- 1Department of Experimental, Clinical and Biomedical Sciences “Mario Serio”, University of Florence, Florence, Italy
- 2Obstetrics and Gynecology, Careggi University Hospital, Florence, Italy
- 3Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
- 4Rheumatology and Scleroderma Unit, Careggi University Hospital, Florence, Italy
Autoimmune and inflammatory rheumatic diseases (RDs) are more prevalent in women and often affect gynecological health. Particularly, heavy menstrual bleeding (HMB) and dysmenorrhea are more common in patients with RD. A link between RDs and endometriosis has been shown, whereas the association with adenomyosis remains unexplored. The present study evaluates the prevalence of adenomyosis in women of reproductive age with RD (n = 76) who were referred to the Gynecology Unit, compared with an age-matched control population (n = 305). A detailed clinical history and pelvic imaging findings obtained via transvaginal ultrasound were collected, excluding menopausal women and those with endometriosis or gynecological malignancies. Adenomyosis was significantly more prevalent in RD patients than in controls (40.8% vs. 19.7%, OR 2.81, 95% CI 1.64–4.82; p < 0.001), whereas the prevalence of uterine fibroids did not differ significantly between groups. These findings highlight the need for greater awareness of adenomyosis among both rheumatologists and gynecologists, as timely and adequate recognition is crucial to improving quality of life and reproductive health in patients with RDs.
Introduction
Autoimmune and inflammatory rheumatic diseases (RDs) occur significantly more often in women than in men (1, 2), largely due to the influence of sex hormones (3), sex-specific immune regulation (4) and epigenetic mechanisms (1, 5). RDs can significantly affect women's health, and gynecological disorders, such as vaginal dryness, impaired sexuality and infertility, are relevant aspects to consider in patient management (6). In fact, contraception, assisted reproductive technologies, preconception and pregnancy management are topics commonly discussed by RD patients with rheumatologists (7).
Recent studies have shown that women with RDs report menstruation-related symptoms, including heavy menstrual bleeding (HMB), dysmenorrhea and dyspareunia, more often than healthy women did, significantly affecting their quality of life (QoL) (8). These symptoms are common in patients with uterine fibroids—the most common uterine benign tumor (9). However, HMB and gynecological pain are also frequently observed in adenomyosis, a condition characterized by abnormal presence of endometrial tissue within the myometrium (10), which shares several similarities with endometriosis (11), including immune changes (12). The prevalence of adenomyosis varies widely in literature, ranging from 8.8%–61.5%, mainly based on pathological examination of hysterectomy specimens (13). More recently, the use of imaging modalities such as magnetic resonance imaging (MRI) and transvaginal ultrasound (TVUS) has enabled adenomyosis diagnosis in women of reproductive age, with studies estimating a prevalence of approximately 30% in this population (14–16).
While robust evidence on the associations between endometriosis and autoimmune conditions have been reported (17), presumably related to immune dysfunction (18, 19), data on adenomyosis in RD patients remain limited. Thus, the present study aimed to evaluate the possible presence of adenomyosis in a group of RD patients. In addition, the presence of uterine fibroids was systematically investigated.
Methods
The present observational cohort study included a group of patients with RD, referred from the Rheumatology Unit to the Gynecology Unit at Careggi University Hospital, Florence, over a 1-year period. As part of the multidisciplinary care model implemented at our institution, gynecological consultations are included in the comprehensive evaluation protocol, aiming to either identify potential gynecologic comorbidities or manage gynecologic symptoms related to RDs. A control group of age-matched women (n = 305), not affected by RD and undergoing routine gynecological checkups, was also included for comparison.
Our primary objective was to compare the prevalence of adenomyosis in women with RDs vs. age-matched controls. Secondary objectives were to assess the association between adenomyosis and menstruation-related symptoms, including abnormal uterine bleeding (AUB), heavy menstrual bleeding (HMB), dysmenorrhea and dyspareunia; and to compare the prevalence of uterine fibroids between groups.
Exclusion criteria included age under 18, overt menopause, pregnancy, and malignant gynecologic diseases. Patients with a known diagnosis of endometriosis or those newly diagnosed with the condition during the gynecological consultation were also excluded from the study. The flow chart outlines the inclusion and exclusion process used to identify eligible participants (Figure 1).
