- Heilongjiang University of Chinese Medicine, Harbin, China
Background: This article reviews the research progress in recent years on the influencing factors for menstrual improvement in patients with moderate to severe Intrauterine Adhesions. To improve the treatment effect, reduce the risk of re-adhesion, optimize the treatment plan, enhance patients' quality of life, and prevent infertility and miscarriage.
Objective: Identify the factors that may affect menstrual improvement in patients with moderate to severe Intrauterine Adhesions.
Method: An in-depth literature search was carried out on four databases to sort out the research results on the influencing factors of menstrual improvement in patients with moderate to severe Intrauterine Adhesions from 2014 to 2024.
Findings: The review incorporated 61 papers and found that the influencing factors for menstrual improvement in patients with moderate to severe Intrauterine Adhesions involve: (1) The development of the Müllerian duct and the levels of Anti-Müllerian Hormone. (2) The interaction and dynamic changes between Mesenchymal Stem Cells and the endocrine system. (3) The impact of hysteroscopic surgery on the endometrium and menstrual improvement, including the effects of the operation method, frequency of implementation, and postoperative management of hysteroscopic surgery. (4) The role of psychological factors.
Discussion: The results of this review highlight the factors influencing menstrual improvement in patients with moderate to severe Intrauterine Adhesions. However, the influencing factors of menstrual improvement are multifaceted and interrelated. Future research needs to further explore the interactions among these factors and how to optimize treatment plans to improve treatment outcomes.
1 Introduction
IUA is an intrauterine lesion resulting from trauma and/or infection of the endometrial basal layer (1, 2). The pathogenesis of IUA remains incompletely understood. Nevertheless, it is commonly thought to be associated with the mutual adhesion between the uterine muscular walls following damage to the basal layer of the endometrium. This process encompasses three stages: the inflammatory stage, the tissue formation stage, and the tissue reconstruction stage (1). Given that the repair of the endometrium mostly involves incomplete regeneration, it eventually results in functional impairment and scar formation. The clinical manifestations of IUA patients mainly include reduced menstrual flow, amenorrhea, dysmenorrhea, infertility, and recurrent miscarriage. The severity of these symptoms is positively correlated with the degree of IUA (3). In severe cases, IUA may have a profound impact on a woman's fertility and mental health (2). Because of this, in recent years, numerous scholars have conducted extensive and in-depth research on the influencing factors of menstrual improvement in patients with moderate to severe IUA (Table 1) (4). This paper aims to review the latest progress of these studies, with the hope of providing more accurate professional guidance for clinical practice.
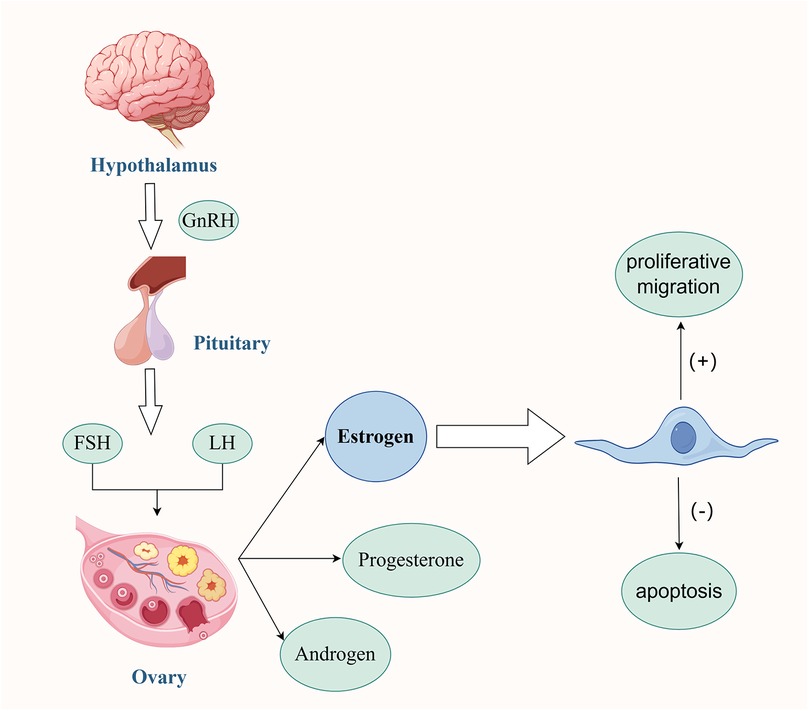
Figure 1. Interaction between MSCs and Estrogen. Under the regulatory mechanism of the human Hypothalamus-Pituitary-Ovary Gonadal Axis, the secreted Estrogen plays several key roles. On one hand, it can effectively promote the proliferation and migration of MSCs, enhancing their cell activity. On the other hand, it can also inhibit the apoptosis of MSCs themselves, maintaining the number of viable cells. Estrogen creates a microenvironment suitable for the survival of MSCs. In this environment, the two work together to promote the angiogenesis process in the damaged endometrial tissue. This angiogenesis effect further significantly improves the menstrual conditions of patients with moderate to severe IUA, contributing to the restoration of the normal physiological functions of the patients. Created with FigDraw.com.
