- Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
Background: The Triple Elimination initiative is a global effort aimed at eliminating vertical transmission of HIV, hepatitis B and syphilis. This paper describes HIV, syphilis and hepatitis B testing and diagnosis in young women and men accessing sexual and reproductive health services and identifies opportunities to integrate prevention interventions.
Methods: The study was conducted in eight primary healthcare and four mobile clinics in South Africa, integrating HIV PrEP within prevention services. Programme data were collected and analysed from women and men ≥15 years accessing services for the first time between June 2023 and March 2024.
Results: Of 10,007 clients, 89.4% were female and 65.5% 18─24 years. Overall, 70.9% were provided HIV PrEP. Among females, 16.8% were provided contraceptives for the first time. HIV was identified in 1.2% of males and 2.2% of females tested and with results available, syphilis in 5.6% and 5.0%, and hepatitis B in 1.7% and 0.9% respectively. An HIV diagnosis was less likely among older age groups and those enrolled in school and more likely among those with part-time relative to full-time employment. Syphilis was less likely among older age groups and those reporting consistent condom use. Hepatitis B was more likely among those who had used oral PrEP before.
Conclusions: Opportunities for integrated prevention interventions, aligned to triple elimination, include condom programming, contraception, point-of-care testing, PrEP and vaccination. Integrated care delivered through HIV prevention programmes provides an opportunity to treat and prevent HIV, syphilis and hepatitis B, and offer contraception to prevent unintended pregnancies.
1 Introduction
Together HIV, viral hepatitis and sexually transmitted infections (STIs) account for 2.5 million deaths and 1.2 million new cases of cancer each year (1). Despite goals to eliminate viral hepatitis in sub-Saharan Africa by 2030, hepatitis-related deaths have increased from 1.1 million in 2019 to 1.3 million in 2022, and the estimated number of deaths due to hepatitis B now exceeds those of HIV (1). The unprecedented global increases in new adult and congenital syphilis cases since the COVID-19 pandemic have raised much alarm, requiring urgent interventions to reverse these trends (1, 2). Despite key achievements and declining HIV-incidence and HIV related deaths, new HIV infections remain disproportionately high in young women in sub-Saharan Africa and require accelerated efforts to achieve targets by 2025 (1). Africa has the highest burden in the world of HIV, syphilis and hepatitis B, yet has fallen behind other regions in reducing vertical transmission of these infections (3). In the African region there were an estimated 172,000 hepatitis B (0.4% of live births) (4) and 110,400 HIV vertical transmission events in 2022 (5), and an estimated 404,000 cases of congenital syphilis in 2016 (6). A global, integrated focus on “Triple Elimination” (7) has been initiated to address these three diseases, given their overlapping burden and modes of transmission, their synergistic effects which increase vertical transmission and adverse birth outcomes, and the similarity and availability of rapid diagnostic tests and effective prevention and treatment interventions (3, 7).
Much focus has been placed on integrating triple elimination interventions through existing maternal and child health (MCH) service delivery platforms, yet opportunities exist for triple elimination interventions beyond MCH settings. Substantial investment has been made in scaling access to oral pre-exposure prophylaxis (PrEP) for the prevention of HIV, particularly for young women. In South Africa, HIV PrEP is available at over 4,000 health facilities, and the country has initiated almost 1.5 million people on oral PrEP as of May 2024 (8). Given the common modes of transmission, HIV prevention services may offer an entry point to integrate key primary prevention interventions for HIV, syphilis and hepatitis B virus (HBV), thereby accelerating primary prevention efforts for all three diseases alongside improved access to screening, diagnosis and management.
The objectives of this paper are to describe the testing and diagnosis of HIV, syphilis and hepatitis B in a population of young women and men accessing sexual and reproductive health (SRH) services in eight primary healthcare clinics and four linked mobile clinics in South Africa; and to identify and discuss opportunities for integration of triple elimination efforts with a focus on HIV prevention and SRH programmes.
2 Methods
2.1 Study setting
This cross-sectional study is embedded within a large, ongoing Unitaid-funded implementation study (Project PrEP), which is evaluating the introduction of PrEP (including new PrEP methods) in primary healthcare settings through integrated HIV prevention and SRH services, with a focus on girls and young women 15─24 years. The project is implemented in eight fixed Department of Health facilities and four linked mobile clinics in four areas of South Africa: Tshwane (peri-urban area) in Gauteng, Gqeberha (peri-urban area) and Mthatha (rural area) in the Eastern Cape, and eThekwini (urban area) in Kwa Zulu Natal. These sites have been supported by Project PrEP to provide integrated, youth-friendly SRH services since 2018. Sites were selected to participate in Project PrEP based on the incidence of HIV and teenage pregnancy in their surrounding communities, as well as their proximity to learning institutions which serve young people. The first phase of the project supported the introduction of oral PrEP in South Africa and has been described elsewhere (9). The second phase is ongoing and has introduced the new PrEP methods dapivirine vaginal ring (DVR), since August 2023, and injectable cabotegravir (CAB-LA) since April 2024. Project service points within participating sites provide integrated HIV prevention and SRH services according to local guidelines, including HIV testing, PrEP, contraception, STI screening and management, with screening and linkage to gender-based-violence and mental health services provided. Clients known to be living with HIV and taking antiretroviral therapy are generally seen at other service points in the clinic, as are clients who are known to be pregnant, who are seen at service points providing antenatal care.
