- 1Division of Vascular and Interventional Radiology, Department of Radiology, University of Virginia, Charlottesville, VA, United States
- 2Division of Vascular and Interventional Radiology, Department of Radiology, University of Washington, Seattle, WA, United States
- 3Division of Vascular and Interventional Radiology, Department of Radiology, University of Southern California, Los Angeles, CA, United States
- 4Division of Vascular and Interventional Radiology, Department of Radiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Interventional radiology (IR) is a unique specialty that incorporates a diverse set of skills ranging from imaging, procedures, consultation, and patient management. Understanding how IR generates value to the healthcare system is important to review from various perspectives. IR specialists need to understand how to meet demands from various stakeholders to expand their practice improving patient care. Thus, this review discusses the domains of value contributed to medical systems and outlines the parameters of success. IR benefits five distinct parties: patients, practitioners, payers, employers, and innovators. Value to patients and providers is delivered through a wide set of diagnostic and therapeutic interventions. Payers and hospital systems financially benefit from the reduced cost in medical management secondary to fast patient recovery, outpatient procedures, fewer complications, and the prestige of offering diverse expertise for complex patients. Lastly, IR is a field of rapid innovation implementing new procedural technology and techniques. Overall, IR must actively advocate for further growth and influence in the medical field as their value continues to expand in multiple domains. Despite being a nascent specialty, IR has become indispensable to modern medical practice.
Main points
• Interventional Radiology is a rapidly evolving specialty that provides value to all healthcare stakeholders through their collaboration, innovation, and dedication to patients.
• IR physicians are uniquely trained to provide minimally invasive procedures that offer safer, faster, and more cost-effective options for patients.
• With the evolution of value-based reimbursement models, IR physicians can adopt clinical patient models to supplement their reimbursement and patient volume.
• IR needs to practice multisystem marketing and advocacy to grow their practice, broaden their public influence, and recruit new talent to the specialty to expand their international impact.
Introduction
Since the inception of interventional radiology (IR) by Charles Dotter in 1963, IR has proven to be an integral part of patient care. Advancements in imaging technology have broadened the scope of visually guided minimally invasive procedures to improve patient outcomes (1). IR's guiding principle is to reduce trauma via minimally invasive access with the goal of reducing patient recovery time, postoperative complications, healthcare costs, and achieving greater clinical outcomes. IR optimizes the “triple aim” of modern healthcare: improving patient experience (quality & quantity), improving the health of populations, and reducing per-capita costs (2). In the delivery of complex medical care IR provides value at all levels of patient care and to the associated stakeholders in the healthcare system.
The practice of IR in medicine has expanded over the past. IR in oncology spans from detection, diagnosis, therapeutic delivery, and continued management (ports, drains, etc.). The value of the IR market is expected to exceed 43 billion by 2029 with an estimated growth rate of 7.13% (3). The IR ecosystem is expanding into mixed clinical care models. Physicians are integrating inpatient and outpatient care with both procedural and longitudinal care. The resulting increase of billing for evaluation and management (E&M) are showing increases of 722% and 669%, respectively (4, 5). IR continues to be one of the most innovative fields of interdisciplinary medicine that is positioned for significant growth, investment, and prominence in the healthcare industry.
IR has a responsibility to demonstrate value at all levels of medical care. As one of the more nascently recognized medical specialties in 1994, IR physicians have identified a growing need to prove its value to external stakeholders, including hospital executives, insurance providers, hybrid IR/DR practices, referring specialists, patients, and to the general public (6). A common critique among healthcare specialists is that IR physicians are “masters of none,” and face increased competition for patient flow among other interventionalists (neurosurgery, vascular, cardiology, pulmonary, etc.). The purpose of this article is to review the domains of IR value and highlight important components of IR's success. This work reviews key functions of the IR physician's practice, outlines the financial value to patients, the profitability within different payment models, infrastructure requirements for successful practice, and defines important initiatives for continued advancement of the field.
Methods
This is a descriptive review on how IR provides value to the medical system. This paper will review the advantages of IR procedures to patient outcomes, their cost-benefit analysis, and how IR practice can succeed in payment models. We also review the necessary factors including infrastructure, facilities, patient networking, and physician recruitment and retainment strategies for IR to be competitive in healthcare. Lastly, this work discusses future efforts that must be taken to communicate this value to healthcare specialists and the public at large.
In the review of comparative studies highlighting the medical or financial benefit of IR specific procedures, only studies in the last 25 years were included for relevancy. The exception to this cutoff was if no more recent comparative study has been performed or identified on literature search for a specific procedure. Studies referencing mixed results of an IR procedure's value or efficacy to surgical or medical alternatives addressed.
Outline
1. Scope of Practice
a. Diagnostics
b. Clinical & Consultation Services
c. Minimally Invasive Procedures
2. Minimally Invasive IR Procedures
a. Nonvascular Procedures
b. Vascular procedures
3. Medical and Financial Value of Minimally Invasive Procedure
a. Medical Benefits
b. Financial Benefits
c. Research & Innovation
4. Financial Contributions
a. Direct Contributions
b. Indirect Contributions
5. Revenue Analysis of IR Reimbursement Models
a. Fee for Service
b. Value Based Care
c. Bundled Payment
d. Capitation
6. Elements of Successful IR Practice
a. Recruitment
b. Marketing
c. Networking
d. Incentives
e. Expectations
f. Infrastructure needs
7. Expanding the Impact of IR
a. Promotion and Awareness
b. Governmental Policy and Healthcare Infrastructure
c. Future Directions
Scope of practice
IR services are diverse and provide interventions at all stages of disease. The role of the IR physician spans beyond the surgical suite, where hospital systems rely on their skills in image analysis, consultation services, and patient clinics.
Diagnostic role in data acquisition and analysis
IR physicians assist in patient data collection. They acquire angiograms for venous and arterial pathology. Nonvascular studies include lymphangiography and arthrography. IR has become the preferred specialist for ultrasound (US), computed tomography (CT), or magnetic resonance (MR) guided biopsies and has significantly reduced the need for open surgical sampling without sacrificing sample quality. Minimally invasive venous sampling collected by IR physicians supplements imaging data for the localization of endocrine tumors, such as parathyroid, pituitary, adrenal glands, and islet cell tumors where imaging is indeterminate, or laterality is uncertain. IR provides valuable information to specialists who rely on high quality images and sampling data for their clinical management.
IR physicians serve the important concomitant responsibility interpreting noninvasive imaging studies. IR confidently interprets MR, percutaneous and endoscopic/intravascular US, CT, angiography, and fluoroscopy. Surgeons and oncologists rely on discussions with trained radiologists for complex patient care and procedural evaluation. This extends to outpatient care where specialists rely on image analysis for disease management including prevention, detection, diagnosis, therapy monitoring, and prognosis. The focus on these diagnostic duties is highly setting dependent. Academic center IR physicians focus more on procedural tasks, where private practice physicians focused more on diagnostic interpretation (7).
Consultation services are essential for advanced disease
In oncology patients, providers are challenged to find a meaningful therapeutic strategy to improve quality of life with minimal risks of intervention. IR serves as a valuable member of the tumor boards to provide their expertise in the development of a care plan. Bringing IR into the conversation can provide additional expertise on when nonsurgical options become viable.
IR can offer unique procedural options for challenging clinical cases. As consultants, IR can address internal referrals for vascular and localized procedures for untrained clinicians or offer experienced advantages over obstetricians and surgeons that do not perform these procedures regularly in their practice. By collaboration on patient care, the referring physician can synergistically benefit patient expense, experience, and outcome.
Patient clinics provide longitudinal patient care
There are many financial and intangible qualities to incorporating patient clinics into IR practice. These allow greater involvement in evaluation and management of their own patient population and greater control over their pre-procedural and post-procedural pipeline. Office visits and follow-up provide an important service in building patient rapport and establish an element of public relations that would otherwise be impossible from procedural referrals alone. This step complements the other functions of IR and elevates the importance of their skills to the public (8).
Specialized minimally invasive alternative therapy
IR's suite of minimally invasive strategies can be applied to an array of anatomical structures to accomplish various goals including tissue drainage, revascularization, reconstruction, repair, reinforcement, embolization, obliteration, and more. These procedures can serve as first-line therapies depending on patient pathology. Importantly, these therapies are alternatives to the traditional surgical and medical treatments that have a unique safety and efficacy profile particularly in poor surgical candidates and critically ill patients.
Minimally invasive IR procedures
The use of MR, US, CT, and fluoroscopy made visually guided procedures the forefront of innovation in patient care. The suite of tools and skills available to the IR physician made a wide variety of organ system interventions both safe, feasible, and practical. Some procedures offer a unique alternative to medical and surgical management for patients, while others are critical interventions that are instrumental to patient survival (Figure 1).
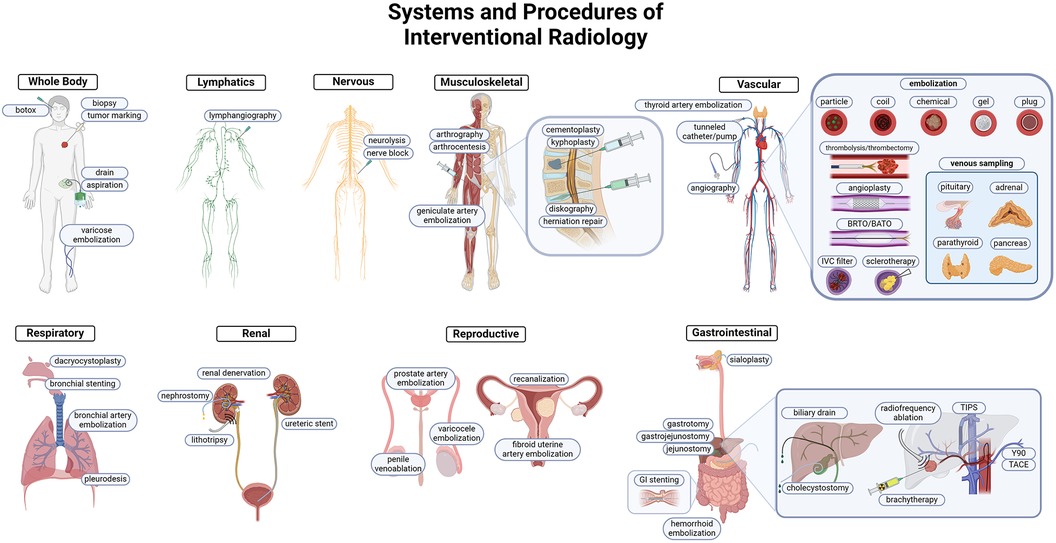
Figure 1 Overview of IR procedures. IR uses minimally invasive techniques to target pathology in every organ system. Each system highlights common and important interventions performed by IR. Targeting methodology commonly utilizes vascular access, and the prominent tools are highlighted in the cardiovascular system schematic. Both arterial and venous embolization, angioplasties, thrombectomy/thrombolysis, and venous sampling are integral to the IR toolkit.
General procedures
Biopsies
IR is the most common operator of biopsy procedures because of their historical success in obtaining quality samples with less risk than open surgical retrieval. Biopsies performed using US, MR, CT, and positron-emission tomography (PET) are extensively documented and findings show high rates of success with few adverse events (9). Needle biopsies are safer, cheaper, and faster and can achieve sufficient samples in most clinical scenarios. While some scenarios benefit from open biopsies with higher rates of diagnostic success, there is often sufficient indication to trial needle biopsies first due to their high safety profile. In biopsy samples where the target is perivascular or difficult to access percutaneously, trans-vascular approaches can be taken to biopsy the liver and kidney (10, 11).
This function has become increasingly important in recent decades in oncological treatment. Initially the value was in cancer staging but has expanded to pathological analysis of molecular biomarker testing including gene expression, biomarker expression, cell signaling pathways, and other tumor indicators of tumor heterogeneity. IR serves as a critical intersection between the oncologist's medical management and the pathological characterization of the tumor (12).
Image guided tumor marking
The precise marking of malignant lesions is a crucial step prior to surgical excision where margins can be challenging to delineate. Indication criteria have been generated based on the frequency of failed tumor localization or positive margins on resection (13). Wire and radioactive seed localization (RSA) are two techniques commonly used in breast cancer, with evidence showing RSA has improved outcomes for breast and lymph node biopsies (14–16). Other procedures where marking is indicated include pulmonary nodules, liver metastasis, hepatocellular carcinomas (HCC), and bone lesions. These IR procedures are valuable steps to minimizing follow-up surgeries and higher rates of complications.
Image guided percutaneous drains and aspirations
With symptomatic abscess formation, image-guided percutaneous drainage can obviate the need for surgical intervention. IR physicians have become particularly useful in the treatment of pelvic, subphrenic, epigastric, urogenital, diverticular, appendiceal, and hepatobiliary abscesses (17). Antibiotic penetration into large confined thick-walled abscesses is poor, and IR is an indispensable resource for the management of localized infections. Their interventions significantly reduce morbidity and mortality of complex septic patients and provide source control.
Hemostasis in trauma
IR has a growing role in providing hemostasis in trauma cases (18, 19). For acute hepatic laceration and hematomas, angioembolization is an approach to reduce the high rate of mortality. With excessive exsanguination, identifying the source of the bleed for operative repair can be impossible, allowing IR to both identify and embolize the source. With severe AAST (American Association for the Surgery of Trauma) liver grade IV or V injuries, IR intervention reduces mortality in conjunction with open surgery (20). In the setting of a hemodynamically stable patient with blunt or penetrating (e.g., gunshot wound) hepatic trauma, angioembolization can be sufficient for treatment for AAST II and III hepatic injuries. Based on this evidence, the Society of IR (SIR) has generated guidelines for the practicing physician to intervene in hepatic trauma (21).
Similarly for splenic lacerations, there is limited data to support either splenectomy or splenic artery embolization and often is dependent on center-to-center preferences. For Grade IV-V lacerations, embolization is often preferred with some evidence showing a decrease in infection risk without a change in mortality (22). Embolization can also be utilized pre-operatively for a planned splenectomy to improve operative time and reduce blood loss (23). However, the therapeutic appropriateness of embolization only applies for patients that are hemodynamically stable.
Pelvic trauma is another injury that commonly presents with organ compromise and associated pelvic vascular injury with high mortality rates. In cases with proven extravasation, selective and super-selective angiography can identify the origin of vascular compromise with simultaneously embolization. Thus, in many trauma centers the involvement of an IR team has become a vital component of their operation (24).
Vascular interventions
IR physicians have demonstrated advanced skills for vascular access and intervention. The ability to obtain high quality angiography for diagnostic purposes, and simultaneously deliver therapeutics offers an efficient healthcare delivery model for patients. There are a wide set of diseases where vascular access is important for treatment. IR consults with oncologists, urologists, gynecologists, gastroenterologists, pulmonologists, hepatologists, and other vascular specialists to address vascular pathology in common diseases in their patient population. IR procedures are often first line therapy for management, and newer procedures are becoming increasingly advantageous to other alternative therapies and expanding their role in patient care.
Central lines and ports
Hospital systems commonly utilize IR services for central venous catheters and tunneled lines/ports. Interventional radiologists can perform these procedures very quickly, cost-effectively, and with fewer complications than surgical placement. This contention is controversial. Given the direct methodology, the procedure can be performed successfully by other specialists and advanced practitioners. The procedure can be done safely in an outpatient setting as well. Many specialists would prefer to manage their patients internally, thus larger studies are required to better study the risks based on operator, setting, and equipment.
Endovascular angioplasty and angiography
IR was the original pioneer of the experimental angioplasty to restore vessel integrity, and since that inception has become a cornerstone of vascular therapies (1). The practice of vascular procedures has shifted over time. With vascular IR growing in scope and capabilities to perform endovascular interventions, some were concerned that they would impede the practice of vascular surgery. Vascular IR physicians are frequently the source of diagnostic arterial and venous angiograms for evaluation of vascular disease. Neither practice was disrupted, as the treatment of peripheral arterial disease still largely lies with vascular surgery. According to Medicare data, IR performs only 25% of these procedures (25). However, IR's role in this space has been growing incrementally over the years in the US (26). In Europe, IR physicians are often the primary interventionalist since they often have the hybrid OR requirements for endovascular procedures (27).
This is not to say that endovascular procedures are not competitive among physician specialties. There are many cases where IR physicians were frontier proceduralists in difficult endovascular diseases including heart and brain catheterization. The primary issue here is that they controlled the patients and once they were trained in the technique it was incentivized to manage the patients internally. Neurosurgeons also argue that they are the most qualified to manage neurovascular procedures and their neurocritical care to have the lowest rates of major complications (28). In practice, there is collaboration between specialties in patient care and intervention may be dictated more by hospital resources and staff (29).
Embolization
Embolization is the primary and essential tool in resolving many systemic diseases. The ability to percutaneously gain vascular access, and to achieve endovascular localization of pathology is essential to the benefits yielding IR. Significant research into embolic agents have provided more tools and options for IR to address unique pathology. Components range from coils, balloons, plugs, particles, liquids, and foams. The sub-specialization of equipment and combinatorial approach has refined the practice to confidently limit perfusion without causing ischemic damage to healthy tissue. IR can apply these agents to both arterial and venous systems as appropriate. While the success of these different agents can be operator dependent, they have exploded in popularity thanks to IR because of the safety profile and high efficacy rates (30).
