- Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Despite increases in prevalence, many cutaneous T-cell lymphoma (CTCL) patients still lack effective and safe therapies for their disease. The most prevalent subtype, mycosis fungoides is usually managed with skin directed treatments in early stages, while advanced stages are often targeted with systemic medications. These treatments are all symptomatic except for allogeneic hematopoietic stem cell transplantation, which is associated with its own risks of relapse and potentially fatal complications. A novel class of drugs termed “JAK inhibitors” (JAKi) has recently been developed primarily for chronic inflammatory diseases, but there is substantial evidence of JAK/STAT pathway overactivation also in CTCL. As of 1 December 2024, 14 JAKis have been collectively approved by the European Medicines Agency, the Food and Drug Administration and the Pharmaceutical and Medical Devices Agency of Japan. Despite some evidence from case reports, the efficacy and safety of JAKi in CTCL remains to be determined in controlled clinical trials. This review summarizes the current evidence on pathogenic JAK activation and its potential therapeutic inhibition in CTCL.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a diverse group of non-Hodgkin lymphomas originating from malignant skin T cells. Although exact estimates fluctuate, a growing body of literature supports an increase in CTCL cases over the last years (1–4). CTCL is more commonly diagnosed in males, individuals of black race, and older adults (1, 2, 4–12, 13). From 2002 to 2021, CTCL incidence was 1.7 times higher in black individuals and males (compared to whites and females respectively), and 4.5 times higher in those aged 65–74 compared to 20–54 years (14). There are many CTCL subtypes, which differ in prevalence, clinical manifestation, and severity (1, 8). Mycosis fungoides (MF) and Sézary syndrome (SS) are the most prominent subtypes and typically stem from malignant mature CD4+ helper T cells. Representing approximately half of all CTCL cases, MF is the most prevalent subtype and generally presents with a more indolent course than SS. SEER18 data (covering ∼28% of the U.S. population) reported that MF and SS respectively accounted for 56.6% and 1.8% of CTCL cases (n=14,942) from 2000 to 2018 (1). More recent SEER22 data (∼48% of the U.S. population) estimate that MF and SS comprise 37.8% and 1.4% of CTCL cases (n=46,433), respectively (14). To assess disease severity and prognosis, MF and SS are staged (IA-IVB) based on ISCL/EORTC guidelines using the TNMB system, which considers skin lesions, and involvement of the lymph nodes, viscera, and blood (8, 16). Skin involvement includes T1 (<10% patches/plaques), T2 (>10% patches/plaques), T3 (tumors), and T4 (erythroderma) (16, 17). Lymph node involvement spans from N0 to N3, and metastasis involves M0 (none) and M1 (visceral involvement). Blood involvement spans from B0 to B2. Overall, early-stage disease encompasses stages IA, IB and IIA, while advanced stage disease includes stages IIB, III, IVA and IVB (7, 16). Approximately 70% of MF cases present with early-stage disease (18–20). Five-year overall survival (OS) rates are high for early-stage disease with rates of 96-100% for stage IA, 73-86% for stage IB, and 49-73% for stage IIA (21). However, OS rates drop significantly in advanced stages, including IIB (40-65%), III (40-57%), IVA (15-40%) and IVB (0-15%) (21). Therapeutically, early-stage CTCL is often managed with topical steroids, phototherapy, and sometimes irradiation (134, 135). On the other hand, advanced-stage disease typically requires systemic treatments (134, 23).
Established treatment approaches
Depending on disease severity, established treatments for CTCL range from skin-directed therapies for early-stage disease to systemic therapies for advanced-stage disease (134, 17). These therapies are generally symptomatic rather than curative. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the only curative option, but it is associated with high relapse rates and serious complications, including graft-versus-host disease (GVHD), limiting its use to carefully selected patients (24, 25).
Topicals
Topical corticosteroids (TCS) are a common first-line treatment for early-stage MF, achieving meaningful clinical responses, although long-term efficacy data are limited (26, 27). In a 1998 prospective study, Zackheim et al. reported response rates of 94% (T1) and 82% (T2) in 79 patients with patch (n=75) or plaque stage (n=4) MF. Complete remission occurred in 63% of T1 and 25% of T2 cases after 3–4 months (28). In a 2021 retrospective analysis, Kartan et al. observed disease improvement in 73% of 37 MF patients, with complete remission in 44% of responders after 18.5 months on average (27). However, TCS are typically inadequate for higher stage disease (IIA and above), with only 33% of patients responding (27). A primary limitation of TCS is the risk of cutaneous atrophy with extended use (29). Topical chlormethine/mechlorethamine, a chemotherapy agent, offers an alternative with 76.7% of MF patients (n=206; stage IA and IB) achieving partial response in a real-world setting (30).
Phototherapy
Phototherapy, most commonly psoralen plus UVA (PUVA) or narrowband UVB (NB-UVB), is a standard treatment for early-stage MF (31). Complete remission rates range from 60–81% for NB-UVB and 62–71% for PUVA. However, PUVA increases the risk of skin cancers, including melanoma, and is less widely available (31, 32).
Radiotherapy
High-energy radiation therapy is used to target malignant cells in individual lesions or the entire body as in total skin electron-beam therapy (TSEB) (33). Radiation therapy, particularly TSEB, can achieve average complete response rates of 81%. However, relapse rates are high, with up to 73% of patients relapsing within five years post-treatment (34–46). Repeated courses of high-dose TSEB increase the risk of cumulative adverse effects. (34–36, 38–41, 47–49). Low-dose radiation therapy (RT) (7-12 Gy) has been utilized as an alternative because it is associated with fewer grade 2 (33% vs. 79%) and grade 3 adverse events (6% vs. 15%) compared to a standard dose (30 Gy) (50). However, low-dose RT (10 to <20 Gy) demonstrates diminished efficacy, achieving complete responses in only 35% of patients compared to 62% in standard dosing (>30 Gy) (51).
Small molecule inhibitors
Small molecule inhibitors (SMIs) frequently used for CTCL treatment include methotrexate, bexarotene, and HDAC inhibitors. Methotrexate (MTX), a folic acid metabolism inhibitor effective in highly proliferative cells, has been used to treat CTCL (52, 53). Oral MTX achieved complete responses in 30% of MF and 5.5% of SS cases, though 57% of responders relapsed after a median time of 11 months after treatment implementation (53, 54). Bexarotene, a synthetic retinoid, is used in patients with refractory advanced-stage MF (52). When administered orally (initial dose: 300 mg/m2/day), bexarotene prompted response rates of 54% in early-stage MF patients (n=28) and 45% in advanced-stage MF patients (n=56) (55, 56). Bexarotene doses above 300 mg/m2/day produced response rates of 67% and 55% in early and advanced MF respectively (55, 56). Two histone deacetylase (HDAC) inhibitors are currently approved to treat CTCL by the FDA. Vorinostat, a hydroxamic acid, inhibits class I and II HDACs in patients with refractory or relapsed CTCL (57, 58). Romidepsin is a bicyclic, class I HDAC inhibitor approved to treat relapsed CTCL (59, 60).
Monoclonal antibodies
Approved monoclonal antibody (mAb) therapies for CTCL include mogamulizumab and brentuximab vedotin. Mogamulizumab is a defucosylated, humanized IgG1 anti-CCR4 mAb that promotes antibody-dependent cellular cytotoxicity (ADCC) to deplete target cells in patients with relapsed or refractory MF or SS (61, 62). Brentuximab vedotin is an anti-CD30 antibody-drug conjugate used to treat CD30+ PTCL and CTCL, including refractory, CD30+ transformed MF (63, 64). Both antibodies demonstrated superior efficacy compared to conventional therapies in controlled trials (65, 66), making them important additions to the therapeutic armamentarium for CTCL.
Allogeneic hematopoietic stem cell transplantation
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), the sole curative measure for MF/SS, is generally used for patients with progressive stage IIB-IV CTCL (including transformed MF) (67, 68). A meta-analysis by Iqbal et al. reported pooled progression-free survival (PFS) and relapse rates of 36% and 47%, respectively (24). Graft-versus-host disease (GVHD) occurs in 40%–60% of patients undergoing allo-HSCT and represents a common, significant adverse event (25) associated with considerable morbidity and mortality (69, 70).
