- 1Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- 2The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 3Hangzhou Women’s Hospital (Hangzhou Maternity and Child Health Care Hospital), Hangzhou, China
- 4Hangzhou Xihu Xixi Community Health Service Center, Hangzhou, China
- 5The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou, China
- 6Traditional Chinese Medicine for Reproductive Health Key Laboratory of Zhejiang Province, Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology and Key Lab Womens Reprod Hlth Zhejiang Prov, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 7Interdisciplinary Research Academy (IRA), Zhejiang Shuren University, Hangzhou, China
Polycystic ovary syndrome (PCOS) is a prevalent endocrine disorder among women, characterized by metabolic abnormalities and infertility. Despite its high prevalence, the etiology and pathogenesis of PCOS remain poorly understood. Emerging evidence suggests that persistent organic pollutants (POPs), known for their detrimental effects on the endocrine and reproductive systems, may play a role in the development and progression of PCOS. Among POPs, organochlorine pesticides (OCPs) are particularly widespread and pose significant health risks. This review examines the potential of OCPs as an environmental factor in the development and progression of PCOS. It highlights the mechanisms through which OCPs may disrupt the hypothalamic-pituitary-ovarian (HPO) axis and impair hormonal regulation, contributing to the onset and exacerbation of PCOS. Evidence links OCPs to insulin resistance, obesity, and type 2 diabetes mellitus. These disruptions may occur via pathways involving hypothyroidism or altered adrenal androgen secretion. While current evidence supports a plausible connection between OCP exposure and PCOS, significant gaps and inconsistencies in the data warrant further investigation. Elucidating the precise mechanisms underlying these associations is crucial for developing targeted prevention and intervention strategies.
1 Introduction
Polycystic ovary syndrome (PCOS) is a prevalent endocrine disorder affecting women of reproductive age and is recognized as the leading cause of infertility. The global prevalence of PCOS ranges from 4% to 21%, varying by diagnostic criteria and population (1). This complex condition is characterized by hyperandrogenism, ovarian dysfunction, and polycystic ovarian morphology. The expression of PCOS is thought to result from a multifactorial etiology involving both genetic and environmental factors (2). Among environmental factors, persistent organic pollutants (POPs) have emerged as potential contributors.
Persistent organic pollutants (POPs) are pervasive environmental contaminants known for bioaccumulation in living organisms. Persistent organic pollutants exhibit bioaccumulation factors >5,000 in adipose tissue (3). A significant proportion function as endocrine-disrupting chemicals (EDCs), interfering with hormone synthesis, secretion, and metabolism. Organochlorine pesticides (OCPs)—a prominent subgroup of POPs—are synthetic chlorine-containing compounds first synthesized in 1874. Historically used as insecticides and for disease control (e.g., malaria), OCPs are highly lipophilic and resistant to biodegradation (4). Due to their persistence and toxicity, global regulatory efforts have targeted OCPs.
The United Nations implemented the Stockholm Convention in 2001 to eliminate POP production and restrict agricultural/industrial use (Stockholm Convention (5). OCPs were included in Annex A given their historical prevalence and environmental persistence (6). OCPs bind to estrogen receptors with affinity constants (Kd) of 10−8–10−9 M (7), classifying them as prototypical EDCs. Despite regulatory action, the endocrine-disrupting potential of OCPs remains a concern for reproductive disorders like PCOS.
The hypothalamic-pituitary-ovarian (HPO) axis regulates female neuroendocrine function, secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the pituitary gland via gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. Dysregulation of this axis—alongside insulin resistance (IR) -is central to PCOS pathophysiology. There is emerging evidence that serum levels of oral contraceptive pills (OCPs) differ significantly between patients with polycystic ovary syndrome (PCOS) and controls (8–10). This evidence implicates OCPs in reproductive/endocrine disruption. This motivates our systematic examination of OCPs as environmental risk factors.
Therefore, the aim of this review is to: (1) provide a summary of PCOS pathogenesis and clinical manifestations; (2) evaluate the effects of OCPs on reproductive and endocrine systems; (3) analyze the epidemiological and mechanistic links between OCPs and PCOS; and (4) discuss OCPs as potential environmental etiological factors that contribute to the dysregulation associated with PCOS.
2 Review strategy
To evaluate the current research perspective on the relationship between PCOS and OCPs, a systematic literature search was conducted using the Web of Science and SCOPUS databases for the period 2004–2024. The search strategy included a combination of topic-specific keywords applied to the title, abstract, and keywords sections of the studies. The search terms included: (i) PCOS, (ii) PCOS and EDC, (iii) OCP, (iv) HPO axis, and (v) endocrine system.
Keywords were as follows:
(i) PCOS: patholo* or mechanism or epidemiolo* or aetiolo*;
(ii) PCOS and EDC: bisphenol A or per- and polyfluoroalkyl substance* or polychlorinated biphenyl*(PCBs) or phthalate* or air pollut* or tributyltin(TBT) or microplastic* or pharmaceuticals and personal care product*(PPCPs) or nanoparticle* or organochlorine pesticide*;
(iii) OCPs: epidemiolog* or soil or plant or food or population risk or estrogen*;
(iv) HPO axis: OCP and hypothalamus or pituitary or ovaries or uterus;
(v) Endocrine system: OCP and insulin resisten* or diabetes or obesity, OCP and thyroid funct*, OCP and adrenal gland or adrenal hormones.
The inclusion criteria were formulated to include articles written in English that explored the pathogenesis, clinical manifestations of PCOS, and the endocrine-disrupting effects of OCPs. While several reviews have examined PCOS from clinical, epidemiological, or pathogenetic perspectives (11–13), this review uniquely emphasizes the potential association between PCOS and a pervasive class of POPs, namely, OCPs. The initial screening process involved reviewing titles and abstracts against the predefined inclusion criteria. Subsequently, full-text articles meeting these criteria were examined in detail. In addition, reference lists of the included articles were manually reviewed for the identification of relevant studies.
3 Endocrine disruptors and PCOS: a mechanistic insight
3.1 Historical context and diagnostic evolution
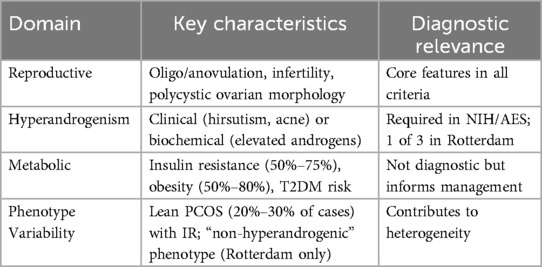
PCOS was first described in 1935 by Stein and Leventhal, who observed symptoms including hirsutism, obesity, amenorrhea, and bilaterally enlarged polycystic ovaries (14). Diagnostic criteria have evolved through three major frameworks: the National Institutes of Health (1990), Rotterdam (2003), and Androgen Excess Society (2006) criteria, reflecting ongoing refinement in PCOS characterization (2, 15).
3.2 Epidemiological trends and clinical findings
The global prevalence of PCOS ranges from 4% to 21%, varying by diagnostic criteria and population (1). Longitudinal data indicate a rising trend, with the global age-standardized incidence increasing by 1.45% from 2007 to 2017 (reaching 82.44 per 100,000 women) and affecting approximately 66 million women by 2019 (16, 17). Geographical disparities exist, with the highest incidence in Ecuador (242.54 per 100,000) and significant increases in countries with medium-high socio-demographic indices (18).
PCOS typically manifests during puberty, with menstrual dysfunction (e.g., oligomenorrhea, amenorrhea) as the primary feature (19). Clinical hallmarks include hyperandrogenism (hirsutism, acne, alopecia) and metabolic complications, with over 50% of patients exhibiting abdominal obesity (20–22). Notably, IR occurs across BMI categories, affecting a subset of lean patients, while infertility due to anovulation remains a major concern (23, 24).
3.3 Core pathophysiological mechanisms
PCOS manifests through three interconnected pathways, summarized in Table 1. The first involves dysregulation of the hypothalamic-pituitary-ovarian (HPO) axis. Increased pituitary sensitivity to gonadotropin-releasing hormone (GnRH) stimulation results in excessive luteinizing hormone (LH) secretion, which promotes androgen production by theca cells and contributes to hyperandrogenemia (25, 26). Concurrently, follicle-stimulating hormone (FSH) levels are relatively low due to negative feedback from estrogen, inhibiting follicular development and leading to a high LH/FSH ratio. This hormonal imbalance further disrupts ovarian follicle maturation and increases anti-Müllerian hormone (AMH) levels (27, 28). Additionally, in a hyperandrogenic environment, the pulsatility of GnRH is impaired, resulting in an increase in LH release and a decrease in FSH release (29).
