- 1Department of Parasitology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil
- 2Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil
- 3Institut Pasteur de São Paulo, São Paulo, Brazil
- 4Institute of Integrated Cell Biology and Physiology, University of Münster, Münster, Germany
In recent years, several viral epidemics and pandemics have emerged, leading to significant increases in both morbidity and mortality rates. This highlights the urgent need for the discovery of effective antiviral agents. A promising alternative approach to treating viral infections is the use of medicinal plants and their secondary metabolites. Plant-derived natural products have long been a valuable source for discovering novel therapeutic agents, owing to their chemical and structural diversity. This mini-review focuses on the antiviral activity of various enriched extracts and phytoconstituents isolated from medicinal plants, which have demonstrated efficacy against viral infections caused by the influenza virus, coronaviruses, arboviruses such as dengue, chikungunya, Zika, and Mayaro, as well as the human immunodeficiency virus (HIV).
Introduction
In recent years, viral infections have emerged as major global public health concerns, with increasing incidence and geographic spread. These infections significantly impact global health and economies due to their epidemic and pandemic potential (1). RNA viruses, in particular, are leading causes of human infectious diseases. Their high mutation rates contribute to the emergence of new viral subtypes and genotypes resistant to existing therapies, posing persistent threats of new outbreaks (2). For instance, Influenza A virus (H1N1), an enveloped, single-stranded RNA virus of the Orthomyxoviridae family, is responsible for most seasonal influenza epidemics (3). Highly pathogenic avian influenza A (H5N1) has also caused recent outbreaks involving zoonotic transmission (4). Similarly, SARS-CoV-2, a member of the Coronaviridae family with a single-stranded RNA genome encoding approximately 29 proteins, caused the COVID-19 pandemic, resulting in over seven million deaths worldwide (5). Although licensed antiviral therapies exist, RNA viruses can rapidly evolve through reassortment and point mutations, leading to resistance against conventional treatments such as neuraminidase inhibitors for Influenza (6) and protease inhibitors targeting SARS-CoV-2 main protease (Mpro) (7).
In addition, four arboviruses Dengue (DENV) and Zika (ZIKV) (Flaviridae family), as well as Chikungunya (CHIKV) and Mayaro (MAYV) (Togaviridae family) primarily transmitted by the mosquito Aedes aegypti or Haemagogus janthinomys (MAYV), are single-stranded RNA representing a significant global public health concern, particularly due to their increasing geographic distribution and potential to cause a wide spectrum of neurological complications in recent years (8). Dengue virus, with its four major serotypes (DEN-1 to DEN-4), infects up to 400 million people annually (9, 10). Although vaccines and therapeutics are available, their efficacy varies widely across serotypes (11). No effective treatments or vaccines currently exist for Zika virus, Chikungunya virus, or Mayaro virus, highlighting the urgent need for new antiviral agents. Therefore, identifying new inhibitors for these arboviruses is imperative. With over 42.3 million deaths reported over the past four decades, HIV continues to affect global populations. Its single-stranded RNA genome encodes 15 proteins essential for viral replication and immune evasion. While antiretroviral therapies have significantly reduced viral loads, they are not curative and often cause adverse side effects. A prophylactic vaccine is still lacking (12).
This scenario raises an intensified focus on medicinal plants since they hold an immense reservoir of bioactive compounds that could lead to the discovering novel antiviral drugs (6, 13). Such compounds include mainly diverse secondary metabolites, isolated, purified, and identified from the crude extracts of various plant parts, harboring rich structural and chemical diversity which allows them to interact with different biological and viral targets (7). These characteristics led to wide functionality of these phytochemicals, favoring a sustained safety and effectiveness blocking multiples viral infections (14, 15). This review presents recent advances in plant-derived extracts and phytochemicals that inhibit various stages of the viral life cycle of Influenza, Dengue, Zika, Chikungunya, Mayaro, Coronavirus, and HIV (Figure 1), underscoring their potential for antiviral drug development.
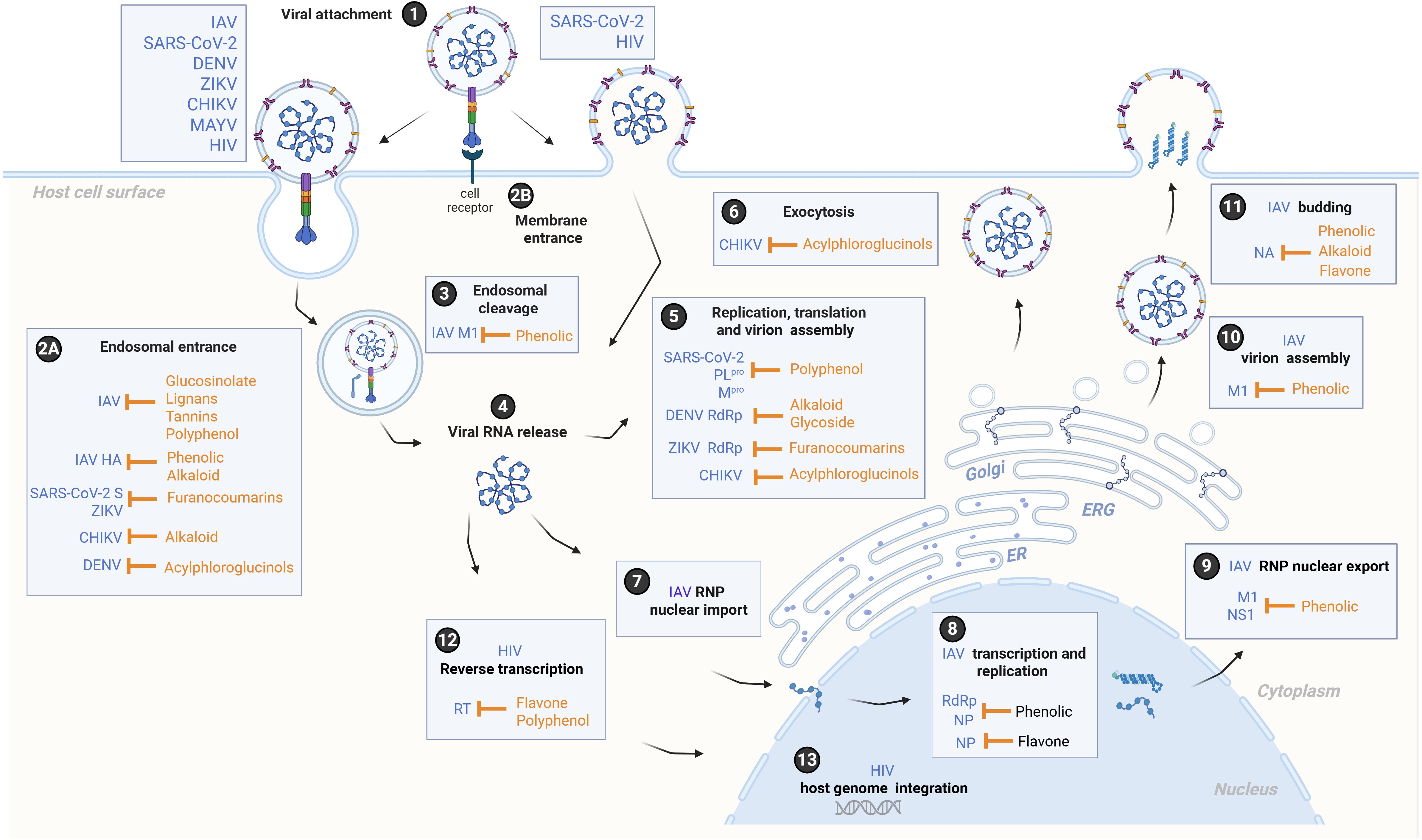