Despite all these treatment options for CTCL, many patients encounter disease relapses, mandating more efficacious, highlighting the need for safer, more effective treatment options for long-term disease control
JAK inhibitors – a novel group of therapeutics for inflammatory and malignant skin diseases
The JAK/STAT signaling pathway plays a key role in regulating immune responses and inflammation, and its dysregulation has been implicated in the pathogenesis of CTCL (71–74). This evolutionarily conserved pathway promotes gene expression following cytokine receptor engagement by interleukins (ILs), interferons (IFNs), and growth factors (75). JAK/STAT signaling is essential for orchestrating both innate and adaptive immune responses to pathogens and malignancies (76–78). All four known JAK family members (JAK1, JAK2, JAK3 and TYK2) share conserved domains (kinase domain, pseudokinase domain, SH2 domain and FERM domain) (74). However, they respond to different cytokines based on their unique tyrosine phosphorylation sites (74). The STAT family includes seven molecules (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6) (74). JAKs recruit and phosphorylate one or more STATs that then dimerize and translocate into the nucleus (74). STATs act as transcription factors (TFs) to promote expression of genes involved in angiogenesis, proliferation, cell differentiation and apoptosis (79). Dysregulation of JAK/STAT signaling has been implicated in autoimmune diseases and cancers, including hematologic malignancies such as CTCL (74, 80, 81). In addition to its role in tumorigenesis and metastasis of various cancers (82 83, 84, 86, 87), JAK/STAT overactivation has been specifically implicated in hematologic malignancies, including CTCL (88). However, the precise role of JAK inhibitors (JAKis) in CTCL remains to be clarified.
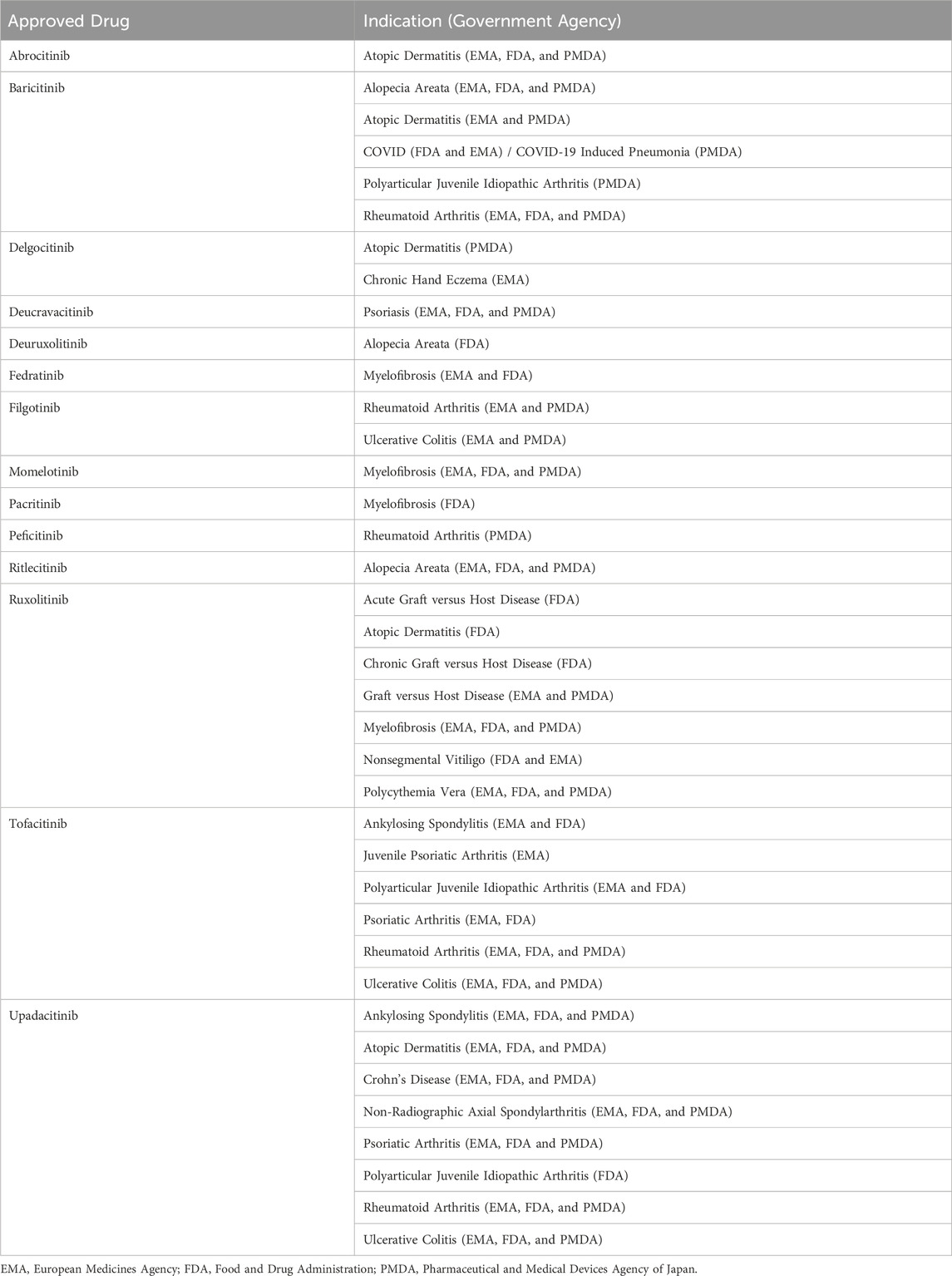
In 2011, the Food and Drug Administration (FDA) approved its first JAKi, ruxolitinib, to treat myelofibrosis (89). Since then, new JAK inhibitors continue to be approved each year by the FDA and other regulatory agencies. As of 1 December 2024, 14 JAKis have been approved by respective administrative bodies in the United States, the European Union and Japan across 17 different indications (Figure 1) (90–92). These include chronic inflammatory skin conditions (atopic dermatitis, psoriasis, chronic hand eczema, nonsegmental vitiligo), arthritis and spondyloarthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis), autoimmune and pediatric conditions (alopecia areata, graft versus host disease, polyarticular juvenile idiopathic arthritis, juvenile psoriatic arthritis), myeloproliferative neoplasms (myelofibrosis, polycythemia vera), inflammatory bowel diseases (ulcerative colitis, Crohn’s disease), and infectious disease (COVID-19 induced pneumonia) (Table 1).
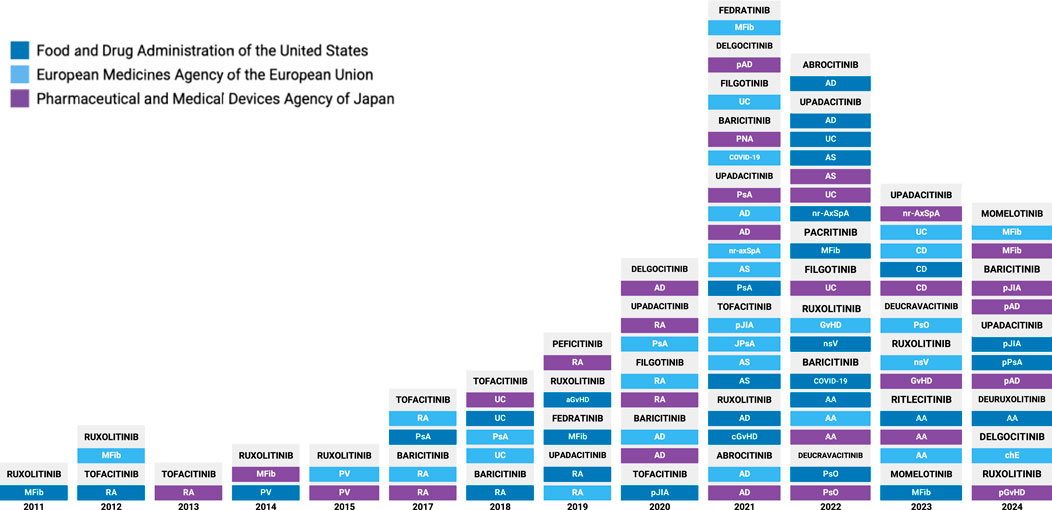
Figure 1. Timeline of JAK Inhibitor approvals by global regulatory agencies. AA, Alopecia areata; AD, Atopic dermatitis; aGvHD, acute graft-vs-host disease; AS, Ankylosing spondylitis; cGvHD, chronic graft-vs-host disease; COVID, Coronavirus-2019; CD, Crohn’s Disease; chE, chronic hand eczema; GvHD, graft-vs-host disease; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MFib, myelofibrosis; pJIA, polyarticular juvenile idiopathic arthritis; nr-AxSpA, non-radiographic axial spondyloarthritis; nsV, nonsegmental vitiligo; pAD, pediatric atopic dermatitis; pGvHD, pediatric graft-vs-host disease; PsA, psoriatic arthritis; PsO, psoriasis; pPsA, pediatric psoriatic arthritis; PNA, Pneumonia (COVID-19 induced); PV, polycythemia vera; RA, rheumatoid arthritis; UC, ulcerative colitis.
