- 1Division of Molecular Biology, School of Life Sciences, JSS Academy of Higher Education and Research, Mysuru, Karnataka, India
- 2Division of Cosmetic Science, School of Life Sciences, JSS Academy of Higher Education and Research, Mysuru, Karnataka, India
- 3Cellular and Molecular Biology, Centre of Excellence in Molecular Biology and Regenerative Medicine, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education and Research, Mysuru, Karnataka, India
- 4Department of Biotechnology, Sri Jayachamarajendra College of Engineering, JSS S&TU, Mysuru, Karnataka, India
The impact of endocrine-disrupting chemicals (EDCs) on public health is growing due to their wide-ranging consequence and likelihood of morbidity on human health. Humans are confronted with EDCs through their skin and drinks, as well as by inhaling. EDCs are extensively dispersed in the environment. EDCs have been shown to primarily influence puberty, the reproductive system, embryonic growth, the hypothalamus-pituitary-gonadal axis (HPG) neuroendocrine axis, and gender differentiation in the foetus, despite their capacity to influence a variety of hormone systems. Treatment for afflicted persons will benefit greatly from an understanding of the several ways that modifiable lifestyle circumstances connected to PCPS impede female infertility. The purpose of this review is to raise awareness of the hidden danger that environmental dyes (EDCs) pose to human health, particularly in terms of their detrimental effects on female reproductive health.
1 Introduction
An essential biological process for all living things is reproduction. Since the reproductive health of the parent species is essential to the continued existence of any species, any danger to reproductive health will provoke a robust response from the scientific community. Reproductive health indices have been reported to be declining globally during the past five to six decades, particularly in industrialized and affluent nations, due to factors associated with lifestyle that may be altered (1). The reproductive system is very susceptible to environmental stimuli. This is due to the fact that it requires energy expenditures, and it makes sense that, as in an organism, the physiological controls of the reproductive axis would be intimately correlated with nutritional condition (2). Scientific evidence has suggested that modifiable lifestyle factors (consumption of fat-rich diets, delayed childbearing/age of starting family, smoking, alcohol misuse, sexual behaviours, anxiety/depression, and perception/beliefs) play important roles in the general health and well-being of individuals, including fertility (3). Lifestyle variables are changeable behaviours and ways of living that have the potential to affect an individual's overall health and wellbeing, including fertility (4). They also have a significant role in determining an individual's exposure to the environment (5). A healthy lifestyle can be created by modifying behaviour and incorporating elements such as health consciousness, practical understanding of health sciences, motivation, and concern for taking action to protect and promote health.
A subset of self-care items known as personal care products (PCPs) is often used for grooming, cleaning, personal hygiene, and beautifying. Products that readily expose individuals include those for hair and skin care, baby care, UV blocking creams, facial cleansers, insect repellents, perfumes, scents, soap, detergents, shampoos, conditioners, toothpaste, and more. The frequency of PCP utilization is a highly varied personal choice that is influenced by lifestyle circumstances and socioeconomic status (6). Furthermore, one of the primary contributors of contaminants that are surfacing in the environment includes PCPs. PCPs prevail in human bodies at all phases of life, including intrauterine development. Inhalation, cutaneous interaction, ingestion, and absorption are the direct modes of exposure; product use and environmental contamination are the direct pathways (7). The most prevalent sort of merchandise found in homes and public areas in PCPs. Globally, between 30 and 40 percent of dermatologist prescriptions comprise at least one PCP, and a single person utilizes at least two PCPs in a 24 h period (8, 9). The total mass loading of PCPs in the Human Province of Southern China was 506.35 mg/d/1,000 people, which contributed to the overall emission of 357.56 mg/d/1,000 people (10). A variety of compounds are released by PCPs. For instance, the only products that emit 49.25 and 9,574 µg of siloxanes per individual per day are shampoo and shower gel. The air was found to be contaminated with decamethylcyclopentasiloxane (D5) and dodecamethylcyclohexasiloxane (D6), with per capita emission levels of 8.33 and 6,109 µg/day, respectively (11). Likewise, phthalates from PCPs contaminate indoor air (12). Monoethanolamine and diethanolamine are commonly found in cleaners, shampoos, hair dyes, and detergents (13). The TESIE study identified phthalates in almost all hand wipes and dust samples, and their metabolites were detected in all children's urine samples, confirming their ubiquitous exposure (14). Several of these compounds are referred to as endocrine-disrupting chemicals (EDCs), and because of their potential for morbidity and wide-ranging impacts on human health, public health is now starting to place greater attention on EDCs. EDCs are widely distributed in the environment, and humans become susceptible to them through their skin, in their consumption of food and drink, and through their respiration. EDCs have been shown to primarily impact puberty, embryonic development, the reproductive system, and sex differentiation in the fetus, despite their capacity to influence a variety of hormone systems. Consequently, the most plausible explanation for their primary mode of action is that they interfere with sex steroid hormones (15). Comprehending the diverse mechanisms through which modifiable lifestyle-associated PCPs hinder female infertility will significantly aid in the treatment of affected individuals. This review attempts to shed light on the unnoticed harm that EDCs cause to human health, particularly as endocrine disruptors hampering female reproductive health.
