- 1Kazakhstan Medical University «KSPH», Almaty, Kazakhstan
- 2Medical Center RAHAT, Almaty, Kazakhstan
- 3Department of Obstetrics and Gynecology № 1, Medical University of Astana, Astana, Kazakhstan
- 4Department of Science and Human Resources, Ministry of Health of the Republic of Kazakhstan, Astana, Kazakhstan
- 5Department of Obstetrics and Gynecology 1, Azerbaijan Medical University, Baku, Azerbaijan
- 6Department of Obstetrics and Gynecology, Baku Branch of the First Moscow State Medical University, Baku, Azerbaijan
Introduction: Tubal factor infertility is one of the most prevalent and diagnostically complex causes of female infertility. Standard imaging modalities, including hysterosalpingography and laparoscopy, frequently fail to detect subtle mucosal or molecular abnormalities. This study investigated whether combining salpingoscopy with immunohistochemical (IHC) profiling improves diagnostic precision and prognostic assessment in women with tubal-factor infertility.
Methods: This retrospective observational cohort study included 197 women who underwent laparoscopy between April 2020 and February 2025. Salpingoscopy was used to grade mucosal pathology severity, and ampullary biopsies were analyzed by IHC for CD138, Ki-67, and estrogen/progesterone receptors. A composite prognosis score incorporated clinical history, duration of conservative therapy, salpingoscopic findings, and IHC results. Spearman's rank correlation assessed associations between diagnostic parameters and pregnancy outcomes (natural or assisted).
Results: Higher salpingoscopic severity scores were significantly associated with reduced pregnancy likelihood (r = −0.457; p < 0.0001). Elevated IHC scores similarly correlated with decreased conception rates (r = −0.263; p = 0.0002). Tubal dilations and adhesions showed negative associations with reproductive outcomes (r = −0.232; p = 0.001). In contrast, the composite prognosis score demonstrated a strong positive correlation with treatment success (r = 0.578; p < 0.0001).
Discussion: Integrating salpingoscopic visualization with IHC profiling enhances detection of subtle mucosal and molecular abnormalities that are frequently missed by conventional diagnostic approaches. This combined modality offers superior prognostic accuracy and may guide personalized surgical and assisted reproductive treatment strategies. Ethical approval was obtained from the Ethics Committee of the Kazakhstan Medical University «KSPH» (IRB-64-2023).
1 Introduction
Infertility affects an estimated 12.6%–17.5% of couples of reproductive age worldwide and is a growing public health issue with complex psychological and socioeconomic consequences (1). Among the causes of female infertility, tubal factor infertility is one of the most prevalent and diagnostically challenging etiologies (2). It accounts for 30%–40% of all women's infertility cases (3). Tubal factor infertility can result from obstruction, scarring, or impaired function of the fallopian tubes (4). The routine assessment of tubal patency typically involves the use of hysterosalpingography (HSG), sonohysterography (SHG), and hysterosalpingo-contrast sonography (HyCoSy). These imaging methods are accessible and provide non-invasive detection of anatomical blockages (5, 6). However, they do not provide information on subtle epithelial or functional abnormalities (7). Even laparoscopy, currently considered the gold standard for evaluating tubal and pelvic pathology, lacks the resolution to identify mucosal damage, ciliary dysfunction, or subclinical inflammation (8). These limitations may account for cases of unexplained infertility and reduce the effectiveness of both natural and assisted reproductive technology (ART) treatments (9, 10).
In clinical practice among underrepresented and resource-constrained populations, such as in Central Asia, such diagnostic limitations are particularly evident (11). In Kazakhstan, female infertility accounts for the majority of reported infertility cases, but many patients undergo empirical treatment without a definitive diagnosis (12, 13). This further highlights the need for improved diagnostic strategies capable of assessing not only tubal patency but also functional and molecular integrity. However, few studies have systematically assessed the combined diagnostic and prognostic value of salpingoscopy and immunohistochemistry (IHC) in tubal factor infertility. While each method has shown promise separately (3, 14), their integration into routine evaluation remains limited in both clinical practice and the literature.
The combination of salpingoscopy and IHC analysis may offer a novel opportunity to bridge this diagnostic gap. Salpingoscopy is an endoscopic technique that allows for the direct visualization of the inside of the fallopian tube (14). Recent studies have indicated that specific salpingoscopic findings, such as loss of mucosal folds or intraluminal adhesions, are associated with reduced fertility potential and poor ART outcomes (15–17). IHC analysis allows for molecular profiling of the tubal epithelium, including markers of inflammation (e.g., CD138), hormone receptor status (e.g., progesterone and estrogen), and cell proliferation (e.g., Ki-67 and p53) (18, 19). Increased expression of CD138+ plasma cells and elevated Ki-67 indices in the tubal epithelium have been linked to chronic inflammation and ciliary dysfunction (20, 21).
