- 1Colorectal Cancer Alliance, Washington, DC, United States
- 2Red Thred Solutions, Nineveh, IN, United States
- 3Montsouris Consilium, Montpellier, France
The colorectal cancer (CRC) screening landscape has rapidly evolved, introducing new technologies alongside established methods. The lack of head-to-head observational studies comparing these diverse options impairs clinicians’ and patients’ ability to make informed choices in CRC screening test selection. This manuscript aims to provide a comprehensive review of existing and emerging CRC screening technologies and develop a practical framework for informed decision-making. We conducted a systematic review of current literature on CRC screening methods, including colonoscopy, fecal immunochemical test (FIT), multi-target stool DNA test (mt-sDNA), the next-generation multi-target stool DNA test, multi-target stool RNA test (mt-sRNA), and blood-based tests. We summarized performance characteristics, adherence rates, follow-up colonoscopy rates, accessibility, and costs for each method. Our review revealed significant variations in test performance, patient adherence, and implementation factors across screening modalities. Blood-based tests showed promise in terms of patient acceptance but currently have lower sensitivity for early-stage cancers with a higher participant adherence when screening navigation is provided. Our review led to the development of a comprehensive framework for evaluating CRC screening options, addressing the critical need for informed decision-making in this area. The framework encompasses five key dimensions: test performance (sensitivity and specificity for CRC and precancerous lesions), patient considerations (invasiveness, preparation, and location preferences), adherence and follow-up (real-world rates and diagnostic colonoscopy completion rates), accessibility and cost (insurance coverage, out-of-pocket expenses, and system integration), and screening interval (recommended frequency and long-term impact). By synthesizing data, the framework enables healthcare providers and patients to navigate the complex landscape of screening options, facilitating personalized recommendations tailored to individual risk factors, preferences, and healthcare system constraints. Future research should validate this framework in diverse clinical settings and update it as new technologies emerge, ensuring continued improvement in CRC screening participation, effectiveness, and outcomes.
Introduction
CRC remains a significant public health concern in the United States (1) due, in part, to the currently stagnant, and below-target screening rates with traditional modalities. The rapid evolution of CRC screening technologies has introduced a wide array of options, creating a lot of opportunities, ranging from traditional methods like colonoscopy to innovative technologies such as multi-target stool RNA/DNA tests and blood-based tests. While these advancements expand the possibilities for prevention and early detection, they also create complexities for healthcare providers and patients tasked with choosing the most suitable options (1).
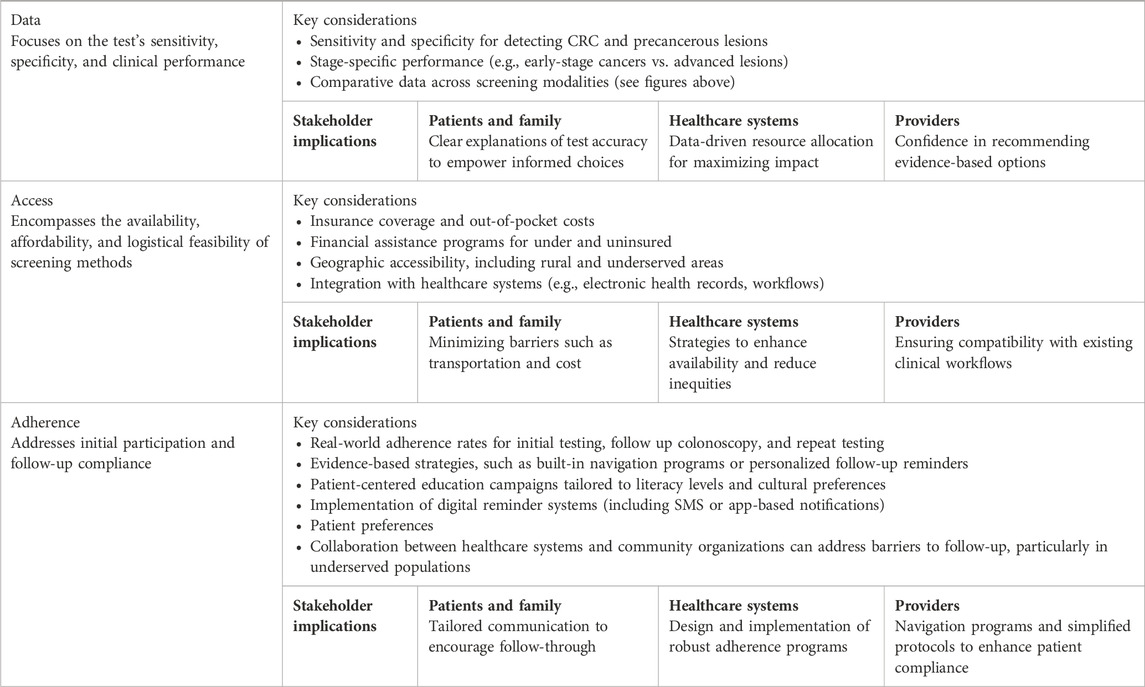
This paper proposes a practical framework centered on three essential pillars: data, access, and adherence; to guide informed decision-making in the selection of CRC screening methods. By evaluating existing and emerging tests through these interconnected lenses, the framework enables healthcare providers to balance performance characteristics, accessibility, and real-world adherence. These pillars ensure that decisions are patient-centric, equitable, and aligned with clinical best practices, ultimately improving screening participation and outcomes.
Through a detailed examination of these pillars, this manuscript aims to offer a structured approach to navigating the evolving landscape of CRC screening. This framework not only addresses the challenges posed by diverse screening modalities but also highlights opportunities to enhance screening rates and patient outcomes. It is designed to simplify complex clinical research for the purpose of making key concepts more accessible to a broad range of stakeholders (notably patients and providers). By integrating these concepts throughout the analysis, we aim to empower stakeholders with actionable insights to optimize screening strategies.
Methodology
To comprehensively evaluate CRC screening technologies and develop an informed decision-making framework, we employed a multi-faceted approach combining systematic literature review, data extraction, and comparative analysis. This methodology aimed to synthesize evidence on screening modalities while considering real-world adherence, accessibility, and performance metrics.
Literature review and data collection
We conducted a systematic review of peer-reviewed literature, clinical guidelines, regulatory documents, and health system reports published up to March 2025. Our search strategy utilized major medical databases, including PubMed, with a combination of controlled vocabulary and free-text terms related to CRC screening methods, test performance, adherence, accessibility, and cost-effectiveness. Specific keywords were used to capture relevant studies, such as “colonoscopy,” “fecal immunochemical test (FIT),” “multi-target stool DNA test (mt-sDNA),” “multi-target stool RNA test (mt-sRNA),” and “blood-based CRC screening.” These terms were combined using Boolean operators to ensure a comprehensive search. Inclusion criteria included studies published in English that evaluated the sensitivity and specificity of CRC screening tests (colonoscopy, FIT, mt-sDNA, mt-sRNA, and blood-based tests), as well as those reporting adherence rates, follow-up colonoscopy completion, accessibility, cost, and coverage in different healthcare settings. The data extraction was performed by two independent reviewers to ensure consistency and accuracy.