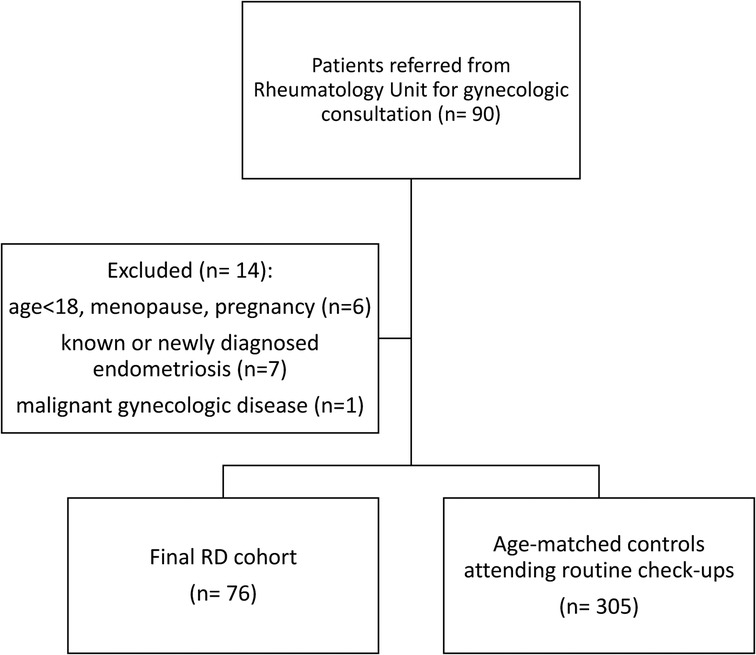
Figure 1. Flow chart of study population, with inclusion and exclusion criteria for eligible participants.
In order to explore the potential diagnosis of adenomyosis, a detailed clinical and gynecological history was obtained from each patient. The presence of AUB, HMB, dysmenorrhea or dyspareunia was assessed. Pain severity was assessed through the Numeric Rating Scale and in the case of 8–10 points, pain was defined as severe. At our institution, ultrasound is routinely performed as part of the standard gynecological consultation. A transvaginal ultrasound (TVUS) pelvic examination was performed by a single operator with expertise in gynecologic imaging (S.V.), using ultrasound machines (Voluson E8, General Electric, GE) equipped with a transvaginal probe (5–7.5 MHz), allowing for two-dimensional (2D), three-dimensional (3D), and Color and Power Doppler evaluation. The diagnosis of adenomyosis was based on a combination of clinical assessment and TVUS findings (10), based the Morphological Uterus Sonographic Assessment (MUSA) consensus. Both direct and indirect MUSA features were considered, including: echogenic subendometrial lines and buds, myometrial cysts, hyperechogenic islands, interrupted or irregular junctional zone, asymmetrical myometrial thickening, globular uterus, translesional vascularity, and fan-shaped shadowing. A diagnosis of adenomyosis was made when at least one direct feature was identified (14, 20). In addition to the assessment for adenomyosis, the presence of uterine fibroids was also evaluated (21, 22). Suspected endometriosis was systematically screened and excluded according to the International Deep Endometriosis Analysis (IDEA) consensus protocol (23).
The study was approved by the local Ethics Committee (protocol number 14558). Informed consent was obtained from the subjects involved in the study.
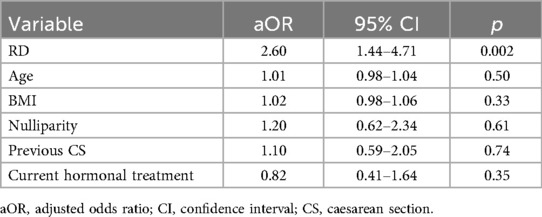
All the data were reported in an electronic database and the statistical analysis was performed by using IBM SPSS Statistics software, version 22 (IBM Corporation, Armonk, NY, United States). Descriptive statistics were performed: continuous variables are expressed as the mean (±standard deviation, SD), whereas for categorical data, absolute and relative frequencies were calculated. Normality of continuous variables was assessed with the Shapiro–Wilk test. Continuous parameters were evaluated by Student's t test for normally distributed data, otherwise the Mann–Whitney test was applied. To evaluate the associations between categorical variables and patient groups a chi-square test or Fisher's exact test were used. Effect sizes for categorical outcomes were expressed as odds ratios (OR) with 95% confidence intervals, derived from 2 × 2 contingency tables. Additionally, a multivariable logistic regression analysis was performed to estimate the association between RD status and adenomyosis, adjusting for potential confounders. Variables included in the model were age, BMI, nulliparity, previous caesarean section, and current hormonal therapy. These covariates were selected a priori based on clinical relevance and data availability. Results are reported as adjusted odds ratios (aOR) with 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant.
