- 1Center for Conservation, Ugyen Wangchuck Institute for Conservation and Environmental Research, Bumthang, Bhutan
- 2Ecosystem Management, University of New England, Armidale, NSW, Australia
- 3Geography and Planning, University of New England, Armidale, NSW, Australia
- 4Wildlife Conservation Research Unit, Department of Zoology, University of Oxford, Abingdon, United Kingdom
- 5Early Learning Center, Thimphu, Bhutan
Most canids face population declines and range contractions worldwide. Although the dhole (Cuon alpinus) is widely distributed across 10 countries in South and Southeast Asia, limited studies exist on this species. Despite its globally “Endangered” status and ecological role as an apex predator, assessments on its distribution are limited to a few landscapes and countries. This explains the lack of a dhole-specific species conservation plan in most range countries, including Bhutan where no current population estimate exists. The species has also recovered from a country-wide poisoning campaign in the 1970s and 80s. In this study, we determine the dhole's distribution pattern and assess the protection and connectivity of dhole habitat in Bhutan. We anticipated dholes to be extant within their habitat well-represented in protected areas (PAs) and biological corridors (BCs). We used 721 georeferenced dhole occurrence records and eight environmental variables in MaxEnt software to model potential dhole distribution and habitat suitability. The model output was overlaid on the spatial layers of PAs and BCs to assess habitat protection and connectivity. As anticipated, we found the dhole widely distributed in all districts, PAs, and BCs in Bhutan. Dholes were recorded at the highest elevation range limit of 4,980 m above sea level, which overlapped with the “Vulnerable” snow leopard (Panthera uncia). Our model identified 72% (27,634 km2) of the country as suitable areas for dholes, of which, 31% (11,899 km2) was highly suitable and 41% (15,735 km2) was moderately suitable. Contrary to our expectation, PAs and BCs encompassed only 29% (8,046 km2) and 12% (3,185 km2) of suitable areas for dholes, respectively. A vast majority of the areas we deemed suitable for dholes currently remain unprotected, thus making dholes more vulnerable to human persecution and local extermination. We recommend adjusting PA boundaries to fully encompass suitable dhole habitat, and also advocate improved livestock husbandry to reduce dhole related livestock predation and minimize conflict, thereby ensuring its long-term survival in Bhutan.
Introduction
With 13 genera encompassing 37 species in 81% of countries, canids are widespread across diverse habitats and environments in all continents except Antarctica (Fleming et al., 2017). As apex predators, large canids can influence prey populations and cause trophic cascades when their populations fluctuate (Newsome and Ripple, 2015; Fleming et al., 2017). Globally, canid distributions overlap with human-modified landscapes (Srivathsa et al., 2019b) to pose conservation challenges (Lamb et al., 2020), resulting in population declines caused by habitat loss, prey decrease, human persecution, disease, and overexploitation (Ripple et al., 2014). The most notable examples include the extermination of the Mexican wolf (Canis lupus baileyi) from its natural range (Brown, 1983), a 93% range contraction of the African wild dog (Lycaon pictus; Woodroffe and Sillero-Zubiri, 2020), eradication of gray wolves (Canis lupus) from most of the United States and Europe (Mech, 1995), and extinction of the Falkland Islands wolf (Dusicyon australis; Sillero-Zubiri, 2015).
The dhole (Cuon alpinus, Pallas 1811), or Asiatic wild dog, is one of the most widely distributed members of the 10 canid species described from Asia (Din et al., 2013). It is an apex social carnivore that preys mostly on ungulates (Kamler et al., 2012, 2020) in forested areas across most of South and Southeast Asia and parts of China (Srivathsa et al., 2014; Kamler et al., 2015). Although largely restricted to protected areas (Kamler et al., 2015), dholes have also been recorded in unprotected secondary forests, multi-use forest fragments, and agro-forest plantations adjacent to protected areas (Srivathsa et al., 2014). Despite being shy and elusive with infrequent contacts with humans (Srivathsa et al., 2020), this canid has been extirpated from ~82% of its original range through human persecution and habitat loss (Wolf and Ripple, 2017). It is currently listed as globally “Endangered” by the IUCN based on an estimated population of 4,500–10,500 individuals comprising <2,500 adults, with India housing substantial populations concentrated to the south of the Ganges River in the Western Ghats and central forested regions (Kamler et al., 2015).
Although endangered, the dhole has received less conservation attention than other charismatic carnivores (Widodo et al., 2020). Much of the focus on dholes is related to their depredation on livestock (e.g., Katel et al., 2015; Srivathsa et al., 2020). While basic information from camera-trapping (presence and relative abundance) has been reported for site-specific dhole populations in India (Datta et al., 2008; Bashir et al., 2014), Myanmar (Rao et al., 2005), Peninsular Malaysia (Kawanishi and Sunquist, 2008), and Laos (Johnson et al., 2006), there remains a lack of country-specific consolidated distribution data and range maps for dholes. This hinders the assessment of their population status (Karanth et al., 2009) for both in-country and regional conservation planning (Srivathsa et al., 2014; Punjabi et al., 2017), because knowledge of suitable sites where species occur and survive can aid in conservation planning (Papeş and Gaubert, 2007).
Several modeling studies on dhole distribution and occupancy have been carried out at varying scales. At local scales, park-wide potential dhole distribution modeling was carried out by Namgyal and Thinley (2017) in Bhutan's Jigme Dorji National Park and by Rahman et al. (2018) in Indonesia's Ujung Kulon National Park, whereas Singh et al. (2020) recently reported on dhole occupancy in India's Dampa Tiger Reserve. At the broader landscape scale, Srivathsa et al. (2014), Punjabi et al. (2017), and Srivathsa et al. (2019a) modeled dhole occupancy across the Western Ghats of Karnataka, India. Similarly, Srivathsa et al. (2019b) also assessed occupancy by dholes across the Pench-Kanha Landscape in Madya Pradesh, India. Recently, Widodo et al. (2020) modeled potential dhole distribution across the Rimbang Baling and Tesso Nilo landscapes in Sumatra. At the country level, Jenks et al. (2012) used maximum entropy modeling to predict potential dhole distribution in Thailand based on dhole occurrence data from 15 protected areas while Karanth et al. (2009) used occupancy modeling to predict areas of dhole occurrence in India.
In Bhutan, dholes are apex predators similar to tigers (Panthera tigris; Thinley et al., 2018) and snow leopards (Panthera uncia; (Leki and Shrestha, 2018). There is no current population estimate for dholes in Bhutan. They were, however, almost extirpated from the country in the 1970s and 80s by mass poisoning campaigns due to blames over persistent livestock depredation (Wang and Macdonald, 2006; Thinley et al., 2011; Namgyal and Thinley, 2017). Because dholes are known to control populations of wild pig (Sus scrofa), the principal crop-raiding species in Bhutan (Wangchuk, 2004; Thinley et al., 2018), it is believed that wild pig populations in Bhutan substantially increased and intensified crop damage after the mass extermination of dholes (Wangchuk, 2004). Despite the dhole population recovering and re-establishing itself in Bhutan from the late 1990s with some probable recolonization from the neighboring Indian states of Assam and West Bengal (Wangchuk, 2004), little is known on its current distribution in Bhutan (Namgyal and Thinley, 2017). The dhole is still not listed as a protected species in Schedule I of Bhutan's Forests and Nature Conservation Act despite being globally endangered (Namgyal and Thinley, 2017). Therefore, it is important to ascertain dhole distribution in Bhutan at the landscape level to promote efficient research and planning decisions (Guisan et al., 2013). The only previous attempt to discern dhole distribution in Bhutan was by Wangchuk (2004) who interviewed 67 field forestry staff members and surveyed residents in 18 villages across seven dzongkhags (districts) of Gasa, Paro, Punakha, Thimphu, Trongsa, Wangduephodrang, and Zhemgang (Figure 1). However, no distribution map was produced in addition to the anecdotes of localities where dholes were present or absent. As such, there is no information on how well dhole habitats are protected within Bhutan's protected area network.