The second key pathway is hyperinsulinemia and insulin resistance (IR). Reduced peripheral insulin sensitivity diminishes its biological efficacy (30). Insulin acts as a potent regulator of androgen production in the ovary, stimulating the growth and secretion of hormones in follicle cells by acting on insulin receptors (31). It also triggers ovarian P450c17 and P450scc enzyme activities to promote ovarian steroidogenesis (32), and in the presence of chorionic gonadotropin, it synergistically increases these activities (33). Excessive insulin levels directly impact pituitary receptors, leading to the release of LH and subsequent stimulation of androgen secretion from the ovaries and adrenal glands. This process increases free testosterone by inhibiting the synthesis of hepatic sex hormone-binding globulin. While IR is a central feature of PCOS (affecting approximately 75% of patients), it is notable that some PCOS patients with normal or low body mass indices develop IR, indicating that the relationship is not absolute (23, 34).
The third pathway encompasses ovarian and endometrial alterations. Compared to women without PCOS, the ovaries of those with the condition are typically bilaterally and uniformly enlarged, ranging from approximately 2–5 times their normal size (35). Additionally, the endometrium in patients with PCOS often exhibits varying degrees of hyperplasia due to estrogen surges associated with prolonged anovulation, which may elevate the risk of developing endometrial cancer (36–38). These pathological changes reflect the systemic impact of hormonal dysregulation on reproductive tissues.
3.4 Genetic and environmental etiology
The etiology of PCOS remains incompletely understood but is recognized to involve complex interactions between genetic and environmental factors. From a genetic perspective, the disorder may arise from cumulative effects of polymorphisms in genes related to steroidogenesis, insulin signaling, and chronic inflammation, alongside epigenetic modifications and altered protein profiles that collectively drive systemic dysfunction (39, 40). Environmentally, increasing attention focuses on pollutants, particularly endocrine-disrupting chemicals (EDCs), that can mimic, antagonize, or disrupt hormonal signaling pathways. These compounds may alter normal endocrine homeostasis through multiple mechanisms, as detailed in the following subsection. Of note, lipophilic EDCs like organochlorine pesticides (OCPs) may accumulate in ovarian tissue, potentially exacerbating hormonal dysregulation and contributing to PCOS pathogenesis.
3.5 Endocrine-Disrupting chemical actions
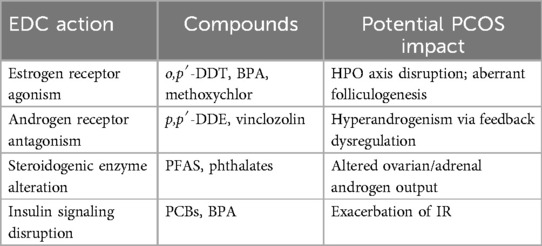
Endocrine-disrupting chemicals interfere with hormonal homeostasis through four primary mechanisms (Table 2): (1) mimicking endogenous steroid hormones by binding to nuclear receptors; (2) antagonizing natural hormone actions; (3) altering the synthesis, metabolism, or clearance of endogenous hormones; and (4) modifying hormone receptor expression in target tissues (41). A growing body of evidence suggests correlations between PCOS and exposure to environmental contaminants acting through these pathways. Notably, OCPs—as prototypical EDCs—exemplify these mechanisms by binding estrogen receptors and inducing estrogen-like responses, thereby disrupting reproductive and metabolic functions relevant to PCOS.
4 Environmental exposure to EDCs and PCOS
4.1 Evidence for key EDC classes
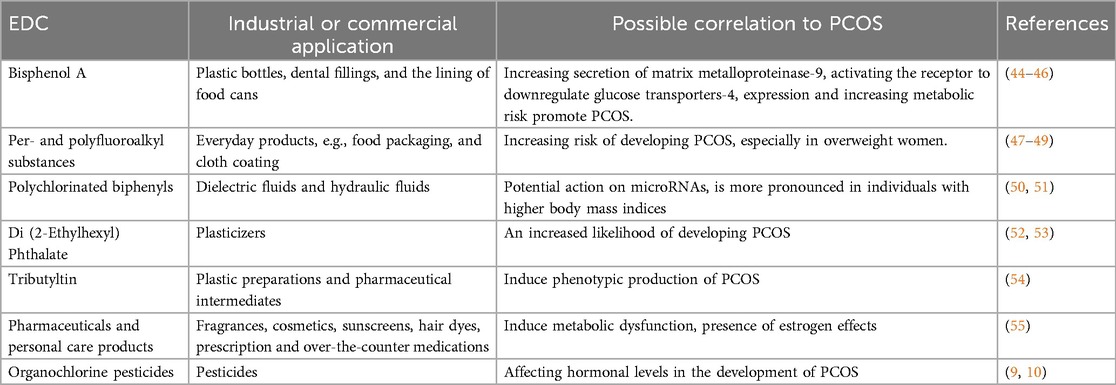
EDCs encompass diverse substances including pesticides, industrial chemicals, plasticizers, and pharmaceuticals that are ubiquitous in modern environments (42). These compounds interfere with sex steroid hormone synthesis, action, and metabolism, contributing to adverse reproductive outcomes including developmental toxicity, infertility, and hormone-related cancers (3). There have also been reports of interference with the hypothalamic-pituitary-thyroid and adrenal axes (43). Four major classes show documented associations with PCOS pathogenesis through distinct pathways (Table 3):
4.1.1 Bisphenol A (BPA)
As the most extensively studied EDC in PCOS contexts, BPA exhibits mild estrogenic and anti-androgenic properties. Human biomonitoring reveals urinary BPA correlates with elevated testosterone in PCOS patients (56), while in vitro studies demonstrate it increases matrix metalloproteinase-9 secretion in granulosa cells (44). Proposed mechanisms include downregulation of glucose transporter-4 via aryl hydrocarbon receptor activation (46) and multigenerational effects evidenced in medaka fish models (57). BPA exposure is associated with visceral obesity, hyperinsulinemia, dyslipidemia, and elevated androgens in PCOS patients (45). While human evidence is robust (n = 1,046 in Milanovic et al.), causal inference is limited by predominantly cross-sectional designs.
4.1.2 Per/Polyfluoroalkyl Substances (PFAS)
PFAS (“forever chemicals”) used in textiles, food packaging, and firefighting foams demonstrate environmental persistence (47). Elevated PFAS levels associate with increased PCOS risk (48, 58), particularly among overweight/obese women. Recent analyses confirm PFAS as significant contributors to PCOS risk profiles (49). Mechanistic evidence remains less developed than for BPA, though obesogenic effects are well-documented.
4.1.3 Polychlorinated Biphenyls (PCBs)
These dielectric fluids accumulate in adipose tissue due to extended half-lives. PCBs have been linked to PCOS through microRNA interactions (50), with stronger correlations observed in higher-BMI individuals (131). Counterevidence exists regarding menstrual cycle hormone impacts (59). Discrepant findings highlight phenotype-specific susceptibility and exposure timing variables.
4.1.4 Phthalates and PPCPs
Di(2-ethylhexyl) phthalate (DEHP) exposure correlates with increased PCOS likelihood (52), with mixture exposure showing stronger effects in urinary metabolite analyses (53). Environmentally obesogenic tributyltin induces PCOS-like reproductive, metabolic, and cardiovascular abnormalities (54), while select pharmaceuticals and personal care products (PPCPs) disrupt metabolic function through estrogenic, anti-estrogenic, and anti-androgenic activities (55). PPCP evidence derives primarily from animal models, warranting human cohort validation.
4.1.5 Organochlorine Pesticides (OCPs)
Emerging human evidence shows higher serum OCP levels (p,p′-DDE, o,p′-DDT) in PCOS patients vs. controls (9, 10). This supports OCPs as environmental contributors to PCOS pathogenesis, though mechanistic research remains limited compared to other EDCs.
4.2 Human exposure to OCPs and EDC effects
Human exposure to organochlorine pesticides occurs primarily through inhalation of contaminated air, ingestion of tainted food and water sources, and direct dermal contact with polluted environmental media, as evidenced by biomonitoring studies across diverse populations (60). Global biomonitoring data confirm widespread body burden, with breast milk samples from four Asian countries showing 100% detection rates for p,p′-DDT and p,p′-DDE (61). In the United States, the National Health and Nutrition Examination Survey (NHANES) reported mean serum p,p′-DDE concentrations of 238 ng/g lipid during 2003–2004 (62), while contemporary Chinese cohorts exhibit particularly elevated levels, including pentachlorophenol concentrations reaching 3.13 μg/L in Beijing residents (51). Australian longitudinal data demonstrate temporal declines across five sampling periods (2002–2013), though persistent detection confirms ongoing exposure despite regulatory restrictions (63).