2.2 Data collection and analysis
This study utilized routine programme monitoring and evaluation data from women and men ≥15 years accessing SRH and HIV prevention services through Project PrEP, at their point of entry to project services. Data encompassed client demographics and socio-behavioural characteristics, documented in clinic files by the consulting healthcare provider. Data on pregnancy, contraception use, presence of STI signs and symptoms and STI treatment in the preceding three months were also documented. Additionally, data on the provision and results of rapid point-of-care HIV testing, pregnancy screening, syphilis testing, HBV testing, the initiation of PrEP and the provision of contraception were recorded. HIV testing was provided to consenting clients, following a two-step serial testing algorithm aligned with national HIV Testing Service guidelines (10), with two positive rapid test results confirming an HIV positive status, and a single negative rapid test confirming an HIV negative status. Pregnancy testing was conducted using a point-of-care (POC) urine human chorionic gonadotropin (hCG) test. In line with national PrEP guidelines (11), clients initiated on oral PrEP underwent baseline laboratory testing for hepatitis B surface antigen (HBsAg) and those pregnant or ≥30 years had their renal function assessed through a baseline serum creatinine and estimated glomerular filtration rate at the time of PrEP initiation. Furthermore, clients seeking services who, based on the clinician's assessment, had symptoms of syphilis, may have been exposed to syphilis, or were from a vulnerable population group (i.e., men who have sex with men, sex workers and their clients, adolescent girls and young women accessing SRH services, pregnant women, mobile workers) underwent syphilis screening using a Rapid Plasma Reagin (RPR) test conducted at local laboratories. Results from laboratory-based testing for Hepatitis B and syphilis were generally available within three days, through an online laboratory results system that could be accessed by the attending clinician. Clients received their laboratory results at their next visit or were recalled to the clinic for treatment or clinical care by the attending clinician if required. Clients with STI symptoms were treated on the same day according to a syndromic management approach (12), and asymptomatic clients on the day they returned for their results if positive for syphilis. Clients testing positive for HIV were initiated on antiretroviral treatment (ART) by the attending nurse, or referred for initiation within the same clinic, on the day of testing. Clients testing positive for HBsAg were referred for further evaluation and management by the doctor at the clinic.
We conducted a cross-sectional, descriptive analysis of routinely collected data from clients accessing clinical services for the first time at project sites between June 2023 and March 2024. To examine the association between independent variables and the binary dependent variables HIV, syphilis, and HBV test results, while holding other variables constant, we fit multivariable firth's logistic regression models. Given the pronounced imbalance in HIV status (195 HIV-positive vs. 9,219 HIV-negative), syphilis status (168 positive vs. 3,136 negative), and hepatitis B status (32 positive vs. 3,218 negative), Firth's penalized logistic regression was used to correct for bias in maximum likelihood estimates that can arise under such conditions and prevent overfitting (13). Statistical significance was assessed at a p-value cutoff of 0.05. Data were analysed using Stata statistical software, version 18.0 (14).
3 Results
Table 1 outlines the characteristics of the study population at their first visit, by biological sex. Of the 10,007 clients, 10.6% (n = 1,064) were male and 89.4% (n = 8,943) female. Most (65.5%, n = 6,556) were aged 18–24 years, with a higher proportion of females 15–17 years (16.4%, n = 1,463) compared to males (10.0%, n = 106), and a higher proportion of males ≥25 years (33.2%, n = 353) compared to females (17.1%, n = 1,529). A higher proportion of females (66.6%, n = 5,958) than males (46.8%, n = 498) were attending school or an educational institute. Most participants had completed secondary or a higher level of education, with a higher proportion of males (14.3%, n = 152) having completed tertiary education than females (8.3%, n = 743). Only 10.3% (n = 1,026) of the participants were employed full-time, higher amongst males (22.6%, n = 240) than females (8.8%, n = 786). Three-quarters of the participants were married or in a relationship (74.6%, n = 7,409). A higher proportion of males (22.3%, n = 202) compared to females (11.3%, n = 886) reported having sex under the influence of alcohol or drugs. Consistent condom use was reported for every sexual encounter by 15.9% (n = 1,349) of participants, similar among both males and females. Among females, a third (30.3%, n = 3,035) were using injectable contraceptives, 8.7% (n = 780) a subdermal contraceptive implant, 4.8% (n = 427) oral contraceptive pills and 0.7% (n = 68) an intrauterine contraceptive device (IUCD) on presentation to project sites. Overall, 1,506 (16.8%) females were provided contraceptives for the first time at their visit. There were 415 (5.1%) females who were pregnant or confirmed to be pregnant at their visit. STI signs or symptoms were documented for approximately a tenth (9.0%, n = 898) of participants, with 2.4% (n = 230) reporting being treated for an STI in the preceding three months. Similar proportions of males (9.5%, n = 98) and females (9.1%, n = 757) tested for HIV for the first time, whereas more females (14.4%, n = 1,203) than males (10.4%, n = 100) had used oral PrEP before. PrEP provision was approximately equal among males and females, at 70.9% (n = 7,019) overall.
Table 2 presents data on the testing and diagnosis of HIV, hepatitis B, and syphilis at first visit, by sex and age. Among male clients, 96.8% (1,030/1,064) were tested for HIV, with 1.2% (n = 12) testing positive. Of the 693 (65.1%) males who were tested for syphilis, an active infection was confirmed in 5.6% (21/371) of those with results available, highest at 7.7% (8/104) among those ≥25 years. Of the 694 (65.2%) males screened for HBV, HBsAg was positive in 6/349 (1.7%) of those with results available, of whom two were co-infected with both syphilis and HBV. Among females, 93.7% (8,384/8,943) were tested for HIV, with 2.2% (n = 183) testing positive. Of the 5,634 (63.0%) females who were tested for syphilis, an active infection was confirmed in 5.0% (147/2,932) of those with results available, highest at 6.6% (33/499) among those 15–17 years. Of the 5,731 (64.1%) females screened for HBV, HBsAg was positive in 26/2,901 (0.9%) of those with results available, of whom five were co-infected with syphilis and HBV. Among clients diagnosed with hepatitis B, 15/32 (46.8%) were born prior to childhood HBV vaccine roll out in South Africa and 17/32 (53.1%) after.
