- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2Hunan Clinical Research Center for Cerebrovascular Disease, Changsha, China
Background: Adipokines have been proven to be associated with atherosclerotic diseases such as ischemic stroke and coronary heart disease. The role of novel adipokines in the development of symptomatic intracranial atherosclerotic stenosis (sICAS) and extracranial atherosclerotic stenosis (sECAS) has not yet been investigated. This study aimed to evaluate the plasma levels of novel adipokines in patients with sICAS and sECAS and their associations with the prognosis of sICAS groups.
Methods: A total of 134 patients with acute ischemic stroke attribute to large-artery atherosclerosis (LAA) and 66 age- and sex-matched controls without atherosclerotic stenosis (NCAS) were included in this study. The LAA group was further sub-classified as sICAS (n = 102) and sECAS (n = 32) according to the location of atherosclerosis. Demographics, clinical parameters, angiographical features and plasma levels of novel adipokines (apelin, visfatin, omentin, RBP-4) were assayed and compared among groups.
Results: LAA patients had significantly lower levels of omentin [39.92 (30.74–52.61) ng/ml vs. 54.42 (34.73–79.91) ng/ml, P < 0.001] and visfatin [11.32 (7.62–16.44) ng/ml vs. 13.01 (9.46–27.54) ng/ml, P < 0.001] than those in the NCAS group. Multiple logistic regression analysis identified that the lowest tertile of omentin was independently associated with LAA (OR, 3.423; 95% CI, 1.267–9.244, when referenced to the third tertile). Levels of omentin, visfatin and RBP-4 showed no significant difference between sICAS and sECAS groups. However, median concentrations of apelin were lower in sECAS [84.94 (46.88–130.41) ng/mL) than in sICAS [118.64 (93.22–145.08) ng/mL, P = 0.002] and NCAS [114.38 (80.56–162.93) ng/mL, P = 0.004]. Logistic regression analysis showed that the lowermost tertile of apelin was independently associated with sECAS (OR, 5.121; 95% CI, 1.597–16.426) when adjusted for risk factors. As for sICAS patients, spearman coefficient analysis showed no significant correlation between these four adipokines and the severity of sICAS or the number of vessels with intracranial stenoses. Patients with severe stroke had lower levels of apelin (P = 0.005), while the other three adipokines showed no such difference. During follow up, no difference was found between these four novel adipokines and short- and long-term outcome of sICAS.
Conclusions: Lower levels of omentin are independent biomarkers of LAA while low apelin plasma levels seem to be risk factors of sECAS.
Introduction
Large-artery atherosclerosis (LAA) stroke is a highly prevalent cause of cerebral infarction according to the classification standard of Trial of Org 10172 in Acute Stroke Treatment (1). LAA affects both intracranial and extracranial arteries and symptomatic intracranial atherosclerotic stenosis (sICAS) has been proposed to be the major cause of LAA among Asian and African patients. On the contrary, symptomatic extracranial atherosclerotic stenosis (sECAS) is more common in Caucasians (2, 3). sICAS is a developing and progressing disease; sICAS patients are at high risk of stroke recurrence and developing other vascular events like myocardial infarction and peripheral vascular disease (4). Even under aggressive medical treatment and risk factor mitigation, sICAS-related recurrence risk continues to be high (5). Given the large burden of post-sICAS, assessment of the presence and prognosis of this disease is crucial. The potential pathogenesis of sICAS and the underlying causes behind the difference between intra- and extracranial stenosis remain unknown. Pathophysiological mechanisms like endothelial injury, lipid deposition, inflammation, angiogenesis and impaired fibrinolysis, etc. may play important roles in the pathological process of sICAS (6–9). Conventional risk factors like smoking, hypertension (HBP), diabetes mellitus (DM), and metabolic syndrome (MetS) have been reported to contribute differently to the development of sICAS and sECAS (7, 10–13).
Several studies have investigated the diagnostic or prognostic value of biomarkers like matrix metalloproteinases (MMP-2), Lipoprotein-associated phospholipase A2 (Lp-PLA2), Vascular Endothelial Growth Factor (VEGF) and Endostatin in patients with sICAS or sECAS (5, 7, 14). However, some conclusions have failed to be validated for these studies. Adipose tissue is an endocrine organ which can produce several adipokines that can modulate insulin sensitivity and contribute to the pathogenesis of MetS, DM, dyslipidemia, inflammation, endothelial dysfunction, coronary artery disease (CAD), and atherosclerotic stroke (15, 16). Adiponectin, leptin, and resistin are the most well-studied adipokines. The role of these traditional adipokines in cerebral vascular atherosclerotic stenosis has been reported in several studies. O.Y. Bang reported that lower adiponectin levels were associated with symptomatic sICAS vs. other ischemic stroke subtypes (17). Another study from South Korea showed that lower levels of adiponectin and higher levels of leptin had significant associations with increased risk of LAA stroke, but not sICAS (18). The Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) Study suggested that resistin was independently associated with combined intracranial-extracranial atherosclerotic disease (19).
