- Infectious Disease, Hai Long Guiyang Public Health Clinical Center, Guiyang, China
Background: This study evaluated the effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) in treatment-naïve adults with HIV-1 and an ultra-high viral load (uVL; HIV-1 RNA ≥500,000 copies/mL)—a population at high risk for suboptimal outcomes.
Methods: A retrospective cohort study was conducted among 75 patients initiating BIC/FTC/TAF. The modified intent-to-treat (mITT) group included all patients, while the per-protocol (PP) group comprised 58 individuals who completed 96 weeks of follow-up. Virological suppression, immunological response, safety, and resistance were assessed.
Results: Virological suppression rates in the PP group were 98.3% at week 48 and 96.6% at week 96, compared to 74.7% and 78.7% in the mITT group—a gap largely due to non-clinical dropouts. Immunological recovery was significant, with median CD4+ counts increasing from 61.5 to 326.0 cells/μL (p < 0.001). No treatment-emergent resistance was detected. Adverse events were mild (6.9%).
Conclusion: BIC/FTC/TAF is highly effective and safe for adherent uVL patients, demonstrating rapid viral suppression and immune recovery. However, real-world effectiveness is significantly limited by socioeconomic barriers, highlighting the need for improved programmatic support to enhance retention and treatment access.
1 Introduction
Acquired immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV), remains a critical global public health challenge, timely initiation of antiretroviral therapy (ART), and sustained adherence are paramount to suppressing viral replication, delaying disease progression, reducing transmission risk, and improving patients’ quality of life and life expectancy (1). Despite the availability of effective ART, a subset of treatment-naïve patients presents with ultra-high viral loads (uVL), commonly defined as HIV-1 RNA ≥500,000 copies/mL. These individuals face unique challenges: they are at significantly higher risk for rapid CD4+cell count decline, accelerated disease progression to AIDS, development of opportunistic infections (OIs), and potentially higher rates of HIV transmission (2, 3)Furthermore, initiating ART in uVL patients raises theoretical concerns about the risk of virological failure and the development of drug resistance, particularly with regimens possessing lower genetic barriers to resistance (4). Therefore, selecting an initial ART regimen with potent and rapid virological suppression, a high genetic barrier to resistance, and a favorable safety profile is crucial for optimizing outcomes in this vulnerable population (5) Bictegravir co-formulated with emtricitabine and tenofovir alafenamide (BIC/FTC/TAF) is a once-daily, single-tablet regimen. Bictegravir, an unboosted integrase strand transfer inhibitor (INSTI), demonstrates potent antiviral activity and a high genetic barrier to resistance (6). Tenofovir alafenamide (TAF) offers improved renal and bone safety compared to tenofovir disoproxil fumarate (TDF) (7). Randomized controlled trials (RCTs) and real-world studies have established the high efficacy, safety, and tolerability of BIC/FTC/TAF in broad populations of treatment-naïve and treatment-experienced people with HIV (PWH), including those with high (but not specifically ultra-high) baseline viral loads (6, 8, 9).
However, dedicated data on the effectiveness and safety of BIC/FTC/TAF specifically in treatment-naïve PWH with confirmed ultra-high viral loads are relatively scarce. While existing trials included participants with high VLs, subgroup analyses specifically focusing on the uVL threshold and real-world evidence in this distinct subgroup are limited (6, 8). Understanding the performance of this preferred regimen in the most immunocompromised and virologically advanced patients is essential for clinical decision-making.