OCPs exert significant adverse effects on female reproductive development and function across the lifespan. Adolescent exposure correlates with delayed physical and sexual maturation (64), while reproductive-age women experience dose-dependent ovarian impacts including follicular atresia, altered steroid secretion patterns, and diminished oocyte viability (65). Transplacental transfer occurs consistently across populations, with maternal serum concentrations exceeding fetal levels despite partial placental barrier function (66), as demonstrated in Wuhan cohort studies where median hexachlorocyclohexane (HCH) and DDT concentrations in umbilical cord serum reached 10.1 ng/g lipid and 35.5 ng/g lipid, respectively (67).
Molecular mechanisms underlying these effects involve multifaceted endocrine disruption pathways. OCPs elicit estrogenic responses through direct binding to estrogen receptors (7) while simultaneously modulating steroidogenesis via transcriptional regulation of aromatase (CYP19A1) in granulosa cells (68). Androgen receptor antagonism has been documented in receptor binding assays (69), and early developmental pathway alterations occur through epigenetic reprogramming effects observed in fish models (70). In a separate study, p,p′-DDE inhibited 17 alpha-methyltestosterone (TES)-induced sult2st3 expression in zebrafish embryos; however, at higher concentrations of TES, p,p′-DDE did not inhibit sult2st3 induction but rather enhanced TES-induced sult2st3 expression (71). These findings collectively demonstrate that OCPs disrupt reproductive physiology through receptor-mediated signaling and enzymatic interference, contributing to PCOS-relevant endocrine dysfunction.
5 Associations between OCPs and the HPO axis
The hypothalamic-pituitary-ovarian (HPO) axis represents a primary target for OCP-induced endocrine disruption, as illustrated in Figure 1. Evidence suggests OCPs impair regulatory functions at three anatomical levels:
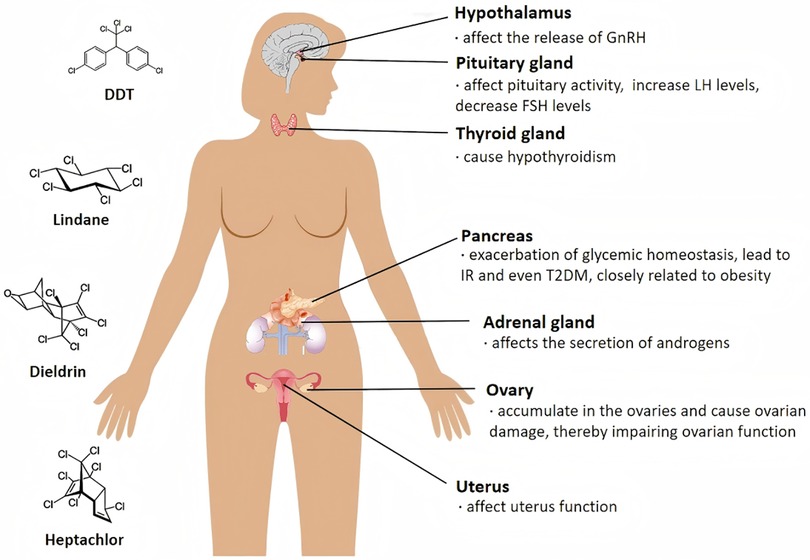
Figure 1. Proposed mechanisms linking environmental EDCs to PCOS development. The schematic diagram represents a hypothetical illustration of the potential adverse effects of OCPs on female internal organs. Notably, the diagram does not represent definitive conclusions but rather provides a hypothetical representation of the potential impact of these compounds.
5.1 Hypothalamic-pituitary dysregulation by OCPs
Previous reports have suggested that exposure to OCPs during neurodevelopment is associated with altered cytokine homeostasis in the hypothalamus, which would favor changes in hormonal communication.
In vitro studies revealed that metolachlor increased GnRH mRNA levels in GT1-7 cells, whereas another study revealed that it reduced them in female mice, with reduced ESR2 (ERβ) expression in the medial preoptic region and no significant change in ESR1 (ERα) expression (72). The results from other studies have also indicated that methoxychlor is associated with an increase in kisspeptin cell expression in the anterior ventral periventricular region of the medial preoptic area, whereas chronic dieldrin exposure alters hypothalamic messenger RNA and protein abundance (73). Endosulfan reduces catfish tryptophan hydroxylase 2 (tph2) and gonadotropin-releasing hormone (cfGnRH) transcript levels and significantly reduces forebrain/preoptic tph2 immunoreactivity (74).
The results from in vivo studies further confirmed that some OCPs, e.g., methoxychlor, have a direct effect on the pituitary gland (75). Moreover, o,p′-DDD, methoxychlor, and HCH can stimulate the growth of the rat pituitary cell line MtT/E-2 both In vitro and in vivo (132). Endosulfan may act as a downstream mediator of LH receptor activation and as an upstream mediator of the steroidogenic enzymes studied, leading to reduced pituitary FSH levels (37). Endosulfan may affect pituitary activity by inducing the gene expression of nitric oxide synthase in rats. It increases plasma LH and GH levels but decreases plasma thyroid-stimulating hormone concentrations (76).
In a highly polluted area in Brazil, human biomonitoring studies have suggested that organochlorine pesticides are negatively correlated with LH and FSH in perimenopausal and menopausal women (77).
5.2 Ovarian-uterine pathological effects of OCPs
A study of stranded pregnant sperm whales revealed that contaminants such as DDT and HCH were present at higher concentrations in the ovaries than in the epidermis. OCPs may accumulate in ovarian tissue through metabolism and trigger adverse effects (78). In vitro studies suggest that β-HCH, DDE, and dieldrin induce reactive oxygen species, proinflammatory responses, and DNA damage in human ovarian surface epithelial cells (79). Chlordane and endrin stimulate the secretion of testosterone and estradiol from the granulosa cells of the ovaries of cows, whereas toxaphene and heptachlor can inhibit this effect (80, 81). Pentachloronitrobenzene can alter MAPK3/1 signaling and progesterone production and accelerate follicular development while increasing AMH in ovarian tissue and serum (82). HCB inhibits lipofuscin secretion and expression in follicle cells by directly inhibiting lipofuscin (83) and decreases lipocalin-stimulated E2 secretion, which may contribute to ovarian dysfunction in obesity-related diseases. Human ovarian cells exposed to the HCB and p,p′-DDE presented a decrease in the proportion of unilamellar follicles, an increase in follicular atresia, and altered expression of LDHA, ATP5A and GPX4 in exposed tissues, as well as altered ATP production in KGN and tissue cultures.
The results from animal studies also indicated that OCPs could affect ovarian/uterus function. Methoxychlor causes follicular atresia by inducing apoptosis in granulosa cells (84), which can damage ovarian epithelial cells and oocytes (85) and reduce serum estradiol and progesterone levels (86). It can also alter the expression of genes related to intracellular and membrane trafficking, intra-ovarian signaling, and intercellular junctions and communication in piglet ovaries (87). Early methoxychlor exposure may result in accelerated initial recruitment of ovarian follicles and impaired cyclic recruitment of sinusoidal follicles. It has also been proposed that both methoxychlor and its metabolite, 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane, could inhibit early ovarian development and stimulate AMH production by granulosa cells in the rat ovary. These effects may further reduce estrogen production and reduce estrogen synthesis through the inhibition of FSH-stimulated estrogen receptors, thereby reducing cAMP production (88). Early methoxychlor exposure may result in accelerated initial recruitment of ovarian follicles and impaired cyclic recruitment of sinusoidal follicles (89), while it has also been proposed that both methoxychlor and its major metabolite inhibit early ovarian development and stimulate AMH production by granulosa cells in the rat ovary (90), which may reduce estrogen synthesis by inhibiting FSH-stimulated estrogen receptors (ESR1 and 2), thereby reducing cAMP production. Dieldrin affects the reproductive endocrine system in buffalo granulosa cells (68) by regulating the proximal promoter, leading to increased specific CYP19A1 transcription and elevated estrogen levels. Lindane (91) can increase the level of the Cx43 gap junction blocker lindane in mouse granulosa cells, leading to ultrastructural damage and apoptosis.
High levels of OCPs in the follicular fluid (92) adversely affect the outcome of intracytoplasmic sperm injection. The higher the OCP concentration measured in samples of human origin was, the lower the sampling rate, fertilization rate and embryo retrieval rate were. Human biomonitoring studies have detected OCPs in ovarian fluid samples from female populations. In 127 follicular fluid samples from randomly selected fertile women in China, 17 OCPs were detected, with chloromethyl chloride being the most common, followed by heptachlor epoxide, hexachlorocyclohexane, endrin, and DDT (93). In Uppsala, Sweden, OCPs were found in both blood and follicular fluid samples collected from 185 women (94). Current knowledge suggests that OCPs have the capacity to disrupt physiological function through exposure, but the response‒dose relationships between OCPs and adverse health outcomes warrant further investigation.