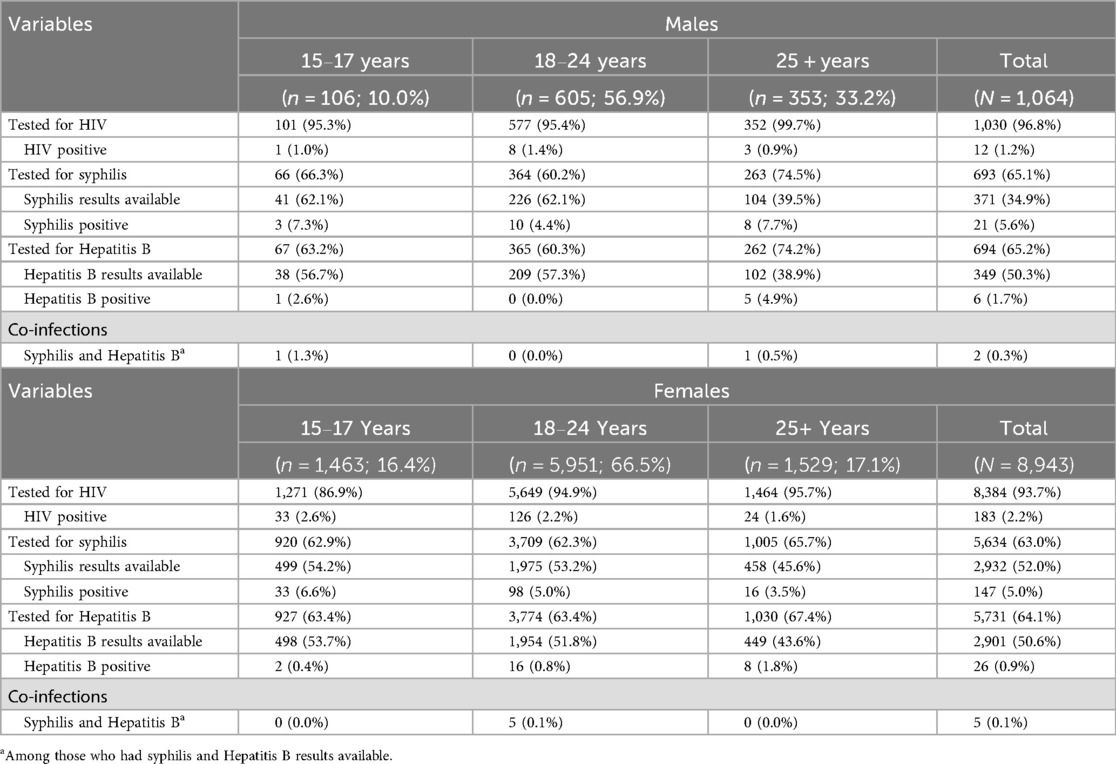
Table 2. HIV, hepatitis B and syphilis testing among clients accessing SRH and HIV prevention services, by sex and age.
In the multivariable regression analysis (Table 3), participants aged 18–24 [adjusted odds ratio (aOR) = 0.47; 95% CI: 0.28–0.78] and those ≥25 years (aOR = 0.30; 95% CI: 0.15–0.61) had 53% and 70% lower odds of an HIV positive diagnosis, respectively, compared to those aged 15–17 years. Being currently enrolled in school or an educational institution was associated with 60% lower odds of an HIV positive diagnosis (aOR = 0.40; 95% CI: 0.26–0.62) relative to non-students and participants employed part-time had more than four times the odds of an HIV positive diagnosis compared with full-time employees (aOR = 4.46; 95% CI: 1.75–11.37). Syphilis diagnosis was associated with younger age and was less likely among consistent condom users (Table 3). Those aged 18–24 years had 38% lower odds of a syphilis diagnosis (aOR = 0.62; 95% CI: 0.39–0.99) and those ≥25 years had 57% lower odds (aOR = 0.43; 95% CI: 0.22–0.82), compared to adolescents aged 15–17 years. Participants who reported using condoms at every sexual encounter were 45% less likely to have a syphilis diagnosis than those who did not (aOR = 0.55; 95% CI: 0.32–0.96). Regarding hepatitis B diagnosis, those who had used oral PrEP before faced 2.5-times higher odds of hepatitis B than PrEP-naïve participants (aOR = 2.52; 95% CI: 1.04–6.11).
