- 1The First School of Medicine, School of Information and Engineering, Wenzhou Medical University, Wenzhou, China
- 2Department of Pulmonary and Critical Care Medicine, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, Zhejiang, China
- 3Department of Gynecology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Cervical cancer (CeCa) remains a significant global health burden, with complex interactions between oxidative stress and immune response playing critical roles in its pathogenesis and progression. This review synthesizes current knowledge on the molecular mechanisms linking oxidative stress pathways and immune evasion, particularly focusing on human papillomavirus oncogenes E6 and E7. We highlight the dual roles of immune components such as Type 17 T helper (Th17) cells and the antioxidant enzyme superoxide dismutase 2 (SOD2), which exhibit context-dependent tumor-promoting and suppressive functions. While extensive mechanistic insights have been gained, translation to clinical practice remains limited, partly due to inconsistent biomarkers and incomplete understanding of therapeutic resistance. Recent advances in targeted therapies, including mitochondrial inhibitors, Immune checkpoint inhibitors (ICIs) (e.g., pembrolizumab, nivolumab), and PARP inhibitors, demonstrate promise but face translational hurdles such as assay variability and immune-related adverse events. Future research must address gaps including predictive biomarker development, noninvasive monitoring via liquid biopsy, and rational combination therapies integrating redox modulation and immunotherapy. Enhanced multi-omics integration and refined preclinical models are essential to advance personalized treatment strategies for CeCa.
1 Introduction
Cervical cancer (CeCa), despite being largely preventable, remains a leading cause of cancer-related mortality among women worldwide. In 2022, an estimated 662,301 new cases and 348,874 deaths were reported globally (1, 2). According to the American Cancer Society, while long-term declines in the US have plateaued, there are notable age-specific differences. Incidence has increased by about 1.7% per year among women 30–44 years old from 2012 to 2019, while it has declined by approximately 11% per year among those 20–24 years old (3, 4).
In China, CeCa is a significant concern, accounting for 150,659 new cases and 55,694 deaths in 2022, which represents roughly 23% of global incidents and 16% of global deaths (1). The situation is further compounded by urban-rural disparities, inadequate screening, and uneven HPV vaccine uptake (5).
The etiology of CeCa is strongly linked to persistent infection with high-risk human papillomavirus (HPV) types, especially human papillomavirus type 16 (HPV16) and human papillomavirus 18 (HPV18). The viral oncogenes E6 and E7 disrupt tumor suppressor pathways by inactivating p53 and retinoblastoma protein (pRb), facilitating uncontrolled cellular proliferation and tumor progression (6–8). Beyond oncogenic transformation, HPV E6/E7 also contribute to immune evasion by modulating antigen presentation and dampening immune surveillance, creating an immunosuppressive microenvironment conducive to tumor growth [(9); Figure 1A].
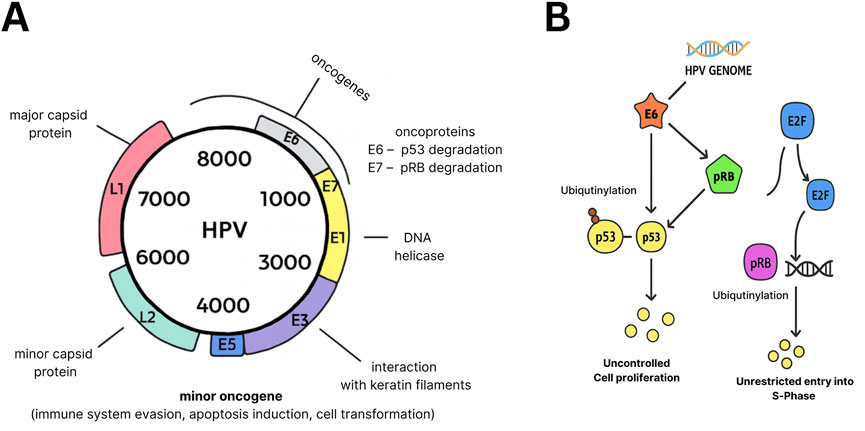
Figure 1. Oncogenic functions of HPV E6 and E7. (A) Genomic organization of the HPV genome, showing capsid proteins (L1, L2) and early genes (E1–E7). (B) Mechanisms of oncogenesis: E6 promotes ubiquitination and degradation of p53, while E7 inactivates pRb to release E2F, driving S-phase entry and uncontrolled proliferation. Both proteins additionally facilitate immune evasion through downregulation of MHC-I and disruption of interferon signaling (not shown).
Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) generation and antioxidant defenses, has emerged as a critical factor in cervical carcinogenesis. Excess ROS can induce DNA damage, lipid peroxidation, and protein oxidation, promoting genetic instability and oncogenic signaling (2). Interestingly, oxidative stress plays a dual role by also activating immune responses, which can either suppress or promote tumor progression depending on the context. The crosstalk between oxidative stress pathways and immune regulation in CeCa is complex and incompletely understood, necessitating further exploration (10).
The tumor microenvironment (TME) in CeCa includes diverse immune cells, such as tumor-associated macrophages (TAMs), T cells, and regulatory T cells (Tregs), which influence cancer development and response to therapy. Immune evasion mechanisms mediated by HPV, coupled with oxidative stress-induced inflammation, contribute to TME remodeling and tumor immune escape (2). However, conflicting evidence exists regarding the role of certain immune subsets, such as Type 17 T helper (Th17) cells, which have been reported to exhibit both pro- and anti-tumorigenic effects in different studies (10). This highlights the need for more detailed investigation into immune dynamics within CeCa.
2 Core pathogenesis: HPV–immunity–redox crosstalk
2.1 HPV-mediated immune evasion mechanisms
Persistent infection with high-risk HPV types drives cervical carcinogenesis through coordinated immune-evasion strategies. The viral oncoproteins E6 and E7 impair antigen presentation by downregulating major histocompatibility complex class I (MHC-I) molecules on infected cell surfaces, limiting recognition by CD8+ T cells (9). Furthermore, these oncogenes interfere with interferon (IFN) signaling pathways, reducing the production of type I IFNs that are crucial for antiviral immunity (11). This suppression of innate immune responses fosters an immunosuppressive TME that promotes viral persistence and tumor progression [(12), Figure 2A].