Results
The study population included 76 patients with RD (mean age 39 ± 8.48 years). RDs were distributed as follows:
1. connective tissue diseases (52%): Sjögren's syndrome, systemic lupus erythematosus, systemic sclerosis, and undifferentiated connective tissue disease;
2. arthritis (41%): rheumatoid arthritis, spondyloarthritis, juvenile idiopathic arthritis, polyarthritis, psoriatic arthritis, other seronegative spondylarthritis;
3. vasculitis (7%): Behçet's disease;
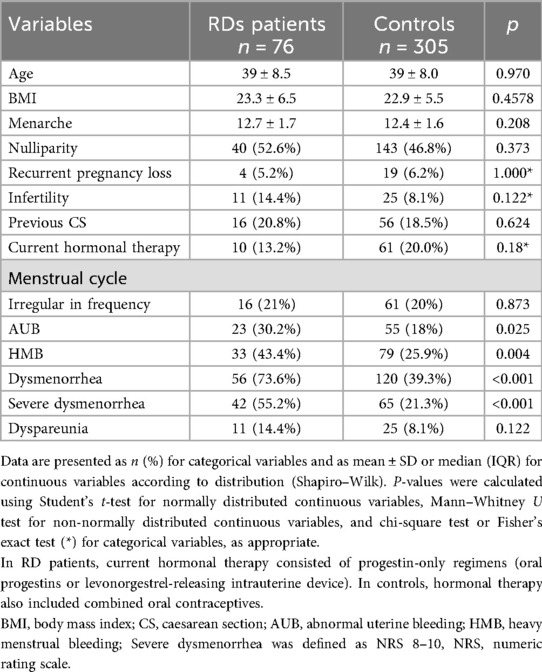
Almost all RD patients (98.6%) were receiving treatment for their underlying disease. Baseline demographic and clinical characteristics of RD patients and controls are summarized in Table 1. No significant differences were observed between the two groups in term of age, nulliparity, infertility, previous caesarean section and current hormonal treatment. However, when evaluating menstrual cycle symptoms and gynecological pain, women with RDs reported significantly higher rates of AUB (39.5% vs. 26.2%; p = 0.025), HMB (43.4% vs. 25.9%; p = 0.004) and severe dysmenorrhea (55.2% vs. 21.3%; p < 0.001) compared to controls (Table 1).