Figure 1. A schematic overview of the life cycle of Influenza A virus (IAV), SARS-CoV-2, Dengue virus (DENV), Zika virus (ZIKV), Chikungunya virus (CHIKV), Mayaro virus (MAYV), and the human immunodeficiency virus (HIV), depicting potential mechanisms of action and targets of phytocompounds. Viruses attach to a host cell (1), and entry is mediated by host receptor binding and fusion at the endosomal membrane (2A) or the cellular membrane (2B). The virions within endosomal compartments undergo viral uncoating (3), resulting in the release of the viral RNA genome into the cytoplasm (4). At the endoplasmic reticulum (ER), the viral RNA genomes of SARS-CoV-2, DENV, ZIKV, CHIKV, and MAYV are translated into viral polyproteins, which are subsequently cleaved by host and viral proteases into non-structural proteins forming the viral replication and transcription complex, and structural proteins that transit through the ER-to-Golgi intermediate compartment (ERGIC) for virion maturation (5). Finally, virions are secreted from the host cell by exocytosis (6). For IAV, viral ribonucleoproteins (RNPs) are transported into the nucleus (7), where viral mRNAs are transcribed by RNA-dependent RNA polymerase (RdRp) and replicated by the viral polymerase along with nucleoprotein (NP) (8). The nuclear export of RNPs is facilitated by matrix protein 1 (M1), which provides structural support and regulates the trafficking of viral RNA segments within the cell, and by nonstructural protein 1 (NS1), which modulates the host nuclear export response and viral mRNA processing (9). Subsequently, RNPs are translated at the ER membrane, trafficked to the Golgi for further processing, and virions are assembled in the cytoplasm (10). Lastly, the expression of viral transmembrane glycoproteins, including neuraminidase (NA), induces lipid raft formation at the host plasma membrane, from which progeny virions bud (11). Regarding HIV, the viral RNA genome is reverse transcribed into DNA by viral reverse transcriptase (RT) to form the provirus (12), which transits through the cytoplasm and nucleus to stably integrate into the host cell genome (13). Subsequent steps involve replication and viral gene expression, followed by the assembly and egress of nascent viral particles (not shown). The figure was created with the help of BioRender.com (2025) (License #2364–1,511, Toronto, ON, Canada).
Family of Orthomyxoviridae
Here we analyze 17 extracts and 16 isolated compounds from species across 12 plant families that exhibit anti-influenza activity, as listed in Table 1.
Extracts of medicinal plants with anti-Influenza activity
For instance, dry extracts from the aerial parts of Spiraea species have demonstrated a pronounced antioxidant effect against Influenza A, as well as cytoprotective activity by reducing the viral cytopathic effect in infected cells (41). Accordingly, the hydroethanolic extract of Caesalpinia mimosoides, primarily containing flavonoids and glycosylated derivatives, showed strong antioxidant and antiviral properties against H1N1. In this context, computational molecular docking studies revealed that multiple derivative metabolites preferentially interacted with viral neuraminidase and the PB2 subunit of RNA polymerase, suggesting potential mechanisms of anti-influenza activity. Molecular dynamics simulations and further in vitro assays are needed to support its therapeutic potential (32). Similarly, Melk (2024) (16) reported that hydroethanolic extracts of Ruellia tuberosa and Ruellia patula, both rich in flavonoids, exhibited antiviral activity against H1N1 by reducing infectious viral particles, likely through molecular interactions between the bioactive compounds quercetin, hesperetin, and rutin with viral neuraminidase (NA), as determined by molecular docking and dynamics simulations. In addition, aqueous extracts of raw Nepeta cataria and Glechoma hederacea showed strong inhibitory effects on H5N1 virus replication. Specifically, the aerial parts of N. cataria were rich in catechin flavonoids, suggesting that this group of phenolic compounds may be responsible for the observed antiviral effects (30).
The butanolic extract of Davallia mariesii, a species used in traditional Chinese medicine for treating osteoporosis and inflammatory conditions, impaired the neuraminidase activity of H1N1 (26). Similarly, butanol extracts of S. glycycarpa and S. sarmentosa inhibited the replication of Influenza H1N1 (42).
A phytochemical investigation revealed that various extracts and fractions of Tilia platyphyllos, Camellia sinensis, and Myrtus communis exhibited in vitro hemagglutination inhibition after H1N1 treatment, possibly due to reduced physical interaction between the extracts and fractions and the virus surface hemagglutinin glycoprotein (36). Finally, Lonicera japonica has been studied for its antiviral properties against H1N1, using extracts from its dry buds and flowers, which are rich in acidic flavonoids. In vivo studies showed that mice treated with 600 mg/kg/day of the acidic extracts for 8 days were protected from influenza-induced death (22).
