- 1School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2School of Medicine, Islamic Azad University of Medical Sciences, Tehran, Iran
- 3Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 4School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 5Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
- 6Pediatric Gastroenterology, Hepatology and Nutrition Research Center, Research Institute for Children’s Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 8Radiology Department, Faculty of Medicine, University of Medical Sciences, Qazvin, Iran
- 9Associate Professor of Radiology, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran
- 10Radiology Department, Mofid Children’s Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background and objective: COVID-19 has emerged as a global pandemic affecting individuals of all ages. The disease can lead to severe complications and even death, particularly due to pulmonary involvement. Contrary to popular belief, children can also experience significant complications from COVID-19. To date, there have been limited studies focusing on pulmonary manifestations in pediatric patients with COVID-19. This study aims to investigate the imaging patterns (CT scans) in children diagnosed with COVID-19 in Iran.
Materials and methods: This retrospective study analyzed data from hospitalized children with COVID-19 in Tehran from March 2020 to September 2020. Information collected included demographic details (sex and age), previous medical history, clinical manifestations, vital signs at admission, laboratory findings, and imaging results, including CT scan and chest x-ray.
Results: 252 patients were included, with a mean age of 71.2 ± 59.42 months; 58.3% were male. Fever was the most prevalent symptom, occurring in 67.4% of cases. The most common underlying condition was oncological disorders, present in 85% of patients. Notably, 52% required admission to the ICU, and 1.8% needed intubation. CT scans revealed that the most frequent lung involvement patterns were mixed patterns and consolidation, with bilateral involvement being the most common. The mean CT score was calculated at 3 ± 4. Abnormal CT findings were associated with a poorer prognosis, and correlations were observed between specific CT findings and clinical manifestations.
Conclusion: Chest CT manifestations offer valuable insights for assessing pediatric patients with COVID-19, especially in severe cases and those with pre-existing health conditions. Integrating clinical evaluations with radiological scoring systems facilitates early identification of disease severity.
Introduction
In January 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the cause of pneumonia cases first reported in Wuhan, China. Soon after, the virus started spreading around the world (1). By March 2020, the World Health Organization declared COVID-19 a pandemic (2). While COVID-19 primarily affects adults, there has been a notable increase in reported cases among children (3). Current data indicates that children generally experience a lower incidence of symptomatic disease and tend to have milder illness (4, 5). Common symptoms in pediatric cases include fever and cough, which are also prevalent in other childhood viral infections (6).
In the United States, the hospitalization rate for children under 18 stands at 8 per 100,000, significantly lower than the 164.5 per 100,000 rate observed in adults (7). This disparity may be attributed to distinct immune responses in children, including elevated levels of innate IgM antibodies and rapid natural antibody production, as highlighted by Carsetti et al. (8). Factors such as variations in T cell responses in adults, differing ACE-2 expression levels in children, and the presence of other respiratory viruses also contribute to this phenomenon (9). Additionally, children typically have fewer underlying health conditions and exhibit better lung regeneration capabilities than adults (10).
Nucleic acid testing using reverse transcriptase-polymerase chain reaction (RT-PCR) remains the gold standard for diagnosing COVID-19. However, delays in result turnaround can occur due to limitations in test kit availability, laboratory capacity, and trained personnel (11). Moreover, test sensitivity can be suboptimal, with detection rates ranging between 50% and 72% for nasal swab samples (12, 13). Consequently, the role of chest imaging in diagnosing COVID-19 has gained attention.
Research indicates that computed tomography (CT) chest imaging may offer higher sensitivity and prognostic value than traditional chest x-rays (14). Some studies have found that patients with normal chest radiographs often exhibit subtle abnormalities on CT scans (15). In fact, individuals who initially test negative via RT-PCR but show abnormal findings on chest CT may later test positive during their hospital stay (16). This suggests that CT imaging could be crucial for early detection and timely isolation of COVID-19 cases.
However, there is limited evidence regarding how COVID-19 manifests in pediatric imaging findings. A systematic review identified ground-glass opacities (GGO) and consolidations or pneumonic infiltrates as the most common abnormalities in pediatric COVID-19 cases (17). Remarkably, over one-third of affected children had normal chest CT scans. Furthermore, data on the relationship between radiological findings indicative of COVID-19 severity and clinical presentation in children remains sparse. A retrospective study by Ali et al. (18) found significant associations between severe-to-critical illness and abnormal radiological findings.
Therefore, this study aims to reveal the clinical characteristics and imaging features of COVID-19 in children. The goal is to establish standardized clinical and imaging approaches that accurately predict disease severity in children.
Materials and methods
Study design and population
This study was a cross-sectional investigation conducted among children under 18 years of age who were confirmed cases of COVID-19 based on clinical findings and RT-PCR test results. The study population comprised patients admitted to the Mofid Children's hospital in Tehran, Iran, from March 2020 to September 2020. Patients with incomplete medical records, a history of respiratory or allergic diseases, recent respiratory infections, and congenital heart and lung disorders were excluded from the study. The minimum required sample size was calculated to be 86 children based on previous findings indicating abnormal pulmonary manifestations in 66% of children with COVID-19 (19).