Emerging evidence suggests a relationship between novel adipokines such as apelin, omentin, visfatin, RBP-4, and vascular risk factors and atherosclerotic diseases. Apelin, encoded by APLN gene, is a peptide that functions as an endogenous ligand of the orphan G-protein-coupled receptor. Apelin serum levels have been found to be increased in diabetic patients and in patients with metabolic syndrome and have been linked with coronary atherosclerosis (20, 21). On the contrary, Kadoglou et al. has supported an inverse relationship between serum apelin concentration and carotid atherosclerosis (22). Omentin, encoded by ITLN1 gene, is a novel adipokine produced by visceral adipose tissue. Omentin has been intimately linked with protective mechanisms against insulin-resistant states, for example, obesity and diabetes (23). Moreover, low omentin-1 levels have been detected in patients with carotid atherosclerosis and ischemic stroke (24, 25), and higher levels of omentin-1 have been associated with poor functional outcome of acute ischemic stroke (IS) patients (26). Visfatin, mainly secreted by visceral adipose tissue, is also known as nicotinamide phosphoribosyl transferase (NAMPT). Elevated visfatin levels have been found in DM, obesity, CAD, and symptomatic carotid stenosis (27–30). Another newly identified adipokine, Retinol-binding protein 4 (RBP-4), has emerged as a possible regulator of insulin resistance, obesity, and inflammation (31). Studies have shown that RBP-4 is positively correlated with the presence of CAD and carotid atherosclerosis (24, 32). To our knowledge, no study has examined the relationship of these four novel adipokines with sICAS or focused on their different roles in sICAS and sECAS. Given the relationship between the four novel adipokines with obesity, diabetes, inflammation, and atherosclerosis, we can infer that they may have additional connections with sICAS.
The purpose of our research was to study the association between plasma levels of four novel adipokines (apelin, visfatin, omentin, RBP-4) in sICAS and sECAS patients. We also examined the relationship between the plasma levels of these adipokines and the prognosis of sICAS patients.
Patients and Methods
Study Population
Study flow chart is shown in Figure 1. We prospectively recruited patients with acute large-artery atherosclerotic stroke (<7 days of onset) with relevant neuroimaging findings from September 2015 to May 2017 in the Department of Neurology, Xiangya Hospital. At the same time, 66 controls without atherosclerotic stenosis (NCAS) who were age- and sex-matched from the Health Examination Center of Xiangya Hospital were included. The exclusion criteria were as follows: infection on admission, autoimmune rheumatic diseases, malignancies, renal and hepatic insufficiency, recent surgery and those who underwent incomplete vascular imaging and laboratory tests, those who had stroke of other determined etiologies and those who did not provide informed consent. Patients who had combined intracranial and extracranial artery stenoses were also excluded. All subjects signed the informed consent and the study protocol was approved by the Ethics Committee of Xiangya Hospital of the Central South University in China.
Figure 1. Study flow chart. sICAS, symptomatic intracranial atherosclerotic stenosis disease; sECAS, symptomatic extracranial atherosclerotic stenosis disease; NCAS, no atherosclerotic stenosis controls; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; ELISA, enzyme-linked immunosorbent assay.
Imaging Assessment
Using our previously published angiographic assessment (7, 33), all patients underwent Brain Magnetic Resonance Imaging (MRI) scans to determine whether cerebral infarction exists. Most patients completed Magnetic Resonance Angiography (MRA) examination on a 1.5 or 3.0 T magnetic resonance scanner and CT angiography was performed in patients with contraindication of MRI. All patients underwent evaluations of extracranial vessels like carotid-enhanced magnetic resonance angiography (CE-MRA), carotid CTA, and ultrasonography of cervical vessels. Digital subtraction angiography (DSA) and high-resolution magnetic resonance imaging (HR-MRI) were used to validate the results of MRA/CEMRA/CTA findings. Intracranial vessels are defined as the proximal portion of internal carotid artery (ICA) and vertebral artery (VA), basilar artery (BA), anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA). Extracranial vessels are the extra-segment of ICA and VA. Intracranial artery stenosis was evaluated by referring to the published method in Warfarin–Aspirin Symptomatic Intracranial Disease Study (34). Stenosis degree of extracranial ICA and VA was calculated by the formula published by Rothwell et al. (35). The number of stenotic arteries of each patient was also included. According to stenosis severity, sICAS were categorized into moderate (50–69%) and severe (≥70%) groups. Finally, 102 patients with sICAS and 32 patients with sECAS were included in this study. Combined cerebral atherosclerotic stenosis (CCAS) was excluded from this study because of the small number of cases.
Clinical Assessment
Traditional risk factors like HBP, DM, dyslipidemia, CAD, smoking, and drinking were collected by clinical questionnaire and were defined as previously published (7, 36–38). Additionally, we also collected data on demographics, blood pressure, FBG (fasting glucose levels), HbA1C (glycated hemoglobin), TC (total cholesterol) levels, LDL (low-density lipoprotein cholesterol) levels, HDL (high-density lipoprotein cholesterol) levels, and TG (triglyceride) levels. All venous blood samples were drawn at baseline after an overnight fast at least for 12 h, the median time of blood sampling was 4 days after stroke onset. Metabolic syndrome was defined as having at least three conditions: men with waist-circumference ≥90 cm or women ≥80 cm; triglycerides >1.7 mmol/L; men with HDL cholesterol <1.03 mmol/L or women <1.30 mmol/L; already taking antihypertensive drugs or blood pressure ≥130/85 mmHg; previously diagnosed with type 2 DM, or taking antidiabetic drugs or current fasting blood glucose ≥5.6 mmol/L (39).
At admission, the National Institutes of Health Stroke Scale (NIHSS) was evaluated to define stroke severity. NIHSS ≤ 8 was defined as mild stroke and >8 was defined as severe stroke. The short-term outcome was measured on the 14th day after stroke according to the modified Rankin Scale (mRS) score. mRS ≤ 2 was defined as favorable outcome and mRS >2 or death was defined as unfavorable outcome. Then all patients were followed-up with 12 months after the discharge, information was obtained by telephone interview.
Determination of Novel Adipokines in Plasma
EDTA tubes were used to collect the venous blood samples at baseline. The median time of blood sampling was 4 days after stroke onset. The blood samples were centrifuged at 3,000 rpm at 4°Cfor 15 min immediately after collection. We collected plasma samples and stored them in an −80°C ultra-low temperature refrigerator until analysis. Plasma concentrations of apelin, omentin, visfatin, and RBP-4 (RayBiotech, Inc., Norcross, GA, USA) were assayed using quantitative sandwich enzyme immunoassay commercially available kits. ELISAs were performed according to the RayBio® ELISA manual strictly. All samples were assayed twice. The intra-assay coefficients of variance (CVs) were <10% for apelin, omentin, visfatin and RBP-4, while the inter-assay CVs for these variables were <15% for apelin, omentin, visfatin and <12% for RBP-4. All absorption readings were performed according to the standard curve.