Transvenous obliteration
In systems of recurrent venous bleeding common in portal hypertension where esophageal and gastric varices are distended, venous obliteration by IR has been shown to produce the most reliable results until the liver disease can be addressed (31). Venous obliteration may also be more effective than TIPS, provided it's a more localized solution to destroy the venous system to prevent further bleeds (32). Further variants based on operator preference, speed, costs, and effectiveness can be selected such as using a plug (plug assisted retrograde transvenous obliteration, PARTO), coils with gel foam (coil-assisted retrograde transvenous obliteration, CARTO), or can be approached from systemic circulation for anterograde obliteration (balloon occluded antegrade transvenous obliteration, BATO). These customization options allow this procedure to have a high rate of success with few complications when sclerosing agent is successfully directed to the target varix.
Balloon venoplasty and venograms
Because endovascular interventions to address venous obstruction have high rates of technical success, IR is offering first line approaches to resolving venous pathologies. Numerous etiologies cause venous fibrosis causing outflow obstruction such as central venous catheters, hemodialysis catheters, radiation exposure, trauma, or strictures. This may be symptomatic or cause interventional issues in gaining central venous access past the obstruction. Commonly IR will intervene in the subclavian, axillary, and brachiocephalic veins with balloon angioplasty or iliofemoral veins with balloon venoplasty with high rates of success (33). In the diagnostic evaluation of chronic venous disease, IR will obtain quality venograms.
Venograms are also important in diagnostic evaluation of portal hypertension to determine if the source of obstruction is prehepatic, intrahepatic, or posthepatic. Where Budd Chiari is identified, a combination of steps involving venoplasty, thrombus maceration, and stent placement is all within the procedural confines of venous reconstruction performed with IR (34). Successful drops in portal hypertension will prevent the patient from having surgical reconstruction and shunt placement.
Endovascular aneurysm repair
Vascular rupture from aneurysms signifies a life-threatening emergency from large vessels in the thorax, abdomen, and cranium. Large aneurysms were traditionally addressed with open surgical repair. Large structural compromise of the aorta or common iliac vessels can be stabilized with the placement of a graft. Endovascular aneurysm repair (EVAR) now represents the mainstay of elective treatment (35, 36). Smaller vessels can be reinforced with coils or clips delivered through intravascularly. Large tube aortic stent grafts via a percutaneous femoral approach have been validated to be a safe and durable alternative to open surgery. However, there is still a lack of comparative studies to create confident guidelines when either open or endoscopic approaches would be appropriate in elective settings.
Inferior vena cava (IVC) filter placement/retrieval
IVC filters commonly used for prior venous thromboembolism with contraindications for anticoagulation have become an IR dominant procedure. While they were traditionally placed by surgeons, advancements in minimally invasive fluoroscopy have made IR aptly trained for efficient and safe placement (37). Compared with surgeon or OR placement, IVC placement by IR has significantly reduced the cost, time, and complications of traditional surgical placement (38). Similarly, IR can apply similar techniques for the snare and retrieval of IVC filters for outpatient surgery.
Respiratory
Bronchial artery embolization
In the control of moderate to severe bronchial hemoptysis where medical management has failed, patients often need to resort to bronchoscopy or surgical treatment to provide hemostasis (39). Bronchial artery embolization has proven to be a safe and effective method to provide reliable hemostasis (40–44). While there is a high rebleed rate, the combination of more permanent embolic agents such as coils and polyvinyl alcohol or N-Butyl-2-Cyanoacrylate glue can ensure both immediate technical success and sustained hemostasis until the underlying pathology can be managed further. In emergent situations, IR is often the only effective bridge toward more definitive therapies.
Bronchial biopsy and stent placement
Interventional pulmonologists have specialized in the use of bronchial scopes for the placement of airway stents to alleviate malignant obstruction (45). IR can fluoroscopically approach an endobronchial biopsy sample for masses proximal to the airway, and if there is sufficient access, it is logical that IR place the stent to maximize efficiency for the patient. This has been found to be safe and effective, demonstrating that IR can save patients from undergoing multiple separate procedures.
Dacryocystoplasty
When the obstruction of the nasolacrimal duct causes epiphora, the definitive treatment is dacryocystorhinostomy. IR can offer an alternative dacryocystoplasty or stent placement that does not require general anesthesia to be performed (46). There are few absolute contraindications where neoplasm or dacryocystitis is observed, suggesting a broad population with epiphora could be offered this therapy as a first line therapy. While the long-term success rate is lower than surgical management, mild cases now have a safe and quick attempt to resolve the obstruction and associated symptoms.
Musculoskeletal
Arthrography
The injection of contrast into the joint space can provide enhanced CT or MR resolution for indeterminate joint disease. IR can inject contrast into the tibiofemoral, glenohumeral, ulnar radiocarpal, and tibiotalar joint spaces to provide increased sensitivity over CT or MR alone to correlate with physical exam finds (47–50). A positive finding using arthrography can sometimes prevent the more invasive arthroscopy. IR is particularly valued in pediatric arthrography with their advanced ultrasound navigation to guide contrast deposition.
Provocative discography
The injection of contrast into the nucleus pulposus to correlate patient symptoms and disc disease has been controversial for its historically high false-positive rates and questionable clinical utility after significant advancements in MR imaging modalities (51, 52). There is also a risk of damaging the annulus and increasing the risk of future herniation (53). Despite this risk, MR findings have a false positives and spinal fusion or laminectomy with discectomy carries significant postoperative complications. With advancements in procedural techniques and tools to control the contrast manometry, IR's role in this complementary test can provide unique insight into the etiology of back pain in the right patient population (54).
Vertebroplasty and kyphoplasty
The use of vertebroplasty for osteoporotic fractures was a common IR procedure used for pain and stabilization, but dropped in prevalence after the release of randomized controlled trials showing no statistical changes in these outcomes against sham placebo controls (55, 56). Further refinement in the procedure and inclusion criteria has allowed for continued use of the procedure and evidence of its effectiveness in reducing pain (57). This result is similar with kyphoplasty, where correction of distorted vertebral height relieve pain and limited mobility (58–60). Whether either procedure is superior is controversial and highly user dependent (61). IR should be consulted about spinal pain and the nature of their vertebral or disc pathology if they are a viable candidate because it can be performed quickly in an outpatient clinic to provide pain relief.
Geniculate artery embolization
In the treatment of medication resistant osteoarthritis, patients with evidence of neovascularization and synovitis can embolize this vasculature to reduce inflammation and further degeneration (62). This is a novel approach receiving some preliminary validation in small cohort studies, that demonstrates technical success of reducing synovitis and improving functionality presumably from pain reduction. While the long-term success of this procedure is unstudied, this could serve as a robust complementary therapy with corticosteroids, hyaline injections, and physical therapy to preserve articular integrity and function prior to arthroplasty.
Hepatobiliary
Tunneled peritoneal catheters
Tunneled peritoneal catheters provide a dialysis option for patients with kidney failure (63). Surgeons and nephrologists can place these catheters, where IR is only utilized around 5% of the time (64). Percutaneous placement has shown to be safe and effective, and also found to be more cost effective than hemodialysis (65, 66). While a minority of IR physicians place these, having additional imaging and fluoroscopic equipment can provide additional confirmation about proper placement (67). This percutaneous peritoneal access can also be used for malignant ascites, where this approach was more cost effective after several paracentesis (68). This another procedural opportunity for IR to demonstrate proficiency in this technique to benefit patients of oncological and kidney diseases.
Transjugular intrahepatic portosystemic shunt (TIPS)
When a patient with elevated portal pressures suffers from life-threatening recurrent variceal bleeding, TIPS is instrumental to minimizing future bleeds used in conjunction with beta-blockers, octreotide, vasopressin, and endoscopic ligation. TIPS can prevent bleeds longer than medications or endoscopic procedures alone due to the ability to equalize portal pressures (69–71). As a result, performed at early stages of cirrhotic disease is a cost saving measure that reduces the chances of a life-threatening bleed (69, 70, 72). While this does significantly increase the risk of hepatic encephalopathy, the clinical team balances the relative risks to mitigate immediate risks as the patient awaits transplant.
Yttrium-90(Y90)/transarterial chemoembolization (TACE)/brachytherapy
Interventional oncology plays a significant role in the treatment and management of hepatobiliary tumors from hepatocellular carcinoma (HCC), cholangiocarcinoma, or metastasis (73–76). Working in conjunction with the surgical oncologist, minimally invasive procedures focus on curative ablation, reducing tumor burden, or inducing contralateral hypertrophic compensation for lobe resection. The choice in using Y90 or chemotherapy transporting beads to tumor sites is variable depending on the institution and the preferences of the oncologist and interventional oncologist, but evolving evidence suggests that radioembolization produces more robust patient outcomes (77). Similarly, the implantation of a radioactive I-125 seed can provide localized radioactive therapy with positive results when used in combination with other therapies.
Tumor ablations
In the setting of hepatocellular carcinoma, minimally invasive techniques can be attempted as a curative therapy (78). Radiofrequency ablation targets a probe with low frequency currents that provide thermal stress to the tumor. Microwave ablations use higher frequency waves that generate heat stress inducing coagulative necrosis. Both techniques are frequently evolving and improving in their overall survival rates and data from several randomized trials are being collected. Current recommendations suggest only reserving this therapy for individuals who are poor surgical candidates, however growing evidence suggests that ablation techniques used in combination with radiation, chemotherapy, and surgical management.
Biliary drain
Percutaneous transhepatic access to the biliary tree offers an alternative route to alleviate malignant obstructive jaundice. Access is traditionally obtained through endoscopic retrograde cholangiopancreatography to drain excess bile and alleviate the obstruction. Currently both options are safe with high success rates and have comparable impact on patient outcomes (79–81). Preferences typically depend more on the hospital system and patient preferences. If the patient has altered surgical anatomy, IR performing biliary endoscopy and drainage may be the only feasible option due to surgically altered anatomy (82).
Endocrine
Traditionally IR has a limited role in treating endocrine related diseases. Recent experimental studies are investigating the utilization of thyroid artery embolization for the treatment of Graves’ disease and multinodular goiter (83). This therapeutic option is high risk with several reported complications including thyroid storm and cerebral infarctions (84). With proper patient selection and technique the procedure can be safe and effective for the treatment of nodular goiter to reduce thyroid volume and thyroid hormone (85). This technique is still under investigation and is not typically recommended unless patients are poor surgical candidates or fail other therapy.
Venous sampling can be an important procedural step when prior imaging by ultrasound and Tc99 scintigraphy fail to adequately localize the source of hypertrophy or hormone secreting tumor. IR physicians can be instrumental in gaining access to the venous drainage of the parathyroid, petrosal sinus of anterior pituitary, adrenal glands, or pancreatic veins of islet cells. This is often a secondary intervention due to additional financial and procedural exposure that would otherwise be avoided with proceeding surgery. In cases where imaging was indeterminate in hyperthyroidism, venous sampling could identify the calcitonin hormone reliably to improve management (86).
For primary aldosteronism, venous sampling has become an important diagnostic step in identifying unilateral or bilateral adrenal disease (87). By using CT and MR alone, there is poor ability to detect laterality of disease (88). Despite incidental adenomas, there is controversy about the utility after the SPARTACUS trial demonstrated similar outcomes to CT alone at one year. Regardless, the practice of adrenal sampling is the gold standard to confirm the source of hormonal secretion (89). Similarly, utilizing pancreatic venous sampling with calcium stimulation can improve the location of endocrine tumors better than angiography alone (90).
Lymphatics
Magnetic resonance lymphangiography plays a key role in various clinical scenarios. Most often there is a need to visualize lymphatic flow and structures such as the thoracic duct or cisterna chyli in plastic bronchitis, chylothorax, chyloperitoneum, and intestinal lymphangiectasia (91). In patients with postoperative lymphatic leaks, lymphangiography with sclerosing oils can be sufficient for reducing the leak (92). Larger leaks involving the thoracic duct can undergo embolization in lieu of surgical ligation with high rates of success without exposing the patient to surgical risks.
In the settings of cancer, visualization of the lymphatics can also help assess staging. Incurring the initial costs of obtaining the imaging can more appropriately stage breast cancer and maximize life expectancy and life extended per dollar spent (93). Similarly, lymphatic imaging can be utilized for proper staging in abdominal lymphomas, though PET-CT scans have become standard due to their increased resolution capabilities (94, 95). Thus, this is another tool in the IR kit to provide to oncologists to better manage complex cancer patients.
Gastrointestinal
Gastrointestinal stent placement
Gastroduodenal stent placement for gastroduodenal obstruction is a therapeutic option for malignant strictures or benign obstruction with other failed therapeutic trials (96). The role of IR for percutaneous placement is often addressed if transoral endoscopic options fail or are poor surgical candidates. The goal of stent placement is to resolve the obstruction and improve quality of life. Percutaneous access yields a high rate of success when performed via transhepatic access if biliary stents are required. IR is well suited for this procedure that can offer quick resolution of symptoms in a cost-effective manner (97). While surgical gastrojejunostomy remains the gold standard for the longest resolution of symptoms, stenting is a therapeutic option that may be beneficial for oncology patients.
IR can also provide obstructive relief for colonic obstruction (96). Endoscopic stent placement is often sufficient in distal obstruction, but factors can prevent adequate access to proximal colonic obstruction that would necessitate urgent surgical intervention. IR can provide a last resort option for fluoroscopic stent placement using wires through the anus with a high level of success in relieving obstruction (98, 99).
Percutaneous radiological gastrotomy (PRG)/gastrojejunostomy/jejunostomy
IR physicians are skilled at placing percutaneous radiological gastrotomy and gastrojejunostomy tubes for enteral nutrition (100). Radiologic placement has proven to be as safe and effective as endoscopic placement with a reduced risk profile relative to surgical placement (101). Whether IR places the gastrotomy tube or not usually depends more on the institutional resources or contraindications, as no clear advantages have been established (102). The comparative costs can also be quite variable, though Percutaneous endoscopic gastrotomy (PEG) is either of similar or higher cost as this is more frequently done in an operating room suite (103). In terms of average CMS reimbursement rate, the 2022 facility CPT codes currently list PEG and PRG tube placement to be $203 and $204 respectively.
Renal
Nephrostomy/nephro-ureteral tube & stent placement
IR physicians are instrumental in the treatment of urinary obstruction. Interventional oncology is frequently consulted as chemotherapies, radiation, and tumors are common etiologies of obstruction. IR use of ultrasound and fluoroscopy allow for high fidelity placement and replacement of nephrostomy tubes. IR performs a significant volume of nephrostomy tubes, comprising over 90% of nephrostomy tube claims according to Medicare data (104). When percutaneous access is gained, IR can do simultaneous pressure measurements with the Whitaker test to complement diuretic renography. Compared with nephrologists or urologists that may be achieving access blind or with ultrasound, the fluoroscopic feedback allows for safer and more cost-effective placement. Urology is more often the operator for the placement of nephro-ureteral tubes and ureteral stents (104). When stent placement requires retrograde access, urology has more expertise to operate, but IR can use similar methodology when tube and stent placement is delivered anterograde with percutaneous access.
Nephrostolithotomy
With large obstructive stones, percutaneous nephrostolithotomy remains the most efficacious technique (105). The stone can be disintegrated such that small pieces can be removed from a dilated nephrostomy tract. Nephrostolithotomy staghorn calculi lead to faster recovery times, fewer infections, and with longer stone free periods (106, 107). Pediatric populations also benefit from this procedure for stone resolution (108). This technique while carrying nontrivial risks of bleeding, an experienced IR physician can minimize complications.
Suprapubic cystostomy
Prolonged bladder outlet obstruction leads to hydronephrosis, kidney failure, and parenchymal fibrosis. In pelvic trauma, posterior urethral injury is a frequent occurrence that prevents foley catheter placement. While attempts are made for early primary realignment, the gold standard to treatment is cystostomy with delayed urethroplasty (109, 110). There is also evidence that percutaneous suprapubic cystostomy by IR may be preferable to repeated catheterization or surgical cystostomy (111). Even in clinical scenarios requiring short-term bladder drainage, suprapubic catheters had less bacteriuria, pain, and recatheterization without reports of elevated risks or complications (112, 113). These benefits to patients are also seen when replacing indwelling catheters, with fewer catheter associated urinary tract infections (114). IR operators are key to keeping complication rates low to make this alternative equally safe to perform.
Renal denervation
Ablation of the nerve plexus of the renal artery is an interventional technique to address resistant hypertension (115). Randomized control trial SIMPLICITY HTN-2 demonstrate efficacy in reducing systolic blood pressure, however SIMPLICITY HTN-3 which included a sham control trial did not show any added benefit (116). The SYMPATHY trial did not show benefits over medication alone, though medication compliance was inconsistent between trial arms (117, 118). While the procedure has been demonstrated to be safe in all trials, the efficacy of the procedure with current techniques lacks the evidence necessary that it is an effective form of hypertensive management.