Development of the evaluation framework
Based on synthesized data, we developed a structured framework encompassing three key pillars: data (test sensitivity, specificity, and clinical performance), access (availability, affordability, and logistical feasibility), and adherence (initial participation and follow-up compliance). Each pillar is thought to provide information through the lens of the patients and their family, the providers and the healthcare systems. The framework is designed to accommodate emerging technologies and evolving evidence.
A draft evaluation framework was presented and debated during a panel discussion at the Screening Dinner, organized by the Colorectal Cancer Alliance in May 2024. Insights from panelists across relevant stakeholder groups were incorporated into the final framework iteration.
Limitations
Our methodology provides a comprehensive assessment of CRC screening options, though it is not without limitations. This work was designed as a structured narrative review with stakeholder input, rather than a full systematic review. As such, it does not include certain systematic review elements. Existing, approved screening tests benefit from real-world performance data, while newer technologies (those not yet approved or widely available) lack such evidence. Study design heterogeneity and variability in adherence definitions may also introduce variability across studies. Moreover, the absence of large-scale head-to-head comparisons among newer screening modalities necessitates cautious interpretation of indirect comparisons. Finally, our synthesis aimed at providing a practical, accessible framework for decision-making, rather than definitive comparative estimates. Future research should aim to validate and refine this framework across diverse populations and healthcare settings, ideally supported by systematic evidence reviews and decision-analytic modeling.
Results
The landscape of CRC screening has evolved significantly in recent years, with several new technologies emerging alongside established methods. This analysis focuses on six key screening modalities: colonoscopy, fecal immunochemical test (FIT), multi-target stool DNA tests (mt-sDNA, including Cologuard and Cologuard Plus), multi-target stool RNA test (mt-sRNA, ColoSense), and cell-free DNA blood-based tests (Shield and PREEMPT CRC).
Performance characteristics
Sensitivity and specificity
Evaluation of the performance characteristics of newer tests, particularly sensitivity and specificity, is crucial for understanding their effectiveness in detecting early-stage cancers and precancerous lesions.
Table 1 presents the sensitivity of each screening method for detecting CRC overall and at different stages, as well as advanced precancerous lesions (APL) and advanced adenomas (AA) with definitions that are not the same in all studies and publications. It is worth noting that most of the data was not retrieved from head-to-head studies.
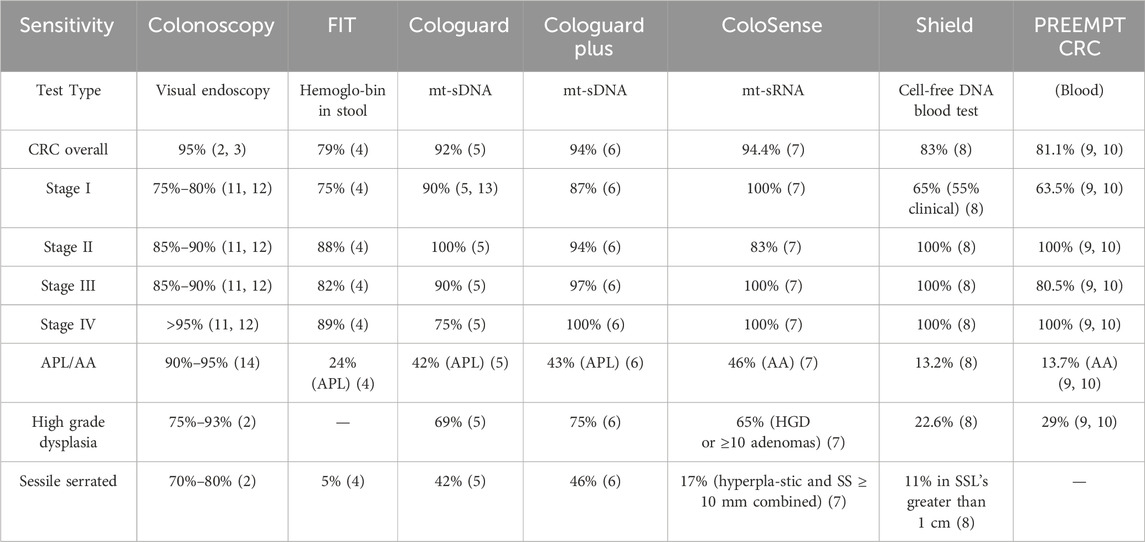
Table 1. Sensitivity of CRC screening methods. It is important to note that the data presented in Table 1 and the subsequent Tables 2–4 derive from heterogeneous study designs, thresholds, and populations. As such, results are not directly comparable across modalities, and indirect comparisons should be interpreted as hypothesis-generating rather than definitive.
Colonoscopy has long been considered the test with the highest sensitivity for CRC overall and is the benchmark against which other tests are compared, though the next-generation mt-sDNA test and the mt-sRNA test has been shown to have similar cancer sensitivity as colonoscopy (2). Stool-based tests offer varying levels of sensitivity for CRC and APL/AA, with multi-target stool DNA (mt-sDNA) and multi-target stool RNA (mt-sRNA) tests generally outperforming FIT, especially for early-stage cancers and advanced precancerous lesions (5–7). Both the mt-sDNA tests and the mt-sRNA test have proven to have higher sensitivity as compared to FIT in head-to-head pivotal trials (6, 7). mt-sDNA tests are more likely to detect sessile serrated lesions (so-called “flat lesions”) than FIT or mt-sRNA. As of March 2025, there is no head-to-head comparison between mt-sDNA and mt-sRNA. The performance for stool-based tests in terms of CRC sensitivity ranged from 74%–94%, while the performance for CRC sensitivity in blood-based test was 83% (8, 10). It is worth noting that recent publications have suggested wide variability in FIT performance depending on test used (15).
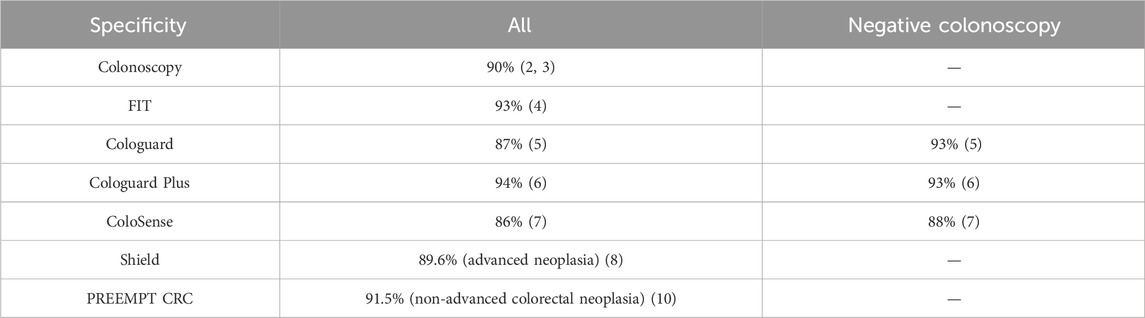
Specificity is generally high across all testing modalities, with most methods demonstrating specificity at or above 90% except for mt-sRNA and mt-DNA.
