Abstract
Background:
Menopausal hormone therapy (MHT) is one of the most recent therapeutic options available for the management of menopausal symptoms. MHT is used in healthy symptomatic women under 60 or within 10 years of menopause without contraindications. Still, as many menopausal women use both MHT and antidepressants, safer alternatives are needed. Herbal remedies like Ashwagandha can offer a safer alternative to existing therapies. Ashwagandha aids in hormonal balance, vitality, and reduces stress and fatigue.
Objective:
The study aimed to assess the efficacy and safety of Ashwagandha root extract (ARE) for managing menopausal symptoms.
Methods:
A randomized, double-blind, placebo-controlled study included 60 women aged 45–55 years who received either ARE or a placebo (PL) for 56 days. The primary outcome was a change in the Menopause Rating Scale (MRS) score from baseline to 56 days. Secondary outcomes were changes in serum hormonal parameters [estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH)], hot flash events, Short Form-12 Health Survey (SF-12) (Quality of Life) score, and Perceived Stress Scale-10 (PSS-10) from baseline to day 56. Tolerability was measured using Patient Global Assessment of Tolerability to Therapy. Safety outcomes, such as change in severity and frequency of adverse events, were also assessed from baseline to day 56.
Results:
At the end of the study, the total MRS score reduced significantly (p < 0.0001) with ARE intervention, in psychological (p < 0.0001), somatic (p < 0.0001), and urogenital (p < 0.0001) domains as compared to the PL group. Similarly, ARE group showed improved serum estradiol (p < 0.001) and progesterone (p < 0.001) levels, and increase in SF-12 scores (p < 0.001), while presenting reduced serum FSH (p < 0.001) and LH (p < 0.001), hot flashes events (p < 0.001) and PSS-10 scores (p < 0.001) compared to PL.
Conclusion:
Ashwagandha root extract can be a potential herbal intervention for managing menopausal symptoms in healthy women.
Clinical Trial Registration:
https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=OTk3Mw==&Enc=&userName=, CTRI/2022/02/040551.
1 Introduction
Menopause is defined as the stoppage of menstrual periods for 12 consecutive months, signaling the end of ovarian function and permanent amenorrhea. A gradual shift from active to inactive ovarian function occurs over several years. Besides its association with aging, menopause involves significant biological and psychological changes in women (1, 2).
The reduction in estrogen and progesterone release during menopause makes women more susceptible to psychosomatic issues. The predominant symptoms are hot flashes, sweating, and libido alterations. However, other specific concerns associated with menopause are vasomotor symptoms, sleep disruptions, urogenital issues, breast and joint pain, cognitive changes, and mood disorders such as depression and anxiety (3, 4).
The primary treatment for menopausal symptoms involves a combination of menopausal hormone therapy (MHT), pharmaceutical antidepressants, and lifestyle changes. However, MHT is linked to a higher risk of venous thromboembolism, stroke, cardiovascular disease, gallstones, and breast cancer. In addition, women frequently discontinue antidepressants due to their significant side effects. It has been suggested that MHT is not effective in addressing psychological manifestations (1, 5–7). So nowadays, women are moving towards alternative options such as herbal remedies. One such herb used traditionally is Ashwagandha.
Ashwagandha, scientifically referred to as Withania somnifera, belonging to the family Solanaceae, has been utilized in Ayurveda for centuries. Ashwagandha has been utilized for millennia as a Rasayana due to its multitude of health benefits. It is a potent adaptogen that enhances the body's resilience to stress, supports cell-mediated immunity, and possesses antioxidant capabilities (8). It is a renowned herb that is highly valued for its ability to balance, energize, rejuvenate, and revitalize the body system (9–11).
Many clinical investigations have shown that Ashwagandha root extract (ARE) has a variety of physiological functions, including anti-inflammatory, neuroprotective, adaptogenic, and cognitive-enhancing properties (12). It has been utilized as an alternative treatment for hormonal disorders, in particular for infertility and sexual dysfunction. ARE has shown benefits in improving sexual desire and sexual dysfunction. A study conducted by Dongre et al. reported that ARE enhanced sexual function, sexual arousal, lubrication and lowered sexual distress in healthy women (13). It has also been shown to relieve mild to moderate climacteric symptoms during perimenopause in women. A study by Gopal et al. demonstrated that Ashwagandha significantly reduces the total MRS score and significantly increases in serum estradiol and reduces follicle stimulating hormone (FSH) and luteinizing hormone (LH) concentrations (10).
Despite this, the mechanism of ARE influence on the reproductive system is not fully understood; it might be related to its adaptogenic, anti-inflammatory, and antioxidant effects. Another possible mechanism could be Ashwagandha's GABA mimetic action, stimulating gonadotropin-releasing hormone and thus improving the hormonal balance (12).
Given these potential effects of ARE supplementation on reproductive health in women, the present study evaluated the efficacy and safety of ARE managing menopausal symptoms in women.
2 Materials & methods
2.1 Study design
This was a 56-day, prospective, randomized, double-blind, placebo-controlled, parallel clinical trial designed to evaluate the efficacy and safety of ARE in women with menopausal symptoms. The trial was conducted at Govt. Medical College & Govt. General Hospital (Old RIMSGGH), Srikakulam, Andhra Pradesh, India, between February 28, 2022, and November 30, 2022. Written informed consent was obtained from all participants in their preferred language before enrollment. A comprehensive explanation of the study objectives and expected outcomes was provided to each participant before obtaining consent. The enrollment of participants commenced on February 28, 2022. The study consisted of two site visits (baseline visit—day 1, end of study—day 56), and one telephonic follow-up visit (day 28). All assessments were carried out at the study center during visit 1 (day 1) and visit 3 (day 56), while a telephonic follow-up visit was conducted to verify drug and protocol compliance and collect details on any adverse events (AEs) without any formal assessment. The 56 days (8 weeks) were deemed to capture meaningful changes in key biochemical and psychological endpoints while ensuring optimal participant compliance and safety (10, 11).
2.2 Ethical approvals
The clinical study protocol was approved by the Institutional Ethics Committee (IEC), Govt. Medical College & Govt. General Hospital (Old RIMSGGH). The trial was prospectively registered with the Clinical Trials Registry of India (CTRI) with registration number CTRI/2022/02/040551. The study was conducted as per the ethical principles outlined in the Declaration of Helsinki (2013 revision), Good Clinical Practice (GCP) guidelines, and the Consolidated Standards of Reporting Trials (CONSORT) statement.
2.3 Study population
Inclusion criteria
Healthy women aged 45–55 years with a clinical diagnosis of menopause with no history of hormone therapy or antidepressant treatment in the past 3 months were included in the study. Demographic parameters included a body mass index (BMI) within the range of 20–30 Kg/m2 and a history of 10 or more hot flash events per week. Participants who were willing to follow the procedures as per the study protocol and agreed to take the investigational product till Day 56, and had the capability of complete compliance and completion of follow-up, were included in the study.
