- Department of Joint and Sports Medicine, Zaozhuang Municipal Hospital Affiliated to Jining Medical University, Zaozhuang, Shandong, China
Studies have indicated that the preservation of joint health and the facilitation of damage recovery are predominantly contingent upon the joint’s microenvironment, including cell-cell interactions, the extracellular matrix’s composition, and the existence of local growth factors. Mesenchymal stem cells (MSCs), which possess the capacity to self-renew and specialize in many directions, respond to cues from the microenvironment, and aid in the regeneration of bone and cartilage, are crucial to this process. Changes in the microenvironment (such as an increase in inflammatory mediators or the breakdown of the extracellular matrix) in the pathological context of arthritis might interfere with stem cell activation and reduce their ability to regenerate. This paper investigates the potential role of joint microenvironmental variables in promoting or inhibiting the development of arthritis by influencing stem cells’ ability to regenerate. The present status of research on stem cell activity in the joint microenvironment is also outlined, and potential directions for developing new treatments for arthritis that make use of these intervention techniques to boost stem cell regenerative potential through altering the intra-articular environment are also investigated. This review’s objectives are to investigate these processes, offer fresh perspectives, and offer a solid scientific foundation for the creation of arthritic treatment plans in the future.
Introduction
A degenerative condition of the joints, osteoarthritis (OA) usually results in pain, stiffness, and decreased joint function (Fingleton et al., 2015; de Rooij et al., 2016; Roos and Arden, 2016). In recent times, it has emerged as a primary source of pain and impairment among the elderly (Vos et al., 2012), mainly impairing the knee’s range of motion and other typical functions (Cao et al., 2020). With osteoarthritis (OA) affecting up to 20% of the global population, the number of patients with this condition will rise as the world’s population ages. This will unavoidably lower patients’ quality of life overall and place a significant financial burden on society’s healthcare system. In orthopaedic clinical work, cartilage lesions are fairly common. However, articular cartilage lacks intrinsic healing potential and is unable to mend itself since it is an avascular, neurogenic tissue (Curl et al., 1997). Osteoarthritis (OA) and other degenerative joint disorders are caused by increasing cartilage detachment and subchondral sclerosis, which are usually the first signs of tissue degradation (Moskowitz, 2007).
Research has shown that cartilage chondrogenic cells, also known as mesenchymal stem cells (MSCs), are highly clonal, multifunctional, and chemotactic cells that live in hyaline tissues (Pittenger et al., 1999; Prockop, 2009; Hilfiker et al., 2011). When an organism sustains damage, these stem cells can precisely locate the site of the lesion while also proliferating and differentiating as necessary to replace the destroyed tissues and finish the healing process (Dupuis et al., 2007; Quesenberry et al., 2007).
In recent years, the microenvironment has become a focus of research, and it is often mentioned more frequently in tumor diseases. Of course, it also plays an important role in the occurrence and development of other diseases (Mayani et al., 1992; Kenny et al., 2007; Arneth, 2019).
Osteoclasts cause the increased subchondral bone resorption seen in the early stages of osteoarthritis (OA), and the microenvironment within the joints has a major impact on osteoclast formation and apoptosis, for example, osteoblasts, osteocytes, or activated T cells in the microenvironment, who are capable of producing osteoclast-inducing factors, which are high in the production of a factor called receptor activator of nuclear factor κB ligand (RANKL). The receptor for RANKL is RANK, and the two of them bind, the latter being the receptor expressed by osteoclast precursors, and lastly, trigger signaling cascades via adaptor proteins like Tumor Necrosis Factor Receptor Associated Factor 6 (TRAF6), which involve the pathways of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) (Tsukasaki and Takayanagi, 2019).The results of many studies have found that osteoclasts that are highly active, which affects the histopathological changes in subchondral bone remodeling, play an important role in the development of OA, and that osteoclasts are affected by the intra-articular microenvironment, which in turn affects the microenvironment once again, resulting in a cycle that contributes to the progression of OA (Pippenger et al., 2015; Hu et al., 2021; Jiang et al., 2022).
Osteoclasts mediate increased subchondral bone resorption in early osteoarthritis. The microenvironment has an important influence on osteoclast formation, and as described in previous studies, osteoblasts, osteoclasts, and activated T cells in the intra-articular microenvironment can influence osteoclasts, as well as the function of MSCs. In addition, it is believed that the effects of the microenvironment on osteoarthritis (OA) may be mediated by stem cells, whereby the microenvironment influences stem cells, which in turn influence the ability of stem cells to repair cartilage tissue in OA.
Studies have demonstrated that the development of specific drugs can improve the animal model and the pathology of arthritis, all of which proves that the interactions between osteoclasts and the microenvironment and stem cells can be a possible avenue for the treatment of OA (Duarte, 2014). Through the secretion of immune factors, mesenchymal stem cells (MSCs) exhibit strong anti-inflammatory effects and possess immunological features that may facilitate tissue healing (Le Blanc and Mougiakakos, 2012).
Significant changes in the microenvironment will lead to changes in stem cell function, further interfering with stem cell cartilage regeneration efficacy (Berenbaum et al., 2018; Sui et al., 2019). Inflammatory variables in the joint can affect MSCs development to regulate tissue remodeling and regeneration as arthritis progresses (Shang et al., 2021). Furthermore, research has revealed that tumor necrosis factor α (TNF-ɑ) stimulates MSCs’ immunosuppressive capacity and mitigates the negative impacts of inflammatory elements in the surrounding milieu, hence facilitating tissue regeneration (Ren et al., 2008; Xu et al., 2022). Several stem/progenitor cells have been detected in bone and cartilage samples, according to the findings of several research (Zhu et al., 2010; Zhu et al., 2015; Li et al., 2020; Wang et al., 2020; He et al., 2021). Potential mechanisms and a delicate link between stem cells and the milieu were indicated by the biological targets of these cells, which included osteoclasts, dendritic cells, and macrophages (Zhu et al., 2015; Li et al., 2020; Li et al., 2021).
