- 1Chacruna Institute for Psychedelic Plant Medicines, San Francisco, CA, United States
- 2Cactus Conservation Institute, Alpine, TX, United States
- 3Independent Researcher, Market Harborough, Leicestershire, United Kingdom
Psychedelics have a profound potential to catalyze psychological transformation and support traditional cultures and ways of life. However, many naturally occurring psychoactive plants and animals are facing threats from climate change, habitat loss and other anthropogenic pressures like overharvesting. In this narrative review we examine the conservation issues pertaining to some of the most well-known naturally occurring psychedelics: peyote (Lophophora williamsii), ayahuasca vine (Banisteriopsis caapi), iboga (Tabernanthe iboga) and the Sonoran Desert toad (Incilius alvarius). For each of the four species we aim to: review their conservation status; assess the sustainability of current sourcing practices; discuss pathways for sustainability of access; examine the efforts underway to preserve these medicines by Indigenous people and local communities; and propose how these efforts could be supported or expanded. This review focuses on an urgent issue of conservation of naturally occurring psychedelic plant and animal species and draws attention to their conservation needs. We conclude that despite biological and ecological differences, all four species face similar knowledge gaps limiting evidence-based conservation. Priorities across species include: long-term ecological and demographic monitoring; genetic and chemical diversity studies; sustainable harvest and management research; integration of Indigenous knowledge and socio-cultural research into conservation frameworks.
Introduction
One class of psychoactive substances, the psychedelics, has come to the forefront of public attention in the last few years. Psychedelics, originating from Greek words meaning “mind manifesting,” are substances that significantly alter consciousness. The “classic” psychedelics include serotonergic compounds (i.e. agonists of the serotonin receptors) such as lysergic acid diethylamide (LSD), psilocybin (the active ingredient in many species of magic mushrooms, notably genus Psilocybe), mescaline (found in several species of cacti, notably genera Echinopsis/Trichocereus and Lophophora) and N,N-dimethyltryptamine (DMT, the primary psychoactive component in ayahuasca) (Nichols, 2016). For millennia, cultures have employed psychedelics in various sociocultural contexts, often as part of spiritual or healing rituals (Hofmann et al., 2001). In recent years, a growing number of researchers, clinicians and members of the general public have become aware of their potential for the treatment of a variety of mental health disorders (McIntyre et al., 2025), most notably depression, anxiety (Kim et al., 2025) and addiction (Vamvakopoulou and Nutt, 2024). With this change in perceptions comes a change in drug laws and policy, an increase in ‘drug tourism’ to the Global South and an increased demand for these substances globally. Changes in drug policies are evident, for example, in the legalization of the medical use of psychedelics by Switzerland and Australia (Nutt et al., 2024) or a wave of decriminalization of naturally-occurring psychedelics across various USA states and cities (Bhave, 2024). This is accompanied by an increase in online retailers and in-person dispensaries, particularly in the USA and Canada (Lorinc, 2024), thriving sales through the dark web (Stringham et al., 2023) and anecdotally, through the deep web (the part of the World Wide Web that is not discoverable by means of standard search engines, instead referring to social media, messaging apps), although exact data for the latter are lacking.
Across the globe (and particularly in the Global South), an increasing number of retreat centers are emerging, offering promises of healing, wellness, and a connection to Indigenous traditions through a variety of substances, such as ayahuasca, San Pedro, peyote and mushrooms (Kamin, 2021)*. These retreats often feature complementary practices like sweat lodges, meditation, yoga, and vegan cuisine, along with various medical and spiritual claims made to generate interest. Prices vary widely, ranging from hundreds to several thousand dollars, catering to diverse budgets and preferences (Kamin, 2021)*. More and more people are turning to psychedelics in search of healing, connection and meaning. Yet some practices aimed at therapy, spirituality or recreation are threatening some of the species being used. This is complicated by a lack of knowledge regarding the conservation and ecology of many of these species, about the rates of resource extraction or impacts thereof, or the effects of current drug policies. For the Indigenous communities around the world that have used plant and fungi medicines since time immemorial, the sudden rush to commodify these entities is both troubling and disruptive, as is the lack of recognition of Indigenous Knowledge (Fotiou, 2016, 2019; Palumbo, 2024). Specifically, many Indigenous Nations have expressed concerns about being excluded from psychedelic spaces that extract their knowledge without benefit sharing, compromise what they regard as their intellectual property, and disconnect their sacred medicines from their spiritual and cultural contexts (Celidwen et al., 2023).
Psychedelics have a profound potential to catalyze psychological change and transformation and support traditional cultures and ways of life (Pollan, 2018)*. However, many naturally occurring psychoactive plants and animals are facing threats from climate change, habitat loss and other anthropogenic pressures like overharvesting. Moreover, cultural traditions associated with these plants are being increasingly appropriated and commodified. It may be beneficial to consider these species in the context of a One Health model, which emphasizes the holistically interconnected health of people, wildlife and the environment (Mumford et al., 2023). They can be viewed as occupying an important and dynamic interface that interweaves ecological integrity, cultural identity and cohesion, and human health and well-being. This makes their conservation of vital importance not just for biodiversity and ecological functioning, but also for the preservation of traditional healing practices, spiritual relationships to the land, and emerging mental health treatments. It is imperative that any conservation strategies centered on these species must not only be ecologically sound, but also bioculturally sensitive, co-managed with the Indigenous communities that have deep relationships with them.
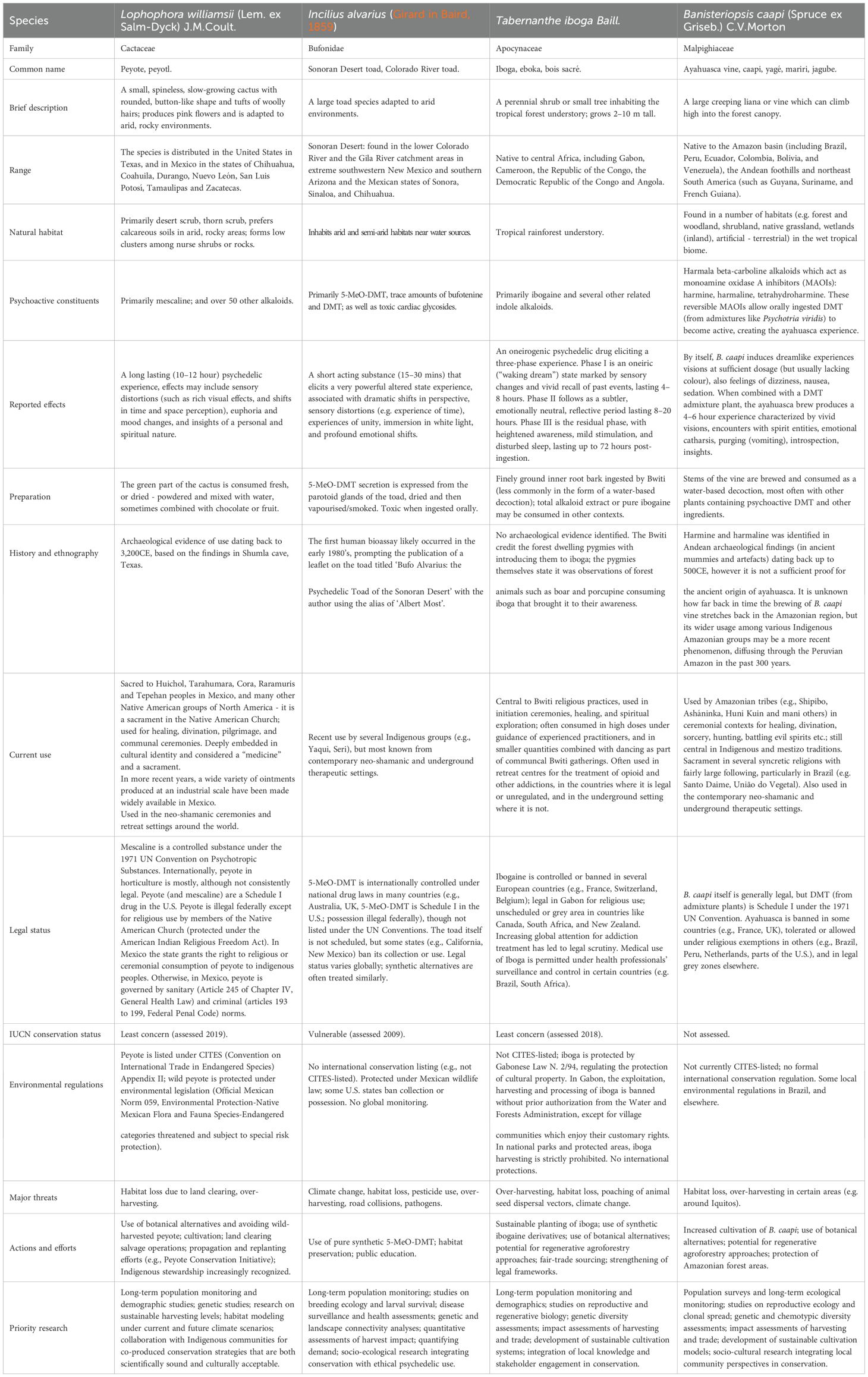
In this article we will dive deep into the conservation issues around some of the most well-known naturally occurring psychedelics: peyote (Lophophora williamsii), ayahuasca vine (Banisteriopsis caapi), the Sonoran Desert toad (Incilius alvarius) and iboga (Tabernanthe iboga) (Figure 1). We are going to examine their biology, ecology and conservation status, and where possible assess the sustainability of current sourcing practices and discuss pathways for sustainability of access. Where known, we will also describe the efforts which are being directed towards preserving these species by Indigenous people and local communities and discuss how we can best support or expand on these efforts.

Figure 1. Four species from our case studies: (a) Lophophora williamsii, photo by A. Ermakova; (b) Incilius alvarius, photo by Rodolpho Vega Littlewood; (c) Tabernanthe iboga, photo by Yann Guignon; (d) Banisteriopsis caapi, photo by Apollo (CC license).
This narrative review’s objective is to synthesize the available literature and provide an overview of the ecology and conservation data about four species commonly used for psychoactive purposes (Table 1). This review is timely and important because of the rapidly changing landscape brought about by the “psychedelic renaissance”, bringing with it a wave of decriminalization and broad change in the attitudes to psychedelics. This is the first review on a very pertinent issue of conservation of naturally occurring psychedelic plant and animal species.
Methods
To identify references for this article, we searched Google Scholar and PubMed for publications published from January 1965 to December 2024 using the current Latin and colloquial names (confirmed from International Plant Names Index and AmphibiaWeb databases): “Lophophora williamsii”, “peyote”, “Tabernanthe iboga”, “iboga”, “Incilius alvarius” or “Bufo alvarius”, “Sonoran Desert toad”, “Colorado river toad”, “Banisteriopsis caapi”, “caapi”. Papers in English or Spanish were included, representing the fluent language proficiencies of the authors. The search was supplemented by additional articles, which were identified during the review of the bibliographies from the papers sourced through PubMed and Google Scholar, by the relevant books on the topic, and also a search of news articles and other grey literature (to clearly differentiate anecdotal from empirical evidence we have marked those with an *). References were then selected on the basis of relevance to the content of review: i.e. we included articles relevant to biology, ecology and conservation of the four species in question. We have also searched biodiversity databases (Global Biodiversity Information Facility, GBIF; IUCN Red list; NatureServe and AmphibiaWeb), and ethnobotanical databases (Dr Duke’s Phytochemical and Ethnobotanical Databases).
Case studies
Ayahuasca vine (Banisteriopsis caapi)
Ecology and biology of ayahuasca vine
Ayahuasca vine (Banisteriopsis caapi (Spruce ex Griseb.) C.V.Morton, 1931) is a woody, evergreen tropical liana of the Malpighiaceae family that can attain 30 m in length, with the girth of old vines attaining up to 1m in diameter. The vine has a reddish-brown stem and produces pink, white and off-white flowers. It will grow in partial shade to full sun, in moist but well-drained soils. It is a large climbing shrub that uses trees and other plants for support when growing (Gates, 1982; Souto and Oliveira, 2012). Research is revealing that woody lianas play a particularly important ecological role in forests in the tropics, influencing forest regeneration, species diversity and ecosystem-level processes (Schnitzer and Bongers, 2002). The precisenative range of this species is difficult to determine due to extensive areas of the Amazon rainforest being under-surveyed, and possible deliberate planting conducted by Indigenous groups (Gates, 1982). However, it is reported from the rainforests of the northwestern Amazon (Brazil, Bolivia, Colombia, Ecuador, Peru), the Orinoco Basin of Venezuela, and Pacific coastal areas of Colombia and Panama (Schultes and Hofmann, 1980).
While B. caapi is currently defined as a single species, this is debated by botanists, and Indigenous groups recognize numerous different varieties with various distinct properties and uses (Schultes, 1986; Sheldrake, 2020; De Oliveira et al., 2023). Variation among these varieties expressed by distinguishing characteristics between them has led some researchers to call for a reassessment of the species’ taxonomy (Oliveira et al., 2021). Biochemical analysis of these varieties has demonstrated marked variation in the relative proportions of harmala alkaloids (Santos et al., 2020) and there are genetic studies underway that will shed more light on their taxonomy, starting with sequencing, assembling, and analyzing the complete genome of B. caapi’s mitochondrion (Chavarro-Mesa et al., 2024). Genetic sequencing centering on Internal Transcribed Spacer (ITS) DNA barcoding can also potentially be effectively paired with traditional knowledge of ethnovarieties of Banisteriopsis spp., with one study demonstrating correspondence of ITS DNA barcoding with traditional designation of different ethnovarieties of these plants (Luz et al., 2023). To ensure the successful conservation of this species, further research is needed to assess this intraspecific diversity, so that the diversity of this liana can be maintained in all of its forms.
History of human use of ayahuasca
B. caapi is referred to as ayahuasca, but this name is also applied to a number of other species in the Malpighiaceae family (De Oliveira et al., 2023), in addition to the shamanic brew of which it may be a foundational ingredient, alongside a number of different plant additives that vary, depending on tradition, intent and locality (Hofmann et al., 2001; Luna and White, 2018; Kaasik et al., 2020). In the Quechuan language, ayahuasca translates to “vine of the soul”, while in the Tukanoan languages B. caapi also being specifically referred to as yagé.