Data collection
Data were collected through a retrospective review of patient records, which included information on patients' backgrounds, clinical manifestations, laboratory findings, and disease outcomes during hospitalization. Vital signs at admission and laboratory findings (e.g., blood cell counts, inflammatory markers, coagulation tests) were documented. The severity of the disease was determined based on the Iranian national guidelines for the diagnosis and management of COVID-19 in pediatric patients (1).
Inclusion and exclusion criteria
Patients with symptoms suggestive of infection but without respiratory distress or abnormal imaging findings are classified as having mild disease. Moderate disease is defined by imaging showing signs of lower respiratory tract involvement while maintaining an SpO2 level of 94% or higher in room air. Severe disease is characterized by tachypnea (rapid breathing), more than 50% lung involvement on imaging, an SpO2 less than 94% in room air, and a FiO2/PaO2 ratio of less than 300 mmHg.
Data collection
CT scans were used to assess the type, pattern of spread, and severity of pulmonary involvement based on the severity score. The lungs were divided into five lobes, with severity scored as follows: Grade 1 for involvement less than 25%, Grade 2 for 25%–50%, Grade 3 for 50%–75%, and Grade 4 for involvement more significant than 75%. These individual scores were then summed to produce a total severity score. CT images were independently reviewed by two pediatric radiologists blinded to each other's interpretations. Common CT manifestations were analyzed about clinical symptoms, laboratory findings, disease severity, and outcomes.
Statistical analysis
Descriptive statistics for qualitative data were reported as frequencies and percentages, while quantitative data were summarized using means, standard deviations, medians, and ranges. Statistical evaluations included the Kruskal–Wallis test, correlation coefficient analysis, and Pearson chi-squared test. Analyses were performed using SPSS software version 25.0, with a p-value of less than 0.05, which is considered statistically significant. Ethical principles for research were strictly adhered to throughout the study, ensuring the confidentiality of patient information.
Results
Study population
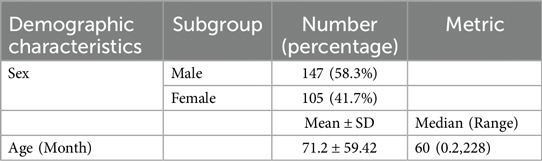
A total of 252 patients were enrolled in the study, comprising 147 boys (58.3%) and 105 girls (41.7%). The mean age of the patients was 71.2 months (±59.42), with an age range from 0.2 to 228 months. The demographic profile of patients is discussed in Table 1.
Clinical outcomes
The most common clinical outcomes observed are shown in Table 2 and were as follows: fever was reported in 153 patients (67.4%), myalgia in 62 patients (27.3%), vomiting in 48 patients (21.1%), and diarrhea in 28 patients (12.3%). Abdominal pain was reported in the same number of cases as diarrhea. Other notable outcomes included poor feeding in 35 patients (15.4%), cough in 47 patients (20.7%), respiratory distress in 53 patients (23.5%), rash in 12 patients (5.3%), diabetic ketoacidosis (DKA) in 4 patients (1.8%), loss of consciousness (LOC) in 10 patients (4.4%), and seizures in 9 patients (4%). Fever was the most prevalent outcome, while DKA was the least common.
Underlying health conditions
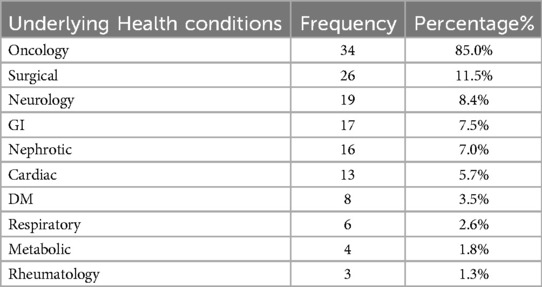
An analysis of underlying health conditions revealed that oncology-related diseases were the most frequently observed, affecting 34 patients (85%). In contrast, rheumatological conditions were the least common, affecting only 3 patients (1.3%). Other underlying conditions, ranked from most to least prevalent, included surgical issues in 26 patients (11.5%), neurological disorders in 19 patients (8.4%), gastrointestinal issues in 17 patients (7.5%), nephrotic syndrome in 16 patients (7%), cardiac conditions in 13 patients (5.7%), diabetes mellitus in 8 patients (3.5%), respiratory issues in 6 patients (2.6%), and metabolic disorders in 4 patients (1.8%). Overall, 121 patients (48%) had at least one underlying health condition, as shown in Table 3.
Severity of illness
Among the children, severe illness was noted, with 118 patients (52%) requiring ICU admission and 4 patients (1.8%) needing intubation. The agreement between ICU admission and intubation was assessed using the Kappa coefficient and Pearson correlation, indicating a very weak association between the two variables, as shown in Table 4. Ultimately, 204 patients (93.2%) were discharged, while 15 patients (6.8%) expired.