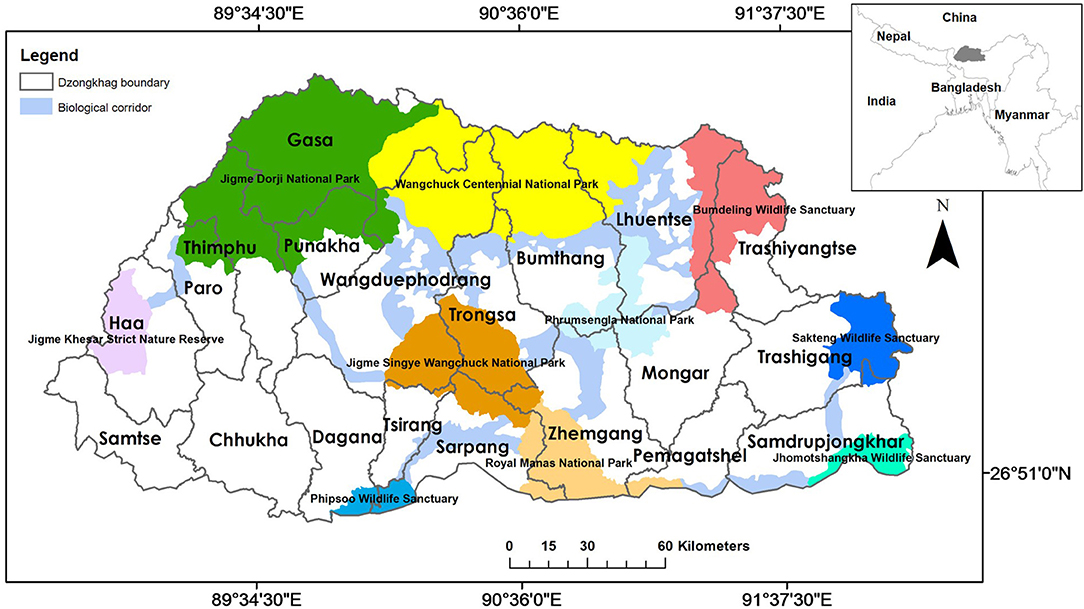
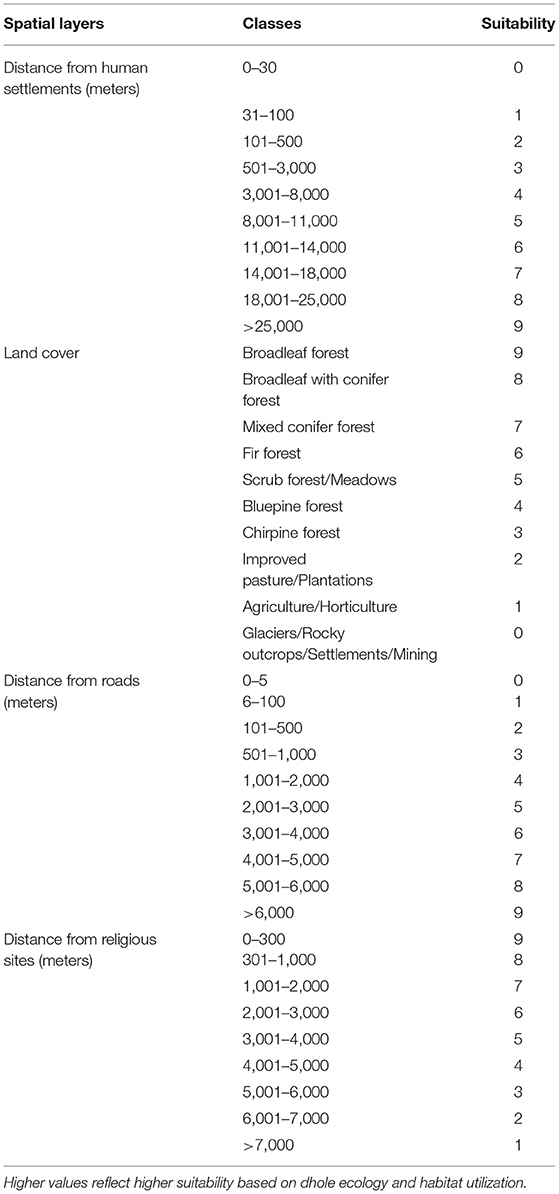
Figure 1. Protected areas, biological corridors, and dzongkhags (districts) of Bhutan. The inset depicts Bhutan's location relative to its neighboring countries in South Asia. Source: Environmental Systems Research, Inc.
An adequate knowledge of distribution, including associated environmental and anthropogenic variables, further enables appropriate modeling to predict additional areas where dholes are likely to occur, both within and outside protected areas, as recently demonstrated by Thinley et al. (2021) for the tiger in Bhutan. Because dholes are prominently linked to livestock predation in Bhutan (Thinley et al., 2011; Katel et al., 2015; Rajaratnam et al., 2016), determining and modeling their distribution further enables an identification of potential human-wildlife conflict hotspots to prioritize mitigation efforts (Sharma et al., 2020). Juxtaposing conflict hotspots and habitat protection against the current and predicted dhole distribution can provide the spatial framework to develop an appropriate dhole conservation plan for Bhutan.
In this study, we investigated dhole distribution based on current presence records across Bhutan and assessed the protection and connectivity of its habitat. Specifically, we modeled potential dhole distribution and suitable habitat coverage in Bhutan's protected areas (PAs) and interconnecting biological corridors (BCs). Based on Bhutan's strong conservation policy and high proportion of land dedicated to nature conservation, we anticipated dholes to be extant in their habitats well-represented in PAs and BCs.
Materials and Methods
Study Area
Bhutan (Figure 1) is one of the least-populated countries of Asia with only 735,553 people (NSB, 2017). Situated in the eastern Himalayas (Figure 1 inset) and administratively divided into 20 dzongkhags (district), Bhutan encompasses an area of 38,394 km2 (NSB, 2018) and is well-known for its rich biodiversity. Approximately 71% of the country is forested (FRMD, 2016) and encompasses 11,248 species of plants and animals, including 129 mammal species (NBC, 2017). This mammalian community includes four wild canid species, namely dhole, Tibetan wolf (Canis lupus chanco), golden jackal (Canis aureus), and red fox (Vulpes vulpes) (Wangchuk et al., 2004). The country's Constitution mandates the government to maintain a minimum of 60% forest cover for eternity. As such, more than half of the country (51%) is designated as a protected area network (Figure 1) comprising protected areas and interconnecting biological corridors (Dorji et al., 2019). The country's topography is mountainous and highly rugged with a pronounced elevation range from 97 m above sea level (a.s.l.) in the southern foothills to 7,750 m a.s.l. in the greater Himalayas near the Chinese border (Tshering et al., 2020).
Modeling Potential Dhole Distribution or Habitat
We modeled potential dhole distribution and habitat suitability in Bhutan using MaxEnt program version 3.4.3 (Phillips et al., 2020) which uses a widely employed maximum entropy method of modeling species distribution based on presence-only data (Phillips et al., 2006). In MaxEnt, georeferenced occurrence points of a target species are associated with environmental variables to yield a spatial layer representing the most widespread probability of its presence, given constraints imposed by these environmental layers (Elith et al., 2011). The resulting layer also constitutes a habitat suitability layer of a species and depicts its realized niche, which is a subset of its fundamental niche (Phillips et al., 2006). MaxEnt modeling was chosen because our dataset solely comprised dhole presence points, thereby, precluding the use of a boosted regression tree based on additional absence data (Yu et al., 2020) and species occupancy modeling based on repeated surveys (MacKenzie et al., 2006).
We utilized a database of 721 georeferenced dhole occurrence points in Bhutan from 2014 to 2019 based on cumulative pooling of dhole records from: (a) a 2014–2015 nationwide camera trapping survey on tigers (Thinley et al., 2015) up to 4,500 m a.s.l.; (b) a 2015–2016 nationwide camera trapping survey on snow leopards (Lham et al., 2016) between 3,500 and 5,500 m a.s.l.; (c) camera trap and sign surveys across protected areas between 2014 and 2019; (d) SMART (Spatial Monitoring and Reporting Tool) patrolling reports submitted by wildlife personnel between 2015 and 2019; and (e) camera trapping of wildlife in catchment studies (Thinley et al., 2020) in Wangchu and Kholongchhu sub-basins between 2018 and 2019.