By integrating anatomical and cellular-level assessment, these methods may increase diagnostic precision, leading to targeted therapeutic strategies and enhancing reproductive outcomes.
This observational retrospective study aimed to evaluate whether combining salpingoscopy and IHC profiling improves diagnostic precision and better predicts pregnancy outcomes compared to conventional assessment methods. We further investigated whether these diagnostic indicators independently predict treatment success, and whether they can identify patients with poorer reproductive prognosis who may benefit from earlier ART or targeted interventions. We hypothesized that the integration of salpingoscopic grading and IHC profiling would offer prognostic value beyond traditional assessment and support more personalized fertility treatment planning.
2 Methods
2.1 Study population and study design
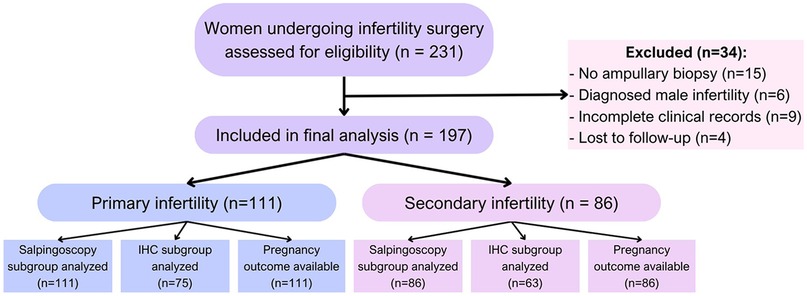
This observational retrospective cohort study included 197 women participants from collaborating reproductive health centers in Kazakhstan between April 2020 and February 2025. The sample size was determined by enrolling all eligible cases between April 2020 and February 2025 in collaborating centers. Inclusion criteria were women aged 18–45 years with a clinical diagnosis of primary or secondary infertility, defined as failure to conceive after 12 months of regular unprotected intercourse. All the patients had an indication for diagnostic laparoscopy based on unexplained infertility, suspected tubal factor, or failed ART attempts. The exclusion criteria included patients with incomplete medical records, absence of ampullary biopsy, loss to follow-up within 12 months, or evidence of male-factor infertility as the sole diagnosis (Figure 1). Patients with missing or incomplete key clinical data, such as ampullary biopsy results or follow-up pregnancy outcomes, were also excluded from the analysis. No data imputation was performed. Selection bias was minimized by the inclusion of multiple centers and protocolized surgical approaches.
The participants were categorized into two groups, namely primary infertility (n = 111), with no prior pregnancies, and secondary infertility (n = 86), with at least one prior pregnancy but failure to conceive again.
2.2 Ethical consideration
The study was approved by the local ethics committee (IRB-64-2023). All the participants provided written informed consent before inclusion. The study was conducted according to the Declaration of Helsinki. All data were anonymized before the analysis to protect patient confidentiality, and the study complied with relevant data protection regulations.
2.3 Diagnostic procedures
During the laparoscopies, salpingoscopies were performed using a microendoscope. Tubal patency was assessed by chromopertubation. Mucosal appearance, adhesion, stenosis, and dilation were systematically documented. Mucosal appearance was graded based on the standardized “F-score” system (22), assigning one point for each of six features (adhesions, loss of folds, rounded fold edges, debris, foreign bodies, and abnormal vessels) observed during salpingoscopy. Each abnormal finding resulted in the addition of 1 point to the F-score, and the maximum was 12 points.
Tissue biopsies from the ampullary region were collected for IHC analysis. The biopsies were taken from the epithelial lining of the ampullary portion of the fallopian tube, mainly from the mucosal surface and occasionally closer to the fimbrial end. The most visibly altered epithelial areas were preferentially sampled. Smooth muscle tissue was not collected, as the fallopian tube wall is thin, prone to bleeding, and considered non-informative for the intended analyses.
Immunohistochemical staining was performed in the affiliated clinical pathology laboratory according to their standard diagnostic protocols; the authors did not perform the IHC procedures themselves. For each patient, the finalized pathology reports and slide assessments were provided to the investigators and reviewed for this study. The standard panels included the expression of various protein markers, such as Ki-67, p53 (23), estrogen and progesterone receptors (24), and CD138 (25), which indicate cell proliferation, inflammatory processes, or the presence of precancerous changes.
Existing pathology reports and slide assessments were reviewed and categorized based on marker expression patterns. The results were grouped into three categories for analysis: (1) IHC not performed, with histologically normal epithelium; (2) positive expression, indicating abnormal findings such as CD138+ plasma cells, elevated Ki-67 index, or aberrant hormone receptor profiles; and (3) negative expression, defined as the absence of pathological immunoreactivity. Discrepancies between reviewers were resolved by consensus.
