- 1Department of Diagnostic Radiology, Tohoku University Hospital, Sendai, Japan
- 2Department of Diabetes, Metabolism and Endocrinology, Tohoku University Hospital, Sendai, Japan
Adrenal vein sampling (AVS) is the gold standard for subtyping primary aldosteronism (PA). However, through conventional AVS, unilateral PA may be misdiagnosed as bilateral PA. Compared with conventional AVS, segmental AVS with additional sampling in adrenal tributaries can detect aldosterone-producing adenomas (APAs) with higher sensitivity. Herein, we describe two cases wherein high aldosterone levels were not detected through initial segmental AVS but were identified in anomalous drainage veins during the second AVS session. In Case 1, computed tomography (CT) during left adrenal arteriovenography revealed a fine renal capsular vein connecting an adrenal nodule to the third lumbar vein. Sampling in this vein during the second AVS revealed high aldosterone levels. The surgical specimen showed the presence of an 11 mm APA. Furthermore, Case 2 presented with bilateral small adrenal nodules; bilateral renal capsular vein sampling was performed during the second AVS session. The samples from the renal capsular vein connected to the renal vein revealed considerably high aldosterone levels. Left adrenalectomy revealed the presence of a 6 mm aldosterone-producing nodule. These cases highlight the importance of anomalous drainage vein sampling, the limitation of conventional and segmental AVS in diagnosing PA, and the utility of CT during adrenal arteriovenography for estimating the drainage route.
1 Introduction
Adrenal vein sampling (AVS) is the gold standard for subtyping primary aldosteronism (PA), a common cause of secondary hypertension (1, 2). Most subtypes include unilateral PA due to aldosterone-producing adenoma (APA) and bilateral PA due to idiopathic hyperaldosteronism or bilateral hyperaldosteronism. Some cases of unilateral APA could be misdiagnosed as bilateral PA through conventional AVS (cAVS) in which samples are obtained from both adrenal veins (3–7).
Compared with cAVS, segmental AVS (sAVS) in which samples are obtained from adrenal tributaries using a microcatheter can detect APA and small aldosterone-producing nodules (APNs) with higher sensitivity (6). However, in rare cases, aldosterone levels may only be detected in anomalous drainage veins (ADV). The sampling techniques in the ADV are not well-known, including the identification of target blood vessels, the choice of catheters, the specific sampling positions, and the expected results. We successfully detected elevated aldosterone levels through this route. Herein, we reported the details of these challenging cases. In Case 1, a 55-year-old woman was diagnosed with hypertension (141/107 mmHg) and hypokalemia (serum potassium level, 3.2 mmol/L) during a health checkup and subsequent further examination at a local hospital. She had a medical history of depression, asthma, and sleep apnea syndrome. Laboratory tests revealed elevated plasma aldosterone concentration (PAC; 17.9 ng/dl) and low plasma renin activity (PRA; 0.5 ng/ml/h), resulting in a high aldosterone-to-renin ratio (ARR; 35.8) with daily administration of amlodipine (5 mg) at a prior hospital. Captopril challenge test showed an ARR of 164 after 90 min, and saline infusion test revealed a PAC of 28.7 ng/dl after 240 min; thus, confirming PA (2). In Case 2, a 57-year-old man with a 7-year history of hypertension presented with hypokalemia (serum potassium level, 2.9 mmol/L) at a local doctor. PA screening test is positive. At the time of admission, the PAC was 10.6 ng/dl, PRA was ≤0.2 ng/ml/h, ARR was ≥53 with daily oral administration of amlodipine (10 mg) and potassium chloride (45 mmol) (1, 2). Captopril challenge test showed an ARR of 45.2 after 90 min, and saline infusion test revealed a PAC of 6.0 ng/dl after 240 min; thus, confirming PA (2).
2 Case description
2.1 Case 1
Before AVS, precontrast and dynamic contrast-enhanced computed tomography (CT) were performed, and an 11 mm adrenocortical adenoma was detected in the left adrenal gland (Figure 1A). In segmental AVS (sAVS), samples were obtained from both adrenal central veins and tributaries to localize excess aldosterone, as previously described (6). Adrenocorticotropic hormone (ACTH) was administered at an initial bolus dose of 200 µg, followed by a continuous intravenous infusion at a rate of 50 µg/h starting 30 min later (6, 7).
