- 1Laboratory of Molecular Medicine, National Institute of Gastroenterology IRCCS “S. de Bellis”, Bari, Italy
- 2Medical Oncology Unit, National Institute of Gastroenterology, IRCCS “S. de Bellis” Research Hospital, Castellana Grotte, Italy
- 3Institute for Chemical-Physical Processes, Italian National Research Council (IPCF)-CNR SS Bari, Bari, Italy
- 4National Interuniversity Consortium of Materials Science and Technology (INSTM), Bari Research Unit, Bari, Italy
- 5Department of Chemistry, University of Bari, Bari, Italy
- 6Department of Medicine and Surgery, University of Parma, Parma, Italy
- 7Scientific Direction, National Institute of Gastroenterology IRCCS “S. de Bellis”, Bari, Italy
Background and aims: Liquid biopsy offers a minimally invasive tool to detect actionable mutations, monitor minimal residual disease (MRD), and guide therapy in gastrointestinal (GI) cancers. We critically review the clinical utility of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and small extracellular vesicles (sEVs) across GI malignancies and propose a framework for their integration into clinical practice.
Methods: We synthesized evidence from over 200 studies, including prospective trials and translational research, to assess diagnostic accuracy, prognostic value, and clinical actionability of each biomarker type in esophageal, gastric, colorectal, pancreatic, hepatocellular, and biliary cancers.
Results: ctDNA has shown strong potential for MRD detection and treatment monitoring, particularly in colorectal and pancreatic cancer. CTCs offer insights into metastatic risk and therapeutic resistance, while sEVs provide molecular cargo relevant to immunomodulation and disease progression. Emerging microfluidics and AI-driven multi-omics approaches may overcome current limitations.
Conclusion: The integration of liquid biopsy technologies into GI oncology holds promise for early detection and precision therapy. We propose a five-phase clinical roadmap and outine the key research gaps that need to be addressed before widespread implementation in routine care.
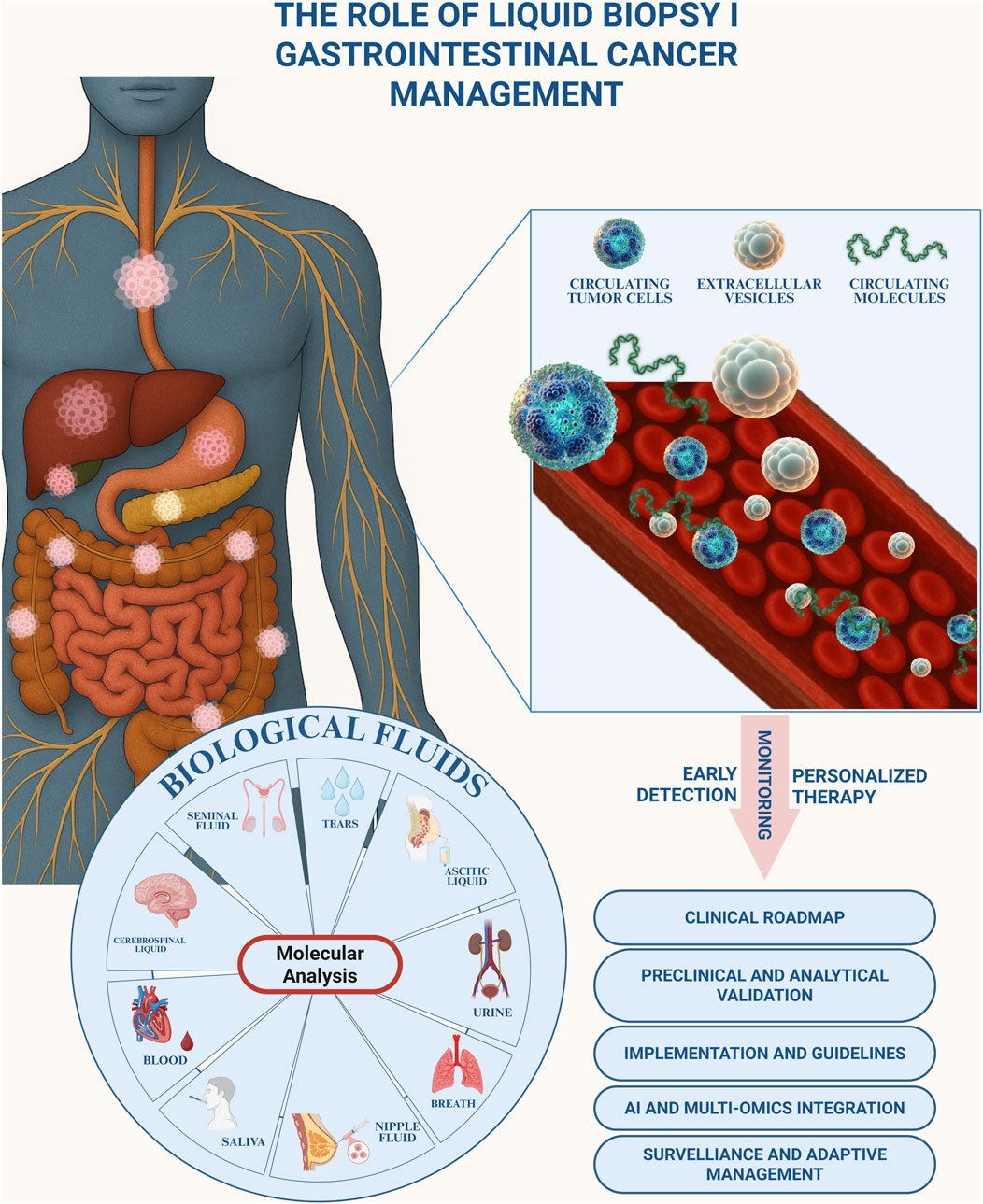
GRAPHICAL ABSTRACT | Graphical abstract illustrating the role of liquid biopsy in the management of gastrointestinal cancer. Tumor-derived biomarkers, including circulating tumor cells, extracellular vesicles, and circulating molecules, can be detected in different biological fluids (blood, saliva, urine, cerebrospinal fluid, seminal fluid, nipple fluid, ascitic liquid, tears, and breath) through molecular analysis. Liquid biopsy enables early detection, longitudinal monitoring, and the development of personalized therapeutic strategies. Key translational aspects include the establishment of a clinical roadmap, preclinical and analytical validation, implementation of standardized guidelines, integration of artificial intelligence and multi-omics approaches, as well as surveillance and adaptive management.
1 Introduction
Cancer is the world’s second-deadliest disease, making early detection vital. While tissue biopsy is still the diagnostic gold standard, it is invasive and often misses tumour diversity or changes over time. Liquid biopsy, by analysing tumour-derived material in blood, saliva, urine, or other fluids, provides a non-invasive, real-time, and more comprehensive picture of tumour biology and progression (1). This innovative diagnostic method minimizes patient discomfort and enables real-time monitoring of tumour evolution and therapeutic responses (Figure 1) (2). Additionally, tissue biopsies may be unsuitable for detecting tumours at early stages (3). In gastrointestinal (GI) cancers, often marked by late diagnoses and limited treatment options, liquid biopsy offers a more precise approach to disease management (4). Key biomarkers include circulating tumour cells (CTCs), extracellular vesicles (EVs), and circulating tumour DNA (ctDNA). CTCs are cancer cells shed into the bloodstream from primary or metastatic sites (5), and their detection often relies on epithelial markers (e.g., EpCAM, Cytokeratin) or size and density differences. Advances in single cell sequencing of CTCs provide valuable insights into genetic heterogeneity and resistance mechanisms (6). EVs constitute a diverse population of membrane-bound vesicles secreted by most cell types and found in biological fluids. Small EVs (sEVs, <200 nm), among them exosomes, are the most extensively studied subclass due to their involvement in both physiological and pathological processes. They play key roles in intercellular communication by transferring bioactive molecules, and increasingly studied for their involvement in disease pathogenesis, diagnostics, and therapeutics (7). In liquid biopsy, sEVs have gained prominence due to their ability to carry proteins, lipids, nucleic acids (DNA, mRNA, non-coding RNA), and metabolites. These molecular cargos reflect the physiological and pathological states of their originating cells, making sEVs valuable biomarkers. They hold significant potential for early cancer detection, prognostic assessment, and therapeutic monitoring, providing insights into tumour biology and aiding in personalized oncology strategies (8). In GI tumourigenesis, sEVs promote cancer progression by remodelling the microenvironment, enhancing angiogenesis, and modulating immune responses, supporting metastasis (9–12). Their non-invasive detection in body fluids enables the monitoring of disease progression, therapeutic responses, and recurrence. sEVs-based assays improve diagnostic accuracy, patient stratification, and clinical decision-making in GI cancers (13). Similarly, ctDNA is an important component of liquid biopsy approaches, consisting of short nucleic acid fragments released into the bloodstream by cancer cells through apoptosis, necrosis, or active secretion (14). ctDNA mirrors the genetic and epigenetic landscape of its tumour, enabling non-invasive liquid biopsy for GI cancers. It supports early detection, surveillance of progression, minimal residual disease (MRD), recurrence, and treatment response. In colorectal, gastric, and pancreatic cancers, ctDNA detects mutations, resistance, and relapse risk, guiding personalized therapy (15–17). This review aims to examine the advances in liquid biopsy technologies and critically assess their significant clinical potential for each type of most common GI cancer. It explores recent developments in these technologies and evaluates their impact on the clinical management of GI cancers (Figure 1).
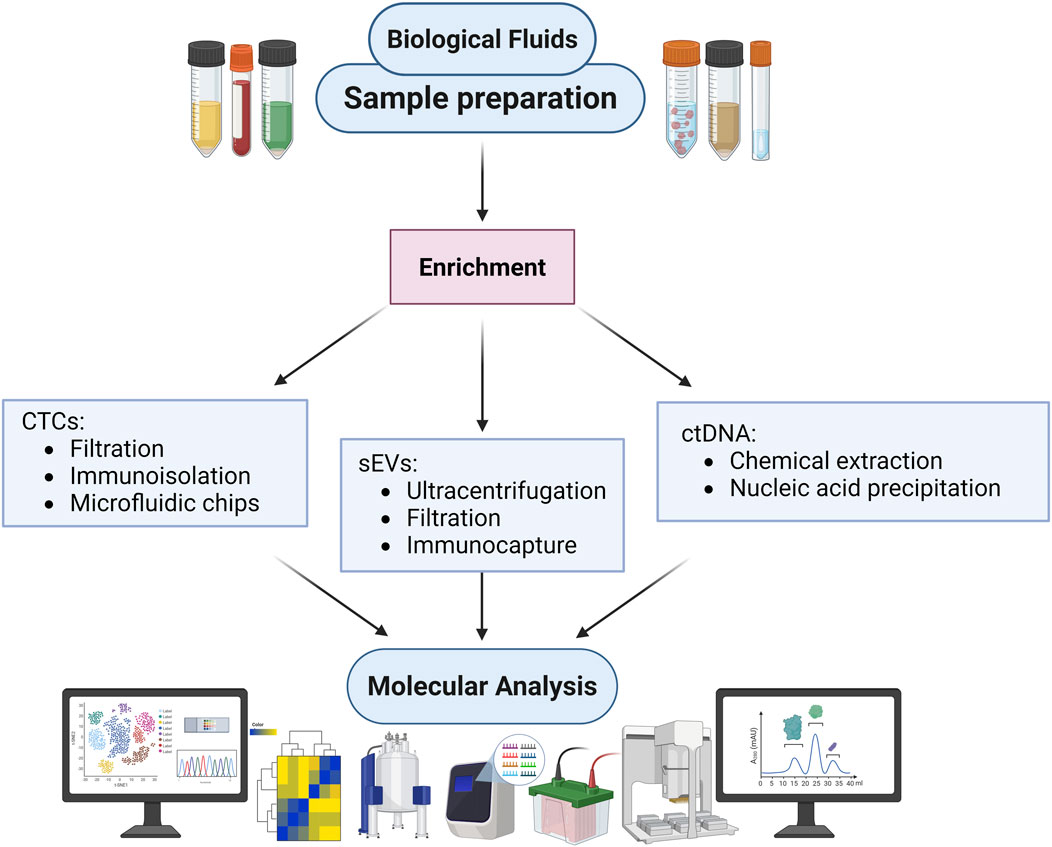
Figure 1. Fraction of Liquid Biopsy derived from body fluids. Overview of biological fluids utilized for diagnostic and research purposes. The outer ring identifies different types of biological fluids, including seminal fluid, tears, ascitic liquid, urine, breath, nipple fluid, saliva, blood, and cerebrospinal fluid. The inner section highlights key circulating components present within these fluids, such as circulating tumour cells (CTCs), circulating molecules (e.g., DNA, RNA, and proteins), and small extracellular vesicles (sEVs), that are obtained from liquid biopsy.
2 Technological landscape and pre-analytical considerations
Fragile circulating biomarkers demand ultrasensitive workflows, combining next-generation sequencing with error-suppression barcodes, digital PCR and droplet digital PCR, each paired with purpose-built enrichment modalities (18). CTC pre-enrichment, microfluidic capture of sEVs and quantitative ctDNA assays now sharpen MRD detection, serial therapeutic monitoring and immediate, data-driven treatment adjustment (19–21). Whole blood must be drawn into stabilising tubes, transported promptly and processed under cold-chain control to preserve biomolecule integrity from deviations accelerate degradation. Pre-analytical disparities collection tubes, centrifugation speeds, storage times and heterogeneous sequencing or PCR platforms foster pronounced inter-laboratory variability, complicating meta-analysis and reproducible standardisation efforts (22, 23). Enrichment exploits physical and biological differences: size exclusion filters eliminate smaller haematologic cells, while immunoisolation seizes tumour cells via EpCAM or other surface markers. Cutting edge microfluidic chips integrate size selective and antigen specific traps within nanoscale channels, achieving high sensitivity and purity for CTC recovery while setting performance benchmarks for liquid biopsy and broader clinical diagnostic adoption (24). Advanced enrichment platforms enhance liquid-biopsy diagnostic power. The CTC-iChip merges size filtration with magnetic immunocapture for label-free CTC recovery (25). Di-electrophoresis separates CTCs by dielectric properties, capturing epithelial and mesenchymal phenotypes without markers (26). Instead, photoacoustic flow cytometry detects and isolates rare CTCs in real time via optical-absorption signatures (27). For sEV isolation, density-based ultracentrifugation, size-exclusion filtration and antibody-based immunocapture remain standard approaches, while acoustic nanofilters have recently emerged as efficient high-throughput methods that preserve veicles integrityduring enrichment (28). Microfluidic chips functionalized with anti-CD63/CD81 nanostructures enhance capture specificity (29), the ExoChip platform integrates isolation and analysis in a single step (30), and tangential-flow filtration enables continuous, high-purity sEVs harvesting (31).
As shown in Figure 2, a unified approach to liquid biopsy begins with biological fluids collection and sample preparation, followed by parallel or sequential processing for CTCs, sEVs, and ctDNA. Each biomarker class demands specific pre-analytical and analytical workflows, from magnetic bead capture to microfluidic enrichment and nucleic acid sequencing. Integrated platforms that consolidate isolation, detection, and quantification steps are critical for improving standardization, reducing operator variability, and enabling routine clinical use.
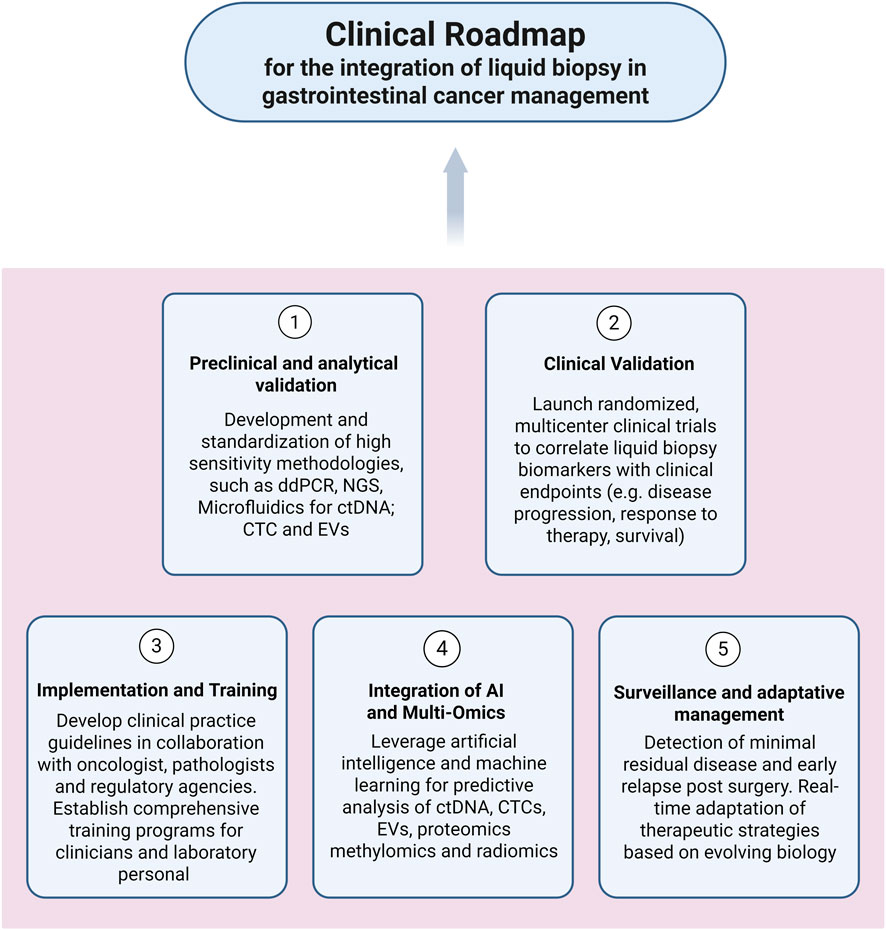
Figure 2. Schematic diagram outlining a unified workflow for isolating circulating tumour cells (CTCs), small extracellular vesicles (sEVs), and circulating tumour DNA (ctDNA) from blood samples. Magnetic beads functionalized with silica or sequence-specific ligands enhance biomarker recovery and shorten processing time. Advances in droplet microfluidics, nanopore sequencing, and integrated microfluidic devices enable sensitive and reproducible detection of rare variants and methylation signatures across all biomarker classes.