JAK inhibition in CTCL–a viable concept?
In recent years, sequencing techniques (including whole exome sequencing, whole genome sequencing, and targeted capture sequencing) have been utilized to investigate JAK-STAT genomic alterations underlying MF and SS (79, 93–104). A review by Garcia-Diaz et al. evaluating NGS publications (88, 96–98, 100, 101, 103–106) in 2021 found that ≥60% of cases showed genetic alterations in JAK/STAT genes (79). STAT3 and STAT5B amplifications were fairly common (60% of patients), in contrast to activating JAK mutations (4%) (79). In addition to missense mutations reported by Song et al. (5% of 55 MF; 3% of 31 SS), amplifications in STAT5B were noted, for instance, by Choi et al. (62.5% of 40 CTCL) and Iyer et al. (18% of 49 MF samples) (93, 98, 100, 104). Missense STAT3 mutations were reported by Song et al. (15% of 55 MF) (93). Amplifications, gain of function mutation or SNVs of STAT3 were observed by Iyer et al. (12% of 49 MF samples), Kiel et al. (3% of 66 SS), and Bastidas-Torres et al. (11% of 9 MF), amongst others (97, 98, 100, 101). STAT5A alterations were noted, for example, by McGirt et al. (103) and Vaqué et al. (96), in addition to STAT1 (96). Wang et al. also noted significant upregulation of STAT1 in SS patients (94). Ligand-independent (constitutive) phosphorylation and activation has respectively been observed in STAT5 (95) and STAT3 (102).
Regarding the JAK family, reports of JAK1 amplifications or gain of function mutations included Iyer et al. (27% of 49 MF) and Kiel et al. (3% of 66 SS) (93, 97, 98, 100). Song et al. identified missense JAK1 mutations in 9% of 55 MF, and Vaqué et al. described a tolerated missense mutation in 1/11 MF cases (93, 96). JAK3 alterations included amplifications and deleterious SNVs in 22% of 9 MF tumors by Bastidas-Torres et al. (101), as well as various mutations observed by Iyer et al. (12% of 49 MF) (100), Koo et al., (35.4% of 65 CTCL) (95), and Kiel et al. (3% of 66 SS) (97). Additional JAK3 tumor SNVs were noted by Woollard et al., as well as missense and multi-hit mutations by Song et al. (13% MF) (93, 98). JAK2 showed focal amplification in 12.5% of 40 CTCL by Choi et al. (104) and 4% of 49 MF by Iyer et al. (100). Woollard et al. reported mixed JAK2 alterations including deletions, amplification, and SNVs (98).
Multiple members of the SOCS (Suppressor of Cytokine Signaling) family, key negative regulators of the JAK/STAT pathway, were recurrently altered, suggesting a loss of pathway inhibition may contribute to malignant T-cell survival. Specifically, SOCS1 deletions were noted in 33% of 27 MF (extension cohort) by Bastidas-Torres et al. (101). SOCS2 deletions were identified in CTCL cases by Choi et al. (104). Vaqué et al. noted a deleterious missense mutation in SOCS5 in 1/11 MF samples (96). SOCS7 showed alterations in SS (Woollard et al.) (98), with additional deletions and mutations reported by Song et al. (93). Mutations in those negative JAK-STAT regulators highlight frequent impairment of negative feedback mechanisms in this pathway. Such an increase in activity of the JAK/STAT pathway might directly promote tumor growth, but will likely also depend on the cellular context within the tumor microenvironment (TME) (109). While the mechanisms underlying CTCL and its progression are still only incompletely understood, differences within the TME are believed to distinguish early and advanced MF. Driven by Th1 (CD4+ helper T cells), Tc1 (CD8+ cytotoxic T cells) and NK (natural killer) cells, the early MF TME promotes type 1 immune skewing (IL-12 and IFN-y) and anti-tumor cytotoxic activity via STAT1 and STAT4 (110, 111). In contrast, the tumorigenic TME of advanced-stage MF utilizes STAT3, STAT5 and STAT6 signaling pathways to promote type 2 inflammation via cytokines (IL-4, IL-5, IL-13) and chemokines (CCL17, CCL18, CCL22, CCL26) (110, 112), but exact mechanisms remain unclear. Thus, clinical trials of JAK inhibitors are necessary to distinguish true disease drivers from bystander or counterregulatory JAK activation in CTCL.
Published case reports
In addition to sequencing results supporting JAK/STAT amplification in CTCL, JAKis have been administered to several CTCL patients as detailed in a few published case reports and clinical trials. Case reports by Castillo et al., Kook et al. and Mo et al. detail noticeable improvement in MF following treatment with systemic upadacitinib (114–116). Similarly, Levy et al. noted improvements to symptoms following initiation of another JAKi, ruxolitinib (117).
Castillo et al. reported a significant clinical response to 15 mg upadacitinib QD in an 87-year-old male with erythrodermic MF (stage 3; T4N0M0B0) and severe pruritus (114). Following at least 6 weeks of treatment (sequential manner: cyclosporine, methotrexate, dupilumab, acitretin and narrowband UV-B therapy), the patient demonstrated no significant response and was started on 15 mg upadacitinib QD for 16 weeks. After 16 weeks of upadacitinib, the patient demonstrated noticeable improvement in generalized itching and redness, as well as diminished scaling (from >80% body surface area to <10% post treatment). Improvements to the abdomen and pubic area (resolved erythematous scaly patches and plaques), as well as the back (diminished erythema and scaling) were noted.
Kook et al. reported significant clinical improvement in a 43-year-old male diagnosed with MF (Stage IB; onset 7 years prior) predominantly on the trunk and lower back (115). The patient was initially misdiagnosed with AD. Following proper diagnosis of MF, NB-UVB therapy and methotrexate (20 mg) were initiated. After 2 months of treatment, the patient had not improved and was started on 15 mg upadacitinib QD for 16 weeks. After 1 week, the patient noted noticeable improvement in perceived pain in addition to improved itch demonstrated by a decrease in Numerical Rating Scale (range 0–10) score from 9 to 3.
Levy et al. treated a 16-year-old male patient with recurrent subcutaneous panniculitis-like T-cell lymphoma (SPTCL) and hemophagocytic lymph histiocytosis (HLH) (117). From age 11 to 16, the patient repeatedly relapsed during various therapies (including corticosteroids, cyclosporine A, etoposide, anakinra and methotrexate). The patient was started on 15 mg ruxolitinib BID and achieved remission after 4 months. Ruxolitinib was ultimately discontinued for 8 months until disease recurred. 10 months following re-initiation of ruxolitinib monotherapy, the patient remained in complete remission.
Mo et al. report a 44-year-old male previously diagnosed with severe generalized eczema and treated with TCS and phototherapy (116). With no symptom improvement noted, he was started on upadacitinib and exhibited partial relief of symptoms. After approximately 7 months, upadacitinib was stopped and rapid deterioration of symptoms was observed, including debilitating generalized pruritus and redness. The patient was ultimately diagnosed with folliculotropic mycosis fungoides and unsuccessfully treated with oral methotrexate, TCS and oral prednisone. With no initial biopsy performed, it is uncertain whether the mycosis fungoides was present and treated successfully with upadacitinib. It is notable, however, that introduction of this JAKi provided symptom relief for this patient until it was ultimately discontinued.
Clinical trials
A clinical trial initiated by Wilcox et al. (NCT03601819) evaluated the JAK2 inhibitor pacritinib in CTCL (118). In this open label phase Ib study, the primary endpoint was the dose limiting toxicity (DLT) rate during the 1st cycle (28 days) of pacritinib (118). However, given low accrual of participants as per ClinicalTrials.gov, this trial was terminated early and does not have published results (118).