Therefore, this retrospective real-world cohort study aimed to evaluate the effectiveness (virological suppression, immunological recovery) and safety of BIC/FTC/TAF in treatment-naïve Chinese HIV-1 patients with ultra-high viral loads, providing crucial evidence to support its optimal use in this high-risk population.
2 Patients and methods
2.1 Study design
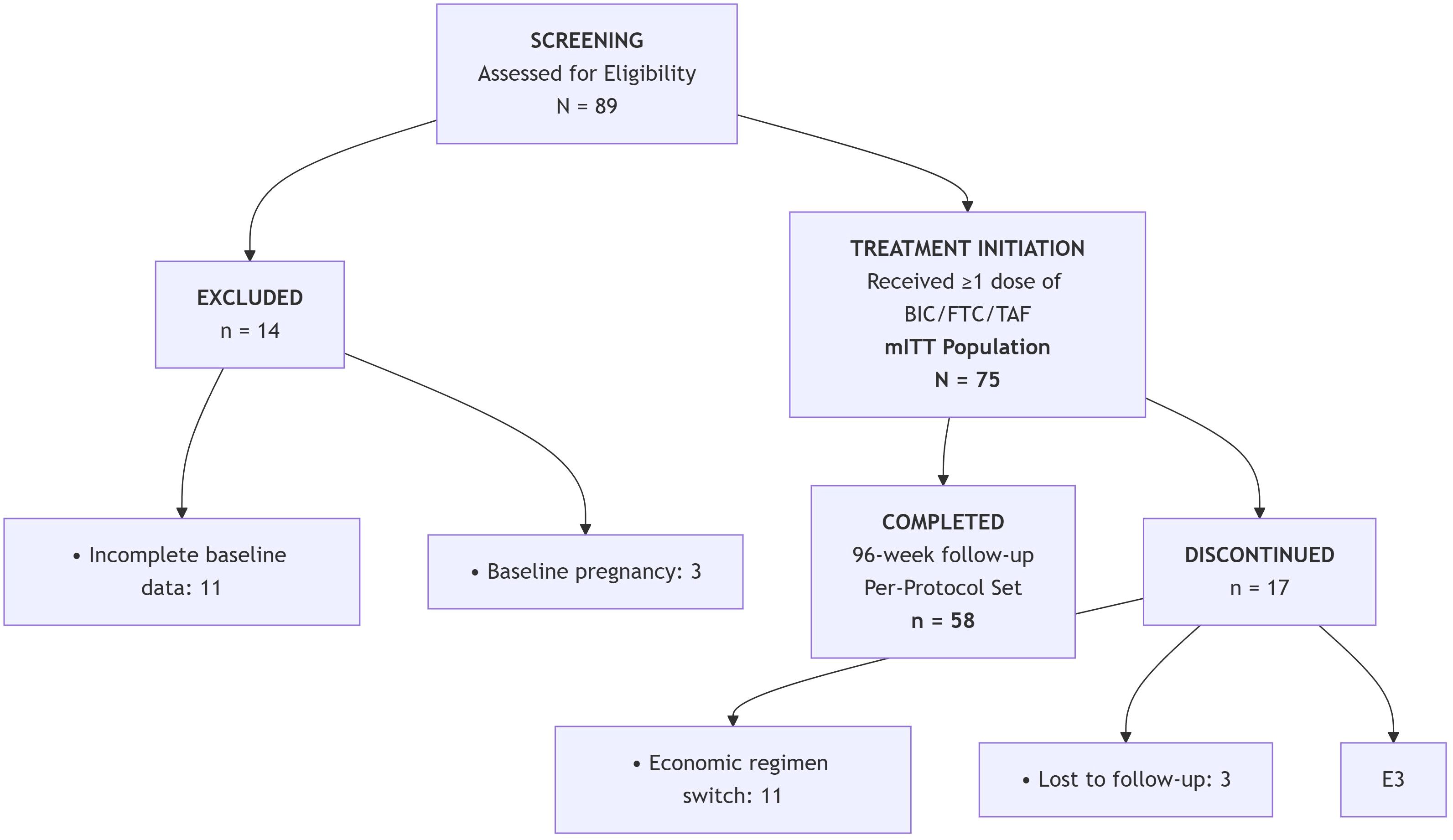
This retrospective cohort study evaluated treatment-naïve HIV-1 patients with ultra-high viral loads (≥500,000 copies/mL) initiating BIC/FTC/TAF at Guiyang Public Health Clinical Center between March 2021 and September 2024. Of 89 patients screened, 14 were excluded at baseline: 11 due to incomplete clinical data and 3 for baseline pregnancy. The remaining 75 patients initiated treatment, forming the Intent-to-Treat (ITT) population for primary analysis. During the 96-week follow-up period, 17 patients discontinued treatment: 11 switched regimens due to economic constraints, 3 were lost to follow-up, and 3 died from pre-existing opportunistic infections. Ultimately, 58 patients completed the study protocol, comprising the Per-Protocol population for sensitivity analysis (Figure 1).
This study was approved by the Ethics Committee of our hospital (approval number: 202504). The researchers informed the patients of relevant knowledge about using this treatment regimen, and the patients signed a written informed consent form. The primary endpoints were the rate of virological suppression, the average decrease log of HIV RNA at 12 weeks of BIC/FTC/TAF treatment, and the proportion of patients with HIV RNA <50 copies/mL at 12, 24, 48, and 96 weeks. Secondary outcomes were CD4+ cell count, CD4+/CD8+ cell ratio, and lipid levels at 48 weeks of treatment, adverse reactions.
2.1.1 Clinical definitions
Dyslipidemia was diagnosed based on the following criteria (1):TG ≥203.6mg/dL was defined as hypertriglyceridemia, and TC >239.9 mg/dL, LDL >120.7 mg/dL and HDL <38.7 mg/dL as hypercholesterolemia, per the guidelines on the prevention and treatment of dyslipidemia in adults in China (2). Liver and renal functions were evaluated periodically in patients undergoing ART and liver dysfunction was evaluated based on established diagnostic criteria (3). Renal dysfunction was defined as estimated glomerular filtration rate (eGFR) <90 mL·min–1 ·1.73 m–2 and/or resence of markers indicative of renal damage persisting for at least 3 months. Virological success was reflected by HIV-RNA <50 copies/ml, while virological failure should be defined as HIV RNA >50 copies/mL in two consecutive measurements at least 6 months after initiation or at any time point during follow-up up to 96 weeks or a single measurement>50 copies/mL followed by treatment discontinuation. Ultra-high viral load was defined as HIV-RNA≥500,000 copies/mL. HIV RNA TND(Target Not Detected)is less than 50 copies/mL.
2.2 Drug resistance monitoring
(1) Virologic failure (HIV RNA >200 copies/mL after initial suppression) (2); Incomplete virologic response (>50 copies/mL at W24) (3);Viral rebound (≥1 log10 increase from nadir).
2.3 Data collection and laboratory analyses
The demographic baseline characteristics and related information of HIV/AIDS patients were collected from the Chinese AIDS Prevention and Control Database and the Hospital Case System. Age, gender, height, weight, CD4+ cell count, HIV RNA level, comorbidities, ALT, AST and lipid profile for the patients were collected.
2.4 Statistical analysis
Statistical analysis was performed using the SPSS 25.0 software. Quantitative data were expressed as mean ± standard deviation in case of normal distribution and compared by the independent t-test or the paired t-test; in case of skewed distribution, median (P25, P75) was used, and comparisons used the Wilcoxon signed-rank test. Categorical data were expressed as number (%). GraphPad Prism 9.0 was used for plotting. P < 0.05 was considered statistically. The hazard ratio (HR) with a 95% confidence interval (CI) was calculated using a Cox proportional hazards model.