Isolated natural compounds with anti-Influenza activity
In a bio-guided assay of the ethanolic extract of Angelica dahurica, four isolated furanocoumarin compounds, isoimperatorin, oxypeucedanin, oxypeucedanin hydrate, and imperatorin, exhibited activity against both H1N1 and H9N2 viruses by inhibiting infection and replication. Notably, oxypeucedanin strongly inhibited H1N1 neuraminidase activity, suppressed the synthesis of NA and nucleoprotein (NP), and exerted an anti-apoptotic effect on virus-infected cells, suggesting multiple roles in preventing H1N1 infection and replication (63).
From the roots of Isatis indigotica, several glucosinolate compounds, epiprogoitrin, progoitrin, epigoitrin, and goitrin, were isolated, showing potent anti-H1N1 activity by interfering with viral adsorption or budding from host cells. However, mechanistic studies indicated that these glucosinolates have limited inhibitory effects on hemagglutinin and neuraminidase (53).
Other studies have shown that berberine, an isoquinoline alkaloid from Berberis vulgaris, blocks the host mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK) signaling pathway, which is essential for the transport of viral ribonucleoproteins into the cytoplasm, thereby inhibiting H1N1 replication (52).
From Elaeocarpus sylvestris (Lour.), distributed in the subtropical regions of Jeju Island (Korea), Japan, and southern China, two polyphenol compounds, 1,2,3,4,6-penta-O-galloyl-β-D-glucose and geraniin, were isolated from the butanol fraction. These compounds significantly inhibited the production of H1N1 RNAs, non-structural proteins, and infectious viral particles in vitro. They also reduced pulmonary viral load and inflammatory cytokines (IFN-γ, TNF-α, and IL-6) in vivo, which are associated with disease severity in influenza infection (57).
Additionally, Camellia sinensis is a promising medicinal source, as its isolated polyphenolic compounds, theaflavins, inhibited both hemagglutinin and neuraminidase of H1N1, exhibiting a virucidal action and indicating a direct effect on the viral particle (62).
Family Coronaviridae
In this study, we investigated 15 species from 12 plant families, along with 9 extracts (Table 1), exhibiting promising medicinal properties against Coronavirus and SARS-CoV-2.
Extracts from medicinal plants with anti-Coronavirus and anti-SARS-CoV-2 activity
Using UPLC-MS/MS coupled with in vitro studies and chemometric analysis, Darwish (2022) (43) demonstrated that ethanol extracts from the flowers and leaves of Lantana camara, native to the tropical regions of the Americas (cultivar Chelsea Gem), and flower extracts from the cultivars Spreading Sunset and Drap d’Or, exhibited robust selectivity indices by inhibiting the expression levels of the viral RNA-dependent RNA polymerase (RdRp) gene. These findings indicate high safety, efficacy, and promising anti-COVID-19 properties. Additionally, in vitro tests were conducted to assess the crude methanolic leaf extract of Byrsonima coccolobifolia against SARS-CoV-2. The extract demonstrated excellent in vitro activity with no cytotoxicity under the tested conditions, confirming its efficacy and safety against SARS-CoV-2 (35).
In a study by Giugliano (2024) (39), ethanolic extracts of Moringa oleifera leaves obtained via microwave-assisted extraction showed significant inhibition of coronavirus HCoV-229E infection, without cytotoxic effects in either of the two cell models used.
Likewise, the methanolic extract of Strobilanthes cusia leaves, traditionally used in Chinese medicine for respiratory viral infections, potently inhibited the cytopathic effect (CPE) and viral RNA yield of human coronavirus NL63 (HCoV-NL63), indicating potential to block viral infection and replication (17). Similarly, antiviral activity was observed with the methanolic extract of Ficus rubiginosa leaves against coronavirus HCoV-229E by impairing viral replication (38).
Azadirachta indica and Melia azedarach, both long used in traditional Indian folk medicine, had total methanolic extracts enriched with phenolic and flavonoid compounds evaluated against SARS-CoV-2. These showed strong antiviral activity and robust safety indices by restraining infectious viral particles (37). Likewise, the methanolic extract of Strychnos mattogrossensis was reported for the first time to have biological activity against SARS-CoV-2, protecting cells from the virus’s cytopathic effects (34). Additionally, methanolic leaf extracts of Vitis vinifera significantly reduced SARS-CoV-2 replication at early stages of infection by directly blocking expression of the spike protein, as confirmed by Real-Time PCR (44). Similarly, Cistus ladanifer, a traditional Moroccan medicinal plant, was shown by Bouothmany (2025) (24) to interfere with both replication and viral entry into SARS-CoV-2-infected host cells. Ethyl acetate and dichloromethane extracts of its leaves were particularly effective against the Omicron variant.
Melissa officinalis, with a long history of use in Mediterranean and Western Asia, showed strong virucidal and antiviral activity in its methanolic extract by inhibiting SARS-CoV-2 infection, with a robust safety profile (selectivity index of 230). The inhibition mechanisms of five key compounds from M. officinalis were investigated via molecular docking, revealing strong binding affinities with the spike receptor-binding domain (RBD) of SARS-CoV-2, indicating promising anti-infective action (29).
Isolated natural compounds with anti-SARS-CoV-2 activity
Numerous studies have evaluated the ability of isolated natural compounds to inhibit viral replication by targeting Mpro and PLpro of SARS-CoV-2. For example, the alkaloids vasicoline, vasicolinone, vasicinone, vasicine, adhatodine, and anisotine, particularly enriched in the leaves of Justicia adhatoda, were examined using molecular docking, dynamics simulations, and molecular mechanics with Generalized Born surface area (MMGBSA) calculations. Notably, anisotine was more effective at inhibiting Mpro than the approved antiviral drugs darunavir and lopinavir, suggesting its potential to block SARS-CoV-2 replication by inhibiting Mpro enzymatic activity (46). Sesquiterpene metabolites claroguaiane A, claroguaianes B and C, and claroeudesmane A, derived from Carpesium abrotanoides (native to Europe, Japan, and the Himalayas), were tested via bio-guided inhibitory activity assay against SARS-CoV-2 Mpro. Claroeudesmane A showed moderate activity, while 1β-hydroxy-8-epi-inuviscolide demonstrated stronger activity. Other compounds showed no noticeable effect (50). Srinivasan (2022) (51) evaluated phenolic compounds for PLpro inhibition. In vitro and structural assays showed that 4-hydroxybenzaldehyde (from Tagetes patula), 3,4-dihydroxybenzoate (from Acalypha torta), and 4-(2-hydroxyethyl)phenol (from Lawsonia alba) effectively inhibited PLpro under non-cytotoxic conditions.