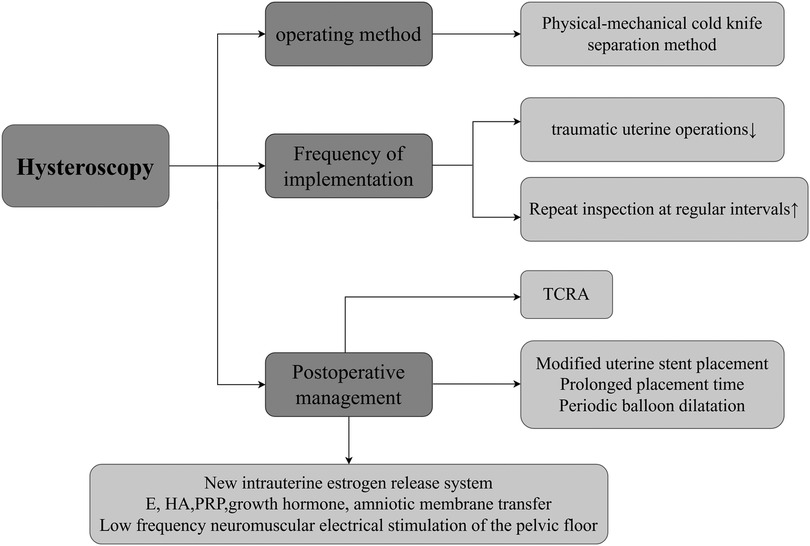
Figure 2. Approaches of hysteroscopic surgery to improve menstruation. This mainly involves the following three aspects: (1) Operation Method. At the level of the operation method, the physical-mechanical cold-knife separation method is the preferred choice. (2) Execution Frequency. Reducing the number of traumatic intrauterine operations during surgery and conducting regular hysteroscopic examinations after surgery jointly constitute a key link in ensuring the effectiveness of the surgery. (3) Postoperative Management, In terms of postoperative management, a modified uterine stent is adopted, and the placement time of the stent is appropriately extended to prevent intrauterine re-adhesion. Periodic balloon dilation is carried out to maintain the shape of the uterine cavity. Adjuvant artificial cycle therapy is used to regulate the endocrine level. Moreover, measures such as the application of Hyaluronic Acid, Platelet-Rich Plasma, pelvic floor low-frequency neuromuscular electrical stimulation, intrauterine perfusion of growth hormone, and amniotic membrane transplantation can promote the repair and regeneration of the endometrium from different perspectives. In patients with moderate to severe IUA, the comprehensive treatment approach centered around hysteroscopy is widely applied in clinical practice. Created with FigDraw.com.
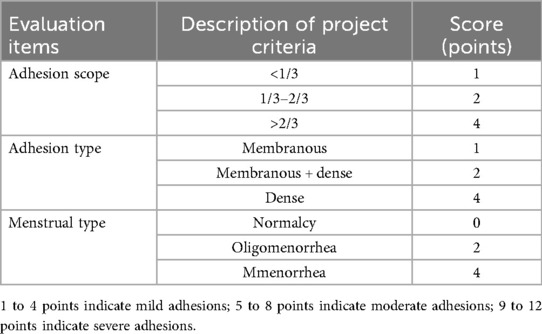
Table 1. Classification and scoring criteria for intrauterine adhesions by the American fertility society.
2 Müllerian duct development and Anti-Müllerian hormone (AMH) levels
The Müllerian duct is the simplest basic duct and the cornerstone of the female reproductive tract. This structure consists of an epithelial lumen and the surrounding mesenchymal layer (5). As the fundamental structure of the female reproductive tract, the development of the Müllerian duct is crucial for the formation of the female reproductive system. The uterine morphological and functional abnormalities associated with Müllerian duct anomalies (6) increase the susceptibility of the endometrium to trauma. During the post-trauma repair process, they are more likely to trigger abnormal extracellular matrix deposition and tissue fibrosis, thus significantly elevating the risk of IUA. AMH, also known as Müllerian inhibiting substance (7), plays a very important role in regulating cell differentiation and inhibiting the development of the Müllerian duct (8). In the bodies of adult females, Follicle-Stimulating Hormone can stimulate the expression of AMH. AMH exerts an inhibitory effect by counteracting follicle-stimulating hormone, thus promoting the gradual regression of the Müllerian duct (9). AMH assesses the ovarian reserve function through the quantity and quality of ovarian follicles in women. Its level is positively correlated with the ovarian reserve function (10) but negatively correlated with age (11, 12). Therefore, among patients with moderate to severe IUA, the improvement in menstruation in young women is generally better than that in middle-aged and elderly women. This may be closely related to the good development of the Müllerian duct and the relatively high level of AMH in young women.