3 Results
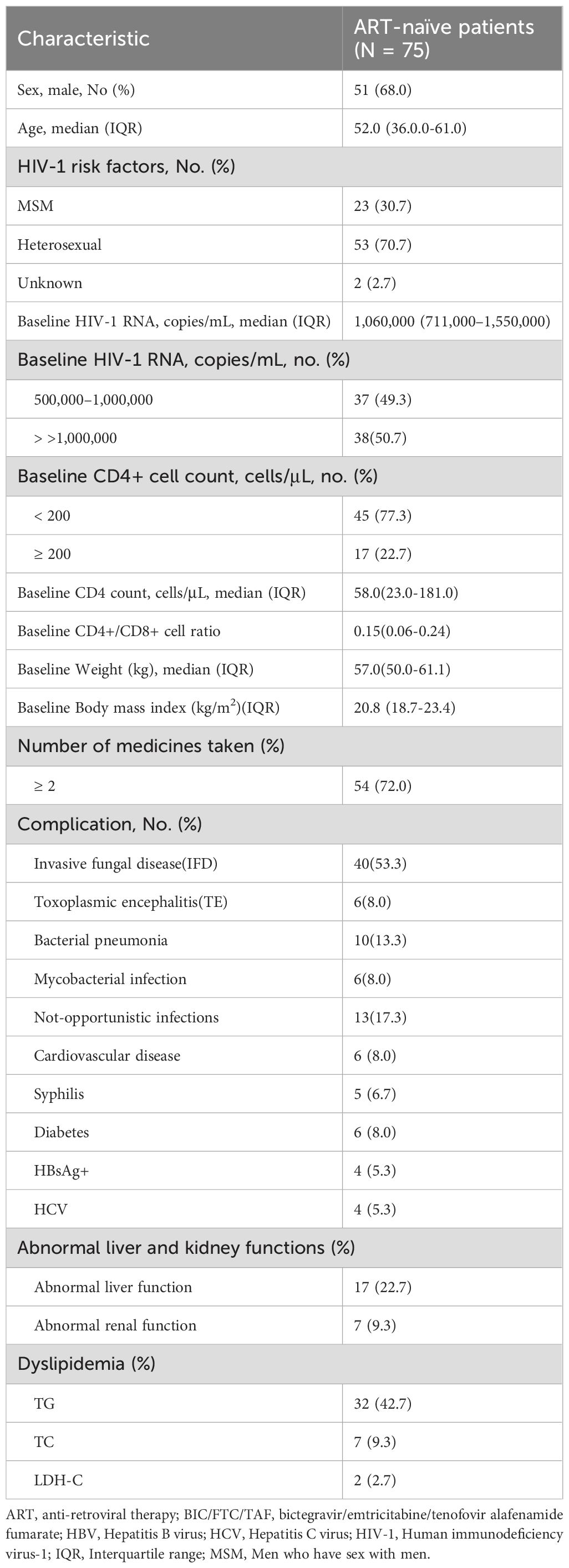
3.1 Baseline characteristics
Table 1 summarizes the baseline characteristics of the participants (Table 1).
3.2 Virologic responses
The mean decrease in virus load was 4 logs at 12 weeks. In the mITT analysis (N = 75), virologic suppression rates were 45.3% (34/75) at Week 12, 56.0% (42/75) at Week 24, 74.7% (56/75) at Week 48, and 78.7% (59/75) at Week 96. Per-protocol analysis (N = 58) demonstrated higher suppression rates: 56.9% (33/58), 63.8% (37/58), 98.3% (57/58), and 96.6% (56/58) at corresponding timepoints. The growing divergence (18% difference at W96) primarily reflects the impact of treatment discontinuations in the mITT cohort (Figure 2).
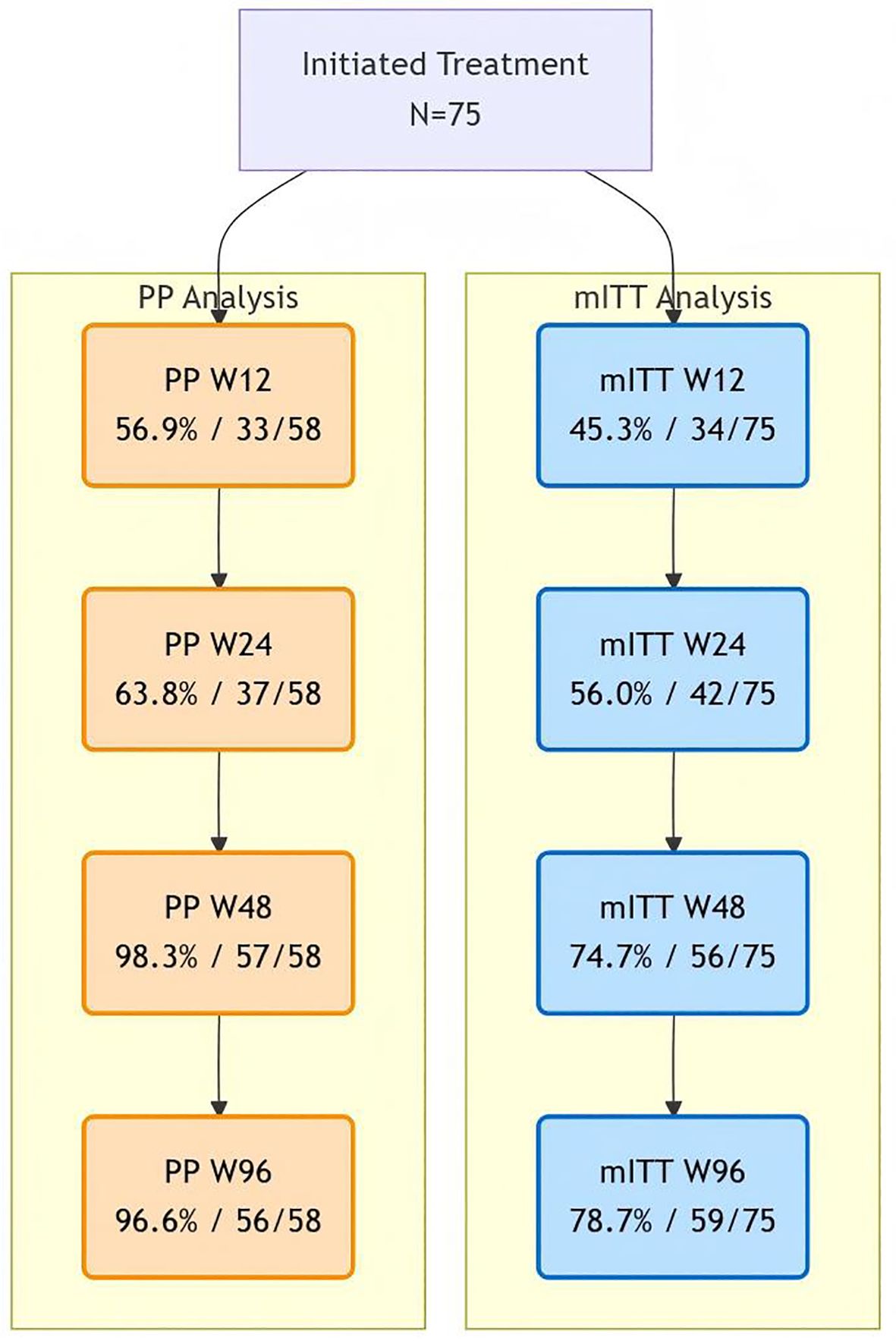
Figure 2. Viral suppression analysis figure. Comparative virologic suppression rates. Blue pathway shows mITT analysis (all initiators; discontinuations = failure). Orange pathway shows per-protocol analysis (completers only). Note: 2 late rebound in per-protocol (W48 98.3% → W96 96.6%).