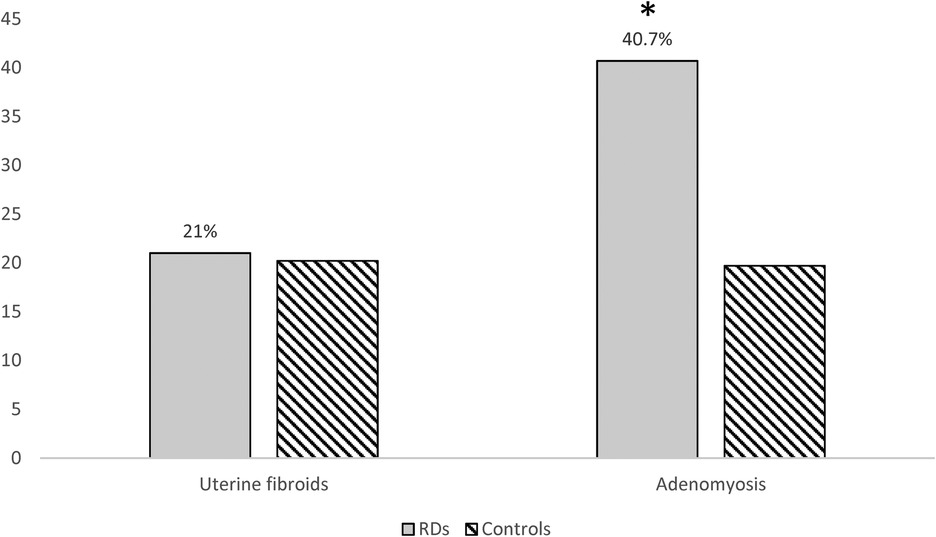
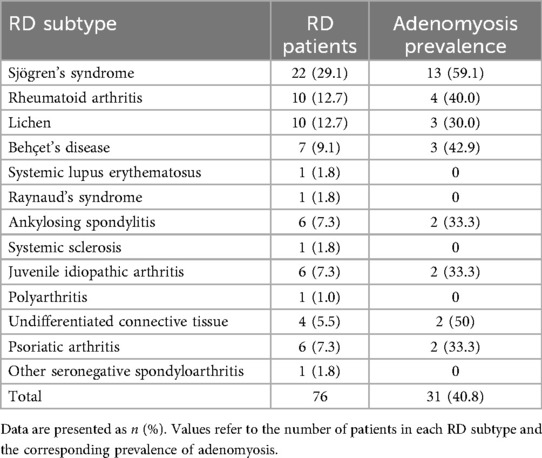
Among the 76 women with RDs and the 305 age-matched controls included, the prevalence of adenomyosis was significantly higher in RD patients (31/76, 40.8% vs. 60/305, 19.7%; OR 2.81, 95% CI 1.64–4.82; p < 0.001) (Figure 2). Table 3 summarizes the frequency of various RDs within the study population and the related prevalence of adenomyosis observed across these groups. After adjustment for age, BMI, nulliparity, previous caesarean section, and current hormonal therapy, RD remained independently associated with adenomyosis (aOR 2.60, 95% CI 1.44–4.71; p = 0.002). None of the covariates showed significant independent associations (Table 2). Adenomyosis was also significantly associated with the clinical coexistence of HMB and severe dysmenorrhea (OR = 5.75 (CI 95%: 2.08–15.88; p = 0.0009).
In contrast, the prevalence of uterine fibroids did not differ between groups (16/76, 21.1% vs. 61/305, 20.2%; p = 0.873), with the coexistence of both conditions in 15.3% cases of RD patients (Figure 2).
Discussion
The primary finding of our study is a significantly higher prevalence of adenomyosis in women with RDs compared to age-matched controls. This association remained significant after multivariable adjustment for age, BMI, nulliparity, previous caesarean section, and current hormonal therapy. This novel association suggests a link between immune-mediated rheumatic conditions and adenomyosis, in line with existing evidence on endometriosis and autoimmune disorders (17). Furthermore, patients with RDs were overall significantly more symptomatic, experiencing higher frequencies of AUB, HMB and severe dysmenorrhea than the control group. Notably, the coexistence of adenomyosis and RDs was significantly associated with both HMB and severe dysmenorrhea.
The interplay between sex hormones and immunity appears to be a central mechanism to explain this pattern. Estrogen and progesterone modulate immune responses by influencing lymphocyte activity, cytokine production, and antibody synthesis. In women with RDs—who already exhibit immune dysregulation—this hormonal–immune interaction could exacerbate menstrual symptoms via heightened inflammatory responses in the endometrium (24). These symptoms, including AUB, HMB, and dysmenorrhea, are also key clinical features of structural uterine disorders such as adenomyosis (10, 16). Nevertheless, bleeding symptoms referred by patients affected by either uterine fibroids or adenomyosis are strongly associated with reduced quality of life and increased perceived stress (25). In our cohort, adenomyosis was significantly more frequent in women with RDs and was itself significantly associated with the coexistence of HMB and severe dysmenorrhea, suggesting a compounding effect of systemic inflammation and uterine pathology.