Statistical Analysis
SPSS 22.0 (IBM SPSS, Chicago, Ill, USA) was used for all statistical analysis. Kolmogorov-Smirnov test was used to evaluate the normality of distribution. Normal distribution data were expressed as mean ± SD, and non-normal distribution data were expressed as median and quartile range. Adipokine levels were not normally distributed in our study. Category variables were expressed as percentages. Classified variables were tested by χ2-test and Fisher exact test to evaluate the statistical significance of differences between groups. One-way ANOVA and independent sample t-test were used to compare the parameters of normal distribution. Kruskal-Wallis test and Mann-Whitney U-test were used to compare the variables of non-normal distribution. Spearman correlation coefficient was used to study the correlation between the number and severity of sICAS and adipokines levels. In further analysis, adipokines levels were divided into several groups according to tertiles. Variables showing P < 0.05 in the univariate analysis and potential confounding factors such as age, gender, hypertension, diabetes, dyslipidemia, and MetS were included in the logistic regression analysis. Continuous variables were converted into categorized variables to get better and more reasonable interpretations of the results. The mRS at 14th days was not included as covariates in the regression analysis because it was the definition of early functional outcome, not the risk factors. The accuracy of biomarkers in disease prediction were measured by receiver operating characteristics (ROC) curves. Due to the small sample size of sECAS, biomarkers did not allow reliable analyses by subgroups. As for multiple comparisons, the significance level was adjusted to 0.05/4 ≈ 0.0125 with the Bonferroni method.
Results
Study Population Characteristics
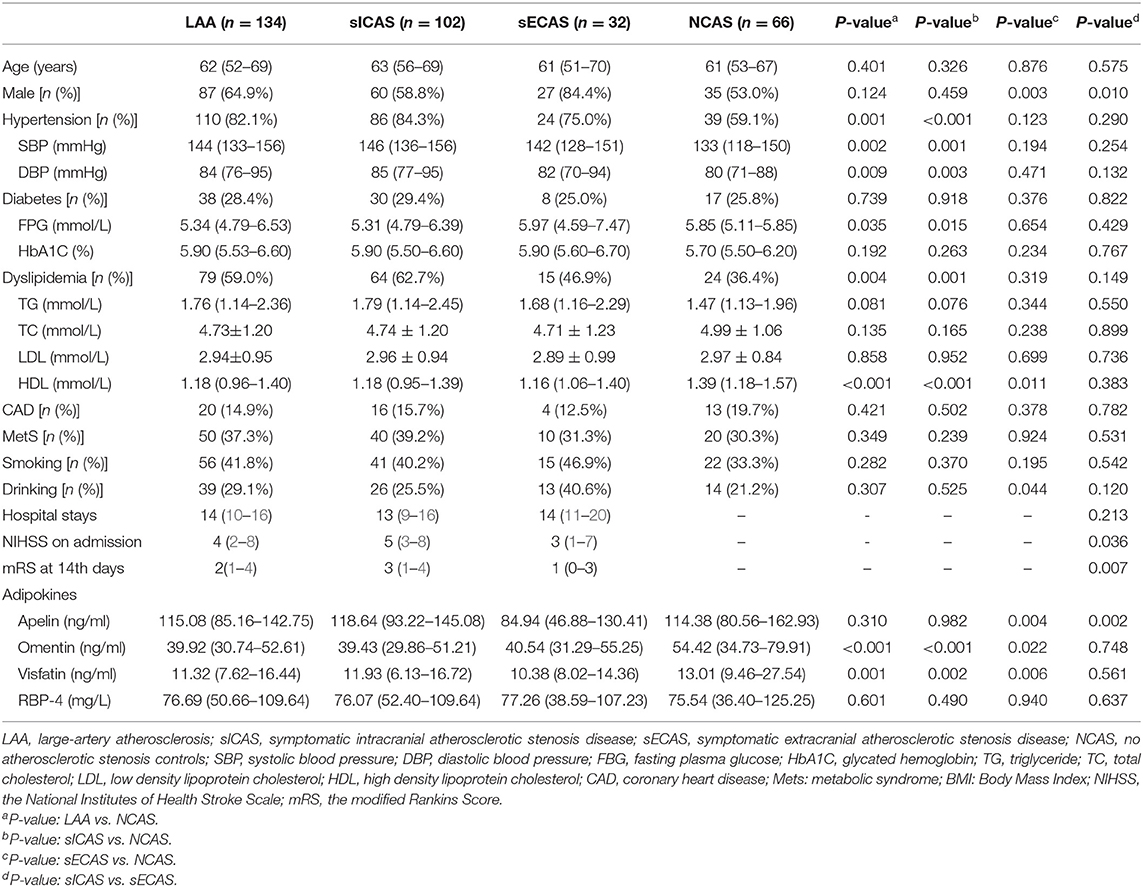
A total of 134 patients with LAA and 66 age and sex-matching NCAS controls were included in our study. The LAA group was further subclassified into 102 patients with sICAS and 32 with sECAS. Baseline demographic data, vascular risk factors, and laboratory test results of study participants are depicted in Table 1. Among all individuals, 149 (74.5%) had hypertension, 55 (27.5%) had diabetes mellitus, 103 (51.5%) had dyslipidemia, and 33 (16.5%) had coronary heart disease. Seventy-eight (39.0%) patients were smokers and 53 (26.5%) patients were drinkers. Median plasma levels of apelin, omentin, visfatin, and RBP-4 were 114.69, 43.25, 12.08 ng/mL, and 76.19 mg/L, respectively.