The composition of intra-articular microenvironment and how microenvironment affects joint function
Extracellular matrix (ECM), various cell types, metabolic components, and certain mechanical and physical characteristics make up the intricately controlled intra-articular microenvironment. Studying joint function and disease, particularly the genesis and progression of arthritis, requires an understanding of the elements that make up the intra-articular microenvironment and how they interact.
The cells that make up the joint are mostly composed of chondrocytes, synoviocytes, stem cells, and cells that are engaged in inflammation, such T cells, neutrophils, and macrophages. While synoviocytes are in charge of producing synovial fluid, which lubricates joints, chondrocytes are crucial for preserving the integrity of cartilage tissue. Mesenchymal stem cells (MSCs), in particular, are essential for joint regeneration and repair.
A wide range of growth factors like TGF-β1 (Zhen et al., 2013; Zhen and Cao, 2014; Zhang et al., 2018a), as well as inflammatory factors like IL1β, (Fujisawa et al., 1999; Kobayashi et al., 2000; Lam et al., 2000; Kudo et al., 2003; Cao et al., 2016; Pearson et al., 2017), VEGF (Plotkin et al., 2015; Cabahug-Zuckerman et al., 2016; Dai et al., 2020) are biochemical elements in the intra-articular milieu that have a significant impact on cell activity, these elements are essential for preserving joint health, encouraging the healing of injuries, and controlling inflammation.
The mechanical and physical characteristics of the joint microenvironment—such as the tension and pressure produced by joint movement and an adequate flow of nutrients and oxygen—are essential for preserving the health of the ECM and cells. These elements work together to create a dynamic and well-balanced system that controls joint function and its capacity to heal and adapt to illness.
Numerous studies have demonstrated that TGF-β1 can inhibit stem cell activation, promote osteoclast activation that leads to bone tissue destruction, induce subchondral angiogenesis, induce hypertrophy and apoptosis of chondrocytes, and induce migration of endothelial progenitor cells and bone progenitor cells (Gerber et al., 1999; Zhen et al., 2013; Zhen and Cao, 2014; Zhang et al., 2018b; Sanchez et al., 2018; Xu et al., 2018).
Furthermore, a number of research have discovered that PGE2 and IL-6 both indirectly control chondrocytes and promote the production of osteoclasts (Liu et al., 2005; Liu et al., 2006; Ni et al., 2011). Other studies have hypothesized that TNF-α, IL-1β, and IL-6 either directly or indirectly increase the differentiation of osteoclasts (Fujisawa et al., 1999; Kobayashi et al., 2000; Lam et al., 2000; Kudo et al., 2003; Cao et al., 2016; Pearson et al., 2017).
Furthermore, it has been demonstrated that VEGF encourages angiogenesis and favorably influences osteoclast recruitment, which in turn influences chondrocyte activity indirectly (Lee et al., 2002; Sanchez et al., 2008).The findings of additional research have also demonstrated that VEGF and TGF-β1 stimulate angiogenesis to indirectly regulate chondrocytes (Plotkin et al., 2015; Cabahug-Zuckerman et al., 2016; Dai et al., 2020). The detailed content is shown in Figure 1.

Figure 1. The development of arthritis is ultimately influenced by microenvironmental TNF-α and IL-1 and inflammatory factors that affect stem cells and then interfere with osteoclasts or osteoblasts. Note: Several examples of inflammatory cytokines that may be produced by inflammation include interleukin-1 (IL-1) and tumor necrosis factor- α (TNF)- α). These cytokines have the ability to activate stem cells and increase or decrease osteoclast activity, ultimately disrupting bone formation. Osteogenic growth factor (BMP) can promote the differentiation of stem cells into bone cells. The behavior of cells in joints may also be influenced by oxygen levels. Low oxygen environment can promote the development of stem cells into chondrocytes, while low oxygen environment can help activate bone cells.
Influence of the intra-articular microenvironment on the function and behavior of stem cells
The maintenance of joint health and the development and progression of joint disorders are two areas where the intra-articular milieu has a significant impact on stem cell activity and behavior. Mesenchymal stem cells (MSCs), in particular, are intra-articular stem cells that are linked to arthritis pathogenesis and cartilage regeneration and repair. Stem cell destiny and activity are influenced by a variety of intra-articular microenvironmental elements, including as extracellular matrix, cellular makeup, metabolic variables, and mechanical and physical circumstances.
Osteoclasts mediate the increased subchondral bone resorption seen in early OA. The generation of osteoclasts is largely reliant on the microenvironments in which osteoblasts produce osteoblasts and osteoclasts as well as activated T cells that express molecules that induce osteoclast formation, such as receptor activators of nuclear factor κB ligand (RANKL). Tumor necrosis factor receptor-associated factor 6 (TRAF6) adapter protein activates many signaling cascades, including as the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. Osteoclast precursors express the receptor RANK, which is bound by RANKL (Tsukasaki and Takayanagi, 2019). The activated osteoclasts will adhere to the bone and release lytic enzymes and acids that will further degrade the bone matrix. The combination of these two mechanisms may eventually lead to arthritis: Stem cell function suppression and osteoclast activation (Pippenger et al., 2015; Hu et al., 2021; Jiang et al., 2022).
Through direct cell-to-cell contact or released cytokines, intra-articular cellular components—such as chondrocytes, synoviocytes, and inflammation-associated cells—influence the proliferation, differentiation, and migration of stem cells. For instance, inflammatory cells’ cytokines may suppress stem cells’ ability to develop into cartilage while also fostering inflammation and degenerative tissue alterations.
Stem cells receive their biochemical cues and physical scaffolding from the extracellular matrix. In addition to providing structural support, extracellular matrix (ECM) constituents like collagen and proteoglycans can affect stem cell development and function by interacting with their surface receptors.
The capacity of stem cells to self-renew and differentiate is significantly impacted by inflammatory factors like IL1β and TNF-α as well as growth factors and cytokines like TGF-β and BMPs in the joint milieu. The differentiation of stem cells into certain cell lines, such chondrocytes or osteoblasts, can be encouraged or inhibited by these variables, which might impact pathological processes and joint healing.
Through our analysis of the influence of the microenvironment, which includes numerous factors, on stem cell regeneration and thus further interferes with the development of arthritis, we have shown as clearly as possible the process by which this mechanism occurs, as shown in Figure 2.