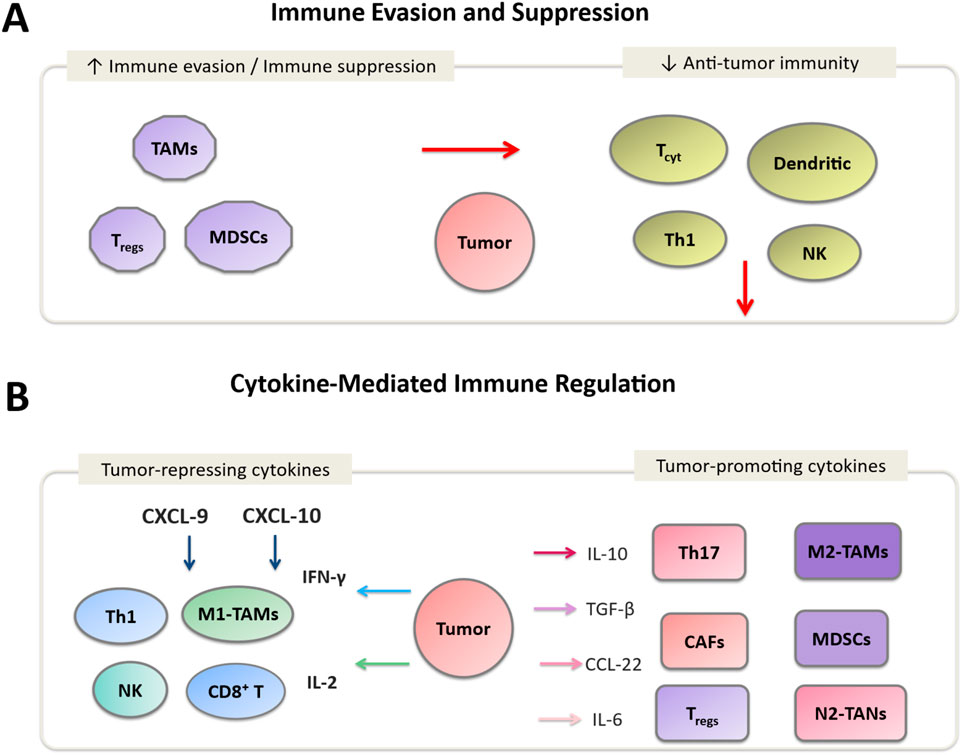
Figure 2. Immune regulation in the cervical cancer tumor microenvironment (TME). (A) Immune evasion mechanisms involving Tregs, MDSCs, and M2-TAMs, along with immunosuppressive cytokines (IL-10, TGF-β, IL-6) that inhibit CD8+ T cells and NK cells. (B) Cytokine-mediated modulation: tumor-repressing cytokines (IFN-γ, IL-2, CXCL-9) activate effector immune cells (CD8+ T cells, NK cells, M1-TAMs), while tumor-promoting cytokines (IL-10, TGF-β, IL-6) enhance angiogenesis, immune escape, and tumor progression.
Innate immunity disruption: HPV employs multiple strategies to evade innate immune responses. β-HPV type 38 E6/E7 proteins have been shown to downregulate Toll-like receptor 9 (TLR9), a DNA sensor important for antiviral responses, in keratinocyte models (13, 14). While these findings are specific to HPV38 and should not be directly generalized to high-risk mucosal types such as HPV16/18, some evidence suggests similar mechanisms may occur. Cell-based experiments also demonstrate that HPV16 E6 interferes with the RIG-I pathway by binding the ubiquitin ligase TRIM25 (15, 16), causing TRIM25 degradation and preventing RIG-I activation via K63-linked ubiquitination (16). The impaired RIG-I pathway further suppresses downstream type I interferon signaling in vitro and requires clinical confirmation in CeCa patients (17–19).
Adaptive immunity suppression: HPV oncoproteins target the cGAS–STING DNA-sensing pathway (20). HPV16/18 oncoproteins downregulate STING transcription in experimental systems (21). This is in line with the lower level of STIING mRNA in HPV-positive cervical lesions compared with normal tissue. These observations are most consistent with transcriptional downregulation by viral proteins rather than established epigenetic silencing in patients (22, 23).
The immune escape facilitated by HPV oncogenes also involves modulation of immune checkpoint molecules such as programmed death ligand-1 (PD-L1), which further inhibits T-cell activation (24, 25). Elevated PD-L1 expression has been observed in CeCa tissues and correlates with poor prognosis and resistance to conventional therapies (26–28).
Clinically, durable responses to anti-PD-1 therapy have been observed in the cervical cancer cohort of the multi-cohort KEYNOTE-158 study, particularly in PD-L1–positive disease (29, 30). HPV oncogenes additionally induce chronic inflammation via COX-2 and PGE2, activating the COX–PG pathway (31, 32).
2.2 HPV-induced oxidative stress
Persistent HPV infection disrupts cellular redox balance through coordinated actions of viral oncoproteins (33). E6 degrades p53, impairing transcriptional activation of antioxidant genes (34, 35), while E7 inactivates pRb, promoting cell-cycle progression under oxidative conditions (36–38). This dual assault creates a pro-oxidant state characterized by mitochondrial dysfunction, in which damaged electron-transport chains generate excessive reactive oxygen species (ROS) (6, 33, 39).
Metabolic reprogramming: HPV further amplifies oxidative stress through metabolic changes. The E4 protein can disrupt mitochondrial–cytoskeletal interactions, reducing membrane potential and promoting apoptosis resistance (40, 41). While E6/E7 upregulate glycolytic enzymes to sustain the Warburg effect (42, 43). This metabolic shift creates a feed-forward loop in which mitochondrial ROS generation perpetuates further oxidative damage (40, 44, 45).
DNA damage and mutagenesis: The resulting oxidative stress induces nuclear and mitochondrial DNA damage, including mutagenic 8-hydroxy-2′-deoxyguanosine (8-OHdG) lesions that contribute to oncogenic transformation. The resulting oxidative stress induces nuclear and mitochondrial DNA damage, including mutagenic 8-hydroxy-2′-deoxyguanosine (8-OHdG) lesions that drive oncogenic transformation (8, 43, 46). These immunosuppressive networks further correlate with both TAM polarization states and advanced disease progression (43, 47). Certain mitochondrial DNA polymorphisms (e.g., C150T) may also exacerbate this process, increasing susceptibility to HPV persistence and cervical cancer development (48).
2.3 Context-dependent immune modulation
The TME in CeCa comprises diverse immune cells that influence tumor development and therapy response (Table 1). Conflicting evidence exists regarding Th17 cells: in early lesions Th17-associated IL-17 can participate in neutrophil recruitment (49).Whereas in advanced disease Th17 cells have been linked to angiogenesis, immunosuppression, and tumor progression (50, 51). Similarly, the antioxidant enzyme superoxide dismutase 2 (SOD2) demonstrates context-dependent functions, acting as both protective and tumor-supportive depending on redox status and treatment exposure (52, 53).
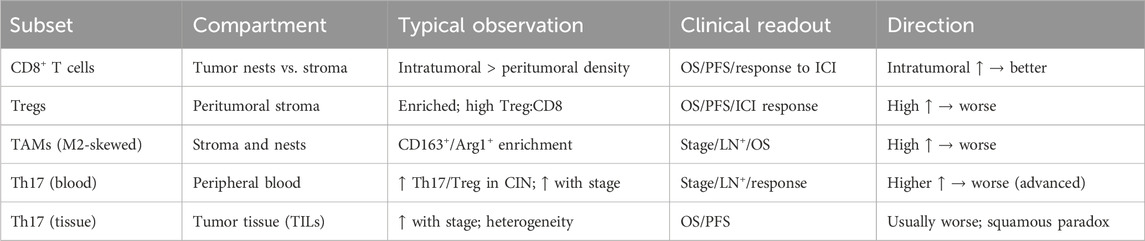
Table 1. Immune cell subsets in cervical cancer: compartmental distribution, stage-specific dynamics, clinical correlations, and direction of prognostic effect.