Horwitz et al. completed a non-randomized, open-label phase 1/2a clinical trial (multi-dose and multi-center) (NCT01994382) investigating the efficacy of cerdulatinib in patients with relapsed or refractory PTCL (n = 65) and CTCL (n = 41) (119). Initiated in August 2013, the study was completed in December 2020 with complete results provided April 2022 (120). Cerdulatinib, an oral small-molecule inhibitor of spleen tyrosine kinase (SYK), JAK1 and JAK3, caused adverse events (AEs) in 100% of PTCL and CTCL patients (120). Serious AEs were noted in 65% of PTCL patients and 51% of CTCL patients (136). In PTCL, common (n ≥ 3) serious AEs were diarrhea, pyrexia, pneumonia, sepsis and neoplasm progression (120). In CTCL, common (n ≥ 2) serious AEs were diarrhea, pneumonia, sepsis, staphylococcal bacteremia, dehydration, and neoplasm progression (120). In phase 2, overall response rates (ORR; both complete and partial responses) were observed in 36% of PTCL patients evaluated (n = 58). (120) Of the PTCL subtypes, patients with angioimmunoblastic T-cell lymphoma/T follicular helper lymphoma (AITL/TFH; n = 29) demonstrated the highest ORR (52%) and a clinical benefit (CB) of 63% (120). The other-PTCL (n = 25) cohort had an ORR of 32% and 73% CB. Lastly, PTCL-NOS (not otherwise specified; n = 11) exhibited 0% ORR and 22% CB. Out of 41 CTCL patients who were started on cerdulatinib, 37 were evaluated for efficacy in 2019 (121). Notably, MF patients exhibited higher ORR (45%) and CR (9%) compared to SS patients (17% ORR; 0% CR) (121).
In November 2016, Moskowitz et al. initiated a non-randomized, open-label Phase 2 clinical trial (multi-center) (NCT02974647) investigating the efficacy of 20 mg BID ruxolitinib in patients with relapsed/refractory peripheral T-cell lymphomas (PTCLs; n = 45) or MF (n = 7). While participant recruitment is ongoing as of 16 January 2025, trial completion is estimated for November of 2025 (126). Preliminary data on the initial cohort (n = 52) was published in Blood in 2021 (122). Common adverse events included febrile neutropenia, fatigue, diarrhea, anemia, as well as decreases in platelet and neutrophil counts (122). All patients were assigned to one of three cohorts based on the presence of JAK/STAT mutations (n = 21), pSTAT3 expression (≥30%; n = 14) or the absence of both (n = 17; 6 presented with incomplete sequencing data). The most common mutated genes during next-generation sequencing were STAT5B (n = 8), STAT3 (n = 7) and JAK3 (n = 6). PTCL patients (n = 19) with JAK/STAT mutations (cohort 1) exhibited clinical benefit rates (CBRs) approximately four times higher (53%) than those negative for biomarker expression (13% for cohort 3; n = 15) (122). Notably, CBRs of patients with JAK/STAT alterations (cohort 1 & 2) were significantly higher (p = 0.02) than those without (cohort 3) (122). While not all biomarker-positive PTCL patients responded to treatment, this result supports the role of JAK/STAT alterations in T-cell lymphomas, including CTCL. Conclusions about ruxolitinib efficacy in MF are limited in this study by the small cohort utilized. Although 71% of all MF cases presented with JAK/STAT mutations or pSTAT3 expression, clinical efficacy was limited (CBR = 14%) (122). These data suggest a potential for subtype specific JAK/STAT involvement and thus consideration for future treatment selection.
Moskowitz et al. are currently conducting a Phase I open-label multi-center clinical trial (NCT05010005) evaluating ruxolitinib in combination with duvelisib (phosphoinositide 3-kinase inhibitor) in patients with relapsed or refractory T- or NK-cell (natural killer cell) lymphoma (n = 49) (124). Patients are to receive 20 mg ruxolitinib BID with duvelisib (25 mg, 50 mg or 75 mg BID). The study includes dose escalation to determine maximum tolerated dose and efficacy evaluation in two cohorts (with and without JAK/STAT pathway activation). The study initiated on 12 August 2021, has terminated recruitment and estimates completion to occur in August 2027 (124). Preliminary results (2024) noted a maximum tolerated dose of 20 mg of ruxolitinib and 25 mg of duvelisib BID (127). The overall response rate (ORR) and complete response rate (CR) of all enrolled patients (n = 49) was 41% and 24% respectively (127). In the JAK/STAT activation cohort, complete responses (29%) occurred twice as often and overall response ratios (52%) were four times greater than those without mutations (14%; 14%) (p = 0.023) (127). Enrolled patients (n=49) presented with various disease subtypes, including T-follicular helper lymphomas (TFH; n = 14), PTCL-NOS (n = 13), and MF (n = 7) (127). The highest ORR (79%) and CR (64%) were observed in patients with TFH lymphomas (127). PTCL-NOS patients exhibited an ORR and CR of 23% and 15% respectively (127). Notably, MF patients had the lowest benefit, with an ORR of 14% and no complete responses (127). Treatment related AEs (grade 3 through 5) included neutropenia (24% G3, 14% G4), anemia (16% G3), thrombocytopenia (6% G3, 6% G4), lung infections (4% G3), hypertension (4% G3), hypertriglyceridemia (4% G3), transaminitis (4% G3), sepsis (2% G3, 2% G5), urinary tract infection (2% G3), diarrhea (2% G3), weight gain (2% G3), leukopenia (2% G3), and mucositis (2% G3) (127).
Wilcox et al. are currently conducting a non-randomized Phase 2, open label, clinical trial (NCT04858256) evaluating pacritinib in patients with relapsed/refractory T-cell neoplasms (goal n = 100) (125). All patients will receive 200 mg BID pacritinib and be grouped by subtype (PTCL-NOS, AITL/TFH PTCL, CTCL, Other PTCL). ORR, the primary outcome measure, will be assessed in CTCL by using the modified Severity Weighted Assessment Tool (mSWAT).
Brunner et al. are currently conducting a Phase 2A, open-label, clinical trial (NCT05879458) evaluating ritlecitinib in patients with CTCL including MF and SS (estimated n = 20) (123). All patients will receive 200 mg QD for 8 weeks followed by 100 mg QD for 16 weeks. The primary endpoint is the change in mSWAT at week 24 compared to baseline. Secondary endpoints include safety and quality of life measures.
JAKi use in CTCL – benefits, limitations and practical applications
Given the abovementioned reasons, JAKis might offer a targeted therapeutic strategy for CTCL. JAKis have exhibited efficacy in improving pruritus, erythema and tumor burden for some patients with advanced or refractory disease. This suggests a potential added benefit of JAKi use in patients who have previously failed traditional therapies. Given that existing evidence is based on a handful of case reports and few clinical trials, it remains speculative whether JAK inhibition will have a role in CTCL treatment, especially when considering specific disease subsets. More data is necessary to further clarify JAKi efficacy in CTCL. JAKi side effects are relatively well characterized in chronic inflammatory diseases and can include opportunistic infections and herpes zoster reactivation, hematologic toxicities (anemia, thrombocytopenia and neutropenia), acne, thromboembolic events, gastrointestinal problems (diarrhea and nausea), liver enzyme elevations, dyslipidemia, fatigue and headache (128). The safety profile in CTCL, however, remains to be determined with robust data from more clinical trials.
Future research directions
To determine a role for JAKi in CTCL treatment, more controlled clinical trials are evidently necessary, that include clinical endpoints such as mSWAT, quality of life and biomarkers from the blood and skin that might help to guide future stratified treatment approaches. Given the difficulty of obtaining repeated skin biopsies in patients, minimally invasive sampling techniques such as tape stripping are a very promising approach to monitor disease biomarkers (129–133), but data in CTCL are still missing. Enlarged datasets will hopefully provide a better understanding of targetable disease subsets in this highly heterogeneous disease spectrum.
Conclusion
While there is evidence of JAK/STAT activation in CTCL, its role in disease initiation and progression remains unclear. While mouse models are indispensable for better understanding basic mechanisms of inflammation and cancer, only clinical trials will be able to assess whether individual JAK or STAT alterations have a role in CTCL pathogenesis.