Following Thinley et al. (2021), we selected eight environmental (geophysical and anthropogenic) variables deemed to influence dhole occurrence based on its ecology (Johnsingh and Acharya, 2013), particularly prey selectivity (Wangchuk, 2004; Wang and Macdonald, 2009; Thinley et al., 2011) and habitat use (Aryal et al., 2015; Namgyal and Thinley, 2017). All environmental variables were processed in ArcMap version 10.7.1 where spatial layers were classified into 10 categories based on suitability to dholes and standardized with a spatial resolution of 30 ×30 m cell corresponding to the spatial resolution of the Digital Elevation Model (DEM), Transverse Mercator projection, DRUKREF 03 coordinate system, and geographic extent of Bhutan. Dhole points co-occurring within the same 30 ×30 m cell were omitted to minimize errors from spatial autocorrelation (Kanagaraj et al., 2011).
Categorization of spatial layers for environmental variables was based on Thinley et al. (2021) as follows:
1) Elevation: reclassified from Bhutan's Digitial Elevation Model (Jarvis et al., 2006) into 10 classes such that lower elevations were ranked more suitable than higher elevations (Table 1);
2) Slope: extracted from the DEM such that lower slope classes were ranked more suitable than higher classes (Table 1);
3) Prey richness: prey densities are ecological determinants of carnivore densities (Karanth et al., 2004) and dholes generally prey on medium to large ungulates (Wang and Macdonald, 2009; Kamler et al., 2020; Srivathsa et al., 2020). We, therefore, merged the distribution of nine potential wild prey species (Wangchuk, 2004; Wang and Macdonald, 2009; Thinley et al., 2011)—wild pig, sambar (Rusa unicolor), muntjac (Muntiacus muntjac), Himalayan goral (Naemorhedus goral), serow (Capricornis sumatraensis), hog deer (Axis porcinus), alpine musk deer (Moschus chrysogaster), gaur (Bos gaurus), and Bhutan takin (Budorcas taxicolor whitei). Spatial layers were obtained from the Field Guide to Mammals of Bhutan (Wangchuk et al., 2004), rasterized, and reclassified with higher suitability values assigned to areas with higher prey richness (Table 1);
4) Distance from rivers and streams: rasterized from the Drainage Map of Bhutan based on Euclidean distance; areas closer to rivers and streams were assigned higher suitability values than those farther away (Table 1);
5) Distance from human settlements: rasterized from the Settlement Map of Bhutan 2006 (OCC, 2005) based on Euclidean distance; assigned suitability scores increasing with distance away from the settlements (Table 2), because dholes avoid human settlements and presence (Srivathsa et al., 2014);
6) Land cover: reclassified from the Land-use Map of Bhutan 2011 (NSSC and NSSC, 2011) with higher suitability values assigned to forested areas compared to open areas (Table 2), because the dhole primarily inhabits forested areas (Srivathsa et al., 2014);
7) Distance from roads: rasterized from the latest Road Map of Bhutan obtained from the Department of Roads based on Euclidean distance; assigned suitability scores increasing with the distance away from roads (Table 2);
8) Distance from religious sites: digitized areas occupied by Buddhist temples, monasteries, meditation centers, and other religiously significant areas from Google EarthTM; areas were rasterized with an inverse relationship between suitability values and Euclidean distance (Table 2), as demonstrated by snow leopards finding safe sanctuaries near Buddhist monasteries in Tibet (Li et al., 2013).
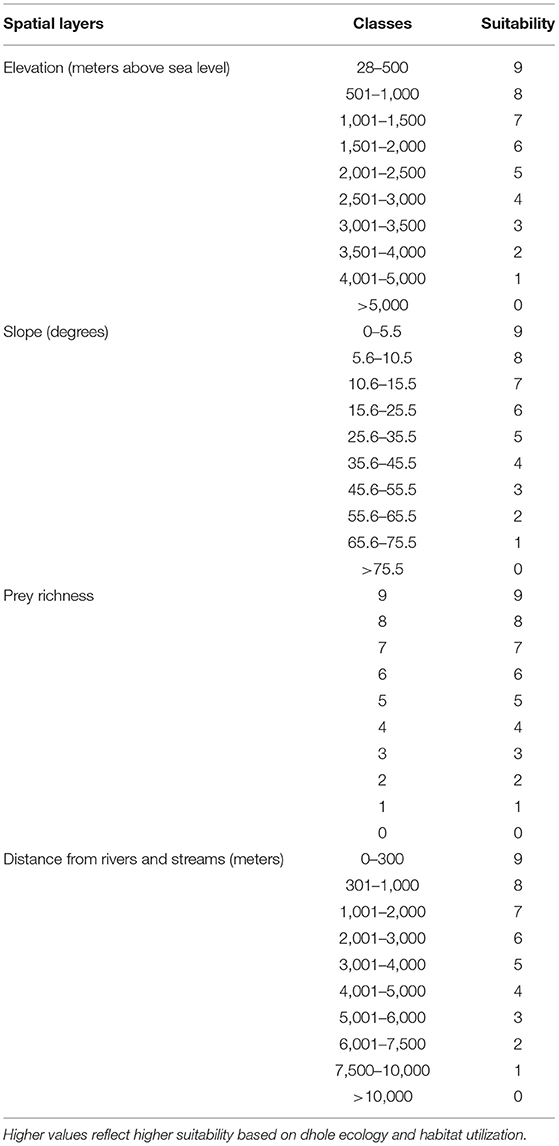
Table 1. Geophysical and biological variables with suitability values for dholes (Cuon alpinus) in Bhutan.
Following Jenks et al. (2012), Namgyal and Thinley (2017), and Thinley et al. (2021), in our generated MaxEnt model we used the default setting of 500 iterations with a convergence threshold of 0.00001, a regularization multiplier of 1, and a maximum background of 10,000 background pseudo-absence points with 50% random tests and 10 replicates. We assessed model performance using AUC (Area under the Receiver Operating Curve) whereby values ≤ 0.5 indicate very poor fit, >0.5 indicate good fit, and equal to 1 indicate perfect fit (Fielding and Bell, 1997). The resulting MaxEnt probability surface was exported to ArcMap and reclassified into the probabilities of dhole occurrence (“highly probable,” “moderately probable,” and “not probable”). These probabilities correspond to the suitability surfaces (“highly suitable,” “moderately suitable,” and “unsuitable”) because a relatively high number of dhole occurrence points collected across a wide range of environmental conditions optimally reflects the dhole's fundamental niche. Following Thinley et al. (2021), we used the Jenks (natural breaks) classification in ArcMap to classify model surface values from 0 to 0.254 as not probable/unsuitable; 0.255 to 0.461 as moderately suitable/probable; and >0.461 as highly suitable/probable.
Assessing Dhole Protection and Habitat Connectivity
We overlaid protected area (PA) and biological corridor (BC) layers on the dhole suitability layer to assess how much of the habitat that we deemed suitable for dholes is encompassed within PAs (assessing dhole protection) and corridors (assessing dhole habitat connectivity between protected areas). Using the Field Calculator tool in ArcMap, we computed areas (km2) for each of the dhole suitability classes falling within and outside PAs and BCs.
Results
Dhole Distribution
Dholes were distributed throughout Bhutan across all 20 districts and in all PAs and BCs (Figure 2), and occurred within a broad elevation range from 110 m a.s.l. in Royal Manas National Park (RMNP) in the southern foothills to 4,980 m a.s.l. in Jigme Dorji National Park (JDNP) in the upper Himalayas (Figure 1). They were also present in almost all habitat types ranging from sub-tropical forests in the lowlands to alpine meadows in the uplands. The highest concentration of dhole occurrence records was observed in JDNP whereas the least was recorded in Wangchuck Centennial National Park (WCNP) (Figure 2).