3 Immune landscape in cervical cancer
The cervical cancer tumor microenvironment (TME) is composed of diverse immune populations whose density, phenotype, and spatial organization influence prognosis and therapeutic response (Figure 1B). The balance between effector and suppressive subsets, as well as their localization within tumor nests, peritumoral stroma, or circulation, critically shapes clinical outcomes.
3.1 Cytotoxic T cells (CD8+)
CD8+ tumor-infiltrating lymphocytes (TILs) constitute a principal effector population in antitumor immunity (54). Their distribution within the TME has prognostic significance: high intratumoral (nest) density is generally associated with favorable outcomes, whereas exclusion to the peritumoral stroma correlates with immune escape and resistance to immune checkpoint inhibitors (ICIs) (55, 56). Spatial metrics, such as proximity to the tumor–stroma interface, are increasingly recognized as key determinants of therapeutic efficacy and should be systematically reported in clinical studies (57). High intratumoral CD8+ density has been shown to be an independent predictor of improved progression-free and overall survival in cervical cancer patients (58). Meta-analyses also confirm the prognostic value of CD8+ TILs, with increased infiltration correlating with significantly better survival outcomes across cervical cohorts (59).
3.2 Regulatory T cells (Tregs)
Regulatory T cells (Tregs) are enriched in the peritumoral stroma of cervical tumors, where they exert suppressive effects on effector T cells via cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) signaling and secretion of inhibitory cytokines such as interleukin-10 (IL-10) and TGF-β: Transforming Growth Factor-β (Figure 1B). High Treg density and elevated Treg:CD8 ratios are consistently associated with poor prognosis and diminished ICI efficacy. This reflects the creation of an exclusionary stromal niche that is unfavorable for effective antitumor immunity (47, 60).
3.3 Tumor-associated macrophages (TAMs)
Cervical tumors frequently exhibit polarization toward an M2-skewed macrophage phenotype (61). These tumor-associated macrophages (TAMs) are characterized by arginase-1 expression, pro-angiogenic activity, and suppression of T-cell effector function [(62, 63), Figure 2B]. Enrichment of M2-like TAMs correlates with advanced disease stage, lymph-node metastasis, and resistance to therapy (64–66). Accurate characterization requires specification of phenotypic markers such as CD68/CD163 and careful annotation of their anatomical compartment within tumor nests or stroma (67).
3.4 Th17 cells—stage- and compartment-specific roles
T helper 17 (Th17) cells, defined by interleukin-17 (IL-17) secretion, display stage- and compartment-specific behaviors in CeCa.
Early disease (CIN/early tumors): Peripheral blood studies demonstrate an elevated Th17: Treg ratio compared with healthy controls, reflecting early immune dysregulation. This imbalance persists into invasive disease, though without substantial systemic amplification (68, 69).
Advanced disease: Th17 accumulation in blood and tumor tissue correlates with higher clinical stage, lymph-node metastasis, and poor outcomes (68, 69). Increased Th17 frequencies under treatment pressure (e.g., chemoradiation) have been linked to poor therapeutic response and early relapse (70).
Notably, data remain heterogeneous. In squamous histologies, higher intratumoral IL-17+ cell density has been associated with improved survival (69), though much of this IL-17 signal has been derived from neutrophils rather than Th17 cells (71, 72). Thus, IL-17 alone cannot be employed as surrogate marker for Th17 activity. The impact of Th17 cells depends on the histological subtype of the tumor. In squamous cell carcinoma, Th17 responses may provide some protective or beneficial effects, whereas in adenocarcinoma they are mostly harmful and contribute to disease progression. In select niches, Th17 cells may also indirectly promote antitumor immunity by recruiting cytotoxic effector cells (69).
Overall, the evidence supports a pro-tumorigenic role for Th17 cells in advanced cervical cancer, while recognizing histological and spatial exceptions.
4 Biomarkers: evidence levels and clinical utility
Biomarkers reflecting oxidative stress and immune contexture are increasingly investigated in context of CeCa. While several biomarkers demonstrate biological and prognostic relevance, most remain exploratory due to methodological variability and lack of clinical validation. A tabulated summary of these methods can be found in Table 2.
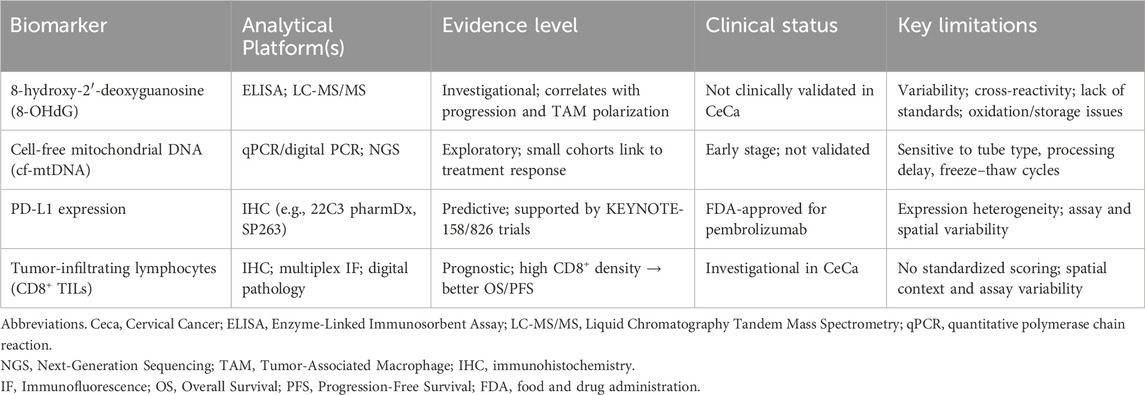
Table 2. Oxidative stress and immune biomarkers in cervical cancer: analytical platforms, evidence levels, clinical status, and key limitations.
4.1 Oxidative stress biomarkers
Among oxidative stress–related biomarkers, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is the most widely studied DNA lesion. It reflects ROS-mediated oxidative damage and has been linked to genomic instability, carcinogenesis, and macrophage polarization (8, 43, 46). Detection methods include enzyme-linked immunosorbent assay (ELISA) and liquid chromatography–tandem mass spectrometry (LC-MS/MS). However, these platforms are not directly comparable, as pre-analytical oxidation, sample storage, and assay-specific cross-reactivity introduce variability. Such inter-assay inconsistency currently precludes standardized application in clinical practice (73, 74). Thus, 8-OHdG should therefore be regarded as investigational, with evidence best interpreted as correlational rather than as a validated decision-making tool.
Circulating cell-free mitochondrial DNA (cf-mtDNA) represents another emerging marker (75). Its potential utility lies in real-time monitoring of treatment response and disease dynamics. Yet cf-mtDNA quantification is also highly sensitive to technical variables, including collection tube type, processing delays, freeze–thaw cycles, extraction chemistry, and amplicon length. Readers are directed to (75) for a detailed review of these quantitative methods (75). Early cohort studies suggest that cf-mtDNA levels may reflect disease activity, but reproducibility challenges underscore its current exploratory status, pending prospective validation (73, 74).
4.2 Immune biomarkers
PD-L1 expression serves as a clinically established predictive biomarker in CeCa, guiding patient selection for immune checkpoint inhibitor (ICI) therapy (76, 77). Clinical benefit has been demonstrated in the CeCa cohort of KEYNOTE-158, though responses occur in only a subset of PD-L1–positive patients (78). PD-L1 utility is constrained by intratumoral heterogeneity, assay variability, and temporal dynamics of expression, which limit its standalone predictive accuracy (78). Consequently, multiplexed or composite biomarker strategies are increasingly advocated.