Chest x-ray findings
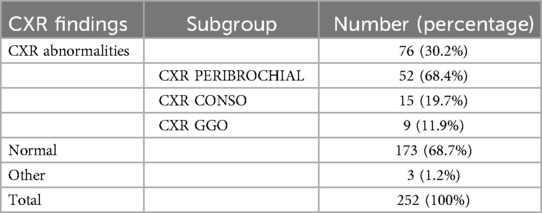
Regarding chest x-ray (CXR) findings (Table 5), among the total data of 252 patients, 76 patients (30.2%) had abnormal CXR results, while 173 patients (68.7%) had normal CXRs, and 3 patients (1.2%) exhibited other findings. Among the 76 patients with abnormal CXRs, peribronchial patterns were the most prevalent, observed in 52 patients (68.4%), followed by ground-glass opacity in 15 patients (19.7%), and consolidation in 9 patients (11.9%).
CT scan findings
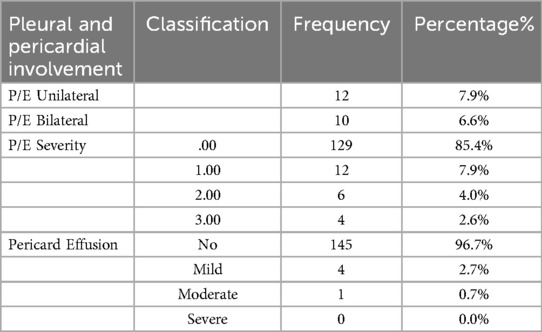
CT scan findings were categorized based on type and location of involvement, detailed in Tables 6A,B. The most common type of involvement was mixed pattern, while cavitation was the least observed. Bilateral involvement was the most frequent location, whereas anterior dominance was the least common. Regarding pleural and pericardial effusions, unilateral pleural effusion was noted in 12 patients (7.9%) and bilateral effusion in 10 patients (6.6%), detailed in Table 7. The highest frequency of pleural effusion severity was mild, with 12 patients (7.9%) affected, while mild pericardial effusion was observed in 4 patients (2.7%). Investigating the associated findings on CT scans also showed that peri-bronchial cuffing was the most frequently observed finding, while LAP was the least common. In the end, we analyzed the findings from CT scans with the diagnostic imaging results for COVID-19. The most common diagnosis was “typical appearance,” observed in 56 cases (37.6%), while the least common was “indeterminate appearance,” noted in 17 cases (11.4%).
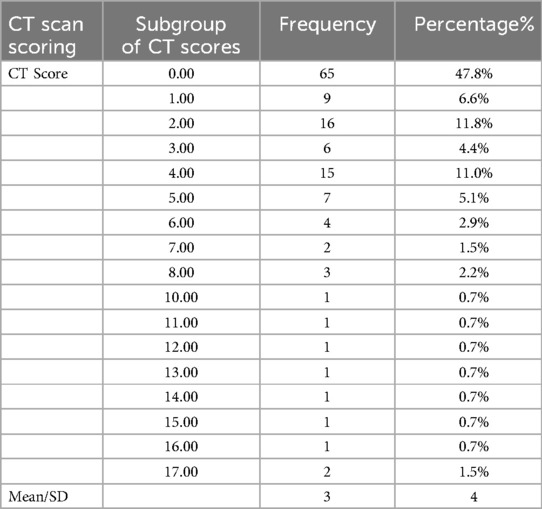
CT scan scoring
CT scan scoring as shown in Table 8, revealed that out of the total 252 patients, 136 had CT scores recorded, with a highest frequency score of 2 seen in 16 patients (11.8%). The mean CT score was found to be 3 ± 4. In recent years, there has been growing interest in automated tools for disease severity assessment based on previously developed algorithms on chest CT, mainly within the context of COVID-19. Thoracic VCAR (Volume Computer-Assisted Reading) is one such tool that is an AI-based software that uses deep learning algorithms to quantify pulmonary involvement. It segments lung regions automatically, identifies abnormalities like ground-glass opacities (GGO), consolidations, and reticular patterns, and generates a severity score aggregating volumetric analysis. AI-based systems are more objective, have higher interobserver agreement and faster assessment time than manual scoring, which makes them attractive for high-throughput clinical settings.
Manual CT severity scoring showed a significant association with disease severity among pediatric COVID-19 patients in our study (p < 0.05). Mean CT severity score was 3 ± 4 (higher in children with ICU admission: 4 ± 5; mild disease: 2 ± 3). Our results align with previous studies that show a relationship between higher lung involvement on CT and worse clinical outcomes, such as intubation and mortality. In addition, certain CT abnormalities including non-lobar consolidation, mixed patterns, reverse halo sign, and multifocal or peripheral involvement were significantly associated with high disease severity (p < 0.05). These findings showcase the role of automated and AI-assisted applications like Thoracic VCAR in augmenting clinical decision-making through the standardized evaluation of severity and generating fast and reproducible assessments. Such AI-based systems integrated into clinical workflows may allow to assess the risk early on, decrease the workload of radiologists, and help improve patient outcomes, particularly in resources limited setups. Such technologies would need to be adapted for application in pediatric imaging, where verification of accuracy in different patient populations would be a must in future studies.