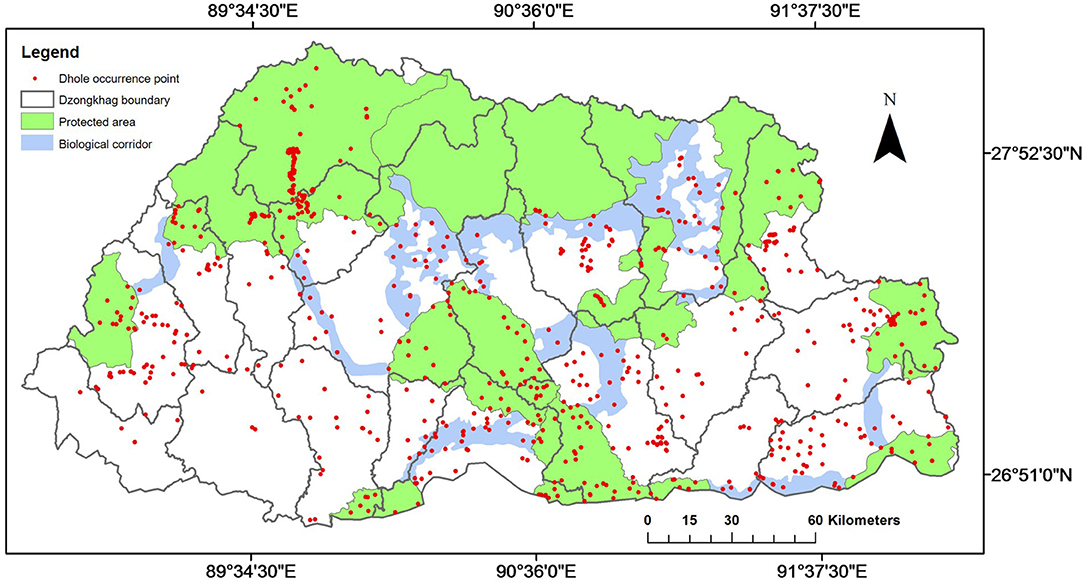
Figure 2. Dhole (Cuon alpinus) presence records overlaid on protected areas, biological corridors, and dzongkhags (districts) of Bhutan.
Suitable Areas for Dholes
The MaxEnt model (AUC = 0.73 for training data; 0.72 for test data) predicted that suitable area for dholes covered 72% (27,634 km2) of Bhutan (Figure 3; Table 3). This comprised 31% (11,899 km2) of highly suitable area and 41% (15,735 km2) of moderately suitable area (Figure 3; Table 3). Among the eight environmental variables, model prediction (or gain) was maximally influenced by slope (26.4%), followed by distance from human settlement (24.3%), elevation (16.5%), and land cover (13.1%; Table 4). Distance from water bodies (Table 4) contributed least (1%) to model prediction. Overall, suitable area for dholes coincided with flat and moderately flat area situated further away from human settlements in forested areas below 5,000 m a.s.l. The remaining 28% (10,760 km2) of modeled area was unsuitable for dholes (Figure 3; Table 3) and overlapped with steep areas closer to human settlements and roads, and with areas above 5,000 m a.s.l. which were either too cold or permanently covered with snow and glaciers.
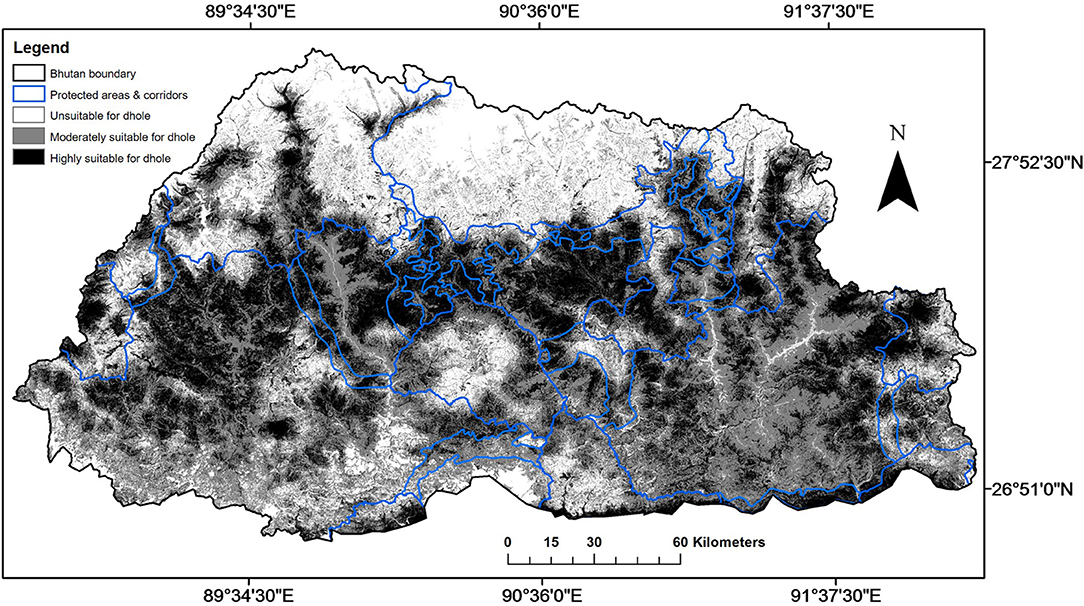
Figure 3. The extent of highly suitable, moderately suitable, and unsuitable areas for dholes (Cuon alpinus) in Bhutan as generated by the MaxEnt model.
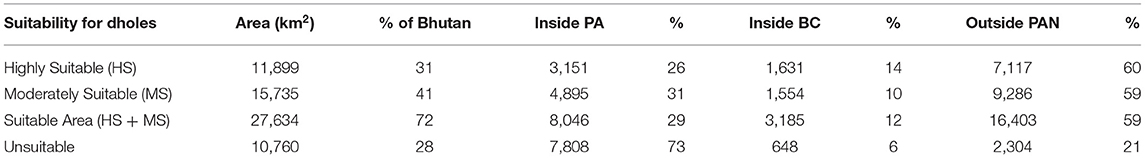
Table 3. Suitable and unsuitable areas for dholes (Cuon alpinus) in Bhutan distributed inside and outside the protected area network (PAN) comprising protected areas (PA) and biological corridors (BC).
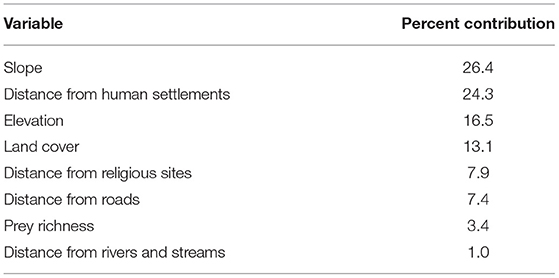
Table 4. Relative contributions of the input variables to the MaxEnt model of dhole (Cuon alpinus) distribution/habitat suitability in Bhutan.
Dhole Protection and Habitat Connectivity
When PA and BC layers were overlaid on the suitability layer for the dhole, PAs encompassed only 29% (8,046 km2) of suitable area for dholes in Bhutan (Figure 3; Table 3), of which, 39% (3,151 km2) was highly suitable and 61% (4,895 km2) was moderately suitable. Similarly, BCs encompassed merely 12% (3,185 km2) of suitable area for dholes (Figure 3; Table 3), which comprised 51% (1,631 km2) of highly suitable and 49% (1,554 km2) of moderately suitable area. In contrast, a substantial proportion of suitable area for dholes (59%; 16,403 km2) occurred outside the protected area network (Figure 3; Table 3), of which, 43% (7,117 km2) and 57% (9,286 km2) were highly and moderately suitable for dholes, respectively.