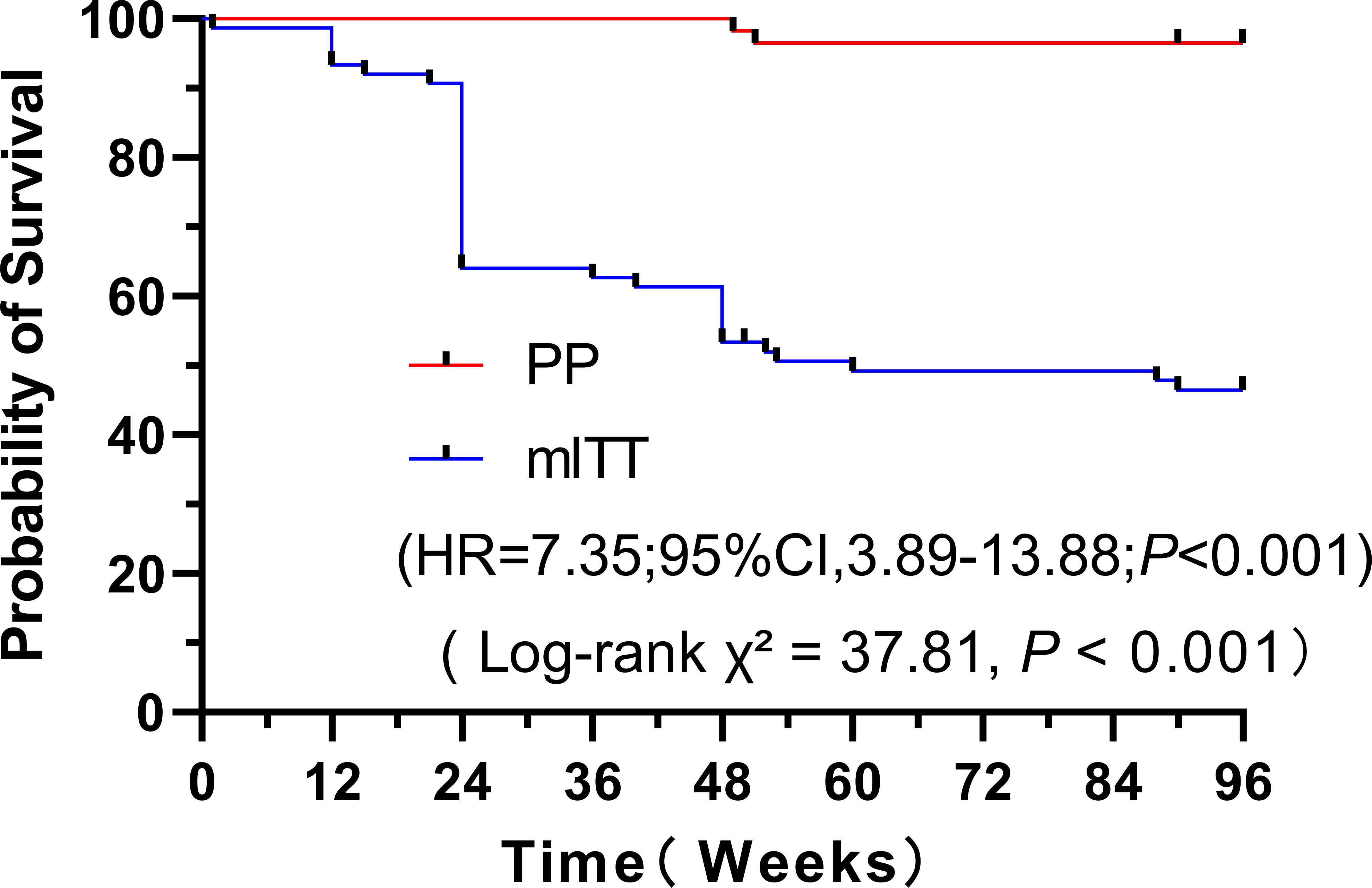
3.3 Time to virological failure
The virological durability of the BIC/FTC/TAF regimen was further assessed using Kaplan-Meier survival analysis in both cohorts (Figure 3). The Kaplan-Meier analysis (Figure 3) evaluated the cumulative probability of remaining free from virological failure or treatment discontinuation (i.e., ‘treatment success’). By week 96, this probability was significantly lower in the mITT cohort compared to the PP cohort (approximately <50% vs. >95%). This corresponds to a virological suppression rate at the Week 96 timepoint of 78.7% (59/75) in the mITT cohort and 96.6% (56/58) in the PP cohort. A significant difference in the risk of treatment failure was observed between the two groups (Hazard Ratio [HR] = 7.35, 95% Confidence Interval [CI]: 3.89 - 13.88; P < 0.001 (Figure 3).
Blue curve (mITT): Modified intention-to-treat cohort (N = 75). Treatment discontinuation for any reason (including regimen switch due to economic constraints, loss to follow-up, or death) was considered as an event. Orange curve (PP): Per-protocol cohort (N = 58). Events represent confirmed virological failure (HIV-1 RNA ≥50 copies/mL) in patients who completed the study protocol. Statistical analysis: The difference between the curves was statistically significant by the log-rank test (Hazard Ratio = 7.35, 95% Confidence Interval: 3.89-13.88; P < 0.001).