Natural compounds may also prevent SARS-CoV-2 infection. Kuwanon C, a flavone from Morus alba, was shown by Kim et al. (2022) (58) to suppress SARS-CoV-2 cell entry. ELISA and in vitro kinetic binding analysis confirmed that kuwanon C effectively blocked spike S1 RBD-ACE2 interaction. In silico docking simulations supported this, making kuwanon C a promising lead compound. Yang (2025) (45) reported that crude extracts from seed embryos of Nelumbo nucifera, as well as the isolated alkaloid neferine, significantly reduced SARS-CoV-2 infectious particles and showed improved virucidal activity and safety when combined with organic salts. The virucidal effect of β-escin, a bioactive constituent in Aesculus hippocastanum seed extract, was also tested. β-escin limited virus infection in vitro and reduced SARS-CoV-2 spike protein expression as seen via immunofluorescence microscopy (60).
Finally, oleandrin, a cardiac glycoside from Nerium oleander, was tested in vitro against SARS-CoV-2, significantly reducing viral replication, likely by blocking ATP binding sites on Na/K-ATPase. In vivo tests on golden Syrian hamsters treated with up to 130 µg/mL oleandrin for 7 days provided preliminary evidence of efficacy (49).
Family Flaviviridae
The potential therapeutic properties of plant extracts from 22 species across 17 families are explored in this section, as outlined in Table 1.
Extracts of medicinal plants with anti-Dengue, anti-Zika, anti-Chikungunya, and anti-Mayaro activity
Alagarasu (2022) (18) reported in vitro anti-Dengue and anti-Chikungunya activities of extracts from several plant species, including Plumeria alba, Ancistrocladus heyneanus, Bacopa monnieri, and Vitex negundo, commonly used in traditional medicine in Belagavi, India. Specifically, chloroform extracts of the bark of P. alba and A. heyneanus, and the hydroalcoholic extract of the whole B. monnieri plant, reduced replication or infection of Dengue and Chikungunya viruses, while the chloroform extract of V. negundo leaves showed activity only against Dengue. In addition, Ocimum sanctum, a traditional Ayurvedic herb known as Vishnu-Priya or Tulsi, containing the isolated compound eugenol (1-hydroxy-2-methoxy-4-allylbenzene), exhibited potent inhibition of Dengue-2 replication, achieving complete inhibition in in vitro assays. Docking analysis showed that eugenol interacts with Dengue non-structural proteins NS1 and NS5 with binding energies of 5.33 and 5.75 kcal/mol, respectively, suggesting potential pharmacological use in Dengue treatment (31). Jayasekara (2024) (33) investigated the antiviral potential of aqueous extracts from the roots of Glycyrrhiza glabra (Leguminosae) against Dengue and found that subfractions of the extract significantly suppressed viral adsorption to cells.
Carvalho (2023) (40) reported that methanolic and hydroalcoholic leaf extracts, as well as a methanolic bark extract from Phyllanthus brasiliensis, showed potent in vitro activity against Zika virus infection. Similarly, Chan (2021) (25) reported in vitro activity of ethyl acetate extracts of Gynura bicolor and Sechium edule against Chikungunya virus replication, and the activity of Ocimum americanum against both infection and replication of this virus.
The ethanolic leaf extract of Fridericia formosa, a Bignoniaceae species rich in xanthones and found in the Brazilian Cerrado biome, exhibited effective activity against Mayaro virus infection (20). In turn, Fridericia chica, rich in flavonoids and traditionally used in Latin American countries to treat infections, showed activity against Dengue-2, Zika, and Mayaro viruses in in vitro assays (21). Lastly (23), reported that ethyl acetate extracts of Maytenus quadrangulata leaves had a virucidal effect against Mayaro virus by acting on viral adsorption and internalization.
Isolated natural compounds with anti-Dengue, anti-Zika, and anti-Chikungunya activity
In this respect, pure phenolic compounds, euparin, 2-oxo-8-deoxyligustrin, and santhemoidine, isolated from Urolepis hecatantha, Stevia alpina, and Stevia satureiifolia, respectively, were able to inhibit Dengue virus infections (19). Furthermore, two major alkaloid compounds, canthin-6-one and 1-hydroxy-11-methoxycanthin-6-one, abundant in the roots of Brucea javanica, a traditional medicinal plant used in Malaysia for treating fever, showed potential binding interactions with the active sites of the NS5 protease and RNA-dependent RNA polymerase (RdRp) by molecular docking analysis using DENV-2. Notably, 1-hydroxy-11-methoxycanthin-6-one reduced viral RNA load in in vitro assays (61).
Phytochemical investigations of Crinum jagus revealed the presence of lycorine and several alkaloids from the cherylline, crinine, and galanthamine groups, which efficiently inhibited both Dengue and Zika viruses. Specifically, cherylline effectively hindered RNA synthesis in both viruses, indicating RNA replication as its main target (47). In turn, lycorine inhibited Zika RNA synthesis by binding to RdRp in vitro and protected against Zika-induced lethality by reducing viral load in vivo (48). Additionally, Phyllanthus phillyreifolius, endemic to Réunion Island and traditionally used to treat fever, venereal diseases, and kidney stones, contains the polyphenol-rich compound geraniin, which prevented RNA production in Zika-infected human cell assays (59). For Chikungunya, fractions of Humulus lupulus containing α-acids, β-acids, cohumulon, canthohumol, and flavonoids were found to affect the entire viral cycle, from entry to the egress of newly formed viral particles, without cytotoxic effects. Notably, the acylphloroglucinol β-acid fraction exhibited the strongest virucidal effect in vitro and caused a significant reduction in viral replication in drug-addition cell experiments (54).
Family Retroviridae
Two species of medicinal plants and four promising isolated compounds (Table 1) with anti-HIV activity are discussed in this section.
