- 1National Institute of Mental Health, Centre of Perinatal Mental Health, Klecany, Czechia
- 2Department of Psychology, Faculty of Arts, Charles University, Prague, Czechia
- 3First Faculty of Medicine, Charles University, Prague, Czechia
- 4Department of Demography and Geodemography, Faculty of Science, Charles University, Prague, Czechia
- 5Department of Epidemiology, Second Faculty of Medicine, Charles University, Prague, Czechia
- 6Third Faculty of Medicine, Charles University, Prague, Czechia
Introduction: In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) patients often experience stress, which exacerbates the burden associated with infertility and may lead to an increased risk of mental-health difficulties. In this systematic review and meta-analysis, we examined the effects of IVF or ICSI on female patients’ mental health.
Methods: A database search was conducted in PubMed, Web of Science, and PsychInfo to select relevant studies. Forty-four studies involving 858,966 participants were included in the systematic review. The results of these studies were very heterogeneous and yielded contradictory findings. Two meta-analyses, comprising a total of seven studies, were conducted. The first calculated the standardized mean difference of symptoms of depression between women who became pregnant through IVF and those who conceived spontaneously. In the second, we compared symptoms of anxiety between these two groups.
Results: Five studies examined depressive symptoms and showed no significant difference between the two groups: SMD = −.15; 95% CI [−.33,.03], p = .10. A meta-analysis of six studies on anxiety symptoms revealed significantly higher levels in the IVF groups compared to the controls: SMD = .33; 95% CI [.17,.49], p < .001.
Discussion: The results suggest that the psychological effects of IVF/ICSI, especially with respect to anxiety, require attention and support from healthcare providers, although the effect size is small. Further studies with adequate sample sizes, including women with both successful and unsuccessful treatment, and adequately controlling for important confounders are needed to fully understand the effects of IVF/ICSI on mental health.
Systematic Review Registration: PROSPERO (CRD42023461472).
Highlights
• Higher levels of anxiety symptoms in the third trimester were found in women after IVF when compared to spontaneously pregnant women, although the effect size is rather small.
• No clear conclusion in terms of effects of IVF or ICSI on other of women's perinatal mental health outcomes could be made.
• Major limitation is omitting women whose treatment was unsuccessful.
Introduction
Global estimates indicate that around one in six people will deal with fertility problems at some point during their lifetimes (1). Furthermore, the prevalence of both male and female infertility is rising by over 1% per year (2), which has led to a dramatic increase in the use of assisted reproductive technologies (ART) over the past three decades (3).
The Centers for Disease Control and Prevention reports that over 230,000 patients underwent ART treatment which resulted in approximately 92,000 of over 3.6 million live births in the United States in 2021 (4, 5). In 2019, 3% of all live births in Europe resulted from pregnancies conceived with ART, and in some countries the rate surpassed 6%. In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are of the most frequently used ART methods (3).
IVF is often perceived as stressful (6), adding to the psychological strain inherently associated with infertility (7–9). Furthermore, in conventional IVF protocols, hormonal ovarian stimulation is used to enhance the production of oocytes (10), and this stimulation can have adverse effects on patients’ mood (11). These effects of ART on mental health may be persistent or lead to further difficulties, even when it results in conception (12).
Moreover, becoming pregnant itself entails significant changes in parents’ lives; even if the pregnancy is desired, this period of life is associated with increased proneness to developing mental health difficulties (13, 14). In the general population, around 15% of pregnant (15) and 14% postpartum (16) women suffer from major depression. The prevalence of any clinically diagnosable anxiety disorder in either pregnancy or postpartum is estimated at 15% (17). These difficulties can contribute to adverse birth outcomes (18), act as risk factors for subsequent mental-health problems (19, 20), impair bonding with the child (21), and result in health and developmental problems in the child (22, 23).
To prevent or mitigate these adverse outcomes, it is essential to understand the long-term effect of IVF on pregnant women's mental health. To our knowledge, the most recent meta-analysis regarding effects of ART on depressive symptoms was published by Chen et al. (24). Its focus, however, was relatively broad, as it included studies with a variety of designs which concerned any ART and measured depressive symptoms that occurred at any time point during the perinatal period. In their recent systematic review on a similar topic, Capuzzi et al. (25) highlighted the need for a meta-analysis to strengthen evidence and resolve their contradictory findings. To our knowledge, no meta-analysis has been published concerning the effects of ART on the symptoms of anxiety. In the present systematic review, we aimed to summarize the empirical evidence of the effects of IVF and ICSI on women's perinatal mental health. In the meta-analytical portion of the study, we assessed the effect of IVF on symptoms of depression and anxiety in the third trimester of pregnancy. This targeted analysis aimed to provide a more refined understanding of the relationship between IVF and perinatal mental health during this critical stage.
Methods
Search strategy
The systematic review and meta-analysis were conducted according to The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (26). A protocol was registered in the PROSPERO register (ID: CRD42023461472). We intended primarily to conduct a systematic review concerning the effects of IVF and ICSI on perinatal mental-health problems with the option of conducting meta-analysis focusing on a more detailed topic if the relevant data were available.
We performed a database search of studies published through September 2023 in PubMed, Web of Science, and PsychInfo. The search terms were: (“IVF” OR “in vitro fertilization” OR “in vitro fertilisation” OR “ICSI” OR “intracytoplasmic sperm injection”) AND (“mental health” OR “mental disorders” OR “distress” OR “anxi*” OR “depress*” OR “psychopathology” OR “emotional distress”). For the selection and data extraction of studies, the Covidence tool (27) was used.
Study selection
The abstracts and titles of the selected studies were screened, and the full texts of studies included in the first step were reviewed independently by two reviewers (HN and TB). In the case of conflict, a third reviewer (AH) decided on the study's inclusion.
The inclusion criteria were: (1) studies concerning female patients 18 years or older; (2) the intervention used was IVF or ICSI; (3) the outcome measures were symptoms of distress or diagnoses or symptoms of mental disorder; (4) the assessment of outcome measures occurred between the start of the treatment and one year postpartum. We included all studies published since 1978, when IVF was first introduced (28). The exclusion criteria were: (1) studies not in English; (2) animal studies; (3) the timing of assessment of outcome was not specified; (4) qualitative studies, reviews, or meta-analyses; (5) studies using solely native IVF cycle.
For the purposes of the meta-analysis, the included studies were screened for the most frequent study design used with participants of similar gestational age or time point postpartum. Consequently, the meta-analysis included studies that used self-reporting scales to assess symptoms of depression and/or state of anxiety. Participants were women in the third trimester of pregnancy who had undergone IVF and were compared to spontaneously pregnant women.
Data extraction
From the chosen studies, the following data were extracted independently by HN and TB using Covidence software (27): name of the lead author; year of publication; country in which the study was conducted; year of data collection; aim; study design; population description; inclusion criteria; exclusion criteria; use of a control group; characteristics of the control group; total number of participants; intervention used; outcome variable; timing of outcome assessment; and findings. For the purposes of statistical analyses, sample sizes, means and standard deviations of scores on scales measuring symptoms of depression and anxiety for both the IVF and control groups were compiled. Afterward, the data extracted by HN and TB were discussed, and a final consensus was reached.