Author contributions
SP: Data curation, Visualization, Writing–original draft, Writing–review and editing. PB: Conceptualization, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
PB is principal investigator of a clinical trial investigating the effects of ritlecitinib on CTCL, supported by Pfizer.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cai, ZR, Chen, ML, Weinstock, MA, Kim, YH, Novoa, RA, and Linos, E. Incidence trends of primary cutaneous T-cell lymphoma in the us from 2000 to 2018: a seer population data analysis. JAMA Oncol (2022) 8(11):1690. doi:10.1001/jamaoncol.2022.3236
2. Bradford, PT, Devesa, SS, Anderson, WF, and Toro, JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood (2009) 113(21):5064–73. doi:10.1182/blood-2008-10-184168
3. Korgavkar, K, Xiong, M, and Weinstock, M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol (2013) 149(11):1295–9. doi:10.1001/jamadermatol.2013.5526
4. Criscione, VD, and Weinstock, MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol (2007) 143(7):854–9. doi:10.1001/archderm.143.7.854
5. Burns, MK, Ellis, CN, and Cooper, KD. Mycosis fungoides-type cutaneous T-cell lymphoma arising before 30 years of age. J Am Acad Dermatol (1992) 27(6):974–8. doi:10.1016/0190-9622(92)70297-S
6. Pope, E, Weitzman, S, Ngan, B, Walsh, S, Morel, K, Williams, J, et al. Mycosis fungoides in the pediatric population: report from an international childhood registry of cutaneous lymphoma. J Cutan Med Surg (2010) 14(1):1–6. doi:10.2310/7750.2009.08091
7. Agar, NS, Wedgeworth, E, Crichton, S, Mitchell, TJ, Cox, M, Ferreira, S, et al. Survival outcomes and prognostic factors in mycosis fungoides/sézary syndrome: validation of the revised international society for cutaneous lymphomas/European organisation for research and treatment of cancer staging proposal. JCO (2010) 28(31):4730–9. doi:10.1200/JCO.2009.27.7665
8. Hristov, AC, Tejasvi, T, and Wilcox, RA. Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management. Am J Hematol (2019) 94(9):1027–41. doi:10.1002/ajh.25577
9. Rodd, AL, Ververis, K, and Karagiannis, TC. Safety and efficacy of pralatrexate in the management of relapsed or refractory peripheral T-cell lymphoma. Clin Med Insights Oncol (2012) 6:305–14. doi:10.4137/CMO.S8536
10. Wilson, LD, Hinds, GA, and Yu, JB. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin Lymphoma Myeloma Leuk (2012) 12(5):291–6. doi:10.1016/j.clml.2012.06.010
11. Scarisbrick, JJ, Prince, HM, Vermeer, MH, Quaglino, P, Horwitz, S, Porcu, P, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and sezary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol (2015) 33(32):3766–73. doi:10.1200/JCO.2015.61.7142
12. Weinstock, MA. Mycosis fungoides in the United States: increasing incidence and descriptive epidemiology. JAMA (1988) 260(1):42. doi:10.1001/jama.1988.03410010050033
13. Ferenczi, K, and Makkar, HS. Cutaneous lymphoma: kids are not just little people. Clin Dermatol (2016) 34(6):749–59. doi:10.1016/j.clindermatol.2016.07.010
14. Surveillance, Epidemiology, and End Results (SEER) Program. SEERStat Database: Incidence - SEER Research Data, 22 Registries, Nov 2023 Sub (2000-2021) - Linked To County Attributes - Time Dependent (2000-2022) Income/Rurality, 1969-2022 Counties. April 2024 ed. Surveillance Research Program, National Cancer Institute. (2024). Bethesda, MD: SEERStat software version 8.4.2. Available from: http://seer.cancer.gov/registries/terms.html (Accessed May 6, 2024).
15. Khan, S, and Sawas, A. Antibody-directed therapies: toward a durable and tolerable treatment platform for CTCL. Front Oncol (2019) 9:645. doi:10.3389/fonc.2019.00645
16. Olsen, E, Vonderheid, E, Pimpinelli, N, Willemze, R, Kim, Y, Knobler, R, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood (2007) 110(6):1713–22. doi:10.1182/blood-2007-03-055749
17. Willemze, R, Cerroni, L, Kempf, W, Berti, E, Facchetti, F, Swerdlow, SH, et al. (2019). The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 133(16):1703–14.
18. Van Doorn, R, Van Haselen, CW, Van Voorst Vader, PC, Geerts, ML, Heule, F, de Rie, M, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol (2000) 136(4):504–10. doi:10.1001/archderm.136.4.504
19. Kim, YH, Bishop, K, Varghese, A, and Hoppe, RT. Prognostic factors in erythrodermic mycosis fungoides and the sézary syndrome. Arch Dermatol (1995) 131(9):1003–8. doi:10.1001/archderm.1995.01690210033005
20. Kim, YH, Liu, HL, Mraz-Gernhard, S, Varghese, A, and Hoppe, RT. Long-term outcome of 525 patients with mycosis fungoides and sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol (2003) 139(7):857–66. doi:10.1001/archderm.139.7.857
21. Whittaker, SJ, Marsden, JR, Spittle, M, Russell Jones, R, British Association of, D, and Group, UKCL. Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br J Dermatol (2003) 149(6):1095–107. doi:10.1111/j.1365-2133.2003.05698.x
22. Horwitz, SM. Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma (2008) 8(Suppl. 5):S187–92. doi:10.3816/CLM.2008.s.015
23. Johnson, WT, Mukherji, R, Kartan, S, Nikbakht, N, Porcu, P, and Alpdogan, O. Allogeneic hematopoietic stem cell transplantation in advanced stage mycosis fungoides and Sézary syndrome: a concise review. Chin Clin Oncol (2019) 8(1):12. doi:10.21037/cco.2018.10.03
24. Iqbal, M, Reljic, T, Ayala, E, Sher, T, Murthy, H, Roy, V, et al. Efficacy of allogeneic hematopoietic cell transplantation in cutaneous T cell lymphoma: results of a systematic review and meta-analysis. Biol Blood Marrow Transplant (2020) 26(1):76–82. doi:10.1016/j.bbmt.2019.08.019
25. de Masson, A, Beylot-Barry, M, Bouaziz, JD, Peffault de Latour, R, Aubin, F, Garciaz, S, et al. (2014). Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on cutaneous lymphomas. Haematologica 99(3):527–34.
26. Lovgren, ML, and Scarisbrick, JJ. Update on skin directed therapies in mycosis fungoides. Chin Clin Oncol (2019) 8(1):7. doi:10.21037/cco.2018.11.03
27. Kartan, S, Shalabi, D, O'Donnell, M, Alpdogan, SO, Sahu, J, Shi, W, et al. Response to topical corticosteroid monotherapy in mycosis fungoides. J Am Acad Dermatol (2021) 84(3):615–23. doi:10.1016/j.jaad.2020.05.043
28. Zackheim, HS, Kashani-Sabet, M, and Amin, S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol (1998) 134(8):949–54. doi:10.1001/archderm.134.8.949
29. Zackheim, HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther (2003) 16(4):283–7. doi:10.1111/j.1396-0296.2003.01639.x
30. Querfeld, C, Nelson, WW, Gor, D, Pashos, CL, Doan, QV, Turini, M, et al. Maintenance and concomitant therapy use with chlormethine gel among patients with stage IA/IB mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL): a real-world evidence study. Dermatol Ther (Heidelb) (2022) 12(12):2781–95. doi:10.1007/s13555-022-00831-w
31. Yonekura, K. Current treatment strategies and emerging therapies for cutaneous lymphoma. The J Dermatol (2022) 49(2):223–31. doi:10.1111/1346-8138.16289
32. Stern, RS, Nichols, KT, and Vakeva, LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). N Engl J Med (1997) 336(15):1041–5. doi:10.1056/NEJM199704103361501
