- Department of Dermatology, American University of Beirut Medical Center, Beirut, Lebanon
A rapid spread of different strains of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to an unprecedented pandemic. Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, the medical body has encountered major obstacles concerning disease management at different levels. Even though patients infected with this virus mainly present with respiratory symptoms, it has been associated with a plethora of well-documented cutaneous manifestations in the literature. However, little investigations have been conducted concerning COVID-19 and its impact on skin disorders mediated by type 2 inflammation leaving multiple dermatologists and other specialists perplexed by the lack of clinical guidelines or pathways. This review focuses on the effects of this pandemic in patients with skin disorders mediated by type 2 inflammation, specifically atopic dermatitis and chronic spontaneous urticaria. In addition, it will provide clinicians a guide on treatment and vaccination considerations for this stated set of patients.
Introduction
Given the rapid spread of the SARS-CoV-2 worldwide, multiple nations adopted a lockdown policy limiting all types of activities including healthcare services. Therefore, this incurred alternative approaches to maintain continuity of care including increasing use of teledermatology and home-based treatments (1). This was simultaneously accompanied by exercising sanitary practices, particularly hand hygiene (2). These measures affected the care of patients with chronic diseases such as atopic dermatitis and chronic urticaria among others and has led to an acute need of developing practical guidelines for the management of these conditions (1, 2). These have been mostly challenging in patients treated with systemic immunotherapies (1–3).
The aim of this review is to highlight the impact of COVID-19 on patients with atopic dermatitis and chronic urticaria as well as provide clinicians with a guide on navigating these patients regarding treatment and vaccinations throughout the pandemic.
Atopic dermatitis
Impact of COVID-19
Atopic dermatitis (AD) is a complex disease caused by the interplay of genes, immune system and the environment. It typically develops during infancy or childhood and is characterized by intense pruritus and a chronic or chronically relapsing dermatosis. Management of AD may be quite challenging and requires adequate continuity of care, especially in the older age group (4, 5).
Literature and real-world experience have indicated that COVID-19 poses a great risk on patients with comorbidities including patients with atopic dermatitis (3). This is attributed to the fact that atopic dermatitis is often associated with other atopic disorders such as asthma and allergic rhino-conjunctivitis (1). Studies did not demonstrate atopic dermatitis as a key independent risk factor to developing severe SARS-CoV-2 infection (5–7). However, more recent studies established that AD is associated with an increased odds of COVID-19 infection even after controlling for common comorbidities (8, 9). A systematic review evaluating cutaneous manifestations in patients infected with COVID-19 have shown that 20% of these patients had an underlying dermatologic disease including atopic dermatitis (10). The most common cutaneous presentation in this cohort of patients has been a vesicular rash with no predilection for location (10). Unfortunately, and until date, it is unclear how this virus pathogenetically affects AD patients (3).
The exaggerated immune innate and impaired adaptive immune inflammatory responses causing a cytokine storm have been postulated as a potential mechanism/s by which SARS-CoV-2 causes severe illness (10). Given the fact that AD is an immune-mediated disease, an associated surge in flares of atopic dermatitis has been observed during the pandemic (11). It is imperative then to assume that strict hygiene practices and increased stress levels imposed by the pandemic will likely contribute to this noted increase in AD flares (2, 5, 12). In addition, the pandemic with the need to quarantine has negatively impacted the quality of life of AD patients with reported increased levels of anxiety and depression in this specific subset (7, 12, 13). Physicians should take into account these issues and should incorporate this psychosocial dimension in their management plan.
Treatment considerations
The lockdown measures put in place during this critical COVID-19 pandemic has markedly decreased clinical activities in dermatology clinics and limited access to care by patients. However, it also gave health care professionals a window of opportunity in the field of teledermatology (web- and phone- counseling) which has significantly surged during the pandemic (14, 15). This platform has been and will likely be an extremely useful mean of reducing patient access to hospital (2, 5). From a patient's perspective, teledermatology has been well-accepted by AD patients who continued to have access to a dermatologist consultation, guaranteeing support and treatment continuation in most cases (1, 5, 16).