Exclusion criteria
Women with severe anemia, previous treatment with hormonal therapy, a history of any bleeding disorders, breast, endometrial, or other gynecological cancer at any time, or any other cancer within the last 5 years were excluded from the study. Participants who had a medical history of smoking, alcoholism, and drug dependence, or hypersensitivity to ARE, were excluded from the study. Participants using vitamin or mineral supplements, nutritional supplements, and or medical foods, estrogen, selective serotonin reuptake inhibitors within 30 days, using prescription medications for acute medical conditions, semi-acute medical conditions, and weight loss were not included in the study. Participants who were pregnant and breastfeeding were excluded. Participants with uncontrolled, unstable comorbidities or taking part in any other clinical trials, or any other condition that the principal investigator believed could compromise the safety of patients, were excluded.
After providing informed consent, 60 women who met the inclusion and exclusion criteria were enrolled in the study.
2.4 Sample size calculation
The sample size was calculated based on the primary endpoint of the study. The sample size calculation incorporated testing the hypothesis with a 5% level of significance and adequate power. The assumptions for the parameters, the effect size, and the standard deviation were based on past similar studies. Assuming the mean change in Menopause Rating Scale (MRS) score as 1.67 and 2.1 in the ARE and placebo (PL) arms respectively, the common standard deviation as 0.48, two-sided level of significance as 5%, and power as 90%, the study needed 54 evaluable subjects (27 subjects in each arm) to show superiority of ARE over PL in terms of change in MRS score. However, 60 women (30 in each group) participated in the study.
2.5 Randomization & blinding
Block Randomization was carried out using an automated random number generation system (Rando version 1.2 R) with 1:1 allotment. Participants underwent assessments at baseline and day 56. To ensure blinding, ARE and PL capsules were identical in appearance, shape, color, and packaging. The randomization codes were securely concealed in separate envelopes and were only accessed by the investigator after assigning a study number to each participant. The investigator and all personnel involved in data collection and statistical analysis remained blinded to the treatment allocation throughout the study.
2.6 Study intervention
Dried roots of Withania somnifera (L.) Dunal [Solanaceae; Withaniae radix] was used in this study. Participants received an ARE capsule (a light brown capsule containing 300 mg Ashwagandha root extract powder, Ixoreal Biomed Inc., Los Angeles, USA) (ARE, n = 30) or an identical PL capsule (a light brown capsule containing 300 mg starch) (Placebo group, PL, n = 30) in a 1:1 ratio as per the randomization schedule. Participants were instructed to take one capsule twice daily after meals (breakfast and dinner) with water for 56 days.
2.7 Investigational product details
The investigational product KSM-66 Ashwagandha was obtained from the manufacturer Ixoreal Biomed Inc., Los Angeles, California, USA. KSM-66 is the commercially available highest concentration root-only extract of Ashwagandha, which is produced through a green chemistry (aqueous-extraction process) method that is devoid of any alcohol or chemical solvents. The product is a light yellowish powder and does not have any carcinogenic, teratogenic, or mutagenic effects. The product contains a root-only extract from Ashwagandha, with an optimum amount of withanolides (>5%) precisely estimated by the High-Performance Liquid Chromatography (HPLC) method (Supplementary Figure S1) with an herb to extract ratio of 12:1. The herbs are grown in regions with optimum rainfall (650 mm–750 mm) and proper soil conditions (pH: 7.5–8.0). The PL contained starch powder. Both the products (placebo and the Ashwagandha root extract) were used in the form of an identical gelatin capsule, which was invariable in color, shape, and size. The dosage used for the present study was 300 mg capsules twice daily, both for the Ashwagandha and PL groups. The Ashwagandha root extract used in this study has been classified as Extract Type A in accordance with the Consensus statement on the reporting of pharmacology and physiology studies in natural product research (ConPhyMP) (14). The classification was confirmed using the ConPhyMP interactive tool (https://ga-online.org/best-practice/#conphymp) under the domain of Phytochemical Characterization of Medicinal Plant Extracts.
2.8 Study outcomes
Participants were assessed at baseline (visit 1) and day 56 (visit 3) by a trained clinician. At each visit, vital signs were recorded, including systolic and diastolic blood pressure, pulse rate, respiratory rate, and body temperature. Along with these, blood samples were collected to evaluate hematological parameters, liver and kidney functions, and lipid profile.
2.8.1 Primary outcome measures
The MRS is a standardized Health-Related Quality of Life Scale (HRQoL) measure with good psychometric characteristics (15). The MRS and the mean change in the MRS from baseline were descriptively summarized for each visit and treatment.
2.8.2 Secondary outcome measures
2.8.2.1 Change in hormonal parameters and hot flashes
Blood samples were collected at baseline and day 56 to measure serum hormone levels. Hormonal evaluations included serum estradiol, progesterone, LH, and FSH. Samples were drawn into both Ethylenediaminetetraacetic Acid (EDTA) and non-EDTA vials, then centrifuged, and the serum was stored at −80 °C for subsequent analysis. The hormone levels were measured using enzyme-linked immunosorbent assay (ELISA) kits.
Hot flashes were evaluated based on their reported frequency and intensity. Participants rated the severity of each symptom on a scale from 0 (not at all bothersome) to 4 (very bothersome), reflecting their experience over the past month. Hot flashes were determined by comparing scores recorded at baseline and at the end of the study.
2.8.2.2 Change in SF-12 (quality of life)
The 12-Item Short Form Health Survey (SF-12) (16) is used to measure quality of life related to health. This survey produced two main scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). A known scoring systems was used to figure out these scores. A score of 50 or more means that health is better than average. A score of 40–49 means that health is slightly worse than average. A score of less than 40 means that health is really bad, either physically or mentally. Scores below 30 show that health-related quality of life is very bad. These interpretations were used to look at and compare the physical and mental health of all the people in the study.
2.8.2.3 Patient global assessment of tolerability to therapy (PGATT)
It was assessed based on a four-point rating scale as follows: 1 = excellent tolerability, 2 = good tolerability, 3 = average tolerability, and 4 = poor tolerability. This scoring was done by the patients. The adherence to the assigned regimen was assessed and verified by study personnel through recording the dosing of the investigational products (17).
2.8.2.4 Perceived stress scale (PSS-10)
The Perceived Stress Scale (17) score is a 10-item questionnaire designed to evaluate the degree to which individuals perceive their lives as stressful over the past month. Each item is rated on a five-point Likert scale ranging from “never” to “very often”.
2.8.3 Safety assessment
Adverse events observed by the investigator or reported by the participants were documented for each participant throughout the study as part of a safety evaluation. Serum biochemical parameters were assessed at baseline and day 56 for any effects on kidney (serum creatinine, blood urea nitrogen) and the liver (serum alanine transaminase [ALT], aspartate transaminase [AST], alkaline phosphatase [ALP], bilirubin). Hematological assessments included haemoglobin, Red Blood Cell count, haematocrit, total leukocyte count, lymphocytes, monocytes, eosinophils, basophils, absolute neutrophils, and platelet count.
2.9 Statistical methods
All relevant statistical calculations were carried out with Stata 13.0 IC (Stata Corp. USA). Since all women completed the study as per the study protocol, efficacy and safety analysis were performed on all patient's dataset (n = 60). The analyses used two-sided tests and a p-value of less than 0.05 was considered statistically significant. For categorical variables, the number and percentage of subjects within each category (with category for missing data as needed) were provided and p-values were calculated and compared using the Chi-square test between the groups (ARE vs. PL). For continuous variables, the number of subjects, mean, median, standard deviation (SD), minimum and maximum values were provided. Paired t-test was used for the within group (Baseline-Day 56) comparison, Two-independent samples t-test was used for the between group (ARE vs. PL) comparison at Baseline and Day 56 and Analysis of Covariance (ANCOVA) was used for between-group comparisons (ARE vs. PL) after controlling for baseline.
