- 1Department of Biochemistry, Faculty of Science, University of Yaounde I, Yaounde, Cameroon
- 2The Biotechnology Centre, University of Yaounde 1, Yaounde, Cameroon
- 3Fobang Institutes for Innovations in Science and Technology, Yaoundé, Cameroon
- 4SIANTOU Higher School of Medical Sciences, Yaounde, Cameroon
- 5University of Yaounde I, Yaounde, Cameroon
- 6Cameroon Academy of Young Scientists (CAYS), Yaounde, Cameroon
Background: Viral infections, particularly hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis Delta virus (HDV), and human immunodeficiency virus (HIV), pose significant public health challenges worldwide, especially in low- and middle-income countries (LMICs) in Africa. The diagnosis and management of these diseases become increasingly complicated when viral co-infections are present. This review aims to explore current trends in the epidemiology and management of viral co-infections and comorbidities in LMICs.
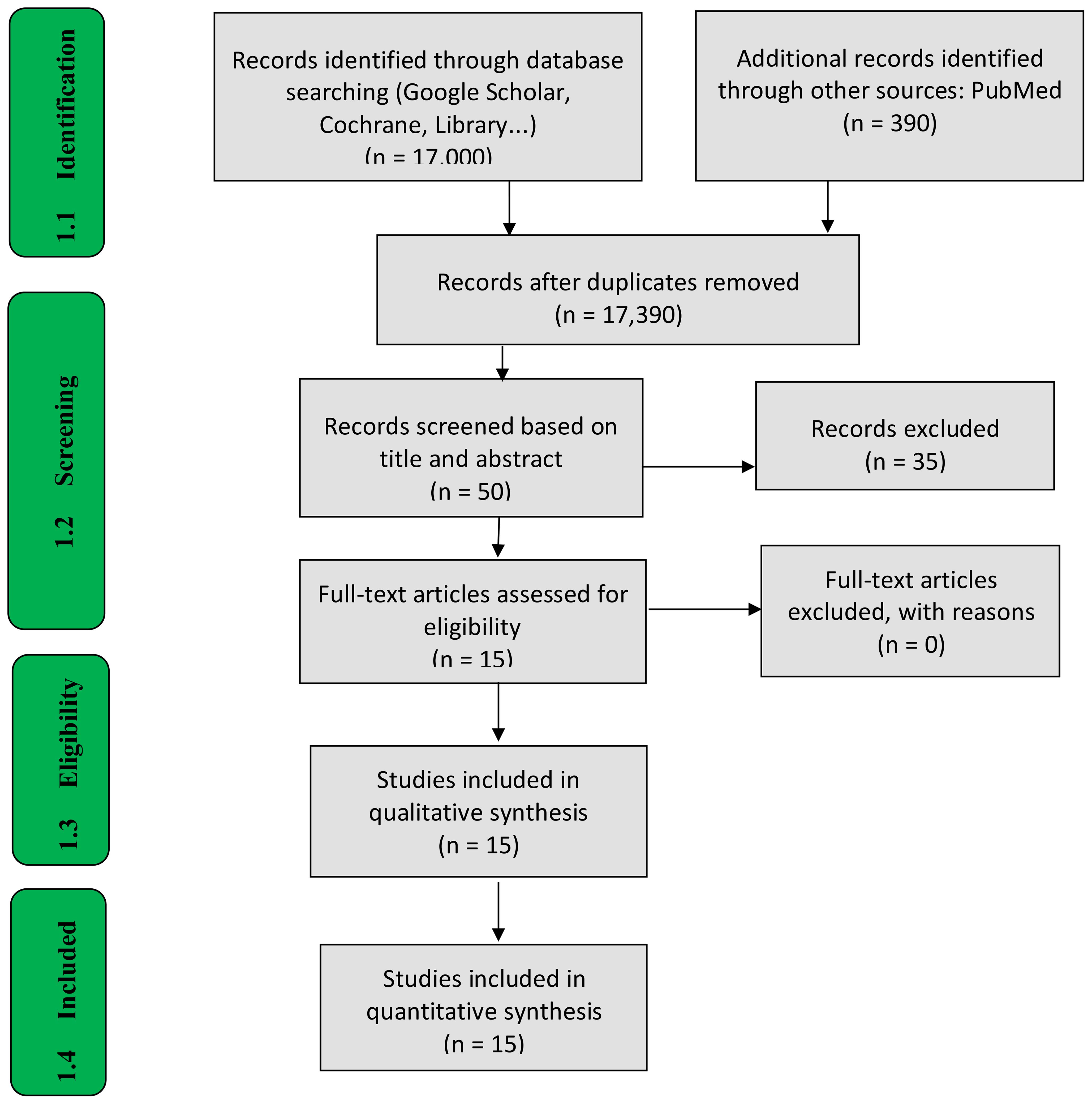
Methods: A systematic review was conducted following the PRISMA-ScR (Preferred Reporting Items for Systematic and Meta-Analyses extension for Scoping Reviews) guidelines. A thorough literature search was performed across two databases: PubMed and Google Scholar. A total of 390 records were identified from PubMed, and approximately 17,000 from Google Scholar for studies published between 2015 and 2025. After removing duplicates and applying eligibility criteria, 50 articles from PubMed were screened based on their titles, abstracts, and full texts. Of these, 15 studies that met the inclusion criteria were selected for data extraction and synthesis, using a data extraction form to organize the relevant information.
Results: Out of the 50 screened studies, 15 met the inclusion criteria and were included in the final analysis. These studies primarily focused on co-infections of HIV with HBV and HCV. The prevalence rates of HBV among individuals living with HIV varied from 1.1% to 9.1% in cross-sectional studies, with notable populations including pre-ART patients and pregnant women. One study reported a particularly high rate of HCV co-infection at 66.6% among intravenous drug users. However, no eligible studies were available for HBV-HDV co-infection or treatment-specific outcomes.