2 Endocrine-disrupting chemicals (EDCs)
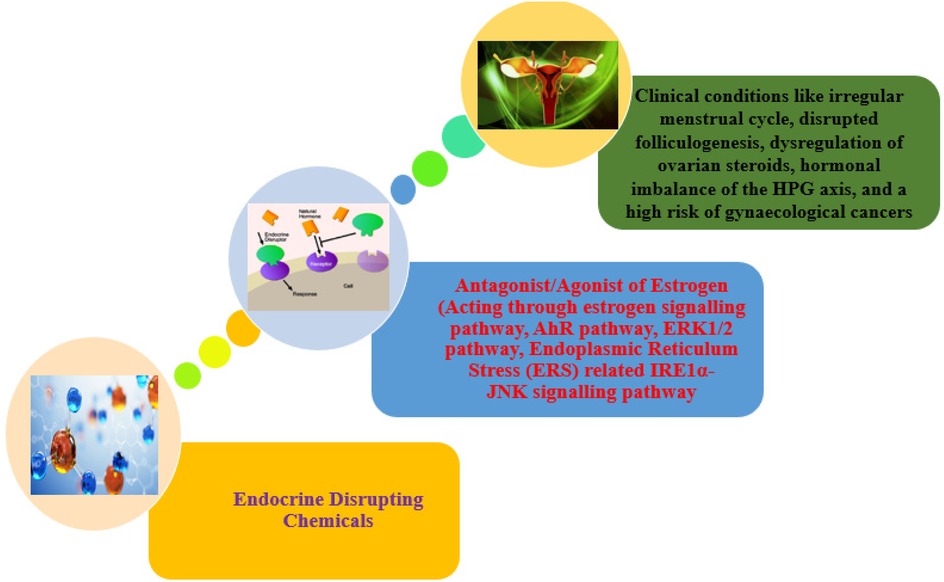
“An exogenous (non-natural) chemical, or a mixture of chemicals, that interferes with any aspect of hormone action” is how the Endocrine Society defines EDCs. By functioning as hormone antagonists, imitating hormones, interfering with hormone production or breakdown, changing the process by which hormone receptors develop, or altering hormone binding, these substances affect the body's hormonal balance in a wide range of ways (15). The manufacture and consumption of man-made chemicals such as flame retardants, chemical pesticides, plastics and plasticizing agents, electronic waste materials, food additives, metallic substances, and personal care products are the main sources of environmental EDCs. These EDCs have the potential to throw off the hormonal balance, which can result in a host of health problems, including immunological system dysfunction, growth abnormalities, and neurodevelopmental delays in children, abnormalities in the reproductive and developmental processes, and hormone-sensitive cancers. It is recognized that EDCs modify the metabolic balance via several methods, such as changes to peroxisome proliferator-modulated pathways (16), adipogenesis (17), pancreatic β-cell function (18–20), and hypothalamic neuropeptides (21, 22). The majority of data on the direct impact of EDCs on fertility is gathered from studies done using animals and in vitro. Although several studies have demonstrated a direct correlation between EDCs and infertility parameters, other studies have associated them with PCOS, a complex endocrinopathy that results in insulin resistance and infertility, concurrently damaging the HPG neuroendocrine axis (Figure 1). The current review discusses how PCPs, as endocrine disruptors, affect female reproductive outcomes.
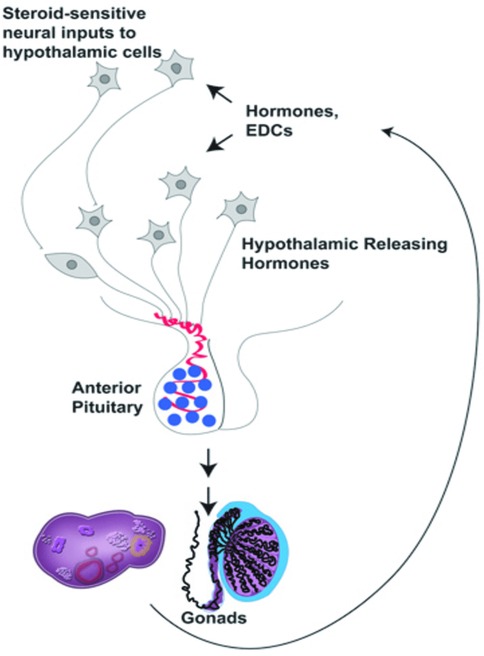
Figure 1. The hypothalamic-pituitary-gonadal (HPG) axis (22).