The precise origins of the human usage of ayahuasca in Amazonia are debated (Ruffell et al., 2023; Greco et al., 2024), although usage has been documented for 150 years (Spruce, 1873), but likely predates this. Usage of the B. caapi vine seems to have spread between different Indigenous groups in Amazonia, and it tends to be regarded as a plant teacher with healing properties by these groups, as well as being used for other purposes like sorcery, hunting, finding lost objects, mediating social disputes, and understanding ecological rhythms and animal behavior (Luna, 1984; Luna and White, 2018). In the 1930s, use of the vine left the rainforest setting to find usage in urban centers in Brazil within religious contexts, adopted by syncretic churches such as the Santo Daime, Barquinha, and União do Vegetal (Labate and Jungaberle, 2011; Labate and Cavnar, 2014). Today there is a growing global interest in ayahuasca, particularly among Westerners seeking it for emotional healing, personal insight and growth, and spiritual experiences, with people travelling to South America to experience it (Winkelman, 2005; Durán, 2023).
B. caapi tends to be considered as the central part of the ayahuasca brew by the Indigenous groups that use it in Amazonia, and some groups consume brews made exclusively with B. caapi vine. It harbors a number of beta-carboline alkaloids that act as monoamine oxidase inhibitor (MAOI) compounds, including harmine, harmaline and tetrahydroharmine. These impart a direct psychoactive effect, while also allowing the psychedelic compound N,N-Dimethyltryptamine (DMT) to be orally activated; otherwise DMT is rapidly broken down by MAOI enzymes (Egger et al., 2024). B. caapi is frequently brewed and ingested alongside DMT-containing admixture plants, including leaves of Psychotria viridis (chacruna) or Diplopterys cabrerana (referred to chaliponga or chagropanga) in different parts of the Amazon. Use of this plant is undergoing a rapid global expansion and is generating increasing biomedical research attention (Ruffell et al., 2023). A recent report indicates that over 4 million people worldwide have consumed ayahuasca in their lifetime, with only about 10% representing Indigenous communities. In 2019, it is estimated that 820,000 people used ayahuasca, with around five and a half million servings being consumed (Álvarez and Mazarrasa, 2023)*.
Conservation of ayahuasca
While B. caapi is not listed or assessed by the International Union for Conservation of Nature (IUCN), there is anecdotal and now academic evidence that its populations are being depleted in various parts of Amazonia (Coe and Gaoue, 2023). In Peru in 2008, ayahuasca (and its ceremonial shamanic usage) was designated as being part of Indigenous cultural heritage, which seeks to protect it and its usage in this context (Giove, 2022). Ayahuasca vine faces a number of threats, including over-harvesting to supply a growing global demand for the plant (including a rise in Amazonian ayahuasca tourism), deforestation of its rainforest habitat (due to conversion to agriculture, resource extraction and infrastructure projects), and an erosion of traditional Indigenous knowledge encompassing sustainable management of the plant due to growing cultural displacement and commercialization. Threats to it also vary by location, with B. caapi in Colombia threatened by coca cultivation, oil extraction, expansion of cattle farming and agriculture and the fragmentation of Indigenous territories (Mowbray, 2022). Commercialized ayahuasca tourism in Peru may also have other detrimental conservation impacts, potentially being a driver of the trade in jaguar body parts (Braczkowski et al., 2019).
B. caapi takes a minimum of five years growing time before it is ready for harvesting or re-harvesting (Kilham, 2018; Meyer, 2021)*, although older and more mature vine is more highly prized to meet the global demand. Interestingly, traditional harvesting methods practiced by the Indigenous peoples throughout Amazon include mainly harvesting smaller lianas (<2.5 cm in diameter) (Coe and Gaoue, 2023). Diminishing wild populations of B. caapi in some regions is evidenced by local shortages, rising prices of harvested vine, harvesters being forced to travel further to locate the vine, and a decreasing size (and age) of the vine being harvested (Kilham, 2018; Álvarez, 2020)*. Concerns about the overharvesting of B. caapi vine were recently raised by over a hundred Shipibo-Conibo shamans from the Ucayali region of Peru at a meeting centered on Indigenous knowledge, forest and territory protection, and the future of spiritual tourism in the region (Carreon, 2024)*.
Only one study in the Peruvian Amazon has examined the effects of harvesting caapi lianas on their demography and population dynamics. Coe and Gaoue (2023) report that B. caapi populations were resilient to low harvesting pressure, indicating that some level of harvesting can be tolerated. They found that harvesting negatively affected size-dependent survival, with larger lianas experiencing higher mortality under high harvest pressure compared to smaller lianas. Under high harvest pressure, smaller lianas had better survival rates than those in low harvest pressure populations. Harvesting did not influence clonal or sexual reproduction, but fertility was size-dependent. Long-term projections estimated in this study suggest a decline of B. caapi populations by 1.3% under high harvest pressure, while populations under low harvest pressure are expected to grow by 3.2%. In the short term, all populations experienced significant declines, with high harvest populations dropping by 26% annually and low harvest populations by 20.4%. They conclude that for sustainable management, prioritizing the survival of larger lianas and vegetative reproducers is critical, especially in high harvest systems facing multiple stressors (Coe and Gaoue, 2023). Further research has highlighted that in some instances, ayahuasca using groups can also potentially play a protective role, with Brazilian ayahuasca-using groups having been reported to play a role in maintaining a significant portion of B. caapi diversity in that region, acting as “guardians” of the plant (De Oliveira et al., 2023).
Iboga (Tabernanthe iboga)
Ecology and biology of iboga
Iboga (Tabernanthe iboga Baill. 1888), sometimes also referred to as eboga or eboka is an evergreen perennial shrub that is a member of the Apocynaceae family, native to the tropical rainforests of central Africa, reported from Gabon, Cameroon, Angola, the Republic of Congo, and the Democratic Republic of Congo. It grows in the understory of these forests, requiring moist soils and partial shade. Iboga is primarily applied to the species Tabernanthe iboga but may also encompass other species such as Tabernanthe manii (Tonye et al., 2000). Iboga has been translated as “to heal” or “to care for” from various Indigenous dialects in the Congo basin (Dickinson, 2016). Mature plants can attain a height of 1–2 meters but can potentially grow to height of 10 m in some cases and they produce yellow blossoms and oblong orange fruits after their first year of growth (Dickinson, 2016). These fruits are consumed by a number of animals such as elephants and gorillas which in turn act as seed dispersal vectors for the plant (Cousins and Huffman, 2002).
The inner root bark of the plant is the most highly prized part of it, harboring the highest concentrations of a range of iboga alkaloids, with ibogaine being considered the primary of these. Ibogaine levels have been found to vary widely from 0.27%–10% by weight of the root bark (Bouso et al., 2020; Iyer et al., 2021). Levels of ibogaine are thought to be influenced by a range of different factors, including age of the iboga plant, soil, symbiotic associations with other trees, and possibly other factors, with further research needed to elucidate these (Ermakova, 2021). Iboga takes a minimum of 5–6 years to mature (but possibly up to 7–10 years), but older plants are far higher yielding, with a 20–30-year-old plant potentially capable of yielding 1-2 kg of root bark in comparison to a five-year-old plant which can yield 250g of root bark (Ermakova, 2021).
History of human use of iboga
Iboga is used as a medicine and sacrament and forms a central aspect of the spiritual tradition of the Bwiti which is most prominent in Gabon (Ravalec and Mallendi, 2007). The emergence of Bwiti among the Fang people of West Africa occurred around the turn of the 20th century, although its use by other groups likely predates this, with the precise origin of usage being unclear. According to Bwiti legends, the Pygmies first discovered iboga by observing animals such as boar digging up and consuming the roots of the plant and observing shifts in their behavior (Pope, 1969). Iboga is used ritualistically by the Bwiti as a single large initiatory dose, for initiates to connect with ancestors and seek guidance on their life, and also in much more modest amounts in weekly masses, or ngoze, where it is tied to ceremonial practices and dances. In small doses it may also be used to counter fatigue and enhance performance (Fernandez and Fernandez, 2001; Ndoua and Vaghar, 2018). It has been referred to as a cultural keystone species, given that it holds exceptional significance to a culture or people (Quiroz and Andel, 2018).
Outside of its central African context of usage, ibogaine’s ability to reduce opioid withdrawal and interrupt addiction was discovered by Howard Lotsof in 1962, with Lotsof being an outspoken proponent for its potential in this regard (Brown and Alper, 2018). There is a growing global interest and demand for iboga, principally due to ibogaine’s application as an addiction interrupting agent (Köck et al., 2022). Beyond its application in addiction treatment, there is also a growing global interest in iboga use for psychospiritual purposes or other mental and physical health problems (Cherian et al., 2024). Depending on the context of usage, it may be ingested in the form of ibogaine hydrochloride (primarily in an addiction treatment context), a total alkaloid or full spectrum alkaloid extract, or inner iboga root bark (the form consumed by the Bwiti) (Faura and Langlois, 2019, 2020, 2021)*.
Conservation of iboga
T. iboga is currently designated on the IUCN Red List of Threatened Species as being of “least concern” (Group, 2019) although there is a lack of detailed biological assessments of its wild populations available, and there is also a lack of knowledge pertaining to other members of the Tabernanthe genus, or what subspecies or varieties of T. iboga there might be (Tonye et al., 2000). Iboga faces several threats, including overharvesting, habitat loss due to deforestation, poaching of animals that act as seed dispersal vectors, and climate change (with associated shifts in temperature and rainfall patterns) (Ermakova, 2021). Complex political and economic factors in the countries where it is found are also a source of concern for its populations, and pressure from growing international export is a growing issue (Towns et al., 2014). Iboga fruits are eaten by elephants, with seeds distributed along their forest trails, and this puts them at risk of poaching to supply the ivory trade by some seeking to harvest iboga (Dickinson, 2016).
A reduction in iboga availability is evidenced by an 800% increase in the price of iboga in Gabon over the previous decade (Nuwer, 2023). The Gabonese branch of the Wildlife Conservation Society in Libreville has expressed concerns about unsustainable harvesting of iboga for export (Towns et al., 2014). A scarcity of iboga within Gabon to supply Bwiti needs has resulted in mixingof iboga root bark with outer root bark or whole root (Dickinson, 2016) and other plants being misrepresented and sold as iboga which can pose health risks (Gicquel et al., 2016). Three reports centered on the status of T. iboga in Gabon led by the International Center for Ethnobotanical Education, Research and Service, ICEERS (Faura and Langlois, 2019, 2020, 2021)*, based on interviews with the international ibogaine community and local Gabonese stakeholders state there has been a sharp rise in the global consumption of iboga and production of ibogaine is a serious concern, leading to issues with poaching and unsustainable harvesting to supply an international export demand. This is confirmed by a recent report by the INTERPOL, where additionally an analysis of the online markets and illegal smuggling networks operating out of Gabon is provided (INTERPOL, 2023). ICEERS in 2019 documented over 80 ibogaine clinics across the world. Mexico is leading in the number of clinics/retreats, where ibogaine treatment is also frequently combined with toad-derived 5-MeO-DMT (Davis et al., 2023).
As of 2019, Gabon’s Ministry of Agriculture designated it illegal to export iboga from the county unless it has been cultivated on private land, supported by permits from the Ministry of Forestry and the Environment (Nuwer, 2023). Moreover, the Gabonese government requested to place iboga under the jurisdiction of the United Nations Convention on Biological Diversity (1992) and, by extension, the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity.
Iboga was freely available in the forests from where it was harvested by the Bwiti, meaning there wasn’t a need to cultivate it, and to date very little assessment of iboga cultivation and propagation practices have been undertaken with T. iboga in Central Africa (Faura and Langlois, 2019, 2020, 2021)*. However, in some instances, iboga plants have been cultivated adjacent to Bwiti temples (Dickinson, 2016). In 2000, under Gabonese president Omar Bongo (himself an iboga initiate) the Council of Ministers of the Republic of Gabon declared T. iboga a national treasure, or “cultural heritage strategic reserve.” It is legally protected by a number of laws including the Convention on Biodiversity (Rio 1992, ratified in Gabon in 1997), Law for the Protection of Cultural Goods (December 10, 1994), and the Nagoya Protocol (Signed in Gabon in July 2012); in spite of this, overharvesting continues (Nuwer, 2023).
The first scalable and economically feasible method for ibogaine synthesis has recently been developed (Iyer et al., 2025). But before its use becomes widespread,any manufacturers of ibogaine derived medications or those seeking iboga for psychospiritual purposes should ensure they source sustainably cultivated iboga, to ensure no further pressure is placed on wild populations of the plant.
Iboga root can potentially be harvested in a more labor-intensive manner without killing the plant, with a portion of the roots excavated, while some are left intact, and the soil cover replaced. The iboga plant will survive, although future growth is likely to be stunted, with a minimum three-year recovery time recommended (Ermakova, 2021)*. However, the roots are commonly harvested in their entirety, with the whole plant uprooted (Tonye et al., 2000). Plants could provide numerous cuttings, which could potentially be propagated using hydroponic or aeroponic techniques for subsequent planting, although there is a lack of research on this form of iboga cultivation, and collaboration between cultivators could be advantageous. This could also be a viable means of propagating any favored or high yielding specimens.
Sonoran Desert toad (Incilius alvarius)
Toad biology and ecology
Incilius alvarius (formerly Bufo alvarius (Girard, 1859), known as the Sonoran Desert toad or Colorado river toad) is native to northern Mexico and the southwestern United States (Frost et al., 2009; Frost, 2025). These toads can live in different habitats from desert to dry meadows and forests in mountain canyons in the riparian areas within the Sonoran Desert (Holycross et al., 2022).
The Sonoran Desert toad is the second largest anuran in North America, after the cane toad (Rhinella marinus). They grow up to 19 cm, weigh up to 900 g and can live for over 15 years. They have prominent cranial crests, relatively smooth skin colored olive, brown or grey on the top of the body, with a creamy-white underside. Recently metamorphosed toadlets are tan to green with orange or red spots on the dorsum. I. alvarius have very distinctive glands: large, kidney-shaped parotoid glands, white glands or tubercles under the parotoid glands, and large, lumpy glands on sides of the limbs (Holycross et al., 2022). I. alvarius is a dietary generalist, known to eat wasps, ants, beetles, centipedes, millipedes, spiders (including tarantulas), scorpions, lizards, mice, and smaller toads (Luccioni et al., 2023).
These anurans have a fascinating natural history: most of their life is spent underground, where they aestivate for nine dry months every year. From May, before the onset of summer rains, they emerge from their burrows. They are nocturnal during the hot summer months. As the rains start, they move towards seasonal or permanent water sources such as springs, pools, cattle tanks, and irrigation ditches to start breeding (Fouquette et al., 2005). Males form choruses and emit low-pitched toots to attract females (Sullivan and Malmos, 1994). The species congregates in large numbers, with nearly all reproduction of a local breeding population occurring in a single night. Females may lay up to around 8,000 eggs in long strands. The time required for hatching and metamorphosis is presumed to be around 6–10 weeks (Frost, 2025).