Conclusion: This review highlights the significant burden and variability of HIV co-infections in Africa, particularly the co-infection of HIV with HBV. The findings emphasize the necessity for integrated screening and management strategies, as well as the need for further research to optimize interventions and improve health outcomes for individuals with co-infections.
Introduction
Viral infections, including hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis Delta virus (HDV), and human immunodeficiency virus (HIV) are a significant public health concern worldwide, particularly in low- and middle-income countries (LMICs) in Africa. Globally, approximately 39 million and 50 million people are living with HIV and HCV infections, respectively (1, 2). According to the World Health Organization, around 5% (approximately 15–20 million people) of individuals with hepatitis B surface antigen (HBsAg), out of a global total of 296 million, are infected with HDV (3, 4). These infections are endemic in LMICs in Africa and can interact with one another due to the similar transmission routes they share (blood exposure accidents, unprotected sexual intercourse, or mother-to-child transmission). Viral penetration (HBV, HCV, HDV) via parenteral or sexual routes is followed by a viremic phase that drives the viruses into liver. Liver damage is characterized by cytopathic action and immune response directed against infected hepatocytes. This promotes co-infections, which exacerbates disease progression and lead to increased morbidity and mortality (5, 6). HIV affects approximately 25.6 million people in Sub-Saharan Africa and was responsible for an estimated 630,000 AIDS-related deaths globally in 2022 (7). Similarly, viral hepatitis presents a major health burden, with the WHO reporting 1.5 million new infections each year for each virus and 1.1 million annual deaths (HBV accounting for 820,000 deaths and HCV 290,000 deaths), primarily due to its role in causing cirrhosis and hepatocellular carcinoma (8). Sub-Saharan Africa bears the highest burden of HBV with HBsAg prevalence estimated at 6.1%, affecting around 60 million people, while HCV and HDV have prevalence rates of 4.17% and 8.39%, respectively (9–11).
Common viral co-infections in LMICs include HIV/HBV, HIV/HCV, HBV/HDV. Hepatitis C and B are liver affections, leading to liver cirrhosis and hepatocellular carcinoma, the risk of which increases with the presence of HIV in the case of co-infection. Co-infections of this type are mostly documented in people already living with HIV. Primary HIV infection after contamination is characterized by explosive viral replication that Leads to progressive decline in CD4 T cells through three mechanisms: the cytopathic effect, the destruction of T helper infected cells by CD8 cells (cytotoxicity mechanism) and apoptosis. Due to this immunosuppression state that gradually sets, HIV patients are at higher risk of being infected with HBV and HCV, with increase liver-related morbidity and mortality compared to HCV or HBV mono-infected persons. HIV increases HBV replication and reactivation (12, 13). It has been reported that around 10% of people living with HIV are co-infected with hepatitis B worldwide. In different geographical areas, prevalence varies and depends on HBV endemicity (14). HIV co-infection has a detrimental effect on HCV progression just as in HBV, leading to higher rates of HCV persistence, higher viral levels and accelerated progression of liver fibrosis and end-stage liver disease.
Globally, 2.3 million individuals are co-infected with HIV and HCV, with the highest prevalence observed among people who inject drugs (PWID) and men who have sex with men (MSM) (14, 15). Co-infections of HIV with HBV or HCV are linked to increased hepatotoxicity from antiretroviral therapy (16). Hepatitis D virus (HDV) relies on the presence of hepatitis B virus (HBV) and cannot exist without an active HBV infection. Superinfection with HDV in individuals already infected with HBV can lead to more severe liver diseases, including hepatic decompensation and hepatocellular carcinoma (HCC). Additionally, the presence of HDV complicates the management of HBV infection (17).
The high prevalence of HIV, HBV, HCV mono-infections as well as co-infections, comorbidities and the considerably high death rate remain a call for concern. Additionally, management poses a constant challenge, due to treatment side-effects, drug toxicity, drug resistance, and mutations that make vaccine designs difficult. This review is aimed at determining the prevalence and burden of HIV/HBV, HIV/HCV, HBV/HDV co- infections in LMICs in Africa.
Methods
A review was conducted in accordance with the JBI methodology for systematic reviews and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR). A systematic review was appropriate as the aim was to describe the epidemiological trends and management of HIV and Hepatitis co-infections among adult patients in Sub-Saharan Africa. A preliminary search of MEDLINE, the Cochrane Database of Systematic Reviews and JBI Evidence Synthesis was conducted and no current or underway systematic reviews or scoping reviews on the topic were identified.
A search was conducted using PubMed, Google Scholar and relevant articles were included. The studies included were those published between 2015 and 2025. The articles included focused on the epidemiology, clinical implications, and management strategies for HIV/HBV, HIV/HCV, HBV/HDV co-infections in LMICs. The review focused on studies that examined the epidemiology (e.g., prevalence, incidence) and management of HIV co-infections with HBV, HCV, and HBV/HDV.
Inclusion and exclusion criteria
This review follows the Participant, Concept, and Context (PCC) framework. Given that our study does not evaluate interventions or comparisons of treatments, the PCC framework was selected as the most appropriate methodological approach for synthesizing the broader conceptual and contextual aspects surrounding viral co-infections in LMICs. Specifically:
Participants (P): The review included studies involving children, adolescents and adult patients who have tested positive for HIV and have co-infections of Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), or Hepatitis D Virus (HDV).
Concept (C): Analytical observational studies, including prospective and retrospective cohort studies, case-control studies, and analytical cross-sectional studies, as well as descriptive observational study designs such as case series, individual case reports, and descriptive cross-sectional studies. Systematic reviews that met the inclusion criteria were also included. We looked at the epidemiological patterns, management strategies, and public health challenges.
Context (C): Only studies conducted within Sub-Saharan African countries and published between 2015 and 2025 were considered for inclusion.