A series of technological innovations has revolutionised the analysis and isolation of circulating biomarkers, improving sensitivity, specificity and operational efficiency. Magnetic beads functionalised with silica or sequence-specific ligands simplify workflows, boost recovery, and cut processing time (32). Nanopore sequencing provides real-time, single-molecule interrogation of ctDNA, detecting rare variants with exceptional sensitivity (33). Instead, droplet microfluidic platforms encapsulate individual fragments, amplify, and sequence them, enabling base-level mutation and methylation profiling (34). Microfluidics and acoustic nanofilters have further enhanced capture specificity; label-free systems now recover both epithelial and mesenchymal CTC phenotypes, overcoming immunoaffinity blind spots (35). While other approaches integrate tangential flow filtration and updated magnetic-bead devices consolidate steps, curtail labour, and lower costs, advantages for resource-limited laboratories (36). Moreover, high-throughput droplet assays and nanopore readers also facilitate continuous tracking of treatment response and MRD, directly informing clinical decisions (37). Automated, microfluidic isolators cement reproducibility and standardisation for routine adoption (38).
3 Clinical applications by tumour type
3.1 Esophageal cancer
Liquid biopsy is a promising non-invasive tool for managing oesophageal carcinoma (EC), reducing reliance on repeated tissue biopsies in monitoring and treatment guidance (39). In gastroesophageal junction (GEJ) adenocarcinoma treated with pembrolizumab and neoadjuvant chemo-radiotherapy, serial ctDNA analysis effectively tracks treatment response and disease progression; post-therapy ctDNA clearance correlates with higher pathological complete response and better outcomes, while persistence indicates recurrence risk (40). Beyond quantity, ctDNA profiling, including TP53 mutations and methylation, may aid early diagnosis. Singh et al. studied the CAPOX-BETR regimen in advanced HER2-positive GE adenocarcinoma (phase II randomized trial), showing ctDNA-detected amplifications in EGFR, FGFR1, MET, and KRAS correlated with clinical outcomes, supporting its use in personalized treatment (41). Recent data also support the role of ctDNA in minimal residual disease detection and early relapse prediction (42). Ongoing trials like the EXPLORING phase II randomized trial are evaluating ctDNA-guided therapy intensification in ctDNA-positive gastric and GEJ cancers using XELOX, anlotinib, and penpulimab (43). Cell free DNA (cfDNA) levels are elevated in EC versus healthy individuals and carry tumour-specific changes, supporting their role in surveillance (44). In a Randomized controlled trial, the CTC counts, reduced after pre-operative chemotherapy in oesophageal squamous cell carcinoma, associate with improved prognosis, highlighting their utility in treatment assessment (45). Additionally, salivary sEVs rich in tRNA-GlyGCC-5 can distinguish malignant from benign conditions, and combined with real-time sequencing, may enhance early diagnosis and monitoring (46).
3.2 Gastric cancer
Gastric cancer (GC) remains a major challenge due to late diagnosis and poor prognosis. Liquid biopsy has transformed non-invasive diagnostics and treatment monitoring, as demonstrated in subsequent studies. In a prospective clinical study, Bai et al. showedthat peritoneal lavage CTCs and ctDNA can predict metachronous peritoneal metastases aftersurgery in patients with advanced GI cancer (47), while Jung et al. confirmed the utility of liquid biopsy for guiding therapy in HER2-positive metastatic GC (48). Izumi et al. validated its use for early-stage GC diagnosis in a prospective study (49), and Modlin et al. demonstrated that multigenomic liquid biopsy markers outperform traditional markers like CgA in neuroendocrine tumours, suggesting relevance in GC (50). Slagter et al. linked higher perioperative ctDNA levels with worse outcomes (CRITICS phase III randomized trial) (51), and phase II trials using CAPOX-bevacizumab-trastuzumab confirmed ctDNA utility in precision oncology (41). In two independent randomized phase III international trials, Lukovic and Rosati provided evidence supporting the feasibility of liquid biopsy markers (52, 53). In GEJ tumours, undetectable ctDNA pre/post-surgery predicted superior survival and correlated with T-cell expansion, highlighting its immunologic role (54). In a phase I study, ctDNA confirmed FGFR2/3 alterations and mirrored response to FGFR inhibitor KIN-3248 (55).
Besides, Cai et al. identified von Willebrand factor-bearing sEVs as diagnostic and therapeutic targets (56), and PD-L1-containing sEVs were linked to poor outcomes post-resection, serving as independent prognostic indicators (57). Contemponary, another study showed that exosomal miR-29b suppressed peritoneal metastases, supporting sEV-based therapy (58), while exosomal miR-92a-3p was also noted as a non-invasive early diagnostic biomarker (59), and BM-MSC-derived exosomes overexpressing miRNA-1228 promoted GC progression via SCAI inhibition (60). Offering new therapeutic avenues, macrophage-derived sEVs from TAMs were shown to promote angiogenesis, metastasis, and resistance (61).
CTCs analysis improved diagnostic accuracy and supported real-time treatment decisions in advanced GC (62). Zhang et al. used CTCs to track trastuzumab resistance in HER2-positive GC (63); Overall, liquid biopsy supports early detection, treatment adjustment, and non-invasive monitoring in GC, with Jung SH et al. reinforcing its value in HER2-targeted therapy (48). ctDNA, CTC, and sEVs aid therapy guidance in neoadjuvant, unresectable, or metastatic GC (52, 53).
3.3 Cholangiocarcinoma
Cholangiocarcinoma (CCA), a rare and aggressive bile duct cancer, has benefited from liquid biopsy advances, which offer minimally invasive detection of tumour-specific alterations through ctDNA analysis, especially important given the difficulty of obtaining tissue biopsies (55). ctDNA enables identification of actionable mutations like FGFR2 fusions and IDH1/2 mutations to guide targeted therapy. Garmezy et al. demonstrated ctDNA utility in a phase I clinical trial of the FGFR inhibitor KIN-3248, confirming FGFR2/3 alterations in 63.3% of cases and correlating ctDNA clearance with radiographic response, supporting its role in patient selection and real-time treatment monitoring (55). CTCs, explored by Reduzzi et al., revealed in an observational study, non-conventional CTCs (ncCTCs) lacking epithelial markers, expanding detection capabilities and improving insights into tumour heterogeneity and progression (64). sEVs further advance diagnostic and monitoring strategies, with serum- and utine-derived miR-21 and miR-221 profiles mirroring tumour RNA signatures, while FGFR2 mRNA carried by sEVs supports early detection (65). Gu et al. identified by a prospective observational study a specific exosomal PIWI-interacting RNA (piRNA) signatures, including piR-10506469, piR-20548188, and piR-01856912, as novel diagnostic biomarkers for early detection and personalized care in CCA (66). Together, ctDNA, CTCs, and sEVs reinforce liquid biopsy as a key tool in early diagnosis, monitoring, and precision oncology for CCA.
3.4 Colorectal cancer
Colorectal cancer (CRC) ranks third in incidence and second in mortality in high-HDI countries, per 2022 GLOBOCAN (67). Metastases are synchronous in 15%–30% and later develop in 20%–50% of localized cases (68). Carcinogenesis involves APC or TP53 loss, RAS/BRAF/PIK3CA activation, or microsatellite instability (69). EGFR, VEGFR, and HER2 signaling drive progression; HER2 is amplified in 5% of metastases, often with RAS mutations (17%) (70–72). ESMO recommends biomarker profiling before anti-EGFR or anti-VEGFR therapy (68), but pathway mutations often cause resistance (73).
ctDNA enables real-time mutation detection, resistance monitoring, and disease tracking, addressing tissue biopsy limitations, as revealed in the SCRUM-Japan GI-SCREEN and GOZILA studies (74–79). Post-operative ctDNA predicts residual disease and relapse, as shown in prospective and randomized trials (e.g., NEJM 2022, stage II colon cancer study) (80–85); positive status supports adjuvant therapy, while negativity may justify omission (86–88). High baseline MAF or on-treatment VAF predicts poor survival (89–92), and serial ctDNA tracks mutational burden and immune changes in microsatellite-stable CRC (93–96). RAS-wild-type ctDNA indicates anti-EGFR benefit, RAS mutations signal resistance in a non-interventional, uncontrolled multicenter study (97–104). Several randomized phase II trials, including CRONOS, IL VELO, Beyond and CAVE have evaluaed the clearance of RAS, BRAF, or EGFR mutations and supported monoclonal antibody rechallenge (105–117). These findings have subsequently refined adjuvant treatment decisions, as confirmed in both phase II and phase III trials (118–123). In the EVICT (Erlotinib and Vemurafenib in combination trial) and NEW BEACON studies, ctDNA has been used to guide therapy for BRAF V600E and KRAS G12C mutations (124–130). Similary, HER2 (ERBB2) levels in ctDNA inform anti-HER2 tretament decisions and monitor therapeutic response, as demonstrated with pertuzumab in a phase2 trial (131), cetuximab or panitumumab in the NSABP FC-7 a phase Ib study (132), and trastuzumab deruxtecan in the DESTINY-CR01 study (133). Methylation of GRIA4, RARB, VIM, WNT5A, SDC2, SLC8A1, and NPY in ctDNA correlates with poor prognosis and may aid early detection (134–136). In the same way, cfDNA-based screening models like GALNT9/UPF3A show high sensitivity and specificity (137). Contemporary, in a multicenter clinical study, Whang et al. reported that the MethyDT test (NTMT1/MAP3K14-AS1) outperforms SEPT9 for CRC diagnosis (138), offering better compliance, though further validation is needed (139). Blood-based MSI burden from ctDNA predicts immunotherapy response (140), though distinguishing tumour from immune DNA remains challenging (141).
CTCs correlate with metastasis, invasiveness, and prognosis in CRC (142, 143), identifying patients for intensified treatment based on FOLFOXIRI and bevacizumab versus FOLFOX, in a randomised phase III VISNÚ-1 trial (144), and in an observational cohort study (145). CTC enumeration assesses surgery or stent outcomes (146, 147), while Wu et al. in an experimental study demonstratedthat the detection in peritoneal lavage predicts poor outcomes (148). Mesenchymal CTCs signal relapses and high mortality (149, 150). CTCs also correlate with immune dysfunction in MRD and worse survival (151–153), possibly due to MMP-2-mediated immunosuppression (154), though some studies report limited added value (155). EVs offer alternative biomarkers; tumour-derived EVs promote progression, and endothelial EVs predict survival in metastatic CRC (156). CRC-plasma EVs reprogram monocytes and differ by disease stage (157). sEVs associated miRNAs, such as miR-19b, miR-21, miR-222, and miR-92a contribute to early diagnosis with high miR-222 levels predicting worse survival (158), while low sEV-miR-193a-5p is associated with nodal spread (159). Exosomal circ-133 rises with disease stage (160), and circ-HMGCS1 drives invasion via the circ-HMGCS1/miR-34a-5p/SGPP1 axis (161). A multicenter study identified a five-miRNA fecal signature (miR-1246, miR-607-5p, miR-6777-5p, miR-4488, miR-149-3p), with potential to improve non-invasive CRC screening (162).
3.5 Pancreatic cancer
Pancreatic cancer (PC) remains a top cause of cancer mortality, with about 467,000 deaths in 2022 and a 10% survival rate (67, 163). Late diagnosis limits curative options, highlighting the need for early biomarkers. KRAS-mutant ctDNA is scarce and error-prone; combining it with serum protein markers improves diagnostic accuracy (164). Tumour-derived ctDNA is shorter than benign cfDNA, particularly in early-stage PC (165). In PDAC, ctDNA detects BRCA2 mutations for PARP inhibitor use and clonal KRAS/GNAS alterations (166, 167). KRAS-mutant ctDNA signals poor prognosis, while wild-type status relates to better survival, though it does not predict immunotherapy response. KRAS G12D/V mutations expand T-reg cells and suppress antitumour immunity, especially G12V (168). Elevated neutrophil-to-lymphocyte ratios correlate with ctDNA presence, linking inflammation and tumour burden. Serial ctDNA declines during effective therapies correlate with improved survival in advanced disease (169–171). Particularly, Pant et al underligthed the use of ELI-002P vaccine to recude the ctDNA in several patients affected by PC enrolled in the phase 1 AMPLIFY-201 trial (172). Postoperative ctDNA drop predicts longer survival; cfDNA fragmentomics supports this trend (173, 174). Despite limited yield, ctDNA retains prognostic value post-chemotherapy; pre-op ctDNA still reflects tumour status in a non-randomized controlled trial (175). Methylation assays (HOXD8, POU4F1) of circulation tumor DNA enhance prognostication in metastatic PC by a post hoc analyses of two clinical trials (176). In a prospective observational study, named “PASEA” was detected KRAS mutations in 62.4% of PDAC, with ctDNA clearance marking stability and reappearance signaling progression (177). CTCs predict drug response and survival in advanced PDAC (178), track treatment response and early metastasis, and are detectable in early stages via microfluidic devices (179–181). EVs also hold diagnostic promise, though differentiation from benign EVs is needed. A three-module PPI model identified LEP and SSTR5 as key regulators with prognostic value (182). A digital ELISA test (DEST) showed elevated MUC5AC in EVs predicts IPMN progression to carcinoma (183). EVs-TFs trigger pro-thrombotic states, driving progression and chemo-resistance, and independently predict mortality (184). GPC1 and CD82 markers in EVs may support diagnosis (185). EVs RNA studies show miR-200 family upregulation promotes EMT and metastasis, with high diagnostic accuracy (186). PDAC EVs also deliver miR-155-5p, which activates NF-κB, suppresses EHF, and drives invasiveness (187). In a multicenter case-control study, six dysregulated exosomal miRNAs (including miR-21-5p, miR-223-3p) show diagnostic value, especially with CA19-9, though post-treatment dynamics remain unclear (188). A three-miRNA signature (PPP1R12A, SCN7A, SGCD) predicted poor survival (189). Combining cf-miRNA and exo-miRNA yielded a 13-miRNA signature for early detection, even in low CA19-9 cases (multicenter cohort study) (190). Two diagnostic plasma panels include five miRNAs (miR-215-5p, miR-122-5p, miR-192-5p, miR-30b-5p, miR-320b) (191) and three (hsa-miR-1246, hsa-miR-205-5p, hsa-miR-191-5p) (192). CA19-9 remains a standard marker, but protein panels (193), inflammatory markers (FAR, FPR, FLR) (194), or ctDNA (174) enhance its diagnostic range and detect non-threshold cases. The glycan sTRA, combined with CA19-9, may predict chemo-resistance (195). Autotaxin, secreted by CAFs, mediates treatment resistance and tumour growth post-TGFβ inhibition, suggesting value in monitoring therapy (196). The NETest, a multigene blood test, aids early detection and monitoring of neuroendocrine cancers (50, 197, 198). Despite progress, identifying reliable biomarkers for early PC detection and treatment response remains challenging.