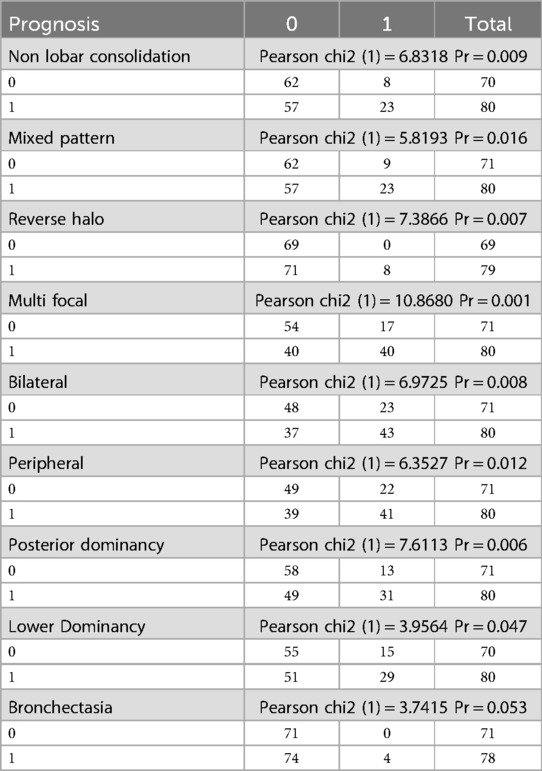
Prognostic factors and CT scan findings
Prognostic factors for COVID-19 in children were analyzed concerning CT scan findings (Table 9). The presence of non-lobar consolidation, mixed pattern, reverse halo sign, multifocal involvement, bilateral involvement, peripheral involvement, posterior dominance, lower dominance, and bronchiectasis were all significantly associated with poor prognosis, indicated by the need for intubation, ICU admission, and mortality (all P-values <0.05).
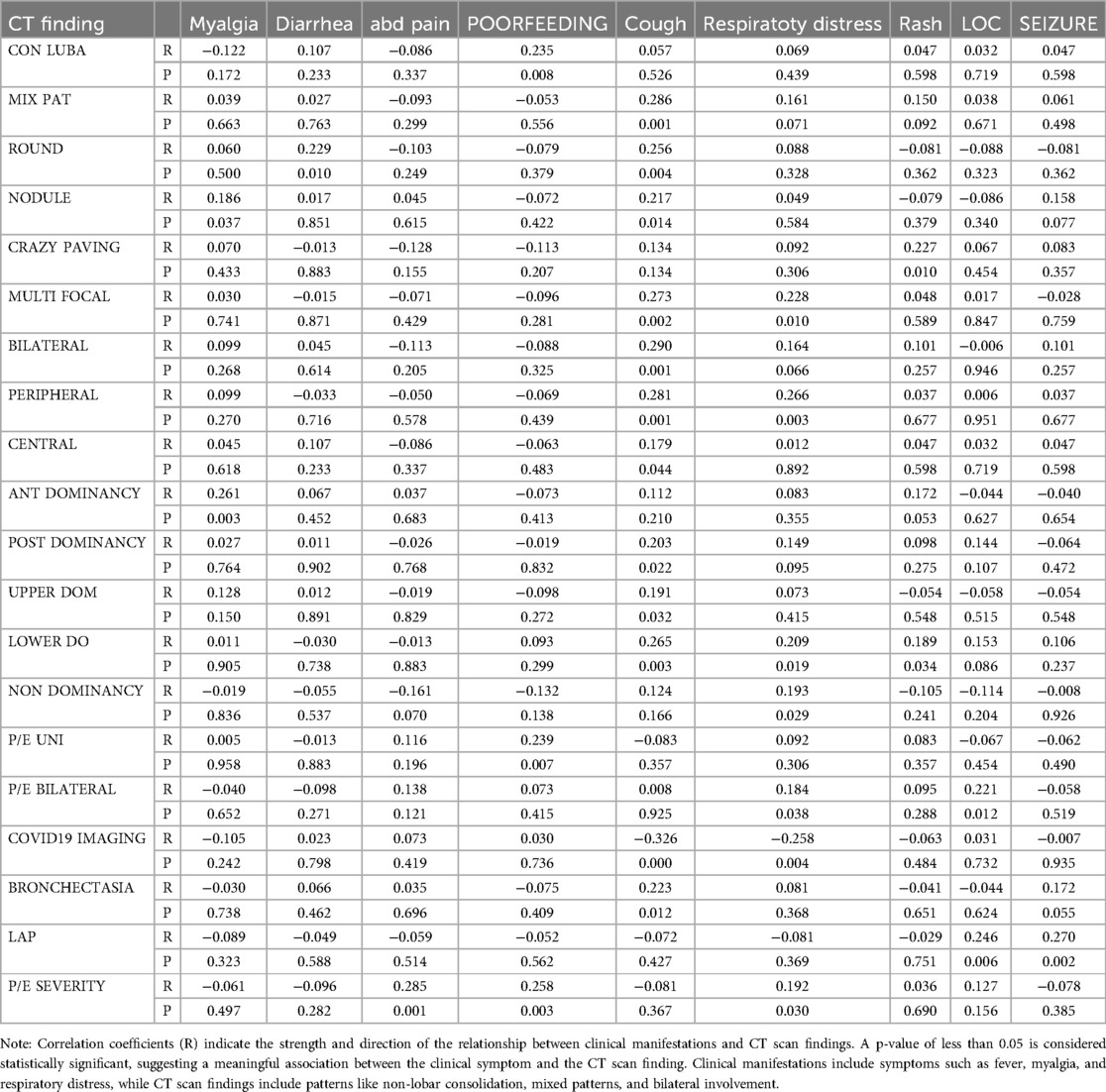
Correlation Between Clinical Manifestations and CT Findings (Table 10)