Integumentary
Cosmetic procedures have been a growing and lucrative field of medicine that has been a growing frontier for IR (119). IR focuses on vascular anomalies that cause undesirable dermatologic changes such as vascular malformations, varicose veins, and spider veins. Cutaneous vascular malformations can be embolized successfully in similar fashion to other vascular procedures where surgical removal is unnecessary or infeasible (120). Varicose vein treatment has been transformed by the rise of percutaneous interventions, where surgical ligation and stripping can be substituted with laser ablation or radiofrequency ablation with similar efficacy (121). There is a dearth of available evidence regarding specialty specific performance, but IR is well suited with the skills to manage these patients.
Reproductive
Uterine fibroid embolization
Embolization of large uterine fibroids has been utilized in the treatment algorithm for uterine-preserving conservative medical management. The procedure has been gaining in relative popularity compared to myomectomy and endometrial ablation (122). Various studies have shown that fertility can also be conserved in the majority of patients despite potential compromise to endometrial vasculature and radiation exposure (123). Thus, embolization competes for utility among myomectomy and hysterectomy as the definitive treatment of abnormal uterine bleeding. While there is a general increase in the re-treatment for embolization, they generally have great responses to the procedure with minimal complications and a shorter hospital stay relative to either procedure (Table 1).
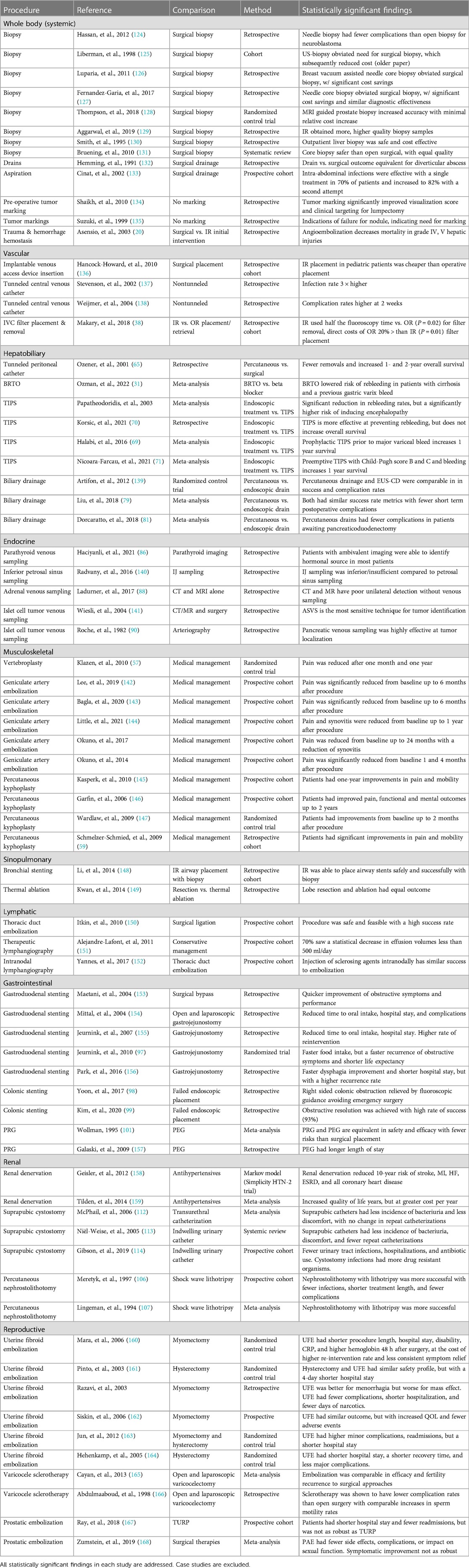
Table 1 Review of comparative studies of IR procedures. Only comparative studies on IR procedures investigating surgical or medical alternatives are included in this review.
Fallopian tube recanalization
Fallopian tube obstructions are caused by tubal spasms, pelvic inflammatory disease, endometriosis, polyps, congenital malformations, salpingitis isthmica nodosa, or intratubal debris is a common primary or secondary etiology in in infertility. Proximal obstructions are easily accessible for recanalization. Hysterosalpingography can successfully identify the origin of the obstruction and catheterization past the obstruction can be sufficient to provide clear passage to the infundibulum. Successful pregnancy and delivery rates have been reported as a result (169, 170).
Varicocele sclerotherapy
The therapeutic options for varicoceles that are painful or causing a reduction in fertility include open and laparoscopic varicocelectomy or anterograde/retrograde sclerotherapy (171). When comparing embolization to surgical options, there is a comparable alleviation of symptoms and increase in fertility rates (165). Sclerotherapy has an advantage in that this can be done quickly and minimally invasively, and therefore have fewer associated costs (172). Surgical options may be better when dealing with severe cases to prevent higher rates of reintervention.
Penile venoablation and angioplasty
Vascular causes of erectile dysfunction can be addressed by IR physicians. In cases of arterial claudication, the delivery of drug-eluting stents in the internal pudendal arteries or common penile arteries can restore erections. In venous pathology, embolization of the dorsal vein can also regain functional erections using ethanol, coils, or balloons (173–177). For patients with severe dysfunction due to venous leakage resistant to medication, endovascular therapeutics are often the best option.
Prostate embolization
There are several potential treatments for benign prostatic hyperplasia (BPH) including medical management, surgical transurethral resection (TURP), laser vaporization, and open prostatectomy. There is a major complication risk of causing erectile and ejaculatory dysfunction as a result (178). Prostatic artery embolization does not carry a similar risk, while also being comparably effective for BPH treatment (179). Compared to surgical options, prostatic embolization had faster recovery times and fewer complications, but was not as robust in alleviating symptoms (167). IR can offer a therapeutic option with fewer risks and symptom relief in milder cases that have failed medical management.
Medical and financial value of minimally invasive procedures
Training in minimally invasive procedures equips the IR physician with a wide toolset in techniques and equipment that enables intervention throughout the whole body. IR consults with specialists ranging from gastroenterologists, nephrologists, gynecologists, urologists, pulmonologists, and neurologists. IR also has advanced relationships with oncologists, serving as a consultant for disease diagnosis, central venous access, monitoring, and therapeutic procedures. There has been a concerted effort in the field to demonstrate the value added to medical care through these procedures in terms of patient outcomes.
Therapeutic value
The procedures performed in IR are valuable to many medical subspecialties. There are many procedures exclusive to IR that offer alternative minimally invasive options to surgical or medical intervention. There is a large library of evidence that these IR procedures provide comparative advantages to these surgical and medical management (Table 1). When there are cross-specialty operators, IR's specialty training can yield better results than alternatives including biopsies, pediatric implantable venous access devices, and IVC filter placement (38, 129, 136). IR's performance in biopsy has made them the predominant operator for all biopsies at most hospital systems over the past 20 years.
Comparative studies and meta-analyses of IR procedures show common therapeutic benefits. Patients have shorter recovery times, commonly due to pain being successfully managed with local anesthetics and moderate sedation alone facilitating timely discharges. Procedures have fewer complications and infections, because even laparoscopic surgery has more sites of bacterial access and risk of damaging internal structures. Procedures can be more robust than conservative medical management, and while procedures like geniculate artery embolization, renal denervation, kyphoplasty, and nephrostolithotomy do introduce bleeding and infection risk, they offer more robust effects without exposing the patient to the risks of full surgical management. In interventional oncology, palliative procedures are faster and safer than surgery.
IR procedures do have their limitations, in that many times their benefits are often temporary. Surgery for uterine fibroids or prostatic hyperplasia offers definitive resolution, where embolization may require repeat trials. In palliative care, placing stents in bronchial tree or within the GI tract may provide immediate relief, but the tumor's growth will quickly obstruct the stent and require surgery. Patients must then choose between a permanent and expedient solution. The value of offering choice to the patient and specialist to treat their illness cannot be understated. The heterogeneity of presentation and physician preferences allow for greater future optimization of patient care and comfort than ever before.
Financial value
The ability of IR to reduce costs is beneficial to all the healthcare stakeholders. There have been studies comparing the financial differences between IR procedures and surgical procedures that generally show greater financial value to patients, hospitals, and payers (Table 2). Similarly, using IR physicians for procedures where they are most competent also saves money relative to other operators (38, 199). However, these benefits may be limited to the organizational structure of the hospital system and reimbursement model (202, 203).
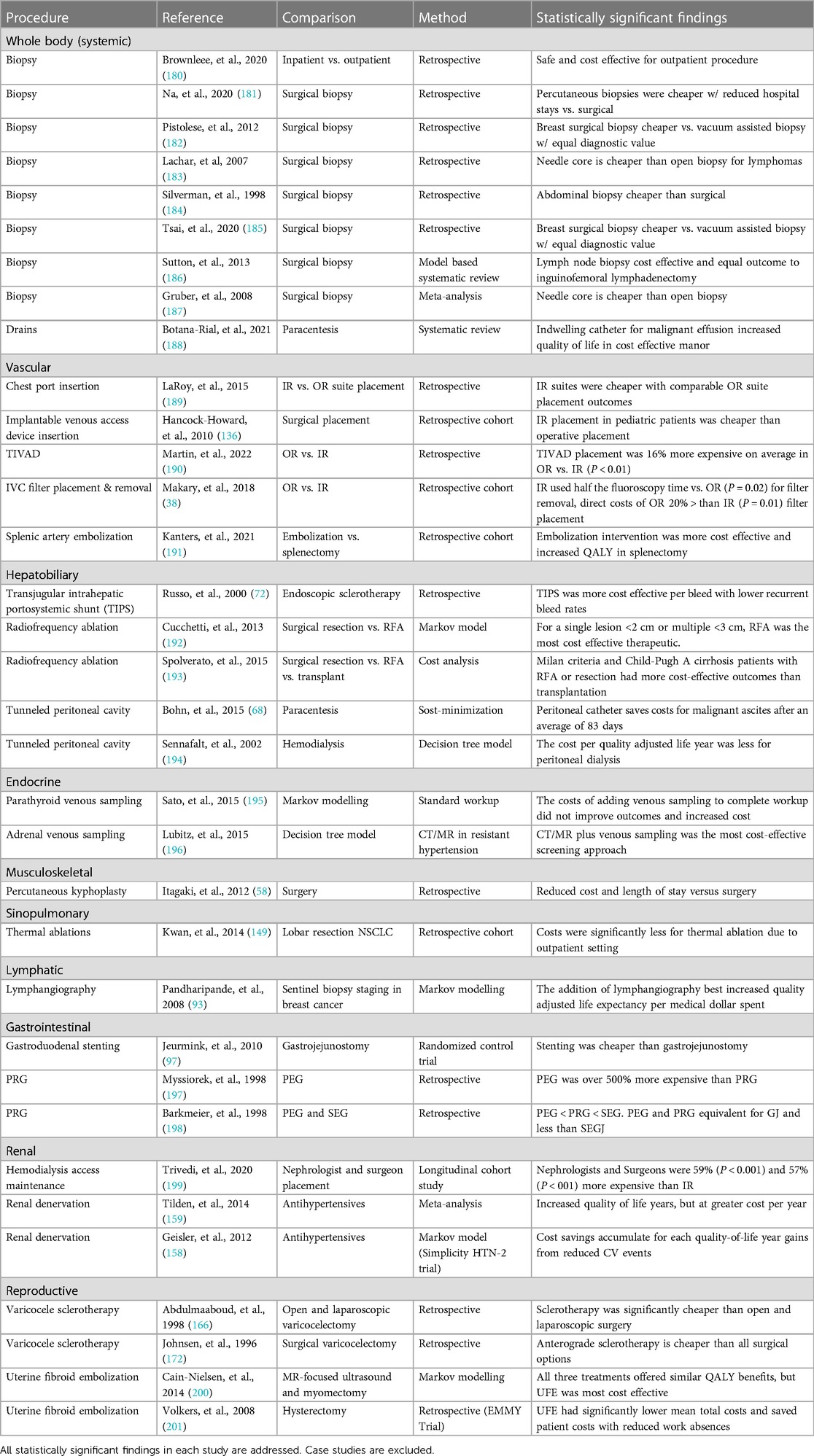
Table 2 Review of comparative economic studies of IR procedures. Only comparative studies on IR procedures investigating surgical or medical alternatives are included in this review.
The common cost saving measures of IR procedures are the facilities. IR suites are typically less expensive than OR suites. Similarly, local anesthetic and moderate sedation is cheaper than having an anesthesiologist present. Because there is less anesthesia and manageable pain, these patients can confidently be outpatient, saving on overall patient costs. IR procedures also achieve greater patient value over medical management because they can be more effective at treating disease over long-term analysis of cost and quality adjusted life years. Collectively, the data demonstrate the utility of the IR physician to be a value driven member of the medical team.
Research and innovation
IR is a young field with a prominent history of procedural and technical innovation. IR's success has been centered on the ability to develop new less invasive strategies to benefit patients. Innovation is one of the cornerstones of IR, with physician motivation, their fascination with novelty, venturesomeness, and mentorship have made this specialty one of leading drivers of change in medicine under the principle that, “less is more” (204). Because of IR's practice touching most other specialties in medicine, they are well positioned to both understand and identify limitations in the management of disease processes and imagine solutions without the burdens of conventional dogma (205).
The current areas of innovation in the field focus on new applications for existing procedures. New “embotherapies” are novel applications of localized ischemia or necrosis in disease treatment (206). Similarly, immuno-oncology will utilize IR for localization of new targeted therapies for chemotherapy delivery and gene therapies. IR is also exploring new ways to visualize their procedures. The expansion of tools available for virtual reality and augmented reality allow further evolution on how anatomy is displayed during the procedure (207). With new visualization comes with new tools for recognition and with the growth of artificial intelligence tools in radiology, there is a growing need to focus efforts on applying these tools to high yield areas (208).
While the initial development of IR procedures was overshadowed by other specialties adopting these techniques, the future is optimistic for the expansion in their value to medicine. The preliminary techniques were easily adaptable relative to open surgery, but the evolution of advanced tools to challenge the limitations of image guided micro procedures will require advanced nontransferable expertise. Novel IR procedures introduced are often initially expensive and difficult to garner support for innovation at an institution. However, advocating for the long-term impact of new procedures that decrease morbidity, mortality, and overall cost will reinforce the goals of modern healthcare systems to reduce healthcare expenses.
Financial contributions
Direct contributions
IR makes direct contributions to patient care. Diagnostic and therapeutic interventions are directly reimbursed by healthcare payers. These procedures generate billable relative value units (RVU) that go toward directly reimbursing the facilities, labor, and equipment costs. The forces guiding these procedures are not only guided by internal factors of the skills of the practitioners in the practice, but also the external needs of the healthcare system and the contractual relationships that are formed (209). These can be quite variable over time, both in volume and in scope of practice. Thus, gaining the appropriate credentialing, equipment, and expertise to serve these obligations best serve patients and generate the most revenue for your responsible parties.
An expanding field for generating revenue has been in the direct contributions in the role of evaluation and management. The utilization of skilled advanced practitioners, such as physician assistants, nurse practitioners, and radiologist assistants, have allowed a longitudinal model to be financially viable with alternative value-based healthcare reimbursement models (210, 211). With their unique billing codes, IR practices can better utilize their physicians for highly skilled tasks, without passing on direct longitudinal clinical activities.
Indirect contributions
The indirect contributions of IR are the collaborative nature of the field. As the microinvasive proceduralist, they can collaborate with most other specialties in the hospital setting to bring new and specialized procedures for their patients. Because of their efficiency and general low risk, low cost, and faster recovery profiles, patients generally have better experiences that can be passed through their facilities. While most procedures could be technically handled by other specialties, expert operation also frees up the other specialists to focus more on disease management and planning. IR also plays a role in this consultation for diagnostic interpretation, to participate in monitoring and assessment. Furthermore, with IR broadening its public accessibility, IR can also work with other specialties to drive patient flow toward their hospital system. Between the expert consultation, specialist collaboration, procedural efficiency, optimizing patient experience, and directive of patient flow, IR makes the whole healthcare system run more effectively by being a flexible participant in patient care.
Revenue analysis of IR reimbursement models
The reimbursement paradigm can have a significant impact on the generated revenue for IR physicians, their department, or their practice. While the standard model of reimbursement has historically been a fee-for-service (FFS) model, the implementation of the Patient Protection and Affordable Care Act (ACA) in 2009 has been a driving force for the adoption of alternative reimbursement models. The ACA developed the National Quality Strategy through the creation of the Agency for Healthcare Research and Quality (AHRQ). This agency dictates the payment policy for the Centers for Medicare and Medicaid services (CMS). These services comprise approximately 36% of all national healthcare spending in the United States (212). Over the last decade, the ACA has been progressively shifting toward “alternative payment models” to de-incentivize high throughput service with complications. These include the following: value-based care (pay for performance), accountable care, bundled payment, patient-centered medical home, and capitation. Each of these models operate in parallel in the modern healthcare system and have a distinct impact on how the IR physician can practice.