To minimize AD flares, the American Academy of Dermatology (AAD) and the European Task Force on Atopic Dermatitis (ETFAD) advocated reinforcing proper skin care regimens. These include the use of fragrance-free soap with warm water for hand washing followed directly by the application of moisturizer. If soap is unavailable, patients with AD should be instructed to use hand sanitizers devoid of sensitizing ingredients to minimize the risk of developing allergic contact dermatitis. Concerning face masks, cotton-woven face cloths are preferred over other potentially irritating face masks. Frequent laundering of these face cloths is recommended though studies on the optimal frequency of washing and the precise make of the fabric are currently lacking and will require additional studies (2, 3).
Treatment of AD may be complex and necessitate the employment of immune-modulating therapies. Such therapies may be of concern if initiated in the setting of SARS-CoV-2 given the dysfunctional and/or exaggerated immune response seen with this infection (10). Additionally, this poses a challenge for AD patients who are maintained on immune-modulating therapies since there is a concern of an increased risk of infection (1, 3). With the multitude of therapeutic modalities currently employed to control moderate to severe AD including systemic corticosteroids, dupilumab, methotrexate, cyclosporine, azathioprine, Janus kinase (JAK) inhibitors and phototherapy among others, recommendations concerning their use during the pandemic have been outlined by international and national societies and are summarized in (Table 1) (17). However, an interdisciplinary approach for severe AD cases is favored (3).
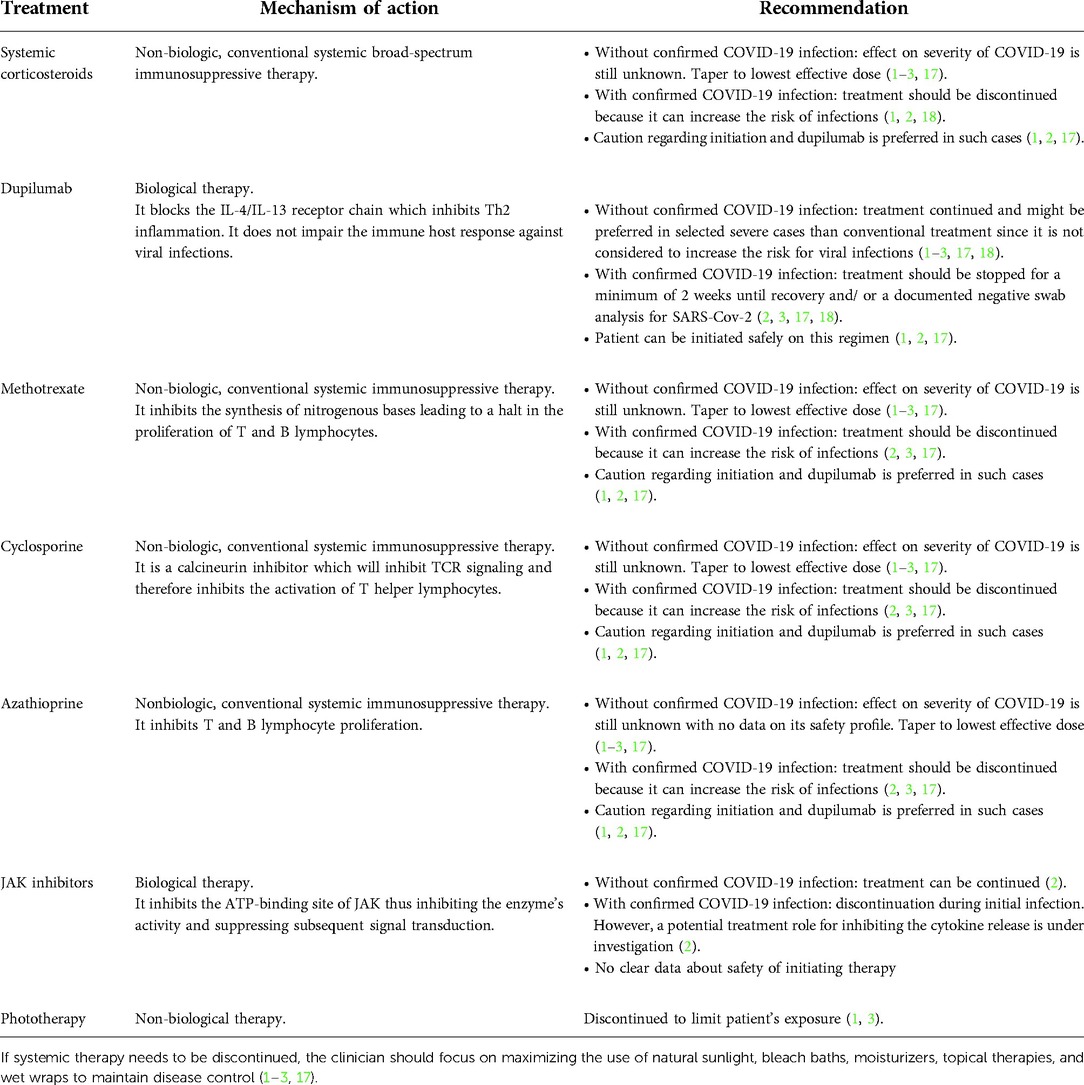
Table 1. Summary of current recommendation for nonbiologic and biologic systemic medications in AD treatment.
An international effort to facilitate and guide clinical decision making during the pandemic has been launched via SECURE-AD (Surveillance Epidemiology of Coronavirus Under Research Exclusion—Atopic Dermatitis) (www.covidderm.org). It is a web-based registry for clinicians to report COVID-19 outcomes in atopic dermatitis patients (20). This large dataset will serve as a reference for health care professionals and will provide counseling and information about treatment/s during the COVID-19 pandemic. Also, it will aid in filling our present knowledge gaps in regards to the management of AD.