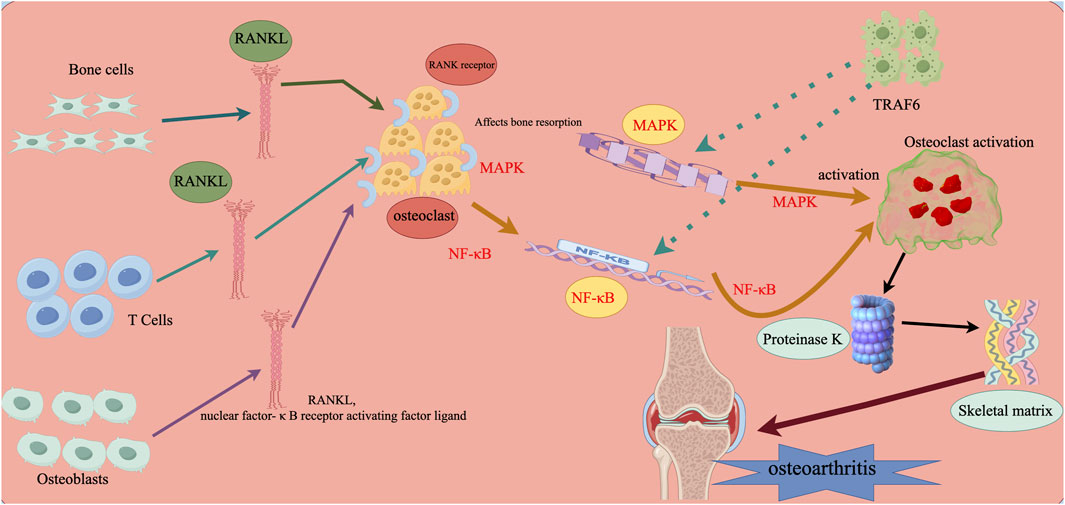
Figure 2. Microenvironmental influences on stem cell regeneration that further interfere with the development of arthritis. Note: RANKL (Nuclear Factor-κB Receptor Activator Ligand) is expressed by osteoblasts, osteoclasts, and activated T cells. It is an inducible factor that stimulates osteoclastogenesis in the microenvironment by attaching to RANK receptors expressed on osteoclast precursors and starting intracellular signaling pathways. Among these pathways are nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK). When tumor necrosis factor receptor-associated factor 6 (TRAF6) activates the relevant signaling pathways, mature osteoclasts adhere to the bone’s surface, release proteinase K, create lysozyme and acid, and degrade the bone matrix.
Stem cells in the intra-articular milieu have been shown to progress through the early, middle, and late phases of the development of arthritis. Researchers discovered signs of anabolic and catabolic metabolism in clusters of cells located in and around the articular cartilage fissures in cases of early arthritis (Lotz et al., 2010; Brack and Rando, 2012). The majority of the cells displayed Notch-1, STRO-1, and other, which are positive stem cell markers. These three stem cell markers are likewise positively stained in the central area of the arthritic cartilage (Grogan et al., 2009; Lotz et al., 2010).
The interference of the inflammatory environment within the joint on stem cell regeneration affects arthritis
Numerous investigations have shown that the local production and release of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), is a crucial component in the pathophysiology of osteoarthritis (OA) (Todhunter et al., 1996; Héraud et al., 2000; Aigner and Kim, 2002). It has been shown that nuclear factor-κB (NF-κB) is activated by TNF-α and IL-1β production. NF-κB subsequently translocates to the nucleus of the cell, where it induces inflammation, apoptosis, and the release of enzymes that degrade extracellular matrix (ECM). The expression of these factors also accelerates the extracellular matrix’s degradation and causes pain, which influences the development and progression of arthritis (Ding et al., 1998). In the intra-articular microenvironment, NF-κB promotes pro-inflammatory catabolic cytokines that in turn cause apoptosis by splitting the DNA repair enzyme poly (ADP-ribose) polymerase (PARP) and activating the pro-apoptotic enzyme caspase-3 (Shakibaei et al., 2007).
Envisioning the future of stem cell therapy for bone and joint by interfering with stem cell regeneration in the intra-articular microenvironment
A comprehensive analysis was conducted in a study that examined the influence of the intra-articular milieu, including aged chondrocytes, on the behavior and potential for regenerative capacity of stem cells. The study underlined how crucial it is to enhance this milieu in order to raise stem cell therapy’s efficacy (Cao et al., 2019). Table 1 shows some influencing factors.
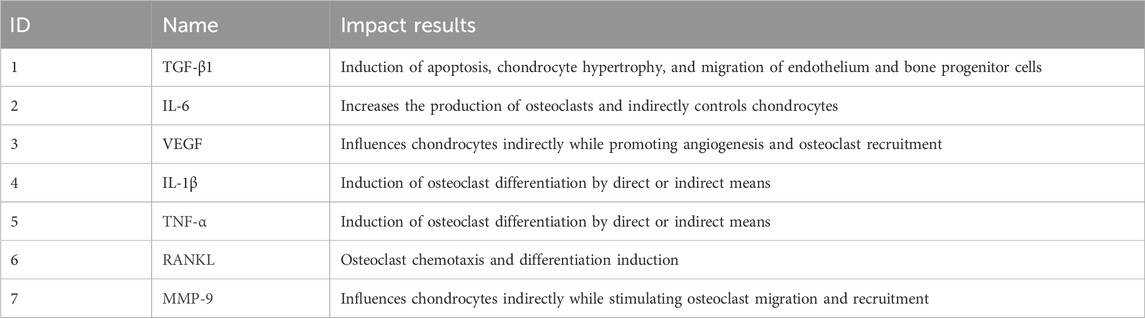
Table 1. Effects of various factors in the intra-articular microenvironment that interfere with the function of stem cells or osteoclasts.
