- Unit of Clinical Microbiology, Department of Health Sciences, “Magna Græcia” University Hospital of Catanzaro, Catanzaro, Italy
Introduction: Human Cytomegalovirus (CMV) infection represents a significant health burden, particularly for immunocompromised patients, including solid-organ transplant (SOT) recipients and people living with human immunodeficiency virus (PLWH). Despite the availability of antiviral prophylaxis and treatment, prolonged therapy can lead to viral drug resistance, complicating disease management. In this study, we present a series of CMV cases in immunocompromised patients, including two SOT recipients and one PLWH patient, focusing on microbiological data, clinical presentation, and therapeutic management.
Methods: CMV serostatus and DNA viral load were carried out by Chemiluminescence Immunoassay (CLIA) and quantitative real-time PCR to monitor patient follow-up.
Results: The three patients had CMV reactivation following an immunocompromised status. The prompted antiviral treatments determined the viral infection resolution, despite CMV-related complications worsening clinical outcomes.
Discussion: The development of a safe and effective CMV vaccine represents a needed challenge, especially for individuals at high risk of severe CMV-related complications. However, it is difficult to achieve high CMV variability. Our findings contribute to the ongoing discussion on the importance of developing vaccines to mitigate CMV-related morbidity in vulnerable populations.
1 Introduction
The Human Cytomegalovirus (CMV), Herpesviridae family, Betaherpesvirinae subfamily, is a widespread Herpesvirus infecting a significant portion of the global population (1). It is known for the ability to establish lifelong latent infections after primary exposure, which predominantly occur during childhood or early adulthood (1). CMV exhibits considerable genetic diversity compared to other human Herpesviruses, largely due to recombination and coinfection events (2–4). The viral seroprevalence ranges from 45% to 100% worldwide, with specific IgG antibody positivity in up to 60% of adults in industrialized countries and over 90% in individuals with lower socioeconomic conditions (5). Typically, primary infection is asymptomatic or causes mild, mononucleosis-like symptoms in immunocompetent individuals (1).
However, CMV poses severe health risks to immunocompromised patients (6) and women who contract primary CMV infection during the first trimester of pregnancy (7, 8). Currently, congenital CMV infection remains the leading cause of non-genetic sensorineural hearing loss and a significant contributor to neurodevelopmental disorders (9). In solid-organ transplant (SOT) recipients, hematopoietic stem cell transplantation (SCT) recipients, and people living with human immunodeficiency virus (PLWH) (9), the virus may reactivate, leading to illnesses that include pneumonia, gastroenteritis, and retinitis (6). Additionally, reinfection with a new CMV strain can occur through direct contact with an infectious individual (1). Although the target populations and the mechanisms of vaccine delivery are not yet defined, it is well known that children born with congenital infection and immunocompromised subjects are the two groups of patients suffering the most serious consequences of CMV contact. Thus, the most suitable vaccination targets could be transplant recipients and pregnant women or seronegative women of childbearing age to prevent congenital infection (10, 11). Antiviral prophylaxis or treatments that prevent serious outcomes of diseases are currently available for SOT, HSCT, and PLWH patients. However, prolonged antiviral therapies can accumulate mutations in CMV DNA hotspots, conferring resistance to antivirals and consequently causing therapy failure (12). The different clinical scenarios (primary infection, reactivation, and reinfection) and treatments cause virus variability (12–14). In particular, the genes encoding envelope glycoproteins may segregate by genetic assortment into distinct genotypes, which have been reported to influence viral virulence, immune response, and disease outcome (15–17). Considering the key role of envelope glycoproteins in CMV infection and antibody (Ab)-mediated neutralization immunity, the development of an effective and safe vaccine for CMV that can provide broad protection against multiple genotypes, without causing severe complications, is considered a major challenge that would, however, address a relevant and yet unsatisfied medical need (18, 19). In this study, we report the clinical and therapeutic management of CMV infection in immunocompromised patients, specifically two SOT recipients and one PLWH. By reviewing antiviral treatments, dosing strategies, and patient outcomes, we reported the effectiveness of current CMV management approaches. Additionally, to address the discussion on the importance of vaccination in immunocompromised populations, we started with the main virological characteristics and infection control, going through the current vaccine research.
2 Materials and methods
2.1 Study design
Accurate diagnosis of CMV and practices preventing CMV infection are of paramount importance to several special populations, such as SOT recipients and PLWH. The prevention strategies and diagnostic tests performed in special populations infected with CMV are summarized in Figure 1.

Figure 1. Schematic overview of CMV infection prevention strategies and diagnostic tests in special population. SOT, solid-organ transplant recipients; PLWH, People living with HIV infection.
CMV serostatus, viral genomic detection (DNA or mRNA), and specific cell-mediated immunity (CMV-CMI) are important factors that determine outcomes after SOT and in PLWH under antiretroviral therapy (ART) (20, 21). To improve the post-operative outcome of transplantation, it is crucial to shift the focus of CMV detection to the donor and achieve early diagnosis, as well as implement effective preventive and therapeutic measures. For CMV prevention, there are two main methods, the first one is universal prophylaxis, which involves giving antiviral medication at prophylaxis dose when either the donor and/or the recipient is seropositive for CMV. The second one is preemptive therapy, which is defined as serial testing done weekly, through quantitative PCR, for the first few months after transplant or after treatment of rejection with a treatment dose of antiviral therapy (22).
2.2 Antiviral treatment and candidate vaccine
At present, the United States Food and Drug Administration (FDA) has approved six drugs, which target specific genomic regions, to treat or prevent CMV disease (11). Two main different approaches, based on patient CMV immune status, clinical conditions, risk factors, and co-morbidities, were adopted for the prevention of primary, reactivated, or recurrent CMV infection: universal prophylaxis and/or pre-emptive therapy. Universal prophylaxis makes it possible to maintain viral latency for CMV infection or reactivation in high-risk SOT and allo-HSCT recipients, as well as in PLWH (23). However, this therapeutic strategy is administered to all patients, even those in whom CMV cannot reactivate, thus needlessly exposing them to side effects. Moreover, this approach does not prevent the virus from reactivating after the discontinuation of the prophylactic therapy (24). Pre-emptive therapy is applied to asymptomatic CMV-infected patients with positive viremia diagnosed by molecular screening tests. Based on the CMV viral load measured for 3 or 6 months, antiviral agents are to be administered weekly (25). To avert the risk of CMV reactivation after prophylaxis discontinuation, a hybrid approach (prophylactic approach for 3 to 6 months, followed by pre-emptive therapy) has also been evaluated (26, 27). In 2017, the introduction of letermovir (LTM), a new anti-CMV molecule that binds to the components of the viral terminase complex (UL51, UL56, and UL89) to block CMV DNA processing and viral particle packaging, provided a novel prophylactic approach (28, 29). In a randomized controlled trial, LTM prophylaxis proved to be superior to pre-emptive therapy in reducing the clinical picture of CMV infections and enhancing 24-week survival rates (30). However, prolonged and repeated use of anti-CMV therapies (which typically last months) can lead to the accumulation of mutations in target regions of the CMV genome; this, in turn, confers resistance to antivirals (Table 1) (29–40).
The CMV management guidelines for patients with persistent symptoms of disease or rising/relapsing viremia recommend genotypic resistance testing (11). Several candidate vaccines for CMV that aim to prevent congenital and post-transplant infections in immunocompromised people, as well as in healthy subjects, are currently in development (Figure 2).

Figure 2. Summary of vaccines in completed or underway clinical trials. Vaccine and Clinical Trial Registration number (NCT identifier) were related to target population and development phase. SOT, solid-organ transplant recipients; HSCT, allogeneic hematopoietic stem cell transplant.
