- Department of Entomology, MOA Key Laboratory of Pest Monitoring and Green Management, College of Plant Protection, China Agricultural University, Beijing, China
Thioredoxins (Trxs), a member of the thioredoxin system, play crucial roles in maintaining intracellular redox homeostasis and protecting organisms against oxidative stress. In this study, we cloned and characterized two genes, GmTrx2 and GmTrx-like1, from Grapholita molesta. Sequence analysis showed that GmTrx2 and GmTrx-like1 had highly conserved active sites CGPC and CXXC motif, respectively, and shared high sequence identity with selected insect species. The quantitative real-time polymerase chain reaction results revealed that GmTrx2 was mainly detected at first instar, whereas GmTrx-like1 was highly concentrated at prepupa day. The transcripts of GmTrx2 and GmTrx-like1 were both highly expressed in the head and salivary glands. The expression levels of GmTrx2 and GmTrx-like1 were induced by low or high temperature, E. coli, M. anisopliae, H2O2, and pesticides (emamectin benzoate). We further detected interference efficiency of GmTrx2 and GmTrx-like1 in G. molesta larvae and found that peroxidase capacity, hydrogen peroxide content, and ascorbate content all increased after knockdown of GmTrx2 or GmTrx-like1. Furthermore, the hydrogen peroxide concentration was increased by emamectin benzoate and the sensitivity for larvae to emamectin benzoate was improved after GmTrx2 or GmTrx-like1 was silenced. Our results indicated that GmTrx2 and GmTrx-like1 played vital roles in protecting G. molesta against oxidative damage and also provided the theoretical basis for understanding the antioxidant defense mechanisms of the Trx system in insects.
Introduction
Reactive oxygen species (ROS) is a collective term for hydrogen peroxide, superoxide anion, and hydroxyl radical. It can be generated during metabolic activities and induced by external factors such as pro-oxidants, heavy metals, pesticides, and other adverse factors (Imlay, 2003). Excessive ROS can disrupt the balance of intracellular redox homeostasis (Droge, 2002) and cause oxidative damage to proteins, lipids, and nucleic acids (Wu et al., 2011). For protecting against the toxicity of excessive ROS, aerobic organisms have developed complex antioxidant enzymatic systems to maintain the intracellular ROS at proper levels (Kobayashi-Miura et al., 2007). The thioredoxin (Trx) is an important part of thioredoxin systems that play important roles in redox-regulatory processes (Lu and Holmgren, 2014).
Thioredoxins (Trxs) are the family of soluble proteins involved in cellular dithiol-disulfide redox of organisms (Rietsch and Beckwith, 1998). The Trxs can protect cells from the damage of ROS generated under stressful conditions by acting as disulfide reductases or electron donors in the reduction of disulfide and dithiol (Stryer et al., 1967; Holmgren, 1979; Nakamura et al., 1997; Kalinina et al., 2008; Mahmood et al., 2012). It plays a crucial role in the maintenance of cellular thiol redox balance (Arnér and Holmgren, 2000; Chen et al., 2002; Myers et al., 2008).
In insects, research on Trx has been focused on a few species of insects, for example, Drosophila melanogaster, Bombyx mori, and Apis cerana cerana. In D. melanogaster, three Trx genes (Trx1, Trx2, and TrxT) have been identified (Kanzok et al., 2001; Bauer et al., 2002; Svensson et al., 2003), and DmTrx2 played important roles in redox regulation, the oxidative defense system, and modulating the lifespan of flies (Bauer et al., 2002; Svensson and Larsson, 2007). In B. mori, BmTrx has been shown to have a major role in resisting oxidative stress caused by extreme temperatures and microbial infection (Kim et al., 2007). In Apis mellifera, AmTrx1 was found in the mitochondria, which is an organelle responsible for aerobic respiration, suggesting AmTrx1 might be involved in scavenging ROS in mitochondria (Corona and Robinson, 2006). In A. cerana cerana, some Trxs, including AccTrx-like1, AccTrx1, and AccTrx2 have been demonstrated to participate in antioxidant defense (Lu et al., 2012; Yao et al., 2013, 2014). In Lepidoptera, SlTrx1 and SlTrx2 from Spodoptera litura and HaTrx2 from Helicoverpa armigera have been identified and demonstrated to participate in antioxidant defense (Kang et al., 2015; Zhang et al., 2015). These studies suggested that Trxs play important roles in maintaining redox homeostasis and resisting adverse circumstances in insects. However, there are no relevant reports in Grapholita molesta.
The G. molesta (Busck) is a worldwide pest of stone and pome fruits in most temperate fruit-growing regions (Kirk et al., 2013). Nowadays, due to the larvae’s habits of drilling, insecticides used for egg and neonate are the main means to control G. molesta. Unfortunately, organophosphate insecticide resistance has occurred in G. molesta (Kanga et al., 2003). It is crucial to understand which genes are involved in the defense mechanism of insects. According to previous studies, Trxs play an important role in elucidating the ROS induced by extreme environments. We hypothesize that Trx2 and Trx-like1 can help to resist the insecticides in G. molesta. To elucidate the functions of Trx system genes in G. molesta, we have identified Trx2 and Trx-like1 and analyzed their spatio-temporal expression patterns. Moreover, the transcript levels of GmTrx2 and GmTrx-like1 were also assessed after various types of stress treatments (low or high temperatures, exposure to Escherichia coli, Metarhizium anisopliae, H2O2, and emamectin benzoate infection). After GmTrx2 or GmTrx-like1 knockdown, we further examined antioxidant enzyme activities and metabolite amounts. Finally, we detected the content of hydrogen peroxide caused by emamectin benzoate and examined the susceptibility of larvae to emamectin benzoate after GmTrx2 or GmTrx-like1 silencing.
Materials and Methods
Ethics Statement
In this study, the larvae of oriental fruit moth G. molesta were originally collected in the Institute of Pomology in Liaoning province, China. No permissions were required for the insect collection, as the orchards are experimental plots belonging to the Institute of Pomology in Liaoning province. The “List of Protected Animals in China” excludes insects.
Insect Rearing
The G. molesta used in this study were reared on fresh apples and an artificial diet at a constant temperature of 25 ± 1°C at 70 ± 10% RH and with a 15 L: 9 D light regime in our laboratory. Adults were reared in beakers (1 L in volume) with fresh apples inside for egg laying and fed with 10% honey solution. The fifth instars larvae picked out from rotten apples were transferred to finger-shaped glass tube (5.5 cm in length × 2.2 cm in diameter) on artificial diet until the pupation.
Total RNA Extraction and cDNA Synthesis
The TRIzol reagent (TaKaRa, Kyoto, Japan) was used to extract total RNA according to the instructions. The quality and quantity of the extracted RNA products were determined by using an ultraviolet spectrophotometer (Abs260) and a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, Kyoto, Japan); the first-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA according to the manufacturer’s instructions.
Primer Design and Quantitative Real-Time Polymerase Chain Reaction Amplification Conditions
The DNAClub and DNAman software were used to design GmTrx2 and GmTrx-like1 primers for reverse transcription PCR (RT-PCR), quantitative real-time PCR (qRT-PCR), and dsRNA synthesis. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH, KJ094948.1) and actin (actin, KF022227.1) were used as internal reference genes for the qRT-PCR to normalize target gene expression. The primers were synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China).
The qRT-PCR was conducted in a 20 μl reaction volume comprising 1 μl cDNA, 10 μl 2 × SYBR Green Supermix (TaKaRa, Kyoto, Japan), 2 μl qRT-PCR primers, and 7 μl ddH2O on a Bio-Rad CFX Connect Real-Time PCR Detection System (Hercules, CA, United States). The amplification was performed following the program as: 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, and 60°C for 30 s. The 2−ΔΔCT method was used for the quantitative analysis (Livak and Schmittgen, 2001).
Cloning, Sequencing, and Sequence Analysis of GmTrx2 and GmTrx-like1
The genes of GmTrx2 and GmTrx-like1 were obtained from G. molesta transcriptome. The full-length cDNA sequences of both Trx genes were amplified with specific primers (Supplementary Table S1) and G. molesta cDNA. According to the manufacturer’s instructions of the DNA fragment purification kit (TaKaRa, Kyoto, Japan) and the pMDTM18-T Vector Cloning Kit (TaKaRa, Kyoto, Japan), the PCR products were sequentially purified and cloned into the PMD 18-T vector. The recombinant plasmid extracted was sequenced by Sangon Biotechnology Co., Ltd. (Shanghai, China).
The online bioinformatics ProtParam tool1 was used to analyze the physicochemical properties of GmTrx2 and GmTrx-like1. The related homologous protein sequences from various species were obtained from NCBI database and analyzed using DNAman 6.0.3 software. The conserved domains in GmTrx2 and GmTrx-like1 were detected using bioinformatics tools available on the NCBI server2. The phylogenetic trees based on amino acid sequence were constructed by using the neighbor-joining method with poisson model, uniform rates, and complete deletion in MEGA 5.10 software. To ensure the accuracy of the tree structure, the tree was created with 1000 replicates.
Developmental Analysis and Tissue Distribution of GmTrx2 and GmTrx-like1
To determine the spatio-temporal expression pattern of GmTrx2 and GmTrx-like1, samples from different developmental stages and different tissues of larvae were collected. The different developmental stages included eggs, first, second, third, fourth, fifth instar, and prepupa; first, third, fifth, and seventh day pupae; and first and third day adults. The stages of pupae and adults were divided into male and female. The different tissues including the head, epidermis, fat body, midgut, Malpighian tubules, and salivary glands were collected from larvae of the fifth instar. All samples were immediately stored at −80°C for total RNA extraction and cDNA synthesis.
