- 1Department of Medical Laboratory Science, College of Health Science, Debre Tabor University, Gondar, Ethiopia
- 2Department of Microbial Sciences and Genetics, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 3Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
- 4Department of Microbiology, Immunology, and Parasitology, School of Medicine, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
- 5Department of Clinical Microbiology, and Mycology, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Introduction: N. gonorrhoeae is the cause of gonorrhea, which is one of the most common public health problems among sexually transmitted infections. The highest incidence of disease occurs in less developed countries, and gonococcal infections are common among adolescents and young adults. Risky sexual behavior (RSB) is also the main concern. It has many consequences on the health system, which is the most risk factor for the transmission of sexually transmitted diseases, particularly gonorrheal diseases. Little is known about the magnitude of gonococcal infection and risky sexual behavior (RSB) in the reproductive age groups.
Objectives: To assess the burden of Neisseria gonorrhoeae, Risky Sexual Behavior, and Associated Risk Factors among Sexually Transmitted Infections in a Resource-Limited Area of Addis Ababa City, Ethiopia.
Methods: A health institution-based cross-sectional study was conducted from April 2023 to December 2024 in Addis Ababa City. A convenient sampling method was used to collect endocervical and urethral sample swabs from 571 study subjects. Samples were cultured onto Thayer Martin Luther agar, and gram staining and biochemical tests were used to confirm the presence of gonococci. A pre-tested and well-structured questionnaire was used to assess risk factors, and data were analyzed using SPSS version 22. Descriptive and logistic analyses were computed. P-values ≤0.05 were considered statistically significant.
Results: Of the total study subjects, 62.2% were females, and 61.6% were urban residents. Moreover, 183 (32.0%) were in the age of >35 years, followed by 170 (29.8%) in the 30–34 years old. The prevalence of N. gonorrhoeae among STI patients was 17.33%, and risky sexual behavior was 56.9%. The odds of N. gonorrhoeae infection were 1.55 times higher among chat users than the non-chat users [AOR = 1.55, 95% CI: (1.32–1.95)]. Similarly, the odds of risky sexual behavior were 10.95 [AOR = 10.95, 95% CI (5.75–20.84)] times higher among STIs who had a new sexual partner than their counterparts.
Conclusion: The prevalence of N. gonorrhoeae and risky sexual behavior among STI patients were high. Gender, watching pornographic films, alcohol consumption, and not participating in religious education have been found to increase the risk of experiencing both N. gonorrheae infections and risky sexual behavior.
Introduction
Sexually transmitted infections (STIs) have been the major causes of morbidity and mortality for many years and are predominantly transmitted via sexual contact. Despite the medical system becoming advanced, STIs continue to pose a threat to health and are a major public health problem with an annual estimate of 374 million people infected with them (1). To address these public health issues, the WHO has adopted a strategy to control STIs that aligns with the Agenda for the Sustainable Development Goals (SDGs) by 2030. To minimize the incidence and prevalence of STIs, targeting the population at greater risk, effective clinical interventions, promoting the use of condoms, and having reliable data are useful means (1, 2).
Neisseria gonorrhoeae (NG) is one of the most common STIs, which causes gonorrhea. The bacterium is a gram-negative, oxidase-positive, non-spore-forming, non-capsulated, kidney-shaped diplococcus. It affects adolescents and infects the newborn infant during delivery (1, 3). According to the World Health Organization (WHO) 2016 report, gonorrhea cases were 30.6 million worldwide, with a prevalence of 0.9% females and 0.7% in males. The highest prevalence of gonorrhea among females and males in the Africa WHO region was 1.9% and 1.6%, respectively (4), whereas a study in sub-Saharan Africa indicated that the pooled prevalence was 2.4% in females and 1.7% in males (5). The summary report of a WHO European region from 1949 to 2021 revealed that the pooled prevalence of gonococcus (GC) among females and males was 3.2% and 12.1%, respectively (6).The disease is one of the main public health problems in Ethiopia. Some of the recent studies in Ethiopia indicated that the pooled prevalence of gonorrhea among STI-suspected patients ranged from 0.4% to 69% (7, 8), Mekelle (10.4%) (9), Gondar (7.6%) (10), Addis Ababa (50%) (11), Hawassa (4.3%) (12), and Jimaa (9.8%) (13). Even though females are more vulnerable to infections during sexual contact than males, the gonorrhea rates in males are mostly higher than in females. That is due to the lower rates of asymptomatic disease in females than in males. It infects the mucosal surfaces of the cervix in women and the urethra in men (4, 14).
The more likely high-risk groups for the infection of gonorrhea are commercial sex workers, those in more densely populated areas, low socioeconomic status, young individuals aged less than 25, and migrants. The main risk factors for acquiring STIs are unsafe sex practices, multiple sexual partners, substance use, early sexual intercourse, and risky sexual behavior (14–16).
Risky sexual behaviors (RSB) are behaviors that include engaging in sexual activity from an early age, inconsistent use of condoms during sexual intercourse, unprotected sexual intercourse, having sex with commercial sex workers, and the tendency to have multiple sexual partners that enhance the transmission of STIs including NG (10, 17). A recent systematic review of RSB in sub-Saharan Africa showed that the prevalence was 36.16% (18), whereas the pooled prevalence in Ethiopia was 42.8% (19). The report of the Ethiopian Demographic and Health Survey (EDHS) indicated that the average percentage of first sexual intercourse in the age group of 18–24-year-olds in 2019 was 40%, and 12% for females and males, respectively (20). The Ethiopian government's Ministry of Health has developed, implemented, and introduced various strategies to prevent and control sexual and reproductive health. For effective prevention and care of risky sexual behavior and STIs, there have been monitoring and evaluation methods (21).
As indicated, the prevalence of N. gonorrhoeae and risky sexual behavior in Ethiopia showed that there was a significant variation in their distributions across the regions. That was due to the availability and/or unavailability of the laboratory settings, the recommended type of culture media, the growth supplements, and the inhibitors for the cultivation of N. gonorrhoeae in each diagnostic laboratory. Addis Ababa is one of the regions where the prevalence of STIs is very high (8). It is commonly assumed that urban residents are more educated, have awareness and information, and based on that, they can practice. As a result, they are considered the low-risk population compared to their counterparts. However, practical observation and many articles indicated that the opposite happens (22, 23).
However, the risk factors are common in the city; there is no adequate information on the magnitude of risky sexual behaviors and N. gonorrhoeae. Therefore, this study was done to determine the prevalence of N. gonorrhoeae, risky sexual behavior, and associated risk factors among sexually transmitted infections in a resource-limited area of Addis Ababa City, Ethiopia.
Materials and methods
Study design and setting
A health institution-based cross-sectional study was conducted among sexually transmitted infection-suspected patients from April 2023 to December 2024 at the Addis Ababa City administration, the capital of Ethiopia. The city has over six million residents, twenty one governmental hospitals, and one hundred three health centers.
Sample size determination
A single proportional formula was used to calculate the sample size with a 95% confidence interval, a 4% margin of error, and an 11% contingency. A 69% (8) prevalence from the previous study in Ethiopia was used, and the final estimated sample was 571.
Using a 95% confidence interval with a 4% margin of error sample size was calculated as follows.
Where:
n = No of samples that will be included
= confidence level
P = prevalence from the previous study.
W = margin of error
Study variables
The prevalence of N. gonorrhoeae and risky sexual behavior were the dependent variables.
The independent variables were the socio-demographic variables (sex, age, marital status, occupation, educational status, participation in religious education, use of a condom, living with a spouse), the substance-related factors (alcohol consumption, khat chewing, and cigarette smoking), and risky sexual behavior factors (watching pornographic movies, attending nightclubs, having a new sexual partner, having a sexual partner two or more, previous STI, previous STI drug use, and commercial sex workers).