The development of CMV vaccines began in the 1970s when two strains of the virus, Towne and AD169, were attenuated and used as active immunoprophylaxis in solid organ transplant recipients (41). These strains have several modifications in an area of the genome that consists of sequences spanning CMV ORFs UL128-151. Specifically, UL128, UL130, and UL131 direct the synthesis of three polypeptides that are constituents of the pentameric complex (PC) required for efficient viral tropism in most epithelial and endothelial cell types (10). Despite initial results being seemingly promising, statistical analyses revealed that protection against infection was not significant (42). V160 is the first attenuated vaccine designed to express the PC to be constructed on the backbone of the AD169 strain. V160 can propagate in epithelial cell lines only in the presence of Shield-1, a synthetic stabilizing ligand. In the absence of this ligand, the fusion protein is rapidly degraded and viral replication is inhibited (43). Therefore, given that the Shield-1 ligand does not exist in nature, the V160 attenuated virus should be unable to revert to a replication-competent virus, ensuring an excellent safety profile for this vaccine. In a Phase I study conducted between 2013 and 2017, it was demonstrated that the V160 vaccine can induce neutralizing antibody and T cell responses. Furthermore, after a new dose, there is an increase in cell-mediated immune response to CMV (44). The glycoprotein gB is one of several glycoproteins expressed in the viral envelope. This protein works in combination with both the gH/gL complex to facilitate viral entry into human fibroblasts and with the PC to enter epithelial and endothelial cells (45). A phase I randomized trial was conducted involving a CMV vaccine based on recombinant gB with an adjuvant, MF59, an oil-in-water emulsion of squalene. This study demonstrates that immunization of healthy adults achieved with a subunit CMV vaccine combined with an adjuvant can induce an immune response to the gB neutralizing antibody (46). A Phase 2 trial (NCT00299260) measured, after the administration of the gB/MF59 vaccine, antibody titers and CMV viremia in kidney or liver transplant patients. The results showed a significant increase in the gB-binding antibody titer one month after the second vaccine dose. Furthermore, an increase in neutralizing antibody titers was measured after a similar amount of time in seropositive vaccine recipients. Similarly, seronegative organ recipients who had received the vaccine and had had seropositive organ donors demonstrated reduced viremia. This viremia had a post-transplantation duration that was inversely correlated to gB antibody titers (47). An issue to consider in the gB recombinant subunit vaccine is that the ability to induce immunity to one strain of CMV clearly does not mean immunity to all strains of CMV (48). According to the FDA, DNA vaccines are purified plasmid preparations containing one or more DNA sequences capable of inducing and/or promoting an immune response against a pathogen (49). After preclinical, the ASP0113 contains two plasmids encoding pp65 and gB and is administered with two adjuvants. A phase I clinical trial evaluated the safety of ASP0113 in CMV seropositive and seronegative immunized individuals. Vaccination of seronegative subjects elicited pp65- and gB-specific T-cell responses in addition to gB antibody responses, while seropositive vaccinated groups showed increases only in pp65-specific T-cell responses (50). The second clinical trial of ASP0113 included the vaccination of allogeneic HSCT adult recipients, who then exhibited a significant reduction in viral load endpoints and increased frequencies of pp65-specific interferon-γ-producing T cells (51). Two similar studies evaluating the safety and efficacy of this DNA vaccine in solid organ transplant recipients (NCT01974206) and dialysis patients (NCT02103426) were recently completed. Transplant recipients were randomized (1:1) to receive 5 doses of ASP0113 and showed no statistically significant difference between the ASP0113 group and the placebo group. ASP0113 demonstrated a safety profile similar to the placebo in the prevention of CMV viremia in this CMV-seronegative kidney transplant population. A trivalent, non-adjuvanted DNA vaccine trial (VCL-CT02) is currently in progress. These studies (NCT00370006 and NCT00373412) include the IE1 T-cell target in addition to the gB and pp65 coding sequences and were conducted in CMV seronegative subjects vaccinated intramuscularly or intradermally, followed by Towne immunization. Furthermore, Inovio has recently been developing SynCon, an alternative nucleic acid-based vaccine technology. This model is based on an extensive sequence analysis of the antigen of several target pathogens, in which the most conserved, or dominant, amino acid in the antigen gene sequence is identified. Then, a consensus gene sequence is synthetically created and inserted into a DNA plasmid to create the testing vaccine (52). In addition to DNA vaccines, several RNA vaccines are also being studied, such as an alternative nucleic acid-based vaccine technology. An RNA vaccine uses a synthetic copy of a natural pathogen messenger RNA (mRNA) and leads to the immune system producing responses against its corresponding antigen, using lipid nanoparticles for delivery; these particles can protect the RNA strands and facilitate their absorption into the cells. Several preclinical studies have been carried out with excellent results (53). A CMV vaccine that uses a recombinant vector works by being composed of one or more antigens, which are delivered via a viral vector that is capable of infecting human cells and of expressing the viral proteins without establishing a productive infection (10). One of the candidate vaccines tested in clinical trials was a vaccine called Triplex, based on a modified Vaccinia Ankara encoding three immunodominant CMV antigens, pp65, IE1-exon4, and IE2-exon5. Triplex was tested in a Phase I trial evaluating the CMV serological status at different points in time by using three progressively higher doses of the vaccine (54). Based on these results, a Phase II clinical trial was started in 2015, in which CMV-seropositive HSCT recipients received Triplex two days after HSCT (NCT02506933), and then in 2021, Triplex was evaluated in adults with CMV infection and PLWH (NCT05099965). The first trial confirmed that, in patients who received the Triplex vaccine, the risk for a significant CMV event during the first 100 days after transplant was reduced by half, while the second trial is still ongoing (55). A novel strategy for a vaccine candidate was the use of virus-like particles (VLP), protein structures that mimic viruses without a viral genome. One candidate VLP vaccine against CMV was based on the production of VLP in mammalian cells encoding the truncated sequence of the gB extracellular portion fused with the TM and cytoplasmic domains of the vesicular stomatitis virus (VSV) G protein. In pre-clinical studies, this formulation, named CMV gB-G, demonstrated evidence of trimeric expression of the gB-G ectodomain, which is capable of eliciting higher epithelial neutralizing antibody titers compared to the full-length monomeric gB antigen (56). The high intra-host and inter-strain genetic diversity, together with frequent mixed and coinfections, can substantially reduce the generalizability and real-world effectiveness of CMV candidate vaccines. Human CMV infection sustained by a mixture of genetically distinct strains within the same host, as reported by haplotype reconstruction and longitudinal case series, and recombination events could undermine strain-specific immunity and vaccine-induced protection (57). Case series of primary and congenital infections reported the multiple gB detection of different genotypes, as well as reinfection with heterologous strains despite prior immunity, indicating vaccines protection gaps targeting a single antigen or specific strain (58). Coinfection with multiple CMV genotypes was documented in transplant recipients and correlated to delayed antiviral immune reconstitution and prolonged viremia (59). CMV vaccine trials must accounted for mixed viral strain infections, regional strain variability, and microorganism coinfection when correlates of protection and clinical endpoints are evaluated (60, 61).
2.3 Routinely diagnose
Chemiluminescent immunoassay (CLIA) was performed to detect CMV Immunoglobulin M (IgM) and/or Immunoglobulin G (IgG) antibodies by LIAISON® system (Diasorin S.p.A, Italy). Quantitative viremia was evaluated by NUCLISENS EASYMAG (BIOMERIEUX, Italy) and CMV ELITE MGB KIT QUANTITATIVE (ELITECH GROUP S.P.A, Turin, Italy).