The pathogenetic mechanism linking adenomyosis and RD may involve inflammatory and immune dysregulation (26, 27), as it is reported in endometriosis (28). In adenomyosis, both systemic and local immune changes, through the epithelial-mesenchymal transition mechanism, stimulate the migration of endometrial cells into the myometrium and promote inflammation (12). Adenomyosis is also characterized by hormonal aberrations, such as increased estrogen receptors activity and progesterone resistance (29, 30). Estrogen and progesterone modulate the immune system, affecting the cascade of proinflammatory factors (31–33). Immune dysregulation and inflammation may contribute to menstruation-related disorders in women with RDs (12, 17, 34–36). The RD inflammatory setting may amplify the local inflammation characteristic of adenomyosis and potentially exacerbate menstrual symptoms (37). In RDs neurogenic inflammation may also play a role in amplifying gynecological pain symptoms. By altering neuronal excitability and impairing pain sensitivity, it contributes to heightened pain perception and central sensitization, thereby potentially worsening dysmenorrhea and other pelvic pain manifestations (38). Importantly, almost all RD patients in our cohort were receiving treatment for their underlying disease. However, we did not investigate whether rheumatologic therapy influenced adenomyosis-related symptoms, nor whether adenomyosis management could impact the course of RD. Furthermore, we did not collect data on disease duration or severity, which may also play a role in the association between RDs and adenomyosis. Regarding hormonal therapy, while its overall prevalence did not significantly differ between groups, RD patients were treated exclusively with progestin-only regimens, whereas combined oral contraceptives were also represented among controls. This heterogeneity may have influenced menstrual symptom profiles, despite the relatively small proportion of patients on hormonal treatment in both groups. These important questions remain unexplored in the current literature and warrant further dedicated studies.
Interestingly, the study found a significantly higher incidence of adenomyosis in women with RDs, independent of endometriosis as a comorbidity. It is already well established that RDs are associated with an increased prevalence of endometriosis (17), which is frequently underdiagnosed and may occur alone or in combination with adenomyosis. Indeed, endometriosis shares several pathophysiological and immunological features with adenomyosis (11). To minimize confounding, women with a known or newly diagnosed endometriosis were excluded, allowing a clearer exploration of the relationship between adenomyosis and RDs.
Regarding uterine fibroids, no significant difference in prevalence was observed between women with RDs and controls groups, supporting the concept that fibroids may represent a distinct clinical and biological entity compared to adenomyosis, despite partially overlapping clinical manifestations, including AUB, HMB, and pelvic pain (39). The pathogenesis of fibroids is multifactorial, involving hormonal, genetic, and environmental factors, but growing evidence indicates that immune and inflammatory processes also contribute to their development and progression. Fibroids are associated with chronic, low-grade inflammation in the myometrium, characterized by infiltration of immune cells such as macrophages, mast cells, and T lymphocytes. These cells release mediators that promote fibrosis, angiogenesis, and tissue growth, while impaired immune tolerance and abnormal responses to injury may also contribute. However, unlike adenomyosis, where inflammation is central to disease mechanisms, in fibroids these pathways are secondary, with pathogenesis driven primarily by hormonal signalling and somatic mutations in smooth muscle cells (39). For this reason, the lack of a higher fibroid prevalence in RD patients suggests that systemic autoimmune and inflammatory processes, which strongly intersect with adenomyosis, may not significantly influence fibroid development. Assessing both conditions in the same cohort is nonetheless crucial, as it allows for differentiation between overlapping gynecologic symptoms and provides a clearer understanding of the specific uterine pathologies more closely linked to RDs.
Some limitations of this study should be acknowledged. First, no a priori sample-size calculation was performed, as this was an exploratory study analyzing a consecutive cohort within a multidisciplinary care pathway. Despite that, the observed difference in adenomyosis prevalence between groups was large and statistically robust, suggesting that the available sample size was adequate to detect clinically relevant associations. Nevertheless, the overall number of RD patients was relatively limited, and RDs are highly heterogeneous in their clinical manifestations. The study was therefore not powered to perform subgroup analyses across individual RD subtypes. Moreover, the single-centre design may limit generalizability. Although no specific distribution of uterine disorders was observed across different RDs, the variability within this patient population may be an additional limitation. The potential for selection bias should also be considered, as participation in the gynecological consultation was voluntary. Additionally, the operator was not blinded to RD status, since the examinations were conducted in a real-world multidisciplinary setting. Although the consultation was systematically offered to all women with RDs within a multidisciplinary care setting, it is possible that those experiencing gynecological symptoms were more likely to accept referral, potentially leading to an overrepresentation of symptomatic patients in the study cohort. Age distribution also represents a relevant consideration. All participants were over 30 years of age, reflecting both the typical age at diagnosis of most RDs and the deliberate exclusion of patients with endometriosis. Since endometriosis is often diagnosed earlier in reproductive life, its exclusion may have skewed the population toward older age groups, in whom adenomyosis is more prevalent. As a result, the findings may not fully extend to younger RD patients. Nonetheless, as endometriosis can sometimes be asymptomatic or undetectable on imaging, its complete exclusion cannot be assured. This limitation was partly mitigated by the use of the IDEA protocol, which maximized diagnostic accuracy and reduced, though did not entirely eliminate, the risk of missed or subclinical cases.