Quality assessment
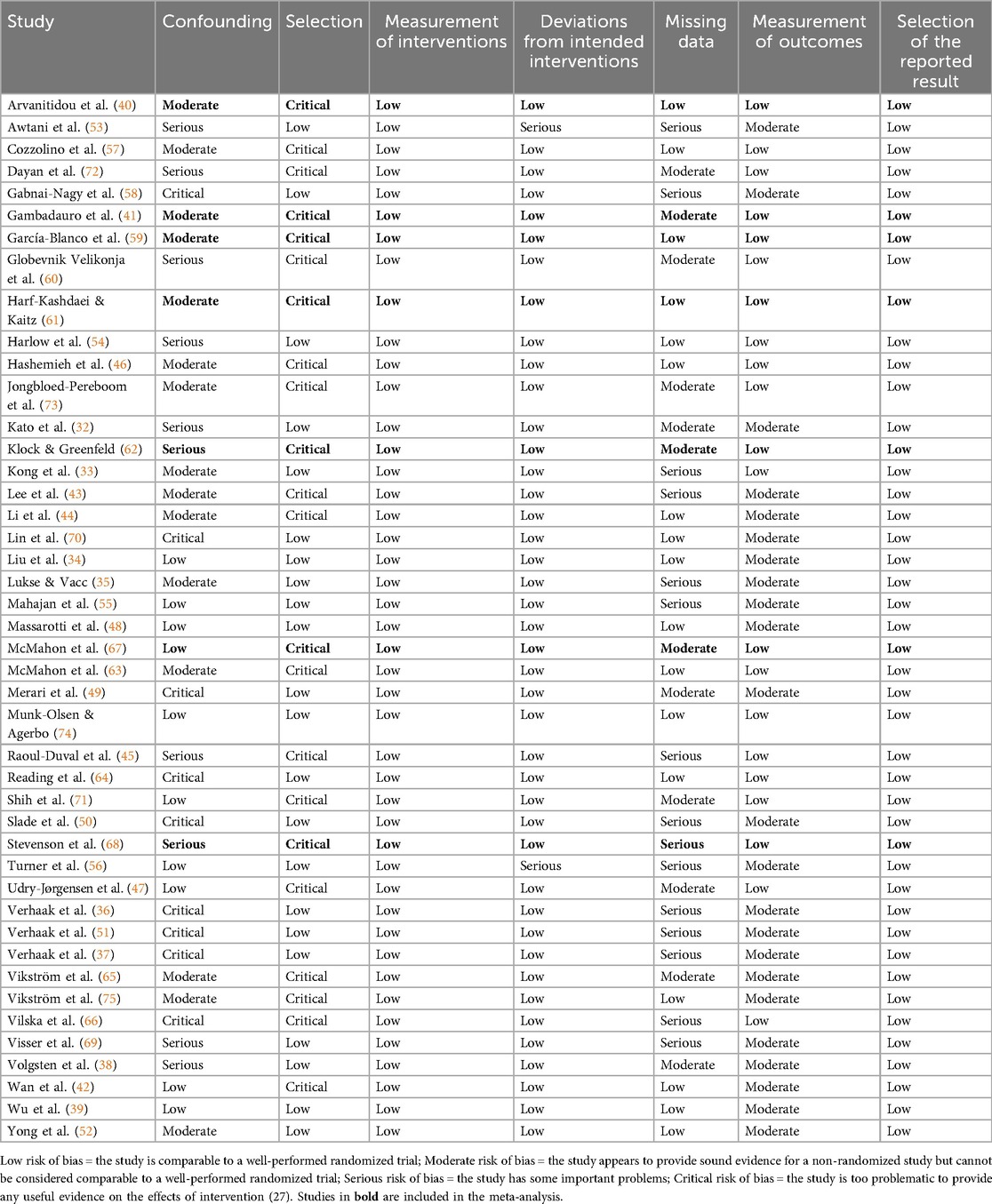
To assess the quality of the studies, the Risk of Bias in Non-randomized Studies of Intervention [ROBINS-I; (29)] was used. This tool is suitable for studies comparing the effects of interventions between groups without employing randomization. The bias of each study was evaluated as low, moderate, serious, or critical in seven domains.
Statistical analysis
To perform the statistical analysis, Stata 17.0 software was used. A Q-test was used to estimate the heterogeneity of included studies. The standardized mean difference between the IVF and control groups was assessed using a random-effects models (30) separately for symptoms of anxiety and symptoms of depression. The risk of publication bias was assessed using a fail-safe N test, Begg and Mazumdar rank correlation test, and Egger's regression test. A funnel plot was not used, as we included fewer than 10 studies (31).
Results
Literature search
As indicated in Figure 1, the database search yielded a total of 3,355 records: 1,816 from Web of Science, 1,064 from PubMed, and 475 from PsychInfo. After excluding 961 duplicate studies, the title and abstract screening involved 2,394 records, of which 2,226 were excluded and 31 did not have their full text available. A full-text assessment was conducted on 137 studies, of which 44 studies were included in the systematic review. Seven of these 44 studies were included in the meta-analyses: five in the meta-analysis on symptoms of depression and six in the meta-analysis on symptoms of anxiety.
Systematic review
Study characteristics
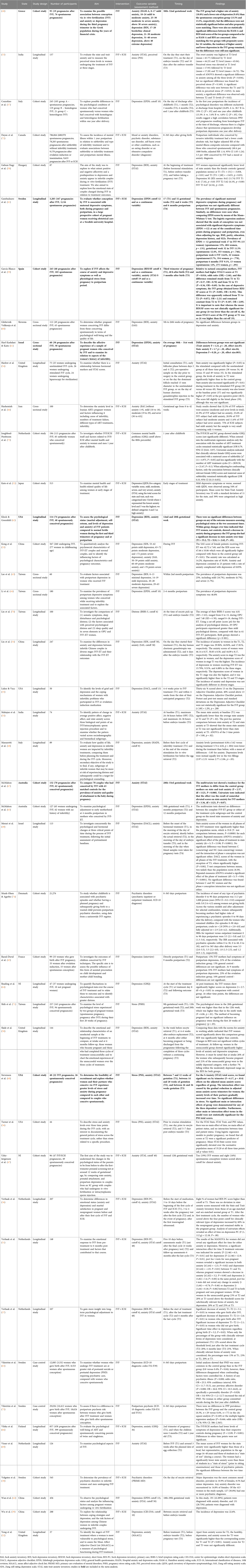
Table 1 presents the characteristics of the included studies. They involved 858,966 participants and sample sizes ranging from 37 to 786,064. Twenty-one studies were conducted in Europe, 14 in Asia, five in North America, and two in Australia; two studies did not provide location information. The most reported outcome variable was symptoms or diagnosis of depression (28 studies). Furthermore, symptoms or diagnosis of anxiety (27 studies), stress (5 studies), any mental health disorder or problems (4 studies), and psychotic disorders (1 study) were also assessed.
The most common tools to measure symptoms of depression were the Beck Depression Inventory (BDI) and the Edinburgh Postpartum Depression Scale (EPDS). Both were used in six studies (21%). Most of the studies concerning anxiety (n = 20.74%) used the State-Trait Anxiety Inventory (STAI). Thirty studies (68%) reported that the intervention was IVF, and in 14 studies (32%) the intervention was a combination of IVF and ICSI.