Toad secretions/5-MeO-DMT
Sonoran Desert toads have extremely potent defensive toxins that are released from several glands (primarily the parotoids) in their skin. When threatened, the toads inflate their body, hiss and orient their glands towards potential predators. Milky-white toxin contains bufadienolides, which are cardiac glycosides, tryptamines (e.g., bufotenine, 5-MeO-DMT), various catecholamines such as epinephrine and norepinephrine, and non-cardioactive sterols (Erspamer et al., 1967; Cei et al., 1972). Eating the toads can be lethal – there are documented cases of death and paralysis in dogs (Stebbins, 2003). In summer months, when the toads are active, these toxic secretions act as a potent deterrent for most predators, except for indigo and garter snakes (which appear resistant to these toxins) or raccoons who have learnt to bypass their strong chemical defenses by flipping the toads on their back, thus avoiding the glands (Wright, 1966; Gutiérrez-González et al., 2006).
What is unique about the Sonoran Desert toad is that it is the only animal species known to secrete a highly potent psychedelic, 5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT). 5-MeO-DMT content of toad “venom” (as toad secretions are colloquially known in psychedelic circles) ranges from 15-45% of dry weight (Erspamer et al., 1967; Cei et al., 1972; Uthaug et al., 2019; Schwelm et al., 2021), making it the most potent known natural source of this compound. A toad yields about 0.25–0.50 g of dried secretion from a “milking session,” or about 75 mg 5-MeO-DMT. Why or how these toads produce 5-MeO-DMT is not known. It could be produced endogenously, as it is arguably produced in humans and rodents (reviewed in (Ermakova et al., 2021a) or acquired via skin microbial symbionts. A recent study by Luccioni et al. (2023) indicates that it is unlikely 5-MeO-DMT is sequestered from specific dietary elements (such as toxic ants and beetles), because this toad’s diet does not differ from other sympatric anurans - unless it was through consumption of particular plants or fungi as the authors could not identify/compare plant or fungal matter in their analysis (Luccioni et al., 2023).
History of human use of toad secretions
5-MeO-DMT is arguably one of the most potent psychedelic substances, with a unique phenomenology, and various epidemiological surveys and field studies of naturalistic use indicate that it has therapeutic potential for a variety of mental health problems (Davis et al., 2018, 2019; Uthaug et al., 2019).
Toads feature a lot in the Meso- and North- American iconography, and remains of various toad species have been found in many archaeological sites, however, they likely have symbolic value or were used as food (Whyte and Compton, 2020). For example, in Aztec and Mayan cultures, toads were associated with rain deities and agricultural fertility due to their amphibious nature, with their breeding coinciding with the arrival of the rains. There is no archaeologically confirmed or documented use of Incilius alvarius for entheogenic purposes (Horák et al., 2019; Bernal, 2024). Human use of “toad venom” for psychedelic purposes dates to the publication by Albert Most (a pseudonym of Ken Nelson) of a booklet titled “Bufo alvarius: The psychedelic toad of the Sonoran Desert” in 1984 (Nelson) 1984; Morris, 2021)*. For several decades after that, smoking toad “venom” remained a very obscure, underground activity known among psychonauts but not the mainstream public – to the extent that 5-MeO-DMT was not classified as a Schedule I drug in the USA until 2011. Ralph Metzner was one of the first practitioners who described the use of toad “venom” for ceremonial/medicinal use (Metzner, 2013). A key player responsible for both increasing the popularity of “toad medicine” and propagating the myth of the ancient indigenous traditional use in Mexico was Octavio Rettig (Greef, 2022)*. In the early 2010s, he started administering 5-MeO-DMT to people with addictions, while falsely marketing it as an ancestral medicine of first the Seri (Comca’ac) people of Mexico, and then the Yaqui (Bello, 2021; Ortíz-Bernal, 2024). The Seri, and several other indigenous communities (e.g. The Tohono, and recently the Mayo) are divided, as some profit from the resulting psychedelic tourism, while others resent this misrepresentation. The Yaqui (Yoeme) are actually the only Indigenous tribe in Sonora that has not incorporated toad secretion into any form of ceremonial or medicinal practice, at least not in any organized, traditional, or community-sanctioned way. While the toad holds symbolic importance within the Yaqui cosmovision, its significance is not tied to its psychoactive secretions, but rather to its ecological and spiritual role within the broader natural world. Yaqui leaders and elders responded by firmly rejecting and resisting these efforts, emphasizing that such practices were not part of their traditional lifeways. This stance reflects a clear refusal to adopt externally imposed narratives that misrepresent or distort their cultural heritage. Unlike other communities in the region that have become divided over this issue, the Yaqui have maintained a unified position of cultural integrity and refusal to commodify the toad in this way (Ortiz Bernal, 2025, personal communication).In the last decade the popularity of “toad medicine” has soared, with celebrities such as Mike Tyson and Hunter Biden publicly acknowledging using 5-MeO-DMT, advertising it as a life-changing experience. At the same time, multiple clinical trials with synthetic 5-MeO-DMT are ongoing, testing it as a potential treatment for a range of mental health conditions, such as depression, addiction or bipolar disorder, with promising preliminary results (Reckweg et al., 2022). However, it might take decades for sufficient evidence of safety and efficacy to be obtained before it is re-scheduled or becomes accessible legally via healthcare providers. In the meantime, countless retreat centers operating in Mexico and around the world offer ceremonies with “toad venom”. Likewise, there is a proliferation of psychedelic churches, claiming religious freedom to use it as a sacrament (Londoño, 2024)*. Bufotoxin is available for purchase from the dark web, and through other underground channels (Stringham et al., 2023). The growing demand for toad secretions leads to all sorts of potentially unsustainable harvesting practices that could have disastrous effects on the toads (Villa, 2023).
Unfortunately, due to the clandestine nature of the market, it is very difficult to estimate the extent of harvesting practices, the amount of toad “venom” available on the market, or even the number of people consuming 5-MeO-DMT derived from the toad in comparison to synthetic (as most population surveys, assuming they specify 5-MeO-DMT at all, do not distinguish its origins).
Conservation
Although the International Union for Conservation of Nature lists this species as of “least concern,” the lowest category of risk, the latest assessment was conducted in 2004 (Group. ISAS, 2022), and very likely does not reflect the reality of the current situation. In the USA, the toads are likely extirpated from California, declining in New Mexico and vulnerable in Arizona (NatureServe, 2002; Thompson et al., 2016). This species is not listed in Mexico among species at risk or protected by the NOM-059 (Domínguez-Sánchez, 2020).
Amphibians are the most threatened vertebrate class, imperiled by a variety of interacting threats: climate change, habitat loss, a global pandemic of chytrid fungus (Batrachochytrium dendrobatidis) and ranavirus, water contamination and urbanization. Many of these threats affect the Sonoran Desert toad. I. alvarius is vulnerable to the effects of climate change, as the species depends on the presence of permanent and temporary water sources for their breeding and tadpole habitat – and climate in the Sonoran Desert is predicted to become hotter and drier over time (Albuquerque et al., 2024). Through the effects of climate change alone the range of I. alvarius is forecasted to shrink by 23% (Stefanick and Miles, 2023)*. Many toads are killed by road traffic because they are often found at night on the roads, catching insects attracted by streetlights (Blais et al., 2024). Chytrid fungus has been devastating amphibian populations worldwide, however, until recently it was assumed that the environment of the Sonoran Desert was too hot and dry for it to survive. Unfortunately, recent studies confirmed the widespread presence of chytrid in parts of the Sonoran Desert, although the infection rate of I. alvarius in the populations sampled was low, at ~6% (Roth et al., 2023).
On top of everything else that threatens toad populations, the demand for the toad’s secretion and resulting poaching could lead to its population collapse, as already happened in at least one location in Sonora (Villa, 2023). “Milking” toads for their secretions is often presented as a sustainable practice, because they can regenerate their secretions in a few weeks. At a cursory glance catching toads, expressing their glands and then releasing them is not the same as killing them – but it is still a stressful event that can negatively influence reproduction, immune function and growth (Walls and Gabor, 2019). In the past, when demand was much lower, and toads were mostly caught for personal use or very small-scale business operations, it could have been argued that the impact of this on the individuals was small - however, these practices do not scale well. While there is no quantitative data regarding the extent of ongoing harvesting, nor about the demand for toad secretions, the reports from the journalists and researchers working in the field indicate alarming trends (Romero, 2022*; Peterson, 2023; Ortíz-Bernal, 2024). Thousands of toads are likely removed from their habitat every year. This is often done by low-paid workers who drive around and collect toads, put them in sacks and deliver them to a centralized place where they are milked repeatedly. Toads from different populations and geographical areas are mixed together, and kept in suboptimal conditions, thus facilitating the spread of the pathogens and intensifying their stress. Eventually toads are released outside, often into some random site far away from where they were harvested. Toads, like many amphibians, exhibit breeding site fidelity. When relocated, even to a suitable habitat, they need to reorient themselves, find water and shelter and compete with all the other toads already present, all the while being defenseless from predators – so relocation is often fatal for a long-lived, territorial animal (Germano and Bishop, 2009).
Is there an ethical way to obtain “toad venom”? There are commercial initiatives (often operating in the grey areas of legality) advertising ‘ethical’ breeding programs – but there are no standards to evaluate these claims due to their clandestine nature. Moreover, unlike with plants, captive breeding and care for these toads is complicated (Villa, 2023).
Peyote (Lophophora williamsii)
Peyote biology and ecology
The genus Lophophora (family Cactaceae) consists of the four accepted species that are morphologically and chemically distinct (Chan et al., 2021). Only one of them, L. williamsii (peyote in English and Spanish, peyotl in Nahuatl, hikuli in Huichol, huaname in Tarahumara) contains the psychoactive alkaloid mescaline, and it is this species we are going to focus on here.
Lophophora williamsii (Lem. ex J.F.Cels) J.M.Coult., 1894, is a small, spineless cactus native to Texas and Mexico. The plant can grow alone, or in clusters of multiple crowns. Where it is abundant, it forms carpet-like “planchas”. The cacti are about 2–7 cm in height and 2–12 cm in diameter. They often feature prominent vertical ribs, in a Fibonacci progression – 5, 8 or 13. The number of ribs correlates with the age and size of the plant: young plants usually have 5 ribs, while adults have 8 or 13. The growth rate of the plants of L. williamsii is very slow and they require more than 5 years to reach a diameter of 15 mm in the natural environment. Peyote is a very long-lived plant and can survive decades. The growth rates of the plant vary markedly with environmental conditions, and when cultivated optimally it is possible to accelerate it considerably. Stems are blue-green in color, although stressed plants sometimes have a purplish tint. From the cusp areoles, a tuft of soft, yellowish, or whitish woolly hairs emerges. Their flowers are pink, occasionally reddish, and bloom during the day. Flowering occurs sporadically during March-August and is followed by the appearance of small pink fruits measuring 1.5 to 2 cm in length. The fruits contain black, pear-shaped seeds, which are 1 to 1.5 mm long and 1 mm wide (Rojas-Aréchiga and Flores, 2016). Peyote seeds have the potential to maintain a persistent seed bank. Germination of the seeds requires hot, humid conditions and shade (Mandujano et al., 2020). In addition to sexual reproduction, peyote can reproduce vegetatively. In the latter case, another crown grows from the base of the stem. This usually happens in response to some kind of damage, like herbivory or cutting of the crown of the plant during harvesting. Peyote plants germinate and grow in the shade of shrubs which provide a favorable microclimate (these are known as nurse plants, or in the absence of plants, nurse rocks must suffice) (Schwertner-Charão et al., 2022). No studies have examined pollination and seed dispersal in the wild populations.
Despite the body of research pertaining to this plant (in the field of anthropology and studies of its traditional use) the studies on its ecology are rare and very little is known about its natural populations, so we do not have a clear picture of the population dynamics, structure or spatial interactions (Rojas-Aréchiga and Flores, 2016).
The geographical range of L. williamsii extends across the Chihuahuan Desert, covering much of central and northern Mexico and reaching the Trans-Pecos region of western Texas. It also occurs within several Texas border counties in the ecological region known as the Tamaulipan Thornscrub of South Texas. However, it is important to note that despite this seemingly wide range, in practice it grows only in certain parts of that range – its distribution is very clustered (Anaya and Rubio, 2010; Ermakova et al., 2021b). Anthropogenic pressure has considerably reduced the number of peyote cacti in the wild, to the extent that the species is now depleted from several areas, and many populations along its distribution range no longer exist (Briseño-Sánchez et al., 2020).
Only one study to date has examined peyote’s response to harvesting (Terry et al., 2011, 2012, 2014), indicating that although peyote can regenerate well because it produces more crowns in response to harvesting, even if the harvesting has been done correctly, i.e. leaving subterranean stem and root intact, it takes about 8 years for the re-growth to reach the same size as the originally harvested crown.
Mescaline
Peyote contains over fifty different alkaloids, including pellotine, anhalonidine, tyramine, and hordenine (Anderson, 1996). Certain alkaloids appear to lack direct pharmacological activity but amplify the effects of mescaline (Dinis-Oliveira et al., 2019). Some of these provide a bitter deterrent to herbivory (quite successfully, as most animals, except for certain terrestrial snails and humans avoid it), and evoke various unpleasant physiological effects like vomiting, dizziness, sleepiness and others (Poulie et al., 2023). Peyote’s psychoactive properties are primarily attributed to the alkaloid mescaline, with about 90% concentrated in the chlorophyll-rich (chlorenchyma) layer of the cactus’s aerial stem, or crown. In L. williamsii, the highest mescaline concentration is found in the crown, decreasing tenfold in the subterranean stem and a further tenfold in the root structures (Klein et al., 2015). It is widely believed that mescaline concentration usually correlates with the size/age of the plant. Thus, 13-ribbed plants are more desirable for harvesting – however at least one of the studies looking at Texan peyote did not find this to be true (Newbold et al., 2020). The concentration of mescaline in peyote crowns ranges from 1-6%, and a typical dose is 4–12 crowns (Anderson, 1996).
Cultural history/Indigenous use
The ritual use of peyote in the Americas dates back over 5,000 years to prehistoric times. Evidence of peyote in ceremonial contexts has been discovered at Cuatro Ciénegas in Coahuila, Mexico, dating back to 810 to 1070 A.C. It was used in Monte Alban (Oaxaca) in 200 B.C. and in Colima in 100 B.C (Hofmann et al., 2001). The earliest records come from Shumla Cave in Texas (El-Seedi et al., 2005; Terry et al., 2006), where plant remains were discovered alongside other shamanic artifacts such as ritual deer scapula rattles, bone rods, scrapers, and incense-filled tubes. Numerous Mesoamerican cultures, including the Maya and Aztecs, were known to use peyote (Carod-Artal, 2015). Peyote can rightly be considered a ‘cultural keystone species’, i.e. a species of exceptional significance that can influence social systems and culture and be a key feature of a community identity (Garibaldi and Turner, 2004).