Vaccination considerations
To achieve herd immunity against the virus, safe and effective vaccines have been developed. The current available vaccines are either mRNA or virus vectored vaccines. There is no evidence to suggest that any will pose a risk of disease flares in patients with AD receiving any of the SARS-CoV-2 vaccine given the fact that response to such vaccines is primarily a Th1 response (21). Even though the chronic phase of AD is a Th2-mediated process, there is currently no data on Th2 response following vaccination (22). Hence, there is no contraindication to vaccination in the setting of AD (6, 21).
In regards to patients on systemic immunosuppressants and JAK-inhibitors, these medications may attenuate or blunt the expected immune response (6, 23). Therefore, it has been recommended to either pause therapy or reduce dosing depending on the systemic therapeutic modality used during vaccination to achieve an optimal immune response (Table 2) (6, 23). Although dupilumab is a biologic therapy for AD, no attenuation is expected (6, 21, 23). Noteworthy is the ETFAD recommendation to schedule the vaccine in the week interval between dupilumab injections (6). Moreover, it is prudent to have the injection of dupilumab at least 48–72 h apart from that of the vaccine in order to distinguish any potential adverse reaction occurring after dupilumab from those occurring after the vaccine. As for the pediatric population, the Food and Drug Administration (FDA) and EMA (European Medicines Agency) have approved the administration of COVID-19 vaccines (Pfizer-BioNTech, Moderna, Novanax) from the age of 6 months to 17 years. At present, there are no studies assessing the impact of the vaccine/s on AD in terms of triggering it or affecting its course in patients who have it.
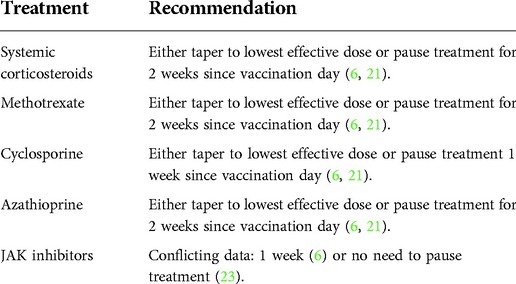
Table 2. Summary of current recommendation for SARS-CoV-2 vaccination in AD patients maintained on systemic therapy.