6 Associations between OCPs and the endocrine system
6.1 Associations between OCPs and IR and obesity
Insulin, which is secreted by beta cells in the pancreas, works with glucagon to regulate blood glucose levels (95). It induces glucose storage in the liver, muscles and adipose tissue, leading to weight gain (96, 97). IR is physiologically defined as a state of reduced responsiveness of insulin target tissues to high physiological levels of insulin (98). IR is one of the major symptoms of PCOS, as approximately 75% of patients with PCOS will develop it (99), and a higher percentage of patients with PCOS who are obese will suffer from IR (100).
Insulin has two main pathways. The metabolic pathway is mainly mediated by phosphatidylinositol 3-kinase and protein kinase B, also known as the phosphatidylinositol 3-kinase pathway. The second pathway is the mitogenic pathway, which refers to the mitogen-activated protein kinase-extracellular signal-regulated kinase pathway. Increased serine phosphorylation and decreased tyrosine phosphorylation of the insulin receptor and IRS terminate insulin action, leading to insulin dysfunction in women with PCOS (100, 101).
OCPs can lead to IR and even diabetes. Exposure to lindane (102) and DDT (103) at sub-toxic concentrations could induce oxidative stress in mouse myotubular cells, activating receptor-sensitive kinases, impairing insulin signaling pathways and promoting IR effects. An In vitro study revealed that p,p′-DDE causes pancreatic islet beta cells to become dysfunctional and destroyed (104). Exposure to DDE alters the plasma lipid metabolomic profile while impairing glucose homeostasis and even inducing dysbiosis of the gut flora (105). p,p′-DDE may play a role in the regulation of immune cell function, leading to immune system dysfunction resulting in IR or metabolic syndromes (106).
Studies suggest that human exposure to OCPs may cause biological, metabolic, and endocrine disruption. Reports from cross-sectional studies have shown a positive correlation between OCP and the risk of metabolic syndromes, such as abdominal obesity and dysglycemia (51, 107–109). Longitudinal studies have also shown that long-term exposure to OCPs could worsen people's glycemic homeostasis (110, 111) and increase the risk of metabolic syndrome (111, 112). Serum OCP concentrations play an important role in the development of human metabolic phenotypes, and this conclusion also applies to healthy individuals of normal weight (113, 114). The vast majority of PCOS patients have abdominal obesity and IR, leading to the hypothesis that OCP accumulation is more likely in PCOS patients than in the general population and that OCP may be one of the environmental factors contributing to PCOS patients with IR or obesity.
A strong association between exposure to OCPs and type 2 diabetes mellitus (T2DM) has been reported in epidemiological studies. For example, a study in northern India reported significantly higher levels of β-HCH, dieldrin and p,p′-DDE in a pre-diabetic group and a newly identified diabetic group than in a normal population (115). Serum concentrations of OCPs are significantly associated with the risk of T2DM in adults in Saudi Arabia exposed to OCPs (116). The risk of T2DM was found to be greatly increased in people exposed to higher levels of OCPs via contaminated drinking water (117) and/or living environments (118). OCPs, such as the HCBs in adipose tissue or fat, have also been reported to be significantly associated with IR and diabetes (119).
OCPs are also associated with obesity, another major symptom of PCOS. The results from animal studies have shown that Endrin promotes early adipogenesis in 3T3-L1 cells through the activation of mammalian target of rapamycin (120). Another study revealed that exposure to DDE increased body weight and adiposity in mice (105). P,p′-DDE was also found to be able to induce the expression of macrophage surface markers in mouse adipose tissue and affect macrophage reactivity In vitro, thereby promoting fat synthesis (106). Similar findings have been reported in human biomonitoring. The results of a cross-sectional study revealed that waist circumference was strongly associated with OCPs in 117 patients who underwent non oncological surgery (109). OCPs may also be linked to the worsening of obesity-related clinical complications (121). There was a strong correlation between OCPs and two of the characteristic symptoms of PCOS, namely, IR and obesity. However, the underlying mechanisms of these effects require further in vitro, in vivo and in silico studies.
6.2 OCP and the thyroid gland
There is evidence to suggest that an increased incidence of thyroid disorders, including autoimmune thyroiditis and subclinical hypothyroidism, is associated with PCOS (122). Animal studies have suggested that pre-pregnancy exposure to HCB causes hypothyroidism, which has serious implications for female reproduction by severely affecting the functional connectivity of the thyroid and ovaries (123), and a study in Belgium revealed that residual organochlorine contaminants in umbilical cord blood were associated with thyroid hormones in newborns and the risk of maternal hypothyroidism (124). Existing studies suggest a link between thyroid disease and PCOS, but the causal relationship is unclear, and more research is needed (122).
6.3 OCP and adrenal glands
The adrenal glands can secrete androgens, and excessive secretion can cause PCOS in women. A possible link between OCPs and the adrenal glands is therefore also worthy of investigation. Animal studies have shown that methoxychlor reduces adrenal secretion of DHEA, StAR, CYP11A1, and HSD3B while increasing the expression of CYP17A1 and SULT2A1; i.e., OCPs may downregulate genes involved in the first step of adrenal androgen synthesis (125). Methoxychlor increased the number of fetal testicular mesenchymal stromal cells and stimulated CYP11A1 expression in mice exposed in utero. However, testosterone production was inhibited at high doses of methoxychlor (126). Disruption of the secretory function of chromaffin cells in the adrenal gland by prenatal and postnatal exposure to DDT in male Wistar rats (127). Studies suggest that paternal exposure to p,p′-DDE may have some effect on the offspring of male rats (128).
A biomonitoring study in humans revealed that dieldrin is negatively associated with serum testosterone levels in a Chinese male population, whereas p,p′-DDD is positively associated with serum testosterone levels (129). The effects of OCPs on the adrenal glands and their possible associations with PCOS warrant further investigation.
6.4 Genetic susceptibility modifiers
While studies directly linking detoxification SNPs to OCP metabolism in PCOS remain limited, preliminary evidence suggests genetic variants may modulate susceptibility. For example, CYP1A1 polymorphisms are associated with altered organochlorine pesticide clearance in general populations (130), though PCOS-specific pharmacogenomic investigations are scarce. Future studies should prioritize genotyping in well-characterized PCOS cohorts with OCP exposure biomarkers. This represents a critical research frontier, understanding how genetic variation in detox pathways (e.g., GST deletions, CYP polymorphisms) interacts with OCP accumulation to potentially amplify PCOS risk requires targeted investigation.
7 Current knowledge gaps and perspectives
7.1 Limitations
The following limitations must be considered when interpreting the findings of this review: Firstly, the heterogeneity in PCOS diagnostic criteria (Rotterdam vs. NIH) complicates cross-study comparisons. Secondly, there is a clear geographical bias in favour of data on Asian and European exposure, with limited representation of the Global South. Thirdly and finally, causal inference in this domain is constrained by the preponderance of cross-sectional human studies. To advance our understanding, it is essential to employ longitudinal designs that systematically track the accumulation of OCPs in the period preceding the onset of PCOS.
7.2 Research gaps and perspectives
PCOS is a prevalent condition that affects women's health and is increasingly recognized as a significant public health concern, with its incidence rising each year. There is growing evidence that OCP may be detrimental to the female reproductive system and could be one of the environmental factors contributing to the etiology of PCOS.
Available literature suggests that OCPs may affect the HPO axis by affecting the hypothalamus and pituitary gland. This could lead to elevated production of GnRH and LH, alongside a decrease in FSH, resulting in endocrine disruption within the HPO axis. However, studies examining the effects of OCPs on the hypothalamus and pituitary are limited and often yield conflicting results, highlighting a significant gap in our understanding.
Furthermore, it has been suggested that OCPs can accumulate in the ovaries and cause ovarian damage, impairing ovarian function. These chemicals may also contribute to endocrine dysregulation among women, potentially causing or exacerbating the onset and progression of PCOS. Additionally, there is a possibility that some OCPS could lead to hypothyroidism and influence androgen production by the adrenal glands. While it is conceivable that various OCPs may have cumulative effects on ovarian function, the precise mechanisms by which specific OCPs or combinations of them affect the ovaries remain unclear. The existing evidence regarding the effects of OCPs on thyroid and adrenal function is inconsistent, indicating that further research is essential to elucidate these mechanisms.