3.6 Liver cancer
Liver cancer is the third leading cause of cancer-related death globally, with 865,000 new cases in 2022 (67). Hepatocellular carcinoma (HCC), mostly caused by chronic HBV or HCV infection, represents 75%–85% of cases (199). Due to poor early detection, diagnosis often occurs at advanced stages (200). Liquid biopsy is gaining value for early diagnosis and monitoring. The Hepa-AiQ ctDNA methylation test outperformed AFP and DCP for early-stage HCC and relapse prediction, though limited to CHB/LC-related cases in Chinese patients (prospective validation study) (201). In the PETAL phase Ib study, D.J. Pinato and colleagues demonstrated that ctDNAeffectively tracked responses to neoantigen vaccines and revealed tumor heterogeneity; however, the immune response was insufficient to fully eradicate residual disease (202). In another phase II clinical trial, Y.Xia et al. estabilished that the changes in ctDNA levels reflected radiological response to TACE with PD-1 inhibitors (203) and post-op increases predicted recurrence during immunosuppressive therapy (204). In HBV-related cases, vh-DNA tracked tumour burden and recurrence risk but lacks general applicability (205). cfDNA concentrations post-resection independently predicted recurrence better than AFP (206). cSMART-detected mutations (TERT, TP53, CTNNB1), combined with AFP, AFP-L3, and PIVKA-II, created a model superior to AFP alone, especially for early HCC (207). The mt-HBT test, combining cfDNA methylation markers, AFP, and gender, showed 88% overall and 82% early-stage sensitivity, outperforming AFP and GALAD (208). The PreCar Score, based on cfDNA features, enhanced detection in non-cirrhotic, HBV-related cases, especially when paired with ultrasound (209). CTC counts and mesenchymal traits predicted recurrence risk and informed resection strategy (210), while the anterior approach reduced intraoperative CTC spread and early relapse (211). CTC-based models, incorporating size, nodule count, and MVI, accurately predicted recurrence (212), and high CTC levels before/after surgery indicated poorer survival and metastasis risk (213). EV size >145.65 nm before TACE was associated with worse prognosis (214). sEV proteins like A2MG and PIGR showed better diagnostic accuracy than AFP, while others (Fetuin-A, Meprin A) indicated progression in the SORAMIC trial study (215). In non-viral HCC, EV markers (GPX3, ACTR3, ARHGAP1) predicted SIRT and sorafenib outcomes (216). EV lncRNAs such as SENP3-EIF4A1, FAM72D-3, EPC1-4, and a panel (MALAT1, DLEU2, HOTTIP, SNHG1) showed diagnostic and prognostic potential (217, 218). A combined EV purification and RT-ddPCR test achieved high sensitivity and specificity in early HCC detection (219). MYCN correlated with liver function and fibrosis, outperforming AFP in predicting progression (220). A five-protein panel (OPN, GDF15, NSE, TRAP5, OPG) effectively detected early-stage HCC (221), and a seven-autoantibody panel showed greater sensitivity than AFP (222). Spectroscopy proved useful for early detection in obese cirrhotic patients where ultrasound fails (223), and platelet mRNA markers have been proposed for early-stage HCC detection (224).
4 Clinical decision framework: matching liquid biopsy tools to clinical objectives
To translate emerging evidence into actionable clinical strategies, we propose a decision-oriented framework that aligns each liquid biopsy modality ctDNA, CTCs, and EVs with specific oncologic goals in gastrointestinal (GI) cancers. For instance, EV-based profiling, especially in saliva or plasma, offers promise for early detection or screening, particularly when combined with miRNA signatures. Furthermore, sEVs represent a unique biomarker class in liquid biopsy, offering molecular cargo that captures complex tumor biology beyond genetic mutations alone. Unlike ctDNA, which primarily reflects tumor-specific genetic alterations, and CTCs, which provide phenotypic and genomic information on intact circulating tumor cells, EVs carry a diverse set of bioactive molecules including miRNAs, proteins, lipids, and metabolites. This cargo influences tumor progression, immune evasion, and metastatic niche formation, thus providing insights into tumor microenvironment interactions and systemic disease processes. For example, specific EV-derived miRNAs such as miR-21, miR-29b, and miR-92a-3p have been linked to tumor growth, chemoresistance, and prognosis in gastrointestinal cancers, while proteins like PD-L1 carried on EVs correlate with immune checkpoint modulation and therapy response. These molecular signatures offer distinct clinical advantages, especially in scenarios where ctDNA levels are low or CTC capture is challenging, such as in early-stage disease or certain upper GI cancers. Furthermore, sEVs are highly stable in bodily fluids, making them suitable for repeated sampling and longitudinal monitoring. Emerging evidence also supports their role in predicting treatment outcomes and immune responses, thereby complementing the information obtained from ctDNA and CTC analyses and enriching personalized oncology strategies. Conversely, ctDNA analysis via serial plasma sampling is the most robust tool for MRD detection, treatment response monitoring, and molecular relapse prediction. CTC enumeration and phenotyping, on the other hand, may be particularly informative for predicting metastatic spread, immune evasion, and drug resistance, especially in cancers such as colorectal and pancreatic carcinoma.
The choice of biomarker is also informed by tumour location and disease extent. In upper GI malignancies (esophageal, gastric, cholangiocarcinoma), CTCs and sEVs often yield higher diagnostic utility due to anatomical sampling limitations. In lower GI cancers (colorectal, pancreatic, hepatocellular carcinoma), ctDNA tends to be more abundant and clinically actionable, especially in the metastatic setting. Finally, advanced-stage disease or patients under active systemic therapy may benefit most from real-time ctDNA tracking, while drug resistance can be further evaluated by combining ctDNA mutation profiling with dynamic CTC analysis.
4.1 Overview of clinical trials
A systematic analysis of ongoing clinical trials was conducted using the ClinicalTrials.gov database (accessed on 22 September 2025). Each gastrointestinal malignancy (oesophageal, gastric, colorectal, pancreatic, hepatic, and biliary tumours) was searched in combination with terms related to liquid biopsy (CTCs, ctDNA, EVs, and “liquid biopsy”). The total number of active studies is presented in Table 1, while the complete list, including identifiers, project titles, and URLs, is provided in Supplementary Table S1.
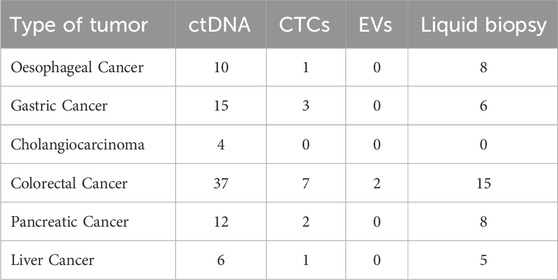
Table 1. Number of ongoing clinical trials on liquid biopsy in gastrointestinal cancers (ClinicalTrials.gov, accessed 22 September 2025).
The distribution of ongoing clinical trials indicates significant trends in the translational adoption of liquid biopsy in gastrointestinal cancers. Colorectal cancer emerges as the leading field, with a predominance of ctDNA-based studies, reflecting its central role in the identification of minimal residual disease, therapeutic monitoring, and clinical decision-making. Gastric and oesophageal cancers also show a growing number of ctDNA- and liquid biopsy-oriented studies, in line with their clinical necessity to improve early diagnosis and response assessment. In contrast, cholangiocarcinoma and liver cancer remain underrepresented, with only a handful of ctDNA-focused initiatives, underscoring the limited clinical translation of liquid biopsy in these settings. In detail, studies specifically investigating EVs are virtually non-existent, suggesting that while preclinical evidence is expanding, its incorporation into large-scale clinical protocols is still in its early stages. On an overall basis, the current study landscape highlights the greater clinical readiness of ctDNA compared to CTCs and VEs, who remain in the early stages of translational validation.
5 From bench to bedside: a clinical integration roadmap for liquid biopsy in GI oncology
Liquid biopsy holds strong promise across GI cancers, but its clinical translation remains incomplete. To move from experimental utility to standard of care, a structured roadmap is needed one that aligns assay development, regulatory validation, and clinical adoption. We propose a five-phase integration model to guide the systematic implementation of liquid biopsy platforms across GI malignancies (Figure 3). This framework emphasizes harmonization of technologies, validation through clinical endpoints, and interdisciplinary collaboration among oncologists, pathologists, and laboratorians.
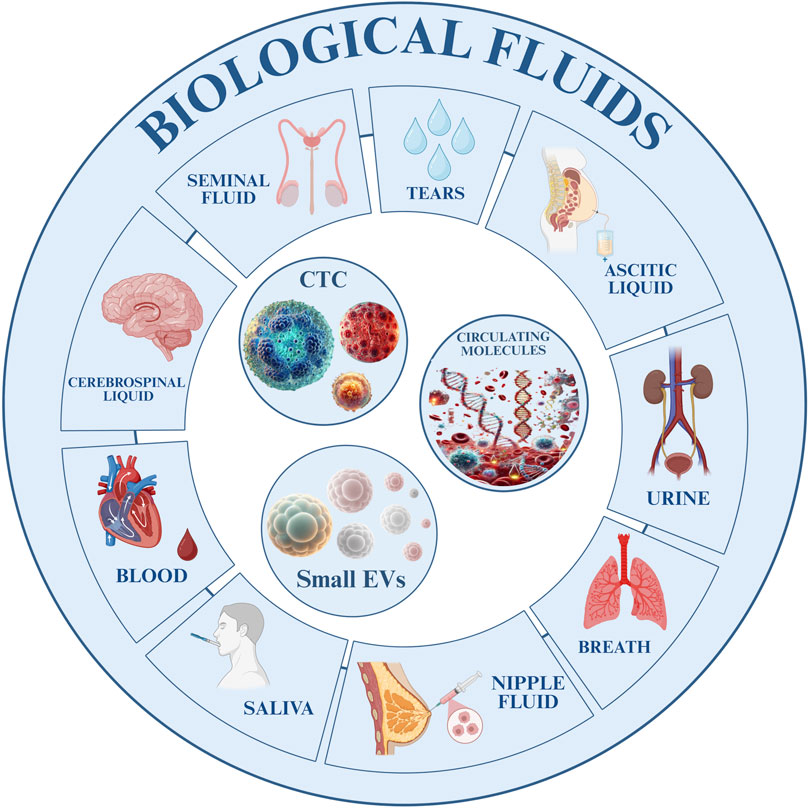
Figure 3. Flowchart illustrating the proposed clinical roadmap for integrating liquid biopsy in gastrointestinal cancer management. The diagram outlines five sequential phases: (1) analytical validation; (2) clinical validation and threshold definition; (3) guideline development and training; (4) multi-omics and AI integration; (5) adaptive surveillance and therapeutic adjustment.
5.1 Phase 1: analytical and preclinical validation
The first step toward clinical translation of liquid biopsy is the development of high-fidelity, reproducible assays for ctDNA, CTCs, and EVs. A critical requirement at this stage is the implementation of standardized workflows, capable of reducing inter-laboratory variability and ensuring clinical comparability.
Recent studies in various cancer types have shown the feasibility of standardized liquid biopsy workflows. For example, Sathyanarayana et al. reported an automated cfDNA extraction and quantification protocol validated across multiple centers, ensuring reproducibility and minimizing pre-analytical variability (225). The International Society of Liquid Biopsy (ISLB) recently issued minimal quality control requirements for ctDNA analysis, stressing harmonization across pre-analytical, analytical, and post-analytical phases (226). Pantel and Alix-Panabières highlighted key barriers to CTC adoption and advocated inter-laboratory trials with robust benchmarking to speed translation (227). In the pre-analytical setting, Grölz et al. showed that collection tubes, transport time, and storage conditions critically affect cfDNA integrity and downstream analyses (228).
Building on these experiences, we propose a GI-specific analytical workflow, aimed at addressing the unique challenges of GI tumors:
• Pre-analytical standardization. Use of cfDNA-stabilizing blood collection tubes to reduce leukocyte lysis. Strict limits for transport (<24 h) and processing times, under controlled temperature. Defined centrifugation protocols (two-step processing with standardized speeds).
• Controlled extraction and enrichment. Automated bead-based methods for cfDNA isolation with internal QC metrics (yield, size distribution). Microfluidic or immunoaffinity platforms for reproducible CTC and EV enrichment. Validation of devices such as ExoChip or CTC-iChip in GI-specific clinical settings.
• Analytical performance benchmarking
• Definition of thresholds for sensitivity, specificity, and limit of detection (LOD) through the use of reference standards and spike-in controls. Inclusion of both positive and negative process controls in every analytical run.
• Inter-laboratory harmonization. Establishment of ring trials among reference centers to assess reproducibility of ctDNA allele frequency quantification and CTC counts. Development of shared databases and consensus reporting templates.
• Bioinformatics and reporting. Adoption of error-corrected sequencing pipelines, including molecular barcoding, to reduce false positives. Transparent reporting of quality metrics (e.g., read depth, fragment size distribution, variant allele frequency confidence). Harmonization of output into clinically interpretable reports for integration into tumor boards.
5.2 Phase 2: clinical validation
This phase focuses on demonstrating the correlation between liquid biopsy metrics and meaningful clinical endpoints:
• Launch of prospective, multi-center trials to assess ctDNA, CTCs, and EVs in early diagnosis, treatment response, and MRD detection.
• Definition of actionable thresholds (e.g., ctDNA mutant allele frequency, CTC count cutoffs).
• Cross-comparison with conventional markers such as CEA, CA19-9, AFP, and radiologic imaging.
• Integration with histology, tumour stage, and therapy type to refine biomarker interpretation.
5.3 Phase 3: implementation, and training
For clinical integration, three parallel initiatives must occur:
• Development of practical guidelines, consensus statements, and diagnostic algorithms for biomarker use in specific GI tumour types.
• Training programs for clinicians, lab personnel, and oncology teams on interpretation and use of liquid biopsy data.
• Inter-laboratory standardization networks to ensure reproducibility, quality assurance, and data interoperability.
5.4 Phase 4: integration of AI and multi-omics
As datasets grow in complexity, artificial intelligence and machine learning will be essential to:
• Integrate liquid biopsy data with proteomics, methylomics, radiomics, and clinical variables.
• Develop predictive models for recurrence, response, and resistance.
• Identify novel biomarker signatures using pattern recognition from high-dimensional data.
5.5 Phase 5: surveillance and adaptive management
The final phase positions liquid biopsy as a cornerstone of precision surveillance:
• Use of serial ctDNA and CTC analysis to detect early relapse and MRD.
• Real-time biomarker feedback to guide therapy escalation, de-escalation, or rechallenge strategies.
• Incorporation into adaptive trial designs and tumour board decision-making.
6 Limitations and future directions
Recent advances in liquid biopsy research are promising; however, significant limitations continue to constrain the robustness and generalizability of the evidence in GI oncology. Many studies involve small and heterogeneous patient cohorts, which limits statistical power and hampers meaningful subgroup analyses, particularly for less common malignancies such as cholangiocarcinoma, where data remain notably sparse. Methodological variability remains a critical barrier. Differences in pre-analytical procedures—including blood collection tubes, centrifugation protocols, and storage conditions—combined with inconsistencies in analytical platforms, such as sequencing technologies, PCR assays, and enrichment methods, contribute to significant inter-laboratory variability. This lack of standardization hinders the establishment of clinically relevant thresholds for biomarkers like ctDNA allele frequency, CTC counts, and EV signatures.
Furthermore, the geographic and institutional concentration of existing studies limits external validity, as much of the evidence originates from single-centre investigations or cohorts from East Asia and selected European institutions. These limitations raise concerns about the broader applicability of findings across diverse populations and healthcare systems. Additionally, much of the current data is descriptive or exploratory. Although retrospective analyses and early-phase prospective trials offer valuable proof-of-concept insights, large, randomized, multi-centre studies demonstrating improvements in overall survival, progression-free survival, or cost-effectiveness are still scarce.
Biological complexities also pose substantial challenges to clinical translation. Intratumourally heterogeneity, clonal evolution, and variability in biomarker shedding contribute to false negatives and inconsistent results, while distinguishing tumour-derived signals from background circulating material remains particularly difficult in early-stage disease when biomarker abundance is low. Looking forward, the field must prioritize harmonized protocols, broad international collaboration, and the incorporation of artificial intelligence–driven analytic frameworks. Only through rigorously designed, globally representative clinical trials can liquid biopsy transition from an experimental adjunct to a validated, standardized component of routine oncological care. Addressing economic factors, regulatory heterogeneity, inter-laboratory variability, and educational needs in parallel with technological and clinical advances is essential to ensure the effective integration of liquid biopsy into everyday clinical practice.