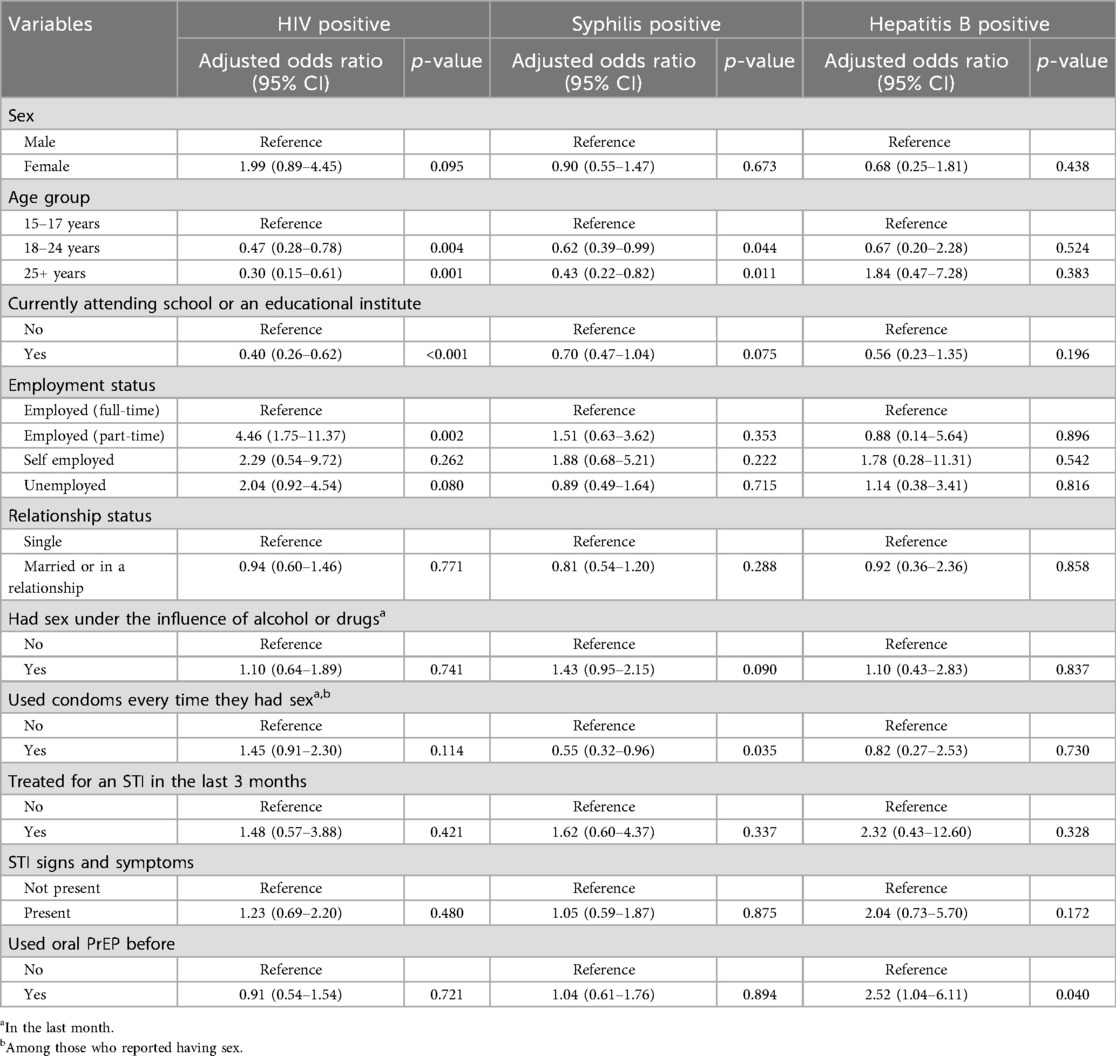
Table 3. Multivariable firth's logistic regression models of factors associated with HIV, syphilis, or hepatitis B positive test results.
4 Discussion
In this large implementation study, focusing on integrated HIV prevention service delivery in primary care settings in South Africa, we identified HIV in 2%, syphilis in 5% and HBV in 1% of clients tested when accessing SRH services. The likelihood of testing positive for HIV and syphilis decreased with age, highlighting the importance of accessible prevention interventions for adolescents. In keeping with prior studies reporting the protective effect of school completion on HIV acquisition (15, 16), clients in this study who were attending a school or educational institute were less likely to be diagnosed with HIV than those who were not, whilst those who were employed part-time were more likely to be diagnosed with HIV than those employed full-time. Although condom use was not associated with HIV or hepatitis B in this study, those who consistently used condoms were less likely to test positive for syphilis than those who did not, underscoring the importance of condoms in the prevention of STIs. Prior use of oral PrEP was associated with testing positive for hepatitis B, possibly due to clients with known vulnerabilities and exposures self-identifying their need for prevention interventions. Almost all clients were tested for HIV at their first engagement with project services, including 9% of clients who tested for HIV for the first time. In addition, 17% of women were provided with contraception for the first time. Our results highlight the following opportunities for integration of triple elimination efforts within integrated SRH and HIV prevention programmes, focusing on the first pillar of triple elimination which looks at primary prevention interventions.
4.1 Condoms
Condoms are the only currently available multipurpose prevention technology, preventing pregnancy, HIV and other STIs; they are cost effective and should be the cornerstone of any integrated prevention package (17). However, consistent use of condoms remains a challenge and was observed to be low in this population, not dissimilar to reports from other PrEP programmes (18, 19). Low use of condoms among young women has been reported to be influenced by difficulty in negotiating use, fear of being perceived as unfaithful, and wanting to please partners (20, 21); with men reporting that forgetfulness and condoms being a barrier to pleasure impede consistent use (22). Condom use has been reported to increase among young women exposed to PrEP (18, 23), highlighting the benefits that may be achieved through strengthened integration, ongoing non-judgemental counselling, and incorporation of innovative approaches such as pleasure based messaging (17).
4.2 Contraception
Among women presenting for SRH services, opportunity exists to conduct pregnancy screening and provide contraceptive services. As highlighted through our study, almost half of women accessing services were not using any contraceptive at their first visit, but 17% were provided with contraception for the first time through integrated services. South Africa has a high unmet need for contraception, reported at 19% among women of reproductive age in a 2016 Demographic Health Survey (24); higher at 31% among adolescent girls and women 15─19 years and 28% among those 20─24 years of age (24, 25). There is also evidence of unintended pregnancy being high, especially in girls and young women. As shown in an analysis of the South African National HIV Prevalence, Incidence and Behaviour Survey, only a third of women reporting pregnancy in the last five years had desired the pregnancy; even lower at 10.1% among adolescent girls and women 15─19 years and 20.9% among those 20─24 years of age. Contraception use was low, particularly in younger women, with only 12.8% of those 15─19 years and 19.7% of those 20─24 years reporting using contraception (26, 27). In addition to well-known benefits relating to improved maternal and child health, contraception has been highlighted as a key, cost-effective intervention to prevent new infant HIV transmissions, even in the context of increased antiretroviral therapy (ART) coverage (28, 29). The risk of acquiring HIV during pregnancy and the postpartum period more than doubles as compared to non-pregnant women (30). A high prevalence of STIs and a high incidence of HIV have been reported among women seeking contraceptive services (31), highlighting the need and potential to address multiple health needs through integrated service provision. This project has previously reported the increased access to contraceptives that is achieved when integrated with HIV prevention services (32).