Author contributions
SY: Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. WY: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. FL: Writing – review & editing. MJ: Writing – original draft. YF: Writing – original draft. YC: Writing – original draft. XB: Writing – original draft. YD: Writing – original draft. SM: Funding acquisition, Writing – original draft. KH: Writing – review & editing. XJ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Dr. S Yin acknowledges the support from National Natural Science Foundation of China (22276166), and Basic Scientific Research Special Funds of Zhejiang Shuren University (2021XZ017). Dr. X Jin acknowledges the support from Natural Science Foundation of Zhejiang Province (LY20H040001), Key Science and Technology Project of Hangzhou Medical and Health Bureau (ZD20210048), and Hangzhou Agricultural and Social Development Research Project (202204B21). Dr. S Mao acknowledges the support from Key Research and Development Project of Zhejiang Province (2024C03232), and National Natural Science Foundation of China (No. 42307512).
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author's contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003
2. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14(5):270–84. doi: 10.1038/nrendo.2018.24
3. Giulivo M, Lopez de Alda M, Capri E, Barcelo D. Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. (2016) 151:251–64. doi: 10.1016/j.envres.2016.07.011
4. Keswani C, Dilnashin H, Birla H, Roy P, Tyagi RK, Singh D, et al. Global footprints of organochlorine pesticides: a pan-global survey. Environ Geochem Health. (2022) 44(1):149–77. doi: 10.1007/s10653-021-00946-7
5. Secretariat of the Stockholm Convention on Persistent Organic Pollutants. Stockholm convention on persistence organic pollutants. In: U.N.E.P. (UNEP), editor. Secretariat of the Stockholm Convention on Persistent Organic Pollutants. (2009 ed.) Geneva: Secretariat of the Stockholm Convention on Persistent Organic Pollutants (2009).
6. UNEP. Annex A of the Stockholm Convention. Genève: Secretariat of the Stockholm Convention (2017). Available online at: http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (Accessed September 19, 2024).
7. Combarnous Y, Nguyen TMD. Membrane hormone receptors and their signaling pathways as targets for endocrine disruptors. J Xenobiot. (2022) 12(2):64–73. doi: 10.3390/jox12020007
8. Vagi SJ, Azziz-Baumgartner E, Sjodin A, Calafat AM, Dumesic D, Gonzalez L, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC Endocr Disord. (2014) 14:86. doi: 10.1186/1472-6823-14-86
9. Yang Q, Zhao Y, Qiu X, Zhang C, Li R, Qiao J. Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): a case-control study. Hum Reprod. (2015) 30(8):1964–73. doi: 10.1093/humrep/dev123
10. Guo Z, Qiu H, Wang L, Wang L, Wang C, Chen M, et al. Association of serum organochlorine pesticides concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Chemosphere. (2017) 171:595–600. doi: 10.1016/j.chemosphere.2016.12.127
11. Myers SH, Russo M, Dinicola S, Forte G, Unfer V. Questioning PCOS phenotypes for reclassification and tailored therapy. Trends Endocrinol Metab. (2023) 34(11):694–703. doi: 10.1016/j.tem.2023.08.005
12. Vatier C, Christin-Maitre S. Epigenetic/circadian clocks and PCOS. Hum Reprod. (2024) 39(6):1167–75. doi: 10.1093/humrep/deae066
13. Yang J, Chen C. Hormonal changes in PCOS. J Endocrinol. (2024) 261(1):14. doi: 10.1530/JOE-23-0342
14. Azziz R, Adashi EY. Stein and leventhal: 80 years on. Am J Obstet Gynecol. (2016) 214(2):247.e1–e11. doi: 10.1016/j.ajog.2015.12.013
15. Azziz R, Kintziger K, Li R, Laven J, Morin-Papunen L, Merkin SS, et al. Recommendations for epidemiologic and phenotypic research in polycystic ovary syndrome: an androgen excess and PCOS society resource. Hum Reprod. (2019) 34(11):2254–65. doi: 10.1093/humrep/dez185
16. Liu J, Wu Q, Hao Y, Jiao M, Wang X, Jiang S, et al. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: global burden of disease study 2017. Hum Reprod. (2021) 36(4):1108–19. doi: 10.1093/humrep/deaa371
17. Safiri S, Noori M, Nejadghaderi SA, Karamzad N, Carson-Chahhoud K, Sullman MJM, et al. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990–2019. Hum Reprod. (2022) 37(8):1919–31. doi: 10.1093/humrep/deac091
19. Steegers-Theunissen RPM, Wiegel RE, Jansen PW, Laven JSE, Sinclair KD. Polycystic ovary syndrome: a brain disorder characterized by eating problems originating during puberty and adolescence. Int J Mol Sci. (2020) 21(21):20. doi: 10.3390/ijms21218211
20. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 1. Endocr Pract. (2015) 21(11):1291–300. doi: 10.4158/EP15748.DSC
21. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. American Association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 2. Endocr Pract. (2015) 21(12):1415–26. doi: 10.4158/EP15748.DSCPT2
22. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metab Clin Exp. (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
23. Genazzani AD, Genazzani AR. Polycystic ovary syndrome as metabolic disease: new insights on insulin resistance. touchREV Endocrinol. (2023) 19(1):71–7. doi: 10.17925/EE.2023.19.1.71
24. Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. (2023) 108(10):2447–69. doi: 10.1210/clinem/dgad463
25. Patel K, Coffler MS, Dahan MH, Malcom PJ, Deutsch R, Chang RJ. Relationship of GnRH-stimulated LH release to episodic LH secretion and baseline endocrine-metabolic measures in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). (2004) 60(1):67–74. doi: 10.1111/j.1365-2265.2004.01945.x
26. Trent M, Gordon CM. Diagnosis and management of polycystic ovary syndrome in adolescents. Pediatrics. (2020) 145(Suppl 2):S210–8. doi: 10.1542/peds.2019-2056J
27. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. (2008) 14(5):539–539. doi: 10.1093/humupd/dmn028
28. Crisosto N, Ladron de Guevara A, Echiburu B, Maliqueo M, Cavada G, Codner E, et al. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil Steril. (2019) 111(2):381–8. doi: 10.1016/j.fertnstert.2018.10.011
29. Burt Solorzano CM, McCartney CR. Polycystic ovary syndrome: ontogeny in adolescence. Endocrinol Metab Clin North Am. (2021) 50(1):25–42. doi: 10.1016/j.ecl.2020.10.003
30. Greenwood EA, Huddleston HG. Insulin resistance in polycystic ovary syndrome: concept versus cutoff. Fertil Steril. (2019) 112(5):827–8. doi: 10.1016/j.fertnstert.2019.08.100
31. He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. (2020) 13(1):73. doi: 10.1186/s13048-020-00670-3
32. Ibanez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. (2017) 88(6):371–95. doi: 10.1159/000479371
33. Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. (2020) 502:214–21. doi: 10.1016/j.cca.2019.11.003
34. Siddiqui S, Mateen S, Ahmad R, Moin S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. (2022) 39(11):2439–73. doi: 10.1007/s10815-022-02625-7
35. Crisosto N, Echiburu B, Maliqueo M, Perez V, Ladron de Guevara A, Preisler J, et al. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. (2012) 97(1):218–24. doi: 10.1016/j.fertnstert.2011.10.002
36. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Australian Ovarian Canc Study G, et al. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. (2010) 21(12):2303–8. doi: 10.1007/s10552-010-9658-7
37. Da Cuna RH, Rey Vazquez G, Dorelle L, Rodriguez EM, Guimaraes Moreira R, Lo Nostro FL. Mechanism of action of endosulfan as disruptor of gonadal steroidogenesis in the cichlid fish cichlasoma dimerus. Comp Biochem Physiol C Toxicol Pharmacol. (2016) 187:74–80. doi: 10.1016/j.cbpc.2016.05.008
38. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. (2016) 2:14. doi: 10.1186/s40738-016-0029-2