Quality assessment
Table 2 shows the results of ROBINS-I. Most of the studies (n = 30.68%) received a critical risk of bias rating in at least one category, thus the overall rating is critical (29). To show the difference between the studies, we only present ratings in each domain. All studies in the meta-analysis received a critical rating in the selection category, as they included only women who conceived after IVF and omitted those who did not.
Results of included studies
Table 1 shows the results of all studies, which are summarized below according to their focus and study design.
Studies assessing the prevalence of symptoms of depression
Eight studies (32–39) addressed the prevalence of symptoms of depression at different time points during IVF/ICSI treatment. This prevalence ranged from 7% (34) to 54% (32). Furthermore, six different instruments were used to assess symptoms of depression.
Three studies (40–42) focused on the prevalence of symptoms of depression in pregnancy conceived after IVF. The assessment was conducted at different time points, between the 17th and 32nd gestational weeks. The reported rates ranged from 9% (41) to 29% (42). Two studies used the EPDS with different cutoffs: 12 (41) and 13 (42). The third study (40) used the BDI.
Four studies (41, 43–45) concerned the prevalence of symptoms of depression after childbirth following an IVF/ICSI pregnancy. The assessment was conducted at different time points up to nine months postpartum. The reported rates ranged from 9% (41) to 47% (44). Two studies used EPDS with different cutoffs: 10 (44) and 12 (41). One study (43) used the BDI, and the remaining one (45) used interviews to assess the symptoms of depression.
Studies assessing the prevalence of symptoms of anxiety
Five studies (32–34, 37, 38) analyzed the prevalence of symptoms of anxiety during IVF/ICSI treatment. These were measured at various time points during the intervention and ranged from 14% to 45%. Two studies (32, 37) used the STAI, two (33, 34) used the Zung Self-Rating Anxiety Scale, one used the PRIME-MD (38), and one (32) did not disclose the cutoff it used.
Four studies (40, 42, 46, 47) focused on the prevalence of symptoms of anxiety in pregnancy conceived after IVF. Two studies (40, 47) found that their prevalence was 19%. Arvanitidou et al. (40) assessed outcomes between the 30th and 32nd gestational weeks using the Hamilton Anxiety Rating Scale (HAM-A); this study did not identify the cutoff used. Udry-Jørgensen et al. (47) conducted their assessment in approximately the 12th gestational week using the STAI with a cutoff of 40. Wan et al. (42) estimated prevalence of symptoms of anxiety between 18th and 29th gestational weeks at 42% using the STAI with a cutoff of 50. Hashemieh et al. (46) found that 32.5% of women had moderate to severe anxiety when measured between the eighth and 42nd gestational weeks using the BAI.
Longitudinal studies of depression trajectories
Seven studies (35, 37, 48–52) explored the trajectories of the symptoms of depression throughout IVF/ICSI treatment. Two (35, 48) found no significant difference between the measurement time points. Merari et al. (49) found no significant difference between the time points before the treatment and before pregnancy tests. Yong et al. (52) found a significant increase in depressive symptoms between the start of the treatment and the embryo transfer and between the embryo transfer and the pregnancy test.
Verhaak et al. (37, 51) found a significant decrease in levels of depressive symptoms before the treatment and six months after the last cycle but only in women whose treatment was successful. On the contrary, in women who did not give birth after IVF, the levels of symptoms of depression significantly increased. Similarly, Slade et al. (50) observed a significant increase in symptoms of depression in women who did not get pregnant.
Longitudinal studies of anxiety trajectories
Ten studies (37, 48–56) explored the trajectories of symptoms of anxiety throughout IVF/ICSI treatment. All but one study (56) found significantly different levels of anxiety between the observed time points. Harlow et al. (54), Mahajan et al. (55), and Yong et al. (52) observed a significant increase in anxiety levels between pretreatment and the later stages of treatment. On the contrary, Massarotti et al. (48) found a significant decrease in symptoms of anxiety between the time points.
Verhaak et al. (37, 51) found a significant decrease in symptoms of anxiety between pretreatment and the end of the treatment cycle but only in women who subsequently gave birth. In women who did not conceive, the levels of anxiety increased between these time points. This tendency was also reported by Slade et al. (50). Merari et al. (49) found significant differences in the levels of symptoms of anxiety among the four time points, but the levels did not increase or decrease continuously. Awtani et al. (53) found a significant main effect of time on levels of anxiety, but the only significant difference between two time points was an increase between the day of embryo transfer and 10 days afterward.
Comparative studies assessing depression
Sixteen studies (33, 40, 41, 45, 49, 50, 57–66) compared symptoms of depression throughout the IVF/ICSI treatment, resulting pregnancy, and postpartum periods with control groups or norms. Four (33, 49, 58, 64) assessed symptoms of depression during various phases of the IVF treatment. Two (33, 49) observed higher symptoms of depression in the IVF group when compared to controls, although Merari et al. (49) found an exception at one time point of measurement (the morning before embryo transfer). On the contrary, Gabnai-Nagy et al. (58) observed lower symptoms of depression in the IVF group. Reading et al. (64) found higher symptoms of depression in the IVF group compared to controls only post-treatment but found no significant differences in the beginning or on day eight of the treatment. Furthermore, Slade et al. (50) observed significantly higher symptoms of depression in IVF women compared to adult norms.
Five studies (40, 41, 59, 61, 62) compared symptoms of depression in the third trimester of pregnancy conceived by IVF with those in a control group of spontaneously pregnant women. Our meta-analysis describes them in more detail. Two other studies focused on symptoms of depression in pregnancy. Vilska et al. (66) observed lower symptoms of depression in the second trimester of pregnancy in the IVF/ICSI group. Globevnik Velikonja et al. (60) found no significant difference between pregnant women who conceived by IVF and those who conceived spontaneously in terms of either the levels or the incidence of depression.
Six studies (41, 45, 57, 63, 65, 66) compared symptoms of depression in IVF and control groups at various time points in the postpartum period. Only one study (65) found significantly more women with a diagnosis of depression in the control group. These differences disappeared, however, when controlling for confounders.
Comparative studies assessing anxiety
Fifteen studies (33, 40, 49, 50, 54, 56, 58–63, 67–69) compared symptoms of anxiety at various time points of IVF/ICSI treatment and resulting pregnancy with control groups or norms. Seven assessed the symptoms of anxiety at a minimum of one time point throughout the IVF treatment. Five (33, 49, 50, 54, 56) found higher levels of anxiety in the IVF groups. The remaining two studies (58, 69) found higher levels of symptoms of anxiety in the control groups. Furthermore, Harlow et al. (54) found higher levels of anxiety in a group undergoing IVF with hormonal stimulation compared to a group without stimulation.
Six studies (40, 59, 61, 62, 67, 68) assessed anxiety symptoms in the third trimester of pregnancy; the meta-analysis below describes these in more detail. The study by Globevnik Velikonja et al. (60) was the only other study measuring anxiety in pregnancy, but its time range was too wide to be included in the meta-analysis. Nevertheless, this study found no significant differences between the IVF and control groups. Only McMahon et al. (63) assessed levels of anxiety postpartum. They found no significant difference between groups at either four or 12 months postpartum.