3 Results
A total of 73 participants were screened for eligibility for enrollment. Of these, 60 participants met the inclusion criteria and were randomized in a 1:1 ratio to receive either ARE (n = 30) or PL (n = 30).
During the study period, no participants from either group were withdrawn due to follow-up loss or failure of medication adherence (Figure 1). Thus, for efficacy and safety data analysis, the Per protocol (PP) population and Intent to treat (ITT) population are ARE (n = 30) and PL (n = 30), respectively. A consort flow diagram illustrating the participant disposition is presented in Figure 1.
Figure 1
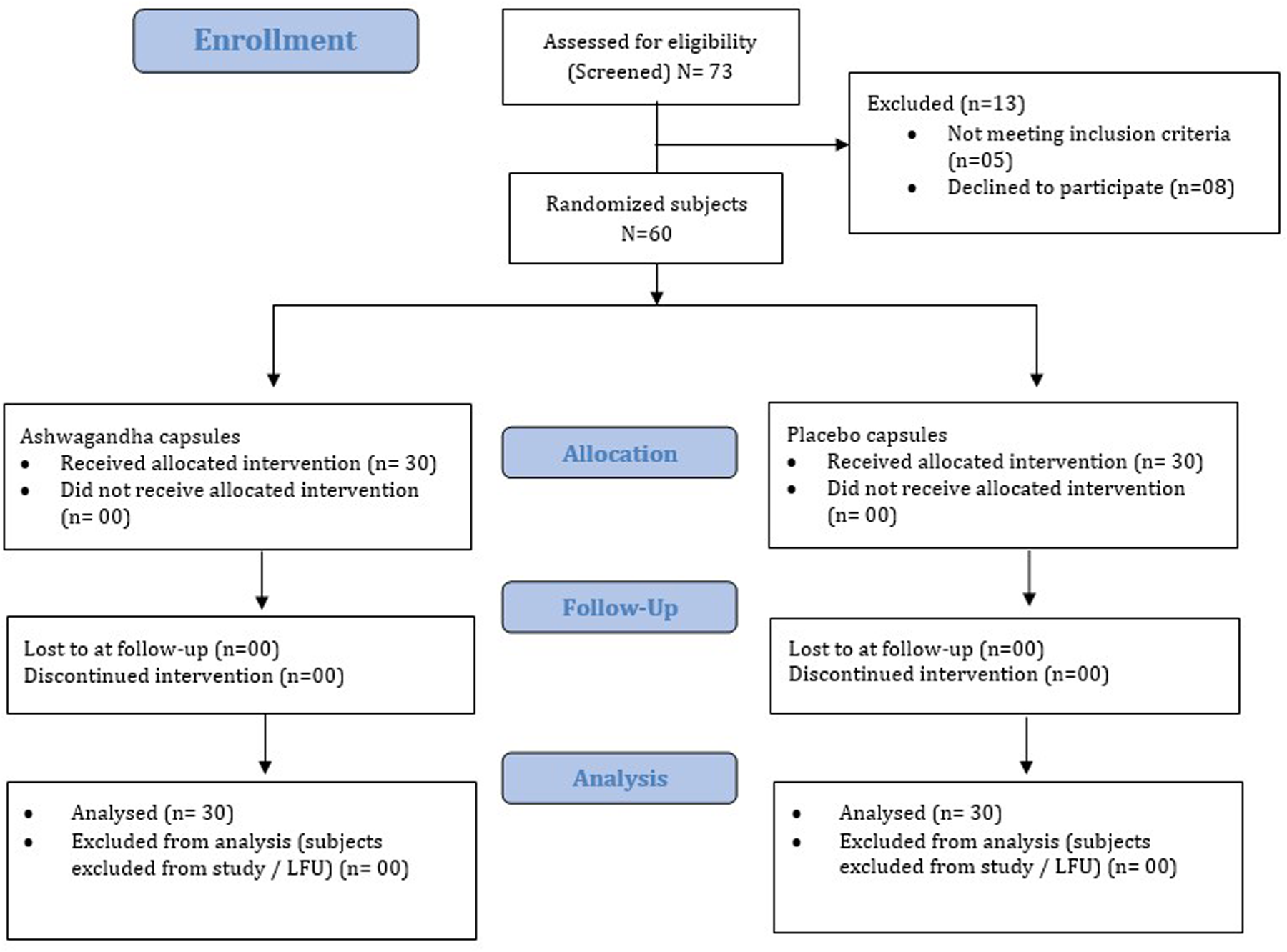
Consort flow representation of the patient enrollment, allocation, follow-up and analysis. LFU, lost to follow-up.
3.1 Demographics and baseline data (ITT dataset)
Table 1 presents the baseline demographic and medical history profile for randomized participants (n = 60) included in the ITT analysis. No statistically significant difference between ARE and PL groups was detected, for any measurable parameters at baseline. The mean age for ARE was 49.8 ± 2.4 years and for PL was 50.0 ± 2.3 years (p = 0.700), indicating no significant difference. The mean BMI for ARE was 24.9 ± 2.4 and for PL was 25.0 ± 2.5 (p = 0.955), also showing no significant difference. No statistically significant difference between ARE and PL groups was detected in the prevalence of co-morbidities such as anxiety (ARE: 6.7%, PL: 3.3%, p = 0.549), constipation (ARE: 3.3%, PL: 0%, p = 0.319), diabetes (ARE: 6.7%, PL: 3.3%, p = 0.549), hypertension (ARE: 26.7%, PL: 16.7%, p = 0.351), and obesity (ARE: 50%, PL: 43.3%, p = 0.605).
Table 1
| Characteristic | ARE group (n = 30) | PL group (n = 30) | p Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 49.8 ± 2.4 | 50.0 ± 2.3 | 0.700 |
| BMI (kg/m2) | 24.9 ± 2.4 | 25.0 ± 2.5 | 0.955 |
| Medical History | |||
| Anxiety, n (%) | 2 (6.7%) | 1 (3.3%) | 0.549 |
| Constipation, n (%) | 1 (3.3%) | 0 (0%) | 0.319 |
| Diabetes, n (%) | 2 (6.7%) | 1 (3.3%) | 0.549 |
| Hypertension, n (%) | 8 (26.7%) | 5 (16.7%) | 0.351 |
| Obesity, n (%) | 15 (50.0%) | 13 (43.3%) | 0.605 |
Demographic and medical history characteristics of participants at baseline.
Values are presented as mean ± standard deviation or number (%). ARE, Ashwagandha Root Extract; PL, Placebo Group.
3.2 Vital parameters
Table 2 shows the vital parameters at baseline (day 1) and end of study period (day 56) with no significant difference between the two groups (p > 0.05). The physical examination of patients in both groups was found to be within normal limits.
