- 1Department of Medicine, Division of Infectious Diseases, University of the Philippines-Manila, Philippine General Hospital, Manila, Philippines
- 2Department of Medicine, Division of Public Health, Infectious Diseases and Occupational Medicine, and The William J Von Liebig Center for Transplantation and Clinical Regeneration, Mayo Clinic College of Medicine and Sciences, Rochester, MN, United States
Background: We systematically reviewed the published literature to describe the epidemiology and outcomes of human herpes virus 6 (HHV-6) syndromes and diseases after solid organ transplantation (SOT), hematopoietic transplantation (HCT), and chimeric antigen receptor T-cell (CAR-T) therapy.
Methods: PubMed, Scopus, Embase, and Ovid/Medline were reviewed from inception through May 31, 2024, using the keywords HHV-6 and transplantation or CAR-T. Abstracts, case reports, and cohort and case–control studies among adults that were published or translated into English were included.
Results: A total of 136 case reports or series contributed 268 unique cases—225 HCT, 37 SOT, and 6 CAR-T—while 39 cohort studies on HCT (28), SOT (9), CAR-T (1), and mixed SOT/HCT (1) recipients were included. The HHV-6 incidence varied widely from 1% to 95% among cohort studies on HCT and SOT but was low in CAR-T recipients (5.6%). Among the case reports, the median age was 46 years (range, 18–73 years), and most were men (159/236, 67.4%). HHV-6 subtyping was performed only in 66 cases, and 46 were variant B. There were 10 cases that were chromosomally integrated (ciHHV-6). Among the 268 cases with detailed clinical information, fever was reported only in 89 patients (33.2%). The most common clinical syndrome was neurological (204/268, 76.1%), followed by viral syndrome (28/268, 10.4%) and disseminated disease (14/268, 5.2%). The initial therapy was ganciclovir (87/234, 37.2%) or foscarnet (82/234, 35%). At least a third of patients developed neurological sequelae (45/151, 29.8%). HHV-6-attributable mortality was 20.6% (22/107).
Conclusions: Neurological disease is the most frequent clinical syndrome of HHV-6 infection. Early recognition of limbic involvement either through the triad of confusion, amnesia, and seizures or through compatible MRI findings may help with early identification. Diagnosis is secured through molecular methods, although an extremely high viral load needs to be interpreted in the context of ciHHV-6. The neurological sequelae of HHV-6 can be disabling and cause significant morbidity.
1 Introduction
Human herpes virus-6 (HHV-6) is a β-herpes virus that was first described in children in 1988 as the causative agent of exanthem subitum (1). HHV-6 infects nearly all children by 3 years of age (2) and is now classified into two closely related but distinct species: HHV-6A and HHV-6B (3). This recognition as separate viruses was made by the International Committee on Taxonomy of Viruses in 2012, primarily due to their different epidemiological, biological, and immunological distinctions. Primary HHV-6B infection may be asymptomatic and present with nonspecific symptoms such as fever, fussiness, rash, diarrhea, and seizures in children (1). The epidemiology of HHV-6A, on the other hand, is still largely uncertain, although it is thought to be acquired later in life and may be more commonly associated with severe neurological disease compared with HHV-6B (4). In adults, the virus has been associated with a broad range of clinical syndromes, ranging from mild undifferentiated rashes, temporal lobe epilepsy (5) to multiple sclerosis and encephalitis (6, 7). Unique to HHV-6 is its ability to integrate into chromosomes (8, 9). As a consequence of the presence of the viral genome in every nucleated human cell, patients with chromosomally integrated HHV-6 (ciHHV-6) are characterized by very high levels of HHV-6 DNA in the blood and tissues (10). ciHHV-6 has been misinterpreted as “active” infection, leading to unnecessary antiviral treatment.
The pathogenicity of HHV-6A, HHV-6B, and ciHHV-6 in immunocompromised hosts, such as recipients of hematopoietic cell transplantation (HCT), solid organ transplantation (SOT), and chimeric antigen receptor T-cell (CAR-T) therapy, has been reported. It is assumed that HHV-6B is the more prevalent species, while HHV-6A is suspected as the etiology of most cases of HHV-6 encephalitis with some outliers (11). However, there are also sporadic reports of other clinical syndromes including myelitis, myocarditis, hepatitis, and hemophagocytic syndromes. ciHHV-6, on the other hand, has been associated with an increased risk of acute graft-versus-host disease (aGvHD) in allogeneic hematopoietic transplants, and it has been most often mistaken for active infection (10).
Earlier reviews have focused only on specific hosts (12), virus types (13), or an aspect of HHV-6 (14). Hence, we aimed to perform a more encompassing systematic review to comprehensively examine the epidemiology of HHV-6 in all these populations by describing its different syndromes and patient outcomes.
2 Methods
With the help of a professional librarian, we searched multiple databases (i.e., PubMed, Embase, Scopus, and Medline/Ovid) and identified all cases of HHV-6 after SOT, HCT, and CAR-T from inception to May 31, 2024. Search terms included the keywords “human herpes virus 6 or HHV-6” and “solid organ or hematopoietic transplant” or “encephalitis.” The complete search strategy is shown in Appendix 1. The article references were reviewed for additional cases. Our search was limited to publications in English or those with an English translation. All reports of adult SOT, HCT, and CAR-T recipients who developed HHV-6 after transplantation within the study period were eligible for study inclusion and were screened by two authors (CLA and RRR). Only those cases where the symptoms were attributed to HHV-6 were included. HHV-6 infection was defined as evidence of viremia or DNAemia, while disease was confirmed in the presence of a compatible clinical syndrome [e.g., fever and rash, post-transplant acute limbic encephalitis (PALE), myelitis, myocarditis, and hepatitis, among others] and/or isolation of HHV-6 from culture, shell vial assay, or via molecular methods (e.g., polymerase chain reaction or metagenomic sequencing). The abstracts of case reports were included if sufficient clinical information was provided. Recipients with ciHHV-6 were included only if there was clinical disease. All case reports or series included symptomatic HHV-6, but incidences of asymptomatic (e.g., viremic) or symptomatic HHV-6 as reported by cohort studies were captured and recorded. We excluded pediatric cases and cases diagnosed prior to transplantation or upon removal of the transplant allograft. Reports on HHV-6 where clinical information was inadequate or lacking, could not be extracted in detail, or had mixed populations (e.g., predominantly pediatric or non-transplant) were excluded. Data were extracted (CLA) and coded into an Excel spreadsheet. Informed consent was not required as these cases have already been previously reported. The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) was used to help generate the four-phase flow diagram.
2.1 Statistical analysis
Detailed case reports, case series, and cohorts of SOT, HCT, and CAR-T recipients were described and analyzed separately or in combination, as appropriate. Frequency statistics (e.g., median, range, and percent) were used to describe categorical and continuous variables.
3 Results
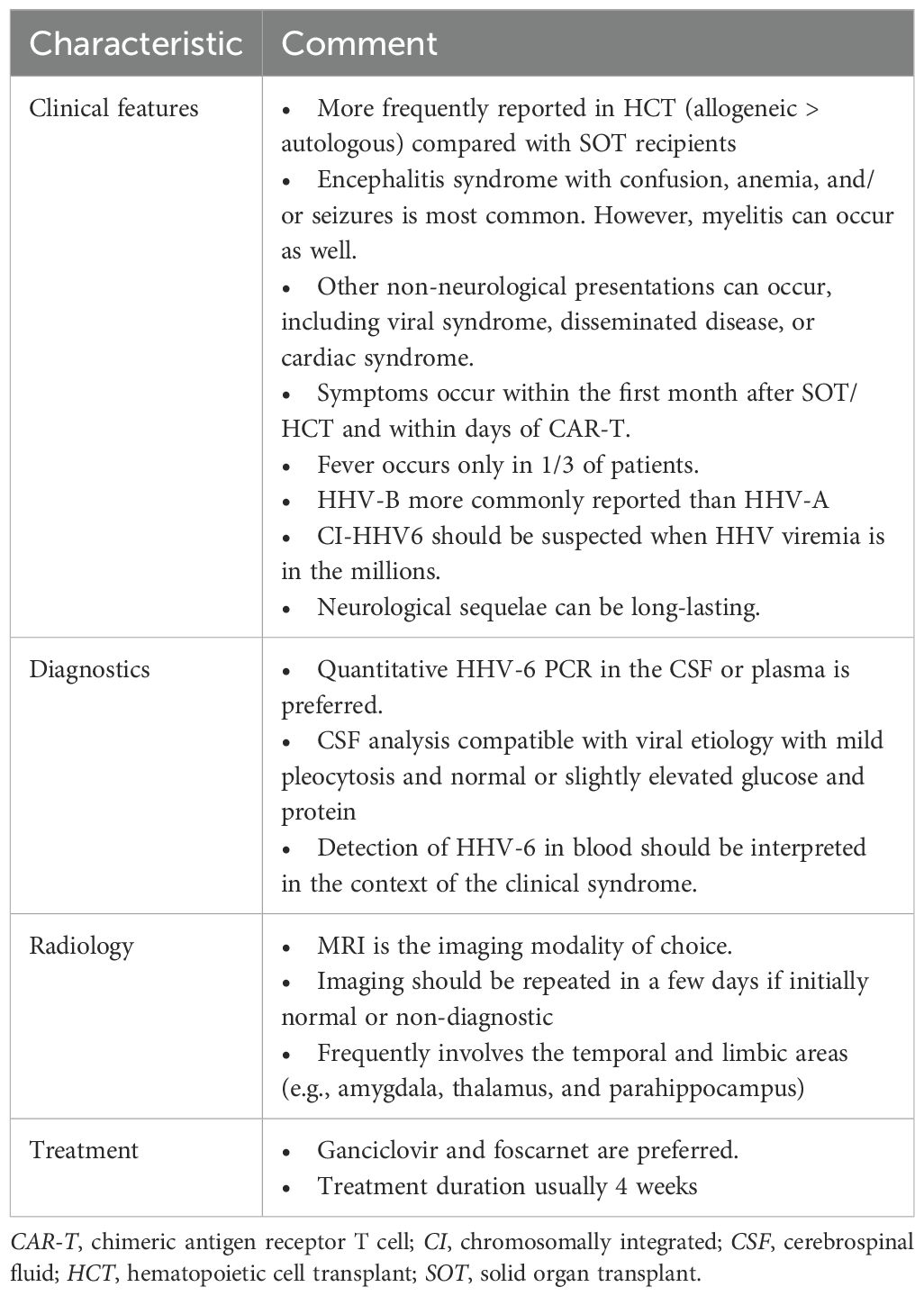
Our search identified a total of 1,239 studies across multiple databases (PubMed, n = 122; Embase, n = 987; Medline, n = 85; and Scopus, n = 45). After the reference review (n = 25) and the exclusion of duplicates (n = 24), a total of 164 studies were included in the final review (Figure 1). There were 136 (32 SOT, 100 HCT, 3 CAR-T, and 1 both SOT/HCT) case reports or series (11, 15–149) that contributed a total of 268 unique cases: 225 HCT, 37 SOT, and 6 CAR-T recipients.
A total of 39 cohort studies (34, 42, 45, 51, 58, 94, 126, 131, 138, 143, 146, 147, 149–175) on HCT (28), SOT (9), CAR-T (1), and mixed SOT/HCT (1) recipients were also included. Of these, 13 studies provided detailed clinical cases that were included in our systematic review of case reports or series (34, 42, 45, 51, 58, 94, 126, 131, 138, 143, 146, 147, 149).
3.1 Case reports/series
3.1.1 Hematopoietic transplantation
3.1.1.1 Epidemiology and patient characteristics
A total of 101 reports (11, 15, 17, 19, 22, 24–26, 28–36, 41–49, 51, 53–59, 61–66, 70–76, 78–81, 83–85, 87–95, 97, 98, 102, 103, 106, 110–113, 115, 117, 118, 121–127, 129–131, 135–149, 165) described 225 cases of HHV-6 among 223 HCT recipients (e.g., two recipients were retransplanted and developed another episode of HHV-6 after the second transplant) (64, 84). Of these reports, the majority were from the Asia-Pacific region (n = 41, 40.6%) (15, 24, 29, 45, 51, 54, 55, 57–59, 62, 63, 65, 75, 79, 80, 83–85, 88–90, 92–94, 118, 121, 122, 124–127, 135–138, 141, 145–147, 165) and the USA or Canada (n = 33, 32.7%) (11, 17, 26, 28, 31–33, 41, 42, 46–49, 53, 64, 70–72, 78, 81, 95, 97, 106, 110, 112, 113, 130, 139, 140, 142, 144, 148, 149). Information on sex was provided in some case reports, and the majority of the patients were men (125/188, 66.5%). The median age was 46 years (range, 18–73 years) (Table 1). Of 225 transplant cases, the vast majority occurred after allogeneic stem cell transplantation [including 139 (61.8%) allogeneic bone marrow or stem cell recipients (11, 15, 19, 24, 28, 29, 31–33, 35, 36, 41, 42, 44, 46, 49, 51, 53, 54, 56–59, 62, 64–66, 70–74, 81, 83, 85, 87–89, 91, 97, 98, 102, 103, 106, 110–113, 118, 121, 123–125, 129, 131, 135–143, 146–149, 165), and 64 (28.4%) umbilical cord blood (UCB) recipients (25, 30, 43, 45, 47, 48, 55, 63, 75, 79, 80, 84, 90, 92–95, 122, 126, 130, 145)], while 21 (9.3%) were autologous stem cell recipients (17, 22, 26, 34, 76, 78, 115, 117, 127, 144) and 1 (0.44%) was not specified (61). The donor characteristics were described as fully matched related or unrelated in half of the cohort (54/107, 50.5%), mismatched or unmatched related or unrelated in 19 (17.8%), haploidentical in 7 (6.5%), unrelated in 24 (22.4%), and related in 3 (2.8%). The underlying hematological disorder requiring transplantation was highly varied, with acute myelogenous leukemia (AML) being the most frequent (37/181, 20.4%) (25, 36, 43, 58, 59, 64, 66, 72, 75, 79, 84, 97, 111, 122, 139, 142, 149, 165), followed by acute lymphocytic leukemia (ALL) or mantle cell lymphoma (30/181,16.6%) (15, 19, 33, 44, 47, 48, 62, 83, 85, 88, 89, 102, 115, 135, 136) and myelodysplastic syndrome (MDS) (19/181, 10.5%) (26, 32, 45, 54, 55, 63, 73, 80, 81, 118, 143, 145, 148). The conditioning regimens were diverse and dependent on the underlying disease and the institutional protocol. Information regarding the aGvHD prophylaxis regimens were provided in less than half (95/225, 42.2%) of the recipients (15, 17, 19, 24, 25, 30, 32, 36, 45–47, 51, 55, 57–59, 62, 63, 65, 66, 75, 79, 80, 83, 84, 87–89, 91, 93, 97, 102, 103, 111, 113, 121, 122, 125, 126, 129, 135–137, 140–142, 145, 148, 149, 165). Of these, tacrolimus was the most frequent drug for prophylaxis (n = 54, 56.8%), followed by cyclosporine (n = 42, 44.2%), methotrexate (n = 36, 37.9%), mycophenolate mofetil (n = 24, 25.3%), and corticosteroids (n = 12, 12.6%).