Sampling method and data collection
The study sites were Addis eray health center, Kuas meda health center, Millennium health center, Afenchobere health center, and Yeka health center. They were selected based on the previous STI - suspected patient flow in the health institutions, mass population living areas, and expected low living standards of the community near the health center. A convenient sampling technique was used to include the study subjects.
Clinicians and the primary investigator participated in the data collection, and sample processing was done via the principal investigator. The study subjects were those of all the reproductive age groups with any one of the signs and symptoms of gonorrhea who attended the health institutions during the study period. Individuals who had no signs and symptoms of STIs, were on recent antibiotic treatment, and were outside the reproductive age group were excluded from the study group. All eligible participants who attended the health center OPD were informed about the research objective and asked for their permission. After obtaining the participants' consent, a face-to-face interview using a structured questionnaire was conducted to collect the socio-demographic data and the risk factor variables related to GC and RSB.
Specimen collection
Two sterile swabs were collected from the endocervical canal of females using a speculum with warm water, and urethral samples were collected from males. One swab was for gram staining, and the other was for culturing. During the collection of the samples from the females, swabs were inserted into the cervix up to 2–3 cm, rotated for 5–10 s, and urethral samples were collected directly from the discharges.
Transport of the samples
After collecting the sample from the study subject, it was immediately delivered to the Amines transport media, which were suitable for 6–12 h of collection. The semisolid Amines transport media with charcoal can neutralize the toxic byproducts and other inhibitory substances and also contain sodium chloride to preserve these viable NG organisms. The samples were transported to the nearby reference laboratories of the Ethiopian Public Health Institute and the Institute of Biotechnology, Addis Ababa University. The transport media were labeled with the sample number, date, name of the health center, and time of collection. Ideally, the recommendation is that the samples of the NG are to be inoculated directly into their selective culture media to preserve the NG organisms. However, in this case, the sample collection sites were in different health centers, which had to be transported to the reference laboratories (14, 24).
Isolation and identification of N. gonorrhoeae
Microscopy
In the reference laboratories, one swab was used for gram staining to look for a bean-shaped gram-negative diplococcus under a microscope, which was important for the presumptive diagnosis of NG.
Culture
Gonococci are fastidious organisms; they need an enriched culture medium to support their growth and development, and selectives to suppress the growth of other organisms. The swabs taken from individual patients were inoculated onto selective Modified Thayer-Martin medium (MTM) with the supplements and incubated at 37°C in a moist atmosphere enriched with 5%–10% CO2 for 24–48 h. The MTM media were supplemented with IsovitaleX to support the growth of NG and VCNT (vancomycin, colistin, nystatin, and trimethoprim) inhibitors for other organisms. Culture plates were examined within 18–24 h after incubation and again after 48 h of incubation. The positive cultures produced small, raised, shiny, grey colonies that were sub-cultured on enriched media of chocolate agar, and all positive cultures were identified by their characteristic appearance on the media. Suspected NG in culture media were identified using gram stain, oxidase test, superoxol test, and carbohydrate utilization tests.
Gram staining was performed on the suspected colonies from the culture media to confirm a kidney-shaped gram-negative diplococcus. In the oxidase test, a suspected colony was made into a smear on the filter paper, and a positive result showed purple color development. In the superoxol test (30% w/v hydrogen peroxide), the suspected colonies from the culture media were mixed with 30% w/v hydrogen peroxide, and positive results showed strong bubble formation. For the carbohydrate utilization test, the suspension of the Ng isolates from the culture media was added to each of the glucose, sucrose, and maltose tube media. The color change observed in the glucose media from red to yellow, but not in the sucrose and maltose media, was considered as Ng isolates. The isolated NG were stored at −80°C in tryptic soy broth (TSB) with glycerol (14, 24, 25).
Data quality
The reliability of the study findings was ensured by implementing quality control (QC) measures throughout the entire laboratory work process. All materials, equipment, and procedures were adequately enrolled, and culture media were tested for sterility and performance. Pre-analytical, analytical, and post-analytical stages of quality assurance that were incorporated in standard operating procedures (SOPs) of the Microbiology laboratory at the Institute of Microbiology laboratory were strictly followed. The standard reference strains of N. gonorrhoeae ATCC 49226 (26) were used as controls for culture and biochemical tests (26).
Data analysis
Data were entered and analyzed using SPSS statistical software version 22. Frequencies and cross-tabulations were used to summarize descriptive statistics. Tables were used for data presentation. The odd ratio and adjusted odds ratio, both bivariate and multiple logistic regression, were employed to assess the association between outcome and explanatory variables. P-values <0.05 were considered statistically significant.
Operational definition
Commercial sex worker
Study participants who had sex for the exchange of money on commercial sites, hotels, streets, and residences (27).
Early sexual intercourse
Study participants who had had sexual intercourse before the age of 18 years old (28).
Illiterate
Individuals who didn't attend formal education
Literate
Individuals who attended formal education.
Multiple sexual partners
Study participants who had sexual intercourse with two or more sexual partners (19).
Risky sexual behavior (RSB)
Those who are reported having sexual intercourse with multiple sexual partners in the past year, sex with commercial sex workers, early initiation of sex, and unprotected sex (19).
Sexual experience
Having sexual intercourse practice in their life.
Substance use
Individuals who have used these substances in the last year (alcohol, khat, and cigarette use) in any amount (29).
Ethical considerations
Ethical clearance was obtained from the ethical review committee of the College of Natural and Computational Sciences, Addis Ababa University, with reference no CNCSDO/622/15/2023. Written informed consent was obtained from the study participants. The study was conducted following the Declaration of Helsinki.
Result
Socio-demographic characteristics of the study participants
The study includes 571 suspected STI patients in Addis Ababa City from April 2023 to December 2024, ranging from 15 to 67 years. Most of the study participants were in the age group of >35 years (32%), followed by 30–34 years (29.8%), with a mean age of 32.76 years (SD 8.24). Moreover, many participants were from an urban area 352 (61.6%). Three hundred twenty (56.0%) were unmarried, 459 (80.4%) were literate, and 329 (57.6%) were unemployed (Table 1).
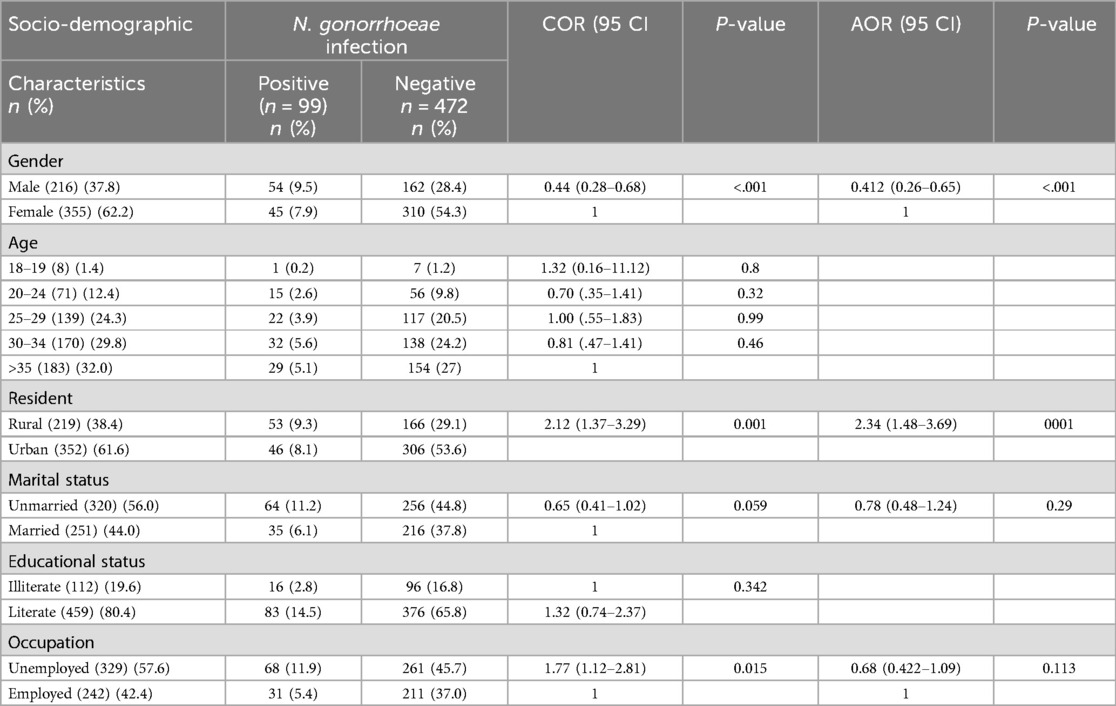
Table 1. Socio-demographic characteristics of the study participants among STI-suspected patients (n = 571) in Addis Ababa, Ethiopia, 2024.