Fee-for service model
FFS is still the most common reimbursement model that exists today. Most healthcare organizations today still use this model to generate over 50% of their revenue, and private practices often generating over 75% of revenue from FFS (213). The reimbursement model is based off the current procedural terminology (CPT) codes that assign relative value units (RVUs) to compensate the physician work, practice expense, and professional liability insurance (214). RVUs are adjusted by a geographic practice cost index, and multiplied by the annually updated conversion factor ($34.61 est. 2022) to determine the reimbursement for each CPT. In IR, the bulk of the FSS billing codes are procedural, and heavily rely on these RVUs for compensation. There has been a consistent downtrend in reimbursement rates due to reductions in the conversion factor, as well as reducing the total RVUs for surgical procedures across many surgical subspecialties. IR is no exception, with −2.8% inflation adjusted annual decreases in compensation averaged across common IR procedures (215). This change has had a direct impact on the revenue IR physicians generate when focusing heavily on surgical interventions.
In IR, the RVU rates are frequently not representative of their institutions' realized costs. There is a current shift away from the RVU model for calculation to a Time-Driven Activity-Based Costing model adopted from other industries to approximate costs bottom up (216). When observing ports, biopsies, or clinic visits, a process map is generated through various observational and retrospective analysis to estimate costs for personnel, equipment, and consumables for each event. While a newer development, these studies are currently taking place, such as evaluation of hepatocellular carcinoma treatments for interventional oncology (217). These models highlight how some of the actual costs could be off by over 50% to where the RVU value is currently set (218).
IR physicians have also been impacted by the current FSS schedule for their imaging interpretations, as currently only the most expensive image is reimbursed in full, while every following scan by the same provider on the same day is provided 75% of the standard rate (219). This can be a common occurrence when evaluating for intervention. Similarly, the ICD-10 codes are expansive to allow for diagnostic specificity, but this can also lead to a significant increase in reimbursement delays or denials if coded improperly. Because these codes are frequently evolving and growing, inaccurate coding is one of the most common causes of delayed revenue. There are clear benefits to the IR physician and their group to understand the expected expenses and revenue for different procedures to project gross margin and optimize the efficiency of the team.
Value-based payment
The value-based reimbursement model was designed to target healthcare waste and reduce the per-capita cost of equivalent healthcare outcomes. In this model, the creation of accountable care organizations are dedicated healthcare professionals that work with providers to investigate current practice to minimize costs while optimizing care. Any shared savings to the Medicare program accrued by participating organizations would then share a proportion of the savings. This has been an opportunity for IR physicians to demonstrate that their procedural expertise will result in significant cost savings that will benefit the healthcare system. There has been an active effort over the past 15 years among radiological societies such as SIR, to ensure that IR physicians have a voice in the restructuring of healthcare payments (220, 221).
There has been an increasing push for more research to demonstrate the financial and clinical value of IR procedures (222). The comparative effectiveness research initiatives have been undertaken to train, publish, and disseminate findings of how IR procedures impact patients and the healthcare system. The American Recovery and Reinvestment Act of 2009 offers extramural sources of funding for physicians to conduct effectiveness research to better inform the implementation of a value-based model. There have since been consensus panels to help guide a standardization of the process for future investigation to ensure findings are reproducible and comparable (223). Specifically, IR costs in healthcare delivery has also been previously addressed in consensus panels to both review past work and further conceptualize how effectiveness research will benefit the field (224). SIR, Radiological Society of North America, and the American Society of Neuroradiology have cosponsored international training efforts to provide IR physicians to have the skills to perform comparative research.
With a shift from an FFS model to a value model, there is increased importance on finding more ways to meaningfully interact with patients outside of an operating room to provide value to the healthcare system. This has been the increasing push for clinical IR practice, where more face to face clinical interactions with the patient provide increased value (8, 222). With the help of advanced practitioners, there is increased revenue to be acquired in a value-based healthcare system. This function in addition to interdisciplinary collaboration has a greater opportunity for monetary reward, especially with prior practices potentially undervalued or simply not compensated in an FFS model (225).
Bundled payment
The bundled payment mode driven by the Bundled Payments for Care Improvement (BPCI) Initiative is designed to provide a prearranged payment for an episode of care. All services provided to the beneficiaries would be linked to this payment. Depending on the model, this includes prospective or retrospective payment to cover inpatient stay and related services up to 90 days post-hospital discharge. If IR procedures are reimbursed with this model, the practice's gross margin will be dependent on that patients' co-morbidities and procedural risk. Studies of readmission rates for common IR procedures have shown that there is a high 30- and 90-day readmission rate (15%–50%) (226). A set payment will encourage high-quality care to minimize the exposure to unnecessary complications. This may conversely provide perverse incentives to avoid patients with co-morbidities and high-risk procedures because of the large financial downside risk and result in patient neglect.
IR physicians should strive to optimize their utilization of outpatient procedures. For example, angiography can be done safely as an outpatient and reduce the average number of patients filling hospital beds overnight (227). New research into safety should be evaluated periodically to find new ways to expand the value delivered to patients. Similarly, IR physicians have a responsibility to challenge the status quo regarding the use of OR suites when appropriate. For example, placing a femoral venous catheter could be done safely and effectively at a patient's bedside. Cost savings include the facility, time, and improved patient experience through convenience (228). Similarly, applying procedural techniques at bedside for IVC filters, PEG tubes, and dilatational tracheostomy can be done faster, cheaper, and with immeasurable changes in risk for the patient (229–231). An explicit goal of IR includes the discovery of new methodology to improve efficiency in patient care.
Capitation
The capitation model is when a fixed payment is provided for all necessary patient care costs over a set period. While initially implemented in health maintenance organizations (HMOs) managed by insurance companies, the ACA payment system adopted the system that includes quarterly adjustments for clinical outcomes and patient satisfaction (232, 233). IR has been diversifying the setting in which procedures can be safely performed, including outpatient clinics. If IR physicians offer a therapeutic option that is cheaper and safer than the alternative surgical options, physicians may be in a position where they will be capable to thrive in a capitation model (6).
Environment for successful practice
For those looking to build or establish a successful department of interventional radiology or private practice, there are factors that should be addressed to fulfill best practice expectations. There have been published standards of management and care that have been proposed as helpful guidance for practitioners in patient management and associated tools required (234). These published sources represent educational tools and not legal standards by which healthcare professionals can be adjudicated. Thus, providing updated and evolving reviews of important parameters is important to continued success of the field.
Team members
The IR physician is responsible for patient clinical management and procedural performance. Supporting team members include advanced practice providers (APP), nurses, registered radiologist assistant, radiologic technologist, a certified medical assistant, and an administrator. APPs consisting of nurse practitioners and physician assistants have been growing in their scope of practice to facilitate care in both patient care and procedures, and under CMS can bill patients under unique ID numbers. This has been shown to boost productivity for the physician for other tasks (210, 211). With sufficient patient volume, APPs can make a substantial impact on patient care and generate revenue.
Interventional radiology technologists are essential for procedures, with certifications to act as a scrub technician and radiologic technician. They serve the role of a surgical assistant by procurement and organization of surgical tools and wires, while also being proficient in the operation of the C-arm Cone-beam CT scanner, powered injectors, and associated software. The addition of a float technician to retrieve additional tools is required as many procedures are dynamic and are best suited to communicate and retrieve the proper equipment. Similarly Registered Radiologist Assistants can provide similar value from the imaging services. Under supervision, they can perform tasks related to patient management, assessment, and preliminary imaging observations. They can help protocol or coordinate with medical imaging technologists to streamline the process of acquiring proper imaging studies of diagnostic quality.
Nurses serve essential functions as a liaison of patient care and communication. For operations, they can gather history, screening, and vitals. For operations requiring sedation, they provide medications and monitor the patient's status. Lastly, they can follow-up with the patient regarding education, wound management, and updating family members. The managerial role of coordinators includes triage referrals, assist with research protocols, and general scheduling or consultations. Certified medical assistants are an adjuvant for nursing functions. These individuals require less advanced education and certifications to provide basic functions that may become challenging due to the volume of activities performed if there is high patient volume. IR teams should staff reflexively to procedural demand and have feedback from nursing staff accordingly to ensure the safety and completeness of patient care.
Lastly, administrative functions are extremely important and responsible for scheduling, precertification, procedural coding, claim submission, structured reporting, and quality improvement. While these functions are fluid, physicians need to provide training in these operations to ensure limited down-time or interruptions in reimbursement. The administrative burden will vary based on inpatient or outpatient clinical practice, and the IR physician can prospectively plan to the expected workload.
Facility requirements for clinical and operational practice
To provide interventional procedures, an operating room with the addition of imaging equipment creates a hybrid operating space. Several guidelines have been published and construction is usually dictated by local regulatory standards, and hybrid models for minimally invasive procedures have proven to be safe and efficient (235). Specific equipment that are important for most vascular procedures include: large image monitor, Cone-beam CT scanner with biplane imaging and 3-D angiography, C-arm compatible table, pressure injectors, and ultrasound. For biopsy procedures availability of a wide bore CT-scanner with CT fluoroscopy can prove to be beneficial based on physician preference and patient size. With this equipment, software functions involve image capture, image modification, and digital compatibility with the picture archiving and communication system (PACS). Radiation safety equipment is also required, including radiation dosimeter, lead vests with thyroid cover and lead glasses, and radiation shields proven to reduce scatter exposure (236–238). With procedures requiring moderate sedation or general anesthesia, there should also be a post-anesthesia recovery space equipped appropriately to manage complications associated with sedation (239).
In the clinical or outpatient setting, the requirements needed for practice are less specialized than other medical practices. Most of the consultation and follow-up functions can be served by standard clinical offices. Additional tools important for function may also include the close access of ultrasound machines with doppler, vein light, high resolution monitors with multiple displays, dictation/transcript capabilities, and PACS integration.
Physician recruitment and retainment
If you are building a practice or department, you will need to recruit new IR physicians in a competitive market. Thus, these elements are important for your team to communicate to the IR community such that interested qualified candidates would be interested in joining your team (Figure 3). Beyond entering your team, you want them to be satisfied and be a part of the future growth of that team.
Expectations
IR daily activities can be highly variable and dependent on their training and the priorities of the hosting institution. All parties would benefit from clarity in teaching obligations, group governance, mentoring, incentives, benefits, and scope of practice. Having a detailed conversation about the details of the job obligations will guide performance and satisfaction from the physician. When discussing the clinical duties, there should also be details for logistics including patient population, case logs, call schedule, case mix, and site locations. A discussion about telemedicine can offer a viable alternative to outreach facilities if such methods exist in your program. This can be an appealing feature, as there can be an increase in work efficiency for certain contexts. If there are research interests from a faculty member, the funding sources, available startup funds, and protected time should be clearly stated.
Compensation and incentives
Having clear incentive structures will function to both attract and retain new talent. The salary structure should clearly articulate whether this is a straight salary, salary plus bonus, equal shares, pay-for-performance, productivity wRVU based compensation, or a blended model. New physicians want to know how their salary would be at risk for extenuating circumstances such as reduced patient volume, disability, childcare, or illness. If joining a private practice, there should be a discussion about partnership, and what the buy-in requirements would involve. Additionally, the IR team needs to discuss the conditions for a new physician to be eligible for other financial incentives that involve payments from call coverage, committee work, or other non-clinical activities. Other non-salary benefits could also include disability protection, signing bonus, malpractice coverage, CME (Continuing Medical Education) credits, relocation reimbursement, and debt repayment.
Exploring Non salary quality of life benefits may also overvalue standard financial offerings. Avoiding seven-day weekly call coverage can be desirable even in low volume settings. For junior attendings, having mentorship options available help them feel more secure when consultation is available for patient and career decisions. Selling the setting of the practice can appeal to physicians with families where the cost of living, real estate, education and recreational opportunities, ease of commute, or popular family career options. Lastly, offering vacation scheduling for major holidays and ease of scheduling can mean that physicians can derive more value out of the vacation days that are provided.
Career opportunities
IR physicians seek positions where there is support for career growth. In academia, having discussions with the team and department heads about mentorship and professional development should be established early in the physician's new position. Clear expectations about joining committees, additional training, and certifications. Furthermore, offering time allocation and infrastructure support by the team will help launch new investigative projects and yield more productivity from your team. Private practice physician groups must clearly delineate the requirements for that physician to earn partnership rights.
Facilities and infrastructure
It is important that physicians feel that they have sufficient equipment and facilities to practice. Present the supporting equipment and facilities that your practice has available, including the basics discussed in this paper and any additional unique assets, such as office procedure rooms, hybrid OR equipment, fluoroscopy suites, and extensive endovascular equipment inventory. This data is valuable to provide, as the physician will need to know if the resources available will support his desired procedural workload. If the department shares space with other specialties, anesthesia is short staffed, or the time can be difficult to schedule, it may be challenging to promote junior attendings to join where limited resources will be disbursed based on seniority. Similarly, the quality of life can be negatively impacted for the IR physician if available block times are limited to Friday afternoons or weekends.
Personnel allocated for both administrative and clinical capacities should be available for ancillary support for new physicians. Given program variability, stating the presence of scribes, IT support, electronic medical record system and training, and coding responsibilities can provide an edge for your program's recruitment efforts. For value-based reimbursement systems, quality support for reimbursement will also impact income or administrative workload for physicians.
Lifestyle
Promoting a healthy lifestyle can benefit the happiness of junior physicians while also benefiting the IR practice. Offering IR suites only at unpopular times, a disproportionate call allocation, poor schedule flexibility, and limited vacation time will have an impact on that individual's moral and team synergy. If they are highly desired by the new team member, the practice can leverage other financial incentives that will reduce expenses. The physician will be happier and perform better with the team.
Inclusive hiring practices
The advertising and position details should be designed to be inclusive and appealing to all qualified applicants. IR has historically been a male dominated specialty, and a 2015 UK census has shown female labor force participation around 10% (240). The commonly cited concerns about focusing on IR were work/life balance, risks of radiation exposure, effect of pregnancy on training, and the male-dominated work environment (241). Advertising efforts to promote the position should make efforts to address what is being done in these areas of concern such that any concerned party can be aware how these obstacles can be overcome. Some non-financial benefits that improve quality of life can also attract unique qualified applicants that have other personal obligations that could be better balanced with a more flexible schedule.
Environment
The cultural environment impacts team performance and ability to recruit and retain physicians. Poor morale can develop due to deficits in any listed domain. The hostile attitude will infect the expanded team including nurses, advanced practitioners, technicians, and trainees. Dedicated efforts must be made to audit the individuals and factors that corrupt overall morale because it will significantly hinder current performance, ability to grow the staff, and influence of the practice. Outside the department, greater hospital incentives such as collaboration facilitate IR ability to add value to patient care. If specialties are protective of their patients, it will be challenging to maintain growing patient flow. Lastly, having a positive active community with the public helps the physician feel fulfilled. Highlighting how new members of the team can enjoy the city culture can ensure that time spent outside of patient care makes them appreciate their work.
IR marketing
Like other businesses, there needs to be a concerted effort to get patients through your practice to grow your influence and provide work and revenue for your physician team (Figure 2). However, there are unique methodologies to growing an IR practice that are less draining on finances compared to paid advertising (242). First, in promotional opportunities be sure to promote the whole hospital system and the suite of programs that is offered. Overall awareness and recognition may lead to future referrals. If patients learn about your hospital system and utilize bariatric surgery, they might be good candidates for bariatric embolization, vertebroplasty from joint degeneration, or candidates for fibroid embolization. Similarly, oncological referrals can be high yield opportunities to intervene and help this population, as broadening the awareness of treatment options will make them more apt for Y-90 referrals. Experience has shown that utilizing your available network of physicians is an effective marketing option.
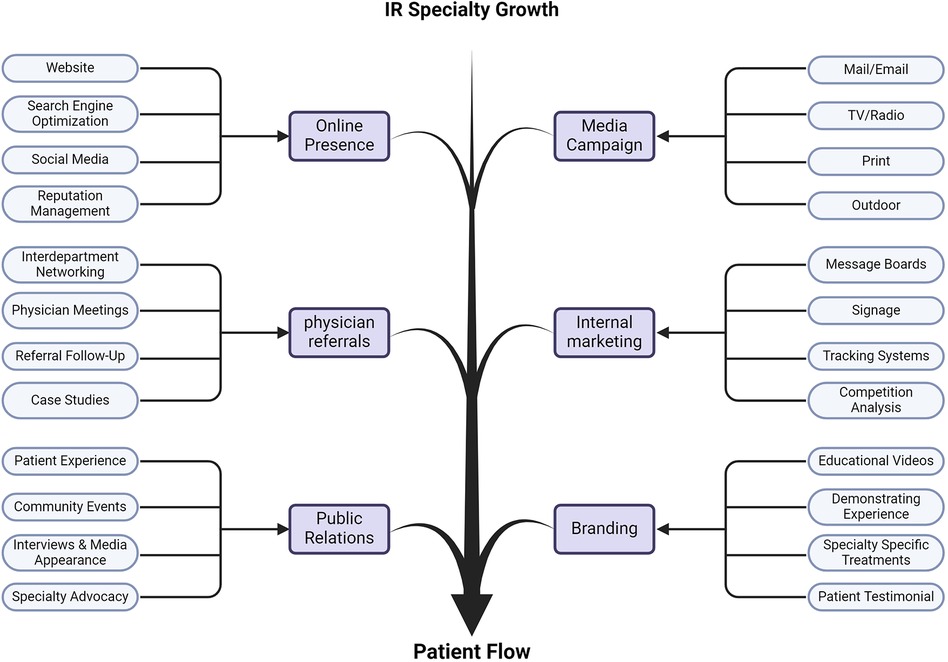
Figure 2 Expanding the value of IR. Figure outlines the various dimensions IR physicians and practices should address to grow the influence and importance of IR in the medical system. Approaches range from maximizing your visibility, to general societal and cultural branding. These dedicated foci require active participation to be successful and drive more patient flow through IR to benefit from IR innovation.
