- 1Division of RCN, Indian Council of Medical Research, New Delhi, India
- 2Sir Ganga Ram Hospital, New Delhi, India
- 3Sher-i-Kashmir Institute of Medical Sciences, SKIMS, Srinagar, Jammu & Kashmir, India
- 4Government Medical College, Thiruvananthapuram, Kerala, India
Background: Polycystic Ovary Syndrome is an endocrine disorder that affects reproductive, metabolic, and mental health. In LMICs, PCOS management is hindered by late diagnosis, lack of awareness, and high treatment costs which leads to long-term complications.
Objective: The aim of the review is to document the challenges in PCOS diagnosis and management in LMICs and provide public health solution to overcome these barriers in accordance with SDG goals.
Methods: A narrative review synthesizing existing literature on PCOS epidemiology, barriers to diagnosis and treatment, and potential solutions relevant to LMICs.
Results: Key challenges include lack of uniformity in diagnosis and treatment, lack of trained HR and equipment. High cost of care, stigma and fragmented health care.
Outcomes/proposed solutions: Develop national PCOS guidelines, bring the management of PCOS under the reproductive health program, shift some of the tasks to primary health workers, like generating awareness and screening for symptoms. Invest in research to find public health solutions.
Conclusion: Addressing PCOS in LMICs requires a multi-sectoral public health approach, including prevention, early detection, and affordable care. Strengthening healthcare systems through policy reforms and community-based interventions can improve outcomes for affected women.
1 Background
Polycystic Ovary Syndrome (PCOS) is one of the most common disorders among reproductive-age women worldwide. The Rotterdam criteria defines it as a combination of features like hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology (1). PCOS is characterized by a heterogeneous range of clinical features, including oligomenorrhea, anovulation and infertility on the one end, and metabolic disorders such as hyperinsulinemia, insulin resistance and obesity on the other (2). These symptoms often overlap with other health conditions, complicating the diagnosis and management of the disorder. Globally, the prevalence of PCOS is estimated to range between 6% and 20%, depending on the diagnostic criteria and population studied. In India, however, the prevalence is alarmingly high, with reports suggesting rates as high as 36% among women of reproductive age (2). This disparity is often attributed to rapid urbanization, increasing rates of obesity, and lifestyle changes among Indian women. The higher prevalence of PCOS in urban settings compared to rural areas further underscores the role of environmental and lifestyle factors in its pathogenesis (3). The impact of PCOS extends beyond reproductive health, significantly affecting metabolic and psychological well-being. Women with PCOS are at a higher risk of developing cardiovascular diseases and non-alcoholic fatty liver disease as well (4). In addition, the psychological burden of PCOS, including increased rates of anxiety, depression, and body image issues, often remains underdiagnosed and untreated (5). These complications highlight the need for holistic approaches to the diagnosis and management of PCOS, particularly in low-resource settings where healthcare systems are already overburdened.
The complexities involved in the diagnosis and management of PCOS are more pronounced in low—resource settings than in developed ones. Low and middle income countries (LMICs) are part of a classification system developed by World bank that classifies based on gross national income capita, where healthcare resources are limited (6). These countries have issues like poor developed healthcare, insufficiently trained medical personal, higher costs of tests and drugs because of reduced availability (7). These include problems like lack of recognition of the condition by women and health workers as well as the socio-economic strains and the underdeveloped health care system (8). It is common to see a late diagnosis of PCOS since many women go to the doctor only when they have infertility or other advanced complications of PCOS (9). A study in Bangladesh reiterates the problem of delayed diagnosis in PCOS lack of awareness about PCOS among women (10). Health disparities between urban and rural areas also cause the problem to become more complex. While metropolis may offer women professionals and advanced testing equipment, rural women depend on primary healthcare providers, who may not have the necessary training or resources to diagnose and treat PCOS effectively (9). A study from Nigeria discusses issues with awareness, and poor health infrastructure affecting PCOS care (11). This disparity is further exacerbated by social taboos on discussions concerning menstrual and reproductive health, and thus, the society hinders the opportunity for quick medical assistance. One of the socioeconomic aspects also in fact that the cost involved in acquiring these diagnostic tests is one of the major barriers to accessing medical care. The costs of diagnostic tests like hormone assays and ultrasound imaging that are done on the ovary, and which are not covered by insurance, make the tests unaffordable to many in low-income situations (12–14). Lack of integrated care also affects PCOS management, due to which only selective management of PCOS manifestations gets treated due to their obvious presentation, while long term complications get neglected (13, 15, 16).
Efforts to address PCOS in these settings require a comprehensive approach that includes preventive measures, early diagnosis, and community-based interventions (1, 8). Community-based healthcare models have shown promise in bridging the gap between urban and rural healthcare disparities (17). Telemedicine platforms also offer a viable solution for connecting women in remote areas with specialists, reducing the need for travel and associated costs (18). The 2015 Sustainable development goals (SDG) 3, 5, 10 talk about achieving health for all, gender equality and reducing barriers to health across for all groups by 2030, PCOS being an illness that disproportionately affects a woman of all ages across the world, needs to be tackled head on to achieve those targets (7, 8). This review tries to bridge the gaps of clinician led approach to tacking this illness by suggesting public health and policy solutions by highlighting varies models and approaches that have been used across the globe. The aim of the review is to document the challenges in PCOS diagnosis and management in LMICs and provide public health solution to overcome these barriers in accordance with SDG goals.
2 Challenges to PCOS management in LMIC
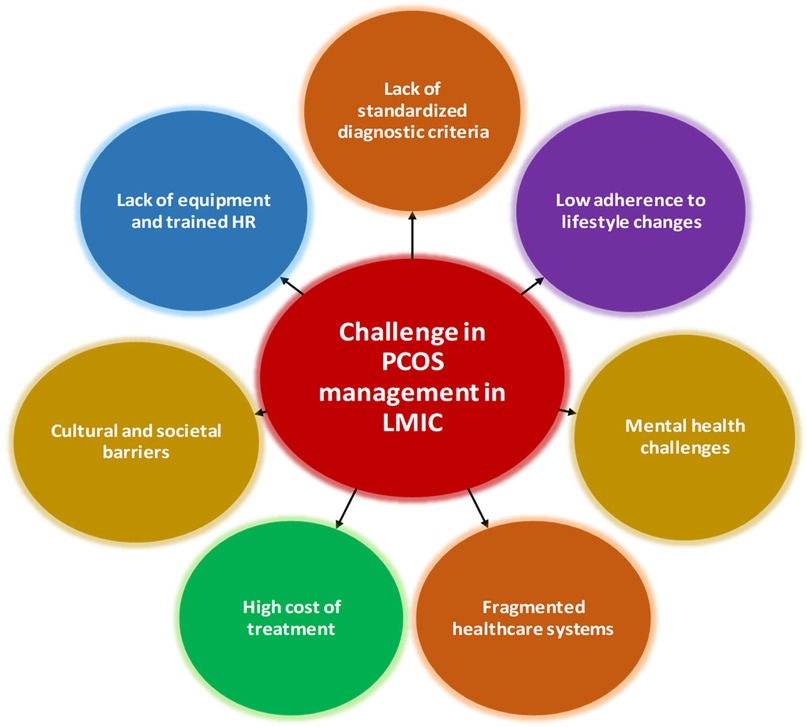
This section describes the various bottle necks that prevent PCOS women of LMICs from getting holistic care, which includes challenges in diagnosis and treatment (Figure 1). Addressing these challenges requires a multifaceted approach. Standardizing diagnostic criteria, improving access to diagnostic tools, training healthcare providers, and integrating multidisciplinary care are critical steps (19, 20).