Prevalence of N. gonorrhoeae infection
Of the 571 study participants, 99 (17.33%) had a confirmed gonococcal (GC) infection. The highest prevalence of GC infection was observed among those aged 30–34 years (5.6%), followed by those older than 35 years (5.1%), compared to a low occurrence in the 15–19 age group (0.2%) (Figure 1). The rate of GC infections was lower among married individuals (6.1%) than among unmarried individuals (11.2%), and among literate participants (14.5%) compared to illiterate ones (2.8%). Additionally, the infection rate was lower among employed individuals (5.4%) compared to unemployed participants (11.9%).
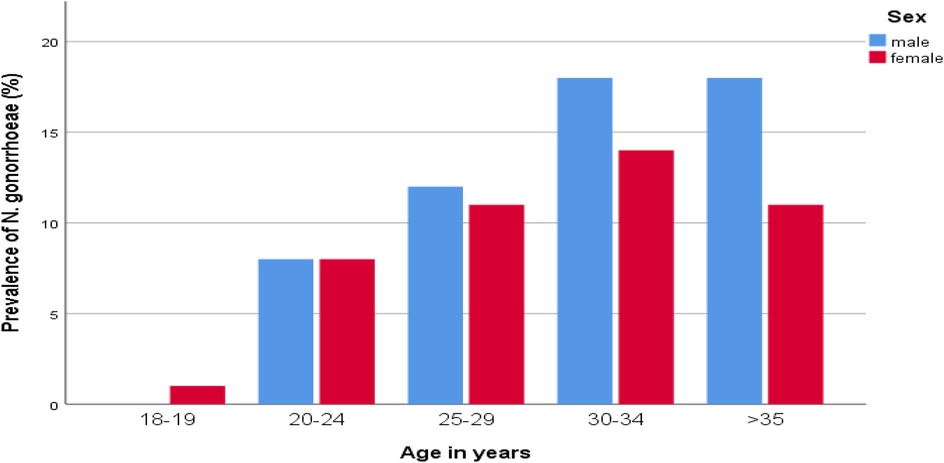
Figure 1. Distribution of N. gonorrhoeae by age groups and sex of STI suspected patients in Addis Ababa, Ethiopia.
The burden of GC infection in males (9.5%) was higher than in females (7.9%). The odds of GC infections in males were found to be 4.12 times higher than in females (AOR = 4.12, 95% CI: (0.26–0.65). The prevalence of GC infections in rural residents (9.3%) was higher than in urban residents (8.1%). Those participants who were rural dwellers had 2.34 times higher GC infection than their counterparts (AOR = 2.34, 95% CI: 48–3.69) (Table 1).
Factors associated with N. gonorrhoeae infections
Of the respondents, 163 (28.5%) were used condoms, 151(26.4%) bought condom, and 121 (22.2%) were had a condom in their home. One hundred sixty-eight (29.4%) were previous STI drug users, 211(37.0%) had a previous STI, and (193) (33.8) had STI in the last five years (Table 2).
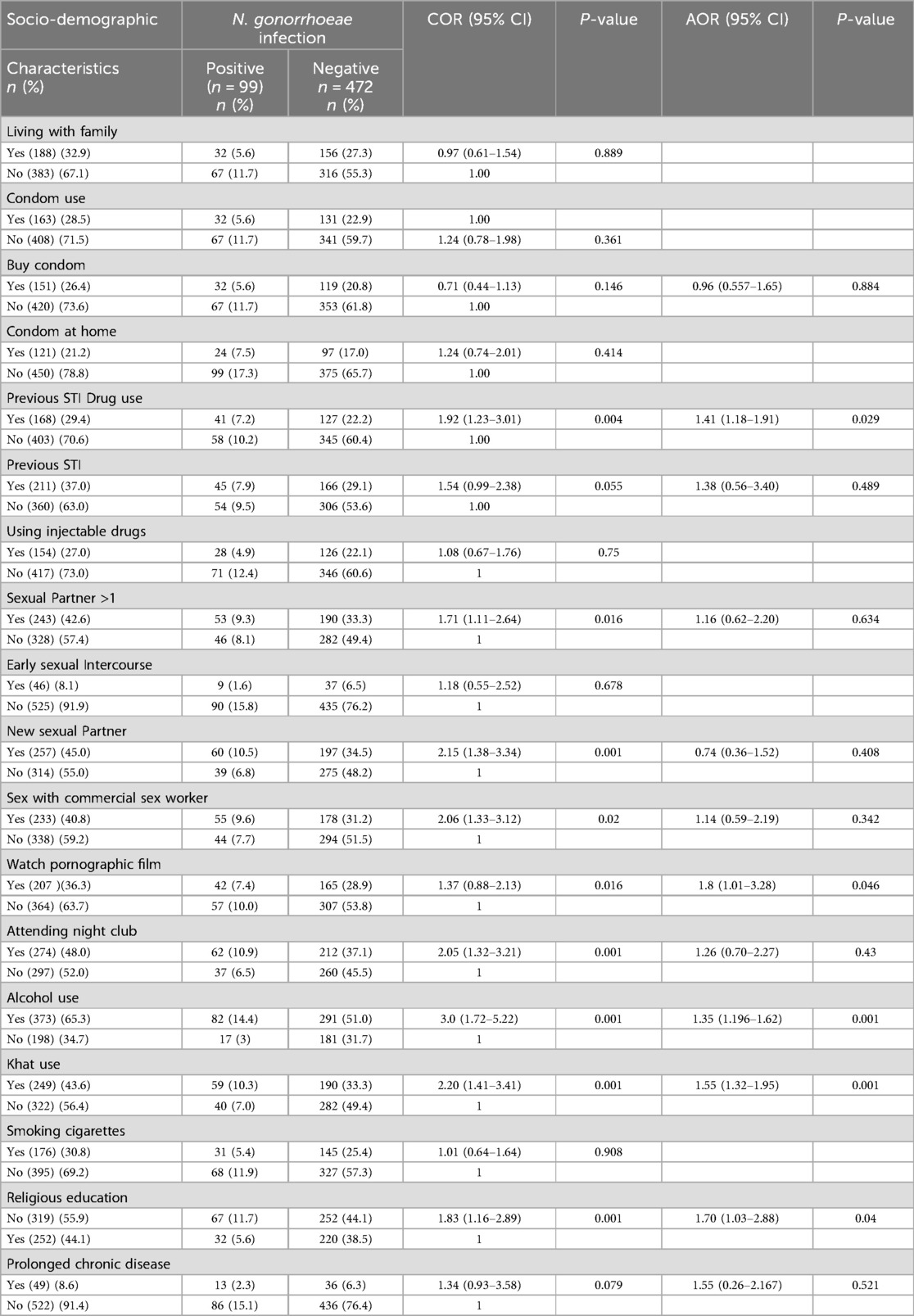
Table 2. Factors associated with N. gonorrhoeae infections among STI-suspected patients (n = 571) in Addis Ababa, Ethiopia, 2024.