1. Patients with nodules significantly reported myalgia (P-value = 0.037).
2. Patients with round opacities had a significant incidence of diarrhea (P-value = 0.01).
3. Higher severity of pleural effusion was associated with increased abdominal pain (P-value = 0.001).
4. Patients with unilateral pleural effusion and non-lobar consolidation exhibited poor feeding symptoms.
5. Patients with mixed patterns and other specific findings exhibited increased cough frequency.
6. Dyspnea was significantly more prevalent among those with multifocal and peripheral involvement.
7. Rash was more commonly reported among patients with crazy paving patterns.
8. Loss of consciousness (LOC) was more prevalent among those with bilateral pleural effusion.
9. Seizures were notably higher in patients with bronchiectasis.
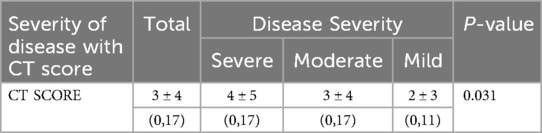
CT scan scoring and disease severity
There is a significant link between the CT score and disease severity (p-value=0.03). As shown in Table 11, The average disease score in patients with severe disease is 4 ± 5, while in those with mild disease, it is 2 ± 3.
Discussion
In the present study, we aimed to investigate the imaging patterns observed through CT scans in pediatric patients with COVID-19 in Iran. A total of 252 children were included, with an average age of 71.2 ± 59.42 months and a slight male predominance, as 58.3% of the participants were boys. This male-to-female ratio is consistent with findings from studies conducted in China (4) and the United States (3).
Fever emerged as the most prevalent symptom, reported in 67.4% of cases, followed by myalgia (27.3%), respiratory distress (23.5%), vomiting (21.1%), cough (20.7%), poor feeding (15.4%), and other gastrointestinal symptoms such as abdominal pain and diarrhea (12.3% each). Other symptoms were less common, each occurring in fewer than 10% of patients. These findings are comparable to previous research; for instance, Xia et al. (20) found fever (60%) and cough (65%) to be the most common symptoms among 13 evaluated children. Similarly, Du et al. (21) reported fever (43.4%) and dry cough (44.5%) among 182 patients, with gastrointestinal symptoms accounting for 11% of cases, including diarrhea, abdominal discomfort, and vomiting. A systematic review encompassing 43 articles and 158 confirmed pediatric COVID-19 cases also identified fever, cough, and vomiting as the most frequently reported symptoms (22). Notably, our study did not include any asymptomatic patients, as all participants were hospitalized due to symptomatic presentation.
In terms of clinical severity, 52% of the children required admission to the Intensive Care Unit (ICU), with 1.8% needing intubation. In comparison, a systematic review by Patel et al. (23) indicated that 26 out of 382 hospitalized children (6.8%) required ICU care, and among studies providing intubation data, 7 out of 327 hospitalized children (2.1%) with COVID-19 required this intervention. The high rate of ICU admissions in our study may be attributed to the underlying health conditions of our patients, as well as the impact of COVID-related social restrictions, which limited access to medical centers to only those with severe symptoms. The observed mortality rate in our study was 7.3%. In contrast, Patel et al. (23) reported a mortality rate of approximately 0.18%. The higher mortality rate in our study may be attributed to the fact that we focused specifically on hospitalized patients, whereas the systematic review included all children diagnosed with COVID-19. Consistent with our findings, the CDC has noted that while children generally experience milder symptoms, severe cases do occur within this population, and fatalities have been documented (3).
In our study, we found that the most common underlying condition among participants was oncology-related diseases, affecting 34 children (85%). This was followed by 26 children (11.5%) who had undergone surgical procedures, while rheumatological diseases were the least prevalent, affecting only 3 children (1.3%). These findings are consistent with a large multicenter retrospective study conducted in the United States, which indicated that pediatric patients with cancer face a greater risk of severe COVID-19 infection compared to their peers in the general pediatric population (24).