Pregnancy outcomes were identified retrospectively through medical record review of the 12 months following surgery. A pregnancy was considered confirmed if documented as either a biochemical pregnancy (positive β-hCG) or a clinical pregnancy (ultrasound-confirmed intrauterine gestation), achieved either through natural conception or ART. Treatment success was defined as any documented pregnancy within the follow-up period.
2.4 Data collection
Clinical data were retrospectively collected from medical records and included patient age, type of infertility (primary or secondary), gynecological comorbidities, history of STIs, spermogram results of male partners, and morphological findings noted during salpingoscopy. Two coordinators independently abstracted key variables, and a third reviewer resolved any discrepancies.
2.5 Statistical analysis
The statistical analyses were performed using GraphPad Prism 10.4.2. Continuous variables are reported as medians with interquartile ranges (IQRs). Group comparisons were performed using the chi-square (χ2) test. Spearman's rank correlation was used to explore the relationship between the tubal pathology features and pregnancy outcome. A p-value < 0.05 was considered statistically significant.
2.6 Reporting guidelines
This study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. A completed checklist is provided in the Supplementary Materials.
3 Results
3.1 Age distribution
The age range of the participants was 23–43 years (n = 197). The IQR was 28–37 years, with a median age of 31 years. The 25th percentile was 28 years, and the 75th percentile was 37 years, while the minimum and maximum ages were 23 and 43 years, respectively. Thus, 50% of the participants were between 28 and 37 years old. The distribution appears symmetrical, as the median (31 years) is close to the midpoint of the IQR. The study participants predominantly belonged to the young and middle-aged groups, which may reflect the characteristics of the study or the target population.
3.2 Clinical characteristics
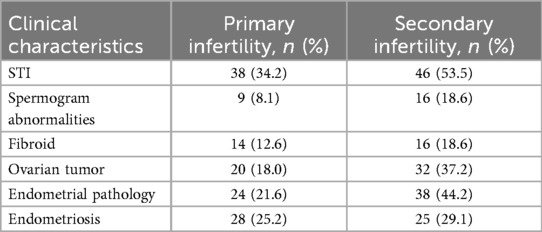
Participants were categorized into two groups, namely, primary infertility (n = 111) and secondary infertility (n = 86). Clinical data were collected retrospectively from the medical records of the patients, including comorbid gynecological diseases, the presence of STIs, and spermogram abnormalities in their male partners (Table 1). These clinical characteristics were then compared between the two groups to determine any significant differences (Figure 2).
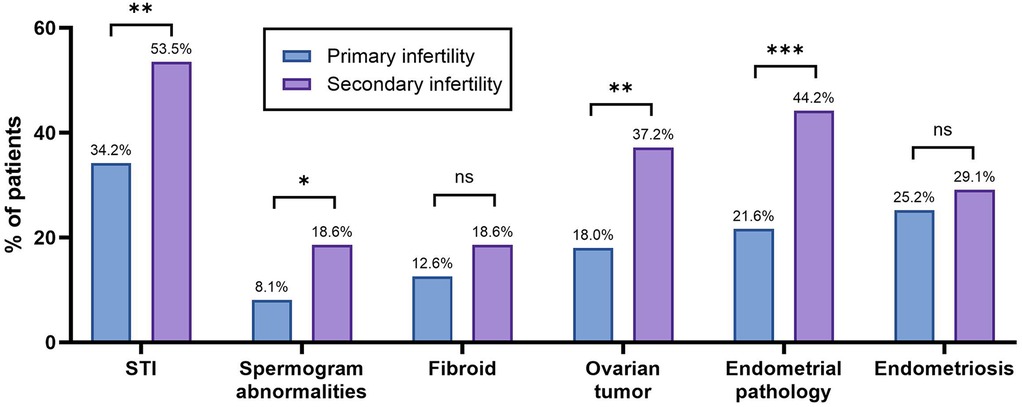
Figure 2. Clinical characteristics of the patients with primary or secondary infertility. The bar graph shows the percentage of patients with specific conditions across infertility types. Statistical significance was identified using the chi-square (χ2) test, with * denoting p < 0.05, ** denoting 0.001 < p < 0.01, *** denoting 0.0001 < p < 0.001, **** denoting p < 0.0001, and ns denoting no statistically significant difference.
The prevalence of ovarian tumors and endometrial pathologies was statistically significantly higher (p = 0.0024 and p = 0.0007, respectively) among the patients with secondary infertility compared to those with primary infertility. In the primary infertility group, STIs were identified in 34.2% (n = 38), while in the secondary infertility group, STIs were identified in 53.5% (n = 46). The difference was found to be statistically significant (p = 0.0067). Male factor infertility was also analyzed, as spermogram abnormalities were found in 8.1% and 18.6% of the male partners in the primary and secondary infertility groups, respectively. This was significantly higher in the secondary infertility group (p = 0.0282).