Studies assessing stress
Five studies (53, 56, 68, 70, 71) assessed differences between stress levels at various time points of the treatment and pregnancy. Three studies (53, 56, 70) focused on the treatment period. While Lin et al. (70) observed significantly more participants experiencing distress during embryo transfer than during oocyte pickup, Awtani et al. (53) reported that, on the day of embryo transfer, women felt less stressed than at the beginning of the treatment. Turner et al. (56) found no significant difference between three time points of assessment. Both Shih et al. (71) and Stevenson et al. (68) found no significant differences in stress levels between pregnant women who conceived after IVF and those who conceived spontaneously. Shih et al. (71), however, found that stress levels increased throughout pregnancy.
Studies assessing other mental health outcomes
Four studies (72–75) focused on mental-health outcomes other than depression, anxiety, or stress. A Canadian register study by Dayan et al. (72) addressed the risk of receiving any psychiatric diagnosis within one year postpartum. Compared to women who conceived spontaneously, women undergoing IVF had a lower crude absolute risk but a higher adjusted relative risk of mental illness. Furthermore, 3.5 per 1,000 postpartum women who conceived by IVF had mood or anxiety diagnoses. In a Danish register study, Munk-Olsen and Agerbo (74) estimated the incidence of any mental-health disorder within 90 days postpartum in women who conceived after IVF at 11.3 per 1,000 compared to 3.6 per 1,000 within 90–365 days postpartum. In women who did not give birth following IVF, the incidence was 3.8 per 1,000.
Jongbloed-Pereboom et al. (73) compared the common mental-health problems (defined as >80th of the General Health Questionnaire) of women within one year of giving birth following IVF with women who conceived spontaneously and found no significant difference. Vikström et al. (75) found no significant difference when comparing the incidence of postpartum psychosis in women who gave birth after IVF and those who had a spontaneous pregnancy.
Meta-Analyses
Meta-Analysis on the effect of in vitro fertilization on symptoms of depression
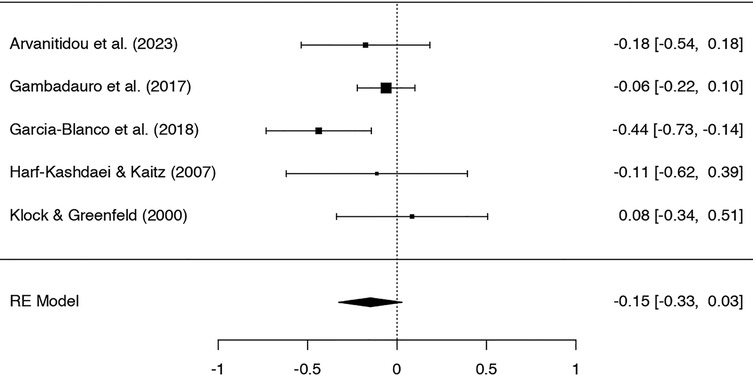
Five studies were included in the meta-analysis on the effect of IVF on symptoms of depression, with 340 participants in IVF groups and 3,503 in control groups. A Q-test showed a moderate but not significant heterogeneity in the included studies (Q = 5.97, p = .20, tau² = .01, I² = 36.91%). Rosenthal's failsafe N showed a potential risk of publication bias (N = 4, p = .018), which was not detected by the Begg and Mazumdar rank correlation test (p = 1.0) or Egger's regression (p = 0.91).
The standardized mean difference (SMD) ranged from −.44 to.08; the average SMD based on the random-effects model was −.15; 95% CI [−.33,.03]. Thus, women in IVF groups had lower symptoms of depression than those in the control groups, but the average SMD did not differ significantly from zero (Z = −1.65, p = .10). See Figure 2 for the forest plot.
Meta analysis on the effect of in vitro fertilization on symptoms of anxiety
Six studies were included in the meta-analysis on the effect of IVF on symptoms of anxiety, with 280 participants in the IVF groups and 744 in the control groups. A Q-test showed no significant heterogeneity in the included studies (Q = 3.97, p = .55, tau² = .00, I² = .00%). Rosenthal's fail-safe N showed a potential risk of publication bias (N = 31, p < .001), which was not confirmed by the Begg and Mazumdar rank correlation test (p = 0.72) or Egger's regression (p = 0.73).
The SMD ranged from.09 to.66; the average SMD based on the random-effects model was.33; 95% CI [.17,.49]. Thus, women in the IVF groups showed significantly higher symptoms of anxiety than those in the control groups (Z = 4.09, p < .001). See Figure 3 for the forest plot.
Discussion
In this systematic review and meta-analysis, we assembled empirical evidence about effects of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment on women's mental health in the perinatal period. We included 44 studies that covered 858,966 participants and were conducted in 20 countries. The substantial number of studies using various study designs, together with the broad inclusion criteria, have contributed to the notable heterogeneity of the results.
Most frequently, the included studies assessed symptoms or diagnoses of depression as an outcome measure. Estimates of the prevalence of depression throughout the treatment and perinatal period varied substantially, ranging from 7% to 54% compared to general estimates of 15% in pregnancy (15) and 14% postpartum (16). The prevalence of anxiety during the treatment and pregnancy ranged from 14% to 45% compared to 15% in the general population of pregnant women (17).
One reason for the evident discrepancy between included studies might be the use of various assessment tools and different cutoffs, such as 10 (44), 12 (41) and 13 (42) in studies using the EPDS and 40 (47), 48 (37), and 50 (42) in studies using the STAI. Furthermore, the rates of incidence of any psychiatric disorder, defined by diagnostic codes in two register studies, were very low: according to Dayan et al. (72), 3.5 per 1,000 postpartum women experienced a new onset of mood or anxiety disorder after IVF; Munk-Olsen and Agerbo (74) estimated the incidence of any new psychiatric disorder at 3.8 per 1,000 postpartum women after IVF. Although the outcomes of these studies focusing on incidence are not fully comparable with those of the studies assessing prevalence, it can be assumed that the rates of mental difficulties are higher when measured by self-reported questionnaires than the rates of psychiatric diagnoses. This implies the need to interpret the results of this systematic review and meta-analysis in terms of the symptoms of mental disorders, not mental disorders per se.
The alarmingly high prevalence of depressive symptoms found in some studies were surprising in the context of comparative studies, most of which found no significant differences between women conceiving after IVF and in the control groups. During the treatment, the results of studies were heterogeneous, some indicating significant differences between the two groups. These differences were observed in both directions, however, making it challenging to draw clear conclusions. No statistically significant mean difference in the third trimester was confirmed by the meta-analytical part of this study. This is in line with a meta-analysis by Chen et al. (24) with a broader focus on the whole perinatal period and another by Gressier et al. (76) analyzing depression in the postpartum period.
Studies comparing the symptoms of anxiety in IVF/ICSI and control groups yield more consistent results: most have found higher levels of anxiety in the IVF/ICSI groups. This effect was also confirmed by the meta-analytical part of this study, although the standardized mean difference is rather low. According to Schaller et al. (77), the major stressors leading to anxiety in women undergoing IVF are fear of obtaining a negative pregnancy test, disappointment after anticipation of pregnancy, and childlessness at an older age.