Active plant extracts with anti-HIV activity
The hydroalcoholic and aqueous extracts from the roots of Withania somnifera, commonly used in traditional Indian medicine, were investigated for their bioactive potential against HIV-1 replication. In vitro enzymatic analysis revealed that the hydroalcoholic extract inhibited HIV-1 integrase activity by 86.18%, while the aqueous extract achieved 93.98% inhibition. For HIV-1 protease activity, the hydroalcoholic and aqueous extracts showed inhibition rates of 91.77% and 84.84%, respectively. Regarding HIV-1 reverse transcriptase (RT) activity, the hydroalcoholic extract exhibited 76.82% inhibition, whereas the aqueous extract demonstrated a lower inhibition rate of 58.53%. All results were obtained within the sub-cytotoxic concentration range.
Confirmatory cell-based assays showed that both hydroalcoholic and aqueous extracts effectively inhibited infectious virus release, as indicated by HIV-1 p24 detection, even at lower dosages, with EC50 values of 17 µg/mL and 59 µg/mL, respectively. Additionally, in silico molecular docking studies revealed the highest binding affinity against HIV-1 integrase by the compounds 12-deoxywithastramonolide and 27-hydroxywithanone; against HIV-1 protease by ashwagandhanolide and withacoagin; and against HIV-1 reverse transcriptase by ashwagandhanolide and withanolide B. These findings suggest potential mechanisms for the inhibition of HIV-1 replication (12). Another species, Croton dichogamus, traditionally used in African medicine, exhibited significant anti-HIV activity in its methanolic extract. This extract inhibited more than 90% of infectious viral particles in cell lines (IC50 value of 0.06 µg/mL) and demonstrated a high safety profile, with a selectivity index (SI) of 318.5 (27).
Isolated natural compounds with anti-HIV activity
Anogeissus acuminata, an Asian species found in the Bandarban, Chattogram, Cox’s Bazar, Khagrachari, and Rangamati regions of Bangladesh, produces two dibenzylbutadiene lignans: anolignan A and anolignan B. Both compounds showed significant inhibitory activity against the HIV-1-RT. Furthermore, the two phytocompounds exhibited a synergistic effect against this enzyme (55). Similarly, from the leaf extract of Shepherdia argentea, the tannins shephagenin A and B were isolated and also demonstrated inhibitory activity against the HIV-1-RT, highlighting the importance of these compounds as potential HIV-1 reverse transcription inhibitors (56).
Future perspectives
The ongoing discovery of bioactive compounds in plants represents a valuable source for identifying new, potent antiviral agents and selective compounds. These compounds not only offer potential as standalone treatments but can also complement or enhance existing therapies, especially when they exhibit synergistic interactions with other drugs. This can increase the overall effectiveness and potentially reduce adverse side effects. However, the connection between traditional knowledge and future research on plant-derived products with potential pharmacological properties should be further strengthened.
This mini-review provides an overview of recent literature (from the last five years) on medicinal plant extracts and isolated natural compounds with potential antiviral effects. A major limitation in exploring the bioactivity of plant-derived products lies in the absence of standardized methodologies for extraction, fractionation, and characterization. This is due to the immense diversity of extracts and phytochemicals found in nature, which ultimately affects the development of new antiviral agents (64). For instance, isolating alkaloid phytocompounds presents several challenges, such as solvent use, low extraction efficiency, and variations in plant genotype, all of which complicate the process of obtaining consistent yields. Moreover, the current understanding of the antiviral activity of plant-derived extracts and compounds is mainly based on in vitro studies, limiting their clinical applicability.
In this context, emerging technologies such as artificial intelligence (AI) have proven effective in predicting and optimizing the chemical, physical, and biological properties of phytocompounds. AI contributes to accelerating the identification of bioactive molecules that can target viral pathogens. Similarly, CRISPR-Cas technologies are increasingly being employed in plants to speed up the screening of phytocompounds and their targets through functional characterization. Additionally, these strategies enhance and optimize the biosynthesis of compounds in plants, offering a scalable production (65).
By applying these advanced techniques, well-characterized plant-derived compounds can be incorporated into modern medicinal chemistry. This approach could potentially alter their activity and selectivity, enabling them to target both emerging and established viral threats.
Author contributions
GR: Writing – review & editing, Writing – original draft, Investigation, Visualization, Validation. EES: Conceptualization, Writing – original draft, Validation, Writing – review & editing. GP: Validation, Supervision, Writing – review & editing. ED: Writing – review & editing, Conceptualization. EL: Writing – review & editing, Writing – original draft. CW: Supervision, Writing – review & editing, Conceptualization, Writing – original draft, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), grant numbers 2025/03616-0 (G.J.G.R.), 2020/12277-0, 2025/07228-4 (E.E.S), 2018/18257-1, 2018/15549-1, 2020/04923-0 (G.P.) and 2023/07746-0 (C.W.) for financial support. Additionally, the authors would like to thank the DAAD (Deutscher Akademischer Austauschdienst) within the “Integrated International Degree Programs with Double Degrees” entitled SãMBio between the Universities of Münster, Germany, and São Paulo, Brazil for support. C.W. and G.P.are CNPq Productivity Research fellows.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dai J, Jiang X, da Silva-Júnior EF, Du S, Liu X, and Zhan P. Recent advances in the molecular design and applications of viral RNA-targeting antiviral modalities. In: Drug Discovery Today, vol. 29. Elsevier Ltd (2024). doi: 10.1016/j.drudis.2024.104074
2. Du S, Hu X, Menéndez-Arias L, Zhan P, and Liu X. Target-based drug design strategies to overcome resistance to antiviral agents: opportunities and challenges. In: Drug Resistance Updates, vol. 73. Churchill Livingstone (2024). doi: 10.1016/j.drup.2024.101053
3. Arumugam H, Wong KH, Low ZY, Lal S, and Choo WS. Plant extracts as a source of antiviral agents against influenza A virus. In: Journal of Applied Microbiology, vol. 136. Oxford University Press (2025). doi: 10.1093/jambio/lxaf056
4. Capelastegui F and Goldhill DH. H5N1 2.3.4.4b: a review of mammalian adaptations and risk of pandemic emergence. J Gen Virol. (2025) 106:002109. doi: 10.1099/jgv.0.002109
5. WHO. Reported COVID-19 cases. In: COVID-19 Cases, World (2025). World Health Organization (WHO), p. 1–30. Available onlin at: https://covid19.who.int/ (Accessed June 16, 2025).