33. Smith, BD, and Wilson, LD. Cutaneous lymphomas. Semin Radiat Oncol (2007) 17(3):158–68. doi:10.1016/j.semradonc.2007.02.001
34. Navi, D, Riaz, N, Levin, YS, Sullivan, NC, Kim, YH, and Hoppe, RT. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol (2011) 147(5):561–7. doi:10.1001/archdermatol.2011.98
35. Lindahl, LM, Kamstrup, MR, Petersen, PM, Wirén, J, Fenger-Grøn, M, Gniadecki, R, et al. Total skin electron beam therapy for cutaneous T-cell lymphoma: a nationwide cohort study from Denmark. Acta Oncologica (2011) 50(8):1199–205. doi:10.3109/0284186X.2011.585999
36. Shouman Na, T, El-Taher, Z, Rasheed, H, and Barsoum, M. Total skin electron beam therapy (TSEBT) in the management of mycosis fungoides: single institution experience. J Egypt Natl Cancer Inst (2003) 15:275–83. doi:10.21873/anticanres.16945
37. Jones, GL, Wittmann, G, Yokosawa, EB, Yu, H, Mercer, AJ, Lechan, RM, et al. Selective restoration of pomc expression in glutamatergic POMC neurons: evidence for a dynamic hypothalamic neurotransmitter network. eNeuro (2019) 6(2):0400–18.2019. doi:10.1523/ENEURO.0400-18.2019
38. Quiros, PA, Jones, GW, Kacinski, BM, Braverman, IM, Heald, PW, Edelson, RL, et al. Total skin electron beam therapy followed by adjuvant psoralen/ultraviolet-A light in the management of patients with T1 and T2 cutaneous T-cell lymphoma (mycosis fungoides). Int J Radiat Oncology*Biology*Physics (1997) 38(5):1027–35. doi:10.1016/s0360-3016(97)00127-2
39. Ysebaert, L, Truc, G, Dalac, S, Lambert, D, Petrella, T, Barillot, I, et al. Ultimate results of radiation therapy for T1-T2 mycosis fungoides (including reirradiation). Int J Radiat Oncology*Biology*Physics (2004) 58(4):1128–34. doi:10.1016/j.ijrobp.2003.08.007
40. Micaily, B, Miyamoto, C, Kantor, G, Lessin, S, Rook, A, Brady, L, et al. Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncology*Biology*Physics (1998) 42(2):361–4. doi:10.1016/s0360-3016(98)00218-1
41. Hoppe, RT, Fuks, Z, and Bagshaw, MA. The rationale for curative radiotherapy in mycosis fungoides. Int J Radiat Oncology*Biology*Physics (1977) 2(9-10):843–51. doi:10.1016/0360-3016(77)90182-1
42. Vloten, W, Vroome, H, and Noordijk, EM. Total skin electron beam irradiation for cutaneous T-cell lymphoma (mycosis fungoides). Br J Dermatol (1985) 112(6):697–702. doi:10.1111/j.1365-2133.1985.tb02340.x
43. Kim, YH, Jensen, RA, Watanabe, GL, Varghese, A, and Hoppe, RT. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol (1996) 132(11):1309–13. doi:10.1001/archderm.1996.03890350051009
44. Wilson, LD, Licata, AL, Braverman, IM, Edelson, RL, Heald, PW, Feldman, AM, et al. Systemic chemotherapy and extracorporeal photochemotherapy for T3 and T4 cutaneous T-cell lymphoma patients who have achieved a complete response to total skin electron beam therapy. Int J Radiat Oncology*Biology*Physics (1995) 32(4):987–95. doi:10.1016/0360-3016(95)00073-8
45. Chinn, DM, Chow, S, Kim, YH, and Hoppe, RT. Total skin electron beam therapy with or without adjuvant topical nitrogen mustard or nitrogen mustard alone as initial treatment of T2 and T3 mycosis fungoides. Int J Radiat Oncology*Biology*Physics (1999) 43(5):951–8. doi:10.1016/s0360-3016(98)00517-3
46. Wilson, LD, Jones, GW, Kim, D, Rosenthal, D, Christensen, IR, Edelson, RL, et al. Experience with total skin electron beam therapy in combination with extracorporeal photopheresis in the management of patients with erythrodermic (T4) mycosis fungoides. J Am Acad Dermatol (2000) 43(1 Pt 1):54–60. doi:10.1067/mjd.2000.105510
47. Aral, IP, Göçer Gürok, N, Konuk, AO, and Ucer, O. Ultra-low-dose radiotherapy for palliation of mycosis fungoides. Case Rep Dermatol Med (2020) 2020:4216098. doi:10.1155/2020/4216098
48. Jones, GW, Tadros, A, Hodson, DI, Rosenthal, D, Roberts, J, and Thorson, B. Prognosis with newly diagnosed mycosis fungoides after total skin electron radiation of 30 or 35 GY. Int J Radiat Oncology*Biology*Physics (1994) 28(4):839–45. doi:10.1016/0360-3016(94)90103-1
49. Kazmierska, J. Clinical results of the total skin electron irradiation of the mycosis fungoides in adults. Conventional fractionation and low dose schemes. Rep Pract Oncol and Radiother (2014) 19(2):99–103. doi:10.1016/j.rpor.2013.08.008
50. Kroeger, K, Elsayad, K, Moustakis, C, Haverkamp, U, and Eich, HT. Low-dose total skin electron beam therapy for cutaneous lymphoma: minimal risk of acute toxicities. Strahlenther Onkol (2017) 193(12):1024–30. doi:10.1007/s00066-017-1188-8
51. Harrison, C, Young, J, Navi, D, Riaz, N, Lingala, B, Kim, Y, et al. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncology*Biology*Physics (2011) 81(4):e651–7. doi:10.1016/j.ijrobp.2011.01.023
52. Hamminga, L, Hermans, J, Noordijk, EM, Meijer, CJ, Scheffer, E, and Vloten, W. Cutaneous T-cell lymphoma: clinicopathological relationships, therapy and survival in ninety-two patients. Br J Dermatol (1982) 107(2):145–56. doi:10.1111/j.1365-2133.1982.tb00332.x
53. Zackheim, HS. Cutaneous T cell lymphoma: update of treatment. Dermatology (1999) 199(2):102–5. doi:10.1159/000018214
54. Alenezi, F, Girard, C, Bessis, D, Guillot, B, Du-Thanh, A, and Dereure, O. Benefit/risk ratio of low-dose methotrexate in cutaneous lesions of mycosis fungoides and sezary syndrome. Acta Derm Venereol (2021) 101(2):adv00384. doi:10.2340/00015555-3719
55. Duvic, M, Martin, AG, Kim, Y, Olsen, E, Wood, GS, Crowley, CA, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol (2001) 137(5):581–93.
56. Duvic, M, Hymes, K, Heald, P, Breneman, D, Martin, AG, Myskowski, P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol (2001) 19(9):2456–71. doi:10.1200/JCO.2001.19.9.2456
57. Zhang, Q, Wang, S, Chen, J, and Yu, Z. Histone deacetylases (HDACs) guided novel therapies for T-cell lymphomas. Int J Med Sci (2019) 16(3):424–42. doi:10.7150/ijms.30154
58. Drugs@FDA. Zolinza (vorinostat): labeling-package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021991s009lbl.pdf (Accessed April 24, 2024).
59. Frye, R, Myers, M, Axelrod, KC, Ness, EA, Piekarz, RL, Bates, SE, et al. Romidepsin: a new drug for the treatment of cutaneous T-cell lymphoma. Clin J Oncol Nurs (2012) 16(2):195–204. doi:10.1188/12.CJON.195-204
60. Drugs@FDA. Istodax (romidepsin). Efficacy-labeling change with clinical data. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022393s017lbl.pdf (Accessed April 24, 2024).
61. Remer, M, Al-Shamkhani, A, Glennie, M, and Johnson, P. Mogamulizumab and the treatment of CCR4-positive T-cell lymphomas. Immunotherapy (2014) 6(11):1187–206. doi:10.2217/imt.14.94
62. Drugs@FDA. Poteligeo (mogamulizumab-kpkc). Labeling-package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761051s015lbl.pdf (Accessed April 24, 2024).
63. Kamijo, H, and Miyagaki, T. Mycosis fungoides and sezary syndrome: updates and review of current therapy. Curr Treat Options Oncol (2021) 22(2):10. doi:10.1007/s11864-020-00809-w
64. Drugs@FDA. Adcetris (brentuximab vedotin). Efficacy-labeling change with clinical data. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125388s107lbl.pdf (Accessed April 24, 2024).