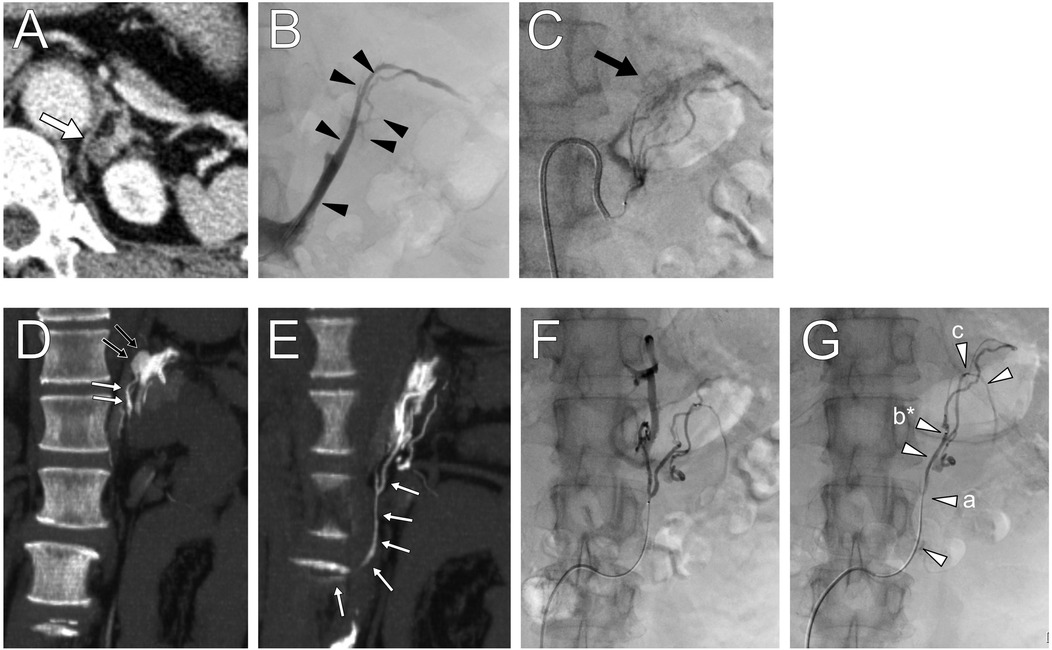
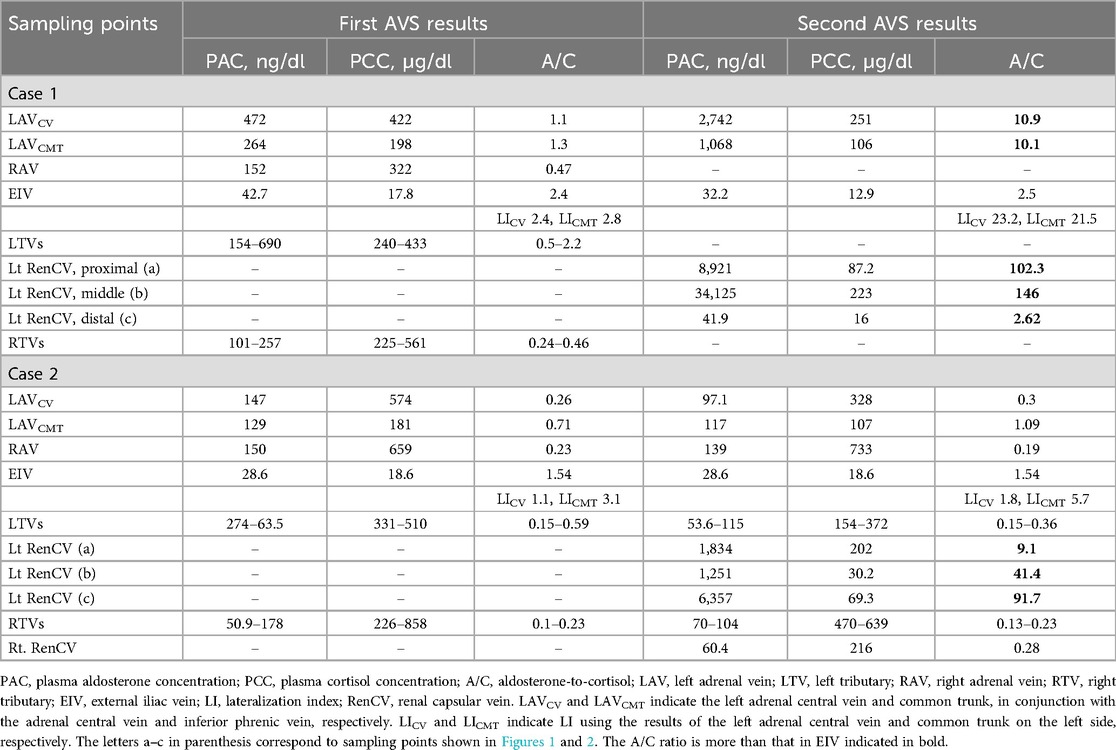
Figure 1. Contrast-enhanced computed tomography (CT) images showing (A) a low-density left adrenal nodule (white arrow). (B) Left adrenal venography image highlighting the locations of segmental adrenal venous sampling in the tributaries (black arrowheads). (C) The left inferior adrenal arteriography depicting a tumor stain. (D,E) CT during adrenal arteriovenography revealed tumor enhancement (black small arrows) and a drainage vein (white small arrows) connected to the third lumbar vein. (F,G) Anomalous drainage vein sampling was performed at several sites (white arrowheads) from the vein. The letters a–c in parenthesis shown in Table 1 correspond to sampling points, and those marked with an asterisk indicate the maximum aldosterone concentration.