Bivariate and multivariable logistic regression were used to identify the possible risk factors for the infection of N. gonorrhoeae. The odds of N. gonorrhoeae infection were found to be 1.41 times higher among the previous drug users than the nonusers [AOR = 1.41, 95% CI: 1.41(1.18–1.91)]. Those participants who were watching pornographic films were more vulnerable to N. gonorrhoeae infections than their counterparts (AOR = 1.8, 95% CI: (1.01–3.28); however, those who had more sexual partners, new sexual partners, had sex with a commercial sex worker, and had an early sexual intercourse history were not statistically associated with N. gonorrhoeae infections. Participants who had an alcohol use history were statistically associated with N. gonorrhoeae infections [AOR = 1.35, 95% CI: 1.35 (1.196–1.62)]. Moreover, the odds of N. gonorrhoeae infection were 1.55 times higher among chat users than the non-chat users [AOR = 1.55, 95% CI: (1.32–1.95)], however, having a smoking cigarette history was not statistically significant with N. gonorrhoeae infection. Participants who didn't attend the religious sectors were more vulnerable to the infection than their counterparts [AOR = 1.70, 95% CI: (1.03–2.88)] (Table 2).
Prevalence of risky sexual behavior
Of the male respondents, 157 (27.5%) were having sexual intercourse. Being males were statistically more significant than females for RSB (p < 0.001). Of the participants, the likelihood of being sexually active rose with the increase of age. At the age of 15–29 (0.4%), 25–29 (16.3%), 30–34 (13.5%), and >35 (17.7%) reported having sex with two or more however, age were not statistically significant with RSB. One hundred ninety-five (34.2%) respondents among urban had two or more sexual partners and 250(43.8%) of literates had a sexual history with two or more (p < 0.001). Moreover, 222(38.9%) of the employers had two or more sexual partners (p < 0.001). The prevalence of RSB among STI patients was 56.9% (Table 3).
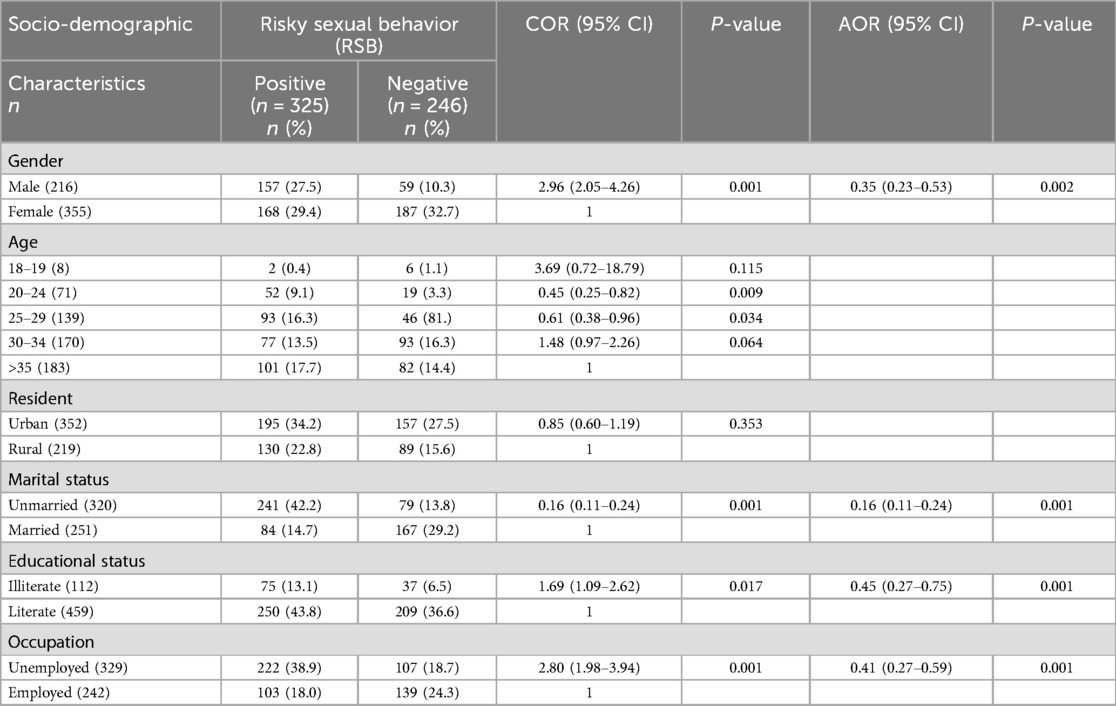
Table 3. Socio-demographic characteristics of the study participants with risky sexual behavior among STI-suspected patients (n = 571) in Addis Ababa, Ethiopia, 2024.
The percentage of participants watching pornographic films among study subjects was 36.3% (n = 207). Of these participants, females (53.1%, n = 110) had more experience watching the films than males (46.9%, n = 97). Among the 207 study subjects who had experience watching pornographic films, a higher level of experience was observed in singles (58.9%, n = 122), followed by married individuals (20.8%, n = 43), and divorced individuals (18.8%, n = 39), compared to the widowed group (1.4%, n = 3) (Figure 2).
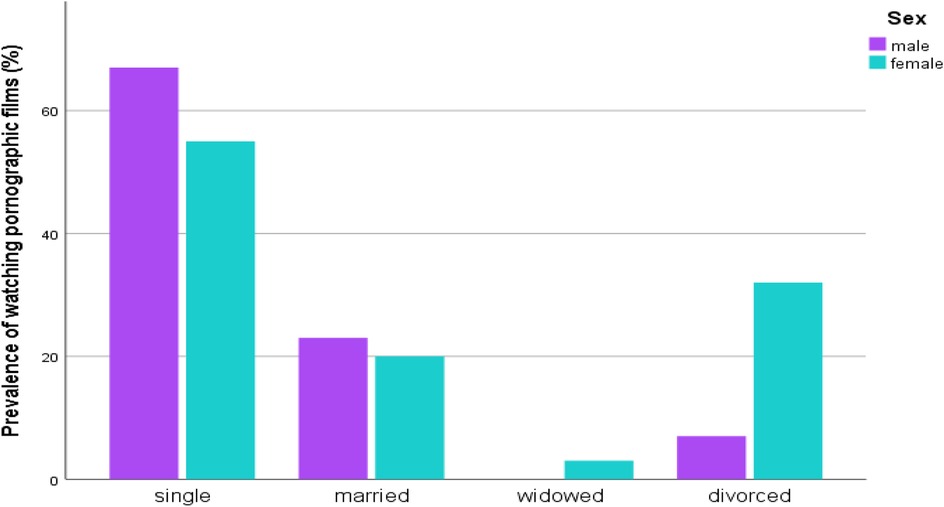
Figure 2. Distribution of watching pornographic films by marital status and sex of STI suspected patients in Addis Ababa, Ethiopia.
Factors influencing the acquisition of risky sexual behavior
One hundred eighty-eight respondents were living with their family; of them, 89 (15.6%) had an RSB. Of the positive RSB response individuals, 127 (22.2%) used condoms during sexual intercourse, 118 (20.7%) bought condoms, and 94 (16.5%) had a condom at their home. Moreover, 145 (25.4%) had a previous history of usage of STI drugs, 182 (31.9%) had a previous history of STI (P < 0.001), and 164 (28.7%) had a history of STI within the last five years.