Based on this framework, we formulated a structured Population, Concept, and Context (PCC) research question: “What are the current trends in the epidemiology and management of viral co-infections and comorbidities among individuals living with HIV in Africa?”
Types of sources and search strategy
A comprehensive three-step search strategy was employed to identify relevant studies.
Step 1: Initial Limited Search An initial limited search was conducted using MEDLINE (PubMed) and Google Scholar to identify key articles and relevant search terms. The following preliminary search string was used in PubMed: (“HIV”[Mesh] OR “Human Immunodeficiency Virus” OR “acquired immunodeficiency syndrome”) AND (“Hepatitis B”[Mesh] OR “Hepatitis B virus” OR “HBV”) AND “Africa”[Mesh]. The keywords and MeSH terms identified in this initial search—along with variations and synonyms—were then refined to develop a comprehensive search strategy.
Step 2: Comprehensive Search Using an optimized combination of MeSH terms and keywords, a broader search strategy was designed to capture studies on HIV, Hepatitis B, Hepatitis C, Hepatitis D, and related topics concerning African countries. Search strings were adapted for individual databases to accommodate differences in indexing and functionality. For example, the Google Scholar search string included: Allin title: HIV Hepatitis B Africa OR HIV Hepatitis C Africa OR HIV HBV HCV Africa. Additional keywords incorporated included: co-infection, prevalence, epidemiology, management, LMIC, Sub-Saharan Africa. Relevant filters were applied where available, including publication date (2015–2025) and study type (observational studies).
Step 3: Supplementary Searching To ensure comprehensive coverage, reference lists from all included studies and relevant reviews were manually screened to identify additional sources. Forward and backward citation searching was conducted using Google Scholar.
Study/source of evidence selection
Following the search, all identified citations were collated and uploaded into Zotero citation manager and duplicates removed. Following a pilot test, titles and abstracts were then be screened by two or more independent reviewers for assessment against the inclusion criteria for the review. The full text of selected citations was assessed in detail against the inclusion criteria by two or more independent reviewers. Reasons for exclusion of sources of evidence at full text that do not meet the inclusion criteria were recorded and reported in the scoping review. Any disagreements that arise between the reviewers at each stage of the selection process were resolved through discussion, or with an additional reviewer. The results of the search and the study inclusion process were reported in full in the review and presented in a PRISMA flow diagram (Figure 1).
Data extraction
Data was extracted from papers included in the scoping review by two independent reviewers using a data extraction tool developed by the reviewers. The data extracted include, authors name, year of publication, title of study, aim, study design, sample size, population, prevalence, management strategies and interventions. Any disagreements that arouse between the reviewers will be resolved through discussion, or with an additional reviewer.
Given the scoping nature of this review, which aims to map the available evidence rather than synthesize findings to make recommendations, a formal quality appraisal of included studies was not conducted.
Results
This systematic review of studies on HIV co-infections in Africa reveals a complex landscape, with a predominant focus on HIV and Hepatitis B Virus (HBV) co-infection. A key observation is the significant variability in HBV prevalence rates, ranging from 0.011 in Ethiopia to 10.4% in Zambia, highlighting the heterogeneity of the HIV/HBV co-infection burden across the continent and the need for region-specific interventions.
Several studies focused on HBV co-infection among pregnant women, reflecting concerns about vertical transmission and its implications for maternal and child health. These studies provide valuable insights into the epidemiology of the co-infection in this critical population and underscore the importance of integrating HBV screening into maternal health programs.
Beyond pregnant women, the research also encompasses other key populations, including people living with HIV, individuals who inject drugs, and blood donors. Studies investigated HBV prevalence within HIV-positive individuals, and the co-infection of HIV, HBV, and Hepatitis C Virus (HCV) among people who inject drugs. Additionally, a study assessed the seroprevalence of HIV, HBV, HCV, and syphilis among blood donors.
Overall, the findings emphasize the multifaceted nature of HIV co-infections in Africa and highlight the necessity of implementing comprehensive and targeted public health strategies to address these challenges effectively.
Regarding specific prevalence findings: Studies among pregnant women found HIV-HBV co-infection prevalence rates of 0.041, 2.8, and 0.126 in certain countries. Studies among HIV-infected adults showed HBV infection prevalence of 0.091 in Mozambique, 0.055 in Ethiopia, 0.023 in Tanzania and 0.085 in South Africa. One cross-sectional study on HBV infected people found a prevalence of 0.011 of HIV-HCV co-infection in pregnant women in Ethiopia. One study described the prevalence of HIV-HBV among children born in the pre- and post-vaccine eras and investigated the short term treatment outcomes of pediatric HIV-HBV in Zambia. One study assessed the seroprevalence of HIV1, hepatitis B and syphilis co-infection of 0.095 among newly diagnosed HIV individuals aged 16 to 65 years initiating antiretroviral therapy. One study in Tanzania, investigated the epidemiology of HIV, hepatitis B and hepatitis C and co infection of these virus among people who inject drugs with the prevalence of 0.09. In Ethiopia, one study determined the seroprevalence of HIV of 0.034 among blood donors over period of five years. The results are presented in the table below (Table 1).