7 Conclusion
Liquid biopsy offers a sensitive, minimally invasive complement to tissue sampling in GI malignancies. Its components CTCs, ctDNA, and EVs capture intratumour heterogeneity, support early detection and enable real-time therapeutic monitoring (229). In EC and GC, combining ctDNA with CTCs enumeration refines neoadjuvant decision-making (4), while early detection of resistance allows rapid therapy adjustment. Protein- or miRNA-enriched sEVs sharpen prognosis and may predict immunotherapy benefit (230–232). CCA presents unique diagnostic and therapeutic challenges, often due to the difficulty of obtaining adequate tissue samples. In this setting, ctDNA profiling for actionable alterations in genes such as FGFR and IDH not only circumvents the limitations of tissue biopsy but also informs the selection of targeted therapies. Longitudinal monitoring of ctDNA can guide modifications in dosage or therapeutic agents over the course of treatment, supporting a more adaptive and responsive approach to disease management (233, 234). CRC has been at the forefront of liquid biopsy adoption, with post-operative ctDNA detection serving as a highly sensitive indicator of MRD. Dynamic changes in ctDNA mutation profiles can herald impending relapse, while the emergence of Neo-RAS wild-type status may reopen eligibility for anti-EGFR therapies, thereby expanding treatment options (235, 236). Furthermore, analysis of sEVs cargo has been shown to provide additional risk stratification for metastatic disease, particularly in cases where conventional markers are inconclusive (237). In PC, the diagnostic sensitivity of liquid biopsy is enhanced by integrating ctDNA analysis with serum protein markers or by employing fragment omics approaches to detect subclinical disease. The identification of KRAS-associated regulatory T cell enrichment and chemoresistance-associated circulating tumour-initiating cells provides valuable insights for guiding immunological and pharmacological interventions (238, 239). Exosomal miRNAs—miR-200 family, miR-155-5p—augment diagnostic and prognostic panels (240). HCC studies show ctDNA methylation assays (e.g., HepaAiQ) and virus-host DNA hybrids enhance early detection, while combining ctDNA with AFP, AFP-L3 and PIVKA-II yields superior accuracy (201, 208). Counting, CTCs alongside MET markers and EVs-derived molecules yields strong prognostic value and sharper post-operative surveillance. Yet broad clinical use of liquid biopsy still depends on assay standardisation, inter-laboratory reproducibility and the management of biomarker heterogeneity. In addition to the discussion above, Table 2 provides a comprehensive summary of the key studies referenced throughout the manuscript, detailing the application of liquid biopsy techniques, across a spectrum of GI cancers such as oesophageal, gastric, cholangiocarcinoma, colorectal, pancreatic, and liver malignancies. Each entry in the table specifies the tumour type, the liquid biopsy component investigated, and the corresponding references that support the diagnostic, prognostic, and therapeutic insights discussed. This compilation further underscores the expanding evidence base for the integration of liquid biopsy into routine cancer management and highlights its potential to transform clinical practice as standardization and validation efforts advance. Large, prospective, multi-center trials are needed to validate biomarkers and cement uniform protocols. Growing data nevertheless show that liquid biopsy improves early detection, patient stratification, treatment guidance and disease monitoring in GI cancers. As ongoing research resolves current obstacles, this sensitive, dynamic and non-invasive approach is likely to become a mainstay of GI oncology, raising the standard of care and patient outcomes.
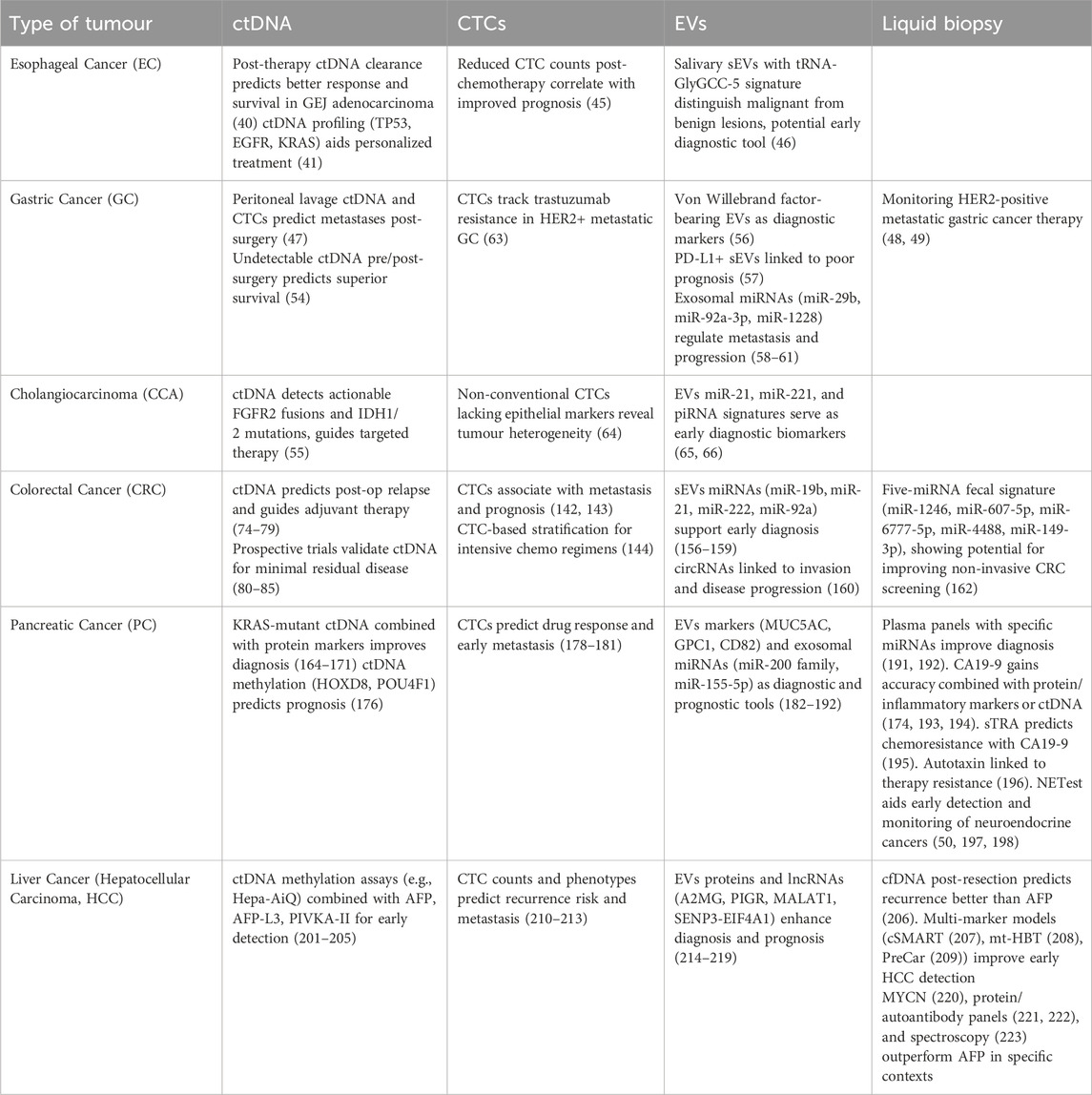
Table 2. Summary table presenting the key studies cited throughout the manuscript, highlighting liquid biopsy techniques—including ctDNA, CTCs, EVs and generally liquid biopsy, across various GI cancers. Each entry specifies tumour type, liquid biopsy component studied, and the associated references supporting the discussed diagnostic, prognostic, therapeutic insights, and promising biomarkers. This compilation underscores the growing evidence base for integrating liquid biopsy into cancer management.
Liquid biopsy has emerged as a transformative tool in GI oncology, enabling minimally invasive diagnosis, real-time monitoring, and dynamic treatment adaptation. While ctDNA, CTCs, and EVs have each demonstrated clinical relevance, their true potential will be unlocked through integration with multi-omics and AI-driven analytics. Such approaches will allow the simultaneous incorporation of genomic, epigenomic, transcriptomic, proteomic, and radiomic features into predictive models, refining patient stratification and guiding precision therapies. Looking forward, certain biomarkers appear particularly promising in specific GI cancers: ctDNA for minimal residual disease detection in colorectal and pancreatic cancers; CTCs for predicting metastasis and therapeutic resistance in esophageal and gastric cancers; EV-derived signatures (miRNAs, proteins) for early detection and immunomodulation in gastric and liver cancers; and ctDNA for actionable mutations in cholangiocarcinoma and hepatocellular carcinoma. As large-scale prospective studies validate these applications and standardization improves, liquid biopsy—augmented by multi-omics and AI—will become a cornerstone of precision oncology, offering more tailored and adaptive management strategies for patients with GI malignancies.
This study highlights several promising biomarkers reported in Table 2 with potential applications in early diagnosis, prognosis, and as therapeutic targets in GIcancers. Given the continuous evolution and dynamic nature of this research field, these biomarkers represent valuable tools not only for improving clinical decision-making but also for guiding the development of innovative therapeutic strategies.
Author contributions
RP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. MD: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. FB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. GP: Investigation, Software, Writing – original draft, Methodology. CL: Data curation, Formal Analysis, Supervision, Validation, Writing – original draft. FR: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. AR: Data curation, Investigation, Methodology, Supervision, Writing – original draft. RM: Data curation, Formal Analysis, Software, Writing – original draft. MC: Investigation, Supervision, Validation, Visualization, Writing – review and editing. LL: Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing. GG: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review and editing. ND: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing. MS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the European Union - Next-Generation EU - PNRR-MCNT2-2023-12377885- Handling of mesenchymal-like circulating pancreatic cancer cells as an innovative approach to restrain disease progression, and Italian Ministry of Health, grant number Ricerca Corrente 2025 (RC 2025). Funded by the European Union - Next Generation EU - NRRP M6C2. Investment 2.1 Enhancement and strengthening of biomedical research in the NHS + CUP G23C24000830006.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/or.2025.1702932/full#supplementary-material
References
1. Ho, HY, Chung, KS, Kan, CM, and Wong, SC. Liquid biopsy in the clinical management of cancers. Int J Mol Sci (2024) 25:8594. doi:10.3390/IJMS25168594
2. Adhit, KK, Wanjari, A, Menon, S, and K, S. Liquid biopsy: an evolving paradigm for non-invasive disease diagnosis and monitoring in medicine. Cureus (2023) 15:e50176. doi:10.7759/CUREUS.50176
3. Vaidyanathan, R, Soon, RH, Zhang, P, Jiang, K, and Lim, CT. Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip (2018) 19:11–34. doi:10.1039/C8LC00684A
4. David, P, Mittelstädt, A, Kouhestani, D, Anthuber, A, Kahlert, C, Sohn, K, et al. Current applications of liquid biopsy in gastrointestinal cancer disease-from early cancer detection to individualized cancer treatment. Cancers (Basel) (2023) 15:1924. doi:10.3390/CANCERS15071924
5. Loy, C, Ahmann, L, De Vlaminck, I, and Gu, W. Liquid biopsy based on cell-free DNA and RNA. Annu Rev Biomed Eng (2024) 26:169–95. doi:10.1146/ANNUREV-BIOENG-110222-111259
6. Lin, D, Shen, L, Luo, M, Zhang, K, Li, J, Yang, Q, et al. Circulating tumor cells: biology and clinical significance. Signal Transduction Targeted Ther (2021) 6:404. doi:10.1038/S41392-021-00817-8
7. Yáñez-Mó, M, Siljander, PRM, Andreu, Z, Zavec, AB, Borràs, FE, Buzas, EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles (2015) 4:1–60. doi:10.3402/JEV.V4.27066
8. Schirizzi, A, Contino, M, Carrieri, L, Riganti, C, De Leonardis, G, Scavo, MP, et al. The multiple combination of paclitaxel, Ramucirumab and Elacridar reverses the paclitaxel-mediated resistance in gastric cancer cell lines. Front Oncol (2023) 13:1129832. doi:10.3389/FONC.2023.1129832
9. Scavo, MP, Depalo, N, Rizzi, F, Ingrosso, C, Fanizza, E, Chieti, A, et al. FZD10 carried by exosomes sustains cancer cell proliferation. Cells (2019) 8:777. doi:10.3390/CELLS8080777
10. Scavo, MP, Rizzi, F, Depalo, N, Fanizza, E, Ingrosso, C, Curri, ML, et al. A possible role of FZD10 delivering exosomes derived from Colon cancers cell lines in inducing activation of epithelial-mesenchymal transition in normal Colon epithelial cell line. Int J Mol Sci (2020) 21:6705–15. doi:10.3390/IJMS21186705
11. Zhang, X, Yuan, X, Shi, H, Wu, L, Qian, H, and Xu, W. Exosomes in cancer: small particle, big player. J Hematol Oncol (2015) 8:83. doi:10.1186/S13045-015-0181-X
12. Fang, T, Lv, H, Lv, G, Li, T, Wang, C, Han, Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun (2018) 9:191. doi:10.1038/S41467-017-02583-0
13. Yee, NS. Liquid biopsy: a biomarker-driven tool towards precision oncology. J Clin Med (2020) 9:2556–3. doi:10.3390/JCM9082556
14. Parikh, AR, Leshchiner, I, Elagina, L, Goyal, L, Levovitz, C, Siravegna, G, et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat Med (2019) 25:1415–21. doi:10.1038/S41591-019-0561-9
15. Mencel, J. ctDNA guided diagnosis and management of gastrointestinal cancers (2023). Available online at: https://repository.icr.ac.uk/handle/internal/5879 (Accessed March 11, 2025).