6. Kupferschmidt K. Why hasn’t the bird flu pandemic started? Sci (1979). (2024) 386:1205–6. doi: 10.1126/science.adv2422
7. Iketani S, Mohri H, Culbertson B, Hong SJ, Duan Y, Luck MI, et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature. (2023) 613:558–64. doi: 10.1038/s41586-022-05514-2
8. Srichawla BS, Manan MR, Kipkorir V, Dhali A, Diebel S, Sawant T, et al. Neuroinvasion of emerging and re-emerging arboviruses: A scoping review. SAGE Open Med. (2024) 12:20503121241229847. doi: 10.1177/20503121241229847
9. Yang X, Quam MBM, Zhang T, and Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med. (2021) 28:taab146. doi: 10.1093/jtm/taab146
10. Bos S, Zambrana JV, Duarte E, Graber AL, Huffaker J, Montenegro C, et al. Serotype-specific epidemiological patterns of inapparent versus symptomatic primary dengue virus infections: a 17-year cohort study in Nicaragua. Lancet Infect Dis. (2025) 25:346–56. Available online at: https://linkinghub.elsevier.com/retrieve/pii/S1473309924005668 (Accessed June 16, 2025).
11. Zambrana JV, Hasund CM, Aogo RA, Bos S, Arguello S, Gonzalez K, et al. Primary exposure to Zika virus is linked with increased risk of symptomatic dengue virus infection with serotypes 2, 3, and 4, but not 1. Sci Transl Med. (2024) 16:eadn2199. doi: 10.1126/scitranslmed.adn2199
12. Jadaun P, Harshithkumar R, Gaikwad SY, Seniya C, Borse S, Gawai AA, et al. Withania somnifera extracts induced attenuation of HIV-1: a mechanistic approach to restrict viral infection. Virol J. (2023) 20:173. doi: 10.1186/s12985-023-02130-y
13. Ma Y, Frutos-Beltrán E, Kang D, Pannecouque C, De Clercq E, Menéndez-Arias L, et al. Medicinal chemistry strategies for discovering antivirals effective against drug-resistant viruses. In: Chemical Society Reviews, vol. 50. Royal Society of Chemistry (2021). p. 4514–40. doi: 10.1039/d0cs01084g
14. Mehta SK and Pradhan RB. Phytochemicals in antiviral drug development against human respiratory viruses. Drug Discov Today. (2024) 29:104107. doi: 10.1016/j.drudis.2024.104107
15. Ben-Shabat S, Yarmolinsky L, Porat D, and Dahan A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Delivery Transl Res. (2020) 10:354–67. doi: 10.1007/s13346-019-00691-6
16. Melk MM and El-Sayed AF. Phytochemical profiling, antiviral activities, molecular docking, and dynamic simulations of selected Ruellia species extracts. Sci Rep. (2024) 14:15381. doi: 10.1038/s41598-024-65387-5
17. Tsai YC, Lee CL, Yen HR, Chang YS, Lin YP, Huang SH, et al. Antiviral action of tryptanthrin isolated from strobilanthes cusia leaf against human coronavirus nl63. In: Biomolecules, vol. 10. MDPI AG (2020). doi: 10.3390/biom10030366
18. Alagarasu K, Patil P, Kaushik M, Chowdhury D, Joshi RK, Hegde HV, et al. In vitro antiviral activity of potential medicinal plant extracts against dengue and Chikungunya viruses. Front Cell Infect Microbiol. (2022) 12. doi: 10.3389/fcimb.2022.866452
19. Borgo J, Wagner MS, Laurella LC, Elso OG, Selener MG, Clavin M, et al. Plant extracts and phytochemicals from the asteraceae family with antiviral properties. Molecules. (2024) 29:814. doi: 10.3390/molecules29040814
20. Vaz LBA, Amparo TR, Reis ACC, de Mello Silva B, de Brito Magalhães CL, Kohlhoff M, et al. Identification, characterization and quantification of xanthones from Fridericia formosa leaves extract with antiviral activity. Sci Rep. (2024) 14:2258. doi: 10.1038/s41598-024-51881-3
21. da Cruz AFG, Reis ACC, Sousa JAC, Vaz LBA, de Mello Silva B, de Brito Magalhães CL, et al. High-resolution mass spectrometry identification and characterization of flavonoids from Fridericia chica leaves extract with anti-arbovirus activity. Molecules. (2022) 27:6043. doi: 10.3390/molecules27186043
22. Li M, Wang Y, Jin J, Dou J, Guo Q, Ke X, et al. Inhibitory activity of honeysuckle extracts against influenza A virus in vitro and in vivo. Virol Sin. (2021) 36:490–500. doi: 10.1007/s12250-020-00302-6
23. Nunes DA de F, Lopes GFM, Nizer WS da C, de Aguilar MG, Santos FR da S, de Sousa GF, et al. Virucidal antiviral activity of Maytenus quadrangulata extract against Mayaro virus: Evidence for the presence of catechins. J Ethnopharmacol. (2023) 311:116436. doi: 10.1016/j.jep.2023.116436
24. Bouothmany K, Bourhia M, Chebaibi M, Rhazzar Z, Addoum B, Khallouki F, et al. Uncovering antiviral potential of cistus ladanifer extracts against herpes simplex virus 1 and severe acute respiratory syndrome coronavirus 2 by in vitro and in silico analysis. Chem Biodivers. (2025) 5. doi: 10.1002/cbdv.202402661
25. Chan SM, Khoo KS, Sekaran SD, and Sit NW. Mode-dependent antiviral activity of medicinal plant extracts against the mosquito-borne chikungunya virus. Plants. (2021) 10:1658. doi: 10.3390/plants10081658
26. Chen YL, Chao PY, Hsieh CF, Hsieh PW, and Horng JT. Novel Anti-Viral Properties of the Herbal Extract of Davallia mariesii against Influenza A Virus. Viruses. (2024) 16:523. doi: 10.3390/v16040523
27. Terefe EM, Okalebo FA, Derese S, Batiha GES, Youssef A, Alorabi M, et al. Cytotoxicity and anti-HIV activities of extracts of the twigs of Croton dichogamus Pax. BMC Complement Med Ther. (2022) 22:49. doi: 10.1186/s12906-022-03532-1
28. Simo Nemg FB, De S, Keshry SS, Mamidi P, Njayou FN, Demanou M, et al. Plants extracts from Cameroon pharmacopeia strongly inhibit the Chikungunya virus infection by targeting entry and replication steps. J Ethnopharmacol. (2022) 296:115458. doi: 10.1016/j.jep.2022.115458
29. Alsahafi T, Bouback T, Albeshri A, Alnhhas S, Ali M, Moatasim Y, et al. Antiviral potential of Melissa officinalis extracts against influenza and emerging coronaviruses. Sci Rep. (2025) 15:12118. doi: 10.1038/s41598-025-96417-5
30. Protsenko MA, Mazurkova NA, Filippova EI, Kukushkina TA, Lobanova IE, Pshenichkina YA, et al. Anti-influenza activity of extracts from plants of the lamiaceae family. Russ J Bioorg Chem. (2022) 48:1534–41. doi: 10.1134/S1068162022070238
31. Kaushik S, Kaushik S, Dar L, and Yadav JP. Eugenol isolated from supercritical fluid extract of Ocimum sanctum: a potent inhibitor of DENV-2. AMB Express. (2023) 13:105. doi: 10.1186/s13568-023-01607-x
32. Klamrak A, Rahman SS, Nopkuesuk N, Nabnueangsap J, Narkpuk J, Janpan P, et al. Integrative computational analysis of anti-influenza potential in Caesalpinia mimosoides Lamk hydroethanolic extract. Sci Rep. (2025) 15:3988. doi: 10.1038/s41598-025-87585-5
33. Jayasekara KG, Suresh S, Goonasekara C, Soyza P, Perera N, and Gunasekera K. Anti-dengue viral activity of Glycyrrhiza glabra roots in Vero cells. Sci Rep. (2024) 14:25922. Available online at: https://www.nature.com/articles/s41598-024-76184-5 (Accessed June 16, 2025).