It's important to note that lower specificity may lead to more follow-up colonoscopies, which can impact healthcare resources and patient experience. However, the high specificity across all testing modalities suggests that false positives are relatively rare (4–14, 5% (6, 7, 16)), which is crucial for maintaining patient trust and minimizing unnecessary procedures.
Testing intervals and adherence rates
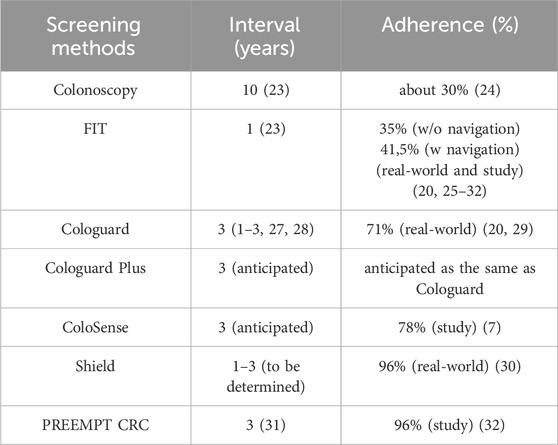
The interval and adherence rates (Table 3) for different CRC screening methods vary significantly. Colonoscopy has the longest recommended interval at 10 years (18), with adherence rates of about 30% in real-world settings. Fecal immunochemical test (FIT) has an annual interval, with adherence rates of 35% without navigation and 41.5% in real-world and study settings. Cologuard, a multi-target stool DNA test, has a recommended interval of 3 years (with a range of 1–3 years), and shows adherence rates of 71.3% overall with commercial insurance at 72.3%, Medicare Advantage at 70.2%, Medicare at 69.9%, and Medicaid at 52% (19).
Cologuard Plus, the next-generation, will maintain a 3-year interval and is expected to mirror that of Cologuard, since ordering processes and patient experience will not change with study adherence reported at 71% (20). ColoSense, a multi-target stool RNA test, also has an anticipated 3-year interval and does not yet have real world adherence. It demonstrated an 78% adherence rate in study settings. The blood-based test, Shield, has an adherence of 96% based on real world clinical usage and integrating a blood-based test into CRC screening discussion leads to a twofold increase of the screening rates (21), with Shield’s interval to be determined (estimated 1–3 years) while PREEMPT CRC’s proposed at 3 years. It is worth noting that adherence rates for newer tests are often based on study data and may differ in real-world applications. A recent systematic literature review across 36 million patients undergoing routine USPSTF recommended blood-based screening tests, adherence rates ranged from 34%–68% indicating that outside of clinical trials, real-world adherence to recommended blood-based screening may be suboptimal (22).
Follow up colonoscopy
Colonoscopy follow-up, access, and costs are critical factors in evaluating the effectiveness of CRC screening programs. According to the data presented in Table 4, follow-up colonoscopy rates vary significantly across different screening modalities. For FIT, follow-up rates range from 47% to 83%, highlighting the importance of robust tracking and reminder systems (33). Cologuard demonstrates higher follow-up rates of 71.5%–84.9% in real-world settings. While neither ColoSense nor Shield have conducted studies with endpoints that look at follow up colonoscopy adherence, ColoSense showed an 88% follow-up rate in study settings and Shield had a rate of 44% in a real world, claims analysis for individual with a colonoscopy within 6 months of the positive test (34, 35), emphasizing the need for improved follow-up strategies for blood-based tests.
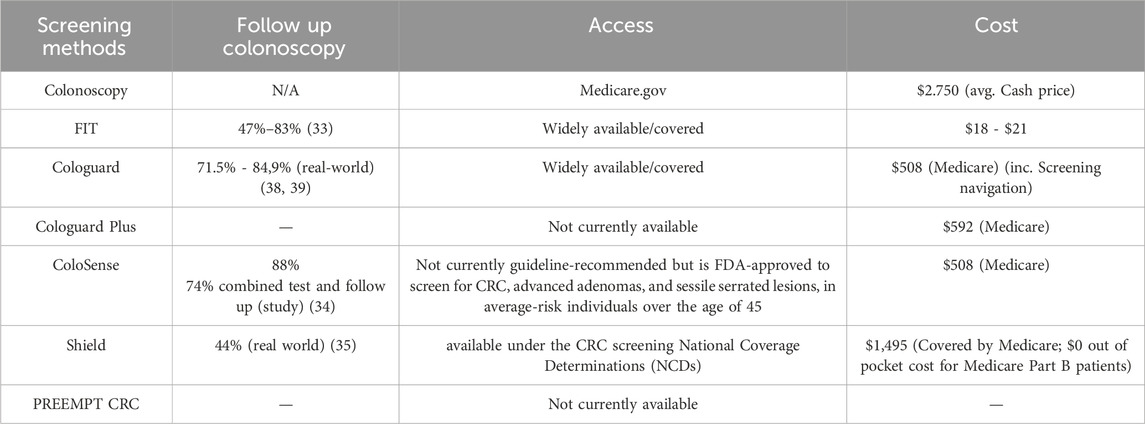
Table 4. Follow-up colonoscopy, access and cost of the different CRC screening methods (7, 8, 17–25, 33–36).
Access and cost
Access to screening tests varies, with colonoscopy, FIT and Cologuard being widely available and covered by most insurance plans. Shield is available under the CRC screening National Coverage Determinations (NCDs). However, newer tests like ColoSense are not currently guideline-recommended but is FDA-approved to screen for CRC, advanced adenomas, and sessile serrated lesions, in average-risk individuals over the age of 45. Cost considerations play a significant role in screening implementation. Colonoscopy has the highest cost at an average cash price of $2,750, which may present a barrier for uninsured or underinsured individuals. FIT is the most affordable option, ranging from $18 to $21 with an estimation of $153 per screening cycle when including the patient support costs (37). Cologuard and ColoSense have a Medicare reimbursement rate of $508, and $592 for Cologuard Plus, positioning them as mid-range options that also have screening navigation programs built into the price of the tests. Further, there is no charge for Cologuard or ColoSense until resulted, meaning the manufacturers bear the burden of ensuring a kit’s return, which is not the case with most FIT kits. The announced cash price for Shield is $1,495, which may limit its accessibility without adequate insurance coverage. It is available with no out-pocket costs for patients eligible for Medicare Part B. There are other considerations as well. As newer tests enter the market, their cost and coverage will be crucial factors in determining their accessibility and impact on overall screening rates and health system resources.
Factors influencing test choice
The choice of CRC screening test is influenced by various factors related to test performance, characteristics, and contextual elements. Understanding these factors is crucial for improving screening rates and outcomes.