The Tarahumara, Tepehúan, and Huichol peoples of northern Mexico use peyote in ritual and healing ceremonies. Peyote rituals remain integral to Huichol culture, highlighted by their annual pilgrimage from the western Sierra Madre to Wirikuta, the sacred peyote site in Potosí. This journey involves spiritual purification, abstinence rites, and ceremonial practices, such as shooting arrows into the first harvested cactus and offering it corn as a tribute (Schaefer and Furst, 1996). In the USA and Canada, peyote is used in the context of a pan-tribal syncretic Native American religion with the cactus playing a central sacramental role (Stewart, 1987).
Complexity of peyote politics
Mescaline has been known and synthesized since the late 19th century. Since then, in particular in the 1920s-1950s, it was used in multiple clinical trials, until it was eclipsed in research by a better-tolerated and more potent LSD in the 50s and subsequently banned together with many other psychedelics (Jay, 2019). Both mescaline, and the peyote cactus are categorized by the U.S. Drug Enforcement Agency (DEA) as Schedule I controlled substances, which is defined as those substances with a high potential for abuse and no currently accepted medical use. An exception to this has been granted to the Native American Church (NAC), and its members are the only people legally permitted to buy, transport and consume peyote as part of “bona fide religious ceremonies”. It took Native Americans decades of legal struggle and court cases to defend their rights to this medicine and sacrament, and was only passed into law in 1978, with the American Indian Religious Freedom Act Amendments in 1994 providing legislative protection for religious practices of the NAC (Maroukis, 2012; Feeney, 2016). The Diné roadman Steven Benally recently estimated that there are presently over 50 tribes and ~400,000 NAC members who use peyote as a sacrament, and each chapter of the NAC has its own regulations (Pollan, 2021). It is worth noting that these laws only allow federally recognized Native American tribes to use peyote and don’t apply to the broader group of Indigenous people in the US.
In Texas, a curious system exists where peyote is harvested and sold to the NAC by the dealers licensed by the DEA (for detailed description and historical outline of how it came to be see (Terry and Trout, 2017), and there are currently ~2–5 dealers in operation (the number changes slightly on a year by year basis). Sales records indicate that approximately two million crowns of peyote have been sold per year from 1986 until 2000, and that numbers were declining to about 1 million per year by 2016. Data on sales and distributors were easily accessible from the Texas Department of Public Safety until 2016, when keeping of these records was transferred to the DEA (making them virtually impossible to obtain) (Ermakova et al., 2022). In Texas, all legal harvesting happens in the Tamaulipan thorn scrub ecoregion. There are also anecdotal reports of Mexican peyote being smuggled into the USA to then be sold legally via distributors to the NAC or illegally to whoever else.
UN drug conventions only list mescaline, thus in most of the world except for the USA, it is legal to grow and cultivate L. williamsii as long as it is for horticultural purposes. In Mexico peyote regulation occurs at the intersection of several legislations: environmental protection, drug control and Indigenous interests (Labate and Feeney, 2016; Guzmán and Labate, 2019).
Conservation
Despite its wide distribution, L. williamsii has been declared as a vulnerable species by the International Union for Conservation of Nature (IUCN) Red List (Terry, 2017), and in Texas it is considered imperiled (threatened) (NatureServe, 2020)*. The Government of Mexico classifies peyote as a species subject to special protection through the Official Mexican Standard, the fourth and most lax category (NOM-059-SEMARNAT-2010). The whole family of Cactaceae (with several exceptions) is included in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) appendix II, making trade of wild-harvested plants illegal (Goettsch et al., 2015).
While Mexico hosts larger peyote populations than Texas, these are not immune to anthropogenic pressures, where they face similar threats, such as habitat loss, climate change, and overharvesting Rojas-Aréchiga and Flores, 2016). Moreover, Indigenous communities in Mexico, such as the Wixárika, depend on peyote for their cultural practices (Schaefer and Furst, 1996), adding to the urgency of conservation efforts. Without significant action, both Texan and Mexican peyote populations are at risk of further decline, threatening both ecological and cultural heritage.
Texas
Possibly the greatest threat to peyote in Texas is land-use change. The native Tamaulipan thorn scrub, where peyote thrives, is being rapidly cleared for urban development, agriculture, pasture improvement, and energy infrastructure, including oil, gas, and wind power. Overharvesting is another major pressure, particularly if the harvesting is done improperly, by cutting too deep into the subterranean stem or taking the whole cactus with the root attached (Terry and Mauseth, 2006). In Texas, the situation is dire, with many wild peyote populations depleted - shortages have been reported since the 1980s. Entire populations have been eradicated in some areas due to land clearing, often involving bulldozers that remove not just the peyote but all of the native brush.
Native/Indigenous peyote practitioners are concerned about the recent wave of decriminalization of psychedelics, and some have actively campaigned to have peyote (and even mescaline) explicitly excluded. They cite concerns that decriminalization could lead to an increase in poaching of this already scarce cactus (e.g. see Navajo Nation Council’s Resolution 0141-24, 2024). However, this is unlikely to happen, at least when it concerns infrequent non-ceremonial peyote users for various reasons: a) peyote in Texas is found almost exclusively on private land, trespassing on which can be very dangerous if the owners are present; b) it is hard to find unless one has inside information on where it grows; c) peyote tourism can be conducted much more cheaply, easily and safely in Mexico (and this is where it happens in practice, regardless of legality) (Guzmán and Labate, 2019; Basset, 2016). In contrast, in Texas, when poaching occurs, it is done by the locals, who have extensive knowledge of where it can be found. However, cultural appropriation and commercialization are a very valid concern, it being disrespectful when people organize ceremonies for personal profit and gain. Many Native Americans oppose recreational use, sale of the ceremonies and appropriation of their rituals, but especially commercial entities like pharmaceutical companies or commercial growers coming to cash in on their sacred plant. Many Native Americans regard existing criminal legislation as a means to safeguard their sacred plant and cultural traditions (Sahagún, 2020)* and prefer to implement their own nation-wide strategy, driven by Native American people, to protect, conserve and ensure the spiritual and ecological sustainability of peyote. This is considered preferable rather than having to rely on the existing environmental protection pathways (e.g. listing a species under the Endangered Species Act), although specific details of it have not been publicly known (Jaeger, 2021; Golden, 2022)*.
Mexico
In Mexico, peyote faces the expansion of agro-businesses, increased pesticide and fertilizer use, and growing interest in peyote tourism. Tourists are driven by Mexico’s strong cultural association with psychoactive medicine traditions. Peyote tourists have been flocking to San Luis Potosí since the late 1960s, with numbers increasing. Peyote-centered retreats are plentiful, as well as ‘pick-your-own’ tours in the desert, or one can simply purchase peyote at the local markets (Basset, 2016). In Mexico, peyote is also used in folk medicine, particularly in topical analgesic ointments, though the actual peyote content of such products is unclear (LeBlanc et al., 2021). In folk medicine it is used to treat arthritis, infections, asthma and to treat snake and scorpion bites, and it is also used as a stimulant (Rojas-Aréchiga and Flores, 2016). Under Mexican law, only Indigenous groups are authorized to harvest and ingest peyote, but that doesn’t seem to stop the “peyote tourism” industry (Basset, 2016).
Despite its larger populations, Mexican peyote is under pressure from land-use change, climate change, and unregulated harvesting on common lands. The Huichol or Wixárika are among the oldest continuously surviving Indigenous cultural traditions known for their intimate connection to peyote, and their yearly pilgrimage in search of peyote in Wirikuta has been dated back to 200 AD. Each year their communities make an extensive pilgrimage to the sacred land of Wirikuta spanning over 500km, to the desert area of the Sierra de Catorce in San Luis Potosí, Mexico. In 1999, UNESCO recognized Wirikuta as one of the world’s 14 Sacred Natural Sites. In 2010 the Huichol communities discovered that over 70% of land there has been allocated to mining concessions, which presents a threat to peyote and biodiversity as a whole in this part of the Chihuahuan desert. The concern led to the creation of the Wixárika Regional Council for the Defense of Wirikuta (CRW) in 2011, aimed at safeguarding, protecting, and defending the sacred land of Wirikuta. Since then, a prolonged legal battle has been ongoing, with suspension of all mining activities until it is resolved (Arellano, 2024)*. Climate change is another important issue, along with the rapid regional expansion of agro-businesses such as factories for eggs, chicken, pork, tomatoes, cucumbers, chili, and bell peppers. These large-scale projects have been implemented despite state and national decrees designating this region as a protected area for flora and fauna. For example, around 2000 hectares from the 140,000-hectare Wirikuta cultural and ecological reserve has been converted to tomato plantations (Negrín, 2021)*. Much of the land in Wirikuta was previously owned communally by ejidatarios (shareholders in common land). However, in recent years the Mexican government has created laws that permit privatization. This process of privatization of the water and the desert by agribusinesses, sometimes financed “with resources from illicit origins” is a huge threat – and is potentially harder to defend against than mining (Negrín, 2021; Busby and Virdi, 2022). Several Huichol leaders have been killed in Mexico, for defending rights on behalf of their sacred land: Margarito Díaz, Vázquez Torres and Agustín Torres (Dinechin, 2024)*.
Potential conservation actions
Alternative sources of alkaloids
One supplementary means of reducing pressure on wild populations of psychedelicspecies is to tap into alternative sources of alkaloids, potentially achieved through botanical alternatives, or through other means of producing the desired alkaloids. Outside of its Amazonian context, various ayahuasca analogues have been concocted, resulting in effects emulating those of ayahuasca, using different botanical sources (Ott, 1999)*. Syrian rue (Peganum harmala) offers a more sustainable source of beta-carboline MAOI compounds. The seeds of the plants are a far more potent source of these alkaloids than the ayahuasca vine (Hemmateenejad et al., 2006), and these seeds can be harvested without damaging the plant, unlike harvesting the B. caapi vine. Syrian rue has a cosmopolitan distribution, having become invasive in some parts of the world) and it is a tenacious and easily grown plant (USDA, 2014; Invasive Plants of the United States, n.d.; Navajo Nation Council, 2024; Weeds Australia, n.d.)*.
Other species of Tabernanthe and related genera of plants comprising the Apocynaceae family to which T. iboga belongs such as Tabernaemontana and Voacanga also produce iboga alkaloids (Kombian et al., 1997; Krengel et al., 2019a). Greater investigation and cultivation of other related species could play a role in taking the pressure off wild populations of T. iboga (Krengel et al., 2019b; Iyer et al., 2021). Voacanga africana holds promise, being much more abundant than T. iboga, with the alkaloid voacangine it produces acting as a viable precursor for the semi-synthesis of ibogaine (Jenks, 2002). Development of synthetic ibogaine derivatives such as 18-methoxycoronaridine (18-MC) (Glick et al., 1996) may provide an additional option. However, iboga is still likely to remain in demand for the therapeutic potential of its particular alkaloid profile, so cultivation of other plants and synthetic ibogaine derivatives such as 18-MC aren’t likely to fully accommodate this demand (Dickinson, 2016). Another potential means of producing ibogaine and other iboga alkaloids would be its transgenic biosynthesis via yeast or E. coli, as is already possible for psilocybin and DMT and their related tryptamine derivatives (Milne et al., 2020; Friedberg et al., 2023).
Aside from peyote, several species of currently abundant, fast-growing and easily cultivated columnar cacti are a more sustainable mescaline alternative. These species belong to the genus Echinopsis (although taxonomy is unresolved), and include Echinopsis lageniformis (syn. Trichocereus bridgesii), Echinopsis macrogonus (esp. var. pachanoi), Echinopsis peruviana (syn. Trichocereus peruvianus) (Trout, 2005)*.
As for Sonoran Desert toads, pure synthetic 5-MeO-DMT is a viable alternative. A few other possible and relatively novel 5-MeO-DMT synthesis options to consider could be transgenic biosynthesis via E. coli (Friedberg et al., 2023) or cell-based biosynthesis of I. alvarius parotoid gland secretions (including 5-MeO-DMT) (Lerer et al., 2023).
Education and changing behaviors of psychedelic consumers
It is important to raise awareness about ecology and conservation among potential consumers of naturally occurring psychedelics, hopefully reducing demand. Although there will inevitably be people who will always believe that natural “plant and toad medicine” is always better than the “synthetic drugs, maybe the idea of reciprocity and highlighting an inherent imbalance of seeking personal healing by harming another living being (and threatening the survival of its species) may be enough to make at least some people reconsider. A survey of mescaline consumers (n=284) revealed a strong preference for naturally derived sources, with most respondents indicating they consumed plant-based mescaline (56.7% had consumed San Pedro-based mescaline, 22.9% had consumed peyote-based mescaline), while only 16.6% reported using synthetic mescaline; moreover, 20.0% reported consuming peyote collected from native habitats (Engel et al., 2023). In a more recent survey with a large sample of psychedelic consumers (Global Drug Survey), 56% of 6379 respondents reported preference for mescaline-containing cacti, 9% preferred synthetic mescaline, 21% had no preference, and the rest had no interest in the substance. However, many of the mescaline-cacti consumers (67.7% of the respondents, n = 3691) indicated they were conscious about the potential environmental impact and would happily switch to synthetic substances (Syed et al., 2024). Interestingly, across five naturally occurring psychedelic substances, the highest preference among respondents for using synthetic sources was for 5-MeO-DMT, and qualitative data additionally confirmed the concern for I. alvarius toads (Syed et al., 2024). Of course, this survey, however large, was still a convenience sample of the English-speaking psychedelic users, so the responses obtained may not necessarily reflect those of the broader psychedelic consuming population. Nevertheless, these data indicate that while there is a clear preference for naturally derived psychedelics, potential consumers could be open to switching to synthetic substances; another natural alternative (e.g. substituting peyote for San Pedro); or an ethically grown plant (for example grown in a greenhouse or intentionally planted rather than looted from the wild).