Discussion
Dhole Distribution
We mapped the first-ever nation-wide distribution of dholes in Bhutan (Figure 2), which also constituted the first study of its kind amongst dhole range countries in the Himalayan Mountains. Dholes were present in high elevation alpine meadows and screes, and their distribution overlapped with “Vulnerable” snow leopards which are known to range between 3,400 and 5,186 m a.s.l. (Thinley et al., 2016). Dholes are reported to be sympatric with snow leopards in the arid region of the Altun Mountains in western China (Xue et al., 2015). We documented the highest elevation occurrence (4,980 m a.s.l.) for dholes in Bhutan, which also constitutes the highest across its entire distribution range, thus representing an uppermost range limit for dholes. Whether this range extreme is attributed to climate change or prey availability, including livestock such as yak (Bos grunniens), needs further investigation. To date, high altitude livestock predation in Bhutan has been mainly attributed to snow leopards (Rajaratnam et al., 2016). Further investigation is required on a potential dietary overlap with the snow leopard (Namgyal and Thinley, 2017) to ascertain the competitive impact of dholes on this iconic flagship carnivore species for the montane Himalayas.
As anticipated, we also discovered that the dhole has the widest distribution amongst large carnivores in Bhutan. It was previously not reported in the three eastern districts of Trashigang, Samdrupjongkhar and Pemagatashel (Wangchuk, 2004), but is now confirmed to be present in all 20 districts of Bhutan. Its wide distribution also indicates the possibility of frequent conflicts with humans due to livestock depredation (Namgyal and Thinley, 2017) given that dholes are principal livestock predators in western Bhutan (Katel et al., 2015; Tshering and Thinley, 2017) and neighboring Arunachal Pradesh in India (Lyngdoh et al., 2014). Increased prevalence of livestock predation by dholes is a distinct possibility in Jigme Dorji National Park (Thinley et al., 2011) which harbored the highest concentrations of dhole occurrence records. Livestock loss presents a significant socioeconomic setback for rural farmers in Bhutan's predominantly agrarian society because the loss of yak results in sizable income loss to upland pastoralists, while the loss of cattle compromises agricultural production and nutrition for lowland agro-pastoralists (Sangay and Vernes, 2008; Rajaratnam et al., 2016).
In relation to agricultural crop loss, wild pigs are responsible for more crop damage compared to any other species in Bhutan. Such losses have major negative impacts on subsistence farmers throughout Bhutan. In fact, the crop damage caused by wild pigs is so severe that wild pigs are considered a national pest with significant funding allocated by the government to control this species. Consequently, the controlling effect by dhole on wild pig populations (Wangchuk, 2004) suggests that increasing the number and expanding the distribution of dholes in Bhutan may lead to lowered numbers of wild pig, which ultimately, would benefit local farmers.
Slope was the top contributor to dhole distribution and habitat suitability (Table 4), indicating the influence of Himalayan rugged topography on dhole distribution in Bhutan. Aryal et al. (2015) also determined slope to be the most significant factor influencing dhole presence and occupancy in Nepal's Dhorpatan Hunting Reserve, whereby dholes used gently sloped land more than steep areas. This is likely related to the cursorial hunting strategy of dholes which is more effective on flatter terrain and gentle slopes compared to more rugged terrain or areas with steeper slopes (Kamler et al., 2012). In contrast to Jenks et al. (2012), contribution of prey richness to modeled potential dhole distribution in our study was minimal and much lower than that of land cover. This is possibly because forested habitats throughout Bhutan naturally harbor a high richness of potential prey species (Wangchuk et al., 2004). We do, however, acknowledge the greater role of prey density in influencing dhole distribution. This variable was excluded due to the lack of its spatial layer, which constitutes a limitation to our study.
Dhole Conservation Mismatch
We assessed whether protected areas and biological corridors are adequate for conserving dhole habitats. Although protected areas in Bhutan were designated for multiple species, we found them inadequate for protecting and connecting wide-ranging species like the dhole, similar to that observed for the tiger (Thinley et al., 2021). Contrary to expectations, only 29% of total suitable habitat for dholes was available in protected areas, reflecting an increased vulnerability of the species to further human persecution and habitat loss through livestock depredation and land-use changes outside protected areas. Similarly, only 30% of potential habitat for dholes in Thailand was encompassed within protected areas (Jenks et al., 2012) while 41% of areas occupied by dhole were inside protected wildlife reserves in the Western Ghats landscape within Karnataka, India (Srivathsa et al., 2014). As such, the current level of landscape protection may be insufficient to support functional dhole meta-populations (Bargelt et al., 2020) across Bhutan as equally demonstrated by the meager 33–35% of suitable areas captured by Bhutan's protected areas for the wide ranging tiger (Thinley et al., 2021).
Dhole as a Potential Umbrella Species
Dholes are also estimated to require five times more land area than large-bodied carnivores such as tigers (Kamler et al., 2012), mainly because of the social structure of populations living in exclusive territories (Johnsingh, 1982), unlike solitary tigers with overlapping territories (Carter et al., 2015). Large space requirements in conjunction with a hypercarnivorous diet (Van Valkenburgh, 1991) make dholes more vulnerable to extirpation, as evidenced by their disappearance from more reserves than tigers (Woodroffe and Ginsberg, 1998). Most reserves in Asia are typically focused on conserving umbrella species such as tigers (e.g., Wikramanayake et al., 2011) and greater one-horned rhinoceros (Rhinoceros unicornis) in Nepal (e.g., Aryal et al., 2017) to highlight conservation efforts. As exemplified above, umbrella species are typically large-bodied animals which require large areas to ensure species persistence. They are good surrogates for overall biodiversity but are more sensitive than other species to human activities, ecosystem changes, and habitat destruction whilst having the largest land requirements and most stringent ecological needs (Woodroffe and Ginsberg, 2005). Recently, Kaszta et al. (2020) advocated habitat prioritization for the medium-sized clouded leopard (Neofelis nebulosa) as a good indicator and focal species to address limited resources for immediate biodiversity conservation actions to conserve forest ecosystems and forest-dependent biodiversity in Southeast Asia. Based on this premise and the results of our study, we suggest that the dhole, given its unusually high requirements for space and prey, could constitute a more effective umbrella species in Asia and a driver for future designation of protected areas and/or expansion of current reserves with dhole metapopulations.
Dhole as a Keystone Species
In most terrestrial ecosystems, large carnivores have been identified as “keystone species” (Terborgh et al., 1999; Ripple et al., 2014) based on the premise that keystone species exert disproportionately larger influence on an ecosystem relative to their abundance (Power et al., 1996). Although the top-down effects of dholes are loosely described (see Thinley et al., 2018), previous research showed that dholes killed more ungulates than sympatric leopards (Panthera pardus) and tigers (Venkataraman, 1999), indicating that dholes have a greater impact on ungulate numbers relative to other large Asian carnivore species. As such, there may be a parallel to pack-living wolves in North America which cause trophic cascades primarily via predation on large ungulates which, in turn, affects vegetation growth patterns across the landscape (Ripple et al., 2001; Beschta and Ripple, 2009). Further repercussions from the decline of dholes are exemplified in Bhutan, whereby wild pig populations thrived and increased crop depredation after dholes were exterminated from many areas (Wangchuk, 2004). The dhole may, therefore, be a top keystone carnivore in Bhutan, and probably across its range. Consequently, their presence may have a greater impact on biodiversity in Asia, compared to other large carnivores. Thus, if protected areas in Asia are to preserve entire ecosystem functions, adequate protection and conservation of dholes should be equally considered.