3.3 Tubal patency
Hysterosalpingography was performed in 197 patients for diagnostic assessment of tubal patency (Figure 3). Bilateral contrast passage, indicating open fallopian tubes, was observed in 48.8% of the women with primary infertility and in 39.6% of those with secondary infertility. The remaining patients exhibited unilateral or bilateral tubal obstruction. However, the statistical analysis revealed no significant difference between the two groups (Figure 4).
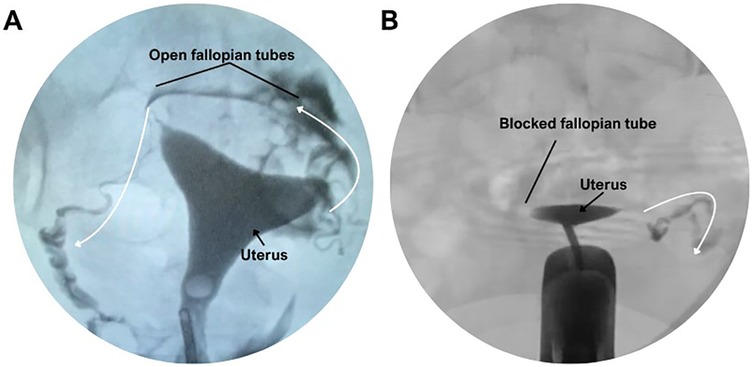
Figure 3. Hysterosalpingographic evaluation of fallopian tube patency. (A) Normal hysterosalpingogram showing bilateral contrast passage through open fallopian tubes. (B) Hysterosalpingogram demonstrating a blocked fallopian tube with no contrast spill.
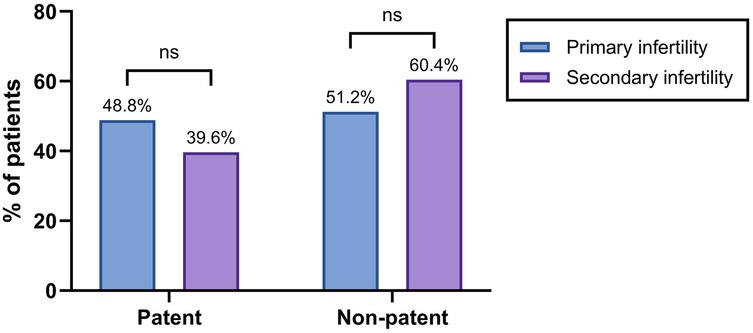
Figure 4. Tubal patency in the infertility groups. The bar graph shows the percentage of patients with patent or non-patent fallopian tubes based on hysterosalpingography. Statistical significance was identified using the chi-square (χ2) test, with * denoting p < 0.05, ** denoting 0.001 < p < 0.01, *** denoting 0.0001 < p < 0.001, **** denoting p < 0.0001, and ns denoting no statistically significant difference.
3.4 Duration of conservative infertility treatment
Among 197 patients, a total of 51.3% (n = 101) underwent conservative therapy for less than 5 years, while 48.7% (n = 96) received treatment for more than 5 years. In the primary infertility group, 53.2% (n = 59) had conservative infertility treatment for more than 5 years, with 43.0% (n = 37) in the secondary infertility group receiving the same. However, the statistical analysis showed no significant difference between the two groups (Supplementary Figure S1).
3.5 Immunohistochemical analysis
A positive IHC result indicated abnormal findings, such as the presence of CD138-positive plasma cells, an elevated Ki-67 proliferation index, p53 overexpression, or aberrant estrogen and progesterone receptor profiles, reflecting inflammatory or precancerous alterations in the tubal epithelium. A negative result denoted the absence of pathological immunoreactivity, with normal marker expression consistent with histologically healthy tissue.
According to the results of the IHC profiling, 51.2% of the patients with primary infertility had a positive result, 22.1% had a negative result, and 26.7% did not undergo the assessment due to having a completely healthy tube. Among the patients with secondary infertility, 41.4% had a positive result, 26.1% had a negative result, and 32.4% did not undergo the assessment due to having a completely healthy tube (Figure 5).
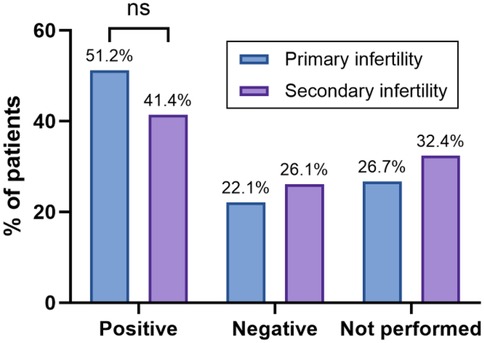
Figure 5. The bar graph shows the percentage of patients with positive, negative, or unperformed IHC findings, stratified by infertility type. Statistical significance was identified using the chi-square (χ2) test, with * denoting p < 0.05, ** denoting 0.001 < p < 0.01, *** denoting 0.0001 < p < 0.001, **** denoting p < 0.0001, and ns denoting no statistically significant difference.
