robert kelley
BobCoBiologics Consulting LLC
Petaluma, United States
794
Total views and downloads
Submit your idea
You will be redirected to our submission process.
Submission deadlines
Manuscript Summary Submission Deadline 31 March 2026 | Manuscript Submission Deadline 31 July 2026
This Research Topic is currently accepting articles.
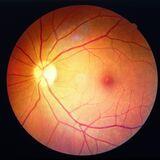
In this research topic we would like to address the question of what improvements are needed in the treatment of posterior eye disease. These improvements likely include safer and more efficacious therapeutics, changes that improve the patient experience through less invasive or less frequent dosing, while also decreasing the burden on the health care system that is associated with frequent intravitreal administration of large molecule drugs. A desired outcome of this research topic is that the collection of manuscripts will help influence future treatment paradigms for back of the eye diseases.
Treatments for posterior eye diseases, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and posterior uveitis, have seen significant advancements, particularly with the widespread use of intravitreal injections (e.g., anti-VEGF therapies for wet AMD and diabetic macular edema). Protein-based therapeutics such as aflibercept and ranibizumab have been critical components of wet AMD treatment. More recently, peptidic complement inhibitors administered via intravitreal injection have gained approval (US) for treatment of geographic atrophy (GA), an advanced form of dry AMD, and have shown modest therapeutic benefit. Substantial improvement in treatment options is necessary to enhance patient outcomes and quality of life. Less frequent intravitreal injections, or less invasive routes of administration, would increase availability of vision sparing treatments to new patients worldwide and enhance the treatment experience for existing patients. Improved gain in vision beyond what can be obtained with anti-VEGF alone in patients that respond to anti-VEGF therapy, and efficacy for patients that are non-responders to anti-VEGF, would have an impact on quality of life. More effective treatment options for GA, with better durability of effect than current approved therapeutics, likely would result in a greater sparing of vision for those suffering from this disease. Manuscripts that address these concepts, with scope as described below, are requested for consideration to be included in a research topic collection on treatment improvements for posterior eye disease to be published in Frontiers in Drug Delivery.
Current treatments often require frequent intravitreal injections, which can be burdensome, uncomfortable, and carry risks like infection, hemorrhage, and retinal detachment. Patient access to treatment has been limited since health care professionals (HCPs) trained in intravitreal injection are required to administer the drugs. Demand for anti-VEGF therapies has created logistical challenges for facilities with capabilities and staffing for performing intravitreal injections. Nucleic acid based medicines (NABM) have shown promise for durable treatment but efficacy in pivotal clinical trials has yet to be demonstrated. While anti-VEGF therapies have revolutionized treatment for neovascular conditions, many posterior eye diseases involve complex inflammatory, degenerative, or genetic pathways beyond VEGF such that different and/or additional drugs are required. Further research is needed to meet these challenges.
Here are key areas where advances are needed, and where submissions can shape future treatment paradigms for posterior eye diseases. Manuscripts describing original research, reviews, and commentaries are welcome:
• Drug Delivery Systems: There is a need for therapies with longer duration to reduce injection frequency, as well as alternative and less invasive delivery routes (e.g., sustained-release implants, topical or systemic administration). Novel approaches to overcome ocular barriers are encouraged.
• Treatment Efficacy: New drugs targeting pathways beyond anti-VEGF, combination therapies, and improved bioavailability are critical. Neuroprotective strategies and pre-clinical/clinical models that better predict human response are also sought.
• Early Detection: Enhanced diagnostic tools and personalized treatment strategies can enable earlier intervention, improving outcomes and minimizing side effects. Prophylactic treatments are also valuable.
• Safety: Safer therapies with fewer ocular and systemic side effects are essential, including better delivery methods and risk assessment.
• Regenerative Medicine and Gene Therapy: Advances in stem cell therapies, gene therapy, and gene editing (like CRISPR) hold promise for restoring vision, not just preserving it.
Future therapies will focus on being less invasive, more effective, and safer, with a strong emphasis on regeneration and disease modification.
Topic Editor Dr. Susan Crowell and Dr. Whitney Shatz-Binder are employed by Genentech, Inc. Topic Editor Dr. Robert Kelley declares no competing interest.
This Research Topic accepts the following article types, unless otherwise specified in the Research Topic description:
Articles that are accepted for publication by our external editors following rigorous peer review incur a publishing fee charged to Authors, institutions, or funders.
Article types
This Research Topic accepts the following article types, unless otherwise specified in the Research Topic description:
Keywords: Posterior Eye Disease, Retinal Disease, Posterior Uveitis, Intravitreal Injection, Non-invasive Delivery
Important note: All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Manuscripts can be submitted to this Research Topic via the main journal or any other participating journal.
Submit your idea
You will be redirected to our submission process.
Share on WeChat
Scan with WeChat to share this article
