- 1Department of Osteonecrosis of the Femoral Head, Luoyang Orthopedic Hospital of Henan Province, Luoyang, China
- 2Department of Osteoarthritis, Luoyang Orthopedic Hospital of Henan Province, Luoyang, China
Aims: The purpose of this study was to assess the relationship between genetic variants and steroid-induced osteonecrosis of the femoral head (SONFH) in steroid use populations.
Methods: We searched the public databases up to April 15, 2018. This study analyzed only the single-nucleotide polymorphisms (SNPs) that have appeared in more than three studies and assessed the level of evidence by classifying the outcomes according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Results: The ABCB1 rs1045642 C>T mutation had a protective effect against SONFH in the allelic model (I2 = 50.2%; OR: 0.74; 95% CI: 0.55–1.00; p = 0.046). The rs2032582 mutation in the ABCB1 gene showed no relationship to SONFH (allelic model: I2 = 63.4%; OR: 0.85; 95% CI: 0.58–1.23; p = 0.382). In ApoB rs693, four models showed that mutations can increase SONFH risk, but the allelic model did not. The ApoB rs1042031 mutation increased SONFH risk in the dominant model (I2 = 50.3%; OR: 2.90; 95% CI: 1.49–5.66; p = 0.002).
Conclusion: An allelic model of ABCB1 rs1045642 showed that mutations have a protective effect against SONFH at a very low level of evidence. The mutations in ApoB rs693 and rs1042031 increase the SONFH risk with moderate levels of evidence.
Introduction
Osteonecrosis of the femoral head (ONFH) is caused by the obstruction or interruption of local blood supply, resulting in tissue ischemia, necrosis, and finally bone collapse (Kim et al., 2011). The core decompression method only relieved the pressure of the pulp cavity and delayed the progression of necrosis (Yu et al., 2018). At present, long-term and/or large-dose use of steroids have become the most important risk factors for non-traumatic ONFH (Shigemura et al., 2011). The pathogenesis of steroid-induced ONFH (SONFH) is not yet clear and may be related to an imbalance in lipid metabolism and abnormal microcirculation (Johnson et al., 2004). The abnormal blood supply leads to apoptosis of osteocytes and osteoblasts, which causes bone loss and reduces bone mineral density (Luo et al., 2018). In addition, disordered lipid metabolism is another crucial pathogenesis that leads to increases in circulating lipid levels, microvascular fat embolisms, and lipid accumulation in the pulp cavity and ultimately causes osteocyte death.
Genetic polymorphisms have been found to be related to SONFH due to individual differences. Studies exploring the association between single-nucleotide polymorphisms (SNPs) and SONFH will help to identify the high SONFH risk population who use steroids. For these patients, it is necessary to avoid steroid application or to change the application strategy, increase the frequency of detection of SONFH, and enable early intervention. Furthermore, genetic variant-related studies can help to understand the pathogenesis of SONFH. Presently, several meta-analyses have been published that indicate that PAI-1 rs1799768 (Gong et al., 2013), ABCB1 rs1045642 (Gong et al., 2013; Li et al., 2014; Zhou et al., 2015), and CYP3A variants (Guo and Deng, 2017) are related to the occurrence of SONFH. However, there is still a dispute over ABCB1 rs2032582 (Gong et al., 2013; Li et al., 2014; Zhou et al., 2015). This study will explicitly exclude studies in which the control group includes a healthy population or other types of ONFH; only comparison studies between ONFH and non-ONFH in the steroid use population are included. Because all studies are based on case-control and cohort designs with low evidence levels, we also used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines to assess the outcomes (Guyatt et al., 2008). Overall, the relationship between genetic variants and steroid-induced osteonecrosis of the femoral head in steroid use populations will be assessed in this study.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Moher et al., 2009).
Data Source and Search Strategy
Two authors independently performed a literature search using PubMed, Embase, the Cochrane Library, and the Chinese public databases, including the China National Knowledge Infrastructure (CNKI), the China Biology Medicine (CBM) Database, the China Science Periodical Database (CSPD, Wanfang Database), and the VIP Journal Integration Platform (VJIP). Searches were performed for studies published up to April 15, 2018. The following terms were used in the search strategy: hormone, glucocorticoid, steroid, corticosteroid, osteonecrosis, necrosis, femoral, femur, femoris, whirlbone, polymorphism, SNP, genetic, mutation, genotype, allele, allelic, and variation. We also conducted manual searches of the reference lists of relevant reviews to avoid omissions.
Selection Criteria
The following studies were included in our meta-analysis if they fulfilled the inclusion criteria: (1), study has a case-control or cohort study design; (2), steroid-using patients are included; (3), study compares ONFH and non-ONFH patients after steroid use; (4), study assesses the association between SNPs and SONFH; and (5), study indicates the frequencies of specific alleles or the effect sizes of individual genotypes between cases and controls.
The exclusion criteria included the following: (1), study compares patients with SONFH to healthy populations or populations with other types of ONFH; (2), study is about family heredity; (3), study is not SNP-related; and (4), study does not report data pertaining to allelic frequencies or calculable effect size. In addition, conference reports, editor comments, reviews, and academic dissertations were also excluded from the analysis.