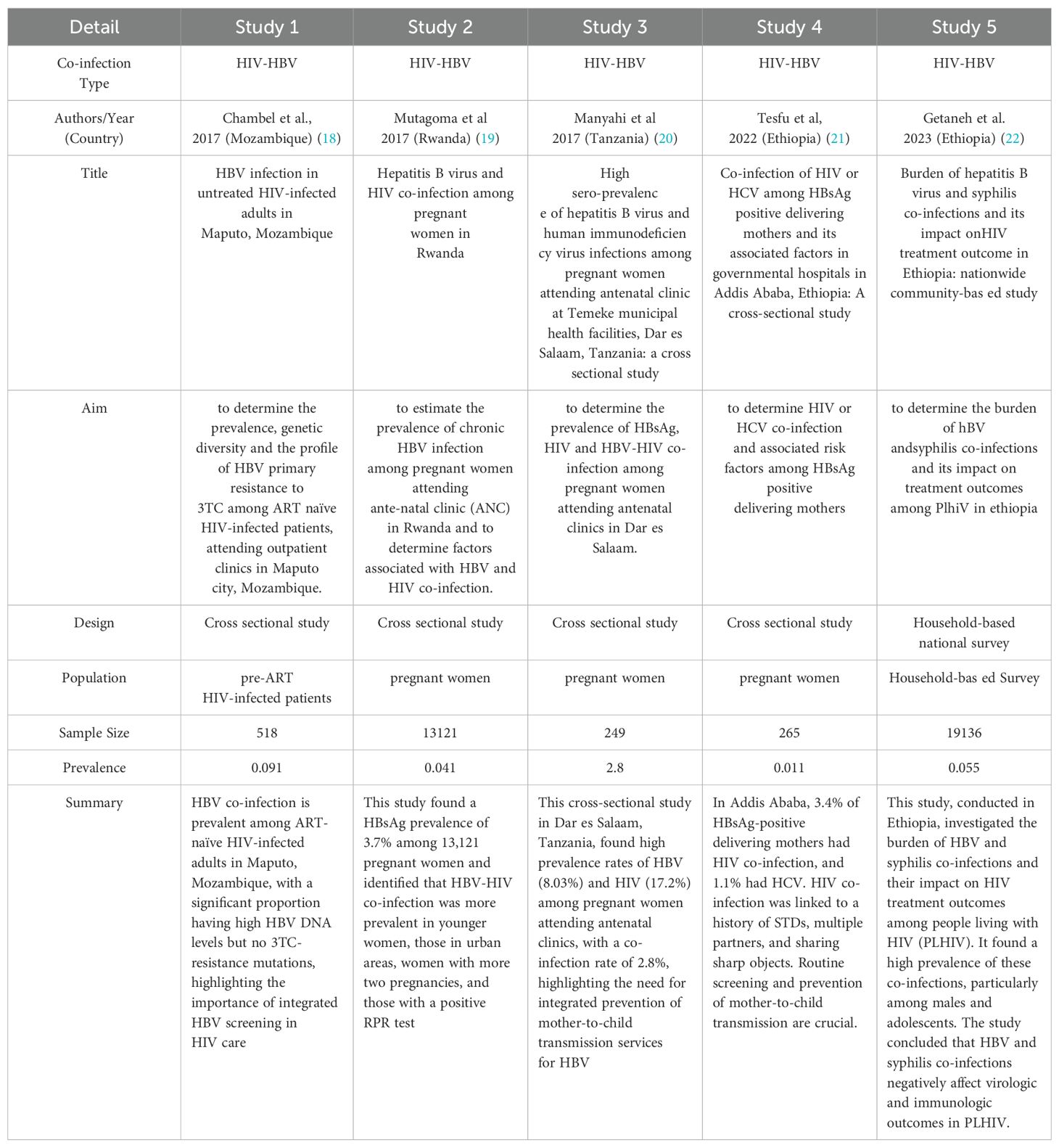
Table 1A. Summary of studies on HIV co-infections in Africa: Prevalence, characteristics, and population focus.
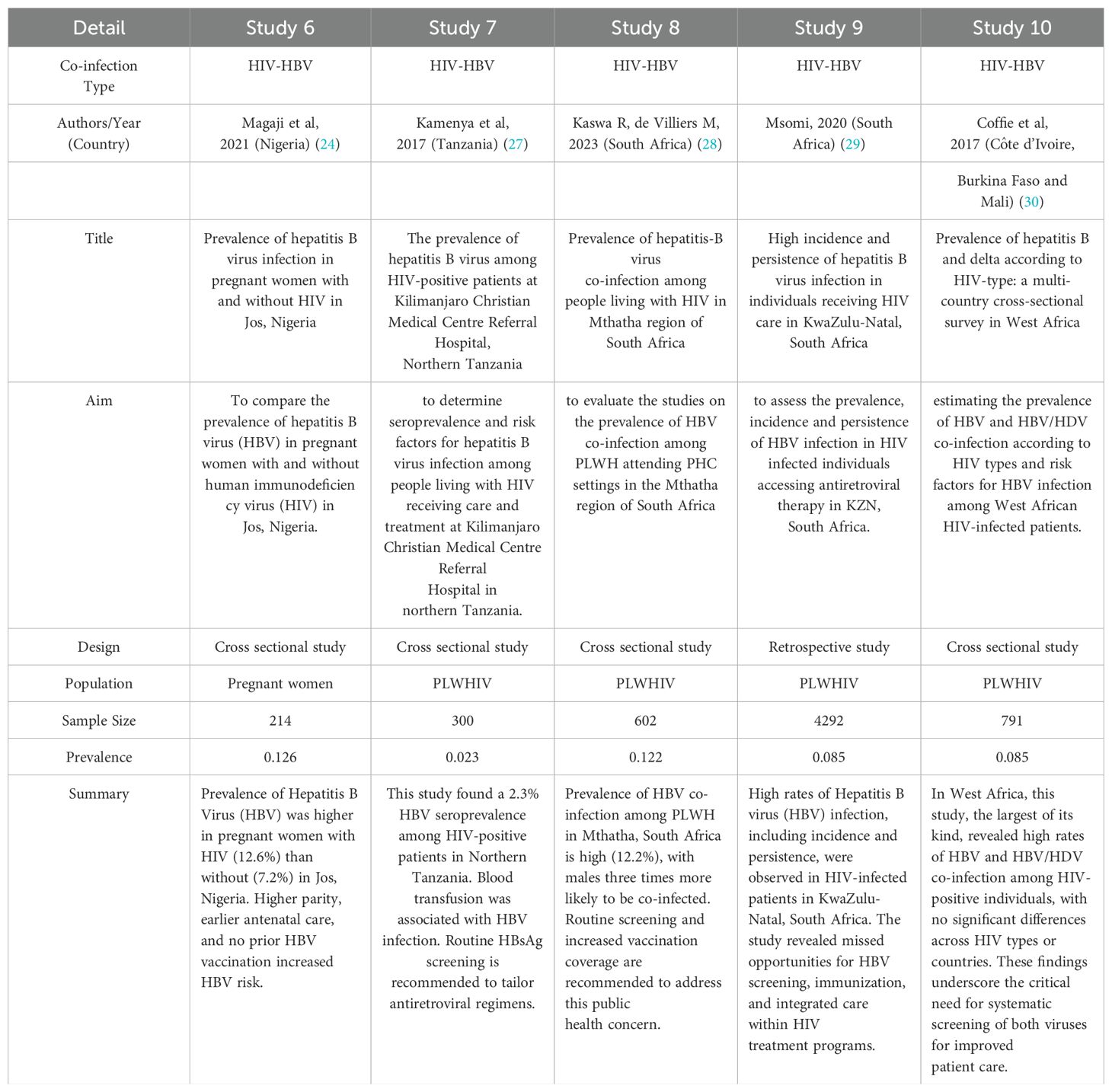
Table 1B. Summary of studies on HIV co-infections in Africa: Prevalence, characteristics, and population focus.