Despite these limitations, the study has several notable strengths. To our knowledge, this is the first report to systematically evaluate menstruation-related disorders other than endometriosis in RD patients, providing novel insights into a relatively unexplored area of women's health in rheumatology. In addition, the inclusion of an age-matched control group represents a methodological strength, as both adenomyosis and uterine fibroids increase with age. Matching minimized the potential confounding effect of age and strengthened the reliability of comparisons between RD patients and controls. Finally, all ultrasound assessments were performed by an experienced sonographer using strict protocols (23, 25) ensuring high diagnostic accuracy and consistency across the study.
In conclusion, this study highlights a significantly higher prevalence of adenomyosis in women with RDs, with important implications for both clinical practice and future research. These findings suggest a potential role of systemic inflammation and immune dysregulation in adenomyosis pathophysiology and underscore the value of interdisciplinary management. Greater awareness of this association is crucial for rheumatologists and gynecologists, as early recognition of menstrual symptoms such as HMB or severe dysmenorrhea should prompt timely gynecologic referral, given that RD patients are at increased risk for adenomyosis. Considering the well-documented impact of adenomyosis on fertility and pregnancy outcomes (40), its coexistence with RDs may further exacerbate reproductive risks already present in this population (41, 42). Strengthening collaboration between rheumatology and gynecology, alongside continued investigation into shared mechanisms, may lead to earlier diagnosis, improved patient care, and the development of targeted therapeutic strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato Etico Regione Toscana—Area Vasta Centro (CEAVC)—protocol number 14558. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SV: Methodology, Conceptualization, Writing – original draft, Investigation. MO: Methodology, Writing – review & editing. FL: Writing – review & editing, Investigation, Data curation. EG: Data curation, Investigation, Writing – review & editing. MF: Writing – review & editing, Supervision. MM: Writing – review & editing, Supervision. FP: Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by institutional research funds (ex 60%—RICATEN2024) from University of Florence, Italy, specifically allocated to cover publication fees.
Acknowledgments
We are grateful for the participation of the subjects and we thank the clinical staff who supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fairweather D, Beetler DJ, McCabe EJ, Lieberman SM. Mechanisms underlying sex differences in autoimmunity. J Clin Invest. (2024) 134:e180076. doi: 10.1172/JCI180076
2. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. (2014) 35:347–69. doi: 10.1016/j.yfrne.2014.04.004
3. Cutolo M, Straub RH. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol. (2020) 16:628–44. doi: 10.1038/s41584-020-0503-4
4. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
5. Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. (2007) 6:366–72. doi: 10.1016/j.autrev.2006.10.001
6. Østensen M. Sexual and reproductive health in rheumatic disease. Nat Rev Rheumatol. (2017) 13:485–93. doi: 10.1038/nrrheum.2017.102
7. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. (2020) 72:529–56. doi: 10.1002/art.41191
8. Orlandi M, Vannuccini S, El Aoufy K, Melis MR, Lepri G, Sambataro G, et al. Menstruation-related disorders—dysmenorrhea and heavy bleeding—as significant epiphenomena in women with rheumatic diseases. Front Pharmacol. (2022) 13:807880. doi: 10.3389/fphar.2022.807880