2.1 Bisphenol A (BPA)
Found in a variety of plastics, food containers, and receipts. Bisphenol A (BPA) is a chemical that has been widely utilized in the production of different plastics and epoxy resins since the 1960s. Consumer products, including water bottles, food storage containers, dental sealants, and canned food lining, are common places to find it. The ability of BPA to harden polymers and increase the toughness of various materials is well documented. However, concerns about the potential health effects of BPA exposure have been highlighted. Because it can interfere with the body's hormonal system, BPA is regarded as an endocrine disruptor. The BPA is a xenoestrogen that interacts with the estrogen receptor and acts as an antagonist or agonist of estrogen, involved in the estrogen-signalling pathway and disrupts the endocrine system (Table 1) (23). Numerous health concerns, such as those pertaining to reproduction, development, and an elevated risk of certain ailments, have been associated with it (24). BPA was discovered to be linked to a lower ovarian reserve in previous investigations, along with (25) lower antral follicle count (26) and PCOS (27, 28) in infertile women. Additionally, another study elsewhere has demonstrated that higher quartiles of urine BPA content are linked to a higher risk of implantation failure (29). A case study report on BPA/BPB has pointed out the definite association between endometriosis prevalence and BPA and/or BPB levels in the blood (30). Another case-control study conducted in China on BPA implies that BPA may impact ovarian follicles in PCOS women, hence lowering ovarian reserve (25). Additionally, BPA could reduce ovarian maturation, and this reduction could be recovered after BPA treatment withdrawal (Figure 2) (31). The United Kingdom case-control studies have identified BPA as an endocrine disruptor that may play an essential role in the pathophysiology of PCOS, as being identified by a statistically significant positive association between androgens and BPA, along with the greater BPA levels in PCOS women compared to controls (27).
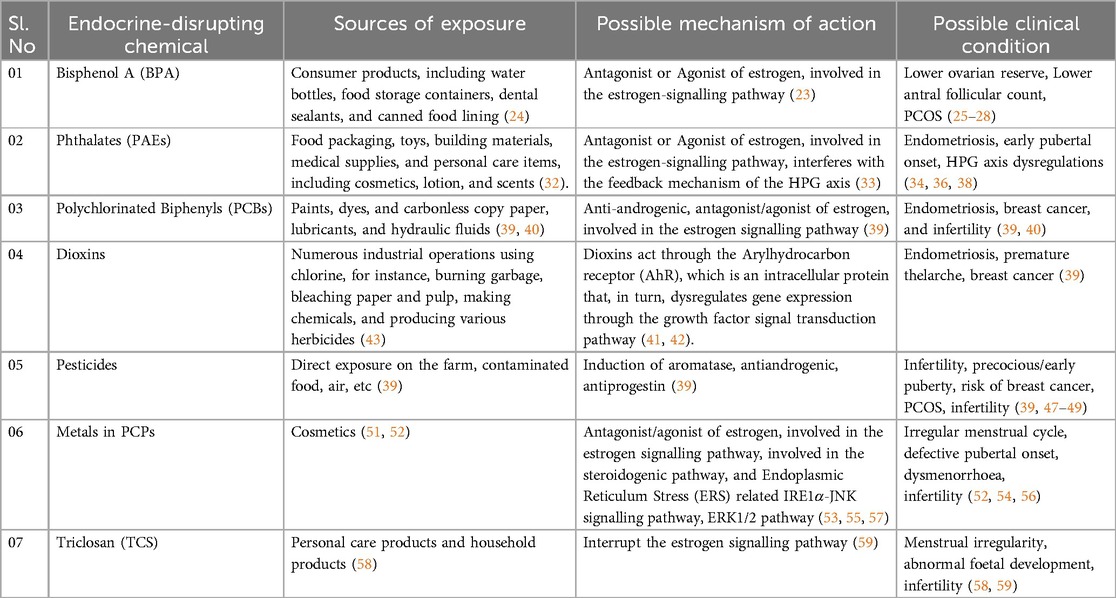
Table 1. EDCs, their sources of exposure, possible mechanism of action, and their possible clinical outcomes in females.
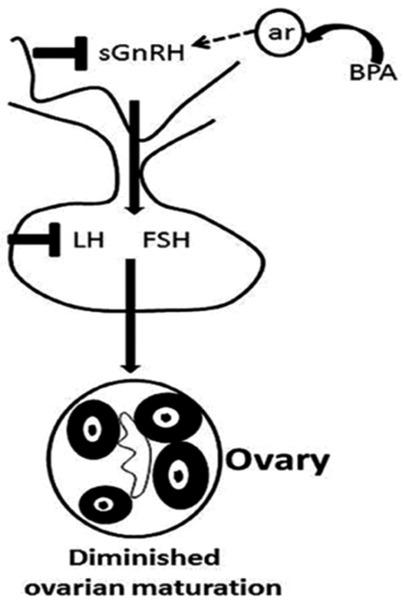
Figure 2. BPA reduced ovarian maturation by affecting the HPG axis (31).