Subgroup analysis was performed according to the presence or absence of opportunistic infections. The proportions of patients with HIV RNA <50 copies/mL at 12 and 24 weeks were significantly higher in the opportunistic infection group compared with the non-opportunistic infection group (55.8% vs. 33.3%, P < 0.001; 76.4% vs. 53.3%, P < 0.001, respectively). At 48 weeks, the non-opportunistic infection group had significantly higher proportion than the opportunistic infection group (99.3 vs. 88.4, P < 0.001)(Table 2).
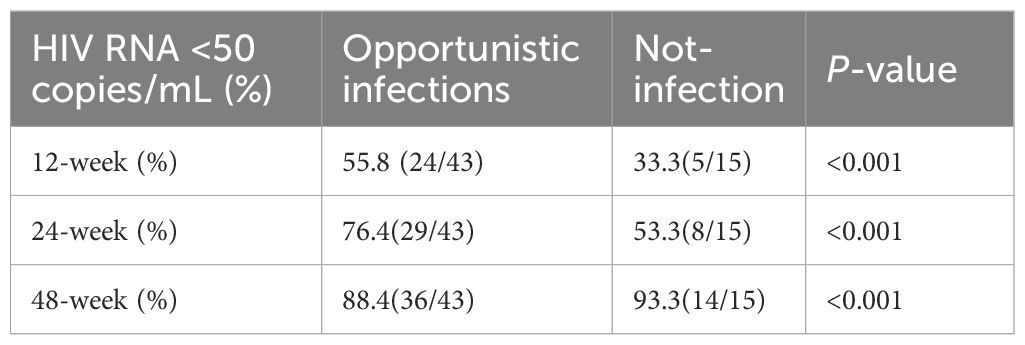
Table 2. Proportions of patients with HIV-1 RNA <50 copies/mL in the opportunistic infection and non-opportunistic infection groups.
3.3 Immunological responses
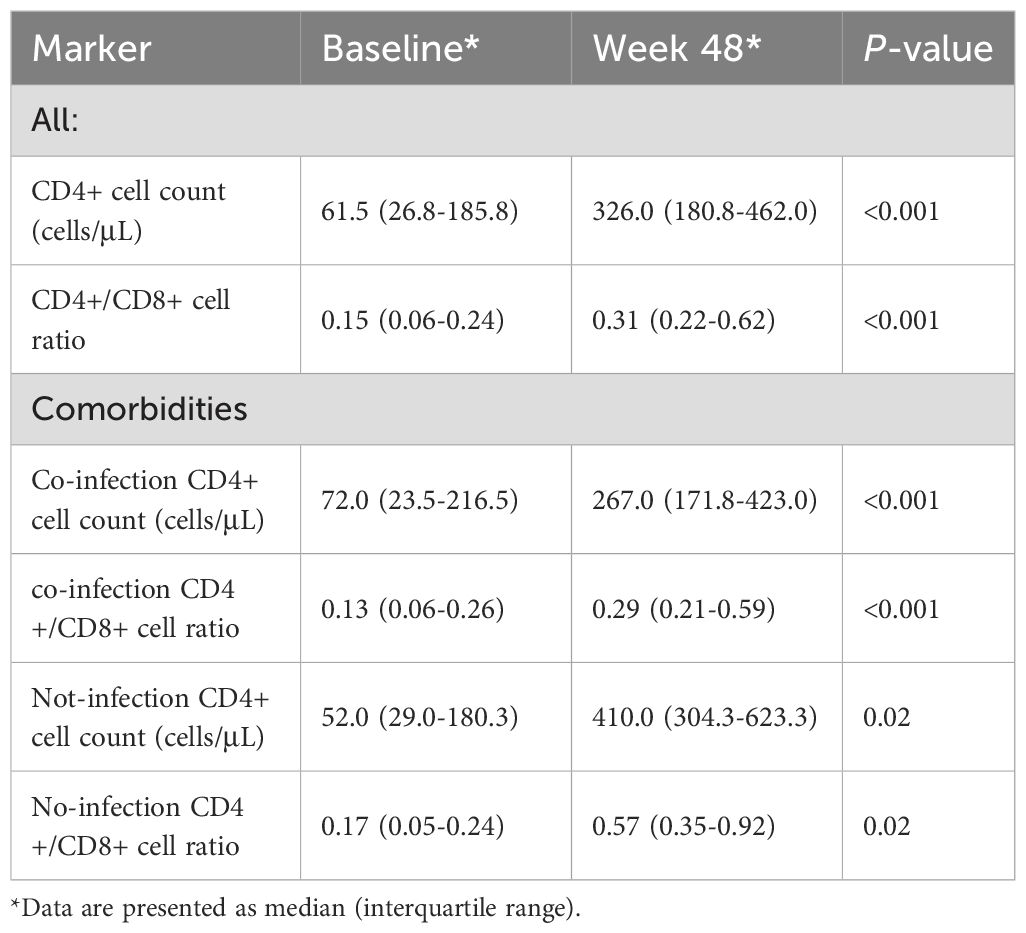
At 48 weeks, the CD4+ cell count increased from a median of 61.5 (268-185.8) cells/μL at baseline to 326.0 (180.8-462.0) cell/μL (P < 0.001) (Table 3). The CD4+/CD8+ cell ratio also increased from a median of 0.15 (0.06-0.38) at baseline to 0.31 (0.22-062) (P < 0.001) (Table 3). The CD4+ cell count in the opportunistic infection group increased from a median of 72.0 (23.5-216.5 cells/μL at baseline to 267.0 (171.8-423.0) cells/μL (P < 0.01) (Table 3), and the CD4+/CD8+ cell ratio also increased from a median of 0.13 (0.06-026) at baseline to 0.29 (0.21-0.59) (P < 0.001) (Table 3). CD4+ cell count in the non-opportunistic infection group increased from a median of 52.0 (29-180.3) cells/μL at baseline to 410.0 (304.3-623.3) cells/μL (P < 0.001) (Table 3), and the CD4+/CD8+ cell ratio also increased from a median of 0.17 (0.05-0.24) at baseline to 0.57 (0.35-0.92) (P < 0.001) (Table 3).