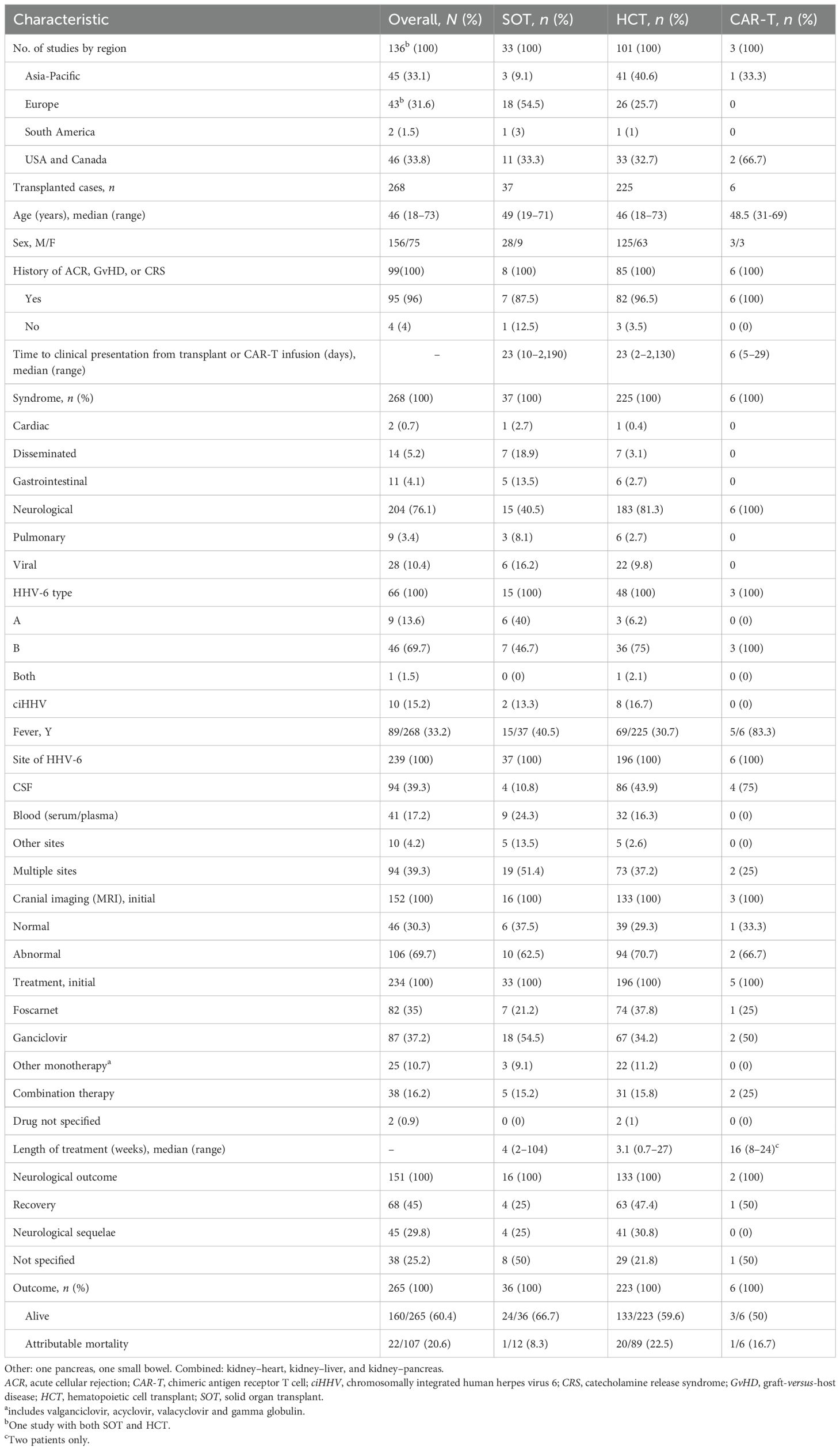
Table 1. Clinical and demographic characteristics of patients with human herpes virus 6 (HHV-6) infection after CAR-T infusion and transplantation.
3.1.1.2 Clinical presentation and syndromes
The overall time to presentation had a median of 23 days (range, 2–2,130 days) after transplantation. Fever was reported only in 69/225 (30.7%) transplant cases (17, 19, 26, 29–34, 36, 44, 46, 48, 55, 63, 64, 71, 72, 74, 79, 87, 102, 111, 115, 117, 123–125, 127, 136, 144, 145, 147, 165). Subtyping of HHV-6 was performed in only 48 cases (11, 19, 22, 31, 33, 36, 44, 46, 53, 59, 62, 63, 66, 70, 76, 79, 83, 87, 89, 91, 93, 94, 98, 102, 110–113, 118, 141, 143, 145, 165), and the majority (36/48, 75%) were classified as HHV-6 variant B. There were eight identified cases of symptomatic ciHHV-6 (31, 70, 76, 91, 98, 102, 143) (Table 1) who presented at a median time of 148 days (range, 11–750 days) after transplantation. Of those with ciHHV-6, six were men, and the median age was 48.5 years (range, 18–66 years).
The most common clinical syndrome was neurological (183/225, 81.3%) (11, 15, 17, 19, 25, 28–33, 35, 36, 41–45, 47, 49, 51, 54–59, 61–63, 70–73, 75, 78–80, 83, 89, 90, 92–94, 97, 98, 102, 103, 110–112, 118, 121–127, 129–131, 136–142, 146–149, 165), described as encephalitis (137/183, 74.9%), mixed encephalomyelitis (10/183, 5.5%) (89), or myelitis (18/183, 9.8%) (15, 126). Subsets of those with encephalitis were categorized as PALE (15/183, 8.2%), Guillain–Barré syndrome (GBS) (2/183, 1.1%), or posterior reversible encephalopathy syndrome (PRES) (1/183, 0.5%). The initial symptoms were confusion (n = 58), disorientation (n = 26), mental status impairment (n = 11), hallucination (n = 4), amnesia (n = 27), memory impairment or loss (n = 48), seizures (n = 36), headache (n = 9), or loss of consciousness (n = 4). Those with myelitis presented with pain around the affected nerves (n = 12), pruritus (n = 9), dysesthesia/paresthesia (n = 6), bladder disturbance (n = 4), or epigastralgia (n = 3).
Magnetic resonance imaging (MRI) of the brain was the initial imaging modality (n = 133) (11, 15, 17, 19, 22, 25, 29, 32, 33, 35, 36, 41–43, 47, 49, 51, 54–59, 61–63, 65, 70, 71, 73, 75, 76, 80, 83, 85, 89, 90, 92, 93, 95, 98, 102, 103, 106, 111, 112, 118, 121–125, 127, 129, 130, 136–138, 140–142, 147–149, 165) and had abnormal findings in 70.7% (94/133), with signal abnormalities typically involving either the hippocampus (41/94, 45.6%) and/or the temporal lobe (22/94 24.4%). In some instances (n = 30), the initial CT imaging was normal (32, 34, 43, 49, 51, 56, 62, 63, 71, 73, 121, 123, 125, 127, 129, 142, 148), but on MRI imaging (n = 12) (17, 32, 43, 49, 51, 56, 71, 121, 142) or repeat imaging days later (n = 18) (49, 57, 58, 62, 121, 122, 127, 129, 148) eventually showed abnormal radiographic findings. Electroencephalogram (EEG) was performed in only a few cases (n = 35) (17, 25, 28, 32, 36, 41, 63, 71, 73, 111, 112, 122, 125, 130, 131, 136, 139, 142, 146, 148), but the majority (32/35, 91.4%) showed abnormal activity often described as increases or spikes in theta activity (n = 10) or bursts of sharp waves with diffuse or bitemporal slowing (n = 9). Other descriptions included mild or severe diffuse abnormalities (n = 3), periodic lateralized epileptiform discharge (n = 2), pronounced pathologic episodes (n = 1), left temporal spikes (n = 1), flat waves (n = 1), low frequencies of background activity (n = 3), metabolic encephalopathy (n = 1), or epileptiform activity (n = 1).
A viral-like syndrome with fever, rashes, and cytopenias was the next most common clinical syndrome, occurring in 22/225 (9.8%) transplant cases (29, 34, 64, 66, 76, 84, 106, 113, 115, 117, 135, 143–145). The time to presentation of this viral syndrome was a median of 21 days (range, 2–629 days) after transplantation. Other clinical syndromes occurred much less frequently and are summarized in Table 2.
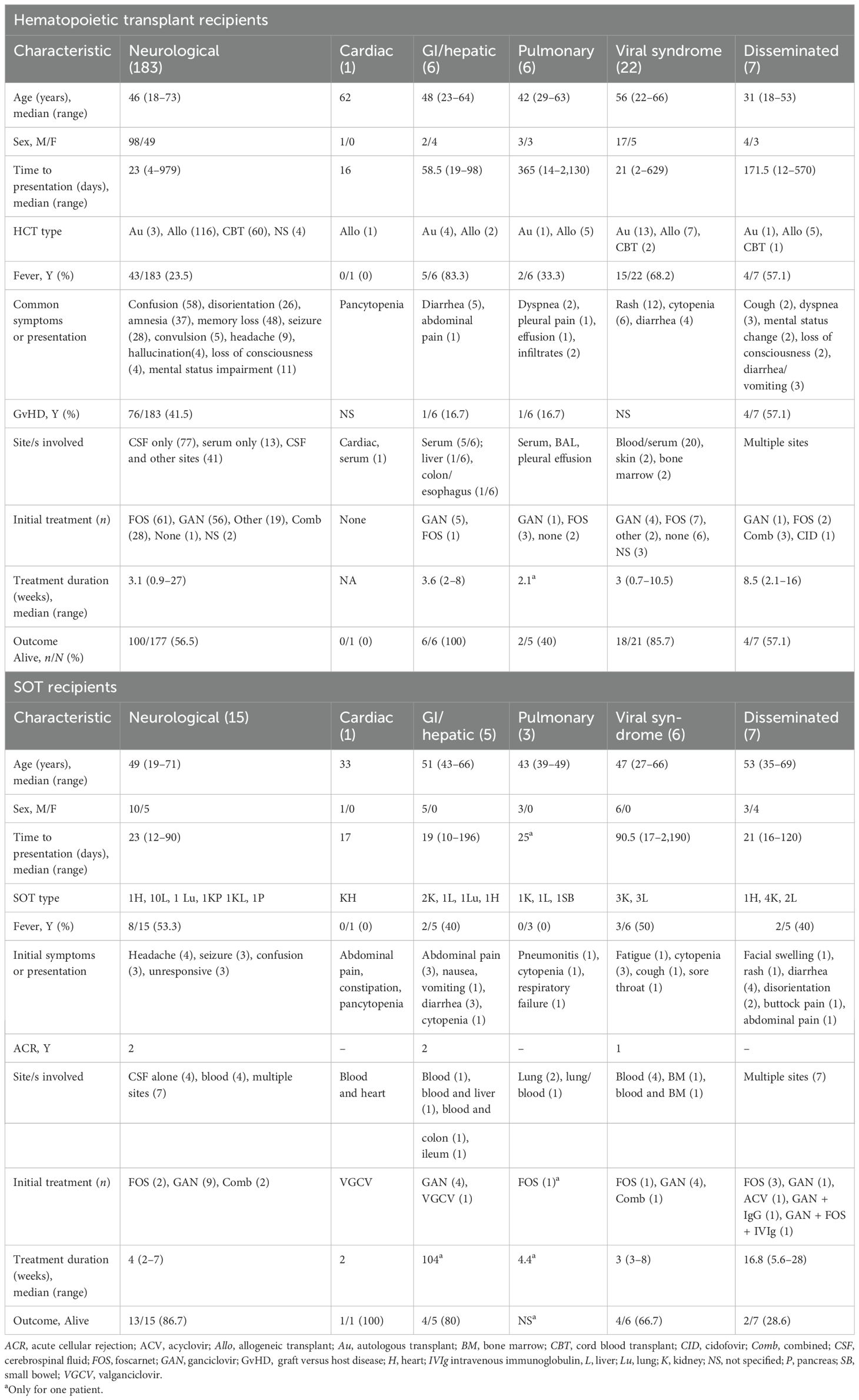
Table 2. Clinical syndromes of human herpes virus 6 (HHV-6) among hematopoietic cell transplant (HCT) and solid organ transplant (SOT) recipients.
3.1.1.3 Diagnosis
Laboratory diagnosis of HHV-6 was primarily through quantitative (n = 125) or qualitative (n = 63) polymerase chain reaction (PCR) DNA-based tests. Other less common tests included immunohistochemical (IHC) staining (n = 4) (11, 24, 26, 91), shell vial assay (n = 1) (33), metagenomic sequencing (n = 1) (141), and fluorescent in situ hybridization (n = 1) (91). The sites of HHV-6 DNA positivity included the cerebrospinal fluid (CSF) (n = 86), blood/serum (n = 32), lung (n = 1), and bone marrow (n = 1) alone or a combination of sites (n = 73).