Test performance and characteristics
Test performance is a key consideration in CRC screening. Colonoscopy, sometimes referred to as the gold standard, has high sensitivity (95%) for detecting CRC and advanced precancerous lesions. Stool-based tests show sensitivity for CRC detection at 79% (FIT), 92% (mt-sDNA) and up to 94% for ColoSense and Cologuard Plus, though lower sensitivity for advanced precancerous lesions (precancerous lesion performance is highly heterogeneous, and was not systematically assessed due to the lack of available data and should be interpreted with caution). Emerging blood-based tests, while promising, currently show similar sensitivity for advanced adenomas compared to stool-based tests (40). Test characteristics significantly impact patient preferences. Stool-based tests are often preferred over colonoscopy due to their non-invasive nature, convenience, and ability to be completed at home. A national survey found that 65.4% of respondents preferred mt-sDNA tests over colonoscopy, and 61% preferred FIT/gFOBT over colonoscopy (41). Handling stool samples may be a barrier for some patients, making blood-based tests a potential alternative for those patients who would not otherwise get screened, however the patients need to travel to a venipuncture site and some patients may fear needles. Test interval is another important characteristic, with patients generally preferring longer intervals between screenings (41).
Contextual factors
Contextual factors such as travel distance, insurance coverage, health system test, geography, access availability of colonoscopy impact screening decisions. A study in rural Southern Illinois found that long-distance travel for care was normalized but not necessarily preferable for patients. Providers identified distance-related challenges specific to CRC screening, including transportation issues and the need for patients to take time off work (42). Insurance coverage also influences screening choices. Among adults aged 45–49 years, those with Medicare or Medicaid were more likely to prefer colonoscopy (62.8%) compared to those with private insurance (54.8%) or no insurance (46.7%) (41). Uninsured individuals are more likely to prefer stool-based tests over colonoscopy due to cost considerations.
Geographic location plays a role, with rural residents facing unique barriers to CRC screening. In rural Southern Illinois, providers reported that distance to care remains a significant challenge to increasing CRC screening and contributes to disparities in rural communities (42–44). They suggested various solutions to reduce distance and transportation barriers, such as mobile screening units and telemedicine options for pre-screening consultations.
Patient preferences
Patient preferences are shaped by multiple factors. Convenience and ease of use are primary considerations, with many patients citing forgetfulness or procrastination as reasons for not completing stool-based tests (45, 46). A study on pharmacy-based CRC screening programs found that 93% of participants preferred completing the test at home, and 85% valued the ability to drop off the completed test at a convenient location. Perceived accuracy also influences choice, with some patients believing blood tests to be more accurate than stool tests, despite lacking information on actual test characteristics. In a qualitative study, patients were receptive about completing a blood test for CRC screening, citing simplicity, ease, convenience, and high perceived accuracy (47, 48). Cultural factors can impact screening preferences. A study focusing on Latino patients found varying levels of awareness about FIT testing, with some expressing doubts about its efficacy compared to colonoscopy (49). This underscores the need for culturally tailored education and outreach efforts.
Healthcare provider preferences
Healthcare provider preferences are influenced by factors such as test accuracy, patient compliance, and system integration. A survey of 1,281 primary care providers found that 82.9% rated colonoscopy as very effective for patients aged 50-74, compared to 59.6% for FIT (50). Moreover, 26.3% rated colonoscopy as more effective than FIT, despite some modeling studies suggesting comparable effectiveness. Providers express concerns about patient adherence to different screening modalities. The same survey found that providers recommended colonoscopy every 10 years for 77.9% of patients aged 50-74, while 92.4% recommended FIT annually. System integration is crucial, with providers favoring options that are easy to implement within their healthcare systems. The ability to integrate screening programs with electronic health records and existing workflows is an important consideration for many healthcare organizations (51).
Education and communication
Education and communication play vital roles in test choice and screening adherence. Both patients and providers express a need for clear, comprehensive information about different screening options (52–54). A study on Latino patients’ perceptions of FIT testing found that awareness levels varied based on prior screening experiences, highlighting the need for targeted education efforts, especially for underserved populations (49). Providers may benefit from education about the comparative effectiveness of different screening modalities. The survey of primary care providers revealed discrepancies between provider perceptions and evidence from modeling studies regarding the effectiveness of FIT compared to colonoscopy (50). Addressing these knowledge gaps could influence provider recommendations and, subsequently, patient choices. Effective communication strategies and decision aids can help patients make informed choices aligned with their preferences and values. A study on older adults found that a targeted patient decision aid influenced screening preferences, particularly among those in poorer health states (55). This suggests that decision aids can be valuable tools for facilitating shared decision-making about CRC screening.
Emerging technologies and future directions
As technology advances, new screening options are emerging that may further influence test choices. Artificial intelligence (AI) is being explored to enhance the accuracy of existing screening methods. For instance, AI-assisted colonoscopy has shown promise in improving adenoma detection rates (56, 57). These emerging technologies may address some of the current barriers to screening, such as the invasiveness of colonoscopy or the discomfort associated with stool-based tests. However, their implementation will require careful consideration of factors such as cost-effectiveness, accessibility, and integration into existing healthcare systems (58, 59).
Policy implications
Policies that address barriers to screening, such as lack of insurance coverage or limited access to healthcare facilities, can significantly impact screening rates and test choices. For instance, the Affordable Care Act’s requirement for insurance plans to cover preventive services, including CRC screening, has been associated with increased screening rates (60).
The choice of CRC screening test is influenced by a complex interplay of factors related to test performance, characteristics, context, and stakeholder preferences. Understanding and addressing these multifaceted influences is key to optimizing CRC screening strategies and outcomes. Future efforts to increase screening rates should focus on addressing barriers related to access, education, and communication, while considering the diverse needs and preferences of different patient populations. As new technologies emerge and our understanding of CRC screening evolves, it will be crucial to continually reassess and adapt screening strategies. This may involve integrating new screening modalities, refining risk-stratification approaches, or developing more personalized screening recommendations based on individual risk factors and preferences through early detection and prevention. By carefully considering the various factors that influence screening test choices and working to optimize these choices for different populations and contexts, we can make significant strides towards this important public health goal.
Modeling studies to inform CRC screening policies
Simulation modeling has emerged as a valuable tool in evaluating CRC screening strategies, offering insights into the complex interplay of factors that influence screening effectiveness and cost-efficiency. These models provide decision-makers with essential information to guide policy and healthcare system choices in the absence of feasible large-scale clinical trials (61, 62). However, it is crucial to understand both the strengths and limitations of these modeling studies in the context of CRC screening methods.