2.1 Challenges in diagnosis
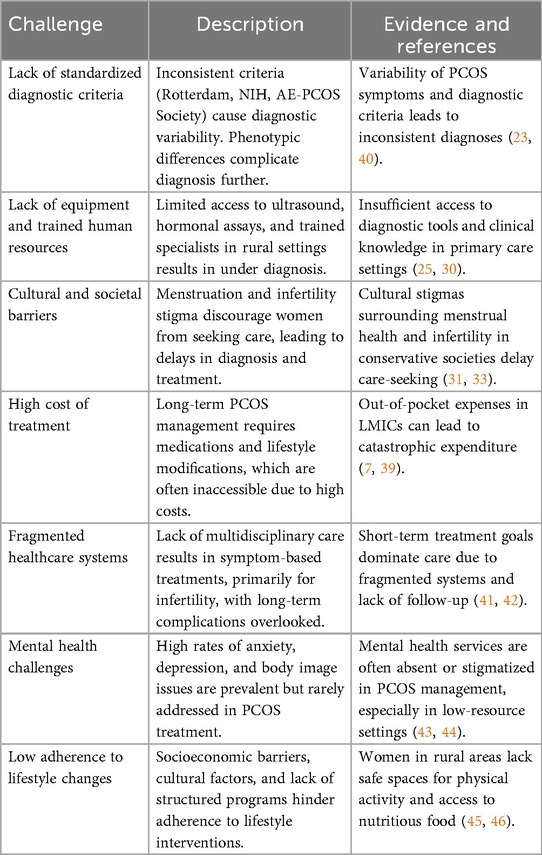
2.1.1 Lack of standardised diagnostic criteria
The absence of uniform diagnostic criteria for PCOS the world is one of the barriers for PCOS diagnosis. The 3 most common guidelines are the 2003 Rotterdam Consensus and the criteria of the National Institutes of Health (NIH) and the Androgen Excess and PCOS Society (AE-PCOS) (21).
Rotterdam criteria: Claims that two of the following three features should be hyperandrogenism (HA), oligo/anovulation (OA), and polycystic ovarian morphology (PCOM) on ultrasound for a woman to full fill the PCOS classification, while the absence of the remaining feature is being compensated by two other features (22).
NIH: Specifies that the diagnosis should include hyperandrogenism and oligo/anovulation but exclude the ultrasound findings (23).
AE-PCOS Criteria: States that women should have hyperandrogenism and at least one other feature to be diagnosed with PCOS (24).
Due to the variability of the disease, the symptoms of PCOS it is often a matter of controversy among medical practitioners and sometimes the diagnosis is inconsistent especially in primary care settings. Women are often treated without adequate diagnosis which leads to inappropriate treatment. Furthermore, the condition can worsen (1).
The diagnosis of PCOS is further complicated by varying phenotypic presentations such as phenotype-A which includes all the three criteria for diagnosis, phenotype-B includes hyper androgenic and oligo anovulatory features (HA + OA), phenotype-C has features of hyper androgenism and polycystic ovaries (HA + PCOM), and phenotype-D (OD + PCOM). Phenotype-A is more common in subjects identified in clinical populations, whereas phenotype-C is more common in unselected populations (22, 25).
In low-resource settings, a simpler diagnostic method based on clinical features like oligomenorrhoea and hirsutism might be a practical alternative to the full Rotterdam criteria. This is particularly true when hormonal tests or pelvic ultrasounds are not possible. These models have been suggested as useful tools for early detection and referral from primary care (24, 26).
2.1.2 Lack of equipment and trained human resources (HR)
In rural and poor settings, access to diagnostic tools such as high-resolution ultrasound and hormonal assays is limited and knowledge on how to diagnose and screen this disorder is limited (27, 28). Polycystic ovarian morphology, a key diagnostic criterion in the Rotterdam guidelines, cannot be assessed without ultrasound imaging, trained health care provides who can perform ultrasounds are limited in primary care set up. Hormonal assays for measuring androgen levels are equally challenging to access due lack of specialised technicians in rural healthcare facilities (29). As a result, diagnosis in these settings often relies on clinical evaluations, which are insufficiently sensitive (30).
Symptoms related to puberty like irregular periods, acne, and hirsutism may be misinterpreted as normal development or other health issues. This can lead to delay in the right diagnosis and effective treatment (30). Therefore, it is more important to recognize Polycystic Ovary Syndrome (PCOS) at an early stage among adolescents (31, 32). On the other hand, a study in India showed that many adolescent PCOS cases were not diagnosed due to the failure of healthcare providers to identify the vague presentation of the syndrome (2).
PCOS also has symptoms that are common with other medical conditions entitled, thyroid diseases, hyperprolactinemia, and adrenal hyperplasia (33, 34). This shared symptom presentation further hinders the diagnosis process (4).
2.1.3 Cultural and societal barriers
In many cultures, menstruation and infertility are stigmatized topics, discouraging women from seeking medical attention (31, 33). The stigma surrounding menstrual irregularities leads to delays in diagnosis and treatment, particularly in conservative societies (33). Additionally, a lack of awareness about PCOS among women themselves compounds the problem, as symptoms are often normalized or ignored (8). Studies from Myanmar, Nepal and Lebanon talk about disaster scenarios in the region where women hardly have access to menstrual education, or menstrual products (35, 36).
2.2 Challenges in PCOS treatment
2.2.1 High cost of treatment
PCOS management often involves a combination of pharmacological and non-pharmacological approaches for several years. Medications like oral contraceptives, metformin, and clomiphene citrate are frequently prescribed in tandem (37). Long term complications of PCOS also require muti disciplinary specialised care, these services are expensive and often unavailable in low-income settings. Lifestyle modification programs, a cornerstone of PCOS treatment, also require resources such as dieticians and fitness professionals, which are inaccessible to many women (38). Many LMICs lack universal healthcare, leading to them having to send from their own pockets, this leads to catastrophic expenditure (39) (Table 1).
Metformin is one of the most affordable medications for diabetes, PCOS and other metabolic syndrome (47). It significantly reduces insulin resistance and high androgen levels while improving ovulation (48). Its availability as a generic drug and low monitoring needs makes it a great choice for health systems in low to middle-income countries.
2.2.2 Fragmented healthcare systems
The treatment of PCOS requires a team of gynaecologists, endocrinologists, dietitians, and mental health professionals work together, but many a case, these are not available in rural as well as in other low resource settings, the specialists if present tend to work on only one aspect of the syndrome and do not take sufficient care to council with rest of the other specialists to provide comprehensive care (41). Due to which a symptom-based approach to treatment is followed, catering predominantly to patients with problems like infertility, which is more commonly known and hence the collaborative approach to treatment is not followed (49).
PCOS is associated with long-term issues such as type 2 diabetes, cardiovascular diseases, and endometrial cancer. Despite this, treatment in many settings remains focused on short-term goals like regulating menstrual cycles or inducing ovulation. Long-term complications are often overlooked, particularly in low-resource settings where regular follow-up and preventive care are unavailable (1).
2.2.3 Mental health challenges
Anxiety, depression, and body image issues are prevalent among women with PCOS, with studies indicating significantly higher rates compared to the general population (50). Despite this, mental health is rarely addressed in PCOS treatment plans, particularly in low-resource settings where psychological services are either unavailable or stigmatized (43).
2.2.4 Low adherence to lifestyle changes
Lifestyle modification is a cornerstone of PCOS management, yet adherence remains low due to socioeconomic barriers, lack of support, and cultural factors (45). For instance, women in rural areas may not have access to safe spaces for physical activity or affordable, nutritious food. Additionally, the absence of structured lifestyle intervention programs in low-resource settings further limits their effectiveness.
Summary of the challenges in PCOS diagnosis and management, in LMIC context, in Nepal, sociocultural norms often discourage adolescent girls from seeking reproductive health care, compounding delays in PCOS diagnosis (35). In Ethiopia, PCOS is underdiagnosed due to a lack of trained gynaecologists outside urban centers (23). In Brazil and Mexico, PCOS awareness is significantly lower in rural communities compared to urban counterparts, leading to delayed care and higher disease burden (9). These insights have been incorporated to demonstrate how public health responses must be tailored to reflect each country's epidemiological profile, service availability, and cultural context.