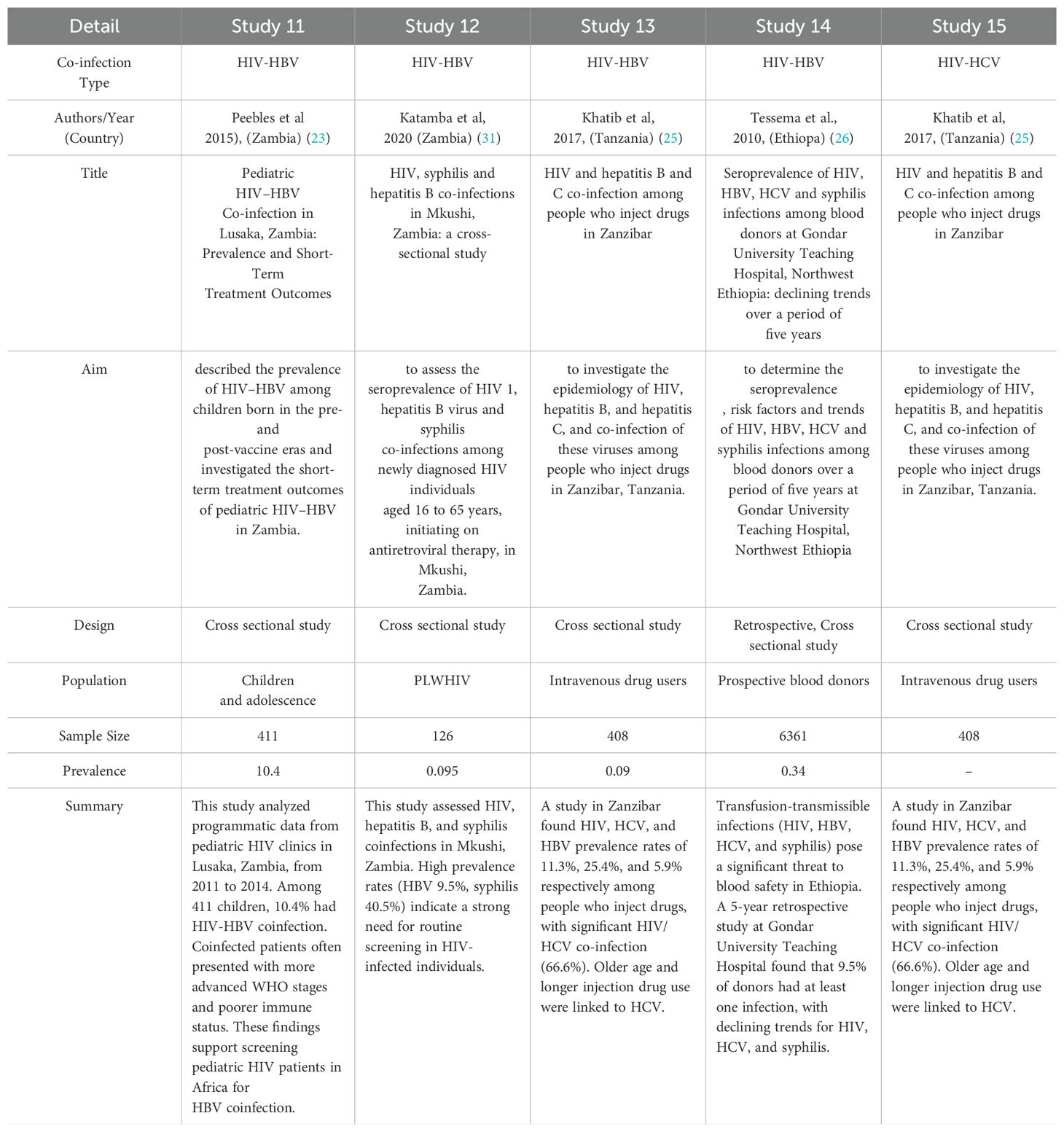
Table 1C. Summary of studies on HIV co-infections in Africa: Prevalence, characteristics, and population focus.
Discussion
From this systematic review, the results were synthesized from 15 studies conducted in African countries. These studies examined the prevalence and characteristics of HIV co-infections. This work highlights the significant burden of these co-infections, with a particular focus on HIV and Hepatitis B Virus (HBV), and underscores the variability in prevalence across different regions and populations. Cross-sectional studies were the most prominent in this review, and through their methodologies, they allowed for the assessment of the extent of co-infections within various specific groups.