The use of stem cells and microenvironment regulation in the treatment of osteoarthritis, as well as the application of bioengineering technology to improve the intra-articular stem cells and microenvironment, are likely to be explored further in this field of study. Potential treatment approaches that involve altering the microenvironment of intra-articular stem cells, such as regulating inflammatory responses, enhancing the extracellular matrix’s composition, and encouraging targeted differentiation of stem cells, could be examined. The use of nanotechnology in managing the microenvironment of stem cells will also include future studies on augmenting the repair and regeneration of intra-articular stem cells by delivering growth factors, gene editing tools, and other bioactive chemicals.
In the future, with the continuous advancement of medical technology, personalized treatment will become a trend in the treatment of bone and joint diseases. By analyzing the patient’s genetic background, gene expression profile, and other information, targeted stem cell therapy plans can be implemented to maximize treatment effectiveness and prevent the occurrence of complications. Although stem cell therapy has shown great potential in the treatment of bone and joint diseases, it still faces many challenges, including uncertainty in treatment efficacy, safety considerations, and treatment costs. In the future, it is necessary to further strengthen basic research, explore the mechanisms of stem cells in bone and joint tissue regeneration, continuously improve treatment techniques and plans, in order to achieve the widespread promotion and application of stem cell therapy in clinical applications.
We visualize the keywords appearing in the research field of microenvironmental interference with intra-articular stem cell regeneration affecting arthritis and find the top 10 keywords with the highest frequency of keywords appearing in the research in this field, as in Table 2, from which we can find the hotspots of the research in this field and the development focuses of the attention of the researchers in the recent years, and we can intuitively find out the cooperation between the keywords appearing in the research field of microenvironmental interference with intra-articular stem cell regeneration affecting arthritis in Figure 3, where the larger the dots indicate the greater frequency of the appearances, and the number of the lines connecting the dots indicate the degree of the close relationship between the cooperating relationships.
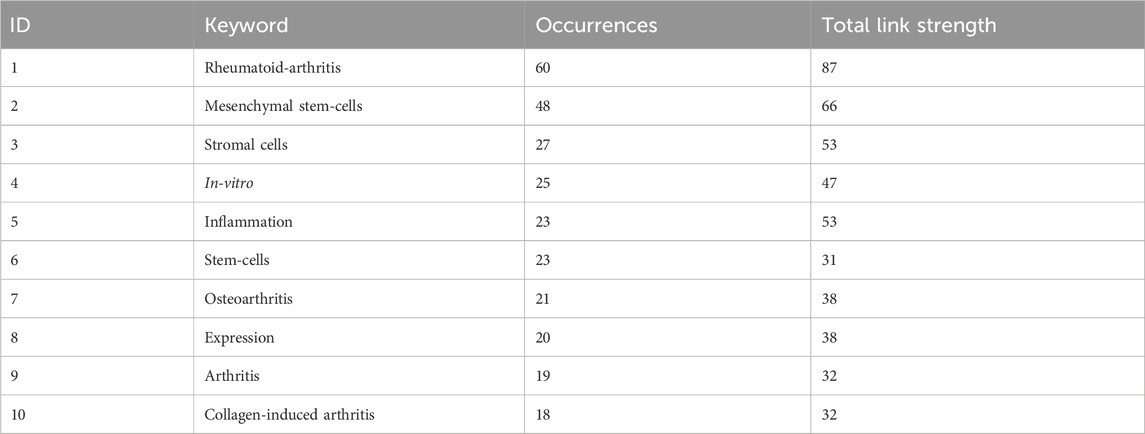
Table 2. Microenvironmental interference with intra-articular stem cell regeneration affects the top 10 most frequently occurring keywords in the field of arthritis research.
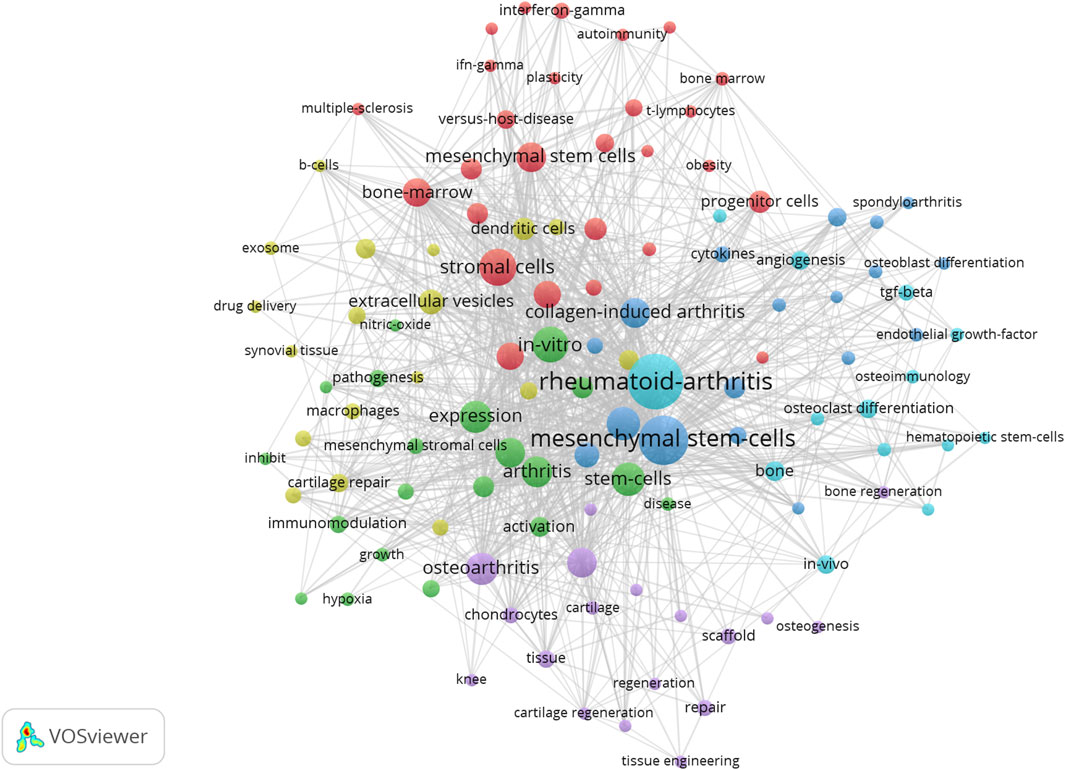
Figure 3. Microenvironmental disruption of intra-articular stem cell regeneration affecting arthritis Collaboration between keywords in the field of research on arthritis.