3 Public health solutions
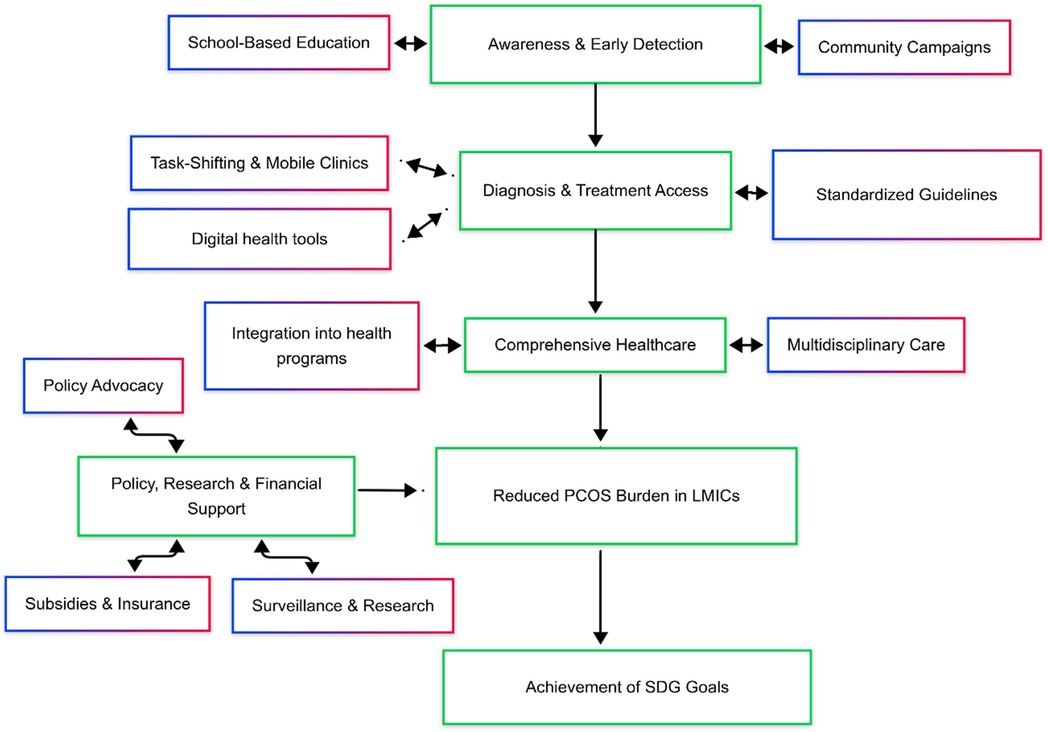
This section discusses, public health solutions like, prevention and early intervention approaches for PCOS, models of cost-effective care and policy changes (Figure 2).
3.1 Prevention and early intervention in PCOS
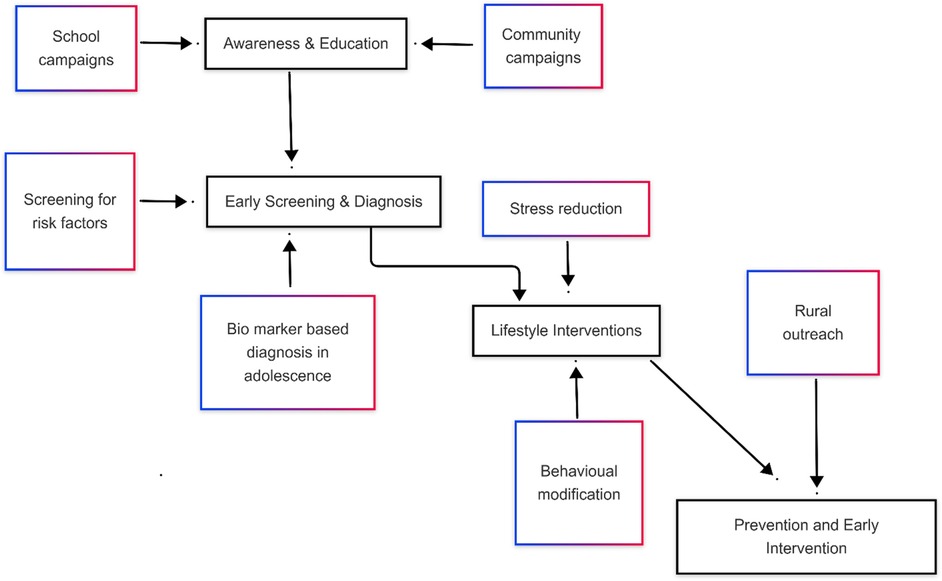
Promoting lifestyle modifications, implementing early diagnosis and treatment are effective strategies contribute holistic care for those diagnosed with PCOS, consequently healthcare costs can be reduced (51, 52). Early intervention in PCOS management is crucial to the mitigation of type 2 diabetes, cardiovascular disease, and infertility (53). Furthermore, achieving these goals will require joint action by governments, healthcare providers, educators, and community leaders (54). The screening for risk factors will facilitate lifestyle interventions in time, which are more effective when started soon (55). All three mode of prevention the primary, secondary and tertiary plan an important role in reducing the burden of PCOS and its complications in the community (Figure 3).
3.1.1 Early screening and diagnosis
The timely detection of PCOS is important (56). The period of adolescence is the golden time when it comes to the detection of the earliest symptoms of PCOS that are mostly related to menstrual disturbance, acne, and facial hair (55, 57). Another intervention that can assist in reaching more people in the community is through the engagement of local leaders and organizations. The roll out of mobile health units with primary diagnostic tools means the screening service can be brought to remote areas and the barriers of geography and finance can be overcome. Biomarkers such as AMH, INH-A, and INSL3 are found to be useful in some studies in the early diagnosis of PCOS in adolescents, there is currently no consensus on their diagnostic thresholds (58).
3.1.2 School-based preventive approaches
Educating adolescent girls about menstrual health and the risk factors of PCOS is a cost-effective strategy for recognition of risk factors related to PCOS and encouraging healthy behaviours (59). School-based programs can include workshops and sessions that normalize discussions about menstrual health can help dispel myths and reduce stigma (25). Educating young women can emphasize the importance of a balanced diet, regular exercise, and stress management. Incorporating these evidence-based practices into school curricula ensures that students receive consistent support mitigate lifestyle diseases like PCOS (12). Parents and teachers often play a crucial role in supporting the children, hence sensitizing them is also important for the sustainability of primary prevention strategies (35).
3.1.3 Early interventions
Lifestyle interventions like exercise and dietary modification, is an important cornerstone of PCOS prevention and management (55, 56). Evidence showcases the important role of dietary changes, regular physical activity, and stress management in alleviating symptoms and reducing the risk of complications (60). Nutritional interventions are important in managing insulin resistance, a common feature of PCOS. Diets rich in whole grains, lean proteins, and vegetables, along with reduced intake of processed foods and sugary beverages, have been shown to improve metabolic and reproductive outcomes (61). However, implementing such dietary changes can be challenging in low-resource settings due to food insecurity and limited access to nutritional counselling (62).
Regular exercise has been proven to improve insulin sensitivity, reduce androgen levels, and promote weight loss in women with PCOS. Even moderate-intensity activities, such as brisk walking or yoga, can yield significant benefits (63). Community-based initiatives that create safe spaces for exercise and promote group activities can help overcome barriers to physical activity in rural areas (24).
Chronic stress exacerbates PCOS symptoms by disrupting hormonal balance. Stress-reduction techniques, such as mindfulness, meditation, and cognitive-behavioural therapy, are effective in improving mental health and overall quality of life (64). Integrating these practices into community programs can make stress management accessible to women in low-resource settings.
In low-resource settings, dietary change programs can be supported by school-based initiatives, women-led self-help groups, and nutritional counselling included in primary healthcare systems. In India, similar models have shown success in tackling child and maternal under nutrition (65–67).
3.2 Cost-effective models of care for tackling PCOS
3.2.1 Task-shifting models
Task-shifting models represent a strategy of redistributing responsibilities from specialists to trained nurses and primary care physicians (68). These models can play an important role in improving accessibility to PCOS management (43). Such methods have the potential to reduce the burden placed on specialists by giving primary care providers the knowledge they need to diagnose and manage PCOS effectively (69). Training courses aimed at nurses and primary care physicians consist of identifying main symptoms including the irregular menstruation, hyperandrogenism, and polycystic ovarian morphology, as well as offering lifestyle interventions and pharmacological treatments (70) (Table 2 and Figure 3).
Adding task-shifting models can also result in earlier diagnosis and management, thus preventing such long-term complications as infertility, type 2 diabetes, and cardiovascular disease (69). Of note, care to guarantee geographical and economic access is also obtained by these models, and thereby it enables PCOS management to be brought within reach of women in rural and the most underserved regions (50). The implementation plan consists of rigorous training programs, regular supervision, and the embedding of evidence-based guidelines into primary care settings (70).
Successful case studies of utilization of task shifting models for non-communicable disease care has been reported in South Africa. Where screening for hypertension which was traditionally done by physician were reallocated to physician assistants (78). Similar examples have also reported in HIV care, where nurses took over the roles traditionally done by physicians (79).