2.2 Phthalates (PAEs)
A class of chemical substances known as phthalates is frequently added to plastic as plasticizers-substance that give polymers more flexibility, transparency, resilience, and lifespan. They are frequently present in an extensive variety of commodities, including food packaging, toys, building materials, medical supplies, and personal care items, including cosmetics, lotion, and scents. Urinary metabolite concentrations were demonstrated to have a negative correlation with serum inhibin B levels, indicating that phthalates have a deleterious influence on the growth of antral follicles (Table 1) (32). Phthalates act as the xenohormone for both androgen and estrogen, which blocks the corresponding receptors, which interferes with the feedback mechanism of the HPG axis (33). Korea case-control Research phthalate study has demonstrated that individuals with advanced-stage endometriosis had considerably higher plasma levels of monoethylhexyl phthalate and DEHP (34). Particularly, phthalates may have detrimental effects on reproductive and developmental health, according to a specific study. As a precaution, some people opt to minimize their exposure to products that contain phthalates, especially pregnant women and young children who are more susceptible than other populations. USA Research using case-control phthalate suggests that phthalates might alter a woman of reproductive age and risk of hormone-mediated illness (35). Thailand cross-sectional research phthalates, increased concentration of mono-ethyl-phthalates (MEP) was associated with girls who have reached puberty early (36). USA cohort study Longitudinal phthalates Research indicates that at some crucial stages of in utero development, female reproductive development may be more susceptible to the negative effects of phthalate or BPA exposure (37). As an essential endocrine axis that regulates the reproductive system, whether dysfunction of the hypothalamus-pituitary-gonadal (HPG) axis is involved in reproductive toxicity mediated by environmental endocrine disruptors, PAEs has become a hot topic of widespread concern (Figure 3) (38).
2.3 Polychlorinated biphenyls (PCBs)
The stable, non-flammable, and electrically insulating properties of polychlorinated biphenyls, or PCBs, are highly valued in synthetic organic molecules. To create PCBs, a biphenyl molecule, a substance with two benzene rings linked to chlorine atoms. PCBs were commonly discovered in electrical equipment, including lubricants and hydraulic fluids, as well as transformers and capacitors. They were also used in a number of industrial operations, including the creation of paints, dyes, and carbonless copy paper. The PCBs act as agonists or antagonists of estrogen, and also show antiandrogenic activity (Table 1) (39). In a case-control study, France found that persons with deep infiltrating endometriosis (DIE) had greater internal exposure levels of various PCBs, BFRs, OCPs, and dioxins in adipose tissue, particularly in severe instances of endometriosis (Stages III–IV) (40).
2.4 Dioxins
Dioxins are a category of extremely toxic chemicals that are persistent environmental pollutants. They belong to the categories of chemical compounds known as polychlorinated dibenzofurans (PCDFs) and polychlorinated dibenzo-p-dioxin (TCDD). Numerous industrial operations using chlorine, for instance, burning garbage, bleaching paper and pulp, making chemicals, and producing various herbicides, accidentally result in the production of dioxins. Dioxins may reach the food chain through polluted soil, water, or air and gather in the animal's fatty tissues. Humans are most exposed through the consumption of contaminated food, particularly animal products like meat, dairy, and seafood. Dioxins act through the Arylhydrocarbon receptor (AhR), which is an intracellular protein that, in turn, dysregulates gene expression through the growth factor signal transduction pathway (41, 42). In the Spanish case-control study, individuals with DIE had substantially higher levels of dioxins and PCBs in their adipose tissue compared to the control group (p < 0.05) (Table 1) (43).
2.5 Pesticides
Chemicals identified as pesticides are used to regulate or eliminate the presence of pests that can destroy crops, kill livestock, or cause additional issues. Many pesticides can interact with the endocrine systems of both humans and animals because they are endocrine disruptors. Hormones, which are essential for metabolism, growth, development, and reproduction, are regulated by the endocrine system. Certain pesticides can alter, suppress, or mimic the body's hormones from within, which may cause problems with the endocrine system. Numerous health issues, including abnormal growth, trouble becoming pregnant, and modification to the usual operation of the hormone-regulated organs, might result from this interference. Sources of drinking water have been found to contain pesticides, some of which are known to be harmful to reproduction. Most pesticides act as antiandrogenic, antiprogestin agents. They also act as aromatase inducers and reduce the production of insulin-like growth factors (39). For instance, reduced sperm counts and negative pregnancy results in both humans and non-human primates are linked to pesticide exposure (Table 1) (44–46). According to an investigation, inadequate outcomes from embryological intracytoplasmic sperm injection were connected with high quantities of PCB and pesticides in the follicular fluid (47). According to another study, women with PCOS had greater blood concentration of phthalate metabolism and perfluorinated chemicals (48). DDT may have a part in the pathophysiology of PCOS in Chinese women with the condition due to its association with altered hormone levels. An early menopausal onset was linked to EDCs. According to a large cross-sectional study that included over 30,000 women (49). Additionally, they found 15 EDCs (comprising 3 pesticides, 2 phthalates, 1 furan, and 9 PCBs) that required additional assessment to rule out any potential detrimental effects on ovarian function (50).