For those with neurological disease, CSF cytologic analysis was reported in approximately half (83/183, 45.4%) of the cases (11, 15, 17, 19, 25, 28–33, 35, 36, 41–43, 47, 49, 51, 56–59, 62, 63, 70, 71, 73, 75, 79, 83, 89, 97, 98, 102, 103, 111, 112, 122–125, 129, 131, 136, 137, 140–142, 149). The CSF white blood cell (WBC) count (67/83, 80.7%) had a median of 6 cells/mm3 (range, 0–81 cells/mm3). CSF protein and glucose were carried out in 71/91 (78%) and 46/91 (50.5%) cases, respectively. The median CSF protein was 59.5 mg/dl (range, 71–384 mg/dl), while the median CSF glucose was 67 mg/dl (range, 46–133 mg/dl).
3.1.1.4 Treatment and outcomes
Treatment was described for 196/225 (87.1%) HCT recipients (11, 15, 17, 19, 22, 24–26, 28–36, 41–43, 45–49, 51, 53–59, 62, 63, 65, 66, 70–76, 78–81, 83–85, 87–89, 91–95, 97, 98, 102, 103, 110–113, 115, 117, 121–127, 129, 131, 135–143, 146–148, 165), including eight cases of symptomatic ciHHV-6. The initial drug of choice was either foscarnet (74/196, 37.8%) or ganciclovir (67/196, 34.2%). A smaller proportion of patients were started on combination therapy (31/196, 15.8%), which was commonly ganciclovir with foscarnet (n = 25). Other combinations included one each of ganciclovir with intravenous immunoglobulin (IVIg); ganciclovir, foscarnet, and IVIg; ganciclovir and acyclovir; foscarnet, plasmapheresis, and IVIg; foscarnet and IVIg; and foscarnet with valganciclovir. Other monotherapies (22/196, 11.2%) included acyclovir (n = 13, 58.6%), valganciclovir (n = 4, 18.2%), cidofovir (n = 3, 13.6%), valacyclovir (n = 1, 4.5%), and gamma globulin (n = 1, 4.5%). Treatment was not specified in two recipients (73, 139), while nine recipients were not treated (26, 144, 145). Overall, the median length of treatment was 3 weeks (range, 0.7–27 weeks). Of the 223 patients with reported outcomes, 133 (59.6%) were alive at the last follow-up, but 41/133 (30.8%) had persistent neurological deficits. HHV-6-attributed mortality was reported in 20/89 (22.5%) cases.
3.1.2 Solid organ transplantation
3.1.2.1 Epidemiology and patient characteristics
There were 33 reports (16, 18, 20, 21, 23, 27, 37–40, 50, 60, 67, 69, 77, 86, 96, 99–101, 104, 105, 108, 109, 114, 116, 119, 120, 128, 132–134, 143) that described 37 SOT recipients with HHV-6 infection. The majority of the reports originated from Europe (n = 17, 51.5%) (18, 21, 23, 27, 37, 39, 60, 67, 77, 96, 100, 108, 109, 114, 116, 133, 143) or the USA (n = 11, 33.3%) (16, 38, 40, 69, 86, 99, 104, 105, 119, 120, 128). Most of the SOT recipients were men (28/37, 75.7%), and the median age was 49 years (range, 19–71 years) (Table 1). The majority were recipients of liver (n = 17, 45.9%) (16, 23, 38, 40, 50, 77, 99, 104, 109, 119, 120, 128, 132, 133) or kidney (n = 10, 27%) (20, 37, 60, 69, 96, 100, 101, 108, 114, 116) transplants. Heart (n = 3, 8.1%) (27, 86, 105), lung (n = 2, 5.4%) (21, 67), small bowel (n = 1, 2.7%) (143), pancreas (n = 1, 2.7%) (134), and combined transplants (n = 3, 8.1%) (18, 39, 67) were less common. Maintenance immunosuppression was detailed in 25 recipients, of whom 18 (72%) were on tacrolimus, 5 (20%) on cyclosporine, and 2 (8%) on azathioprine.
3.1.2.2 Clinical presentation and syndromes
Less than half of the SOT recipients (15/37, 40.5%) with HHV-6 infection developed fever (18, 21, 39, 60, 69, 86, 96, 99, 104, 108, 109, 114, 120, 128, 133). The median time to presentation was 23 days (range, 10–2,190 days) after transplantation. Acute cellular rejection (ACR) prior to infection was reported in 7/8 (87.5%) recipients; for four patients, ACR occurred 0–83 days prior to symptom onset. The HHV-6 subtype was described in 15 patients (23, 40, 50, 67, 100, 101, 104, 105, 109, 114, 120, 132, 134, 143) and, of these, 7 (46.7%) were variant B, 6 (40%) were variant A, and 2 (13.3%) were symptomatic ciHHV-6A (23, 100). The most common clinical syndrome was neurological (15/37, 40.5%) (16, 18, 21, 38–40, 50, 77, 86, 99, 120, 128, 132–134), followed by disseminated disease (7/37, 18.9%) (23, 100, 101, 105, 109, 114, 116) (Table 1).
Those who developed encephalitis had a median age of 49 years (range, 19–71 years) and were mostly men (10/15, 66.7%). Two-thirds (10/15, 66.7%) presented with neurological symptoms at the onset, including headache (n = 4), confusion (n = 3), unresponsiveness (n = 4), or seizures (n = 3). The remaining five patients (33.3%) initially presented with fever and rash before subsequently developing neurological symptoms. Fever occurred in approximately half (8/15, 53.3%) of the patients. The median time to presentation was 23 days (range, 12–90 days) after transplantation. Brain MRI was the initial diagnostic imaging in 11 patients, with the majority (9/11, 81.8%) showing abnormalities including non-enhancing lesions in the temporal lobe or the hippocampus (n = 5) (16, 18, 21, 77, 128), cortical and subcortical hyperintensity (n = 1) (40), ischemic changes (n = 2) (50, 132), or nonspecific subcortical white matter areas of abnormal intensity, most prominent in the right parietal region (n = 1) (86). In three patients, CT was reported as normal (18, 21, 77), but abnormal findings were observed on MRI performed on the same day.
Those who had disseminated disease (7/37, 18.9%) had a median age of 53 years (range, 35–69 years). The presenting symptoms were varied, and HHV-6 was isolated from multiple sites including the CSF (5), the visceral (e.g., duodenal, colonic, and cardiac) tissues (6), and the blood (5). Other less common syndromes are summarized in Table 2.
3.1.2.3 Diagnosis
HHV-6 was diagnosed using different methods, including qualitative (n = 13) or quantitative (n = 16) PCR, IHC staining (n = 6), shell vial assay or culture (n = 7), and next-generation sequencing (n = 2). In some recipients, HHV-6 was positive from a single site, including the CSF (4/37, 10.8%), the blood/serum (9/37, 24.3%), the lung (2/37, 5.4%), the bone marrow (1/37, 2.7%), and the gastrointestinal (GI) tract (2/37, 5.4%). In others, HHV-6 was identified from multiple sites (19/37, 51.3%), including the CSF (n = 11), the blood/serum (n = 11), and in body tissues such as the colon (n = 4), the duodenum (n = 2), the bone marrow (n = 3), and the liver (n = 2).
CSF analysis was performed in a few studies (15/33, 45.4%) (16, 18, 21, 23, 50, 77, 86, 99, 100, 109, 114, 116, 128, 132, 134). The median CSF WBC, protein, and glucose were 11 cells/mm3 (range, 0–90 cells/mm3), 28 mg/dl (range, 87–101 mg/dl), and 75 mg/dl (64–78 mg/dl), respectively.
3.1.2.4 Treatment and outcomes
Antiviral treatment was provided to 33/37 (89.2%) SOT recipients (16, 18, 21, 23, 27, 37, 38, 40, 50, 60, 67, 69, 77, 86, 96, 99–101, 104, 105, 108, 109, 114, 116, 119, 120, 128, 132–134, 143). Initial monotherapy with ganciclovir (18/33, 54.5%) was the most common antiviral therapy, followed by foscarnet (7/33, 21.2%). Combination therapy with both drugs was started in 5/33 (15.2%) patients. The median length of treatment was 4 weeks (range, 2–104 weeks). Many were alive on the last follow-up (24/36, 66.7%), but at least 4/16 (25%) had neurological sequelae. The outcome was unknown for one patient (20). HHV-6-attributable mortality was reported only in one patient (1/12, 8.3%).
3.1.3 CAR-T
3.1.3.1 Epidemiology and patient characteristics
There were six cases (52, 68, 107) of HHV-6 reported after CAR-T therapy for underlying diffuse large B-cell lymphoma (DLBCL). Half of the CAR-T recipients were men. The median age was 48.5 years (range, 31–69 years) (Table 1). All patients experienced cytokine release syndrome (CRS), of whom two were given tocilizumab and one dexamethasone. Four developed immune effector cell-associated neurotoxicity syndrome (ICANS).
3.1.3.2 Clinical presentation and syndromes
The time to presentation after CAR-T was described only in three patients, with a median time of 6 days (range, 5–29 days). The majority presented with fever (5/6, 83.3%); for 3/6 (50%) patients, delirium and memory loss occurred later. All were reported to have neurological involvement, with 2/6 (33.3%) developing myelitis.
3.1.3.3 Diagnosis
HHV-6 subtyping was performed in 3/6 (50%) patients, all with sub-type B. HHV-6 was detected in the CSF in all cases, either by quantitative DNA PCR (n = 2), qualitative PCR (n = 1), or next-generation sequencing (n = 3). HHV-6 in the blood or plasma was elevated in two patients. MRI was performed in 3/6 (50%), which showed T2 increased activity in the limbic system (2/3, 66.7%) or hyperintensity in the white matter and brainstem (1/3, 33.3%). Two other patients had CT imaging, the results of which were normal. EEG was reported in only one patient and showed diffuse generalized slowing.
3.1.3.4 Treatment and outcomes
Treatment with ganciclovir was initiated in 2/6 (33.3%) patients, who both survived. One patient who was given foscarnet and IVIg died on day 24, while another, who was on foscarnet prophylaxis at the time of illness, survived. This patient (69) completed a course of 24-day ganciclovir and foscarnet with additional IVIg for 1 week. The remaining two patients who were not on prophylaxis at the time of HHV-6 disease died before antiviral treatment could be initiated.
3.2 Cohorts
3.2.1 Hematopoietic transplant
There were 25 cohort (34, 42, 45, 51, 58, 126, 138, 146, 147, 149, 151, 154, 157, 158, 161, 162, 164–166, 168–170, 173–175) and three case–control studies (94, 131, 153) that reported on HHV-6. Of these, the majority (22/28, 78.6%) were single-center studies from Japan (n = 9) (51, 58, 126, 138, 146, 164, 169, 173, 175), the USA (n = 7) (42, 149, 153, 154, 157, 170, 174), Sweden (n = 3) (131, 161, 162), France (n = 2) (34, 158), or Egypt (168). The remaining papers were multicenter studies from Japan (45, 94, 147, 165, 166) and France (151). Almost all included only allogeneic HCT recipients, but three studies (153, 158, 168) included autologous HCT recipients. Most of the studies (n = 24) (34, 45, 51, 58, 94, 126, 131, 138, 146, 147, 149, 151, 153, 154, 157, 158, 161, 162, 166, 169, 170, 173–175) reported on the incidence of asymptomatic HHV-6 reactivation or clinical disease, which ranged from 1% to 95%. The median time to HHV-6 diagnosis also varied, with most occurring within the first 30 days of transplantation, except in three studies where the median values were 40 (153), 60 (149), and 70 days (161), respectively. Most of the studies described encephalitis or encephalomyelitis (15/18) (45, 58, 94, 126, 131, 147, 149, 157, 162, 164–166, 169, 173, 175) or possible central nervous system (CNS) dysfunction (174), while a few reported cases of pneumonitis (153), bone marrow suppression (154), hepatitis (162), and rashes (151) among their cohorts of patients (Table 3). Concomitant cytomegalovirus (CMV) reactivation was reported in 10 studies (138, 146, 151, 153, 154, 158, 161, 162, 170, 175), of whom eight had confirmed disease (one pneumonitis and seven not specified) and 91 were viremic.
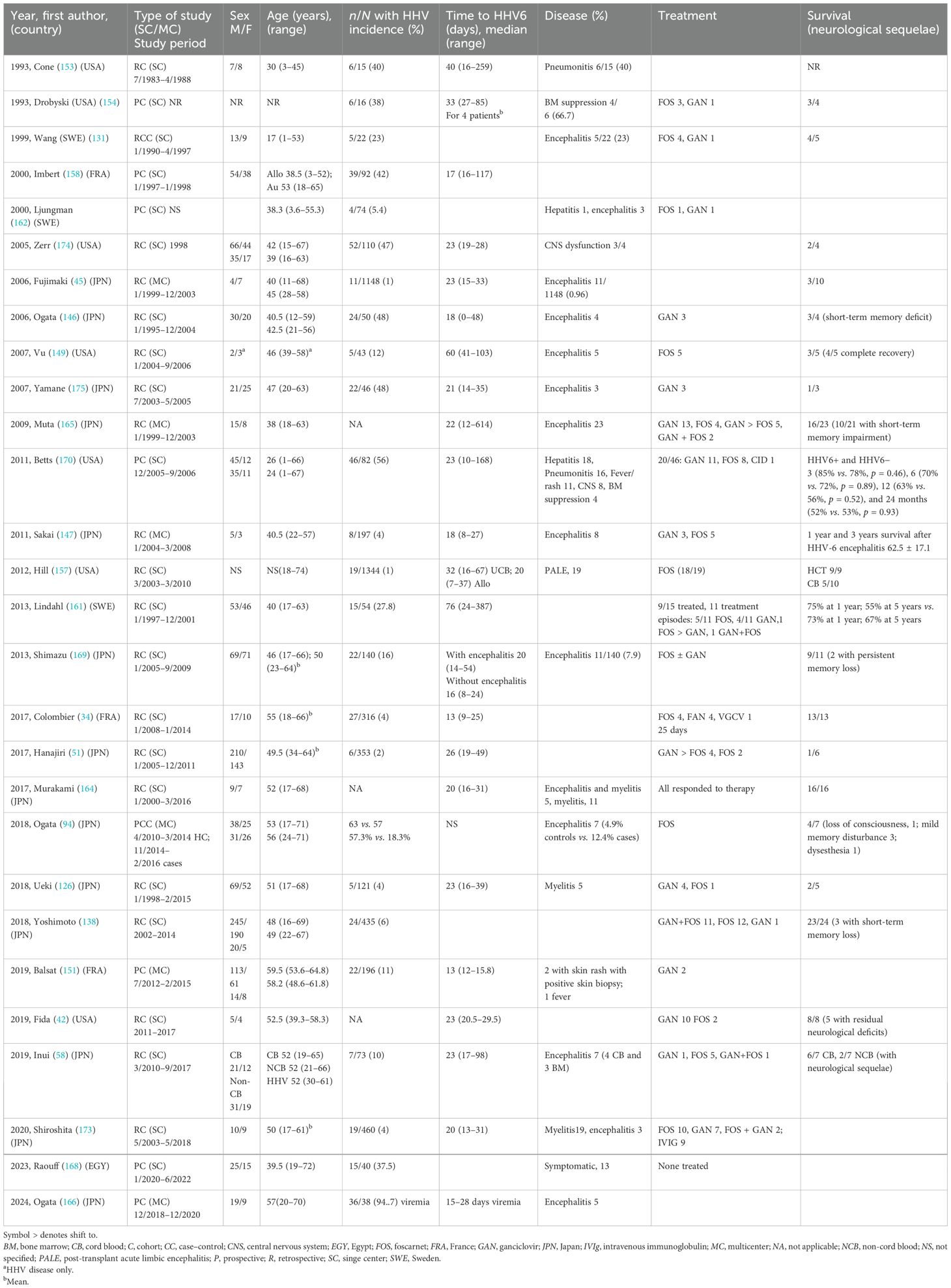
Table 3. Characteristics of cohort studies of human herpes virus 6 (HHV-6) among hematopoietic transplant recipients.