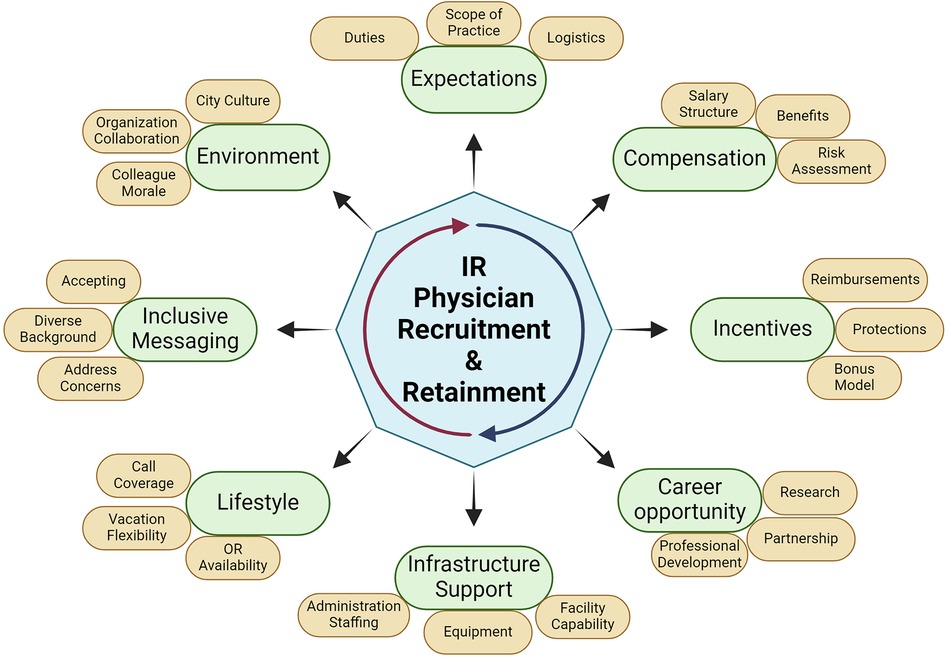
Figure 3 Model of IR physician recruitment and retainment. Each domain addresses an essential element in growing an IR practice that can operate successfully in the modern healthcare system. These elements begin with clear communication (expectations), followed by individual needs (compensation, incentives, career opportunity), followed by sociocultural desires (support, lifestyle, messaging, environment). Teams must periodically re-address these domains to accommodate change. Efforts in these areas ensure the team can focus on their collective mission of helping patients.
To best utilize marketing efforts, there are a few essential components (242). Having dedicated physicians who dedicate time to promote the program and the treatment opportunities to physicians and other public outreach. Have specific staff with marketing expertise to gain more visibility including social media, advertisements in traditional and alternative media, search engine optimization, email promotion, online presence, and networking management. Not only does this gain more visibility but also drives the prestige of your practice. Once all this work is focused on generating attention, internal validation should be done so that points of contact facilitate patient follow-up. Make sure that public lines have staff capable of answering questions regarding the staff, procedures, scheduling, and benefits that can be provided.
Expanding the impact of IR
Promotion and awareness
There are recent efforts in the field of IR research and development to expand the methodology for minimally invasive procedures. Importantly, IR needs to expand its development of new efforts to expand the visibility and influence of the field on medicine and the public (243–245). More efforts have been directed toward academia trying to influence the medical curriculum to broaden the exposure to IR in clinical rotation (246). This ensures that training programs for integrated and early specialization are filled and can expand new spots for talented new physicians. Dedicated efforts to spread the amazing work of IR should also expand to popular media, as the name “interventionalist” doesn't carry the same perceptual connotation as “surgeon.” Despite several high-profile cases, such as Melania Trump and Steve Scalise, there is little public recognition of IR contribution (247). While IR should reserve the use of “surgeon” for physicians who are board certified in surgery for descriptive accuracy, there is still an unresolved general branding issue.
Governmental policy and healthcare infrastructure
Governmental advocacy and lobbying for pro-IR positions ensure the financial security of the field. If IR can justify its RVUs and still provide sufficient patient throughput to be profitable, hospital administrations will network and allocate resources to provide them with a stable source of revenue. Similarly, because of the challenges of other specialties evolving expertise in minimally invasive and endovascular procedures, sufficient funding from the National institutes of Health. Professional societies focus on these fronts, but they are small compared to other specialties. By increasing our value to all the stakeholders in the modern medical landscape, the synergistic effects of common goals will lead to a strong future for IR physicians and new developments to drive more efficient patient care.
Future directions
The types of minimally invasive procedures continue to expand as modern technology improves current techniques. The fundamental principle of remaining a valuable specialty is developing new methodologies to improve patient outcomes while reducing morbidity and mortality. The inception of the field was based on integrating innovative ideas into practice. Some of these include advances in robotics and artificial intelligence (AI). Robotics can assist in a wide array of procedures from improving the speed, accuracy, and radiation exposure of localized tumor ablation. Alternatively, robotic systems can improve intravascular wire navigation with similar advantages (1).
AI's impact will broadly impact the practice of medicine, including IR. AI tools can streamline clinical practice and reduce inefficiencies in workflow from scheduling, consenting, or monitoring patient messages. AI can provide direct medical assistance in the rapidly improving capability to communicate scientific literature, evaluate patient pre-procedural imaging, as well as complex procedural recommendations (2). While this technology is still prone to significant errors limiting its role in clinical decision making, AI will only continue to improve. Physicians should be open to exploring these tools to maximize the advantages IR practice and benefit to patients.
Conclusions
IR provides value for diverse needs within the medical system. Patients are the primary focus and beneficiary of the techniques and innovations to medicine. IR developments offer new alternative procedures that demonstrate reduced cost, recovery times, and fewer complications relative to historical medical and surgical therapies. Hospitals and healthcare payers benefit by achieving similar outcomes more efficiently. Regardless of how the reimbursement system is structured, IR is a diverse medical specialty involving diagnostics and procedures capable of providing financial value. While benefits are gained on the patient level, collective efforts must be invested into expanding IR's market capitalization. This starts with optimizing a personal practice with the right team and equipment. The expanded growth of IR is accomplished with a focus on recruiting, networking, marketing, and public advocacy. IR has a responsibility to foster leaders in the healthcare space to support the mission to revolutionize how medicine is practiced (248, 249).
Author contributions
WC: Writing – original draft, Writing – review & editing. JC: Conceptualization, Writing – review & editing. DS: Writing – review & editing. MM: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baum RA, Baum S. Interventional radiology: a half century of innovation. Radiology. (2014) 273(2S):S75–91. doi: 10.1148/radiol.14140534
2. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). (2008) 27(3):759–69. doi: 10.1377/hlthaff.27.3.759
3. Interventional Radiology Market Size, Share, Overview, Value, Growth Drivers, & Trends By 2029. Available online at: https://www.databridgemarketresearch.com/reports/global-interventional-radiology-market (Accessed July 15, 2022).
4. White SB, Dybul SL, Patel PJ, Hohenwalter EJ, Hieb RA, Shah SP, et al. A single-center experience in capturing inpatient evaluation and management for an IR practice. J Vasc Interv Radiol. (2015) 26(7):958–62. doi: 10.1016/j.jvir.2015.03.013
5. Misono A, Mueller P, Hirsch J, Harbaugh A, Sheridan R, Liu R. Outpatient interventional radiology clinic: financial modeling predicts revenues and profitability. J Vasc Interv Radiol. (2016) 27(3):S45. doi: 10.1016/j.jvir.2015.12.127
6. Hawkins CM, Duszak R, Hughes DR, Liu R, Resnick AS, Kooby DA, et al. Defining the value of interventional radiology to healthcare stakeholders: proceedings from a society of interventional radiology research consensus panel. J Vasc Interv Radiol. (2021) 32(7):1088.e1–.e8. doi: 10.1016/j.jvir.2021.04.011
7. Balthazar P, Hawkins CM, Vijayasarathi A, Loehfelm TW, Duszak R. Current clinical practice patterns of self-identified interventional radiologists. AJR Am J Roentgenol. (2018) 210(3):663–8. doi: 10.2214/AJr17.18592
8. Soares GM. The value of clinical interventional radiology. J Am Coll Radiol. (2011) 8(5):318–24. doi: 10.1016/j.jacr.2010.11.010
9. Sheth RA, Baerlocher MO, Connolly BL, Dariushnia SR, Shyn PB, Vatsky S, et al. Society of interventional radiology quality improvement standards on percutaneous needle biopsy in adult and pediatric patients. J Vasc Interv Radiol. (2020) 31(11):1840–8. doi: 10.1016/j.jvir.2020.07.012
10. Thompson BC, Kingdon E, Johnston M, Tibballs J, Watkinson A, Jarmulowicz M, et al. Transjugular kidney biopsy. Am J Kidney Dis. (2004) 43(4):651–62. doi: 10.1053/j.ajkd.2004.01.001
11. Zhuo L, Wang H, Chen D, Lu H, Zou G, Li W. Alternative renal biopsies: past and present. Int Urol Nephrol. (2018) 50(3):475–9. doi: 10.1007/s11255-017-1668-x
12. Tam AL, Lim HJ, Wistuba II, Tamrazi A, Kuo MD, Ziv E, et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol. (2016) 27(1):8–19. doi: 10.1016/j.jvir.2015.10.019
13. Tsoumakidou G, Saltiel S, Villard N, Duran R, Meuwly JY, Denys A. Image-guided marking techniques in interventional radiology: a review of current evidence. Diagn Interv Imaging. (2021) 102(12):699–707. doi: 10.1016/j.diii.2021.07.002
14. Lovrics PJ, Goldsmith CH, Hodgson N, McCready D, Gohla G, Boylan C, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol. (2011) 18(12):3407. doi: 10.1245/s10434-011-1699-y
15. Gray RJ, Pockaj BA, Karstaedt PJ, Roarke MC. Radioactive seed localization of nonpalpable breast lesions is better than wire localization. Am J Surg. (2004) 188(4):377–80. doi: 10.1016/j.amjsurg.2004.06.023
16. Hughes JH, Mason MC, Gray RJ, McLaughlin SA, Degnim AC, Fulmer JT, et al. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J. (2008) 14(2):153–7. doi: 10.1111/j.1524-4741.2007.00546.x
17. Hynes D, Aghajafari P, Janne d’Othée B. Role of interventional radiology in the management of infection. Semin Ultrasound CT MRI. (2020) 41(1):20–32. doi: 10.1053/j.sult.2019.10.006
18. Pereira BMT. Non-Operative management of hepatic trauma and the interventional radiology: an update review. Indian J Surg. (2013) 75(5):339–45. doi: 10.1007/s12262-012-0712-4
19. Lee JT, Slade E, Uyeda J, Steenburg SD, Chong ST, Tsai R, et al. American Society of emergency radiology multicenter blunt splenic trauma study: CT and clinical findings. Radiology. (2021) 299(1):122–30. doi: 10.1148/radiol.2021202917
20. Asensio JA, Roldán G, Petrone P, Rojo E, Tillou A, Kuncir E, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma Acute Care Surg. (2003) 54(4):647–54. doi: 10.1097/01.TA.0000054647.59217.BB
21. Padia SA, Ingraham CR, Moriarty JM, Wilkins LR, Bream PR, Tam AL, et al. Society of interventional radiology position statement on endovascular intervention for trauma. J Vasc Interv Radiol. (2020) 31(3):363–369.e2. doi: 10.1016/j.jvir.2019.11.012
22. Aiolfi A, Inaba K, Strumwasser A, Matsushima K, Grabo D, Benjamin E, et al. Splenic artery embolization versus splenectomy: analysis for early in-hospital infectious complications and outcomes. J Trauma Acute Care Surg. (2017) 83(3):356. doi: 10.1097/TA.0000000000001550
23. Wu Z, Zhou J, Pankaj P, Peng B. Comparative treatment and literature review for laparoscopic splenectomy alone versus preoperative splenic artery embolization splenectomy. Surg Endosc. (2012) 26(10):2758–66. doi: 10.1007/s00464-012-2270-z
24. Kos S, Gutzeit A, Hoppe H, Liu DM, Jacob AL. Diagnosis and therapy of acute hemorrhage in patients with pelvic fractures. Semin Musculoskelet Radiol. (2013) 17(4):396–406. doi: 10.1055/s-0033-1356469
25. Powell R, Menard M, Farber A, Rosenfield K, Goodney P, Gray B, et al. Comparison of specialties participating in the BEST-CLI trial to specialists treating peripheral arterial disease nationally. J Vasc Surg. (2019) 69(5):1505–9. doi: 10.1016/j.jvs.2018.08.188
26. Keller E, Crowley-Matoka M, Collins J, Chrisman H, Milad M, Vogelzang R. Why vascular surgeons and interventional radiologists collaborate or compete: a look at endovascular stent placements. J Vasc Interv Radiol. (2017) 28(2):S155. doi: 10.1016/j.jvir.2016.12.979
27. Kok HK, Rodt T, Fanelli F, Hamady M, Müller-Hülsbeck S, Santiago MC, et al. Clinical and endovascular practice in interventional radiology: a contemporary European analysis. CVIR Endovasc. (2018) 1(1):8. doi: 10.1186/s42155-018-0010-8
28. Fennell VS, Martirosyan NL, Palejwala SK, Lemole GM, Dumont TM. Morbidity and mortality of patients with endovascularly treated intracerebral aneurysms: does physician specialty matter? J Neurosurg. (2016) 124(1):13–7. doi: 10.3171/2014.11.JNS141030
29. Montanera W. Editorial: does physician specialty matter? J Neurosurg. (2016) 124(1):7–8. doi: 10.3171/2015.1.JNS142794
30. Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A. Endovascular embolization: review of currently available embolization agents. Curr Probl Diagn Radiol. (2014) 43(1):35–53. doi: 10.1067/j.cpradiol.2013.10.003
31. Osman KT, Nayfeh T, Abdelfattah AM, Alabdallah K, Hasan B, Firwana M, et al. Secondary prophylaxis of gastric variceal bleeding: a systematic review and network meta-analysis. Liver Transplant. (2022) 28(6):945–58. doi: 10.1002/lt.26383
32. Wang ZW, Liu JC, Zhao F, Zhang WG, Duan XH, Chen PF, et al. Comparison of the effects of TIPS versus BRTO on bleeding gastric varices: a meta-analysis. Can J Gastroenterol Hepatol. (2020) 2020:5143013. doi: 10.1155/2020/5143013
33. Lauten A, Strauch J, Jung C, Goebel B, Krizanic F, Baer FM. Endovascular treatment of superior vena cava syndrome by percutaneous venoplasty. Heart Lung Circ. (2010) 19(11):681–3. doi: 10.1016/j.hlc.2010.07.007
34. Ferral H, Behrens G, Lopera J. Budd-Chiari syndrome. Am J Roentgenol. (2012) 199(4):737–45. doi: 10.2214/AJR.12.9098
35. Rosenblum JM, Chen EP. Thoracoabdominal aortic aneurysm repair: open, endovascular, or hybrid? Gen Thorac Cardiovasc Surg. (2019) 67(1):175–9. doi: 10.1007/s11748-017-0820-y
36. Abdul Jabbar A, Chanda A, White CJ, Jenkins JS. Percutaneous endovascular abdominal aneurysm repair: state-of-the art. Catheter Cardiovasc Interv. (2020) 95(4):767–82. doi: 10.1002/ccd.28576
37. Yunus TE, Tariq N, Callahan RE, Niemeyer DJ, Brown OW, Zelenock GB, et al. Changes in inferior vena cava filter placement over the past decade at a large community-based academic health center. J Vasc Surg. (2008) 47(1):157–165.e4. doi: 10.1016/j.jvs.2007.08.057
38. Makary MS, Kapke J, Yildiz V, Pan X, Dowell JD. Outcomes and direct costs of Inferior vena cava filter placement and retrieval within the IR and surgical settings. J Vasc Interv Radiol. (2018) 29(2):170–5. doi: 10.1016/j.jvir.2017.09.005
39. Zheng Z, Zhuang Z, Yang M, Luo J, Zhang W, Yan Z, et al. Bronchial artery embolization for hemoptysis: a systematic review and meta-analysis. J Interv Med. (2021) 4(4):172–80. doi: 10.1016/j.jimed.2021.08.003
40. Anuradha C, Shyamkumar NK, Vinu M, Babu NRSS, Christopher DJ. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol Ank Turk. (2012) 18(1):96–101. doi: 10.4261/1305-3825.DIR.3876-11.2
41. Ayx I, Müller-Wille R, Wohlgemuth WA, Pfeifer M, Lepiorz M, Hubauer H, et al. Treatment of acute hemoptysis by bronchial artery embolization with the liquid embolic agent ethylene vinyl alcohol copolymer. J Vasc Interv Radiol JVIR. (2017) 28(6):825–31. doi: 10.1016/j.jvir.2016.12.1226
42. Daliri A, Probst NH, Jobst B, Lepper PM, Kickuth R, Szucs-Farkas Z, et al. Bronchial artery embolization in patients with hemoptysis including follow-up. Acta Radiol Stockh Swed 1987. (2011) 52(2):143–7. doi: 10.1258/ar.2010.100302
43. Lee SH, Lee JH, Chang JH, Kim SJ, Yoon HY, Shim SS, et al. Hemoptysis requiring bronchial artery embolization in patients with nontuberculous mycobacterial lung disease. BMC Pulm Med. (2019) 19(1):117. doi: 10.1186/s12890-019-0881-z
44. Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. (2017) 23(4):307–17. doi: 10.5152/dir.2017.16454
45. Guibert N, Saka H, Dutau H. Airway stenting: technological advancements and its role in interventional pulmonology. Respirology. (2020) 25(9):953–62. doi: 10.1111/resp.13801
46. Patella F, Panella S, Zannoni S, Jannone ML, Pesapane F, Angileri SA, et al. The role of interventional radiology in the treatment of epiphora. Gland Surg. (2018) 7(2):103–10. doi: 10.21037/gs.2017.09.16