2.6 Metals in PCPs
Chemical bleaching chemicals and possibly hazardous ingredients are found in skin-lightening cosmetics, according to a comprehensive literature review (Table 1) (51). According to 25 research studies that the group reviewed, 12 of the research studies examined mercury alone, while 13 of them examined mercury in conjunction with other widely used active ingredients for skin lightening (such as betamethasone, clobetasol propionate, kojic acid, hydroquinone, and corticosteroids) or trace elements (such as bismuth, cadmium, chromium, cobalt, copper, lead, iron, nickel, manganese, palladium, thallium, titanium, titanium dioxide, zinc, and arsenic). Mercury (Hg) has demonstrated an increased incidence of irregular menstrual periods (52). Hg acts as an antagonist or an agonist of estrogen, interfering with the estrogen-signalling pathway (53). Lead (Pb) is associated with a delay in pubertal development and growth of girls (54). A study conducted on rats shows a downregulation of steroidogenic genes such as STAR, CYP17A1, and HSD3B1, upregulation of FSHR and CYP19A1 upon Pb exposure. The study has also noticed the stimulation of the apoptotic pathway along with the stimulation of Endoplasmic Reticulum Stress (ERS) related IRE1α-JNK signalling pathway members, which leads to the dysregulation of the HPG axis hormonal profile, folliculogenesis, and delayed pubertal onset upon juvenile exposure to Pb (55). Cadmium (Cd) has been reported to be associated with abnormal menstrual cycle, dysmenorrhoea in unmarried women, and sterility in married women (56). Studies have observed a decreased folliculogenesis upon Cd exposure in animals. It is well demonstrated that Cd exposure leads to an accumulation of hydrogen peroxide with a decrease in antioxidant enzymes in the ovaries, which in turn causes the apoptosis of ovarian cells, and results in the downregulation of ovarian steroids. It is also shown that Cd exposure causes the rapid activation of the ERK1/2 pathway and thereby interferes with the estrogen signalling pathway (57).
2.7 Triclosan (TCS)
2,4,4-trichloro-2′-hydroxydiphenyl ether, commonly known as triclosan (TCS), is an antibacterial agent used in personal care and household items that has brought attention as it affects female reproductive health. Emerging research on TCS concerning female reproductive health has shown its effect on endocrine disruption, which causes the hormonal dysregulation resulting in irregular menstruation, infertility, and abnormalities in the development of the foetus (Table 1) (58). Studies have shown that the TCA will bind to the estrogen receptor, which may lead to irregular menses and hormonal disruption (59). Animal studies have also demonstrated the bioaccumulation of TCA in the ovarian and uterine tissues, which leads to a compromise in the reproductive function (60). Further research is recommended to unveil the major effects of TCA as an endocrine disruptor.
3 Conclusion
“Endocrine disruptors” are compounds that have the ability to interfere with the endocrine system, an intricate web of hormones and glands that control a wide range of bodily physiological functions. These disturbances can mimic, hinder, or inhibit endogenous hormone generation, binding, action, metabolism, release, transport, or excretion. As a result, they may disrupt the normal function of the endocrine system, which may have detrimental effects on growth and well-being. Endocrine disruptor exposure has been linked to several health issues, including immune system malfunction, abnormal development, reproductive abnormalities, and an elevated risk of multiple cancers. It is believed that young toddlers, neonates, and pregnant women are most vulnerable to the effects of these disruptors. The hazards and exposure levels to parabens and bisphenols associated with Indian women's consumption of personal care products (PCPs) are not well recognized. It is imperative that individuals follow safety protocols while handling or applying pesticides and be aware of the potential risks associated with pesticide exposure. Furthermore, consuming organic food, adopting integrated pest management strategies, and supporting sustainable agriculture practices can all help reduce exposure to chemicals that have endocrine-disrupting properties. Global regulatory agencies, including the Environmental Protection Agency (EPA) in the US and the European Food Safety Authority (EFSA) in Europe, analyse the potential of pesticides to interfere with the endocrine system before approving their usage. A lot of work goes into determining safe exposure thresholds and limiting or outlawing the use of pesticides that seriously endanger human health. To reduce the effects of endocrine disruptors on the environment and public health, regulatory organizations from all over the world are trying to discover and control their use. The possible dangers are consequence of exposure to these compounds are still being investigated. Additionally, people can lessen their exposure by using glass or stainless-steel containers instead of plastic ones, choosing items that are labelled as “BPA-free” or “Phthalates-free”, and paying attention to the products they use regularly. PCBs may still be present in some older materials and equipment despite the bans and regulations, creating ongoing problems for public health and environmental management. Concerns over PCB contamination persist, and clean-up initiatives are being undertaken in affected areas. Cleansing techniques include the containment or removal of polluted soil, water, and silt. Effective disposal of products containing PCBs is an essential aspect of remediation. Studies have shown that EDCs, such as phthalates, bisphenols, and parabens, may have negative health impacts on people, yet these chemicals are widely regarded as harmless and are present in a variety of goods. The fundamental reason is that the applicable regulation requires companies to keep trace quantities of suspicious chemical substances on reserve. That being said, it's crucial to consider the possibility of combination effects (synergism, additivity, inhibition) when there are several endocrine disruptors. Considering that the actual chemical combination cannot be safely exposed to at theoretically acceptable concentrations of separate substances. The majority of research on the negative effects of EDCs comes from studies conducted on animals. Along with the manufacturing and dispersion of EDCs into the surroundings, potable water supplies, and eventually the food chain, global industrialization is contributing to the sharp increase in the prevalence of numerous diseases, most notably HPG neuroendocrine axis disruption associated with reproductive endocrine disorders. The anterior pituitary gonadotrophs are the specific organs targeted by the release of GnRH from hypothalamic neurons to produce/secrete LH and FSH. From that point, gonadal steroidogenesis and gametogenesis are triggered by these circulating gonadotropins acting on receptors in the gonad, ovary, or testis. In addition to acting on peripheral targets, steroid hormones in the bloodstream also feedback on steroid-sensitive brain neurons, which provide inputs to the hypothalamus. Henceforth, EDC exposure during development can disrupt HPG systems by interfering with any unexplored mechanisms. Consequently, additional investigation is required to identify the EDC threshold concentrations in human matrices below which negative effects do not manifest. Comprehensive, systematic epidemiological studies that consider the combination and low-dose effects of EDCs are required.