Initial sAVS was technically successful, and samples were collected from both adrenal veins, several tributaries (Figure 1B), and the left inferior phrenic vein. Table 1 shows the blood sampling results. The selectivity index after ACTH stimulation confirmed successful AVS (2). Aldosterone secretion was mildly predominant on the left side, with a lateralization index [LI; defined as the aldosterone-to-cortisol ratio (A/C) of the dominant side over the contralateral side] of 2.4. The common cutoff for LI is four, and in this case, the result suggested bilateral PA. However, the A/C ratios of the right and left adrenal veins were 0.47 and 1.12, respectively, which were lower than the peripheral A/C ratio of 2.4, indicating apparent bilateral aldosterone suppression (ABAS) or double-down state (5, 8–11). Moreover, the A/C ratio in adrenal tributaries was ≤2.2. This leads to the possibility of anomalous blood drainage from an aldosterone-producing lesion.
Considering abnormal aldosterone-rich blood drainage from the left adrenal cortical adenoma, we reanalyzed the preoperative 0.25-mm-thick CT images using an ultra-high resolution multidetector CT scanner (Aquilion Precision, Canon Medical Systems Corporation, Otawara, Japan), which revealed a barely identifiable fine extra-adrenal vein connected to the adenoma. The total drainage route was not identified, and we attempted to elucidate the route using CT during adrenal arteriovenography (CTAV), which may be used to detect the right adrenal vein during AVS (12, 13) and obtain samples from this vein.
A 5 Fr sheath introducer and a 5 Fr Shepherd hook-shaped diagnostic catheter were inserted from the right femoral artery to identify the left superior and inferior adrenal artery branching from the inferior phrenic artery and accessory renal artery, respectively. Using a 1.7–2.8 Fr tapered microcatheter (Asahi Veloute, Asahi Intecc, Tokyo, Japan) and a 0.016-inch micro guidewire (Asahi Meister, Asahi Intecc, Tokyo, Japan), we cannulated the left superior and inferior adrenal artery and performed CTAV (Figure 1C). The fivefold diluted contrast media was injected at the minimum speed of 0.3 ml/s by a power injector, with scanning starting 10 s after injection began. CTAV images from the inferior adrenal artery showed an enhanced adrenal nodule and a fine renal capsular vein connected to the nodule, draining into the third lumbar vein (Figures 1D,E).
The left third lumbar vein and distal renal capsular vein were cannulated using a 5 Fr Cobra-shaped diagnostic catheter, 2–2.9 Fr tapered split-tip microcatheter (Goldcrest NEO type OM, Medicos Hirata, Osaka, Japan), and micro guidewire from the femoral vein. After ACTH stimulation, blood was sampled from several sites within the vein (Figures 1F,G) under heparinization. Blood sampling required fine positioning due to insufficient blood flow and venous spasms. Finally, blood from the adrenal central vein was collected on the left side. The samples were sequentially submitted to the laboratory for intraoperative measurement, which confirmed elevated aldosterone levels, and the procedure was concluded. The total procedure time was 216 min, with a fluoroscopy time of 67.6 min and radiation dose of 431.2 mGy; the volume of contrast medium used was 77 ml.