9. Mension E, Carmona F, Vannuccini S, Chapron C. Clinical signs and diagnosis of fibroids from adolescence to menopause. Fertil Steril. (2024) 122:12–9. doi: 10.1016/j.fertnstert.2024.05.003
10. Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. (2020) 26:392–411. doi: 10.1093/humupd/dmz049
11. Donnez J, Stratopoulou CA, Dolmans M-M. Endometriosis and adenomyosis: similarities and differences. Best Pract Res Clin Obstet Gynaecol. (2024) 92:102432. doi: 10.1016/j.bpobgyn.2023.102432
12. Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. (2021) 27:108–29. doi: 10.1093/humupd/dmaa038
13. Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. (2020) 38:089–107. doi: 10.1055/s-0040-1718920
14. Harmsen MJ, Van den Bosch T, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L, et al. Consensus on revised definitions of morphological uterus sonographic assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol. (2022) 60:118–31. doi: 10.1002/uog.24786
15. Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, et al. Transvaginal sonographic features of diffuse adenomyosis in 18–30-year-old nulligravid women without endometriosis: association with symptoms: adenomyosis in young women. Ultrasound Obstet Gynecol. (2015) 46:730–6. doi: 10.1002/uog.14834
16. Vannuccini S, Meleca C, Toscano F, Mertino P, Pampaloni F, Fambrini M, et al. Adenomyosis diagnosis among adolescents and young women with dysmenorrhoea and heavy menstrual bleeding. Reprod Biomed Online. (2024) 48:103768. doi: 10.1016/j.rbmo.2023.103768
17. Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:486–503. doi: 10.1093/humupd/dmz014
18. Khan KN, Guo S-W, Ogawa K, Fujishita A, Mori T. The role of innate and adaptive immunity in endometriosis. J Reprod Immunol. (2024) 163:104242. doi: 10.1016/j.jri.2024.104242
19. Riccio LDGC, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. (2018) 50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010
20. Van Den Bosch T, De Bruijn AM, De Leeuw RA, Dueholm M, Exacoustos C, Valentin L, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gyne. (2019) 53:576–82. doi: 10.1002/uog.19096
21. Munro MG, Critchley HOD, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. (2011) 113:3–13. doi: 10.1016/j.ijgo.2010.11.011
22. Van den Bosch T, Dueholm M, Leone FPG, Valentin L, Rasmussen CK, Votino A, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the morphological uterus sonographic assessment (MUSA) group. Ultrasound Obstet Gynecol. (2015) 46:284–98. doi: 10.1002/uog.14806
23. Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FPG, Van Schoubroeck D, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the international deep endometriosis analysis (IDEA) group. Ultrasound Obstet Gynecol. (2016) 48:318–32. doi: 10.1002/uog.15955
24. Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther. (2009) 11:252. doi: 10.1186/ar2825
25. Vannuccini S, Clemenza S, Cassioli E, Rossi E, Castellini G, Ricca V, et al. Uterine fibroids, perceived stress, and menstrual distress: a key role of heavy menstrual bleeding. Reprod. Sci. (2023) 30:1608–15. doi: 10.1007/s43032-022-01126-3
26. Di Franco M, Bazzichi L, Casale R, Sarzi-Puttini P, Atzeni F. Pain in systemic connective tissue diseases. Best Pract Res Clin Rheumatol. (2015) 29:53–62. doi: 10.1016/j.berh.2015.05.006
27. Ysrraelit MC, Correale J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology. (2019) 156:9–22. doi: 10.1111/imm.13004
28. Wei L, Wang S, Xu S, Zhang C. The interplay between systemic inflammatory factors and endometriosis: a bidirectional mendelian randomization study. J Reprod Immunol. (2024) 165:104293. doi: 10.1016/j.jri.2024.104293
29. Vannuccini S, Petraglia F. Adenomyosis: is an endocrine-related uterine dysfunction? Gynecol Endocrinol. (2022) 38:1017–8. doi: 10.1080/09513590.2023.2172156
30. Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. (2020) 38:129–43. doi: 10.1055/s-0040-1716687
31. Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: a comprehensive review. Pain. (2014) 155:2448–60. doi: 10.1016/j.pain.2014.08.027
32. Lombardo G, Mondelli V, Dazzan P, Pariante CM. Sex hormones and immune system: a possible interplay in affective disorders? A systematic review. J Affect Disord. (2021) 290:1–14. doi: 10.1016/j.jad.2021.04.035
33. Zhang L, Zhao Y, Liu X, Chen J, Sun M, Zhang J, et al. Changes in sex hormones and their interactions are related to pain perception between different menstrual subphases. Am J Physiol Regul Integr Comp Physiol. (2023) 325:R280–9. doi: 10.1152/ajpregu.00275.2022
34. Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. (2014) 20:386–402. doi: 10.1093/humupd/dmt052
35. Carrarelli P, Yen C-F, Funghi L, Arcuri F, Tosti C, Bifulco G, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci. (2017) 24:369–75. doi: 10.1177/1933719116657192
36. Zervou MI, Tarlatzis BC, Grimbizis GF, Spandidos DA, Niewold TB, Goulielmos GN. Association of endometriosis with Sjögren’s syndrome: genetic insights (review). Int J Mol Med. (2024) 53:20. doi: 10.3892/ijmm.2024.5344
37. Affaitati G, Costantini R, Tana C, Cipollone F, Giamberardino MA. Co-occurrence of pain syndromes. J Neural Transm (Vienna). (2020) 127:625–46. doi: 10.1007/s00702-019-02107-8
38. Seifert O, Baerwald C. Interaction of pain and chronic inflammation. Z Rheumatol. (2021) 80:205–13. doi: 10.1007/s00393-020-00951-8
39. Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev. (2022) 43:678–719. doi: 10.1210/endrev/bnab039
40. Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, Spaanderman M, Kramer BW, Mueller MD, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online. (2021) 42:185–206. doi: 10.1016/j.rbmo.2020.09.023
41. Crisafulli F, Lazzaroni MG, Nalli C, Orabona R, Franceschini F, Tincani A. Reproductive health in scleroderma, vasculitis, and sjögren syndrome. J Clin Rheumatol. (2024) 30:S49–55. doi: 10.1097/RHU.0000000000002128
Keywords: adenomyosis, dysmenorrhea, heavy menstrual bleeding, pelvic pain, rheumatic diseases, uterine fibroids
Citation: Vannuccini S, Orlandi M, La Torre F, Gallucci E, Fambrini M, Matucci Cerinic M and Petraglia F (2025) Adenomyosis in patients with rheumatic diseases: a cross-disciplinary clinical observation. Front. Reprod. Health 7:1697567. doi: 10.3389/frph.2025.1697567
Received: 2 September 2025; Accepted: 31 October 2025;
Published: 18 November 2025.
Edited by:
Carlo Ticconi, Policlinico Tor Vergata, ItalyReviewed by:
Ashish Ashish, Banaras Hindu University, IndiaFrancesco Giuseppe Martire, University of Rome Tor Vergata, Italy
David Lukanovic, University Medical Centre Ljubljana, Slovenia
Nitish Kumar Singh, Banaras Hindu University, India
Copyright: © 2025 Vannuccini, Orlandi, La Torre, Gallucci, Fambrini, Matucci Cerinic and Petraglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Vannuccini, c2lsdmlhLnZhbm51Y2NpbmlAdW5pZmkuaXQ=
†PRESENT ADDRESSES Martina Orlandi, Rheumatologic Unit, Department of Medical and Surgical Sciences for Children and Adults, University of Modena and Reggio Emilia, Modena, Italy Marco Matucci Cerinic, Unit of Immunology, Rheumatology, Allergy and Rare diseases (UnIRAR), Scleroderma Unit, IRCCS San Raffaele Hospital, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy;Inflammation Fibrosis and Ageing Initiative (INFLAGE), Division of Genetics and Cell Biology, IRCCS San Raffaele Scientific Institute, Milan, Italy
‡ORCID:
Silvia Vannuccini
orcid.org/0000-0001-5790-587X
Martina Orlandi
orcid.org/0000-0001-6784-2235
Felice Petraglia
orcid.org/0000-0002-8851-625X
 Silvia Vannuccini
Silvia Vannuccini Martina Orlandi
Martina Orlandi Francesco La Torre
Francesco La Torre Ernesto Gallucci1,2
Ernesto Gallucci1,2 Massimiliano Fambrini
Massimiliano Fambrini Marco Matucci Cerinic
Marco Matucci Cerinic Felice Petraglia
Felice Petraglia