16. Sun, X, Huang, T, Cheng, F, Huang, K, Liu, M, He, W, et al. Monitoring colorectal cancer following surgery using plasma circulating tumor DNA. Oncol Lett (2018) 15:4365–75. doi:10.3892/OL.2018.7837
17. Parikh, AR, Mojtahed, A, Schneider, JL, Kanter, K, Van Seventer, EE, Fetter, IJ, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res (2020) 26:1877–85. doi:10.1158/1078-0432.CCR-19-3467
18. Abouali, H, Hosseini, SA, Purcell, E, Nagrath, S, and Poudineh, M. Recent advances in device engineering and computational analysis for characterization of cell-released cancer biomarkers. Cancers (Basel) (2022) 14:288. doi:10.3390/CANCERS14020288
19. Pantel, K, and Alix-Panabières, C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol (2019) 16:409–24. doi:10.1038/S41571-019-0187-3
20. Giannopoulou, L, Zavridou, M, Kasimir-Bauer, S, and Lianidou, ES. Liquid biopsy in ovarian cancer: the potential of circulating miRNAs and exosomes. Translational Res (2019) 205:77–91. doi:10.1016/J.TRSL.2018.10.003
21. De Rubis, G, Rajeev Krishnan, S, and Bebawy, M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci (2019) 40:172–86. doi:10.1016/J.TIPS.2019.01.006
22. Heitzer, E, Haque, IS, Roberts, CES, and Speicher, MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet (2019) 20:71–88. doi:10.1038/S41576-018-0071-5
23. Rolfo, C, Cardona, AF, Cristofanilli, M, Paz-Ares, L, Diaz Mochon, JJ, Duran, I, et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: perspective of the international Society of Liquid Biopsy (ISLB). Crit Rev Oncology/Hematology (2020) 151:102978. doi:10.1016/J.CRITREVONC.2020.102978
24. Alix-Panabières, C, and Pantel, K. Challenges in circulating tumour cell research. Nat Rev Cancer (2014) 14:623–31. doi:10.1038/NRC3820
25. Ozkumur, E, Shah, AM, Ciciliano, JC, Emmink, BL, Miyamoto, DT, Brachtel, E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med (2013) 5:179ra47. doi:10.1126/SCITRANSLMED.3005616
26. Russo, GI, Musso, N, Romano, A, Caruso, G, Petralia, S, Lanzanò, L, et al. The role of dielectrophoresis for cancer diagnosis and prognosis. Cancers (Basel) (2021) 14:198. doi:10.3390/CANCERS14010198
27. Galanzha, EI, Menyaev, YA, Yadem, AC, Sarimollaoglu, M, Juratli, MA, Nedosekin, DA, et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med (2019) 11:eaat5857. doi:10.1126/SCITRANSLMED.AAT5857
28. Wu, M, Ouyang, Y, Wang, Z, Zhang, R, Huang, PH, Chen, C, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A (2017) 114:10584–9. doi:10.1073/PNAS.1709210114
29. Chen, Y, Zhu, Q, Cheng, L, Wang, Y, Li, M, Yang, Q, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods (2021) 18:212–8. doi:10.1038/S41592-020-01034-X
30. Kanwar, SS, Dunlay, CJ, Simeone, DM, and Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip (2014) 14:1891–900. doi:10.1039/C4LC00136B
31. Haraszti, RA, Didiot, MC, Sapp, E, Leszyk, J, Shaffer, SA, Rockwell, HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracellular Vesicles (2016) 5:32570. doi:10.3402/JEV.V5.32570
32. Bronkhorst, AJ, Aucamp, J, and Pretorius, PJ. Cell-free DNA: preanalytical variables. Clinica Chim Acta (2015) 450:243–53. doi:10.1016/J.CCA.2015.08.028
33. Van Dessel, LF, Beije, N, Helmijr, JCA, Vitale, SR, Kraan, J, Look, MP, et al. Application of circulating tumor DNA in prospective clinical oncology trials - standardization of preanalytical conditions. Mol Oncol (2017) 11:295–304. doi:10.1002/1878-0261.12037
34. Liang, N, Li, B, Jia, Z, Wang, C, Wu, P, Zheng, T, et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat Biomed Eng (2021) 5(6 5):586–99. doi:10.1038/s41551-021-00746-5
35. Iliescu, FS, Poenar, DP, Yu, F, Ni, M, Chan, KH, Cima, I, et al. Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics (2019) 13:041503. doi:10.1063/1.5087690
36. Vasu, S, Johnson, V, Archana, M, Reddy, KA, and Sukumar, UK. Circulating extracellular vesicles as promising biomarkers for precession diagnostics: a perspective on lung cancer. ACS Biomater Sci Eng (2025) 11:95–134. doi:10.1021/ACSBIOMATERIALS.4C01323
37. Cumbo, C, Anelli, L, Specchia, G, and Albano, F. Monitoring of Minimal Residual Disease (MRD) in chronic myeloid leukemia: recent advances. Cancer Management Res (2020) 12:3175–89. doi:10.2147/CMAR.S232752
38. Sekhwama, M, Mpofu, K, Sivarasu, S, and Mthunzi-Kufa, P. Applications of microfluidics in biosensing. Discover Appl Sci (2024) 6:303. doi:10.1007/S42452-024-05981-4
39. Matsushita, D, Arigami, T, Okubo, K, Sasaki, K, Noda, M, Kita, Y, et al. The diagnostic and prognostic value of a liquid biopsy for esophageal cancer: a systematic review and meta-analysis. Cancers (Basel) (2020) 12:3070–33. doi:10.3390/CANCERS12103070
40. Zhu, M, Chen, C, Foster, NR, Hartley, C, Mounajjed, T, Salomao, MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res (2022) 28:3021–31. doi:10.1158/1078-0432.CCR-22-0413
41. Singh, H, Lowder, KE, Kapner, K, Kelly, RJ, Zheng, H, McCleary, NJ, et al. Clinical outcomes and ctDNA correlates for CAPOX BETR: a phase II trial of capecitabine, oxaliplatin, bevacizumab, trastuzumab in previously untreated advanced HER2+ gastroesophageal adenocarcinoma. Nat Commun (2024) 15:6833. doi:10.1038/S41467-024-51271-3
42. Iacob, R, Mandea, M, Iacob, S, Pietrosanu, C, Paul, D, Hainarosie, R, et al. Liquid biopsy in squamous cell carcinoma of the esophagus and of the head and neck. Front Med (Lausanne) (2022) 9:827297. doi:10.3389/FMED.2022.827297
43. Chen, Y, Zhang, J, Han, G, Tang, J, Guo, F, Li, W, et al. Efficacy and safety of XELOX combined with anlotinib and penpulimab vs XELOX as an adjuvant therapy for ctDNA-positive gastric and gastroesophageal junction adenocarcinoma: a protocol for a randomized, controlled, multicenter phase II clinical trial (EXPLORING study). Front Immunol (2023) 14:1232858. doi:10.3389/FIMMU.2023.1232858
44. Yuan, Z, Wang, X, Geng, X, Li, Y, Mu, J, Tan, F, et al. Liquid biopsy for esophageal cancer: is detection of circulating cell-free DNA as a biomarker feasible? Cancer Commun (2021) 41:3–15. doi:10.1002/CAC2.12118
45. Zhao, Y, Han, L, Zhang, W, Shan, L, Wang, Y, Song, P, et al. Preoperative chemotherapy compared with postoperative adjuvant chemotherapy for squamous cell carcinoma of the thoracic oesophagus with the detection of circulating tumour cells randomized controlled trial. Int J Surg (2020) 73:1–8. doi:10.1016/J.IJSU.2019.11.005
46. Li, K, Lin, Y, Luo, Y, Xiong, X, Wang, L, Durante, K, et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer (2022) 21:21. doi:10.1186/S12943-022-01499-8
47. Bai, L, Guan, Y, Zhang, Y, Gu, J, Ni, B, Zhang, HY, et al. Effectiveness of peritoneal lavage fluid circulating tumour cells and circulating tumour DNA in the prediction of metachronous peritoneal metastasis of gastric cancer (pT4NxM0/pT1-3N+M0) after radical resection: protocol of a prospective single-centre clinical study. BMJ Open (2024) 14:e083659. doi:10.1136/BMJOPEN-2023-083659
48. Jung, SH, Lee, CK, Kwon, WS, Yun, S, Jung, M, Kim, HS, et al. Monitoring the outcomes of systemic chemotherapy including immune checkpoint inhibitor for HER2-Positive metastatic gastric cancer by liquid biopsy. Yonsei Med J (2023) 64:531–40. doi:10.3349/YMJ.2023.0096
49. Izumi, D, Zhu, Z, Chen, Y, Toden, S, Huo, X, Kanda, M, et al. Assessment of the diagnostic efficiency of a liquid biopsy assay for early detection of gastric cancer. JAMA Netw Open (2021) 4:E2121129. doi:10.1001/JAMANETWORKOPEN.2021.21129
50. Modlin, IM, Kidd, M, Falconi, M, Filosso, PL, Frilling, A, Malczewska, A, et al. A multigenomic liquid biopsy biomarker for neuroendocrine tumor disease outperforms CgA and has surgical and clinical utility. Ann Oncol (2021) 32:1425–33. doi:10.1016/J.ANNONC.2021.08.1746
51. Slagter, AE, Vollebergh, MA, Caspers, IA, van Sandick, JW, Sikorska, K, Lind, P, et al. Prognostic value of tumor markers and ctDNA in patients with resectable gastric cancer receiving perioperative treatment: results from the CRITICS trial. Gastric Cancer (2022) 25:401–10. doi:10.1007/S10120-021-01258-6
52. Lukovic, J, Moore, AJ, Lee, MT, Willis, D, Ahmed, S, Akra, M, et al. The feasibility of quality assurance in the TOPGEAR international phase 3 clinical trial of neoadjuvant chemoradiation therapy for gastric cancer (an intergroup trial of the AGITG/TROG/NHMRC CTC/EORTC/CCTG). Int J Radiat Oncology*Biology*Physics (2023) 117:1096–106. doi:10.1016/J.IJROBP.2023.06.011
53. Rosati, G, Cella, CA, Cavanna, L, Codecà, C, Prisciandaro, M, Mosconi, S, et al. A randomized phase III study of fractionated docetaxel, oxaliplatin, capecitabine (low-tox) vs epirubicin, oxaliplatin and capecitabine (eox) in patients with locally advanced unresectable or metastatic gastric cancer: the lega trial. Gastric Cancer (2022) 25:783–93. doi:10.1007/S10120-022-01292-Y
54. Kelly, RJ, Landon, BV, Zaidi, AH, Singh, D, Canzoniero, JV, Balan, A, et al. Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: a phase Ib trial and ctDNA analyses. Nat Med (2024) 30:1023–34. doi:10.1038/S41591-024-02877-Z
55. Garmezy, B, Borad, MJ, Bahleda, R, Perez, CA, Chen, LT, Kato, S, et al. A phase I Study of KIN-3248, an irreversible small-molecule Pan-FGFR inhibitor, in patients with advanced FGFR2/3-driven solid tumors. Cancer Res Commun (2024) 4:1165–73. doi:10.1158/2767-9764.CRC-24-0137
56. Cai, W, Wang, M, Wang, Cy., Zhao, Cy., Zhang, Xy., Zhou, Q, et al. Extracellular vesicles, hyperadhesive von willebrand factor, and outcomes of gastric cancer: a clinical observational study. Med Oncol (2023) 40(2023):140. doi:10.1007/S12032-023-01950-W
57. Li, G, Wang, G, Chi, F, Jia, Y, Wang, X, Mu, Q, et al. Higher postoperative plasma EV PD-L1 predicts poor survival in patients with gastric cancer. J Immunother Cancer (2021) 9:e002218. doi:10.1136/JITC-2020-002218
58. Kimura, Y, Ohzawa, H, Miyato, H, Kaneko, Y, Kuchimaru, T, Takahashi, R, et al. Intraperitoneal transfer of microRNA-29b-containing small extracellular vesicles can suppress peritoneal metastases of gastric cancer. Cancer Sci (2023) 114:2939–50. doi:10.1111/cas.15793
59. Lu, X, Lu, J, Wang, S, Zhang, Y, Ding, Y, Shen, X, et al. Circulating serum exosomal miR-92a-3p as a novel biomarker for early diagnosis of gastric cancer. Future Oncol (2021) 17:907–19. doi:10.2217/FON-2020-0792
60. Chang, L, Gao, H, Wang, L, Wang, N, Zhang, S, Zhou, X, et al. Exosomes derived from miR-1228 overexpressing bone marrow-mesenchymal stem cells promote growth of gastric cancer cells. Aging (2021) 13:11808–21. doi:10.18632/AGING.202878
61. Zheng, P, Luo, Q, Wang, W, Li, J, Wang, T, Wang, P, et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis (2018) 9:434. doi:10.1038/S41419-018-0465-5
62. Ning, D, Cui, K, Liu, M, Ou, Y, Wang, Z, Zou, B, et al. Comparison of CellSearch and circulating tumor cells (CTC)-Biopsy systems in detecting peripheral blood circulating tumor cells in patients with gastric cancer. Med Sci Monit (2021) 27:e926565. doi:10.12659/MSM.926565
63. Zhang, J, Qiu, W, Zhang, W, Chen, Y, Shen, H, Zhu, H, et al. Tracking of trastuzumab resistance in patients with HER2-positive metastatic gastric cancer by CTC liquid biopsy. Am J Cancer Res (2023) 13:5684–5697. Available online at: https://pubmed.ncbi.nlm.nih.gov/38058840/ (Accessed February 27, 2025).
64. Reduzzi, C, Vismara, M, Silvestri, M, Celio, L, Niger, M, Peverelli, G, et al. A novel circulating tumor cell subpopulation for treatment monitoring and molecular characterization in biliary tract cancer. Int J Cancer (2020) 146:3495–503. doi:10.1002/IJC.32822
65. Lapitz, A, Arbelaiz, A, O’Rourke, CJ, Lavin, JL, Casta, AL, Ibarra, C, et al. Patients with Cholangiocarcinoma present specific RNA profiles in serum and urine extracellular vesicles mirroring the tumor expression: novel liquid biopsy biomarkers for disease diagnosis. Cells (2020) 9:721. doi:10.3390/CELLS9030721
66. Gu, X, Wang, C, Deng, H, Qing, C, Liu, R, Liu, S, et al. Exosomal piRNA profiling revealed unique circulating piRNA signatures of cholangiocarcinoma and gallbladder carcinoma. Acta Biochim Biophys Sinica (2020) 52:475–84. doi:10.1093/ABBS/GMAA028
67. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians (2024) 74:229–63. doi:10.3322/CAAC.21834
68. Cervantes, A, Adam, R, Roselló, S, Arnold, D, Normanno, N, Taïeb, J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34:10–32. doi:10.1016/J.ANNONC.2022.10.003
69. Brenner, H, Kloor, M, and Pox, CP. Colorectal cancer. The Lancet (2014) 383:1490–502. doi:10.1016/S0140-6736(13)61649-9
70. Herrera, R, and Sebolt-Leopold, JS. Unraveling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends Mol Med (2002) 8:S27–S31. doi:10.1016/S1471-4914(02)02307-9
71. Davies, H, Bignell, GR, Cox, C, Stephens, P, Edkins, S, Clegg, S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417:949–54. doi:10.1038/NATURE00766
72. Ross, JS, Fakih, M, Ali, SM, Elvin, JA, Schrock, AB, Suh, J, et al. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer (2018) 124:1358–73. doi:10.1002/CNCR.31125
73. Misale, S, Di Nicolantonio, F, Sartore-Bianchi, A, Siena, S, and Bardelli, A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov (2014) 4:1269–80. doi:10.1158/2159-8290.CD-14-0462
74. Bredno, J, Lipson, J, Venn, O, Aravanis, AM, and Jamshidi, A. Clinical correlates of circulating cell-free DNA tumor fraction. PLoS One (2021) 16:e0256436. doi:10.1371/JOURNAL.PONE.0256436
75. Nakamura, Y, Taniguchi, H, Ikeda, M, Bando, H, Kato, K, Morizane, C, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med (2020) 26:1859–64. doi:10.1038/S41591-020-1063-5
76. Nakamura, Y, Watanabe, J, Akazawa, N, Hirata, K, Kataoka, K, Yokota, M, et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med (2024) 30:3272–83. doi:10.1038/S41591-024-03254-6
77. Wang, F, Huang, YS, Wu, HX, Wang, ZX, Jin, Y, Yao, YC, et al. Genomic temporal heterogeneity of circulating tumour DNA in unresectable metastatic colorectal cancer under first-line treatment. Gut (2022) 71:1340–9. doi:10.1136/GUTJNL-2021-324852
78. Valladares-Ayerbes, M, Safont, MJ, González Flores, E, García-Alfonso, P, Aranda, E, Muñoz, AML, et al. Sequential RAS mutations evaluation in cell-free DNA of patients with tissue RAS wild-type metastatic colorectal cancer: the PERSEIDA (Cohort 2) study. Clin Transl Oncol (2024) 26:2640–51. doi:10.1007/S12094-024-03487-4
79. Jafri, H, Mushtaq, S, Baig, S, Bhatty, A, and Siraj, S. Comparison of KRAS gene in circulating tumor DNA levels vs histological grading of colorectal cancer patients through liquid biopsy. Saudi J Gastroenterol (2023) 29:371–5. doi:10.4103/SJG.SJG_85_23
80. Lygre, KB, Forthun, RB, Høysæter, T, Hjelle, SM, Eide, GE, Gjertsen, BT, et al. Assessment of postoperative circulating tumour DNA to predict early recurrence in patients with stage I-III right-sided colon cancer: prospective observational study. BJS Open (2024) 8:zrad146. doi:10.1093/BJSOPEN/ZRAD146