The occurrence of GvHD was reported only in 14 studies (42, 58, 94, 126, 146, 149, 157, 161, 165, 166, 168–170, 173) and ranged from none (grade 0) to severe (grade IV). Treatment choices were described in 22 studies (34, 42, 51, 58, 94, 126, 131, 138, 146, 147, 149, 151, 154, 157, 161, 162, 164, 165, 169, 170, 173, 175), with ganciclovir or foscarnet being the most often used. The duration of therapy was specified in only eight studies (34, 42, 58, 131, 147, 151, 157, 173), and the median length of treatment was as short as 6 days (157) to as long as 41 days (147). The survival outcomes were reported in 21/28 (75%) studies (34, 42, 45, 51, 58, 94, 126, 131, 138, 146, 147, 149, 154, 157, 161, 162, 164, 165, 169, 170, 175) (Table 3).
3.2.2 Solid organ transplant
There were eight single-center cohorts [four prospective (160, 167, 171, 172) and four retrospective (152, 155, 156, 163)] and one case–control study (150) on SOT recipients. Many studies included liver transplant recipients only (152, 163, 167, 171, 172), while the remainder included kidney (150, 155), kidney–pancreas (155), or lung and heart–lung (160) allografts (Table 4).
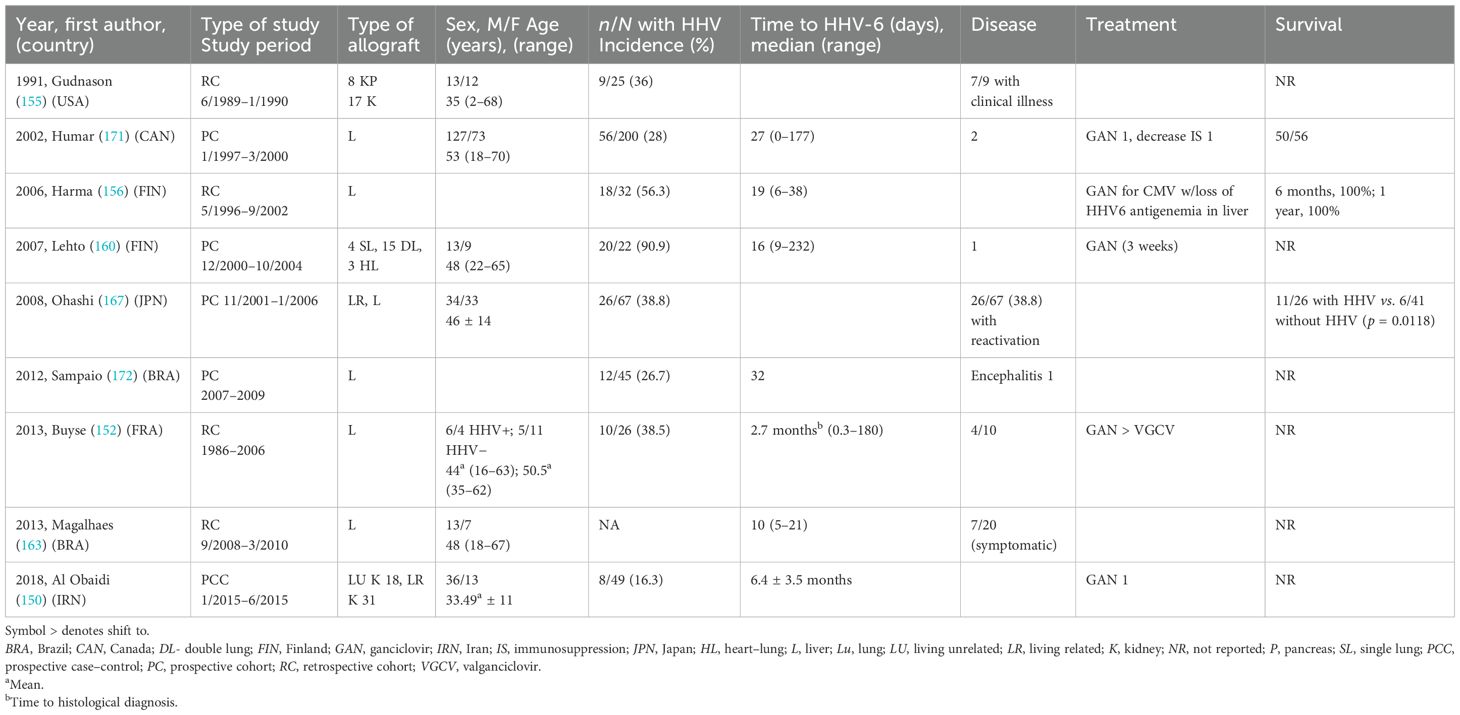
Table 4. Characteristics of cohort studies of human herpes virus 6 (HHV-6) among solid organ transplant recipients.
All except one study (163) provided information on the incidence of HHV-6 infection (which were mostly asymptomatic reactivation), which ranged from 26.7% to 91%. The median time to HHV-6 infection for the majority (156, 160, 163, 171, 172) was within the first month of transplantation, except in two studies where the median times were 2.7 (152) and 6.4 months (150). ACR that occurred in close proximity to the HHV-6 infection was described in five studies (150, 156, 167, 171, 172), as was CMV co-infection (155, 156, 160, 171, 172).
Specific HHV-6 treatment was reported in only four studies (150, 152, 160, 171), and the duration was mentioned only in one (160). Overall survival was described in three studies (156, 167, 171) and appeared high, ranging from 42% (11/26) (167) to 100% (156).
3.2.3 Other cohorts
Two other cohorts described HHV-6 infections in mixed SOT and allogeneic HCT recipients (143) and among CAR-T recipients (159).
The study by Potenza et al. (143) was the only single-center prospective study that evaluated the prevalence of ciHHV-6 among 343 mixed SOT (303 liver, 15 kidney–liver, 1 heart–liver, 3 liver–small bowel, and 21 small bowel) and 78 HCT recipients. Prevalence was reported in 7/52 (13.4%) SOT and 3/16 (18%) HCT recipients. In addition, CMV co-infection occurred in 19/52 (36.5%) SOT and 4/16 (25%) HCT recipients.
The only study that evaluated HHV-6 among CAR-T recipients (159) included both a prospective and a retrospective cohort, which comprised 89 and 626 recipients, respectively. Of the prospective cohort, 5/89 (5.6%) developed HHV-6 variant B within a median of 21 days (range, 14–42 days) of CAR-T. All five patients with HHV-6 developed CRS, and three of the five experienced ICANS. In the retrospective cohort, CSF testing was carried out in 34 patients due to suspicion of neurological disease. Of these, there was only one positive HHV-6 PCR test with a viral load of 1,100 copies/ml, giving an overall prevalence of 0.16%. The patient was given steroids and responded well; he was not treated with an antiviral due to the lack of typical features of HHV-6 encephalitis.
4 Discussion
Through this systematic review of HHV-6 in SOT, HCT, and CAR-T recipients, we synthesized information from more than 200 cases and 38 cohort studies and draw attention to the following observations: 1) the rates of clinical and asymptomatic reactivation are highly variable; 2) encephalitis is the most common but not the only clinical syndrome; 3) fever occurrence is highly variable, ranging from 30% to 80%; 4) diagnosis is most often secured using molecular methods in addition to compatible clinical disease; 5) MRI is the imaging modality of choice for neurological disease; 6) antiviral drug preference differs between HCT and SOT; and 7) there is a high rate of long-term neurological sequelae.
Based on our review, the incidence of HHV-6 reactivation is variable and can be as high as 95% in either the SOT or the HCT populations. This is not surprising as HHV-6, similarly to other human herpes viruses, exhibits lifelong latency and tends to reactivate with immunosuppression. The high rate of HHV-6 reactivation is documented in studies from centers that have systematically performed HHV-6 PCR testing, either prospectively or retrospectively in stored clinical samples (146, 174, 175). The vast majority of HHV-6 reactivation that was detected through surveillance is asymptomatic and transient. Hence, the monitoring of HHV-6 is controversial, as asymptomatic or subclinical reactivation happens very often, occurring only transiently, with no impact on the clinical outcomes, and leading to unnecessary treatment with antivirals. As shown in Tables 3 and 4, clinical HHV-6 disease did not occur (138) or occurred in a much smaller proportion of patients, despite the high overall incidence of HHV-6 reactivation in both HCT (94, 140, 146, 151) and SOT recipients (155, 160, 171, 172). Thus, the clinical utility of routine HHV-6 surveillance is debated. More research is needed to identify patients at high risk of clinical disease and to define the optimal timing and frequency of monitoring for HHV-6 infections in these high-risk patients.
This review confirms that the majority of clinical HHV-6 disease (204/268, 76.1%) in transplant and CAR-T populations manifests as a neurological syndrome, primarily encephalitis. These patients presented within the first month of transplantation with varied neurological symptoms, including confusion, amnesia, and seizures, and the majority had abnormal findings on initial imaging. We propose that HHV-6 viremia in the presence of confusion, amnesia, and seizures plus limbic involvement seen on MRI in a post-transplant or CAR-T recipient is defined as probable HHV-6, until proven otherwise. Notably, many of those who developed a neurological syndrome were HHV-6B. While this could be due to reporting bias and the overall higher prevalence of HHV-6B, it highlights that it has a similar neurotrophic property to that of HHV-6A (176). Indeed, in one study, it was proposed that both viruses are equally neurotrophic but that HHV-6A may potentially be more virulent (177). Whether these in vitro data translate into worse outcomes for those infected with HHV-6A remains to be seen. Interestingly, only a small subset of patients (15 HCT and 1 SOT) was specifically reported as HHV-6 PALE. HHV-6 PALE was first described in HCT recipients (11) and has not been formally defined but is the primary involvement of the limbic system driven by HHV-6 infection. One study (148) proposed characterizing it as a distinct entity with acute alterations in mental status, prominent amnesia, and unexplained seizures. Based on this definition, and compatible with the MRI findings, at least 14 other cases fit the criteria for HHV-6 PALE; however, these cases were simply described as “encephalitis.” This is worth mentioning as it highlights the under-recognition of this entity, as well as the need to more clearly define this category of patients. Early recognition of this specific syndrome may have clinical implications for treating clinicians—such as the association with HHV-6, the prevention of progressive disease, and the potential avoidance of neurological sequelae with early antiviral therapy. Among the HCT recipients, the majority with encephalitis were either allogeneic or cord blood recipients, which may be due to their greater level of immunosuppression compared with autologous transplants. The risk factors for HHV-6 reactivation and disease included the use of a mismatched donor and steroids (146, 174). For the patients in this review, approximately half were mismatches or unrelated donors (50/92, 54.3%) or were given steroids for GvHD (76/183, 41.5%). There may be other host factors related to the complex immunological CD8 T-cell responses, which are dampened by transplant-related immunosuppression that may have significantly influenced the HHV-6 reactivation (178); however, these were not explored in this review.
When there is suspicion of encephalitis, we advocate MRI as the preferred imaging of choice. MRI was able to capture findings not initially seen on the CT scan, even when done on the same day. Imaging evidence involving one or both hippocampi and adjacent medial temporal lobe structures of the limbic system, including the amygdalae and parahippocampal gyri, is suggestive of “limbic encephalitis” and should help narrow the differential diagnosis (148). Although other causes such as paraneoplastic malignancies, neurosyphilis, herpes simplex, and varicella zoster have been implicated in limbic encephalitis, HHV-6 should be a leading impression given the right clinical context.
Other syndromes of HHV-6 occurred but were much less common. Disseminated disease was infrequently seen among both SOT and HCT recipients. For HCT recipients, the median time to presentation of disseminated disease was beyond the period of engraftment (e.g., 171 days). Of the reported cases, 5/8 (62.5%) developed aGvHD and were treated with steroids. We propose that this increased level of immunosuppression contributed to the risk of disseminated disease. However, given the limited number, more definite conclusions cannot be made. In contrast, disseminated disease among SOT recipients occurred within the first month of transplantation. Although it is unclear what drove early disseminated disease in these patients, we hypothesize that the choice of induction immunosuppression played a role.
