- 1Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, Bethesda, MD, United States
- 2Department of Virology/Immunology, National Institute of Health, Park Road, Chak Shahzad, Islamabad, Pakistan
- 3Department of Biochemistry, Faculty of Science, University of Yaounde I, Yaounde, Cameroon
Editorial on the Research Topic
Epidemiology and control of blood-borne viruses in low- and middle-income countries: genomic insights and public health strategies
Blood-borne viruses (BBVs), including human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and human T-cell lymphotropic virus (HTLV), continue to impose a staggering burden on global health, with a disproportionate impact on low- and middle-income countries (LMICs). These regions face a dual challenge of the highest prevalence of infections and the most significant constraints on resources needed for effective public health responses. Ambitious global targets, such as the World Health Organization’s (WHO) goal to eliminate viral hepatitis as a public health threat by 2030, underscore the urgency of the situation (1). However, achieving these goals requires a nuanced understanding of the complex interplay between viral evolution, transmission dynamics, socio-economic factors, and the challenges of healthcare delivery in resource-limited settings.
It is precisely this need that our Research Topic, “Epidemiology and Control of Blood-Borne Viruses in Low and Middle-Income Countries: Genomic Insights and Public Health Strategies” was conceived to address. The objective was to collate a broad collection of studies that leverage molecular epidemiology, data-driven modeling, and implementation science to illuminate the path toward controlling BBVs in LMICs. We are pleased to present a collection of five articles that provide a compelling, multi-faceted perspective on these critical issues.
A multi-layered view of a complex challenge
The initial aim of this Research Topic was to explore the landscape of BBV control from multiple perspectives, from global burden estimates to implementation. The five articles in this Research Topic have successfully met this objective, each providing a unique and vital perspective on this multifaceted challenge.
At the highest level, the work by Zhang et al. provides a sweeping analysis of the global burden of acute viral hepatitis using the Global Burden of Disease (GBD) Study 2021. Their findings confirm that while incidence rates are declining globally, the burden remains immense and is tightly, and inversely, correlated with the socio-demographic index (SDI). This establishes a foundational theme of the Research Topic: lack of fair access to health care is a primary driver of the BBV epidemic.
Zooming into a continental focus, the systematic review by Ambassa et al. addresses the critical issue of viral co-infections in Africa. By synthesizing data on HIV-HBV and HIV-HCV co-infections, they highlight the necessity for integrated screening and management strategies, reminding us that patient care must be all-inclusive.