3 The interrelationship between mesenchymal stem cells (MSCs) and the endocrine system
MSCs are a type of multipotent stem cells, renowned for their self-renewal and multilineage differentiation potential (13). MSCs have a rich variety of sources, mainly including umbilical cord mesenchymal stem cells, bone marrow mesenchymal stem cells, adipose tissue-derived stromal cells, human amniotic mesenchymal stem cells, placental mesenchymal stem cells, etc. These are all hot topics in research and application (13). Some studies have shown that MSCs can not only promote the repair and regeneration of the endometrium through paracrine action (14) but also increase the vitality of the damaged endometrium via exosomes (15, 16), inhibit and even reverse the process of endometrial fibrosis (17, 18). The specific mechanisms of action may involve multiple aspects such as promoting angiogenesis (19), regulating the inflammatory response (20), and providing nutritional support. These characteristics endow MSCs with unique advantages and potential in the restoration of menstruation in IUA patients. In addition, MSCs can maintain the homeostasis of the uterine internal environment. The balance between self-differentiation and self-renewal of stem cells is crucial for the cyclic regeneration of the endometrium (13). Under physiological conditions, the human body is precisely regulated by the Hypothalamic-Pituitary-Ovarian-Gonadal Axis. As a result, the endometrium undergoes cyclic exfoliation and repair, demonstrating a high regenerative potential. Among them, the level of Estrogen is one of the key factors promoting the repair and regeneration of the endometrium. Some studies have confirmed (21) that the estrogen secreted by MSCs plays a decisive role in therapeutic applications. Estrogen not only promotes the proliferation and migration of MSCs (22) but also inhibits their apoptosis, providing a suitable microenvironment for the survival of MSCs (13). This interaction synergistically promotes angiogenesis in damaged tissues, thereby facilitating the repair and regeneration of the endometrium (23, 24). The endometrium is divided into the superficial functional layer and the deep basal layer. The integrity and continuity of the basal layer are the keys to the cyclic repair and regeneration of the endometrium (25). Some studies have found (26) that when the deep basal layer of the endometrium suffers severe damage, the vast majority of the basal layer is often replaced by a single-layer epithelium and fibrous tissue. This leads to difficulty in the regeneration of the functional layer, a decline in the endometrium's self-repair ability, and the endometrium being in an abnormal state. In this case, even if the Estrogen level in the body is high, due to the reduction in the number of Estrogen receptors or the saturation of their functions, the response to Estrogen stimulation weakens, resulting in an unsatisfactory treatment effect (27). Therefore, for patients with moderate to severe IUA, an adequate Estrogen level and an endometrial basal layer that has not suffered severe damage are two indispensable conditions for achieving menstrual improvement (Figure 1) (28).
4 Impact of hysteroscopic surgery on endometrium and menstrual improvement
With the continuous development and increasing maturity of hysteroscopic technology, the comprehensive treatment method mainly based on hysteroscopy is being increasingly widely applied in patients with moderate to severe IUA (1). Therefore, the impact of hysteroscopic surgery on endometrial and menstrual improvement has become a hot topic in academic and clinical practice.
Hysteroscopic technology (3) is an innovative technique in the field of minimally invasive gynecological diagnosis and treatment (29), its core advantage is in using fiber-source endoscopes for precise inspection and treatment of the uterine cavity. The main operating mechanism of this technology is to insert an endoscope with a light source into the uterine cavity via the vagina and cervix, enabling direct and clear observation of multiple aspects such as the shape of the uterine cavity, the condition of the endometrium, the presence of endometrial polyps, the distribution of abnormal blood vessels, and the state of the bilateral fallopian tube openings (30). Meanwhile, surgical instruments can be introduced through their working channel to perform operations like tissue cutting, separation, and electrocoagulation for hemostasis to treat various intrauterine diseases. Additionally, specimens can be accurately collected under direct vision for pathological examination, providing a reliable basis for diagnosis and treatment (31). Given that the fiber-optic and lens design of the hysteroscope is extremely precise, the damage it causes to the endometrium is minimal, and theoretically, it should not significantly affect a woman's menstrual function. During Transcervical Resection of Adhesion (TCRA) performed under hysteroscopy, the mechanical cold-knife technique is often used to physically separate the adhesive tissue in patients with moderate to severe IUA. Although this process may cause a certain degree of damage to the endometrium, after a period of recovery following the surgery, the endometrial function can return to normal completely, and it will not have a long-term impact on menstrual improvement (32). One study mentioned (33) that it conducted a detailed analysis of the outcomes of hysteroscopic surgery in patients with different degrees of IUA. The results showed that 100% of patients with severe IUA experienced an increase in menstrual volume after hysteroscopic surgery (including a return to normal levels). This result may be closely related to multiple factors such as Surgical Technique, Follow-up Frequency, Barrier Methods, Hormone Therapy, Biological Adjuncts, Innovations (e.g., estrogen stents, HA, PRP, and smart medicine). The following will explore these influencing factors in depth (Figure 2).
4.1 Surgical technique
As a refined minimally invasive technique, TCRA surgery has been widely proven to be a safe, effective, scientific, and feasible primary method for improving the postoperative menstrual function of patients with moderate to severe IUA (34). TCRA enables precise diagnosis of the location, extent, nature, and severity of IUA under direct visualization (4). It then selectively separates adhesive bands and excises scar-like adhesive tissues.The aim is to protect the remaining endometrium and restore the anatomical shape of the uterine cavity. Relatively speaking, the surgical safety is high. TCRA significantly improves the menstrual conditions of IUA patients. On one hand, it can eliminate the occurrence of IUA. On the other hand, it can minimize damage to the endometrium, thus facilitating the restoration of the uterine shape and function to the optimal state (35). According to the different instruments used during the operation, TCRA can be divided into two types: mechanical surgery and energy-based surgery, that is, the traditional cold-knife separation method and the hot-knife separation method. At the histological level, cold knife surgery is based on purely mechanical sharp cutting without thermal effects, with clear sections and no coagulation necrosis at the edges, which facilitates pathological evaluation and maximizes the protection of the endometrial basal layer, thus reducing the inflammatory response of the body and promoting endometrial regeneration and uterine cavity morphology restoration (36). In contrast, energy devices, which use a “thermal spreading band” to synchronize coagulation, may involve adjacent tissues and increase the risk of delayed injury. Meta-analysis showed (37) that bipolar vessel sealers reduced blood loss and operative time in laparoscopic hepatectomies, but in hysteroscopy, cold knife microsurgery was still better than plasma electrosurgery for improving menstrual abnormalities. plasma electrosurgery (35). Therefore, to more effectively improve the menstrual conditions of patients with moderate to severe IUA, the physical and mechanical cold-knife separation method in TCRA surgery should be regarded as the preferred surgical approach. Its scientific nature, safety, and effectiveness have been widely recognized (38). However, in the future, there is still a need for local spraying or infiltration of hemostatic agents in the operative field, combined with microenergetic punctual coagulation and real-time visualization and navigation, which can significantly reduce bleeding and shorten the operation time of cold knife surgery without introducing obvious thermal damage, to better applying cold knife surgery to a wide range of clinical work.