Community health workers (CHWs) have long been instrumental in delivering primary healthcare services in underserved regions (80). The potential of CHWs Like CHOs, MPWs, ANM to bridge gaps in PCOS care by leveraging their trusted position within communities is promising and has been proven effective in other health programs in India (81, 82)Empowering CHWs with portable diagnostic tools, such as glucometers and blood pressure monitors, they can assist in the early identification, health education and follow up of associated metabolic disorders, thereby improving treatment adherence (78, 49). Such initiatives require comprehensive training modules, adequate remuneration, and support systems to maintain CHW motivation and effectiveness (79).
The cost-effectiveness of these three approaches, task-shifting to primary care physicians, structured training for nurses, and CHW-led interventions, has been clearly shown in LMIC health systems. In Uganda, training nurses to conduct cardiovascular and metabolic risk screening cut per-patient costs by 30% compared to physician-led services, without affecting diagnostic accuracy (83). In Tanzania, shifting PMTCT (Prevention of Mother-to-Child Transmission) tasks to nurses and midwives reduced nurse-to-patient time by 25% and brought notable cost savings for the health system (84). CHW-based hypertension management programs in Cameroon demonstrated that giving CHWs portable diagnostic devices and referral protocols was both cost-effective and sustainable. This approach lowered uncontrolled hypertension prevalence at a cost of less than USD 3 per patient each year (85). These findings highlight that when such models include structured supervision, evidence-based protocols, and performance-linked incentives, they can provide high-quality, affordable PCOS-related services in resource-limited settings.
Simple body measurements such as waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) strongly relate to insulin resistance and metabolic syndrome in PCOS. They offer a practical, low-cost option for early risk assessment in field situations (86).
3.2.2 Digital health in primary care
Advancement in technology even brought telemedicine which is a game changer in the way we should approach PCOS care for those women who live in the countryside and accessible areas of the country (87). Telemedicine is the provider's vehicle to the patients through virtual check-ups, which in return decrease the need for travel and lessen the costs (88).
Moreover, telemedicine assists patients’ interaction through educating materials and guiding on the modification of their lifestyles according to their needs (52). Telemedicine would enrich an already established healthcare infrastructure by combining a primary care provider with specialists. For instance, general practitioners can communicate with endocrinologists or gynaecologists virtually for confirmations of diagnoses, or treatments refinements (88). The main advantage of telemedicine is its capability carter to a larger population of the people without a need to correspondingly to increase the workforce. But problems such as digital literacy, internet connection, and data protection concerns must be resolved in the desire to realize equality of access and usage (53).
Studies in Asia has shown that the use of mobile application in helping improve patient adherence to lifestyle intervention in NCD care. A study in New Zealand has showcased the use of digital health in risk profiling in cardiovascular illness, using real time data, which helps make personalised treatment strategies and improve health outcomes (89). A study has found that use of remainders telephone messages improve adherence to medications (90).
New Zealand's CONNECT trial showcased the viability of app-based follow-ups and health coaching for chronic conditions, which includes work with metabolic disorders (75). In the same vein, India's e-Sanjeevani platform has expanded the delivery of digital health services to rural primary health centres (PHCs). Nevertheless, digital literacy and infrastructural gaps need to be bridged (87). In Pakistan, a mobile health intervention focused on reproductive disorders improved symptom tracking and adherence to follow-up (74). Brazil's “Telessaúde” program, designed for remote consultation and education, demonstrated high user satisfaction among women with hormonal disorders, including PCOS (88). India's e-Sanjeevani telemedicine platform has successfully delivered NCD and reproductive health consultations across rural and urban areas, including states with limited specialist availability (91, 92). We have also acknowledged critical barriers such as low digital literacy, inconsistent internet access, and privacy concerns, which could hinder PCOS-specific digital health interventions in LMICs unless addressed through supportive infrastructure and training (93).
3.2.3 Community mobilization
Raising awareness about PCOS through mass campaigns is essential to reduce stigma, encourage early diagnosis, and promote health-seeking behaviour (94). It is also important to use culturally sensitive materials and local languages to engage diverse populations effectively. Awareness campaigns can address misconceptions about PCOS, highlight its symptoms, and educate women about the benefits of timely intervention (8).
Such campaigns can leverage various media, including radio, television, social media, and community events, to disseminate information (61). Collaborations with schools, workplaces, and community organizations can amplify the reach and impact of these efforts. For example, organizing workshops and seminars targeting adolescent girls and young women can foster early recognition of symptoms and encourage preventive measures. Additionally, involving male family members in awareness programs can help build supportive environments for women seeking care (62).
Mass campaigns also play a pivotal role in advocating for policy changes and increasing public investment in PCOS care. By generating widespread awareness and demand for services, these initiatives can drive the expansion of healthcare infrastructure and resources dedicated to PCOS management (1). However, sustained funding and strategic planning are critical to ensuring the long-term success of awareness campaigns (63).
3.3 Public health policy needs for PCOS management
3.3.1 Integration into reproductive health programs
Embedding PCOS diagnosis and management into existing reproductive and maternal health programs can optimize resource utilization and improve accessibility. Training and incentivising existing public health human resources like PHC medical officers, Accredited Social Health Activists (ASHAs) and Auxiliary Nurse Midwives (ANMs) to screen for PCOS symptoms and provide basic counselling can be a cost-effective solution (95, 96). This approach leverages existing healthcare infrastructure, particularly in rural areas, to identify women at risk and provide timely interventions. US veteran affairs have incorporated PCOS into their program, they have trained their primary health care professionals to diagnose and treat minor manifestations of PCOS. Addressing PCOS requires collaborative efforts involving government agencies, non-governmental organizations (NGOs), and private healthcare providers (64). Multi-sectoral partnerships enhance the reach and efficacy of PCOS management programs (97).
Integrating chronic disease management into reproductive health services has shown clear success in low- and middle-income countries and sets a strong example for managing PCOS. In Tanzania, including hypertension and diabetes screening in HIV and antenatal clinics cut patient travel time by 40% and increased detection rates for non-communicable diseases without needing additional staff (98). In Bangladesh, reproductive health workers trained to provide combined maternal health and non-communicable disease services saw a 25% rise in early diagnosis of metabolic disorders among women of reproductive age (99).
3.3.2 National guidelines for PCOS management
The establishment of standardized national guidelines tailored to low-resource settings is essential for consistent and effective PCOS management (100). A simplified diagnostic criteria and accessible treatment protocols, including lifestyle interventions and affordable pharmacological options. Although international guidelines are available, national guidelines are realistic, culturally sensitive and should prioritise the unique challenges faced by underserved populations, such as limited access to healthcare and financial constraints (100) Australia's National Health and Medical Research Council, has developed a guideline for comprehensive management of PCOS (101).
Nationally adapted guidelines for chronic disease care in low- and middle-income countries show that standardization can improve consistency and quality in settings with limited resources. In South Africa, the Primary Care 101 guideline incorporates algorithms for non-communicable diseases into primary health care practice (102). This allows nurses to start evidence-based management with little input from specialists, resulting in better adherence to clinical protocols and fewer inappropriate referrals. In India, the NPCDCS program standardizes the management of hypertension and diabetes across states with simple flowcharts and training modules for non-specialist providers (103). Adapting PCOS guidelines at the country level, with straightforward diagnostic criteria and culturally relevant lifestyle recommendations, would increase feasibility in resource-limited environments.
3.3.3 Subsidized healthcare services
Subsidizing diagnostic tests, medications, awareness generation through mass campaigns and lifestyle counselling can alleviate the financial burden of PCOS management for low-income populations (56, 104, 105). The policy interventions that make essential healthcare services more affordable and accessible. Providing incentives to private healthcare providers to offer low-cost services can further expand the availability of care (50). Subsidized programs can ensure that economic constraints do not hinder women from seeking timely diagnosis and treatment, ultimately improving health outcomes at the population level (57). In countries like Canada, the provincial health insurance plans cover consultations, diagnostic tests, and treatments of PCOS management.
In Kenya, government-funded non-communicable disease clinics offer free screening for diabetes and hypertension, reducing out-of-pocket costs and improving follow-up (99). In Sri Lanka, essential medicines for non-communicable diseases are fully subsidized through the public health system, with free metabolic screening available in government hospitals (15). Applying this model to PCOS could include subsidizing hormone tests, ultrasound imaging, and metformin, along with community-based lifestyle counselling, to reduce financial barriers for low-income women.