The odds of having multiple sexual partners and early sexual intercourse were 10.95 (95% CI 5.75–20.84) times higher among STI-suspected patients who had a new sexual partner than their counterparts. The odds of risky sexual behavior were 3.31 times higher in those attending nightclubs than their counterparts [AOR = 3.31, 95% CI (1.82–1.90)] and also religious education had a positive impact on the prevention of risky sexual behavior [AOR = 0.5, 95% CI (0.29–0.86)]; however using a chat 59 (10.3%), smoking cigarette 144 (25.2%) and having a prolonged chronic disease 36 (6.3) hadn't had a statistically significant association with risky sexual behavior.
Study participants who had a previous STI history were 2.85 times [AOR = 1.58, 95% CI (1.17–6.91)] more likely to have a risky sexual behavior than those who didn't have a previous STI disease history. Similarly, study subjects who watched pornographic films regularly were also more exposed to risky sexual behavior than those who weren't involved in watching pornographic films [AOR = 2.32, 95% CI (1.84–5.14)] (Table 4).
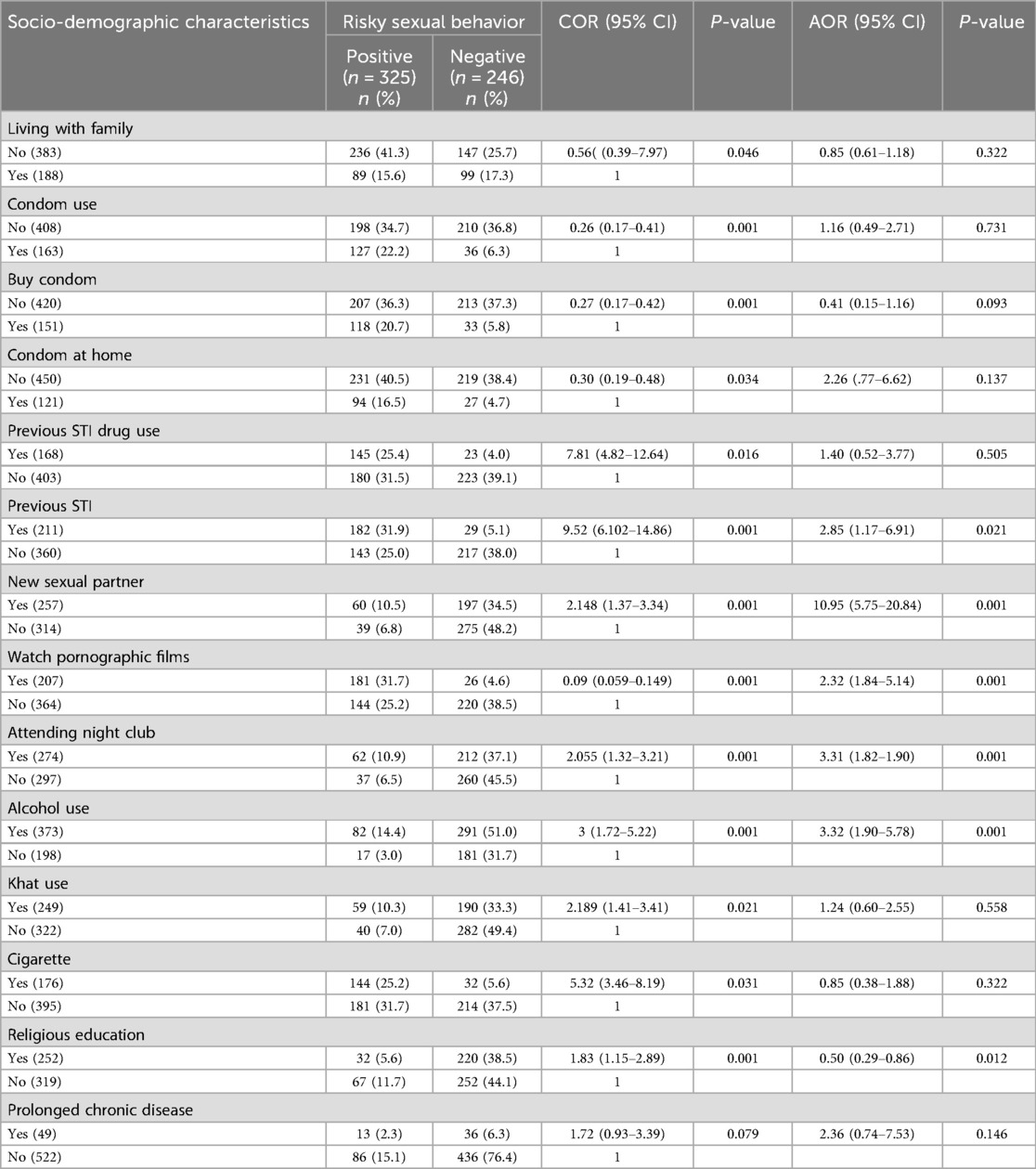
Table 4. Factors associated with risky sexual behavior among STI-suspected patients (n = 571) in Addis Ababa, Ethiopia, 2024.
Discussion
STIs are one of the major public health problems. Reducing the STI burden is one of the WHO's third Sustainable Development Goals, and it is planned to minimize NG infection by 90% globally by 2030 (30). The WHO plans to develop new methods for the diagnosis, prevention, management, and epidemiology of STIs, including NG. They plan to design a new detection test that have a low-cost, rapid, and accessible test with self-sampling and detection to reduce the transmission rate, incidence, and prevalence of NG infection, which is especially helpful in the low-resource settings of developing countries where the burden of the disease is high (31).
The present study aimed to determine the distribution of NG infections among suspected STI patients, which helps to reduce the impact of disease causations and improve public health.
In this study, the prevalence of N. gonorrhoeae among suspected STI patients was 17.3%, which was higher than studies done in Mekele (10.04%) (9), Jimma (9.8%) (13), Gondar (7.6%) (10), Hawassa (3.3%) (32), Kenya (6.3%) (33), India (5.7%) (34), Latin America in low-risk groups (1.46%), and high-risk groups (5.68%) (35), and WHO European region (1.0%) (6). This relatively increased rate of gonococcal infections seen in the present study might be due to multicultural practice in the city where commercial sex work was high (22, 36). The city is the capital city, it is the center for business, education, employment, medication, and it is also a transition center to travel from one area to another, and residence of many people coming from different corners of the country to the city including migrant workers displaced from rural and urban areas of other regions of Ethiopia (37, 38). Additionally, areas with rare male circumcision practices are also represented in the city, with a prevalence of 53% of males not circumcised in the Gambela region (39). However, the association between male uncircumcision and the development of NG infection has not been adequately studied. Studies have shown that uncircumcised men are more likely to develop NG infection than circumcised men (40, 41). Such practices increase the risk factors for acquiring STI and N. gonorrhoeae infections. The relative increase of gonococcal infections in the present study area may also indicate the presence of HIV (23, 42) and other STIs that increase the risk of acquiring N. gonorrhoeae. In contrast, the prevalence in the present study was lower than in studies done in Addis Ababa (69%) (8), Ethiopia (20%) (7), Ghana (27.4%) (43), and Nigeria (19.1%) (44). The difference could be variations in study populations, geographical differences, sample size, specimen type, and social beliefs.