Conservation Implications
Based on our study, Bhutan is a stronghold for dholes in the eastern Himalayas due to their widespread distribution and availability of large tracts of suitable habitat. In order to ensure long-term survival of the dhole in Bhutan, we recommend replicating the recommendations of Thinley et al. (2021) for tigers in terms of readjusting PA boundaries to encompass prime dhole habitats and extending dhole conservation efforts outside the PAs. However, local people exhibit resentment to dholes and their conservation due to persistent dhole-related livestock predation (Katel et al., 2015). This is principally driven by socio-economic losses because pastoral communities experiencing human-carnivore conflict tend to have low income with low tolerance to carnivores and their conservation (Ahmad et al., 2016). As such, there is likelihood for retaliation against dholes reminiscent of historic poisoning efforts against the species in the 1970–80s (Wangchuk, 2004). Therefore, dhole conservation efforts both within and outside the PAs need to incorporate efforts to improve livestock husbandry like grazing livestock in and around villages, including stall-feeding and cooperative herding of livestock in forests during the day (Katel et al., 2015). Tshering and Thinley (2017) further recommended stock improvement, fodder development, pasture development, and livestock insurance schemes to reduce livestock predation by dholes, while Sangay and Vernes (2008) advocated non-grazing of livestock in depredation hotspots. Dhole-specific livestock insurance schemes are also feasible to alleviate socio-economic loss from livestock predation by dholes. Local people should also be educated about the positive impacts that dholes have on ecosystems, including suppression of major crop-destroying species such as wild pigs. Based on this control of crop depredators, rural farmers might be inclined to adopt livestock protection measures against dholes as well. This will alleviate livestock losses in addition to a reduction in crop damage. We further advocate the listing of the dhole in Schedule I of Bhutan's Forests and Nature Conservation Act, urging for increased legal protection of this globally endangered canid. Lastly, we recommend other dhole range countries to conduct a similar study on the role of protected areas in conserving dhole populations to ensure species viability across its distribution area.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The study was reviewed and approved by Department of Forests and Park Services, Bhutan.
Author Contributions
PT: conceptualization, data collection, data curation, formal analysis, and writing—original draft. RR: conceptualization, validation, writing—review, and editing. JK: validation, writing—review, and editing. CW: data collection, data curation, formal analysis, and writing—review. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Royal Government of Bhutan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was conducted as part of the annual performance agreement (APA) of the Department of Forests and Park Services (DoFPS) that was duly approved by the Royal Government of Bhutan (reference number UWICER/ADM-18/2019/Camp Thimphu dated 10/01/2019). We thank Lobzang Dorji, the Director of DoFPS, for administrative approval and support. We also thank relevant wildlife officials and Chief Forest Officers of all territorial forest divisions and protected areas for sharing dhole occurrence data.
References
Ahmad, S., Hameed, S., Ali, H., Khan, T. U., Mehmood, T., and Nawaz, M. A. (2016). Carnivores' diversity and conflicts with humans in Musk Deer National Park, Azad Jammu and Kashmir, Pakistan. Eur. J. Wildl. Res. 62, 565–576. doi: 10.1007/s10344-016-1029-6
Aryal, A., Acharya, K. P., Shrestha, U. B., Dhakal, M., Raubenhiemer, D., and Wright, W. (2017). Global lessons from successful rhinoceros conservation in Nepal. Conserv. Biol. 31, 1494–1497. doi: 10.1111/cobi.12894
Aryal, A., Panthi, S., Barraclough, R. K., Bencini, R., Adhikari, B., Ji, W., et al. (2015). Habitat selection and feeding ecology of dhole (Cuon alpinus) in the Himalayas. J. Mammal. 96, 47–53. doi: 10.1093/jmammal/gyu001
Bargelt, L., Fortin, M.-J., and Murray, D. L. (2020). Assessing connectivity and the contribution of private lands to protected area networks in the United States. PLoS ONE 15:e0228946. doi: 10.1371/journal.pone.0228946
Bashir, T., Bhattacharya, T., Poudyal, K., Roy, M., and Sathyakumar, S. (2014). Precarious status of the endangered dhole Cuon alpinus in the high elevation Eastern Himalayan habitats of Khangchendzonga Biosphere Reserve, Sikkim, India. Oryx 48, 125–132. doi: 10.1017/S003060531200049X
Beschta, R. L., and Ripple, W. J. (2009). Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142, 2401–2414. doi: 10.1016/j.biocon.2009.06.015
Brown, D.E., (ed.). (1983). The Wolf in the Southwest: The Making of an Endangered Species. Tucson, AZ: University of Arizona Pres.
Carter, N., Levin, S., Barlow, A., and Grimm, V. (2015). Modeling tiger population and territory dynamics using an agent-based approach. Ecol. Model. 312, 347–362. doi: 10.1016/j.ecolmodel.2015.06.008
Datta, A., Anand, M. O., and Naniwadekar, R. (2008). Empty forests: large carnivore and prey abundance in Namdapha National Park, north-east India. Biol. Conserv. 141, 1429–1435. doi: 10.1016/j.biocon.2008.02.022
Din, J., Hameed, S., Shah, K., Khan, M., Khan, S., Ali, M., et al. (2013). Assessment of canid abundance and conflict with humans in the Hindu Kush Mountain Range of Pakistan. Wildl. Biol. Pract. 9, 20–29. doi: 10.2461/wbp.2013.9.5
Dorji, S., Rajaratnam, R., and Vernes, K. (2019). Mammal richness and diversity in a Himalayan hotspot: the role of protected areas in conserving Bhutan's mammals. Biodivers. Conserv. 28, 3277–3297. doi: 10.1007/s10531-019-01821-9
Elith, J., Phillips, S. J., Hastie, T., Dudi'k, M., En Chee, Y., and Yates, C. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Fielding, A. H., and Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. doi: 10.1017/S0376892997000088
Fleming, P. J., Nolan, H., Jackson, S. M., Ballard, G.-A., Bengsen, A., Brown, W. Y., et al. (2017). Roles for the Canidae in food webs reviewed: where do they fit? Food Webs 12, 14–34. doi: 10.1016/j.fooweb.2017.03.001
FRMD (Forest Resources Management Divsion) (2016). National Forest Inventory Report (Volume I). Thimphu: Forest Resources Management Division, Department of Forests and Park Services, Ministry of Agriculture and Forests.
Guisan, A., Tingley, R., Baumgartner, J. B., Naujokaitis-Lewis, I., Sutcliffe, P. R., Tulloch, A. I., et al. (2013). Predicting species distributions for conservation decisions. Ecol. Lett. 16, 1424–1435. doi: 10.1111/ele.12189
Jarvis, A., Reuter, H. I., Nelson, A., and Guevara, E. (2006). International Centre for Tropical Agriculture (CIAT). Available online at: http://srtm.csi.cgiar.org (accessed April 12, 2016).
Jenks, K. E., Kitamura, S., Lynam, A. J., Ngoprasert, D., Chutipong, W., Steinmetz, R., et al. (2012). Mapping the distribution of dholes, Cuon alpinus (Canidae, Carnivora), in Thailand. Mammalia 76, 175–184. doi: 10.1515/mammalia-2011-0063
Johnsingh, A. (1982). Reproductive and social behaviour of the dhole, Cuon alpinus (Canidae). J. Zool. 198, 443–463. doi: 10.1111/jzo.1982.198.4.443
Johnsingh, A. J. T., and Acharya, B. (2013). “Asiatic wild dog (Cuon alpinus),” in Mammals of South Asia, Vol. 1, eds A. J. T. Johnsingh and M. Manjreakar (Noida: Universities Press (India) Private Ltd.), 392–415.
Johnson, A., Vongkhamheng, C., Hedemark, M., and Saithongdam, T. (2006). Effects of human-carnivore conflict on tiger (Panthera tigris) and prey populations in Lao PDR. Anim. Conserv. 9, 421–430. doi: 10.1111/j.1469-1795.2006.00049.x
Kamler, J. F., Johnson, A., Vongkhamheng, C., and Bousa, A. (2012). The diet, prey selection, and activity of dholes (Cuon alpinus) in northern Laos. J. Mammal. 93, 627–633. doi: 10.1644/11-MAMM-A-241.1
Kamler, J. F., Songasen, N., Jenks, K., Srivathsa, A., Sheng, L., and Kunkel, K. E. (2015). Cuon alpinus. The IUCN Red List of Threatened Species. Version 2015: e.T5953A72477893. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T5953A72477893.en (accessed April 01, 2020).