3.6 Natural conception prognosis
Considering the patients’ medical history, results of conservative treatment, and examination findings from IHC and salpingoscopy, a prognosis score (from 0 to 10) for the likelihood of natural conception was generated. Thus, 51 women were assigned a “favorable” prognosis, 62 an “unfavorable” prognosis, and 84 an “uncertain” prognosis (Figure 6). Pregnancy outcomes were reported as not pregnant, ART, or natural conception.
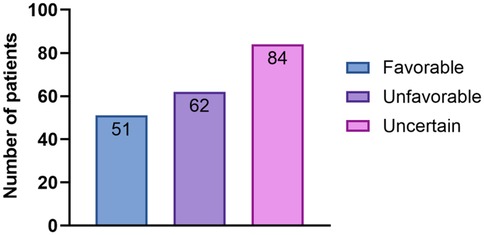
Figure 6. The bar chart shows the distribution of the prognosis categories for spontaneous pregnancy. A total of 197 patients were categorized as having a favorable (n = 51), unfavorable (n = 62), or uncertain (n = 84) prognosis for natural conception, based on clinical and diagnostic criteria.
In the “favorable” prognosis group, 21 patients conceived naturally, and 30 patients conceived with the help of ART. In the “unfavorable” prognosis group, two patients conceived naturally, 35 patients conceived with the help of ART, and 25 were unable to become pregnant. In the “uncertain” prognosis group, 30 patients conceived naturally, 49 patients conceived with the help of ART, and eight were unable to become pregnant. In the “favorable” group, the pregnancy rate was 100% (21 natural, 30 ART). In the “unfavorable” group, 60.5% achieved pregnancy (2 natural, 35 ART), while in the “uncertain” group, 94% conceived (30 natural, 49 ART). Overall, 167 of the 197 patients (84.8%) achieved pregnancy within 12 months, including 53 (26.9%) via natural conception and 114 (57.9%) via ART.
A comparison of pregnancy outcomes between the women with primary and secondary infertility revealed no statistically significant differences. While the natural conception and ART pregnancy rates were slightly higher in the secondary infertility group, a chi-square test indicated that these differences were not statistically significant (p = 0.111). This suggests that, within this cohort, infertility type alone was not a strong predictor of treatment outcome.
3.7 Correlation of diagnostic parameters with pregnancy outcome
The tube severity score was calculated based on salpingoscopic grading (“F-score” system), ranging from normal to severely pathological (from 0 to 12) (22). Spearman's rank correlation analysis (Table 2) showed that higher severity scores in the right fallopian tube were significantly associated with a lower likelihood of achieving pregnancy, both naturally and with ART (r = −0.457, p < 0.0001). A similar but weaker relationship was observed for the left tube severity score (r = −0.243, p = 0.0005). The presence of dilations or adhesions is also associated with significantly lower chances of treatment success.
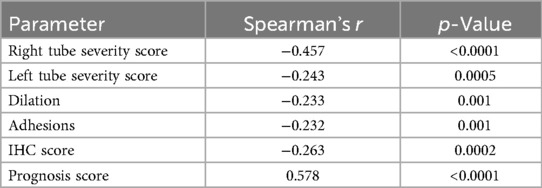
Table 2. Spearman's rank correlation analysis of the association between the diagnostic parameters and pregnancy outcome.
The IHC findings also demonstrated a slight negative correlation with pregnancy outcome (r = −0.263, p = 0.0002). In contrast, the prognosis score showed a strong positive association with pregnancy (r = 0.578, p < 0.0001), indicating that higher predicted probabilities were related to observed treatment success.
Among all the diagnostic parameters, the right tube severity score demonstrated the strongest negative correlation with pregnancy outcome (r = –0.457), while the composite prognosis score was the strongest positive predictor (r = 0.578).
4 Discussion
In this retrospective cohort study of 197 patients who underwent surgical evaluation for infertility, the integration of salpingoscopy and IHC analysis demonstrated a significant association with treatment outcomes. These diagnostic techniques provided complementary anatomical and molecular information in addition to conventional assessments. To our knowledge, this is one of the first studies to integrate salpingoscopic grading and IHC profiling of the fallopian tube epithelium in a large cohort, and to demonstrate their combined predictive value for fertility outcomes.
It was observed that higher salpingoscopic severity scores in both the right and left fallopian tubes correlated negatively with the likelihood of achieving pregnancy (Spearman’s r = −0.457 and −0.243, respectively; p < 0.0001 and p = 0.0005). Moreover, the presence of tubal dilations and adhesions was linked to poorer reproductive treatment outcomes (r = −0.233 and −0.232, both p = 0.001), highlighting the prognostic value of detailed mucosal evaluation. Several studies have already indicated the importance of salpingoscopy in fertility prognosis. It was demonstrated that the addition of salpingoscopy to laparoscopy significantly increased the accuracy when predicting short-term pregnancy (15, 26). Our findings mirror this, with higher mucosal severity scores correlating strongly with reduced pregnancy rates.