Plasma Adipokines Between Groups
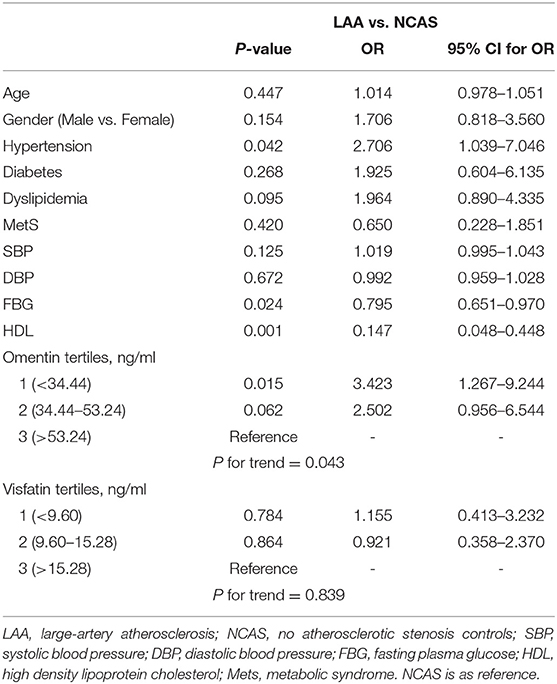
As shown in Table 1, when compared with NCAS controls, patients with LAA had significantly decreased levels of HDL and higher levels of SBP and DBP (P < 0.0125, Bonferroni correction). Patients with LAA showed higher frequency of hypertension and dyslipidemia than individuals in the control group. Moreover, the LAA group displayed lower plasma concentrations of omentin [39.92 (30.74–52.61) ng/ml vs. 54.42 (34.73–79.91) ng/ml, P < 0.001] and visfatin [11.32 (7.62–16.44) ng/ml vs. 13.01 (9.46–27.54) ng/ml, P = 0.001] than control counterparts, while the two study groups had non-significant differences between apelin and RBP-4 levels (shown in Figure 2). The levels of omentin and visfatin were then grouped according to tertiles. Variables showing P < 0.05 in the univariate analysis were selected for entry into logistic regression analysis. And we also adjusted with age, gender (male vs. female), hypertension, diabetes, dyslipidemia, and MetS. Logistic regression analysis revealed that the lowest tertile of omentin was independently associated with LAA (OR, 3.423; 95% CI, 1.267–9.244, when referenced to the third tertile). Tertiles of visfatin showed no significance when adjusted with their confounding factors (as shown in Table 2). With ROC curves analysis, omentin had reasonable accuracy for prediction of LAA (AUC: 0.657, Youden Index: 0.33, P < 0.001). The optimal cut-off value of omentin was ≤ 49.97 ng/mL (sensitivity: 0.606; specificity: 0.724) (as Figure 3 showed).
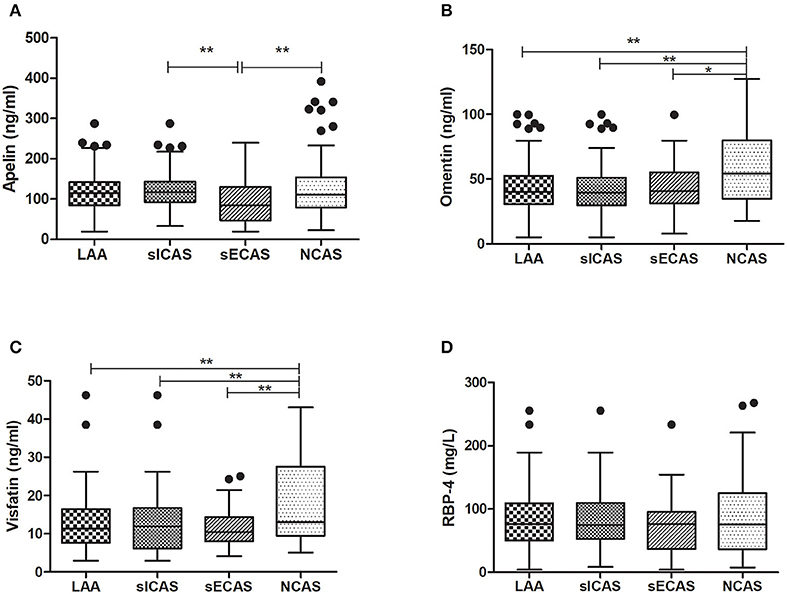
Figure 2. Levels of plasma adipokines in different groups. *P < 0.05 and **P < 0.01. n = 134 in LAA, n = 102 in sICAS, n = 32 in sECAS, n = 66 in NCAS. LAA, large-artery atherosclerosis; sICAS, symptomatic intracranial atherosclerotic stenosis disease; sECAS, symptomatic extracranial atherosclerotic stenosis disease; NCAS, no atherosclerotic stenosis controls.
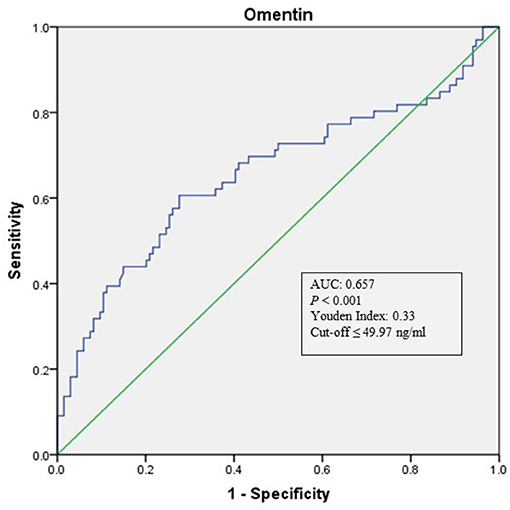
Figure 3. ROC curve analysis of Omentin cutoff point for the presence of LAA (vs. NCAS). AUC, area under the curve; ROC, receiver operating characteristic; LAA, large-artery atherosclerosis; NCAS, no atherosclerotic stenosis controls.