Kamler, J. F., Thatdokkham, K., Rostro-García, S., Bousa, A., Caragiulo, A., Crouthers, R., et al. (2020). Diet and prey selection of dholes in evergreen and deciduous forests of Southeast Asia. J. Wildl. Manag. 84, 1396–1405. doi: 10.1002/jwmg.21931
Kanagaraj, R., Wiegand, T., Kramer-Schadt, S., Anwar, M., and Goyal, S. P. (2011). Assessing habitat suitability for tiger in the fragmented Terai Arc Landscape of India and Nepal. Ecography 34, 970–981. doi: 10.1111/j.1600-0587.2010.06482.x
Karanth, K. K., Nichols, J. D., Hines, J. E., Karanth, K. U., and Christensen, N. L. (2009). Patterns and determinants of mammal species occurrence in India. J. Appl. Ecol. 46, 1189–1200. doi: 10.1111/j.1365-2664.2009.01710.x
Karanth, K. U., Nichols, J. D., Kumar, N. S., Link, W. A., and Hines, J. E. (2004). Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl. Acad. Sci. U.S.A. 101, 4854–4858. doi: 10.1073/pnas.0306210101
Kaszta, Z., Cushman, S., and Macdonald, D. (2020). Prioritizing habitat core areas and corridors for a large carnivore across its range. Anim. Conserv. 23, 607–616. doi: 10.1111/acv.12575
Katel, O. N., Pradhan, S., and Schmidt-Vogt, D. (2015). A survey of livestock losses caused by Asiatic wild dogs, leopards and tigers, and of the impact of predation on the livelihood of farmers in Bhutan. Wildl. Res. 41, 300–310. doi: 10.1071/WR14013
Kawanishi, K., and Sunquist, M. E. (2008). Food habits and activity patterns of the Asiatic golden cat (Catopuma temminckii) and dhole (Cuon alpinus) in a primary rainforest of Peninsular Malaysia. Mammal Study 33, 173–177. doi: 10.3106/1348-6160-33.4.173
Lamb, C. T., Ford, A. T., McLellan, B. N., Proctor, M. F., Mowat, G., Ciarniello, L., et al. (2020). The ecology of human–carnivore coexistence. Proc. Natl. Acad. Sci. U.S.A. 117, 17876–17883. doi: 10.1073/pnas.1922097117
Leki T.hinley, Rajaratnam, P., and Shrestha, R. (2018). Establishing baseline estimates of blue sheep (Pseudois nayaur) abundance and density to sustain populations of the vulnerable snow leopard (Panthera uncia) in Western Bhutan. Wildl. Res. 45, 38–46. doi: 10.1071/WR16218
Lham, D., Thinley, P., Wangchuk, S., Wangchuk, N., Lham, K., Namgay, T., et al. (2016). National Snow Leopard Survey 2014–2016 (Phase II): Camera Trap Suvey for Population Estimation. Thimphu: Department of Forests and Parks Services, Ministry of Agriculture and Forests.
Li, J., Wang, D., Yin, H., Zhaxi, D., Jiagong, Z., Schaller, G. B., et al. (2013). Role of Tibetan Buddhist monasteries in snow leopard conservation. Conserv. Biol. 28, 87–94. doi: 10.1111/cobi.12135
Lyngdoh, S., Gopi, G. V., Selvan, K. M., and Habib, B. (2014). Effect of interactions among ethnic communities, livestock, and wild dogs (Cuon alpinus) in Arunachal Pradesh, India. Eur. J. Wildl. Res. 60, 771–780. doi: 10.1007/s10344-014-0846-8
MacKenzie, D. L., Nichols, J. D., Royle, J. A., Pollok, K. H., Bailey, L. L., and Hines, J. E. (2006). Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Cccurrence. London: Academic Press.
Mech, L. D. (1995). The challenge and opportunity of recovering wolf populations. Conserv. Biol. 9, 270–278. doi: 10.1046/j.1523-1739.1995.9020270.x
Namgyal, C., and Thinley, P. (2017). Distribution and habitat use of the endangered Dhole Cuon alpinus (Pallas, 1811) (Mammalia: Canidae) in Jigme Dorji National Park, western Bhutan. J. Threat. Taxa 9, 10649–10655. doi: 10.11609/jott.3091.9.9.10649-10655
National Biodiversity Centre (NBC) (2017). Biodiversity Statistics of Bhutan 2017: A Preliminary Baseline. Thimphu: National Biodiversity Centre.
National Statistics Bureau (NSB) (2017). Population and Housing Census of Bhutan 2017. Thimphu: National Statistics Bureau, Royal Government of Bhutan.
National Statistics Bureau (NSB) (2018). Bhutan at a Glance 2018. Thimphu: National Statistics Bureau.
Newsome, T. M., and Ripple, W. J. (2015). A continental scale trophic cascade from wolves through coyotes to foxes. J. Anim. Ecol. 84, 49–59. doi: 10.1111/1365-2656.12258
NSSC and PPD. (2011). Bhutan Land Cover Assessment 2010. Thimphu: National Soil Service Centre, Department of Agriculture, MoAF.
OCC (Office of the Census Commissioner) (2005). Population and Housing Census of Bhutan 2005. Thimphu: National Statistics Bureau.
Papeş, M., and Gaubert, P. (2007). Modelling ecological niches from low numbers of occurrences: assessment of the conservation status of poorly known viverrids (Mammalia, Carnivora) across two continents. Divers. Distrib. 13, 890–902. doi: 10.1111/j.1472-4642.2007.00392.x
Phillips, S. J., Anderson, R. P., and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Dudík, M., and Schapire, R. E. (2020). Maxent Software for Modeling Species Niches and Distributions (Version 3.4.3). Available online at: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed November 13, 2020).
Power, M. E., Tilman, D., Estes, J. A., Menge, B. A., Bond, W. J., Mills, L. S., et al. (1996). Challenges in the quest for keystones: identifying keystone species is difficult—but essential to understanding how loss of species will affect ecosystems. BioScience 46, 609–620. doi: 10.2307/1312990
Punjabi, G. A., Edgaonkar, A., and Srivathsa, A. (2017). Distribution of the dhole in its northern range limits in the western fgats India. Canid Biol. Conserv. 20, 7–13.
Rahman, D. A., Rianti, P., Muhiban, M., Muhtarom, A., Rahmat, U. M., Santosa, Y., et al. (2018). Density and spatial partitioning of endangered sympatric Javan leopard (Felidae) and dholes (Canidae) in a tropical forest landscape. Folia Zool. 67, 207–219. doi: 10.25225/fozo.v67.i3-4.a8.2018
Rajaratnam, R., Vernes, K., and Sangay, T. (2016). “A review of livestock predation by large carnivores in the Himalayan Kingdom of Bhutan,” in Problematic Wildlife: A Cross-Disciplinary Approach, ed F. M. Angelici (Cham: Springer), 143–171.
Rao, M., Myint, T., Zaw, T., and Htun, S. (2005). Hunting patterns in tropical forests adjoining the Hkakaborazi National Park, north Myanmar. Oryx 39, 292–300. doi: 10.1017/S0030605305000724
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world's largest carnivores. Science 343:1241484. doi: 10.1126/science.1241484
Ripple, W. J., Larsen, E. J., Renkin, R. A., and Smith, D. W. (2001). Trophic cascades among wolves, elk, and aspen on Yellowstone National Park's northern range. Biol. Conserv. 102, 227–234. doi: 10.1016/S0006-3207(01)00107-0
Sangay, T., and Vernes, K. (2008). Human-wildlife conflict in the Kingdom of Bhutan: patterns of livestock predation by large mammalian carnivores. Biol. Conserv. 141, 1272–1282. doi: 10.1016/j.biocon.2008.02.027
Sharma, P., Chettri, N., Uddin, K., Wangchuk, K., Joshi, R., Tandin, T., et al. (2020). Mapping human–wildlife conflict hotspots in a transboundary landscape, Eastern Himalaya. Glob. Ecol. Conserv. 24:e01284. doi: 10.1016/j.gecco.2020.e01284
Sillero-Zubiri, C. (2015). Dusicyon Australis. The IUCN Red List of Threatened Species 2015: e.T6923A82310440. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T6923A82310440.en (accessed July 27, 2020).