Table 2
| Vital parameters and laboratory values | ARE (n = 30) | PL (n = 30) | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 56 | p | Day 1 | Day 56 | p | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| SBP (mmHg) | 142.50 (20.5) | 140.8 (19.6) | 0.748 | 129.8 (16.8) | 130.0 (16.4) | 0.969 |
| DBP (mmHg) | 90.0 (10.7) | 88.8 (9.3) | 0.653 | 84.5 (7.8) | 84.3 (7.2) | 0.932 |
| Pulse Rate (/min) | 95.3 (15.4) | 94.1 (15.4) | 0.770 | 84.7 (7.0) | 84.9 (7.0) | 0.927 |
| Respiration Rate (/min) | 20.5 (2.8) | 20.4 (2.9) | 0.858 | 19.4 (2.7) | 19.4 (2.7) | >0.999 |
| Temperature (˚C) | 36.5 (0.4) | 36.5 (0.4) | >0.999 | 36.6 (0.4) | 36.6 (0.4) | 0.812 |
| Haemoglobin (g/dL) | 12.2 (0.8) | 12.6 (1.0) | 0.177 | 11.7 (0.9) | 11.7 (0.9) | 0.753 |
| RBC count (x10^6/µL) | 4.7 (0.5) | 4.8 (0.5) | 0.336 | 4.7 (0.6) | 4.6 (0.5) | 0.823 |
| Haematocrit (PCV) (%) | 42.9 (3.5) | 43.2 (3.2) | 0.79 | 43.0 (3.4) | 41.1 (4.7) | 0.084 |
| TLC (cells/µL) | 4,740.0 (447.7) | 4,736.7 (426.3) | 0.997 | 4,846.7 (337.1) | 4,693.3 (865.4) | 0.37 |
| Lymphocytes (%) | 29.2 (5.7) | 29.1 (5.4) | 0.926 | 32.3 (6.6) | 32.9 (6.4) | 0.752 |
| Monocytes (%) | 4.7 (1.7) | 4.7 (1.7) | 0.958 | 5.5 (1.3) | 5.4 (1.3) | 0.775 |
| Eosinophils (%) | 3.3 (1.3) | 3.4 (1.2) | 0.757 | 3.1 (1.0) | 3.2 (1.0) | 0.518 |
| Basophils (%) | 0.5 (0.4) | 0.5 (0.3) | 0.848 | 0.5 (0.2) | 0.5 (0.2) | 0.533 |
| Absolute Neutrophils (%) | 3,930.0 (490.0) | 3,933.3 (488.0) | 0.979 | 3,916.7 (472.2) | 3,893.3 (441.1) | 0.844 |
| Platelet Count (cells/µL) | 1,59,833.3 (10,627.2) | 1,60,350.0 (9,150.2) | 0.841 | 1,59,566.7 (12,475.3) | 1,60,100.0 (0.5) | 0.861 |
| Total bilirubin (mg/dL) | 0.5 (0.3) | 0.5 (0.3) | 0.897 | 0.6 (0.2) | 0.6 (0.2) | 0.46 |
| AST/SGOT (U/L) | 12.2 (3.9) | 11.7 (3.5) | 0.553 | 12.3 (3.4) | 13.0 (3.2) | 0.44 |
| ALT/SGPT (U/L) | 10.3 (3.5) | 10 (3.2) | 0.7 | 12.4 (3.2) | 13.1 (3.4) | 0.393 |
| ALP (U/L) | 101.4 (13.6) | 103.9 (15.5) | 0.51 | 101.5 (17.3) | 103.5 (20.1) | 0.681 |
| BUN (mg/dL) | 9.5 (2.0) | 9.5 (2.0) | >0.999 | 9.4 (1.5) | 9.4 (1.5) | >0.999 |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.8 (0.2) | 0.803 | 0.7 (0.2) | 0.7 (0.2) | 0.702 |
| Total Cholesterol (mg/dL) | 192.0 (30.3) | 192.0 (30.3) | >0.999 | 190.8 (16.2) | 191.8 (16.3) | 0.824 |
Vital parameters and laboratory values at baseline and end of study.
ARE, Ashwagandha Root Extract; PL, Placebo; SD, Standard Deviation; PCV, Packed Cell Volume; TLC, Total Leukocyte Count; AST/SGOP, Aspartate aminotransferase; ALT/SGPT: Alanine aminotransferase; ALP, Alkaline phosphatase; BUN, Blood Urea Nitrogen.
3.3 Efficacy assessment
3.3.1 Menopause rating scale
Table 3 presents the MRS and its sub-domain scores at baseline (day 1) and end of study (day 56). A significant reduction in all sub-domain scores, such as psychological, somatic, and urogenital scores, was seen in the ARE group as compared to the PL group (p < 0.0001). There was a significant reduction in mean (SD) of the total MRS scores with ARE from 31.37 (1.45) at baseline to 18.53 (2.29) at day 56, compared to PL from 30.73 (2.50) to 30.03 (2.55), with a p value of <0.0001. The effect sizes (Cohen's d) observed across all domains of the MRS indicate a consistently strong and clinically meaningful impact of the intervention in the ARE group compared to the PL group over 56 days. Figure 2 presents the percentage change from baseline to Day 56 for the individual domain and the total score.
Table 3
| MRS and sub-domain scores | ARE (N = 30) | PL (N = 30) | Difference | Effect size Cohen’s “d” | p-Value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Psychological score | =d/SD | ||||
| Baseline | 12.77 (1.41) | 12.58 (1.42) | 0.19 | 0.13 | 0.605 |
| Day 56 | 7.07 (1.05) | 12.33 (1.52) | −5.26 | −5.01 | <0.0001* |
| Change from baseline | −5.70 (1.97) | −0.25 (0.55) | −5.45 | −2.76 | <0.0001* |
| Somatic score | |||||
| Baseline | 12.03 (1.25) | 11.87 (1.45) | 0.16 | 0.13 | 0.6488 |
| Day 56 | 6.97 (1.07) | 11.77 (0.90) | −4.80 | −4.49 | <0.0001* |
| Change from baseline | −5.06 (0.99) | −0.10 (3.01) | −4.96 | −1.65 | <0.0001* |
| Urogenital score | |||||
| Baseline | 6.57 (0.82) | 6.28 (1.13) | 0.29 | 0.35 | 0.2599 |
| Day 56 | 4.50 (1.01) | 5.93 (1.26) | −1.43 | −1.42 | <0.0001* |
| Change from baseline | −2.07 (1.04) | −0.35 (0.71) | −1.72 | −1.65 | <0.0001* |
| MRS total score | |||||
| Baseline | 31.37 (1.45) | 30.73 (2.50) | 0.64 | 0.44 | 0.235 |
| Day 56 | 18.53 (2.29) | 30.03 (2.55) | −11.50 | −5.02 | <0.0001* |
| Change from baseline | −12.84 (4.60) | −0.35 (0.71) | −12.14 | −2.64 | <0.0001* |
Menopause rating scale scores.
ARE, Ashwagandha Root Extract; PL, Placebo; SD, Standard Deviation; MRS, Menstrual Rating Scale.
Statistically significant at <0.0001.