Tumor-infiltrating lymphocytes (TILs), particularly CD8+ T cells, do not also retain universally consistent prognostic value across studies and different types of cancers (79). Higher intratumoral CD8+ density is associated with improved overall survival (OS) and progression-free survival (PFS), reflecting a more favorable immune contexture (80–82). To maximize reproducibility, standardized immunohistochemistry or multiplex immunofluorescence assays with attention to spatial localization (tumor nests vs. stroma) are recommended (47, 83).
5 Therapeutics: current standards and emerging combinations
The therapeutic landscape of CeCa has evolved with the advent of immune checkpoint inhibitors (ICIs, e.g., PDL-1) and exploratory redox-targeting agents. However, clinical benefit remains heterogeneous, underscoring the need for biomarker-guided selection and rational combinations.
5.1 Current standards: immune checkpoint inhibitors
Immune checkpoint blockade has transformed the management of recurrent and metastatic CeCa, though responses remain confined to subsets of patients.
The KEYNOTE-158 trial established pembrolizumab as a therapeutic option in recurrent/metastatic CeCa. In the dedicated cervical cohort (n:98), the overall response rate (ORR) was 12.2%, with a modest increase to 14.6% among PD-L1–positive tumors. Responses were not frequent in PD-L1–negative disease. While durable responses were achieved in some patients, most non-responders progressed, reflecting the persistence of an immunosuppressive TME. Immune-related adverse events occurred in ∼12% of patients, consistent with the broader safety profile of pembrolizumab (84).
The phase III KEYNOTE-826 trial redefined the standard of care for persistent, recurrent, or metastatic disease. Pembrolizumab combined with platinum-based chemotherapy ± bevacizumab significantly improved both overall survival (OS) and progression-free survival (PFS). At 22 months of median follow-up, OS reached 24.4 months compared with 16.5 months in the control arm, with a hazard ratio for death of 0.64 (95% CI 0.50–0.81) in PD-L1–positive patients. These results established pembrolizumab plus chemotherapy (with or without bevacizumab) as the global first-line standard for PD-L1–positive advanced CeCa (85).
5.2 Other immunotherapeutic approaches
Therapeutic HPV vaccines represent an area of ongoing development. The ISA101 peptide vaccine combined with nivolumab demonstrated an ORR of ∼33% in a cohort of HPV16-positive solid tumors, predominantly head-and-neck squamous cell carcinoma. Only a minority of patients had CeCa, limiting generalizability of the results (86). Additional vaccine modalities, including DNA- and viral vector–based platforms, are under early-phase evaluation, but no vaccine has yet achieved regulatory approval forCeCa.
5.3 Redox-targeting agents and repurposed drugs
Given the role of oxidative stress in HPV-driven carcinogenesis, redox modulators are under investigation. BMX-001, a manganese porphyrin radiomodulator, has entered clinical trials primarily in glioblastoma and head-and-neck cancer (87). Its application in CeCa remains investigational with no disease-specific data available (39).
Mdivi-1, a mitochondrial division inhibitor widely employed in preclinical studies, has demonstrated mechanistic utility but possesses off-target effects precluding clinical suitability (88, 89, 102).
Drug repurposing represents an additional therapeutic avenue. For instance, Metformin has exhibited antiproliferative and redox-modulating effects in CeCa cell lines and xenograft models (90, 91), though randomized clinical evidence in CeCa is lacking. Similarly, imipramine and nelfinavir have shown ROS-modulating and anti-HPV effects in preclinical models (92, 93), with early-phase trials underway in other malignancies (Table 3). However, cervical-specific clinical validation remains absent, which positions these agents as hypothesis-generating rather than clinically actionable (94, 95).

Table 3. Therapeutic landscape in advanced cervical cancer: mechanisms, clinical trial phases, endpoints, and outcomes of current and investigational agents.
5.4 Mechanistic rationale for combinations
Resistance to ICIs arises from multiple mechanisms, including activation of the adenosine/CD73 axis, compensatory upregulation of inhibitory receptors (LAG-3, TIM-3, TIGIT), and cancer-associated fibroblasts (CAFs)-CXCL12 signaling (96, 97). Preclinical studies suggest that redox normalization can reduce PD-L1 expression, restore antigen presentation, and enhance tumor immunogenicity (98, 99). These insights provide a rationale for combining redox-targeted therapies with ICIs (100). However, no patient-level CeCa data currently validates this approach, highlighting a key translational gap.
6 Future directions and conclusion
Despite significant progress in elucidating the molecular underpinnings of cervical cancer (CeCa), translation of mechanistic insights into clinical benefit remains limited. The interplay between HPV oncogenes, oxidative stress, and immune dysregulation presents both challenges and opportunities for therapeutic innovation. Several key priorities emerge for future research.
1. Development of predictive biomarkers. Reliable biomarkers are essential to stratify patients and guide therapy. While PD-L1 expressions and tumor-infiltrating lymphocytes (TILs) offer partial predictive value, their wider application is restricted following lack of multiplexed or composite biomarker strategies. Integration of oxidative stress markers (e.g., 8-OHdG, cf-mtDNA) with immune profiling may improve patient selection for immune checkpoint inhibitors (ICIs) and combination therapies. Nonetheless, standardization of assay platforms and pre-analytical workflows is critical for reproducibility and clinical translation.
2. Advancement of noninvasive monitoring. Liquid biopsy approaches, particularly cf-mtDNA and circulating immune signatures, represent promising tools for real-time monitoring of disease progression and therapeutic response. Rigorous prospective validation in large, clinically annotated cohorts is required before clinical adoption.
3. Rational therapeutic combinations. Evidence suggests that redox modulation may enhance immunotherapy efficacy by restoring antigen presentation and reducing PD-L1 expression. Rational design of trials integrating ICIs with redox-targeted agents, therapeutic HPV vaccines, or repurposed drugs could provide synergistic benefit. However, careful attention must be paid to potential antagonistic interactions and context-dependent effects.
4. Preclinical model refinement. Current in vitro and in vivo models incompletely recapitulate the complexity of the CeCa tumor microenvironment (TME). Integration of multi-omics platforms, single-cell technologies, and spatial profiling will be essential to capture the dynamic crosstalk between HPV-driven pathways, oxidative stress, and immune regulation. Such models will accelerate discovery of therapeutic vulnerabilities and facilitate translational research.
7 Conclusion
Cervical cancer remains a major global health challenge, with pathogenesis shaped by intricate interactions between HPV oncogenes, oxidative stress, and immune modulation. While immune checkpoint inhibitors and emerging redox-targeting strategies provide new therapeutic avenues, clinical benefit is limited to subsets of patients. Current evidence underscores the context-dependent duality of immune subsets such as Th17 cells and antioxidant enzymes like superoxide dismutase 2 (SOD2), reflecting the complexity of redox–immunity crosstalk.