An important finding of this scoping review is the notable heterogeneity in the prevalence of HIV-HBV co-infection among the included studies. Prevalence rates varied significantly, demonstrating the complex epidemiological landscape of these co-infections in Africa. Studies conducted in Ethiopia by Tesfu et al. (2022) showed a relatively low co-infection rate of 0.011 among mothers carrying the hepatitis B surface antigen (HBsAg) (21). This result does not align with the higher prevalence (10.4%) observed by Peebles et al. (2015) among children and adolescents in Lusaka, Zambia (23). Several factors could contribute to this variability, such as differences in HBV endemicity, risk behaviors, vaccination coverage, access to healthcare, and the populations studied. These differences, therefore, highlight the need for context-specific interventions and public health strategies tailored to the epidemiological situation in these countries.
The implications of this variability in prevalence are critical for the design and implementation of public health interventions. A one-size-fits-all approach is unlikely to be effective across all settings given the diverse epidemiological contexts. Interventions should therefore be adapted to local realities, taking into account the prevalence and specific risk factors identified in each population. For instance, regions with higher co-infection prevalence may require more intensive screening programs, targeted health education, and expanded access to integrated care services. Conversely, in areas with lower prevalence, resources might be more effectively utilized in maintaining surveillance and ensuring continued vaccination coverage. Furthermore, the heterogeneity suggests the necessity of strengthening regional data collection systems to enable real-time, location-specific monitoring and evaluation of co-infection trends.
Beyond the factors already discussed, differences in diagnostic methodologies used across the included studies could also contribute significantly to the observed variability in prevalence estimates. Some studies may have relied on rapid diagnostic tests with lower sensitivity and specificity, while others may have used more advanced laboratory techniques such as enzyme-linked immunosorbent assays (ELISA) or nucleic acid testing (NAT), which can detect infections with greater accuracy. Variations in diagnostic algorithms, test kits used, and laboratory infrastructure can lead to under- or over-estimation of co-infection prevalence. Moreover, inconsistencies in defining and reporting co-infection cases—for example, whether only chronic HBV carriers or all individuals with any HBV markers are included—may further affect comparability across studies. Addressing these methodological discrepancies through standardization of diagnostic criteria and laboratory protocols is essential for improving data reliability and guiding policy decisions.
Regarding HIV-HBV co-infection in Specific Populations, this review highlights the focus on HIV-HBV co-infection in several key populations, including people living with HIV (PLHIV), pregnant women, and individuals with specific risk factors.
Among pregnant women, numerous studies have examined the prevalence of HIV-HBV co-infection in this group, which is particularly concerning due to the risk of mother-to-child transmission of HBV. Studies conducted by Mutagoma et al. (2017) estimated the prevalence of HBV and HIV co-infection among pregnant women in Rwanda at 0.041; and 2.8% in Tanzania among pregnant women attending prenatal clinics in Dar es Salaam (19). Similarly, Magaji et al. (2021) also examined HBV prevalence among HIV+ and HIV– pregnant women in Nigeria, reporting an overall prevalence of 0.126 (24). These findings highlight the importance of integrating HBV screening into prenatal care services while aiming to implement effective interventions to prevent vertical (mother-to-child) transmission, such as HBV vaccination for newborns.
A significant number of studies focused on the prevalence of HBV co-infection among PLHIV. These studies provide valuable insights into the burden of co-infection among people already living with HIV, who may face increased morbidity and mortality due to the synergistic effects of both viruses. Studies conducted in South Africa reported HIV-HBV co-infection prevalence rates of 8.5% and 12.2% among PLHIV. Similarly, studies carried out in Nigeria and Tanzania also examined HBV co-infection among PLHIV, with prevalence rates ranging from 2.3% to 12.6%. These results emphasize the need for systematic HBV screening and management in HIV care programs, including access to antiretroviral therapy that is effective against both HBV and HIV.
This review also included other studies that examined HIV co-infections in other specific populations. Khatib et al. (2017) investigated co-infection with HIV, HBV, and Hepatitis C Virus (HCV) among people who inject drugs in Zanzibar, Tanzania. A 9% prevalence of HIV-HBV co-infection was reported (25). In 2010, Tessema and collaborators assessed the seroprevalence of HIV, HBV, HCV, and syphilis infections among blood donors in Ethiopia, finding an HIV-HBV co-infection rate of 0.34% (26). These studies emphasize the importance of considering co-infections in various populations, including those with high-risk behaviors or specific health conditions.
A range of methodological approaches was used for the studies included in this review, with cross-sectional studies being the predominant design. Cross-sectional studies provide a snapshot of co-infection prevalence at a specific point in time, offering valuable information on the current disease burden. However, they do not provide information on the temporal relationship between HIV infection and the acquisition of other viral infections such as HBV and HCV. Some studies, on the other hand, used retrospective approaches involving the analysis of existing data. Retrospective studies can be useful for examining trends over time but may be limited by the quality and completeness of available data. Sample sizes varied considerably across the different studies, which could influence the accuracy and generalizability of prevalence estimates.
The results of this review have important implications for health policy and practice in the African region. The high prevalence of HIV-HBV co-infection highlights the urgent need for integrated screening and management strategies. This includes implementing systematic HBV screening for all PLHIV, pregnant women, and other at-risk populations, ensuring access to HBV vaccination, and providing appropriate antiviral treatment.
It is important to note that this review identified a limited number of studies focusing on certain viral co-infections, particularly HBV-HDV co-infection in the context of HIV. Despite the known significance of HDV as a complication of HBV infection, few studies in our review explicitly examined this triple co-infection. This gap in the literature may reflect underreporting, limited testing for HDV in many African settings, or a focus on the more commonly studied HIV/HBV and HIV/HCV co-infections. Further research is needed to understand the burden and clinical implications of HBV-HDV co-infection, particularly in individuals living with HIV in LMICs.