At the national and sub-national level, two articles use distinct methodologies to dissect epidemic drivers. Brites et al. conducted a multicenter cross-sectional study of HTLV-1 in Brazil, an often-neglected virus that is endemic in the country. Their work provides crucial prevalence data and identifies key risk factors, older age, female gender, and HCV co-infection, pointing toward sexual transmission as a primary driver and demonstrating the importance of surveillance in specific, at-risk populations (Figure 1A). In parallel, Abbas et al. employed mathematical modeling to create a data-driven comparison of the HIV epidemics in Pakistan and the USA. Their model illustrates the consequences of different access to resources, with a reproduction number (R0) well above 2 in Pakistan versus below 1 in the USA, attributing this difference to lower rates of screening and treatment.
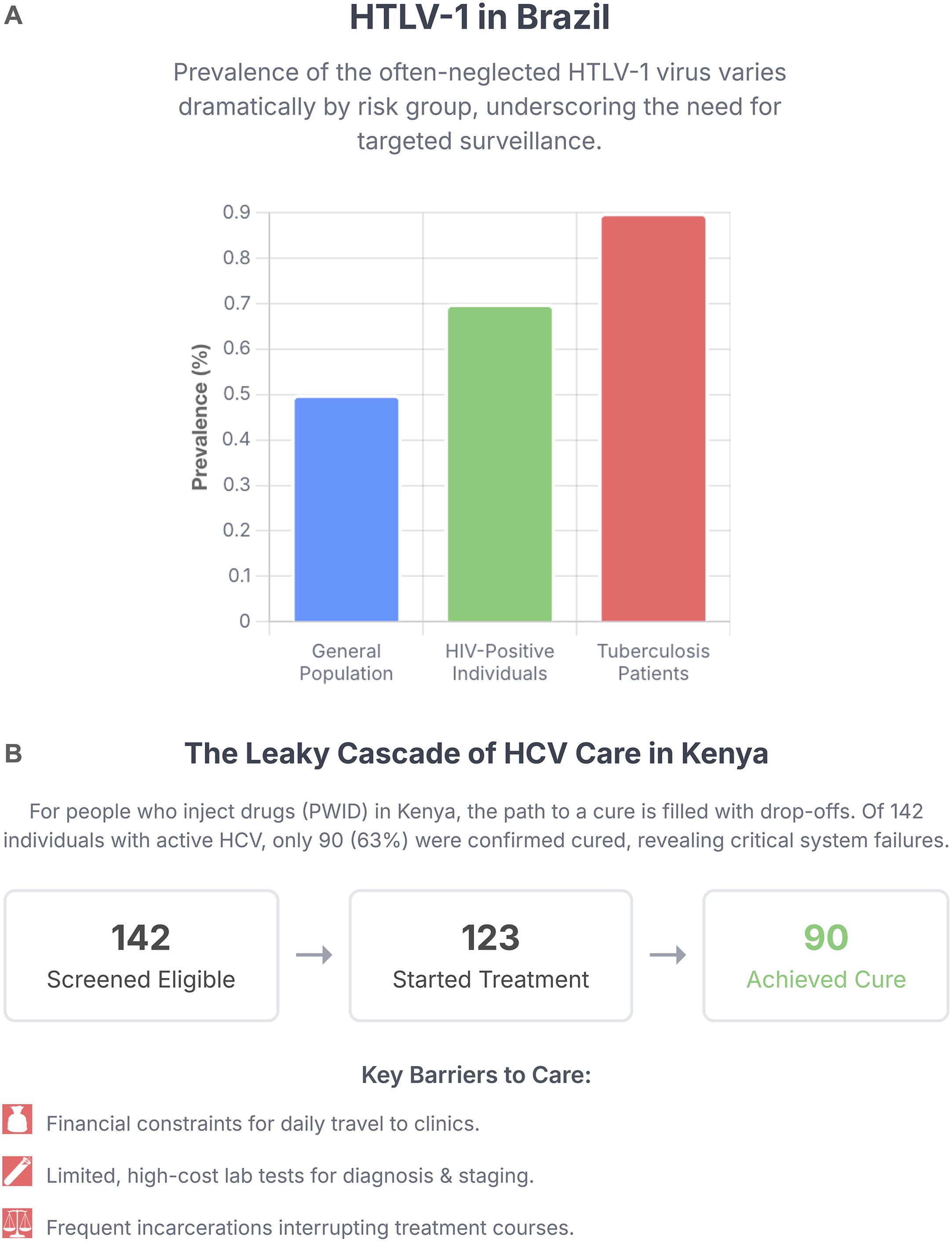
Figure 1. Challenges in the cascade of care and the need for targeted surveillance in low- and middle-income countries. (A) Varying prevalence of human T-cell Lymphotropic Virus type 1 (HTLV-1) among different risk populations in Brazil, highlighting the high burden in those with HIV and Tuberculosis co-infections and the need for integrated screening. Data from Brites et al. (B) The cascade of care for Hepatitis C Virus (HCV) treatment among people who inject drugs (PWID) in Kenya, illustrating significant patient drop-off between screening and cure. Data from Boke et al..
Finally, bringing us to the front lines of healthcare delivery, the community case study by Boke et al. details the experiences and challenges of providing curative HCV treatment to people who inject drugs (PWID) in Kenya. This work moves beyond epidemiology to the practicalities of implementation, identifying critical barriers such as limited laboratory capacity and financial constraints for patients, that lead to significant patient drop-off in the cascade of care (Figure 1B), while also showcasing the promise of peer-led, integrated care models
Emerging themes and key insights
Synthesizing the findings from these diverse articles reveals several cross-cutting themes that are central to advancing the fight against BBVs in LMICs.
First is the pervasive and defining role of socio-economic disparity. This was quantified on a global scale by Zhang et al., modeled mechanistically by Abbas et al., and observed at the patient level by Boke et al., where a patient’s inability to afford transportation could mean the difference between treatment adherence and failure. This Research Topic reinforces that biomedical interventions alone are insufficient; they must be embedded within strategies that address the structural determinants of health.
A second major theme is the critical need for integrated and targeted public health strategies. The “one-size-fits-all” approach is demonstrably inadequate. Ambassa et al. call for the integration of hepatitis care with HIV programs, a sentiment echoed by the findings of Brites et al. regarding HTLV-1/HCV co-infection. Furthermore, the importance of targeting key populations is paramount. The focus on PWID by Boke et al. aligns with micro-elimination strategies that can provide stepping stones toward broader national goals. Similarly, the modeling by Abbas et al. identifies the undiagnosed infected population as the primary engine of HIV transmission, making targeted screening the most effective intervention.