3.3.4 Multi-disciplinary care
PCOS being a muti faceted causes manifestations like the reproductive, metabolic, dermatological and psychological. These might require care from various specialists like obstetricians, endocrinologists, dermatologists and psychiatrist for comprehensive care. Centres like University of California, San Francisco, National institute for research in reproductive health conduct multi-disciplinary clinics to treat PCOS cases (16, 64). There should be a policy to develop infrastructure for multi-disciplinary clinics all tertiary care hospitals across LMICs.
In South Africa, multidisciplinary NCD clinics bring together reproductive, metabolic, and mental health services in one place. This setup lowers patient drop-out rates and helps people stick to lifestyle changes (13). In Brazil, the Family Health Strategy connects community health workers with specialists. This approach ensures ongoing support and early treatment for women with reproductive and metabolic issues (14). These models show that even in areas with limited resources, teamwork among different specialties, backed by referral networks and shared care plans, can lead to better results for PCOS while making the most of available specialist resources.
3.4 Research priorities for comprehensive management of PCOS
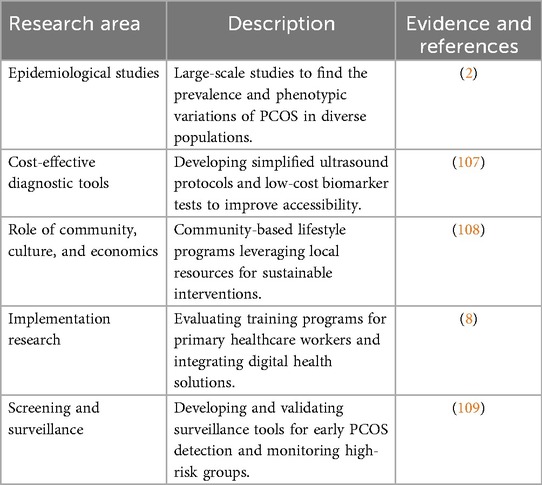
3.4.1 Epidemiological studies
Large-scale epidemiological studies are crucial to understanding the true prevalence and phenotypic variations of PCOS in India (106). There is a need for comprehensive research that includes both rural and urban populations, providing insights into the disease burden and its socio-demographic correlates. Such studies can inform public health policies and resource allocation, ensuring that interventions are tailored to the needs of diverse populations (3) (Table 3).
3.4.2 Cost-effective diagnostic tools development
Developing low-cost diagnostic tools, such as simplified ultrasound protocols or biomarker-based tests, can significantly improve accessibility in resource-constrained settings (110). Affordable one prick diagnostic tools are particularly important in rural areas, where access to advanced healthcare facilities is often limited (17) (Table 3). More affordable markers of insulin resistance and hepatic steatosis in PCOS, such as the triglyceride-glucose index, TG/HDL ratio, HOMA-IR, and ALT/AST ratio, have been validated in numerous LMIC populations (107).
3.4.3 Role of community, culture and economics
Pilot studies evaluating community-based lifestyle intervention programs can provide evidence for scalable solutions to PCOS management (55). Community, school and workplace-based approaches are effective for helping sustain dietary modifications, exercise regimens, and mental health counselling in improving metabolic and reproductive outcomes. Community-based interventions leverage local resources and social networks, making them cost-effective and culturally appropriate (59).
Research exploring the cultural and socioeconomic determinants of PCOS can inform the development of targeted interventions (58). The importance of understanding barriers to healthcare access and adherence in different communities. Addressing these factors can enhance the acceptability and effectiveness of public health programs, ensuring that they meet the unique needs of diverse populations (51).
3.4.4 Implementation research
Implementation research is critical to integrating PCOS management into existing healthcare systems. There is a need to evaluate training programs for primary healthcare providers and community health workers and assessing effectiveness of improving delivery of these using CHWs lead community-based interventions (54).
The role of digital health tools, such as mobile apps for symptom tracking and telemedicine for remote consultations, should be explored as cost-effective alternatives for PCOS management (68). The digital platforms can enhance access to care, particularly in remote and underserved areas. These tools also promote patient engagement and self-management, empowering women to take an active role in their health (64).
3.4.5 Screening and surveillance
Screening and surveillance strategies and tools can help in early detection of PCOS risk factors and symptoms among adolescents and young women, studies can be made on developing and validating such surveillance tools and algorithms (111, 112). Sentinel surveillance sites can be developed at facilities where people with high PCOS risk show up regularly to monitor the trends of the risks, disease and its complications among the target group.
4 Conclusion
To improve PCOS management in low resource setting where there is inadequate availability of trained health professionals, certain components of it can be merged into primary healthcare systems to improve early identification and comprehensive care. Task shifting models that have proven effective for other programs including diabetes mellitus control program, which involves training community health workers, such as ASHAs and ANMs, can increase awareness reduce stigma and promoting early detection. Embedding PCOS management into reproductive and maternal health programs can utilize existing infrastructure for better resource utilization. Simultaneously, establishing national guidelines tailored to resource-constrained settings ensures equitable and consistent care delivery.
While lowcost options like metformin are available, care should be taken to prescribe those cost-efficient medications though primary care centres, and subsidized tertiary care can ensure access to care, and catastrophic expenditure for low-income populations. Research into cost-effective diagnostic tools, scalable lifestyle interventions, and digital health solutions is vital for addressing gaps in PCOS management. A coordinated effort involving government, NGOs, and private stakeholders is crucial for comprehensive management of PCOS to achieve SDG by 2030. By prioritizing prevention, affordability, and accessibility, public health policies can reduce the burden of PCOS and help mitigate the burden of PCOS and its complications.
Author contributions
SV: Conceptualization, Methodology, Writing – original draft. RG: Data curation, Writing – original draft. PM: Data curation, Writing – original draft. AA: Conceptualization, Writing – review & editing. MG: Conceptualization, Investigation, Writing – review & editing. JP: Supervision, Validation, Writing – review & editing. TA: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The author (s) declare financial support was received from ICMR (Indian Council of Medical Research). Grant Id-5/7/103/PCOS-phaseII/NTF/2020RBMCH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Artificial intelligence assistance (Chat GPT) was used to correct grammar and spelling.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. (2018) 110(3):364–79. doi: 10.1016/j.fertnstert.2018.05.004
2. Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. (2011) 24(4):223–7. doi: 10.1016/j.jpag.2011.03.002
3. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003
4. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. (2011) 7(4):219–31. doi: 10.1038/nrendo.2010.217
5. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14(5):270–84. doi: 10.1038/nrendo.2018.24
6. World Bank Country and Lending Groups. World bank data help desk. Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (Accessed May 01, 2025).
7. Berni TR, Morgan CL, Rees DA. Rising incidence, health resource utilization, and costs of polycystic ovary syndrome in the United Kingdom. J Clin Endocrinol Metab. (2025) 110(5):e1580–9. doi: 10.1210/clinem/dgae518
8. Baheiraei A, Bakouei F, Mohammadi E, Montazeri A, Hosseni M. The social determinants of health in association with women’s health Status of reproductive age: a population-based study. Iran J Public Health. (2015) 44(1):119. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4449998/26060783
9. Chen X, Orom H, Hay JL, Waters EA, Schofield E, Li Y, et al. Differences in rural and urban health information access and use. J Rural Heal. (2019) 35(3):405–17. doi: 10.1111/jrh.12335
10. Wasata R, Chertok IRA, Kingori C, Haile ZT. Exploratory study of knowledge and experience of polycystic ovary syndrome (PCOS) among PCOS-diagnosed Bangladeshi women. J Women Health Care Issues. (2020) 3(1):1–9. Available online at: www.auctoresonline.org
11. Makwe CC, Olamijulo JA, Balogun MR, Akinkugbe AO, Samuel A, Udenze K, et al. SAT363 The prevalence and phenotype of polycystic ovary syndrome (PCOS) in A community-based population in sub-sahara Africa: the Nigeria-PEP study. J Endocr Soc. (2023) 7(Suppl 1):bvad114.1668. doi: 10.1210/jendso/bvad114.1668
12. Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab. (2022) 107(2):575–85. doi: 10.1210/clinem/dgab613
13. Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV-AIDS on a collision course: relevance for health care delivery, particularly in low-resource settings—insights from South Africa. Am J Clin Nutr. (2011) 94(6):1690S–6. doi: 10.3945/ajcn.111.019075
14. Macinko J, Harris MJ. Brazil’s family health strategy—delivering community-based primary care in a universal health system. N Engl J Med. (2015) 372(23):2177–81. doi: 10.1056/NEJMp1501140
15. Dabare PRL, Wanigatunge CA, Beneragama BH. A national survey on availability, price and affordability of selected essential medicines for non communicable diseases in Sri Lanka. BMC Public Health. (2014) 14(1):817. doi: 10.1186/1471-2458-14-817
16. University of California San Francisco. UCSF multidisciplinary clinic for women with polycystic ovary syndrome. Available online at: https://pcos.ucsf.edu/ (Accessed May 01, 2025).