39. Sagvekar P, Dadachanji R, Patil K, Mukherjee S. Pathomechanisms of polycystic ovary syndrome: multidimensional approaches. Front Biosci (Elite Ed). (2018) 10(3):384–422. doi: 10.2741/e829
40. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. (2022) 23(2):33. doi: 10.3390/ijms23020583
41. Bonefeld-Jorgensen EC, Ghisari M, Wielsoe M, Bjerregaard-Olesen C, Kjeldsen LS, Long MH. Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin Pharmacol Toxicol. (2014) 115(1):118–28. doi: 10.1111/bcpt.12263
42. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. (2017) 13(3):161–73. doi: 10.1038/nrendo.2016.186
43. Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. (2020) 21(1):127–47. doi: 10.1007/s11154-019-09521-z
44. Dominguez MA, Petre MA, Neal MS, Foster WG. Bisphenol A concentration-dependently increases human granulosa-lutein cell matrix metalloproteinase-9 (MMP-9) enzyme output. Reprod Toxicol. (2008) 25(4):420–5. doi: 10.1016/j.reprotox.2008.05.059
45. Milanovic M, Milosevic N, Sudji J, Stojanoski S, Krstonosic MA, Bjelica A, et al. Can environmental pollutant bisphenol A increase metabolic risk in polycystic ovary syndrome? Clin Chim Acta. (2020) 507:257–63. doi: 10.1016/j.cca.2020.05.009
46. Shi J, Hu KL, Li XX, Ge YM, Yu XJ, Zhao J. Bisphenol a downregulates GLUT4 expression by activating aryl hydrocarbon receptor to exacerbate polycystic ovary syndrome. Cell Commun Signal. (2024) 22(1):28. doi: 10.1186/s12964-023-01410-y
47. Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. (2011) 7(4):513–41. doi: 10.1002/ieam.258
48. Hammarstrand S, Jakobsson K, Andersson E, Xu YY, Li Y, Olovsson M, et al. Perfluoroalkyl substances (PFAS) in drinking water and risk for polycystic ovarian syndrome, uterine leiomyoma, and endometriosis: a Swedish cohort study. Environ Int. (2021) 157:8. doi: 10.1016/j.envint.2021.106819
49. Zhang Y, Martin L, Mustieles V, Ghaly M, Archer M, Sun Y, et al. Per- and polyfluoroalkyl substances exposure is associated with polycystic ovary syndrome risk among women attending a fertility clinic. Sci Total Environ. (2024) 950:175313. doi: 10.1016/j.scitotenv.2024.175313
50. Brennan E, Butler AE, Drage DS, Sathyapalan T, Atkin SL. Serum polychlorinated biphenyl levels and circulating miRNAs in non-obese women with and without polycystic ovary syndrome. Front Endocrinol (Lausanne). (2023) 14:1233484. doi: 10.3389/fendo.2023.1233484
51. Zhang M, Wang L, Li X, Song L, Luo D, Li Q, et al. Individual and mixtures of polychlorinated biphenyls and organochlorine pesticides exposure in relation to metabolic syndrome among Chinese adults. Sci Total Environ. (2023) 877:162935. doi: 10.1016/j.scitotenv.2023.162935
52. Al-Saleh I. The relationship between urinary phthalate metabolites and polycystic ovary syndrome in women undergoing in vitro fertilization: nested case-control study. Chemosphere. (2022) 286(Pt 1):131495. doi: 10.1016/j.chemosphere.2021.131495
53. Zhang M, Liu C, Yuan XQ, Cui FP, Miao Y, Yao W, et al. Individual and joint associations of urinary phthalate metabolites with polycystic ovary and polycystic ovary syndrome: results from the TREE cohort. Environ Toxicol Pharmacol. (2023) 102:104233. doi: 10.1016/j.etap.2023.104233
54. Merlo E, Silva IV, Cardoso RC, Graceli JB. The obesogen tributyltin induces features of polycystic ovary syndrome (PCOS): a review. J Toxicol Environ Health B Crit Rev. (2018) 21(3):181–206. doi: 10.1080/10937404.2018.1496214
55. Hamid N, Junaid M, Wang Y, Pu SY, Jia PP, Pei DS. Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish. Environ Pollut. (2021) 273:116494. doi: 10.1016/j.envpol.2021.116494
56. Zhan W, Tang W, Shen X, Xu H, Zhang J. Exposure to bisphenol A and its analogs and polycystic ovarian syndrome in women of childbearing age: a multicenter case-control study. Chemosphere. (2023) 313:137463. doi: 10.1016/j.chemosphere.2022.137463
57. Chakraborty S, Anand S, Coe S, Reh B, Bhandari RK. The PCOS-NAFLD multidisease phenotype occurred in medaka fish four generations after the removal of bisphenol a exposure. Environ Sci Technol. (2023) 57(34):12602–19. doi: 10.1021/acs.est.3c01922
58. Zhan W, Qiu W, Ao Y, Zhou W, Sun Y, Zhao H, et al. Environmental exposure to emerging alternatives of per- and polyfluoroalkyl substances and polycystic ovarian syndrome in women diagnosed with infertility: a mixture analysis. Environ Health Perspect. (2023) 131(5):57001. doi: 10.1289/EHP11814
59. Butler AE, Brennan E, Drage DS, Sathyapalan T, Atkin SL. Exploration of the correlation of serum polychlorinated biphenyl levels with luteal phase hormonal parameters and infertility in women with or without polycystic ovary syndrome. Front Endocrinol (Lausanne). (2023) 14:1270949. doi: 10.3389/fendo.2023.1270949
60. Achour A, Derouiche A, Driss MR, Tebourbi O. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in adipose tissue of women from grand Tunis and their association with demographic factors and dietary habits. Chemosphere. (2023) 338:139600. doi: 10.1016/j.chemosphere.2023.139600
61. Haraguchi K, Koizumi A, Inoue K, Harada KH, Hitomi T, Minata M, et al. Levels and regional trends of persistent organochlorines and polybrominated diphenyl ethers in Asian breast milk demonstrate POPs signatures unique to individual countries. Environ Int. (2009) 35(7):1072–9. doi: 10.1016/j.envint.2009.06.003
62. Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells US about our toxic burden and how it assists environmental medicine physicians. Altern Med Rev. (2010) 15(2):101–8.20806995
63. Thomas A, Toms LL, Harden FA, Hobson P, White NM, Mengersen KL, et al. Concentrations of organochlorine pesticides in pooled human serum by age and gender. Environ Res. (2017) 154:10–8. doi: 10.1016/j.envres.2016.12.009
64. Bapayeva G, Issayeva R, Zhumadilova A, Nurkasimova R, Kulbayeva S, Tleuzhan R. Organochlorine pesticides and female puberty in south Kazakhstan. Reprod Toxicol. (2016) 65:67–75. doi: 10.1016/j.reprotox.2016.06.017
65. Venkidasamy B, Subramanian U, Samynathan R, Rajakumar G, Shariati MA, Chung IM, et al. Organopesticides and fertility: where does the link lead to? Environ Sci Pollut Res Int. (2021) 28(6):6289–301. doi: 10.1007/s11356-020-12155-3
66. Yin SS, Zhang JY, Guo FJ, Zhao L, Poma G, Covaci A, et al. Transplacental transfer of organochlorine pesticides: concentration ratio and chiral properties. Environ Int. (2019) 130:8. doi: 10.1016/j.envint.2019.104939
67. Fang J, Liu HX, Zhao HZ, Xu SQ, Cai ZW. Concentrations of organochlorine pesticides in cord serum of newborns in Wuhan, China. Sci Total Environ. (2018) 636:761–6. doi: 10.1016/j.scitotenv.2018.04.337
68. Sharma D, Kumari S, Rani P, Onteru SK, Roy P, Tyagi RK, et al. Organochlorine pesticide dieldrin upregulate proximal promoter (PII) driven CYP19A1 gene expression and increases estrogen production in granulosa cells. Reprod Toxicol. (2021) 106:103–8. doi: 10.1016/j.reprotox.2021.10.009
69. Lemaire G, Terouanne B, Mauvais P, Michel S, Rahmani R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol Appl Pharmacol. (2004) 196(2):235–46. doi: 10.1016/j.taap.2003.12.011
70. Martyniuk CJ, Mehinto AC, Colli-Dula RC, Kroll KJ, Doperalski NJ, Barber DS, et al. Transcriptome and physiological effects of toxaphene on the liver-gonad reproductive axis in male and female largemouth bass (Micropterus salmoides). Comp Biochem Physiol Part D Genomics Proteomics. (2020) 36:100746. doi: 10.1016/j.cbd.2020.100746
71. Chen X, Hirano M, Ishibashi H, Lee JS, Kawai YK, Kubota A. Efficient in vivo and in silico assessments of antiandrogenic potential in zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. (2023) 264:109513. doi: 10.1016/j.cbpc.2022.109513