The negative correlation between IHC score, which reflects markers of inflammation, hormonal receptor status, and proliferation, and treatment outcome (r = −0.263, p = 0.0002) suggests that subclinical epithelial or inflammatory changes may significantly affect tubal function even when gross patency is maintained. These findings align with other studies that indicated that chronic endometritis and increased CD138+ cell counts adversely affect fertility and in vitro fertilization (IVF) treatment outcomes (27, 28). By directly sampling ampullary biopsies, our combined approach detects functional impairment that may be missed by HSG, SHG, or laparoscopy alone.
Importantly, a composite prognosis score, incorporating medical history, conservative therapy duration, and combined diagnostic findings, was calculated. It showed a strong positive association with treatment success (r = 0.578, p < 0.0001), validating its potential application as a clinical decision-making aid. The patients who were classified according to the composite prognosis score as having a favorable prognosis were more likely to conceive, either naturally or via ART, compared to those with unfavorable or uncertain prognoses.
Although some studies suggest different treatment responses in patients with primary or secondary infertility, particularly in response to ART or expectant management, the evidence remains mixed (29, 30). In our study, we did not observe a statistically significant difference in pregnancy outcomes between the patients with primary and secondary infertility (p = 0.111), suggesting that the combined diagnostic approach may be more predictive than infertility type alone.
These results support the need to add salpingoscopic visualization and molecular profiling to standard tubal assessments. Salpingoscopy allows for a high-resolution investigation of mucosal integrity, leading to the discovery of possible stenosis, dilation, and adhesions. IHC provides an understanding of the inflammatory and proliferative processes that influence ciliary function and epithelial health. Combined, these tools permit more accurate diagnoses and better-tailored treatment strategies. In particular, this approach may reduce time to pregnancy by identifying the patients who are unlikely to benefit from further empirical treatment and prioritizing them for ART. It may also help avoid unnecessary surgical interventions in patients with unremarkable mucosal and molecular findings.
Several limitations need to be acknowledged. The retrospective, multicenter design can introduce selection bias, and the cohort predominantly reflects practices in Kazakhstan, which may limit its generalizability. To avoid these limitations, data were collected from multiple large centers that follow standard assessment protocols. In addition, all the participating centers were located in Kazakhstan, and while they followed standardized diagnostic and treatment protocols, generalizability may be limited to similar healthcare settings in Central Asia or countries with comparable reproductive health infrastructure. Another limitation is the potential variability in salpingoscopic image interpretation and IHC scoring across the centers. Although our coordinators and pathologists followed standard protocols, interobserver reproducibility was not formally assessed. Future work should focus on developing training modules and digital tools to support diagnostic consistency. Moreover, future studies should validate these findings in larger, more diverse populations and evaluate the cost-effectiveness of the combined diagnostic protocol. Adding genomic or proteomic biomarkers to this salpingoscopic and IHC protocol may further improve its prognostic potential. A comprehensive multimodal diagnostic protocol could optimize personalized treatment, improve pregnancy rates, and reduce the weaknesses of empirical infertility therapies.
5 Conclusions
Integrating salpingoscopy and IHC enhances diagnostic and prognostic accuracy in tubal factor infertility. The proposed composite model reliably identifies the patients who are likely to benefit from surgical repair vs. those who require early ART intervention. Prospective validation of this multimodal approach may further optimize personalized treatment and improve reproductive outcomes.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: the datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Adalyat Alenova,cGhkLnN1cmdlb24uYWxlbm92YUBnbWFpbC5jb20=.
Ethics statement
This study involving humans was approved by the Ethics Committee of the Kazakhstan Medical University «KSPH» under protocol number IRB-64-2023. This study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AA: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. AK: Methodology, Supervision, Validation, Writing – review & editing. AK: Formal analysis, Resources, Supervision, Visualization, Writing – review & editing. IM: Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2025.1701315/full#supplementary-material
Supplementary Figure S1 | Duration of conservative therapy among the patients with primary or secondary infertility. The bar chart shows the percentage of patients who received conservative infertility treatment for less than or more than 5 years. Statistical significance was confirmed using the chi-square (χ2) test, with * denoting p < 0.05, ** denoting 0.001 < p < 0.01, *** denoting 0.0001 < p < 0.001, **** denoting p < 0.0001, and ns denoting no statistically significant difference.
Abbreviations
HSG, hysterosalpingography; SHG, sonohysterography; HyCoSy, hysterosalpingo-contrast sonography; IHC, immunohistochemical; ART, assisted reproductive technology; STI, sexually transmitted infection; IQR, interquartile range.