Effect of Different Types of Stress on the Expression of GmTrx2 and GmTrx-like1
To clarify the effect of various stress on GmTrx2 and GmTrx-like1 expression, fifth instar larvae were treated by low temperature (15°C) or high temperature (35°C), E. coli, M. anisopliae, H2O2, and emamectin benzoate. In the temperature treatments, the larvae were exposed to 15 and 35°C and larvae in 25°C were as a control; For E. coli, M. anisopliae, and H2O2 treatments, the larvae were injected with 1 μL of 1.0 × 105 E. coli cells, M. anisopliae (1.0 cfu/mL × 105 cfu/mL), and hydrogen peroxide (concentration of 100 mM), respectively; control larvae were injected with an equal volume of phosphate buffered saline. The samples from each group were collected at 0, 6, 12, and 24 h after injection. In insecticide treatments, larvae were soaked in emamectin benzoate (active ingredient concentration: 16.42 mg/L), purchased from Hansen Biologic Science Co., Ltd. (Qingdao, China) for 5 s and samples were collected at 2, 4, and 8 h. The larvae soaked in water were used as the controls. There were 13 larvae in each treatment group and 10 larvae in the control group in one replicate and three replicates were used for each treatment. All samples were immediately stored at −80°C for later total RNA extraction.
Synthesis of dsRNA and Detecting of RNAi Efficiency
The DNA fragment purification kit (TaKaRa Biotechnology, Dalian, China) was used to purify PCR products, which were synthesized with the primers containing a T7 polymerase promoter sequence (Supplementary Table S1) and cDNA of G. molesta. The purified PCR products were used as templates and MEGAscript RNAi Kit (Ambion) was used to synthesize the dsRNA of EGFP, GmTrx2, and GmTrx-like1, according to the manufacturer’s instructions. The synthesized dsRNA products were purified by using MEGAclear columns (Ambion) and redissolved with diethyl pyrocarbonate-treated nuclease-free water. The purity and concentration of dsRNA were measured with ultraviolet spectrophotometry and gel electrophoresis. To determine the interference efficiency of GmTrx2 and GmTrx-like1, fifth instar larvae were injected with approximately 3 μg of dsRNA into proleg using capillary microsyringe. The larvae were injected with dsEGFP as a control. One replicate of the treatments injected with dsRNA of GmTrx2 or GmTrx-like1 contained 13 larvae, while the treatments injected with dsEGFP had 10 larvae. Three replicates were used for each treatment. All samples were collected at 24, 48, and 72 h and stored at −80°C for later detection of gene expression.
Analysis of Enzymatic Activity and Metabolite Content After Knockdown of GmTrx2 and GmTrx-like1
The samples were collected at 48 and 72 h after dsRNA injection of EGFP, GmTrx2, and GmTrx-like1. The BCA Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to extract and quantify total protein. The capacity of superoxide dismutase and peroxidase were assayed by using superoxide dismutase test kit and peroxidase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. The hydrogen peroxide, and ascorbate test kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to quantify the contents of hydrogen peroxide and ascorbate, respectively, according to the manufacturer’s protocols.
Determination of H2O2 Contents and Survival Assay
Whole body larval samples were collected at 4 and 8 h after soaking in the emamectin benzoate (active ingredient concentration: 16.42 mg/L) for 5 s. The method for detecting hydrogen peroxide concentration was the same as earlier. In the survival assay, after injecting the 3 μg dsRNA of GmTrx2 or GmTrx-like1 for 12 h, the larvae were immersed in emamectin benzoate (active ingredient concentration: 1.642 mg/L) for 5 s. Non-injected and injection of dsEGFP larvae were as the controls. The number of dead larvae was recorded in each group for 96 h. The tested insects used for the groups of emamectin benzoate, emamectin benzoate + dsEGFP, emamectin benzoate + dsTrx2, and emamectin benzoate + dsTrx-like1 were 75, 73, 69, and 70, respectively.
Statistical Analyses
The data are presented as means ± standard error (SE) with three independent replicates. Statistically significant differences in gene expression, the results of enzymatic activity, and metabolite contents were evaluated with pair-wise Student’s t-test analysis using SPSS 17.0 software and denoted by ∗(0.01 < P < 0.05) and ∗∗(P < 0.01). The data for spatio-temporal expression pattern and mortality were analyzed using ANOVA, followed by a Tukey’s HSD multiple comparison test, and the letters were used to indicate significant differences at P < 0.05. Survival curves were analyzed by the method of Kaplan-Meier and statistical significance between survival curves was determined using the log-rank test, when P-values were <0.05.
Results
Sequence Analysis of GmTrx2 and GmTrx-like1
Sequence analysis showed the GmTrx2 gene was 321 bp in length and encoded 106 amino acids with a predicted molecular weight of 11.8 kDa and an isoelectric point of 4.74. Multiple amino acid alignments showed GmTrx2 had 64–84% identity with homologous sequences of selected species (Figure 1A). The N-terminal portion of the GmTrx2 had a highly conserved active site sequence of CGPC among all of the selected insect species (Figure 1A). The phylogenetic tree indicated that GmTrx2 was most closely related to genes from Pieris rapae (Figure 1B), which was also consistent with the results of multiple amino acid alignments. The GmTrx-like1 had an open reading frame of 861 bp and encoded 286 amino acids. The predicted weight of protein was 31.4 kDa and the isoelectric point was 5.48. Multiple alignment analysis of the amino acid sequence showed that GmTrx-like1 shared amino acid identity (61–88%) with Trx-like1 sequences from selected insect species and the high conserved CXXC motif was found in N-terminal portion (Figure 2A). Phylogenetic analysis showed that GmTrx-like1 was more closely related to the SlTrx-like1 and HaTrx-like1 homolog (Figure 2B).
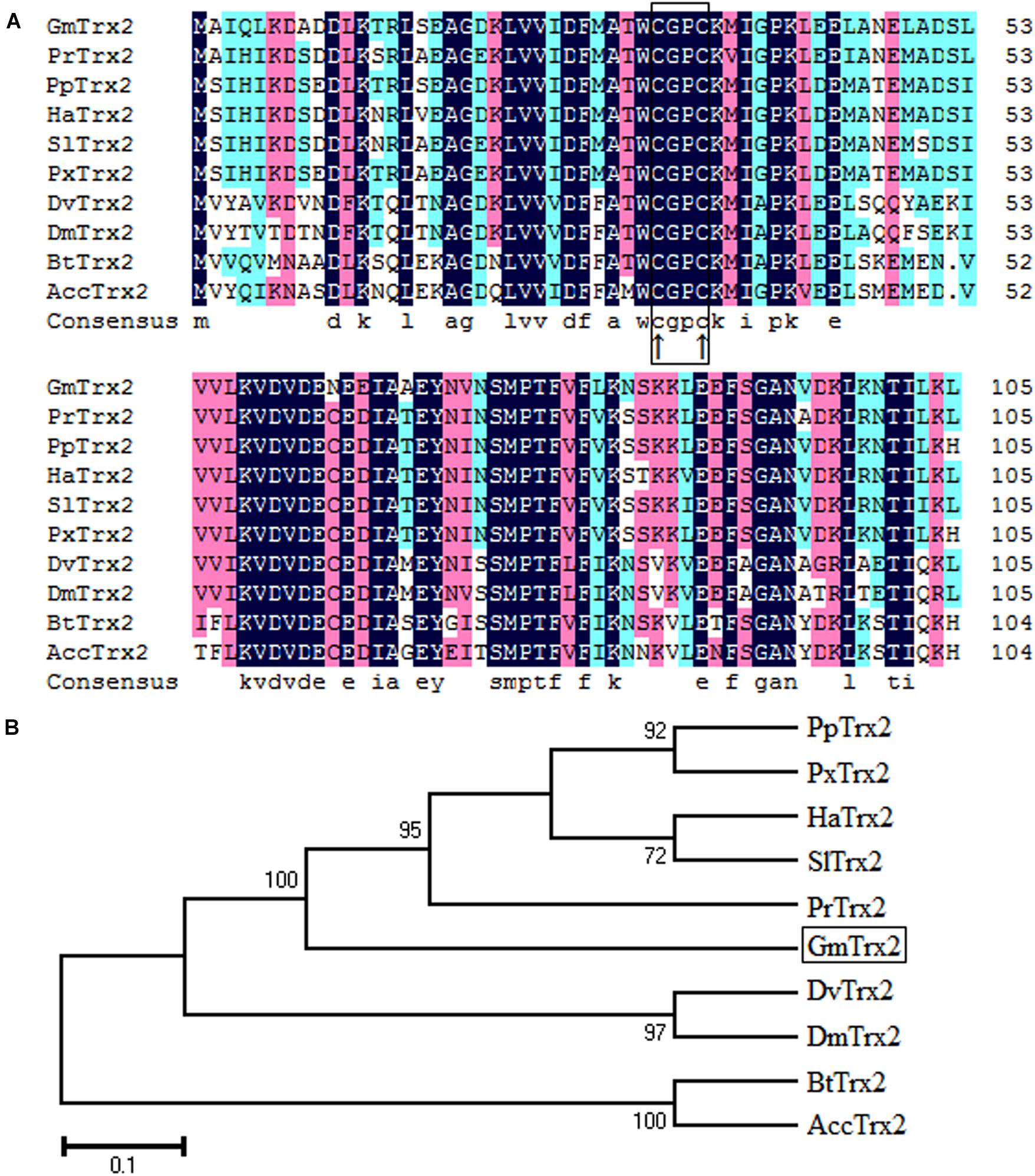
FIGURE 1. Sequence characterization of Trx2 from various species. (A) Multiple alignments of the amino acid sequence of GmTrx2 with homologs from other insect species. Black represents 100% identity, red represents ≥75% identity, green represents ≥50% identity, and white represents <50% identity. The conserved CGPC motif is boxed and the active sites are marked by ↑. GmTrx2 (Grapholita molesta, MH443001), PrTrx2 (Pieris rapae, XP_022125058.1), PpTrx2 (Papilio polytes, NP_001298667.1), HaTrx2 (Helicoverpa armigera, XP_021198961.1), SlTrx2 (Spodoptera litura, XP_022828547.1), PxTrx2 (Papilio xuthus, NP_001299755.1), DvTrx2 (Drosophila virilis, XP_002057775.1), DmTrx2 (Drosophila mojavensis, XP_002002787.1), BtTrx2 (Bombus terrestris, XP_012164907.1), and AccTrx2 (Apis cerana cerana, AFU83101.1). (B) Phylogenetic tree analysis of GmTrx2 and its homologs in insects.