In the present study, the overall magnitude of risky sexual behavior among sexually transmitted infections was 56.9%, which was lower than reports found in Haramaya University students (65.8%) (45), Southern Ethiopia (75.5%) (46) and Southern Ethiopia (78%) (47). On the other hand, the finding was much higher than studies done at Injibara University (38%) (48), Ethiopian public university students (19.5%) (49), Southern Ethiopia (25.9%) (50), Gondar (27.5%) (51) and Addis Ababa (40.6%) (52). The variations could be differences in the target study populations.
According to the present findings, culture-confirmed positive N. gonorrhoeae isolates were higher in males than in females, and being male showed a statistically significant association for N. gonorrhoeae infections and risky sexual behavior. A study done in Uganda (17) also supports these findings. This could be because females might have been more likely to have public clinic follow-ups that enabled them to know STI prevention and control methods.
The present study showed that RSB was higher among the non-employed than the employed. The study was supported by the previous studies done in Addis Ababa (52). This might be due to an individual who hadn't employed maybe feeling more levels of independence and less levels of social expectations and responsibilities, which can lead to having multiple sexual partners and committing sexual intercourse. The educational levels of the participants were also statistically significantly associated with the RSB. Individuals who were not educated were more at risk for RSB than those who were educated, which was supported by studies done in Gondar (51), Addis Ababa (52), and Southern Ethiopia (47). That might be due to illiterates having less awareness of sexually transmitted diseases.
In the present study, marital status was not statistically associated with the prevalence of N. gonorrhoeae infection; however, being unmarried was statistically significantly associated with risky sexual behavior. In contrast, a study done in Jimma (13) on sexually transmitted disease patients indicated that marital status was significantly associated with the prevalence of N. gonorrhoeae infections. Additionally, the current study showed that respondents who were rural residents were more likely to have N. gonorrhoeae infections than urban dwellers, which was supported by a study done at Jimma (13), whereas being urban and rural were not statistically different for risky sexual behavior. This difference might be due to geographical location variations; being urban has a chance of getting information from health professionals regarding the negative effects of risky sexual behavior and the burden of sexually transmitted infections.
The current study indicated that respondents who had a previous STI drug use history had significant associated risk factors for the acquisition of N. gonorrhoeae infection than those who hadn't had a history of STI drug use, but having a previous drug use history was not a risk factor for the RSB. However, individuals who had a previous STI and who didn't take the drug were statistically significant with RSB, which was supported by a study done in Mekele (9).
In our findings, the odds of risky sexual behavior and N. gonorrhoeae infection were higher among STIs who drank alcohol as compared with STIs who didn't drink alcohol. Similar findings were reported in the previous studies of Gondar (10) and India (34). Also, drinking alcohol was a statistically significant factor for the occurrence of risky sexual behavior, which was supported by studies done in Addis Ababa (52) and Southern Ethiopia (50). That could be due to excessive drinking of alcohol, which affects personal judgment and behavior, altering decisions, and creating a conflict of interest between desire and inhibitions that leads to risky sexual practices.
The use of khat chewing was another risk factor in the present study. The finding indicated that individuals who chewed khat were at 1.55 times higher risk of engaging in N. gonorrhoeae infections than non-khat chewers, which has been supported by studies done in Mekele (9). However, chewing khat was not an associated factor for risky sexual behavior in contrast with this a study done in Bahir dar city showed that chewing khat was a factor for risky sexual behavior (53).
According to our report, there was a positive association between religious beliefs and practices and a lower prevalence of N. gonorrhoeae infections and RSB. This finding was supported and stated that adolescents and young adults who associated with their religion throughout their lives had shown lower rates of RSB, which was supported by studies done in (54, 55) and N. gonorrhoeae infections. Religious practice had a protective effect on risky sexual behavior.
The current study showed that study participants who watched pornographic movies were more likely to have N. gonorrhoeae infections and RSB as compared with their counterparts, which was supported by a study done in Addis Ababa (52) and Southern Ethiopia (47). Similarly, attending nightclubs was also a statistically significant association with risky sexual behavior, but not for N. gonorrhoeae compared with those who weren't. Studies done in Gondar (51) and Southern Ethiopia support this finding (50). It could be due to watching pornographic films, and attending nightclubs may increase the motivations for sexual needs that were prone to risky sexual behavior.
Conclusion
The prevalence of N. gonorrhoeae and risky sexual behavior among STI patients in Addis Ababa was high. Gender, watching pornographic films, alcohol consumption, and not participating in religious education have been found to increase the risk of experiencing both N. gonorrhoeae infections and risky sexual behavior. Moreover, a previous drug use history, residence, and chat chewing were the risk factors for the prevalence of N. gonorrhoeae infections, whereas marital status, educational status, occupational status, having a previous STI, and having a new sexual partner were the risk factors for risky sexual behavior. Therefore, health education has to be given to the community to create awareness of sexuality and risky sexual behaviors, as the risk factors indicate that knowledge and attitude gaps exist.
Limitations of the study
The limitations of the present study were important factors like social norms, other risky sexual behaviors, and beliefs that were not included, which would have an impact on risky sexual behavior and sexually transmitted diseases. Moreover, because of the sensitive nature of risky sexual behavior and sexually transmitted diseases, response bias of the participants may be present, and the data in the present study were not also nationally representative. Therefore, further studies should be undertaken at the national level and include important factors to explore more on N. gonorrhoeae and risky sexual behavior.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by College of Natural Science, Addis Ababa University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TA: Supervision, Writing – review & editing, Methodology, Formal analysis, Writing – original draft, Data curation, Investigation, Visualization, Conceptualization, Software. GB: Resources, Writing – review & editing, Funding acquisition, Visualization, Software, Formal analysis, Methodology, Conceptualization, Validation, Supervision, Data curation, Investigation. AD: Software, Data curation, Investigation, Methodology, Funding acquisition, Conceptualization, Writing – review & editing, Formal analysis, Supervision. HN: Conceptualization, Writing – review & editing, Validation, Supervision, Data curation, Software, Project administration, Investigation, Methodology, Visualization. WM: Investigation, Resources, Writing – review & editing, Visualization, Validation, Data curation, Methodology, Conceptualization. AD: Investigation, Validation, Resources, Conceptualization, Writing – review & editing, Visualization, Methodology, Data curation. GT: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing, Data curation, Project administration, Methodology. YM: Investigation, Conceptualization, Funding acquisition, Writing – review & editing, Supervision, Project administration, Methodology, Data curation. DB: Data curation, Investigation, Project administration, Methodology, Resources, Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was received from Addis Ababa University.
Acknowledgments
We would like to acknowledge our study participants for giving their consent and participation. The authors also gratefully acknowledge both the Ethiopian Public Health Institute and the Department of Health Biotechnology, Institute of Biotechnology, Addis Ababa University, for the laboratory setting and materials. Finally, we are pleased to thank Addis Ababa University, College of Natural and Computational Science, Department of Microbial Sciences and Genetics for the approval of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; GC, gonococcal; HIV, human immunodeficiency virus; MTM, Thayer Martin medium; NG, N. gonorrheae; RSB, risky sexual behavior; STI, sexually transmitted infection; WHO, World Health Organization.
References
1. World Health Organization WHO. The Diagnostics Landscape for Sexually Transmitted Infections. Geneva: World Health Organization (2023).