47. Llopis E, Fernandez E, Cerezal L. MR and CT arthrography of the hip. Semin Musculoskelet Radiol. (2012) 16(1):42–56. doi: 10.1055/s-0032-1304300
48. O’Kane JW, Toresdahl BG. The evidenced-based shoulder evaluation. Curr Sports Med Rep. (2014) 13(5):307–13. doi: 10.1249/JSR.0000000000000090
49. Roy JS, Braën C, Leblond J, Desmeules F, Dionne CE, MacDermid JC, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. (2015) 49(20):1316–28. doi: 10.1136/bjsports-2014-094148
50. Bhure U, Roos JE, Pérez Lago M del S, Steurer I, Grünig H, Hug U, et al. SPECT/CT arthrography. Br J Radiol. (2018) 91(1082):20170635. doi: 10.1259/bjr.20170635
51. Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. (2009) 34(10):1066–77. doi: 10.1097/BRS.0b013e3181a1390d
52. Practice guidelines for chronic pain management: an updated report by the American Society of anesthesiologists task force on chronic pain management and the American Society of regional anesthesia and pain medicine*. Anesthesiology. (2010) 112(4):810–33. doi: 10.1097/ALN.0b013e3181c43103
53. Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Carrino J, et al. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. (2009) 34(21):2338–45. doi: 10.1097/BRS.0b013e3181ab5432
54. Provenzano DA. Diagnostic discography: what is the clinical utility? Curr Pain Headache Rep. (2012) 16(1):26–34. doi: 10.1007/s11916-011-0239-6
55. Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. (2009) 361(6):557–68. doi: 10.1056/NEJMoa0900429
56. Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. (2009) 361(6):569–79. doi: 10.1056/NEJMoa0900563
57. Klazen CAH, Lohle PNM, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (vertos II): an open-label randomised trial. Lancet Lond Engl. (2010) 376(9746):1085–92. doi: 10.1016/S0140-6736(10)60954-3
58. Itagaki MW, Talenfeld AD, Kwan SW, Brunner JWM, Mortell KE, Brunner MC. Percutaneous vertebroplasty and kyphoplasty for pathologic vertebral fractures in the medicare population: safer and less expensive than open surgery. J Vasc Interv Radiol. (2012) 23(11):1423–9. doi: 10.1016/j.jvir.2012.08.010
59. Schmelzer-Schmied N, Cartens C, Meeder PJ, Dafonseca K. Comparison of kyphoplasty with use of a calcium phosphate cement and non-operative therapy in patients with traumatic non-osteoporotic vertebral fractures. Eur Spine J. (2009) 18(5):624–9. doi: 10.1007/s00586-008-0880-x
60. Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech. (2005) 18(3):238–42. PMID: 15905767.15905767
61. Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. (2010) 21(2):359–64. doi: 10.1007/s00198-009-0952-8
62. Heller DB, Beggin AE, Lam AH, Kohi MP, Heller MB. Geniculate artery embolization: role in knee hemarthrosis and osteoarthritis. RadioGraphics. (2022) 42(1):289–301. doi: 10.1148/rg.210159
63. Teitelbaum I. Peritoneal dialysis. N Engl J Med. (2021) 385(19):1786–95. doi: 10.1056/NEJMra2100152
64. Crabtree JH. Who should place peritoneal dialysis catheters? Perit Dial Int J Int Soc Perit Dial. (2010) 30(2):142–50. doi: 10.3747/pdi.2009.00066
65. Özener Ç, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant. (2001) 16(9):1893–9. doi: 10.1093/ndt/16.9.1893
66. Sloan CE, Coffman CJ, Sanders LL, Maciejewski ML, Lee SYD, Hirth RA, et al. Trends in peritoneal dialysis use in the United States after medicare payment reform. Clin J Am Soc Nephrol. (2019) 14(12):1763–72. doi: 10.2215/CJN.05910519
67. Morris CS. Interventional radiology placement and management of tunneled peritoneal dialysis catheters: a pictorial review. RadioGraphics. (2020) 40(6):1789–806. doi: 10.1148/rg.2020200063
68. Bohn KA, Ray CE. Repeat large-volume paracentesis versus tunneled peritoneal catheter placement for malignant ascites: a cost-minimization study. AJR Am J Roentgenol. (2015) 205(5):1126–34. doi: 10.2214/AJR.15.14484
69. Halabi SA, Sawas T, Sadat B, Jandali A, Halabi HA, Halabi FA, et al. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. (2016) 31(9):1519–26. doi: 10.1111/jgh.13303
70. Korsic S, Stabuc B, Skok P, Popovic P. TIPS Vs. endoscopic treatment for prevention of recurrent variceal bleeding: a long-term follow-up of 126 patients. Radiol Oncol. (2021) 55(2):164–71. doi: 10.2478/raon-2021-0006
71. Nicoara-Farcau O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: a meta-analysis of individual patient data. Gastroenterology. (2021) 160(1):193–205.e10. doi: 10.1053/j.gastro.2020.09.026
72. Russo MW, Zacks SL, Sandler RS, Brown RS Jr. Cost-effectiveness analysis of transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic therapy for the prevention of recurrent esophageal variceal bleeding. Hepatology. (2000) 31(2):358–63. doi: 10.1002/hep.510310215
73. Makary MS, Ramsell S, Miller E, Beal EW, Dowell JD. Hepatocellular carcinoma locoregional therapies: outcomes and future horizons. World J Gastroenterol. (2021) 27(43):7462–79. doi: 10.3748/wjg.v27.i43.7462
74. Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional therapy approaches for hepatocellular carcinoma: recent advances and management strategies. Cancers (Basel). (2020) 12(7):E1914. doi: 10.3390/cancers12071914
75. Zane KE, Nagib PB, Jalil S, Mumtaz K, Makary MS. Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol. (2022) 14(5):885–95. doi: 10.4254/wjh.v14.i5.885
76. Zane KE, Cloyd JM, Mumtaz KS, Wadhwa V, Makary MS. Metastatic disease to the liver: locoregional therapy strategies and outcomes. World J Clin Oncol. (2021) 12(9):725–45. doi: 10.5306/wjco.v12.i9.725
77. Zhang Y, Li Y, Ji H, Zhao X, Lu H. Transarterial Y90 radioembolization versus chemoembolization for patients with hepatocellular carcinoma: a meta-analysis. Biosci Trends. (2015) 9(5):289–98. doi: 10.5582/bst.2015.01089
78. Shi Y, Zhai B. A recent advance in image-guided locoregional therapy for hepatocellular carcinoma. Gastrointest Tumors. (2016) 3(2):90–102. doi: 10.1159/000445888
79. Liu J guo, Wu J, Wang J, Shu G ming, Wang Y jun, Lou C, et al. Endoscopic biliary drainage versus percutaneous transhepatic biliary drainage in patients with resectable hilar cholangiocarcinoma: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech. (2018) 28(9):1053–60. doi: 10.1089/lap.2017.0744
80. Duan F, Cui L, Bai Y, Li X, Yan J, Liu X. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: a systematic review and meta-analysis. Cancer Imaging. (2017) 17(1):27. doi: 10.1186/s40644-017-0129-1
81. Dorcaratto D, Hogan NM, Muñoz E, Garcés M, Limongelli P, Sabater L, et al. Is percutaneous transhepatic biliary drainage better than endoscopic drainage in the management of jaundiced patients awaiting pancreaticoduodenectomy? A systematic review and meta-analysis. J Vasc Interv Radiol JVIR. (2018) 29(5):676–87. doi: 10.1016/j.jvir.2017.12.027
82. Makary MS, Farrell JJ, Khayat M, Chick JFB, Srinivasa RN. Biliary endoscopy for benign and malignant biliary strictures. Tech Vasc Interv Radiol. (2019) 22(3):135–8. doi: 10.1053/j.tvir.2019.04.005
83. Xiao H, Zhuang W, Wang S, Yu B, Chen G, Zhou M, et al. Arterial embolization: a novel approach to thyroid ablative therapy for Graves’ disease. J Clin Endocrinol Metab. (2002) 87(8):3583–9. doi: 10.1210/jcem.87.8.8723
84. Safety of Thyroidal Artery Embolization - ClinicalKey. Available online at: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1051044321014792?returnurl=Available online at: https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1051044321014792%3Fshowall%3Dtrue&referrer=Available online at: https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (Accessed February 16, 2024).
85. Yilmaz S, Habibi HA, Yildiz A, Altunbas H. Thyroid embolization for nonsurgical treatment of nodular goiter: a single-center experience in 56 consecutive patients. J Vasc Interv Radiol JVIR. (2021) 32(10):1449–56. doi: 10.1016/j.jvir.2021.06.025
86. Gücek Haciyanli S, Acar N, Balli Ö, Erdoğan N, Haciyanli M. Selective venous sampling in primary hyperparathyroidism: is it worth doing? Turk J Med Sci. (2022) 52(1):144–9. doi: 10.3906/sag-2108-151
87. Loberg C, Antoch G, Stegbauer J, Dringenberg T, Steuwe A, Fürst G, et al. Update: selective adrenal venous sampling (AVS) - indication, technique, and significance. RoFo Fortschritte Auf Dem Geb Rontgenstrahlen Bildgeb Verfahr. (2021) 193(6):658–66. doi: 10.1055/a-1299-1878
88. Ladurner R, Sommerey S, Buechner S, Dietz A, Degenhart C, Hallfeldt K, et al. Accuracy of adrenal imaging and adrenal venous sampling in diagnosing unilateral primary aldosteronism. Eur J Clin Invest. (2017) 47(5):372–7. doi: 10.1111/eci.12746
89. Dekkers T, Prejbisz A, Kool LJS, Groenewoud HJMM, Velema M, Spiering W, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. (2016) 4(9):739–46. doi: 10.1016/S2213-8587(16)30100-0
90. Roche A, Raisonnier A, Gillon-Savouret MC. Pancreatic venous sampling and arteriography in localizing insulinomas and gastrinomas: procedure and results in 55 cases. Radiology. (1982) 145(3):621–7. doi: 10.1148/radiology.145.3.6292994
91. Chavhan GB, Lam CZ, Greer MLC, Temple M, Amaral J, Grosse-Wortmann L. Magnetic resonance lymphangiography. Radiol Clin North Am. (2020) 58(4):693–706. doi: 10.1016/j.rcl.2020.02.002
92. Kim PH, Tsauo J, Shin JH. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol. (2018) 29(2):194–202.e4. doi: 10.1016/j.jvir.2017.10.006
93. Pandharipande PV, Harisinghani MG, Ozanne EM, Specht MC, Hur C, Lee JM, et al. Staging MR lymphangiography of the axilla for early breast cancer: cost-effectiveness analysis. AJR Am J Roentgenol. (2008) 191(5):1308–19. doi: 10.2214/AJR.07.3861
94. Pera A, Capek M, Shirkhoda A. Lymphangiography and CT in the follow-up of patients with lymphoma. Radiology. (1987) 164(3):631–3. doi: 10.1148/radiology.164.3.3615860
95. Gong Y, Guo Z, Tang X, Li C, Wang Q. Performance of different imaging techniques for detection of para-aortic lymph node metastasis from gynecological malignancies: a systematic review and meta-analysis. Gynecol Obstet Invest. (2020) 85(1):53–71. doi: 10.1159/000502821
96. Kim GH, Shin JH, Zeng CH, Park JH. Recent updates in gastrointestinal stent placement from the esophagus to the colon: a radiological perspective. Cardiovasc Intervent Radiol. (2022) 45(4):425–37. doi: 10.1007/s00270-022-03067-5
97. Jeurnink SM, Steyerberg EW, Hooft JE van, Eijck CHJ van, Schwartz MP, Vleggaar FP, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. (2010) 71(3):490–9. doi: 10.1016/j.gie.2009.09.042
98. Yoon J, Kwon SH, Lee CK, Park SJ, Oh JY, Oh JH. Radiologic placement of uncovered stents for the treatment of malignant colonic obstruction proximal to the descending colon. Cardiovasc Intervent Radiol. (2017) 40(1):99–105. doi: 10.1007/s00270-016-1474-3
99. Kim DR, Yoon CJ, Lee JH, Choi WS. Fluoroscopic rescue of failed endoscopic stent placement for obstructing colorectal malignancy. Am J Roentgenol. (2020) 214(1):213–7. doi: 10.2214/AJR.19.21744
100. Given MF, Lyon SM, Lee MJ. The role of the interventional radiologist in enteral alimentation. Eur Radiol. (2004) 14(1):38–47. doi: 10.1007/s00330-003-1911-y
101. Wollman B, D’Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. (1995) 197(3):699–704. doi: 10.1148/radiology.197.3.7480742
102. Yuan Y, Zhao Y, Xie T, Hu Y. Percutaneous endoscopic gastrostomy versus percutaneous radiological gastrostomy for swallowing disturbances. Cochrane Database Syst Rev. (2016) 2:CD009198. doi: 10.1002/14651858.CD009198.pub2
103. Özmen MN, Akhan O. Percutaneous radiologic gastrostomy. Eur J Radiol. (2002) 43(3):186–95. doi: 10.1016/S0720-048X(02)00155-9
104. von Ende E, Gayou EL, Dowell JD, Waid M, Chaves L, Rula E, et al. Nationwide trends in tube-related genitourinary interventions for medicare beneficiaries. J Am Coll Radiol JACR. (2021) 18(9):1289–96. doi: 10.1016/j.jacr.2021.04.015
105. Knoll T, Daels F, Desai J, Hoznek A, Knudsen B, Montanari E, et al. Percutaneous nephrolithotomy: technique. World J Urol. (2017) 35(9):1361–8. doi: 10.1007/s00345-017-2001-0
106. Meretyk S, Gofrit ON, Gafni O, Pode D, Shapiro A, Verstandig A, et al. Complete staghorn calculi: random prospective comparison between extracorporeal shock wave lithotripsy monotherapy and combined with percutaneous nephrostolithotomy. J Urol. (1997) 157(3):780–6. doi: 10.1016/S0022-5347(01)65039-0
107. Lingeman JE, Siegel YI, Steele B, Nyhuis AW, Woods JR. Management of lower pole nephrolithiasis: a critical analysis. J Urol. (1994) 151(3):663–7. doi: 10.1016/S0022-5347(17)35042-5
108. Bhageria A, Nayak B, Seth A, Dogra PN, Kumar R. Paediatric percutaneous nephrolithotomy: single-centre 10-year experience. J Pediatr Urol. (2013) 9(4):472–5. doi: 10.1016/j.jpurol.2013.02.004
109. Light A, Gupta T, Dadabhoy M, Daniel A, Nandakumar M, Burrows A, et al. Outcomes following primary realignment versus suprapubic cystostomy with delayed urethroplasty for pelvic fracture-associated posterior urethral injury: a systematic review with meta-analysis. Curr Urol. (2019) 13(3):113–24. doi: 10.1159/000499282
110. Barrett K, Braga LH, Farrokhyar F, Davies TO. Primary realignment vs suprapubic cystostomy for the management of pelvic fracture–associated urethral injuries: a systematic review and meta-analysis. Urology. (2014) 83(4):924–9. doi: 10.1016/j.urology.2013.12.031
111. Lee MJ, Papanicolaou N, Nocks BN, Valdez JA, Yoder IC. Fluoroscopically guided percutaneous suprapubic cystostomy for long-term bladder drainage: an alternative to surgical cystostomy. Radiology. (1993) 188(3):787–9. doi: 10.1148/radiology.188.3.8351348
112. McPhail MJW, Abu-Hilal M, Johnson CD. A meta-analysis comparing suprapubic and transurethral catheterization for bladder drainage after abdominal surgery. Br J Surg. (2006) 93(9):1038–44. doi: 10.1002/bjs.5424
113. Niël-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. (2005) (3):CD004203. doi: 10.1002/14651858.CD004203.pub2
114. Gibson KE, Neill S, Tuma E, Meddings J, Mody L. Indwelling urethral versus suprapubic catheters in nursing home residents: determining the safest option for long-term use. J Hosp Infect. (2019) 102(2):219–25. doi: 10.1016/j.jhin.2018.07.027
115. Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Interv. (2019) 12(12):1095–105. doi: 10.1016/j.jcin.2019.02.050
116. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. (2014) 370(15):1393–401. doi: 10.1056/NEJMoa1402670
117. Vink EE, de Beus E, de Jager RL, Voskuil M, Spiering W, Vonken EJ, et al. The effect of renal denervation added to standard pharmacologic treatment versus standard pharmacologic treatment alone in patients with resistant hypertension: rationale and design of the SYMPATHY trial. Am Heart J. (2014) 167(3):308–314.e3. doi: 10.1016/j.ahj.2013.11.010
118. de Jager RL, de Beus E, Beeftink MMA, Sanders MF, Vonken EJ, Voskuil M, et al. Impact of medication adherence on the effect of renal denervation. Hypertension. (2017) 69(4):678–84. doi: 10.1161/HYPERTENSIONAHA.116.08818
119. Carrafiello G, Laganà D, Fontana F, Mangini M, Mariani D, De Bernardi IC, et al. Cosmetic treatments: an emerging field of interest for interventional radiologists. Int J Surg. (2008) 6:S70–4. doi: 10.1016/j.ijsu.2008.12.016
120. Yakes WF, Haas DK, Parker SH, Gibson MD, Hopper KD, Mulligan JS, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. (1989) 170(3):1059–66. doi: 10.1148/radiology.170.3.2916057
121. Al-Khouja F, Macherla A, Crawford M, Ramachandran V, Rupasinghe M, Kabutey N, et al. Abstract No. 63 comparative cost effectiveness of varicose vein treatment: a meta-analysis. J Vasc Interv Radiol. (2021) 32(5):S28. doi: 10.1016/j.jvir.2021.03.484
122. Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (2006). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK349622/ (Accessed August 9, 2022).