Chronic spontaneous urticaria
Impact of COVID-19
Chronic spontaneous urticaria (CSU) is an autoimmune/autoallergic condition with a fluctuating course and may be difficult to control. It is characterized by the appearance of recurrent evanescent wheals lasting for more than 6 weeks and variable degree of pruritus. During the COVID-19 pandemic, several case reports, and series have been published on acute urticaria as a cutaneous manifestation of the SARS-CoV-2 infection (24). A third of patients with CSU experiencing exacerbation of their condition have been reported when infected with the virus (24–26). This exacerbation may most likely be seen in patients with moderate-to-severe forms of the infection (25). In terms of pathogenesis, the SARS-CoV-2 virus is postulated to increase pro-inflammatory cytokines causing direct mast cell degranulation (27) and therefore explains the possible exacerbation experienced by CU patients (19, 28, 29). Furthermore, the direct viral cytopathic effect on keratinocytes through the ACE2 receptor of the basal layer of the epidermis has been postulated as an added hypothesis in the pathophysiology (27). It is important to emphasize the role of psychological stress faced by most people during the current sanitary crisis as a potential cause of these exacerbations since it has been previously demonstrated that there is a close association between CSU and anxiety (24, 26, 30). Although COVID-19 infection may result in flares of CSU in some patients, current evidence does not demonstrate that CSU is an independent predictor of the severity of COVID-19 infection or influences the course of the disease (24).
Treatment considerations
Similar to patient with AD, patients with CSU reported great satisfaction with teledermatology (24, 25). The mainstay treatment for CSU consists of second-generation non-sedating H1 antihistamines, cyclosporine and omalizumab. There is no consensus between clinicians regarding the discontinuation of biologics or immune suppressants during the pandemic. Recently, the European Academy of Allergy and Clinical Immunology (EAACI) has published an expert-led consensus on practical recommendations for the management of patients with urticaria (24). Treatment with antihistamines should be maintained throughout the pandemic regardless of the infection status of the patient. As for cyclosporine, an immunosuppressant, there is no clear data whether it should be continued or be avoided (31). If the immunosuppressant cannot be replaced, it has been advised to decrease it to the lowest possible dose to avoid or minimize complications (31). Concerning omalizumab, expert opinion advises maintaining the treatment in patients with mild-to-moderate COVID-19 infection based on findings extrapolated from a meta-analysis of randomized controlled trials in CSU where similar rates of upper respiratory tract infections in omalizumab and placebo-treated individuals were detected (31, 32). Such recommendation has been substantiated by recent reports demonstrating the safety of omalizumab in COVID-19 patients with urticaria (28, 31, 33). In patients with severe COVID-19 infection, it has been agreed to prolong intervals between the omalizumab injections or pause therapy (24). Omalizumab is usually administered in office for the first third doses and home administration can be considered for the fourth. Early transition to home/self-administered omalizumab injections during the COVID-19 pandemic has been recently recommended to eliminate hospital visits and mitigate the risk of exposure to COVID-19 infection (34, 35). This can be achieved via training at the second dose which includes an action plan in the event of anaphylaxis (35, 36). Additionally, telemedicine may be provided to ensure proper patient education.
Vaccination considerations
The literature is scarce regarding SARS-CoV-2 vaccination in patients with CSU. On one hand, a small case series reported flares of CSU induced by the mRNA vaccine (37). Most of these patients experienced a resolution of their symptoms within a week. Hence, it may be beneficial for this subset of patient to be evaluated by a physician prior to receiving the vaccine (37). On the other hand, the Canadian Society of Allergy and Clinical Immunology state that this condition is unlikely to interfere with the immune response triggered by the vaccine. Therefore, all patients including those maintained on omalizumab should proceed with their SARS-CoV-2 vaccine/s. Unfortunately, there is no consensus on what dosing schedule of omalizumab with respect to vaccine scheduling.