72. Mahoney MM, Padmanabhan V. Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol Appl Pharmacol. (2010) 247(2):98–104. doi: 10.1016/j.taap.2010.05.017
73. Martyniuk CJ, Kroll KJ, Doperalski NJ, Barber DS, Denslow ND. Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration? Toxicol Sci. (2010) 117(1):190–9. doi: 10.1093/toxsci/kfq192
74. Chakrabarty S, Rajakumar A, Raghuveer K, Sridevi P, Mohanachary A, Prathibha Y, et al. Endosulfan and flutamide, alone and in combination, target ovarian growth in juvenile catfish, clarias batrachus. Comp Biochem Physiol C Toxicol Pharmacol. (2012) 155(3):491–7. doi: 10.1016/j.cbpc.2011.12.007
75. Lafuente A, Gonzalez-Carracedo A, Romero A, Esquifino AI. Methoxychlor modifies the ultradian secretory pattern of prolactin and affects its TRH response. Med Sci Monit. (2003) 9(5):PI37–42.12761465
76. Caride A, Lafuente A, Cabaleiro T. Endosulfan effects on pituitary hormone and both nitrosative and oxidative stress in pubertal male rats. Toxicol Lett. (2010) 197(2):106–12. doi: 10.1016/j.toxlet.2010.05.006
77. Freire C, Koifman RJ, Sarcinelli PN, Rosa ACS, Clapauch R, Koifman S. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int J Hyg Environ Health. (2014) 217(2–3):370–8. doi: 10.1016/j.ijheh.2013.07.012
78. Zhan F, Yu X, Zhang X, Chen L, Sun X, Yu RQ, et al. Tissue distribution of organic contaminants in stranded pregnant sperm whale (Physeter microcephalus) from the Huizhou coast of the South China Sea. Mar Pollut Bull. (2019) 144:181–8. doi: 10.1016/j.marpolbul.2019.05.005
79. Shah HK, Sharma T, Banerjee BD. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: an in vitro study. Chemosphere. (2020) 246:125691. doi: 10.1016/j.chemosphere.2019.125691
80. Wrobel MH, Mlynarczuk J. Secretory function of ovarian cells and myometrial contractions in cow are affected by chlorinated insecticides (chlordane, heptachlor, mirex) in vitro. Toxicol Appl Pharmacol. (2017) 314:63–71. doi: 10.1016/j.taap.2016.11.011
81. Wrobel MH, Mlynarczuk J. Chlorinated insecticides (toxaphene and endrin) affect oxytocin, testosterone, oestradiol and prostaglandin secretion from ovarian and uterine cells as well as myometrial contractions in cow in vitro. Chemosphere. (2018) 198:432–41. doi: 10.1016/j.chemosphere.2018.01.143
82. Kuai Y, Gao X, Yang H, Luo H, Xu Y, Liu C, et al. Pentachloronitrobenzene alters progesterone production and primordial follicle recruitment in cultured granulosa cells and rat ovarydagger. Biol Reprod. (2020) 102(2):511–20. doi: 10.1093/biolre/ioz195
83. Rak A, Zajda K, Gregoraszczuk EL. Endocrine disrupting compounds modulates adiponectin secretion, expression of its receptors and action on steroidogenesis in ovarian follicle. Reprod Toxicol. (2017) 69:204–11. doi: 10.1016/j.reprotox.2017.03.004
84. Bhardwaj JK, Saraf P. N-acetyl cysteine-mediated effective attenuation of methoxychlor-induced granulosa cell apoptosis by counteracting reactive oxygen species generation in caprine ovary. Environ Toxicol. (2017) 32(1):156–66. doi: 10.1002/tox.22221
85. Cheng L, Song W, Rao Q, Zhou J, Zhao Z. Bioaccumulation and toxicity of methoxychlor on Chinese mitten crab (Eriocheir sinensis). Comp Biochem Physiol C Toxicol Pharmacol. (2019) 221:89–95. doi: 10.1016/j.cbpc.2019.04.002
86. El-Sharkawy EE, Kames AOG, Sayed SM, Nisr N, Wahba NM, Elsherif WM, et al. The ameliorative effect of propolis against methoxychlor induced ovarian toxicity in rat. Exp Toxicol Pathol. (2014) 66(9–10):415–21. doi: 10.1016/j.etp.2014.06.003
87. Knapczyk-Stwora K, Nynca A, Swigonska S, Paukszto L, Jastrzebski JP, Witek P, et al. Effects of neonatal methoxychlor exposure on the ovarian transcriptome in piglets. Anim Reprod Sci. (2022) 238:106956. doi: 10.1016/j.anireprosci.2022.106956
88. Harvey CN, Chen JC, Bagnell CA, Uzumcu M. Methoxychlor and its metabolite HPTE inhibit cAMP production and expression of estrogen receptors alpha and beta in the rat granulosa cell in vitro. Reprod Toxicol. (2015) 51:72–8. doi: 10.1016/j.reprotox.2014.12.001
89. Witek P, Grzesiak M, Koziorowski M, Slomczynska M, Knapczyk-Stwora K. Long-term changes in ovarian follicles of gilts exposed neonatally to methoxychlor: effects on oocyte-derived factors, anti-mullerian hormone, follicle-stimulating hormone, and cognate receptors. Int J Mol Sci. (2022) 23(5):18. doi: 10.3390/ijms23052780
90. Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-mullerian hormone production in the rat ovary. J Endocrinol. (2006) 191(3):549–58. doi: 10.1677/joe.1.06592
91. Palmerini MG, Zhurabekova G, Balmagambetova A, Nottola SA, Miglietta S, Belli M, et al. The pesticide lindane induces dose-dependent damage to granulosa cells in an in vitro culture. Reprod Biol. (2017) 17(4):349–56. doi: 10.1016/j.repbio.2017.09.008
92. Al-Hussaini TK, Abdelaleem AA, Elnashar I, Shabaan OM, Mostafa R, El-Baz MAH, et al. The effect of follicullar fluid pesticides and polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological and clinical outcome. Eur J Obstet Gynecol Reprod Biol. (2018) 220:39–43. doi: 10.1016/j.ejogrb.2017.11.003
93. Zhu YD, Huang B, Li QX, Wang J. Organochlorine pesticides in follicular fluid of women undergoing assisted reproductive technologies from central China. Environ Pollut. (2015) 207:266–72. doi: 10.1016/j.envpol.2015.09.030
94. Bjorvang RD, Hallberg I, Pikki A, Berglund L, Pedrelli M, Kiviranta H, et al. Follicular fluid and blood levels of persistent organic pollutants and reproductive outcomes among women undergoing assisted reproductive technologies. Environ Res. (2022) 208:112626. doi: 10.1016/j.envres.2021.112626
95. Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol (Lausanne). (2018) 9:613. doi: 10.3389/fendo.2018.00613
96. Jarosinski MA, Dhayalan B, Chen YS, Chatterjee D, Varas N, Weiss MA. Structural principles of insulin formulation and analog design: a century of innovation. Mol Metab. (2021) 52:101325. doi: 10.1016/j.molmet.2021.101325
97. White MF, Kahn CR. Insulin action at a molecular level—100 years of progress. Mol Metab. (2021) 52:101304. doi: 10.1016/j.molmet.2021.101304
98. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46(1):15–37. doi: 10.4093/dmj.2021.0280
99. Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. (2021) 44(2):233–44. doi: 10.1007/s40618-020-01351-0
100. Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. (2019) 236:116940. doi: 10.1016/j.lfs.2019.116940
101. Tong C, Wu Y, Zhang LL, Yu Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: association with PI3K signaling pathway. Front Endocrinol (Lausanne). (2022) 13:10. doi: 10.3389/fendo.2022.1091147
102. Singh VK, Sarkar SK, Saxena A, Koner BC. Sub-toxic exposure to lindane activates redox sensitive kinases and impairs insulin signaling in muscle cell culture: the possible mechanism of lindane-induced insulin resistance. Toxicol in Vitro. (2019) 54:98–104. doi: 10.1016/j.tiv.2018.09.014
103. Singh VK, Sarkar SK, Saxena A, Koner BC. Effect of subtoxic DDT exposure on glucose uptake and insulin signaling in rat L6 myoblast-derived myotubes. Int J Toxicol. (2019) 38(4):303–11. doi: 10.1177/1091581819850577
104. Bresson SE, Isom S, Jensen ET, Huber S, Oulhote Y, Rigdon J, et al. Associations between persistent organic pollutants and type 1 diabetes in youth. Environ Int. (2022) 163:107175. doi: 10.1016/j.envint.2022.107175
105. Liang Y, Liu D, Zhan J, Luo M, Han J, Wang P, et al. New insight into the mechanism of POP-induced obesity: evidence from DDE-altered microbiota. Chemosphere. (2020) 244:125123. doi: 10.1016/j.chemosphere.2019.125123