The results of studies assessing the longitudinal trajectories of the symptoms of depression, anxiety, and stress were also very heterogeneous. Notably, studies that separately compared the levels of symptoms in women who conceived after IVF/ICSI and women who did not found a significant decrease of symptoms in the successful group and a significant increase in the unsuccessful group between the time point before the treatment and during pregnancy (37, 50, 51). The reason for such an effect might be quite straightforward: couples dealing with fertility problems are often affected by psychological strain, which might lessen when pregnancy is conceived and persist or deepen when the treatment is unsuccessful. It has been proven that planned pregnancy is a protective factor in developing perinatal depression (15).
A contributing factor to the discrepancies between the results of the mentioned studies might be the difference in the social context of ART in different countries. Kato et al. (32) argue that the high price of the treatment (up to US$6,700) together with difficulties coordinating the treatment with long work hours in a corporate climate in Japan might be a stressor that contributes to mental difficulties in treated women. Furthermore, the social pressure to have children and the persistent preference for male offspring in some Asian countries might also augment the susceptibility to psychopathology. Higher levels of depressive symptoms in the perinatal period in Asian countries were also reported by a meta-analysis by Chen et al. (24). On the contrary, in Sweden, where up to three IVF treatment cycles are funded by regional health services, the social stigma of ART subsides, and the patient-friendliness of the whole treatment process increases (41).
In many countries, IVF/ICSI treatment is expensive and is paid by the patients (78–80). It can be assumed that the treatment might not be accessible to infertile patients with low socioeconomic status, which might contribute to the overrepresentation of participants with higher socioeconomic status in the studies included in this review. It is well known that low socioeconomic status is a risk factor for depressive symptoms in the perinatal period (76), which might also lessen depressive symptoms in IVF women in the included studies.
It is also important to interpret our findings within the broader context of empirical evidence on the psychotropic consequences of women's hormonal environments and other interventions that alter them. Hormonal fluctuations across the menstrual cycle, pregnancy, the postpartum period, and during menopause are known to influence women's well-being and vulnerability to mood disturbances, although the specific effects of these hormones are not clear (81–83). Likewise, exposure to exogenous reproductive hormones through various medical treatments has been associated with changes in mental health (84, 85). Considering the effects of IVF procedures in this context would provide valuable insight into the complex interplay between hormonal environments and women's psychological outcomes.
Strengths and limitations
In terms of the number of included studies, this study was larger than other systematic reviews and meta-analyses on similar topics (24, 25, 76), resulting in a broader scope of evidence. On the other hand, as we focused solely on the effects of IVF/ICSI and not ART more generally, our study provides more precise information on this kind of treatment. To our knowledge, the meta-analysis concerning symptoms of anxiety is the first of its kind.
A major limitation of the studies included in this systematic review and meta-analysis is that they almost completely omitted women whose treatment was unsuccessful. As indicated in studies by Slade et al. (50), Verhaak et al. (51), and Verhaak et al. (37), this experience might have adverse effect on female patients’ mental health. Thus, the generalizability of our results is strongly limited by this exclusion and very likely underestimates the effects of IVF/ICSI on perinatal mental health.
A significant bias might have been caused by the way confounding factors were approached in the included studies. In some, confounding factors were controlled for well, while other studies did not use proper controlling techniques or failed to control for the most important variables known to be associated with perinatal mental difficulties, such as a history of psychopathology, low income, or social support. Similarly, in the comparative studies included in our meta-analysis, not all control groups were matched according to possible confounders.
Some of the limitations are also reflected in the risk of bias assessment: most of the included studies (n = 30.68%) received a critical rating in at least one category, implying that the overall risk of bias in these studies is critical. Most frequently, the category of selection of the participants was rated as critical, a categorization linked to the noted omission of women who did not get pregnant after IVF/ICSI.
Further limitations should be considered when interpreting the results of both meta-analyses. The studies were fairly homogeneous, but their number was rather small, as were, for the most part, the sample sizes. Most failed to disclose the results of the power analysis indicating the number of participants needed for robust results, and, in some, the sample size was so small that it could have led to biased outcomes. Furthermore, it is likely that the distributions of data in the individual studies were skewed, which can lead to inaccurate outcomes in the meta-analysis. This can be improved by the transformation of the individual participants’ data, which we were unable to access; thus, we consider this a possible limitation. Similar methodological challenges to those described above have been found in previous reviews and meta-analyses of reproductive outcomes after ART, particularly regarding study designs, outcomes, and confounding measures (86, 87).
Conclusion
The studies included in this systematic review and meta-analysis show considerable heterogeneity in terms of their study designs and the time points of their data collection, and they reached very heterogeneous results. We found, however, significantly higher levels of anxiety in women in the third trimester of pregnancy conceived by IVF compared to women who conceived spontaneously. On the other hand, the standardized mean difference is rather low, and, considering the limitations of the included studies, this result should be interpreted cautiously. Our meta-analysis focused on symptoms of depression only in the third trimester of pregnancy. It would be valuable to conduct such analyses at different time points throughout the perinatal period; the limited number of studies per subgroup, however, would prevent us from drawing meaningful conclusions. Some of the studies assessing the prevalence of symptoms of mental disorders in the perinatal period after IVF/ICSI found alarming results, with this prevalence found to be as high as 54%. This implies the need for increased awareness of possible difficulties among medical staff providing ART. Ideally, mental-health screening with subsequent care by mental-health professionals should be a standard service of fertility clinics.
To reach robust results, future studies should engage larger samples of participants when comparing mental difficulties between women pregnant after IVF and those pregnant spontaneously. To obtain unbiased results, women whose treatment was unsuccessful should not be excluded from the studies. It is crucial for future studies to use standardized, validated tools to provide valid results. There is also a lack of studies concerning such comparison in women in the first two trimesters of pregnancy and postpartum: notably, only one study focusing on symptoms of anxiety in the postpartum period was identified. Future research should also focus on factors contributing to the prevalence of symptoms of depression and anxiety in this population, as the rates vary substantially among the published studies. If these factors are known, it will be easier to predict and prevent mental difficulties in this population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HN: Conceptualization, Writing – review & editing, Methodology, Data curation, Writing – original draft, Visualization, Formal analysis. TB: Writing – review & editing, Data curation, Writing – original draft, Project administration. AH: Writing – review & editing. MK: Writing – review & editing, Formal analysis, Data curation. KH: Writing – review & editing. AS: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Infertility Prevalence Estimates, 1990-2021. (2023). Available online at: https://www.who.int/publications/i/item/978920068315 (Accessed January 10, 2024).
2. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1859–922. doi: 10.1016/S0140-6736(18)32335-3
3. The European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), Smeenk J, Wyns C, De Geyter C, Kupka M, Bergh C, et al. ART in Europe, 2019: results generated from European registries by ESHRE. Hum Reprod. (2023) 38(12):2321–38. doi: 10.1093/humrep/dead197
4. Centers for Disease Control and Prevention. Assisted Reproductive Technology (ART). (2024). Available online at: https://www.cdc.gov/art/artdata/index.html (Accessed January 10, 2024).
5. Hamilton BE, Martin JA, Osterman MJK. Centers for Disease Control and Prevention. Births: Provisional data for 2021 (2022). Available online at: https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf (Accessed January 10, 2024).