3 Case series of immunocompromised patients
3.1 Clinical case 1: CMV reactivation in PLWH recipient
A 36-year-old male was admitted to the “Renato Dulbecco” University Hospital of Catanzaro from August 05 to October 10, 2024. Medical history included HIV infection, first diagnosed in 2012, recurrent prostatitis, anorexia-related malnutrition, and previous AntiRetroviral Treatment (ART) regimens with poor adherence. Currently, the patient is on Highly Active AntiRetroviral Therapy (HAART) treatment to bictegravir/emtricitabine/tenofovir (50 + 200 + 25 mg cp; 1cp/die), exhibiting poor compliance. In the first evaluation, the immune status was moderately compromised, with a CD4+ T-cell count of 458/μL (26.5%, R: 0.45). The patient presented with fever (37.5 °C), mandibular pain with hypoesthesia and paresthesia along the second and third branches of the trigeminal nerve, and a pruritic erythematous rash on the trunk and extremities. The clinical presentation as well as current/past seroimmunological investigations, were consistent with secondary syphilis (rapid plasma reagin titer of 1:512). Additionally, quantitative real-time-RT-PCR revealed HIV RNA relapse from <20 to 23,600 copies/mL, with genotypic drug resistance sequencing confirming sensitivity to all antiretroviral drug classes. Diagnostic imaging demonstrated osteoarthritis involving the left mandibular condyle and temporal bone, which needed surgical management for recurrent temporomandibular joint dislocation. Throughout the hospitalization, the patient exhibited severe malnutrition, sarcopenia, and persistent gastrointestinal symptoms, including mucorrhea and poorly formed stools. A colonoscopy demonstrated chronic nonspecific inflammation, benign lymphoid hyperplasia, and anal condyloma. Biopsies revealed no evidence of malignancy. On August 28, CMV reactivation was detected with an initial DNA viral load of 1,929 copies/mL. It was promptly initiated with intravenous ganciclovir at a dose of 500 mg every 24 hours. The treatment was continued for three weeks, resulting in a progressive reduction of CMV DNAemia, until 192 copies/mL on October 10, 2024 (Figure 3). The immunological condition was re-evaluated, showing a decrease of the CD4+ T-cell count of 407/μL (26.5%, R: 0.38). HIV viral suppression (HIV-RNA <20 copies/mL) was achieved by directly observed therapy (DOT) with bictegravir/emtricitabine/tenofovir on September 6, 2024. However, adherence to HAART therapy remained suboptimal, leading to another virological relapse (HIV RNA viral load 7,540 copies/mL) on October 14, 2024. The patient’s hospital course was complicated by episodes of candidemia and a bloodstream infection caused by Klebsiella pneumoniae with New Delhi metallo-beta-lactamase (NDM) resistance phenotype. Caspofungin at a dose of 50 mg daily was administered for 15 days. For the NDM K. pneumoniae infection, cefiderocol (2g x 3/die) and fosfomycin (4g x 4/die) were administered until September 11, 2024. Further microbiological investigations excluded other opportunistic pathogens, including Cryptococcus neoformans. Due to severe anorexia and malnutrition, parenteral nutrition was initiated to stabilize the patient’s nutritional status (Table 2).
3.2 Clinical case 2: CMV reactivation in kidney transplant patient
A 25-year-old woman was admitted to the Nephrology-Dialysis Unit of the “Renato Dulbecco” University Hospital of Catanzaro from April 13 to 15, 2023, with symptoms of dyspnea and fever. The patient’s medical history included opsismodysplasia, chronic obstructive pulmonary disease, chronic pancreatitis, and retinitis pigmentosa. In October 2017, the patient was diagnosed with initial renal failure, with a diagnosis of “familial polycystosis”. On 23 January 2023, the patient underwent living donor kidney transplantation (mother) at Policlinico Gemelli in Rome. After transplantation, she started immunosuppressive and steroid treatment with Tacrolimus and Bactrim. Before transplantation, the patient’s serological data showed only IgG positivity for CMV (103 UA/ml) with undetected DNA, suggesting previous infection. Two months after transplantation, on 16 March 2023, the patient showed negative IgM and IgG>180 UA/ml with positivity for CMV DNA (22,992 copies/ml) (Figure 3). The patient then discontinued immunosuppressive therapy and started treatment with Valganciclovir 450 mg 1 capsule twice daily. On April 11 she attended a follow-up visit at the Transplant Outpatient Clinic with a clinical-radiological-laboratory picture suggesting a lower respiratory tract infection. After two days, she presented to the Nephrology OU with suspected H. influenzae pneumonia treated with levofloxacin 500 mg every 24 hours, with a Protein C Reactive (PCR) value of 88 and negative procalcitonin (PCT). During hospitalization, antibiotic therapy was continued by oral administration following IgM positivity for Chlamydophila pneumoniae, resulting in a marked improvement in thoracic objectivity and respiratory symptoms, with a reduction in inflammatory indices and normalization of the leukocyte formula. At the third negative determination of CMV DNA on plasma, in agreement with the referring Transplant Center, Valganciclovir therapy was discontinued. The patient is voluntarily discharged in good clinical condition, apiretic and eupnoic, with advice to continue infectious evaluation at the referral clinic and continue nephrologic follow-up (Table 2).
3.3 Clinical case 3: CMV reactivation in lung transplant recipient
A 29-year-old Caucasian man is admitted to the thoracic surgical unit in Padua for a double lung transplant on April 30, 2023. The diagnosis revealed Langerhans cell histiocytosis. The patient’s medical history includes pulmonary emphysema and idiopathic pulmonary fibrosis. After transplantation, the patient started on tacrolimus 3 mg 1 cp x 2/day as an immunosuppressant. During the hospital stay, positive blood cultures for S. epidermidis, K. oxytoca in the presence of vascular abscesses and CMV-DNA replication were reported. Previous administration of ganciclovir was replaced with oral prophylaxis of valganciclovir 450mg 2cp x 2/day. Rejection monitoring TB biopsies were performed on 6 June and found to be absent. On June 28, 2023, CMV DNAemia was under 130 copies/ml. Bronchial aspirate was positive for Escherichia coli and persisted for several months. On July 26, 2023, at the last check-up at the hospital in Padua, Cytomegatect 2000 IU was administered. Immunoglobulin administration was recommended for one year. The patient was admitted to the pneumology operating unit of the Renato Dulbecco hospital in Catanzaro as an outpatient. After two weeks, CMV DNA was not detectable, while serology was: anti-CMV-IgG of 42 UA/mL, anti-CMV-IgM of 25 UA/mL. After one month, on August 30, 2023, the patient was compatible with reactive CMV infection (anti-CMV-IgG: 44 UA/mL, anti-CMV-IgM: 26 UA/mL), confirmed by PCR with detectable CMV viremia (6576 copies/mL). Additionally, sputum was positive for Aspergillus niger and became negative after 4 months. After 5 months of follow-up, viremia decreased (<130 copies/ml), and the bronchial aspirate was negative. On March 7, 2024, DNAemia was 212 copies/mL (anti-CMV-IgG: 60 UA/mL, anti-CMV-IgM: 49 UA/mL) (Figure 3). The patient is still under control in the pneumology unit (Table 2).