Fever was more frequent among CAR-T recipients and occurred in a third of other patients. As such, for individuals without a fever, other concomitant symptoms of HHV-6-related disease should be sought. Rashes, diarrhea, and cytopenias were commonly reported with other HHV-6 syndromes. Rashes from a viral exanthem such as HHV-6 are difficult to distinguish from aGvHD based on clinical appearance. In most cases, a biopsy is necessary to determine the etiology of the rashes as the approach to treatment differs.
The diagnosis of HHV-6 was secured primarily using molecular methods (e.g., DNA PCR) in the majority of patients. Confirmation via tissue diagnosis was rarely done, even among SOT recipients. Although the isolation of HHV-6 in cell cultures is the reference method and unambiguously demonstrates the presence of infectious viral particles, it is poorly sensitive, time-consuming, expensive, and unavailable in most centers and thus cannot be used for routine diagnosis (179). However, HHV-6 DNAemia by itself can be exceptionally tricky to interpret in the context of ciHHV-6. ciHHV-6 diagnosis previously required hair follicle or molecular cytogenetic analysis, which is impractical in the clinical setting. Experts propose that ciHHV-6 can be assumed in the presence of >1 million genomic copies of HHV-6 per milliliter of whole blood (10). In our review, the 10 ciHHV-6 cases were treated because all had symptoms consistent with clinical disease. However, the literature is replete with reports of asymptomatic high-level HHV-6 viremia (10, 180–182), with delayed realization of the possibility of ciHHV-6 and the need for treatment (10). As such, careful consideration for ciHHV-6 should be made when there is high-level viremia in an asymptomatic patient.
Interestingly, ganciclovir was the primary therapy used in 54.5% (18/33) of SOT recipients, while foscarnet (74/196, 37.8%) and ganciclovir (67/196, 34.2%) were almost equally used in HCT recipients. Ganciclovir, a nucleoside analogue, is likely preferred in SOT as it has fewer nephrotoxic effects than foscarnet. Foscarnet also causes multiple electrolyte imbalances and QTc prolongation, which poses increased difficulty in terms of monitoring, particularly among kidney allografts. In contrast, ganciclovir and valganciclovir are generally avoided in HCT recipients due to their myelosuppressive effects, which can delay marrow recovery. Unfortunately, the optimal drug of choice and the duration of therapy against HHV-6 are unknown and need to be defined. To highlight the key features of HHV-6, a summary is provided (Table 5).
Although the attributable mortality from HHV-6 was modest (22/107, 20.6%), at least a third of patients (45/151, 29.8%) had some neurological deficits at the last follow-up, long after resolution of the clinical disease. The incidence may even be higher, as the neurological outcome was unspecified in a fourth of cases (38/151, 25.2%). Morbidity from HHV-6 disease can be debilitating; as such, HHV-6 neurological involvement should be recognized early and treated aggressively.
Other unique observations from this systematic review deserve mention. Firstly, the higher proportion of men could suggest that they are disproportionately affected by HHV-6. Although this may be a reflection of the overall gender disparity of the transplant population, which is predominantly male, this merits further exploration. Secondly, CMV co-infection was more frequently reported in hematopoietic than in SOT recipients, which mirrors the expectations in the larger transplant population. This review also encompasses a period where CMV surveillance and preemptive therapy comprise a more common CMV prevention strategy in hematopoietic stem cell transplants compared with universal prophylaxis among SOT recipients. However, most case reports did not explicitly state whether CMV was evaluated, and the risk of CMV reactivation may have been underreported overall. Finally, HHV-6A appears to be as prevalent as HHV-6B among SOT recipients, which challenges the notion that HHV-6B is more common in the transplant population. However, as the numbers are limited, no definitive conclusions can be made, and this must be interpreted with caution.
Our study has several limitations. Firstly, there are duplications across studies (e.g., case reports and cohorts) as some cases from larger cohorts were described in detail. Secondly, we excluded asymptomatic HHV-6 and ciHHV-6, but these could have been inadvertently misclassified as asymptomatic when true disease was present. Alternatively, other viral infections (e.g., CMV and EBV) or syndromes (e.g., ICANS) can present similarly, and symptoms attributable to HHV-6 could have been from these other causes. Important publications in non-English journals may have been missed as the language was limited to English. Reports lacking patient-level data, pediatric transplants, and abstracts of cohort studies were also excluded; thus, our numbers likely underestimated the true magnitude of HHV-6. Finally, due to the heterogeneity of cohort and case–control studies, we were unable to perform a meta-analysis. A formal assessment of risk factors predictive of HHV-6 disease, for example, may be helpful for transplant clinicians, and a future analysis needs to be performed. Despite these, our study is the first to provide cumulative data on HHV-6 for transplant and CAR-T recipients.
5 Conclusions
Our review highlights that HHV-6 subclinical reactivation frequently occurs. HHV-6 clinical disease is more commonly reported among HCT compared with SOT or CAR-T recipients. However, only six CAR-T cases and one cohort study were included, prohibiting conclusions for this group. Neurological disease in the form of encephalitis is the most frequent clinical syndrome. Early recognition of limbic involvement either through the triad of confusion, amnesia, and seizures or through compatible MRI findings may help with the early identification of HHV-6. Diagnosis is secured through molecular methods, although HHV-6 detection per se may not necessarily indicate disease. Extremely high viremia needs to be interpreted with caution in the context of ciHHV-6, which may not warrant treatment. HHV-6-associated mortality is modest; however, the neurological sequelae can be disabling and cause significant morbidity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CA: Data curation, Writing – review & editing, Conceptualization, Writing – original draft. RR: Writing – original draft, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Michell McGinnis, Mayo Clinic librarian, for her invaluable assistance with the literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2025.1641157/full#supplementary-material
References
1. Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. (1988) 1:1065–7. doi: 10.1016/S0140-6736(88)91893-4
2. Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. (1989) 27:651–3. doi: 10.1128/jcm.27.4.651-653.1989
3. Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. (2014) 159:863–70. doi: 10.1007/s00705-013-1902-5
4. De Bolle L, Naesens L, and De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. (2005) 18:217–45. doi: 10.1128/CMR.18.1.217-245.2005
5. Uesugi H, Shimizu H, Maehara T, Arai N, and Nakayama H. Presence of human herpesvirus 6 and herpes simplex virus detected by polymerase chain reaction in surgical tissue from temporal lobe epileptic patients. Psychiatry Clin Neurosci. (2000) 54:589–93. doi: 10.1046/j.1440-1819.2000.00758.x
6. Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, Armstrong RW, et al. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. (2005) 40:890–3. doi: 10.1086/427944
7. Torre D, Speranza F, Martegani R, Ferrante P, Omodeo-Zorini E, Mancuso R, et al. Meningoencephalitis caused by human herpesvirus-6 in an immunocompetent adult patient: case report and review of the literature. Infection. (1998) 26:402–4. doi: 10.1007/BF02770845
8. Hall CB, Caserta MT, Schnabel K, Shelley LM, Marino AS, Carnahan JA, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. (2008) 122:513–20. doi: 10.1542/peds.2007-2838
9. Hall CB, Caserta MT, Schnabel KC, Shelley LM, Carnahan JA, Marino AS, et al. Transplacental congenital human herpesvirus 6 infection caused by maternal chromosomally integrated virus. J Infect Dis. (2010) 201:505–7. doi: 10.1086/650495
10. Lee SO, Brown RA, and Razonable RR. Chromosomally integrated human herpesvirus-6 in transplant recipients. Transplant Infect Disease. (2012) 14:346–54. doi: 10.1111/j.1399-3062.2011.00715.x
11. Drobyski WR, Knox KK, Majewski D, and Carrigan DR. Brief report: Fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. New Engl J Med. (1994) 330:1356–60. doi: 10.1056/NEJM199405123301905
12. Toomey D, Phan TL, Phan T, Hill JA, and Zerr DM. Viral encephalitis after hematopoietic cell transplantation: A systematic review. Transplant Cell Ther. (2023) 29:636.e1–.e9. doi: 10.1016/j.jtct.2023.06.022
13. Kampouri E, Handley G, and Hill JA. Human herpes virus-6 (HHV-6) reactivation after hematopoietic cell transplant and chimeric antigen receptor (CAR)- T cell therapy: A shifting landscape. Viruses. (2024) 16:1–18. doi: 10.3390/v16040498
14. Stathis CJ, Zhu H, Carlin K, Phan TL, Toomey D, Hill JA, et al. A systematic review and meta-analysis of HHV-6 and mortality after hematopoietic cell transplant. Bone Marrow Transpl. (2024) 59:1683–93. doi: 10.1038/s41409-024-02398-w
15. Aoki K, Arima H, Kato A, Hashimoto H, Tabata S, Matsushita A, et al. Human herpes virus 6-associated myelitis following allogeneic bone marrow transplantation. Ann Hematol. (2012) 91:1663–5. doi: 10.1007/s00277-012-1444-z
16. Baldwin K. Ganciclovir-resistant Human herpesvirus-6 encephalitis in a liver transplant patient: A case report. J NeuroVirology. (2011) 17:193–5. doi: 10.1007/s13365-011-0019-4
17. Belford A, Myles O, Magill A, Wang J, Myhand RC, and Waselenko JK. Thrombotic microangiopathy (TMA) and stroke due to human herpesvirus-6 (HHV-6) reactivation in an adult receiving high-dose melphalan with autologous peripheral stem cell transplantation. Am J Hematology. (2004) 76:156–62. doi: 10.1002/ajh.20068
18. Benito N, Ricart MJ, Pumarola T, Marcos MA, Oppenheimer F, and Moreno Camacho A. Infection with human herpesvirus 6 after kidney-pancreas transplant. Am J Transplantation. (2004) 4:1197–9. doi: 10.1111/j.1600-6143.2004.00449.x
19. Bethge W, Beck R, Jahn G, Mundinger P, Kanz L, and Einsele H. Successful treatment of human herpesvirus-6 encephalitis after bone marrow transplantation. Bone Marrow Transplantation. (1999) 24:1245–8. doi: 10.1038/sj.bmt.1702065
20. Bisso IC, Rey LN, Anaya JG, Niveyro PXI, Ceballos IF, and Heras ML. Herpesvirus 6 tracheobronchitis. Medicina (Argentina). (2024) 84:185.
21. Bollen AE, Wartan AN, Krikke AP, and Haaxma-Reiche H. Amnestic syndrome after lung transplantation by human herpes virus-6 encephalitis [2. J Neurol. (2001) 248:619–20. doi: 10.1007/s004150170142
22. Bommer M, Pauls S, and Greiner J. Challenging complications of treatment–human herpes virus 6 encephalitis and pneumonitis in a patient undergoing autologous stem cell transplantation for relapsed Hodgkin’s disease: a case report. Virol J. (2009) 6:111. doi: 10.1186/1743-422X-6-111
23. Bonnafous P, Marlet J, Bouvet D, Salamé E, Tellier AC, Guyetant S, et al. Fatal outcome after reactivation of inherited chromosomally integrated HHV-6A (iciHHV-6A) transmitted through liver transplantation. Am J Transplantation. (2018) 18:1548–51. doi: 10.1111/ajt.14657
24. Brennan Y, Gottlieb DJ, Baewer D, and Blyth E. A fatal case of acute HHV-6 myocarditis following allogeneic haemopoietic stem cell transplantation. J Clin Virol. (2015) 72:82–4. doi: 10.1016/j.jcv.2015.09.013
25. Camus V, Bouwyn JP, Chamseddine A, Lenain P, Ahtoy P, Stamatoullas A, et al. Human herpesvirus-6 acute limbic encephalitis after unrelated umbilical cord blood transplantation successfully treated with ganciclovir. Bone Marrow Transplantation. (2015) 50:1385–7. doi: 10.1038/bmt.2015.159
26. Carrigan DR, Drobyski WR, Russler SK, Tapper MA, Knox KK, and Ash RC. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. (1991) 338:147–9. doi: 10.1016/0140-6736(91)90137-E
27. Cervera C, Marcos MA, Linares L, Roig E, Benito N, Pumarola T, et al. A prospective survey of human herpesvirus-6 primary infection in solid organ transplant recipients. Transplantation. (2006) 82:979–82. doi: 10.1097/01.tp.0000229938.12722.ee
28. Chamberlain MC and Chowdhary S. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. (2008) 70:491–2; author reply 2-3. doi: 10.1212/01.wnl.0000304028.19061.46
29. Chan PKS, Peiris JSM, Yuen KY, Liang RHS, Lau YL, Chen FE, et al. Human herpesvirus-6 and human herpesvirus-7 infections in bone marrow transplant recipients. J Med Virol. (1997) 53:295–305. doi: 10.1002/(SICI)1096-9071(199711)53:3<295::AID-JMV20>3.0.CO;2-F
30. Chapuis A, Chabrot C, Mirand A, Poirier P, and Nourrisson C. Encephalitis caused by an unusual human herpes virus type 6 and Toxoplasma gondii co-infection in a cord blood transplant recipient. Int J Infect Diseases. (2016) 46:79–81. doi: 10.1016/j.ijid.2016.04.002
31. Cheema A, Katta J, Velez AP, Medveczky M, Medveczky PG, Quilitz R, et al. Encephalitis and inherited HHV-6: Encephalitis case report. Infect Dis Clin Practice. (2012) 20:419–21. doi: 10.1097/IPC.0b013e3182506ec7
32. Chordia P and Chandrasekar P. Status epilepticus due to severe HHV-6 encephalitis in an allogeneic stem cell transplant recipient. Mediterr J Hematol Infect Diseases. (2014) 6. doi: 10.4084/mjhid.2014.008
33. Cole PD, Stiles J, Boulad F, Small TN, O’Reilly RJ, George D, et al. Successful treatment of human herpesvirus 6 encephalitis in a bone marrow transplant recipient. Clin Infect Diseases. (1998) 27:653–4. doi: 10.1086/517145
34. Colombier MA, Amorim S, Salmona M, Thieblemont C, Legoff J, and Lafaurie M. HHV-6 reactivation as a cause of fever in autologous hematopoietic stem cell transplant recipients. J Infection. (2017) 75:155–9. doi: 10.1016/j.jinf.2017.05.011
35. Cury RG and Lopez WO. Bilateral striatal lesion due to herpesvirus-6 infection. J Neurological Sci. (2015) 358:538–9. doi: 10.1016/j.jns.2015.10.015
36. de Labarthe A, Gauthert-Dejean A, Bossi P, Vernant JP, and Dhedin N. HHV-6 variant A meningoencephalitis after allogeneic hematopoietic stem cell transplantation diagnosed by quantitative real-time polymerase chain reaction. Transplantation. (2005) 80:539. doi: 10.1097/01.tp.0000168339.08246.73
37. Delbridge MS, Karim MS, Shrestha BM, and McKane W. Colitis in a renal transplant patient with human herpesvirus-6 infection. Transplant Infect Disease. (2006) 8:226–8. doi: 10.1111/j.1399-3062.2006.00143.x
38. DeRon N and Hunter LK. Hhv-6 infection in a 19-year-old liver transplant recipient - much more than Roseola! J Gen Internal Med. (2023) 38:S474–S5. doi: 10.1016/j.idcr.2023.e01863
39. Dharancy S, Crombe V, Copin MC, Boleslawski E, Bocket L, Declerck N, et al. Fatal hemophagocytic syndrome related to human herpesvirus-6 reinfection following liver transplantation: A case report. Transplant Proc. (2008) 40:3791–3. doi: 10.1016/j.transproceed.2008.05.083
40. Dougherty A, DeRon N, and Hunter L. HHV-6 infection in a 19-year-old liver transplant recipient - much more than roseola! IDCases. (2023) 33:1–4. doi: 10.1016/j.idcr.2023.e01863
41. El-Jawahri AR, Schaefer PW, El Khoury JB, and Martinez-Lage M. Case 5-2018: A 63-year-old man with confusion after stem-cell transplantation. New Engl J Med. (2018) 378:659–69. doi: 10.1056/NEJMcpc1707556
42. Fida M, Hamdi AM, Bryson A, Razonable RR, and Abu Saleh O. Long-term outcomes of patients with human herpesvirus 6 encephalitis. Open Forum Infect Diseases. (2019) 633:1–4. doi: 10.1093/ofid/ofz269
43. Forest F, Duband S, Pillet S, Stachowicz ML, Cornillon J, Dumollard JM, et al. Lethal human herpesvirus-6 encephalitis after cord blood transplant. Transplant Infect Disease. (2011) 13:646–9. doi: 10.1111/j.1399-3062.2011.00642.x
44. Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, et al. Detection of active human herpesvirus-6 infection in the brain: Correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Diseases. (2007) 195:450–4. doi: 10.1086/510757
45. Fujimaki K, Mori T, Kida A, Tanaka M, Kawai N, Matsushima T, et al. Human herpesvirus 6 meningoencephalitis in allogeneic hematopoietic stem cell transplant recipients. Int J Hematology. (2006) 84:432–7. doi: 10.1532/IJH97.06072
46. Galan A, McNiff JM, Choi JN, and Lazova R. Fatal HHV6 infection in an immunocompromised patient presenting with skin involvement. J Cutan Pathol. (2010) 37:277–81. doi: 10.1111/j.1600-0560.2009.01291.x
47. Gannamani V, Varma A, Nathan S, and Ustun C. Human herpesvirus 6 (HHV-6) associated permanent hyponatremia in umbilical cord blood transplant recipient. Transplant Immunol. (2023) 76:1–2. doi: 10.1016/j.trim.2022.101742
48. Glockenberg K, Crawford C, and Otaki F. Serum-negative, site specific HHV-6 infection causes nausea and vomiting following double umbilical cord stem cell transplantation. Am J Gastroenterology. (2012) 1):S358. doi: 10.14309/00000434-201210001-00872
49. Gorniak RJT, Young GS, Wiese DE, Marty FM, and Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. Am J Neuroradiology. (2006) 27:887–91.