Data Extraction and Quality Assessment
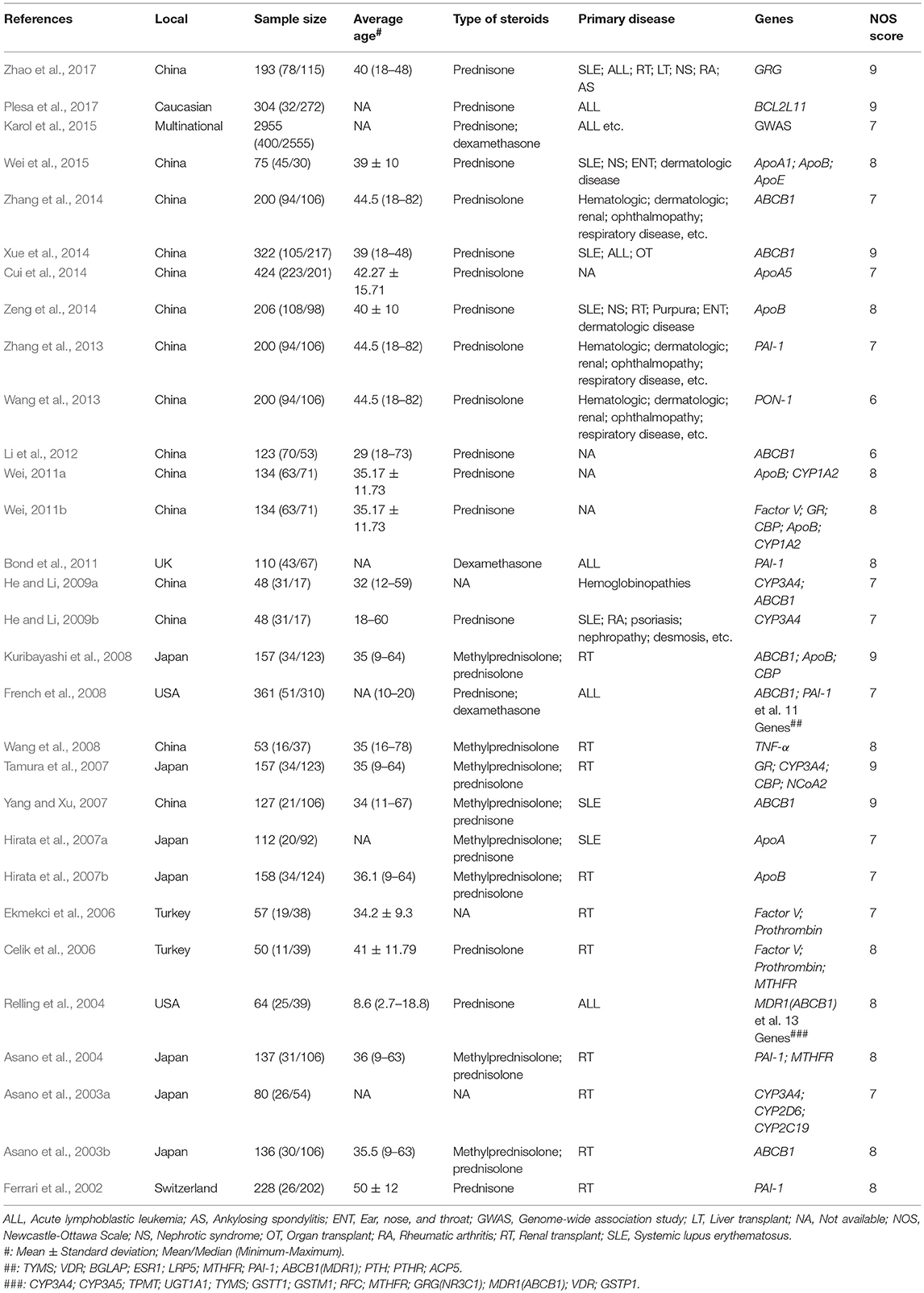
Two authors independently extracted the following information from each eligible study: the first author's name, publication year, research location, sample size, average age, primary disease, diagnostic mode, and genes of interest. The methodological quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS), a validated tool for evaluating the quality of observation studies that includes the following 3 subscales: selection, comparability, and exposure (Stang, 2010). We assessed all results for the level of evidence using the GRADE approach (Andrews et al., 2013), the average NOS score, and the number of patients included and then presented the data in a three-dimensional Manhattan plot.
Statistical Analysis
The Hardy-Weinberg equilibrium (HWE) of the included populations was calculated to assess the consistencies of allele frequencies between generations (Chen and Chatterjee, 2007). Association analysis was performed using five genetic models (Areeshi et al., 2013). The effect size was estimated by calculating the summary odds ratio (OR) and its 95% confidence intervals (CIs). The I2 statistic was used to estimate the degree of heterogeneity among the studies. If the I2 ≥ 50% (Q test, p < 0.1), a random-effect model was used; otherwise, a fixed-effect model was used. Subgroup analysis was also performed according to the ethnicity and location of the population. We assessed publication bias using the Begg and Egger tests. All tests were two-tailed, and a p-value of less than 0.05 was deemed statistically significant. We analyzed the data using the R program (Version 3.3.1) and STATA (Version 14.0).
We used GRADE to assess the level of evidence with four levels graded from high (best) to very low (worst). All objective studies used an observational study design, which downgrades the quality of evidence. Inconsistency was evaluated using the I2 and p-values of the Q test, and the outcome was imprecise if the standard error (SE) was larger than 0.2. Publication bias (reporting bias) also reduced the level of evidence. Finally, a large (OR > 2 or <0.5) or very large (OR > 5 or <0.2) effect upgraded the quality of evidence.
Results
Our research returned 278 English articles and 285 Chinese articles after removing duplications. After screening the titles and abstracts, 482 of these articles were excluded. The full texts of 81 articles were assessed, among which the control group did not receive steroid therapy (25); the article was a review (7); the study was non-SNP-related (5); the research was basic (3); the study did not report frequencies or effect size results (2); the study did not include steroid-related ONFH (2); the study was a case report (2); the research was microRNA related (2); the report was a duplicate (2); and the research was of heredity SONFH (1). Finally, we collected 30 trials assessing 7,553 patients for our meta-analysis (Table 1, Figure 1).