6. Cui Y, Yu H, Meng F, Liu J, Yang F. Prospective study of pregnancy outcome between perceived stress and stress-related hormones. J Obstet Gynaecol Res. (2020) 46(8):1355–63. doi: 10.1111/jog.14278
7. Bagade T, Mersha AG, Majeed T. The social determinants of mental health disorders among women with infertility: a systematic review. BMC Women’s Health. (2023) 23(1):668. doi: 10.1186/s12905-023-02828-9
8. Doyle M, Carballedo A. Infertility and mental health. Adv Psychiatr Treat. (2014) 20(5):297–303. doi: 10.1192/apt.bp.112.010926
9. Keisswetter M, Marsoner H, Luehwink A, Fistarol M, Mahlknecht A, Duschek S. Impairments in life satisfaction in infertility: associations with perceived stress, affectivity, partnership quality, social support and the desire to have a child. Behav Med. (2020) 46(2):130–41. doi: 10.1080/08964289.2018.1564897
10. Casper RF. Basic understanding of gonadotropin-releasing hormone-agonist triggering. Fertil Steril. (2015) 103(4):867–9. doi: 10.1016/j.fertnstert.2014.12.129
11. González-Rodríguez A, Cobo J, Soria V, Usall J, Garcia-Rizo C, Bioque M, et al. Women undergoing hormonal treatments for infertility: a systematic review on psychopathology and newly diagnosed mood and psychotic disorders. Front Psychiatry. (2020) 11:479. doi: 10.3389/fpsyt.2020.00479
12. Hammarberg K, Fisher JRW, Wynter KH. Psychological and social aspects of pregnancy, childbirth and early parenting after assisted conception: a systematic review. Hum Reprod Update. (2008) 14(5):395–414. doi: 10.1093/humupd/dmn030
13. Lee AM, Lam SK, Sze Mun Lau SM, Chong CSY, Chui HW, Fong DYT. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. (2007) 110(5):1102–12. doi: 10.1097/01.AOG.0000287065.59491.70
14. McCrory C, McNally SC. The effect of pregnancy intention on maternal prenatal behaviors and parent and child health: results of an Irish cohort study. Pediatr Perinat Epidemiol. (2013) 27(2):208–15. doi: 10.1111/ppe.12027
15. Yin X, Sun N, Jiang N, Xu X, Gan Y, Zhang J, et al. Prevalence and associated factors of antenatal depression: systematic reviews and meta-analyses. Clin Psychol Rev. (2021) 83:101932. doi: 10.1016/j.cpr.2020.101932
16. Liu X, Wang S, Wang G. Prevalence and risk factors of postpartum depression in women: a systematic review and meta-analysis. J Clin Nurs. (2022) 31(19–20):2665–77. doi: 10.1111/jocn.16121
17. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. (2017) 210(5):315–23. doi: 10.1192/bjp.bp.116.187179
18. Loomans EM, van Dijk AE, Vrijkotte TGM, van Eijsden M, Stronks K, Gemke RJBJ, et al. Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi-ethnic community-based birth cohort. Eur J Public Health. (2013) 23(3):485–91. doi: 10.1093/eurpub/cks097
19. Clayborne ZM, Colman I, Kingsbury M, Torvik FA, Gustavson K, Nilsen W. Prenatal work stress is associated with prenatal and postnatal depression and anxiety: findings from the Norwegian mother, father and child cohort study (MoBa). J Affect Disord. (2022) 298:548–54. doi: 10.1016/j.jad.2021.11.024
20. Faisal-Cury A, Menezes PR. Antenatal depression strongly predicts postnatal depression in primary health care. Rev Bras Psiquiatr. (2012) 34(4):446–50. doi: 10.1016/j.rbp.2012.01.003
21. Tichelman E, Westerneng M, Witteveen AB, van Baar AL, van der Horst HE, de Jonge A, et al. Correlates of prenatal and postnatal mother-to-infant bonding quality: a systematic review. PLoS One. (2019) 14(9):e0222998. doi: 10.1371/journal.pone.0222998
22. Caparros-Gonzalez RA, de la Torre-Luque A, Romero-Gonzalez B, Quesada-Soto JM, Alderdice F, Peralta-Ramírez MI. Stress during pregnancy and the development of diseases in the offspring: a systematic-review and meta-analysis. Midwifery. (2021) 97:102939. doi: 10.1016/j.midw.2021.102939
23. Slomian J, Honvo G, Emonts P, Reginster JY, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Women’s Health. (2019) 15:1745506519844044. doi: 10.1177/1745506519844044
24. Chen S, Wang T, Zhang S, Zhao L, Chen L. Association between infertility treatment and perinatal depressive symptoms: a meta-analysis of observational studies. J Psychosom Res. (2019) 120:110–7. doi: 10.1016/j.jpsychores.2019.03.016
25. Capuzzi E, Caldiroli A, Ciscato V, Zanvit FG, Bollati V, Barkin JL, et al. Is in vitro fertilization (IVF) associated with perinatal affective disorders? J Affect Disord. (2020) 277:271–8. doi: 10.1016/j.jad.2020.08.006
26. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
27. Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. (2023). Available online at: www.covidence.org
28. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. (1978) 2(8085):366. doi: 10.1016/s0140-6736(78)92957-4
29. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 12(355):i4919. doi: 10.1136/bmj.i4919
30. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1(2):97–111. doi: 10.1002/jrsm.12
31. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. (2011) 22(343):d4002. doi: 10.1136/bmj.d4002
32. Kato T, Sampei M, Saito K, Morisaki N, Urayama KY. Depressive symptoms, anxiety, and quality of life of Japanese women at initiation of ART treatment. Sci Rep. (2021) 11(1):7538. doi: 10.1038/s41598-021-87057-6
33. Kong L, Shao Y, Xia J, Han J, Zhan Y, Liu G, et al. Quantitative and qualitative analyses of psychological experience and adjustment of in vitro fertilization-embryo transfer patients. Med Sci Monit. (2019) 25:8069–77. doi: 10.12659/MSM.916627
34. Liu YF, Fu Z, Chen SW, He XP, Fan LY. The analysis of anxiety and depression in different stages of in vitro fertilization-embryo transfer in couples in China. Neuropsychiatr Dis Treat. (2021) 17:649–57. doi: 10.2147/NDT.S287198
35. Lukse MP, Vacc NA. Grief, depression, and coping in women undergoing infertility treatment. Obstet Gynecol. (1999) 93(2):245–51. doi: 10.1016/S0029-7844(98)00432-3
36. Verhaak CM, Smeenk JMJ, Eugster A, van Minnen A, Kremer JAM, Kraaimaat FW. Stress and marital satisfaction among women before and after their first cycle of in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. (2001) 76(3):525–31. doi: 10.1016/S0015-0282(01)01931-8
37. Verhaak CM, Smeenk JMJ, Nahuis MJ, Kremer JAM, Braat DDM. Long-term psychological adjustment to IVF/ICSI treatment in women. Hum Reprod. (2007) 22(1):305–8. doi: 10.1093/humrep/del355
38. Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist Ö, Sundström Poromaa I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum Reprod. (2008) 23(9):2056–63. doi: 10.1093/humrep/den154
39. Wu G, Yin T, Yang J, Xu W, Zou Y, Wang Y, et al. Depression and coping strategies of Chinese women undergoing in vitro fertilization. Eur J Obstet Gynecol Reprod Biol. (2014) 183:155–8. doi: 10.1016/j.ejogrb.2014.10.019
40. Arvanitidou O, Kalaitzopoulos DR, Samartzis N, Athanasiadis A, Ierodiakonou-Benou I, Daniilidis A. Is conception by means in vitro fertilization associated with increased risk of antenatal anxiety and depression? Curēus. (2023) 15(3):e36659. doi: 10.7759/cureus.36659
41. Gambadauro P, Iliadis S, Bränn E, Skalkidou A. Conception by means of in vitro fertilization is not associated with maternal depressive symptoms during pregnancy or postpartum. Fertil Steril. (2017) 108(2):325–32. doi: 10.1016/j.fertnstert.2017.06.006
42. Wan F, Li A, Hao G, Zhao Z, Li W, Zhi K, et al. Psychological status and influencing factors among pregnant women undergoing in vitro fertilization. Altern Ther Health Med. (2023) 29(6):393–9.37442184
43. Lee SH, Liu LC, Kuo PC, Lee MS. Postpartum depression and correlated factors in women who received in vitro fertilization treatment. J Midwifery Womens Health. (2011) 56(4):347–52. doi: 10.1111/j.1542-2011.2011.00033.x
44. Li CC, Hwang JL, Ko YL, Chen HH, Chien LY. Factors associated with postpartum depressive symptoms among women who conceived with infertility treatment. Acta Psychol. (2023) 238:103987. doi: 10.1016/j.actpsy.2023.103987
45. Raoul-Duval A, Bertrand-Servais M, Frydman R. Comparative prospective study of the psychological development of children born by in vitro fertilization and their mothers. J Psychosom Obstet Gynaecol. (1993) 14(2):117–26. doi: 10.3109/01674829309084435
46. Hashemieh C, Neisani Samani L, Taghinejad H. Assessment of anxiety in pregnancy following assisted reproductive technology (ART) and associated infertility factors in women commencing treatment. Iran Red Crescent Med J. (2013) 15(12):e14465. doi: 10.5812/ircmj.14465
47. Udry-Jørgensen L, Darwiche J, Germond M, Wunder D, Vial Y. Anxiety, depression, and attachment before and after the first-trimester screening for down syndrome: comparing couples who undergo ART with those who conceive spontaneously. Prenat Diagn. (2015) 35(13):1287–93. doi: 10.1002/pd.4688
48. Massarotti C, Gentile G, Ferreccio C, Scaruffi P, Remorgida V, Anserini P. Impact of infertility and infertility treatments on quality of life and levels of anxiety and depression in women undergoing in vitro fertilization. Gynecol Endocrinol. (2019) 35(6):485–9. doi: 10.1080/09513590.2018.1540575
49. Merari D, Feldberg D, Elizur A, Goldman J, Modan B. Psychological and hormonal changes in the course of in vitro fertilization. J Assist Reprod Genet. (1992) 9(2):161–9. doi: 10.1007/BF01203757
50. Slade P, Emery J, Lieberman BA. A prospective, longitudinal study of emotions and relationships in in vitro fertilization treatment. Hum Reprod. (1997) 12(1):183–90. doi: 10.1093/humrep/12.1.183
51. Verhaak CM, Smeenk JMJ, van Minnen A, Kremer JAM, Kraaimaat FW. A longitudinal, prospective study on emotional adjustment before, during and after consecutive fertility treatment cycles. Hum Reprod. (2005) 20(8):2253–60. doi: 10.1093/humrep/dei015
52. Yong P, Martin C, Thong J. A comparison of psychological functioning in women at different stages of in vitro fertilization treatment using the mean affect adjective check list. J Assist Reprod Genet. (2000) 17(10):553–6. doi: 10.1023/A:1026429712794
53. Awtani M, Kapoor G, Kaur P, Saha J, Crasta D, Banker M. Anxiety and stress at different stages of treatment in women undergoing in vitro fertilization-intracytoplasmic sperm injection. J Hum Reprod Sci. (2019) 12(1):47–52. doi: 10.4103/jhrs.JHRS_23_18
54. Harlow CR, Fahy UM, Talbot WM, Wardle PG, Hull MGR. Stress and stress-related hormones during in vitro fertilization treatment. Hum Reprod. (1996) 11(2):274–9. doi: 10.1093/HUMREP/11.2.274
55. Mahajan NN, Turnbull DA, Davies MJ, Jindal UN, Briggs NE, Taplin JE. Changes in affect and state anxiety across an in vitro fertilization/intracytoplasmic sperm injection cycle. Fertil Steril. (2010) 93(2):517–26. doi: 10.1016/j.fertnstert.2008.12.054
56. Turner K, Reynolds-May MF, Zitek EM, Tisdale RL, Carlisle AB, Westphal LM. Stress and anxiety scores in first and repeat IVF cycles: a pilot study. PLoS One. (2013) 8(5):e63743. doi: 10.1371/journal.pone.0063743
57. Cozzolino M, Troiano G, Coccia ME. Spontaneous pregnancy versus assisted reproductive technologies: implications on maternal mental health. Women Health. (2021) 61(3):303–12. doi: 10.1080/03630242.2021.1881025
58. Gabnai-Nagy E, Bugán A, Bodnár B, Papp G, Nagy BE. Association between emotional state changes in infertile couples and outcome of fertility treatment. Geburtshilfe Frauenheilkd. (2020) 80(2):200–10. doi: 10.1055/a-0854-5987
59. García-Blanco A, Diago V, Hervás D, Ghosn F, Vento M, Cháfer-Pericás C. Anxiety and depressive symptoms, and stress biomarkers in pregnant women after in vitro fertilization: a prospective cohort study. Hum Reprod. (2018) 33(7):1237–46. doi: 10.1093/humrep/dey109
60. Globevnik Velikonja V, Lozej T, Leban G, Verdenik I, Vrtačnik Bokal E. The quality of life in pregnant women conceiving through in vitro fertilization. Zdr Varst. (2016) 55(1):1–10. doi: 10.1515/sjph-2016-0001
61. Harf-Kashdaei E, Kaitz M. Antenatal moods regarding self, baby, and spouse among women who conceived by in vitro fertilization. Fertil Steril. (2007) 87(6):1306–13. doi: 10.1016/j.fertnstert.2006.11.035
62. Klock SC, Greenfeld DA. Psychological status of in vitro fertilization patients during pregnancy: a longitudinal study. Fertil Steril. (2000) 73(6):1159–64. doi: 10.1016/S0015-0282(00)00530-6
63. McMahon CA, Ungerer JA, Tennant C, Saunders D. Psychosocial adjustment and the quality of the mother-child relationship at four months postpartum after conception by in vitro fertilization. Ferti. Steril. (1997) 68(3):492–500. doi: 10.1016/S0015-0282(97)00230-6