Discussion
Numerous research have emphasized the impact of the body’s microenvironment on stem cells, including how it affects MSCs’ paracrine signaling (Kusuma et al., 2017), the impact of the microenvironment on stem cells’ ability to treat (Tsai et al., 2020). Stem cells have been shown in a number of studies to have an impact on arthritis, which can promote repair and regeneration of articular cartilage (Jiang et al., 2021). It can also play a role in synovial joint inflammation by itself (Bedoui et al., 2020). This suggests that the microenvironment can influence the development of arthritis and even slow down the progression of arthritic disease through its effect on stem cells.
By altering the intra-articular milieu, stem cells’ ability for regeneration can be markedly increased. For instance, by boosting certain growth factors or altering the nature of the extracellular matrix, it is feasible to promote the proliferation and differentiation of stem cells and speed up the repair of bone and cartilage (Cattaneo and McKay, 1990; Tarasenko et al., 2004; Danišovič et al., 2012; Eom et al., 2014; Qian et al., 2017). It has also been demonstrated that guided stem cell differentiation and tissue regeneration may be facilitated by using biomaterials to replicate the natural cellular milieu (Singh and Elisseeff, 2010; Griffin et al., 2015; Kuo and Rajesh, 2017).
Specific growth factors can be introduced, or the chemical and physical circumstances in the microenvironment can be altered, to encourage stem cell differentiation into chondrocytes or bone cells, which will aid in tissue regeneration and repair (Hao et al., 2017; Maisani et al., 2017; Xing et al., 2019; Zhu et al., 2021). Biomaterials, or biocompatible materials, are scaffolds that mimic the natural cellular milieu and help stem cell proliferation and differentiation (Przekora, 2019; Zhao et al., 2021a; Zhao et al., 2021b). Gene editing technology: To improve stem cells’ capacity for regrowth, the expression of certain genes is changed using CRISPR/Cas9 and other gene editing techniques (Zhang et al., 2017; Ben et al., 2018).
Stem cell research has made progress in recent years, but some obstacles remain. For example, properly regulating the activities of stem cells, including their migration, differentiation and proliferation, remains a technical challenge. There is also the fact that current research focuses on how to ensure the biocompatibility and biosafety of biomaterials, as well as how to design and prepare these materials to match the natural microenvironment. Finally, more investigations and evaluations are needed to determine the long-term effects and any adverse consequences of stem cell therapy.
Researchers remain convinced that the use of stem cell therapies to treat bone and joint disorders still holds great promise. Future advances in bioengineering and materials science, coupled with a deeper understanding of stem cells and the mechanisms that regulate their microenvironment, should pave the way for the creation of more effective therapies that will improve patient outcomes and quality of life. A major concern for researchers in this field is how to accurately regulate the activities of stem cells in the body, including their proliferation, differentiation and localization. The long-term safety and efficacy of stem cell therapy requires more research, especially the possible risks of immune rejection and tumorigenesis. Many researchers expect to try to develop standardized and reproducible stem cell therapy techniques in the near future, as well as to promote these techniques to fit clinical needs. Stem cell therapies are likely to be used more often in the future to treat bone and joint disorders. An interesting future will eventually emerge as research on stem cells and the intra-articular microenvironment deepens.
Conclusion
In this work, we highlight the pivotal function of the intra-articular milieu in controlling stem cell renewal and its noteworthy influence on the onset and course of arthritis. The capacity of stem cells, especially mesenchymal stem cells (MSCs), to regenerate and repair injured joint tissues is dependent on how these cells interact with their surroundings. Stem cell activity can be hindered by microenvironmental changes brought on by inflammation, extracellular matrix breakdown, and disruption of cellular signaling. This can exacerbate the symptoms and course of arthritis.
Furthermore, we think that modifying the intra-articular milieu promotes stem cell-mediated regeneration, opening up exciting new therapeutic options for arthritis. Our ability to better understand these intricate relationships and create focused interventions to maximize the regenerative potential of intra-articular stem cells will open the door to novel and more successful treatments for arthritic patients, which will ultimately improve their prognosis and quality of life.
Author contributions
ZS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BW: Data curation, Formal Analysis, Methodology, Project administration, Supervision, Validation, Writing–review and editing. HG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing–review and editing. SZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aigner, T., and Kim, H. A. (2002). Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 46 (8), 1986–1996. Epub 2002/09/05. doi:10.1002/art.10554