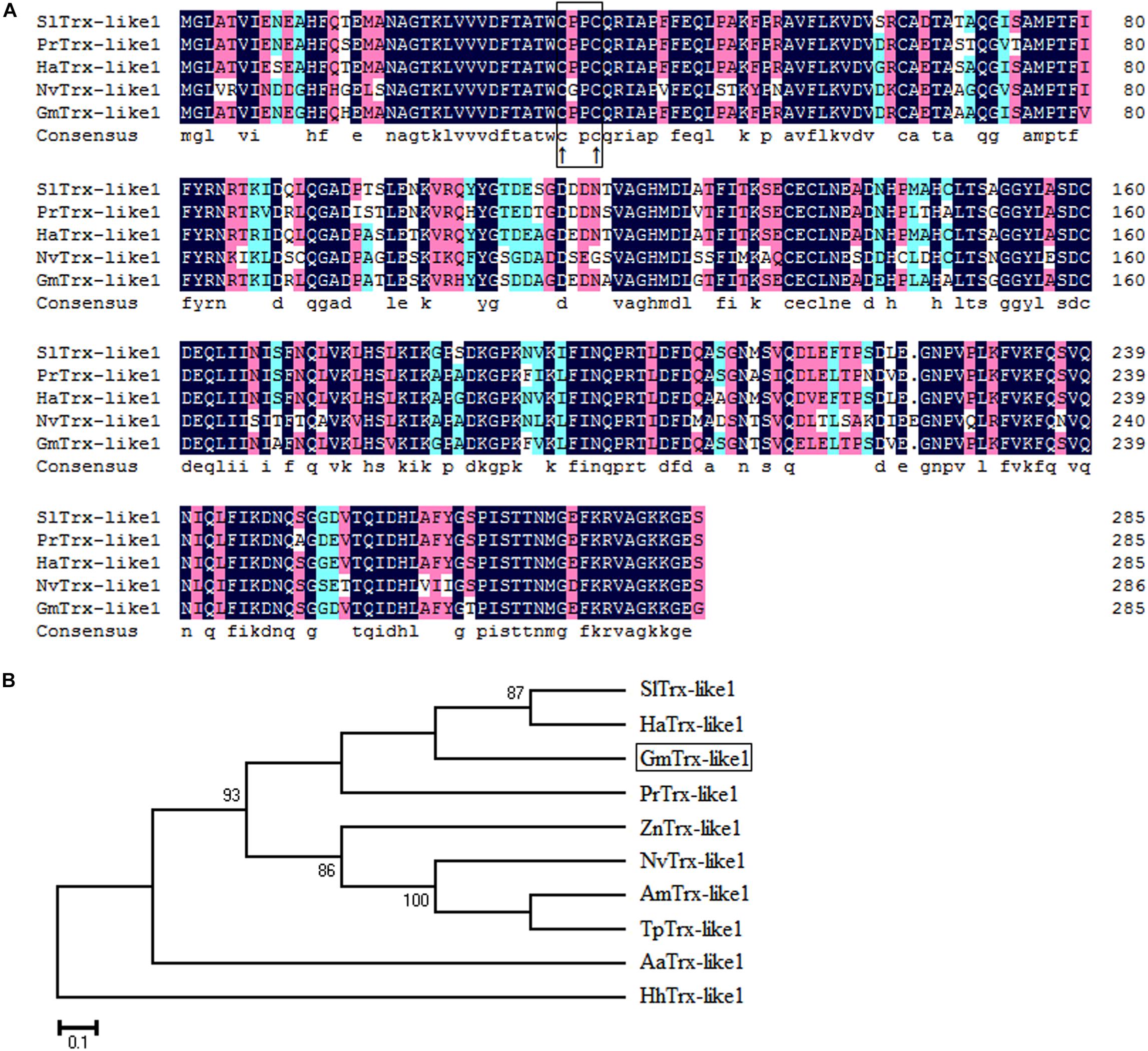
FIGURE 2. Sequence characterization of Trx-like1 from various species. (A) Multiple alignments of the amino acid sequence of GmTrx-like1 with homologs from other insect species. Black represents 100% identity, red represents ≥75% identity, green represents ≥50% identity, and white represents <50% identity. The conserved CXXC motif is boxed and the active sites are marked by ↑. (B) Phylogenetic tree analysis of GmTrx-like1 and its homologs in insects. GmTrx-like1 (Grapholita molesta, MH443002), SlTrx-like1 (Spodoptera litura, XP_022818589.1), PrTrx-like1 (Pieris rapae, XP_022127554.1), HaTrx-like1 (Helicoverpa armigera, XP_021194378.1), ZnTrx-like1 (Zootermopsis nevadensis, XP_021934497.1), HhTrx-like1 (Halyomorpha halys, XP_014270327.1), AmTrx-like1 (Apis mellifera, XP_623128.1), NvTrx-like1 (Nasonia vitripennis, XP_001608075.1), AaTrx-like1 (Aedes aegypti, XP_001659108.1), and TpTrx-like1 (Trichogramma pretiosum, XP_014235091.1).
Temporal and Spatial Expression Profiles of GmTrx2 and GmTrx-like1
The GmTrx2 was detected in all developmental stages and mainly expressed at stage L1 (first larval day) and stage P1 in female (first day of female pupa) compared to other stages (Figure 3A). For spatial expression, GmTrx2 had the highest levels of expression in the salivary glands, followed by the head and Malpighian tubules (Figure 3B). The mRNA expression of GmTrx-like1 was highest at stage prep (prepupa day) and the spatial expression profiles revealed GmTrx-like1 transcripts were expressed the highest in the head and salivary glands (Figures 3C,D).