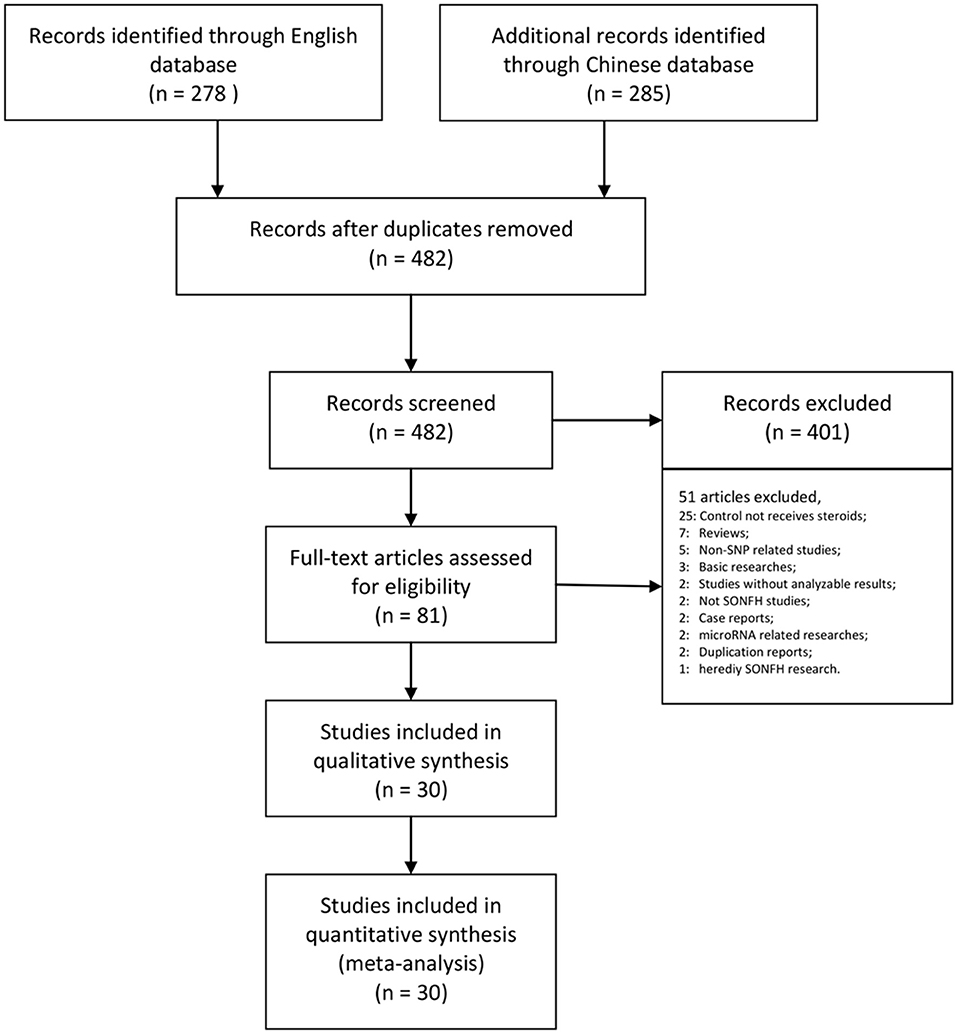
Figure 1. PRISMA flow chart illustrating the selection process of the studies included in our analysis. SNP, single-nucleotide polymorphism; SONFH, steroid-induced osteonecrosis of the femoral head.
All studies used a case-control design except one that used a cohort design (Karol et al., 2015). In addition, Weibao Fang's two studies (Wei, 2011a,b), and Kuribayashi and Tamura's studies (Tamura et al., 2007; Kuribayashi et al., 2008) included the same patient population but did not assess the same SNPs. We analyzed different SNP results and excluded duplicated results. The NOS scores were six to nine points, and the overall quality was ideal in observational studies (Table 1).
Six SNPs in four genes were included in the meta-analysis. In the ABCB1 gene, rs1045642 is also known as C3435T, is located in the coding region, and is a synonymous mutation. In this research, the pooled results for rs1045642 showed that the C > T mutation protected against SONFH in the allelic model (I2 = 50.2%; OR: 0.74; 95% CI: 0.55–1.00; p = 0.046) (Figure 2, Table 2). In the ApoB gene, rs693 is located in the coding region. The results showed that there was no significant relationship between this mutation and SONFH in the allelic model (I2 = 58%; OR: 2.63; 95% CI: 0.92–7.54; p = 0.072). However, other models showed that this mutation could increase SONFH risk (heterozygous model: I2 = 54.5%; OR: 2.46; 95% CI: 1.27–4.77; p = 0.008; homozygous model: I2 = 24.4%; OR: 7.70; 95% CI: 1.23–48.16; p = 0.029; dominant model: I2 = 31.4%; OR: 2.99; 95% CI: 1.71–5.21; p < 0.001; recessive model: I2 = 32.1%; OR: 7.16; 95% CI: 1.19–43.02; p = 0.031). rs1042031 could cause a missense mutation of glutamic acid to lysine or stop-gain mutation. Only the dominant model was analyzed, and the results showed that there was a significant relationship between this mutation and SONFH (I2 = 50.3%; OR: 2.90; 95% CI: 1.49–5.66; p = 0.002). Three studies were from China, and subgroup analysis results also indicated a significant relationship (I2 = 0%; OR: 4.81; 95% CI: 2.05–11.31; p < 0.001) (Figure 2, Table 2).
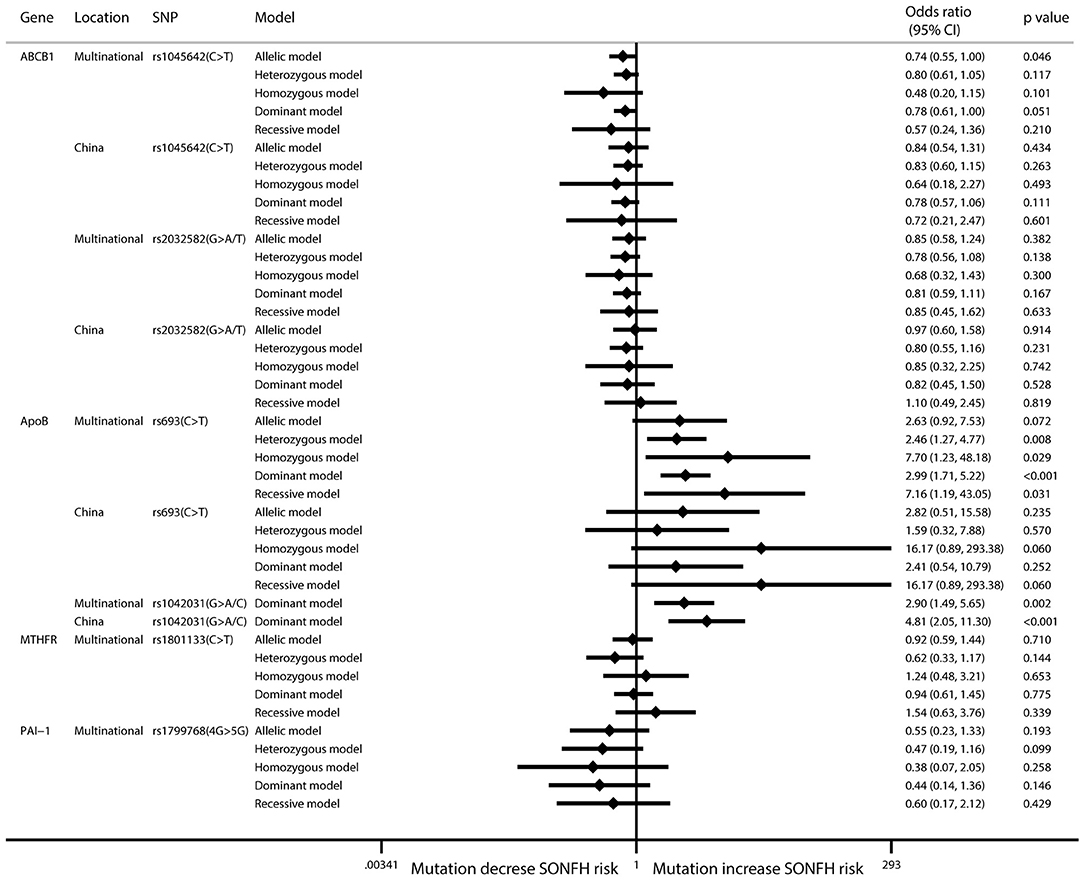
Figure 2. Forest plot of associations between mutations and SONFH risk. SNP, single-nucleotide polymorphism; SONFH, steroid-induced osteonecrosis of the femoral head.