2. Gottlieb SL, Spielman E, Abu-Raddad L, Aderoba AK, Bachmann LH, Blondeel K, et al. WHO Global research priorities for sexually transmitted infections. Lancet Glob Health. (2024) 12(9):e1544–51. doi: 10.1016/S2214-109X(24)00266-3
3. Guglielmino CJD, Sandhu S, Lau CL, Buckely C, Trembizki E, Whiley DM, et al. Molecular characterisation of Neisseria gonorrhoeae associated with disseminated gonococcal infections in Queensland, Australia: a retrospective surveillance study. BMJ Open. (2022) 12(8):e061040. doi: 10.1136/bmjopen-2022-061040
4. Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of gonorrhoea: a global perspective. Sex Health. (2019) 16(5):401–11. doi: 10.1071/SH19061
5. Michalow J, Hall L, Rowley J, Anderson RL, Hayre Q, Chico RM, et al. Prevalence of chlamydia, gonorrhoea, and trichomoniasis among male and female general populations in sub-Saharan Africa from 2000 to 2024: A systematic review and meta-regression analysis. medRxiv: the preprint server for health sciences. (2024).
6. Chidiac O, AlMukdad S, Harfouche M, Harding-Esch E, Abu-Raddad LJ. Epidemiology of gonorrhoea: systematic review, meta-analyses, and meta-regressions, world health organization European region, 1949–2021. Euro Surveill. (2024) 29(9):2300226. doi: 10.2807/1560-7917.ES.2024.29.9.2300226
7. Gobezie MY, Tesfaye NA, Solomon T, Demessie MB, Kassa TD, Wendie TF, et al. Neisseria gonorrhea in Ethiopia, prevalence among STI suspected patients and its antimicrobial susceptibility: a systematic review and meta-analysis. Front Microbiol. (2024) 15:1390001. doi: 10.3389/fmicb.2024.1390001
8. Fentaw S, Abubeker R, Asamene N, Assefa M, Bekele Y, Tigabu E. Antimicrobial susceptibility profile of gonococcal isolates obtained from men presenting with urethral discharge in Addis Ababa, Ethiopia: implications for national syndromic treatment guideline. PLoS One. (2020) 15(6):e0233753. doi: 10.1371/journal.pone.0233753
9. Kahsay AG, Mezgebo TA, Gebrekidan GB, Desta BL, Mihretu HG, Dejene TA. Prevalence, antibiotic resistance and associated factors of Neisseria gonorrhoeae among patients attending non-profitable private clinics in Mekelle, Tigrai, Ethiopia. Infect Drug Resist. (2023) 16:4065–72. doi: 10.2147/IDR.S416344
10. Demissie E, Amare A, Birhanu M, Gizachew M. Neisseria gonorrhoeae antimicrobial resistance patterns and associated risk factors in women of childbearing potential in northwestern Ethiopia. BMC Womens Health. (2024) 24(1):82. doi: 10.1186/s12905-024-02898-3
11. Ayalew E, Fentaw S, Ebrahim S, Seyoum E, Woldesenbet Z, Wolde M. Comparison of syndromic versus laboratory-confirmed diagnosis of Neisseria gonorrhoeae and Treponema palladium, infections at the selected health centers in Addis Ababa, Ethiopia. Reprod Health. (2022) 19(1):88. doi: 10.1186/s12978-022-01395-w
12. Zenebe MH, Mekonnen Z, Loha E, Padalko E. Prevalence, risk factors and association with delivery outcome of curable sexually transmitted infections among pregnant women in southern Ethiopia. PLoS One. (2021) 16(3):e0248958. doi: 10.1371/journal.pone.0248958
13. Sahile A, Teshager L, Fekadie M, Gashaw M. Prevalence and antimicrobial susceptibility patterns of Neisseria gonorrhoeae among suspected patients attending private clinics in Jimma, Ethiopia. Int J Microbiol. (2020) 2020:7672024. doi: 10.1155/2020/7672024
14. World Health Organization WHO. WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the Management of Symptomatic Sexually Transmitted Infections. Geneva: World Health Organization (2021).
15. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. (2021) 70(4):1–187. doi: 10.15585/mmwr.rr7004a1
16. World Health Organization WHO. WHO Guidelines Approved by the Guidelines Review Committee. Consolidated Guidelines on HIV, Viral Hepatitis and STI Prevention, Diagnosis, Treatment and Care for key Populations. Geneva: World Health Organization © World Health Organization (2022).
17. Mabonga E, Manabe YC, Elbireer A, Mbazira JK, Nabaggala MS, Kiragga A, et al. Prevalence and predictors of asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae in a Ugandan population most at risk of HIV transmission. Int J STD AIDS. (2021) 32(6):510–6. doi: 10.1177/0956462420979799
18. Wondmeneh TG, Wondmeneh RG. Risky sexual behaviour among HIV-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. BioMed Res Int. (2023) 2023:6698384. doi: 10.1155/2023/6698384
19. Muche AA, Kassa GM, Berhe AK, Fekadu GA. Prevalence and determinants of risky sexual practice in Ethiopia: systematic review and meta-analysis. Reprod Health. (2017) 14(1):113. doi: 10.1186/s12978-017-0376-4
20. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini Demographic and Health Survey 2019: Final Report. Rockville M, USA: EPHI and ICF (2021).
21. Federal Democratic Republic of Ethiopia Ministry of Health Ngftmostiusa. (2015). Available online at: https://platform.who.int/docs/default-source/mca-documents/policy-documents/guideline/ETH-RH-43-01-GUIDELINE-2015-eng-Management-of-STIs.pdf (Accessed March 14, 2025).
22. Berhe TT, Asfaw EA, Tedla GW. Assessment of acceptance and associated factors of HIV pre-exposure prophylaxis among commercial female sex workers in drop-in centers selected sub-cities of Addis Ababa, Ethiopia. Front Public Health. (2024) 12:1462648. doi: 10.3389/fpubh.2024.1462648
23. Tesfie TK, Yismaw GA, Yirsaw BG, Abuhay HW, Alemayehu MA, Derseh NM, et al. Prevalence and associated factors of HIV among female sex workers in eastern and Southern Africa: systematic review and meta-analysis. PLoS One. (2024) 19(12):e0313868. doi: 10.1371/journal.pone.0313868
24. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. (2014) 63(Rr-02):1–19. https://pubmed.ncbi.nlm.nih.gov/24622331/
25. Perilla MJ. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World: Haemophilus Influenzae, Neisseria meningitidis, Streptococcus Pneumoniae, Neisseria gonorrhoeae, Salmonella Serotype Typhi, Shigella, and Vibrio cholerae. WHO/CDS/CSR/RMD/2003.6. Geneva: World Health Organization (2003).
26. Centers for Disease Control and Prevention CDC, Prevention. Neisseria gonorrhoeae Reference Strains for Antimicrobial Susceptibility Testing. Atlanta: Centers for Disease Control and Prevention (2005).
27. O’Doherty T, Bowen R. Commercial sex as valuable? Policy implications of sex Workers’ perspectives on the contributions of their labor. Sexuality Research and Social Policy. (2024) 21(2):527–42. doi: 10.1007/s13178-023-00900-5
28. Kassahun EA, Gelagay AA, Muche AA, Dessie AA, Kassie BA. Factors associated with early sexual initiation among preparatory and high school youths in Woldia town, northeast Ethiopia: a cross-sectional study. BMC Public Health. (2019) 19(1):378. doi: 10.1186/s12889-019-6682-8
29. Shegute T, Wasihun Y. Prevalence of substance use in university students, Ethiopia. Subst Abuse Res Treat. (2021) 15:11782218211003558. doi: 10.1177/11782218211003558
30. World Health Organization WHO. Implementing the Global Health Sector Strategies on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2022–2030: Report on Progress and Gaps 2024. Geneva: World Health Organization (2024).