The samples showed elevated aldosterone levels in the capsular vein via the lumbar vein, with a maximum PAC of 34,125 ng/dl and an A/C ratio of 146 (Table 1). This led to the diagnosis of left unilateral PA due to a CT-detectable APA, warranting surgery. Additionally, the left adrenal vein PAC and A/C ratio were higher during the second AVS session (2,742 ng/dl and 10.9, respectively) than during the first AVS session. An LI of 23.2 was obtained based on the results of the first right AVS (PAC, 152 ng/dl; A/C ratio, 0.47), which indicated left unilateral PA based on cAVS.
Histopathologically, robotic-assisted left adrenalectomy revealed a CYP11B2-positive aldosterone-producing adrenocortical adenoma (11 mm × 7 mm) (14). Hypertension and hypokalemia were cured, with an ARR of <20, achieving complete biochemical and clinical success 6 months postoperatively, based on the primary aldosteronism surgery outcome (PASO) criteria (15).
2.2 Case 2
Preoperative CT revealed small adrenal nodules of ≤6 mm diameter on both sides (Figures 2A,B). sAVS was successfully performed (Figures 2C,D) (2). Table 1 shows the blood sampling results. The LI was 1.1, suggesting bilateral PA; however, the A/C ratios in the adrenal veins and tributaries were ≤0.71; this ratio was lower than that of the iliac vein (1.54). This indicated an ABAS/double-down state (5, 8–11), suggesting anomalous blood drainage of a PA lesion.
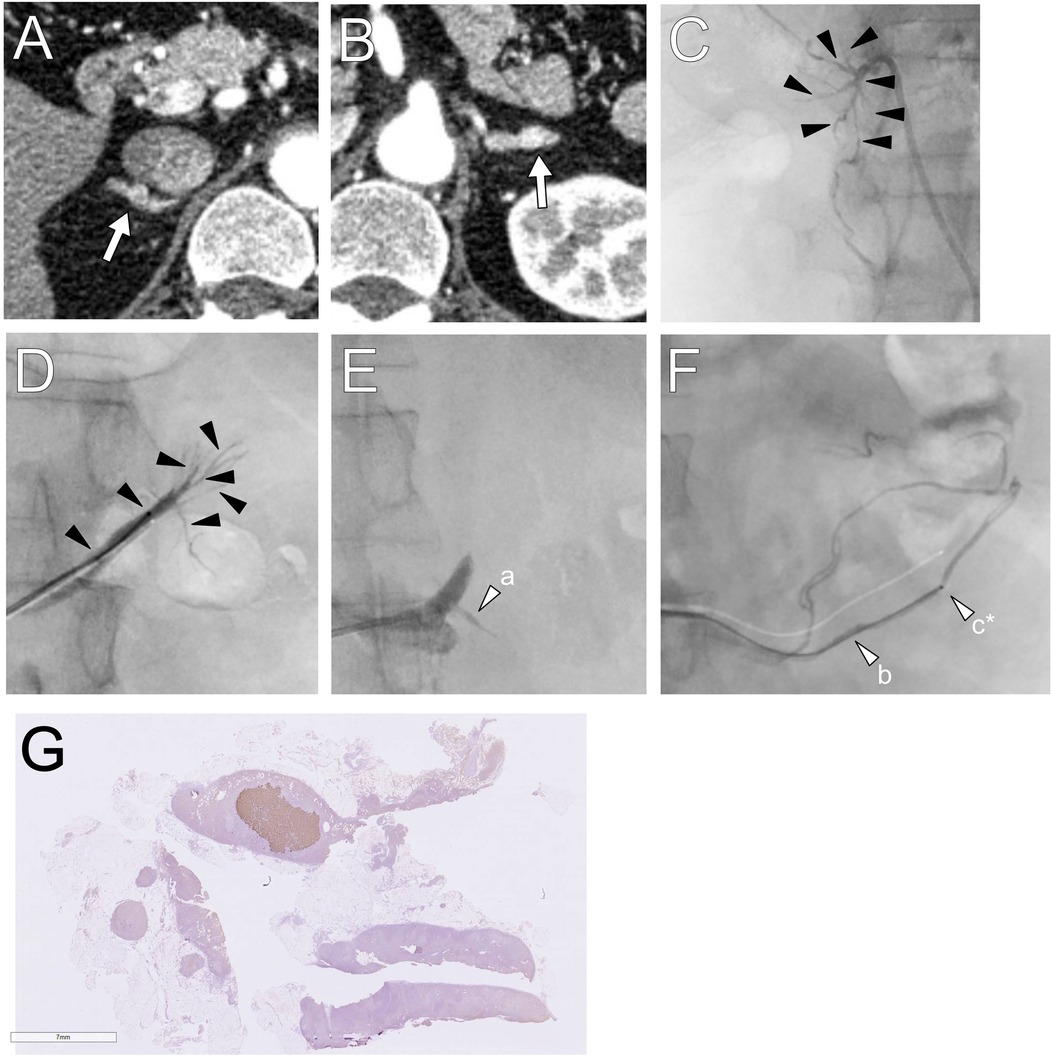
Figure 2. (A,B) Computed tomography images showing small adrenal nodules are detected in the bilateral adrenal glands. (C,D) In the first session, segmental adrenal tributary venous sampling was performed in the tributaries (black arrowheads). (E,F) In the second session, left renal capsular vein sampling was conducted in the fine veins connected to the proximal portion of the adrenal vein and the renal vein (white arrows). The letters a–c in parenthesis shown in Table 1 correspond to sampling points, and those marked with an asterisk indicate the maximum aldosterone concentration. (G) Histopathologically, a 6 mm × 4 mm CYP11B2-positive adrenocortical nodule was revealed.