3.4 Laboratory indexes
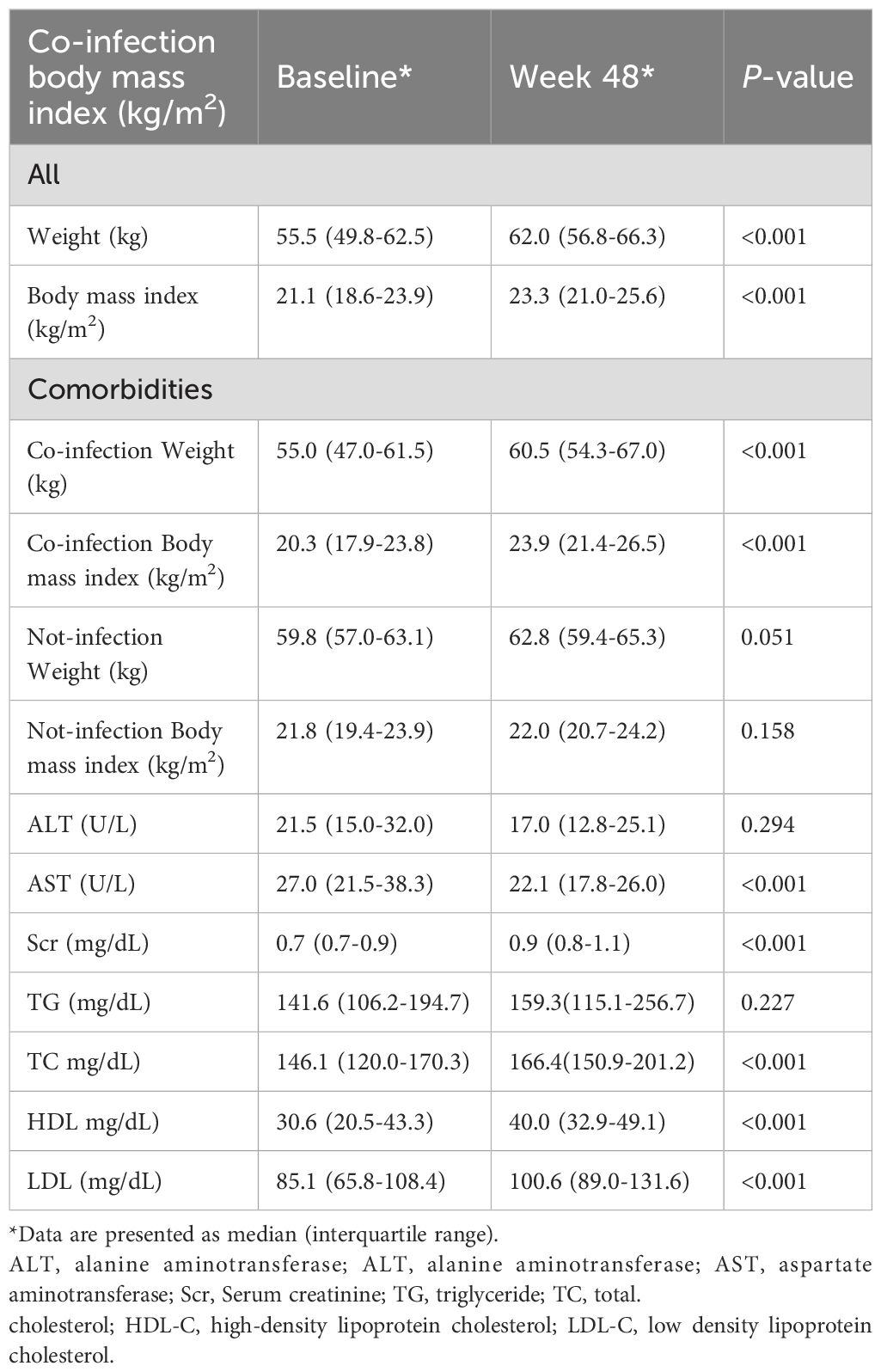
Table 4 lists the weight, body mass index, and laboratory indicators of patients at baseline and 48 weeks. The weight increased from a median of 55.5 (49.8-62.5) kg at baseline to 62.0 (56.8-6.3) kg, and the body mass index increased from a median of 21.1 (18.6-23.9) kg/m2 at baseline to 23.3 (21.0-25.6) kg/m2. Patient weights in both the opportunistic infection and non-opportunistic infection groups were higher than baseline values (55.0 vs 60.5 kg, P < 0.001; 59.8 vs 62.8 kg, P < 0.001), and body mass index values in both groups increased (20.3 vs 23.9 kg/m2, P < 0.001; 21.8 vs 22.0 kg/m2, P < 0.001). Alanine aminotransferase (21.5 vs 17.0 U/L, P < 0.001) and aspartate aminotransferase (27.0 vs 25.1 U/L, P < 0.001) were lower at 48 weeks than at baseline. Serum creatinine (58.1 vs 82.0, μmol/L, P < 0.001) was higher at 48 weeks than at baseline. Triglycerides (1.6 vs 1.8 μmol/L, P = 0.227), total cholesterol (3.8 vs 4 μmol/L, P < 0.001), high-density lipoprotein (0.79 vs 1.06 μmol/L, P < 0.001), and low-density lipoprotein (2.2 vs 2.6 μmol/L, P < 0.001) levels were higher at 48 weeks than at baseline (Table 4).
3.5 Drug resistance and maintenance treatment
No resistance was detected at 96 weeks of follow-up, and 5.2% (3/58) of patients stopped taking the drugs due to poor compliance after 48 weeks, resulting in virological rebound. After education and counseling, the patients resumed taking the medications, and the virus was under control. At 96 weeks, 3.5% (2/58) of patients experienced virological rebound due to poor compliance.
3.6 Adverse events
During the follow-up period, 2 cases of mild skin allergic itching, 1 of mild dizziness and 1 of mild chest tightness, accounting for a total of 6.9% (4/58). These adverse events were considered to be related to BIC/FTC/TAF, and no patients discontinued the medication as a result. There was an obvious increase in blood lipids in 1.7% (1/58) of patients. This patient had hyperlipidemia at the baseline. After adjusting the diet and engaging in appropriate exercise, the patient’s lipid levels returned to normal, and the treatment plan was changed.