50. Guo Y, Zhu Z, Cai W, Tao S, and Yin D. Intracerebral opportunistic infections caused by immunosuppressants after orthotopic liver transplantation: Report of two cases and literature review. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1003254
51. Hanajiri R, Kobayashi T, Yoshioka K, Watanabe D, Watakabe K, Murata Y, et al. Central nervous system infection following allogeneic hematopoietic stem cell transplantation. Hematology/Oncology Stem Cell Ther. (2017) 10:22–8. doi: 10.1016/j.hemonc.2016.08.008
52. Handley G, Khawaja F, Kondapi DS, Lee HJ, Kaufman GP, Neelapu SS, et al. Human herpesvirus 6 myelitis after chimeric antigen receptor T-cell therapy. Int J Infect Diseases. (2021) 112:327–9. doi: 10.1016/j.ijid.2021.09.061
53. Hill JA, Myerson D, Sedlak RH, Jerome KR, and Zerr DM. Hepatitis due to human herpesvirus 6B after hematopoietic cell transplantation and a review of the literature. Transplant Infect Disease. (2014) 16:477–83. doi: 10.1111/tid.12208
54. Hino Y, Doki N, Sekiya N, Takaki Y, and Ohashi K. Optic neuritis as an initial manifestation of human herpesvirus 6 reactivation after unrelated bone marrow transplantation. Br J Haematol. (2016) 172:654. doi: 10.1111/bjh.13826
55. Hirabayashi K, Nakazawa Y, Katsuyama Y, Yanagisawa T, Saito S, Yoshikawa K, et al. Successful ganciclovir therapy in a patient with human herpesvirus-6 encephalitis after unrelated cord blood transplantation: usefulness of longitudinal measurements of viral load in cerebrospinal fluid. Infection. (2013) 41:219–23. doi: 10.1007/s15010-012-0329-3
56. Holden SR and Vas AL. Severe encephalitis in a haematopoietic stem cell transplant recipient caused by reactivation of human herpesvirus 6 and 7. J Clin Virol. (2007) 40:245–7. doi: 10.1016/j.jcv.2007.08.011
57. Imataki O and Uemura M. Ganciclovir-resistant HHV-6 encephalitis that progressed rapidly after bone marrow transplantation. J Clin Virol. (2015) 69:176–8. doi: 10.1016/j.jcv.2015.06.097
58. Inui Y, Yakushijin K, Okamura A, Tanaka Y, Shinzato I, Nomura T, et al. Human herpesvirus 6 encephalitis in patients administered mycophenolate mofetil as prophylaxis for graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplant Infect Dis. (2019) 21:1–8. doi: 10.1111/tid.13024
59. Ito Y, Toyama K, Honda A, Nakazaki K, Arai S, and Kurokawa M. Posterior reversible encephalopathy syndrome concurrent with human herpesvirus-6B encephalitis after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. (2020) 26:265–8. doi: 10.1016/j.jiac.2019.07.016
60. Jacobs U, Ferber J, and Klehr HU. Severe allograft dysfunction after OKT3-induced human herpes virus-6 reactivation. Transplant Proc. (1994) 26:3121.
61. Kapur N and Brooks DJ. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. (1999) 9:247–54. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W
62. Kawaguchi T, Takeuchi M, Kawajiri C, Abe D, Nagao Y, Yamazaki A, et al. Severe hyponatremia caused by syndrome of inappropriate secretion of antidiuretic hormone developed as initial manifestation of human herpesvirus-6-associated acute limbic encephalitis after unrelated bone marrow transplantation. Transplant Infect Disease. (2013) 15:E54–7. doi: 10.1111/tid.12029
63. Kawamoto S, Hatanaka K, Imakita M, and Tamaki T. Central diabetes insipidus in an HHV6 encephalitis patient with a posterior pituitary lesion that developed after tandem cord blood transplantation. Internal Med. (2013) 52:1107–10. doi: 10.2169/internalmedicine.52.9432
64. Knox KK and Carrigan DR. Chronic myelosuppression associated with persistent bone marrow infection due to human herpesvirus 6 in a bone marrow transplant recipient. Clin Infect Diseases. (1996) 22:174–5. doi: 10.1093/clinids/22.1.174
65. Kuribayashi K, Matsunaga T, Iyama S, Takada K, Sato T, Murase K, et al. Human herpesvirus-6 hepatitis associated with cyclosporine-A encephalitis after bone marrow transplantation for chronic myeloid leukemia. Internal Med. (2006) 45:475–8. doi: 10.2169/internalmedicine.45.1507
66. Lagadinou ED, Marangos M, Liga M, Panos G, Tzouvara E, Dimitroulia E, et al. Human herpesvirus 6-related pure red cell aplasia, secondary graft failure, and clinical severe immune suppression after allogeneic hematopoietic cell transplantation successfully treated with foscarnet. Transplant Infect Disease. (2010) 12:437–40. doi: 10.1111/j.1399-3062.2010.00515.x
67. Lamoth F, Jayet PY, Aubert JD, Rotman S, Mottet C, Sahli R, et al. Case report: Human herpesvirus 6 reactivation associated with colitis in a lung transplant recipient. J Med Virol. (2008) 80:1804–7. doi: 10.1002/jmv.21268
68. Li N, Zhang R, Wang J, Zhu X, Meng F, Cao Y, et al. Case report: Acute HHV6B encephalitis/myelitis post CAR-T cell therapy in patients with relapsed/refractory aggressive B-cell lymphoma. Front Neurol. (2024) 15. doi: 10.3389/fneur.2024.1334000
69. Lin WC, Moore JO, Mann KP, Traweek ST, and Smith C. Post transplant CD8+ gammadelta T-cell lymphoma associated with human herpes virus-6 infection. Leukemia Lymphoma. (1999) 33:377–84. doi: 10.3109/10428199909058439
70. Hill JA, Sedlak RH, Zerr DM, Huang ML, Yeung C, Myerson D, et al. Prevalence of chromosomally integrated human herpesvirus 6 in patients with human herpesvirus 6-central nervous system dysfunction. Biol Blood Marrow Transplantation. (2015) 21:371–3. doi: 10.1016/j.bbmt.2014.09.015
71. MacLean HJ and Douen AG. Severe amnesia associated with human herpesvirus 6 encephalitis after bone marrow transplantation. Transplantation. (2002) 73:1086–9. doi: 10.1097/00007890-200204150-00012
72. Mancone S, Selvadurai C, Baehring J, and Patel A. Choreoathetosis in the setting of human herpesvirus-6 infection in a transplant recipient. Tremor Other Hyperkinetic Movements. (2021) 11:1–5. doi: 10.5334/tohm.657
73. Marcelis S, Bossche SV, and Dekeyzer S. Human herpesvirus-6 encephalitis. J Belgian Soc Radiol. (2022) 106:1–3. doi: 10.5334/jbsr.2885
74. Mariotte E, Schnell D, Scieux C, Agbalika F, Legoff J, Ribaud P, et al. Significance of herpesvirus 6 in BAL fluid of hematology patients with acute respiratory failure. Infection. (2011) 39:225–30. doi: 10.1007/s15010-011-0114-8
75. Mata S, Guidi S, Nozzoli C, Orsi A, Pratesi A, Mascalchi M, et al. Human herpesvirus 6-associated limbic encephalitis in adult recipients of unrelated umbilical cord blood transplantation. Bone Marrow Transplantation. (2008) 42:693–5. doi: 10.1038/bmt.2008.233
76. Mineri R, Mariotti J, Sarina B, Morabito L, Crocchiolo R, Bramanti S, et al. Genomic integration of HHV-6 mimicking viral reactivation after autologous stem cell transplantation. Mediterr J Hematol Infect Dis. (2018) 10:1–4. doi: 10.4084/mjhid.2018.013
77. Montejo M, Ramon Fernandez J, Testillano M, Valdivieso A, Aguirrebengoa K, Varas C, et al. Encephalitis caused by human herpesvirus-6 in a liver transplant recipient. Eur neurol. (2002) 48:234–5. doi: 10.1159/000066172
78. Mookerjee BP and Vogelsang G. Human herpes virus-6 encephalitis after bone marrow transplantation: Successful treatment with ganciclovir. Bone Marrow Transplantation. (1997) 20:905–6. doi: 10.1038/sj.bmt.1700988
79. Mori T, Mihara A, Yamazaki R, Shimizu T, Aisa Y, Suzuki S, et al. Myelitis associated with human herpes virus 6 (HHV-6) after allogeneic cord blood transplantation. Scandinavian J Infect Diseases. (2007) 39:276–8. doi: 10.1080/00365540600904803
80. Morita D, Hirabayashi K, Katsuyama Y, Morokawa H, Motobayashi M, Kurata T, et al. Viral load and ganciclovir (GCV) concentration in cerebrospinal fluid of patients successfully treated with GCV or valGCV for human herpesvirus 6 encephalitis/myelitis following umbilical cord blood transplantation. Transplant Infect Disease. (2016) 18:773–6. doi: 10.1111/tid.12579
81. Moukhachen H, Rajendram P, Chawla S, Raoof N, Hale K, Voigt L, et al. Pulmonary arterial hypertension after hematopoeitic stem cell transplant. Chest Conference: CHEST. (2012) 142:118A. doi: 10.1378/chest.1387803
82. Muta T, Kamo M, Gondo H, Kato K, Eto T, Shibuya T, et al. Human herpesvirus-6 encephalitis followed by severe acute GVHD after a stem cell transplant from a microchimeric non-inherited maternal antigen (NIMA)-mismatched sibling. Bone Marrow Transplantation. (2005) 35:411–3. doi: 10.1038/sj.bmt.1704770
83. Nagaie T, Itamura H, Nishihara M, Mihashi T, Fujita M, Sano H, et al. Role of arterial spin labeling perfusion in diagnosis and follow-up of HHV-6 encephalitis following post-hematopoietic stem cell transplantation: A case report. Transplant Cell Ther. (2024) 30:S428. doi: 10.1016/j.jtct.2023.12.614
84. Nakamura Y, Tanaka Y, Ando T, Sato Y, Yujiri T, and Tanizawa Y. Successful engraftment of the second reduced-intensity conditioning cord blood transplantation (CBT) for a patient who developed graft rejection and infectious complications after the first CBT for AML [3. Bone Marrow Transplantation. (2007) 40:395–6. doi: 10.1038/sj.bmt.1705732
85. Nakayama T, Okada F, Ando Y, Honda K, Ogata M, Goto K, et al. A case of pneumonitis and encephalitis associated with human herpesvirus 6 (HHV-6) infection after bone marrow transplantation. Br J Radiol. (2010) 83:e255–e8. doi: 10.1259/bjr/19375793
86. Nash PJ, Avery RK, Tang WHW, Starling RC, Taege AJ, and Yamani MH. Encephalitis owing to human herpes virus-6 after cardiac transplant. Am J Transplantation. (2004) 4:1200–3. doi: 10.1111/j.1600-6143.2004.00459.x
87. Neumann T, Krüger WH, Zimmermann K, Kiefer T, Schüler F, and Dölken G. Successful treatment of an HHV6B-induced diarrhea with ganciclovir in a patient after PBSCT. Bone Marrow Transplantation. (2009) 43:87–8. doi: 10.1038/bmt.2008.272
88. Nishimaki K, Okada S, Miyamura K, Ohno I, Ashino Y, Sugawara T, et al. The possible involvement of human herpesvirus type 6 in obliterative bronchiolitis after bone marrow transplantation [1. Bone Marrow Transplantation. (2003) 32:1103–5. doi: 10.1038/sj.bmt.1704269
89. Nishimoto M, Nakamae H, Hayashi Y, Koh H, Nakane T, Yoshida M, et al. Prolonged sinus tachycardia caused by human herpesvirus 6 (HHV6) encephalomyelitis after allogeneic bone marrow transplantation. Internal Med. (2012) 51:1265–7. doi: 10.2169/internalmedicine.51.6640
90. Noguchi T, Mihara F, Yoshiura T, Togao O, Atsumi K, Matsuura T, et al. MR imaging of human herpesvirus-6 encephalopathy after hematopoietic stem cell transplantation in adults. Am J Neuroradiol. (2006) 27:2191–5.