A second sAVS was performed, and samples were obtained from the bilateral adrenal tributaries and renal capsular veins connected to the proximal portion of the adrenal vein and renal vein (Figures 2E,F). Intraoperative laboratory testing revealed no elevation of aldosterone levels in the right samples, and the left samples were not examined immediately because it was outside of service hours. This was guided by preoperative contrast-enhanced CT and digital subtraction adrenal venography images, which barely depicted fine renal capsular veins. CTAV was not performed because the highly suspected APA was absent. Blood was also collected from the bilateral adrenal veins. The total procedure time was 284 min, with fluoroscopy time of 67.6 min and radiation dose of 1,144 mGy; the volume of contrast medium used was 85 ml.
The maximum PAC and A/C values in the left renal capsular vein were 6,357 ng/dl and 91.7, respectively (Table 1). The second AVS confirmed left unilateral PA due to an APN, indicating surgery. The PAC in the right or left adrenal central vein and the common trunk (conjunction of the left central vein and phrenic vein) were similar; however, the A/C was relatively higher in the common trunk, with an LI of 5.7.
Robotic-assisted left adrenalectomy was performed, revealing a CYP11B2-positive aldosterone-producing nodule (6 mm × 4 mm) histopathologically (14). Hypertension and hypokalemia were resolved after surgery, with an ARR of <20. The patient achieved biochemical and clinical complete success 6 months postoperatively, based on the PASO criteria (15).
3 Discussion
Tumor blood from APAs or APNs might primarily drain out to ADVs rather than the adrenal vein. Herein, we reported two extremely rare cases in which high aldosterone levels were not detected in initial sAVS but were identified in the ADV, specifically the fine renal capsular vein via the lumbar vein and renal vein, including the technical details of their identification. These cases highlight the importance of recognizing anomalous venous drainage routes and the utility of advanced imaging techniques, such as CTAV for precise PA subtyping.
Unilateral APA or APN can be misclassified as bilateral PA through cAVS, because of the following reasons: (1) limited or lack of tumor blood drainage via the adrenal vein; (2) dilution of tumor blood in the adrenal vein due to contamination of normal adrenal blood; (3) technical errors, such as unintentional super-selective sampling beyond the tumor's draining tributary; (4) sampling during the quiescent phase of aldosterone secretion; (5) ectopic APAs; and (6) presence of cortisol-producing lesions, where subtyping with A/C ratio and LI is strongly influenced by cortisol suppression in the adrenal gland (3, 4, 6, 10, 16). sAVS, which includes additional sampling from adrenal tributaries, can diagnose APA and APN more sensitively (3, 4, 6). However, as observed in the current cases, sAVS can still yield false-negative results of APA and APN due to the first reason, although rarely (estimated at <1% of APAs and APNs).
The adrenal gland has an extensive venous network (17–20). Meikos described anatomical extraglandular adrenal veins in addition to the central vein through cadaver studies and revealed that these veins connect to the inferior phrenic vein, adrenal central vein, inferior vena cava, and renal vein (18). These pathways can be visualized via adrenal venography (21) and potentially act as ADV. In fact, previous studies have reported high aldosterone levels in the renal capsular vein, inferior phrenic vein, and lumbar vein in the context of APAs (10, 22, 23). ADV sampling is not always essential for the diagnosis of APAs because some amount of tumor blood usually flows into the adrenal vein. However, as observed in the present study, it may be necessary for diagnosing APA or APN in rare cases.