4 Discussion
In the GS-US-380-1489 and 1490 clinical trials (4, 5). Our per-protocol analysis demonstrated high virologic efficacy of BIC/FTC/TAF in treatment-naïve patients with ultra-high viral loads, achieving 96.6% (56/58) suppression at week 96. However, the mITT analysis revealed a significant efficacy-effectiveness gap, with only 78.7% (59/75) achieving virologic suppression at the same timepoint. This 17.9% divergence (Figure 2) primarily reflects real-world implementation challenges, where socioeconomic factors accounted for 64.7% (11/17) of treatment discontinuations.
Early response (W12-W24): Both cohorts showed similar trajectories (mITT: 45.3%→56.0%; PP: 56.9%→63.8%), confirming BIC/FTC/TAF’s rapid antiviral activity regardless of baseline characteristics (6). Mid-treatment divergence (W48): While PP analysis showed near-perfect suppression (98.3%), mITT results plateaued at 74.7%, coinciding with median discontinuation time. Late outcomes (W96): The PP cohort maintained high suppression (96.6%), but the single rebound case (W48: 57/58 → W96: 56/58) underscores the need for ongoing adherence monitoring even in virologically controlled patients (7). These findings align with recent real-world evidence from the BICSTaR study (8), which reported 12-month suppression rates of 94.0% in optimal conditions but noted 22% lower effectiveness in economically vulnerable populations. Our data extend these observations by quantifying the economic attrition penalty: patients who switched regimens due to financial constraints had achieved virologic suppression before discontinuation, suggesting treatment efficacy wasn’t the limiting factor.
Our Kaplan-Meier analysis (Figure 3) provides the most compelling statistical evidence for the central finding of this study: the chasm between drug efficacy and real-world effectiveness. The significant divergence of the survival curves (HR = 7.35, 95% CI: 3.89–13.88; P<0.001) quantifies the dramatic impact of non-virological attrition, demonstrating that the risk of treatment failure was over 7 times higher in the all-initiator mITT cohort. The tight 95% CI, excluding 1.0, underscores the precision of this estimate.
This observed “efficacy-effectiveness gap” is corroborated by other real-world studies. The global BICSTaR study, which included both treatment-naïve and treatment-experienced PWH, reported a high overall virological suppression rate of 94.0% at 12 months, but similarly identified patient-related factors, including socioeconomic challenges, as predictors of suboptimal outcomes (6). Furthermore, the Asia cohort of the BICSTaR study observed slightly lower real-world effectiveness compared to clinical trial results, reinforcing the universal challenge of translating ideal trial conditions into routine clinical practice, particularly in our region (7). Our study quantifies this gap with striking clarity in a vulnerable uVL population and specifically identifies overwhelming economic constraints as the predominant driver, rather than drug-related issues. This visualization powerfully reframes the outcome: the primary driver of suboptimal effectiveness was not a failure of the drug regimen, but a failure of the socioeconomic ecosystem supporting the patient. The steepest decline in our mITT curve coincides with the median time to discontinuation due to economic constraints, pinpointing a critical window for intervention. Therefore, while BIC/FTC/TAF proves to be a potent therapeutic agent capable of achieving rapid suppression and immune reconstitution even in uVL patients—as demonstrated by our high PP rates and robust CD4+ recovery—its population-level benefit is contingent on parallel programmatic strategies that address affordability and sustain retention (16).
Our stratified analysis revealed a paradoxical virologic response pattern: patients with baseline opportunistic infections (OIs) demonstrated significantly higher early suppression rates at Weeks 12-24 compared to non-OI counterparts (W12: 55.8% vs. 33.3%; W24: 76.4% vs. 53.3%; both P < 0.01), but this advantage reversed by Week 48 (non-OI: 93.3% vs. OI: 88.4%; P = 0.04). This biphasic pattern suggests two distinct mechanisms at play. Rapid initial viral suppression may reflect enhanced immune activation from concurrent antimicrobial therapies (anti-TB/anti-fungal agents) that synergize with BIC/FTC/TAF’s potency.
The lower the CD4+ cell count and CD4+/CD8+ cell ratio, the higher the odds to develop AIDS-related opportunistic infections, which severely affect the patient’s life span. In this study, CD4+ cell count increased from a median of 61.5 cells/μL at baseline to 26.0 cells/μL at 48 weeks, and the CD4+/CD8+ cell ratio also increased from a median of 0.15 to 0.31, which corroborates previous findings (4, 5, 9). In patients with severe opportunistic infections, CD4+ cell count and CD4+/CD8+ cell ratio were significantly increased after treatment with BIC/FTC/TAF (from a median of 72 cells/μL to 267 cells/μL, and the CD4+/CD8+ cell ratio also increased from a median of 0.13 at baseline to 0.29), indicating that BIC/FTC/TAF can significantly improve the immune system of patients with advanced AIDS, which is essential for preventing opportunistic infections.
Patients experienced significant weight gain at week 48 (median +6.5kg; BMI 21.1→23.3 kg/m²), particularly pronounced in the opportunistic infection subgroup (BMI 20.3→23.9 kg/m²). This aligns with expected ART-associated health recovery (9). Hepatic and Renal Safety: Baseline hepatic abnormalities (27.6%, 16/58), all OI-related, normalized post-ART. Serum creatinine elevations remained within normal limits, matching TAF’s known renal profile (10–13). Lipid parameters showed modest increases, consistent with established BIC/FTC/TAF profiles (14–17).
No treatment-emergent resistance was observed through 96 weeks, confirming BIC/FTC/TAF’s high genetic barrier in ultra-high viral load patients (6). Virologic rebounds occurred in 3.5% (2/58) due to non-adherence, with prompt re-suppression after intervention. Mild adverse events (pruritus, dizziness; 6.9%) were transient and unrelated to treatment (18, 19).