Future progress hinges on the development of predictive biomarkers, noninvasive monitoring strategies, and rationally designed therapeutic combinations. Integration of multi-omics data with advanced preclinical models will be pivotal in bridging mechanistic insights into personalized treatment. By addressing these gaps, it may be possible to translate biological understanding into durable clinical benefit and improve outcomes for women with CeCa.
Author contributions
AM: Conceptualization, Writing – original draft, Writing – review and editing. SS: Writing – review and editing. QD: Writing – review and editing. YZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vargas-Cardona, HD, Rodriguez-Lopez, M, Arrivillaga, M, Vergara-Sanchez, C, García-Cifuentes, JP, Bermúdez, PC, et al. Artificial intelligence for cervical cancer screening: scoping review, 2009-2022. Int J Gynecol and Obstet (2024) 165:566–78. doi:10.1002/ijgo.15179
2. Wang, L, Yi, S, Teng, Y, Li, W, and Cai, J. Role of the tumor microenvironment in the lymphatic metastasis of cervical cancer (review). Exp Ther Med (2023) 26:486. doi:10.3892/etm.2023.12185
3. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA: A Cancer J Clinicians (2024) 74:12–49. doi:10.3322/caac.21820
4. Cervical cancer statistics. Available online at: https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html (Accessed August 22, 2025).
5. Hu, S, Xu, X, Zhang, Y, Liu, Y, Yang, C, Wang, Y, et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: implications for vaccine roll-out in mainland China. Vaccine (2021) 39:35–44. doi:10.1016/j.vaccine.2020.11.029
6. Brooks, LA, Sullivan, A, O’Nions, J, Bell, A, Dunne, B, Tidy, JA, et al. E7 proteins from oncogenic human papillomavirus types transactivate p73: role in cervical intraepithelial neoplasia. Br J Cancer (2002) 86:263–8. doi:10.1038/sj.bjc.6600033
7. Allison, SJ, Jiang, M, and Milner, J. Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells. Aging (Albany NY) (2009) 1:316–27. doi:10.18632/aging.100028
8. Pal, A, and Kundu, R. Human Papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol (2020) 10:3116. doi:10.3389/fmicb.2019.03116
9. Evans, M, Borysiewicz, LK, Evans, AS, Rowe, M, Jones, M, Gileadi, U, et al. Antigen processing defects in cervical carcinomas limit the presentation of a CTL epitope from human papillomavirus 16 E6. The J Immunol (2001) 167:5420–8. doi:10.4049/jimmunol.167.9.5420
10. Ma, B, Ren, C, Yin, Y, Zhao, S, Li, J, and Yang, H. Immune cell infiltration and prognostic index in cervical cancer: insights from metabolism-related differential genes. Front Immunol (2024) 15:1411132. doi:10.3389/fimmu.2024.1411132
11. Karsten, CB, Buettner, FFR, Cajic, S, Nehlmeier, I, Neumann, B, Klippert, A, et al. Exclusive decoration of simian immunodeficiency virus env with high-mannose type N-glycans is not compatible with mucosal transmission in rhesus macaques. J Virol (2015) 89:11727–33. doi:10.1128/JVI.01358-15
12. Georgescu, SR, Mitran, CI, Mitran, MI, Caruntu, C, Sarbu, MI, Matei, C, et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. J Immunol Res (2018) 2018:1–10. doi:10.1155/2018/5315816
13. Pacini, L, Savini, C, Ghittoni, R, Saidj, D, Lamartine, J, Hasan, UA, et al. Downregulation of toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J Virol (2015) 89:11396–405. doi:10.1128/JVI.02151-15
14. Pacini, L, Ceraolo, MG, Venuti, A, Melita, G, Hasan, UA, Accardi, R, et al. UV radiation activates toll-like receptor 9 expression in primary human keratinocytes, an event inhibited by human papillomavirus 38 E6 and E7 oncoproteins. J Virol (2017) 91:e01123-17. doi:10.1128/JVI.01123-17
15. Okamoto, M, Kouwaki, T, Fukushima, Y, and Oshiumi, H. Regulation of RIG-I activation by K63-linked polyubiquitination. Front Immunol (2018) 8:1942. doi:10.3389/fimmu.2017.01942
16. Chiang, C, Pauli, E-K, Biryukov, J, Feister, KF, Meng, M, White, EA, et al. The human papillomavirus E6 oncoprotein targets USP15 and TRIM25 to suppress RIG-I-mediated innate immune signaling. J Virol (2018) 92:e01737-17. doi:10.1128/JVI.01737-17
17. Hemmat, N, and Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog Dis (2019) 77:ftz048. doi:10.1093/femspd/ftz048
18. Li, J-X, Zhang, J, Li, C-H, Zhang, Q, Kong, B, and Wang, P-H. Human papillomavirus E2 proteins suppress innate antiviral signaling pathways. Front Immunol (2025) 16:1555629. doi:10.3389/fimmu.2025.1555629
19. Moody, CA. Regulation of the innate immune response during the human papillomavirus life cycle. Viruses (2022) 14:1797. doi:10.3390/v14081797
20. Lou, M, Huang, D, Zhou, Z, Shi, X, Wu, M, Rui, Y, et al. DNA virus oncoprotein HPV18 E7 selectively antagonizes cGAS-STING-triggered innate immune activation. J Med Virol (2023) 95:e28310. doi:10.1002/jmv.28310
21. Miyauchi, S, Kim, SS, Jones, RN, Zhang, L, Guram, K, Sharma, S, et al. Human papillomavirus E5 suppresses immunity via inhibition of the immunoproteasome and STING pathway. Cell Rep (2023) 42:112508. doi:10.1016/j.celrep.2023.112508
22. MacLennan, SA, and Marra, MA. Oncogenic viruses and the epigenome: how viruses hijack epigenetic mechanisms to drive cancer. Int J Mol Sci (2023) 24:9543. doi:10.3390/ijms24119543
23. Da Silva, MLR, De Albuquerque, BHDR, Allyrio, TADMF, De Almeida, VD, Cobucci, RNDO, Bezerra, FL, et al. The role of HPV‑induced epigenetic changes in cervical carcinogenesis (review). Biomed Rep (2021) 15:60. doi:10.3892/br.2021.1436
24. Zhang, L, Zhao, Y, Tu, Q, Xue, X, Zhu, X, and Zhao, K-N. The roles of programmed cell death ligand-1/programmed cell death-1 (PD-L1/PD-1) in HPV-Induced cervical cancer and potential for their use in blockade therapy. Curr Med Chem (2021) 28:893–909. doi:10.2174/0929867327666200128105459
25. Aghbash, PS, Hemmat, N, Baradaran, B, Mokhtarzadeh, A, Poortahmasebi, V, Oskuee, MA, et al. The effect of Wnt/β-catenin signaling on PD-1/PDL-1 axis in HPV-Related cervical cancer. Oncol Res (2022) 30:99–116. doi:10.32604/or.2022.026776
