin infusion solutions in 0.9% sodium chloride in polyvinyl chloride bags. J Oncol Pharm Pract (2016) 22(1):31–6. doi:10.1177/1078155214546016
53. Diaz Amador, F, Sevilla Azzati, E, and Herreros de Tejada y Lopez-Coterilla, A. Stability of carboplatin in polyvinyl chloride bags. Am J Health Syst Pharm (1998) 55(6):602–4. doi:10.1093/ajhp/55.6.602
54. André, P, Cisternino, S, Roy, AL, Chiadmi, F, Schlatter, J, Agranat, P, et al. Stability of oxaliplatin in infusion bags containing 5% dextrose injection. Am J Health Syst Pharm (2007) 64(18):1950–4. doi:10.2146/ajhp060369
55. Spindeldreier, K, Thiesen, J, Lipp, H-P, and Krämer, I. Physico-chemical stability of eribulin mesylate containing concentrate and ready-to-administer solutions. J Oncol Pharm Pract (2013) 20(3):183–9. doi:10.1177/1078155213492449
Keywords: administered infusion solution, cytotoxic drugs, physical stability, chemical stability, sterility
Citation: Wang S, Zhang F-Y, Dou X, Pan X-L, Su C, Tian J-L and Mu D-P (2025) Status analysis of quality control of administered infusion solution with cytotoxic drugs. Oncol. Rev. 18:1415677. doi: 10.3389/or.2024.1415677
Received: 11 April 2024; Accepted: 22 November 2024;
Published: 06 January 2025.
Edited by:
Deepa Kushwaha, Rare Genomics Institute, United StatesReviewed by:
Zhifen Cui, Duke University, United StatesLing Ding, University of Pittsburgh Medical Center, United States
Copyright © 2025 Wang, Zhang, Dou, Pan, Su, Tian and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dian-Ping Mu, cGluZ19tZDAyMDhAMTYzLmNvbQ==
 Shan Wang
Shan Wang Feng-Ying Zhang1
Feng-Ying Zhang1