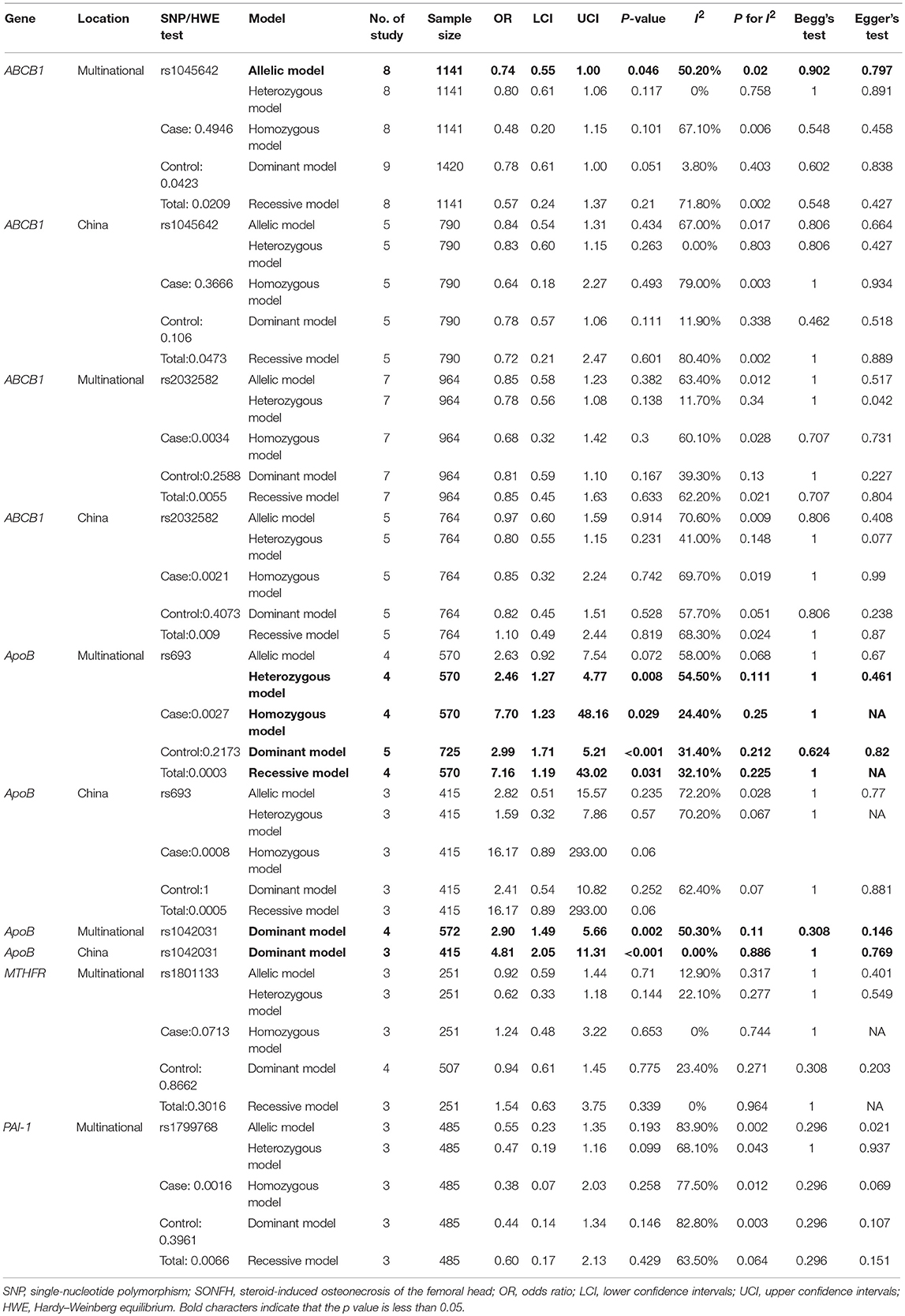
Table 2. Meta-analysis of the associations between SNPs and SONFH in the SNP results in more than three studies.
The level of evidence of the above positive results was assessed according to GRADE guidelines. The allelic model of ABCB1 rs1045642 showed a protective effect against SONFH. In these results, 1,141 patients were included; the average NOS score was 7.88. Although the SE was relatively small, there was heterogeneity (I2 = 50.2%, p = 0.02). Thus, the level of evidence was very low (Figure 3). The heterozygous model and dominant model of ApoB rs693 showed that the mutation increases SONFH risk. The SE of the two models was smaller than 0.2, and heterogeneity was not obvious (heterozygous model: I2 = 54.5%, p = 0.111; dominant model: I2 = 31.40%, p = 0.212). The level of evidence was moderate because of the large effect (OR>2) (Figure 3). The homozygous and recessive models of ApoB rs693 also showed that the mutation increases disease risk without heterogeneity (homozygous model: I2 = 24.4%, p = 0.25; recessive model: I2 = 32.1%, p = 0.225). Although the SEs were larger than 0.2, the OR was greater than 5. Thus, the evidence levels were also moderate (Figure 3). In addition, the average NOS scores of these four models were 8 to 8.25. The dominant model of ApoB rs1042031 indicated that the mutation increases SONFH risk without heterogeneity (I2 = 50.3%, p = 0.11). The SE of the result was less than 0.2, and the OR was greater than 2. Thus, the level of evidence was moderate (Figure 3). However, the genetic linkage disequilibrium that exists in the above positive results might affect stability.