The situation with psychoactive plants is complicated because they exist at the complex intersection between law enforcement and drug regulation; environmental regulations (or lack thereof); Indigenous rights; and religious and spiritual beliefs. How people navigate this complexity very much depends on one’s worldview, belief system, culture, traditions, values and the intention for consuming the psychedelic substance in question. For example, it would be highly unethical to suggest replacing a sacrament central to one’s spiritual beliefs with another plant. However, this suggestion can be perfectly acceptable for a ludibund user. Likewise, one can recommend growing one’s own plants - but the legality of this depends on where one is based. For example, San Pedro cultivation is legal in the USA and illegal in Canada, and vice versa for peyote. All of the above are also highly region-dependent, so it is difficult to provide recommendations that are applicable across different geographical areas and to multiple substances. That caveat aside, it is important to advocate for the availability of alternative sources of psychoactive substances over harvesting them from the wild. In many cases cultivation is the most obvious and readily available option [e.g. for discussion on peyote cultivation see (Ermakova et al., 2022)].
It is possible to use the ‘harm reduction’ approach and come up with the environmentally informed ethical guidelines for each of the naturally-occurring psychedelics. For example, a non-profit organization Entheogenesis Australis has created an identification guide to common acacias (that can be harvested for their DMT content), in order to protect rarer, more vulnerable species and other useful guides (Engel, 2025)*. Figure 2 contains an infographic with synthesis of available information about harm reduction practices during harvesting for our case species. It is important to highlight that there are no sustainable harvesting guidelines for any of the species, and many recommendations are distilled from the reports by various NGOs or journalistic articles, or anecdotal evidence discovered in conversations with people working in the field, and the main recommendation is to discourage wild-harvesting where possible. Evidence-based guidelines for sustainable harvesting need to be developed in the future, as more research is published.
Local and Indigenous-led initiatives
Community-led cultivation initiatives of these psychedelic plants offer a pathway to sustainable production of these species. The Indigenous Medicine Conservation Fund deserves recognition for its work to safeguard all four species. It is an Indigenous-led philanthropic organization supporting efforts that seeks the sovereign protection and regeneration of these medicines, the ecologies in which they are embedded, and the traditional knowledge informed practices they form an integral part among the various Indigenous communities with which these species have a deep relationship (Indigenous Medicine Conservation Fund, 2025)*. Given that a growing international demand for ayahuasca threatens wild populations in Amazonia, cultivation outside of the Amazonian region may be a necessity, and such efforts are underway in such areas (Kilham, 2018; Meyer, 2021)*. B. caapi vines do not require maintenance when growing in an optimal setting, and they can be harvested in a sustainable manner, with regeneration possible if the rootstock of the plants remain in the ground (Kilham, 2018)*. New ayahuasca plants can also be propagated by the planting of vine cuttings (Coe and Gaoue, 2023). B. caapi is a tenacious plant, and fairly easily grown in the tropics and subtropics, e.g. in Hawaii (Álvarez, 2019)*. Local people based around the city of Iquitos in Peru (which could be considered as an epicenter of ayahuasca tourism in the Amazon) have already begun cultivating B. caapi vine, with it being viewed as a ‘cash crop’ (Álvarez, 2019)*. A number of ayahuasca retreat centers around Iquitos have also been practicing ayahuasca cultivation, with some seeking to be totally self-sufficient in time (Kilham, 2018)*. However, existing populations around Iquitos are not sufficient to meet a growing international demand (Álvarez, 2019)*.
The growing global demand for iboga and the resulting overharvesting has inspired some recent efforts to establish iboga cultivation in Gabon, in addition to Cameroon, Ghana and the Ivory Coast, with some manufacturers of iboga-derived medicines supporting some of the latter efforts. One notable initiative being spearheaded in Gabon is being undertaken by the NGO Blessings of the Forest, which is committed to preserving Gabon’s natural and cultural heritage. Through its initiatives it seeks to protect biodiversity and promote sustainable resource management while improving the living conditions of rural communities, in addition to facilitating collaboration between local stakeholders and international partners. It has supported the establishment of iboga plantation by rural communities, with 13 village associations having planted 24,000 iboga trees on 14 plantations as of early 2023 (Nuwer, 2023)*.
For peyote, some Native American tribes have begun working on legal pathways to cultivate peyote for their NAC members (Muneta, 2020), and there are sustainable harvesting, cultivation and repopulation efforts undertaken by the Indigenous Peyote Conservation Initiative in South Texas (IPCI, 2025)*. Such models could be replicated elsewhere.
Regenerative agroforestry approaches
Both B. caapi and T. iboga are plants that inhabit the forest understory, tolerant of partial shade. This makes both plant species amenable to regenerative agroforestry or conservation orientated approaches to cultivation. Given that B. caapi is tolerant of growing in full sun or partial shade, it can be cultivated in open forest settings, or as part of sustainable agroforestry practices (Porro et al., 2012), potentially using successional poly-cropping to ensure efficiency. B. caapi (and P. viridris) are already grown in this manner by members of the UDV, with the B. caapi vine requiring structural support of trees and other vegetation, with this more ecologically holistic approach to cultivation potentially partly motivated by the deepened spiritual connection to nature that consumption of the ayahuasca brew may elicit (Thevenin, 2017). Cultivation of these plants has also been found to be potentially beneficial for the maintenance and restoration of forests in degraded areas, while supporting biodiversity (Thevenin and Sambuichi, 2020). One approach to sustainable or regenerative cultivation well suited to B. caapi is syntropic agroforestry, which blends Indigenous and scientific knowledge, follows a no-impact to low-impact approach that is underpinned by ecological principles, and offers a scalable means of achieving agricultural productivity that can support biodiversity and ecological restoration. This approach is being increasingly adopted in Brazil and elsewhere (Andrade et al., 2020).
A similar approach to iboga cultivation could be adopted as is already being applied to ‘forest coffee’ cultivation as practiced in the Bale Mountains National Park in Ethiopia in the Afromontane forests that comprise coffee’s native habitat (Senbeta and Denich, 2006), with Coffea arabica being a forest understory shrub, similar to iboga. This would allow for the cultivation of iboga in the forest understory while helping maintain the forest habitat and its associated biodiversity. Alternatively, given its preference for forest understory and partial shade, iboga cultivation could potentially be integrated into sustainable agroforestry practices (Hetemäki et al., 2023)*. One such approach is dynamic agroforestry, which is underpinned by ecological principles, and seeks to combine agricultural productivity as part of natural forest-like systems that support biodiversity and provide a range of beneficial ecosystem services (Malka, 2023)*.
Discussion
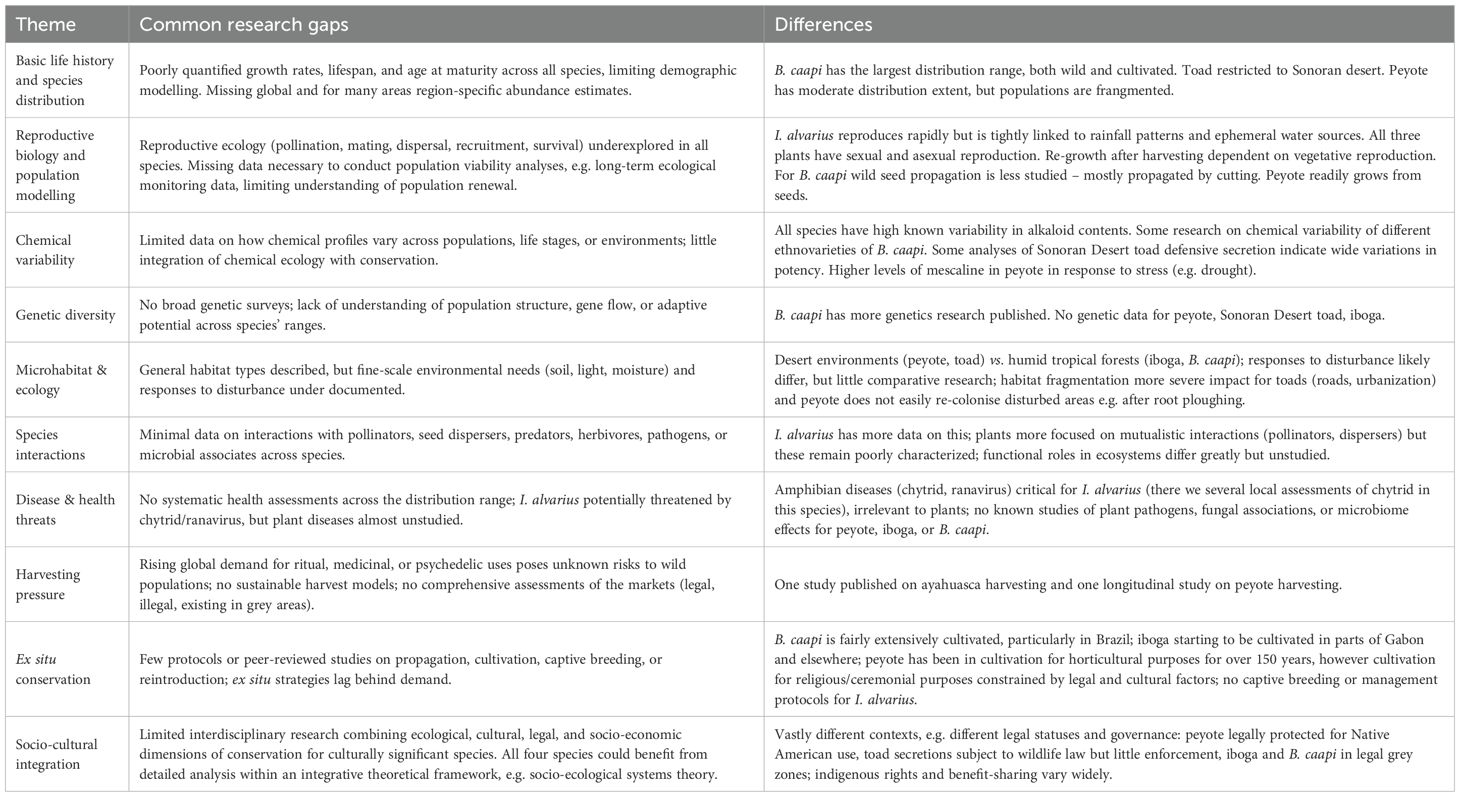
Despite the variety of species and their encompassing context, they have one notable feature in common – a lack of recent comprehensive research on their biology, ecology and conservation. Multiple books and articles have been written about their chemistry, pharmacology and potential clinical applications, patterns of recreational use of their psychoactive alkaloids and their Indigenous usage and cultural traditions within the discipline of anthropology. In comparison, ecological and biological research on these species is lagging behind (see Table 2 for the research gaps). Yet, for any evidence-based conservation actions it is crucial to have knowledge of the species’ distribution, population dynamics, reproductive ecology, and response to varying levels of harvesting pressures, in addition to the optimal cultivation and propagation techniques for the plants. Thus, a critical first step toward figuring out what is going on with these species is to establish a population-monitoring program to determine both geographical extent and population sizes, ideally leading towards a comprehensive management plan for the species. These measures could help to determine if there is a need for conservation initiatives that safeguard these species in their natural habitats. This could be achieved through an implementation of natural protected areas, restoration management and repopulation programs, conducted with transparency and according to IUCN guidelines.
To ensure the long-term survival of these species their conservation and sustainable use is essential, as each plays an important ecological role, enriching the ecosystems they are part of. Beyond this, these species form a central aspect of the cultural identity of the Indigenous groups that use them, comprising a form of biocultural heritage for these cultures of profound importance (Hofmann et al., 2001). There is also a growing body of evidence to suggest that the psychedelic alkaloids these species harbor may be beneficial for mental health, including in the treatment of otherwise intractable conditions (Griffin and Knight, 2024; Vamvakopoulou and Nutt, 2024). It is important to address the ethics of using these species or psychedelic substances derived from them, exploring the issues of bioprospecting, intellectual property rights and benefit-sharing in line with the Nagoya protocol. Given the complicated legal landscapes (locally and internationally) these species exist in, due to the presence of scheduled psychoactive substances and variable conservation policies, it is important to investigate how these policies fail or support species conservation. These issues are complex, context-specific and require in-depth analysis beyond the scope of this review.
Experience with psychedelics has also been associated with a deepened sense of nature connectedness (otherwise referred to as nature relatedness in the literature) (Kettner et al., 2019) and found to predict enhanced pro-environmental awareness and behaviors (Forstmann and Sagioglou, 2017). Nature connectedness is considered a strong psychological predictor of environmental concern and pro-environmental and pro-conservation behaviors (Mackay and Schmitt, 2019; Martin et al., 2020; Richardson et al., 2020; Whitburn et al., 2020) having been described as a ‘core conservation concern’ (Zylstra et al., 2014). This capacity of psychedelics to enhance nature connectedness is made all the more salient given a notable lack of effective interventions for reducing people’s environmentally destructive behavior (Fransson and Gärling, 1999; Prescott and Logan, 2017). This gives us hope that popularization of psychedelics could not only have negative consequences (like commercialization and overexploitation), but bring a positive change, and even potentially be a new vehicle for conservation action. This can be partially achieved through increasing awareness about naturally occurring psychedelics and the ecosystems in which they are found. Reciprocity and interconnectedness should be central to all education approaches, emphasizing respect for the plant or animal species, its origins, native ecosystem and its traditional stewards.
The main limitation of this research is that it is a narrative review and thus has a higher risk of bias due to the subjective nature of the selection and interpretation of studies. Another limitation is that in many cases there were no peer-reviewed studies identified through the publicly available databases, and we had to rely on the reports, journalistic investigations and other grey literature sources. We encourage further research to fill the knowledge gap identified, and to provide material for subsequent systematic reviews going into more depth for each species described.
Conclusion
In conclusion, while the resurgence of global interest in psychedelics offers exciting potential for mental health treatment, spiritual exploration, and cultural revival, it also brings potential conservation challenges for the plant and animal species that produce these fascinating compounds. Peyote, ayahuasca vine, iboga, and the Sonoran Desert toad all face mounting pressures from overharvesting, habitat loss, climate change, and cultural commodification, yet ecological research on their biology, population dynamics, and sustainable use remains strikingly inadequate. Yet, without population viability analyses, long-term ecological monitoring data, and region-specific abundance estimates it would be impossible to substantiate conservation claims. Moreover, all these species exist in a peculiar nexus of criminal, environmental and healthcare issues, and require interdisciplinary approaches combining ecological, cultural, legal, and socio-economic dimensions of conservation. Effective conservation strategies must go beyond ecological protection alone, embracing a biocultural approach that respects Indigenous knowledge systems, supports local stewardship, and balances human well-being with environmental integrity.
Author contributions
AE: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing. SG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research or authorship of this article. The Cactus Conservation Institute supported the publication fee for the article.