Author contributions
NK: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. MN: Data curation, Software, Visualization, Writing – review & editing. AS: Conceptualization, Supervision, Writing – review & editing. JB: Data curation, Investigation, Writing – review & editing. SM: Formal analysis, Supervision, Writing – review & editing, Validation. BJ: Formal analysis, Supervision, Writing – review & editing, Investigation. RN: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgements
The authors would like to thank JSS Academy of Higher Education & Research, Mysuru, for their constant support for the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar S, Thaker R, Verma V, Gor M, Agarwal R, Mishra V. Occupational, environmental exposure, and lifestyle factors: declining male reproductive health. J Gynaecol Infertil. (2018) 1(1):1–29.
2. Giahi L, Mohammad Moradi S, Javidan A, Sadeghi MR. Nutritional modifications in male infertility: a systematic review covering 2 decades. Nutr Rev. (2016) 74(2):118–30. doi: 10.1093/nutrit/nuv059
3. Emokpae MA, Brown SI. Effects of lifestyle factors on fertility: practical recommendations for modification. Reprod Fertil. (2021) 2(1):13–26. doi: 10.1530/RAF-20-0046
4. Acharya S, Gowda CR. Lifestyle factors associated with infertility in a rural area: a cross-sectional study. Int J Med Sci Public Health. (2017) 6(3):502–7. doi: 10.5455/ijmsph.20170852309092016
5. Hemati Z, Heidari-Beni M, Kelishadi R. Exposure to endocrine disrupting chemicals, part of lifestyle factors related to growth disorders in childhood and chronic diseases in adulthood. In: Kelishadi R, editor. Healthy Lifestyle Integrated Science, vol 3. London: Springer Nature (2022). p. 265–75. doi: 10.1007/978-3-030-85357-0_14
6. Khalid M, Abdollahi M. Environmental distribution of personal care products and their effects on human health. Iran J Pharm Res. (2021) 20(1):216–53. doi: 10.22037/ijpr.2021.114891.15088
7. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. (2007) 210(5):623–34. doi: 10.1016/j.ijheh.2007.07.011
8. Pandey A, Jatana GK, Sonthalia S. Cosmeceuticals. Treasure Island (FL): StatPearls Publishing (2020).
9. Hart LB, Walker J, Beckingham B, Shelley A, Alten Flagg M, Wischusen K, et al. A characterization of personal care product use among undergraduate female college students in South Carolina, USA. J Expo Sci Environ Epidemiol. (2020) 30(1):97–106. doi: 10.1038/s41370-019-0170-1
10. Mao H, Li H, Li Y, Li L, Yin L, Yang Z. Four typical personal care products in a municipal wastewater treatment plant in China: occurrence, removal efficiency, mass loading and emission. Ecotoxicol Environ Saf. (2020) 188:1–9. doi: 10.1016/j.ecoenv.2019.109818
11. Capela D, Alves A, Homem V, Santos L. From the shop to the drain—volatile methylsiloxanes in cosmetics and personal care products. Environ Int. (2016) 92:50–62. doi: 10.1016/j.envint.2016.03.016
12. Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Rüden H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air. (2004) 14(3):188–95. doi: 10.1111/j.1600-0668.2004.00223.x
13. Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. (2012) 120(7):935–43. doi: 10.1289/ehp.1104052
14. Hammel SC, Levasseur JL, Hoffman K, Phillips AL, Lorenzo AM, Calafat AM, et al. Children’s exposure to phthalates and non-phthalate plasticizers in the home: the TESIE study. Environ Int. (2019) 132:1–27. doi: 10.1016/j.envint.2019.105061
15. Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Prakash A, et al. Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front Public Health. (2020) 8:1–28. doi: 10.3389/fpubh.2020.553850
16. Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol. (2009) 304(1-2):43–8. doi: 10.1016/j.mce.2009.02.017
17. Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, et al. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator–activated receptor gamma-independent mechanism. Environ Health Perspect. (2012) 120(7):984–9. doi: 10.1289/ehp.1205063
18. Ropero AB, Alonso-Magdalena P, Garcia-Garcia E, Ripoll C, Fuentes E, Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl. (2008) 31(2):194–200. doi: 10.1111/j.1365-2605.2007.00832.x
19. Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, et al. Developmental exposure to di (2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. (2011) 301(3):527–38. doi: 10.1152/ajpendo.00233.2011