Figure 2
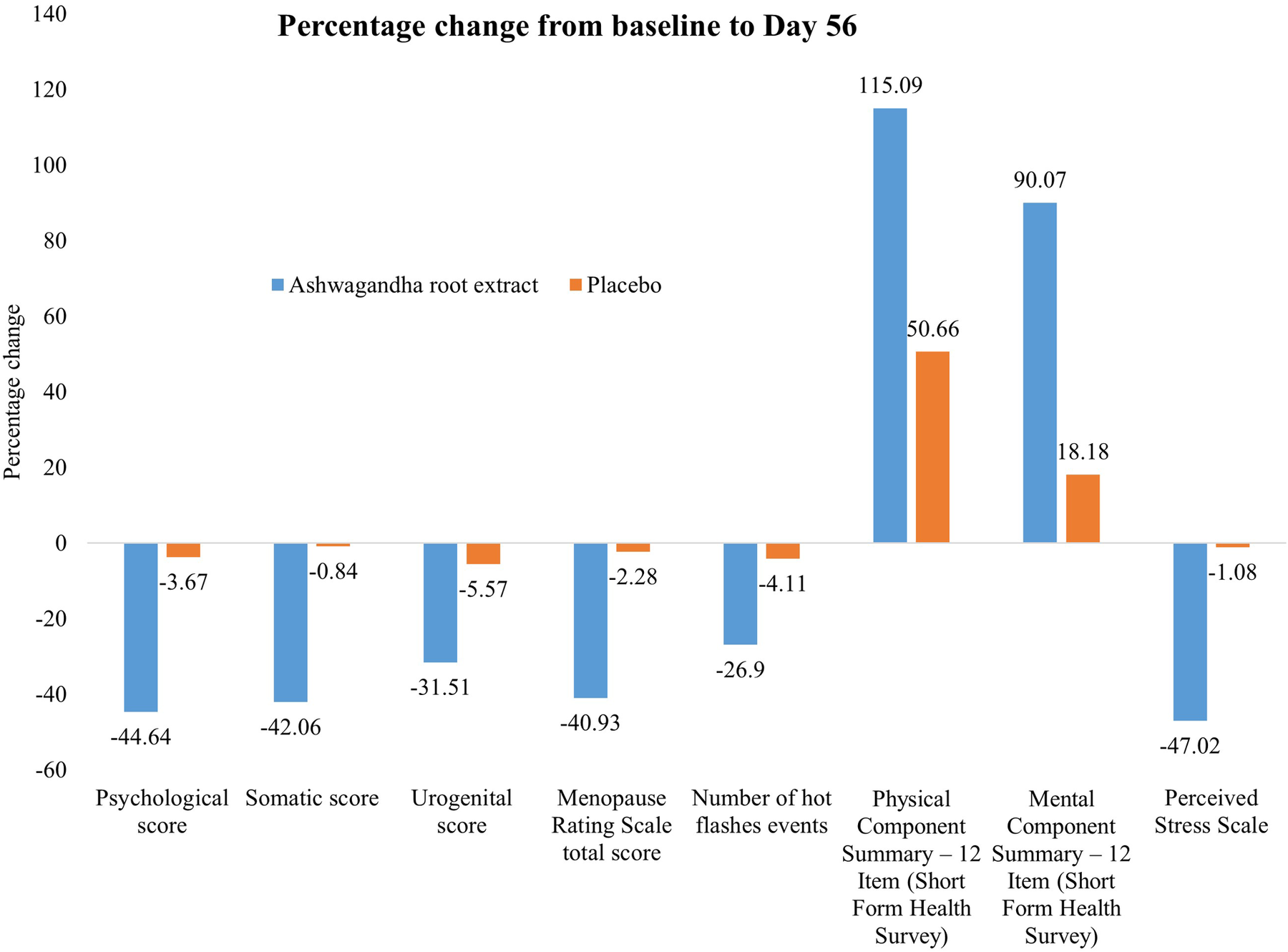
Effect of Ashwagandha on menopausal symptoms and quality of life over 56 days.
3.3.2 Hormone levels and hot flashes
Tables 4 presents serum hormone levels and the occurrence of hot flashes. There were statistically significant changes observed in all parameters between the two groups (p < 0.001). Serum estradiol and serum progesterone levels increased in the ARE group, while luteinizing hormone (LH) and FSH increased in the PL group. In both groups, there was no significant decrease in hot flashes score (number of events) at day 56 as compared to baseline (p > 0.05). Nevertheless, this reduction was statistically significant when compared between groups (p < 0.001). The effect size analysis across hormonal biomarkers, and hot flash frequency indicates a strong and consistent treatment effect in the ARE group compared to the PL group over 56 days. Figure 2 presents the percentage change from baseline to Day 56 for hot flashes.
Table 4
| Hormones (units) | ARE (N = 30) | PL (N = 30) | Difference | Effect size Cohen's “d” | “p” value between group | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||
| Serum estradiol (pg/mL) | Baseline—Mean (SD) | 22.20 (3.80) | 22.20 (3.80) | 0.00 | 0.00 | >0.999 |
| Day 56—Mean (SD) | 23.02 (3.50) | 21.6 0 (3.30) | 1.60 | 0.46 | 0.068 | |
| Change—Mean change ± SD (“p” value Within group) | 1.00 ± 6.00 (<0.001)* | −0.60 ± 6.00 (0.012)* | 1.60 | 0.27 | <0.001* | |
| Serum progesterone (ng/mL) | Baseline—Mean (SD) | 0.28 (0.08) | 0.30 (0.07) | −0.02 | −0.30 | 0.229 |
| Day 56—Mean (SD) | 0.43 (0.19) | 0.30 (0.08) | 0.13 | 0.70 | 0.001* | |
| Change—Mean change ± SD (“p” value Within group) | 0.15 ± 0.96 (<0.001)* | −0.01 ± 0.39 (0.601) | 0.16 | 0.16 | <0.001* | |
| Serum LH (mIU/mL) | Baseline—Mean (SD) | 36.80 (8.60) | 34.80 (7.70) | 2.00 | 0.23 | 0.328 |
| Day 56—Mean (SD) | 35.50 (8.60) | 35.60 (7.70) | −0.10 | −0.01 | 0.948 | |
| Change—Mean change ± SD (“p” value Within group) | −1.30 ± 0.60 (<0.0001)* | 0.80 ± 0.60 (0.001)* | −2.10 | −0.35 | <0.001* | |
| Serum FSH (mIU/mL) | Baseline—Mean (SD) | 61.90 (16.10) | 58.20 (15.30) | 3.70 | 0.23 | 0.362 |
| Day 56—Mean (SD) | 60.90 (15.90) | 58.60 (15.40) | 2.30 | 0.14 | 0.574 | |
| Change—Mean change ± SD (“p” value Within group) | −1.00 ± 0.60 (<0.001)* | 0.40 ± 6.00 (0.034)* | −1.40 | −0.23 | <0.001* | |
| No. of hot flashes events | Baseline—Mean (SD) | 14.50 (2.00) | 14.60 (2.20) | −0.10 | −0.05 | 0.855 |
| Day 56—Mean (SD) | 10.60 (2.4) | 14.00 (2.30) | −3.40 | −1.42 | 0.078 | |
| Change—Mean change ± SD (“p” value Within group) | −3.90 ± 15.00 (<0.001)* | −0.60 ± 6.00 (0.001)* | −3.30 | −0.22 | <0.001* | |
Hormone levels and scores for hot flashes.