17. Srinivas V, Choubey U, Motwani J, Anamika F, Chennupati C, Garg N, et al. Synergistic strategies: optimizing outcomes through a multidisciplinary approach to clinical rounds. Proc (Bayl Univ Med Cent). (2023) 37(1):144. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC10761132/38174031
18. Haleem A, Javaid M, Singh RP, Suman R. Telemedicine for healthcare: capabilities, features, barriers, and applications. Sensors Int. (2021) 2:100117. doi: 10.1016/j.sintl.2021.100117
19. Almhmoud H, Alatassi L, Baddoura M, Sandouk J, Alkayali MZ, Najjar H, et al. Polycystic ovary syndrome and its multidimensional impacts on women’s mental health: a narrative review. Medicine (Baltimore). (2024) 103(25):e38647. doi: 10.1097/MD.0000000000038647
20. Mennella C, Maniscalco U, De Pietro G, Esposito M. Ethical and regulatory challenges of AI technologies in healthcare: a narrative review. Heliyon. (2024) 10(4):e26297. doi: 10.1016/j.heliyon.2024.e26297
21. Calcagno M, Serra P, Etrusco A, Margioula-Siarkou C, Terzic S, Giannini A, et al. A bitter pill to swallow: adjustments to oral contraceptive pill use in polycystic ovary syndrome. Expert Opin Pharmacother. (2024) 25(9):1137–43. doi: 10.1080/14656566.2024.2371977
22. Christ JP, Cedars MI. Current guidelines for diagnosing PCOS. Diagnostics (Basel, Switzerland). (2023) 13(6):1113. doi: 10.3390/diagnostics13061113
23. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. (2015) 36(5):487–525. doi: 10.1210/er.2015-1018
24. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2013) 98(12):4565–92. doi: 10.1210/jc.2013-2350
25. Chang S, Dunaif A. Diagnosis of polycystic ovary syndrome: which criteria to use and when? Endocrinol Metab Clin North Am. (2021) 50(1):11–23. doi: 10.1016/j.ecl.2020.10.002
26. Joham AE, Tay CT, Laven J, Louwers YV, Azziz R. Approach to the patient: diagnostic challenges in the workup for polycystic ovary syndrome. J Clin Endocrinol Metab. (2025) 110(7):e2298–308. doi: 10.1210/clinem/dgae910
27. Vasudevan S. Menstrual health and hygiene practices of adolescent girls attending school in rural parts of south India and its effect on school attendance in the year 2020: a descriptive cross-sectional study. Int J Med Sci Nurs Res. (2024) 4(3):6–14. doi: 10.55349/ijmsnr.202443614
28. Pascoal E, Wessels JM, Aas-Eng MK, Abrao MS, Condous G, Jurkovic D, et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet Gynecol. (2022) 60(3):309–27. doi: 10.1002/uog.24892
29. Peterman NJ, Yeo E, Kaptur B, Smith EJ, Christensen A, Huang E, et al. Analysis of rural disparities in ultrasound access. Cureus. (2022) 14(5):e25425. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC9236672/35774712
30. Meczekalski B, Niwczyk O, Kostrzak A, Maciejewska-Jeske M, Bala G, Szeliga A. PCOS In adolescents-ongoing riddles in diagnosis and treatment. J Clin Med. (2023) 12(3):1221. doi: 10.3390/jcm12031221
31. Roberts L, Renati S, Solomon S, Montgomery S. Women and infertility in a pronatalist culture: mental health in the slums of mumbai. Int J Womens Health. (2020) 12:993–1003. doi: 10.2147/IJWH.S273149
32. Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med. (2020) 18(1):1–16. doi: 10.1186/s12916-020-01516-x
33. Olson MM, Alhelou N, Kavattur PS, Rountree L, Winkler IT. The persistent power of stigma: a critical review of policy initiatives to break the menstrual silence and advance menstrual literacy. PLOS Glob Public Heal. (2022) 2(7):e0000070.
34. Yesiladali M, Yazici MGK, Attar E, Kelestimur F. Differentiating polycystic ovary syndrome from adrenal disorders. Diagnostics. (2022) 12(9):1–14. doi: 10.3390/diagnostics12092045
35. Myers A, Sami S, Onyango MA, Karki H, Anggraini R, Krause S. Facilitators and barriers in implementing the Minimum initial services package (MISP) for reproductive health in Nepal post-earthquake. Confl Health. (2018) 12(1):1–9. doi: 10.1186/s13031-018-0170-0
36. Schmitt ML, Clatworthy D, Ratnayake R, Klaesener-Metzner N, Roesch E, Wheeler E, et al. Understanding the menstrual hygiene management challenges facing displaced girls and women: findings from qualitative assessments in Myanmar and Lebanon. Confl Health. (2017) 11(1):1–11. doi: 10.1186/s13031-017-0121-1
37. Gautam R, Jyoti A, Bhateja A, Malhotra N, Arora T. Pharmacological management of PCOS: trends and insights from a 10-year bibliometric analysis. Expert Opin Pharmacother. (2025) 26(11–12):1351–8. doi: 10.1080/14656566.2025.2535175
38. Gautam R, Bhateja A, Malhotra N, Arora T. Mapping the research landscape of lifestyle modification in PCOS management: a 10 year bibliometric analysis. Clin Epidemiol Glob Heal. (2025) 33:102024. doi: 10.1016/j.cegh.2025.102024
39. Kodali PB. Achieving universal health coverage in low- and middle-income countries: challenges for policy post-pandemic and beyond. Risk Manag Healthc Policy. (2023) 16:607–21. doi: 10.2147/RMHP.S366759
40. Joham AE, Piltonen T, Lujan ME, Kiconco S, Tay CT. Challenges in diagnosis and understanding of natural history of polycystic ovary syndrome. Clin Endocrinol (Oxf). (2022) 97(2):165–73. doi: 10.1111/cen.14757
41. Shukla A, Ashraf GM, Sudharsan V, Arora T, Rather KUI, Chowdhury S, et al. Trends of age at onset of menarche among Indian women of reproductive age and its association with the presence of PCOS and related features: a multicentric cross sectional study. J Obstet Gynecol India. (2025) 75(1):70–7. doi: 10.1007/s13224-024-01994-6
42. Rajkumar E, Ardra A, Prabhu G, Pandey V, Sundaramoorthy J, Manzoor R, et al. Polycystic ovary syndrome: an exploration of unmarried women’s knowledge and attitudes. HELIYON. (2022) 8(7):e09835. doi: 10.1016/j.heliyon.2022.e09835
43. Dewani D, Karwade P, Mahajan KS. The invisible struggle: the psychosocial aspects of polycystic ovary syndrome. Cureus. (2023) 15(12):1–8.