4.3 Point of care screening and testing
POC testing for STIs facilitates earlier diagnosis and linkage to treatment, and is acceptable and feasible in low and middle income settings (33). However, the availability and affordability of quality-assured point of care tests remain a barrier to access. The WHO recommends supply-side interventions, in conjunction with national policy updates and funding, to improve early detection and treatment, resulting in greater impact in ending the epidemics and promoting health coverage (34). Our study highlights the benefits of POC screening and testing, with almost all clients who accessed services tested for HIV, and 9% of clients testing for HIV for the first time. The high burden of syphilis identified in this study points to the impact that may be achieved through the integration of POC syphilis tests within SRH programmes. The limitations of laboratory-based testing, including the loss to follow-up observed in our analysis (indicated by the low proportion of available results), are notable. Although only currently implemented in antenatal care settings in South Africa, increased syphilis screening and treatment may be achieved with the integration of the currently available dual HIV/syphilis POC test within HIV prevention services. These tests are acceptable, feasible and more cost effective than single tests (35), although implementation data from the region, outside of antenatal care, is limited (36).
South African national guidelines recommend HBV surface antigen screening for all pregnant women during the first trimester (37) and for all clients initiating oral PrEP (11). However, this is currently conducted through laboratory-based testing, is not routinely implemented for pregnant women, and POC rapid tests are not currently available within the national health system in South Africa (38). Integrating HBV screening with HIV prevention programmes could facilitate progress towards HBV elimination efforts (39), and could be strengthened with POC testing. Scaling testing to those who may benefit has been identified as one of the greatest challenges in achieving elimination targets for viral hepatitis (40). Use of a POC HBV tests has been shown to be feasible in antenatal care settings in South Africa (41, 42). However, further implementation research on the integration of rapid POC HBV testing within HIV prevention programmes in our context may be required.
The use of self-testing may provide further opportunity to reach partners and social networks with screening and testing services, optimising identification, and early treatment of infection to prevent transmission and reinfection. HIV self-test kits are available in South Africa, and secondary distribution has been acceptable in antenatal care settings (43). Further opportunities for integration of syphilis self-testing, when available, also exist (44).
4.4 Pre-exposure prophylaxis
In keeping with other implementation studies in the region (45, 46), and as reported previously for this project (9), we observed a high uptake of HIV PrEP when offered within a package of integrated prevention services. PrEP has been shown to be highly effective in preventing HIV and is a key component of combination HIV prevention and Triple Elimination efforts (7, 47). Initiatives to accelerate access to meet global prevention targets are, however, still required (48). The introduction of newer, long-acting PrEP methods has the potential to further enhance PrEP uptake and effective use for the prevention of HIV. One advantage of oral PrEP over long-acting PrEP methods, however, is its activity against HBV. Tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) within the oral PrEP combination of TDF and emtricitabine (FTC), are potent inhibitors of HBV viral replication and effective at both preventing HBV acquisition and suppressing HBV replication (39, 49). Globally, challenges with testing and treatment for chronic HBV remain, with less than 3% of people living with chronic hepatitis B globally treated in 2022 (50). Oral PrEP services thus provide an opportunity to facilitate integrated prevention interventions for both HIV and HBV, whilst improving access to treatment for people with HBV (39, 49). It has however been recommended, due to concerns about hepatitis reactivation, or “flares”, in people with chronic HBV who discontinue TDF/FTC, that there is closer follow up and monitoring of those with HBV infection who initiate TDF/FTC PrEP (49). The lack of benefit against HBV with long-acting PrEP methods could be overcome through integrated HBV screening regardless of PrEP method and ensuring availability of a choice of PrEP methods to suit different individual needs (49).
4.5 HBV vaccination
HBV vaccination has been a key success in combatting hepatitis infection, with widespread adoption of childhood immunization globally (40). In South Africa, substantial declines in hepatitis B incidence have been achieved since the inclusion of HBV vaccination for children as part of the expanded programme for immunization (EPI) since 1995 (51, 52). Whilst most programming is focused on childhood vaccination, and intensified focus and strengthening of birth dose vaccination more recently (40), there is opportunity to provide catch up vaccination to non-immune adults, especially those with HIV, who inject drugs or who may be exposed through sexual transmission, as is recommended in national guidelines (37). Within our study population, approximately half of the cases of hepatitis B identified were amongst clients born after the introduction of childhood immunization. Where HBV screening is integrated within HIV prevention programmes, additional laboratory testing would need to be conducted to confirm immunity (37), but the three dose HBV vaccination schedule can be aligned to PrEP follow up visits to facilitate integration (39).
4.6 Linkage to treatment
Linkage to care and treatment for individuals diagnosed with HIV, HBV, or syphilis through SRH programmes should be prioritized and is the second pillar of triple elimination efforts. Ideally this should be provided on the same day, by the same healthcare provider and at the same service point, to facilitate integrated care. National guidelines in South Africa recommend a first line antiretroviral therapy (ART) regimen which includes TDF and lamivudine (3TC), which has the benefit of treating both HIV and hepatitis B (39, 53).