4.2 Follow-up frequency
In the field of hysteroscopic surgery, existing research has pointed out (39) that an increase in the frequency of traumatic intrauterine operations during surgery, especially multiple repeated operations in a short period, may significantly increase the risk of postoperative infection (39). Based on this, it is of particular importance to minimize the number of traumatic intrauterine procedures during surgery and to actively adopt preventive measures after surgery to reduce the risk of infection. In the research of Yang Jianghua and other scholars (40), by strengthening hysteroscopic reexamination every month after surgery and combining with the comprehensive preventive treatment of injecting sodium hyaluronate gel, estradiol valerate gel, and periodic progesterone into the uterine cavity, significant clinical effects have been demonstrated. On one hand, this research achieves local hemostasis by inhibiting the activation and aggregation of inflammatory cells, thus reducing the inflammatory exudation of the wound surface. On the other hand, it can significantly suppress the generation of fibroblasts and reduce the hyperplasia of collagen fibers, thereby reducing the formation of surgical scars. This can effectively prevent re-adhesion in IUA patients, thereby shortening the recovery time of the endometrium. The research results indicate (40) that this comprehensive treatment strategy has enabled the menstrual improvement rate of patients with moderate to severe IUA to reach 94.3% after hysteroscopic surgery. This finding shows a statistically significant difference, providing a new perspective and method in the field of intrauterine adhesion treatment. In addition, Wang X, Duan H (41) also reported a remarkable finding in their research: the cure rate of TCRA secondary treatment is as high as 100%. This result may suggest that, for all the patients involved in the study, after the second-round treatment, the intrauterine adhesions were completely resolved, their menstrual functions returned to normal, and no recurrence of adhesions was observed. In short, for patients with moderate to severe IUA undergoing hysteroscopic surgery, minimizing traumatic intrauterine procedures during the operation and conducting regular hysteroscopic examinations after the surgery are the key factors in ensuring surgical effectiveness and patient recovery.
4.3 Barrier methods
Precise hysteroscopic placement of modified uterine stents has been confirmed by several studies to synchronize postoperative menstrual flow and endometrial thickness (42), initially establishing its central position in the functional reconstruction of moderate-to-severe IUA. On this basis, moderately prolonged balloon stent retention or cyclic balloon dilatation (43, 44) can continue to isolate the trauma and stabilize the uterine cavity morphology in the critical postoperative window, thereby depressing the recurrence rate of IUA in the long term and consistently amplifying menstrual benefit (45), providing a direct and quantifiable evidence-based barrier strategy for moderate-to-severe IUA.
4.4 Hormone therapy
Artificial cycles constructed with high-dose transdermal estradiol gel (6 mg/d) (46) in combination with vaginal estradiol (47) and high-dose transdermal estradiol gel (6 mg/d) in combination with vaginal estrogens can safely and effectively remodel the endothelium after moderate-to-severe IUA: the improvement rate of menstruation was 67% (46), and was comparable to that of the oral formulation. Transdermal and transvaginal administration bypasses the first-pass elimination effect in the liver and has a lower risk of thrombosis, providing a highly effective and safe exogenous estrogen regimen for postoperative endothelial repair.
4.5 Biological adjunct
In patients with moderate-to-severe IUA who had failed previous hysteroscopy, amniotic membrane transplantation (48) immediately after microscopic separation induced crawling regeneration of the basement membrane and rapid reconstruction of the functional layer; G-CSF intrauterine perfusion was initiated within the seventh postoperative day, and the sequential regimen of combined growth hormone and growth factor could synchronize the promotion of neovascularization, cell proliferation, and the inhibition of apoptosis (49). Multicenter data showed (49) that this strategy can thicken the endothelium by an average of 2.36 mm, significantly reduce intraoperative bleeding and shorten the menstrual cycle, and provide a highly efficient and reproducible sequential pathway for functional reconstruction.
4.6 Innovations (e.g., estrogen stents, HA, PRP, and smart medicine)
Multicenter RCT demonstrated (50) that immediate postoperative insertion of the new intrauterine extended-release estrogen system (IERS) could reduce the recurrence rate of moderate-to-severe IUA to <10%, which was significantly better than that of the traditional balloon, while the local steady release of E₂ improved the menstrual flow score by ≥2 points in 72% of the patients in 3 months, realizing the dual benefit of “mechanical barrier + high estrogen concentration”.Sodium hyaluronate gel (Hyaluronic Acid, HA) (51)forms a biodegradable protective layer on the trauma and reduces bleeding, exudation, and inflammation, thus systematically decreasing the incidence and severity of adhesions (52). Intrauterine injections of platelet-rich plasma (PRP) (53–55) improve the prognosis of fertility by concentrating growth factors and anti-inflammatory factors, synergizing with hormones and physical barriers to rapidly rebuild the basal layer and inhibit fibrosis. In addition, low-frequency neuromuscular stimulation of the pelvic floor, as an intelligent means of rehabilitation, further promotes local vascularization and enhances the rate of menstrual recovery (56). Although there is a consensus that the more severe the IUA, the worse the prognosis, multimodal combined interventions are gradually reversing this dilemma (39). Therefore, 7 d postoperative balloon or 1–2 months IUD with mechanical barrier rapidly depresses the AFS score, the balloon is slightly better in reconstructing the uterine cavity morphology, and the IUD is additional anti-inflammatory with copper ions; on top of this (42–45), the self-crosslinked HA gel resides in semi-solid state for 7–14 d, which is confirmed by Meta-analysis to reduce the rate of moderate-to-severe adhesion recurrence and to achieve zero adverse effects (51, 52). The synergistic effect of PRP intrauterine administration further amplified the pro-restorative effects of HA and estrogen scaffolds, and microscopic endothelial injections reduced AFS scores and improved menstrual flow rates compared with perfusion alone (53–55). Taking into account the available evidence, the combination of “mechanical barrier + HA + PRP” can be recommended as the first-line optimization strategy after moderate-to-severe IUA.