34. Ledoux A, Leka K, Bonnet O, Blanquer A, Alembert TT, da Silva Mirowski P, et al. In vitro antiviral activity against SARS-CoV-2 of 28 Strychnos extracts. In: Phytotherapy Research, vol. 36. John Wiley and Sons Ltd (2022). p. 1061–3. doi: 10.1002/ptr.7394
35. Rodrigues CM, Bento CC, Moraes CB, Gomes C, Ioshino RS, Freitas-Junior LH, et al. A potential antiviral against COVID-19 obtained from Byrsonima coccolobifolia leaves extract. Fitoterapia. (2024) 173:105820. doi: 10.1016/j.fitote.2024.105820
36. Mehrbod P, Safari H, Mollai Z, Fotouhi F, Mirfakhraei Y, Entezari H, et al. Potential antiviral effects of some native Iranian medicinal plants extracts and fractions against influenza A virus. BMC Complement Med Ther. (2021) 21:246. doi: 10.1186/s12906-021-03423-x
37. Hemdan BA, Mostafa A, Elbatanony MM, El-Feky AM, Paunova-Krasteva T, Stoitsova S, et al. Bioactive Azadirachta indica and Melia azedarach leaves extracts with anti-SARS-CoV-2 and antibacterial activities. PloS One. (2023) 18:e0282729. doi: 10.1371/journal.pone.0282729
38. Dell’Annunziata F, Sellitto C, Franci G, Marcotullio MC, Piovan A, Della Marca R, et al. Antiviral Activity of Ficus rubiginosa Leaf Extracts against HSV-1, HCoV-229E and PV-1. Viruses. (2022) 14:2257. doi: 10.3390/v14102257
39. Giugliano R, Ferraro V, Chianese A, Della Marca R, Zannella C, Galdiero F, et al. Antiviral Properties of Moringa oleifera Leaf Extracts against Respiratory Viruses. Viruses. (2024) 16:1199. doi: 10.3390/v16081199
40. Carvalho ARV, Reis JDE, Gomes PWP, Ferraz AC, Mardegan HA, Menegatto MB da S, et al. Untargeted-based metabolomics analysis and in vitro/in silico antiviral activity of extracts from Phyllanthus brasiliensis (Aubl.) Poir. Phytochemical Analysis. (2023) 34:869–83. doi: 10.1002/pca.3259
41. Kostikova VA, ZArubaev VV, Esaulkova IL, Sinegubova EO, Kadyrova RA, Shaldaeva TM, et al. The antiviral, antiradical, and phytochemical potential of dry extracts from Spiraea hypericifolia, S. media and S. salicifolia (Rosaceae). South Afr J Botany. (2022) 147:215–22. doi: 10.1016/j.sajb.2022.01.013
42. Leal CM, Simas RC, Miranda M, Campos MF, Gomes BA, Siqueira MM, et al. Amazonian Siparuna extracts as potential anti-influenza agents: Metabolic fingerprinting. J Ethnopharmacol. (2021) 270:113788. doi: 10.1016/j.jep.2021.113788
43. Darwish RS, El-Banna AA, Ghareeb DA, El-Hosseny MF, Seadawy MG, and Dawood HM. Chemical profiling and unraveling of anti-COVID-19 biomarkers of red sage (Lantana camara L.) cultivars using UPLC-MS/MS coupled to chemometric analysis, in vitro study and molecular docking. J Ethnopharmacol. (2022) 291:115038. doi: 10.1016/j.jep.2022.115038
44. Zannella C, Giugliano R, Chianese A, Buonocore C, Vitale GA, Sanna G, et al. Antiviral activity of vitis vinifera leaf extract against sars-cov-2 and hsv-1. Viruses. (2021) 13:1263. doi: 10.3390/v13071263
45. Yang DD, Chutiwitoonchai N, Wang F, Tian P, Sureram S, Lei X, et al. Effects of organic salts of virucidal and antiviral compounds from Nelumbo nucifera and Kaempferia parviflora against SARS-CoV-2. Sci Rep. (2025) 15:6380. doi: 10.1038/s41598-025-89736-0
46. Ghosh R, Chakraborty A, Biswas A, and Chowdhuri S. Identification of alkaloids from Justicia adhatoda as potent SARS CoV-2 main protease inhibitors: An in silico perspective. J Mol Struct. (2021) 5:1229. doi: 10.1016/j.molstruc.2020.129489
47. Ka S, Merindol N, Sow AA, Singh A, Landelouci K, Plourde MB, et al. Amaryllidaceae alkaloid cherylline inhibits the replication of dengue and Zika viruses. Antimicrob Agents Chemother. (2021) 65:e0039821. doi: 10.1128/AAC.00398-21
48. Chen H, Lao Z, Xu J, Li Z, Long H, Li D, et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology. (2020) 546:88–97. doi: 10.1016/j.virol.2020.04.009
49. Plante KS, Dwivedi V, Plante JA, Fernandez D, Mirchandani D, Bopp N, et al. Antiviral activity of oleandrin and a defined extract of Nerium oleander against SARS-CoV-2. Biomed Pharmacother. (2021) 138:111457. doi: 10.1016/j.biopha.2021.111457
50. Fan YW, Ao ZY, Zhang WJ, Chen JY, Lian X, Chen Pan Y, et al. The sesquiterpenes with the COVID-19 Mpro inhibitory activity from the Carpesium abrotanoides L. Nat Prod Res. (2024) 38:1909–17. doi: 10.1080/14786419.2023.2230609