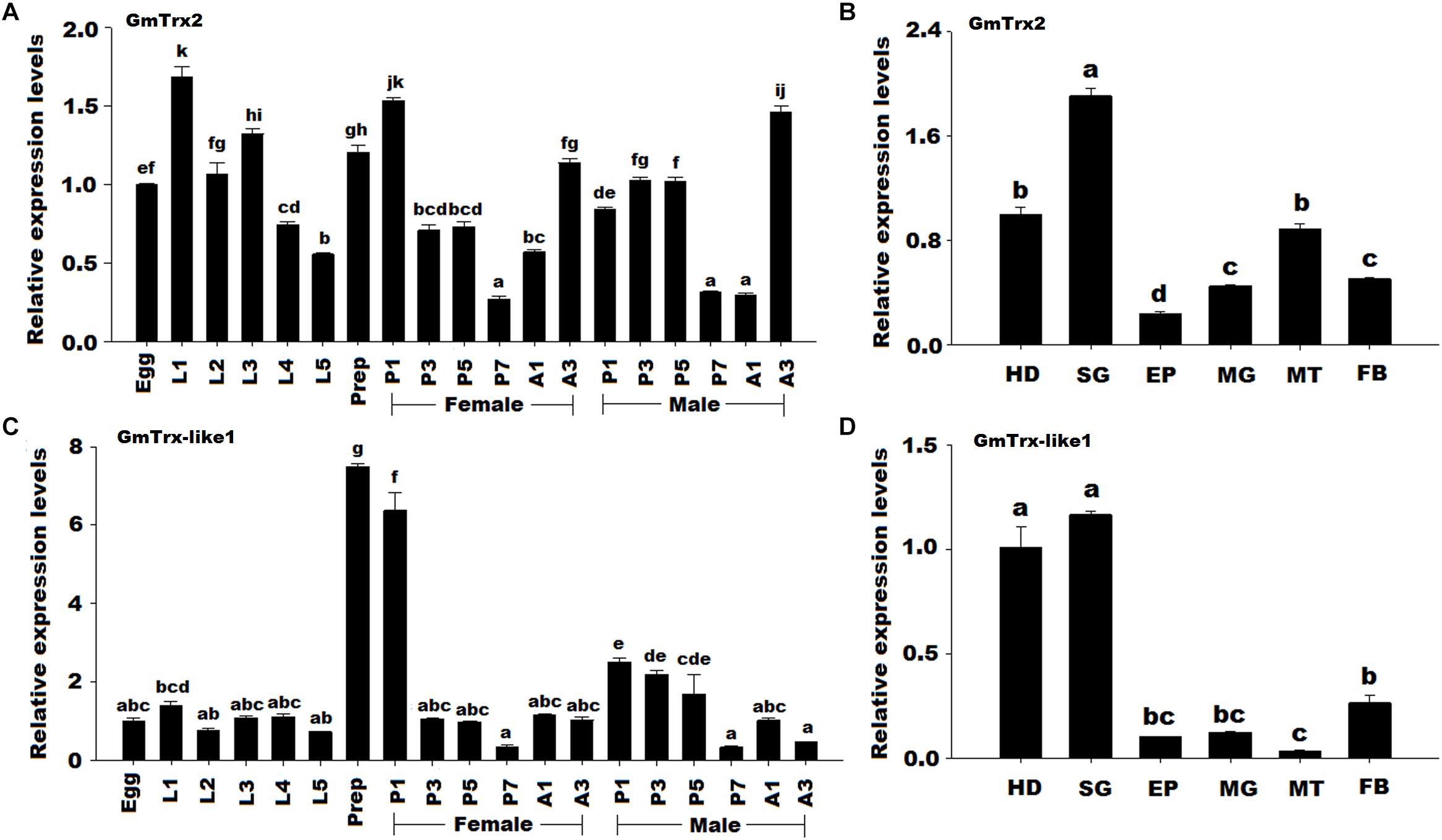
FIGURE 3. Expression profile of GmTrx2 and GmTrx-like1 in different developmental stages and different larval tissues. (A) Transcript levels of GmTrx2 during different developmental stages. Eggs; first, second, third, fourth, fifth instar, and prepupa; first, third, fifth, and seventh day pupae; first, and third day adults. The stages of pupae and adults were divided into male and female. The same as the following. (B) Transcript levels of GmTrx2 in the tissues of fifth instar larvae. HD, heads; EP, epidermis; FB, fat body; MG, midgut; MT, Malpighian tubule; SG, salivary glands; The same as the following. (C) Transcript levels of GmTrx-like1 during different developmental stages. (D) Transcript levels of GmTrx-like1 in the tissues of fifth instar larvae. The data represent the mean ± SE from three biological samples.
Expression Profiles of GmTrx2 and GmTrx-like1 Under Various Oxidative Stresses
The results of qRT-PCR revealed that GmTrx2 was obviously induced by 15 and 35°C, E. coli, M. anisopliae, and H2O2 treatments at 6, 12, and 24 h, and then being markedly upregulated at 2, 4, and 8 h after emamectin benzoate immersion (Figure 4). The expression of GmTrx-like1 was significantly induced at 6 h after 15°C and H2O2 exposure treatments (Figures 5A,E). At 35°C, GmTrx-like1 was markedly increased at 6 and 12 h, whereas was suppressed at 24 h (Figure 5B). Under stress of E. coli, GmTrx-like1 transcription was dramatically induced at 12 and 24 h, in addition to being signally enhanced at 6 and 12 h after M. anisopliae exposure treatment and obviously induced at 2, 4, and 8 h in response to emamectin benzoate treatment (Figures 5C,D,F).
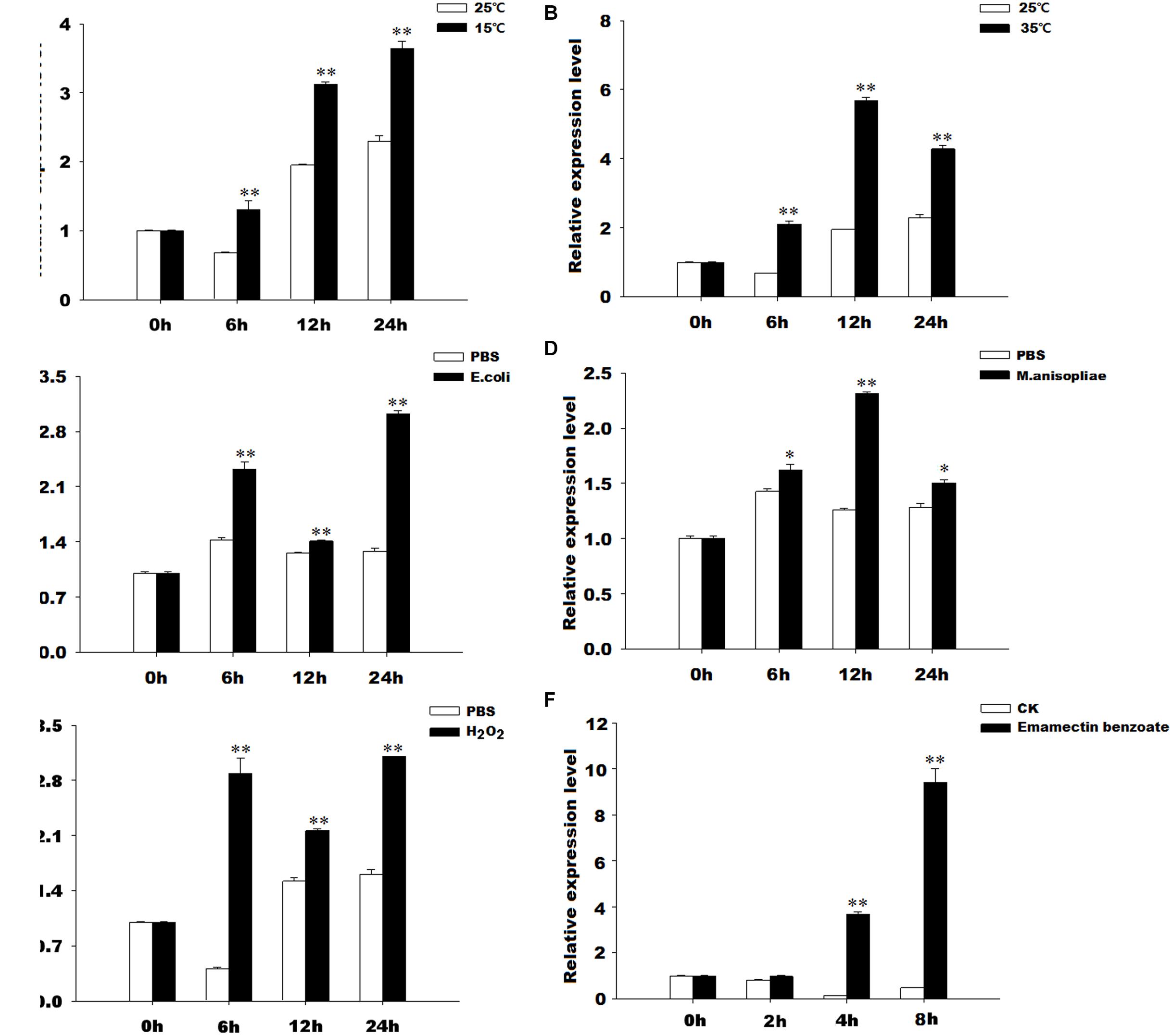
FIGURE 4. Expression profiles of GmTrx2 under various stresses. Total RNA was extracted from G. molesta samples under different stress challenges, including low temperature (15°C), high temperature (35°C), E. coli, M. anisopliae, H2O2, and emamectin benzoate treatments, and then subjected to real-time PCR analysis. (A–F) Indicated the expression profiles of GmTrx-like1 under 15°C, 35°C, E. coli, M. anisopliae, H2O2, and emamectin benzoate, respectively. The data represent the mean ± SE of three biological samples. ∗0.01 < P < 0.05; ∗∗P < 0.01.