5 Psychological factors
Psychological factors play a pivotal role in the improvement of menstrual function in patients with moderate to severe IUA. Due to the long treatment cycles, complex treatment procedures, uncertain treatment efficacy, heavy economic burden, and high recurrence rate of adhesions that IUA patients face, these patients often experience negative emotions such as anxiety and depression (57). Therefore, it is particularly important to develop and implement targeted psychological intervention measures to alleviate patients' psychological burden and improve their quality of life (58). Research shows (57) that a harmonious partner relationship can encourage both spouses to participate in and cooperate during the treatment of IUA, which can effectively relieve the patient's psychological stress. A study by Yuqing Chen, Huan Yang, et al. has found (59) that negative emotions may interfere with sex hormone levels, affect the repair of the endometrium, and thus influence the improvement of menstruation. Mindfulness-Based Stress Reduction, as a method of psychological self-regulation training, has been proven to significantly improve negative emotions such as anxiety and depression in IUA patients during treatment by enhancing the state of mindfulness and adjusting the cognitive level, thus improving their quality of life. Si Jingge and Wang Sha also pointed out that disease-specific follow-up can provide effective guidance and supervision for IUA patients, especially in terms of postoperative adjuvant drug therapy. It can enhance patients' awareness and attention to the disease, avoid unfavorable factors, and standardize subsequent treatment plans. As a result, patients with IUA who receive disease-specific follow-up tend to experience a shorter time to menstrual resumption, a longer menstrual period, and better improvement in menstrual flow (60). In conclusion, psychological factors play a crucial role in the diagnosis and treatment of moderate to severe IUA. The emotional changes in patients are not only triggered by the occurrence of IUA but also, in turn, affect the treatment outcome of IUA (61).
6 Conclusions
In summary, in the research of the past decade, significant progress has been made in studies on improving menstruation in patients with moderate to severe IUA. This progress is not limited to the common clinical manifestations of IUA patients in traditional cognition, such as reduced menstruation or amenorrhea. The current research horizons are constantly expanding, covering but not limited to the following key areas: exploring the association between Müllerian duct development and AMH levels, the interaction and dynamic changes between MSCs and the endocrine system, and the impact of hysteroscopic surgery on the improvement of the endometrium and menstruation, and the crucial role of psychological factors in the treatment process. These multi-dimensional influencing factors jointly act on the process of improving the menstrual function of patients with moderate to severe IUA. Thus, in young patients with moderate-to-severe IUA, good restoration of menstrual patterns is generally achieved, even if the surgical and subsequent intervention regimen tends to be simplified, because of the maturation of the mullerian ducts, the relatively high levels of AMH and E, and the limited damage to the basal layer of the endothelium. The influencing factors for improving menstruation in patients with moderate to severe IUA involve multiple fields such as biology, endocrinology, and psychology. Considering these factors comprehensively and formulating individualized treatment plans are of great clinical significance in many aspects, including improving treatment efficacy, reducing the risk of re-adhesion, optimizing treatment regimens, enhancing the quality of life of patients, and preventing infertility and miscarriage. However, it cannot be ignored that the research on the influencing factors of menstrual improvement in patients with moderate to severe IUA still faces many challenges. At present, a clear theoretical framework has not been formed regarding the specific directions for improving menstrual function, which restricts the prediction and optimization of treatment effects. Additionally, there is a lack of adequate literature and data to support research on the effects of Müllerian duct development and AMH content on the improvement of menstruation, indicating that further research is needed to fill this knowledge gap. Thus, for patients with moderate to severe IUA, the influencing factors of menstrual improvement are multifaceted and interrelated. Future research needs to further explore the interactions among these factors and how to optimize treatment plans to improve treatment outcomes. At the same time, focusing on the development of individualized treatment strategies is also a key direction for enhancing treatment effectiveness.
Author contributions
XZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IUA, intrauterine adhesions; AMH, anti-müllerian hormone; MSCs, mesenchymal stem cells; TCRA, transcervical resection of adhesion.
References
1. Zhao G, Hu Y, editors. Mechanistic Insights into Intrauterine Adhesions. Seminars in Immunopathology. Berlin, Heidelberg: Springer (2025).
2. Jing Jianfa YH, Xingping Z, Shi T, Dabao X. You zhaoling Chinese expert consensus on integrated diagnosis and treatment of intrauterine adhesions (2024 edition). Chin J Pract Gynecol Obstet. (2024) 40(08):819–25. doi: 10.19538/j.fk2024080111
3. Berman JM, editor. Intrauterine Adhesions. Seminars in Reproductive Medicine. New York City: © Thieme Medical Publishers (2008).