References
1. Cox CM, Thoma ME, Tchangalova N, Mburu G, Bornstein MJ, Johnson CL, et al. Infertility prevalence and the methods of estimation from 1990 to 2021: a systematic review and meta-analysis. Hum Reprod Open. (2022) 2022(4):hoac051. doi: 10.1093/hropen/hoac051
2. Zou Y, Li S, Ming L, Yang Y, Ye P, Zou J. The correlation between chronic endometritis and tubal-factor infertility. J Clin Med. (2022) 12(1):285. doi: 10.3390/jcm12010285
3. Ambildhuke K, Pajai S, Chimegave A, Mundhada R, Kabra P. A review of tubal factors affecting fertility and its management. Cureus. (2022) 14(11):e30990. doi: 10.7759/cureus.30990
4. Sun YF, Zhang J, Xu YM, Luo ZY, Sun Y, Hao GM, et al. Effects of age on pregnancy outcomes in patients with simple tubal factor infertility receiving frozen-thawed embryo transfer. Sci Rep. (2020) 10(1):18121. doi: 10.1038/s41598-020-75124-3
5. Xu Z, Wang Y, Sun J, Chen S, Yan Z, Lin C, et al. Evaluation of tubal patency by hysterosalpingo-contrast sonography (HyCoSy): controversies, dilemmas and considerations. Heliyon. (2023) 9(7):e17461. doi: 10.1016/j.heliyon.2023.e17461
6. Devine K, Dolitsky S, Ludwin I, Ludwin A. Modern assessment of the uterine cavity and fallopian tubes in the era of high-efficacy assisted reproductive technology. Fertil. Steril. (2022) 118(1):19–28. doi: 10.1016/j.fertnstert.2022.05.020
7. Van Welie N, Van Rijswijk J, Dreyer K, Van Hooff MHA, De Bruin JP, Verhoeve HR, et al. Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial. Hum Reprod. (2022) 37(5):969–79. doi: 10.1093/humrep/deac034
8. Watrelot A. Is laparoscopy still the gold standard in infertility assessment? A comparison of fertiloscopy versus laparoscopy in infertility: results of an international multicentre prospective trial: the “FLY” (fertiloscopy-LaparoscopY) study. Hum Reprod. (2003) 18(4):834–9. doi: 10.1093/humrep/deg180
9. Pascoal E, Wessels JM, Aas-Eng MK, Abrao MS, Condous G, Jurkovic D, et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet Gynecol. (2022) 60(3):309–27. doi: 10.1002/uog.24892
10. Ferrari D, Violante T, Novelli M, Starlinger PP, Smoot RL, Reisenauer JS, et al. The death of laparoscopy. Surg Endosc. (2024) 38(5):2677–88. doi: 10.1007/s00464-024-10774-2
11. Njagi P, Groot W, Arsenijevic J, Dyer S, Mburu G, Kiarie J. Economic costs of infertility care for patients in low-income and middle-income countries: a systematic review protocol. BMJ Open. (2020) 10(11):e042951. doi: 10.1136/bmjopen-2020-042951
12. Aimagambetova G, Sakko Y, Ukybassova T, Terzic M, Marat A, Kamzayeva N, et al. Epidemiology of endometriosis in Kazakhstan: a national population-based cohort analysis (2014–2019) using data from the national electronic healthcare system. Front. Med. (2025) 11:1436458. doi: 10.3389/fmed.2024.1436458
13. Suleimenova M, Lokshin V, Glushkova N, Karibayeva S, Terzic M. Quality-of-life assessment of women undergoing in vitro fertilization in Kazakhstan. Int J Environ Res Public Health. (2022) 19(20):13568. doi: 10.3390/ijerph192013568
14. Wang T, Dong T, Nie F. Clinical applications, advances, and future directions in hysterosalpingography. Front. Med. (2025) 12:1537506. doi: 10.3389/fmed.2025.1537506
15. Mishra VV, Nawal R, Aggarwal RS, Choudhary S, Singh T, Sharma U, et al. Salpingoscopy: an adjuvant to laparoscopy in evaluation of infertile women. J Obstet Gynecol India. (2017) 67(1):48–52. doi: 10.1007/s13224-016-0903-1
16. Patra S, Dave A, Manchanda R, Lukacs L, Török P. The role of salpingoscopy and falloposcopy in current clinical practice: a review. Ital J Gynaecol Obstet. (2024) 36(2):196. doi: 10.36129/jog.2023.140
17. El-Kharoubi A-F. Tubal pathologies and fertility outcomes: a review. Cureus. (2023) 15(5):e38881. doi: 10.7759/cureus.38881
18. Li Y, Xu S, Yu S, Huang C, Lin S, Chen W, et al. Diagnosis of chronic endometritis: how many CD138+ cells/HPF in endometrial stroma affect pregnancy outcome of infertile women? Am J Reprod Immunol. (2021) 85(5):e13369. doi: 10.1111/aji.13369