Longitudinal studies are needed in the future to better understand the natural history of HIV co-infections, including the incidence, persistence, and clinical outcomes of co-infected individuals. More research is also needed on the cost-effectiveness of different interventions and strategies aimed at optimizing HIV co-infection management in resource-limited settings. Furthermore, studies should examine the impact of co-infections on the progression of HIV and co-infecting viruses, as well as the potential for drug interactions and cross-toxicities of antiretroviral and antiviral medications.
This systematic review provides a comprehensive overview of the available evidence on HIV co-infections in several African countries. The inclusion of a range of studies involving diverse populations and contexts strengthens the generalizability of the findings. However, there are also some limitations to consider. This review revealed a heterogeneous distribution of studies across Sub-Saharan Africa. While some regions were well-represented, others had limited data on HIV co-infections. For instance, there were fewer studies from Central Africa compared to East and Southern Africa. This geographic disparity may limit the generalizability of our findings to the entire continent and highlights the need for more research in underrepresented regions to provide a more comprehensive understanding of the epidemiology of HIV co-infections.
Conclusion
This systematic review delivers a comprehensive overview of the existing evidence on HIV co-infections across various African countries. By incorporating a diverse array of studies that represent different populations and contexts, this review significantly enhances the generalizability of its findings. However, it is crucial to acknowledge some limitations. The variability in study design, population, and methodology among the included studies poses challenges to result comparability. Furthermore, the review is subject to the inherent limitations of primary studies, including potential biases in data collection and reporting.
This review unequivocally underscores the substantial burden and variability of HIV co-infections in Africa, with a particular focus on HIV-HBV co-infection. The findings reveal an urgent need for integrated screening and management strategies and call for further research to optimize interventions and improve health outcomes for individuals facing co-infections.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
AA: Writing – original draft, Validation, Methodology, Investigation, Writing – review & editing. FC: Writing – original draft, Investigation, Data curation, Methodology, Writing – review & editing. EG: Writing – review & editing, Validation, Investigation. BL: Investigation, Writing – review & editing. IA: Investigation, Writing – review & editing. MD: Methodology, Conceptualization, Supervision, Writing – original draft, Project administration, Writing – review & editing, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. In addition to the research bonuses from the University of Yaoundé 1 and the Cameroon Ministry of Higher Education, this review is funded entirely by the corresponding author and their team; consequently, there are no external funds for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. UNAIDS. Global HIV & AIDS statistics — Fact sheet 2023. UNAIDS (2023) Geneva, Switzerland. Available online at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (Accessed April 1, 2025).
2. World Health Organization. Hepatitis C (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed April 1, 2025).
3. World Health Organization. Hepatitis B (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (Accessed April 1, 2025).
4. World Health Organization. Global hepatitis report, 2017 (2017). Available online at: http://www.who.int/news-room/fact-sheets/detail/hepatitis-d (Accessed April 1, 2025).
5. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. (2006) 44:S6–9. doi: 10.1016/j.jhep.2005.11.004
6. UNAIDS. Global HIV & AIDS statistics — Fact sheet 2023. UNAIDS (2024) Geneva, Switzerland. Available online at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (Accessed April 1, 2025).
7. World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: Accountability for the global health sector strategies 2016–2021: actions for impact. World Health Organization (Geneva, Switzerland) (2021). p. 112. Available online at: https://www.who.int/publications/i/item/9789240027077 (Accessed April 1, 2025).
8. Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol hepatology. (2017) 2:900–9. doi: 10.1016/S2468-1253(17)30295-9
9. Kassa GM, Walker JG, Alamneh TS, Tamiru MT, Bivegete S, Adane A, et al. Prevalence, trends, and distribution of hepatitis C virus among the general population in sub-Saharan Africa: A systematic review and meta-analysis. Liver international: Off J Int Assoc Study Liver. (2024) 44:3238–49. doi: 10.1111/liv.16102
10. Stockdale AJ, Chaponda M, Beloukas A, Phillips RO, Matthews PC, Papadimitropoulos A, et al. Prevalence of hepatitis D virus infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Global Health. (2017) 5:e992–e1003. doi: 10.1016/S2214-109X(17)30298-X
11. Cheng Z, Lin P, and Cheng N. HBV/HIV coinfection: impact on the development and clinical treatment of liver diseases. Front Med. (2021) 8:713981. doi: 10.3389/fmed.2021.713981
12. Weldemhret L. Epidemiology and Challenges of HBV/HIV Co-Infection amongst HIV-Infected Patients in Endemic Areas: review. HIV/AIDS: Res Palliative Care. (2021) 13:485–90. doi: 10.2147/hiv.s273649