In our study, 152 children underwent CT scans, revealing that 43.4% exhibited bilateral lung involvement, while GGOs were observed in 19.7% of the cases. The halo sign appeared in 3.3% of patients, and a combination of lobar and non-lobar consolidation was present in 30% of the participants. Unilateral pleural effusion was noted in 12 children (7.9%), and 10 children (6.6%) had bilateral pleural effusion. Additionally, mild pericardial effusion was detected in four patients (2.7%). It is hypothesized that due to the generally milder manifestations of COVID-19 in children, the associated chest imaging findings tend to be less pronounced and may even appear normal in some cases. For instance, a study involving 30 pediatric patients found that all nine asymptomatic individuals had normal chest CT results (25). In a systematic review and meta-analysis conducted by Nino et al. (17) it was reported that 27.7% exhibited bilateral lung lesions. The predominant CT findings among pediatric patients with COVID-19 was similar to our study, and included GGOs (37.2%) and consolidations or pneumonic infiltrations (22.3%). Notably, common imaging features associated with viral respiratory infections in children, such as perihilar symptoms and hyperinflation, were absent in those diagnosed with COVID-19. Pleural effusion or adjacent pleural thickening was detected in only 0.5% of cases, with no instances of pericardial effusion reported. Similar to the previous study, perihilar involvement and hyperinflation were not observed in our participants.
Similarly, Ghodsi et al. (26), also found GGOs as the predominant chest CT finding, observed in 71.43% of cases. This was followed by peribronchial thickening (60%), linear or band opacities (32.8%), consolidation (28.57%), nodules (18.57%), effusion (7.14%), and focal lucency (7.14%). Consistent with previous data, the majority of cases presented with bilateral abnormalities. The authors highlighted distinct patterns of pulmonary involvement compared to other studies focused on the pediatric population, which may be attributed to the age factor, as their study exclusively examined children under one year of age.
Additionally, a review of thirty-nine observational studies examining chest CT scans in children with COVID-19 revealed abnormal findings in 717 out of 1,028 subjects (27). This review noted a higher prevalence of lower lobe involvement and unilateral lung lesions, contrasting with our findings and those of Nino et al. While the authors did not disclose the mean age of their participants, Yoon et al. (22) reported that typical GGOs were more frequently observed in older children (83.3%) compared to only 20.8% in younger children. This suggests that age variations within the pediatric population may influence the typical presentations seen on CT scans. Overall, these findings align with the lung abnormalities documented in adult COVID-19 patients (28); however, they were observed to be less frequent and less severe in children.
Several factors may account for the observed age-related differences in lung findings associated with COVID-19 (17). Variations in the pathobiology of COVID-19 between adults and children could lead to distinct disease patterns. For example, children with SARS-CoV-2 infection are significantly less likely to experience involvement of the pleural space. Also, older adults with COVID-19 often have pre-existing chronic lung conditions that are typically absent in previously healthy children infected with SARS-CoV-2.
Chest x-ray (CXR) abnormalities were observed in only 39.7% of cases, while the remaining images appeared normal. The most frequently noted abnormality was peribronchial involvement, accounting for 25.2%, followed by GGOs at 19.7% and consolidation at 11.9%. Previous studies have identified common CXR findings such as opacities, pneumonic infiltrates, and lung haziness, often referred to as “white lung” (29–31). These radiographic features may represent the GGOs associated with SARS-CoV-2 infection.
Our analysis indicates that abnormalities identified in CT scans are correlated with a poor prognosis, including increased likelihood of intubation, ICU admission, and mortality. These findings suggest that the presence of certain abnormalities on CT scans can serve as a marker for worse disease outcomes. Furthermore, CT scan results are closely linked to the severity of the clinical condition. Notably, children exhibiting higher CT scores tend to present with more severe cases of the disease, highlighting the need for increased monitoring and potential hospitalization in ICU settings for these patients. Our results align with those of Ali et al. (18), who found that abnormal radiological findings were significantly more prevalent among severe and critical cases, and that radiological severity scores correlated strongly with symptom severity. This is consistent with prior studies on COVID-19, which demonstrated that patients classified as severe often had elevated CT involvement scores (32). Li et al. (33) and Sun et al. (30) also reported that high CT scores were characteristic of children with severe or critical COVID-19 conditions. In our study, the average CT scan score for patients in severe condition was 4 ± 5, compared to 2 ± 3 for those with mild disease, which is similar to the mean CT score of 6.5 reported for severe cases by Ali et al. (18). Moreover, we explored the relationship between specific CT scan presentations and clinical symptoms. For instance, nodules were associated with muscle pain, completely round lesions with clear boundaries correlated with diarrhea, severe pleural effusions were linked to abdominal pain, unilateral pleural effusions coincided with poor feeding, and bilateral pleural effusions and lymphadenopathy were associated with decreased levels of consciousness. Bronchiectasis and lymphadenopathy were related to seizures. Additionally, we found that most complications observed on CT scans were tied to pulmonary symptoms such as cough and shortness of breath. In line with this, Du et al. (21) reported that patients with pneumonia exhibited a higher incidence of fever and cough. To further elucidate the relationship between CT scan abnormalities and non-respiratory symptoms, additional research is necessary.