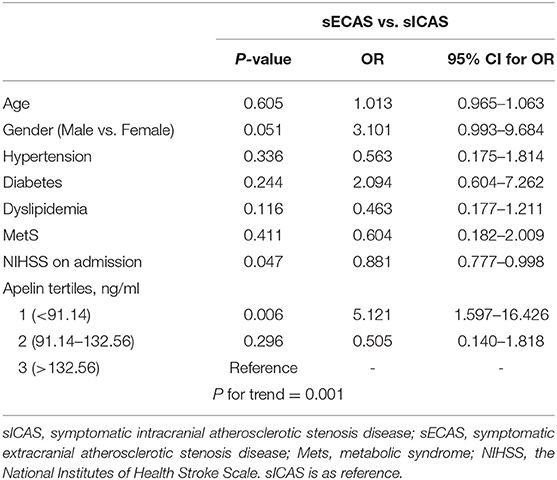
When compared with NCAS controls, patients with sICAS had significantly decreased levels of HDL and higher levels of SBP and DBP (P < 0.0125, Bonferroni correction). Patients with sICAS showed higher frequency of hypertension and dyslipidemia than control individuals. Moreover, sICAS patients appeared to have lower plasma concentrations of omentin [39.43 (29.86–51.21) ng/ml vs. 54.42 (34.73–79.91) ng/ml, P < 0.001] and visfatin [11.93 (6.13–16.72) ng/ml vs. 13.01 (9.46–27.54) ng/ml, P < 0.001] than control counterparts, while the two study groups had non-significant differences between apelin and RBP-4 levels (shown in Figure 2). Differences between sECAS and NCAS included gender, HDL levels, and the levels of apelin and visfatin (P < 0.0125, Bonferroni correction). There were more male patients in sECAS than sICAS. The prevalence of traditional vascular risk factors and laboratory biomarkers showed no differences between the two groups. However, NIHSS at admission and mRS at discharge of sECAS were lower than sICAS. As for the four novel adipokines analyzed in our study, median plasma concentrations of apelin were lower in the sECAS (84.94, 46.88–130.41 ng/mL) than in the sICAS (118.64, 93.22–145.08 ng/mL) group (P = 0.002) (shown in Figure 2). However, levels of omentin, visfatin, and RBP-4 showed no significant differences between these two groups. This data shows that lower levels of apelin seem to be more associated with sECAS.
To explore whether apelin could differentiate sECAS from sICAS, we performed logistic regression analysis. It showed that the lowermost tertile of apelin was independently associated with sECAS (OR, 5.121; 95% CI, 1.597–16.426) when adjusted for age, gender, hypertension, diabetes, dyslipidemia, MetS, and NIHSS at admission (as shown in Table 3). In ROC curves analysis, apelin had reasonable accuracy for prediction of sECAS (AUC: 0.684, Youden Index: 0.408, P = 0.002). The optimal cut-off value of omentin was ≤ 86.57 ng/mL (sensitivity: 0.814; specificity: 0.594) (as Figure 4 showed).
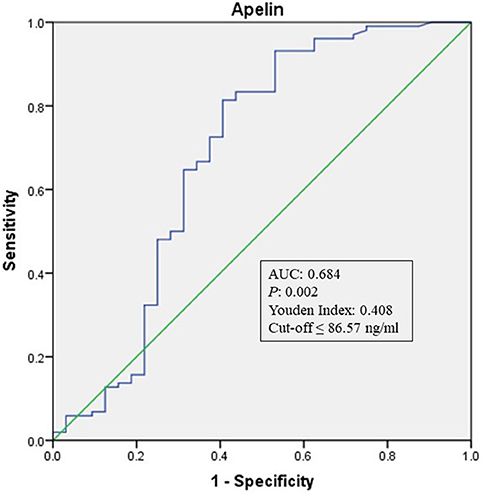
Figure 4. ROC curve analysis of Apelin cutoff point for the presence of sECAS (vs. sICAS). AUC, area under the curve; ROC, receiver operating characteristic; sICAS, symptomatic intracranial atherosclerotic stenosis disease; sECAS, symptomatic extracranial atherosclerotic stenosis disease.
Adipokines and the Severity of sICAS
Among 102 patients with sICAS, 27 (26.5%) patients had moderate stenosis and 75 (73.5%) had severe stenosis. Additionally, according to the number of stenotic vessels, 30 patients (29.4%) had single sICAS, 30 (29.4%) had two affected vessels and 42 (41.2%) had multi-stenoses. Spearman coefficient analysis showed no significant association between the four adipokines and the severity of intracranial atherosclerosis or the number of vessels with intracranial stenoses (data not shown).
Stroke Severity and Short-term Prognosis of sICAS
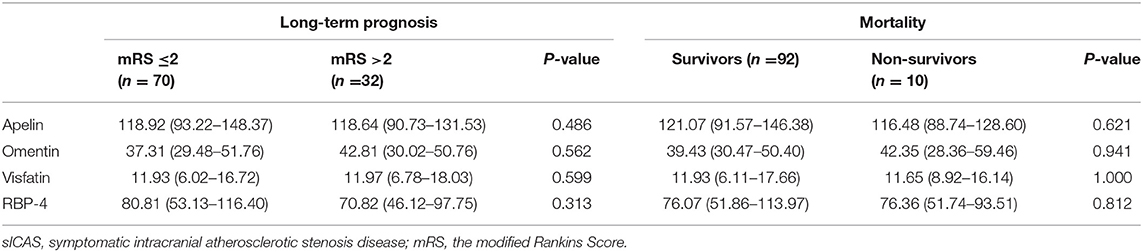
Patients with severe stroke had lower levels of apelin (P = 0.005); the other three adipokines showed no such differences. Individuals with unfavorable outcome in the acute phase (14 days) of sICAS also showed no difference between these four adipokines when compared with the favorable group (shown in Table 4).