Modeling studies have been instrumental in comparing various CRC screening modalities, assessing their cost-effectiveness, and determining the potential impact of different screening strategies on population health outcomes (63). These studies have consistently shown that CRC screening, regardless of the method, is cost-effective compared to no screening (64, 65). They have also provided valuable insights into the relative effectiveness of different screening methods, such as colonoscopy, sigmoidoscopy, and stool-based tests, under various assumptions and scenarios (62, 63). One of the key strengths of modeling studies is their ability to simulate long-term outcomes and evaluate the impact of screening strategies over extended periods, which would be impractical or impossible in real-world trials (61, 63). This capability allows researchers to estimate the potential reduction in CRC incidence and mortality rates associated with different screening approaches, helping to inform policy decisions and resource allocation (62). Moreover, modeling studies can incorporate a wide range of variables, including screening and follow-up colonoscopy adherence rates, test characteristics, and cost parameters, to provide a comprehensive assessment of screening strategies. This flexibility allows for the evaluation of various “what-if” scenarios, helping decision-makers understand the potential impact of changes in screening policies or technologies (61, 65).
Despite their utility, modeling studies have limitations that must be considered when interpreting their results. Firstly, the validity and reliability of these models heavily depend on the quality and accuracy of the input data and assumptions used (62, 66). Many studies fail to report full model validation, with only 58% of reviewed studies conducting any validation at all (63). This lack of thorough validation can lead to uncertainties in the model’s predictions and potentially misleading conclusions. Secondly, while modeling studies can simulate various scenarios, they cannot capture the full complexity of real-world implementation challenges. Factors such as patient preferences, real-world adherence to initial and longitudinal screening, healthcare provider behavior, and system-level barriers to screening implementation are often difficult to accurately represent in models (61). As a result, the actual impact of a screening strategy may differ from model predictions when implemented in practice. Furthermore, modeling studies typically focus on average-risk populations and may not adequately address the needs of specific subgroups or individuals with varying risk profiles. This limitation can lead to oversimplified recommendations that may not be optimal for all segments of the population (62, 65). Lastly, while modeling studies can provide valuable evidence for decision-making, they often fall short in reporting their actual impact on health system or policy decisions. A systematic review found that while 91% of models were developed to address specific health system or policy questions, only 12% reported on the model’s impact on these decisions (63). This gap highlights the need for better integration of modeling results into the decision-making process and improved communication between researchers and policymakers.
Modeling studies offer valuable insights into the potential effectiveness and cost-efficiency of CRC screening methods. However, their limitations, including validation concerns, real-world implementation challenges, and the gap between model development and policy impact, must be carefully considered. To maximize the utility of these models in informing CRC screening policies, there is a need for more rigorous validation processes, improved representation of real-world complexities, and better mechanisms for translating model findings into practice.
A practical framework for evaluating CRC testing options
Discussion
Framework for evaluating CRC screening options
The proposed framework evaluates three primary attributes: data strength, patient adherence, and access. Given the heterogeneity of study designs and outcome definitions across screening modalities, caution is warranted when interpreting indirect comparisons. These data provide a useful overview of trends but should not be considered as head-to-head evidence. While no single attribute dominates, the effort required to address each varies. If one were to prioritize these attributes, performance data would be a logical starting point. Understanding the sensitivity of tests for detecting polyps, stage I and II cancers, and advanced lesions is essential for maximizing mortality prevention. Currently, the majority of data focuses on sensitivity and specificity for CRC. Decisions based solely on data are impractical. Tests must meet minimum sensitivity and specificity thresholds; however, even high-performing tests lose utility if patients do not adhere. Adherence encompasses not only initial screening but also follow-up. For example, blood-based tests may reduce barriers to initial implementation but may disproportionately reach individuals who are less likely to complete necessary follow-up colonoscopies. This demonstrates the importance of evaluating adherence holistically.
Challenges and opportunities in access and capacity
Access-related factors, such as colonoscopy availability and capacity, play a pivotal role in decision-making. Colonoscopy capacity must account for follow-up needs arising from other screening modalities. While the ability to offer choice is beneficial, effective population management necessitates clear protocols. FIT testing, for instance, can be paired with outreach strategies, such as phone or text follow-ups, to increase adherence. However, significant barriers remain, especially for populations with limited access to colonoscopy.
Perspectives on decision-making
Decision-making priorities in healthcare vary significantly among different stakeholders. Patients typically prioritize practical considerations such as time away from work, transportation issues, out-of-pocket expenses, and insurance coverage when making healthcare decisions. Healthcare providers, on the other hand, often emphasize the practicality of tests, adhering to the principle that “The best test is the one that gets done” (67). This mindset may lead them to favor non-invasive options like FIT stool-based tests or blood-based tests in certain situations due to their accessibility, although concerns exist about newer options that may have lower sensitivity for detecting pre-neoplastic, high-risk lesions, or early cancers. Health systems and public health entities face their own set of challenges, primarily driven by logistical and financial constraints. These organizations, particularly public health programs, often prioritize cost-effective, non-colonoscopy options such as stool-based tests for outreach initiatives, especially when targeting populations that are less likely to engage with traditional screening methods. Moving forward, greater collaboration among stakeholders is needed to refine screening strategies and improve both adherence and outcomes.
Innovation’s limitations in detecting precancerous lesions
An important limitation of current CRC screening modalities, particularly non-colonoscopy tests, is their variable and often suboptimal ability to detect precancerous lesions such as advanced adenomas and sessile serrated polyps. While colonoscopy remains the reference standard for identifying and removing these precursor lesions, stool- and blood-based tests have demonstrated significantly lower sensitivity. This shortcoming has major implications for prevention at the population level, as failure to reliably detect precancerous states undermines the long-term effectiveness of screening programs. Addressing this gap represents a key research priority, with innovation urgently needed to improve early lesion detection and thereby enhance the preventive potential of CRC screening.
Although this framework was developed with a focus on the U.S. context, future research should explore its application and adaptation in international settings, including Europe, Asia, and LMICs, where health system structures, access barriers, and patient preferences may differ substantially.
Conclusion
The rapid evolution of CRC screening modalities highlights the need for a structured approach to navigate the complexities of decision-making. The proposed framework, grounded in the core principles of data, access, and adherence, serves as a robust model for assessing both established and novel CRC screening methods. By methodically evaluating performance metrics, accessibility barriers, and adherence challenges, this framework aims to foster screening strategies that are not only clinically effective but also equitable and centered on patient needs.
Each of the three pillars plays a unique and vital role in optimizing screening outcomes. The pillar of “Data” provides the foundational basis for clinical confidence by prioritizing accuracy and reliability in detecting CRC and its precursors. “Access” ensures that screening options are made equitably available, addressing systemic barriers and resource disparities that could otherwise limit the reach of screening programs. Finally, the “Adherence” pillar targets one of the most critical aspects of effective screening programs: the consistent participation of patients, from initial testing to necessary follow-up procedures. These pillars function synergistically, empowering patients, healthcare providers, and health systems alike to make evidence-informed decisions that align with public health priorities and individual needs.