Conflict of interest
AE is a consultant to Beckley Psytech a biotech company developing synthetic 5-MeO-DMT for mental health treatments, but this has no connection to this research. AE is also a board member of the Cactus Conservation Institute, a non-profit organization dedicated to the study and preservation of vulnerable cacti.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Authors used ChatGPT to generate the imagery used in Figure 2.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albuquerque F. S., Bateman H. L., and Johnson J. (2024). Amphibians at risk: Effects of climate change in the southwestern North American drylands. Glob. Ecol. Conserv. 51, e02944. doi: 10.1016/j.gecco.2024.e02944
Álvarez C. S. (2019). Pieces of Ayahuasca, a cash crop. Available online at: https://chacruna.net/pieces-of-ayahuasca-a-cash-crop/ (Accessed January 22, 2025).
Álvarez C. S. (2020). We Are Harvesting Ayahuasca at an Alarming Rate. Available online at: https://kahpi.net/harvesting-ayahuasca-exploitation (Accessed January 22, 2025).
Álvarez C. S. and Mazarrasa J. (2023). Ayahuasca, Global Consumption & Reported Deaths in the Media (Barcelona, Catalonia, Spain: ICEERS).
Anaya D. M. and Rubio O. R. G. (2010). Análisis espacial por índices de distancia (sadie) de Lophophora williamsii en tres parcelas con diferente grado de perturbación en San Luis Potosí. VII Simposio Internacional sobre la Flora Silvestre en Zonas Áridas. Universidad de Sonora (Sonora, México: Universidad de Sonora Hermosillo).
Andrade D., Pasini F., and Scarano F. R. (2020). Syntropy and innovation in agriculture. Curr. Opin. Environ. Sustain 45, 20–24. doi: 10.1016/j.cosust.2020.08.003
Arellano A. (2024). The Wixárika community’s thirteen-year legal battle to stop mining in their sacred territory. Mongabay.
Basset V. (2016). “New age tourism in Wirikuta: conflicts and rituals,” in Peyote: History, Tradition, Politics, and Conservation. Eds. Labate B. C. and Cavnar C. (Santa Barbara, California: ABC-CLIO/Praeger Publishers).
Bello E. A. C. (2021). “Medicinas ancestrales” en la Ciudad de México. Reesemantización de sus usos en el siglo XXI (Mexico City: UAM, Unidad Cuajimalpa, División de Ciencias Sociales y Humanidades).
Bernal A. M. O. (2024). 5-MeO-DMT as a Mental Health Tool: Exploring Possible Mechanisms of Action, the Reactivation Phenomenon, and Historical-Cultural Perspectives on Its Use (Madison, Wis: University of Wisconsin–Madison).
Bhave A. (2024). Magic of the mushrooms: effects of psilocybin decriminalization. J. Psychoact. Drugs 2024, 1–10. doi: 10.1080/02791072.2024.2376755
Blais B. R., Shaw C. J., Brocka C. W., Johnson S. L., Lauger K. K., et al. (2024). Anthropogenic, environmental and temporal associations with vertebrate road mortality in a wildland–urban interface of a biodiverse desert ecoregion. R Soc. Open Sci. 11, 240439. doi: 10.1098/rsos.240439
Bouso J. C., Fornís I., Ventura M., De Loenen B., Sainz-Cort A., Jiménez-Garrido D. F., et al. (2020). An analytical study of iboga alkaloids contained in Tabernanthe iboga-derived products offered by ibogaine treatment providers. Arch. Clin. Psychiatry São Paulo 47, 51–54. doi: 10.1590/0101-60830000000231
Braczkowski A., Ruzo A., Sanchez F., Castagnino R., Brown C., Guynup S., et al. (2019). The ayahuasca tourism boom: An undervalued demand driver for jaguar body parts? Conserv. Sci. Pr 1, e126. doi: 10.1111/csp2.126
Briseño-Sánchez M. I., Martínez-Peralta C., and Mandujano M. C. (2020). Population structure and reproductive biology of peyote (Lophophora diffusa, Cactaceae), a threatened species with pollen limitation1,2. J. Torrey Botanical Soc. 147, 243–257. doi: 10.3159/TORREY-D-18-00055.1
Brown T. K. and Alper K. (2018). Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes. Am. J. Drug Alcohol Abus 44, 24–36. doi: 10.1080/00952990.2017.1320802
Busby M. and Virdi J. (2022). Peyote in Mexico threatened by big business and tourism. Available online at: https://www.opendemocracy.net/en/oureconomy/peyote-mescaline-psychedelic-Mexico-agroindustry-wirikuta/ (Accessed January 28, 2025).
Carod-Artal F. J. (2015). Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurol. Engl. Ed 30, 42–49. doi: 10.1016/j.nrleng.2011.07.010
Carreon M. (2024). Shamans fear the extinction of Ayahuasca in the Amazon. Available online at: https://doubleblindmag.com/shamans-fear-extinction-of-ayahuasca/ (Accessed January 22, 2025).
Cei J. M., Erspamer V., and Roseghini M. (1972). “Biogenic amines,” in Evolution in the genus Bufo. Ed. Blair F. W. (University of Texas Press, Austin).
Celidwen Y., Redvers N., Githaiga C., Calambás J., Añanos K., Chindoy M. E., et al. (2023). Ethical principles of traditional Indigenous medicine to guide western psychedelic research and practice. Lancet Reg. Heal Am. 18, 100410. doi: 10.1016/j.lana.2022.100410
Chan C. B., Poulie C. B. M., Wismann S. S., Soelberg J., and Kristensen J. L. (2021). The alkaloids from Lophophora diffusa and other “False peyotes. J. Nat. Prod. 84, 2398–2407. doi: 10.1021/acs.jnatprod.1c00381
Chavarro-Mesa E., Almeida JV dos A., Silva S. R., Lopes S. S., Barbosa J. B. F., Oliveira D., et al. (2024). The mitogenomic landscape of Banisteriopsis caapi (Malpighiaceae), the sacred liana used for ayahuasca preparation. Genet. Mol. Biol. 47, e20230301. doi: 10.1590/1678-4685-GMB-2023-0301
Cherian K. N., Keynan J. N., Anker L., Faerman A., Brown R. E., Shamma A., et al. (2024). Magnesium–ibogaine therapy in veterans with traumatic brain injuries. Nat. Med. 30, 373–381. doi: 10.1038/s41591-023-02705-w
Coe M. A. and Gaoue O. G. (2023). Increased clonal growth in heavily harvested ecosystems failed to rescue ayahuasca lianas from decline in the Peruvian Amazon rainforest. J. Appl. Ecol. 60, 2105–2117. doi: 10.1111/1365-2664.14488
Cousins D. and Huffman M. A. (2002). Medicinal properties in the diet of gorillas. Afr. study Monogr. 23, 65–89. doi: 10.14989/68214
Davis A. K., Barsuglia J. P., Lancelotta R., Grant R. M., and Renn E. (2018). The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J. Psychopharmacol. 32, 779–792. doi: 10.1177/0269881118769063
Davis A. K., So S., Lancelotta R., Barsuglia J. P., and Griffiths R. R. (2019). 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am. J. Drug Alcohol Abus 45, 161–169. doi: 10.1080/00952990.2018.1545024
Davis A. K., Xin Y., Sepeda N., and Averill L. A. (2023). Open-label study of consecutive ibogaine and 5-MeO-DMT assisted-therapy for trauma-exposed male Special Operations Forces Veterans: prospective data from a clinical program in Mexico. Am. J. Drug Alcohol Abus 49, 587–596. doi: 10.1080/00952990.2023.2220874
De Oliveira R. C., Behrens C. S. B., Nagamine-Pinheiro N., Fagg C. W., e Silva M. S., Martins-Silva T., et al. (2023). Ethnobotany and Wood Anatomy of Banisteriopsis caapi Ethnotaxa and Diplopterys cf. pubipetala, Components of Ayahuasca in Brazilian Rituals. Econ. Bot. 77, 18–47. doi: 10.1007/s12231-023-09567-w
Dickinson J. (2016). Iboga root: dynamics of Iboga’s African origins and modern medical use. HerbalGram 109, 48–57.
Dinechin P. (2024). Murder, drought and peyote: the deadly struggle for Mexico’s water (Kings Place, London: The Guardian).
Dinis-Oliveira R. J., Pereira C. L., and da Silva D. D. (2019). Pharmacokinetic and pharmacodynamic aspects of peyote and mescaline: clinical and forensic repercussions. Curr. Mol. Pharmacol. 12, 184–194. doi: 10.2174/1874467211666181010154139
Domínguez-Sánchez S. (2020). List of species of Mexico at risk and protected by the NOM-059. Available online at: https://library.ucsd.edu/dc/object/bb6865510g (Accessed January 1, 2025).
Durán A. T. (2023). Vine of the soul: the untold consequences of Ayahuasca tourism. Available online at: https://www.latamkcl.co.uk/elcortao/vine-of-the-soul-the-untold-consequences-of-ayahuasca-tourism (Accessed January 21, 2025).
Egger K., Aicher H. D., Cumming P., and Scheidegger M. (2024). Neurobiological research on N,N-dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors. Cell Mol. Life Sci. 81, 395. doi: 10.1007/s00018-024-05353-6
El-Seedi H. R., Smet PAGMD, Beck O., Possnert G., and Bruhn J. G. (2005). Prehistoric peyote use: Alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. J. Ethnopharmacol. 101, 238–242. doi: 10.1016/j.jep.2005.04.022
Engel L. (2025). Learn to identify common acacias. Available online at: https://www.entheogenesis.org/ega-news/common-wattles (Accessed January 28, 2025).
Engel L., Barratt M., Ferris J., Puljevic C., and Winstock A. (2023). Mescaline, Peyote and San Pedro: Is sustainability important for cacti consumers? J. Psychedelic Stud. 7, 135–142. doi: 10.1556/2054.2023.00252
Ermakova A. O. (2021). Iboga conservation. Available online at: https://chacruna.net/iboga_conservation/ (Accessed January 27, 2025).
Ermakova A. O., Dunbar F., Rucker J., and Johnson M. W. (2021a). A narrative synthesis of research with 5-MeO-DMT. J. Psychopharmacol. 36 (3), 026988112110505. doi: 10.1177/02698811211050543
Ermakova A. O., Terry M. K., and Trout K. (2022). Cultivation as a conservation tool for cacti: review of the botanical evidence and a case study of Lophophora williamsii. Bradleya 2022, 71–82. doi: 10.25223/brad.sp40.2022.a8
Ermakova A. O., Whiting C. V., Trout K., Clubbe C., Terry M. K., Fowler N., et al. (2021b). Densities, plant sizes, and spatial distributions of six wild populations of Lophophora williamsii (Cactaceae) in Texas, U.S.A. JBRIT 15, 149–160. doi: 10.17348/jbrit.v15.i1.1057
Erspamer V., Vitali T., Roseghini M., and Cei J. M. (1967). 5-Methoxy- and 5-Hydroxyindoles in the skin of Bufo alvarius. Biochem. Pharmacol. 16, 1149–1164. doi: 10.1016/0006-2952(67)90147-5
Faura R. and Langlois A. (2019). Visions on iboga/ine from the international community. Phase I report (Barcelona, Catalonia, Spain: International Center for Ethnobotanical Education, Research and Service (ICEERS).
Faura R. and Langlois A. (2020). The future of iboga: perspectives from Central Africa. Phase II report (Barcelona, Catalonia, Spain: International Center for Ethnobotanical Education, Research and Service (ICEERS).
Faura R. and Langlois A. (2021). Charting a path forward for iboga: conclusions and recommendations. Phase III report (Barcelona, Catalonia, Spain: International Center for Ethnobotanical Education, Research and Service (ICEERS).
Feeney K. (2016). “Peyote, conservation, and Indian rights in the United States,” in Peyote: History, Tradition, Politics, and Conservation (Santa Barbara, California: ABC-CLIO/Praeger Publishers).
Fernandez J. W. and Fernandez R. L. (2001). Chapter 13 “Returning to the path”: The use of iboga[ine] in an equatorial African ritual context and the binding of time, space, and social relationships. Alkaloids Chem. Biol. 56, 235–247. doi: 10.1016/S0099-9598(01)56017-4
Forstmann M. and Sagioglou C. (2017). Lifetime experience with (classic) psychedelics predicts pro-environmental behavior through an increase in nature relatedness. J. Psychopharmacol. 31, 975–988. doi: 10.1177/0269881117714049
Fotiou E. (2016). The globalization of Ayahuasca shamanism and the erasure of indigenous shamanism. Anthr Conscious 27, 151–179. doi: 10.1111/anoc.12056
Fotiou E. (2019). The role of Indigenous knowledges in psychedelic science. J. Psychedelic Stud. 4, 16–23. doi: 10.1556/2054.2019.031
Fouquette M. J., Painter C. W., and Nanjappa P. (2005). “Bufo alvarius girard 1859: Colorado River toad,” in Amphibian declines: the conservation status of United States species. Ed. Lannoo M. (Berkeley, California: University of California Press).
Fransson N. and Gärling T. (1999). Environmental concern: Conceptual definitions, measurement methods, and research findings. J. Environ. Psychol. 19, 369–382. doi: 10.1006/jevp.1999.0141
Friedberg L. M., Sen A. K., Nguyen Q., Tonucci G. P., Hellwarth E. B., Gibbons W. J. Jr., et al. (2023). In vivo biosynthesis of N,N-dimethyltryptamine, 5-MeO-N,N-dimethyltryptamine, and bufotenine in E. coli. Metab. Eng. 78, 61–71. doi: 10.1016/j.ymben.2023.05.006
Frost D. R. (2025). Incilius alvarius. Available online at: https://amphibiansoftheworld.amnh.org/Amphibia/Anura/Bufonidae/Incilius/Incilius-alvarius (Accessed January 27, 2025).
Frost D. R., Mendelson J. R., and Pramuk J. (2009). Further notes on the nomenclature of Middle American toads (Bufonidae). Copeia 2009, 418–418. doi: 10.1643/CH-08-204
Garibaldi A. and Turner N. (2004). Ecology and society: cultural keystone species: implications for ecological conservation and restoration. Ecol. Soc. 9. doi: 10.5751/ES-00669-090301
Germano J. M. and Bishop P. J. (2009). Suitability of amphibians and reptiles for translocation. Conserv. Biol. 23, 7–15. doi: 10.1111/j.1523-1739.2008.01123.x
Gicquel T., Hugbart C., Le Devehat F., Lepage S., Baert A., Bouvet R., et al. (2016). Death related to consumption of Rauvolfia sp. powder mislabeled as Tabernanthe iboga. Forensic Sci. Int. 266, e38–e42. doi: 10.1016/j.forsciint.2016.06.014
Giove R. A. (2022). The Ayahuasca ritual: Peruvian national cultural heritage and its possible integration into the primary health system. Cult y Drog 27, 17–41. doi: 10.17151/culdr.2022.27.33.2
Girard in Baird S. F. (1859). “Reptiles of the boundary, with notes by the naturalists of the survey," in Report of the United States and Mexican Boundary Survey, Under the Order of Lieut. Col. W.H. Emory, Major First Cavalry, and United States Commissioner. Volume 2, Part II, Reptiles: 1–35. (Washington, D. C.: Department of the Interior).