123. Ludwig PE, Huff TJ, Shanahan MM, Stavas JM. Pregnancy success and outcomes after uterine fibroid embolization: updated review of published literature. Br J Radiol. (2020) 93(1105):20190551. doi: 10.1259/bjr.20190551
124. Thurmond AS, Machan LS, Maubon AJ, Rouanet JP, Hovsepian DM, Van Moore A, et al. Needle core vs open biopsy for diagnosis of intermediate- and high-risk neuroblastoma in children. J Pediatr Surg. (2012) 47(6):1261–6. doi: 10.1016/j.jpedsurg.2012.03.040
125. Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology. (1998) 208(3):717–23. doi: 10.1148/radiology.208.3.9722851
126. Luparia A, Durando M, Campanino P, Regini E, Lucarelli D, Talenti A, et al. Efficacy and cost-effectiveness of stereotactic vacuum-assisted core biopsy of nonpalpable breast lesions: analysis of 602 biopsies performed over 5 years. Radiol Med (Torino). (2011) 116(3):477–88. doi: 10.1007/s11547-011-0625-x
127. Fernández-García P, Marco-Doménech SF, Lizán-Tudela L, Ibáñez-Gual MV, Navarro-Ballester A, Casanovas-Feliu E. The cost effectiveness of vacuum-assisted versus core-needle versus surgical biopsy of breast lesions. Radiol Engl Ed. (2017) 59(1):40–6. doi: 10.1016/j.rxeng.2016.09.002
128. Thompson J, Stricker P. MRI Improves cost and accuracy of prostate cancer biopsy. Nat Rev Urol. (2018) 15(1):6–8. doi: 10.1038/nrurol.2017.185
129. Aggarwal S, Siddiqui WJ, Shahid N, Baynes J, Khattak MW, Ahmed I, et al. A comparison between kidney allograft biopsies performed by nephrologists and surgeons versus interventional radiologists. Cureus. (2019) 11(12):e6315. doi: 10.7759/cureus.6315
130. Smith BC, Desmond PV. Outpatient liver biopsy using ultrasound guidance and the biopty gun is safe and cost effective. Aust N Z J Med. (1995) 25(3):209–11. doi: 10.1111/j.1445-5994.1995.tb01524.x
131. Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. (2010) 152(4):238–46. doi: 10.7326/0003-4819-152-1-201001050-00190
132. Hemming A, Davis NL, Robins RE. Surgical versus percutaneous drainage of intra-abdominal abscesses. Am J Surg. (1991) 161(5):593–5. doi: 10.1016/0002-9610(91)90907-U
133. Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. (2002) 137(7):845–9. doi: 10.1001/archsurg.137.7.845
134. Shaikh T, Chen T, Khan A, Yue NJ, Kearney T, Cohler A, et al. Improvement in interobserver accuracy in delineation of the lumpectomy cavity using fiducial markers. Int J Radiat Oncol. (2010) 78(4):1127–34. doi: 10.1016/j.ijrobp.2009.09.025
135. Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. (1999) 115(2):563–8. doi: 10.1378/chest.115.2.563
136. Hancock-Howard R, Connolly BL, McMahon M, Menon A, Woo G, Wales PW, et al. Cost-effectiveness analysis of implantable venous access device insertion using interventional radiologic versus conventional operating room methods in pediatric patients with cancer. J Vasc Interv Radiol. (2010) 21(5):677–84. doi: 10.1016/j.jvir.2010.01.014
137. Stevenson KB, Hannah EL, Lowder CA, Adcox MJ, Davidson RL, Mallea MC, et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. (2002) 39(3):549–55. doi: 10.1053/ajkd.2002.31405
138. Weijmer MC, Vervloet MG, ter Wee PM. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant. (2004) 19(3):670–7. doi: 10.1093/ndt/gfg581
139. Artifon ELA, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. (2012) 46(9):768–74. doi: 10.1097/MCG.0b013e31825f264c
140. Radvany MG, Quinones-Hinojosa A, Gallia GL, Wand GS, Salvatori R. Venous sampling for cushing disease: comparison of internal jugular vein and inferior petrosal sinus sampling. Endocr Pract. (2016) 22(9):1057–61. doi: 10.4158/EP161266.OR
141. Wiesli P, Bràndle M, Schmid C, Kràhenbühl L, Furrer J, Keller U, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol. (2004) 15(11):1251–6. doi: 10.1097/01.RVI.0000140638.55375.1E
142. Lee SH, Hwang JH, Kim DH, So YH, Park J, Cho SB, et al. Clinical outcomes of transcatheter arterial embolisation for chronic knee pain: mild-to-moderate versus severe knee osteoarthritis. Cardiovasc Intervent Radiol. (2019) 42(11):1530–6. doi: 10.1007/s00270-019-02289-4
143. Bagla S, Piechowiak R, Hartman T, Orlando J, Gaizo DD, Isaacson A. Genicular artery embolization for the treatment of knee pain secondary to osteoarthritis. J Vasc Interv Radiol. (2020) 31(7):1096–102. doi: 10.1016/j.jvir.2019.09.018
144. Little MW, Gibson M, Briggs J, Speirs A, Yoong P, Ariyanayagam T, et al. Genicular artEry embolizatioN in patiEnts with oSteoarthrItiS of the knee (GENESIS) using permanent microspheres: interim analysis. Cardiovasc Intervent Radiol. (2021) 44(6):931–40. doi: 10.1007/s00270-020-02764-3
145. Kasperk C, Grafe IA, Schmitt S, Nöldge G, Weiss C, Da Fonseca K, et al. Three-year outcomes after kyphoplasty in patients with osteoporosis with painful vertebral fractures. J Vasc Interv Radiol JVIR. (2010) 21(5):701–9. doi: 10.1016/j.jvir.2010.01.003
146. Garfin SR, Buckley RA, Ledlie J. Balloon kyphoplasty outcomes group. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine. (2006) 31(19):2213–20. doi: 10.1097/01.brs.0000232803.71640.ba
147. Wardlaw D, Cummings SR, Meirhaeghe JV, Bastian L, Tillman JB, Ranstam J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. (2009) 373(9668):1016–24. doi: 10.1016/S0140-6736(09)60010-6
148. Li ZM, Wu G, Han XW, Ren KW, Zhu M. Radiology-guided forceps biopsy and airway stenting in severe airway stenosis. Diagn Interv Radiol. (2014) 20(4):349–52. doi: 10.5152/dir.2014.12118
149. Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non–small cell lung cancer. J Vasc Interv Radiol. (2014) 25(1):1–9.e1. doi: 10.1016/j.jvir.2013.10.018
150. Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. (2010) 139(3):584–90. doi: 10.1016/j.jtcvs.2009.11.025
151. Alejandre-Lafont E, Krompiec C, Rau WS, Krombach GA. Effectiveness of therapeutic lymphography on lymphatic leakage. Acta Radiol. (2011) 52(3):305–11. doi: 10.1258/ar.2010.090356
152. Yannes M, Shin D, McCluskey K, Varma R, Santos E. Comparative analysis of intranodal lymphangiography with percutaneous intervention for postsurgical chylous effusions. J Vasc Interv Radiol. (2017) 28(5):704–11. doi: 10.1016/j.jvir.2016.12.1209
153. Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. (2004) 36(01):73–8. doi: 10.1055/s-2004-814123
154. Mittal A, Windsor J, Woodfield J, Casey P, Lane M. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg. (2004) 91(2):205–9. doi: 10.1002/bjs.4396
155. Jeurnink SM, Steyerberg EW, Hof GT, van Eijck CHJ, Kuipers EJ, Siersema PD. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol. (2007) 96(5):389–96. doi: 10.1002/jso.20828
156. Park JH, Song HY, Yun SC, Yoo MW, Ryu MH, Kim JH, et al. Gastroduodenal stent placement versus surgical gastrojejunostomy for the palliation of gastric outlet obstructions in patients with unresectable gastric cancer: a propensity score-matched analysis. Eur Radiol. (2016) 26(8):2436–45. doi: 10.1007/s00330-015-4106-4
157. Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol J Can Gastroenterol. (2009) 23(2):109–14. doi: 10.1155/2009/801925
158. Geisler BP, Egan BM, Cohen JT, Garner AM, Akehurst RL, Esler MD, et al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. (2012) 60(14):1271–7. doi: 10.1016/j.jacc.2012.07.029
159. Tilden D, McBride M, Whitbourn R, Krum H, Walton T, Gillespie J. Cost effectiveness of catheter-based renal denervation for treatment resistant hypertension – an Australian payer perspective. Value Health. (2014) 17(7):A762. doi: 10.1016/j.jval.2014.08.266
160. Mara M, Fucikova Z, Maskova J, Kuzel D, Haakova L. Uterine fibroid embolization versus myomectomy in women wishing to preserve fertility: preliminary results of a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. (2006) 126(2):226–33. doi: 10.1016/j.ejogrb.2005.10.008
161. Pinto I, Chimeno P, Romo A, Paúl L, Haya J, de la Cal MA, et al. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment—a prospective, randomized, and controlled clinical trial. Radiology. (2003) 226(2):425–31. doi: 10.1148/radiol.2262011716
162. Siskin GP, Shlansky-Goldberg RD, Goodwin SC, Sterling K, Lipman JC, Nosher JL, et al. A prospective multicenter comparative study between myomectomy and uterine artery embolization with polyvinyl alcohol microspheres: long-term clinical outcomes in patients with symptomatic uterine fibroids. J Vasc Interv Radiol. (2006) 17(8):1287–95. doi: 10.1097/01.RVI.0000231953.91787.AF
163. Jun F, Yamin L, Xinli X, Zhe L, Min Z, Bo Z, et al. Uterine artery embolization versus surgery for symptomatic uterine fibroids: a randomized controlled trial and a meta-analysis of the literature. Arch Gynecol Obstet. (2012) 285(5):1407–13. doi: 10.1007/s00404-011-2065-9
164. Hehenkamp WJK, Volkers NA, Donderwinkel PFJ, Blok S de, Birnie E, Ankum WM, et al. Uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids (EMMY trial): peri- and postprocedural results from a randomized controlled trial. Am J Obstet Gynecol. (2005) 193(5):1618–29. doi: 10.1016/j.ajog.2005.05.017
165. Çayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. (2009) 30(1):33–40. doi: 10.2164/jandrol.108.005967
166. Abdulmaaboud MR, Shokeir AA, Farage Y, El-Rahman AA, El-Rakhawy MM, Mutabagani H. Treatment of varicocele: a comparative study of conventional open surgery, percutaneous retrograde sclerotherapy, and laparoscopy. Urology. (1998) 52(2):294–300. doi: 10.1016/S0090-4295(98)00178-2
167. Ray AF, Powell J, Speakman MJ, Longford NT, DasGupta R, Bryant T, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. (2018) 122(2):270–82. doi: 10.1111/bju.14249
168. Zumstein V, Betschart P, Vetterlein MW, Kluth LA, Hechelhammer L, Mordasini L, et al. Prostatic artery embolization versus standard surgical treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol Focus. (2019) 5(6):1091–100. doi: 10.1016/j.euf.2018.09.005
169. Thurmond AS, Machan LS, Maubon AJ, Rouanet JP, Hovsepian DM, Van Moore A, et al. A review of selective salpingography and fallopian tube catheterization. RadioGraphics. (2000) 20(6):1759–68. doi: 10.1148/radiographics.20.6.g00nv211759
170. Kohi MP. Interventional radiologist’s approach to fallopian tube recanalization. Tech Vasc Interv Radiol. (2021) 24(1):100736. doi: 10.1016/j.tvir.2021.100736
171. Iaccarino V, Venetucci P. Interventional radiology of male varicocele: current status. Cardiovasc Intervent Radiol. (2012) 35(6):1263–80. doi: 10.1007/s00270-012-0350-z
172. Johnsen N, Tauber R. Financial analysis of antegrade scrotal sclerotherapy for men with varicoceles. Br J Urol. (1996) 77(1):129–32. doi: 10.1046/j.1464-410X.1996.78622.x
173. Courtheoux P, Maiza D, Henriet JP, Vaislic CD, Evrard C, Theron J. Erectile dysfunction caused by venous leakage: treatment with detachable balloons and coils. Radiology. (1986) 161(3):807–9. doi: 10.1148/radiology.161.3.3786738
174. Bookstein JJ, Lurie AL. Transluminal penile venoablation for impotence: a progress report. Cardiovasc Intervent Radiol. (1988) 11(4):253–60. doi: 10.1007/BF02577012
175. Schwartz AN, Lowe M, Harley JD, Berger RE. Preliminary report: penile vein occlusion therapy: selection criteria and methods used for the transcatheter treatment of impotence caused by venous-sinusoidal incompetence. J Urol. (1992) 148(3, Part 1):815–20. doi: 10.1016/S0022-5347(17)36731-9
176. Schild HH, Müller SC, Mildenberger P, Strunk H, Kaltenborn H, Kersjes W, et al. Percutaneous penile venoablation for treatment of impotence. Cardiovasc Intervent Radiol. (1993) 16(5):280–6. doi: 10.1007/BF02629158
177. Peşki˙rci˙oğlu L, Teki˙n İ, Boyvat F, Karabulut A, Özkardeş H. Embolization of the deep dorsal vein for the treatment of erectile impotence due to veno-occlusive dysfunction. J Urol. (2000) 163(2):472–5. doi: 10.1016/S0022-5347(05)67904-9
178. Wong T, Tembelis M, Acharya V, Hoffmann JC. Prostatic artery embolization and sexual function: literature review and comparison to other urologic interventions. Tech Vasc Interv Radiol. (2020) 23(3):100693. doi: 10.1016/j.tvir.2020.100693
179. Uflacker A, Haskal ZJ, Bilhim T, Patrie J, Huber T, Pisco JM. Meta-analysis of prostatic artery embolization for benign prostatic hyperplasia. J Vasc Interv Radiol. (2016) 27(11):1686–1697.e8. doi: 10.1016/j.jvir.2016.08.004
180. Brownlee AR, Mitzman B, Cyzman R, Ferguson MK. Outpatient thoracoscopic mediastinal biopsy: a safe and cost-effective approach. Ann Thorac Surg. (2020) 110(5):1726–9. doi: 10.1016/j.athoracsur.2020.04.056
181. Na KJ, Park IK, Park S, Kang CH, Kim YT. Efficacy and cost-effectiveness of surgical biopsy for histologic diagnosis of indeterminate nodules suspected for early stage lung cancer: comparison with percutaneous needle biopsy. J Korean Med Sci. (2020) 35(28):e261. doi: 10.3346/jkms.2020.35.e261
182. Pistolese CA, Ciarrapico A, Perretta T, Cossu E, della Gatta F, Giura S, et al. Cost-effectiveness of two breast biopsy procedures: surgical biopsy versus vacuum-assisted biopsy. Radiol Med (Torino). (2012) 117(4):539–57. doi: 10.1007/s11547-011-0735-0
183. Lachar WA, Shahab I, Saad AJ. Accuracy and cost-effectiveness of core needle biopsy in the evaluation of suspected lymphoma: a study of 101 cases. Arch Pathol Lab Med. (2007) 131(7):1033–9. doi: 10.5858/2007-131-1033-AACOCN
184. Silverman SG, Deuson TE, Kane N, Adams DF, Seltzer SE, Phillips MD, et al. Percutaneous abdominal biopsy: cost-identification analysis. Radiology. (1998) 206(2):429–35. doi: 10.1148/radiology.206.2.9457196
185. Tsai HY, Huang ST, Chao MF, Kan JY, Hsu JS, Hou MF, et al. Cost-effectiveness of stereotactic vacuum-assisted biopsy for nonpalpable breast lesions. Eur J Radiol. (2020) 127:108982. doi: 10.1016/j.ejrad.2020.108982
186. Sutton AJ, Barton P, Sundar S, Meads C, Rosenthal AN, Baldwin P, et al. Cost-effectiveness of sentinel lymph node biopsy vs inguinofemoral lymphadenectomy in women with vulval cancer. Br J Cancer. (2013) 109(10):2533–47. doi: 10.1038/bjc.2013.631