64. Reading AE, Chang LC, Kerin JF. Psychological state and coping styles across an IVF treatment cycle. J Reprod Infant Psychol. (1989) 7(2):95–103. doi: 10.1080/02646838908403580
65. Vikström J, Josefsson A, Hammar M, Bladh M, Sydsjö G. Risk of postnatal depression or suicide after in vitro fertilisation treatment: a nationwide case–control study. BJOG. (2017) 124(3):435–42. doi: 10.1111/1471-0528.13788
66. Vilska S, Unkila-Kallio L, Punamäki RL, Poikkeus P, Repokari L, Sinkkonen J, et al. Mental health of mothers and fathers of twins conceived via assisted reproduction treatment: a 1-year prospective study. Hum. Reprod. (2009) 24(2):367–77. doi: 10.1093/humrep/den427
67. McMahon CA, Ungerer JA, Beaurepaire J, Tennant C, Saunders D. Anxiety during pregnancy and fetal attachment after in vitro fertilization conception. Hum Reprod. (1997) 12(1):176–82. doi: 10.1093/humrep/12.1.176
68. Stevenson EL, Cebert M, Silva S. Stress and anxiety in couples who conceive via in vitro fertilization compared with those who conceive spontaneously. J Obstet Gynecol Neonatal Nurs. (2019) 48(6):635–44. doi: 10.1016/j.jogn.2019.09.001
69. Visser AP, Haan G, Zalmstra H, Wouters I. Psychosocial aspects of in vitro fertilization. J Psychosom Obstet Gynaecol. (1994) 15(1):35–43. doi: 10.3109/01674829409025627
70. Lin YH, Chueh KH, Lin JL. Somatic symptoms, sleep disturbance and psychological distress among women undergoing oocyte pick-up and in vitro fertilisation-embryo transfer. J Clin Nurs. (2016) 25(11–12):1748–56. doi: 10.1111/jocn.13194
71. Shih FF, Chen CH, Chiao CY, Li CR, Kuo PC, Lai TJ. Comparison of pregnancy stress between in vitro fertilization/embryo transfer and spontaneous pregnancy in women during early pregnancy. J Nurs Res. (2015) 23(4):280–9. doi: 10.1097/JNR.0000000000000089
72. Dayan N, Velez MP, Vigod S, Pudwell J, Djerboua M, Fell DB, et al. Infertility treatment and postpartum mental illness: a population-based cohort study. CMAJ open. (2022) 10(2):E430–8. doi: 10.9778/cmajo.20210269
73. Jongbloed-Pereboom M, Middelburg KJ, Heineman MJ, Bos AF, Haadsma ML, Hadders-Algra M. The impact of IVF/ICSI on parental well-being and anxiety 1 year after childbirth. Hum Reprod. (2012) 27(8):2389–95. doi: 10.1093/humrep/des163
74. Munk-Olsen T, Agerbo E. Does childbirth cause psychiatric disorders? A population-based study paralleling a natural experiment. Epidemiology. (2015) 26(1):79–84. doi: 10.1097/EDE.0000000000000193
75. Vikström J, Josefsson A, Hammar M, Bladh M, Sydsjö G. Risk of postpartum psychosis after IVF treatment: a nationwide case-control study. Hum Reprod. (2017) 32(1):139–46. doi: 10.1093/humrep/dew302
76. Gressier F, Letranchant A, Cazas O, Sutter-Dallay AL, Falissard B, Hardy P. Post-partum depressive symptoms and medically assisted conception: a systematic review and meta-analysis. Hum Reprod. (2015) 30(11):2575–86. doi: 10.1093/humrep/dev207
77. Schaller MA, Griesinger G, Banz-Jansen C. Women show a higher level of anxiety during IVF treatment than men and hold different concerns: a cohort study. Arch Gynecol Obstet. (2016) 293(5):1137–45. doi: 10.1007/s00404-016-4033-x
78. Maeda E, Ishihara O, Saito H, Kuwahara A, Toyokawa S, Kobayashi Y. Age-specific cost and public funding of a live birth following assisted reproductive treatment in Japan. J Obstet Gynaecol Res. (2014) 40(5):1338–44. doi: 10.1111/jog.12337
79. Chambers GM, Hoang VP, Illingworth PJ. Socioeconomic disparities in access to ART treatment and the differential impact of a policy that increased consumer costs. Hum Reprod. (2013) 28(11):3111–7. doi: 10.1093/humrep/det302
80. Berg Brigham K, Cadier B, Chevreul K. The diversity of regulation and public financing of IVF in Europe and its impact on ulitization. Hum Reprod. (2013) 28(3):666–75. doi: 10.1093/humrep/des418
81. Frokjaer VG. Pharmacological sex hormone manipulation as a risk model for depression. J. Neurosci. Res. (2020) 98(7):1283–92. doi: 10.1002/jnr.24632
82. Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. (2015) 172(3):227–36. doi: 10.1176/appi.ajp.2014.14070918
83. Takeda T. Premenstrual disorders: premenstrual syndrome and premenstrual dysphoric disorder. JORG. (2023) 49(2):510–8. doi: 10.1111/jog.15484
84. Larsen SV, Mikkelsen AP, Lidegaard Ø, Frokjaer VG. Depression associated with hormonal contraceptive use as a risk indicator for postpartum depression. JAMA Psychiatry. (2023) 80(7):682. doi: 10.1001/jamapsychiatry.2023.0807
85. Zhang J, Yin J, Song X, Lai S, Zhong S, Jia Y. The effect of exogenous estrogen on depressive mood in women: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. (2023) 162:21–9. doi: 10.1016/j.jpsychires.2023.04.002
86. Riemma G, De Franciscis P, Torella M, Narciso G, La Verde M, Morlando M, et al. Reproductive and pregnancy outcomes following embryo transfer in women with previous cesarean section: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2021) 100(11):1949–60. doi: 10.1111/aogs.14239
Keywords: in vitro fertilization, intracytoplasmic sperm injection, perinatal mental health, depression, anxiety
Citation: Nemcova H, Blaskova T, Horakova A, Kuklova M, Hrdlickova K and Sebela A (2025) Effects of in vitro fertilization and intracytoplasmic sperm injection treatment on female patients' perinatal mental health: systematic review and meta-analysis. Front. Reprod. Health 7:1668831. doi: 10.3389/frph.2025.1668831
Received: 18 July 2025; Accepted: 8 September 2025;
Published: 23 September 2025.
Edited by:
Andrea Etrusco, University of Palermo, ItalyReviewed by:
Sujata Kar, Ravenshaw University, IndiaSana Chtourou, Aziza Othmana Hospital, Tunisia
Gaetano Riemma, University of Campania Luigi Vanvitelli, Italy
Evangelia Deligeoroglou, Aristotle University of Thessaloniki, Greece
Copyright: © 2025 Nemcova, Blaskova, Horakova, Kuklova, Hrdlickova and Sebela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hana Nemcova, aGFuYS5uZW1jb3ZhQG51ZHouY3o=
†ORCID:
Hana Nemcova
orcid.org/0000-0001-6114-6191
Anna Horakova
orcid.org/0000-0001-9582-9900
Marie Kuklova
orcid.org/0000-0003-4561-9571
Kristyna Hrdlickova
orcid.org/0000-0002-8039-290X
Antonin Sebela
orcid.org/0000-0002-1063-7772
 Hana Nemcova
Hana Nemcova Tereza Blaskova1
Tereza Blaskova1 Anna Horakova
Anna Horakova Antonin Sebela
Antonin Sebela