Glick S. D., Kuehne M. E., Maisonneuve I. M., Bandarage U. K., and Molinari H. H. (1996). 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 719, 29–35. doi: 10.1016/0006-8993(96)00056-X
Goettsch B., Hilton-Taylor C., Cruz-Piñón G., Duffy J. P., Frances A., Hernández H. M., et al. (2015). High proportion of cactus species threatened with extinction. Nat. Plants 1, 15142. doi: 10.1038/nplants.2015.142
Golden H. (2022). Inside the battle to save the sacred peyote ceremony: ‘We’re in dire straits.’. Guardian.
Greco E., Rivier L., Samorini G., and D’Arienzo A. (2024). Archaeology of psychotropic substances: The problem of analytical detection of ayahuasca. Archaeometry 66, 1328–1342. doi: 10.1111/arcm.12965
Griffin C. and Knight A. (2024). Treatment and therapy of mental health conditions in the Global South using psychedelics: A scoping review and narrative synthesis. J. Psychedelic Stud. 8 (3), 368–379. doi: 10.1556/2054.2024.00333
Group BGCI (BGCI) & ISGTS (2019). Tabernanthe iboga. In: The IUCN Red List of Threatened Species. Available online at: https://www.iucnredlist.org/species/120678584/143718006.
Group. ISAS (2022). “Incilius alvarius,” in The IUCN Red List of Threatened Species 2022. Available online at: https://www.iucnredlist.org/species/54567/53948448.
Gutiérrez-González C. E., Gómez-Ramírez M. A., Gutierrez-Garcia D., Valenzuela J., Rorabaugh J. C., et al. (2006). Interactions between Sonoran Desert Toads (Incilius alvarius) and Mammalian Predators at the Northern Jaguar Reserve, Sonora, Mexico. Sonoran Herpetologist 2, 26–27.
Guzmán M. G. and Labate B. (2019). Reflexiones sobre la expansión y legalidad del campo peyotero en México. Frontera Norte 31, 1–24. doi: 10.33679/rfn.v1i1.2060
Hemmateenejad B., Abbaspour A., Maghami H., Miri R., and Panjehshahin M. R. (2006). Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts. Anal. Chim. Acta 575, 290–299. doi: 10.1016/j.aca.2006.05.093
Hetemäki L., Tegegne Y. T., and Ochieng R. M. (2023). Outlook for Sustainable Forest Bioeconomy in Gabon, Kenya, Nigeria, South Africa and Tanzania (Circular Bioeconomy Alliance, London: Circular Bioeconomy alliance). Available online at: https://circularbioeconomyalliance.org/wp-content/uploads/2023/12/CBA_Outlook_Sustainable_Forest_Bioeconomy_2023.pdf.
Hofmann R. E. S. A., Hofmann A., and Rätsch C. (2001). Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers (Rochester, Vermont: Inner Traditions/Bear).
Holycross A., Brennan T., and Babb R. (2022). A Field Guide to Amphibians and Reptiles in Arizona (Arizona Game and Fish Department, Phoenix, Arizona: AZG&F).
Horák M., Segovia E. M., and Bello A. C. (2019). Bufo alvarius: evidencias literarias y controversias en torno a su uso tradicional. Medicina Naturista 1, 43–49.
Indigenous Medicine Conservation Fund. (2025). Impact report 2023-2024. Available online at: https://docsend.com/view/ynfz7j4qzag5dkxq (Accessed July 24, 2025).
INTERPOL (2023). Illicit trafficking of natural psychotropics from Gabon: special focus on Iboga. ENACT.Invasive Plants Canada. African-rue - Peganum harmala - inspection.Canada.ca. Available online at: https://inspection.Canada.ca/en/plant-health/invasive-species/invasive-plants/invasive-plants/african-rue (Accessed July 24, 2025).
Invasive Plants of the United States African rue: Peganum harmala (Sapindales: Zygophyllaceae): Invasive Plant Atlas of the United States. Available online at: https://www.invasiveplantatlas.org/subject.cfm?sub=6158 (Accessed July 24, 2025).
IPCI (2025). Indigenous peyote conservation initiative. Available online at: https://www.ipci.life/ (Accessed January 28, 2025).
Iyer R. N., Favela D., Domokos A., Zhang G., Avanes A. A., Carter S. J., et al. (2025). Efficient and modular synthesis of ibogaine and related alkaloids. Nat. Chem. 17 (3), 1–9. doi: 10.1038/s41557-024-01714-7
Iyer R. N., Favela D., Zhang G., and Olson D. E. (2021). The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs. Nat Prod Rep. 38 (2), 307–329. doi: 10.1039/d0np00033g
Jay M. (2019). Mescaline: A Global History of the First Psychedelic (New Haven, Connecticut: Yale University Press).
Jenks C. (2002). Extraction studies of Tabernanthe Iboga and Voacanga Africana. Nat. Prod. Lett. 16, 71–76. doi: 10.1080/1057563029001/4881
Kaasik H., Souza R. C. Z., Zandonadi F. S., Tófoli L. F., and Sussulini A. (2020). Chemical composition of traditional and analog Ayahuasca. J. Psychoactive Drugs 53, 1–11. doi: 10.1080/02791072.2020.1815911
Kamin D. (2021). The Rise of Psychedelic Retreats - The New York Times (New York City, New York: The New York Times).
Kettner H., Gandy S., Haijen E. C. H. M., and Carhart-Harris R. L. (2019). From egoism to ecoism: psychedelics increase nature relatedness in a state-mediated and context-dependent manner. Int. J. Environ. Res. Pu 16, 5147. doi: 10.3390/ijerph16245147
Kilham C. (2018). Ayahuasca sustainability field report. Available online at: https://www.medicinehunter.com/ayahuasca-sustainability-field-report-chris-kilham (Accessed January 21, 2025).
Kim V., Wilson S. M., and Woesner M. E. (2025). The use of classic psychedelics for depressive and anxiety-spectrum disorders. J. Clin. Psychopharmacol. 45, 37–45. doi: 10.1097/jcp.0000000000001941
Klein M. T., Kalam M., Trout K., Fowler N., and Terry M. (2015). Mescaline concentrations in three principal tissues of Lophophora williamsii (Cactaceae): implications for sustainable harvesting practices. Haseltonia 20, 34–42. doi: 10.2985/026.020.0107
Köck P., Froelich K., Walter M., Lang U., and Dürsteler K. M. (2022). A systematic literature review of clinical trials and therapeutic applications of ibogaine. J. Subst. Abus Treat 138, 108717. doi: 10.1016/j.jsat.2021.108717
Kombian S. B., Saleh T. M., Fiagbe N. I. Y., Chen X., Akabutu J. J., Buolamwini J. K., et al. (1997). Ibogaine and a total alkaloidal extract of Voacanga africana modulate neuronal excitability and synaptic transmission in the rat parabrachial nucleus in vitro. Brain Res. Bull. 44, 603–610. doi: 10.1016/S0361-9230(97)00284-0
Krengel F., Chevalier Q., Dickinson J., Herrera Santoyo J., and Reyes Chilpa R. (2019a). Metabolite profiling of anti-addictive alkaloids from four mexican tabernaemontana species and the entheogenic African shrub Tabernanthe iboga (Apocynaceae). Chem. Biodivers. 16, e1800506. doi: 10.1002/cbdv.201800506
Krengel F., Mijangos M. V., Reyes-Lezama M., and Reyes-Chilpa R. (2019b). Extraction and conversion studies of the antiaddictive alkaloids coronaridine, ibogamine, voacangine, and ibogaine from two Mexican tabernaemontana species (Apocynaceae). Chem. Biodivers. 16, e1900175. doi: 10.1002/cbdv.201900175
Labate B. C. and Cavnar C. (Eds.) (2014). Ayahuasca Shamanism in the Amazon and Beyond (Oxford, Oxfordshire: Oxford University Press).
Labate B. C. and Feeney K. (2016). “Paradoxes of peyote regulation in Mexico: drug conventions and environmental laws,” in Peyote: History, Tradition, Politics, and Conservation. Eds. Labate B. C. and Cavnar C. (Santa Barbara, California: ABC-CLIO/Praeger Publishers).
Labate B. C. and Jungaberle H. (Eds.) (2011). The Internationalization of Ayahuasca (Münster, North Rhine-Westphalia, Germany: LIT Verlag Münster).
LeBlanc R., Silva S. D., and Terry M. (2021). Analysis of over-the-counter analgesics purported to contain mescaline from the peyote cactus (Lophophora williamsii: Cactaceae). J. Botanical Res. Inst. Tex 15, 125–137. doi: 10.17348/jbrit.v15.i1.1055
Lerer L., Reynolds E., Varia J., Blakolmer K., and Lerer B. (2023). Incilius alvarius cell-based synthesis of 5-methoxy-N,N-dimethyltryptamine. Psychedelic Med. 1 (1), 38–42. doi: 10.1089/psymed.2022.0001
Londoño E. (2024). Drugs, sacraments or medicine? Psychedelic churches blur the line. New York Times.
Lorinc J. (2024). Magic mushroom boom in Vancouver, Toronto is a challenge for police. Bloomberg News.
Luccioni M. D., Wyman J. T., Espinoza E. O., and O’Connell L. A. (2023). Diet and chemical defenses of the Sonoran Desert toads. bioRxiv 2023. doi: 10.1101/2023.10.06.561297
Luna L. E. (1984). The concept of plants as teachers among four mestizo shamans of iquitos, Northeastern Peru. J. Ethnopharmacol. 11, 135–156. doi: 10.1016/0378-8741(84)90036-9
Luna L. E. and White S. F. (Eds.) (2018). Ayahuasca Reader: Encounters with the Amazon’s Sacred Vine. Santa Fe, New Mexico: Synergetic Press.
Luz T. Z., Cunha-MaChado A. S., and Batista J da S. (2023). First DNA barcode efficiency assessment for an important ingredient in the Amazonian ayahuasca tea: mariri/jagube, Banisteriopsis (Malpighiaceae). Genet. Resour. Crop Evol. 70, 1605–1616. doi: 10.1007/s10722-022-01522-3
Mackay C. M. L. and Schmitt M. T. (2019). Do people who feel connected to nature do more to protect it? A meta-analysis. J. Environ. Psychol. 65, 101323. doi: 10.1016/j.jenvp.2019.101323
Malka S. (2023). A short-term interdisciplinary study on the adoption and diffusion of dynamics agroforestry for cocoa small-scale farmers in Ghana Western North District. Master’s thesis. Université de Geneve, Geneva, Switzerland.
Mandujano M. C., Naranjo A. G., Rojas-Aréchiga M., and Golubov J. (2020). “Conservation Status, Germination, and Establishment of the Divine Cactus, Lophophora williamsii (Lem. Ex Salm-Dyck) J. M. Coult., at Cuatro Ciénegas,” in Plant Diversity and Ecology in the Chihuahuan Desert, Emphasis on the Cuatro Ciénegas Basin. Eds. Mandujano M. C., Pisanty I., and Eguiarte L. E. (Greer, South Carolina: Springer).
Maroukis T. C. (2012). The Peyote Road: Religious Freedom and the Native American Church (Norman, Oklahoma: University of Oklahoma Press).
Martin L., White M. P., Hunt A., Richardson M., Pahl S., and Burt J. (2020). Nature contact, nature connectedness and associations with health, wellbeing and pro-environmental behaviours. J. Environ. Psychol. 68, 101389. doi: 10.1016/j.jenvp.2020.101389
McIntyre R. S., Kwan A. T. H., Mansur R. B., Oliveira-Maia A. J., Teopiz K. M., Maletic V., et al. (2025). Psychedelics for the treatment of psychiatric disorders: interpreting and translating available evidence and guidance for future research. Am. J. Psychiatry 182, 21–32. doi: 10.1176/appi.ajp.20230902
Metzner R. (2013). Toad and the Jaguar a Field Report of Underground Research on a Visionary Medicine: Bufo Alvarius And 5-Methoxy-Dimethyltryptamine (Berkeley, California: Regent Press).
Meyer M. (2021). Global ayahuasca trend drives deforestation in Brazil’s Acre state. Available online at: https://news.mongabay.com/2021/12/global-ayahuasca-trend-drives-deforestation-in-Brazils-acre-state/ (Accessed January 21, 2025).
Milne N., Thomsen P., Knudsen N. M., Rubaszka P., Kristensen M., and Borodina I. (2020). Metabolic engineering of Saccharomyces cerevisiae for the de novo production of psilocybin and related tryptamine derivatives. Metab. Eng. 60, 25–36. doi: 10.1016/j.ymben.2019.12.007
Mowbray S. (2022). All coked up: The global environmental impacts of cocaine. Available online at: https://news.mongabay.com/2022/04/all-coked-up-the-global-environmental-impacts-of-cocaine/:~:text=Production%2C%20transit%20and%20consumption%20of,poorly%20understood%2C%20with%20many%20unresearched (Accessed January 21, 2025).
Mumford E. L., Martinez D. J., Tyance-Hassell K., Cook A., Hansen G. R., Labonté R., et al. (2023). Evolution and expansion of the One Health approach to promote sustainable and resilient health and well-being: A call to action. Front. Public Heal 10, 1056459. doi: 10.3389/fpubh.2022.1056459
Muneta J. D. (2020). Peyote crisis confronting modern indigenous peoples: the declining peyote population and a demand for conservation. Am. Indian Law J. 9.
NatureServe (2002). Incilius alvarius. Available online at: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.104462/Incilius_alvarius (Accessed January 27, 2025).
NatureServe (2020). Lophophora williamsii. Available online at: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.139920/Lophophora_williamsii (Accessed January 2, 2025).
Navajo Nation Council (2024). Opposing Decriminalizing the use of Peyote and Urging all States to Abide by the American Indian Religious Freedom Act Amendments of 1994. LEGISLATION NO: _0141-24 (2024). Available online at: https://www.navajonationcouncil.org/wp-content/uploads/2024/07/0141-24.pdf (Accessed July 24, 2025).
Ndoua P. D. N. and Vaghar K. (2018). Bwiti, iboga, trance and healing in Gabon. Ment. Heal Relig Cult 21, 755–762. doi: 10.1080/13674676.2018.1504012
Negrín D. (2021). Water and power in Wirikuta. Available online at: https://www.wixarika.org/water-and-power-wirikuta (Accessed January 2, 2025).