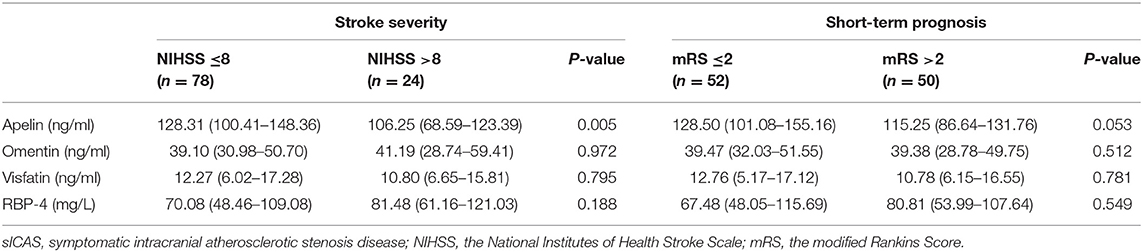
Table 4. Associations between novel adipokines and stroke severity or short-term prognosis of sICAS group.
Mortality and Long-Term Prognosis of sICAS
Long-term follow-ups of sICAS patients were conducted in the 12 months after discharge. During follow-up, a total of ten patients died, eight of whom died due to cerebrovascular reasons and two patients due to pneumonia. Seventy patients had favorable long-term outcome and 32 patients had unfavorable outcome. However, we found no differences of these four novel adipokines between groups (shown in Table 5).
Discussion
Our study showed that admission plasma omentin levels were significantly decreased in LAA patients when compared with NCAS subjects. Furthermore, lower admission plasma apelin levels were identified as reliable and independent markers to predict patients with sECAS, substantiating their potential role as a new diagnostic biomarker. However, we failed to find the prognostic values of these novel adipokines for short- and long-term outcomes and sICAS mortality. To our knowledge, this is the first study to assess the diagnostic and prognostic relationship of novel adipokines, apelin, omentin, visfatin, and RBP-4 with sICAS and sECAS.
In our study, LAA risk increased when levels of omentin decreased, supporting a potential protective role of omentin. As an anti-inflammatory adipokine, decreased levels of omentin-1 have been found in obesity, DM and MetS (40, 41). Previous researchers have found a negative correlation between omentin-1 levels and carotid atherosclerosis. Yoo et al. proved that low levels of omentin-1 were an independent factor of carotid plaque existence among patients with type 2 DM (42). The study by Kadoglou et al. demonstrated that patients with carotid atherosclerosis had lower omentin-1 levels when compared with patients without atherosclerosis (24). Our study adds evidence to the protective role of omentin by demonstrating that patients with LAA had decreased omentin-1 levels when compared to NCAS. The possible mechanisms underlying the protective effect of omentin in LAA can be summarized as follows: (1) omentin attenuates vascular inflammation through a variety of signaling pathways like the AMPK pathway. Research shows that through the AMPK signaling pathway, omentin deficiency can accelerate the formation of atherosclerotic plaque by promoting inflammatory response (2, 41) Omentin promotes angiogenesis by activating eNOS-dependent signaling pathway and enhances vascular dilatation of vascular endothelial cells in response to ischemia (3, 43, 44) omentin can improves insulin sensitivity by activating the Akt protein kinase B in adiposity as it increases the insulin transduction (45). Reduced insulin resistance and inflammation response caused by omentin may resist the formation of atherogenesis, and as a result, lower the risk of LAA.
Intracranial vessels differ from extracranial vessels by having a high antioxidant capacity, low inflammatory response, and a lack of vasa vasorum. They are also surrounded by cerebrospinal fluid (46). As we described in a recent paper (7), lower levels of vascular endothelial growth factor were more correlated with the presence of sICAS than sECAS, which suggests that angiogenesis in sICAS might be suppressed. In this study, lower apelin plasma levels are more associated with sECAS than with sICAS, and sICAS patients with severe stroke had lower levels of apelin. The role of apelin in the development of atherosclerotic diseases is not entirely clear. One study has demonstrated lower apelin levels in patients with carotid atherosclerosis. Since plaque vulnerability has been shown to correlate with an increase local number of inflammatory cells within plaques, a possible underlying mechanism could be that apelin limits macrophage infiltration (47). Apelin is also involved in the development of angiogenesis. It can promote angiogenesis response and cerebral reperfusion through the vascular endothelial growth factor-vascular endothelial growth factor receptor 2 (VEGF-VEGFR2) signaling pathway (48). In acute phase of sICAS, as a “good” adipokine, apelin can facilitate the angiogenesis reaction to increased oxygen supply. On the other hands, the lower levels of apelin means poor collateral circulations, causing neurological deficit symptoms to worsern.
In this study, we failed to discover the relationship between these novel adipokines and short- or long-term outcome of sICAS. This could be due to the fact that adipokines levels were only measured once at admission.
There are also some limitations in our research. Firstly, this study was a single- center cohort, and the sample size was relatively small. The external validity of the findings is limited. Secondly, adipokines levels in our study were only measured once on admission, as discussed above. Furthermore, other novel adipokines, such as vaspin, ghrelin, were not detected in our study.
Conclusion
In conclusion, our present study demonstrated that lower levels of omentin are an independent biomarker of LAA while lower apelin plasma levels are more associated with sECAS. Omentin, apelin, visfatin, and RBP-4 had no prognostic value of sICAS. Our findings may be useful in assessing different atherosclerotic stenosis, detecting possible mechanisms of stroke, and may be of practical significance in the study of specific prevention strategies for sICAS and sECAS.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects have signed the informed consent and the study protocol was approved by the Ethics Committee of Xiangya Hospital of the Central South University in China.