4 Discussion
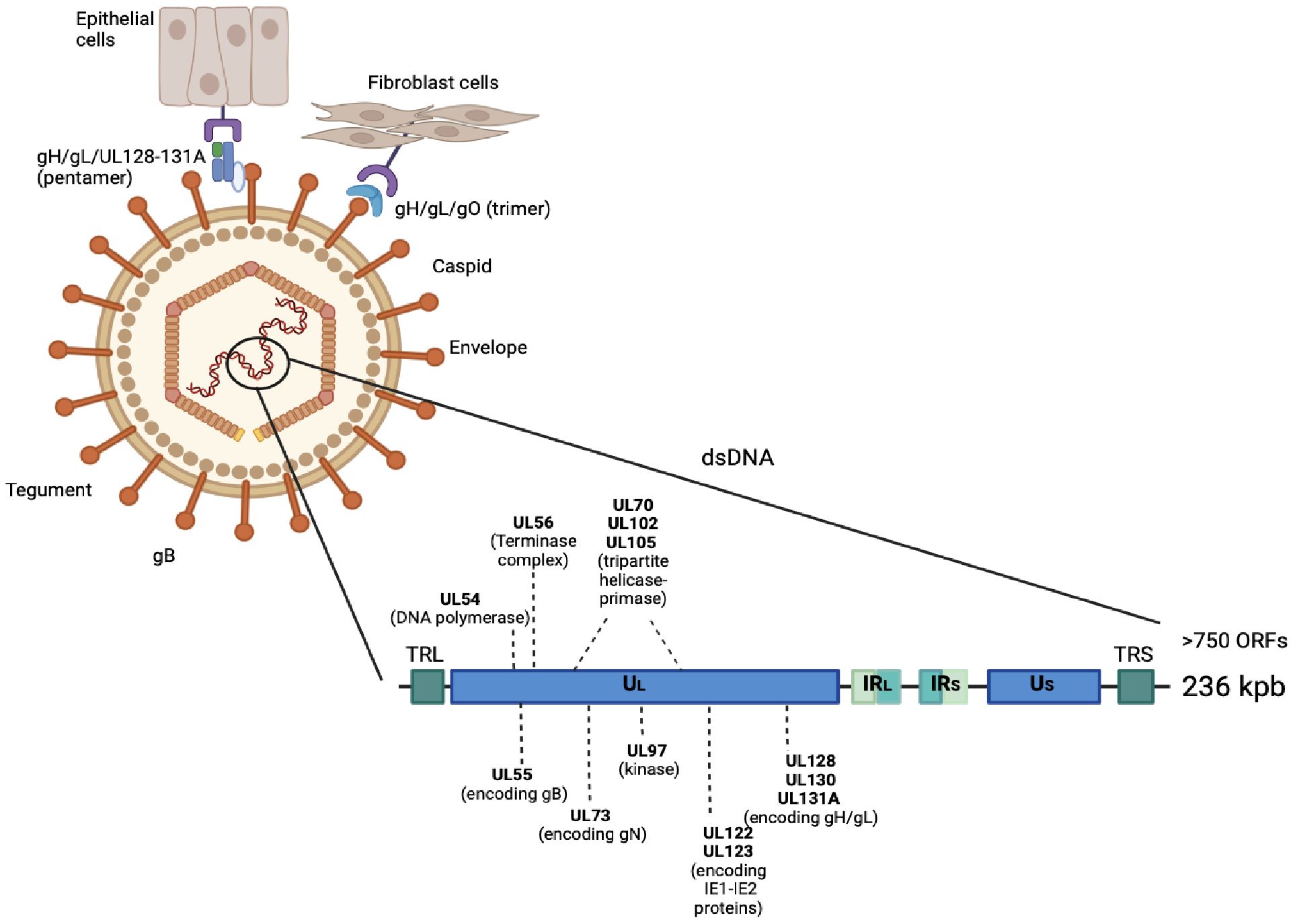
The vaccine for CMV infection was classified as a high priority due to the consequences of congenital infection and disease severity in immunocompromised subjects, the emergence of resistance, and the side effects of drugs, which limited the use of antiviral therapies (6, 11). In this paper, we referred to three cases of immunocompromised patients. In the first case, we illustrated the multifaceted consequences of CMV reactivation in an HIV-positive patient with poor ART adherence, contributing to persistent gastrointestinal symptoms, systemic infections, and treatment challenges. CMV is a common virus that frequently establishes latency and reactivates in immunocompromised hosts (62). CMV has a linear double-stranded DNA (dsDNA) containing 236 kb of information and more than 750 open reading frames (ORFs) packaged inside an icosahedral nucleocapsid, a large layer of tegument proteins, and an envelope containing glycoprotein complexes (20). The genome is divided into two large domains called long (L) and short (S), each of which is constituted by the central unique (UC), unique long (UL), and unique short (US) regions (21). Several CMV genotypes were defined, according to the distribution of polymorphisms along the viral genome (22). Considering the variability of gB (gB1, gB2, gB3, gB4, gB5, gB6, gB7) and gN (gN1, gN2, gN3a, gN3b, gN4a, gN4b, gN4c), it is possible to distinguish seven genotypes for both glycoproteins. The gH encoded by the UL75 gene is also able to identify two different genotypes (gH1 and gH2). Polymorphic genes are usually analyzed to evaluate the prevalence of viral genotype circulation among humans (2, 20, 21, 23). To determine the entry spot of the virions, the glycoproteins of the CMV envelope combine into two different complexes based on the host cellular type. The first one is composed by a trimer formed by a glycoprotein H (gH), glycoprotein L (gL), and glycoprotein O (gO) complex that binds to platelet-derived growth factor receptor α (PDGFRα) to induce pH-independent entry into the fibroblasts (24). The gN is an extensively glycosylated envelope type I glycoprotein. It is a component of the gM/gN complex that plays an essential role in CMV replication. This gM/gN complex is the most abundant protein complex in the virion envelope (23). Pentamer-mediated entry requires the presence of gO, as well as a low endosome pH. A crucial role in viral entry is played by glycoprotein B (gB) (25). The high tropism of CMV for different cell types requires the interaction between several virus-encoded glycoproteins and receptors on the host cell surface (26). The CMV gB is encoded by the UL55 gene and is synthesized as a polypeptide composed of about 900 amino acids that consists of 5 structural domains (I-V) and undergoes cleavage by furin (26). To infect epithelial, endothelial, and likely many other cell types, the gH, gL, UL128, UL130, and UL131A pentamer can bind to Olfactory Receptor family 14 subfamily I Member 1 (OR14I1) or Neuropilin 2 (NRP2) on their cell surface, inducing virion endocytosis (Figure 4) (27).
Among PLWH, CMV seropositivity has been associated with increased epithelial gut damage, microbial translocation, and systemic inflammation, even in individuals receiving long-term ART. These processes are hypothesized to contribute to chronic immune activation and the development of non-AIDS comorbidities (63–65). In the present case, CMV reactivation was identified through the detection of CMV DNAemia, coinciding with exacerbation of gastrointestinal symptoms and chronic colonic inflammation. These findings align with recent evidence demonstrating that CMV replication is a significant contributor to gut permeability and the subsequent translocation of microbes into the circulation, leading to systemic inflammation. CMV replication in the gastrointestinal tract can compromise intestinal barrier integrity by disrupting tight junctions of polarized intestinal cells and enhancing transepithelial permeability, contributing to microbial translocation in PLWH patients (65–67). Studies have shown that CMV coinfection and reactivation are associated with elevated markers of gut damage, such as intestinal fatty acid-binding protein (I-FABP), and microbial translocation markers, including lipopolysaccharide (LPS) and (1→3)-β-d-glucan (BDG) (63). Complications such as candidemia and NDM resistance phenotype K. pneumoniae bloodstream infections highlight the susceptibility of this patient to opportunistic infections. Persistent microbial translocation, secondary to CMV-associated gut damage, may have contributed to these bloodstream infections (63, 68, 69). The patient’s poor adherence to ART further complicated his clinical course, leading to intermittent virological failure and suboptimal immune reconstitution (70). The successful reduction of CMV DNAemia following ganciclovir therapy underscores the potential benefits of targeted antiviral strategies. However, the recurrent nature of CMV reactivation and its long-term impact on immune health necessitate preventive measures beyond pharmacological management (71). The development and deployment of an effective CMV vaccine could represent a transformative advancement in the care of PLWH (11). By preventing CMV infection and reactivation, such a vaccine would reduce epithelial gut damage, mitigate chronic immune activation, and ultimately decrease the incidence of non-AIDS comorbidities (71). In particular, a safe CMV vaccine would be crucial for individuals with poor ART adherence or compromised immune status, as they remain at high risk for CMV-related complications. In people with compromised immune systems, such as SOT patient (case 2), where patients undergo pharmacological immunosuppression to prevent rejection, CMV has the potential to cause severe disease. Because of these adverse effects, CMV prevention is part of the standard of care (72). This can be accomplished through antiviral prophylaxis with Valganciclovir for at least 3 months after SOT. Or as a preventive therapy, which involves the administration of antiviral drugs only after CMV replication is detected. With this strategy, CMV nucleic acid amplification testing monitors patients regularly at close time points during the first 3 months after SOT. If CMV DNA is detected, antiviral drugs such as Valganciclovir are administered to treat patients until the virus is no longer detectable in the blood (73). Immune dysregulation caused by immunosuppressive drugs and CMV reactivation may have favored or facilitated a possible interaction with secondary C. pneumoniae infection, potentially contributing to the severity of respiratory symptoms. This interpretation is consistent with clinical observations that CMV reactivation in transplant and critically ill patients is often accompanied by bacterial and fungal co-infections and with experimental data showing enhanced pro-inflammatory responses during certain viral–bacterial co-infections, which may amplify lung injury and impair pathogen clearance (74–76). Sustained exposure to immunosuppressive agents may predispose patients to subsequent CMV reactivation, despite prior effective antiviral therapy. Moreover, prolonged use of antiviral drugs, such as Valganciclovir, may have side effects such as neutropenia, gastrointestinal problems, and renal toxicity. Therefore, vaccination in patients with a compromised immune system would be considered necessary. Finally, Lung transplantation (LTx) is the definitive treatment option for patients with severe pulmonary diseases (case 3) (77). Cytomegalovirus is one of the most prevalent viral pathogens contributing to morbidity after SOT (78). In immunosuppressed patients, CMV infection can be asymptomatic but more frequently presents as CMV syndrome, characterized by fever, malaise, leukopenia, thrombocytopenia, and elevated serum transaminases, or as organ-specific CMV diseases, such as pneumonia, gastroenteritis, or hepatitis (79). Transplant-associated CMV infection may occur as a primary infection in seronegative recipients, as a reactivation of latent CMV, or because of reinfection with a new CMV strain (80). Due to its immunomodulatory effects, CMV infection may increase susceptibility to other opportunistic infections, such as Aspergillus species, Pneumocystis jirovecii, Nocardia, and Epstein–Barr virus, which could contribute to the overall burden of infection-related complications (81–83). Further, lung transplant recipients are particularly at risk for severe infections from common community-acquired respiratory viruses: Respiratory syncytial virus (RSV), Parainfluenza virus (PIV), Rhinovirus, Coronavirus, Human Metapneumovirus, Influenza, and Enterovirus (84). The early identification and management of CMV viremia have been shown to improve clinical outcomes, underscoring the need for integrated monitoring strategies targeting both viral and fungal pathogens (82, 83). The treatment of symptomatic cases involves a combination of antiviral therapy and immunosuppression reduction. Both valganciclovir and ganciclovir are effective in treating symptomatic CMV disease (81). In CMV infections, intravenous immunoglobulin (IVIG) is administered for the treatment or prevention (85). However, vaccination of recipients significantly reduced severe symptoms and the risk of graft rejection (86). Although acquired immunity does not consistently prevent reinfection (58).