As the sampling and identification methods for ADVs of APAs remain largely unknown, this report might be highly valuable. Previous reports have demonstrated the utility of CTAV for visualizing the right adrenal vein (12, 13), which is sometimes difficult to cannulate and is a common cause of technical failure during AVS. Case 1 showed the utility of this CTAV for identifying the route of drainage, which was impossible via contrast-enhanced CT. Conversely, in Case 2, CTAV was not utilized because of the presence of bilateral small adrenal nodules on CT; none of these nodules were highly suspected to be APA. This case highlights the possibility of sampling from the anomalous pathways by referring to thin-section CT images and adrenal venography. However, detection of small vessels at approximately 1–2 mm in size on imaging, as well as cannulation and sampling, is challenging.
Predicting the need for ADV sampling before or during AVS is difficult. Even when an ADV appears connected to the tumor on CT, adrenal vein or tributary sampling often captures nearly all APA-derived blood. Rapid aldosterone and cortisol measurements can help verify whether tumor blood is present in the sample and assist in determining the intraprocedural endpoint during a second AVS, as demonstrated in the present cases, and potentially during the first AVS, although this approach requires additional time. In the ABAS or double-down state, where the A/C ratio is lower in the bilateral adrenal venous blood than in peripheral blood, ADV sampling should be considered to be performed after the first AVS because one of the causes is tumor blood drainage into the ADV. DePietro et al. (5) described 10 patients with APAs who exhibited ABAS in the first cAVS and underwent repeated AVS. Among these patients, three showed bilateral PA twice. Thus, further examination, such as sampling from ADVs or sAVS, might be needed for accurate subtyping. To our knowledge, ADV sampling is technically challenging and rarely necessary; therefore, sAVS, which is easier to perform, is preferable.
However, the seven remaining patients had unilateral PA with an LI of ≥3.5 from repeated cAVS, with caution of incidental deep sampling (5). Thus, only repeated precise cAVS alone might be sufficient for accurate subtyping. We routinely perform segmental AVS and ensure not to go beyond the branching points of all tributaries, making technical causes unlikely. Nevertheless, the calculated LI based on the second AVS becomes higher than on the first AVS. However, it has no supporting evidence. In Case 1, the LI of 23.2 in the second AVS indicated unilateral PA. However, the vasospasm of the renal capsular vein during sampling might have altered the drainage pathway of the APA blood to the adrenal vein, which blood was sampled after the onset of the vasospasm. In Case 2, the LI was 1.8 and 5.7 based on the results of the left adrenal central vein and the common trunk, the conjunction between the left adrenal central vein and inferior phrenic vein, respectively. The LI of 5.7 indicated unilateral PA; however, equal levels of aldosterone in the left common trunk and right adrenal veins (PAC, 117 and 139 ng/dl, respectively) might cause hesitation in determining unilateral PA and indication for surgery. Thus, the detection of high aldosterone levels in ADV would be particularly beneficial to determining the treatment plan.
In ABAS cases, other possible causes, which are similar to the reasons for APA and APN misclassification, should also be considered, except for the cortisol-producing lesion mentioned above. Cases of blood sampling during a quiescent period of aldosterone secretion reportedly have decreased with ACTH stimulation (9–11). Therefore, repeated cAVS under ACTH stimulation should be considered. The possibility is unintended super-selected sampling, which is a technical issue where blood is sampled beyond the branches where tumor blood is being discharged. Anatomical variations, such as duplicate adrenal veins, should also be considered (17, 24, 25). The possibility of ectopic APAs could exist but it is extremely rare.
In recent years, non-invasive lateralization techniques such as 11C-metomidate positron emission tomography (PET) and 68Ga-Pentixafor PET/CT have been investigated at select centers (26, 27). For APAs ≥1 cm, 68Ga-Pentixafor PET has demonstrated high sensitivity, with a detection rate of 97.3% (36/37) (27). However, false negatives for APAs and false positives for non-functioning adrenal adenomas can still occur. A concordance rate of 77% between 68Ga-Pentixafor PET and AVS findings has been reported (28), indicating that neither modality is definitively superior. Similar to other PET tracers, PET/CT imaging is limited by spatial resolution. Although PA lesions smaller than 1 cm can occasionally be detected, the detection rate for subcentimeter lesions was only 60% (3/5) (27), indicating potentially lower sensitivity for small lesions. Access to these advanced imaging techniques remains extremely limited, including at our institution. Although the approach presented in this report is not necessarily simple, it is considered feasible and clinically valuable.