Bedoui, Y., Lebeau, G., Guillot, X., Dargai, F., Guiraud, P., Neal, J. W., et al. (2020). Emerging roles of perivascular mesenchymal stem cells in synovial joint inflammation. J. Neuroimmune Pharmacol. 15 (4), 838–851. Epub 2020/09/24. doi:10.1007/s11481-020-09958-z
Ben, J. R., Shemer, Y., and Binah, O. (2018). Genome editing in induced pluripotent stem cells using crispr/cas9. Stem Cell. Rev. Rep. 14, 323–336. doi:10.1007/s12015-018-9811-3
Berenbaum, F., Wallace, I. J., Lieberman, D. E., and Felson, D. T. (2018). Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 14 (11), 674–681. Epub 2018/09/14. doi:10.1038/s41584-018-0073-x
Brack, A. S., and Rando, T. A. (2012). Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell. Stem Cell. 10 (5), 504–514. Epub 2012/05/09. doi:10.1016/j.stem.2012.04.001
Cabahug-Zuckerman, P., Frikha-Benayed, D., Majeska, R. J., Tuthill, A., Yakar, S., Judex, S., et al. (2016). Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte rankl production and subsequent resorption of cortical and trabecular bone in mice femurs. J. Bone Min. Res. 31 (7), 1356–1365. Epub 2016/02/08. doi:10.1002/jbmr.2807
Cao, P., Li, Y., Tang, Y., Ding, C., and Hunter, D. J. (2020). Pharmacotherapy for knee osteoarthritis: current and emerging therapies. Expert Opin. Pharmacother. 21 (7), 797–809. Epub 2020/02/27. doi:10.1080/14656566.2020.1732924
Cao, X., Luo, P., Huang, J., Liang, C., He, J., Wang, Z., et al. (2019). Intraarticular senescent chondrocytes impair the cartilage regeneration capacity of mesenchymal stem cells. Stem Cell. Res. Ther. 10 (1), 86. Epub 2019/03/15. doi:10.1186/s13287-019-1193-1
Cao, Y., Jansen, I. D., Sprangers, S., Stap, J., Leenen, P. J., Everts, V., et al. (2016). IL-1β differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets. J. Leukoc. Biol. 100 (3), 513–523. Epub 2016/03/10. doi:10.1189/jlb.1A1215-543R
Cattaneo, E., and McKay, R. (1990). Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 347 (6295), 762–765. doi:10.1038/347762a0
Curl, W. W., Krome, J., Gordon, E. S., Rushing, J., Smith, B. P., and Poehling, G. G. (1997). Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 13 (4), 456–460. Epub 1997/08/01. doi:10.1016/s0749-8063(97)90124-9
Dai, G., Xiao, H., Liao, J., Zhou, N., Zhao, C., Xu, W., et al. (2020). Osteocyte TGFβ1‑Smad2/3 is positively associated with bone turnover parameters in subchondral bone of advanced osteoarthritis. Int. J. Mol. Med. 46 (1), 167–178. Epub 2020/04/23. doi:10.3892/ijmm.2020.4576
Danišovič, Ľ., Varga, I., and Polák, Š. (2012). Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell. 44 (2), 69–73. doi:10.1016/j.tice.2011.11.005
de Rooij, M., van der Leeden, M., Heymans, M. W., Holla, J. F., Häkkinen, A., Lems, W. F., et al. (2016). Prognosis of pain and physical functioning in patients with knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. Hob. 68 (4), 481–492. Epub 2015/09/01. doi:10.1002/acr.22693
Ding, G. J., Fischer, P. A., Boltz, R. C., Schmidt, J. A., Colaianne, J. J., Gough, A., et al. (1998). Characterization and quantitation of nf-kappab nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J. Biol. Chem. 273 (44), 28897–28905. Epub 1998/10/24. doi:10.1074/jbc.273.44.28897
Duarte, J. H. (2014). Osteoarthritis: alendronate treatment improves pathology in animal model of oa by blocking osteoclastic bone resorption. Nat. Rev. Rheumatol. 10 (8), 446. Epub 2014/07/09. doi:10.1038/nrrheum.2014.107
Dupuis, J., Préfontaine, A., Villeneuve, L., Ruel, N., Lefebvre, F., and Calderone, A. (2007). Bone marrow-derived progenitor cells contribute to lung remodelling after myocardial infarction. Cardiovasc Pathol. 16 (6), 321–328. Epub 2007/11/17. doi:10.1016/j.carpath.2007.04.006
Eom, Y. W., Oh, J.-E., Lee, J. I., Baik, S. K., Rhee, K.-J., Shin, H. C., et al. (2014). The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. biophysical Res. Commun. 445 (1), 16–22. doi:10.1016/j.bbrc.2014.01.084
Fingleton, C., Smart, K., Moloney, N., Fullen, B. M., and Doody, C. (2015). Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 23 (7), 1043–1056. Epub 2015/03/10. doi:10.1016/j.joca.2015.02.163
Fujisawa, T., Hattori, T., Takahashi, K., Kuboki, T., Yamashita, A., and Takigawa, M. (1999). Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J. Biochem. 125 (5), 966–975. Epub 1999/04/30. doi:10.1093/oxfordjournals.jbchem.a022376
Gerber, H. P., Vu, T. H., Ryan, A. M., Kowalski, J., Werb, Z., and Ferrara, N. (1999). Vegf couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5 (6), 623–628. Epub 1999/06/17. doi:10.1038/9467
Griffin, M. F., Butler, P. E., Seifalian, A. M., and Kalaskar, D. M. (2015). Control of stem cell fate by engineering their micro and nanoenvironment. World J. stem cells 7 (1), 37–50. doi:10.4252/wjsc.v7.i1.37
Grogan, S. P., Miyaki, S., Asahara, H., D'Lima, D. D., and Lotz, M. K. (2009). Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res. Ther. 11 (3), R85. Epub 2009/06/09. doi:10.1186/ar2719
Hao, Z., Song, Z., Huang, J., Huang, K., Panetta, A., Gu, Z., et al. (2017). The scaffold microenvironment for stem cell based bone tissue engineering. Biomaterials Sci. 5 (8), 1382–1392. doi:10.1039/c7bm00146k
He, J., Yan, J., Wang, J., Zhao, L., Xin, Q., Zeng, Y., et al. (2021). Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses. Cell. Res. 31 (7), 742–757. Epub 2021/01/22. doi:10.1038/s41422-021-00467-z
Héraud, F., Héraud, A., and Harmand, M. F. (2000). Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 59 (12), 959–965. Epub 2000/11/23. doi:10.1136/ard.59.12.959