4. Brown J. AAGL Advancing minimally invasive gynecology worldwide: statement to the FDA on power morcellation. J Minim Invasive Gynecol. (2014) 21(6):970–1. doi: 10.1016/j.jmig.2014.08.780
5. Santana Gonzalez L, Rota IA, Artibani M, Morotti M, Hu Z, Wietek N, et al. Mechanistic drivers of müllerian duct development and differentiation into the oviduct. Front Cell Dev Biol. (2021) 9:605301. doi: 10.3389/fcell.2021.605301
6. Tan Jiahong FY. Analysis of risk factors for intrauterine adhesion and prevention. J Pract Obstet Gynecol. (2024) 40(04):241–3.
7. Cate RL. Anti-Müllerian hormone signal transduction involved in Müllerian duct regression. Front Endocrinol. (2022) 13:905324. doi: 10.3389/fendo.2022.905324
8. Russell N, Gilmore A, Roudebush WE. Clinical utilities of anti-Müllerian hormone. J Clin Med. (2022) 11(23):7209. doi: 10.3390/jcm11237209
9. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. (2016) 22(6):709–24. doi: 10.1093/humupd/dmw027
10. Şükür YE, Aslan B, Kaplan NB, Doğru M, Özmen B, Sönmezer M, et al. Inter-cycle variability of anti-M ü llerian hormone: implications for predicting controlled ovarian stimulation cycle outcomes. J Ovarian Res. (2024) 17(1):209. doi: 10.1186/s13048-024-01517-x
11. Hochberg A, Esteves SC, Yarali H, Vuong LN, Dahan MH. Antimüllerian hormone and antral follicle count thresholds for hyperresponse risk assessment in in vitro fertilization: a hyperresponse risk assessment consensus study. Fertil Steril. (2024) 123(5):827–37. doi: 10.1016/j.fertnstert.2024.11.021
12. Huang Y, Kuang X, Jiangzhou H, Li M, Yang D, Lai D. Using anti-müllerian hormone to predict premature ovarian insufficiency: a retrospective cross-sectional study. Front Endocrinol. (2024) 15:1454802. doi: 10.3389/fendo.2024.1454802
13. Siwen Z, Houmei W, Mingyue X. Research progress on mesenchymal stem cell combined with estrogen for the treatment of intrauterine adhesion. Guangxi Med J. (2023) 45(07):837–42.
14. Huang X, Yang X, Huang J, Wei L, Mao Y, Li C, et al. Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation. Cell Commun Signal. (2024) 22(1):1–13. doi: 10.1186/s12964-024-01656-0
15. Li L, An J, Wang Y, Liu L, Wang Y, Zhang X, et al. Exosomes derived from mesenchymal stem cells increase the viability of damaged endometrial cells via the miR-99b-5p/PCSK9 axis. Stem Cells Dev. (2024) 33(11–12):290–305. doi: 10.1089/scd.2023.0259
16. Wang S, Liu T, Nan N, Lu C, Liang M, Wang S, et al. Exosomes from human umbilical cord mesenchymal stem cells facilitates injured endometrial restoring in early repair period through miR-202-3p mediating formation of ECM. Stem Cell Rev Rep. (2023) 19(6):1954–64. doi: 10.1007/s12015-023-10549-7
17. Chen J-M, Huang Q-Y, Chen W-H, Wu J-X, Zheng L-T, You H-J, et al. Transcriptomics of tissue exosomes to investigate miR-195-5p’s amelioration of endometrial fibrosis via the YAP-Smad7 pathway: an animal study. J Transl Med. (2024) 22(1):1–21. doi: 10.1186/s12967-024-05871-8
18. Song M, Ma L, Zhu Y, Gao H, Hu R. Umbilical cord mesenchymal stem cell-derived exosomes inhibits fibrosis in human endometrial stromal cells via miR-140-3p/FOXP1/Smad axis. Sci Rep. (2024) 14(1):8321. doi: 10.1038/s41598-024-59093-5
19. Yang Y, Wang Y, Huang Y, Song J, Ma X. Interceed combined with bone marrow mesenchymal stem cells improves endometrial receptivity of intrauterine adhesion. Regen Ther. (2024) 27:445–54. doi: 10.1016/j.reth.2024.04.007
20. Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. (2022) 12(1):2776. doi: 10.1038/s41598-022-06824-1
21. Li J, Peng X, Zeng X, Liu B, Hao Q, Yu X, et al. Estrogen secreted by mesenchymal stem cells necessarily determines their feasibility of therapeutical application. Sci Rep. (2015) 5(1):15286. doi: 10.1038/srep15286
22. Wang Q, Xu C, Zhao Y, Xu Z, Zhang Y, Jiang J, et al. miR-26b-3p regulates human umbilical cord-derived mesenchymal stem cell proliferation by targeting estrogen receptor. Stem Cells Dev. (2016) 25(5):415–26. doi: 10.1089/scd.2015.0267
23. Liu H-y, Zhu Z-y, Chen X-m, Lu J-q, Song Y, Xia W. A review of the effects of estrogen and epithelial-mesenchymal transformation on intrauterine adhesion and endometriosis. Transpl Immunol. (2023) 79:101679. doi: 10.1016/j.trim.2022.101679
24. Yuan L, Cao J, Hu M, Xu D, Li Y, Zhao S, et al. Bone marrow mesenchymal stem cells combined with estrogen synergistically promote endometrial regeneration and reverse EMT via Wnt/β -catenin signaling pathway. Reprod Biol Endocrinol. (2022) 20(1):121. doi: 10.1186/s12958-022-00988-1
25. Hooker AB, de Leeuw RA, Emanuel MH, Mijatovic V, Brolmann HA, Huirne JA, et al. The link between intrauterine adhesions and impaired reproductive performance: a systematic review of the literature. BMC Pregnancy Childbirth. (2022) 22(1):837. doi: 10.1186/s12884-022-05164-2
26. Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. (2014) 125(2):121–4. doi: 10.1016/j.ijgo.2013.10.026
27. Wanlin Z, Daner Q, Ruonan T, Reproductive Medical Center DoOaG, Tangdu Hospital, The Second Affiliated Hospital of Air Force Medical University. Correlation analysis between preoperative Serum estradiol levels and postoperative pregnancy outcomes in patients with moderate-to-severe intrauterine adhesion. J Pract Obstet Gynecol. (2024) 40(07):586–90.