26. Gu, X, Dong, M, Liu, Z, Mi, Y, Yang, J, Zhang, Z, et al. Elevated PD-L1 expression predicts poor survival outcomes in patients with cervical cancer. Cancer Cell Int (2019) 19:146. doi:10.1186/s12935-019-0861-7
27. Meng, Y, Liang, H, Hu, J, Liu, S, Hao, X, Wong, MSK, et al. PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer (2018) 9:2938–45. doi:10.7150/jca.22532
28. Ishikawa, M, Nakayama, K, Nakamura, K, Yamashita, H, Ishibashi, T, Minamoto, T, et al. High PD-1 expression level is associated with an unfavorable prognosis in patients with cervical adenocarcinoma. Arch Gynecol Obstet (2020) 302:209–18. doi:10.1007/s00404-020-05589-0
29. Rischin, D, Gil-Martin, M, González-Martin, A, Braña, I, Hou, JY, Cho, D, et al. PD-1 blockade in recurrent or metastatic cervical cancer: data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol Oncol (2020) 159:322–8. doi:10.1016/j.ygyno.2020.08.026
30. Li, G, Li, X, Yin, R, Feng, M, Zuo, J, Wei, S, et al. Phase II study of enlonstobart (SG001), a novel PD-1 inhibitor in patients with PD-L1 positive recurrent/metastatic cervical cancer. Gynecol Oncol (2024) 191:165–71. doi:10.1016/j.ygyno.2024.10.001
31. Parida, S, and Mandal, M. Inflammation induced by human papillomavirus in cervical cancer and its implication in prevention. Eur J Cancer Prev (2014) 23:432–48. doi:10.1097/cej.0000000000000023
32. Kan, X, Zhou, Z, Liu, L, Aiskikaer, R, and Zou, Y. Significance of non-steroidal anti-inflammatory drugs in the prevention and treatment of cervical cancer. Heliyon (2025) 11:e42055. doi:10.1016/j.heliyon.2025.e42055
33. Cruz-Gregorio, A, and Aranda-Rivera, AK. Redox-sensitive signalling pathways regulated by human papillomavirus in HPV-Related cancers. Rev Med Virol (2021) 31:e2230. doi:10.1002/rmv.2230
34. Li, Q, Xie, B, Chen, X, Lu, B, Chen, S, Sheng, X, et al. SNORD6 promotes cervical cancer progression by accelerating E6-mediated p53 degradation. Cell Death Discov (2023) 9:192. doi:10.1038/s41420-023-01488-w
35. Hadami, K, Saby, C, Dakka, N, Collin, G, Attaleb, M, Khyatti, M, et al. Degradation of p53 by HPV16-E6 variants isolated from cervical cancer specimens of Moroccan women. Gene (2021) 791:145709. doi:10.1016/j.gene.2021.145709
36. Marullo, R, Werner, E, Zhang, H, Chen, GZ, Shin, DM, and Doetsch, PW. HPV16 E6 and E7 proteins induce a chronic oxidative stress response via NOX2 that causes genomic instability and increased susceptibility to DNA damage in head and neck cancer cells. Carcinogenesis (2015) 36:1397–406. doi:10.1093/carcin/bgv126
37. Xu, A, Yang, X, Zhao, J, Kong, S, Tang, Q, Li, X, et al. KAT8 facilitates the proliferation of cancer cells through enhancing E7 function in HPV-Associated cervical cancer. Acta Biochim. Biophys. Sin. (2025). doi:10.3724/abbs.2025022
38. Engeland, K. Cell cycle regulation: P53-P21-RB signaling. Cell Death Differ (2022) 29:946–60. doi:10.1038/s41418-022-00988-z
39. Cruz-Gregorio, A, Aranda-Rivera, AK, and Pedraza-Chaverri, J. HPV proteins as therapeutic targets for phytopharmaceuticals related to redox state in HPV-Related cancers. Future Pharmacol (2024) 4:716–30. doi:10.3390/futurepharmacol4040038
40. Cruz-Gregorio, A, Aranda-Rivera, AK, Roviello, GN, and Pedraza-Chaverri, J. Targeting mitochondrial therapy in the regulation of HPV infection and HPV-Related cancers. Pathogens (2023) 12:402. doi:10.3390/pathogens12030402
41. Chen, B, Wang, Y, Wu, Y, and Xu, T. Effect of HPV oncoprotein on carbohydrate and lipid metabolism in tumor cells. Curr Cancer Drug Targets (2024) 24:987–1004. doi:10.2174/0115680096266981231215111109
42. Peng, Q, Wang, L, Zuo, L, Gao, S, Jiang, X, Han, Y, et al. HPV E6/E7: insights into their regulatory role and mechanism in signaling pathways in HPV-Associated tumor. Cancer Gene Ther (2024) 31:9–17. doi:10.1038/s41417-023-00682-3
43. Zahra, K, Patel, S, Dey, T, Pandey, U, and Mishra, SP. A study of oxidative stress in cervical cancer-an institutional study. Biochem Biophys Rep (2021) 25:100881. doi:10.1016/j.bbrep.2020.100881
44. Kowaltowski, AJ, de Souza-Pinto, NC, Castilho, RF, and Vercesi, AE. Mitochondria and reactive oxygen species. Free Radic Biol Med (2009) 47:333–43. doi:10.1016/j.freeradbiomed.2009.05.004
45. Letafati, A, Taghiabadi, Z, Zafarian, N, Tajdini, R, Mondeali, M, Aboofazeli, A, et al. Emerging paradigms: unmasking the role of oxidative stress in HPV-Induced carcinogenesis. Infect Agents Cancer (2024) 19:30. doi:10.1186/s13027-024-00581-8
46. Ebrahimi, S, Soltani, A, and Hashemy, SI. Oxidative stress in cervical cancer pathogenesis and resistance to therapy: EBRAHIMI et al. J Cell Biochem (2019) 120:6868–77. doi:10.1002/jcb.28007
47. Adurthi, S, Mukherjee, G, Krishnamurthy, H, Sudhir, K, Bafna, UD, Umadevi, K, et al. Functional tumor infiltrating TH1 and TH2 effectors in large early-stage cervical cancer are suppressed by regulatory T cells. Int J Gynecol Cancer (2012) 22:1130–7. doi:10.1097/igc.0b013e318262aa53
48. Zhai, K, Chang, L, Zhang, Q, Liu, B, and Wu, Y. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion (2011) 11:559–63. doi:10.1016/j.mito.2011.02.005
49. Anvar, MT, Rashidan, K, Arsam, N, Rasouli-Saravani, A, Yadegari, H, Ahmadi, A, et al. Th17 cell function in cancers: immunosuppressive agents or anti-tumor allies? Cancer Cell Int (2024) 24:355. doi:10.1186/s12935-024-03525-9
50. Asadzadeh, Z, Mohammadi, H, Safarzadeh, E, Hemmatzadeh, M, Mahdian-Shakib, A, Jadidi-Niaragh, F, et al. The paradox of Th17 cell functions in tumor immunity. Cell Immunol (2017) 322:15–25. doi:10.1016/j.cellimm.2017.10.015
51. Joshi, N, Hajizadeh, F, Ansari Dezfouli, E, Zekiy, AO, Nabi Afjadi, M, Mousavi, SM, et al. Silencing STAT3 enhances sensitivity of cancer cells to doxorubicin and inhibits tumor progression. Life Sci (2021) 275:119369. doi:10.1016/j.lfs.2021.119369