By integrating this comprehensive framework, stakeholders have the potential to significantly enhance CRC screening strategies, addressing gaps in access and adherence while promoting equity across diverse populations. Furthermore, the implementation of this framework invites opportunities for iterative improvement. Future research efforts should aim to validate the framework’s utility in varied clinical settings and among diverse populations, while policy initiatives should strive to operationalize its principles within healthcare systems. Engaging all relevant stakeholders in this process (patients, providers, payers, and policymakers) will be crucial to sustaining progress. As the landscape of CRC screening continues to evolve, this framework provides a dynamic foundation for advancing prevention and early detection, ultimately reducing the global burden of CRC. Moreover, the scientific community should explore ways to assigning weights and testing the framework in a formal decision-analytic or scoring model to assess its applicability in real-world clinical decision-making.
Author contributions
MS: Conceptualization, Funding acquisition, Writing – review and editing. CD: Conceptualization, Data curation, Writing – review and editing. MB: Conceptualization, Data curation, Writing – review and editing, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is an outcome of the 2024 Screen Smart Dinner, organized by the Colorectal Cancer Alliance and sponsored by Exact Sciences, Guardant Health, Braintree, Genentech, and Natera. The sponsors had no role in the study design, data analysis and interpretation. The comprehensive list of sponsors of the Colorectal Cancer Alliance can be found at https://colorectalcancer.org/get-involved/advocate/partner-us/sponsorship. Red Thred Solutions received funding from the Colorectal Cancer Alliance to support the organization of the event and the subsequent development of this review.
Acknowledgments
The authors thank heartfully the following contributors for their review of the manuscript: Djenaba Joseph (Centers for Disease Control and Prevention), Robert Smith (American Cancer Society), Steven Itzkowitz (Icahn School of Medicine at Mount Sinai), Richard Wender (Perelman School of Medicine at the University of Pennsylvania), Erica Barnell (Geneoscopy), Todd Kelley (polymedco), Theodore R Levin (Kaiser Permanente), Lance Baldo (Freenome) and Craig Eagle (Guardant Health).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel, RL, Miller, KD, Goding Sauer, A, Fedewa, SA, Butterly, LF, Anderson, JC, et al. Colorectal cancer statistics, 2020. CA: A Cancer J Clinicians (2020) 70(3):145–64. doi:10.3322/caac.21601
2. Rex, DK, Schoenfeld, PS, Cohen, J, Pike, IM, Adler, DG, Fennerty, MB, et al. Quality indicators for colonoscopy. Gastrointest Endosc (2015) 81(1):31–53. doi:10.1016/j.gie.2014.07.058
3. Brenner, H, Chang-Claude, J, Seiler, CM, Rickert, A, and Hoffmeister, M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med (2011) 154(1):22–30. doi:10.7326/0003-4819-154-1-201101040-00004
4. Niedermaier, T, Tikk, K, Gies, A, Bieck, S, and Brenner, H. Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Clin Gastroenterol Hepatol (2020) 18(13):2920–8.e6. doi:10.1016/j.cgh.2020.01.025
5. Imperiale, TF, Ransohoff, DF, Itzkowitz, SH, Levin, TR, Lavin, P, Lidgard, GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med (2014) 370(14):1287–97. doi:10.1056/nejmoa1311194
6. Imperiale, TF, Porter, K, Zella, J, Gagrat, ZD, Olson, MC, Statz, S, et al. Next-generation multitarget stool DNA test for colorectal cancer screening. New Engl J Med (2024) 390(11):984–93. doi:10.1056/nejmoa2310336
7. Barnell, EK, Wurtzler, EM, La Rocca, J, Fitzgerald, T, Petrone, J, Hao, Y, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA (2023) 330(18):1760–8. doi:10.1001/jama.2023.22231
8. Chung, DC, Gray, DM, Singh, H, Issaka, RB, Raymond, VM, Eagle, C, et al. A cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med (2024) 390(11):973–83. doi:10.1056/nejmoa2304714
9. Freenome Freenome announces topline results for PREEMPT CRC® to validate the first version of its blood-based test for the early detection of colorectal cancer [press release]. (2024).
10. Shaukat, A, Burke, CA, Chan, AT, Grady, WM, Gupta, S, Katona, BW, et al. Clinical validation of a circulating tumor dna–based blood test to screen for colorectal cancer. JAMA (2025) 334(01):56–63. doi:10.1001/jama.2025.7515
11. Baxter, NN, Goldwasser, MA, Paszat, LF, Saskin, R, Urbach, DR, and Rabeneck, L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med (2009) 150(1):1–8. doi:10.7326/0003-4819-150-1-200901060-00306
12. Rex, DK, Overhiser, AJ, Chen, SC, Cummings, OW, and Ulbright, TM. Estimation of impact of American college of radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol (2009) 104(1):149–53. doi:10.1038/ajg.2008.35
13. Dominitz, JA, Robertson, DJ, Ahnen, DJ, Allison, JE, Antonelli, M, Boardman, KD, et al. Colonoscopy vs. fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM): rationale for study design. Am J Gastroenterol (2017) 112(11):1736–46. doi:10.1038/ajg.2017.286
14. Rex, DK, Ahnen, DJ, Baron, JA, Batts, KP, Burke, CA, Burt, RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol (2012) 107(9):1315–29. doi:10.1038/ajg.2012.161
15. Levy, BT, Xu, Y, Daly, JM, Hoffman, RM, Dawson, JD, Shokar, NK, et al. Comparative performance of common fecal immunochemical tests: a cross-sectional study. Ann Intern Med (2024) 177(10):1350–60. doi:10.7326/m24-0080
16. Young, GP, Symonds, EL, Allison, JE, Cole, SR, Fraser, CG, Halloran, SP, et al. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci (2015) 60(3):609–22. doi:10.1007/s10620-014-3445-3
17. Goshgarian, G, Sorourdi, C, May, FP, Vangala, S, Meshkat, S, Roh, L, et al. Effect of patient portal messaging before mailing fecal immunochemical test kit on colorectal cancer screening rates: a randomized clinical trial. JAMA Netw Open (2022) 5(2):e2146863. doi:10.1001/jamanetworkopen.2021.46863
18. Baron, TH, Smyrk, TC, and Rex, DK. Recommended intervals between screening and surveillance colonoscopies. Mayo Clinic Proc (2013) 88(8):854–8. doi:10.1016/j.mayocp.2013.04.023
19. Le, QA, Greene, M, Gohil, S, Ozbay, AB, Dore, M, Fendrick, AM, et al. Adherence to multi-target stool DNA testing for colorectal cancer screening in the United States. Int J Colorectal Dis (2025) 40(1):16. doi:10.1007/s00384-025-04805-0
20. Weiser, E, Parks, PD, Swartz, RK, Thomme, JV, Lavin, PT, Limburg, P, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen (2021) 28(1):18–24. doi:10.1177/0969141320903756
21. Coronado, GD, Jenkins, CL, Shuster, E, Johnson, C, Amy, D, Cook, J, et al. Blood-based colorectal cancer screening in an integrated health system: a randomised trial of patient adherence. Gut (2024) 73(4):622–8. doi:10.1136/gutjnl-2023-330980
22. Le, QA, Kiener, T, Johnson, HA, Khan, RS, Kong, J, Li, KH, et al. Systematic review of real-world adherence to blood-based laboratory tests for average-risk disease screening: potential implications for emerging colorectal cancer screening modalities. Amer assoc cancer research 615 chestnut st, 17TH floor. Clinical cancer research (2024).