Author Contributions
JX, BX, and QY involved in the study design. XZ, ZLi, and XF were responsible for the data collection. ZLiu, DL, QH, and XL were responsible for the sample collection. FY conducted the experiments and wrote the manuscript. JX modified and revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81671166 and 81400972) and Clinical and rehabilitation research fund of Sinobioway Biomedicine Co., Ltd. (xywm2015I30), and Independent Exploration and Innovation project for postgraduate of Central South University (No. 2019zzts902).
Conflict of Interest
The authors declare that this study received funding from Clinical and rehabilitation research fund of Sinobioway Biomedicine Co., Ltd.
Acknowledgments
The authors thank Dr. Omar Abdihamid, Department of Medical Oncology, Xiangya School of Medicine and Dr. Jennifer Zhang, Department of Human Genetics, Emory University School of Medicine, for language editing.
References
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
2. Mak W, Cheng TS, Chan KH, Cheung RT, Ho SL. A possible explanation for the racial difference in distribution of large-arterial cerebrovascular disease: ancestral European settlers evolved genetic resistance to atherosclerosis, but confined to the intracranial arteries. Med Hypotheses. (2005) 65:637–48. doi: 10.1016/j.mehy.2005.05.017
3. Uehara T, Bang OY, Kim JS, Minematsu K, Sacco R. Risk factors. Front Neurol Neurosci. (2016) 40:47–57. doi: 10.1159/000448301
4. Kim JS, Bang OY. Medical treatment of intracranial atherosclerosis: an update. J Stroke. (2017) 19:261–70. doi: 10.5853/jos.2017.01830
5. Kim BS, Chung PW, Park KY, Won HH, Bang OY, Chung CS, et al. Burden of intracranial atherosclerosis is associated with long-term vascular outcome in patients with ischemic stroke. Stroke. (2017) 48:2819–26. doi: 10.1161/STROKEAHA.117.017806
6. Chung JW, Oh MJ, Cho YH, Moon GJ, Kim GM, Chung CS, et al. Distinct roles of endothelial dysfunction and inflammation in intracranial atherosclerotic stroke. Eur Neurol. (2017) 77:211–9. doi: 10.1159/000460816
7. Yu F, Lu J, Li Z, Zhou X, Zeng S, Zhan Q, et al. Correlation of plasma vascular endothelial growth factor and endostatin levels with symptomatic intra- and extracranial atherosclerotic stenosis in a Chinese han population. J Stroke Cerebrovasc Dis. (2017) 26:1061–70. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.021
8. Shimizu K, Shimomura K, Tokuyama Y, Sakurai K, Isahaya K, Takaishi S, et al. Association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:211–7. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.019
9. Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. (2008) 39:1456–63. doi: 10.1161/STROKEAHA.107.498600
10. Li Y, Cai Y, Zhao M, Sun J. Risk factors between intracranial-extracranial atherosclerosis and anterior-posterior circulation stroke in ischaemic stroke. Neurol Res. (2017) 39:30–5. doi: 10.1080/01616412.2016.1250856
11. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. (2012) 43:3313–8. doi: 10.1161/STROKEAHA.112.658500
12. Ding X, Li C, Yu K, Gao A, Xiao L, Peng F, et al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: a systematic review and meta-analysis. Int J Neurosci. (2014) 124:834–40. doi: 10.3109/00207454.2013.879580
13. Liu CY, Chen CQ. Intra- and extracranial atherosclerotic stenosis in China: epidemiology, diagnosis, treatment and risk factors. Eur Rev Med Pharmacol Sci. (2014) 18:3368–79.
14. Wang Y, Zhang J, Qian Y, Tang X, Ling H, Chen K, et al. Association of Lp-PLA2 Mass and aysmptomatic intracranial and extracranial arterial stenosis in hypertension patients. PLoS ONE. (2015) 10:e0130473. doi: 10.1371/journal.pone.0130473
15. Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. (2013) 227:216–21. doi: 10.1016/j.atherosclerosis.2012.12.029
16. Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabo Clin Exp. (2013) 62:1513–21. doi: 10.1016/j.metabol.2013.06.004
17. Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. (2007) 68:1931–7. doi: 10.1212/01.wnl.0000263186.20988.9f
18. Jeon SB, Chun S, Choi-Kwon S, Chi HS, Nah HW, Kwon SU, et al. Biomarkers and location of atherosclerosis: matrix metalloproteinase-2 may be related to intracranial atherosclerosis. Atherosclerosis. (2012) 223:442–7. doi: 10.1016/j.atherosclerosis.2012.04.013
19. Lopez-Cancio E, Galan A, Dorado L, Jimenez M, Hernandez M, Millan M, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. (2012) 43:2712–9. doi: 10.1161/STROKEAHA.112.661702
20. Habchi M, Duvillard L, Cottet V, Brindisi MC, Bouillet B, Beacco M, et al. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin Endocrinol. (2014) 81:696–701. doi: 10.1111/cen.12404
21. Karbek B, Bozkurt NC, Topaloglu O, Aslan MS, Gungunes A, Cakal E, et al. Relationship of vaspin and apelin levels with insulin resistance and atherosclerosis in metabolic syndrome. Minerva Endocrinol. (2014) 39:99–105.