65. Kim, YH, Bagot, M, Pinter-Brown, L, Rook, AH, Porcu, P, Horwitz, SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. The Lancet Oncol (2018) 19(9):1192–204. doi:10.1016/S1470-2045(18)30379-6
66. Prince, HM, Kim, YH, Horwitz, SM, Dummer, R, Scarisbrick, J, Quaglino, P, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. The Lancet (2017) 390(10094):555–66. doi:10.1016/S0140-6736(17)31266-7
67. Dumont, M, Peffault de Latour, R, Ram-Wolff, C, Bagot, M, and de Masson, A. Allogeneic hematopoietic stem cell transplantation in cutaneous T-cell lymphomas. Cancers (2020) 12(10):2856. doi:10.3390/cancers12102856
68. Atilla, PA, and Atilla, E. Are we there yet? cellular therapies for cutaneous T cell lymphoma. Curr Res Translational Med (2023) 71(2):103390. doi:10.1016/j.retram.2023.103390
69. Wu, PA, Kim, YH, Lavori, PW, Hoppe, RT, and Stockerl-Goldstein, KE. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sezary syndrome. Biol Blood Marrow Transplant (2009) 15(8):982–90. doi:10.1016/j.bbmt.2009.04.017
70. Duarte, RF, Canals, C, Onida, F, Gabriel, IH, Arranz, R, Arcese, W, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol (2010) 28(29):4492–9. doi:10.1200/JCO.2010.29.3241
71. Aldana, A., Morales-Raya, C., Traves, V., Viqueira, A., and Llamas-Velasco, M. (2024). JAK inhibitors in cutaneous T-cell lymphoma: friend or foe? a systematic review. Cancers. 16(5), 780. doi:10.3390/cancers16050780
72. Bousoik, E, and Montazeri Aliabadi, H. “Do we know jack” about JAK? A closer look at JAK/STAT signaling pathway. Front Oncol (2018) 8:287. doi:10.3389/fonc.2018.00287
73. Harrison, DA. The JAK/STAT pathway. Cold Spring Harbor Perspect Biol (2012) 4(3):a011205. doi:10.1101/cshperspect.a011205
74. Hu, X, Li, J, Fu, M, Zhao, X, and Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduction Targeted Ther (2021) 6(1):402. doi:10.1038/s41392-021-00791-1
75. Xue, C, Yao, Q, Gu, X, Shi, Q, Yuan, X, Chu, Q, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduction Targeted Ther (2023) 8(1):204. doi:10.1038/s41392-023-01468-7
76. Benveniste, EN, Liu, Y, McFarland, BC, and Qin, H. Involvement of the janus kinase/signal transducer and activator of transcription signaling pathway in multiple sclerosis and the animal model of experimental autoimmune encephalomyelitis. J Interferon and Cytokine Res (2014) 34(8):577–88. doi:10.1089/jir.2014.0012
77. O'Shea, J, and Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity (2012) 36(4):542–50. doi:10.1016/j.immuni.2012.03.014
78. O'Shea, JJ, Schwartz, DM, Villarino, AV, Gadina, M, McInnes, IB, and Laurence, A. The JAK-STAT pathway: impact on human disease and therapeutic intervention (2014).
79. Garcia-Diaz, N, Piris, MA, Ortiz-Romero, PL, and Vaque, JP. Mycosis fungoides and sezary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers (Basel) (2021) 13(8):1931. doi:10.3390/cancers13081931
80. O’Shea, JJ, Schwartz, DM, Villarino, AV, Gadina, M, McInnes, IB, and Laurence, A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med (2015) 66:311–28. doi:10.1146/annurev-med-051113-024537
81. Leonard, WJ, and O'Shea, JJ. Jaks and STATs: biological implications. Annu Rev Immunol (1998) 16:293–322. doi:10.1146/annurev.immunol.16.1.293
82. Macha, MA, Rachagani, S, Gupta, S, Pai, P, Ponnusamy, MP, Batra, SK, et al. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett (2013) 341(2):166–77. doi:10.1016/j.canlet.2013.07.037
83. Slattery, ML, Lundgreen, A, Kadlubar, SA, Bondurant, KL, and Wolff, RK. JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol Carcinogenesis (2013) 52(2):155–66. doi:10.1002/mc.21841
84. Kowshik, J, Baba, AB, Giri, H, Deepak Reddy, G, Dixit, M, and Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE (2014) 9(10):e109114. doi:10.1371/journal.pone.0109114
85. Li, X, Zuo, X, Jing, J, Ma, Y, Wang, J, Liu, D, et al. Small-molecule-Driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell (2015) 17(2):195–203. doi:10.1016/j.stem.2015.06.003
86. Li, Y-J, Zhang, C, Martincuks, A, Herrmann, A, and Yu, H. STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer (2023) 23(3):115–34. doi:10.1038/s41568-022-00537-3
87. Xin, P, Xu, X, Deng, C, Liu, S, Wang, Y, Zhou, X, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacology (2020) 80:106210. doi:10.1016/j.intimp.2020.106210
88. Perez, C, Gonzalez-Rincon, J, Onaindia, A, Almaraz, C, Garcia-Diaz, N, Pisonero, H, et al. Mutated JAK kinases and deregulated STAT activity are potential therapeutic targets in cutaneous T-cell lymphoma. Haematologica (2015) 100(11):e450–3. doi:10.3324/haematol.2015.132837
89. Shawky, AM, Almalki, FA, Abdalla, AN, Abdelazeem, AH, and Gouda, AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics (2022) 14(5):1001. doi:10.3390/pharmaceutics14051001
90. European-Medicines-Agency (2024) Committee for medicinal products for human use (CHMP): summary of opinion.
91. Drugs FDA. FDA-approved drugs: highlights of prescribing information (2022). Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (Accessed December 10, 2024).
92. Pharmaceutical and Medical Devices Agency. Approved medical devices [Internet]. Tokyo, Japan: PMDA. (2024). Available from: https://www.pmda.go.jp/.
93. Song, X, Chang, S, Seminario-Vidal, L, de Mingo Pulido, A, Tordesillas, L, Song, X, et al. Genomic and single-cell landscape reveals novel drivers and therapeutic vulnerabilities of transformed cutaneous T-cell lymphoma. Cancer Discov (2022) 12(5):1294–313. doi:10.1158/2159-8290.CD-21-1207
94. Wang, L, Ni, X, Covington, KR, Yang, BY, Shiu, J, Zhang, X, et al. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet (2015) 47(12):1426–34. doi:10.1038/ng.3444
95. Koo, GC, Tan, SY, Tang, T, Poon, SL, Allen, GE, Tan, L, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov (2012) 2(7):591–7. doi:10.1158/2159-8290.CD-12-0028
96. Vaque, JP, Gomez-Lopez, G, Monsalvez, V, Varela, I, Martínez, N, Pérez, C, et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood (2014) 123(13):2034–43. doi:10.1182/blood-2013-05-504308
97. Kiel, MJ, Sahasrabuddhe, AA, Rolland, DCM, Velusamy, T, Chung, F, Schaller, M, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sezary syndrome. Nat Commun (2015) 6:8470. doi:10.1038/ncomms9470
98. Woollard, WJ, Pullabhatla, V, Lorenc, A, Patel, VM, Butler, RM, Bayega, A, et al. Candidate driver genes involved in genome maintenance and DNA repair in Sezary syndrome. Blood (2016) 127(26):3387–97. doi:10.1182/blood-2016-02-699843
99. Ungewickell, A, Bhaduri, A, Rios, E, Reuter, J, Lee, CS, Mah, A, et al. Genomic analysis of mycosis fungoides and Sezary syndrome identifies recurrent alterations in TNFR2. Nat Genet (2015) 47(9):1056–60. doi:10.1038/ng.3370
100. Iyer, A, Hennessey, D, O'Keefe, S, Patterson, J, Wang, W, Wong, GKS, et al. Branched evolution and genomic intratumor heterogeneity in the pathogenesis of cutaneous T-cell lymphoma. Blood Adv (2020) 4(11):2489–500. doi:10.1182/bloodadvances.2020001441
101. Bastidas Torres, AN, Cats, D, Mei, H, Szuhai, K, Willemze, R, Vermeer, MH, et al. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosomes Cancer (2018) 57(12):653–64. doi:10.1002/gcc.22679
102. Sommer, VH, Clemmensen, OJ, Nielsen, O, Wasik, M, Lovato, P, Brender, C, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia (2004) 18(7):1288–95. doi:10.1038/sj.leu.2403385
103. McGirt, LY, Jia, P, Baerenwald, DA, Duszynski, RJ, Dahlman, KB, Zic, JA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood (2015) 126(4):508–19. doi:10.1182/blood-2014-11-611194
104. Choi, J, Goh, G, Walradt, T, Hong, BS, Bunick, CG, Chen, K, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet (2015) 47(9):1011–9. doi:10.1038/ng.3356