23. Davidson, KW, Barry, MJ, Mangione, CM, Cabana, M, Caughey, AB, Davis, EM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA (2021) 325(19):1965–77. doi:10.1001/jama.2021.6238
24. Vijan, S, Vijan, S, Janz, NK, Fagerlin, A, Thomas, JP, Lin, YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med (2012) 172(7):575–82. doi:10.1001/archinternmed.2012.332
25. Jensen, CD, Corley, DA, Quinn, VP, Doubeni, CA, Zauber, AG, Lee, JK, et al. Fecal Immunochemical Test Program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med (2016) 164(7):456–63. doi:10.7326/m15-0983
26. Fleming, TJ, Benitez, MG, and Weintraub, MLR. Evaluating the effectiveness of one-on-one conversations to increase colorectal cancer screening in a community-based clinical setting. J Osteopathic Med (2018) 118(1):26–33. doi:10.7556/jaoa.2018.005
27. Wolf, AMD, Fontham, ETH, Church, TR, Flowers, CR, Guerra, CE, LaMonte, SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer society. CA: A Cancer J Clinicians (2018) 68(4):250–81. doi:10.3322/caac.21457
28. Patel, SG, May, FP, Anderson, JC, Burke, CA, Dominitz, JA, Gross, SA, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. multi-society task force on colorectal cancer. Gastroenterology (2022) 162(1):285–99. doi:10.1053/j.gastro.2021.10.007
29. Miller-Wilson, LA, Rutten, LJF, Van Thomme, J, Ozbay, AB, and Limburg, PJ. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a large, nationally insured cohort. Int J Colorectal Dis (2021) 36(11):2471–80. doi:10.1007/s00384-021-03956-0
30. Raymond, V, Foster, GRN, Hong, Y, Hoang, T, Liu, J, Burke, J, et al. Implementation of blood-based colorectal cancer screening: real-world clinical experience. Am J Gastroenterol. (2023) 118(10S):S218. doi:10.14309/01.ajg.0000950820.05087.2c
31. CMS. National coverage determination (NCD) - colorectal cancer screening tests (2023). Available online at: https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=281#:∼:text=Effective%20January%201%2C%202023%2C%20the%20minimum%20age%20for%20blood%2D,to%2045%20years%20and%20older.&text=All%20other%20indications%20for%20colorectal,above%20remain%20nationally%20non%2Dcovered
32. Raymond, V, Foster, G, Hong, Y, Hoang, T, Liu, J, Burke, J, et al. S295 implementation of blood-based colorectal cancer screening: real-world clinical experience. Am J Gastroenterol (2023) 118:S218. doi:10.14309/01.ajg.0000950820.05087.2c
33. Mohl, JT, Ciemins, EL, Miller-Wilson, LA, Gillen, A, Luo, R, and Colangelo, F. Rates of Follow-up colonoscopy after a positive stool-based screening test result for colorectal cancer among health care organizations in the US, 2017-2020. JAMA Netw Open (2023) 6(1):e2251384. doi:10.1001/jamanetworkopen.2022.51384
34. Ciemins, EL, Mohl, JT, Moreno, CA, Colangelo, F, Smith, RA, and Barton, M. Development of a Follow-Up measure to ensure complete screening for colorectal cancer. JAMA Netw Open (2024) 7(3):e242693. doi:10.1001/jamanetworkopen.2024.2693
35. Zaki, TNJ, Zhang, N, Raymond, VM, Ioannou, N, Forbes, SP, Das, AK, et al. New screening tests, same challenge: an early look at colonoscopic follow-up after abnormal blood-based colorectal cancer screening results in a real-world setting. Dig Dis Week (2024). Available online at: https://eposters.ddw.org/ddw/2024/ddw-2024/412939/timothy.zaki.new.screening.tests.same.challenge.an.early.look.at.colonoscopic.html?f=listing%3D4%2Abrowseby%3D8%2Asortby%3D2%2Aspeaker%3D994008.
36. Austin, G, Kowalkowski, H, Guo, Y, Miller-Wilson, LA, DaCosta Byfield, S, Verma, P, et al. Patterns of initial colorectal cancer screenings after turning 50 years old and follow-up rates of colonoscopy after positive stool-based testing among the average-risk population. Curr Med Res Opin (2023) 39(1):47–61. doi:10.1080/03007995.2022.2116172
37. Ladabaum, U, and Mannalithara, A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology (2016) 151(3):427–39.e6. doi:10.1053/j.gastro.2016.06.003
38. Cooper, GS, Grimes, A, Werner, J, Cao, S, Fu, P, and Stange, KC. Barriers to Follow-Up colonoscopy after positive FIT or multitarget stool DNA testing. J Am Board Fam Med (2021) 34(1):61–9. doi:10.3122/jabfm.2021.01.200345
39. Finney Rutten, LJ, Jacobson, DJ, Jenkins, GD, Fan, C, Weiser, E, Parks, P, et al. Colorectal cancer screening completion: an examination of differences by screening modality. Prev Med Rep (2020) 20:101202. doi:10.1016/j.pmedr.2020.101202
40. Rex, DK, Boland, RC, Dominitz, JA, Giardiello, FM, Johnson, DA, Kaltenbach, T, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Am J Gastroenterol (2017) 112(7):1016–30. doi:10.1038/ajg.2017.174
41. Zhu, X, Parks, PD, Weiser, E, Fischer, K, Griffin, JM, Limburg, PJ, et al. National survey of patient factors associated with colorectal cancer screening preferences. Cancer Prev Res (2021) 14(5):603–14. doi:10.1158/1940-6207.capr-20-0524
42. Lee, KMN, Hunleth, J, Rolf, L, Maki, J, Lewis-Thames, M, Oestmann, K, et al. Distance and transportation barriers to colorectal cancer screening in a rural community. J Prim Care Community Health (2023) 14:21501319221147126. doi:10.1177/21501319221147126
43. Greenwald, ZR, El-Zein, M, Bouten, S, Ensha, H, Vazquez, FL, and Franco, EL. Mobile screening units for the early detection of cancer: a systematic review. Cancer Epidemiol Biomarkers and Prev (2017) 26(12):1679–94. doi:10.1158/1055-9965.epi-17-0454
44. Chua, CY, Stoll, C, Maki, J, Colditz, G, and James, A. Abstract A011: opportunities for connection: a scoping review of telemedicine interventions in colorectal cancer screening in rural and nonrural settings. Cancer Epidemiol Biomarkers and Prev (2020) 29(6_Suppl. ment_1):A011–A. doi:10.1158/1538-7755.disp18-a011
45. Bae, N, Park, S, and Lim, S. Factors associated with adherence to fecal occult blood testing for colorectal cancer screening among adults in the Republic of Korea. Eur. J. Oncol. Nurs. (2014) 18(1):72–77. doi:10.1016/j.ejon.2013.09.001