81. van ’t Erve, I, Medina, JE, Leal, A, Papp, E, Phallen, J, Adleff, V, et al. Metastatic colorectal cancer treatment response evaluation by ultra-deep sequencing of cell-free DNA and matched white blood cells. Clin Cancer Res (2023) 29:899–909. doi:10.1158/1078-0432.CCR-22-2538
82. Bolhuis, K, van ’t Erve, I, Mijnals, C, Delis – Van Diemen, PM, Huiskens, J, Komurcu, A, et al. Postoperative circulating tumour DNA is associated with pathologic response and recurrence-free survival after resection of colorectal cancer liver metastases. EBioMedicine (2021) 70:103498. doi:10.1016/J.EBIOM.2021.103498
83. Slater, S, Bryant, A, Aresu, M, Begum, R, Chen, HC, Peckitt, C, et al. Tissue-Free liquid biopsies combining genomic and methylation signals for minimal residual disease detection in patients with early colorectal cancer from the UK TRACC part B Study. Clin Cancer Res (2024) 30:3459–69. doi:10.1158/1078-0432.CCR-24-0226
84. Li, Y, Mo, S, Zhang, L, Ma, X, Hu, X, Huang, D, et al. Postoperative circulating tumor DNA combined with consensus molecular subtypes can better predict outcomes in stage III colon cancers: a prospective cohort study. Eur J Cancer (2022) 169:198–209. doi:10.1016/J.EJCA.2022.04.010
85. Benhaim, L, Bouché, O, Normand, C, Didelot, A, Mulot, C, Le Corre, D, et al. Circulating tumor DNA is a prognostic marker of tumor recurrence in stage II and III colorectal cancer: multicentric, prospective cohort study (ALGECOLS). Eur J Cancer (2021) 159:24–33. doi:10.1016/J.EJCA.2021.09.004
86. Jakobsen, A, Andersen, RF, Hansen, TF, Jensen, LH, Faaborg, L, Steffensen, KD, et al. Early ctDNA response to chemotherapy. A potential surrogate marker for overall survival. Eur J Cancer (2021) 149:128–33. doi:10.1016/J.EJCA.2021.03.006
87. Wang, DS, Pat Fong, W, Wen, L, Cai, YY, Ren, C, Wu, XJ, et al. Safety and efficacy of adjuvant FOLFOX/FOLFIRI with versus without hepatic arterial infusion of floxuridine in patients following colorectal cancer liver metastasectomy (HARVEST trial): a randomized controlled trial. Eur J Cancer (2025) 214:115154. doi:10.1016/J.EJCA.2024.115154
88. Tie, J, Cohen, JD, Lahouel, K, Lo, SN, Wang, Y, Kosmider, S, et al. Circulating Tumor DNA analysis guiding adjuvant therapy in stage II Colon cancer. N Engl J Med (2022) 386:2261–72. doi:10.1056/NEJMOA2200075
89. Bachet, JB, Laurent-Puig, P, Meurisse, A, Bouché, O, Mas, L, Taly, V, et al. Circulating tumour DNA at baseline for individualised prognostication in patients with chemotherapy-naïve metastatic colorectal cancer. An AGEO prospective study. Eur J Cancer (2023) 189:112934. doi:10.1016/J.EJCA.2023.05.022
90. Lee, S, Kim, J-W, Kim, H-G, Hwang, S-H, Kim, K-J, Lee, JH, et al. Longitudinal comparative analysis of circulating tumor DNA and matched tumor tissue DNA in patients with metastatic colorectal cancer receiving palliative first-line systemic anti-cancer therapy. Cancer Res Treat (2024) 56:1171–82. doi:10.4143/CRT.2024.016
91. Xu, X, Ai, L, Hu, K, Liang, L, Lv, M, Wang, Y, et al. Tislelizumab plus cetuximab and irinotecan in refractory microsatellite stable and RAS wild-type metastatic colorectal cancer: a single-arm phase 2 study. Nat Commun (2024) 15:7255. doi:10.1038/S41467-024-51536-X
92. Jin, Km., Bao, Q, Zhao, Tt., Wang, Hw., Huang, Lf., Wang, K, et al. Comparing baseline VAF in circulating tumor DNA and tumor tissues predicting prognosis of patients with colorectal cancer liver metastases after curative resection. Clin Res Hepatol Gastroenterol (2024) 48:102464. doi:10.1016/J.CLINRE.2024.102464
93. Loree, JM, Titmuss, E, Topham, JT, Kennecke, HF, Feilotter, H, Virk, S, et al. Plasma versus tissue tumor mutational burden as biomarkers of durvalumab plus tremelimumab response in patients with metastatic colorectal cancer in the CO.26 trial. Clin Cancer Res (2024) 30:3189–99. doi:10.1158/1078-0432.CCR-24-0268
94. Chen, EX, Loree, JM, Titmuss, E, Jonker, DJ, Kennecke, HF, Berry, S, et al. Liver metastases and immune checkpoint inhibitor efficacy in patients with refractory metastatic colorectal cancer: a secondary analysis of a randomized clinical trial. JAMA Netw Open (2023) 6:E2346094. doi:10.1001/JAMANETWORKOPEN.2023.46094
95. Diaz, LA, Shiu, KK, Kim, TW, Jensen, BV, Jensen, LH, Punt, C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. The Lancet Oncol (2022) 23:659–70. doi:10.1016/S1470-2045(22)00197-8
96. Crisafulli, G, Sartore-Bianchi, A, Lazzari, L, Pietrantonio, F, Amatu, A, Macagno, M, et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients. Cancer Discov (2022) 12:1656–75. doi:10.1158/2159-8290.CD-21-1434
97. Holm, M, Andersson, E, Osterlund, E, Ovissi, A, Soveri, LM, Anttonen, AK, et al. Detection of KRAS mutations in liquid biopsies from metastatic colorectal cancer patients using droplet digital PCR, Idylla, and next generation sequencing. PLoS One (2020) 15:e0239819. doi:10.1371/JOURNAL.PONE.0239819
98. Max Ma, X, Bendell, JC, Hurwitz, HI, Ju, C, Lee, JJ, Lovejoy, A, et al. Disease monitoring using post-induction circulating tumor DNA analysis following first-line therapy in patients with metastatic colorectal cancer. Clin Cancer Res (2020) 26:4010–7. doi:10.1158/1078-0432.CCR-19-1209
99. Stein, A, Simnica, D, Schultheiß, C, Scholz, R, Tintelnot, J, Gökkurt, E, et al. PD-L1 targeting and subclonal immune escape mediated by PD-L1 mutations in metastatic colorectal cancer. J Immunother Cancer (2021) 9:e002844. doi:10.1136/JITC-2021-002844
100. Watanabe, J, Maeda, H, Nagasaka, T, Yokota, M, Hirata, K, Akazawa, N, et al. Multicenter, single-arm, phase II study of the continuous use of panitumumab in combination with FOLFIRI after FOLFOX for RAS wild-type metastatic colorectal cancer: exploratory sequential examination of acquired mutations in circulating cell-free DNA. Int J Cancer (2022) 151:2172–81. doi:10.1002/IJC.34184
101. Unseld, M, Belic, J, Pierer, K, Zhou, Q, Moser, T, Bauer, R, et al. A higher ctDNA fraction decreases survival in regorafenib-treated metastatic colorectal cancer patients. Results from the regorafenib’s liquid biopsy translational biomarker phase II pilot study. Int J Cancer (2021) 148:1452–61. doi:10.1002/IJC.33303
102. Yang, L, Zhang, W, Fan, N, Cao, P, Cheng, Y, Zhu, L, et al. Efficacy, safety and genomic analysis of SCT200, an anti-EGFR monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type metastatic colorectal cancer: a phase Ⅱ study. EBioMedicine (2024) 100:104966. doi:10.1016/J.EBIOM.2024.104966
103. Pastor, B, André, T, Henriques, J, Trouilloud, I, Tournigand, C, Jary, M, et al. Monitoring levels of circulating cell-free DNA in patients with metastatic colorectal cancer as a potential biomarker of responses to regorafenib treatment. Mol Oncol (2021) 15:2401–11. doi:10.1002/1878-0261.12972
104. Tsai, HL, Lin, CC, Sung, YC, Chen, SH, Chen, LT, Jiang, JK, et al. The emergence of RAS mutations in patients with RAS wild-type mCRC receiving cetuximab as first-line treatment: a noninterventional, uncontrolled multicenter study. Br J Cancer (2023) 129:947–55. doi:10.1038/S41416-023-02366-Z
105. Aparicio, J, Virgili Manrique, AC, Capdevila, J, Muñoz Boza, F, Galván, P, Richart, P, et al. Randomized phase II trial of FOLFIRI-panitumumab compared with FOLFIRI alone in patients with RAS wild-type circulating tumor DNA metastatic colorectal cancer beyond progression to first-line FOLFOX-panitumumab: the BEYOND study (GEMCAD 17-01). Clin Transl Oncol (2022) 24:2155–65. doi:10.1007/S12094-022-02868-X
106. Sartore-Bianchi, A, Pietrantonio, F, Lonardi, S, Mussolin, B, Rua, F, Crisafulli, G, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med (2022) 28:1612–8. doi:10.1038/S41591-022-01886-0
107. Sorah, JD, Moore, DT, Reilley, MJ, Salem, ME, Triglianos, T, Sanoff, HK, et al. Phase II single-arm Study of palbociclib and cetuximab rechallenge in patients with KRAS/NRAS/BRAF wild-type colorectal cancer. The Oncologist (2022) 27:E1006–E930. doi:10.1093/ONCOLO/OYAC222
108. van ’t Erve, I, Greuter, MJE, Bolhuis, K, Vessies, DCL, Leal, A, Vink, GR, et al. Diagnostic strategies toward clinical implementation of liquid biopsy RAS/BRAF circulating tumor DNA analyses in patients with metastatic colorectal cancer. The J Mol Diagn (2020) 22:1430–7. doi:10.1016/J.JMOLDX.2020.09.002
109. Rossini, D, Germani, MM, Pagani, F, Pellino, A, Dell’Aquila, E, Bensi, M, et al. Retreatment with Anti-EGFR antibodies in metastatic colorectal cancer patients: a multi-institutional analysis. Clin Colorectal Cancer (2020) 19:191–9.e6. doi:10.1016/J.CLCC.2020.03.009
110. Kourie, HR, Zouein, J, Zalaquett, Z, Chebly, A, Nasr, L, Karak, FE, et al. Liquid biopsy as a tool for KRAS/NRAS/BRAF baseline testing in metastatic colorectal cancer. Clin Res Hepatol Gastroenterol (2024) 48:102417. doi:10.1016/J.CLINRE.2024.102417
111. Napolitano, S, Ciardiello, D, De Falco, V, Martini, G, Martinelli, E, Della Corte, CM, et al. Panitumumab plus trifluridine/tipiracil as anti-EGFR rechallenge therapy in patients with refractory RAS wild-type metastatic colorectal cancer: overall survival and subgroup analysis of the randomized phase II VELO trial. Int J Cancer (2023) 153:1520–8. doi:10.1002/IJC.34632
112. Raghav, K, Ou, FS, Venook, AP, Innocenti, F, Sun, R, Lenz, HJ, et al. Acquired genomic alterations on first-line chemotherapy with cetuximab in advanced colorectal cancer: circulating tumor DNA analysis of the CALGB/SWOG-80405 Trial (Alliance). J Clin Oncol (2023) 41:472–8. doi:10.1200/JCO.22.00365
113. Ciardiello, D, Famiglietti, V, Napolitano, S, Esposito, L, Pietrantonio, F, Avallone, A, et al. Final results of the CAVE trial in RAS wild type metastatic colorectal cancer patients treated with cetuximab plus avelumab as rechallenge therapy: neutrophil to lymphocyte ratio predicts survival. Clin Colorectal Cancer (2022) 21:141–8. doi:10.1016/J.CLCC.2022.01.005
114. Fukuda, K, Osumi, H, Yoshinami, Y, Ooki, A, Takashima, A, Wakatsuki, T, et al. Efficacy of anti-epidermal growth factor antibody rechallenge in RAS/BRAF wild-type metastatic colorectal cancer: a multi-institutional observational study. J Cancer Res Clin Oncol (2024) 150:369. doi:10.1007/S00432-024-05893-1
115. Napolitano, S, De Falco, V, Martini, G, Ciardiello, D, Martinelli, E, Della Corte, CM, et al. Panitumumab plus Trifluridine-Tipiracil as anti-epidermal growth factor receptor rechallenge therapy for refractory RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol (2023) 9:966–70. doi:10.1001/JAMAONCOL.2023.0655
116. Martinelli, E, Martini, G, Famiglietti, V, Troiani, T, Napolitano, S, Pietrantonio, F, et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE trial. JAMA Oncol (2021) 7:1529–35. doi:10.1001/JAMAONCOL.2021.2915
117. Ciardiello, D, Martinelli, E, Troiani, T, Mauri, G, Rossini, D, Martini, G, et al. Anti-EGFR rechallenge in patients with refractory ctDNA RAS/BRAF wt metastatic colorectal cancer: a nonrandomized controlled trial. JAMA Netw Open (2024) 7:E245635. doi:10.1001/JAMANETWORKOPEN.2024.5635
118. Sunakawa, Y, Satake, H, Usher, J, Jaimes, Y, Miyamoto, Y, Nakamura, M, et al. Dynamic changes in RAS gene status in circulating tumour DNA: a phase II trial of first-line FOLFOXIRI plus bevacizumab for RAS-mutant metastatic colorectal cancer (JACCRO CC-11). ESMO Open (2022) 7:100512. doi:10.1016/J.ESMOOP.2022.100512
119. Palmer, CD, Rappaport, AR, Davis, MJ, Hart, MG, Scallan, CD, Hong, SJ, et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat Med (2022) 28:1619–29. doi:10.1038/S41591-022-01937-6
120. Ahn, DH, Barzi, A, Ridinger, M, Samuëlsz, E, Subramanian, RA, Croucher, PJP, et al. Onvansertib in combination with FOLFIRI and Bevacizumab in second-line treatment of KRAS-Mutant metastatic colorectal cancer: a phase Ib clinical Study. Clin Cancer Res (2024) 30:2039–47. doi:10.1158/1078-0432.CCR-23-3053
121. Lueong, SS, Herbst, A, Liffers, ST, Bielefeld, N, Horn, PA, Tannapfel, A, et al. Serial circulating tumor DNA mutational status in patients with KRAS-Mutant metastatic colorectal cancer from the phase 3 AIO KRK0207 trial. Clin Chem (2020) 66:1510–20. doi:10.1093/CLINCHEM/HVAA223
122. Osumi, H, Shinozaki, E, Nakamura, Y, Esaki, T, Yasui, H, Taniguchi, H, et al. Clinical features associated with NeoRAS wild-type metastatic colorectal cancer A SCRUM-Japan GOZILA substudy. Nat Commun (2024) 15:5885. doi:10.1038/S41467-024-50026-4
123. Wu, FTH, Topham, JT, O'Callaghan, CJ, Feilotter, H, Kennecke, HF, Drusbosky, L, et al. Kinetic profiling of RAS mutations with circulating tumor DNA in the Canadian Cancer Trials Group CO.26 trial suggests the loss of RAS mutations in Neo- RAS-Wildtype metastatic colorectal cancer is transient. JCO Precis Oncol (2024) 8:e2400031. doi:10.1200/PO.24.00031
124. Ueda, K, Yamada, T, Ohta, R, Matsuda, A, Sonoda, H, Kuriyama, S, et al. BRAF V600E mutations in right-side colon cancer: heterogeneity detected by liquid biopsy. Eur J Surg Oncol (2022) 48:1375–83. doi:10.1016/J.EJSO.2022.01.016
125. Tan, L, Tran, B, Tie, J, Markman, B, Ananda, S, Tebbutt, NC, et al. A phase Ib/II trial of combined BRAF and EGFR inhibition in BRAF V600E positive metastatic colorectal cancer and other cancers: the EVICT (erlotinib and vemurafenib in combination trial) study. Clin Cancer Res (2023) 29:1017–30. doi:10.1158/1078-0432.CCR-22-3094
126. Yaeger, R, Uboha, NV, Pelster, MS, Bekaii-Saab, TS, Barve, M, Saltzman, J, et al. Efficacy and safety of adagrasib plus cetuximab in patients with KRASG12C-Mutated metastatic colorectal cancer. Cancer Discov (2024) 14:982–93. doi:10.1158/2159-8290.CD-24-0217
127. Eriksen, M, Pfeiffer, P, Rohrberg, KS, Yde, CW, Petersen, LN, Poulsen, LØ, et al. A phase II study of daily encorafenib in combination with biweekly cetuximab in patients with BRAF V600E mutated metastatic colorectal cancer: the NEW BEACON study. BMC Cancer (2022) 22:1321. doi:10.1186/S12885-022-10420-X
128. Kopetz, S, Murphy, DA, Pu, J, Ciardiello, F, Desai, J, Van Cutsem, E, et al. Molecular profiling of BRAF-V600E-mutant metastatic colorectal cancer in the phase 3 BEACON CRC trial. Nat Med (2024) 30:3261–71. doi:10.1038/S41591-024-03235-9
129. Kopetz, S, Guthrie, KA, Morris, VK, Lenz, HJ, Magliocco, AM, Maru, D, et al. Randomized trial of Irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol (2021) 39:285–94. doi:10.1200/JCO.20.01994
130. Sacher, A, LoRusso, P, Patel, MR, Miller, WH, Garralda, E, Forster, MD, et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N Engl J Med (2023) 389:710–21. doi:10.1056/NEJMOA2303810