Conclusion
The COVID-19 pandemic has largely affected clinicians' and patients' daily practices forcing both groups to quickly adapt to a new model of modus operandi. Current evidence indicates that patients with underlying skin allergies may be treated nearly like the general population. Special considerations should however be applied. These include modifying immune-modulating therapies and vaccination considerations. This sanitary emergency has also permitted the implementations of teledermatology on a large scale. Such a consultative platform is now widely accepted by patients with AD or CSU and provides continuity of care to these patients. The general recommendations published thus far and reviewed in this paper are largely derived from expert opinions and lack robust evidence for final recommendations. A collective worldwide effort is mandatory and is underway to obtain data from web-registries and design the appropriate management strategies needed in patients with skin allergies during this unabating pandemic.
Author contributions
All authors have contributed equally to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chiricozzi A, Talamonti M, De Simone C, Galluzzo M, Gori N, Fabbrocini G, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. (2021) 76(6):1813–24. doi: 10.1111/all.14767
2. Shah M, Sachdeva M, Alavi A, Shi VY, Hsiao JL. Optimizing care for atopic dermatitis patients during the COVID-19 pandemic. J Am Acad Dermatol. (2020) 83(2):e165–7. doi: 10.1016/j.jaad.2020.05.027
3. Wollenberg A, Flohr C, Simon D, Cork MJ, Thyssen JP, Bieber T, et al. European task Force on Atopic Dermatitis statement on severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection and atopic dermatitis. J Eur Acad Dermatol Venereol. (2020) 34(6):e241–2. doi: 10.1111/jdv.16411
4. Chello C, Carnicelli G, Sernicola A, Gagliostro N, Paolino G, Di Fraia M, et al. Atopic dermatitis in the elderly Caucasian population: diagnostic clinical criteria and review of the literature. Int J Dermatol. (2020) 59(6):716–21. doi: 10.1111/ijd.14891
5. Ragamin A, de Wijs LEM, Hijnen DJ, Arends NJT, Schuttelaar MLA, Pasmans S, et al. Care for children with atopic dermatitis in the Netherlands during the COVID-19 pandemic: lessons from the first wave and implications for the future. J Dermatol. (2021) 48(12):1–8. doi: 10.1016/j.jaip.2022.08.032
6. Thyssen JP, Vestergaard C, Barbarot S, Bruin-Weller M, Bieber T, Taieb A, et al. European task Force on Atopic Dermatitis: position on vaccination of adult patients with atopic dermatitis against COVID-19 (SARS-CoV-2) being treated with systemic medication and biologics. J Eur Acad Dermatol Venereol. (2021) 35(5):e308–11. doi: 10.1111/jdv.17167
7. Zhang J, Loman L, Kamphuis E, Schuttelaar MLA. Lifelines Corona research I. Impact of the COVID-19 pandemic on adults with moderate-to-severe atopic dermatitis in the Dutch general population. JAAD Int. (2022) 6:86–93. doi: 10.1016/j.jdin.2021.12.006
8. Fan R, Leasure AC, Damsky W, Cohen JM. Association between atopic dermatitis and COVID-19 infection: a case-control study in the All of Us research program. JAAD Int. (2022) 6:77–81. doi: 10.1016/j.jdin.2021.12.007
9. Wu JJ, Martin A, Liu J, Thatiparthi A, Ge S, Egeberg A, et al. The risk of COVID-19 infection in patients with atopic dermatitis: a retrospective cohort study. J Am Acad Dermatol. (2022) 86(1):243–5. doi: 10.1016/j.jaad.2021.09.061