22. Kadoglou NP, Fotiadis G, Lambadiari V, Maratou E, Dimitriadis G, Liapis CD. Serum levels of novel adipokines in patients with acute ischemic stroke: potential contribution to diagnosis and prognosis. Peptides. (2014) 57:12–6. doi: 10.1016/j.peptides.2014.04.008
23. Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes. (2017) 42:446–56.e1. doi: 10.1016/j.jcjd.2017.10.040
24. Kadoglou NP, Lambadiari V, Gastounioti A, Gkekas C, Giannakopoulos TG, Koulia K, et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis. (2014) 235:606–12. doi: 10.1016/j.atherosclerosis.2014.05.957
25. Yue J, Chen J, Wu Q, Liu X, Li M, Li Z, et al. Serum levels of omentin-1 association with early diagnosis, lesion volume and severity of acute ischemic stroke. Cytokine. (2018) 111:518–22. doi: 10.1016/j.cyto.2018.05.026
26. Xu T, Zuo P, Wang Y, Gao Z, Ke K. Serum omentin-1 is a novel biomarker for predicting the functional outcome of acute ischemic stroke patients. Clin Chem Lab Med. (2018) 56:350–5. doi: 10.1515/cclm-2017-0282
27. Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. (2001) 280:E827–47. doi: 10.1152/ajpendo.2001.280.6.E827
28. Owczarek AJ, Olszanecka-Glinianowicz M, Kocelak P, Bozentowicz-Wikarek M, Brzozowska A, Mossakowska M, et al. The relationship between circulating visfatin/nicotinamide phosphoribosyltransferase, obesity, inflammation and lipids profile in elderly population, determined by structural equation modeling. Scandinav J Clin Lab Investig. (2016) 76:632–40. doi: 10.1080/00365513.2016.1230884
29. Yu F, Li J, Huang Q, Cai H. Increased peripheral blood visfatin concentrations may be a risk marker of coronary artery disease: a meta-analysis of observational studies. Angiology. (2018): 69:825–34. doi: 10.1177/0003319718771125
30. Yang W, Li Y, Wang JY, Han R, Wang L. Circulating levels of adipose tissue-derived inflammatory factors in elderly diabetes patients with carotid atherosclerosis: a retrospective study. Cardiovasc Diabetol. (2018) 17:75. doi: 10.1186/s12933-018-0723-y
31. Korek E, Krauss H. Novel adipokines: their potential role in the pathogenesis of obesity and metabolic disorders. Postepy Hig Med Dosw. (2015) 69:799–810. doi: 10.5604/17322693.1161415
32. Sun Q, Kiernan UA, Shi L, Phillips DA, Kahn BB, Hu FB, et al. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses' health study. Circulation. (2013) 127:1938–47. doi: 10.1161/CIRCULATIONAHA.113.002073
33. Feng X, Zhou X, Yu F, Liu Z, Wang J, Li Z, et al. Low-normal free triiodothyronine and high leukocyte levels in relation to stroke severity and poor outcome in acute ischemic stroke with intracranial atherosclerotic stenosis. Int J Neurosci. (2019) 129:635–41. doi: 10.1080/00207454.2018.1503179
34. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. (2000) 21:643–6.
35. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. (2003) 361:107–16. doi: 10.1016/S0140-6736(03)12228-3
36. Li Z, Yu F, Zhou X, Zeng S, Zhan Q, Yuan M, et al. Promoter hypomethylation of microRNA223 gene is associated with atherosclerotic cerebral infarction. Atherosclerosis. (2017) 263:237–43. doi: 10.1016/j.atherosclerosis.2017.06.924
37. Long Y, Zhan Q, Yuan M, Duan X, Zhou J, Lu J, et al. The Expression of microRNA-223 and FAM5C in cerebral infarction patients with diabetes mellitus. Cardiovasc Toxicol. (2017) 17:42–8. doi: 10.1007/s12012-015-9354-7
38. Zhou X, Yu F, Feng X, Wang J, Li Z, Zhan Q, et al. Immunity and inflammation predictors for short-term outcome of stroke in young adults. Int J Neurosci. (2017) 128:634–9. doi: 10.1080/00207454.2017.1408614
39. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
40. Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. (2010) 20:143–8. doi: 10.1016/j.tcm.2010.12.002
41. Tan YL, Zheng XL, Tang CK. The protective functions of omentin in cardiovascular diseases. Clin Chim Acta. (2015) 448:98–106. doi: 10.1016/j.cca.2015.05.019
42. Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Seo JA, et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol. (2011) 10:103. doi: 10.1186/1475-2840-10-103
43. Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, et al. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. (2012) 287:408–17. doi: 10.1074/jbc.M111.261818
44. Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. (2010) 393:668–72. doi: 10.1016/j.bbrc.2010.02.053
45. Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. (2006) 290:E1253–61. doi: 10.1152/ajpendo.00572.2004
46. Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. (2014) 130:1407–14. doi: 10.1161/CIRCULATIONAHA.114.011147
47. Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Gerasimidis T, Kostakis A, et al. Adipokines: a novel link between adiposity and carotid plaque vulnerability. Eur J Clin Investig. (2012) 42:1278–86. doi: 10.1111/j.1365-2362.2012.02728.x
Keywords: symptomatic intracranial atherosclerotic stenosis, symptomatic extracranial atherosclerotic stenosis, apelin, omentin, visfatin, RBP-4
Citation: Yu F, Zhou X, Li Z, Feng X, Liao D, Liu Z, Huang Q, Li X, Yang Q, Xiao B and Xia J (2019) Diagnostic Significance of Plasma Levels of Novel Adipokines in Patients With Symptomatic Intra- and Extracranial Atherosclerotic Stenosis. Front. Neurol. 10:1228. doi: 10.3389/fneur.2019.01228
Received: 16 April 2019; Accepted: 04 November 2019;
Published: 19 November 2019.
Edited by:
Anna Rosell, Vall d'Hebron Research Institute (VHIR), SpainReviewed by:
Seung-Hun Oh, CHA University, South KoreaJordi Martorell, Institut Químic de Sarrià, Spain
Dolors Giralt, Hipra Scientific, Spain
Copyright © 2019 Yu, Zhou, Li, Feng, Liao, Liu, Huang, Li, Yang, Xiao and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xia, xjian1216@csu.edu.cn
 Fang Yu
Fang Yu Xiaoqing Zhou1
Xiaoqing Zhou1 Bo Xiao
Bo Xiao Jian Xia
Jian Xia