44. Jannink T, Bordewijk EM, Aalberts J, Hendriks J, Lehmann V, Hoek A, et al. Anxiety, depression, and body image among infertile women with and without polycystic ovary syndrome. Hum Reprod. (2024) 39(4):784–91. doi: 10.1093/humrep/deae016
45. Cowan S, Lim S, Alycia C, Pirotta S, Thomson R, Helm MG, et al. Lifestyle management in polycystic ovary syndrome—beyond diet and physical activity. BMC Endocr Disord. (2023) 23:1–33. doi: 10.1186/s12902-022-01208-y
46. Serour M, Alqhenaei H, Al-Saqabi S, Mustafa AR, Ben-Nakhi A. Cultural factors and patients’ adherence to lifestyle measures. Br J Gen Pract. (2007) 57(537):291. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2043336/17394732
47. Solini A, Tricò D. Clinical efficacy and cost-effectiveness of metformin in different patient populations: a narrative review of real-world evidence. Diabetes Obes Metab. (2024) 26(S3):20–30. doi: 10.1111/dom.15729
48. Maan P, Gautam R, Vasudevan S, Menon GR, Arora A, Nair A, et al. Pharmacological and non-pharmacological interventions for polycystic ovary syndrome (PCOS) in Indian women: a systematic review and meta-analysis. Pharm. (2025) 18(5):680. Available online at: https://www.mdpi.com/1424-8247/18/5/680/htm
49. Balaji S, Amadi C, Prasad S, Kasav JB, Upadhyay V, Singh AK, et al. Urban rural comparisons of polycystic ovary syndrome burden among adolescent girls in a hospital setting in India. Biomed Res Int. (2015) 2015:158951. doi: 10.1155/2015/158951
50. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
51. Schwarz T, Schmidt AE, Bobek J, Ladurner J. Barriers to accessing health care for people with chronic conditions: a qualitative interview study. BMC Health Serv Res. (2022) 22(1):1–15. doi: 10.1186/s12913-022-08426-z
52. El-Tallawy SN, Pergolizzi JV, Vasiliu-Feltes I, Ahmed RS, LeQuang JAK, Alzahrani T, et al. Innovative applications of telemedicine and other digital health solutions in pain management: a literature review. Pain Ther. (2024) 13(4):791–812. doi: 10.1007/s40122-024-00620-7
53. Kichloo A, Albosta M, Dettloff K, Wani F, El-Amir Z, Singh J, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Heal. (2020) 8(3):e000530. doi: 10.1136/fmch-2020-000530
54. Ismayilova M, Yaya S. What can be done to improve polycystic ovary syndrome (PCOS) healthcare? Insights from semi-structured interviews with women in Canada. BMC Womens Health. (2022) 22(1):1–15. doi: 10.1186/s12905-022-01734-w
55. Boyle J, Hollands G, Beck S, Hampel G, Wapau H, Arnot M, et al. Process evaluation of a pilot evidence-based polycystic ovary syndrome clinic in the Torres strait. Aust J Rural Health. (2017) 25(3):175–81. doi: 10.1111/ajr.12288
56. Tehrani FR, Amiri M. Polycystic ovary syndrome in adolescents: challenges in diagnosis and treatment. Int J Endocrinol Metab. (2019) 17(3):e91554.31497042
57. Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Heal. (2018) 6(11):e1196–252. doi: 10.1016/S2214-109X(18)30386-3
58. Merkin SS, Azziz R, Seeman T, Calderon-Margalit R, Daviglus M, Kiefe C, et al. Socioeconomic status and polycystic ovary syndrome. J Women’s Health. (2011) 20(3):413–9. doi: 10.1089/jwh.2010.2303
59. Okube OT, Kimani S, Mirie W. Community-based lifestyle intervention improves metabolic syndrome and related markers among Kenyan adults. J Diabetes Metab Disord. (2022) 21(1):607–21. doi: 10.1007/s40200-022-01023-1
60. Gautam R, Maan P, Jyoti A, Kumar A, Malhotra N, Arora T. The role of lifestyle interventions in PCOS management: a systematic review. Nutrients. (2025) 17(2):310. doi: 10.3390/nu17020310
61. Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet (London, England). (2010) 376(9748):1261–71. doi: 10.1016/S0140-6736(10)60809-4
62. Castillo EG, Ijadi-Maghsoodi R, Shadravan S, Moore E, Menash OM III, Docherty M, et al. Community interventions to promote mental health and social equity. Focus (2020) 18(1):60–70. doi: 10.1176/appi.focus.18102
63. Seymour J. The impact of public health awareness campaigns on the awareness and quality of palliative care. J Palliat Med. (2018) 21(S1):S30–6. doi: 10.1089/jpm.2017.0391
64. Patil AD, Vaidya RA, Begum S, Chauhan SL, Mukherjee S, Kokate PP, et al. An integrated multidisciplinary model of care for addressing comorbidities beyond reproductive health among women with polycystic ovary syndrome in India. Indian J Med Res. (2022) 156(3):449–58. doi: 10.4103/ijmr.IJMR_2497_19
65. Kumar N, Raghunathan K, Quisumbing A, Scott S, Menon P, Thai G, et al. Women improving nutrition through self-help groups in India: does nutrition information help? Food Policy. (2024) 128:102716. doi: 10.1016/j.foodpol.2024.102716
66. Menezes R, Lelijveld N, Wrottesley SV, Brennan E, Mates E, James PT. Integrating women and Girls’ nutrition services into health systems in low- and middle-income countries: a systematic review. Nutrients. (2022) 14(21):4488. doi: 10.3390/nu14214488
67. Hazra A, Das A, Ahmad J, Singh S, Chaudhuri I, Purty A, et al. Matching intent with intensity: implementation research on the intensity of health and nutrition programs with women’s self-help groups in India. Glob Health Sci Pract. (2022) 10(2):e37213.
68. Maroju RG, Choudhari SG, Shaikh MK, Borkar SK, Mendhe H. Role of telemedicine and digital technology in public health in India: a narrative review. Cureus. (2023) 15(3):1–12.
69. Sydora BC, Wilke MS, McPherson M, Chambers S, Ghosh M, Vine DF. Challenges in diagnosis and health care in polycystic ovary syndrome in Canada: a patient view to improve health care. BMC Women’s Health. (2023) 23(1):1–13. doi: 10.1186/s12905-023-02732-2
70. Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, et al. Task shifting for non-communicable disease management in low and middle income countries—a systematic review. PLoS One. (2014) 9(8):e103754. doi: 10.1371/journal.pone.0103754
71. Tesema AG, Mabunda SA, Chaudhri K, Sunjaya A, Thio S, Yakubu K, et al. Task-sharing for non-communicable disease prevention and control in low- and middle-income countries in the context of health worker shortages: a systematic review. PLOS Glob Public Heal. (2025) 5(4):e0004289. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12002516/
72. Hartzler AL, Tuzzio L, Hsu C, Wagner EH. Roles and functions of community health workers in primary care. Ann Fam Med. (2018) 16(3):240–5. doi: 10.1370/afm.2208
73. Grave RD, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes Metab Syndr Obes. (2010) 3:373–85. doi: 10.2147/DMSO.S13860
74. Pérez-Jover V, Sala-González M, Guilabert M, Mira JJ. Mobile apps for increasing treatment adherence: systematic review. J Med Internet Res. (2019) 21(6):e12505. doi: 10.2196/12505
75. Redfern J, Coorey G, Mulley J, Scaria A, Neubeck L, Hafiz N, et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. npj Digit Med. (2020) 3(1):1–9. doi: 10.1038/s41746-020-00325-z
76. Malhotra K, Pan CSC, Davitadze M, Kempegowda P. Identifying the challenges and opportunities of PCOS awareness month by analysing its global digital impact. Front Endocrinol (Lausanne). (2023) 14:1109141. doi: 10.3389/fendo.2023.1109141
77. Torres-Zegarra C, Sundararajan D, Benson J, Seagle H, Witten M, Walders-Abramson N, et al. Care for adolescents with PCOS: development and prescribing patterns of a multidisciplinary clinic. J Pediatr Adolesc Gynecol. (2021) 34(5):617–25. doi: 10.1016/j.jpag.2021.02.002
78. Parasuraman G, Jeemon P, Thankappan KR, Ali MK, Mahal A, McPake B, et al. Community control of hypertension and diabetes (CoCo-HD) program in the Indian states of kerala and tamil nadu: a study protocol for a type 3 hybrid trial. BMC Public Health. (2024) 24(1):2275. doi: 10.1186/s12889-024-19746-6
79. Asweto CO, Alzain MA, Andrea S, Alexander R, Wang W. Integration of community health workers into health systems in developing countries: opportunities and challenges. Fam Med Community Heal. (2016) 4(1):37–45. doi: 10.15212/FMCH.2016.0102