Singh, P., Srivathsa, A., and Macdonald, D. W. (2020). Conservation status of the dhole Cuon alpinus in north-east India, with a focus on Dampa Tiger Reserve, Mizoram. Oryx 54, 873–877. doi: 10.1017/S0030605319000255
Srivathsa, A., Karanth, K. K., Jathanna, D., Kumar, N. S., and Karanth, K. U. (2014). On a dhole trail: examining ecological and anthropogenic correlates of dhole habitat occupancy in the Western Ghats of India. PLoS ONE 9:e98803. doi: 10.1371/journal.pone.0098803
Srivathsa, A., Karanth, K. U., Kumar, N. S., and Oli, M. K. (2019a). Insights from distribution dynamics inform strategies to conserve a dhole Cuon alpinus metapopulation in India. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-39293-0
Srivathsa, A., Puri, M., Karanth, K. K., Patel, I., and Kumar, N. S. (2019b). Examining human–carnivore interactions using a socio-ecological framework: sympatric wild canids in India as a case study. R. Soc. Open Sci. 6:182008. doi: 10.1098/rsos.182008
Srivathsa, A., Sharma, S., and Oli, M. K. (2020). Every dog has its prey: range-wide assessment of links between diet patterns, livestock depredation, and human interactions for an endangered carnivore. Sci. Tot. Environ. 714:136798. doi: 10.1016/j.scitotenv.2020.136798
Terborgh, J., Estes, J. A., Paquet, P., Ralls, K., Boyd-Herger, D., Miller, B. J., et al. (1999). “The role of top carnivores in regulating terrestrial ecosystems,” in Continental Conservation, eds M. E. Soule and J. Terborgh (Washington, DC: Island Press), 39–64.
Thinley, P., Dendup, T., Rajaratnam, R., Vernes, K., Tempa, K., Chophel, T., et al. (2020). Tiger reappearance in Bhutan's Bumdeling Wildlife Sanctuary: a case for maintaining effective corridors and metapopulations. Anim. Conserv. 23, 629–631. doi: 10.1111/acv.12580
Thinley, P., Dorji, S., Tempa, T., Wangchuk, N., Namgyel, U. S., et al. (2015). Counting the Tigers in Bhutan: Report on the National Tiger Survey of Bhutan 2014–2015. Thimphu: Department of Forests and Parks Services, Ministry of Agriculture and Forests.
Thinley, P., Kamler, J. F., Wang, S. W., Lham, K., Stenkewitz, U., and Macdonald, D. W. (2011). Seasonal diet of dholes (Cuon alpinus) in northwestern Bhutan. Mamm. Biol. 76, 518–520. doi: 10.1016/j.mambio.2011.02.003
Thinley, P., Lham, D., Wangchuk, S., and Wangchuk, N. (2016). National Snow Leopard Survey 2014–2016 (Phase I): Sign and Prey Base Survey. Thimphu: Department of Forests and Parks Services, Ministry of Agriculture and Forests.
Thinley, P., Rajaratnam, R., Lassoie, J. P., Morreale, S. J., Curtis, P. D., Vernes, K., et al. (2018). The ecological benefit of tigers (Panthera tigris) to farmers in reducing crop and livestock losses in the eastern Himalayas: implications for conservation of large apex predators. Biol. Conserv. 219, 119–125. doi: 10.1016/j.biocon.2018.01.015
Thinley, P., Rajaratnam, R., Morreale, S. J., and Lassoie, J. P. (2021). Assessing the adequacy of a protected area network in conserving a wide-ranging apex predator: the case for tiger (Panthera tigris) conservation in Bhutan. Conserv. Sci. Pract. 3:e318. doi: 10.1111/csp2.318
Tshering, K., and Thinley, P. (2017). Assessing livestock herding practices of agro-pastoralists in western Bhutan: livestock vulnerability to predation and implications for livestock management policy. Pastoralism Res. Policy Pract. 7:5. doi: 10.1186/s13570-017-0077-1
Tshering, K., Thinley, P., Shafapour Tehrany, M., Thinley, U., and Shabani, F. (2020). A comparison of the qualitative analytic hierarchy process and the quantitative frequency ratio techniques in predicting forest fire-prone areas in Bhutan using GIS. Forecasting 2, 36–58. doi: 10.3390/forecast2020003
Van Valkenburgh, B. (1991). Iterative evolution of hypercarnivory in canids (Mammalia: Carnivora): evolutionary interactions among sympatric predators. Paleobiology 17, 340–362. doi: 10.1017/S0094837300010691
Wang, S. W., and Macdonald, D. W. (2006). Livestock predation by carnivores in Jigme Singye Wangchuck National Park, Bhutan. Biol. Conserv. 129, 558–565. doi: 10.1016/j.biocon.2005.11.024
Wang, S. W., and Macdonald, D. W. (2009). Feeding habits and niche partitioning in a predator guild composed of tigers, leopards, and dholes in a temperate ecosystem in central Bhutan. J. Zool. 277, 275–283. doi: 10.1111/j.1469-7998.2008.00537.x
Wangchuk, T. (2004). Predator-prey dynamics: the role of predators in the control of problem species. J. Bhutan Stud. 10, 68–89.
Wangchuk, T., Thinley, P., Tshering, K., Tshering, C., Yonten, D., Pema, B., et al. (2004). A Field Guide to the Mammals of Bhutan. Thimphu: Royal Government of Bhutan.
Widodo, F. A., Sunarto, Hartoyo, D., Gunawan, Fadhli, N., Sukmantoro, W., et al. (2020). Preliminary assessment of abundance and distribution of Dholes Cuon alpinus in Rimbang Baling and Tesso Nilo landscapes, Sumatra. Raffles Bull. Zool. 68, 387–395. doi: 10.26107/RBZ-2020-0055
Wikramanayake, E., Dinerstein, E., Seidensticker, J., Lumpkin, S., Pandav, B., Shrestha, M., et al. (2011). A landscape-based conservation strategy to double the wild tiger population. Conserv. Lett. 4, 219–227. doi: 10.1111/j.1755-263X.2010.00162.x
Wolf, C., and Ripple, W. J. (2017). Range contractions of the world's large carnivores. R. Soc. Open Sci. 4:170052. doi: 10.1098/rsos.170052
Woodroffe, R., and Ginsberg, J. R. (1998). Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128. doi: 10.1126/science.280.5372.2126
Woodroffe, R., and Ginsberg, J. R. (2005). “King of the beasts? evidence for guild redundancy among large mammalian carnivores,” in Large Carnivores and the Conservation of Biodiversity, eds J. C. Ray, K. H. Redford, R. S. Steneck, and J. Berger (Washington, DC: Island Press), 154–175.
Woodroffe, R., and Sillero-Zubiri, C. (2020). Lycaon pictus (amended version of 2012 assessment). The IUCN Red List of Threatened Species 2020: e.T12436A166502262. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2020-1.RLTS.T12436A166502262.en (accessed December 21, 2020).
Xue, Y., Li, D., Xiao, W., Zhang, Y., Feng, B., and Jia, H. (2015). Records of the dhole (Cuon alpinus) in an arid region of the Altun Mountains in western China. Eur. J. Wildl. Res. 61, 903–907. doi: 10.1007/s10344-015-0947-z
Keywords: Bhutan, biological corridors, dhole conservation, dhole distribution, endangered canid, protected areas
Citation: Thinley P, Rajaratnam R, Kamler JF and Wangmo C (2021) Conserving an Endangered Canid: Assessing Distribution, Habitat Protection, and Connectivity for the Dhole (Cuon alpinus) in Bhutan. Front. Conserv. Sci. 2:654976. doi: 10.3389/fcosc.2021.654976
Received: 18 January 2021; Accepted: 18 March 2021;
Published: 16 April 2021.
Edited by:
William J. McShea, Smithsonian Conservation Biology Institute (SI), United StatesReviewed by:
Arjun Srivathsa, University of Florida, United StatesIgor Khorozyan, University of Göttingen, Germany
Copyright © 2021 Thinley, Rajaratnam, Kamler and Wangmo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phuntsho Thinley, Y2hldHNobzc4QGdtYWlsLmNvbQ==
 Phuntsho Thinley
Phuntsho Thinley Rajanathan Rajaratnam
Rajanathan Rajaratnam Jan F. Kamler
Jan F. Kamler Cheten Wangmo
Cheten Wangmo