106. Mangum LH, Crow JA, Stokes JV, Howell GE 3rd, Ross MK, Pruett SB, et al. Exposure to p,p′-DDE alters macrophage reactivity and increases macrophage numbers in adipose stromal vascular fraction. Toxicol Sci. (2016) 150(1):169–77. doi: 10.1093/toxsci/kfv315
107. Lee YM, Kim KS, Kim SA, Hong NS, Lee SJ, Lee DH. Prospective associations between persistent organic pollutants and metabolic syndrome: a nested case-control study. Sci Total Environ. (2014) 496:219–25. doi: 10.1016/j.scitotenv.2014.07.039
108. Rosenbaum PF, Weinstock RS, Silverstone AE, Sjodin A, Pavuk M. Metabolic syndrome is associated with exposure to organochlorine pesticides in Anniston, AL, United States. Environ Int. (2017) 108:11–21. doi: 10.1016/j.envint.2017.07.017
109. Reina-Perez I, Artacho-Cordon F, Mustieles V, Castellano-Castillo D, Cardona F, Jimenez-Diaz I, et al. Cross-sectional associations of persistent organic pollutants measured in adipose tissue and metabolic syndrome in clinically diagnosed middle-aged adults. Environ Res. (2023) 222:115350. doi: 10.1016/j.envres.2023.115350
110. Suarez-Lopez JR, Lee DH, Porta M, Steffes MW, Jacobs DR Jr. Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Environ Res. (2015) 137:485–94. doi: 10.1016/j.envres.2014.11.001
111. Mustieles V, Fernandez MF, Martin-Olmedo P, Gonzalez-Alzaga B, Fontalba-Navas A, Hauser R, et al. Human adipose tissue levels of persistent organic pollutants and metabolic syndrome components: combining a cross-sectional with a 10-year longitudinal study using a multi-pollutant approach. Environ Int. (2017) 104:48–57. doi: 10.1016/j.envint.2017.04.002
112. Lind L, Salihovic S, Lampa E, Lind PM. Mixture effects of 30 environmental contaminants on incident metabolic syndrome-A prospective study. Environ Int. (2017) 107:8–15. doi: 10.1016/j.envint.2017.06.005
113. Gasull M, Castell C, Pallarès N, Miret C, Pumarega J, Téllez-Plaza M, et al. Blood concentrations of persistent organic pollutants and unhealthy metabolic phenotypes in normal-weight, overweight, and obese individuals. Am J Epidemiol. (2018) 187(3):494–506. doi: 10.1093/aje/kwx267
114. Ha KH, Kim SA, Lee YM, Kim DJ, Lee DH. Can persistent organic pollutants distinguish between two opposite metabolic phenotypes in lean Koreans? Diabetes Metab. (2018) 44(2):168–71. doi: 10.1016/j.diabet.2017.12.008
115. Tyagi S, Mishra BK, Sharma T, Tawar N, Urfi AJ, Banerjee BD, et al. Level of organochlorine pesticide in prediabetic and newly diagnosed diabetes mellitus patients with varying degree of glucose intolerance and insulin resistance among north Indian population. Diabetes Metab J. (2021) 45(4):558–68. doi: 10.4093/dmj.2020.0093
116. Al-Othman A, Yakout S, Abd-Alrahman SH, Al-Daghri NM. Strong associations between the pesticide hexachlorocyclohexane and type 2 diabetes in Saudi adults. Int J Environ Res Public Health. (2014) 11(9):8984–95. doi: 10.3390/ijerph110908984
117. Tyagi S, Siddarth M, Mishra BK, Banerjee BD, Urfi AJ, Madhu SV. High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: a study in north India. Environ Pollut. (2021) 271:116287. doi: 10.1016/j.envpol.2020.116287
118. Mansouri E, Reggabi M. Association between type 2 diabetes and exposure to chlorinated persistent organic pollutants in Algeria: a case-control study. Chemosphere. (2021) 264:9. doi: 10.1016/j.chemosphere.2020.128596
119. Barrios-Rodriguez R, Perez-Carrascosa FM, Gomez-Pena C, Mustieles V, Salcedo-Bellido I, Requena P, et al. Associations of accumulated selected persistent organic pollutants in adipose tissue with insulin sensitivity and risk of incident type-2 diabetes. Environ Int. (2021) 155:106607. doi: 10.1016/j.envint.2021.106607
120. Seok JW, Park JY, Park HK, Lee H. Endrin potentiates early-stage adipogenesis in 3T3-L1 cells by activating the mammalian target of rapamycin. Life Sci. (2022) 288:120151. doi: 10.1016/j.lfs.2021.120151
121. Dusanov S, Ruzzin J, Kiviranta H, Klemsdal TO, Retterstol L, Rantakokko P, et al. Associations between persistent organic pollutants and metabolic syndrome in morbidly obese individuals. Nutr Metab Cardiovasc Dis. (2018) 28(7):735–42. doi: 10.1016/j.numecd.2018.03.004
122. Fan H, Ren Q, Sheng Z, Deng G, Li L. The role of the thyroid in polycystic ovary syndrome. Front Endocrinol (Lausanne). (2023) 14:1242050. doi: 10.3389/fendo.2023.1242050
123. Dhaibar HA, Patadia H, Mansuri T, Shah R, Khatri L, Makwana H, et al. Hexachlorobenzene, a pollutant in hypothyroidism and reproductive aberrations: a perceptive transgenerational study. Environ Sci Pollut Res Int. (2021) 28(9):11077–89. doi: 10.1007/s11356-020-11278-x
124. Dufour P, Pirard C, Seghaye MC, Charlier C. Association between organohalogenated pollutants in cord blood and thyroid function in newborns and mothers from Belgian population. Environ Pollut. (2018) 238:389–96. doi: 10.1016/j.envpol.2018.03.058
125. Knapczyk-Stwora K, Kozlowska A, Jastrzabek D, Grzesiak M, Slomczynska M, Koziorowski M. Impact of endocrine-active compounds on adrenal androgen production in pigs during neonatal period. Environ Toxicol Pharmacol. (2024) 107:104435. doi: 10.1016/j.etap.2024.104435
126. Liu S, Li C, Wang Y, Hong T, Song T, Li L, et al. In utero methoxychlor exposure increases rat fetal Leydig cell number but inhibits its function. Toxicology. (2016) 370:31–40. doi: 10.1016/j.tox.2016.09.009
127. Yaglova NV, Obernikhin SS, Tsomartova DA, Yaglov VV, Nazimova SV, Tsomartova ES, et al. Impact of prenatal and postnatal exposure to endocrine disrupter DDT on adrenal medulla function. Int J Mol Sci. (2022) 23(9):14. doi: 10.3390/ijms23094912
128. Lu L, Cheng Y, Wu W, Wang L, Li S, Li Q, et al. Paternal p,p′-DDE exposure and pre-pubertal high-fat diet increases the susceptibility to fertility impairment and sperm Igf2 DMR2 hypo-methylation in male offspring. Ecotoxicol Environ Saf. (2024) 271:115999. doi: 10.1016/j.ecoenv.2024.115999
129. Zeng JY, Miao Y, Liu C, Deng YL, Chen PP, Zhang M, et al. Serum multiple organochlorine pesticides in relation to testosterone concentrations among Chinese men from an infertility clinic. Chemosphere. (2022) 299:134469. doi: 10.1016/j.chemosphere.2022.134469
130. Coumoul X, Diry M, Robillot C, Barouki R. Differential regulation of cytochrome P450 1A1 and 1B1 by a combination of dioxin and pesticides in the breast tumor cell line MCF-7. Cancer Res. (2001) 61(10):3942–8.11358810
131. Brennan E, Kumar N, Drage DS, Cunningham TK, Sathyapalan T, Mueller JF, et al. A case-control study of polychlorinated biphenyl association with metabolic and hormonal outcomes in polycystic ovary syndrome. J Environ Sci Health C Toxicol Carcinog. (2022) 40(1):86–105. doi: 10.1080/26896583.2022.2043135
Keywords: adrenal glands, hypothalamic-pituitary-ovarian axis, insulin resistance, metabolic abnormalities, organochlorine pesticides, polycystic ovary syndrome
Citation: Yin S, Yang W, Lin F, Jia M, Feng Y, Chen Y, Bai X, Dong Y, Mao S, Hayat K and Jin X (2025) Polycystic ovary syndrome and organochlorine pesticides: exploring potential links and mechanisms. Front. Reprod. Health 7:1563414. doi: 10.3389/frph.2025.1563414
Received: 21 January 2025; Accepted: 11 August 2025;
Published: 2 September 2025.
Edited by:
Arturo Bevilacqua, Sapienza University of Rome, ItalyReviewed by:
Antonio Simone Laganà, University of Palermo, ItalyPankaj Prabhakar, Indira Gandhi Institute of Medical Sciences, India
Copyright: © 2025 Yin, Yang, Lin, Jia, Feng, Chen, Bai, Dong, Mao, Hayat and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejing Jin, ODk3NzE5MTRAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Shanshan Yin
Shanshan Yin Wanjia Yang2,†
Wanjia Yang2,† Xiaoxia Bai
Xiaoxia Bai