Reinfection and mixed events are directly related to CMV genome high variability, which can facilitate immune evasion, increase viral replication, and decrease antiviral efficacy. CMV exhibits recombination events, among other evolutionary phenomena, due to the multitude of infections (2). High level of within-host CMV strains diversity is not related to mutational rates, but rather to frequent mixed infections (about 61%) identified using serial and different samples, particularly in immunocompromised subjects. Infection of different viral strains determined recombination events, regarded as putative drivers of CMV evolution, influencing its pathogenesis by promoting viral cell entry (87). The interaction between host and virus generates a positive selection in specific CMV genomic regions, such as surface glycoproteins, avoiding immune recognition (3). The gB and gH/gL proteins elicit serum neutralizing antibodies, blocking entry into target cells (88). Following natural infection, gH-specific neutralizing antibodies were detected in convalescent serum samples and were initially considered for the treatment and prophylaxis of infections. Recently, the strain-specific neutralization capacity of gH antibodies has been demonstrated. Considering three monoclonal antibodies (2B10, 6E3, 3C11), two were effective at blocking distinct CMV strains, while 2B10 was strictly strain-specific against a single residue on the gH surface. This underlines the importance of protective immunity against gH polymorphic sites (88). The strain-specific serological methods could be useful in determining CMV strain diversity, multiple infections, and reinfection, thus identifying the appearance of new antibodies over time against the antigenic determinants on gH and gB envelope glycoproteins (89). CMV genotypes can be identical in different geographic areas, although new/rare viral strains can be detected in restricted areas of the world or in specific risk groups: gB2 was foremost present among PLWH and children with congenital infection, gB1 was the most prevalent among SOT recipients and immunocompromised patients, and finally, gB4 was found in newborns with sepsis-like syndrome (90). CMV genomic evolution occurred by genetic drift within geographically distinct populations. The CMV genome variations interacted to cause geographical differences and their spread to specific areas (91). Interestingly, the CMV population appeared to be relatively stable within tissue compartments in a single host, while it rapidly evolved during the colonization of different compartments. Each compartment reportedly exhibited a unique selective pressure, mutation, or polymorphism on specific genes that can affect the tropism of the viral population, increasing its fitness (92). The analysis, performed on the UL55, UL73, UL75, US28, and UL144 genomic regions in urine, saliva, and plasma samples, showed the presence of multiple genotypes in primary infection. The detection of several CMV viral strains in different specimens from the same subject was common (10/15) (57). In this study, 10 subjects had gN2 only in saliva, four subjects had unique gB genotypes found in saliva or urine, while different gH types were detected in different samples, and one subject displayed a unique US28 genotype only in saliva. New genotypes within the same specimen were observed with a median time of 5 months. The frequency of each genotype was probably related to transmission variability or infection reactivation (57). Hypothetically, the different genotypes of CMV may influence its virulence, though its impact on disease severity is still debated, and there are conflicting results (93). Pathogenesis may be directly dependent on tissue tropism and the local site of replication. In PLWH and immunocompromised patients, the frequency of gB2 in patients with retinitis compared to those without was not higher. On the contrary, a higher frequency of gB4 was found in semen samples from the genitourinary tract: this tissue specificity was not revealed for gB1, gB2, and gB3 (59). However, the extensively reported possibility of mixed infection within a patient underlined the difficulty in studying the association between pathogenesis and a particular genotype, such as potential virulence or symptomatic disease (59). Genetic variation could affect clinical outcome, especially in immunocompromised people. In patients receiving marrow transplants, complications of neutropenia and subsequently death appeared significantly associated with gB3 and gB4, possibly because they escape immune recognition and persist in the marrow, causing more damage (94). The increased susceptibility to reinfection was related to the recognition of gB structural differences by antibodies, suggesting CMV variants and naturally occurring mutations as responsible for a less effective immune response (95). The sequencing techniques highlighted the compartmentalization of gB epitopes, even if the major viral populations were identical between host anatomic compartments. The presence of low-frequency viral variants could explain the partial efficacy of vaccines (96). GB-specific antibodies limited the dissemination of viruses between tissue compartments, selecting the more advantageous CMV strains in each compartment and the subsequent formation of genetically distinct viral variants (96). Mixed CMV infection and viral strain compartmentalization (cervical and breast milk) were also identified in HIV-infected women. In this situation, the congenitally transmitted virus was the one with the highest abundance in the cervix and was genetically distinct from the breast milk strains (97). The gB/MF59 vaccine, constituted with the Towne gB1 genotype strain, showed a lack of protection in vaccine recipients infected with gB1 within 3 months of primary infection. Strain-specific immunity was suggested as being protective against CMV infection (57). Recombinant viral strains are usually positively selected and occur between superinfecting as well as reactivating genomes. Studies based on the analysis of reconstructing haplotypes from short-read sequenced data showed that the most parsimonious explanation of high within-host CMV diversity is the frequent presence of mixed infections, whereas CMV in non-mixed infections is no more diverse than other DNA viruses (58). The availability of CMV sequences from around the world can be used to evaluate viral antigens or gB-specific strains to induce cross-protection against globally circulating variants (91). Evolutionary phenomena during CMV infection, such as multiple CMV strains coinfection and recombination events, were related to viral pathogenesis and to the complexity of developing an effective vaccine.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Calabria Region (#357, approved on December 20, 2024). Written informed consent for participation was not required for these case series because all patient data were provided in an anonymized form, and no identifiable information was included. The laboratory data were produced from routine diagnostic procedures. On human samples, no additional tests were performed for research purposes. Accordingly, the study did not involve any intervention beyond standard clinical care.