91. Oevermann L, Zimmermann C, Voigt S, Kunkele A, Lobitz S, Eggert A, et al. Transmission of chromosomally integrated human herpes virus-6A via haploidentical stem cell transplantation poses a risk for virus reactivation and associated complications. Bone Marrow Transplantation. (2020) 55:260–4. doi: 10.1038/s41409-019-0530-4
92. Ogata M, Satou T, Inoue Y, Takano K, Ikebe T, Ando T, et al. Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: Breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplantation. (2013) 48:257–64. doi: 10.1038/bmt.2012.121
93. Ogata M, Satou T, Kawano R, Goto K, Ikewaki J, Kohno K, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplantation. (2008) 41:279–85. doi: 10.1038/sj.bmt.1705907
94. Ogata M, Takano K, Moriuchi Y, Kondo T, Ueki T, Nakano N, et al. Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: A prospective multicenter trial with an historical control group. Biol Blood Marrow Transplantation. (2018) 24:1264–73. doi: 10.1016/j.bbmt.2018.02.008
95. Olson AL, Dahi PB, Zheng J, Devlin SM, Lubin M, Gonzales AM, et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transpl. (2014) 20:787–93. doi: 10.1016/j.bbmt.2014.02.010
96. Oniszczuk J, Garrouste C, Aniort J, Tiple A, Kemeny JL, Deschelotte P, et al. Fatal human herpesvirus-6 infection 5 months after renal transplantation. Transplant Int. (2013) 3:25.
97. Otoole J, Ades S, Waters BL, Agarwal Z, and Lamba G. Delayed human herpes virus 6 encephalitis in a patient with allogeneic stem cell transplant. Leukemia Lymphoma. (2015) 56:2709–10. doi: 10.3109/10428194.2014.1003054
98. Pasca M, Picchioni A, Mazzeo S, Terenzi F, Prestipino E, Fratangelo R, et al. A case of recurrent progressive multifocal leukoencephalopathy after human stem cell transplant, with detection of John Cunningham virus and human herpesvirus 6 on cerebrospinal fluid, treated with Mirtazapine, Olanzapine and Foscarnet. Intractable Rare Dis Res. (2019) 8:275–8. doi: 10.5582/irdr.2019.01107
99. Paterson DL, Singh N, Gayowski T, Carrigan DR, and Marino IR. Encephalopathy associated with human herpesvirus 6 in a liver transplant recipient. Liver Transplant Surg. (1999) 5:454–5. doi: 10.1002/lt.500050504
100. Petit V, Bonnafous P, Fages V, Gautheret-Dejean A, Engelmann I, Baras A, et al. Donor-to-recipient transmission and reactivation in a kidney transplant recipient of an inherited chromosomally integrated HHV-6A: Evidence and outcomes. Am J Transplantation. (2020) 20:3667–72. doi: 10.1111/ajt.16067
101. Pilmore H, Collins J, Dittmer I, Williams L, Carpenter L, Thomas S, et al. Fatal human herpesvirus-6 infection after renal transplantation. Transplantation. (2009) 88:762–5. doi: 10.1097/TP.0b013e3181b4749f
102. Piras E, Caocci G, Pisano V, Orrù F, Murgia F, Sanna M, et al. Guillain-Barré syndrome after human herpesvirus-6 reactivation in unrelated hematopoietic stem cell transplantation. Leukemia Lymphoma. (2013) 54:1332–3. doi: 10.3109/10428194.2012.740560
103. Pohlmann C, Schetelig J, Reuner U, Bornhauser M, Illmer T, Kiani A, et al. Cidofovir and foscarnet for treatment of human herpesvirus 6 encephalitis in a neutropenic stem cell transplant recipient. Clin Infect Dis. (2007) 44:e118–20. doi: 10.1086/518282
104. Potenza L, Luppi M, Barozzi P, Rossi G, Cocchi S, Codeluppi M, et al. HHV-6A in syncytial giant-cell hepatitis. New Engl J Med. (2008) 359:593–602. doi: 10.1056/NEJMoa074479
105. Randhawa PS, Jenkins FJ, Nalesnik MA, Martens J, Williams PA, Ries A, et al. Herpesvirus 6 variant a infection after heart transplantation with giant cell transformation in bile ductular and gastroduodenal epithelium. Am J Surg Pathol. (1997) 21:847–53. doi: 10.1097/00000478-199707000-00014
106. Rangarajan S and Lalefar N. LB1010 Cutaneous manifestations post stem cell transplant. J Invest Dermatol. (2022) 142:B31. doi: 10.1016/j.jid.2022.05.1037
107. Rebechi MT, Bork JT, and Riedel DJ. HHV-6 encephalitis after chimeric antigen receptor T-cell therapy (CAR-T): 2 case reports and a brief review of the literature. Open Forum Infect Diseases. (2021) 8:1–6. doi: 10.1093/ofid/ofab470
108. Reuter S, Schluter MA, Wolters H, and Suwelack B. Severe aphthous stomatitis related to human herpes virus 6 infection in a renal transplant patient. Transplant Int. (2013) 26(Suppl. 1):28–32.
109. Revest M, Camus C, D’Halluin PN, Cha S, Compagnon P, Boudjema K, et al. Fatal human herpes virus 6 primary infection after liver transplantation [1. Transplantation. (2007) 83:1404–5. doi: 10.1097/01.tp.0000261705.78595.98
110. Reynolds J. Rare case of HHV6 encephalitis in post allogeneic transplant recipient. Biol Blood Marrow Transplant. (2017) 23:S107. doi: 10.1016/j.bbmt.2016.12.128
111. Rieux C, Gautheret-Dejean A, Challine-Lehmann D, Kirch C, Agut H, and Vernant JP. Human herpesvirus-6 meningoencephalitis in a recipient of an unrelated allogeneic bone marrow transplantation. Transplantation. (1998) 65:1408–11. doi: 10.1097/00007890-199805270-00024
112. Rodrigues GDA, Nagendra S, Lee CK, and De Magalhaes-Silverman M. Human herpes virus 6 fatal encephalitis in a bone marrow recipient. Scandinavian J Infect Diseases. (1999) 31:313–5. doi: 10.1080/00365549950163644
113. Rosenfeld CS, Rybka WB, Weinbaum D, Carrigan DR, Knox KK, Andrews DF, et al. Late graft failure due to dual bone marrow infection with variants A and B of human Herpesvirus-6. Exp Hematol. (1995) 23:626–9.
114. Rossi C, Delforge ML, Jacobs F, Wissing M, Pradier O, Remmelink M, et al. Fatal primary infection due to human herpesvirus 6 variant A in a renal transplant recipient. Transplantation. (2001) 71:288–92. doi: 10.1097/00007890-200101270-00021
115. Roux J, Battistella M, Fornecker L, Legoff J, Deau B, Houhou N, et al. Human herpesvirus-6 cytopathic inclusions: An exceptional and recognizable finding on skin biopsy during hhv6 reactivation after autologous stem-cell transplantation. Am J Dermatopathol. (2012) 34:e73–e6. doi: 10.1097/DAD.0b013e31825667ed
116. Saraiva S, Simoes C, MaChado MV, Freitas C, Baldaia C, Valente A, et al. Human herpesvirus 6 reactivation associated with intestinal pseudo-obstruction in a renal transplant recipient. Exp Clin Transplantation. (2022) 20:209–12. doi: 10.6002/ect.2021.0315
117. Schlaweck S, Bragelmann J, Brossart P, and Mayer K. Exanthem subitum (human herpesvirus-6 reactivation) after autologous stem cell transplantation. Transplant Infect Disease. (2016) 18:255–6. doi: 10.1111/tid.12514
118. Shintaku M, Kaneda D, Tada K, Katano H, and Sata T. Human herpes virus 6 encephalomyelitis after bone marrow transplantation: Report of an autopsy case. Neuropathology. (2010) 30:50–5. doi: 10.1111/j.1440-1789.2009.01020.x
119. Singh N, Carrigan DR, Gayowski T, and Marino IR. Human herpesvirus-6 infection in liver transplant recipients: documentation of pathogenicity. Transplantation. (1997) 64:674–8. doi: 10.1097/00007890-199709150-00002
120. Singh N, Carrigan DR, Gayowski T, Singh J, and Marino IR. Variant B human herpesvirus-6 associated febrile dermatosis with thrombocytopenia and encephalopathy in a liver transplant recipient. Transplantation. (1995) 60:1355–7.
121. Tanaka M, Taguchi J, Hyo R, Kawano T, Hashimoto C, Motomura S, et al. Human herpesvirus-6 encephalitis after unrelated cord blood transplantation. Leukemia Lymphoma. (2005) 46:561–6. doi: 10.1080/10428190400029882
122. Tasaka T, Matsuhashi Y, Sadhira K, Matsuoka A, Ohnishi H, Kubota Y, et al. Diabetes insipidus following HHV-6 encephalitis after cord blood transplantation in acute myeloid leukemia. Leukemia Res. (2009) 33:202–4. doi: 10.1016/j.leukres.2008.07.001
123. Tiacci E, Luppi M, Barozzi P, Gurdo G, Tabilio A, Ballanti S, et al. Fatal herpesvirus-6 encephalitis in a recipient of a T-cell-depleted peripheral blood stem cell transplant from a 3-loci mismatched related donor. Haematologica. (2000) 85:94–7.
124. Tomaszewska A, Nasilowska-Adamska B, Dzieciatkowski T, and Marianska B. Simultaneous human herpesvirus 6-associated encephalitis and Guillain-Barre syndrome in a patient after matched unrelated donor haematopoietic stem cell transplantation. Arch Med Sci. (2010) 6:288–90. doi: 10.5114/aoms.2010.13912
125. Tsujimura H, Iseki T, Date Y, Watanabe J, Kumagai K, Kikuno K, et al. Human herpesvirus-6 encephalitis after bone marrow transplantation: Magnetic resonance imaging could identify the involved sites of encephalitis [4. Eur J Haematol. (1998) 61:284–5. doi: 10.1111/j.1600-0609.1998.tb01718.x
126. Ueki T, Hoshi K, Hiroshima Y, Sumi M, Ichikawa N, Ogata M, et al. Analysis of five cases of human herpesvirus-6 myelitis among 121 cord blood transplantations. Int J Hematol. (2018) 107:363–72. doi: 10.1007/s12185-017-2347-5
127. Veni K, Ahmed R, and Shaikh R. Human herpesvirus 6 reactivation after autologous stem cell transplantation in multiple myeloma. Indian J Hematol Blood Transfusion. (2023) 39:S155–S6.