20. Soriano S, Alonso-Magdalena P, Garcia-Arevalo M, Novials A, Muhammed SJ, Salehi A, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One. (2012) 7(2):1–9. doi: 10.1371/journal.pone.0031109
21. Asakawa A, Toyoshima M, Fujimiya M, Harada K, Ataka K, Inoue K, et al. Perfluorooctane sulfonate influences feeding behavior and gut motility via the hypothalamus. Int J Mol Med. (2007) 19(5):733–9. doi: 10.3892/ijmm.19.5.733
22. Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. (2014) 28(1):99–115. doi: 10.1210/me.2013-1270
23. Shafei A, Ramzy MM, Hegazy AI, Husseny AK, El-Hadary UG, Taha MM, et al. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene. (2018) 647:235–43. doi: 10.1016/j.gene.2018.01.016
24. Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the longitudinal investigation of fertility and the environment (LIFE) study. Fertil Steril. (2014) 101(5):1359–66. doi: 10.1016/j.fertnstert.2014.01.022
25. Zhou W, Fang F, Zhu W, Chen ZJ, Du Y, Zhang J. Bisphenol A and ovarian reserve among infertile women with polycystic ovarian syndrome. Int J Environ Res Public Health. (2017) 14(1):1–17. doi: 10.3390/ijerph140.10018
26. Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. (2013) 42:224–31. doi: 10.1016/j.reprotox.2013.09.008
27. Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. (2011) 96(3):480–4. doi: 10.1210/jc.2010-1658
28. Rashidi BH, Amanlou M, Lak TB, Ghazizadeh M, Haghollahi F, Bagheri M, et al. The association between bisphenol A and polycystic ovarian syndrome: a case-control study. Acta Med Iran. (2017) 55(12):759–64.29373882
29. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. (2012) 120(7):978–83. doi: 10.1289/ehp.1104307
30. Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. (2009) 23(11):1186–90. doi: 10.1002/bmc.1241
31. Wang Q, Yang H, Yang M, Yu Y, Yan M, Zhou L, et al. Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere. (2019) 221:235–45. doi: 10.1016/j.chemosphere.2019.01.033
32. Du YY, Fang YL, Wang YX, Zeng Q, Guo N, Zhao H, et al. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol. (2016) 61:142–50. doi: 10.1016/j.reprotox.2016.04.005
33. Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A. Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: a literature review. Int J Environ Res Public Health. (2020) 17(18):6811. doi: 10.3390/ijerph17186811
34. Kim SH, Chun S, Jang JY, Chae HD, Kim CH, Kang BM. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case-control study. Fertil Steril. (2011) 95(1):357–9. doi: 10.1016/j.fertnstert.2010.07.1059
35. Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, et al. Phthalates and risk of endometriosis. Environ Res. (2013) 126:91–7. doi: 10.1016/j.envres.2013.07.003
36. Srilanchakon K, Thadsri T, Jantarat C, Thengyai S, Nosoognoen W, Supornsilchai V. Higher phthalate concentrations are associated with precocious puberty in normal weight Thai girls. J Pediatr Endocrinol Metab. (2017) 30(12):1293–8. doi: 10.1515/jpem-2017-0281
37. Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, et al. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res. (2017) 159:143–51. doi: 10.1016/j.envres.2017.07.051
38. Zhang Y, Yang Y, Tao Y, Guo X, Cui Y, Li Z. Phthalates (PAEs) and reproductive toxicity: hypothalamic-pituitary-gonadal (HPG) axis aspects. J Hazard Mater. (2023) 459:132182. doi: 10.1016/j.jhazmat.2023.132182
39. Anne B, Raphael R. Endocrine disruptor chemicals. (2021). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK569327/ (Accessed June 20, 2025).
40. Ploteau S, Cano-Sancho G, Volteau C, Legrand A, Vénisseau A, Vacher V, et al. Associations between internal exposure levels of persistent organic pollutants in adipose tissue and deep infiltrating endometriosis with or without concurrent ovarian endometrioma. Environ Int. (2017) 108:195–203. doi: 10.1016/j.envint.2017.08.019
41. Matsumura F. Mechanism of action of dioxin-type chemicals, pesticides, and other xenobiotics affecting nutritional indexes. Am J Clin Nutr. (1995) 61(3):695S–701. doi: 10.1093/ajcn/61.3.695S
42. Katz TA, Walker CL. Implications for chromatin biology in toxicology. In: McCullough SD, Dolinoy DC, editors. Toxicoepigenetics. Amsterdam: Academic Press (2019). p. 105–24.