Hilfiker, A., Kasper, C., Hass, R., and Haverich, A. (2011). Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch. Surg. 396 (4), 489–497. Epub 2011/03/05. doi:10.1007/s00423-011-0762-2
Hu, W., Chen, Y., Dou, C., and Dong, S. (2021). Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 80 (4), 413–422. Epub 2020/11/08. doi:10.1136/annrheumdis-2020-218089
Jiang, S., Tian, G., Yang, Z., Gao, X., Wang, F., Li, J., et al. (2021). Enhancement of acellular cartilage matrix scaffold by wharton's jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater 6 (9), 2711–2728. Epub 2021/03/06. doi:10.1016/j.bioactmat.2021.01.031
Jiang, W., Jin, Y., Zhang, S., Ding, Y., Huo, K., Yang, J., et al. (2022). Pge2 activates Ep4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 10 (1), 27. Epub 2022/03/10. doi:10.1038/s41413-022-00201-4
Kenny, P. A., Lee, G. Y., and Bissell, M. J. (2007). Targeting the tumor microenvironment. Front. Biosci. a J. virtual Libr. 12, 3468–3474. doi:10.2741/2327
Kobayashi, K., Takahashi, N., Jimi, E., Udagawa, N., Takami, M., Kotake, S., et al. (2000). Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the odf/rankl-rank interaction. J. Exp. Med. 191 (2), 275–286. Epub 2000/01/19. doi:10.1084/jem.191.2.275
Kudo, O., Sabokbar, A., Pocock, A., Itonaga, I., Fujikawa, Y., and Athanasou, N. A. (2003). Interleukin-6 and interleukin-11 support human osteoclast formation by a rankl-independent mechanism. Bone 32 (1), 1–7. Epub 2003/02/14. doi:10.1016/s8756-3282(02)00915-8
Kuo, Y.-C., and Rajesh, R. (2017). Guided differentiation and tissue regeneration of induced pluripotent stem cells using biomaterials. J. Taiwan Inst. Chem. Eng. 77, 41–53. doi:10.1016/j.jtice.2017.04.043
Kusuma, G. D., Carthew, J., Lim, R., and Frith, J. E. (2017). Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 26 (9), 617–631. Epub 2017/02/12. doi:10.1089/scd.2016.0349
Lam, J., Takeshita, S., Barker, J. E., Kanagawa, O., Ross, F. P., and Teitelbaum, S. L. (2000). Tnf-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of rank ligand. J. Clin. Investig. 106 (12), 1481–1488. Epub 2000/12/20. doi:10.1172/jci11176
Le Blanc, K., and Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12 (5), 383–396. Epub 2012/04/26. doi:10.1038/nri3209
Lee, S. E., Woo, K. M., Kim, S. Y., Kim, H. M., Kwack, K., Lee, Z. H., et al. (2002). The phosphatidylinositol 3-kinase, P38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone 30 (1), 71–77. Epub 2002/01/17. doi:10.1016/s8756-3282(01)00657-3
Li, P. L., Wang, Y. X., Zhao, Z. D., Li, Z. L., Liang, J. W., Wang, Q., et al. (2021). Clinical-grade human dental pulp stem cells suppressed the activation of osteoarthritic macrophages and attenuated cartilaginous damage in a rabbit osteoarthritis model. Stem Cell. Res. Ther. 12 (1), 260. Epub 2021/05/03. doi:10.1186/s13287-021-02353-2
Li, X., Ding, L., Wang, Y. X., Li, Z. L., Wang, Q., Zhao, Z. D., et al. (2020). Skeletal stem cell-mediated suppression on inflammatory osteoclastogenesis occurs via concerted action of cell adhesion molecules and osteoprotegerin. Stem Cells Transl. Med. 9 (2), 261–272. Epub 2019/11/28. doi:10.1002/sctm.19-0300
Liu, X. H., Kirschenbaum, A., Yao, S., and Levine, A. C. (2005). Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear Factor-{Kappa}B (rank) ligand/rank system. Endocrinology 146 (4), 1991–1998. Epub 2004/12/25. doi:10.1210/en.2004-1167
Liu, X. H., Kirschenbaum, A., Yao, S., and Levine, A. C. (2006). Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the opg/rankl/rank system. Ann. N. Y. Acad. Sci. 1068, 225–233. Epub 2006/07/13. doi:10.1196/annals.1346.047
Lotz, M. K., Otsuki, S., Grogan, S. P., Sah, R., Terkeltaub, R., and D'Lima, D. (2010). Cartilage cell clusters. Arthritis Rheum. 62 (8), 2206–2218. Epub 2010/05/28. doi:10.1002/art.27528
Maisani, M., Pezzoli, D., Chassande, O., and Mantovani, D. (2017). Cellularizing hydrogel-based scaffolds to repair bone tissue: how to create a physiologically relevant micro-environment? J. Tissue Eng. 8, 2041731417712073. doi:10.1177/2041731417712073
Mayani, H., Guilbert, L. J., and Janowska-Wieczorek, A. (1992). Biology of the hemopoietic microenvironment. Eur. J. Haematol. 49 (5), 225–233. doi:10.1111/j.1600-0609.1992.tb00053.x
Moskowitz, R. W. (2007) Osteoarthritis: diagnosis and medical/surgical management. China: Lippincott Williams & Wilkins.
Ni, G. X., Zhan, L. Q., Gao, M. Q., Lei, L., Zhou, Y. Z., and Pan, Y. X. (2011). Matrix metalloproteinase-3 inhibitor retards treadmill running-induced cartilage degradation in rats. Arthritis Res. Ther. 13 (6), R192. Epub 2011/11/26. doi:10.1186/ar3521
Pearson, M. J., Herndler-Brandstetter, D., Tariq, M. A., Nicholson, T. A., Philp, A. M., Smith, H. L., et al. (2017). Il-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 7 (1), 3451. Epub 2017/06/16. doi:10.1038/s41598-017-03759-w
Pippenger, B. E., Duhr, R., Muraro, M. G., Pagenstert, G. I., Hügle, T., and Geurts, J. (2015). Multicolor flow cytometry-based cellular phenotyping identifies osteoprogenitors and inflammatory cells in the osteoarthritic subchondral bone marrow compartment. Osteoarthr. Cartil. 23 (11), 1865–1869. Epub 2015/11/03. doi:10.1016/j.joca.2015.07.021
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284 (5411), 143–147. Epub 1999/04/02. doi:10.1126/science.284.5411.143
Plotkin, L. I., Gortazar, A. R., Davis, H. M., Condon, K. W., Gabilondo, H., Maycas, M., et al. (2015). Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κb ligand (rankl) but does not stop bone resorption or the loss of bone induced by unloading. J. Biol. Chem. 290 (31), 18934–18942. Epub 2015/06/19. doi:10.1074/jbc.M115.642090