28. Gianfaldoni A, Roa C, dos Santos Simões R, Baracat MCP, Maggio da Fonseca A, Bagnoli VR, et al. Association of intrauterine synechiae with pituitary gonadotrophin pulse patterns: a pilot study. PLoS One. (2023) 18(12):e0289075. doi: 10.1371/journal.pone.0289075
29. Gupta N, Gupta A. Complications during hysteroscopy for gynecological procedures: prevention is better than cure!. Korean J Anesthesiol. (2020) 73(1):79–80. doi: 10.4097/kja.19339
30. ACOG Technology Assessment No. 13 Summary: hysteroscopy. J Obstet Gynecol. (2018) 131(5):952–3. doi: 10.1097/AOG.000000000000002629
31. Ignaszak-Kaus N, Chmaj-Wierzchowska K, Nowak A, Wszołek K, Wilczak MJC, Obstetrics E, et al. An overview of outpatient hysteroscopy. Clin Exp Obstet Gynecol. (2022) 49(8):181. doi: 10.31083/j.ceog4908181
32. Qiao X, Liu D, Liu C, Pei T, Ouyang YJ. Reproductive outcomes after hysteroscopic adhesiolysis in patients experiencing recurrent pregnancy loss and intrauterine adhesions. J Minim Invasive Gynecol. (2025) 32(1):57–63. doi: 10.1016/j.jmig.2024.09.009
33. Yiping Z, Bei L, Lingfei H, Shanghai First Maternity and Infant Hospital TUSoM. Analysis of the outcomes of patients with different degrees of intrauterine adhesion after hysteroscopy. Acta Medicin Univ Sci Technol Huazhong. (2016) 45(05):551–4.
34. He M, Chen Q, He J, Zhao Q, Jiang H, Xia YJM. Reproductive outcomes of women with moderate to severe intrauterine adhesions after transcervical resection of adhesion: a systematic review and meta-analysis. Medicine. (2023) 102(11):e33258. doi: 10.1097/MD.0000000000033258
35. Lin L. Comparison of hysteroscopic plasma resection and hysteroscopic cold knife micro—shearing in treatment of intrauterine adhesions. Zhejiang Med J. (2023) 45(05):504–7+13.
36. Jin X, Ye J, Zhang L, Chen L. Efficacy of hysteroscopic cold knife separation on intrauterine adhesions. Am J Transl Res. (2021) 13(7):8351.34377327
37. Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms–a meta-analysis. Surgery. (2007) 141(2):203–11. doi: 10.1016/j.surg.2006.06.035
38. Yang J, Pan J, Chen Y, Chen C, Huang Z. Advancing intrauterine adhesion severity prediction: integrative machine learning approach with hysteroscopic cold knife system, clinical characteristics and hematological parameters. Comput Biol Med. (2024) 177:1085. doi: 10.1016/j.compbiomed.2024.108599
39. Zhang Hui-ling CJ-Y, Huang H-y. Comparison of hysteroscopic adhesions separation effects on recurrence and pregnancy outcome in patients with different degrees of intrauterine adhesion. Biomed Eng Clin. (2020) 24(03):302–5. doi: 10.13339/j.cnki.sglc.20200415.003
40. Fan Y. Effect comparison of two different treatment methods on the prevention of re-adhesion after treatment of hysteroscopy. Chin J Hum Sex. (2016) 25(12):53–6. doi: 10.3969/j.issn.1672-1993.2016.12.017
41. Wang X, Duan H. Clinical evaluation of amniontic products after transcervical resection of intensive degree of intrauterine adhesions. Zhonghua Fu Chan Ke Za Zhi. (2016) 51(1):27–30. doi: 10.3760/cma.j.issn.0529-567X.2016.01.007
42. Cao C, Chen Y, Li J, Xu Q, Liu X, Zhao R, et al. Short-term reproductive outcomes analysis and prediction of the modified uterine stent treatment for mild to moderate intrauterine adhesions: experience at a single institution. BMC Womens Health. (2024) 24(1):252. doi: 10.1186/s12905-024-03098-9
43. Ding H, Zhang H, Qiao R, Sun N, Ji Y, Pang W, et al. Comparing the efficacy and pregnancy. Outcome of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis in infertile women: a prospective, randomized, controlled trial study. Reprod Biol Endocrinol. (2024) 22(1):49. doi: 10.1186/s12958-024-01222-w
44. Luo Y, Liu Y, Xie W, Guo Y, Xiao Y. Extended balloon stent placement for reducing intrauterine adhesion recurrence: a retrospective cohort study. Reprod Biomed Online. (2024) 49(2):103947. doi: 10.1016/j.rbmo.2024.103947
45. Hu Y, Ma Y, Li W, Qu J. Long-term effects of hysteroscopic adhesiolysis on postoperative pregnancy rates and fertility outcomes in patients with intrauterine adhesions. Am J Transl Res. (2024) 16(10):5605. doi: 10.62347/GRAK9062
46. Yi T, Zhang X, Gupta V, Li L, Zhong Q. Transdermal estrogen gel vs oral estrogen after hysteroscopy for intrauterine adhesion separation: a prospective randomized study. Front Endocrinol. (2023) 14:1066210. doi: 10.3389/fendo.2023.1066210