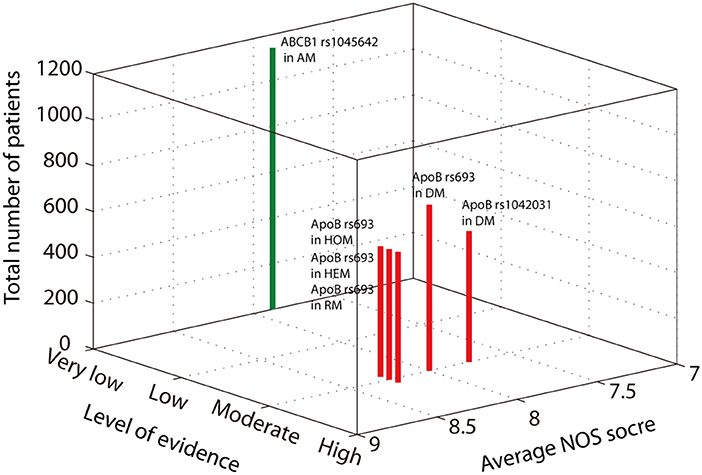
Figure 3. A three-dimensional Manhattan plot showing the average NOS score, the number of included patients, and the level of evidence. The green bar indicates the protection effect, and the red bar indicates the promotion effect.
Discussion
Our study analyzed the association between genetic polymorphisms and SONFH risk by comparing ONFH and non-ONFH in steroid use populations. In the SNP results of the included studies, more than three allelic models of ABCB1 rs1045642 showed that mutations protect against SONFH. The ApoB rs693 and rs1042031 mutations increase SONFH risk. According to the GRADE guidelines, the evidence levels for ApoB rs693 and rs1042031 were moderate, and ABCB1 rs1045642 was very low.
In a previous meta-analysis, Gong et al analyzed 23 studies and 35 genes and indicated that PAI-1 rs1799768 and ABCB1 rs1045642, but not MTHFR rs1801133 or ABCB1 rs2032582, were related to the risk of osteonecrosis (Gong et al., 2013). However, in this study, the control population included a healthy group and a population with other types of ONFH, which might affect the accuracy. In our study, there was no relationship between PAI-1 rs1799768 after adjusting for the control population. Guo et al assessed the correlation between SONFH and hepatic CYP3A activity and high found that CYP3A activity could reduce SONFH risk. However, the conclusion was based on animal model results, and it is unclear whether the risk is reduced in humans (Guo and Deng, 2017).
ABCB1 is a member of a superfamily of ATP-binding cassette transporters that transport variant molecules across cellular membranes. Zhou et al. researched the association between ABCB1 polymorphisms and SONFH and found that rs1045642 and rs2032582 are associated with SONFH (Zhou et al., 2015). However, this study also included a healthy population in the control population. In contrast, a Chinese review showed that rs1045642 could reduce SONFH risk, while rs2032582 is not related to SONFH (Yang J. et al., 2016). For rs1045642, our results are the same as those of a previous study, but with a very low level of evidence. The mutation does not cause changes in the amino acid sequence; thus, the specific mechanism of SONFH risk change caused by this mutation is still unknown. However, this mutation has been shown to be related to the metabolism of some drugs and to the prognosis of some cancers. This relationship may be related to differences in the characteristics of non-expressed proteins, such as codon bias, expression efficiency, and differences in mRNA characteristics. Therefore, this mutation is more suitable for clinical use as a predictor of SONFH risk for steroid users to guide individual drug application. People with the rs1045642 T allele have a low risk of SONFH with steroid application, while individuals with C allele have a high SONFH risk and should avoiding long-term high-dose steroid application, with increased duration of follow-up and frequency of diagnosis. For rs2032582, our results found that the mutation is not related to SONFH (allelic model: p = 0.382) with statistical heterogeneity (I2 = 63.4%, p = 0.012).
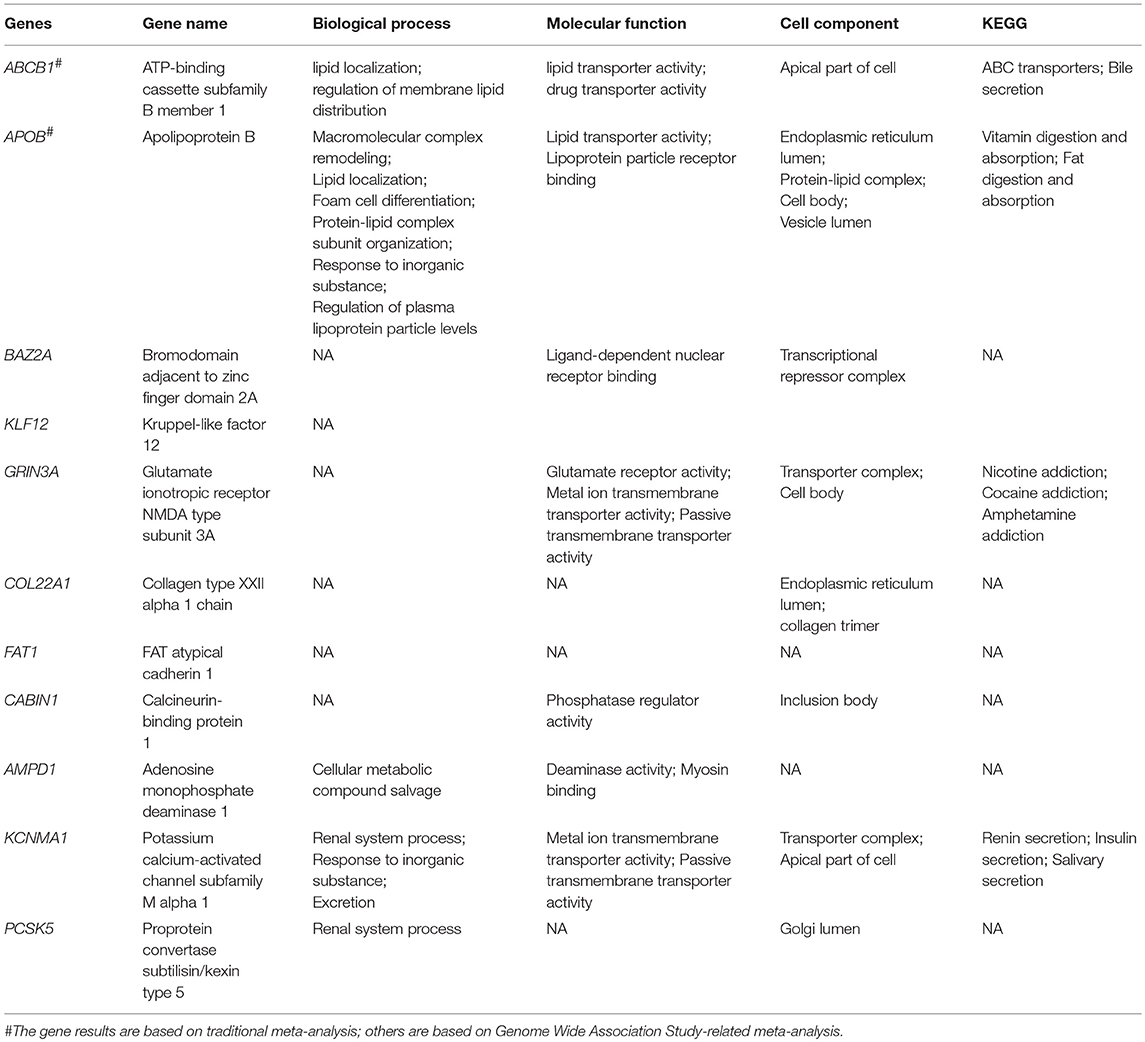