The third theme is the power of data and diagnostics to guide policy and practice. From the GBD data used by Zhang et al. to inform global priorities, to the mathematical model of Abbas et al. that provides a specific, actionable threshold for treatment rates needed to control an epidemic, data are the bedrock of an effective response. This extends to the individual level, where the work of Boke et al. highlights that a lack of access to affordable and reliable confirmatory tests (like HCV RNA) creates a fundamental bottleneck in the cascade of care. Without the ability to diagnose, we cannot treat.
Future directions: from insight to impact
The insights gleaned from this Research Topic illuminate a path forward for both research and public health policy.
First, there must be a concerted focus on bridging the implementation gap. We have highly effective tools, such as direct-acting antivirals for HCV and antiretroviral therapy for HIV. Yet, as the study in Kenya by Boke et al. vividly illustrates, the journey from drug availability to patient cure is fraught with obstacles. Future research must prioritize implementation science to develop, test, and scale up simplified service delivery models. This includes decentralizing care, task-shifting to community health workers and peers, and advocating for policies that reduce out-of-pocket costs for patients (2).
Second, we must fully harness the power of genomic and molecular epidemiology. Future work should expand on the use of molecular diagnostics seen in the study by Brites et al. to include phylogenetic and phylodynamic analysis for tracking transmission networks, monitoring for the emergence of drug resistance, and understanding the distribution of viral genotypes that may impact treatment or vaccine efficacy. Such data provide the high-resolution intelligence needed for precision public health interventions (3, 4).
Third, we must redouble efforts to address neglected infections and co-infections. The inclusion of HTLV-1 by Brites et al. is a crucial step, but a broader effort is required to integrate other neglected infections into mainstream surveillance, research, and clinical care. The syndemic nature of BBVs with each other and with other conditions like tuberculosis requires a fundamental shift away from vertical, disease-specific programs toward integrated, person-centered care. In particular, hepatitis D virus (HDV), the most severe form of viral hepatitis, remains almost entirely unaddressed in many LMICs and represents a major gap in surveillance and care (5).
In conclusion, the articles in this Research Topic collectively argue that while the challenges of controlling BBVs in LMICs are immense, they are not insurmountable. The path forward requires commitment to fair access to health care and related underlying socioeconomic conditions, the strategic integration of services, the intelligent use of data to guide interventions, and a relentless focus on overcoming the real-world barriers that that prevent people from achieving good health. We hope that this Research Topic will serve as a valuable resource and a catalyst for research and innovation, needed to turn the tide against these devastating epidemics.
Author contributions
NT: Writing – original draft, Writing – review & editing, Visualization. ST: Writing – review & editing. MD: Writing – review & editing, Project administration, Supervision.
Acknowledgments
The opinions expressed in this article are those of the authors and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. Geneva: World Health Organization (2022). Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies (Accessed July 28, 2025)).
2. Dore GJ, Ward J, and Thursz M. Hepatitis C disease burden and strategies to manage the burden. J Viral Hepat. (2014) 21 Suppl 1:1–4. doi: 10.1111/jvh.12208
3. Rich SN, Richards V, Mavian C, Rife Magalis B, Grubaugh N, Rasmussen SA, et al. Application of phylodynamic tools to inform the public health response to COVID-19: qualitative analysis of expert opinions. JMIR Form Res. (2023) 7:e39409. doi: 10.2196/39409
4. Phylogenetic and phylodynamic approaches to understanding and combating the early SARS-CoV-2 pandemic | Nature Reviews Genetics . Available online at: https://www.nature.com/articles/s41576-022-00483-8.
Keywords: blood-borne viruses, LMICs, surveillance, implementation science, co-infection, molecular epidemiology, resource-limited settings
Citation: Trovao NS, Tamim S and Djuidje Ngounoue M (2025) Editorial: Epidemiology and control of blood-borne viruses in low- and middle-income countries: genomic insights and public health strategies. Front. Virol. 5:1696495. doi: 10.3389/fviro.2025.1696495
Received: 31 August 2025; Accepted: 01 October 2025;
Published: 13 October 2025.
Edited and reviewed by:
Akio Adachi, Tokushima University, JapanCopyright © 2025 Trovao, Tamim and Djuidje Ngounoue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidia S. Trovao, bmlkaWEudHJvdmFvQG5paC5nb3Y=; Marceline Djuidje Ngounoue, ZGpuZ21hcmNlYXVAeWFob28uZnI=
 Nidia S. Trovao
Nidia S. Trovao Sana Tamim
Sana Tamim Marceline Djuidje Ngounoue
Marceline Djuidje Ngounoue