Author contributions
NM: Visualization, Writing – original draft, Conceptualization. GP: Visualization, Writing – original draft. CM: Writing – original draft. MP: Writing – original draft. EG: Writing – original draft, Data curation. MM: Writing – original draft, Data curation. SG: Writing – original draft, Data curation. GB: Methodology, Writing – original draft. CP: Methodology, Writing – original draft. GM: Supervision, Writing – review & editing. AQ: Visualization, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mussi-Pinhata MM and Yamamoto AY. Natural history of congenital cytomegalovirus infection in highly seropositive populations. J Infect Dis. (2020) 221:S15–22. doi: 10.1093/infdis/jiz443
2. Vankova OE, Brusnigina NF, and Novikova NA. NGS technology in monitoring the genetic diversity of cytomegalovirus strains. Sovrem Tekhnologii V Meditsine. (2023) 15:41–6. doi: 10.17691/stm2023.15.2.04
3. Sijmons S, Thys K, Mbong Ngwese M, Van Damme E, Dvorak J, Van Loock M, et al. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J Virol. (2015) 89:7673–95. doi: 10.1128/JVI.00578-15
4. Hage E, Wilkie GS, Linnenweber-Held S, Dhingra A, Suárez NM, Schmidt JJ, et al. Characterization of human cytomegalovirus genome diversity in immunocompromised hosts by whole-genome sequencing directly from clinical specimens. J Infect Dis. (2017) 215:1673–83. doi: 10.1093/infdis/jix157
5. Fowler K, Mucha J, Neumann M, Lewandowski W, Kaczanowska M, Grys M, et al. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health. (2022) 22:1659. doi: 10.1186/s12889-022-13971-7
6. Ong DSY, Chong GLM, Chemaly RF, and Cremer OL. Comparative clinical manifestations and immune effects of cytomegalovirus infections following distinct types of immunosuppression. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2022) 28:1335–44. doi: 10.1016/j.cmi.2022.05.034
7. Kenneson A and Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. (2007) 17:253–76. doi: 10.1002/rmv.535
8. Cannon MJ, Hyde TB, and Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. (2011) 21:240–55. doi: 10.1002/rmv.695
9. Davis NL, King CC, and Kourtis AP. Cytomegalovirus infection in pregnancy. Birth Defects Res. (2017) 109:336–46. doi: 10.1002/bdra.23601
10. Anderholm KM, Bierle CJ, and Schleiss MR. Cytomegalovirus vaccines: current status and future prospects. Drugs. (2016) 76:1625–45. doi: 10.1007/s40265-016-0653-5
11. Chiavarini M, Genga A, Ricciotti GM, D’Errico MM, and Barbadoro P. Safety, immunogenicity, and efficacy of cytomegalovirus vaccines: A systematic review of randomized controlled trials. Vaccines. (2025) 13:85. doi: 10.3390/vaccines13010085
12. Mallory MA, Hymas WC, Simmon KE, Pyne MT, Stevenson JB, Barker AP, et al. Development and validation of a next-generation sequencing assay with open-access analysis software for detecting resistance-associated mutations in CMV. J Clin Microbiol. (2023) 61:e0082923. doi: 10.1128/jcm.00829-23
13. Brennan DC. Cytomegalovirus in renal transplantation. J Am Soc Nephrol JASN. (2001) 12:848–55. doi: 10.1681/ASN.V124848
14. Huang ES, Alford CA, Reynolds DW, Stagno S, and Pass RF. Molecular epidemiology of cytomegalovirus infections in women and their infants. N Engl J Med. (1980) 303:958–62. doi: 10.1056/NEJM198010233031702
15. Dong N, Cao L, Zheng D, Su L, Lu L, Dong Z, et al. Distribution of CMV envelope glycoprotein B, H and N genotypes in infants with congenital cytomegalovirus symptomatic infection. Front Pediatr. (2023) 11:1112645. doi: 10.3389/fped.2023.1112645
16. Gomes AC, Baraniak IA, Lankina A, Moulder Z, Holenya P, Atkinson C, et al. The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread. Nat Commun. (2023) 14:1041. doi: 10.1038/s41467-023-36683-x
17. Zehner M, Alt M, Ashurov A, Goldsmith JA, Spies R, Weiler N, et al. Single-cell analysis of memory B cells from top neutralizers reveals multiple sites of vulnerability within HCMV Trimer and Pentamer. Immunity. (2023) 56:2602–20. doi: 10.1016/j.immuni.2023.10.009
18. Jiang XJ, Zhang J, Xiong Y, Jahn G, Xiong HR, Yang ZQ, et al. Human cytomegalovirus glycoprotein polymorphisms and increasing viral load in AIDS patients. PloS One. (2017) 12:e0176160. doi: 10.1371/journal.pone.0176160
19. Vasiljevic T, Jankovic M, Tomic A, Bakrac I, Radenovic S, Miljanovic D, et al. Significance of cytomegalovirus gB genotypes in adult patients undergoing hematopoietic stem cell transplantation: insights from a single-centre investigation. Pharm Basel Switz. (2024) 17:428. doi: 10.3390/ph17040428
20. Cytomegalovirus Disease: Adult and Adolescent OIs (2021). NIH. Available online at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/cytomegalovirus (Accessed July 21, 2025).
21. Meesing A and Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. (2018) 78:1085–103. doi: 10.1007/s40265-018-0943-1
22. Red Book. 2015 Report of the Committee on Infectious Diseases (2015). American Academy of Pediatrics. Available online at: https://publications.aap.org/aapbooks/book/498/Red-Book-2015-2015-Report-of-the-Committee-on (Accessed July 22, 2025).
23. Kotton CN. CMV: prevention, diagnosis and therapy. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2013) 13 Suppl 3:24–40; quiz 40. doi: 10.1111/ajt.12006
24. Gagelmann N, Ljungman P, Styczynski J, and Kröger N. Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: A systematic review and meta-analysis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transpl. (2018) 24:2101–9. doi: 10.1016/j.bbmt.2018.05.017
25. Siberry GK, Abzug MJ, Nachman S, Brady MT, Dominguez KL, Handelsman E, et al. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. (2013) 32(0 2):1–KK4. doi: 10.1097/01.inf.0000437856.09540.11
26. van der Beek MT, Berger SP, Vossen ACTM, van der Blij-de Brouwer CS, Press RR, de Fijter JW, et al. Preemptive versus sequential prophylactic-preemptive treatment regimens for cytomegalovirus in renal transplantation: comparison of treatment failure and antiviral resistance. Transplantation. (2010) 89:320–6. doi: 10.1097/TP.0b013e3181bc0301
27. Boillat Blanco N, Pascual M, Venetz JP, Nseir G, Meylan PR, and Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. (2011) 91:251–5. doi: 10.1097/TP.0b013e318200b9f0
28. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. (2017) 377:2433–44. doi: 10.1056/NEJMoa1706640
29. Chou S. A third component of the human cytomegalovirus terminase complex is involved in letermovir resistance. Antiviral Res. (2017) 148:1–4. doi: 10.1016/j.antiviral.2017.10.019
30. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological Malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19:e260–72. doi: 10.1016/S1473-3099(19)30107-0
31. Lurain NS and Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. (2010) 23:689–712. doi: 10.1128/CMR.00009-10
32. Chou S. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antiviral Res. (2020) 176:104711. doi: 10.1016/j.antiviral.2020.104711
33. Wagstaff AJ and Bryson HM. Foscarnet. Drugs. (1994) 48:199–226. doi: 10.2165/00003495-199448020-00007
34. Gilbert C, Azzi A, Goyette N, Lin SX, and Boivin G. Recombinant phenotyping of cytomegalovirus UL54 mutations that emerged during cell passages in the presence of either ganciclovir or foscarnet. Antimicrob Agents Chemother. (2011) 55:4019–27. doi: 10.1128/AAC.00334-11