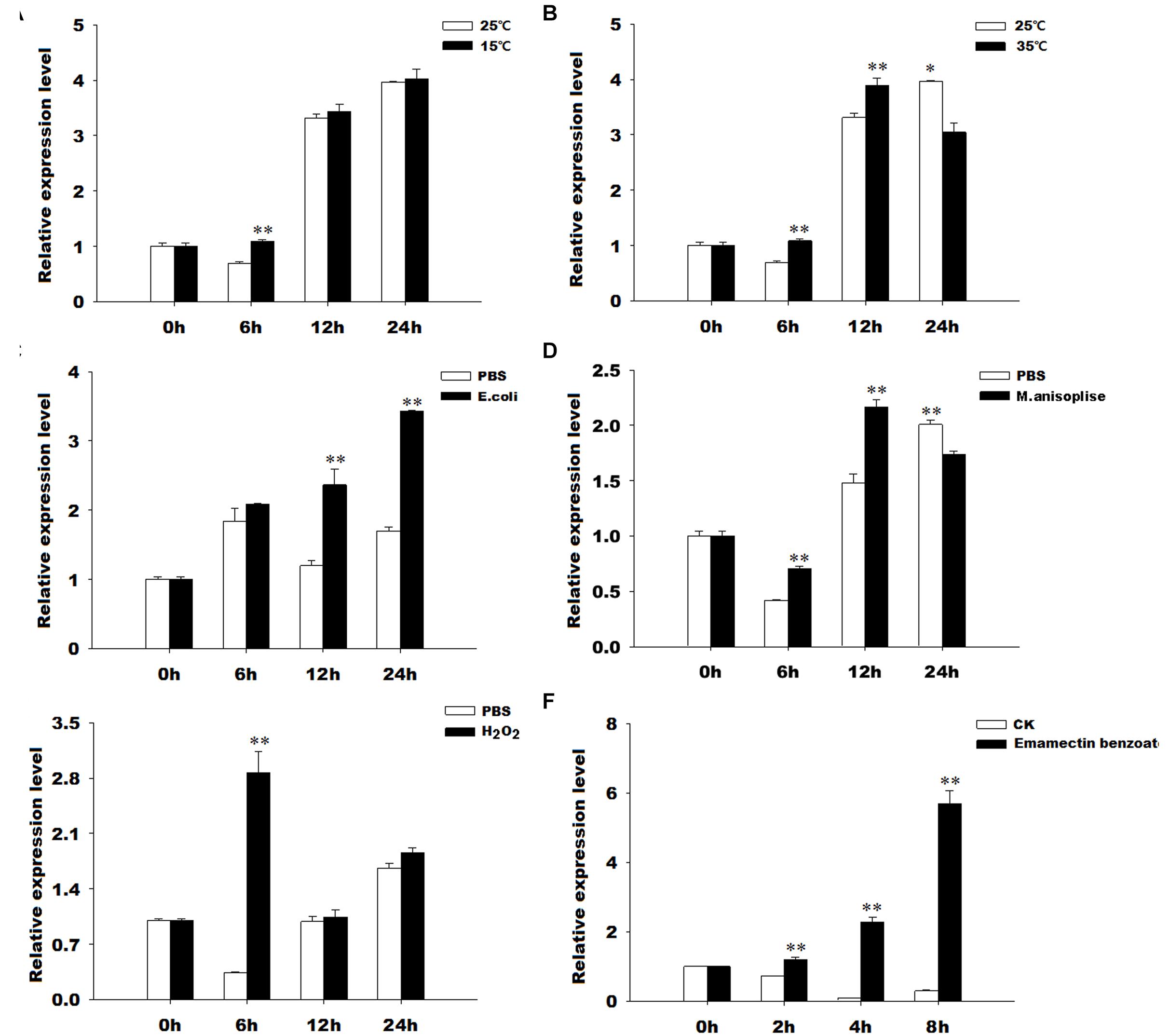
FIGURE 5. Expression profiles of GmTrx-like1 under various stresses. Total RNA was extracted from G. molesta samples under different stress challenges, including low temperature (15°C), high temperature (35°C), E. coli, M. anisopliae, H2O2, and emamectin benzoate treatments, and then subjected to real-time PCR analysis. (A–F) Indicated the expression profiles of GmTrx-like1 under 15°C, 35°C, E. coli, M. anisopliae, H2O2, and emamectin benzoate, respectively. The data represent the mean ± SE of 3 biological samples. ∗0.01 < P < 0.05; ∗∗P < 0.01.
Knockdown of GmTrx2 and GmTrx-like1 and Effects on Enzymatic Activities and Metabolite Contents After Silencing of the Two Genes
The GmTrx2 and GmTrx-like1 expressions were significantly inhibited at 24, 48, and 72 h when both the genes were knocked down compared to with EGFP dsRNA injection (Figure 6). The interference efficiency of GmTrx2 was 53.55, 39.06, and 32.06% at 24, 48, and 72 h (Figure 6A), while GmTrx-like1 was 46.93, 43.57, and 81% at 24, 48, and 72 h (Figure 6B) after the injection of GmTrx2 or GmTrx-like1 dsRNA, respectively.
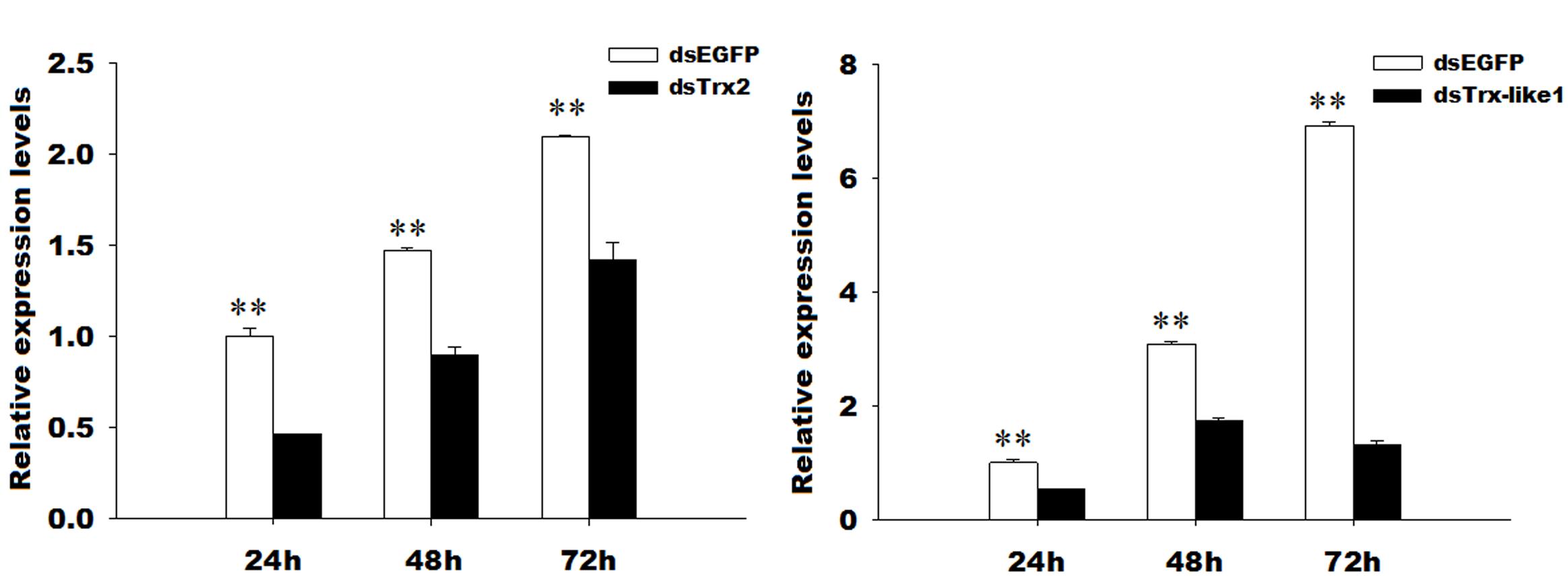
FIGURE 6. RNA interference efficiency of GmTrx2 or GmTrx-like1 dsRNA. (A) RNAi-induced reduction of GmTrx2 expression levels. (B) RNAi-induced reduction of GmTrx-like1 expression levels. The data represent the mean ± SE of three biological samples. ∗0.01 < P < 0.05; ∗∗P < 0.01.
The peroxidase capacity after silencing of GmTrx2 or GmTrx-like1 was higher than that in the control groups (Figure 7A), while there was no significant change in enzymatic activities of superoxide dismutase (Figure 7B). The contents of hydrogen peroxide and ascorbate were increased at the measured time after knockdown of GmTrx2 or GmTrx-like1, compared with control groups (Figures 7C,D).
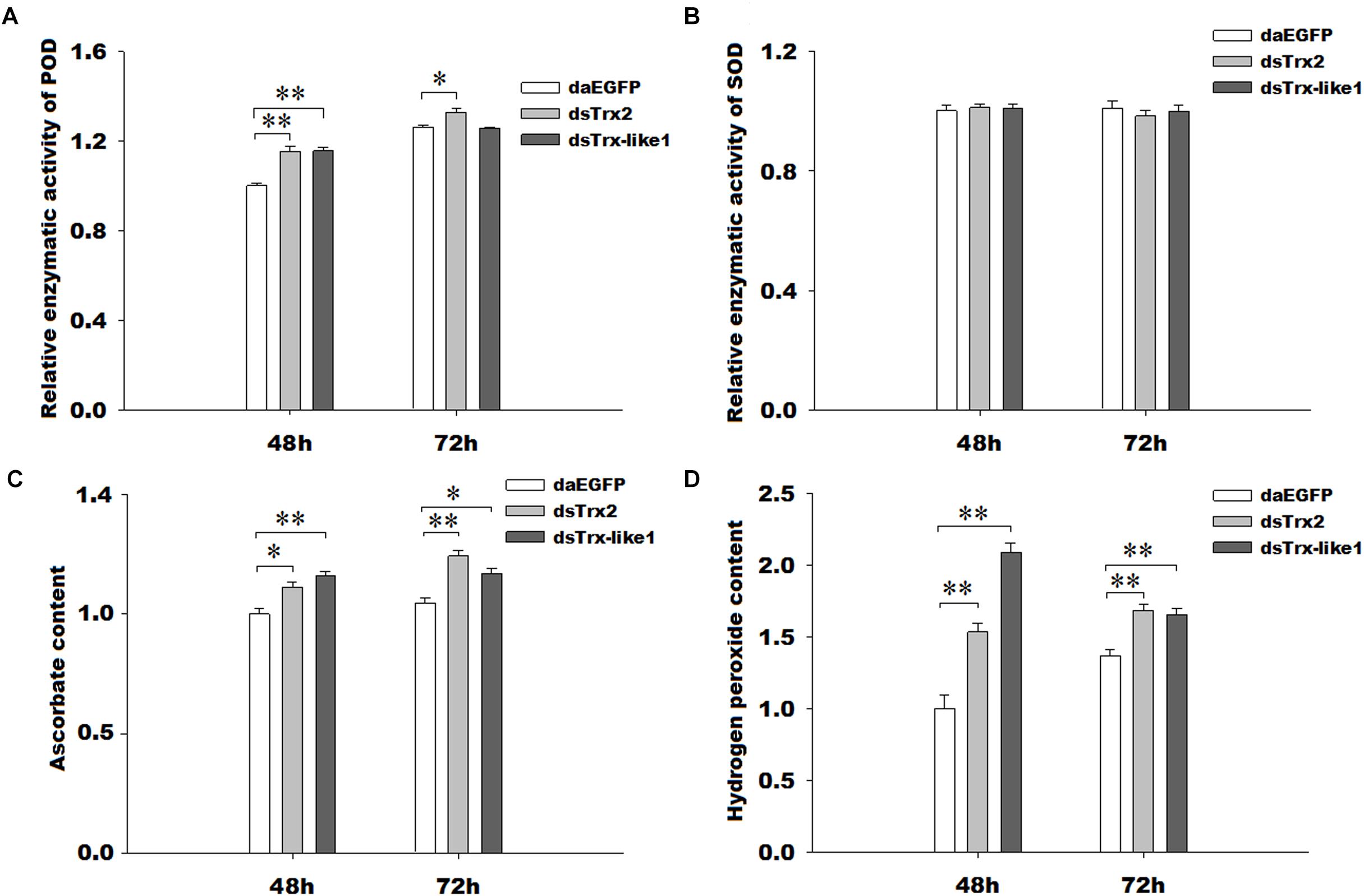
FIGURE 7. Effects of GmTrx2 or GmTrx-like1 knockdown on enzymatic activity of antioxidants (A) POD and (B) SOD and on the metabolites (C) ascorbate and (D) hydrogen peroxide. Whole larvae were collected at 48 and 72 h after dsRNA injection of EGFP, GmTrx2, and GmTrx-like1 and then analyzed using the corresponding kits according to the manufacturer’s specifications. Each value represents the mean ± SE of three biological samples. ∗0.01 < P < 0.05; ∗∗P < 0.01.