ARE, Ashwagandha Root Extract; PL, Placebo; SD, Standard Deviation; FSH, Follicle stimulating hormone; LH, Luteinizing hormone; IU, International units; dL, Deciliter.
Statistically significant.
3.3.3 Short form survey (SF-12)
Table 5 presents the SF-12 (PCS-12, MCS-12) scores at baseline (day 1) and the end of study period (day 56). There was marked improvement in all SF-12 scores in both groups and between the groups (p < 0.001). Assessment of quality of life (SF-12) over 56 days revealed significant and clinically meaningful improvements in the ARE group compared to PL, as reflected in large between-group effect sizes and statistically robust differences. Figure 2 presents the percentage change from baseline to Day 56.
Table 5
| Parameters (scales) | ARE (N = 30) | PL (N = 30) | Difference | Effect size Cohen’s “d” | “p” value between group | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||
| PCS-12 (SF-12) | Baseline—Mean (SD) | 23.20 (1.50) | 22.70 (1.10) | 0.50 | 0.33 | 0.193 |
| Day 56—Mean (SD) | 49.90 (2.00) | 34.20 (3.90) | 15.70 | 7.85 | <0.001* | |
| Change—Mean change ± SD (“p” value Within group) | 26.7 ± 15.00 (<0.001*) | 11.5 ± 21.00 (<0.001*) | 15.20 | 0.72 | <0.001* | |
| MCS-12 (SF-12) | Baseline—Mean (SD) | 28.20 (7.9) | 30.80 (6.60) | -2.60 | -0.33 | 0.175 |
| Day 56—Mean (SD) | 53.60 (2.5) | 36.40 (6.50) | 17.20 | 6.88 | <0.001* | |
| Change—Mean change ± SD (“p” value Within group) | 25.50 ± 45.00 (<0.001*) | 5.60 ± 36.00 (<0.001*) | 19.90 | 0.44 | <0.001* | |
| PSS | Baseline—Mean (SD) | 28.50 (3.10) | 27.70 (3.10) | 0.80 | 0.26 | 0.281 |
| Day 56—Mean (SD) | 15.10 (3.00) | 27.40 (3.00) | -12.30 | -4.10 | <0.001* | |
| Change—Mean change ± SD (“p” value Within group) | -13.40 ± 21.00 (<0.001*) | -0.20 ± 6.00 (0.326) | -13.20 | -0.63 | <0.001* | |
SF-12 (PCS-12, MCS-12) and PSS scores.
ARE, Ashwagandha Root Extract; PL, Placebo; SD, Standard Deviation; PCS-12; Physical component score; MCS-12; Mental component score; PSS, Perceived stress scale.
Statistically significant.
3.3.4 Patients global assessment of tolerability to therapy (PGATT)
Table 6 presents the Patient's Global Assessment of Tolerability to Therapy (PGATT) parameters. Most of the patients in the ARE group reported good to excellent tolerance for ARE compared to PL (p < 0.001). A total of twenty-eight patients (93.3%) in the ARE group rated the tolerability of ARE as “good” to “excellent”. Poor tolerability was reported in 15 participants (50.0%) in the PL group. Figure 3 presents the percentage tolerability of ARE in comparison to PL.
Table 6
| PGATT parameters | ARE (N = 30) | PL (N = 30) | Chi-square Test | |
|---|---|---|---|---|
| No. (%) | No. (%) | χ2 | “p” | |
| Excellent Tolerability | 13 (43.3%) | 0 (-) | 22.941 | <0.001* |
| Good Tolerability | 15 (50.0%) | 15 (50.0%) | ||
| Average Tolerability | 0 (-) | 0 (-) | ||
| Poor Tolerability | 2 (6.7%) | 15 (50.0%) | ||
PGATT parameters at baseline and end of study period.
ARE, Ashwagandha Root Extract; PL, Placebo.
Statistically significant.
Figure 3
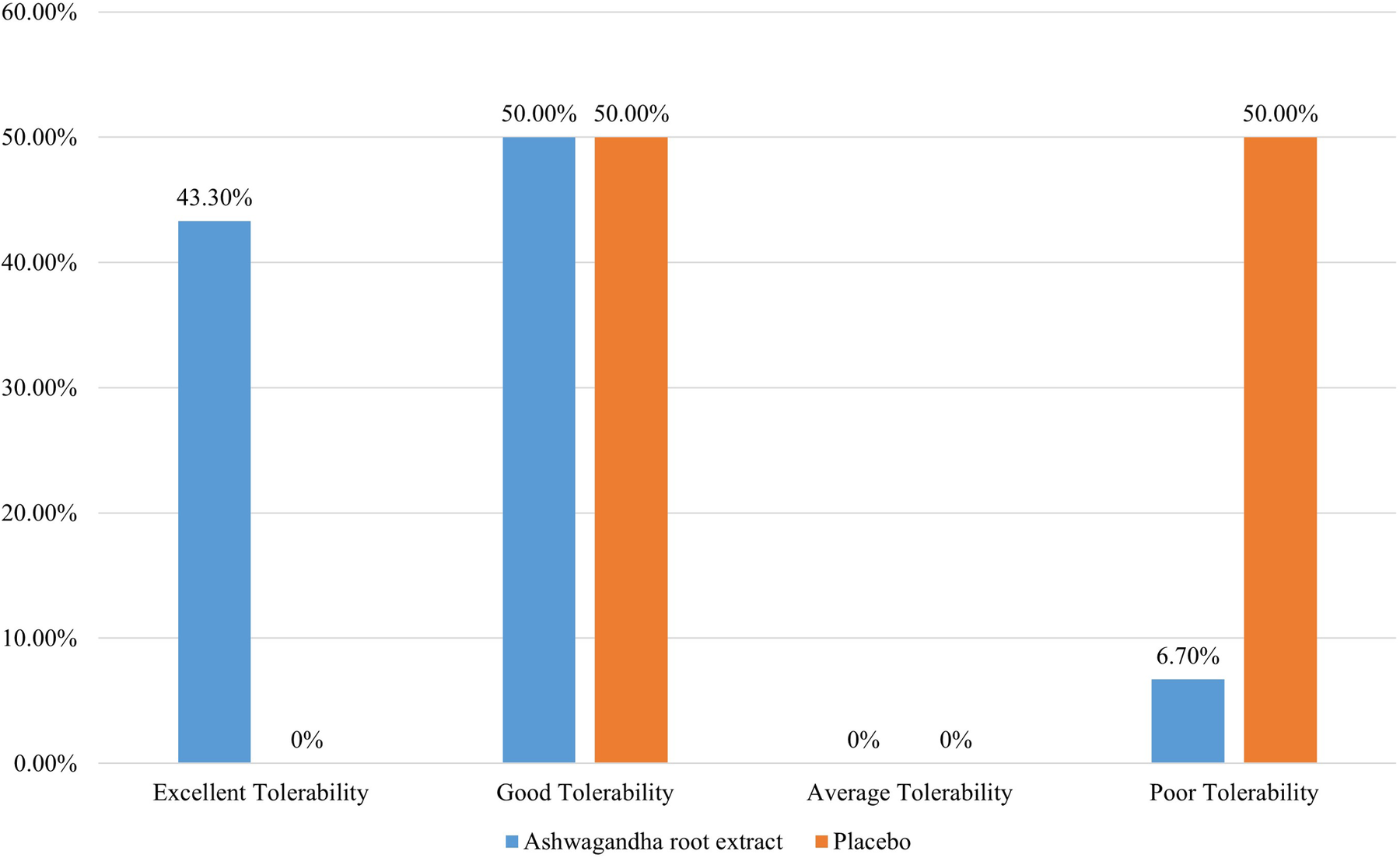
Patients global assessment of tolerability to therapy.