35. Campos AB, Ribeiro J, Boutolleau D, and Sousa H. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: current state of the art. Rev Med Virol. (2016) 26:161–82. doi: 10.1002/rmv.1873
36. Lea AP and Bryson HM. Cidofovir. Drugs. (1996) 52:225–30; discussion 231. doi: 10.2165/00003495-199652020-00006
37. Komatsu TE, Pikis A, Naeger LK, and Harrington PR. Resistance of human cytomegalovirus to ganciclovir/valganciclovir: a comprehensive review of putative resistance pathways. Antiviral Res. (2014) 101:12–25. doi: 10.1016/j.antiviral.2013.10.011
38. Topalis D, Gillemot S, Snoeck R, and Andrei G. Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase. Nucleic Acids Res. (2016) 44:9530–54. doi: 10.1093/nar/gkw875
39. Maertens J, Cordonnier C, Jaksch P, Poiré X, Uknis M, Wu J, et al. Maribavir for preemptive treatment of cytomegalovirus reactivation. N Engl J Med. (2019) 381:1136–47. doi: 10.1056/NEJMoa1714656
40. Chou S, Alain S, Cervera C, Chemaly RF, Kotton CN, Lundgren J, et al. Drug resistance assessed in a phase 3 clinical trial of maribavir therapy for refractory or resistant cytomegalovirus infection in transplant recipients. J Infect Dis. (2024) 229:413–21. doi: 10.1093/infdis/jiad293
41. Plotkin SA, Furukawa T, Zygraich N, and Huygelen C. Candidate cytomegalovirus strain for human vaccination. Infect Immun. (1975) 12:521–7. doi: 10.1128/iai.12.3.521-527.1975
42. Plotkin SA, Starr SE, Friedman HM, Brayman K, Harris S, Jackson S, et al. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann Intern Med. (1991) 114:525–31. doi: 10.7326/0003-4819-114-7-525
43. Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AGL, and Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. (2006) 126:995–1004. doi: 10.1016/j.cell.2006.07.025
44. Adler SP, Lewis N, Conlon A, Christiansen MP, Al-Ibrahim M, Rupp R, et al. Phase 1 clinical trial of a conditionally replication-defective human cytomegalovirus (CMV) vaccine in CMV-seronegative subjects. J Infect Dis. (2019) 220:411–9. doi: 10.1093/infdis/jiz141
45. Isaacson MK and Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol. (2009) 83:3891–903. doi: 10.1128/JVI.01251-08
46. Pass RF, Duliegè AM, Boppana S, Sekulovich R, Percell S, Britt W, et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis. (1999) 180:970–5. doi: 10.1086/315022
47. Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet Lond Engl. (2011) 377:1256–63. doi: 10.1016/S0140-6736(11)60136-0
48. Schleiss MR. Recombinant cytomegalovirus glycoprotein B vaccine: Rethinking the immunological basis of protection. Proc Natl Acad Sci U S A. (2018) 115:6110–2. doi: 10.1073/pnas.1806420115
49. Research C for BE and. Considerations for Plasmid DNA Vaccines for Infectious Disease Indications (2024). FDA. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-plasmid-dna-vaccines-infectious-disease-indications (Accessed August 1, 2025).
50. Wloch MK, Smith LR, Boutsaboualoy S, Reyes L, Han C, Kehler J, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. (2008) 197:1634–42. doi: 10.1086/588385
51. Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. (2012) 12:290–9. doi: 10.1016/S1473-3099(11)70344-9
52. Ramanathan MP, Kuo YC, Selling BH, Li Q, Sardesai NY, Kim JJ, et al. Development of a novel DNA SynCon tetravalent dengue vaccine that elicits immune responses against four serotypes. Vaccine. (2009) 27:6444–53. doi: 10.1016/j.vaccine.2009.06.061
53. Brito LA, Chan M, Shaw CA, Hekele A, Carsillo T, Schaefer M, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther J Am Soc Gene Ther. (2014) 22:2118–29. doi: 10.1038/mt.2014.133
54. La Rosa C, Longmate J, Martinez J, Zhou Q, Kaltcheva TI, Tsai W, et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood. (2017) 129:114–25. doi: 10.1182/blood-2016-07-729756
55. Aldoss I, La Rosa C, Baden LR, Longmate J, Ariza-Heredia EJ, Rida WN, et al. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: A phase 2, randomized clinical trial. Ann Intern Med. (2020) 172:306–16. doi: 10.7326/M19-2511
56. Kirchmeier M, Fluckiger AC, Soare C, Bozic J, Ontsouka B, Ahmed T, et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol CVI. (2014) 21:174–80. doi: 10.1128/CVI.00662-13
57. Ross SA, Pati P, Jensen TL, Goll JB, Gelber CE, Singh A, et al. Cytomegalovirus genetic diversity following primary infection. J Infect Dis. (2020) 221:715–20. doi: 10.1093/infdis/jiz507
58. Plotkin SA and Boppana SB. Vaccination against the human cytomegalovirus. Vaccine. (2019) 37:7437–42. doi: 10.1016/j.vaccine.2018.02.089
59. Rasmussen L, Hong C, Zipeto D, Morris S, Sherman D, Chou S, et al. Cytomegalovirus gB genotype distribution differs in human immunodeficiency virus-infected patients and immunocompromised allograft recipients. J Infect Dis. (1997) 175:179–84. doi: 10.1093/infdis/175.1.179
60. Mokomane M, Tate JE, Steenhoff AP, Esona MD, Bowen MD, Lechiile K, et al. Evaluation of the influence of gastrointestinal coinfections on rotavirus vaccine effectiveness in Botswana. Pediatr Infect Dis J. (2018) 37:e58–62. doi: 10.1097/INF.0000000000001828
61. Simsek C, Bloemen M, Jansen D, Beller L, Descheemaeker P, Reynders M, et al. High prevalence of coinfecting enteropathogens in suspected rotavirus vaccine breakthrough cases. J Clin Microbiol. (2021) 59:e0123621. doi: 10.1128/JCM.01236-21
62. Forte E, Zhang Z, Thorp EB, and Hummel M. Cytomegalovirus latency and reactivation: an intricate interplay with the host immune response. Front Cell Infect Microbiol. (2020) 10:130/full. doi: 10.3389/fcimb.2020.00130/full
63. Ramendra R, Isnard S, Lin J, Fombuena B, Ouyang J, Mehraj V, et al. Cytomegalovirus seropositivity is associated with increased microbial translocation in people living with human immunodeficiency virus and uninfected controls. Clin Infect Dis Off Publ Infect Dis Soc Am. (2019) 71:1438–46. doi: 10.1093/cid/ciz1001
64. Smith DM, Nakazawa M, Freeman ML, Anderson CM, Oliveira MF, Little SJ, et al. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis Off Publ Infect Dis Soc Am. (2016) 63:1517–24. doi: 10.1093/cid/ciw612
65. Maidji E, Somsouk M, Rivera JM, Hunt PW, and Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PloS Pathog. (2017) 13:e1006202. doi: 10.1371/journal.ppat.1006202
66. Routy JP, Royston L, and Isnard S. Aging with grace for people living with HIV: strategies to overcome leaky gut and cytomegalovirus coinfection. J Acquir Immune Defic Syndr 1999. (2022) 89:S29–33. doi: 10.1097/QAI.0000000000002838
67. Royston L, Isnard S, Berini CA, Bu S, Lakatos PL, Bessissow T, et al. Influence of letermovir treatment on gut inflammation in people living with HIV on antiretroviral therapy: protocol of the open-label controlled randomised CIAO study. BMJ Open. (2023) 13:e067640. doi: 10.1136/bmjopen-2022-067640
68. Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. (2014) 210:1228–38. doi: 10.1093/infdis/jiu238
69. Quirino A, Scaglione V, Marascio N, Mazzitelli M, Garofalo E, Divenuto F, et al. Role of the T2Dx magnetic resonance assay in patients with suspected bloodstream infection: a single-centre real-world experience. BMC Infect Dis. (2022) 22:113. doi: 10.1186/s12879-022-07096-w