Nelson) AM (Ken (1984). Bufo alvarius: The psychedelic toad of the Sonoran Desert (Denton: Venom Press).
Newbold R., Silva S. D., and Terry M. (2020). Correlation of mescaline concentrations in Lophophora williamsii (Cactaceae) with rib numbers and diameter of crown (U.S.A.). J. Botanical Res. Inst. Tex 14, 103–120. doi: 10.17348/jbrit.v14.i1.901
Nutt D. J., Hunt P., Schlag A. K., and Fitzgerald P. (2024). The Australia story: Current status and future challenges for the clinical applications of psychedelics. Br. J. Pharmacol. doi: 10.1111/bph.17398
Nuwer R. (2023). The psychoactive drug ibogaine could save lives—and everyone wants to cash in. Natl. Geographic.
Oliveira R. C., Sonsin-Oliveira J., Santos T. A. C., e Silva M. S., Fagg C. W., Sebastiani R., et al. (2021). Lectotypification of Banisteriopsis caapi and B. quitensis (Malpighiaceae), names associated with an important ingredient of Ayahuasca. Taxon 70, 185–188. doi: 10.1002/tax.12407
Ortíz-Bernal A. (2024). 5-MeO-DMT as a mental health tool: Exploring possible mechanisms of action, the reactivation phenomenon, and some ethical considerations regarding the Sonoran Desert Toad. Doctoral dissertation. University of Wisconsin, Madison.
Palumbo S. (2024). Preserving the Sacred Medicine: Why Peyote Should Be Excluded from Entheogenic Plant Decriminalization Efforts. Ohio: Ohio State Legal Studies Research Paper No. 862, Ohio State University.
Peterson K. (2023). The Sonoran Desert toad can get you high. Poachers have taken notice. Natl. Geographic.
Pollan M. (2018). How to Change Your Mind: The New Science of Psychedelics (Nine Elms, London: Penguin UK).
Pope H. G. (1969). Tabernanthe iboga: an African narcotic plant of social importance. Econ. Bot. 23, 174–184. doi: 10.1007/BF02860623
Porro R., Miller R. P., Tito M. R., Donovan J. A., Vivan J. L., Trancoso R., et al. (2012). “Agroforestry in the Amazon Region: A Pathway for Balancing Conservation and Development,” in Agroforestry - The Future of Global Land Use, eds. Nair P. and Garrity D. (Dordrecht: Springer). Adv. Agrofor. 9. 391–428. doi: 10.1007/978-94-007-4676-3_20
Poulie C. B. M., Chan C. B., Parka A., Lettorp M., Vos J., Raaschou A., et al. (2023). In vitro and in vivo evaluation of pellotine: A hypnotic lophophora alkaloid. ACS Pharmacol. Transl. Sci. 6 (10), 1492–1507. doi: 10.1021/acsptsci.3c00142
Prescott S. L. and Logan A. C. (2017). Down to earth: planetary health and biophilosophy in the symbiocene epoch. Challenges 8, 19. doi: 10.3390/challe8020019
Quiroz D. and Andel T.v. (2018). The cultural importance of plants in western African religions. Econ. Bot. 72, 251–262. doi: 10.1007/s12231-018-9410-x
Ravalec V. and Mallendi A. P. (2007). Iboga: the Visionary Root of African Shamanism (Rochester, Vermont: Inner traditions/Bear).
Reckweg J. T., Uthaug M. V., Szabo A., Davis A. K., Lancelotta R., Mason N. L., et al. (2022). The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT). J. Neurochem. 162, 128–146. doi: 10.1111/jnc.15587
Richardson M., Dobson J., Abson D. J., Lumber R., Hunt A., Young R., et al. (2020). Applying the pathways to nature connectedness at a societal scale: a leverage points perspective. Ecosyst. People 16, 387–401. doi: 10.1080/26395916.2020.1844296
Rojas-Aréchiga M. and Flores J. (2016). “An overview of cacti and the controversial peyote,” in Peyote: History, Tradition, Politics, and Conservation. Eds. Labate B. C. and Cavnar C. (Santa Barbara, California: Praeger).
Romero S. (2022). Demand for This Toad’s Psychedelic Toxin Is Booming. Some Warn That’s Bad for the Toad (New York City, New York: The New York Times).
Roth S. A., Griffis-Kyle K. L., and Barnes M. A. (2023). Batrachochytrium dendrobatidis in the arid and thermally extreme Sonoran Desert. EcoHealth 20, 370–380. doi: 10.1007/s10393-023-01668-1
Ruffell S. G. D., Crosland-Wood M., Palmer R., Netzband N., Tsang W., Weiss B., et al. (2023). Ayahuasca: A review of historical, pharmacological, and therapeutic aspects. Psychiatry Clin. Neurosci. Rep. 2, e146. doi: 10.1002/pcn5.146
Sahagún L. (2020). Why are some Native Americans fighting efforts to decriminalize peyote? The Los Angeles Times, Los Angeles, California.
Santos B. W. L., Oliveira R. C. de, Sonsin-Oliveira J., Fagg C. W., Barbosa J. B. F., and Caldas E. D. (2020). Biodiversity of β-carboline profile of Banisteriopsis caapi and Ayahuasca, a plant and a brew with neuropharmacological potential. Plants 9, 870. doi: 10.3390/plants9070870
Schaefer S. B. and Furst P. R. (1996). People of the Peyote: Huichol Indian History, Religion and Survival (Albuquerque, New Mexico: UNM Press).
Schnitzer S. A. and Bongers F. (2002). The ecology of lianas and their role in forests. Trends Ecol. Evol. 17, 223–230. doi: 10.1016/S0169-5347(02)02491-6
Schultes R. E. (1986). Recognition of variability in wild plants by Indians of the Northwest Amazon: An enigma. J. Ethnobiol 6, 229–238.
Schultes R. E. and Hofmann A. (1980). The Botany and Chemistry of Hallucinogens (New York City, New York: Thomas).
Schwelm H. M., Zimmermann N., Scholl T., Penner J., Autret A., Auwärter V., et al. (2021). Qualitative and quantitative analysis of tryptamines in the poison of Incilius alvarius (Amphibia: bufonidae). J. Anal. Toxicol. 46, 540–548. doi: 10.1093/jat/bkab038
Schwertner-Charão L., Delgado-Martínez R., Treviño-Carreón J., Jiménez-Sierra C. L., Astudillo-Sánchez C. C., Osorio-Hernández E., et al. (2022). Interactions between facilitator species and Lophophora williamsii (Lem. ex Salm-Dyck) J.M.Coult. (Cactaceae) in a rosetophyllus desert scrub in México. J. Arid Environ. 205, 104824. doi: 10.1016/j.jaridenv.2022.104824
Senbeta F. and Denich M. (2006). Effects of wild coffee management on species diversity in the Afromontane rainforests of Ethiopia. For Ecol. Manag. 232, 68–74. doi: 10.1016/j.foreco.2006.05.064
Sheldrake M. (2020). The ‘enigma’ of Richard Schultes, Amazonian hallucinogenic plants, and the limits of ethnobotany. Soc. Stud. Sci. 50, 345–376. doi: 10.1177/0306312720920362
Souto L. S. and Oliveira D. M. T. (2012). Pericarp structure in Banisteriopsis C.B.Rob. and Diplopterys A.Juss. (Malpighiaceae): new data supporting generic segregation. Acta Bot. Bras. 26, 527–536. doi: 10.1590/S0102-33062012000300003
Spruce R. (1873). On some remarkable narcotics of the Amazon Valley and Orinoco. Geogr. Rev. 1, 184–193.
Stebbins R. C. (2003). A Field Guide to Western Reptiles and Amphibians (Peterson Field Guide Series) (Boston, Massachusetts: Houghton Mifflin Harcourt).
Stefanick E. R. and Miles D. B. (2023). The effects of environment on the Sonoran Desert Toad (Incilius alvarius) in a changing climate. Student expo poster. Biological Sciences Department (Athens: Ohio University).
Stringham O. C., Maher J., Lassaline C. R., Wood L., Moncayo S., Toomes A., et al. (2023). The dark web trades wildlife, but mostly for use as drugs. People Nat. 5, 999–1009. doi: 10.1002/pan3.10469
Sullivan B. K. and Malmos K. B. (1994). Call variation in the Colorado River toad (Bufo alvarius): behavioral and phylogenetic implications. Herpetologica 50 (2), 146–156. doi: 10.1007/BF00690963
Syed O. A., Petranker R., Fewster E. C., Sobolenko V., Beidas Z., Husain M. I., et al. (2024). Preferences, perceptions, and environmental considerations of natural and synthetic psychedelic substances: findings from the global psychedelic survey. J. Psychoact. Drugs 1–11. doi: 10.1080/02791072.2024.2446445
Terry M. (2017). “Lophophora williamsii (amended version of 2013 assessment),” in The IUCN Red List of Threatened Species 2017. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T151962A121515326.en (Accessed January 4, 2025).
Terry M. and Mauseth J. D. (2006). Root-shoot anatomy and post-harvest vegetative clonal development in Lophophora williamsii (Cactaceae: Cacteae): implications for conservation. SIDA Contrib. to Bot. 22, 565–592.
Terry M., Steelman K. L., Guilderson T., Dering P., and Rowe M. W. (2006). Lower Pecos and Coahuila peyote: new radiocarbon dates. J. Archaeol Sci. 33, 1017–1021. doi: 10.1016/j.jas.2005.11.008
Terry M. K. and Trout K. (2017). Regulation of peyote (Lophophora williamsii: Cactaceae) in the U.S.A.: A historical victory of religion and politics over science and medicine. JBRIT 11, 147–156. doi: 10.17348/jbrit.v11.i1.1146
Terry M., Trout K., Williams B., Herrera T., and Fowler N. (2011). Limitations to natural production of Lophophora williamsii (Cactaceae) I. Regrowth and survivorship two years post harvest in a South Texas population. JBRIT 2, 661–675.
Terry M., Herrera T., Trout K., Williams B., and Fowler N. (2012). Limitations to natural production of Lophophora williamsii (Cactaceae) II. Effects of repeated harvesting at two-year intervals in a South Texas population. JBRIT 2, 567–577.
Terry M., Williams B., Trout K., Herrera T., and Fowler N. (2014). Limitations to natural production of Lophophora williamsii (Cactaceae) III. Regrowth and survivorship two years post harvest in a South Texas population. J. JBRIT 2, 541–550.
Thevenin J. M. R. (2017). Nature in the Paths of Ayahuasca: territoriality, institutional arrangements and phytogeographic aspects of forest conservation in the Amazon. Thesis (Phd in Geography). Universidade Estadual Paulista, São Paulo, Brazil “Júlio de Mesquita Filho”, Presidente Prudente, 2017.
Thevenin J. M. R. and Sambuichi R. H. R. (2020). Phytogeography and floristics of the arbor component in União do Vegetal territories intended for the cultivation of Banisteriopsis caapi and Psychotria viridis in Rondonia. Raega O Espaço Geográfico em Análise 49, 42–63. doi: 10.5380/raega.v49i0.67208
Thompson R. C., Wright A. N., and Shafffer B. H. (2016). “California amphibian and reptile species of special concern,” in Sonoran Desert Toad Species Account (University of California Press, Oakland, California).
Tonye M. M., Asaha S., Ndam N., and Blackmore P. (2000). State of knowledge study on Tabernanthe iboga: A report for the Central African regional program for the Environment (Washington, DC: United States Agency for International Development (USAID).
Towns A. M., Quiroz D., Guinee L., de Boer H., and van Andel T. (2014). Volume, value and floristic diversity of Gabon’s medicinal plant markets. J. Ethnopharmacol. 155, 1184–1193. doi: 10.1016/j.jep.2014.06.052
Trout K. (2005). Trout’s Notes on San Pedro & Related Trichocereus Species (Austin, Texas: Mydriatic Productions).
USDA (2014). Field Guide for Managing African Rue in the Southwest (Washington, D.C., Washington: United States Department of Agriculture).
Uthaug M. V., Lancelotta R., van Oorsouw K., Kuypers K. P. C., Mason N., Rak J., et al. (2019). A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology 236, 2653–2666. doi: 10.1007/s00213-019-05236-w
Vamvakopoulou I. A. and Nutt D. J. (2024). Psychedelics: from cave art to 21st-century medicine for addiction. Eur. Addict. Res. 30 (5), 1–19. doi: 10.1159/000540062
Villa R. A. (2023). Toad in the road: Biocultural history and conservation challenges of the Sonoran Desert Toad. J. Psychedelic Stud. 7 (S1), 68–79. doi: 10.1556/2054.2023.00269
Walls S. C. and Gabor C. R. (2019). Integrating behavior and physiology into strategies for amphibian conservation. Front. Ecol. Evol. 7, 234. doi: 10.3389/fevo.2019.00234
Weeds Australia African Rue, Syrian Rue, Wild Ru, Peganum, Harmal Peganum, Harmal, Harmala. Available online at: https://weeds.org.au/profiles/african-rue-Syrian/ (Accessed July 24, 2025).
Whitburn J., Linklater W., and Abrahamse W. (2020). Meta-analysis of human connection to nature and proenvironmental behavior. Conserv. Biol. 34, 180–193. doi: 10.1111/cobi.13381
Whyte T. R. and Compton J. M. (2020). Explaining toad bones in southern appalachian archaeological deposits. Am. Antiquity 85, 305–330. doi: 10.1017/aaq.2019.104
Winkelman M. (2005). Drug tourism or spiritual healing? Ayahuasca seekers in Amazonia. J. Psychoact. Drugs 37, 209–218. doi: 10.1080/02791072.2005.10399803
Wright J. W. (1966). Predation on the Colorado River toad, Bufo alvarius. Herpetologica 22 (2), 127–128.
Keywords: psychedelic conservation, Lophophora williamsii, Banisteriopsis caapi, Incilius alvarius, Tabernanthe iboga, peyote, iboga, Sonoran Desert toad
Citation: Ermakova AO and Gandy S (2025) Of shrub, cactus, vine and toad: psychedelic species of conservation concern. Front. Conserv. Sci. 6:1569528. doi: 10.3389/fcosc.2025.1569528
Received: 01 February 2025; Accepted: 27 August 2025;
Published: 29 September 2025; Corrected: 03 October 2025.
Edited by:
Anurag Dhyani, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, IndiaReviewed by:
Sumit Chakravarty, Uttar Banga Krishi Viswavidyalaya, IndiaOlamide Fasakin, Federal University of Technology, Nigeria
Copyright © 2025 Ermakova and Gandy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna O. Ermakova, YW55YUBjaGFjcnVuYS5uZXQ=
 Anna O. Ermakova
Anna O. Ermakova Sam Gandy
Sam Gandy