3.3.5 Perceived stress scale (PSS-10)
Table 5 shows the PSS-10 scores at baseline (day 1) and end of study (day 56). There was a significant improvement in all PSS-10 score in the ARE group and changes were statistically significant between the groups (p < 0.001). There was reduction (improvement) in mean (SD) PSS-10 score from baseline to day 56 in ARE group (p < 0.001) with a negligible, statistically non-significant, change from baseline to day 56. Assessment of perceived stress using the PSS over 56 days showed a significant and clinically relevant reduction in the ARE group compared to the PL group. Figure 2 presents the percentage change from baseline to Day 56.
3.4 Safety assessment and treatment compliance
A total of 3 women (1 in the ARE group and 2 in the PL group) reported adverse events. One woman in the ARE group reported a cough and cold, while two women in the PL group reported stomachache and indigestion, respectively. All events were of mild severity, were not associated with the study treatments, and completely resolved with or without symptomatic treatment. None of the women discontinued treatment due to adverse events, and treatment compliance was 100% in both groups.
4 Discussion
Menopause typically occurs between the ages of 45–52 years and is identified by changes in hormones that lead to the stoppage of menstrual cycles (18, 19). By 2030, almost 1.2 billion women globally will be menopausal or postmenopausal, with 47 million new entrants annually. Menopause lasts for one-third of a woman's life (20, 21). Vasomotor symptoms, experienced by over 80% of women, can last from 5 to 15 years, significantly impacting sleep, mood, cognition, and quality of life (22–24). MHT has historically been the primary treatment, while beneficial effects on vasomotor dysfunction have been reported with estrogen, gabapentin, paroxetine and clonidine, but health concerns lead many women to seek alternative choices (25).
A study conducted by Gopal et al. demonstrated the effects of 300 mg of Ashwagandha root extract twice daily on climacteric symptoms in perimenopausal women for eight weeks (10). Of these 91 participants, 46 women received Ashwagandha and showed significant improvements in symptoms within four weeks, including hot flashes. At 8 weeks, the Ashwagandha group showed a decrease in overall MRS scores, and a statistically significant increase in serum estradiol and a decrease in serum FSH and LH levels compared to the PL group (11). A study by Modi et al., evaluated Ashokarishta, Ashwagandha Churna, and Praval Pishti in managing menopausal symptoms, with the result showing reductions in MRS and Menopause Specific Quality of Life (MENQoL) questionnaire scores in 51 women (26). One of the study reported better results with Shatavari when compared to Ashwagandha (27). The current study aligns with similar findings, where a statistically significant reduction in mean MRS score from 31.37 to 18.53 was noted (p < 0.001). This was associated with a statistically significant increase in serum estradiol (p < 0.001) and progesterone (p < 0.001), and a significant reduction in serum FSH (p < 0.001) and LH (p < 0.001) concentrations, as compared with the PL. The statistically significant hormonal changes observed in this study may result from both a direct endocrine effect and an indirect effect mediated by a reduction in physiological stress (23, 24).
There was also a statistically significant reduction in hot flash events (p < 0.05) in the ARE group, as compared to the PL group. While previous studies used MENQoL for quality-of-life assessment, the present study used SF-12 and PSS-10 scales to focus on physical, mental, and psychological well-being. The present study results showed improvement in the quality of life of menopausal women in the ARE group, reflecting statistically significant improvements in PCS-12, MCS-12, and PSS-10 scores (p < 0.001).
ARE is reported to be safe for human use with a daily dosage of up to 1,000 mg (27). The present data support this finding, as no serious adverse events related to ARE were noted and more than 90% of the participants reported tolerability of ARE as good to excellent. Further, treatment compliance was 100% in the present study. These findings add crucial evidence to the ARE safety and efficacy assessment in addressing menopause symptoms.
The observed benefits of ARE in menopausal women may be attributed to its multifaceted mechanisms of action. Ashwagandha is known for its adaptogenic properties, which help the body cope with physical and psychological stress, potentially through modulation of the hypothalamic-pituitary-adrenal axis (28). Additionally, its GABA-mimetic activity may contribute to improvements in sleep, mood, and anxiety, while anti-inflammatory and antioxidant pathways could alleviate systemic stress-related symptoms (7–9).
The strength of the present study lies in its robust randomized, double-blind, and placebo-controlled design, which enhances the reliability of the findings. The intervention used in this study was a branded ARE (KSM-66®). Therefore, the results may not be directly extrapolated to other ARE preparations that have different phytochemical profiles or standardization methods.
The limitations of the study include the short duration, small sample size, and limited ability to detect smaller effects. The cohort was homogeneous and well-defined, drawn from a specific cross-section of society, which, along with the study setting, may restrict the generalizability of the findings. Finally, blinding effectiveness was not formally assessed, although the intervention was low-risk and the outcomes were largely objective. While ARE demonstrated beneficial effects on stress and well-being in this study, it is important to consider the potential contribution of PL effects, which are commonly observed in interventions involving subjective outcomes such as perceived stress and quality of life. Future research conducted in a real-world setting with participants representing a wider range of demographics, occupations, and socio-economic statuses is necessary to validate and extend the applicability of the findings. In addition, the study's eight-week duration may limit the ability to conclude long-term benefits, warranting consideration in future investigations.
5 Conclusions
Ashwagandha (Withania somnifera) appears to be a promising herbal intervention for the management of menopausal symptoms. The present study demonstrated statistically significant improvement in the MRS scale, selected hormonal parameters, hot flash, SF-12 scale, and perceived stress, over a duration of 56 days without adverse effects. While these findings support the therapeutic potential of ARE, further well-powered, long-term clinical trials are warranted to confirm efficacy, elucidate the mechanism of action, and establish its use as an effective and safe alternative to hormone-based therapies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee (IEC), Govt. Medical College & Govt. General Hospital (Old RIMSGGH). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. GM: Data curation, Formal analysis, Project administration, Resources, Writing – review & editing. BRB: Data curation, Formal analysis, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Ixoreal BioMed Inc., Los Angeles, California, USA, for supplying the KSM-66 high-concentration root extract used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2025.1647721/full#supplementary-material
Abbreviations
ARE, ashwagandha root extract; PL, placebo; MHT, Menopausal Hormone Therapy; MRS, Menopause Rating Scale; HRQoL, Health-Related Quality of Life; MCS-12, Mental Component Score (from SF-12); PCS-12, Physical Component Score (from SF-12); SF-12, Short Form Survey-12; PSS-10, Perceived Stress Scale-10; FSH, Follicle-Stimulating Hormone; LH, Luteinizing Hormone.