31. Gul I, Raheem MA, Lian L, Karim AM, Heyat BB, Yu D. CRISPR diagnostics for WHO high-priority sexually transmitted infections. Trends Anal Chem. (2025) 182:118054. doi: 10.1016/j.trac.2024.118054
32. Tadele A, Hussen S, Shimelis T. Prevalence and associated factors of Chlamydia trachomatis and Neisseria gonorrhoeae among female commercial sex workers in Hawassa city, southern Ethiopia. BMC Infect Dis. (2019) 19(1):61. doi: 10.1186/s12879-019-3698-8
33. Abdullahi A, Nzou SM, Kikuvi G, Mwau M. Neisseria gonorrhoeae infection in female sex workers in an STI clinic in Nairobi, Kenya. PLoS One. (2022) 17(2):e0263531. doi: 10.1371/journal.pone.0263531
34. Sharma D, Muralidhar S, Lachyan AS, Khunger N. Risk factors associated with increasing prevalence of gonorrhea and the antimicrobial susceptibility profiles of Neisseria gonorrhoeae among adolescents: a decade-long, hospital-based study from India. Indian J Sex Transm Dis AIDS. (2024) 45(1):15–8. doi: 10.4103/ijstd.ijstd_74_23
35. Bardach A, Alconada T, Palermo C, Rojas-Roque C, Sandoval MM, Gomez J, et al. Burden of disease of gonorrhoea in Latin America: systematic review and meta-analysis. Infect Dis Ther. (2023) 12(6):1505–25. doi: 10.1007/s40121-023-00814-0
36. Kura Z. Early Engagement to Sex Work and Associated Factors Among Commercial Sex Workers in Addis Ketema Subcity Addis Ababa. Ethiopia: University of Gondar Gondar (2015).
37. Kebu H, Berisso O, Mulugeta M. Drivers of migration and determinants of wellbeing among internal youth migrants in Ethiopia: towns along Addis Ababa -Adama route in focus. Heliyon. (2023) 9(3):e13780. doi: 10.1016/j.heliyon.2023.e13780
38. Erulkar A, Hailu E. Young female migrants and job placement brokers in Addis Ababa, Ethiopia. Front Reprod Health. (2024) 6:1241571. doi: 10.3389/frph.2024.1241571
39. Patrick DM, Schneiderman J, Kinahan T, Pollock N, Ma’ayan S. Integrating Male Circumcision (MC) into HIV Prevention Efforts: Our Learning in Ethiopia, Kenya and Rwanda. Canada: BC Center for Disease Control, Canadian Institute for Health Research (2009).
40. Diseker RA 3rd, Peterman TA, Kamb ML, Kent C, Zenilman JM, Douglas JM Jr, et al. Circumcision and STD in the United States: cross sectional and cohort analyses. Sex Transm Infect. (2000) 76(6):474–9. doi: 10.1136/sti.76.6.474
41. Olesen TB, Munk C, Mwaiselage J, Kahesa C, Rasch V, Frederiksen K, et al. Male circumcision and the risk of gonorrhoea, syphilis, HIV and human papillomavirus among men in Tanzania. Int J STD AIDS. (2019) 30(14):1408–16. doi: 10.1177/0956462419874593
42. Adal M. Systematic review on HIV situation in Addis Ababa, Ethiopia. BMC Public Health. (2019) 19(1):1544. doi: 10.1186/s12889-019-7885-8
43. Acheampong DO, Opoku R, Adokoh CK, Boye A, Asiamah EA, Armah FA, et al. Prevalence and antimicrobial susceptibility pattern of Neisseria gonorrhoeae in Kumasi, Ghana. J Adv Microbiol. (2018) 11(2).
44. Keshinro B, Crowell TA, Nowak RG, Adebajo S, Peel S, Gaydos CA, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc. (2016) 19(1):21270. doi: 10.7448/IAS.19.1.21270
45. Derese A, Seme A, Misganaw C. Assessment of substance use and risky sexual behaviour among Haramaya university students, Ethiopia. Sci J Public Health. (2014) 2(2):102–10. doi: 10.4236/jss.2023.118009
46. Bekele Z, Mussa I, Assefa Y, Abera N, Amerga EW, Girma A, et al. Risky sexual practice and associated factors among adult people living with HIV/AIDS in public hospitals of Kembata Tambaro zone, southern Ethiopia: a cross-sectional study. BMJ open. (2023) 13(7):e072505. doi: 10.1136/bmjopen-2023-072505
47. Wondimagegne YA, Anbese AT. Risky sexual behaviors and associated factors among adolescent in Gedeo zone, south Ethiopia: a community based cross-sectional study. Sci Rep. (2024) 14(1):19908. doi: 10.1038/s41598-024-67944-4
48. Adal MA, Abiy SA, Reta MM, Asres MS, Animut Y. Prevalence of risky sexual behavior and associated factors among Injibara university students, northwest Ethiopia. Front Reprod Health. (2024) 6:1356790. doi: 10.3389/frph.2024.1356790
49. Belihu WB, Amogne MD, Herder T, Sundewall J, Agardh A. Risky sexual behavior and associated factors among university students in Ethiopia: a cross-sectional national survey. BMC Public Health. (2024) 24(1):1701. doi: 10.1186/s12889-024-19213-2
50. Asresie MB, Worede DT. Factors associated with risky sexual behavior among reproductive-age men in Ethiopia: evidence from Ethiopian demography and health survey 2016. HIV/AIDS (Auckland, NZ). (2023) 15:549–57. doi: 10.2147/hiv.s426379
51. Geremew AB, Gelagay AA, Yeshita HY, Azale Bisetegn T, Habitu YA, Abebe SM, et al. Youth risky sexual behavior: prevalence and socio-demographic factors in north-west Ethiopia: a community-based cross-sectional study. Community Health Equity Res Policy. (2022) 42(2):145–54. doi: 10.1177/0272684X20976519
52. Sifer SD, Getachew MS. Risky sexual behavior and associated factors among out-of-school youths in Addis Ababa, Ethiopia; mixed methods study. Reprod Health. (2024) 21(1):77. doi: 10.1186/s12978-024-01808-y
53. Abate A, Tareke M, Tirfie M, Semachew A, Amare D, Ayalew E. Chewing khat and risky sexual behavior among residents of Bahir Dar City administration, northwest Ethiopia. Ann Gen Psychiatry. (2018) 17:26. doi: 10.1186/s12991-018-0194-2
54. Rosmarin DH, Pirutinsky S. Problematic sexual behavior and religion among adult Jewish males: an initial study. Ame J Mens Health. (2019) 13(1):1557988318823586. doi: 10.1177/1557988318823586
Keywords: gonorrhea, N. gonorrhoeae, risk factors, risky sexual behavior, sexually transmitted infection
Citation: Andualem T, Belay G, Desta AF, Nigussie H, Mulu W, Desalegn A, Taddesse G, Mekonen Y and Beyene D (2025) The burden of Neisseria gonorrhoeae infection, risky sexual behavior, and associated risk factors among sexually transmitted infections in a resource-limited setting area of Addis Ababa City, Ethiopia. Front. Reprod. Health 7:1601088. doi: 10.3389/frph.2025.1601088
Received: 28 March 2025; Accepted: 14 July 2025;
Published: 29 August 2025.
Edited by:
Teiichiro Shiino, National Center For Global Health and Medicine, JapanReviewed by:
Ijaz Gul, Tsinghua University, ChinaMa. Guadalupe Aguilera-Arreola, National Polytechnic Institute (IPN), Mexico
Copyright: © 2025 Andualem, Belay, Desta, Nigussie, Mulu, Desalegn, Taddesse, Mekonen and Beyene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tesfaye Andualem, dGVzZmF5ZWFuZHVAZ21haWwuY29t
 Tesfaye Andualem
Tesfaye Andualem Gurja Belay2
Gurja Belay2 Adey F. Desta
Adey F. Desta Helen Nigussie
Helen Nigussie Wondemagegn Mulu
Wondemagegn Mulu