In conclusion, sampling from ADVs is rarely essential for the accurate diagnosis of APAs and APNs. Moreover, CT during adrenal arteriovenography might be useful for precisely estimating the drainage routes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tohoku University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HT: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. SO: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. HK: Writing – review & editing. YT: Resources, Writing – review & editing. YO: Resources, Writing – review & editing. KO: Conceptualization, Methodology, Resources, Visualization, Writing – review & editing. KT: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by JSPS KAKENHI Grant Number 23K14831.
Acknowledgments
The authors appreciate Mr. Kazutoshi Shirotori (Department of Radiological Technology, Tohoku University Hospital) for their assistance as a radiologic technologist and Enago (https://www.enago.jp) for the English language review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061
2. Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J. (2022) 69(4):327–59. doi: 10.1507/endocrj.EJ21-0508
3. Satoh F, Morimoto R, Seiji K, Satani N, Ota H, Iwakura Y, et al. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. (2015) 173(4):465–77. doi: 10.1530/EJE-14-1161
4. Makita K, Nishimoto K, Kiriyama-Kitamoto K, Karashima S, Seki T, Yasuda M, et al. A novel method: super-selective adrenal venous sampling. J Vis Exp. (2017) (127):55716. doi: 10.3791/55716
5. DePietro DM, Fraker DL, Wachtel H, Cohen DL, Trerotola SO. “Double-down” adrenal vein sampling results in patients with apparent bilateral aldosterone suppression: utility of repeat sampling including super-selective sampling. J Vasc Interv Radiol. (2021) 32(5):656–65. doi: 10.1016/j.jvir.2020.12.029
6. Tannai H, Makita K, Koike Y, Nakai K, Tsurutani Y, Okudela K, et al. Usefulness and accuracy of segmental adrenal venous sampling on localisation and functional diagnosis of various adrenal lesions in primary aldosteronism. Clin Radiol. (2022) 77(8):e652–9. doi: 10.1016/j.crad.2022.05.010
7. Satani N, Ota H, Seiji K, Morimoto R, Kudo M, Iwakura Y, et al. Intra-adrenal aldosterone secretion: segmental adrenal venous sampling for localization. Radiology. (2016) 278(1):265–74. doi: 10.1148/radiol.2015142159
8. Wolley M, Gordon RD, Pimenta E, Daunt N, Slater GJ, Ahmed AH, et al. Repeating adrenal vein sampling when neither aldosterone/cortisol ratio exceeds peripheral yields a high incidence of aldosterone-producing adenoma. J Hypertens. (2013) 31(10):2005–9. doi: 10.1097/HJH.0b013e328362add3
9. Wolley MJ, Ahmed AH, Gordon RD, Stowasser M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin Endocrinol. (2016) 85(5):703–9. doi: 10.1111/cen.13110
10. Shibayama Y, Wada N, Umakoshi H, Ichijo T, Fujii Y, Kamemura K, et al. Bilateral aldosterone suppression and its resolution in adrenal vein sampling of patients with primary aldosteronism: analysis of data from the WAVES-J study. Clin Endocrinol. (2016) 85(5):696–702. doi: 10.1111/cen.13090
11. Shibayama Y, Wada N, Naruse M, Kurihara I, Ito H, Yoneda T, et al. The occurrence of apparent bilateral aldosterone suppression in adrenal vein sampling for primary aldosteronism. J Endocr Soc. (2018) 2(5):398–407. doi: 10.1210/js.2017-00481
12. Oguro S, Nakatsuka S, Jinzaki M, Misu M, Yashiro H, Hashimoto S, et al. Visualization of the right adrenal vein using CT during right inferior phrenic arteriography in hepatocellular carcinoma patients. Jpn J Radiol. (2014) 32(11):630–6. doi: 10.1007/s11604-014-0356-3
13. Oguro S, Nakatsuka S, Yashiro H, Hashimoto S, Miyashita K, Kurihara I, et al. CT during arteriography to visualize the right adrenal vein for adrenal venous sampling. J Vasc Interv Radiol. (2015) 26(6):910–4. doi: 10.1016/j.jvir.2015.02.008
14. Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. (2021) 106(1):42–54. doi: 10.1210/clinem/dgaa484
15. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. (2017) 5(9):689–99. doi: 10.1016/S2213-8587(17)30135-3
16. Tannai H, Makita K, Nakai K, Sato Y, Tsurutani Y, Saito J, et al. Differences between left adrenal vein sampling sites revealed with segmental sampling in primary aldosteronism. Br J Radiol. (2023) 96(1151):20220766. doi: 10.1259/bjr.20220766
17. Cesmebasi A, Du Plessis M, Iannatuono M, Shah S, Tubbs RS, Loukas M. A review of the anatomy and clinical significance of adrenal veins. Clin Anat. (2014) 27(8):1253–63. doi: 10.1002/ca.22374
18. Miekos E. Anatomical basis of radiodiagnosis of the adrenal gland. Int Urol Nephrol. (1979) 11(3):193–200. doi: 10.1007/BF02081960
19. Monkhouse WS, Khalique A. The adrenal and renal veins of man and their connections with azygos and lumbar veins. J Ana. (1986) 146:105–15.