128. Vinnard C, Barton T, Jerud E, and Blumberg E. A report of human herpesvirus 6-associated encephalitis in a solid organ transplant recipient and a review of previously published cases. Liver Transplantation. (2009) 15:1242–6. doi: 10.1002/lt.21816
129. Visser AM, van Doornum GJ, Cornelissen JJ, and van den Bent MJ. Severe amnesia due to HHV-6 encephalitis after allogenic stem cell transplantation. Eur Neurol. (2005) 54:233–4. doi: 10.1159/000090718
130. Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Edward Coleman R, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. (2001) 50:612–9. doi: 10.1002/ana.1251
131. Wang FZ, Linde A, Hagglund H, Testa M, Locasciulli A, and Ljungman P. Human herpesvirus 6 DNA in cerebrospinal fluid specimens from allogeneic bone marrow transplant patients: Does it have clinical significance? Clin Infect Dis. (1999) 28:562–8. doi: 10.1086/515142
132. Wang Y, Wang D, and Tao X. Human herpesvirus 6B encephalitis in a liver transplant recipient: A case report and review of the literature. Transplant Infect Disease. (2021) 23::1–4. doi: 10.1111/tid.13403
133. Ward KN, Gray JJ, and Efstathiou S. Brief report: Primary human herpesvirus 6 infection in a patient following liver transplantation from a seropositive donor. J Med Virol. (1989) 28:69–72. doi: 10.1002/jmv.1890280203
134. Yamamoto T, Watarai Y, Goto N, Horikoshi Y, Yamada S, Yasui K, et al. Encephalitis caused by human herpesvirus-6B in pancreas-after-kidney transplantation. Transplant Infect Disease. (2014) 16:853–8. doi: 10.1111/tid.12270
135. Yokote T, Muroi K, Kawano C, Kirito K, Ohtsuki T, Komatsu N, et al. Human herpesvirus-6-associated generalized vesiculobullous eruptions after allogeneic bone marrow transplantation. Leukemia Lymphoma. (2002) 43:927–9. doi: 10.1080/10428190290017233
136. Yoshida H, Matsunaga K, Ueda T, Yasumi M, Ishikawa J, Tomiyama Y, et al. Human herpesvirus 6 meningoencephalitis successfully treated with ganciclovir in a patient who underwent allogeneic bone marrow transplantation from an HLA-identical sibling. Int J hematol. (2002) 75:421–5. doi: 10.1007/BF02982136
137. Yoshihara S, Kato R, Inoue T, Miyagawa H, Sashihara J, Kawakami M, et al. Successful treatment of life-threatening human herpesvirus-6 encephalitis with donor lymphocyte infusion in a patient who had undergone human leukocyte antigen-haploidentical nonmyeloablative stem cell transplantation. Transplantation. (2004) 77:835–8. doi: 10.1097/01.TP.0000119603.59880.47
138. Yoshimoto G, Mori Y, Kato K, Shima T, Miyawaki K, Kikushige Y, et al. Human herpes virus-6-associated encephalitis/myelitis mimicking calcineurin inhibitor-induced pain syndrome in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplantation. (2018) 24:2540–8. doi: 10.1016/j.bbmt.2018.07.017
139. Zargarian A, Bryant CA, Rademeyer S, Baumgart P, and Fu B. (50) an “Unforgettable” Case of patchy memory loss due to human herpesvirus-6 encephalitis after stem cell transplant for acute myeloid leukemia. J Acad Consultation-Liaison Psychiatry. (2022) 63:S137. doi: 10.1016/j.jaclp.2022.10.053
140. Zerr DM, Gooley TA, Yeung L, Huang ML, Carpenter P, Wade JC, et al. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin Infect Diseases. (2001) 33:763–71. doi: 10.1086/322642
141. Zhang K, Xu J, Chen G, Yang R, Jiang M, and Yuan H. Metagenomic Next-Generation Sequencing (mNGS) of cerebrospinal fluid for diagnosis of human herpesvirus 6B encephalitis following transplantation for severe aplastic anemia. J Infection Developing Countries. (2024) 18:152–7. doi: 10.3855/jidc.18152
142. Zhu H, Ali A, Woan KV, Tam E, Yaghmour G, Flores A, et al. Unique challenges to diagnosing human herpesvirus-6 (HHV-6) encephalitis following post-hematopoietic stem cell transplant: A case and brief review. Cell Transplant. (2022) 31:1–7. doi: 10.1177/09636897221119734
143. Potenza L, Barozzi P, Masetti M, Pecorari M, Bresciani P, Gautheret-Dejean A, et al. Prevalence of human herpesvirus-6 chromosomal integration (CIHHV-6) in italian solid organ and allogeneic stem cell transplant patients: Case report. Am J Transplantation. (2009) 9:1690–7. doi: 10.1111/j.1600-6143.2009.02685.x
144. Russell SJ, Duran J, Fuchs D, and Yeager AM. Atypical CD4+/CD8+ Lymphocytosis and prolonged pancytopenia associated with human herpesvirus 6 reactivation after autologous peripheral blood stem cell transplantation. Clin Lymphoma Myeloma Leuk. (2019) 19:531–5. doi: 10.1016/j.clml.2019.03.010
145. Tomonari A, Takahashi S, Ooi J, Takasugi K, Konuma T, Iseki T, et al. Human herpesvirus 6 variant A infection with fever, skin rash, and liver dysfunction in a patient after unrelated cord blood transplantation. Bone Marrow Transplantation. (2005) 36:1109–10. doi: 10.1038/sj.bmt.1705184
146. Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. (2006) 193:68–79. doi: 10.1086/498531
147. Sakai R, Kanamori H, Motohashi K, Yamamoto W, Matsuura S, Fujita A, et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. (2011) 17:1389–94. doi: 10.1016/j.bbmt.2011.01.014
148. Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. (2007) 69:156–65. doi: 10.1212/01.wnl.0000265591.10200.d7
149. Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, and Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. (2007) 39:705–9. doi: 10.1038/sj.bmt.1705666
150. Al-Obaidi AB, Shamran HA, Abdlameer AS, Hussein AG, Al-Jobori HRJ, and Al-Saedi AJH. Occurrence and risk factors of human herpes virus-6 among renal transplant recipients: A single-center study. J Pharm Sci Res. (2018) 10:1098–102.
151. Balsat M, Pillet S, Tavernier E, Cacheux V, Escuret V, Molucon-Chabrot C, et al. Human herpesvirus 6 infection after autologous stem cell transplantation: A multicenter prospective study in adult patients. J Infection. (2019) 79:36–42. doi: 10.1016/j.jinf.2019.05.001
152. Buyse S, Roque-Afonso AM, Vaghefi P, Gigou M, Dussaix E, Duclos-Vallée JC, et al. Acute hepatitis with periportal confluent necrosis associated with human herpesvirus 6 infection in liver transplant patients. Am J Clin Pathol. (2013) 140:403–9. doi: 10.1309/AJCP0FWI2XAHECBJ
153. Cone RW, Hackman RC, Huang MLW, Bowden RA, Meyers JD, Metcalf M, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. New Engl J Med. (1993) 329:156–61. doi: 10.1056/NEJM199307153290302
154. Drobyski WR, Dunne WM, Burd EM, Knox KK, Ash RC, Horowitz MM, et al. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: Evidence of a marrow-suppressive role for HHV-6 in vivo. J Infect Dis. (1993) 167:735–9. doi: 10.1093/infdis/167.3.735
155. Gudnason T, Dunn DL, Brown NA, and Balfour HH Jr. Human herpes virus 6 infections in hospitalized renal transplant recipients. Clin Transplantation. (1991) 5:359–64. doi: 10.1111/j.1399-0012.1991.tb00113.x
156. Harma M, Hockerstedt K, Lyytikainen O, and Lautenschlager I. HHV-6 and HHV-7 antigenemia related to CMV infection after liver transplantation. J Med Virol. (2006) 78:800–5. doi: 10.1002/jmv.20626
157. Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: A cohort analysis. Biol Blood Marrow Transplantation. (2012) 18:1638–48. doi: 10.1016/j.bbmt.2012.04.016
158. Imbert-Marcille BM, Tang XW, Lepelletier D, Besse B, Moreau P, Billaudel S, et al. Human herpesvirus 6 infection after autologous or allogeneic stem cell transplantation: a single-center prospective longitudinal study of 92 patients. Clin Infect Diseases. (2000) 31:881–6. doi: 10.1086/318142
159. Kampouri E, Krantz EM, Xie H, Ibrahimi SS, Kiem ES, Sekhon MK, et al. Human herpesvirus-6 reactivation and disease are infrequent in chimeric antigen receptor T-cell therapy recipients. Blood. (2024) 18:18. doi: 10.1182/blood.2024024145
160. Lehto JT, Halme M, Tukiainen P, Harjula A, Sipponen J, and Lautenschlager I. Human herpesvirus-6 and -7 after lung and heart-lung transplantation. J Heart Lung Transplantation. (2007) 26:41–7. doi: 10.1016/j.healun.2006.10.017
161. Lindahl JK, Woxenius S, Brune M, and Andersson R. Human herpesvirus type 6 DNAemia and infection following allogeneic stem cell transplantation with a focus on long-term outcome. Scandinavian J Infect Diseases. (2013) 45:557–61. doi: 10.3109/00365548.2013.773069
162. Ljungman P, Wang FZ, Clark DA, Emery VC, Remberger M, Ringden O, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. (2000) 111:774–81. doi: 10.1111/j.1365-2141.2000.02422.x
163. Magalhaes GS, Guardia AC, Sampaio AM, Boin IFSF, and Stucchi RSB. HHV-6: Clinical and laboratory investigations and correlations with encephalitis in liver transplant recipients. Transplant Proc. (2013) 45:1997–9. doi: 10.1016/j.transproceed.2013.01.095
164. Murakami K, Kohashi S, Sakurai M, Kato J, Toyama T, Koda Y, et al. Hyponatremia associated with human herpesvirus-6 (HHV-6) encephalitis after allogeneic hematopoietic stem cell transplantation: A presentation different from HHV-6 myelitis. Int J Hematol. (2017) 106:436–40. doi: 10.1007/s12185-017-2254-9
165. Muta T, Fukuda T, and Harada M. Human herpesvirus-6 encephalitis in hematopoietic SCT recipients in Japan: A retrospective multicenter study. Bone Marrow Transplantation. (2009) 43:583–5. doi: 10.1038/bmt.2008.359
166. Ogata M, Oshima K, Takano K, Kawano R, Ueda Y, Imamura T, et al. Effects of human herpesvirus 6B reactivation on cognitive function in cord blood transplant recipients: a prospective multicenter study. Int J Hematol. (2024) 119:432–41. doi: 10.1007/s12185-024-03714-2
167. Ohashi M, Sugata K, Ihira M, Asano Y, Egawa H, Takada Y, et al. Human herpesvirus 6 infection in adult living related liver transplant recipients. Liver Transplantation. (2008) 14:100–9. doi: 10.1002/lt.21304
168. Raouf MME, Ouf NM, Elsorady MAS, and Ghoneim FM. Human herpesvirus-6 in hematopoietic stem cell transplant recipients: a prospective cohort study in Egypt. Virol J. (2023) 20:1–10. doi: 10.1186/s12985-023-01980-w
169. Shimazu Y, Kondo T, Ishikawa T, Yamashita K, and Takaori-Kondo A. Human herpesvirus-6 encephalitis during hematopoietic stem cell transplantation leads to poor prognosis. Transplant Infect Disease. (2013) 15:195–201. doi: 10.1111/tid.12049
170. Betts BC, Young JA, Ustun C, Cao Q, and Weisdorf DJ. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biol Blood Marrow Transplant. (2011) 17:1562–8. doi: 10.1016/j.bbmt.2011.04.004
171. Humar A, Kumar D, Caliendo AM, Moussa G, Ashi-Sulaiman A, Levy G, et al. Clinical impact of human herpesvirus 6 infection after liver transplantation. Transplantation. (2002) 73:599–604. doi: 10.1097/00007890-200202270-00021
172. Sampaio AM, Guardia AC, Milan A, Sasaki AN, Andrade PD, Bonon SH, et al. Co-infection and clinical impact of human herpesvirus 5 and 6 in liver transplantation. Transplant Proc. (2012) 44:2455–8. doi: 10.1016/j.transproceed.2012.07.034
173. Shiroshita K, Mori T, Kato J, Sakurai M, Koda Y, Abe R, et al. Clinical characteristics of human herpesvirus-6 myelitis after allogeneic hematopoietic stem cell transplantation and its favorable outcome by early intervention. Bone Marrow Transpl. (2020) 55:939–45. doi: 10.1038/s41409-019-0755-2
174. Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, and Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. (2005) 40:932–40. doi: 10.1086/428060
175. Yamane A, Mori T, Suzuki S, Mihara A, Yamazaki R, Aisa Y, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transpl. (2007) 13:100–6. doi: 10.1016/j.bbmt.2006.09.003
176. Hall CB, Caserta MT, Schnabel KC, Long C, Epstein LG, Insel RA, et al. Persistence of human herpesvirus 6 according to site and variant: possible greater neurotropism of variant A. Clin Infect Dis. (1998) 26:132–7. doi: 10.1086/516280
177. Bahramian E, Furr M, Wu JT, and Ceballos RM. Differential impacts of HHV-6A versus HHV-6B infection in differentiated human neural stem cells. Front Immunol. (2022) 13:847106. doi: 10.3389/fimmu.2022.847106
178. Martin LK, Hollaus A, Stahuber A, Hübener C, Fraccaroli A, Tischer J, et al. Cross-sectional analysis of CD8 T cell immunity to human herpesvirus 6B. PloS Pathog. (2018) 14:e1006991. doi: 10.1371/journal.ppat.1006991
179. Agut H, Bonnafous P, and Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. (2015) 28:313–35. doi: 10.1128/CMR.00122-14
180. Hubacek P, Hrdlickova A, Muzikova K, Briksi A, Zelezna I, Spacek M, et al. Chromosomally integrated HHV-6 in healthy donor and patients treated for haematological Malignancy. J Clin Virol. (2015) 1):S125. doi: 10.1016/j.jcv.2015.07.290
181. Katagiri S, Akahane D, Inukai T, Otsuki S, Yamada A, Moriyama M, et al. Elevation of HHV-6 viral load mimicking HHV-6 reactivation after second umbilical cord blood transplantation in chromosomally integrated human herpesvirus-6. J Infect Chemother. (2021) 27:1517–9. doi: 10.1016/j.jiac.2021.05.016
182. Mori T, Tanaka-Taya K, Satoh H, Aisa Y, Yamazaki R, Kato J, et al. Transmission of chromosomally integrated human herpesvirsus 6 (HHV-6) variant A from a parent to children leading to misdiagnosis of active HHV-6 infection. Transplant Infect Disease. (2009) 11:503–6. doi: 10.1111/j.1399-3062.2009.00430.x
Keywords: HHV 6, HHV6-induced post-transplantation acute limbic encephalitis (PALE), solid organ transplant (SOT), hematopoietic (stem cell) transplant (HSCT), viral infection, transplant infections, review - systematic
Citation: Abad CLR and Razonable RR (2025) A systematic review of HHV-6 infections in recipients of organ and tissue transplantation and chimeric antigen receptor T-cell infusions. Front. Virol. 5:1641157. doi: 10.3389/fviro.2025.1641157
Received: 04 June 2025; Accepted: 09 July 2025;
Published: 01 August 2025.
Edited by:
Haider Al-Hello, Finnish Institute for Health and Welfare, FinlandReviewed by:
Hussein Alburkat, University of Helsinki, FinlandRuy D. Chacón Villanueva, University of São Paulo, Brazil
Copyright © 2025 Abad and Razonable. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cybele Lara R. Abad, Y3JhYmFkQHVwLmVkdS5waA==
†ORCID: Cybele Lara R. Abad, orcid.org/0000-0002-5183-8239
Raymund R. Razonable, orcid.org/0000-0001-5248-0227
 Cybele Lara R. Abad
Cybele Lara R. Abad Raymund R. Razonable
Raymund R. Razonable