Additionally, we performed a post hoc analysis of Seth E. Karol's research that excluded SNP results with very low-level evidence (Karol et al., 2015). Then, we matched potential SNPs with GO/KEGG annotations (Table 3). According to a gene set enrichment analysis based on overrepresentation enrichment analysis, only the molecular functions of ABCB1 and ApoB showed obvious relationships with lipid transporter activity (GO: 0005319) (Zhang et al., 2005). This finding indicates that at present, SNPs involved in lipid transporter activity are more related to SONFH risk than other SNPs, which supports the lipid metabolism disorder theory. ApoB is an important factor in normal lipid metabolism, the mutation of which can also be used as a predictor of SONFH occurrence to assess the risk of SONFH and to guide steroid clinical application. Although rs693 is a synonymous variant, rs693 is related to the circulating concentration of LDL cholesterol. T allele carriers have high levels of TG, TC, and LDL-C and low HDL-C (Sandhu et al., 2008). In addition, rs1042031 is a coding sequence variant that causes glutamate to lysine mutation (C > T), or the introduction of a stop codon in the amino acid sequence (C > A). The rs1042031 mutation is important for regulating the binding of apolipoprotein B to the LDL cholesterol receptor (Liu et al., 2013). In this work, these two mutations were found to be related to SONFH. Therefore, we hypothesized that the increase of circulating LDL induced by mutation increased the risk of SONFH, supporting the hypothesis that lipid metabolism disorders are associated with SONFH. Abnormal lipid metabolism leads to bone marrow adipogenesis of the femoral head that is mainly due to hyperlipidemia and inhibition of mitochondrial dehydrogenation by steroid application. The abnormal lipid metabolism then leads to fat embolism, which blocks local blood perfusion and exacerbates local inflammation. Therefore, the question remains: does reducing circulating LDL effectively reduce the risk of SONFH with steroid application? In animal models, it has been suggested that reduced circulating lipid levels can reduce the risk of SONFH (Yang Z. et al., 2016), but the clinical effect remains to be confirmed. Additionally, in the location subgroup analysis of rs1042031 results, the heterogeneity decreased significantly from 50 to 0%. The pool results of the three Chinese studies had low heterogeneity, and the population was concentrated in Guangxi and Shandong in China (Wei, 2011a; Zeng et al., 2014; Wei et al., 2015). Another study analyzed the Japanese population (Hirata et al., 2007b). Therefore, the centralization of the included population may be the main reason for the reduction in heterogeneity.
Limitations
The present study still has several limitations. First, our study was performed at the study level instead of the individual level. Second, our study did not consider the impact of primary disease or steroid therapy strategies on the results. Third, there is a large amount of heterogeneity in the results of this study. Although random effect models are used for heterogeneous results, these heterogeneities can still have a potential impact on the results. Fourth, our study only analyzed the SNPs that were examined in more than three studies, but with new research and evidence, more SNPs may be studied. Therefore, this study was limited by the research available at the time.
Conclusion
An allelic model of ABCB1 rs1045642 showed that mutations had a protective effect against SONFH at a very low level of evidence. The mutations in ApoB rs693 and rs1042031 increased the SONFH risk with moderate levels of evidence.
Author Contributions
XC performed the conception and design of the work. XC and LZ drafted and revised the work critically. DL and JL analyzed data for work. FL and HM acquisited data for this work. All authors provide approval for publication of the content.