20. Scholten A, Cisco RM, Vriens MR, Shen WT, Duh QY. Variant adrenal venous anatomy in 546 laparoscopic adrenalectomies. JAMA Surg. (2013) 148(4):378–83. doi: 10.1001/jamasurg.2013.610
21. Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. (2005) 25(Suppl 1):S143–58. doi: 10.1148/rg.25si055514
22. Tannai H, Koike Y, Matsui S, Saito J, Makita K. A rare independent left inferior phrenic vein sampling in a left adrenal aldosterone-producing adenoma. Radiol Case Rep. (2021) 16(6):1443–6. doi: 10.1016/j.radcr.2021.03.012
23. Hirose R, Tannai H, Nakai K, Makita K, Matsui S, Saito J. High aldosterone levels in the renal capsular vein from the left aldosterone-producing adenoma on adrenal venous sampling. Endocrinol Diabetes Metab Case Rep. (2023) 2023(3):23–0041. doi: 10.1530/EDM-23-0041
24. Sato Y, Shirota G, Makita K, Itoh D, Hayashi TY, Akamatsu N, et al. Anatomical variations of the left adrenal vein encountered during venous sampling. J Vasc Interv Radiol. (2022) 33(1):71–7.e3. doi: 10.1016/j.jvir.2021.09.005
25. Tannai H, Makita K, Matsui S, Koike Y, Tsurutani Y, Saito J. Radiological characteristics and diagnostic impact of duplicated right adrenal veins on adrenal venous sampling in primary aldosteronism. Diagn Interv Radiol. (2021) 27(6):754–61. doi: 10.5152/dir.2021.21388
26. Chen Cardenas SM, Santhanam P. 11C-metomidate PET in the diagnosis of adrenal masses and primary aldosteronism: a review of the literature. Endocrine. (2020) 70(3):479–87. doi: 10.1007/s12020-020-02474-3
27. Gao Y, Ding J, Cui Y, Li T, Sun H, Zhao D, et al. Functional nodules in primary aldosteronism: identification of CXCR4 expression with 68Ga-pentixafor PET/CT. Eur Radiol. (2023) 33(2):996–1003. doi: 10.1007/s00330-022-09058-x
Keywords: adrenal venous sampling, aldosterone-producing adenoma, primary aldosteronism, hypertension, computed tomography
Citation: Tannai H, Oguro S, Kamada H, Tezuka Y, Ono Y, Omata K and Takase K (2025) Case Report: Anomalous drainage vein sampling for diagnosing aldosterone-producing lesions undetectable by segmental adrenal venous sampling in a two-case series. Front. Radiol. 5:1567779. doi: 10.3389/fradi.2025.1567779
Received: 28 January 2025; Accepted: 22 May 2025;
Published: 10 June 2025.
Edited by:
Edit Dósa, Semmelweis University, HungaryReviewed by:
Edwin A. Takahashi, Mayo Clinic, United StatesSanjit Om Tewari, University of Texas MD Anderson Cancer Center, United States
Xiaoxiao Song, Zhejiang University, China
Copyright: © 2025 Tannai, Oguro, Kamada, Tezuka, Ono, Omata and Takase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiromitsu Tannai, aGlyb21pdHN1LnRhbm5haS5jNkB0b2hva3UuYWMuanA=
 Hiromitsu Tannai
Hiromitsu Tannai Sota Oguro1
Sota Oguro1 Yuta Tezuka
Yuta Tezuka Yoshikiyo Ono
Yoshikiyo Ono Kei Omata
Kei Omata