43. Martínez-Zamora MA, Mattioli L, Parera J, Abad E, Coloma JL, Van Babel B, et al. Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis. Hum Reprod. (2015) 30(5):1059–68. doi: 10.1093/humrep/dev026
44. Cai W, Ji Y, Song X, Guo H, Han L, Zhang F, et al. Effects of glyphosate exposure on sperm concentration in rodents: a systematic review and meta-analysis. Environ Toxicol Pharmacol. (2017) 55:148–55. doi: 10.1016/j.etap.2017.07.015
45. Morris A. Exposure to pesticide residues linked to adverse pregnancy outcomes. Nat Rev Endocrinol. (2018) 14(1):4. doi: 10.1038/nrendo.2017.156
46. Ledda C, Fiore M, Santarelli L, Bracci M, Mascali G, D’Agati MG, et al. Gestational hypertension and organophosphorus pesticide exposure: a cross-sectional study. BioMed Res Int. (2015) 2015:1–5. doi: 10.1155/2015/280891
47. Al-Hussaini TK, Abdelaleem AA, Elnashar I, Shabaan OM, Mostafa R, El-Baz MA, et al. The effect of follicullar fluid pesticides and polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological and clinical outcome. Eur J Obstet Gynecol Reprod Biol. (2018) 220:39–43. doi: 10.1016/j.ejogrb.2017.11.003
48. Vagi SJ, Azziz-Baumgartner E, Sjödin A, Calafat AM, Dumesic D, Gonzalez L, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol a in polycystic ovary syndrome: a case–control study. BMC Endocr Disord. (2014) 14:1–2. doi: 10.1186/1472-6823-14-86
49. Guo Z, Qiu H, Wang L, Wang L, Wang C, Chen M, et al. Association of serum organochlorine pesticides concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Chemosphere. (2017) 171:595–600. doi: 10.1016/j.chemosphere.2016.12.127
50. Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in US women. PLoS One. (2015) 10(1):e0116057. doi: 10.1371/journal.pone.0116057
51. Bastiansz A, Ewald J, Rodríguez Saldaña V, Santa-Rios A, Basu N. A systematic review of mercury exposures from skin-lightening products. Environ Health Perspect. (2022) 130(11):116002. doi: 10.1289/EHP10808
52. Rodríguez-Villamizar LA, Jaimes DC, Manquián-Tejos A, Sánchez LH. Human mercury exposure and irregular menstrual cycles in relation to artisanal gold mining in Colombia. Biomedica. (2015) 35:38–45. doi: 10.1590/S0120-41572015000500005
53. Tursunova V, Muratov J, Shriimathi K, Quadri GAR. Impact of mercury on sex hormones (literature review). Вестник Ошского Государственного Университета. (2024) 4:1–2. doi: 10.52754/16948610_2024_4_1
54. Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. Obstet Gynecol Surv. (2003) 58(9):536–92. doi: 10.1056/NEJMoa020880
55. Shan D, Wen X, Guan X, Fang H, Liu Y, Qin M, et al. Pubertal lead exposure affects ovary development, folliculogenesis and steroidogenesis by activation of IRE1α-JNK signaling pathway in rat. Ecotoxicol Environ Saf. (2023) 257:114919. doi: 10.1016/j.ecoenv.2023.114919
56. Wu SY, Tian J, Wang MZ, Pan BJ, Lü HD, Wang ZM, et al. The effect of cadmium pollution on reproductive health in females. Zhonghua liu Xing Bing Xue Za Zhi. (2004) 25(10):852–5.15631738
57. Massányi P, Massányi M, Madeddu R, Stawarz R, Lukáč N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics. (2020) 8(4):94. doi: 10.3390/toxics8040094
58. Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, et al. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. (2006) 80(3):217–27. doi: 10.1016/j.aquatox.2006.08.010
59. Ahmed RG, El-Gareib AW, Shaker HM. Gestational 3, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: fetal thyroid-cytokines dysfunction. Life Sci. (2018) 192:213–20. doi: 10.1016/j.lfs.2017.11.033
Keywords: fertility, endocrine disruptors, personal care, lifestyle, menstrual cycle
Citation: Kalsi Rajashekara N, Natarajan M, Srinivasan A, Babu J, Madhunapantula SV, Jayshankar B and Nataraj R (2025) Role of personal care products as endocrine disruptors affecting reproductive age women. Front. Reprod. Health 7:1514060. doi: 10.3389/frph.2025.1514060
Received: 23 October 2024; Accepted: 23 June 2025;
Published: 11 July 2025.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Alan Marc Hoberman, Charles River Laboratories, United StatesMianqun Zhang, Anhui Agricultural University, China
Margarida Lorigo, University of Beira interior, Portugal
Emre Gezer, Kocaeli University, Türkiye
Copyright: © 2025 Kalsi Rajashekara, Natarajan, Srinivasan, Babu, Madhunapantula, Jayshankar and Nataraj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bindu Jayshankar, YmluZHVqQGpzc3N0dW5pdi5pbg==; Raghu Nataraj, cmFnaHVuQGpzc3VuaS5lZHUuaW4=
 Nitin Kalsi Rajashekara1
Nitin Kalsi Rajashekara1 Madhumitha Natarajan
Madhumitha Natarajan Asha Srinivasan
Asha Srinivasan Bindu Jayshankar
Bindu Jayshankar Raghu Nataraj
Raghu Nataraj