Assay of Oxidant Status in vivo Under Emamectin Benzoate and Survival Assay
1To further determine the functions of GmTrx2 and GmTrx-like1, we detected the contents of H2O2 under emamectin benzoate and the effects of GmTrx2 or GmTrx-like1 knockdown on the susceptibility of G. molesta larvae exposed to emamectin benzoate. The results showed that H2O2 concentration was dramatically increased under emamectin benzoate (Figure 8A). Survival curves revealed that GmTrx2 or GmTrx-like1 knockdown also obviously promoted susceptibility for G. molesta larvae to emamectin benzoate, compared to EGFP dsRNA injection (Figure 8B). The mortality at 96 h of emamectin benzoate (37.26%) and emamectin benzoate + dsEGFP (35.50%) larvae was significantly lower than in emamectin benzoate + dsGmTrx2 (62.49%) or emamectin benzoate + dsGmTrx-like1 (67.94%) larvae (P < 0.05, Figure 8C).
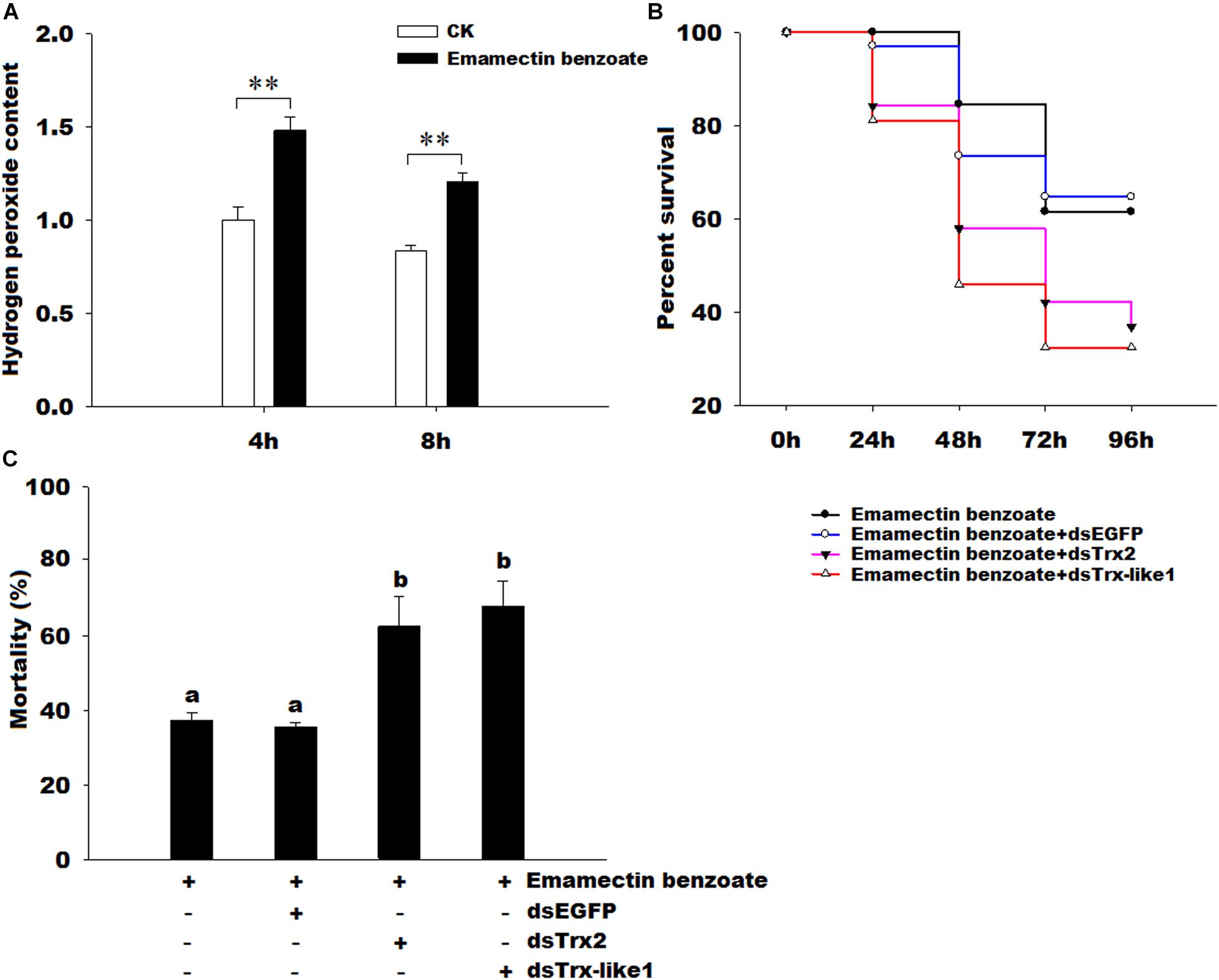
FIGURE 8. Effects of emamectin benzoate on hydrogen peroxide content and effects of GmTrx2 or GmTrx-like1 knockdown on the susceptibility of G. molesta larvae to emamectin benzoate. (A) Effects of emamectin benzoate on hydrogen peroxide content. The samples of G. molesta larvae were collected 4 and 8 h after emamectin benzoate injection and then analyzed using the hydrogen peroxide kits according to the manufacturer’s specifications. (B) Survival curves of the larvae soaked in emamectin benzoate after GmTrx2 or GmTrx-like1 knockdown; P < 0.05. At 12 h after 3 μg dsRNA of EGFP, GmTrx2, and GmTrx-like1 infection, the fifth instar larvae were immersed in emamectin benzoate for 5 s. Survival curves were analyzed by the method of Kaplan-Meier and statistical significance between survival curves was determined using the log-rank test, when P-values were <0.05. (C) The mortality at 96 h after larvae soaked in emamectin benzoate after GmTrx2 or GmTrx-like1 knockdown. The data represent the mean ± SE of three biological samples. ∗0.01 < P < 0.05; ∗∗P < 0.01. a and b: signification difference, P < 0.05.
Discussion
Oxidative stress is involved in many different disease processes and causes alterations in the cellular redox state (Jiménez et al., 2006). The Trxs have functions in resisting oxidative damage caused by ROS and maintaining cellular redox homeostasis (Holmgren, 1985; Arnér and Holmgren, 2000; Myers et al., 2008). Previous studies have focused on model insects, for example, B. mori (Kim et al., 2007) and Apis cerana cerana (Yao et al., 2013). In this study, we focused on the important fruits pest, G. molesta. We have identified and characterized GmTrx2 and GmTrx-like1 from G. molesta. Domain analysis showed that GmTrx2 and GmTrx-like1 possessed highly conserved CGPC and CXXC sequences, respectively, suggesting that the two genes belonged to the Trx families.
Temporal expression profiles showed that both GmTrx2 and GmTrx-like1 expressions appeared as large fluctuations at the physiological processes of incubation, pupation, and emergence whether male or female. These results suggested that the two genes may play important roles in antioxidant defense in these stages, because these periods of intense physical activity may cause excessive accumulation of ROS. Our transcriptional analysis revealed that the GmTrx2 and GmTrx-like1 were expressed at higher levels in the head and salivary glands than other larval tissues. In other insects, BmTrx exhibited higher expression in the fat body and silk gland (Kim et al., 2007); AccTrx1 was mainly expressed in the epidermis (Yao et al., 2014); HaTrx2 gene was expressed at higher levels in the head and epidermis (Zhang et al., 2015). It revealed that the expression of Trx exhibits a tissue-specific pattern. In addition, the brain tissue was very sensitive to oxidative stress (Rival et al., 2004; Ament et al., 2008) and AccTrx2 and AccTrx-like1 had a higher transcript in brain (Lu et al., 2012; Yao et al., 2013), implying that GmTrx2 and GmTrx-like1 may play vital functions in the head.