Several limitations must be acknowledged in this study. First, the cross-sectional design did not allow for differentiation of CT scan findings based on the disease's progression. Previous research indicates that while baseline CT scans may appear normal, subsequent imaging can reveal disease-related lung involvement (34). Second, our analysis did not include diagnostic measures for COVID-19, such as PCR results, which could have provided critical insights into the relationship between serology testing and imaging, as well as the sensitivity of CT scans as a diagnostic tool. Lastly, considering the potential impact of age on imaging patterns, categorizing patients by age group and examining the imaging findings within each group may have yielded additional valuable insights.
Limitation
There are several limitations of this study. First, its retrospective nature may lead to selection bias, favoring only inpatients in whom CT scans are available. Second, disease progression was not controlled, as CT scans were performed at different points of illness. Lastly, this is a single-center study and may limit its generalizability to larger pediatric populations. Prospective designs and multicenter validation, as well as AI-assisted imaging analysis, should be the focus of future research to enhance severity assessment and clinical decision-making.
Conclusion
We found that CT severity scores significantly correlated with clinical outcomes, including ICU admissions, intubation rate, and death (p = 0.031). Of importance, mean CT severity scores were higher among patients with severe disease (4 ± 5) compared to patients with mild disease (2 ± 3). Moreover, certain CT findings, including the presence of non-lobar consolidation, mixed patterns, reverse halo sign, multifocal involvement and peripheral distribution of the lesions, are significantly associated with worse prognosis (p < 0.05). These imaging findings can be used as prognostic factors for assessing patients who are at risk and may eventually need intensive care treatment.
Automated CT scoring tools based on AI (e.g., the software named Thoracic VCAR), could improve the accuracy and performance of severity assessment. Objective, reproducible, and rapid quantification of lung involvement using these pneumonitis and ILD systems could help to standardize disease classification, enable early risk stratification, and potentially optimize treatment strategies—even in resource-limited settings. The next step would be to validate cutting-edge strategies such as AI-assisted severity scoring on the pediatric population, which can help to inform therapeutic and diagnostic decisions. In conclusion, augmenting imaging analytics with clinical evaluations can greatly optimize diagnosis, management and outcomes of children suffering from COVID-19 and other pulmonary diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by This study was approved by the Shahid Beheshti University Ethics Committee (approval number: IR.SBMU.RICH.REC.1398.029). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MA: Writing – original draft, Writing – review & editing. SF: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. RaZ: Writing – original draft, Writing – review & editing. FS: Writing – original draft, Writing – review & editing. YT: Writing – original draft, Writing – review & editing. MH: Writing – original draft, Writing – review & editing. ReZ: Writing – original draft, Writing – review & editing. AN: Writing – original draft, Writing – review & editing. NP: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. MK: Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the Shahid Beheshti University Ethics Committee for their support in approving the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. (2020) 382(13):1199–207. doi: 10.1056/NEJMoa2001316
2. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Med Atenei Parmensis. (2020) 91(1):157.
3. CDC Covid-19 Response Team, Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, et al. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morb Mortal Wkly Rep. (2020) 69(14):422–6. doi: 10.15585/mmwr.mm6914e4
4. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145(6):e20200702. doi: 10.1542/peds.2020-0702
5. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109(6):1088–95. doi: 10.1111/apa.15270
6. Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. (2020) 119(5):982–9. doi: 10.1016/j.jfma.2020.04.007
7. Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged 18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 1–July 25, 2020. Morb Mortal Wkly Rep. (2020) 69:1081–8. doi: 10.15585/mmwr.mm6932e3
8. Carsetti R, Quintarelli C, Quinti I, Piano Mortari E, Zumla A, Ippolito G, et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Health. (2020) 4(6):414–6. doi: 10.1016/S2352-4642(20)30135-8
9. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. (2020) 215:108427. doi: 10.1016/j.clim.2020.108427
10. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: why children fare better than adults? Indian J Pediatr. (2020) 87:537–46. doi: 10.1007/s12098-020-03322-y