46. Jones, RM, Devers, KJ, Kuzel, AJ, and Woolf, SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med (2010) 38(5):508–16. doi:10.1016/j.amepre.2010.01.021
47. Schneider, JL, Johnson, CA, Jenkins, C, Mummadi, R, and Coronado, GD. I was screaming hallelujah: patient and provider perceptions of blood-based testing for colorectal cancer screening. PLoS One (2023) 18(12):e0295685. doi:10.1371/journal.pone.0295685
48. Gwede, CK, Koskan, AM, Quinn, GP, Davis, SN, Ealey, J, Abdulla, R, et al. Patients' perceptions of colorectal cancer screening tests and preparatory education in federally qualified health centers. J Cancer Education (2015) 30(2):294–300. doi:10.1007/s13187-014-0733-8
49. Chablani, SV, Cohen, N, White, D, Itzkowitz, SH, DuHamel, K, and Jandorf, L. Colorectal cancer screening preferences among black and Latino primary care patients. J Immigrant Minor Health (2017) 19(5):1100–8. doi:10.1007/s10903-016-0453-8
50. Ghai, NR, Jensen, CD, Merchant, SA, Schottinger, JE, Lee, JK, Chubak, J, et al. Primary care provider beliefs and recommendations about colorectal cancer screening in four healthcare systems. Cancer Prev Res (2020) 13(11):947–58. doi:10.1158/1940-6207.capr-20-0109
51. Maxwell, AE, DeGroff, A, Hohl, SD, Sharma, KP, Sun, J, Escoffery, C, et al. Evaluating uptake of evidence-based interventions in 355 clinics partnering with the colorectal cancer control program, 2015-2018. Prev Chronic Dis (2022) 19:210258. doi:10.5888/pcd19.210258
52. Baskar, S, Schoeneich, R, Baskar, A, and Grewal, US. Leveraging patient education to amplify colorectal cancer screening in the United States: strategies and implications. J Cancer Education (2024) 40:321–8. doi:10.1007/s13187-024-02482-1
53. Matthias, MS, and Imperiale, TF. A risk prediction tool for colorectal cancer screening: a qualitative study of patient and provider facilitators and barriers. BMC Fam Pract (2020) 21(1):43. doi:10.1186/s12875-020-01113-0
54. JaKa, MM, Henderson, MG, Alch, S, Ziegenfuss, JY, Zinkel, AR, Osgood, ND, et al. Qualitative interviews to add patient perspectives in colorectal cancer screening: improvements in a learning health system. J Cancer Education (2024) 39(1):78–85. doi:10.1007/s13187-023-02378-6
55. Lewis, CL, Golin, CE, DeLeon, C, Griffith, JM, Ivey, J, Trevena, L, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: development and results of an uncontrolled trial. BMC Med Inform Decis Mak (2010) 10:54. doi:10.1186/1472-6947-10-54
56. Huang, D, Shen, J, Hong, J, Zhang, Y, Dai, S, Du, N, et al. Effect of artificial intelligence-aided colonoscopy for adenoma and polyp detection: a meta-analysis of randomized clinical trials. Int J Colorectal Dis (2022) 37(3):495–506. doi:10.1007/s00384-021-04062-x
57. Mori, Y, Kudo, SE, Misawa, M, Saito, Y, Ikematsu, H, Hotta, K, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: a prospective study. Ann Intern Med (2018) 169(6):357–66. doi:10.7326/m18-0249
58. Peterse, EF, Meester, RG, de Jonge, L, Omidvari, A-H, Alarid-Escudero, F, Knudsen, AB, et al. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. JNCI: J Natl Cancer Inst (2021) 113(2):154–61. doi:10.1093/jnci/djaa103
59. Mannucci, A, and Goel, A. Stool and blood biomarkers for colorectal cancer management: an update on screening and disease monitoring. Mol Cancer (2024) 23(1):259. doi:10.1186/s12943-024-02174-w
60. Lee, C, Kushi, LH, Reed, ME, Eldridge, EH, Lee, JK, Zhang, J, et al. Impact of the affordable care act on colorectal cancer incidence and mortality. Am J Prev Med (2022) 62(3):387–94. doi:10.1016/j.amepre.2021.08.025
61. Bespalov, A, Barchuk, A, Auvinen, A, and Nevalainen, J. Cancer screening simulation models: a state of the art review. BMC Med Inform Decis Mak (2021) 21(1):359. doi:10.1186/s12911-021-01713-5
62. Knudsen, AB, Rutter, CM, Peterse, EFP, Lietz, AP, Seguin, CL, Meester, RGS, et al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA (2021) 325(19):1998–2011. doi:10.1001/jama.2021.5746
63. Dj, RCSJV. Simulation modeling validity and utility in colorectal cancer screening: a systematic review. J Am Med Inform Assoc (2020) 27(6):908–18.
64. Wong, CK, Lam, CL, Wan, YF, and Fong, DY. Cost-effectiveness simulation and analysis of colorectal cancer screening in Hong Kong Chinese population: comparison amongst colonoscopy, guaiac and immunologic fecal occult blood testing. BMC Cancer (2015) 15:705. doi:10.1186/s12885-015-1730-y
65. Pokharel, R, Lin, Y-S, McFerran, E, and O’Mahony, JF. A systematic review of cost-effectiveness analyses of colorectal cancer screening in Europe: have studies included optimal screening intensities? Appl Health Econ Health Policy (2023) 21(5):701–17. doi:10.1007/s40258-023-00819-3
66. Escudero, FA, Knudsen, AB, Ozik, J, Collier, N, and Kuntz, KM. Characterization and valuation of uncertainty of calibrated parameters in stochastic decision models. Scanning Electron Microsc Meet (2019). doi:10.3389/fphys.2022.780917
Keywords: colorectal cancer, screening, diagnosis, patients, prevention
Citation: Sapienza M, Davis C and Boudes M (2025) Navigating the evolving landscape of colorectal cancer screening with a practical framework: a comprehensive analysis of existing and emerging technologies for informed decision-making. Oncol. Rev. 19:1653617. doi: 10.3389/or.2025.1653617
Received: 25 June 2025; Accepted: 02 October 2025;
Published: 20 October 2025.
Edited by:
Alessandro Passardi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Gairui Li, Harbin Medical University, ChinaEwan Hunter, Oxford BioDynamics PLC, United Kingdom
Copyright © 2025 Sapienza, Davis and Boudes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathieu Boudes, bWF0aGlldUByZWR0aHJlZHNvbHV0aW9ucy5jb20=
 Michael Sapienza1
Michael Sapienza1 Mathieu Boudes
Mathieu Boudes