13. Kourtis AP, Bulterys M, Hu DJ, and Jamieson DJ. HIV–HBV coinfection — A global challenge. New Engl J Med. (2012) 366:1749–52. doi: 10.1056/nejmp1201796
14. Abiodun OE, Adebimpe O, Ndako JA, Oludoun O, Aladeitan B, and Adeniyi M. Mathematical modeling of HIV-HCV co-infection model: Impact of parameters on reproduction number. F1000Research. (2022) 11:1153. doi: 10.12688/f1000research.124555.2
15. Gobran ST, Ancuta P, and Shoukry NH. A tale of two viruses: Immunological insights into HCV/HIV coinfection. Front Immunol. (2021) 12:726419. doi: 10.3389/fimmu.2021.726419
16. Kenfack-Momo R, Kenmoe S, Takuissu GR, Ebogo-Belobo JT, Kengne-Ndé C, Mbaga DS, et al. Epidemiology of hepatitis B virus and/or hepatitis C virus infections among people living with human immunodeficiency virus in Africa: A systematic review and meta-analysis. PloS One. (2022) 17:e0269250. doi: 10.1371/journal.pone.0269250
17. Ferrante ND and Lo Re V. Epidemiology, natural history, and treatment of hepatitis delta virus infection in HIV/hepatitis B virus coinfection. Curr Hiv/Aids Rep. (2020) 17:405–14. doi: 10.1007/S11904-020-00508-Z
18. Chambal LM, Gudo ES, Carimo A, Corte Real R, Mabunda N, Maueia C, et al. HBV infection in untreated HIV-infected adults in Maputo, Mozambique. PLoS One. (2017) 12(7):e0181836. doi: 10.1371/journal.pone.0181836. Erratum in: PLoS One. (2017) 12(12):e0190460. doi: 10.1371/journal.pone.0190460
19. Mutagoma M, Balisanga H, Malamba SS, Remera E, and Riedel DJ. Hepatitis B virus and HIV co-infection among pregnant women in Rwanda. BMC Infect Dis. (2017) 17:618. doi: 10.1186/s12879-017-2714-0
20. Manyahi J, Msigwa Y, Mhimbira F, and Majigo M. High sero-prevalence of hepatitis B virus and human immunodeficiency virus infections among pregnant women attending antenatal clinic at Temeke municipal health facilities, Dar es Salaam, Tanzania: A cross-sectional study. BMC Pregnancy Childbirth. (2017) 17:109. doi: 10.1186/s12884-017-1299-3
21. Tesfu D, Tadesse M, and Kebede A. Co-infection of HIV or HCV among HBsAg positive delivering mothers and its associated factors in governmental hospitals in Addis Ababa, Ethiopia: A cross-sectional study. PloS One. (2022) 17:e0274341. doi: 10.1371/journal.pone.0273300
22. Getaneh Y, Assefa M, and Melaku T. Burden of hepatitis B virus and syphilis co-infections and its impact on HIV treatment outcome in Ethiopia: Nationwide community-based study. BMC Infect Dis. (2023) 23:45. doi: 10.1080/07853890.2023.2239828
23. Peebles K, Nchimba L, Chilengi R, Bolton Moore C, Mubiana-Mbewe M, and Vinikoor MJ. Pediatric HIV-HBV co-infection in Lusaka, Zambia: Prevalence and short-term treatment outcomes. J Trop Pediatr. (2015) 61:464–7. doi: 10.1093/tropej/fmv058
24. Magaji BA, Musa BM, Mohammed A, and Olayinka AT. Prevalence of hepatitis B virus infection in pregnant women with and without HIV in Jos, Nigeria. Int J Infect Dis. (2021) 104:753–9. doi: 10.1016/j.ijid.2020.12.058
25. Khatib A, Matiko E, Khalid F, Welty S, Ali A, Othman A, et al. HIV and hepatitis B and C co-infection among people who inject drugs in Zanzibar. BMC Public Health. (2017) 17:917. doi: 10.1186/s12889-017-4933-0
26. Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: Declining trends over a period of five years. BMC Infect Dis. (2010) 10:111. doi: 10.1186/1471-2334-10-111
27. Kamenya T, Damian DJ, Ngocho JS, Philemon RN, Mahande MJ, and Msuya SE. The prevalence of hepatitis B virus among HIV-positive patients at Kilimanjaro Christian Medical Centre Referral Hospital, Northern Tanzania. Pan Afr Med J. (2017) 28:275. doi: 10.11604/pamj.2017.28.275.11926
28. Kaswa R and de Villiers M. Prevalence of hepatitis B virus co-infection among people living with HIV in Mthatha region of South Africa. Afr Health Sci. (2023) 23:149–56. doi: 10.4314/ahs.v23i1.17
29. Msomi N, Govender K, Naidoo K, Yende-Zuma N, Mlisana K, and Karim SSA. High incidence and persistence of hepatitis B virus infection in individuals receiving HIV care in KwaZulu-Natal, South Africa. BMC Infect Dis. (2020) 20:468. doi: 10.1186/s12879-020-05575-6
30. Coffie PA, Tchounga BK, Bado G, Kabran M, Minta DK, Wandeler G, et al. Prevalence of hepatitis B and delta according to HIV-type: A multi-country cross-sectional survey in West Africa. BMC Infect Dis. (2017) 17:466. doi: 10.1186/s12879-017-2568-5
Keywords: LMICs, current trends, HCV, HDV, Africa, co-infections and comorbidities, HIV-HBV, epidemiology and management
Citation: Ambassa AC, Chethkwo F, Guiedem E, Lele BG, Fofou IVA and Ngounoue MD (2025) Current trends in the epidemiology and management of viral co-infections and comorbidities in Africa: a systematic review. Front. Virol. 5:1619249. doi: 10.3389/fviro.2025.1619249
Received: 27 April 2025; Accepted: 09 July 2025;
Published: 01 August 2025.
Edited by:
Peter Bai James, Southern Cross University, AustraliaReviewed by:
Mohammed Elfatih Hamida, Orotta College of Medicine and Health Sciences, EritreaSuleiman Adeiza Shuaibu, Ahmadu Bello University, Nigeria
Hamza Khalifa Ibrahim, Higher Institute of Medical Sciences and Technology Bani Waleed, Libya
Copyright © 2025 Ambassa, Chethkwo, Guiedem, Lele, Fofou and Ngounoue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marceline Djuidje Ngounoue, ZGpuZ21hcmNlYXVAeWFob28uZnI=
 Axel Cyriaque Ambassa
Axel Cyriaque Ambassa Fabrice Chethkwo
Fabrice Chethkwo Elise Guiedem4
Elise Guiedem4 Ironne Valdese Ayemfouo Fofou
Ironne Valdese Ayemfouo Fofou Marceline Djuidje Ngounoue
Marceline Djuidje Ngounoue