11. Zhu Y, Zhang M, Jie Z, Tao S. Nucleic acid testing of SARS-CoV-2: a review of current methods, challenges, and prospects. Front Microbiol. (2022) 13:1074289. doi: 10.3389/fmicb.2022.1074289
12. Gietema HA, Zelis N, Nobel JM, Lambriks LJG, van Alphen LB, Oude Lashof AML, et al. CT in relation to RT-PCR in diagnosing COVID-19 in The Netherlands: a prospective study. PLoS One. (2020) 15(7):e0235844. doi: 10.1371/journal.pone.0235844
13. Yang Y, Yang M, Yuan J, Wang F, Wang Z, Li J, et al. Laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infection. Innovation. (2020) 1(3):100061. doi: 10.1016/j.xinn.2020.100061
14. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. (2020) 295(1):202–7. doi: 10.1148/radiol.2020200230
15. Xu G, Yang Y, Du Y, Peng F, Hu P, Wang R, et al. Clinical pathway for early diagnosis of COVID-19: updates from experience to evidence-based practice. Clin Rev Allergy Immunol. (2020) 59:89–100. doi: 10.1007/s12016-020-08792-8
16. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. (2020) 296(2):E115–7. doi: 10.1148/radiol.2020200432
17. Nino G, Zember J, Sanchez-Jacob R, Gutierrez MJ, Sharma K, Linguraru MG. Pediatric lung imaging features of COVID-19: a systematic review and meta-analysis. Pediatr Pulmonol. (2021) 56(1):252–63. doi: 10.1002/ppul.25070
18. Ali HA, Mohammad SA. Pediatric COVID-19: correlations between clinical and imaging perspectives. Pulm Med. (2023) 2023:4159651. doi: 10.1155/2023/4159651
19. Shelmerdine SC, Lovrenski J, Caro-Domínguez P, Toso S, Alexopoulou E, Almanza J, et al. Coronavirus disease 2019 (COVID-19) in children: a systematic review of imaging findings. Pediatr Radiol. (2020) 50:1217–30. doi: 10.1007/s00247-020-04726-w
20. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. (2020) 55(5):1169–74. doi: 10.1002/ppul.24718
21. Du H, Dong X, Zhang J, Cao Y, Akdis M, Huang P, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. (2021) 76(2):510–32. doi: 10.1111/all.14452
22. Yoon S, Li H, Lee K, Hong S, Kim D, Im H, et al. Clinical characteristics of asymptomatic and symptomatic pediatric coronavirus disease 2019 (COVID-19): a systematic review. Medicina (Kaunas). (2020) 56(9):474. doi: 10.3390/medicina56090474
23. Patel NA. Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol. (2020) 41(5):102573. doi: 10.1016/j.amjoto.2020.102573
24. Madhusoodhan PP, Pierro J, Musante J, Kothari P, Gampel B, Appel B, et al. Characterization of COVID-19 disease in pediatric oncology patients: the New York-New Jersey regional experience. Pediatr Blood Cancer. (2021) 68(3):e28843. doi: 10.1002/pbc.28843
25. Steinberger S, Bernheim A, Chung M, Gao Y, Xie Z, Zhao T, et al. CT features of coronavirus disease (COVID-19) in 30 pediatric patients. Am J Roentgenol. (2020) 215:1303–11. doi: 10.2214/AJR.20.23145
26. Ghodsi A, Bijari M, Alamdaran SA, Saberi A, Mahmoudabadi E, Balali MR, et al. Chest computed tomography findings of COVID-19 in children younger than 1 year: a systematic review. World J Pediatr. (2021) 17(3):234–41. doi: 10.1007/s12519-021-00424-1
27. Oraya DB, Militante SK, Dans LF, Lozada MC, Valle AO, Cabaluna IT. Chest CT scan findings in children with COVID-19: a systematic review. Acta Med Philipp. (2024) 58(7):110–28. doi: 10.47895/amp.v58i7.6385
28. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. (2020) 17(6):701–9. doi: 10.1016/j.jacr.2020.03.006
29. Korkmaz MF, Türe E, Dorum BA, Kılıç ZB. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci. (2020) 35(25):e236. doi: 10.3346/jkms.2020.35.e236
30. Sun D, Li H, Lu X-X, Xiao H, Ren J, Zhang F-R, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. (2020) 16:251–9. doi: 10.1007/s12519-020-00354-4
31. Lu Y, Wen H, Rong D, Zhou Z, Liu H. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol. (2020) 75(7):520–5. doi: 10.1016/j.crad.2020.04.010
32. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: relationship to negative RT-PCR testing. Radiology. (2020) 296(2):E41–5. doi: 10.1148/radiol.2020200343
33. Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. (2020) 55(6):327–31. doi: 10.1097/RLI.0000000000000672
Keywords: COVID-19, CT scan, infection, ICU—intensive care unit, pediatric
Citation: Aghabeygiha M, Fahimzad SA, Behzad S, Zadeh RH, Sheikhzadeh F, Tamaddon Y, Hajipour M, Zadeh RH, Neyriz A, Pak N, Shirvani A, Hosseini A and Khalili M (2025) Radiologic analysis of CT imaging patterns and clinical correlations in hospitalized pediatric COVID-19 patients. Front. Radiol. 5:1571672. doi: 10.3389/fradi.2025.1571672
Received: 5 February 2025; Accepted: 24 March 2025;
Published: 24 April 2025.
Edited by:
Guglielmo M. Trovato, European Medical Association (EMA), BelgiumReviewed by:
Carlo Di Donna, University of Rome Tor Vergata, ItalyTeresa Abbattista, Senigallia Hospital, Italy
Copyright: © 2025 Aghabeygiha, Fahimzad, Behzad, Zadeh, Sheikhzadeh, Tamaddon, Hajipour, Zadeh, Neyriz, Pak, Shirvani, Hosseini and Khalili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amirhossein Hosseini, YW1pcjE5ODFob3NzZWluaUBnbWFpbC5jb20=; YWhfaG9zc2VpbmlAc2JtdS5hYy5pcg==; Mitra Khalili, bS5raGFsaWxpNzZAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
 Mehrnoosh Aghabeygiha1,†
Mehrnoosh Aghabeygiha1,† Farzad Sheikhzadeh
Farzad Sheikhzadeh Mahmoud Hajipour
Mahmoud Hajipour Amirhossein Hosseini
Amirhossein Hosseini