131. Nakamura, Y, Okamoto, W, Kato, T, Esaki, T, Kato, K, Komatsu, Y, et al. Circulating tumor DNA-Guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med (2021) 27:1899–903. doi:10.1038/S41591-021-01553-W
132. Jacobs, SA, Lee, JJ, George, TJ, Wade, JL, Stella, PJ, Wang, D, et al. Neratinib-plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) metastatic colorectal cancer resistant to cetuximab or panitumumab: NSABP FC-7, A phase Ib Study. Clin Cancer Res (2021) 27:1612–22. doi:10.1158/1078-0432.CCR-20-1831
133. Siena, S, Raghav, K, Masuishi, T, Yamaguchi, K, Nishina, T, Elez, E, et al. HER2-related biomarkers predict clinical outcomes with trastuzumab deruxtecan treatment in patients with HER2-expressing metastatic colorectal cancer: biomarker analyses of DESTINY-CRC01. Nat Commun (2024) 15:10213. doi:10.1038/s41467-024-53223-3
134. Taieb, J, Taly, V, Henriques, J, Bourreau, C, Mineur, L, Bennouna, J, et al. Prognostic value and relation with adjuvant treatment duration of ctDNA in stage III Colon cancer: a post hoc analysis of the PRODIGE-GERCOR IDEA-France trial. Clin Cancer Res (2021) 27:5638–46. doi:10.1158/1078-0432.CCR-21-0271
135. Brenne, SS, Madsen, PH, Pedersen, IS, Hveem, K, Skorpen, F, Krarup, HB, et al. The prognostic role of circulating tumour DNA detected prior to clinical diagnosis of colorectal cancer in the HUNT study. BMC Cancer (2024) 24:1251. doi:10.1186/S12885-024-13030-X
136. Janssens, K, Vanhoutte, G, Lybaert, W, Demey, W, Decaestecker, J, Hendrickx, K, et al. NPY methylated ctDNA is a promising biomarker for treatment response monitoring in metastatic colorectal cancer. Clin Cancer Res (2023) 29:1741–50. doi:10.1158/1078-0432.CCR-22-1500
137. Gallardo-Gómez, M, Rodríguez-Girondo, M, Planell, N, Moran, S, Bujanda, L, Etxart, A, et al. Serum methylation of GALNT9, UPF3A, WARS, and LDB2 as noninvasive biomarkers for the early detection of colorectal cancer and advanced adenomas. Clin Epigenetics (2023) 15:157. doi:10.1186/S13148-023-01570-1
138. Wang, Z, He, Z, Lin, R, Feng, Z, Li, X, Sui, X, et al. Evaluation of a plasma cell-free DNA methylation test for colorectal cancer diagnosis: a multicenter clinical study. BMC Med (2024) 22:436. doi:10.1186/S12916-024-03662-Y
139. Ladabaum, U, Mannalithara, A, Weng, Y, Schoen, RE, Dominitz, JA, Desai, M, et al. Comparative effectiveness and cost-effectiveness of colorectal cancer screening with blood-based biomarkers (Liquid biopsy) vs fecal tests or colonoscopy. Gastroenterology (2024) 167:378–91. doi:10.1053/J.GASTRO.2024.03.011
140. Veselovsky, E, Lebedeva, A, Kuznetsova, O, Kravchuk, D, Belova, E, Taraskina, A, et al. Evaluation of blood MSI burden dynamics to trace immune checkpoint inhibitor therapy efficacy through the course of treatment. Sci Rep (2024) 14:23454. doi:10.1038/S41598-024-73952-1
141. Mirandola, A, Kudriavtsev, A, Cofre Muñoz, CI, Navarro, RC, Macagno, M, Daoud, S, et al. Post-surgery sequelae unrelated to disease progression and chemotherapy revealed in follow-up of patients with stage III colon cancer. EBioMedicine (2024) 108:105352. doi:10.1016/J.EBIOM.2024.105352
142. Zhu, T, Li, Y, Li, R, Zhang, J, and Zhang, W. Predictive value of preoperative circulating tumor cells combined with hematological indexes for liver metastasis after radical resection of colorectal cancer. Medicine (2025) 104:e41264. doi:10.1097/MD.0000000000041264
143. Sastre, J, Orden, Vd. l., Martínez, A, Bando, I, Balbín, M, Bellosillo, B, et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics in a large cohort of Chemo-naïve Metastatic colorectal cancer patients prospectively collected. Clin Colorectal Cancer (2020) 19:e110–e116. doi:10.1016/J.CLCC.2020.02.014
144. Aranda, E, Viéitez, JM, Gómez-España, A, Gil Calle, S, Salud-Salvia, A, Graña, B, et al. FOLFOXIRI plus bevacizumab versus FOLFOX plus bevacizumab for patients with metastatic colorectal cancer and ≥3 circulating tumour cells: the randomised phase III VISNÚ-1 trial. ESMO Open (2020) 5:e000944. doi:10.1136/ESMOOPEN-2020-000944
145. Guadagni, S, Clementi, M, Mackay, AR, Ricevuto, E, Fiorentini, G, Sarti, D, et al. Real-life multidisciplinary treatment for unresectable colorectal cancer liver metastases including hepatic artery infusion with chemo-filtration and liquid biopsy precision oncotherapy: observational cohort study. J Cancer Res Clin Oncol (2020) 146:1273–90. doi:10.1007/S00432-020-03156-3
146. Ni, Z, Cao, Y, Liu, L, Huang, C, Xie, H, Zhou, J, et al. Impact of endoscopic metallic stent placement and emergency surgery on detection of viable circulating tumor cells for acute malignant left-sided colonic obstruction. World J Surg Oncol (2023) 21:1. doi:10.1186/S12957-022-02879-6
147. Rahbari, NN, Birgin, E, Bork, U, Mehrabi, A, Reißfelder, C, and Weitz, J. Anterior approach vs conventional hepatectomy for resection of colorectal liver metastasis: a randomized clinical trial. JAMA Surg (2021) 156:31–40. doi:10.1001/JAMASURG.2020.5050
148. Wu, Y, He, F, Liu, L, Jiang, W, Deng, J, Zhang, Y, et al. The use of CellCollector assay to detect free cancer cells in the peritoneal cavity of colorectal cancer patients: an experimental Study. Cancer Med (2024) 13:e70378. doi:10.1002/CAM4.70378
149. Shi, DD, Yang, CG, Han, S, Wang, SY, and Xiong, B. Dynamic evaluation of mesenchymal circulating tumor cells in patients with colorectal cancer: clinical associations and prognostic value. Oncol Rep (2020) 44:757–67. doi:10.3892/OR.2020.7629
150. Su, PW, Lai, W, Liu, L, Zeng, Y, Xu, H, Lan, Q, et al. Mesenchymal and phosphatase of regenerating Liver-3 status in circulating tumor cells May serve as a crucial prognostic marker for assessing relapse or metastasis in postoperative patients with colorectal cancer. Clin Transl Gastroenterol (2020) 11:e00265. doi:10.14309/CTG.0000000000000265
151. Murray, NP, Villalon, R, Orrego, S, and Guzman, E. Immune dysfunction as measured by the systemic immune-inflammation index is associated with the sub-type of minimal residual disease and outcome in stage II Colon cancer treated with surgery alone. Asian Pac J Cancer Prev (2021) 22:2391–7. doi:10.31557/APJCP.2021.22.8.2391
152. Murray, NP, Villalon, R, Hartmann, D, Rodriguez, MP, and Aedo, S. Improvement in immune dysfunction after FOLFOX chemotherapy for Stage III colon cancer is associated with improved minimal residual disease prognostic subtype and outcome. Colorectal Dis (2021) 23:2879–93. doi:10.1111/CODI.15899
153. Murray, NP, Villalon, R, Hartmann, D, Rodriguez, PM, and Aedo, S. Improvement in the neutrophil-lymphocyte ratio after combined fluorouracil, leucovorina and oxaliplatino based (FOLFOX) chemotherapy for stage III Colon cancer is associated with improved minimal residual disease and outcome. Asian Pac J Cancer Prev (2022) 23:591–9. doi:10.31557/APJCP.2022.23.2.591
154. Murray, NP, Villalon, R, Aedo, S, Hartmann, D, and Rodriguez, MP. The possible role of matrix Metalloprotienase-2 in the relapse in patients with stage II Colon cancer treated by curative surgery. Asian Pac J Cancer Prev (2023) 24:3373–9. doi:10.31557/APJCP.2023.24.10.3373
155. Sefrioui, D, Beaussire, L, Gillibert, A, Blanchard, F, Toure, E, Bazille, C, et al. CEA, CA19-9, circulating DNA and circulating tumour cell kinetics in patients treated for metastatic colorectal cancer (mCRC). Br J Cancer (2021) 125:725–33. doi:10.1038/S41416-021-01431-9
156. Nanou, A, Mol, L, Coumans, FAW, Koopman, M, Punt, CJA, and Terstappen, LWMM. Endothelium-Derived extracellular vesicles associate with poor prognosis in metastatic colorectal cancer. Cells (2020) 9:2688. doi:10.3390/CELLS9122688
157. Bjørnetrø, T, Steffensen, LA, Vestad, B, Brusletto, BS, Olstad, OK, Trøseid, A, et al. Uptake of circulating extracellular vesicles from rectal cancer patients and differential responses by human monocyte cultures. FEBS Open Bio (2021) 11:724–40. doi:10.1002/2211-5463.13098
158. de Miguel Pérez, D, Rodriguez Martínez, A, Ortigosa Palomo, A, Delgado Ureña, M, Garcia Puche, JL, Robles Remacho, A, et al. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci Rep (2020) 10:3974. doi:10.1038/S41598-020-60212-1
159. Wei, R, Chen, L, Qin, D, Guo, Q, Zhu, S, Li, P, et al. Liquid biopsy of extracellular vesicle-derived miR-193a-5p in colorectal cancer and discovery of its tumor-suppressor functions. Front Oncol (2020) 10:1372. doi:10.3389/FONC.2020.01372
160. Yang, H, Zhang, H, Yang, Y, Wang, X, Deng, T, Liu, R, et al. Hypoxia induced exosomal circRNA promotes metastasis of Colorectal Cancer via targeting GEF-H1/RhoA axis. Theranostics (2020) 10:8211–26. doi:10.7150/THNO.44419
161. He, J, Zhao, H, Liu, X, Wang, D, Wang, Y, Ai, Y, et al. Sevoflurane suppresses cell viability and invasion and promotes cell apoptosis in colon cancer by modulating exosome-mediated circ-HMGCS1 via the miR-34a-5p/SGPP1 axis. Oncol Rep (2020) 44:2429–42. doi:10.3892/OR.2020.7783
162. Pardini, B, Ferrero, G, Tarallo, S, Gallo, G, Francavilla, A, Licheri, N, et al. A fecal MicroRNA signature by small RNA sequencing accurately distinguishes colorectal cancers: results from a multicenter Study. Gastroenterology (2023) 165:582–99.e8. doi:10.1053/J.GASTRO.2023.05.037
163. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics. CA: A Cancer J Clinicians (2022) 72:7–33. doi:10.3322/CAAC.21708
164. Macgregor-Das, A, Yu, J, Tamura, K, Abe, T, Suenaga, M, Shindo, K, et al. Detection of circulating tumor DNA in patients with pancreatic cancer using digital next-generation sequencing. The J Mol Diagn (2020) 22:748–56. doi:10.1016/J.JMOLDX.2020.02.010
165. Zvereva, M, Roberti, G, Durand, G, Voegele, C, Nguyen, MD, Delhomme, TM, et al. Circulating tumour-derived KRAS mutations in pancreatic cancer cases are predominantly carried by very short fragments of cell-free DNA. EBioMedicine (2020) 55:102462. doi:10.1016/J.EBIOM.2019.09.042
166. Renouf, DJ, Loree, JM, Knox, JJ, Topham, JT, Kavan, P, Jonker, D, et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun (2022) 13:5020. doi:10.1038/S41467-022-32591-8
167. Cheng, H, Luo, G, Jin, K, Fan, Z, Huang, Q, Gong, Y, et al. Kras mutation correlating with circulating regulatory T cells predicts the prognosis of advanced pancreatic cancer patients. Cancer Med (2020) 9:2153–9. doi:10.1002/CAM4.2895
168. McLellan, P, Henriques, J, Ksontini, F, Doat, S, Hammel, P, Desrame, J, et al. Prognostic value of the early change in neutrophil-to-lymphocyte ratio in metastatic pancreatic adenocarcinoma. Clin Res Hepatol Gastroenterol (2021) 45:101541. doi:10.1016/J.CLINRE.2020.08.016
169. Du, J, Lu, C, Mao, L, Zhu, Y, Kong, W, Shen, S, et al. PD-1 blockade plus chemoradiotherapy as preoperative therapy for patients with BRPC/LAPC: a biomolecular exploratory, phase II trial. Cell Rep Med (2023) 4:100972. doi:10.1016/J.XCRM.2023.100972
170. Bachet, JB, Blons, H, Hammel, P, Hariry, IE, Portales, F, Mineur, L, et al. Circulating Tumor DNA is prognostic and potentially predictive of eryaspase efficacy in second-line in patients with advanced pancreatic adenocarcinoma. Clin Cancer Res (2020) 26:5208–16. doi:10.1158/1078-0432.CCR-20-0950
171. Rodon, J, Prenen, H, Sacher, A, Villalona-Calero, M, Penel, N, El Helali, A, et al. First-in-human study of AMG 193, an MTA-cooperative PRMT5 inhibitor, in patients with MTAP-deleted solid tumors: results from phase I dose exploration. Ann Oncol (2024) 35:1138–47. doi:10.1016/J.ANNONC.2024.08.2339
172. Pant, S, Wainberg, ZA, Weekes, CD, Furqan, M, Kasi, PM, Devoe, CE, et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat Med (2024) 30:531–42. doi:10.1038/S41591-023-02760-3
173. Hipp, J, Hussung, S, Timme-Bronsert, S, Boerries, M, Biesel, E, Fichtner-Feigl, S, et al. Perioperative cell-free mutant KRAS dynamics in patients with pancreatic cancer. Br J Surg (2021) 108:239–43. doi:10.1093/BJS/ZNAA116
174. Hussung, S, Akhoundova, D, Hipp, J, Follo, M, Klar, RFU, Philipp, U, et al. Longitudinal analysis of cell-free mutated KRAS and CA 19-9 predicts survival following curative resection of pancreatic cancer. BMC Cancer (2021) 21:49. doi:10.1186/S12885-020-07736-X
175. Cecchini, M, Salem, RR, Robert, M, Czerniak, S, Blaha, O, Zelterman, D, et al. Perioperative modified FOLFIRINOX for resectable pancreatic cancer: a nonrandomized controlled trial. JAMA Oncol (2024) 10:1027–35. doi:10.1001/JAMAONCOL.2024.1575
176. Pietrasz, D, Wang-Renault, S, Taieb, J, Dahan, L, Postel, M, Durand-Labrunie, J, et al. Prognostic value of circulating tumour DNA in metastatic pancreatic cancer patients: post-hoc analyses of two clinical trials. Br J Cancer (2022) 126:440–8. doi:10.1038/S41416-021-01624-2
177. Shen, Y, Zhang, X, Zhang, L, Zhang, Z, Lyu, B, Lai, Q, et al. Performance evaluation of a CRISPR Cas9-based selective exponential amplification assay for the detection of KRAS mutations in plasma of patients with advanced pancreatic cancer. J Clin Pathol (2024) 77:853–60. doi:10.1136/JCP-2023-208974
178. Yu, KH, Park, J, Mittal, A, Abou-Alfa, GK, El Dika, I, Epstein, AS, et al. Circulating tumor and invasive cell expression profiling predicts effective therapy in pancreatic cancer. Cancer (2022) 128:2958–66. doi:10.1002/CNCR.34269
179. O’Leary, BR, Alexander, MS, Du, J, Moose, DL, Henry, MD, and Cullen, JJ. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci Rep (2020) 10:17649. doi:10.1038/S41598-020-74806-2
180. Konno, N, Suzuki, R, Takagi, T, Sugimoto, M, Asama, H, Sato, Y, et al. Clinical utility of a newly developed microfluidic device for detecting circulating tumor cells in the blood of patients with pancreatico-biliary malignancies. J Hepato-Biliary-Pancreatic Sci (2021) 28:115–24. doi:10.1002/JHBP.850
181. Padillo-Ruiz, J, Fresno, C, Suarez, G, Blanco, G, Muñoz-Bellvis, L, Justo, I, et al. Effects of the superior mesenteric artery approach versus the no-touch approach during pancreatoduodenectomy on the mobilization of circulating tumour cells and clusters in pancreatic cancer (CETUPANC): randomized clinical trial. BJS Open (2024) 8:zrae123. doi:10.1093/BJSOPEN/ZRAE123
182. Xu, D, Wang, Y, Zhou, K, Wu, J, Zhang, Z, Zhang, J, et al. Identification of an extracellular vesicle-related gene signature in the prediction of pancreatic cancer clinical prognosis. Biosci Rep (2020) 40. doi:10.1042/BSR20201087
183. Yang, KS, Ciprani, D, O’Shea, A, Liss, AS, Yang, R, Fletcher-Mercaldo, S, et al. Extracellular vesicle analysis allows for identification of invasive IPMN. Gastroenterology (2021) 160:1345–58.e11. doi:10.1053/J.GASTRO.2020.11.046
184. Moik, F, Prager, G, Thaler, J, Posch, F, Wiedemann, S, Schramm, T, et al. Hemostatic biomarkers and venous thromboembolism are associated with mortality and response to chemotherapy in patients with pancreatic cancer. Arteriosclerosis, Thromb Vasc Biol (2021) 41:2837–47. doi:10.1161/ATVBAHA.121.316463