The primary limitation stems from the significant divergence between mITT (all initiators; discontinuations = failure) and PP (completers only) analyses, revealing an 17.9% efficacy-effectiveness gap at Week 96 (mITT: 78.7% vs. PP: 96.6%). This highlights real-world implementation challenges, particularly socioeconomic barriers—64.7% of discontinuations were due to cost-driven regimen switches, not treatment failure. Additional constraints include the single-center retrospective design, small sample size (n=58 PP), absence of a control arm, and exclusion of key subgroups (e.g., children).
5 Conclusions
BIC/FTC/TAF demonstrated high efficacy and safety in adherent patients with ultra-high viral loads (96.6% suppression at W96), with no emergent resistance. However, real-world effectiveness was substantially compromised by non-clinical dropouts, emphasizing the critical need for programmatic interventions to ensure affordability and improve retention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Department of Infection, Guiyang Public Health Treatment Center, Reasoning Committee of Guiyang Public Health Treatment Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XH: Data curation, Investigation, Validation, Methodology, Conceptualization, Writing – review & editing, Visualization, Writing – original draft, Project administration. XX: Writing – review & editing, Supervision, Conceptualization. YF: Supervision, Writing – review & editing, Investigation. JH: Validation, Data curation, Writing – review & editing, Formal Analysis. XL: Data curation, Writing – original draft, Methodology, Investigation. HL: Supervision, Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the site staff who supported the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Association CM. Chinese AIDS diagnosis and treatment guide (2024 edition). New Infect Dis Electronic J. (2024) 9:68. doi: 10.13419/j.cnki.aids.2024.08.01
2. Committee CLMGrJE. Chinese guidelines for lipid management (2023). Chin J Cardiovasc Dis. (2023) 51:221–55. doi: 10.3760/cma.j.cn112148-20240102-00002
3. Aithal G, Watkins P, Andrade R, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Therap. (2011) 89:806–15. doi: 10.1038/clpt.2011.58
4. Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. (2017) 390:2063–72. doi: 10.1016/S0140-6736(17)32299-7
5. Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. (2017) 390:2073–82. doi: 10.1016/S0140-6736(17)32340-1
6. Esser S, Brunetta J, Inciarte A, Levy I, D’Arminio Monforte A, Lambert JS, et al. Twelve-month effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide in people with HIV: Real-world insights from BICSTaR cohorts. HIV Med. (2024) 25:440–53. doi: 10.1111/hiv.13593
7. Tseng YT, Yang CJ, Kim YS, Choi JY, Wong CS, Lee KY, et al. Twelve-month effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide in treatment-naïve and treatment-experienced people with HIV: Findings from the Asia cohort of the BICSTaR study. J Microbiol Immunol Infect. (2024) 57:760–70. doi: 10.1016/j.jmii.2024.07.003
8. Lazzaro A, Cacciola EG, Borrazzo C, Innocenti GP, Cavallari EN, Mezzaroma I, et al. Switching to a bictegravir single tablet regimen in elderly people living with HIV-1: data analysis from the BICTEL cohort. Diagnostics. (2022) 12:76. doi: 10.3390/diagnostics12010076
9. Kumar S and Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus. Cardiovasc Disease Mortality. (2018) 9:705. doi: 10.3389/fendo.2018.00705
10. Perrone RD, Madias NE, and Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. (1992) 38:1933–53. doi: 10.1093/clinchem/38.10.1933
11. Ando M and Yanagisawa N. Epidemiology, clinical characteristics, and management of chronic kidney disease in human immunodeficiency virus-infected patients. World J Nephrol. (2015) 4:388–95. doi: 10.5527/wjn.v4.i3.388
12. Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. (2018) 5:e357–e65. doi: 10.1016/S2352-3018(18)30092-4
13. Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. (2013) 207:1359–69. doi: 10.1093/infdis/jit043
14. Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, and Martens F. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta. (2008) 396:49–55. doi: 10.1016/j.cca.2008.06.017
15. Lazzaro A, Bianchini D, Gentilini Cacciola E, Mezzaroma I, Falciano M, Andreoni C, et al. Immune Reconstitution and Safe Metabolic Profile after the Switch to Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among Virologically Controlled PLWH: A 96 Week Update from the BICTEL Cohort. Viruses. (2023) 15:1222. doi: 10.3390/v15061222
16. Sax PE, Arribas JR, Orkin C, Lazzarin A, Pozniak A, DeJesus E, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. EClinicalMedicine. (2023) 59:101991. doi: 10.1016/j.eclinm.2023.101991
17. AIDS Professional Group Society of Tropical Disease and Parasitology of Chinese Medical Association. Chinese expert consensus on integrated lipid management in HIV/AIDS. Zhonghua Nei Ke Za Zhi. (2023) 62:661–72. doi: 10.3760/cma.j.cn112138-20230321-00165
18. Micán R, de Gea Grela A, Cadiñanos J, de Miguel R, Busca C, Bernardino JI, et al. Impact of preexisting nucleos(t)ide reverse transcriptase inhibitor resistance on the effectiveness of bictegravir/emtricitabine/tenofovir alafenamide in treatment experience patients. Aids. (2022) 36:1941–7. doi: 10.1097/QAD.0000000000003311
Keywords: bictegravir/emtricitabine/tenofovir alafenamide fumarate, HIV/AIDS, ultra-high viral load, efficacy, safety
Citation: He X, Xie X, Fu Y, He J, Luo X and Long H (2025) Effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide fumarate for people with ultra-high viral load of HIV-1: a retrospective real-world cohort study. Front. Virol. 5:1653276. doi: 10.3389/fviro.2025.1653276
Received: 04 July 2025; Accepted: 29 October 2025;
Published: 17 November 2025.
Edited by:
Siew Pheng Lim, Singzyme Pte. Ltd., SingaporeReviewed by:
Erica Diani, University of Verona, ItalyMiguel Torralba, Universidad de Alcalá. Ciber de Enfermedades Respiratorias (CIBERES), Spain
Copyright © 2025 He, Xie, Fu, He, Luo and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Long, bG9uZ2xvbmcxMjI1QDEyNi5jb20=
 Xiangxi He
Xiangxi He Xiaoxin Xie
Xiaoxin Xie Hai Long
Hai Long