10. Jamshidi P, Hajikhani B, Mirsaeidi M, Vahidnezhad H, Dadashi M, Nasiri MJ. Skin manifestations in COVID-19 patients: are they indicators for disease severity? a systematic review. Front Med. (2021) 8:634208. doi: 10.3389/fmed.2021.634208
11. COVID-19-associated surge of atopic dermatitis. EBioMedicine. (2021) 64:103268. doi: 10.1016/j.ebiom.2021.103268
12. Pourani MR, Ganji R, Dashti T, Dadkhahfar S, Gheisari M, Abdollahimajd F, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis. Actas Dermosifiliogr. (2022) 113(3): 286–93. doi: 10.1016/j.ad.2021.08.013
13. Sieniawska J, Lesiak A, Ciazynski K, Narbutt J, Ciazynska M. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. (2022) 19(3):1734. doi: 10.3390/ijerph19031734
14. Loh CH, Chong Tam SY, Oh CC. Teledermatology in the COVID-19 pandemic: a systematic review. JAAD Int. (2021) 5:54–64. doi: 10.1016/j.jdin.2021.07.007
15. Dovigi E, Kwok EYL, English JC 3rd, A framework-driven systematic review of the barriers and facilitators to teledermatology implementation. Curr Dermatol Rep. (2020) 9(4):353–61. doi: 10.1007/s13671-020-00323-0
16. Grieco T, Chello C, Sernicola A, Muharremi R, Michelini S, Paolino G, et al. Impact of COVID-19 on patients with atopic dermatitis. Clin Dermatol. (2021) 39(6):1083–7. doi: 10.1016/j.clindermatol.2021.07.008
17. Marko M, Pawliczak R. Can we safely use systemic treatment in atopic dermatitis during the COVID-19 pandemic? Overview of selected conventional and biologic systemic therapies. Expert Rev Clin Immunol. (2021) 17(6):619–27. doi: 10.1080/1744666X.2021.1919511
18. Kridin K, Schonmann Y, Solomon A, Onn E, Bitan DT, Weinstein O, et al. Risk of COVID-19 and its complications in patients with atopic dermatitis undergoing dupilumab treatment-a population-based cohort study. Immunol Res. (2022) 70(1):106–13. doi: 10.1007/s12026-021-09234-z
19. Muntean IA, Pintea I, Bocsan IC, Dobrican CT, Deleanu D. COVID-19 disease leading to chronic Spontaneous urticaria exacerbation: a romanian retrospective study. Healthcare (Basel). (2021) 9(9):1144. doi: 10.3390/healthcare9091144
20. Mahil SK, Yiu ZZN, Mason KJ, Dand N, Coker B, Wall D, et al. Global reporting of cases of COVID-19 in psoriasis and atopic dermatitis: an opportunity to inform care during a pandemic. Br J Dermatol. (2020) 183(2):404–6. doi: 10.1111/bjd.19161
21. Kridin K, Schonmann Y, Onn E, Bitan DT, Weinstein O, Cohen AD. Determinants and effectiveness of BNT162b2 mRNA vaccination among patients with atopic dermatitis: a population-based study. Am J Clin Dermatol. (2022) 2022:1–8. doi: 10.1007/s40257-022-00672-5
22. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. (2020) 586(7830):594–9. doi: 10.1038/s41586-020-2814-7
23. Simonetti O, Radi G, Molinelli E, Rizzetto G, Diotallevi F, Offidani A. Recommendations for dermatologists treating patients with atopic dermatitis during the COVID-19 pandemic: a look into the past for a conscious vaccination management. Hum Vaccines Immunotherapeut. (2021) 17(10):3268–75. doi: 10.1080/21645515.2021.1925502
24. Kocaturk E, Salman A, Cherrez-Ojeda I, Criado PR, Peter J, Comert-Ozer E, et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy. (2021) 76(3):816–30. doi: 10.1111/all.14687
25. Erdem Y, Polat Ekinci A, Altunay IK, Sivaz O, Inal S, Gokalp MO, et al. The impact of COVID-19 pandemic on the management of patients with chronic urticaria: an observational two-center study from Turkey. Dermatol Ther. (2021) 34(1):e14652. doi: 10.1111/dth.14652