4.7. Limitations
Although this study was conducted across four geographical areas of South Africa, the sampling frame did not include a representative sample of all SRH service users and the findings may not be representative of other contexts, particularly where the burden of HIV, HBV and syphilis differs. These data were collected from healthcare users who had presented for SRH and HIV prevention services and may not fully represent populations who have not accessed or experience barriers to accessing healthcare services. It is also notable that clients known to be living with HIV or who were pregnant would likely have received services at other service points in the clinic, and this data would not be representative of these populations. Incomplete laboratory results, particularly for syphilis and hepatitis B, may also have introduced bias. In addition, the lack of data on treatment outcomes for clients diagnosed with HIV, hepatitis B or syphilis and the cross-sectional study design limits analysis of outcomes and trends over time. The proposed opportunities for integration of triple elimination efforts that are discussed, are drawn from the data in our analysis and literature from the field but would be further supported by implementation research to determine their acceptability, feasibility, and cost effectiveness in specific contexts.
5 Conclusion
There are multiple opportunities for integrated prevention interventions, aligned to triple elimination efforts, including condom programming, contraception, POC testing, PrEP and vaccination. Leveraging investments and models of integrated care delivered through HIV prevention programmes focused on girls and young women may provide additional opportunity for triple elimination efforts by treating and preventing HIV, syphilis and hepatitis B prior to women becoming pregnant, in addition to offering integrated contraception services to prevent unintended pregnancies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Human Research Ethics Committees at the University of the Witwatersrand (220604) and by the World Health Organization Ethics Research Committee (ERC.0003784). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because approval was provided for the analysis of routinely collected de-identified program monitoring data.
Author contributions
CM: Conceptualization, Writing – review & editing, Writing – original draft, Investigation. HR: Writing – review & editing, Investigation, Data curation, Formal analysis. NK: Writing – review & editing, Data curation, Formal analysis. MM: Writing – review & editing. SA: Writing – review & editing. MP: Writing – review & editing. SM: Writing – review & editing, Conceptualization, Investigation, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Unitaid [grant number 2017-21-Wits-PrEP].
Acknowledgments
The authors would like to gratefully acknowledge the contribution of the Department of Health South Africa, the project team and field staff, and the communities and clients involved in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ART, antiretroviral therapy; DVR, dapivirine vaginal ring; CAB-LA, injectable long-acting cabotegravir; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; hCG, human chorionic gonadotropin; MCH, maternal and child health; POC, point-of-care; PrEP, pre-exposure prophylaxis; RPR, Rapid Plasma Reagin; SRH, sexual and reproductive health; STIs, sexually transmitted infections; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
References
1. World Health Organisation. Implementing the Global Health Sector Strategies on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2022–2030: Report on Progress and Gaps 2024. Geneva: World Health Organization (2024). Contract No.: Licence: CC BY-NC-SA 3.0 IGO.
2. World Health Organisation. WHO Syphilis Estimates 2016–2022. Geneva: WHO (2024). Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/stis/strategic-information
3. Cohn J, Owiredu MN, Taylor MM, Easterbrook P, Lesi O, Francoise B, et al. Eliminating mother-to-child transmission of human immunodeficiency virus, syphilis and hepatitis B in Sub-Saharan Africa. Bull World Health Organ. (2021) 99(4):287–95. doi: 10.2471/BLT.20.272559
4. Riches N, Henrion MYR, MacPherson P, Hahn C, Kachala R, Mitchell T, et al. Vertical transmission of hepatitis B virus in the WHO African region: a systematic review and meta-analysis. Lancet Glob Health. (2025) 13(3):e447–58. doi: 10.1016/S2214-109X(24)00506-0
5. Joint United Nations Programme on HIV/AIDS. The Path That Ends AIDS: UNAIDS Global AIDS Update 2023. Geneva: Joint United Nations Programme on HIV/AIDS (2023). Contract No.: Licence: CC BY-NC-SA 3.0 IGO.
6. Gilmour LS, Walls T. Congenital syphilis: a review of global epidemiology. Clin Microbiol Rev. (2023) 36(2):e0012622. doi: 10.1128/cmr.00126-22
7. World Health Organisation. Introducing a Framework for Implementing Triple Elimination of Mother-to-child transmission of HIV, Syphilis and Hepatitis B Virus: Policy Brief. Geneva: World Health Organization (2023). Contract No.: Licence: CC BY-NC-SA 3.0 IGO.
8. AVAC. PrEP Watch: The Global PrEP Tracker. AVAC: Global Advocacy for HIV Prevention (2022). Available online at: https://www.prepwatch.org/countries/south-africa/
9. Butler V, Kutywayo A, Martin CE, Pleaner M, Mojapele MV, Ncube S, et al. Implementing differentiated and integrated HIV prevention services for adolescent girls and young women: experiences from oral PrEP rollout in primary care services in South Africa. J Adolesc Health. (2023) 73(6 Suppl):S58–66. doi: 10.1016/j.jadohealth.2023.09.003
10. Department of Health Republic of South Africa. National HIV Testing Services: Policy. Pretoria: National Department of Health (2016).
11. Department of Health Republic of South Africa. Updated Guidelines for the Provision of Oral pre-exposure prophylaxis (PrEP) to Persons at Substantial Risk of HIV Infection. Pretoria: National Department of Health (2021).
12. Department of Health South Africa. Comprehensive STI Clinical Management Guidelines 2021–2025. Pretoria, South Africa: Department of Health South Africa (2021).
13. Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth’s logistic regression with rare events: accurate effect estimates and predictions? Stat Med. (2017) 36(14):2302–17. doi: 10.1002/sim.7273
15. Pettifor AE, Levandowski BA, MacPhail C, Padian NS, Cohen MS, Rees HV. Keep them in school: the importance of education as a protective factor against HIV infection among young South African women. Int J Epidemiol. (2008) 37(6):1266–73. doi: 10.1093/ije/dyn131
16. Lewis L, Kharsany ABM, Humphries H, Maughan-Brown B, Beckett S, Govender K, et al. HIV incidence and associated risk factors in adolescent girls and young women in South Africa: a population-based cohort study. PLoS One. (2022) 17(12):e0279289. doi: 10.1371/journal.pone.0279289
17. World Health Organisation. Condoms. Geneva: World Health Organization (2024). Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/stis/prevention/condoms (Accessed October 23, 2024).
18. Cassidy T, Ntuli N, Kilani C, Malabi N, Rorwana B, Mutseyekwa T, et al. Delivering PrEP to young women in a low-income setting in South Africa: lessons for providing both convenience and support. AIDS Behav. (2022) 26(1):147–59. doi: 10.1007/s10461-021-03366-x
19. de Dieu Tapsoba J, Zangeneh SZ, Appelmans E, Pasalar S, Mori K, Peng L, et al. Persistence of oral pre-exposure prophylaxis (PrEP) among adolescent girls and young women initiating PrEP for HIV prevention in Kenya. AIDS Care. (2021) 33(6):712–20. doi: 10.1080/09540121.2020.1822505
20. Rousseau E, Katz AWK, O'Rourke S, Bekker LG, Delany-Moretlwe S, Bukusi E, et al. Adolescent girls and young women’s PrEP-user journey during an implementation science study in South Africa and Kenya. PLoS One. (2021) 16(10):e0258542. doi: 10.1371/journal.pone.0258542
21. Kayesu I, Mayanja Y, Nakirijja C, Machira YW, Price M, Seeley J, et al. Uptake of and adherence to oral pre-exposure prophylaxis among adolescent girls and young women at high risk of HIV-infection in Kampala, Uganda: a qualitative study of experiences, facilitators and barriers. BMC Womens Health. (2022) 22(1):440. doi: 10.1186/s12905-022-02018-z
22. Cholo FA, Dada S, Martin CE, Mullick S. Experiences of oral pre-exposure prophylaxis use among heterosexual men accessing sexual and reproductive health services in South Africa: a qualitative study. J Int AIDS Soc. (2024) 27(5):e26249. doi: 10.1002/jia2.26249
23. Mathur S, Mishra R, Mahapatra B, Heck CJ, Okal J. Assessing layered HIV prevention programming: optimizing outcomes for adolescent girls and young women. AIDS. (2022) 36(Suppl 1):S75–83. doi: 10.1097/QAD.0000000000003242
24. National Department of Health (NDoH) SSASS, South African Medical Research, Council (SAMRC) aI. South Africa Demographic and Health Survey 2016. Pretoria, South Africa and Rockville. Maryland, USA: NDoH, Stats SA, SAMRC, and ICF (2019).
25. Jonas K, Lombard C, Chirinda W, Govindasamy D, Appollis TM, Kuo C, et al. Participation in an HIV prevention intervention and access to and use of contraceptives among young women: a cross sectional analysis in six South African districts. Contraception. (2022) 116:51–8. doi: 10.1016/j.contraception.2022.07.005
26. Chersich MF, Wabiri N, Risher K, Shisana O, Celentano D, Rehle T, et al. Contraception coverage and methods used among women in South Africa: a national household survey. S Afr Med J. (2017) 107(4):307–14. doi: 10.7196/SAMJ.2017.v107i4.12141
27. Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press (2014).
28. Sherwood J, Lankiewicz E, Roose-Snyder B, Cooper B, Jones A, Honermann B. The role of contraception in preventing HIV-positive births: global estimates and projections. BMC Public Health. (2021) 21(1):536. doi: 10.1186/s12889-021-10570-w
29. Wilcher R, Petruney T, Reynolds HW, Cates W. From effectiveness to impact: contraception as an HIV prevention intervention. Sex Transm Infect. (2008) 84(Suppl 2):ii54–60. doi: 10.1136/sti.2008.030098
30. Joseph Davey DL, Pintye J, Baeten JM, Aldrovandi G, Baggaley R, Bekker LG, et al. Emerging evidence from a systematic review of safety of pre-exposure prophylaxis for pregnant and postpartum women: where are we now and where are we heading? J Int AIDS Soc. (2020) 23(1):e25426. doi: 10.1002/jia2.25426
31. Ahmed K, Baeten JM, Beksinska M, Bekker LG, Bukusi EA, Donnell D, et al. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. (2019) 394(10195):303–13. doi: 10.1016/S0140-6736(19)31288-7
32. Pleaner M, Fipaza Z, Mabetha K, Greener L, Ncube S, Butler V, et al. Uptake of contraception among adolescent girls and young women PrEP clients: leveraging the opportunity to strengthen HIV and sexual and reproductive health integration. Front Reprod Health. (2021) 3:684114. doi: 10.3389/frph.2021.684114
33. Martin K, Wenlock R, Roper T, Butler C, Vera JH. Facilitators and barriers to point-of-care testing for sexually transmitted infections in low- and middle-income countries: a scoping review. BMC Infect Dis. (2022) 22(1):561. doi: 10.1186/s12879-022-07534-9
34. World Health Organization. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. Geneva: World Health Organization (2022). Contract No.: Licence: CC BY-NC-SA 3.0 IGO.
35. Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect. (2017) 93(S4):S3–15. doi: 10.1136/sextrans-2016-053069
36. Kufa T, Tobaiwa O, Cutler E, Singh B, Brukwe Z, Maseko V, et al. Acceptability and performance of dual HIV/syphilis testing in male circumcision clients, 2021. South Afr J HIV Med. (2024) 25(1):1571. doi: 10.4102/sajhivmed.v25i1.1571
37. Department of Health Republic of South Africa. National Guidelines for the Management of Viral Hepatitis. Pretoria: National Department of Health (2020).
38. Van Zyl GU, Maponga T, Rabie H, Taljaard J. The role of new hepatitis B vaccines in South Africa. S Afr Med J. (2024) 114(2):e1473. doi: 10.7196/SAMJ.2024.v114i2.1473
39. Mohareb AM, Kouamé MG, Nouaman M, Kim AY, Larmarange J, Neilan AM, et al. What does the scale-up of long-acting HIV pre-exposure prophylaxis mean for the global hepatitis B epidemic? J Int AIDS Soc. (2024) 27(3):e26218. doi: 10.1002/jia2.26218
40. Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, et al. Accelerating the elimination of viral hepatitis: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. (2019) 4(2):135–84. doi: 10.1016/S2468-1253(18)30270-X
41. Chotun N, Preiser W, van Rensburg CJ, Fernandez P, Theron GB, Glebe D, et al. Point-of-care screening for hepatitis B virus infection in pregnant women at an antenatal clinic: a South African experience. PLoS One. (2017) 12(7):e0181267. doi: 10.1371/journal.pone.0181267
42. Joseph Davey D, Hsiao NY, Wendy Spearman C, Sonderup M, Hu NC, Mashele N, et al. Low prevalence of hepatitis B virus infection in HIV-uninfected pregnant women in Cape Town, South Africa: implications for oral pre-exposure prophylaxis roll out. BMC Infect Dis. (2022) 22(1):719. doi: 10.1186/s12879-022-07697-5
43. Zishiri V, Conserve DF, Haile ZT, Corbett E, Hatzold K, Meyer-Rath G, et al. Secondary distribution of HIV self-test kits by HIV index and antenatal care clients: implementation and costing results from the STAR initiative in South Africa. BMC Infect Dis. (2023) 22(Suppl 1):971. doi: 10.1186/s12879-023-08324-7
44. World Health Organisation. Updated Recommendations for the Treatment of Neisseria gonorrhoeae, Chlamydia trachomatis and Treponema pallidum (syphilis), and new Recommendations on Syphilis Testing and Partner Services. Geneva: World Health Organization (2024).
45. Celum CL, Delany-Moretlwe S, Baeten JM, van der Straten A, Hosek S, Bukusi EA, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc. (2019) 22(Suppl 4):e25298. doi: 10.1002/jia2.25298
46. Kagaayi J, Batte J, Nakawooya H, Kigozi B, Nakigozi G, Strömdahl S, et al. Uptake and retention on HIV pre-exposure prophylaxis among key and priority populations in South-Central Uganda. J Int AIDS Soc. (2020) 23(8):e25588. doi: 10.1002/jia2.25588
47. World Health Organisation. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Geneva: WHO (2021).
48. Bavinton BR, Grulich AE. HIV pre-exposure prophylaxis: scaling up for impact now and in the future. Lancet Public Health. (2021) 6(7):e528–33. doi: 10.1016/S2468-2667(21)00112-2
49. Mohareb AM, Larmarange J, Kim AY, Coffie PA, Kouamé MG, Boyd A, et al. Risks and benefits of oral HIV pre-exposure prophylaxis for people with chronic hepatitis B. Lancet HIV. (2022) 9(8):e585–94. doi: 10.1016/S2352-3018(22)00123-0
50. World Health Organisation. Global Hepatitis Report 2024: Action for Access in low- and Middle-income countries. Geneva: WHO (2024).
51. Amponsah-Dacosta E. Hepatitis B virus infection and hepatocellular carcinoma in sub-saharan Africa: implications for elimination of viral hepatitis by 2030? World J Gastroenterol. (2021) 27(36):6025–38. doi: 10.3748/wjg.v27.i36.6025
52. Moonsamy S, Suchard M, Pillay P, Prabdial-Sing N. Prevalence and incidence rates of laboratory-confirmed hepatitis B infection in South Africa, 2015 to 2019. BMC Public Health. (2022) 22(1):29. doi: 10.1186/s12889-021-12391-3
Keywords: HIV, PrEP, syphilis, hepatitis B, triple elimination, prevention, contraception
Citation: Martin CE, Ramatsoma H, Koloane N, Monametsi M, Arries S, Pleaner M and Mullick S (2025) The contribution of PrEP programmes to triple elimination efforts: a cross-sectional study of status and opportunities. Front. Reprod. Health 7:1637573. doi: 10.3389/frph.2025.1637573
Received: 29 May 2025; Accepted: 1 September 2025;
Published: 18 September 2025.
Edited by:
Phetole Walter Mahasha, Tshwane University of Technology, South AfricaReviewed by:
Haithem Taha Mohammed Ali, University of Zakho, IraqBekezela Siziba, Mpilo Central Hospital, Zimbabwe
Copyright: © 2025 Martin, Ramatsoma, Koloane, Monametsi, Arries, Pleaner and Mullick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine E. Martin, Y21hcnRpbkB3cmhpLmFjLnph
†ORCID:
Maletsatsi Monametsi
orcid.org/0009-0007-4756-3168
 Catherine E. Martin
Catherine E. Martin Hlologelo Ramatsoma
Hlologelo Ramatsoma Nthabiseng Koloane
Nthabiseng Koloane Maletsatsi Monametsi
Maletsatsi Monametsi Sean Arries
Sean Arries Melanie Pleaner
Melanie Pleaner Saiqa Mullick
Saiqa Mullick