Funding
This study was supported by 2017 Special of Scientific Research of Traditional Chinese Medicine of Henan Province (No. 2017ZY2032) and 2016 Luoyang medical and health plan (No. 1603004A-8).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andrews, J., Guyatt, G., Oxman, A. D., Alderson, P., Dahm, P., Falck-Ytter, Y., et al. (2013). GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J. Clin. Epidemiol. 66, 719–725. doi: 10.1016/j.jclinepi.2012.03.013
Areeshi, M. Y., Mandal, R. K., Panda, A. K., Bisht, S. C., and Haque, S. (2013). CD14−159 C>T gene polymorphism with increased risk of tuberculosis: evidence from a meta-analysis. PLoS ONE 8:e64747. doi: 10.1371/journal.pone.0064747
Asano, T., Takahashi, K. A., Fujioka, M., Inoue, S., Okamoto, M., Sugioka, N., et al. (2003a). ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 13, 675–682. doi: 10.1097/00008571-200311000-00003
Asano, T., Takahashi, K. A., Fujioka, M., Inoue, S., Satomi, Y., Nishino, H., et al. (2003b). Genetic analysis of steroid-induced osteonecrosis of the femoral head. J. Orthop. Sci. 8, 329–333. doi: 10.1007/s10776-003-0646-7
Asano, T., Takahashi, K. A., Fujioka, M., Inoue, S., Ueshima, K., Hirata, T., et al. (2004). Relationship between postrenal transplant osteonecrosis of the femoral head and gene polymorphisms related to the coagulation and fibrinolytic systems in Japanese subjects. Transplantation 77, 220–225. doi: 10.1097/01.TP.0000101433.99651.96
Bond, J., Adams, S., Richards, S., Vora, A., Mitchell, C., and Goulden, N. (2011). Polymorphism in the PAI-1 (SERPINE1) gene and the risk of osteonecrosis in children with acute lymphoblastic leukemia. Blood 118, 2632–2633. doi: 10.1182/blood-2011-05-355206
Celik, A., Tekis, D., Saglam, F., Tunali, S., Kabakci, N., Ozaksoy, D., et al. (2006). Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant. Proc. 38, 512–516. doi: 10.1016/j.transproceed.2005.12.062
Chen, J., and Chatterjee, N. (2007). Exploiting Hardy-Weinberg equilibrium for efficient screening of single SNP associations from case-control studies. Hum. Hered. 63, 196–204. doi: 10.1159/000099996
Cui, Y., Kaisaierjiang, A., Cao, P., Wu, Z. Y., and Lv, Q. (2014). Association of apolipoprotein A5 genetic polymorphisms with steroid-induced osteonecrosis of femoral head in a Chinese Han population. Diagn. Pathol. 9:229. doi: 10.1186/s13000-014-0229-1
Ekmekci, Y., Keven, K., Akar, N., Egin, Y., Sengul, S., Kutlay, S., et al. (2006). Thrombophilia and avascular necrosis of femoral head in kidney allograft recipients. Nephrol. Dial. Transplant. 21, 3555–3558. doi: 10.1093/ndt/gfl400
Ferrari, P., Schroeder, V., Anderson, S., Kocovic, L., Vogt, B., Schiesser, D., et al. (2002). Association of plasminogen activator inhibitor-1 genotype with avascular osteonecrosis in steroid-treated renal allograft recipients. Transplantation 74, 1147–1152. doi: 10.1097/00007890-200210270-00016
French, D., Hamilton, L. H., Mattano, L. A. jr., Sather, H. N., Devidas, M., Nachman, J. B., et al. (2008). A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 111, 4496–4499. doi: 10.1182/blood-2007-11-123885
Gong, L. L., Fang, L. H., Wang, H. Y., Peng, J. H., Si, K., Zhu, J., et al. (2013). Genetic risk factors for glucocorticoid-induced osteonecrosis: a meta-analysis. Steroids 78, 401–408. doi: 10.1016/j.steroids.2013.01.004
Guo, F. Q., and Deng, M. (2017). Correlation between steroid-induced osteonecrosis of the femoral head and hepatic CYP3A activity: a systematic review and meta-analysis. J. Invest. Surg. 1, 1–9. doi: 10.1080/08941939.2017.1385663
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi: 10.1136/bmj.39489.470347.AD
He, W., and Li, K. (2009a). Incidence of genetic polymorphisms involved in lipid metabolism among Chinese patients with osteonecrosis of the femoral head. Acta Orthop. 80, 325–329. doi: 10.3109/17453670903025378
He, W., and Li, K. (2009b). Genetic susceptibility of corticosteroid-induced osteonecrosis of femoral head. Chin. Bone Joint Surg. 2, 284–289. doi: 10.3969/j.issn.1674-1439.2009.04.007
Hirata, T., Fujioka, M., Takahashi, K. A., Arai, Y., Asano, T., Ishida, M., et al. (2007b). ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J. Orthop. Sci. 12, 199–206. doi: 10.1007/s00776-007-1110-9
Hirata, T., Fujioka, M., Takahashi, K. A., Asano, T., Ishida, M., Akioka, K., et al. (2007a). Low molecular weight phenotype of Apo(a) is a risk factor of corticosteroid-induced osteonecrosis of the femoral head after renal transplant. J. Rheumatol. 34, 516–522.
Johnson, E. O., Soultanis, K., and Soucacos, P. N. (2004). Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthop. Clin. North Am. 35, 285–291. doi: 10.1016/j.ocl.2004.03.002
Karol, S. E., Yang, W., Van Driest, S. L., Chang, T. Y., Kaste, S., Bowton, E., et al. (2015). Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood 126, 1770–1776. doi: 10.1182/blood-2015-05-643601
Kim, H., Cho, C., Cho, Y., Cho, S., Yoon, K., and Kim, K. (2011). Significant associations of PAI-1 genetic polymorphisms with osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 12:160. doi: 10.1186/1471-2474-12-160
Kuribayashi, M., Fujioka, M., Takahashi, K. A., Arai, Y., Hirata, T., Nakajima, S., et al. (2008). Combination analysis of three polymorphisms for predicting the risk for steroid-induced osteonecrosis of the femoral head. J. Orthop. Sci. 13, 297–303. doi: 10.1007/s00776-008-1244-4
Li, Y., He, W., Zhang, Q. W., Luo, Y. Z., Chen, Q. L., and Zhou, C. (2012). Association between polymorphisms of steroid induced osteonecrosis of the femoral head and traditional Chinese medicine syndrome types. J. New Chin. Med. 44, 87–89. doi: 10.13457/j.cnki.jncm.2012.06.066
Li, Z., Zhao, D., and Wang, B. (2014). ABCB1 gene polymorphisms and glucocorticoid-induced avascular necrosis of the femoral head susceptibility: a meta-analysis. Med. Sci. Monit. 20, 2811–2816. doi: 10.12659/MSM.891286
Liu, X., Wang, Y., Qu, H., Hou, M., Cao, W., Ma, Z., et al. (2013). Associations of polymorphisms of rs693 and rs1042031 in apolipoprotein B gene with risk of breast cancer in Chinese. Jpn. J. Clin. Oncol. 43, 362–368. doi: 10.1093/jjco/hyt018
Luo, P., Gao, F., Han, J., Sun, W., and Li, Z. (2018). The role of autophagy in steroid necrosis of the femoral head: a comprehensive research review. Int. Orthop. 42, 1747–1753. doi: 10.1007/s00264-018-3994-8
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Plesa, M., Gagné, V., Glisovic, S., Younan, M., Sharif-Askari, B., Laverdière, C., et al. (2017). Influence of BCL2L11 polymorphism on osteonecrosis during treatment of childhood acute lymphoblastic leukemia. Pharmacogenomics J. doi: 10.1038/s41397-017-0002-4. [Epub ahead of print].