Previous studies have reported that environmental conditions, such as ultraviolet radiation, pesticides, temperature, and heavy metals, can induce oxidative stress (Lushchak, 2011; Kottuparambil et al., 2012). The Trx is a stress-inducible protein and plays significant roles in the scavenging or quenching of oxidants. In D. melanogaster, Trx2 has been proved to be closely associated with resistance to oxidative stress (Svensson and Larsson, 2007). The cumene hydroperoxide, indoxacarb, and metaflumizone could stimulate the expression levels of SlTrx1 and SlTrx2 in S. litura (Kang et al., 2015). The AccTrx1 and AccTrx2 from A. cerana cerana were upregulated by low or high temperatures, H2O2, and pesticides (acaricide, paraquat, cyhalothrin, and phoxime) treatments (Yao et al., 2013, 2014). In H. armigera, the transcript of HaTrx2 was significantly stimulated by low or high temperatures, UV light, mechanical injury, microorganism [E. coli, M. anisopliae, and nucleopolyhedrovirus (NPV)] (Zhang et al., 2015). In this study, GmTrx2 and GmTrx-like1 were obviously induced by 15 and 35°C, E. coli, M. anisopliae, H2O2, and pesticides-emamectin benzoate treatments, suggesting that GmTrx2 and GmTrx-like1 may play critical roles in resisting oxidative stress caused by these adverse conditions.
It was demonstrated that antioxidant enzymes (such as Grx, Trx, and POD) and antioxidant substances [such as ascorbate, protein carbonyl, and glutathione (GSH)] were used to protect organisms by scavenging excess ROS (Meister, 1994; Corona and Robinson, 2006). The changes in antioxidant enzyme activity and metabolite concentrations after gene silencing were also used to demonstrate the function of genes in resisting oxidative stress. For example, after knockdown of HaGrx, HaGrx3, and HaGrx5 in H. armigera and AccTrx1 in A. cerana cerana, the enzymatic activities of peroxidase, the amount of hydrogen peroxide, and the amount of ascorbate all increased (Yao et al., 2014; Zhang et al., 2016), implying these genes play important roles in resisting oxidative stress. In our study, RNAi mediated knockdown of GmTrx2, and GmTrx-like1 increased the enzymatic activities of POD and the metabolite contents of hydrogen peroxide and ascorbate. It suggested that larvae were exposed to higher oxidative stress after GmTrx2 and GmTrx-like1 were silenced, and the genes of GmTrx2 and GmTrx-like1 may be involved in protecting G. molesta against oxidative stress.
In A. cerana cerana, the H2O2 concentration and the transcripts of AccTpx5 were induced by phoxim and pyriproxyfen, indicating that oxidative stress might be associated with alterations in AccTpx5 expression (Yan et al., 2014). In H. armigera, the larvae injected by NPV increased ROS production and the lipid damage (Zhang et al., 2015). In this study, emamectin benzoate could dramatically increase the content of hydrogen peroxide and the expressions of GmTrx2 and GmTrx-like1. It revealed that the larvae were exposed to higher oxidative stress and the two genes may be involved in the clearance of hydrogen peroxide caused by insecticide. In order to determine the roles of GmTrx2 and GmTrx-like1 involved in protecting organisms from oxidative stress caused by emamectin benzoate, we further examined effects of GmTrx2 or GmTrx-like1 interference on the survival curves of emamectin benzoate to G. molesta and found that the silencing of the two genes increased the sensitivity of larvae to emamectin benzoate and significantly improved mortality at 96 h, suggesting GmTrx2 and GmTrx-like1 were essential in the removal of excessive ROS to protect organisms. At present, the resistance of pests to insecticides was mainly concentrated on important detoxification enzymes such as cytochrome P450. But some antioxidant genes also played an important role in the antagonism of insects, for example, SlTpx inhibited Nomuraea rileyi infection in S. litura (Chen et al., 2014); HaTrx2 was involved in the defense of larvae against NPV infections in H. armigera (Zhang et al., 2015); GmTrx2 and GmTrx-like1 protected larvae against emamectin benzoate in our study. Therefore, some antioxidant genes, included GmTrx2 and GmTrx-like1, may become potential targets for insecticide synergists against G. molesta.
The Trxs are ubiquitously distributed from Archaea to humans (Lu and Holmgren, 2014). Although it has a conserved domain in all species, Trxs from Archaea to humans have only 27–69% sequence identity to that of E. coli Trx1 (Eklund et al., 1991). The sequence inconsistency in a few residues at the amino and carboxyl ends (Eklund et al., 1991) and different mechanisms for reducing Trx (Gleason and Holmgren, 1988) may be responsible for that the physiological functions of Trxs in different types of organisms have evolved from a common fundamental reaction to a large number of different specialized functions. For example, E. coli Trx stabilized complexes of bacteriophage T7 DNA polymerase and primed templates (Huber et al., 1987). In bacteria and yeast, Trx served as electron donors of 30-phosphoadenylsulfate (PAPS) reductase (Schwenn et al., 1988; Lillig et al., 1999). In plants, Trx was involved in regulation of chloroplast photosynthetic enzymes (Buchanan, 1991). In mammals, Trx played an important role in the regulation of redox regulation of transcription factors (Schenk et al., 1994), regulation of apoptosis (Saitoh et al., 1998), and immune regulation (Silberstein et al., 1993; Nakamura et al., 1997; Bertini et al., 1999). Therefore, it is very necessary and useful to further study the protein structures, mechanisms, and specific active sites of Trxs in G. molesta for finding a suitable way to control pests without affecting other organisms.
Conclusion
We have characterized two genes, GmTrx2 and GmTrx-like1, and determined their temporal-spatial expression profiles. The expression levels of GmTrx2 and GmTrx-like1 were induced by low or high temperature, E. coli, M. anisopliae, H2O2, and pesticides such as emamectin benzoate. After knockdown of GmTrx2 or GmTrx-like1, the enzymatic activities of POD and the metabolite contents of hydrogen peroxide and ascorbate all increased. These results revealed that GmTrx2 and GmTrx-like1 may play important roles in resistance to excessive ROS. Emamectin benzoate increased the H2O2 concentration and GmTrx2 or GmTrx-like1 were silenced, which improved the sensitivity of larvae to insecticide-emamectin benzoate, further indicating that GmTrx2 and GmTrx-like1 play vital roles in protecting G. molesta against oxidative damage. These findings may be useful for understanding the antioxidant defense mechanisms of the Trx system in insects.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work. Z-JS and X-XL conceived and designed the experiments. Z-JS and Y-JL performed the experiments. Z-JS, X-ML, S-DZ, and Y-JL analyzed the data. Z-JS, X-ML, S-DZ, and X-HG contributed to reagents, materials, and analysis tools. Z-JS, ZL, Q-WZ, and X-XL wrote the paper.
Funding
This study was supported by the China Modern Agro-industry Technology Research System (Grant no. CARS-28-17).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01421/full#supplementary-material
Footnotes
References
Ament, S. A., Corona, M., Pollock, H. S., and Robinson, G. E. (2008). Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. U.S.A. 105, 4226–4231. doi: 10.1073/pnas.0800630105
Arnér, E. S. J., and Holmgren, A. (2000). Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267, 6102–6909. doi: 10.1046/j.1432-1327.2000.01701.x
Bauer, H., Kanzok, S. M., and Schirmer, R. H. (2002). Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: isolation and characterization of a second thioredoxin in D. Melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J. Biol. Chem. 277, 17457–17463. doi: 10.1074/jbc.M200636200
Bertini, R., Howard, O. M., Dong, H. F., Oppenheim, J. J., Bizzarri, C., Sergi, R., et al. (1999). Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189, 1783–1789. doi: 10.1084/jem.189.11.1783
Buchanan, B. B. (1991). Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch. Biochem. Biophys. 288, 1–9. doi: 10.1016/0003-9861(91)90157-E
Chen, H., Yin, Y., Feng, E., Li, Y., Xie, X., and Wang, Z. (2014). Thioredoxin peroxidase gene is involved in resistance to biocontrol fungus Nomuraea rileyi in Spodoptera litura: gene cloning, expression, localization and function. Dev. Comp. Immunol. 44, 76–85. doi: 10.1016/j.dci.2013.11.012
Chen, Y., Cai, J., Murphy, T. J., and Jones, D. P. (2002). Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J. Biol. Chem. 277, 33242–33248. doi: 10.1074/jbc.M202026200
Corona, M., and Robinson, G. E. (2006). Genes of the antioxidant system of the honeybee: annotation and phylogeny. Insect Mol. Biol. 15, 687–701. doi: 10.1111/j.1365-2583.2006.00695.x
Droge, W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. doi: 10.1152/physrev.00018.2001
Eklund, H., Gleason, F. K., and Holmgren, A. (1991). Structural and functional relations among thioredoxins of different species. Proteins 11, 13–28. doi: 10.1002/prot.340110103
Gleason, F. K., and Holmgren, A. (1988). Thioredoxin and related proteins in prokaryotes. FEMS Microbiol. Rev. 54, 271–298. doi: 10.1111/j.1574-6968.1988.tb02747.x
Holmgren, A. (1985). Thioredoxin. Annu. Rev. Biochem. 54, 237–271. doi: 10.1146/annurev.bi.54.070185.001321
Huber, H. E., Tabor, S., and Richardson, C. C. (1987). Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J. Biol. Chem. 262, 16224–16232.