70. Foka FET and Mufhandu HT. Current ARTs, virologic failure, and implications for AIDS management: A systematic review. Viruses. (2023) 15:1732. doi: 10.3390/v15081732
71. Schnittman SR and Hunt PW. CMV and persistent immune activation in HIV. Curr Opin HIV AIDS. (2021) 16:168–76. doi: 10.1097/COH.0000000000000678
72. Razonable RR and Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl. (2019) 33:e13512. doi: 10.1111/ctr.13512
73. Razonable RR. Cytomegalovirus infection after solid organ transplantation: how I use cell-mediated immune assays for management. Viruses. (2024) 16:1781. doi: 10.3390/v16111781
74. Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis Off Publ Infect Dis Soc Am. (2007) 44:1307–14. doi: 10.1086/514340
75. Yadav SK, Saigal S, Choudhary NS, Saha S, Kumar N, and Soin AS. Cytomegalovirus infection in liver transplant recipients: current approach to diagnosis and management. J Clin Exp Hepatol. (2017) 7:144–51. doi: 10.1016/j.jceh.2017.05.011
76. Girmenia C, Lazzarotto T, Martino M, Bonifazi F, Baldanti F, Clerici P, et al. Management of cytomegalovirus infection in allogeneic hematopoietic stem cell and in solid organ transplantation: updated recommendations by the GITMO, SITO, SIMIT, and AMCLI Italian societies. Clin Transpl. (2025) 39:e70255. doi: 10.1111/ctr.70255
77. Kawashima M, Ma J, Huszti E, Levy L, Berra G, Renaud-Picard B, et al. Association between cytomegalovirus viremia and long-term outcomes in lung transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2024) 24:1057–69. doi: 10.1016/j.ajt.2024.01.027
78. Geris JM, Spector LG, Pfeiffer RM, Limaye AP, Yu KJ, and Engels EA. Cancer risk associated with cytomegalovirus infection among solid organ transplant recipients in the United States. Cancer. (2022) 128:3985–94. doi: 10.1002/cncr.34462
79. Bennett D, Bergantini L, Ferrara P, Cusi MG, Scolletta S, Montagnani F, et al. Cytomegalovirus infection is associated with development of chronic lung allograft dysfunction. Lung. (2022) 200:513–22. doi: 10.1007/s00408-022-00551-0
80. Almaghrabi RS, Omrani AS, and Memish ZA. Cytomegalovirus infection in lung transplant recipients. Expert Rev Respir Med. (2017) 11:377–83. doi: 10.1080/17476348.2017.1317596
81. Patrucco F, Curtoni A, Sidoti F, Zanotto E, Bondi A, Albera C, et al. Herpes virus infection in lung transplantation: diagnosis, treatment and prevention strategies. Viruses. (2023) 15:2326. doi: 10.3390/v15122326
82. Solak Y, Biyik Z, Cizmecioglu A, Genc N, Ozbek O, Gaipov A, et al. Cytomegalovirus and Aspergillus spp. coinfection in organ transplantation: a case report and review of the literature. CEN Case Rep. (2013) 2:59–67. doi: 10.1007/s13730-012-0040-3
83. Heilig L, Bussemer L, Strobel L, Hünniger-Ast K, Kurzai O, Grothey A, et al. Unveiling immune interference: how the dendritic cell response to co-infection with Aspergillus fumigatus is modulated by human cytomegalovirus and its virokine CMVIL-10. mBio. (2025) :e0154125. doi: 10.1128/mbio.01541-25
84. Trachuk P, Bartash R, Abbasi M, and Keene A. Infectious complications in lung transplant recipients. Lung. (2020) 198:879–87. doi: 10.1007/s00408-020-00403-9
85. Aiba N, Shiraki A, Yajima M, Oyama Y, Yoshida Y, Ohno A, et al. Interaction of immunoglobulin with cytomegalovirus-infected cells. Viral Immunol. (2017) 30:500–7. doi: 10.1089/vim.2016.0151
86. Plotkin SA. Preventing infection by human cytomegalovirus. J Infect Dis. (2020) 221:S123–7. doi: 10.1093/infdis/jiz448
87. Cudini J, Roy S, Houldcroft CJ, Bryant JM, Depledge DP, Tutill H, et al. Human cytomegalovirus haplotype reconstruction reveals high diversity due to superinfection and evidence of within-host recombination. Proc Natl Acad Sci U S A. (2019) 116:5693–8. doi: 10.1073/pnas.1818130116
88. Thomas M, Kropff B, Schneider A, Winkler TH, Görzer I, Sticht H, et al. A novel strain-specific neutralizing epitope on glycoprotein H of human cytomegalovirus. J Virol. (2021) 95:e0065721. doi: 10.1128/JVI.00657-21
89. Novak Z, Ross SA, Patro RK, Pati SK, Reddy MK, Purser M, et al. Enzyme-linked immunosorbent assay method for detection of cytomegalovirus strain-specific antibody responses. Clin Vaccine Immunol CVI. (2009) 16:288–90. doi: 10.1128/CVI.00281-08
90. Correa C, Kourí V, Pérez L, Soto Y, and Limia C. Diagnosis, gB genotype distribution and viral load of symptomatic congenitally infected CMV patients in Cuba. J Perinatol Off J Calif Perinat Assoc. (2016) 36:837–42. doi: 10.1038/jp.2016.95
91. Charles OJ, Venturini C, Gantt S, Atkinson C, Griffiths P, Goldstein RA, et al. Genomic and geographical structure of human cytomegalovirus. Proc Natl Acad Sci U S A. (2023) 120:e2221797120. doi: 10.1073/pnas.2221797120
92. Renzette N, Gibson L, Bhattacharjee B, Fisher D, Schleiss MR, Jensen JD, et al. Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PloS Genet. (2013) 9:e1003735. doi: 10.1371/journal.pgen.1003735
93. Bilgilier C, Schneider M, Kührer K, Kilb N, Hartl R, Topakian T, et al. Heterosubtypic, cross-reactive immunity to human Cytomegalovirus glycoprotein B. Clin Exp Immunol. (2022) 208:245–54. doi: 10.1093/cei/uxac031
94. Torok-Storb B, Boeckh M, Hoy C, Leisenring W, Myerson D, and Gooley T. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood. (1997) 90:2097–102. doi: 10.1182/blood.V90.5.2097
95. Valencia SM, Rochat E, Harnois MJ, Dennis M, Webster HS, Hora B, et al. Vaccination with a replication-defective cytomegalovirus vaccine elicits a glycoprotein B-specific monoclonal antibody repertoire distinct from natural infection. NPJ Vaccines. (2023) 8:154. doi: 10.1038/s41541-023-00749-0
96. Nelson CS, Vera Cruz D, Su M, Xie G, Vandergrift N, Pass RF, et al. Intrahost dynamics of human cytomegalovirus variants acquired by seronegative glycoprotein B vaccinees. J Virol. (2019) 93:e01695–18. doi: 10.1128/JVI.01695-18
Keywords: human cytomegalovirus (CMV), case series, vaccine, genetic variability, antiviral therapy
Citation: Marascio N, Pavia G, Mazzei C, Pantanella M, Giorgio E, Manno M, Gigliotti S, Barreca GS, Peronace C, Matera G and Quirino A (2025) Human Cytomegalovirus infection in the era of vaccine development: case series of immunocompromised patients. Front. Virol. 5:1698340. doi: 10.3389/fviro.2025.1698340
Received: 03 September 2025; Accepted: 20 October 2025;
Published: 31 October 2025.
Edited by:
Juan C. Hernandez, Cooperative University of Colombia, ColombiaReviewed by:
Kiran Avula, National Institute of Allergy and Infectious Diseases (NIH), United StatesLing Ding, University of Pittsburgh Medical Center, United States
Copyright © 2025 Marascio, Pavia, Mazzei, Pantanella, Giorgio, Manno, Gigliotti, Barreca, Peronace, Matera and Quirino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grazia Pavia, Z3JhemlhcGF2aWFAdW5pY3ouaXQ=
†ORCID: Grazia Pavia, orcid.org/0000-0002-7685-3419
 Nadia Marascio
Nadia Marascio Grazia Pavia
Grazia Pavia Chiara Mazzei
Chiara Mazzei Marta Pantanella
Marta Pantanella Emanuele Giorgio
Emanuele Giorgio Michele Manno
Michele Manno Simona Gigliotti
Simona Gigliotti Giorgio Settimo Barreca
Giorgio Settimo Barreca Cinzia Peronace
Cinzia Peronace Giovanni Matera
Giovanni Matera Angela Quirino
Angela Quirino