References
1.
Nelson HD . Menopause. Lancet. (2008) 371(9614):760–70. 10.1016/S0140-6736(08)60346-3
2.
Mashiloane CD Bagratee J Moodley J . Awareness of and attitudes toward menopause and hormone replacement therapy in an African community. Int J Gynaecol Obstet. (2002) 76(1):91–3. 10.1016/s0020-7292(01)00565-3
3.
Cobin RH Futterweit W Ginzburg SB Goodman NF Kleerekoper M Licata AA et al American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. (2006) 12(3):315–37. 10.4158/EP.12.3.315
4.
Davis SR Pinkerton J Santoro N Simoncini T . Menopause—biology, consequences, supportive care, and therapeutic options. Cell. (2023) 186(19):4038–58. 10.1016/j.cell.2023.08.016
5.
Martin KA Manson JE . Approach to the patient with menopausal symptoms. J Clin Endocrinol Metab. (2008) 93(12):4567–75. 10.1210/jc.2008-1272
6.
Fait T . Menopause hormone therapy: latest developments and clinical practice. Drugs Context. (2019) 8:212551. 10.7573/dic.212551
7.
Wu CK Tseng PT Wu MK Li DJ Chen TY Kuo FC et al Antidepressants during and after menopausal transition: a systematic review and meta-analysis. Sci Rep. (2020) 10(1):8026. 10.1038/s41598-020-64910-8
8.
Mishra LC Singh BB Dagenais S . Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. (2000) 5(4):334–46.
9.
Singh N Bhalla M de Jager P Gilca M . An overview on ashwagandha: a Rasayana (rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. (2011) 8(5 Suppl):208–13. 10.4314/ajtcam.v8i5S.9
10.
Vetvicka V Vetvickova J . Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N Am J Med Sci. (2011) 3(7):320. 10.4297/najms.2011.3320
11.
Gopal S Ajgaonkar A Kanchi P Kaundinya A Thakare V Chauhan S et al Effect of an ashwagandha (Withania Somnifera) root extract on climacteric symptoms in women during perimenopause: a randomized, double-blind, placebo-controlled study. J Obstet Gynaecol Res. (2021) 47(12):4414–25. 10.1111/jog.15030
12.
Paul S Chakraborty S Anand U Dey S Nandy S Ghorai M et al Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed Pharmacother. (2021) 143:112175. 10.1016/j.biopha.2021.112175
13.
Mikulska P Malinowska M Ignacyk M Szustowski P Nowak J Pesta K et al Ashwagandha (Withania somnifera)—current research on the health-promoting activities: a narrative review. Pharmaceutics. (2023) 15(4):1057. 10.3390/pharmaceutics15041057
14.
Dongre S Langade D Bhattacharyya S . Efficacy and safety of Ashwagandha (Withania somnifera) root extract in improving sexual function in women: a pilot study. BioMed Res Int. (2015) 2015(1):284154. 10.1155/2015/284154
15.
Heinrich M Jalil B Abdel-Tawab M Echeverria J Kulić Ž McGaw LJ et al Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front Pharmacol. (2022) 13:953205. 10.3389/fphar.2022.953205
16.
Heinemann K Ruebig A Potthoff P Schneider HP Strelow F Heinemann LA et al The Menopause Rating Scale (MRS) scale: a methodological review. Health Qual Life Outcomes. (2004) 2:1–8. 10.1186/1477-7525-2-45
17.
Ware J Kosinski M Keller SD . A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34(3):220–33. 10.1097/00005650-199603000-00003
18.
Cohen S Kamarck T Mermelstein R . Perceived stress scale. Measuring stress: a guide for health and social scientists. (1994) 10(2):1–2. 10.1093/oso/9780195086416.001.0001
19.
Dash A Ravat S Srinivasan A . Evaluation of safety and efficacy of zonisamide in adult patients with partial, generalized, and combined seizures: an open labeled, noncomparative, observational Indian study. Ther Clin Risk Manag. (2016) 12:327–334.
20.
Sussman M Trocio J Best C Mirkin S Bushmakin AG Yood R et al Prevalence of menopausal symptoms among mid-life women: findings from electronic medical records. BMC Women’s Health. (2015) 15:1–5. 10.1016/0378-5122(81)90032-3
21.
Hill K . The demography of menopause. Maturitas. (1996) 23(2):113–27. 10.1016/0378-5122(95)00968-x
22.
Thurston RC Joffe H . Vasomotor symptoms and menopause: findings from the study of women’s health across the nation. Obstet Gynecol Clin North Am. (2011) 38(3):489. 10.1016/j.ogc.2011.05.006
23.
Dennerstein L Lehert P Burger HG Guthrie JR . New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update. (2007) 13(6):551–7. 10.1093/humupd/dmm022
24.
Thurston RC Chang Y Mancuso P Matthews KA . Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the study of women’s health across the nation. Fertil Steril. (2013) 100(3):793–800.e1. 10.1016/j.fertnstert.2013.05.005
25.
Utian WH . Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. (2005) 3:47. 10.1186/1477-7525-3-47
26.
Takahashi TA Johnson KM . Menopause. Med Clin North Am. (2015) 99(3):521–34. 10.1016/j.mcna.2015.01.006
27.
Modi MB Donga SB Dei L . Clinical evaluation of Ashokarishta, Ashwagandha Churna and Praval Pishti in the management of menopausal syndrome. Int Q J Res Ayurveda). (2012) 33(4):511–6. 10.4103/0974-8520.110529
28.
Kelgane SB Salve J Sampara P Debnath K . Efficacy and tolerability of Ashwagandha root extract in the elderly for improvement of general well-being and sleep: a prospective, randomized, double-blind, placebo-controlled study. Cureus. (2020) 12(2):e7083. 10.7759/cureus.7083
29.
Pingali U Nutalapati C Wang Y . Ashwagandha and Shatavari extracts dose-dependently reduce menopause symptoms, vascular dysfunction, and bone resorption in postmenopausal women: a randomized, double-blind, placebo-controlled study. J Menopausal Med. (2025) 31(1):21–34. 10.6118/jmm.24025
Summary
Keywords
Ashwagandha root extract, menopause, safety, efficacy, randomized controlled trial
Citation
Vani I, Muralidhar G and Rao BS (2026) A prospective, randomized, double-blind, placebo-controlled study on efficacy and safety of Ashwagandha root extract (Withania somnifera) for managing menopausal symptoms in women. Front. Reprod. Health 7:1647721. doi: 10.3389/frph.2025.1647721
Received
24 June 2025
Revised
23 October 2025
Accepted
26 November 2025
Published
05 January 2026
Volume
7 - 2025
Edited by
Ayman Al-Hendy, The University of Chicago, United States
Reviewed by
Shawn M. Talbott, 3 Waves Wellness, United States
Eleni Memi, National and Kapodistrian University of Athens, Greece
Andi Muh. Maulana, Universitas Muhammadiyah Purwokerto, Indonesia
Ashwinikumar Raut, Medical Research Centre of the Kasturba Health Society (MRC-KHS), India
Updates
Copyright
© 2026 Vani, Muralidhar and Rao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Isukapalli Vani vaniisukapalli6@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.