185. Xiao, D, Dong, Z, Zhen, L, Xia, G, Huang, X, Wang, T, et al. Combined exosomal GPC1, CD82, and serum CA19-9 as multiplex targets: a specific, sensitive, and reproducible detection Panel for the diagnosis of pancreatic cancer. Mol Cancer Res (2020) 18:1300–310. doi:10.1158/1541-7786.MCR-19-0588
186. Liu, DSK, Puik, JR, Patel, BY, Venø, MT, Vahabi, M, Prado, MM, et al. Unlocking the diagnostic power of plasma extracellular vesicle miR-200 family in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res (2024) 43:189. doi:10.1186/S13046-024-03090-Z
187. Wang, S, and Gao, Y. Pancreatic cancer cell-derived microRNA-155-5p-containing extracellular vesicles promote immune evasion by triggering EHF-dependent activation of Akt/NF-κB signaling pathway. Int Immunopharmacology (2021) 100:107990. doi:10.1016/J.INTIMP.2021.107990
188. Yang, G, Qiu, J, Xu, J, Xiong, G, Zhao, F, Cao, Z, et al. Using a microRNA panel of circulating exosomes for diagnosis of pancreatic cancer: Multicentre case-control study. Br J Surg (2023) 110:908–12. doi:10.1093/BJS/ZNAC375
189. Han, Y, Drobisch, P, Krüger, A, William, D, Grützmann, K, Böthig, L, et al. Plasma extracellular vesicle messenger RNA profiling identifies prognostic EV signature for non-invasive risk stratification for survival prediction of patients with pancreatic ductal adenocarcinoma. J Hematol Oncol (2023) 16:7. doi:10.1186/S13045-023-01404-W
190. Nakamura, K, Zhu, Z, Roy, S, Jun, E, Han, H, Munoz, RM, et al. An exosome-based transcriptomic signature for noninvasive, early detection of patients with pancreatic ductal adenocarcinoma: a multicenter cohort Study. Gastroenterology (2022) 163:1252–66.e2. doi:10.1053/J.GASTRO.2022.06.090
191. Khan, IA, Rashid, S, Singh, N, Rashid, S, Singh, V, Gunjan, D, et al. Panel of serum miRNAs as potential non-invasive biomarkers for pancreatic ductal adenocarcinoma. Sci Rep (2021) 11:2824. doi:10.1038/S41598-021-82266-5
192. Shi, W, Wartmann, T, Accuffi, S, Al-Madhi, S, Perrakis, A, Kahlert, C, et al. Integrating a microRNA signature as a liquid biopsy-based tool for the early diagnosis and prediction of potential therapeutic targets in pancreatic cancer. Br J Cancer (2024) 130:125–34. doi:10.1038/S41416-023-02488-4
193. Fahrmann, JF, Schmidt, CM, Mao, X, Irajizad, E, Loftus, M, Zhang, J, et al. Lead-Time trajectory of CA19-9 as an anchor marker for pancreatic cancer early detection. Gastroenterology (2021) 160:1373–83.e6. doi:10.1053/J.GASTRO.2020.11.052
194. Gu, Y, Hua, Q, Li, Z, Zhang, X, Lou, C, Zhang, Y, et al. Diagnostic value of combining preoperative inflammatory markers ratios with CA199 for patients with early-stage pancreatic cancer. BMC Cancer (2023) 23:227. doi:10.1186/S12885-023-10653-4
195. Gao, CF, Wisniewski, L, Liu, Y, Staal, B, Beddows, I, Plenker, D, et al. Detection of chemotherapy-resistant pancreatic cancer using a Glycan biomarker, sTRA. Clin Cancer Res (2021) 27:226–36. doi:10.1158/1078-0432.CCR-20-2475
196. Pietrobono, S, Sabbadini, F, Bertolini, M, Mangiameli, D, De Vita, V, Fazzini, F, et al. Autotaxin secretion is a stromal mechanism of adaptive resistance to TGFβ inhibition in pancreatic ductal adenocarcinoma. Cancer Res (2024) 84:118–32. doi:10.1158/0008-5472.CAN-23-0104
197. Van Treijen, MC, van der Zee, D, Heeres, BC, Staal, FCR, Vriens, MR, Saveur, LJ, et al. Blood molecular genomic analysis predicts the disease course of gastroenteropancreatic neuroendocrine tumor patients: a validation Study of the predictive value of the NETest®. Neuroendocrinology (2021) 111:586–98. doi:10.1159/000509091
198. De Martel, C, Georges, D, Bray, F, Ferlay, J, and Clifford, GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. The Lancet Glob Health (2020) 8:e180–e190. doi:10.1016/S2214-109X(19)30488-7
199. Luo, P, Yin, P, Hua, R, Tan, Y, Li, Z, Qiu, G, et al. A large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology (2018) 67:662–75. doi:10.1002/HEP.29561
200. Guo, D, Huang, A, Wang, Y, Zhou, S, Wang, H, Xing, X, et al. Early detection and prognosis evaluation for hepatocellular carcinoma by circulating tumour DNA methylation: a multicentre cohort study. Clin Translational Med (2024) 14:e1652. doi:10.1002/CTM2.1652
201. Cai, Z, Su, X, Qiu, L, Li, Z, Li, X, Dong, X, et al. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol Cancer (2021) 20:164. doi:10.1186/S12943-021-01467-8
202. Pinato, DJ, D’Alessio, A, Fulgenzi, CAM, Schlaak, AE, Celsa, C, Killmer, S, et al. Safety and preliminary efficacy of pembrolizumab following transarterial chemoembolization for hepatocellular carcinoma: the PETAL phase Ib Study. Clin Cancer Res (2024) 30:2433–43. doi:10.1158/1078-0432.CCR-24-0177
203. Xia, Y, Tang, W, Qian, X, Li, X, Cheng, F, Wang, K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer (2022) 10:e004656. doi:10.1136/JITC-2022-004656
204. Li, CL, Ho, MC, Lin, YY, Tzeng, ST, Chen, YJ, Pai, HY, et al. Cell-Free virus-host chimera DNA from Hepatitis B virus integration sites as a circulating biomarker of hepatocellular cancer. Hepatology (2020) 72:2063–76. doi:10.1002/HEP.31230
205. Long, G, Fang, T, Su, W, Mi, X, and Zhou, L. The prognostic value of postoperative circulating cell-free DNA in operable hepatocellular carcinoma. Scand J Gastroenterol (2020) 55:1441–6. doi:10.1080/00365521.2020.1839127
206. Wu, T, Fan, R, Bai, J, Yang, Z, Qian, YS, Du, LT, et al. The development of a cSMART-based integrated model for hepatocellular carcinoma diagnosis. J Hematol Oncol (2023) 16:1. doi:10.1186/S13045-022-01396-Z
207. Chalasani, NP, Ramasubramanian, TS, Bhattacharya, A, Olson, MC, Edwards V, DK, Roberts, LR, et al. A novel blood-based Panel of methylated DNA and protein markers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol (2021) 19:2597–605.e4. doi:10.1016/J.CGH.2020.08.065
208. Chen, L, Wu, T, Fan, R, Qian, YS, Liu, JF, Bai, J, et al. Cell-free DNA testing for early hepatocellular carcinoma surveillance. EBioMedicine (2024) 100:104962. doi:10.1016/J.EBIOM.2023.104962
209. Qi, LN, Ma, L, Chen, YY, Chen, ZS, Zhong, JH, Gong, WF, et al. Outcomes of anatomical versus non-anatomical resection for hepatocellular carcinoma according to circulating tumour-cell status. Ann Med (2020) 52:21–31. doi:10.1080/07853890.2019.1709655
210. Sasaki, S, Nomura, Y, Sudo, T, Sakai, H, Hisaka, T, Akiba, J, et al. Hematogenous dissemination of tumor cells in hepatocellular carcinoma: comparing anterior and non-anterior approach hepatectomy. Anticancer Res (2022) 42:4129–37. doi:10.21873/ANTICANRES.15911
211. Wei, HW, Qin, SL, Xu, JX, Huang, YY, Chen, YY, Ma, L, et al. Nomograms for postsurgical extrahepatic recurrence prediction of hepatocellular carcinoma based on presurgical circulating tumor cell status and clinicopathological factors. Cancer Med (2023) 12:15065–78. doi:10.1002/CAM4.6178
212. Zhou, J, Zhang, Z, Zhou, H, Leng, C, Hou, B, Zhou, C, et al. Preoperative circulating tumor cells to predict microvascular invasion and dynamical detection indicate the prognosis of hepatocellular carcinoma. BMC Cancer (2020) 20:1047. doi:10.1186/S12885-020-07488-8
213. Schöler, D, Castoldi, M, Jördens, MS, Schulze-Hagen, M, Kuhl, C, Keitel, V, et al. Enlarged extracellular vesicles are a negative prognostic factor in patients undergoing TACE for primary or secondary liver cancer-a case series. PLoS One (2021) 16:e0255983. doi:10.1371/JOURNAL.PONE.0255983
214. Uzzaman, A, Zhang, X, Qiao, Z, Zhan, H, Sohail, A, Wahid, A, et al. Discovery of small extracellular vesicle proteins from human serum for liver cirrhosis and liver cancer. Biochimie (2020) 177:132–41. doi:10.1016/J.BIOCHI.2020.08.013
215. Shuen, TWH, Alunni-Fabbroni, M, Öcal, E, Malfertheiner, P, Wildgruber, M, Schinner, R, et al. Extracellular vesicles May predict response to radioembolization and sorafenib treatment in advanced hepatocellular carcinoma: an exploratory analysis from the SORAMIC trial. Clin Cancer Res (2022) 28:3890–901. doi:10.1158/1078-0432.CCR-22-0569
216. Wang, J, Pu, J, Zhang, Y, Yao, T, Luo, Z, Li, W, et al. Exosome-transmitted long non-coding RNA SENP3-EIF4A1 suppresses the progression of hepatocellular carcinoma. Aging (2020) 12:11550–67. doi:10.18632/AGING.103302
217. Kim, SS, Baek, GO, Son, JA, Ahn, HR, Yoon, MK, Cho, HJ, et al. Early detection of hepatocellular carcinoma via liquid biopsy: panel of small extracellular vesicle-derived long noncoding RNAs identified as markers. Mol Oncol (2021) 15:2715–31. doi:10.1002/1878-0261.13049
218. Sun, N, Lee, YT, Zhang, RY, Kao, R, Teng, PC, Yang, Y, et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun (2020) 11:4489. doi:10.1038/S41467-020-18311-0
219. Qin, XY, Shirakami, Y, Honda, M, Yeh, SH, Numata, K, Lai, YY, et al. Serum MYCN as a predictive biomarker of prognosis and therapeutic response in the prevention of hepatocellular carcinoma recurrence. Int J Cancer (2024) 155:582–94. doi:10.1002/IJC.34893
220. Cheng, K, Shi, J, Liu, Z, Jia, Y, Qin, Q, Zhang, H, et al. A panel of five plasma proteins for the early diagnosis of hepatitis B virus-related hepatocellular carcinoma in individuals at risk. EBioMedicine (2020) 52:102638. doi:10.1016/J.EBIOM.2020.102638
221. Zhang, S, Liu, Y, Chen, J, Shu, H, Shen, S, Li, Y, et al. Autoantibody signature in hepatocellular carcinoma using seromics. J Hematol Oncol (2020) 13:85. doi:10.1186/S13045-020-00918-X
222. Hříbek, P, Vrtělka, O, Králová, K, Klasová, J, Fousková, M, Habartová, L, et al. Efficacy of blood plasma spectroscopy for early liver cancer diagnostics in obese patients. Ann Hepatol (2024) 29:101519. doi:10.1016/J.AOHEP.2024.101519
223. Waqar, W, Asghar, S, and Manzoor, S. Platelets’ RNA as biomarker trove for differentiation of early-stage hepatocellular carcinoma from underlying cirrhotic nodules. PLoS One (2021) 16:e0256739. doi:10.1371/JOURNAL.PONE.0256739
224. Yao, Z, Jia, C, Tai, Y, Liang, H, Zhong, Z, Xiong, Z, et al. Serum exosomal long noncoding RNAs lnc-FAM72D-3 and lnc-EPC1-4 as diagnostic biomarkers for hepatocellular carcinoma. Aging (2020) 12:11843–63. doi:10.18632/AGING.103355
225. Sathyanarayana, SH, Spracklin, SB, Deharvengt, SJ, Green, DC, Instasi, MD, Gallagher, TL, et al. Standardized workflow and analytical validation of cell-free DNA extraction for liquid biopsy using a magnetic bead-based cartridge System. Cells (2025) 14(14):1062. doi:10.3390/cells14141062
226. Fusco, N, Venetis, K, Pepe, F, Shetty, O, Farinas, SC, Heeke, S, et al. International society of liquid biopsy (ISLB) perspective on minimal requirements for ctDNA testing in solid tumors. The J Liquid Biopsy (2025) 8:100301. doi:10.1016/j.jlb.2025.100301
227. Alix-Panabières, C, and Pantel, K. Challenges in circulating tumour cell research. Nat Rev Cancer (2014) 14(9):623–31. doi:10.1038/nrc3820
228. Grölz, D, Hauch, S, Schlumpberger, M, Guenther, K, Voss, T, Sprenger-Haussels, M, et al. Liquid biopsy preservation solutions for standardized pre-analytical workflows-venous whole blood and plasma. Curr pathobiology Rep (2018) 6(4):275–86. doi:10.1007/s40139-018-0180-z
229. Yi, X, Huang, D, Li, Z, Wang, X, Yang, T, Zhao, M, et al. The role and application of small extracellular vesicles in breast cancer. Front Oncol (2022) 12:980404. doi:10.3389/FONC.2022.980404
230. Cheng, HY, Su, GL, Wu, YX, Chen, G, and Yu, ZL. Extracellular vesicles in anti-tumor drug resistance: mechanisms and therapeutic prospects. J Pharm Anal (2024) 14:100920. doi:10.1016/J.JPHA.2023.12.010
231. Li, J, Wang, J, and Chen, Z. Emerging role of exosomes in cancer therapy: progress and challenges. Mol Cancer (2025) 24:13. doi:10.1186/S12943-024-02215-4
232. Angerilli, V, Fornaro, L, Pepe, F, Rossi, SM, Perrone, G, Malapelle, U, et al. FGFR2 testing in cholangiocarcinoma: translating molecular studies into clinical practice. Pathologica (2023) 115:71–82. doi:10.32074/1591-951X-859
233. Ettrich, TJ, Schwerdel, D, Dolnik, A, Beuter, F, Blätte, TJ, Schmidt, SA, et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep (2019) 9:13261. doi:10.1038/S41598-019-49860-0
234. Mauri, G, Vitiello, PP, Sogari, A, Crisafulli, G, Sartore-Bianchi, A, Marsoni, S, et al. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br J Cancer (2022) 127:394–407. doi:10.1038/S41416-022-01769-8
235. Pesola, G, Epistolio, S, Cefalì, M, Trevisi, E, De Dosso, S, and Frattini, M. Neo-RAS wild type or RAS conversion in metastatic colorectal cancer: a comprehensive narrative review. Cancers (Basel) (2024) 16:3923. doi:10.3390/CANCERS16233923
236. Li, Y, Sui, S, and Goel, A. Extracellular vesicles associated microRNAs: their biology and clinical significance as biomarkers in gastrointestinal cancers. Semin Cancer Biol (2024) 99:5–23. doi:10.1016/J.SEMCANCER.2024.02.001
237. Jaworski, JJ, Morgan, RD, and Sivakumar, S. Circulating cell-free tumour DNA for early detection of pancreatic cancer. Cancers (Basel) (2020) 12:3704–16. doi:10.3390/CANCERS12123704
238. Li, Y, Zhang, C, Jiang, A, Lin, A, Liu, Z, Cheng, X, et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J Transl Med (2024) 22:293. doi:10.1186/S12967-024-05104-Y
239. Mok, ETY, Chitty, JL, and Cox, TR. miRNAs in pancreatic cancer progression and metastasis. Clin Exp Metastasis (2024) 41:163–86. doi:10.1007/S10585-023-10256-0
Keywords: liquid biopsy, gastrointestinal cancer (GI), circulating tumor cells (CTC), extracellular vesicles (EVs), circulating tumor DNA (ctDNA)
Citation: Palieri R, De Luca M, Balestra F, Panzetta G, Lotesoriere C, Rizzi F, Ricci AD, Mastrogiacomo R, Curri ML, Laghi LA, Giannelli G, Depalo N and Scavo MP (2025) Liquid biopsy in gastrointestinal oncology: clinical applications and translational integration of ctDNA, CTCs, and sEVs. Oncol. Rev. 19:1702932. doi: 10.3389/or.2025.1702932
Received: 10 September 2025; Accepted: 29 September 2025;
Published: 20 October 2025.
Edited by:
Mauro Cives, University of Bari Aldo Moro, ItalyReviewed by:
Sheefa Mirza, University of Witwatersrand, South AfricaAndrea Giglio, University of Bari Aldo Moro, Italy
Copyright © 2025 Palieri, De Luca, Balestra, Panzetta, Lotesoriere, Rizzi, Ricci, Mastrogiacomo, Curri, Laghi, Giannelli, Depalo and Scavo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Principia Scavo, bWFyaWEuc2Nhdm9AaXJjY3NkZWJlbGxpcy5pdA==
†These authors have contributed equally to this work
 Rita Palieri
Rita Palieri Maria De Luca
Maria De Luca Francesco Balestra
Francesco Balestra Giorgia Panzetta1
Giorgia Panzetta1 Claudio Lotesoriere
Claudio Lotesoriere Angela Dalia Ricci
Angela Dalia Ricci Maria Lucia Curri
Maria Lucia Curri Luigi Andrea Laghi
Luigi Andrea Laghi Gianluigi Giannelli
Gianluigi Giannelli Maria Principia Scavo
Maria Principia Scavo