26. Argolo P, Pereira G, Pereira GF, Kalil J, Motta A, Agondi R. Clinical conditions of patients with chronic urticaria during the pandemic caused by the 2019 Novel Coronavirus Disease (COVID-19). J Allergy Clin Immunol. (2021) 147(2):AB26. doi: 10.1016/j.jaci.2020.12.132
27. Gonzalez Gonzalez F, Cortes Correa C, Penaranda Contreras E. Cutaneous manifestations in patients with COVID-19: clinical characteristics and possible pathophysiologic mechanisms. Actas Dermosifiliogr. (2021) 112(4):314–23. doi: 10.1016/j.adengl.2021.01.024
28. Criado PR, Criado RFJ, Pincelli TP, Yoshimoto TA, Naufal GGA, Abdalla BMZ. Chronic spontaneous urticaria exacerbation in a patient with COVID-19: rapid and excellent response to omalizumab. Int J Dermatol. (2020) 59(10):1294–5. doi: 10.1111/ijd.15134
29. Grieco T, Porzia A, Paolino G, Chello C, Sernicola A, Faina V, et al. IFN-gamma/IL-6 and related cytokines in chronic spontaneous urticaria: evaluation of their pathogenetic role and changes during omalizumab therapy. Int J Dermatol. (2020) 59(5):590–4. doi: 10.1111/ijd.14812
30. Konstantinou GN, Konstantinou GN. Psychological stress and chronic urticaria: a neuro-immuno-cutaneous crosstalk. A systematic review of the existing evidence. Clin Ther. (2020) 42(5):771–82. doi: 10.1016/j.clinthera.2020.03.010
31. Patil A, Godse K, Godse G. Urticaria and its management in the context of coronavirus disease-19 (COVID-19). IP Ind J Clin Experimen Dermatol. (2020) 6(2):102–4. doi: 10.18231/j.ijced.2020.022
32. Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF, et al. Omalizumab for the treatment of chronic spontaneous urticaria: a meta-analysis of randomized clinical trials. J Allergy Clin Immunol. (2016) 137(6):1742–50 e4. doi: 10.1016/j.jaci.2015.12.1342
33. Bostan E, Zaid F, Karaduman A, Dogan S, Gulseren D, Yalici-Armagan B, et al. The effect of COVID-19 on patients with chronic spontaneous urticaria treated with omalizumab and antihistamines: a cross-sectional, comparative study. J Cosmet Dermatol. (2021) 20(11):3369–75. doi: 10.1111/jocd.14484
34. King C, Cox F, Sloan A, McCrea P, Edgar JD, Conlon N. Rapid transition to home omalizumab treatment for chronic spontaneous urticaria during the COVID-19 pandemic: a patient perspective. World Allergy Organ J. (2021) 14(10):100587. doi: 10.1016/j.waojou.2021.100587
35. Vultaggio A, Agache I, Akdis CA, Akdis M, Bavbek S, Bossios A, et al. Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: an EAACI statement. Allergy. (2020) 75(11):2764–74. doi: 10.1111/all.14407
36. Shaker MS, Oppenheimer J, Grayson M, Stukus D, Hartog N, Hsieh EWY, et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol: In Pract. (2020) 8(5):1477–88 e5. doi: 10.1016/j.jaip.2020.03.012
Keywords: skin allergies, atopic dermatitis, chronic urticaria, COVID-19, SARS-CoV-2, pandemic
Citation: Haddad I, Kozman K and Kibbi A-G (2022) Navigating patients with atopic dermatitis or chronic spontaneous urticaria during the COVID-19 pandemic. Front. Allergy 3:809646. doi: 10.3389/falgy.2022.809646
Received: 5 November 2021; Accepted: 12 September 2022;
Published: 4 October 2022.
Edited by:
Anant D. Patil, Padmashree Dr. D.Y. Patil University, IndiaReviewed by:
Jorge Agustin Luna-Pech, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, MexicoAlvise Sernicola, University of Padua, Italy
© 2022 Haddad, Kozman and Kibbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul-Ghani Kibbi agkibbi@aub.edu.lb
Specialty Section: This article was submitted to Skin Allergy, a section of the journal Frontiers in Allergy
 Isabelle Haddad
Isabelle Haddad Kathia Kozman
Kathia Kozman Abdul-Ghani Kibbi
Abdul-Ghani Kibbi