105. da Silva Almeida, AC, Abate, F, Khiabanian, H, Martinez-Escala, E, Guitart, J, Tensen, CP, et al. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat Genet (2015) 47(12):1465–70. doi:10.1038/ng.3442
106. Park, J, Yang, J, Wenzel, AT, Ramachandran, A, Lee, WJ, Daniels, JC, et al. Genomic analysis of 220 CTCLs identifies a novel recurrent gain-of-function alteration in RLTPR (p.Q575E). Blood (2017) 130(12):1430–40. doi:10.1182/blood-2017-02-768234
107. van Kester, MS, Ballabio, E, Benner, MF, Chen, XH, Saunders, NJ, van der Fits, L, et al. miRNA expression profiling of mycosis fungoides. Mol Oncol (2011) 5(3):273–80. doi:10.1016/j.molonc.2011.02.003
108. Netchiporouk, E, Litvinov, IV, Moreau, L, Gilbert, M, Sasseville, D, and Duvic, M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle (2014) 13(21):3331–5. doi:10.4161/15384101.2014.965061
109. Anderson, NM, and Simon, MC. The tumor microenvironment. Curr Biol (2020) 30(16):R921–R925. doi:10.1016/j.cub.2020.06.081
110. Krejsgaard, T, Lindahl, LM, Mongan, NP, Wasik, MA, Litvinov, IV, Iversen, L, et al. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin Immunopathol (2017) 39(3):269–82. doi:10.1007/s00281-016-0594-9
111. Kaiko, GE, Horvat, JC, Beagley, KW, and Hansbro, PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology (2008) 123(3):326–38. doi:10.1111/j.1365-2567.2007.02719.x
112. Catherine, J, and Roufosse, F. What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin Immunopathol (2021) 43(3):439–58. doi:10.1007/s00281-021-00857-w
113. Vahabi, SM, Bahramian, S, Esmaeili, F, Danaei, B, Kalantari, Y, Fazeli, P, et al. JAK inhibitors in cutaneous T-cell lymphoma: friend or foe? A systematic review of the published literature. Cancers (Basel) (2024) 16(5):861. doi:10.3390/cancers16050861
114. Castillo, DE, Romanelli, P, Lev-Tov, H, and Kerdel, F. A case of erythrodermic mycosis fungoides responding to upadacitinib. JAAD Case Rep (2022) 30:91–3. doi:10.1016/j.jdcr.2022.10.010
115. Kook, H, Park, SY, Hong, N, Lee, DH, Jung, HJ, Park, MY, et al. Severely pruritic mycosis fungoides successfully treated with upadacitinib. JDDG: J der Deutschen Dermatologischen Gesellschaft (2024) 22(3):450–1. doi:10.1111/ddg.15325
116. Mo, S, and Friedmann, D. Cutaneous T-cell lymphoma in a JAK inhibitor patient: a case report, 12. SAGE Open Med Case Rep (2024). p. 2050313X241231491. doi:10.1177/2050313X241231491
117. Levy, R, Fusaro, M, Guerin, F, Chetouani, A, Moshous, D, Fischer, A, et al. Efficacy of ruxolitinib in subcutaneous panniculitis-like T-cell lymphoma and hemophagocytic lymphohistiocytosis. Blood Adv (2020) 4(7):1383–7. doi:10.1182/bloodadvances.2020001497
118. Pacritinib in Relapsed/Refractory Lymphoproliferative Disorders (2021). Available at: https://clinicaltrials.gov/study/NCT03601819?term=NCT03601819&rank=1 (Accessed December 10, 2024).
119. Horwitz, SM, Feldman, TA, Hess, BT, Khodadoust, MS, Kim, YH, Munoz, J, et al. The novel SYK/JAK inhibitor cerdulatinib demonstrates good tolerability and clinical response in a phase 2a study in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood (2018) 132(Suppl. 1):1001. doi:10.1182/blood-2018-99-119944
120. Phase 1/2a Dose Escalation Study in Participants With CLL, SLL, or NHL (2022). Available at: https://clinicaltrials.gov/study/NCT01994382?term=NCT01994382%20&limit=10&rank=1 (Accessed January 16, 2025).
121. Horwitz, S, Feldman, T, Hess, B, Khodadoust, MS, Kim, YH, Munoz, J, et al. A phase 2 study of the dual SYK/JAK inhibitor cerdulatinib demonstrates good tolerability and clinical response in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood (2019) 134(Suppl. ment_1):466. doi:10.1182/blood-2019-123986
122. Moskowitz, AJ, Ghione, P, Jacobsen, E, Ruan, J, Schatz, JH, Noor, S, et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood (2021) 138(26):2828–37. doi:10.1182/blood.2021013379
123. Ritlecitinib in CTCL. Available at: https://clinicaltrials.gov/study/NCT05879458?term=NCT05879458&rank=1 (Accessed January 16, 2025).
124. A Study of Ruxolitinib and Duvelisib in People With Lymphoma (2024). Available at: https://clinicaltrials.gov/study/NCT05010005?term=NCT05010005&rank=1 (Accessed January 16, 2025).
125. Pacritinib in Relapsed/Refractory T-cell Lymphoproliferative Neoplasms. Available at: https://clinicaltrials.gov/study/NCT04858256?term=NCT04858256&rank=1 (Accessed January 16, 2025).
126. Study of Ruxolitinib in Relapsed or Refractory T or NK Cell Lymphoma (2025). Available at: https://clinicaltrials.gov/study/NCT02974647?term=NCT02974647%20&rank=1 (Accessed January 16, 2025).
127. Moskowitz, AJ, Ganesan, N, Chang, T, Davey, T, Hancock, H, Smith, M, et al. Dual-targeted therapy with ruxolitinib plus duvelisib for T-cell lymphoma. Blood (2024) 144(1):463. doi:10.1182/blood-2024-206462
128. Yoon, S, Kim, K, Shin, K, Kim, H, Kim, B, Kim, M, et al. The safety of systemic Janus kinase inhibitors in atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol (2024) 38(1):52–61. doi:10.1111/jdv.19426
129. Del Duca, E, He, H, Liu, Y, Pagan, AD, David, E, Cheng, J, et al. Intrapatient comparison of atopic dermatitis skin transcriptome shows differences between tape-strips and biopsies. Allergy (2024) 79:80–92. doi:10.1111/all.15845
130. He, H, Bissonnette, R, Wu, J, Diaz, A, Saint-Cyr Proulx, E, Maari, C, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol (2021) 147(1):199–212. doi:10.1016/j.jaci.2020.05.048
131. Pavel, AB, Renert-Yuval, Y, Wu, J, Del Duca, E, Diaz, A, Lefferdink, R, et al. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy (2021) 76(1):314–25. doi:10.1111/all.14490
132. He, H, Olesen, CM, Pavel, AB, Clausen, ML, Wu, J, Estrada, Y, et al. Tape-strip proteomic profiling of atopic dermatitis on dupilumab identifies minimally invasive biomarkers. Front Immunol (2020) 11:1768. doi:10.3389/fimmu.2020.01768
133. Mikhaylov, D, Del Duca, E, Olesen, CM, He, H, Wu, J, Ungar, B, et al. Transcriptomic profiling of tape-strips from moderate to severe atopic dermatitis patients treated with dupilumab. Dermatitis (2021) 32(1S):S71–S80. doi:10.1097/DER.0000000000000764
134. Mehta-Shah, N, Horwitz, SM, Ansell, S, Ai, WZ, Barnes, J, Barta, SK, et al. (2020). NCCN guidelines insights: primary cutaneous lymphomas, version 2.2020. J Natl Compr Canc Netw. 18(5):522–36.
135. Stuver, R, and Geller, S. (2023). Advances in the treatment of mycoses fungoides and Sézary syndrome: a narrative update in skin-directed therapies and immune-based treatments. Front Immunol. 14, 1284045.
136. Horwitz, SM, Feldman, TA, Ye, JC, Khodadoust, MS, Munoz, J, Hamlin, PA, et al. Results from an open-label phase 2a study of cerdulatinib, a dual spleen tyrosine kinase/janus kinase inhibitor, in relapsed/refractory peripheral T-cell lymphoma. Leuk Lymphoma (2025) 66(6): 1100–1110. doi:10.1080/10428194.2025.2455489
Keywords: CTCL, lymphoma, mycosis fugoides, Sezary syndrome, JAK inhibition
Citation: Packer SE and Brunner PM (2025) Janus kinase inhibitors – a role for the treatment of cutaneous T-cell lymphomas?. Oncol. Rev. 19:1482866. doi: 10.3389/or.2025.1482866
Received: 18 August 2024; Accepted: 17 February 2025;
Published: 11 August 2025.
Edited by:
Cornelis Pieter Tensen, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Mauro Alaibac, University of Padua, ItalyJose A. Sanches, University of São Paulo, Brazil
Copyright © 2025 Packer and Brunner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick M. Brunner, cGF0cmljay5icnVubmVyQG1vdW50c2luYWkub3Jn
 Sarah E. Packer
Sarah E. Packer Patrick M. Brunner
Patrick M. Brunner