51. Srinivasan V, Brognaro H, Prabhu PR, de Souza EE, Günther S, Reinke PYA, et al. Antiviral activity of natural phenolic compounds in complex at an allosteric site of SARS-CoV-2 papain-like protease. Commun Biol. (2022) 5:805. doi: 10.1038/s42003-022-03737-7
52. Botwina P, Owczarek K, Rajfur Z, Ochman M, Urlik M, Nowakowska M, et al. Berberine hampers influenza a replication through inhibition of MAPK/ERK pathway. Viruses. (2020) 12:344. doi: 10.3390/v12030344
53. Nie Lx, Wu Yl, Dai Z, and Ma Sc. Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J Ethnopharmacol. (2020) 251:112550. doi: 10.1016/j.jep.2020.112550
54. Mandova T, Saivish MV, La Serra L, Nogueira ML, and Da Costa FB. Identification of potential antiviral hops compounds against Chikungunya virus. Int J Mol Sci. (2023) 24:3333. doi: 10.3390/ijms24043333
55. El-Ansari MA, Ibrahim LF, and Sharaf M. Anti-HIV activity of some natural phenolics. Herba Polonica. (2020) 66:34–43. doi: 10.2478/hepo-2020-0010
56. Gurjar VK and Pal D. Classification of medicinal plants showing anti-viral activity, classified by family and viral infection types. (2024) Cham: Springer, 97–195. doi: 10.1007/978-3-031-12199-9_3
57. Joo YH, Lee YG, Lim Y, Jeon H, Kim EH, Choi J, et al. Potent antiviral activity of the extract of Elaeocarpus sylvestris against influenza A virus in vitro and in vivo. Phytomedicine. (2022) 97:153892. doi: 10.1016/j.phymed.2021.153892
58. Kim YS, Kwon EB, Kim B, Chung HS, Choi G, Kim YH, et al. Mulberry component Kuwanon C exerts potent therapeutic efficacy in vitro against COVID-19 by blocking the SARS-CoV-2 spike S1 RBD: ACE2 receptor interaction. Int J Mol Sci. (2022) 23:12516. doi: 10.3390/ijms232012516
59. Haddad JG, Grauzdyte D, Koishi AC, Viranaicken W, Venskutonis PR, Duarte dos Santos CN, et al. The geraniin-rich extract from reunion island endemic medicinal plant phyllanthus phillyreifolius inhibits zika and dengue virus infection at non-toxic effect doses in zebrafish. Molecules. (2020) 25:2316. doi: 10.3390/molecules25102316
60. Peñaranda Figueredo FA, Vicente J, Barquero AA, and Bueno CA. Aesculus hippocastanum extract and the main bioactive constituent β-escin as antivirals agents against coronaviruses, including SARS-CoV-2. Sci Rep. (2024) 14:6418. doi: 10.1038/s41598-024-56759-y
61. Yousof NSAM, Afzan A, Zainol M, Bakar SIA, Razak MRMA, Jelas NHM, et al. Molecular networking-based mass spectral identification of Brucea javanica (L.) Merr. metabolites and their selective binding affinities for dengue virus enzymes. Fitoterapia. (2024) 175:105955. doi: 10.1016/j.fitote.2024.105955
62. Mohamed IMA, Ogawa H, and Takeda Y. In vitro virucidal activity of the theaflavin-concentrated tea extract TY-1 against influenza A virus. J Nat Med. (2022) 76:152–60. doi: 10.1007/s11418-021-01568-0
63. Lee BW, Ha TKQ, Cho HM, An JP, Kim SK, Kim CS, et al. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J Ethnopharmacol. (2020) 259:112945. doi: 10.1016/j.jep.2020.112945
64. Mungwari CP, King’ondu CK, Sigauke P, and Obadele BA. Conventional and modern techniques for bioactive compounds recovery from plants: Review. Sci Afr. (2025) 27:e02509. doi: 10.1016/j.sciaf.2024.e02509
Keywords: medicinal plants, antiviral and pharmacological targets, SARS-CoV-2, ZIKV, CHIKV, MAYV, DENV, Influenza
Citation: Ribeiro GdJG, de Souza EE, Palmisano G, Durigon EL, Liebau E and Wrenger C (2025) Plant-derived extracts and natural products with antiviral activity. Front. Virol. 5:1632734. doi: 10.3389/fviro.2025.1632734
Received: 21 May 2025; Accepted: 07 July 2025;
Published: 07 August 2025.
Edited by:
Arif Nur Muhammad Ansori, Universitas Airlangga, IndonesiaReviewed by:
Saikat De, The Scripps Research Institute, United StatesArli Aditya Parikesit, Indonesia International Institute for Life-Sciences (i3L), Indonesia
Ali Jaber, Lebanese American University, Lebanon
Copyright © 2025 Ribeiro, de Souza, Palmisano, Durigon, Liebau and Wrenger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carsten Wrenger, Y3dyZW5nZXJAaWNiLnVzcC5icg==
†These authors have contributed equally to this work
 Giovane de Jesus Gomes Ribeiro1†
Giovane de Jesus Gomes Ribeiro1† Edmarcia Elisa de Souza
Edmarcia Elisa de Souza Giuseppe Palmisano
Giuseppe Palmisano Edison Luiz Durigon
Edison Luiz Durigon Carsten Wrenger
Carsten Wrenger