47. Page K, Guibert J, Weitzen S, Davy C, Fauque P, Olivennes FJF, et al. Administering estradiol vaginally in preparatory artificial cycles for frozen/thawed embryo transfer is advantageous in priming the endometrium for implantation in patients with thin endometria. Fertil Steril. (2005) 84:S181. doi: 10.1016/j.fertnstert.2005.07.449
48. Chung RK, Zhang S, Findley J, Liu J. Amniograft placement at the time of hysteroscopy for intrauterine adhesions (IUA) may improve pregnancy rate (PR) and live birth rate (LBR) in severe cases with history of failed hysteroscopy. Fertil Steril. (2023) 120(4):e91. doi: 10.1016/j.fertnstert.2023.08.753
49. Zhang Y, Chen X, Chen S, Wei C, Li B, Wang Z, et al. Intrauterine administration of G-CSF for promoting endometrial growth after hysteroscopic adhesiolysis: a randomized controlled trial. Hum Reprod. (2022) 37(4):725–33. doi: 10.1093/humrep/deac023
50. Feng L, Sun Y, Zhang S, Qian Y, Fang S, Yang B, et al. A novel intrauterine estrogen-releasing system for preventing the postoperative recurrence of intrauterine adhesion: a multicenter randomized controlled study. BMC Med. (2024) 22(1):395. doi: 10.1186/s12916-024-03608-4
51. Luo Y, Sun Y, Huang B, Chen J, Xu B, Li H, et al. Effects and safety of hyaluronic acid gel on intrauterine adhesion and fertility after intrauterine surgery: a systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Am J Obstet Gynecol. (2024) 231(1):36–50.35. doi: 10.1016/j.ajog.2023.12.039
52. Liu H, Xu Y, Yi N, Yi W. Efficacy and safety of hyaluronic acid gel for the prevention of intrauterine adhesion: a meta-analysis of randomized clinical trials. Gynecol Obstet Invest. (2018) 83:227–33. doi: 10.1159/000486674
53. Wang G, Zhu Y, Duan N, Guo C, Liu N, Huang H. Does platelet-rich plasma improve adhesion recurrence and pregnancy outcomes in women with intrauterine adhesions? A systematic review and meta-analysis. J Minim Invasive Gynecol. (2024) 2(2):133–142.e7. doi: 10.1016/j.jmig.2024.10.013
54. Li Y, Han Y, Su X, Cao J, Liu J, Zhang W. Application of autologous platelet-rich gel formed by calcium gluconate combined with hormone therapy for endometrial repair after hysteroscopic transcervical resection of adhesion surgery and successful pregnancy: case report and literature review. Front Med. (2024) 11:1436089. doi: 10.3389/fmed.2024.1436089
55. Tang R, Xiao X, He Y, Qiu D, Zhang W, Wang X. Clinical evaluation of autologous platelet-rich plasma therapy for intrauterine adhesions: a systematic review and meta-analysis. Front Endocrinol. (2023) 14:1183209. doi: 10.3389/fendo.2023.1183209
56. Liu XD, Feng JL, Wang JM, Yang GX, Chen YQ. Effect of Pelvic Floor Neuromuscular Electrical Stimulation on Endometrial Repair after the Surgery of Moderate to Severe Intrauterine Adhesion. J Sun Yat-Sen.Univ (Med Sci). (2022) 43(01):140–5. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).20211012.001
57. Reisi M, Kazemi A, Maleki S, Sohrabi Z. Relationships between couple collaboration, well-being, and psychological health of infertile couples undergoing assisted reproductive treatment. Reprod Health. (2024) 21(1):119. doi: 10.1186/s12978-024-01857-3
58. Renzi A, Fedele F, Di Trani M, editors. Assisted Reproductive Treatments, Quality of Life, and Alexithymia in Couples. Healthcare. Basel: MDPI (2023).
59. Chen Y, Yang H, Liu L, Fang R. Effects of mindfulness-based stress reduction on the anxiety, depression and quality of life of patients with intrauterine adhesion: a randomized controlled trial. Int J Clin Exp Med. (2017) 10(2):2296–305.
60. Si Jingge WS, Xin Q. Effect of special disease follow-up on postoperative pregnancy inpatients with moderate to severe intrauterine adhesions. Beijing Med J. (2019) 41(03):239–41. doi: 10.15932/j.0253-9713.2019.03.021
Keywords: intrauterine adhesions(IUA), menstrual improvement, hysteroscope, mental health, review, women
Citation: Zhang X-Y (2025) New potential in the treatment of moderate to severe intrauterine adhesions: influencing factors for menstrual improvement. Front. Reprod. Health 7:1608143. doi: 10.3389/frph.2025.1608143
Received: 8 April 2025; Accepted: 18 August 2025;
Published: 8 September 2025.
Edited by:
Vinay Shukla, University of Maryland, United StatesReviewed by:
Richa Dwivedi, Meharry Medical College, United StatesMarcel Vasconcellos, Federal University of Rio de Janeiro, Brazil
Copyright: © 2025 Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Yue Zhang, eGlueXVlemhhbmcwMTI5QG91dGxvb2suY29t
 Xin-Yue Zhang
Xin-Yue Zhang