52. Rabelo-Santos, SH, Termini, L, Boccardo, E, Derchain, S, Longatto-Filho, A, Andreoli, MA, et al. Strong SOD2 expression and HPV-16/18 positivity are independent events in cervical cancer. Oncotarget (2018) 9:21630–40. doi:10.18632/oncotarget.24850
53. Talarico, MCR, Nunes, RAL, Silva, GÁF, Costa, LBEda, Cardoso, MR, Esteves, SCB, et al. High expression of SOD2 protein is a strong prognostic factor for stage IIIB squamous cell cervical carcinoma. Antioxidants (Basel) (2021) 10:724. doi:10.3390/antiox10050724
54. Kumar, S, Singh, SK, Rana, B, and Rana, A. Tumor-infiltrating CD8+ T cell antitumor efficacy and exhaustion: molecular insights. Drug Discov Today (2021) 26:951–67. doi:10.1016/j.drudis.2021.01.002
55. Trontzas, IP, and Syrigos, KN. Immune biomarkers for checkpoint blockade in solid tumors: transitioning from tissue to peripheral blood monitoring and future integrated strategies. Cancers (Basel) (2025) 17:2639. doi:10.3390/cancers17162639
56. Rossi, A, Belmonte, B, Carnevale, S, Liotti, A, De Rosa, V, Jaillon, S, et al. Stromal and immune cell dynamics in tumor associated tertiary lymphoid structures and anti-tumor immune responses. Front Cell Dev Biol (2022) 10:933113. doi:10.3389/fcell.2022.933113
57. Feng, Y, Ma, W, Zang, Y, Guo, Y, Li, Y, Zhang, Y, et al. Spatially organized tumor-stroma boundary determines the efficacy of immunotherapy in colorectal cancer patients. Nat Commun (2024) 15:10259. doi:10.1038/s41467-024-54710-3
58. Shen, X, Wang, C, Li, M, Wang, S, Zhao, Y, Liu, Z, et al. Identification of CD8+ T cell infiltration-related genes and their prognostic values in cervical cancer. Front Oncol (2022) 12:1031643. doi:10.3389/fonc.2022.1031643
59. Ohno, A, Iwata, T, Katoh, Y, Taniguchi, S, Tanaka, K, Nishio, H, et al. Tumor-infiltrating lymphocytes predict survival outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy. Gynecol Oncol (2020) 159:329–34. doi:10.1016/j.ygyno.2020.07.106
60. Ao, C, and Zeng, K. The role of regulatory T cells in pathogenesis and therapy of human papillomavirus-related diseases, especially in cancer. Infect Genet Evol (2018) 65:406–13. doi:10.1016/j.meegid.2018.08.014
61. Choi, Y, Lee, D, Kim, NY, Seo, I, Park, NJ-Y, and Chong, GO. Role of tumor-associated macrophages in cervical cancer: integrating classical perspectives with recent technological advances. Life (Basel) (2024) 14:443. doi:10.3390/life14040443
62. Yang, Q, Guo, N, Zhou, Y, Chen, J, Wei, Q, and Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharmaceutica Sinica B (2020) 10:2156–70. doi:10.1016/j.apsb.2020.04.004
63. Li, A-Q, Huang, F, Talaiti, S, Yang, X, Bi, H, and Fang, J-H. Understanding the complexity of tumor-associated macrophages: druggable and therapeutic insights. Acta Pharmaceutica Sinica B (2025). doi:10.1016/j.apsb.2025.07.021
64. Petrillo, M, Zannoni, GF, Martinelli, E, Pedone Anchora, L, Ferrandina, G, Tropeano, G, et al. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS One (2015) 10:e0136654. doi:10.1371/journal.pone.0136654
65. Guo, F, Kong, W, Zhao, G, Cheng, Z, Ai, L, Lv, J, et al. The correlation between tumor-associated macrophage infiltration and progression in cervical carcinoma. Biosci Rep (2021) 41:BSR20203145. doi:10.1042/BSR20203145
66. Qing, W, Fang, W-Y, Ye, L, Shen, L-Y, Zhang, X-F, Fei, X-C, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid (2012) 22:905–10. doi:10.1089/thy.2011.0452
67. Battaglia, A, Buzzonetti, A, Baranello, C, Ferrandina, G, Martinelli, E, Fanfani, F, et al. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother (2009) 58:1363–73. doi:10.1007/s00262-008-0646-7
68. Zhang, J, Zhan, J, Guan, Z, Lin, X, Li, T, Li, M, et al. The prognostic value of Th17/Treg cell in cervical cancer: a systematic review and meta-analysis. Front Oncol (2024) 14:1442103. doi:10.3389/fonc.2024.1442103
69. Guo, W, Dai, L, and Qiu, L. T cell subsets in cervical cancer tumor microenvironment: advances and therapeutic opportunities. Front Immunol (2025) 16:1612032. doi:10.3389/fimmu.2025.1612032
70. Theobald, L, Stroeder, R, Melchior, P, Iordache, II, Tänzer, T, Port, M, et al. Chemoradiotherapy-induced increase in Th17 cell frequency in cervical cancer patients is associated with therapy resistance and early relapse. Mol Oncol (2021) 15:3559–77. doi:10.1002/1878-0261.13095
71. Punt, S, Fleuren, GJ, Kritikou, E, Lubberts, E, Trimbos, JB, Jordanova, ES, et al. Angels and demons: th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology (2015) 4:e984539. doi:10.4161/2162402X.2014.984539
72. Punt, S, Houwing-Duistermaat, JJ, Schulkens, IA, Thijssen, VL, Osse, EM, de Kroon, CD, et al. Correlations between immune response and vascularization qRT-PCR gene expression clusters in squamous cervical cancer. Mol Cancer (2015) 14:71. doi:10.1186/s12943-015-0350-0
73. Cafforio, P, Palmirotta, R, Lovero, D, Cicinelli, E, Cormio, G, Silvestris, E, et al. Liquid biopsy in cervical cancer: hopes and pitfalls. Cancers (Basel) (2021) 13:3968. doi:10.3390/cancers13163968
74. Shrivastava, A, Mishra, SP, Pradhan, S, Choudhary, S, Singla, S, Zahra, K, et al. An assessment of serum oxidative stress and antioxidant parameters in patients undergoing treatment for cervical cancer. Free Radic Biol Med (2021) 167:29–35. doi:10.1016/j.freeradbiomed.2021.02.037
75. Peng, F, Wang, S, Feng, Z, Zhou, K, Zhang, H, Guo, X, et al. Circulating cell-free mtDNA as a new biomarker for cancer detection and management. Cancer Biol Med (2023) 21:105–10. doi:10.20892/j.issn.2095-3941.2023.0280
76. Hu, T, Wan, X, Wu, H, Cheng, X, and Xu, S. Predictive values of PD-L1 expression for survival outcomes in patients with cervical cancer: a systematic review and meta-analysis. Ginekol Pol (2022) 93:767–74. doi:10.5603/GP.a2022.0071
77. Wang, R, Zhang, Y, and Shan, F. PD-L1: can it be a biomarker for the prognosis or a promising therapeutic target in cervical cancer? Int Immunopharmacology (2022) 103:108484. doi:10.1016/j.intimp.2021.108484