19. Hey-Cunningham AJ, Wong C, Hsu J, Fromm PD, Clark GJ, Kupresanin F, et al. Comprehensive analysis utilizing flow cytometry and immunohistochemistry reveals inflammatory changes in local endometrial and systemic dendritic cell populations in endometriosis. Hum. Reprod. (2021) 36(2):415–28. doi: 10.1093/humrep/deaa318
20. Vitagliano A, Laganà AS, De Ziegler D, Cicinelli R, Santarsiero CM, Buzzaccarini G, et al. Chronic endometritis in infertile women: impact of untreated disease, plasma cell count and antibiotic therapy on IVF outcome—a systematic review and meta-analysis. Diagnostics. (2022) 12(9):2250. doi: 10.3390/diagnostics12092250
21. Dwojak E, Mroczek M, Dworacki G, Dobosz P, Ślubowska A, Stępień M, et al. Plasma cells as the key players of IVF failure? Unlocking the Enigma of infertility and in vitro fertilization failure in the light of uterine inflammation. Int J Mol Sci. (2024) 25(23):13083. doi: 10.3390/ijms252313083
22. Nakagawa K, Inoue M, Nishi Y, Sugiyama R, Motoyama K, Kuribayashi Y, et al. A new evaluation score that uses salpingoscopy to reflect fallopian tube function in infertile women. Fertil Steril. (2010) 94(7):2753–7. doi: 10.1016/j.fertnstert.2010.03.012
23. Silva DC, Gonçalves AK, Cobucci RN, Mendonça RC, Lima PH, Cavalcanti G. Immunohistochemical expression of p16, ki-67 and p53 in cervical lesions—a systematic review. Pathol Res Pract. (2017) 213(7):723–9. doi: 10.1016/j.prp.2017.03.003
24. Mouttet D, Laé M, Caly M, Gentien D, Carpentier S, Peyro-Saint-Paul H, et al. Estrogen-receptor, progesterone-receptor and HER2 status determination in invasive breast cancer. Concordance between immuno-histochemistry and MapQuantTM microarray based assay. PLoS One. (2016) 11(2):e0146474. doi: 10.1371/journal.pone.0146474
25. Kind S, Jaretzke A, Büscheck F, Möller K, Dum D, Höflmayer D, et al. A shift from membranous and stromal syndecan-1 (CD138) expression to cytoplasmic CD138 expression is associated with poor prognosis in breast cancer. Mol Carcinog. (2019) 58(12):2306–15. doi: 10.1002/mc.23119
26. Petrucco OM. Surgery for hydrosalpinx—history, classification, incidence, results, and recommendation for management. Fertil Reprod. (2024) 6(1):1–6. doi: 10.1142/S2661318224300010
27. Fan X, Li X, Li Y, Liao J, Chen H, Li Y, et al. Endometrial CD138 count appears to be a negative prognostic indicator for patients who have experienced previous embryo transfer failure. Fertil Steril. (2019) 112(6):1103–11. doi: 10.1016/j.fertnstert.2019.08.006
28. Bulut H, Yeğinaltay T, Tunç M, Berkkanoğlu M, Töre H, Coetzee K, et al. Chronic endometritis diagnosed using a cut-off of ≥5 CD138 plasma cells significantly affects the reproductive outcomes of frozen embryo transfer: a case-control study. J Turk Ger Gynecol Assoc. (2023) 24(3):165–71. doi: 10.4274/jtgga.galenos.2023.2022-9-20
29. Skjeldestad FE. Minor changes in the incidence of primary and secondary involuntary childlessness across birth cohorts 1916 to 1975, but major differences in treatment success. PLoS One. (2023) 18(7):e0287540. doi: 10.1371/journal.pone.0287540
Keywords: infertility, diagnostic methods, salpingoscopy, immunohistochemistry, reproductive technologies, fallopian tubes
Citation: Alenova A, Korkan A, Kauysheva A and Magalov I (2025) Integrating salpingoscopy and immunohistochemistry to improve tubal infertility diagnosis: a retrospective cohort study. Front. Reprod. Health 7:1701315. doi: 10.3389/frph.2025.1701315
Received: 8 September 2025; Revised: ;
Accepted: 3 November 2025;
Published: 27 November 2025.
Edited by:
Pooja Popli, Baylor College of Medicine, United StatesReviewed by:
Alessandro D'Amuri, Azienda Sanitaria Locale di Brindisi, ItalyYixing Wang, City of Hope National Medical Center, United States
Prasanna K.S, KVASU, India
Deirdre Scully, Case Western Reserve University, United States
Copyright: © 2025 Alenova, Korkan, Kauysheva and Magalov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adalyat Alenova, cGhkLnN1cmdlb24uYWxlbm92YUBnbWFpbC5jb20=
 Adalyat Alenova
Adalyat Alenova Anuar Korkan2,3
Anuar Korkan2,3