Relling, M. V., Yang, W., Das, S., Cook, E. H., Rosner, G. L., Neel, M., et al. (2004). Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J. Clin. Oncol. 22, 3930–3936. doi: 10.1200/JCO.2004.11.020
Sandhu, M. S., Waterworth, D. M., Debenham, S. L., Wheeler, E., Papadakis, K., Zhao, J. H., et al. (2008). LDL-cholesterol concentrations: a genome-wide association study. Lancet 371, 483–491. doi: 10.1016/S0140-6736(08)60208-1
Shigemura, T., Nakamura, J., Kishida, S., Harada, Y., Ohtori, S., Kamikawa, K., et al. (2011). Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology 50, 2023–2028. doi: 10.1093/rheumatology/ker277
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Tamura, K., Nakajima, S., Hirota, Y., Takahashi, K. A., Fujioka, M., Kubo, T., et al. (2007). Genetic association of a polymorphism of the cAMP-responsive element binding protein-binding protein with steroid-induced osteonecrosis after kidney transplantation. J. Bone Miner. Metab. 25, 320–325. doi: 10.1007/s00774-007-0770-z
Wang, S., Wei, M., Han, Y., Zhang, K., He, L., Yang, Z., et al. (2008). Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 8:27. doi: 10.1186/1471-2334-8-27
Wang, Z., Wei, M., Han, Y., Zhang, K., He, L., Yang, Z., et al. (2013). Association of a polymorphism in PON-1 gene with steroid-induced osteonecrosis of femoral head in Chinese Han population. Diagn. Pathol. 8:186. doi: 10.1186/1746-1596-8-186
Wei, B. F. (2011a). Relationship between gene polymorphisms and syndrome types in steroid- induced femoral head osteonecrosis. J. Shandong Med. Coll. 33, 321–325. doi: 10.3969/j.issn.1674-0947.2011.05.001
Wei, B. F. (2011b). Relationship between gene polymorphisms and hereditary susceptibility of steroid-induced femoral head osteonecrosis in Linyi City of Shandong Province. J. Clin. Rehabil. Tissue Eng. Res. 15, 7403–7406. doi: 10.3969/j.issn.1673-8225.2011.39.046
Wei, X. D., Liang, J. C., Zeng, P., Qin, G., and Lv, L. Q. (2015). The correlation between apolipoprotein gene polymorphisms and steroid- induced osteonecrosis of the femoral head. Guangdong Med. J. 36, 1364–1368.
Xue, Y., Zhao, Z. Q., Hong, D., Zhang, H. J., Chen, H. X., and Fan, S. W. (2014). MDR1 gene polymorphisms are associated with glucocorticoid-induced avascular necrosis of the femoral head in a Chinese population. Genet. Test. Mol. Biomarkers 18, 196–201. doi: 10.1089/gtmb.2013.0374
Yang, J., Zhang, A. L., Lu, J. L., Meng, H. Y., and Zhang, X. J. (2016). Correlation between ABCB1C3435T and G2677T/A polymorphism and steroid-induced osteonecrosis of femoral head:a meta-analysis. Chin. J. Hosp. Pharm. 39, 732–737. doi: 10.13286/j.cnki.chinhosppharmacyj.2016.09.08
Yang, X. Y., and Xu, D. H. (2007). MDR1(ABCB1) gene polymorphisms associated with steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus. Pharmazie 62, 930–932. doi: 10.1691/ph.2007.12.7583
Yang, Z., Liu, H., Li, D., Xie, X., Qin, T., Ma, J., et al. (2016). The efficacy of statins in preventing glucocorticoid-related osteonecrosis in animal models: a meta-analysis. Bone Joint Res. 5, 393–402. doi: 10.1302/2046-3758.59.2000500
Yu, X., Zhang, D., Chen, X., Yang, J., Shi, L., and Pang, Q. (2018). Effectiveness of various hip preservation treatments for non-traumatic osteonecrosis of the femoral head: a network meta-analysis of randomized controlled trials. J. Orthop. Sci. 23, 356–364. doi: 10.1016/j.jos.2017.12.004
Zeng, P., Liang, J. C., Wei, X. D., and Wei, B. F. (2014). Relationship between apolipoprotein B gene polymorphism and tendon-vessel stagnation syndrome of steroid induced osteonecrosis of the femoral head. Shandong Med. J. 54, 1–3. doi: 10.3969/j.issn.1002-266X.2014.30.001
Zhang, B., Kirov, S., and Snoddy, J. (2005). WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33, W741–W748. doi: 10.1093/nar/gki475
Zhang, Y., Kong, X., Wang, R., Li, S., Niu, Y., Zhu, L., et al. (2014). Genetic association of the P-glycoprotein gene ABCB1 polymorphisms with the risk for steroid-induced osteonecrosis of the femoral head in Chinese population. Mol. Biol. Rep. 41, 3135–3146. doi: 10.1007/s11033-014-3173-y
Zhang, Y., Wang, R., Li, S., Kong, X., Wang, Z., Chen, W., et al. (2013). Genetic polymorphisms in plasminogen activator inhibitor-1 predict susceptibility to steroid-induced osteonecrosis of the femoral head in Chinese population. Diagn. Pathol. 8:169. doi: 10.1186/1746-1596-8-169
Zhao, Z., Xue, Y., Hong, D., Zhang, H., Hu, Z., Fan, S., et al. (2017). Polymorphisms in the glucocorticoid receptor gene and associations with glucocorticoid-induced avascular osteonecrosis of the femoral head. Genet. Test. Mol. Biomarkers 21, 322–327. doi: 10.1089/gtmb.2016.0260
Keywords: osteonecrosis, steroids, glucocorticoid, genetic polymorphism, meta-analysis
Citation: Chen X, Zhang L, Liang D, Li J, Liu F and Ma H (2018) Lipid Transporter Activity-Related Genetic Polymorphisms Are Associated With Steroid-Induced Osteonecrosis of the Femoral Head: An Updated Meta-Analysis Based on the GRADE Guidelines. Front. Physiol. 9:1684. doi: 10.3389/fphys.2018.01684
Received: 15 August 2018; Accepted: 08 November 2018;
Published: 03 December 2018.
Edited by:
Jue Wang, The University of Texas Health Science Center at Tyler, United StatesReviewed by:
Manit Arora, University of New England, AustraliaMengning Yan, Shanghai Jiao-Tong University School of Medicine, China
Copyright © 2018 Chen, Zhang, Liang, Li, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiantao Chen, Y2hlbnh0MTk3OUAxMjYuY29t
 Xiantao Chen
Xiantao Chen Leilei Zhang1
Leilei Zhang1