78. Kouhen, F, El Ghanmi, A, Inghaoun, H, Miftah, H, Ghazi, B, and Badou, A. The promise of PD1/PDL1 targeted immunotherapy in locally advanced cervical cancer: a game-changer for patients outcome? Front Immunol (2025) 16:1573576. doi:10.3389/fimmu.2025.1573576
79. Clarke, B, Tinker, AV, Lee, C-H, Subramanian, S, van de Rijn, M, Turbin, D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol (2009) 22:393–402. doi:10.1038/modpathol.2008.191
80. Hamanishi, J, Mandai, M, Iwasaki, M, Okazaki, T, Tanaka, Y, Yamaguchi, K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A (2007) 104:3360–5. doi:10.1073/pnas.0611533104
81. Yang, Y, Attwood, K, Bshara, W, Mohler, JL, Guru, K, Xu, B, et al. High intratumoral CD8+ T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. The Prostate (2021) 81:20–8. doi:10.1002/pros.24068
82. Hao, J, Yu, H, Zhang, T, An, R, and Xue, Y. Prognostic impact of tumor-infiltrating lymphocytes in high grade serous ovarian cancer: a systematic review and meta-analysis. Ther Adv Med Oncol (2020) 12:1758835920967241. doi:10.1177/1758835920967241
83. Sheu, BC, Lin, RH, Lien, HC, Ho, HN, Hsu, SM, and Huang, SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. The J Immunol (2001) 167:2972–8. doi:10.4049/jimmunol.167.5.2972
84. Marabelle, A, Le, DT, Ascierto, PA, Di Giacomo, AM, De Jesus-Acosta, A, Delord, J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38:1–10. doi:10.1200/JCO.19.02105
85. Monk, BJ, Colombo, N, Tewari, KS, Dubot, C, Caceres, MV, Hasegawa, K, et al. First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol (2023) 41:5505–11. doi:10.1200/JCO.23.00914
86. Wu, S-Y, Lai, H-T, Sanjib Banerjee, N, Ma, Z, Santana, JF, Wei, S, et al. IDR-Targeting compounds suppress HPV genome replication via disruption of phospho-BRD4 association with DNA damage response factors. Mol Cell (2024) 84:202–20.e15. doi:10.1016/j.molcel.2023.11.022
87. Gad, SC, Sullivan, DW, Spasojevic, I, Mujer, CV, Spainhour, CB, and Crapo, JD. Nonclinical safety and toxicokinetics of MnTnBuOE-2-PyP5+ (BMX-001). Int J Toxicol (2016) 35:438–53. doi:10.1177/1091581816642766
88. Jiang, H, Zuo, J, Li, B, Chen, R, Luo, K, Xiang, X, et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol (2023) 63:102754. doi:10.1016/j.redox.2023.102754
89. Ahn, SI, Choi, SK, Kim, MJ, Wie, J, and You, JS. Mdivi-1: effective but complex mitochondrial fission inhibitor. Biochem Biophysical Res Commun (2024) 710:149886. doi:10.1016/j.bbrc.2024.149886
90. Xia, C, Liu, C, He, Z, Cai, Y, and Chen, J. Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression. J Exp Clin Cancer Res (2020) 39:127. doi:10.1186/s13046-020-01627-6
91. Chen, Y-H, Wang, P-H, Chen, P-N, Yang, S-F, and Hsiao, Y-H. Molecular and cellular mechanisms of metformin in cervical cancer. Cancers (Basel) (2021) 13:2545. doi:10.3390/cancers13112545
92. Reddy, R, Gaiwak, V, Goda, JS, and Teni, T. ’nelfinavir sensitizes a clinically relevant chemo-radioresistant cervical cancer in-vitro model by targeting the AKT-USP15/USP11-HPV16 E6/E7 axis. Biochem Biophys Rep (2025) 42:101987. doi:10.1016/j.bbrep.2025.101987
93. Asensi-Cantó, A, Rodríguez-Braun, E, Beltrán-Videla, A, Hurtado, AM, and Conesa-Zamora, P. Effects of imipramine on cancer patients over-expressing Fascin1; description of the HITCLIF clinical trial. Front Oncol (2023) 13:1238464. doi:10.3389/fonc.2023.1238464
94. Singh, M, Singh, R, Bhui, K, Tyagi, S, Mahmood, Z, and Shukla, Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear Factor-κB and akt activation in human cervical cancer cells. Oncol Res (2011) 19:245–57. doi:10.3727/096504011x13021877989711
95. Subeha, MR, and Telleria, CM. The anti-cancer properties of the HIV protease inhibitor nelfinavir. Cancers (Basel) (2020) 12:3437. doi:10.3390/cancers12113437
96. Zhang, Z, Yu, Y, Zhang, Z, Li, D, Liang, Z, Wang, L, et al. Cancer-associated fibroblasts-derived CXCL12 enhances immune escape of bladder cancer through inhibiting P62-mediated autophagic degradation of PDL1. J Exp Clin Cancer Res (2023) 42:316. doi:10.1186/s13046-023-02900-0
97. Xiao, Y, Li, Z-Z, Zhong, N-N, Cao, L-M, Liu, B, and Bu, L-L. Charting new frontiers: co-Inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Translational Oncol (2023) 38:101794. doi:10.1016/j.tranon.2023.101794
98. Glorieux, C, Xia, X, and Huang, P. The role of oncogenes and redox signaling in the regulation of PD-L1 in cancer. Cancers (Basel) (2021) 13:4426. doi:10.3390/cancers13174426
99. Glorieux, C, Xia, X, He, Y-Q, Hu, Y, Cremer, K, Robert, A, et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-Mediated FGFR1 signaling. Redox Biol (2021) 38:101780. doi:10.1016/j.redox.2020.101780
100. Martinez-Cannon, BA, and Colombo, I. The evolving role of immune checkpoint inhibitors in cervical and endometrial cancer. Cancer Drug Resist (2024) 7:23. doi:10.20517/cdr.2023.120
101. Sousa, LGde, Rajapakshe, K, Rodriguez Canales, J, Chin, RL, Feng, L, Wang, Q, et al. ISA101 and nivolumab for HPV-16+ cancer: updated clinical efficacy and immune correlates of response. J Immunother Cancer (2022) 10:e004232. doi:10.1136/jitc-2021-004232
Keywords: tumor microenvironment, oxidative stress, immune cell infiltration, HPV oncogenes, therapeutic strategies, cervical cancer
Citation: Mlambo A, Su S, Dhlamini Q and Zhang Y (2025) Patterns of immune cell infiltration and oxidative stress in cervical cancer. Oncol. Rev. 19:1570071. doi: 10.3389/or.2025.1570071
Received: 02 February 2025; Accepted: 03 September 2025;
Published: 11 September 2025.
Edited by:
Bastian Czogalla, LMU Munich University Hospital, GermanyReviewed by:
Komsun Suwannarurk, Thammasat University, ThailandXuehai Wang, Karolinska Institutet (KI), Sweden
Copyright © 2025 Mlambo, Su, Dhlamini and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuyang Zhang, emhhbmd5dXlhbmdAd211LmVkdS5jbg==
 Andrea Mlambo
Andrea Mlambo Shuyue Su
Shuyue Su Qhaweni Dhlamini
Qhaweni Dhlamini Yuyang Zhang
Yuyang Zhang