80. Hirschhorn LR, Govender I, Zulu JM. Community health workers: essential in ensuring primary health care for equitable universal health coverage, but more knowledge and action is needed. BMC Prim Care. (2023) 24(1):1–2. doi: 10.1186/s12875-023-02175-6
81. Tripathi N, Parhad P, Garg S, Biswal SS, Ramasamy S, Panda A, et al. Performance of health and wellness centre in providing primary care services in Chhattisgarh, India. BMC Prim Care. (2024) 25(1):360. doi: 10.1186/s12875-024-02603-1
82. Brar S, Purohit N, Prinja S, Singh G, Bahuguna P, Kaur M. What and how much do the community health officers and auxiliary nurse midwives do in health and wellness centres in a block in Punjab? A time-motion study. Indian J Public Health. (2021) 65(3):275–9. doi: 10.4103/ijph.IJPH_1489_20
83. Paier-Abuzahra M, Posch N, Jeitler K, Semlitsch T, Radl-Karimi C, Spary-Kainz U, et al. Effects of task-shifting from primary care physicians to nurses: an overview of systematic reviews. Hum Resour Health. (2024) 22(1):1–14. doi: 10.1186/s12960-024-00956-3
84. Mbaruku G, Bergström S. Reducing maternal mortality in Kigoma, Tanzania. Health Policy Plan. (1995) 10(1):71–8. doi: 10.1093/heapol/10.1.71
85. Dzudie A, Kengne AP, Muna WFT, Ba H, Menanga A, Kouam CK, et al. Prevalence, awareness, treatment and control of hypertension in a self-selected Sub-Saharan African urban population: a cross-sectional study. BMJ Open. (2012) 2(4):e001217. doi: 10.1136/bmjopen-2012-001217
86. Zhu M, Wang K, Feng J, Liu Y, Guan M, Wang Y, et al. The waist-to-height ratio is a good predictor for insulin resistance in women with polycystic ovary syndrome. Front Endocrinol (Lausanne). (2024) 15:1502321. doi: 10.3389/fendo.2024.1502321
87. Arora S, Huda RK, Verma S, Khetan M, Sangwan RK. Challenges, barriers, and facilitators in telemedicine implementation in India: a scoping review. Cureus. (2024) 16(8):e67388. doi: 10.7759/cureus.67388
88. Shende V, Wagh V. Role of telemedicine and telehealth in public healthcare sector: a narrative review. Cureus. (2024) 16(9):e69102. doi: 10.7759/cureus.69102
89. Wells S, Riddell T, Kerr A, Pylypchuk R, Chelimo C, Marshall R, et al. Cohort profile: the PREDICT cardiovascular disease cohort in New Zealand primary care (PREDICT-CVD 19). Int J Epidemiol. (2017) 46(1):22. doi: 10.1093/ije/dyv312
90. Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health. (2014) 14(1):1–15. doi: 10.1186/1471-2458-14-188
91. Parameshwarappa PM, Olickal JJ. Telemedicine awareness and the preferred digital healthcare tools: a community-based cross-sectional study from rural Karnataka, India. Indian J Community Med. (2023) 48(6):915–9. doi: 10.4103/ijcm.ijcm_770_22
92. Dastidar BG, Jani AR, Suri S, Nagaraja VH. Reimagining India’s national telemedicine service to improve access to care. Lancet Reg Heal Southeast Asia. (2024) 30:100480. doi: 10.1016/j.lansea.2024.100480
93. Borges do Nascimento IJ, Abdulazeem H, Vasanthan LT, Martinez EZ, Zucoloto ML, Østengaard L, et al. Barriers and facilitators to utilizing digital health technologies by healthcare professionals. NPJ Digit Med. (2023) 6(1):1–28. doi: 10.1038/s41746-023-00899-4
94. Alotaibi M, Ali Shaman A. Enhancing polycystic ovarian syndrome awareness using private social network. mHealth. (2020) 6:1–7. Available online at: https://pubmed.ncbi.nlm.nih.gov/33209914/32190612
95. Kohli C, Kishore J, Sharma S, Nayak H. Knowledge and practice of accredited social health activists for maternal healthcare delivery in Delhi. J Fam Med Prim Care. (2015) 4(3):359. doi: 10.4103/2249-4863.161317
96. Nandan D, Agarwal D. Human resources for health in India: urgent need for reforms. Indian J Community Med. (2012) 37(4):205–6. doi: 10.4103/0970-0218.103464
97. Jones H, Sprung VS, Pugh CJA, Daousi C, Irwin A, Aziz N, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. (2012) 97(10):3709–16. doi: 10.1210/jc.2012-1382
98. Labhardt ND, Balo JR, Ndam M, Grimm JJ, Manga E. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res. (2010) 10:1–9. doi: 10.1186/1472-6963-10-339
99. Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. (2012) 380(9845):889. doi: 10.1016/S0140-6736(12)60730-2
100. American Society for Reproductive Medicine | ASRM. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome (2023)—practice guidance (2023). Available online at: https://www.asrm.org/practice-guidance/practice-committee-documents/recommendations-from-the-2023-international-evidence-based-guideline-for-the-assessment-and-management-of-polycystic-ovary-syndrome/ (Accessed February 11, 2025).
101. Misso M, Costello M, Dokras A, Laven J, Moran L, Piltonen T, et al. Under section 14A of the national health and medical research council act. Natl Heal Med Res Counc. (2018) 2.
102. Barasa E, Rogo K, Mwaura N, Chuma J. Kenya National hospital insurance fund reforms: implications and lessons for universal health coverage. Heal Syst Reform. (2018) 4(4):346–61. doi: 10.1080/23288604.2018.1513267
103. Ramani VK, Suresh K. Prevalence of hypertension and diabetes morbidity among adults in a few urban slums of bangalore city, determinants of its risk factors and opportunities for control—a cross-sectional study. J Fam Med Prim Care. (2020) 9(7):3264. doi: 10.4103/jfmpc.jfmpc_234_20
104. Malhotra S, Shah R. Women and mental health in India: an overview. Indian J Psychiatry. (2015) 57(Suppl 2):205–11. doi: 10.4103/0019-5545.161479
105. Latha K, Meena KS, Pravitha MR, Dasgupta M, Chaturvedi SK. Effective use of social media platforms for promotion of mental health awareness. J Educ Health Promot. (2020) 9(1):124–124. doi: 10.4103/jehp.jehp_90_20
106. Ganie M, Vasudevan V, Wani I, Baba M, Arif T, Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res. (2019) 150(4):333–44. doi: 10.4103/ijmr.IJMR_1937_17
107. Joshi A. PCOS Stratification for precision diagnostics and treatment. Front Cell Dev Biol. (2024) 12:1358755. doi: 10.3389/fcell.2024.1358755
108. Kepper MM, Stamatakis KA, Deitch A, Terhaar A, Gates E, Cole G, et al. Sustainability planning for a community network to increase participation in evidence-based lifestyle change programs: a mixed-methods approach. Int J Environ Res Public Health. (2024) 21(4):463. doi: 10.3390/ijerph21040463
109. Li M, He Z, Shi L, Lin M, Li M, Cheng Y, et al. Intelligent detection for polycystic ovary syndrome (PCOS): taxonomy, datasets and detection tools. Comput Struct Biotechnol J. (2025) 27:1578. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12032871/40291542
110. Piorino F, Patterson AT, Styczynski MP. Low-cost, point-of-care biomarker quantification. Curr Opin Biotechnol. (2022) 76:102738. doi: 10.1016/j.copbio.2022.102738
111. Sudharsan V, Davidson PD, Lakshmi KV, Amudha VP, Rani MRH. Development and validation of a screening tool for the identification of refractive errors among school going children in tamil nadu. India. Natl J Community Med. (2023) 14(9):581–7. doi: 10.55489/njcm.140920232751
Keywords: PCOS, LMICs, public health, diagnosis, treatment, SDG goals
Citation: Vasudevan S, Gautam R, Maan P, Arora A, Ganie A, Jabbar PK and Arora T (2025) A sustainable public health framework for PCOS management in low- and middle-income countries: a narrative review. Front. Reprod. Health 7:1627670. doi: 10.3389/frph.2025.1627670
Received: 13 May 2025; Accepted: 28 August 2025;
Published: 18 September 2025.
Edited by:
Monica Woll Rosen, University of Michigan, United StatesCopyright: © 2025 Vasudevan, Gautam, Maan, Arora, Ganie, Jabbar and Arora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Puthiyaveettil Khadar Jabbar, ZHJqYWJiYXIxMEBnbWFpbC5jb20=; Taruna Arora, dGFydW5ha2F0eWFsQGdtYWlsLmNvbQ==
 Sudharsan Vasudevan
Sudharsan Vasudevan Rohit Gautam
Rohit Gautam Pratibha Maan
Pratibha Maan Amit Arora2
Amit Arora2 Ashraf Ganie
Ashraf Ganie Puthiyaveettil Khadar Jabbar
Puthiyaveettil Khadar Jabbar Taruna Arora
Taruna Arora