Imlay, J. A. (2003). Pathways of oxidative damage. Annu. Rev. Microbiol. 57, 395–418. doi: 10.1146/annurev.micro.57.030502.090938
Jiménez, A., Pelto-Huikko, M., Gustafsson, J. A., and Miranda-Vizuete, A. (2006). Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett. 580, 960–967. doi: 10.1016/j.febslet.2006.01.025
Kalinina, E. V., Chernov, N. N., and Saprin, A. N. (2008). Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochem. Mosc. 73, 1493–1510. doi: 10.1134/S0006297908130099
Kang, T., Wan, H., Zhang, Y., Shakeel, M., Lu, Y., You, H., et al. (2015). Comparative study of two thioredoxins from common cutworm (Spodoptera litura): cloning, expression, and functional characterization. Comp. Biochem. Physiol. 182, 47–54. doi: 10.1016/j.cbpb.2014.12.004
Kanga, L. H. B., Pree, D. J., van Lier, J. L., and Walker, G. M. (2003). Management of insecticide resistance in oriental fruit moth (Grapholita molesta; Lepidoptera: Tortricidae) populations from Ontario. Pest. Manag. Sci. 59, 921–927. doi: 10.1002/ps.702
Kanzok, S. M., Fechner, A., Bauer, H., Ulschmid, J. K., Müller, H. M., Botella-Munoz, J., et al. (2001). Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 291, 643–646. doi: 10.1126/science.291.5504.643
Kim, Y. J., Lee, K. S., Kim, B. Y., Choo, Y. M., Sohn, H. D., and Jin, B. R. (2007). Thioredoxin from the silkworm, Bombyx mori: cDNA sequence, expression, and functional characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 147, 574–581. doi: 10.1016/j.cbpb.2007.03.012
Kirk, H., Dorn, S., and Mazzi, D. (2013). Worldwide population genetic structure of the oriental fruit moth (Grapholita molesta), a globally invasive pest. BMC Ecol. 13:12. doi: 10.1186/1472-6785-13-12
Kobayashi-Miura, M., Shioji, K., Hoshino, Y., Masutani, H., Nakamura, H., and Yodoi, J. (2007). Oxygen sensing and redox signaling: the role of thioredoxin in embryonic development and cardiac diseases. Am. J. Physiol. Heart Circ. Physiol. 292, H2040–H2050. doi: 10.1152/ajpheart.01316.2006
Kottuparambil, S., Shinb, W., Brownc, M. T., and Han, T. (2012). UV-B affects photosynthesis, ROS production and motility of the freshwater flagellate, Euglena agilis Carter. Aquat. Toxicol. 122, 206–213. doi: 10.1016/j.aquatox.2012.06.002
Lillig, C. H., Prior, A., Schwenn, J. D., Aslund, F., Ritz, D., Vlamis-Gardikas, A., et al. (1999). New thioredoxins and glutaredoxins as electron donors of 30-phosphoadenylsulfate reductase. J. Biol. Chem. 274, 7695–7698. doi: 10.1074/jbc.274.12.7695
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, J., and Holmgren, A. (2014). The thioredoxin antioxidant system. Free Radic. Biol. Med. 66, 75–87. doi: 10.1016/j.freeradbiomed.2013.07.036
Lu, W., Kang, M., Liu, X., Zhao, X., Guo, X., and Xu, B. (2012). Identification and antioxidant characterisation of thioredoxin-like1from Apis cerana cerana. Apidologie 43, 737–752. doi: 10.1007/s13592-012-0148-7
Lushchak, V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 101, 13–30. doi: 10.1016/j.aquatox.2010.10.006
Mahmood, D. F. D., Abderrazak, A., El Hadri, K., Simmet, T., and Rouis, M. (2012). The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Sign. 15, 4757–4848.
Meister, A. (1994). Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 269, 9397–9400.
Myers, J. M., Antholine, W. E., and Myers, C. R. (2008). Hexavalent chromium causes the oxidation of thioredoxin in human bronchial epithelial cells. Toxicology 246, 222–233. doi: 10.1016/j.tox.2008.01.017
Nakamura, H., Nakamura, K., and Yodoi, J. (1997). Redox regulation of cellular activation. Annu. Rev. Immunol. 15, 351–369. doi: 10.1146/annurev.immunol.15.1.351
Rietsch, A., and Beckwith, J. (1998). The genetics of disulfide bond metabolism. Annu. Rev. Genet. 32, 163–184. doi: 10.1146/annurev.genet.32.1.163
Rival, T., Soustelle, L., Strambi, C., Besson, M. T., Iché, M., and Birman, S. (2004). Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 17, 599–605. doi: 10.1016/j.cub.2004.03.039
Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., et al. (1998). Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17, 2596–2606. doi: 10.1093/emboj/17.9.2596
Schenk, H., Klein, M., Erdbrugger, W., Droge, W., and Schulze-Osthoff, K. (1994). Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc. Natl Acad. Sci. U.S.A. 91, 1672–1676. doi: 10.1073/pnas.91.5.1672
Schwenn, J. D., Krone, F. A., and Husmann, K. (1988). Yeast PAPS reductase: properties and requirements of the purified enzyme. Arch. Microbiol. 150, 313–319. doi: 10.1007/BF00408300
Silberstein, D. S., McDonough, S., Minkoff, M. S., and Balcewicz-Sablinska, M. K. (1993). Human eosinophil cytotoxicity-enhancing factor. Eosinophil-stimulating and dithiol reductase activities of biosynthetic (recombinant) species with COOH-terminal deletions. J. Biol. Chem. 268, 9138–9142.
Stryer, L., Holmgren, A., and Reichard, P. (1967). Thioredoxin, a localized conformational change accompanying reduction of the protein to the sulfhydryl form. Biochemistry 6, 1016–1020. doi: 10.1021/bi00856a009
Svensson, M. J., Chen, J. D., Pirrotta, V., and Larsson, J. (2003). The ThioredoxinT and deadhead gene pair encode testis- and ovary-specific thioredoxins in Drosophila melanogaster. Chromosoma 112, 133–143. doi: 10.1007/s00412-003-0253-5
Svensson, M. J., and Larsson, J. (2007). Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas 144, 25–32. doi: 10.1111/j.2007.0018-0661.01990.x
Wu, H. L., Lin, L. R., Giblin, F., Ho, Y. S., and Lou, M. F. (2011). Glutaredoxin2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic. Biol. Med. 51, 2108–2117. doi: 10.1016/j.freeradbiomed.2011.09.011
Yan, Y., Zhang, Y., Huaxia, Y., Wang, X., Yao, P., Guo, X., et al. (2014). Identification and characterisation of a novel 1-Cys thioredoxin peroxidase gene (AccTpx5) from Apis cerana cerana. Comp. Biochem. Physiol. 172–173, 39–48. doi: 10.1016/j.cbpb.2014.04.004
Yao, P., Chen, X., Yan, Y., Liu, F., Zhang, Y., Guo, X., et al. (2014). Glutaredoxin 1, glutaredoxin 2, thioredoxin 1, and thioredoxin peroxidase 3 play important roles in antioxidant defense in Apis cerana cerana. Free Radic. Biol. Med. 68, 335–346. doi: 10.1016/j.freeradbiomed.2013.12.020
Yao, P., Hao, L., Wang, F., Chen, X., Yan, Y., Guo, X., et al. (2013). Molecular cloning, expression and antioxidant characterisation of a typical thioredoxin gene (AccTrx2) in Apis cerana cerana. Gene 527, 33–41. doi: 10.1016/j.gene.2013.05.062
Zhang, S., Li, Z., Nian, X., Wu, F., Shen, Z., Zhang, B., et al. (2015). Sequence analysis, expression profiles and function of thioredoxin 2 and thioredoxin reductase 1 in resistance to nucleopolyhedrovirus in Helicoverpa armigera. Sci. Rep. 5:15531. doi: 10.1038/srep15531
Keywords: Grapholita molesta, Thioredoxins, RNA interference, oxidative stress, antioxidant defense
Citation: Shen Z-J, Liu Y-J, Gao X-H, Liu X-M, Zhang S-D, Li Z, Zhang Q-W and Liu X-X (2018) Molecular Identification of Two Thioredoxin Genes From Grapholita molesta and Their Function in Resistance to Emamectin Benzoate. Front. Physiol. 9:1421. doi: 10.3389/fphys.2018.01421
Received: 18 June 2018; Accepted: 18 September 2018;
Published: 25 October 2018.
Edited by:
Bin Tang, Hangzhou Normal University, ChinaReviewed by:
Aram Megighian, Università degli Studi di Padova, ItalyZhaojiang Guo, Chinese Academy of Agricultural Sciences, China
Copyright © 2018 Shen, Liu, Gao, Liu, Zhang, Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Xia Liu, bGl1eGlhb3hpYTYxMUBjYXUuZWR1LmNu
 Zhong-Jian Shen
Zhong-Jian Shen Yan-Jun Liu
Yan-Jun Liu Xiao-Ming Liu
Xiao-Ming Liu