187. Gruber R, Bernt R, Helbich TH. Cost-effectiveness of percutaneous core needle breast biopsy (CNBB) versus open surgical biopsy (OSB) of nonpalpable breast lesions: metaanalysis and cost evaluation for German-speaking countries. ROFO Fortschr Geb Rontgenstr Nuklearmed. (2008) 180(2):134–42. doi: 10.1055/s-2007-963621
188. Botana-Rial M, Ramos-Hernández C, Lojo-Rodríguez I, Represas-Represas C, Ruano-Raviña A, Leiro-Fernández V, et al. Cost effectiveness of malignant pleural effusion with indwelling catheter: systematic review. J Palliat Med. (2021) 24(8):1206–12. doi: 10.1089/jpm.2020.0695
189. LaRoy JR, White SB, Jayakrishnan T, Dybul S, Ungerer D, Turaga K, et al. Cost and morbidity analysis of chest port insertion: interventional radiology suite versus operating room. J Am Coll Radiol. (2015) 12(6):563–71. doi: 10.1016/j.jacr.2015.01.012
190. Martin B, Witrick B, Sivaraj B, Tyler L, Devane AM, Gimbel RW, et al. Interventional radiologists have equitable outcomes and lower costs from totally implantable venous access device (TIVAD) placement compared to operating room placement. J Vasc Interv Radiol JVIR. (2022) 33(10):1184–90. doi: 10.1016/j.jvir.2022.07.004
191. Kanters TA, Raaijmakers CPAM, Lohle PNM, Vries J de, Roijen LH van, SPLENIQ study group. Cost effectiveness of splenic artery embolization versus splenectomy after trauma in The Netherlands. J Vasc Interv Radiol. (2022) 33(4):392–398.e4. doi: 10.1016/j.jvir.2021.12.011
192. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. (2013) 59(2):300–7. doi: 10.1016/j.jhep.2013.04.009
193. Spolverato G, Vitale A, Ejaz A, Kim Y, Maithel SK, Cosgrove DP, et al. The relative net health benefit of liver resection, ablation, and transplantation for early hepatocellular carcinoma. World J Surg. (2015) 39(6):1474–84. doi: 10.1007/s00268-015-2987-7
194. Sennfält K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis—a cost–utility analysis. Perit Dial Int. (2002) 22(1):39–47. doi: 10.1177/089686080202200107
195. Sato M, Morimoto R, Seiji K, Iwakura Y, Ono Y, Kudo M, et al. Cost-effectiveness analysis of the diagnosis and treatment of primary aldosteronism in Japan. Horm Metab Res. (2015) 47(11):826–32. doi: 10.1055/s-0035-1559645
196. Lubitz CC, Economopoulos KP, Sy S, Johanson C, Kunzel HE, Reincke M, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circ Cardiovasc Qual Outcomes. (2015) 8(6):621–30. doi: 10.1161/CIRCOUTCOMES.115.002002
197. Myssiorek D, Siegel D, Vambutas A. Fluoroscopically placed percutaneous gastrostomies in the head and neck patient. Laryngoscope. (1998) 108(10):1557–60. doi: 10.1097/00005537-199810000-00025
198. Barkmeier JM, Trerotola SO, Wiebke EA, Sherman S, Harris VJ, Snidow JJ, et al. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. (1998) 21(4):324–8. doi: 10.1007/s002709900269
199. Trivedi PS, Jensen AM, Brown MA, Hong K, Borgstede JP, Lindrooth RC, et al. Cost analysis of dialysis access maintenance interventions across physician specialties in U.S. medicare beneficiaries. Radiology. (2020) 297(2):474–81. doi: 10.1148/radiol.2020192403
200. Cain-Nielsen AH, Moriarty JP, Stewart EA, Borah BJ. Cost–effectiveness of uterine-preserving procedures for the treatment of uterine fibroid symptoms in the USA. J Comp Eff Res. (2014) 3(5):503–14. doi: 10.2217/CER.14.32
201. Volkers NA, Hehenkamp WJK, Smit P, Ankum WM, Reekers JA, Birnie E. Economic evaluation of uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids: results from the randomized EMMY trial. J Vasc Interv Radiol. (2008) 19(7):1007–16. doi: 10.1016/j.jvir.2008.03.001
202. Cil BE. Radiological placement of chest ports in pediatric oncology patients. Eur Radiol. (2004) 14(11):2015–9. doi: 10.1007/s00330-004-2380-7
203. Armitage JN, Withington J, Fowler S, Finch WJG, Burgess NA, Irving SO, et al. Percutaneous nephrolithotomy access by urologist or interventional radiologist: practice and outcomes in the UK. BJU Int. (2017) 119(6):913–8. doi: 10.1111/bju.13817
204. Murphy KJ, Elias G, Jaffer H, Mandani R. A study of inventiveness among society of interventional radiology members and the impact of their social networks. J Vasc Interv Radiol. (2013) 24(7):931–7. doi: 10.1016/j.jvir.2013.03.033
205. Steinberger JD, Denend L, Azagury DE, Brinton TJ, Makower J, Yock PG. Needs-based innovation in interventional radiology: the biodesign process. Tech Vasc Interv Radiol. (2017) 20(2):84–9. doi: 10.1053/j.tvir.2017.04.006
206. Midulla M, Pescatori L, Chevallier O, Nakai M, Ikoma A, Gehin S, et al. Future of IR: emerging techniques, looking to the future…and learning from the past. J Belg Soc Radiol. (2019) 103(1):12. doi: 10.5334/jbsr.1727
207. Silva JNA, Southworth M, Raptis C, Silva J. Emerging applications of virtual reality in cardiovascular medicine. JACC Basic Transl Sci. (2018) 3(3):420–30. doi: 10.1016/j.jacbts.2017.11.009
208. Chapiro J, Allen B, Abajian A, Wood B, Kothary N, Daye D, et al. Proceedings from the society of interventional radiology foundation research consensus panel on artificial intelligence in interventional radiology: from code to bedside. J Vasc Interv Radiol JVIR. (2022) 33(9):1113–20. doi: 10.1016/j.jvir.2022.06.003
209. Morrison JJ. Evolution in private practice interventional radiology: data mining trends in procedure volumes. Semin Interv Radiol. (2019) 36(1):17–22. doi: 10.1055/s-0039-1683358
210. Taylor K, Sansivero GE, Ray CE. The role of the nurse practitioner in interventional radiology. J Vasc Interv Radiol. (2012) 23(3):347–50. doi: 10.1016/j.jvir.2011.11.002
211. Stecker MS, Armenoff D, Johnson MS. Physician assistants in interventional radiology practice. J Vasc Interv Radiol JVIR. (2004) 15(3):221–7. doi: 10.1097/01.rvi.0000099529.29957.0b
212. NHE Fact Sheet | CMS. Available online at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet (Accessed July 15, 2022).
213. LLC XHM. Xtelligent Healthcare Media. Available online at: https://www.xtelligentmedia.com/insights/value-based-care-assessment (Accessed July 15, 2022).
214. Beck DE, Margolin DA. Physician coding and reimbursement. Ochsner J. (2007) 7(1):8–15. PMID: 21603473; PMCID: PMC3096340.21603473
215. Schartz D, Young E. Medicare reimbursement trends for interventional radiology procedures: 2012 to 2020. J Vasc Interv Radiol. (2021) 32(3):447–52. doi: 10.1016/j.jvir.2020.12.007
216. Oklu R, Haas D, Kaplan RS, Brinegar KN, Bassoff N, Harvey HB, et al. Time-driven activity-based costing in IR. J Vasc Interv Radiol. (2015) 26(12):1827–31. doi: 10.1016/j.jvir.2015.07.007
217. Ljuboja D, Ahmed M, Ali A, Perez E, Subrize MW, Kaplan RS, et al. Time-driven activity-based costing in interventional oncology: cost measurement and cost variability for hepatocellular carcinoma therapies. J Am Coll Radiol. (2021) 18(8):1095–105. doi: 10.1016/j.jacr.2021.03.027
218. Anzai Y, Heilbrun ME, Haas D, Boi L, Moshre K, Minoshima S, et al. Dissecting costs of CT study: application of TDABC (time-driven activity-based costing) in a tertiary academic center. Acad Radiol. (2017) 24(2):200–8. doi: 10.1016/j.acra.2016.11.001
219. Baadh A, Peterkin Y, Wegener M, Flug J, Katz D, Hoffmann JC. The relative value unit: history, current use, and controversies. Curr Probl Diagn Radiol. (2016) 45(2):128–32. doi: 10.1067/j.cpradiol.2015.09.006
220. Kwan SW, Talenfeld AD, Brunner MC. The top three health care developments impacting the practice of interventional radiology in the next decade. Am J Roentgenol. (2016) 207(4):731–6. doi: 10.2214/AJR.16.16435
221. David Prologo J, Meltzer CC. Health care reform in the United States: an opportunity for interventional radiologists. J Vasc Interv Radiol. (2014) 25(6):881–7. doi: 10.1016/j.jvir.2014.02.030
222. Liu R. The value of interventional radiology: an imperative to understand costs. J Vasc Interv Radiol. (2021) 32(4):614–5. doi: 10.1016/j.jvir.2020.10.032
223. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. (2016) 316(10):1093–103. doi: 10.1001/jama.2016.12195
224. Sarwar A, Hawkins CM, Bresnahan BW, Carlos RC, Guimaraes M, Krol KL, et al. Evaluating the costs of IR in health care delivery: proceedings from a society of interventional radiology research consensus panel. J Vasc Interv Radiol. (2017) 28(11):1475–86. doi: 10.1016/j.jvir.2017.07.024
225. McGinty G. Is value-driven health care an unfunded mandate for radiologists? Am J Roentgenol. (2016) 206(2):280–2. doi: 10.2214/AJR.15.15399
226. Sarwar A, Zhou L, Chakrala N, Brook OR, Weinstein JL, Rosen MP, et al. The relevance of readmissions after common IR procedures: readmission rates and association with early mortality. J Vasc Interv Radiol. (2017) 28(5):629–36. doi: 10.1016/j.jvir.2017.01.008
227. Gradinscak DJ, Young N, Jones Y, O’Neil D, Sindhusake D. Risks of outpatient angiography and interventional procedures: a prospective study. Am J Roentgenol. (2004) 183(2):377–81. doi: 10.2214/ajr.183.2.1830377
228. Chau A, Hernandez JA, Pimpalwar S, Ashton D, Kukreja K. Equivalent success and complication rates of tunneled common femoral venous catheter placed in the interventional suite vs. at patient bedside. Pediatr Radiol. (2018) 48(6):889–94. doi: 10.1007/s00247-018-4090-3
229. Van Natta TL, Morris JAJ, Eddy VA, Nunn CR, Rutherford EJ, Neuzil D, et al. Elective bedside surgery in critically injured patients is safe and cost-effective. Ann Surg. (1998) 227(5):618–26. doi: 10.1097/00000658-199805000-00002
230. Nunn CR, Neuzil D, Naslund T, Bass JG, Jenkins JM, Pierce R, et al. Cost-effective method for bedside insertion of vena caval filters in trauma patients. J Trauma Acute Care Surg. (1997) 43(5):752–8. doi: 10.1097/00005373-199711000-00004
231. Rosenthal D, Wellons ED, Levitt AB, Shuler FW, O’Conner RE, Henderson VJ. Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma. J Vasc Surg. (2004) 40(5):958–64. doi: 10.1016/j.jvs.2004.07.048
232. Langwell KM, Hadley JP. Capitation and the medicare program: history, issues, and evidence. Health Care Financ Rev. (1986) 1986(Suppl):9–20. PMID: 10311935; PMCID: PMC4195086.10311935
233. Capitated Model | CMS. Available online at: https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/FinancialAlignmentInitiative/CapitatedModel (Accessed July 19, 2022).
234. Practice parameter for interventional clinical practice and management. J Vasc Interv Radiol. (2015) 26(8):1197–204. doi: 10.1016/j.jvir.2015.05.017
235. Jin H, Liu J. Application of the hybrid operating room in surgery: a systematic review. J Investig Surg Off J Acad Surg Res. (2022) 35(2):378–89. doi: 10.1080/08941939.2020.1838004
236. Karatasakis A, Brilakis ES. Shields and garb for decreasing radiation exposure in the cath lab. Expert Rev Med Devices. (2018) 15(9):683–8. doi: 10.1080/17434440.2018.1510771
237. Goldsweig AM, Abbott JD, Aronow HD. Physician and patient radiation exposure during endovascular procedures. Curr Treat Options Cardiovasc Med. (2017) 19(2):10. doi: 10.1007/s11936-017-0507-9
238. d’Othée BJ, Lin PJP. The influence of angiography table shields and height on patient and angiographer irradiation during interventional radiology procedures. Cardiovasc Intervent Radiol. (2007) 30(3):448–54. doi: 10.1007/s00270-006-0063-2
239. De Gaudio AR, Rinaldi S. Sedation in PACU: indications, monitoring, complications. Curr Drug Targets. (2005) 6(7):729–40. doi: 10.2174/138945005774574542
240. Benson H. Clinical radiology UK workforce census 2015 report. 57. (2015). Available online at: https://www.rcr.ac.uk/media/3wme412w/rcr-publications_clinical-radiology-uk-workforce-census-2015-report_september-2016.pdf (Accessed April 16, 2024).
241. Wah TM, Belli AM. The interventional radiology (IR) gender gap: a prospective online survey by the cardiovascular and interventional radiological society of Europe (CIRSE). Cardiovasc Intervent Radiol. (2018) 41(8):1241–53. doi: 10.1007/s00270-018-1967-3
242. IRQ Articles - e-IRQ. Available online at: https://connect.sirweb.org/e-irq/participate/viewirqarticle?DocumentKey=c13863f3-bf1e-471f-b77c-f0b426ac928b (Accessed June 20, 2022).
243. Sweeney AM, Wadhwa V, Farrell JJ, Makary MS. Interventional radiology education for improving primary care provider awareness. Curr Probl Diagn Radiol. (2022) 51(3):308–12. doi: 10.1067/j.cpradiol.2021.05.003
244. Makary MS, Gage D, Elliott ED, Dowell JD. Primary care provider awareness of IR: a single-center analysis. J Vasc Interv Radiol JVIR. (2019) 30(9):1420–7. doi: 10.1016/j.jvir.2019.04.001
245. Rodgers B, Rodgers KA, Chick JFB, Makary MS. Public awareness of interventional radiology: population-based analysis of the current state of and pathways for improvement. J Vasc Interv Radiol. (2023) 34(6):960–7.e6. doi: 10.1016/j.jvir.2023.01.033
246. Bassuner J, Duncan D, Molloy C, Makary MS, Bodell B, Assael D, et al. Recruiting medical students to interventional radiology: current state of affairs. Acad Radiol. (2019) 26(9):1274–7. doi: 10.1016/j.acra.2019.01.015
247. ‘Interventional What?’ Available online at: https://www.radiologytoday.net/archive/WebEx0219.shtml (Accessed August 10, 2022).
248. Lanza C, Carriero S, Buijs EFM, Mortellaro S, Pizzi C, Sciacqua LV, et al. Robotics in interventional radiology: review of current and future applications. Technol Cancer Res Treat. (2023) 22:15330338231152084. doi: 10.1177/15330338231152084
Keywords: interventional radiology, IR, value, scope of practice review, successful practices, comparative analysis, practice growth, IR payment models
Citation: Campbell WA, Chick JFB, Shin DS and Makary MS (2024) Value of interventional radiology and their contributions to modern medical systems. Front. Radiol. 4: 1403761. doi: 10.3389/fradi.2024.1403761
Received: 19 March 2024; Accepted: 25 June 2024;
Published: 17 July 2024.
Edited by:
Edit Dósa, Semmelweis University, HungaryReviewed by:
Edward Kuoy, University of California, Irvine, United StatesMo Hamady, Imperial College Healthcare NHS Trust, Imperial College London, United Kingdom
© 2024 Campbell IV, Chick, Shin and Makary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mina S. Makary, bWluYS5tYWthcnlAb3N1bWMuZWR1
 Warren A. Campbell IV
Warren A. Campbell IV Jeffrey F. B. Chick2
Jeffrey F. B. Chick2 Mina S. Makary
Mina S. Makary