Prockop, D. J. (2009). Repair of tissues by adult stem/progenitor cells (mscs): controversies, myths, and changing paradigms. Mol. Ther. 17 (6), 939–946. Epub 2009/04/02. doi:10.1038/mt.2009.62
Przekora, A. (2019). The summary of the most important cell-biomaterial interactions that need to Be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 97, 1036–1051. doi:10.1016/j.msec.2019.01.061
Qian, Y., Han, Q., Chen, W., Song, J., Zhao, X., Ouyang, Y., et al. (2017). Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front. Chem. 5, 89. doi:10.3389/fchem.2017.00089
Quesenberry, P. J., Colvin, G., Dooner, G., Dooner, M., Aliotta, J. M., and Johnson, K. (2007). The stem cell continuum: cell cycle, injury, and phenotype lability. Ann. N. Y. Acad. Sci. 1106, 20–29. Epub 2007/03/16. doi:10.1196/annals.1392.016
Ren, G., Zhang, L., Zhao, X., Xu, G., Zhang, Y., Roberts, A. I., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell. Stem Cell. 2 (2), 141–150. Epub 2008/03/29. doi:10.1016/j.stem.2007.11.014
Roos, E. M., and Arden, N. K. (2016). Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 12 (2), 92–101. Epub 2015/10/07. doi:10.1038/nrrheum.2015.135
Sanchez, C., Deberg, M. A., Bellahcène, A., Castronovo, V., Msika, P., Delcour, J. P., et al. (2008). Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 58 (2), 442–455. Epub 2008/02/02. doi:10.1002/art.23159
Sanchez, C., Mazzucchelli, G., Lambert, C., Comblain, F., DePauw, E., and Henrotin, Y. (2018). Comparison of secretome from osteoblasts derived from sclerotic versus non-sclerotic subchondral bone in oa: a pilot study. PLoS One 13 (3), e0194591. Epub 2018/03/17. doi:10.1371/journal.pone.0194591
Shakibaei, M., John, T., Schulze-Tanzil, G., Lehmann, I., and Mobasheri, A. (2007). Suppression of nf-kappab activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem. Pharmacol. 73 (9), 1434–1445. Epub 2007/02/13. doi:10.1016/j.bcp.2007.01.005
Shang, F., Yu, Y., Liu, S., Ming, L., Zhang, Y., Zhou, Z., et al. (2021). Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater 6 (3), 666–683. Epub 2020/10/03. doi:10.1016/j.bioactmat.2020.08.014
Singh, A., and Elisseeff, J. (2010). Biomaterials for stem cell differentiation. J. Mater. Chem. 20 (40), 8832–8847. doi:10.1039/c0jm01613f
Sui, B. D., Hu, C. H., Liu, A. Q., Zheng, C. X., Xuan, K., and Jin, Y. (2019). Stem cell-based bone regeneration in diseased microenvironments: challenges and solutions. Biomaterials 196, 18–30. Epub 2017/11/11. doi:10.1016/j.biomaterials.2017.10.046
Tarasenko, Y. I., Yu, Y., Jordan, P. M., Bottenstein, J., and Wu, P. (2004). Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J. Neurosci. Res. 78 (5), 625–636. doi:10.1002/jnr.20316
Todhunter, P. G., Kincaid, S. A., Todhunter, R. J., Kammermann, J. R., Johnstone, B., Baird, A. N., et al. (1996). Immunohistochemical analysis of an equine model of synovitis-induced arthritis. Am. J. Vet. Res. 57 (7), 1080–1093. Epub 1996/07/01. doi:10.2460/ajvr.1996.57.07.1080
Tsai, A. C., Jeske, R., Chen, X., Yuan, X., and Li, Y. (2020). Influence of microenvironment on mesenchymal stem cell therapeutic potency: from planar culture to microcarriers. Front. Bioeng. Biotechnol. 8, 640. Epub 2020/07/17. doi:10.3389/fbioe.2020.00640
Tsukasaki, M., and Takayanagi, H. (2019). Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19 (10), 626–642. Epub 2019/06/13. doi:10.1038/s41577-019-0178-8
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (ylds) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 380 (9859), 2163–2196. Epub 2012/12/19. doi:10.1016/s0140-6736(12)61729-2
Wang, Y. X., Zhao, Z. D., Wang, Q., Li, Z. L., Huang, Y., Zhao, S., et al. (2020). Biological potential alterations of migratory chondrogenic progenitor cells during knee osteoarthritic progression. Arthritis Res. Ther. 22 (1), 62. Epub 2020/03/29. doi:10.1186/s13075-020-2144-z
Xing, F., Li, L., Zhou, C., Long, C., Wu, L., Lei, H., et al. (2019). Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem cells Int. 2019, 1–16. doi:10.1155/2019/2180925
Xu, C., Feng, C., Huang, P., Li, Y., Liu, R., Liu, C., et al. (2022). Tnfα and ifnγ rapidly activate pi3k-akt signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell. Res. Ther. 13 (1), 491. Epub 2022/10/05. doi:10.1186/s13287-022-03178-3
Xu, R., Yallowitz, A., Qin, A., Wu, Z., Shin, D. Y., Kim, J. M., et al. (2018). Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 24 (6), 823–833. Epub 2018/05/23. doi:10.1038/s41591-018-0020-z
Zhang, R. K., Li, G. W., Zeng, C., Lin, C. X., Huang, L. S., Huang, G. X., et al. (2018a). Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1). Bone Jt. Res. 7 (11), 587–594. Epub 2018/12/26. doi:10.1302/2046-3758.711.Bjr-2018-0057.R1
Zhang, Y., Sastre, D., and Wang, F. (2018b). Crispr/Cas9 genome editing: a promising tool for therapeutic applications of induced pluripotent stem cells. Curr. Stem Cell. Res. Ther. 13 (4), 243–251. doi:10.2174/1574888X13666180214124800
Zhang, Z., Zhang, Y., Gao, F., Han, S., Cheah, K. S., Tse, H.-F., et al. (2017). Crispr/Cas9 genome-editing system in human stem cells: current status and future prospects. Mol. Therapy-Nucleic Acids 9, 230–241. doi:10.1016/j.omtn.2017.09.009
Zhao, X., Hu, D. A., Wu, D., He, F., Wang, H., Huang, L., et al. (2021a). Applications of biocompatible scaffold materials in stem cell-based cartilage tissue engineering. Front. Bioeng. Biotechnol. 9, 603444. doi:10.3389/fbioe.2021.603444
Zhao, X., Li, Q., Guo, Z., and Li, Z. (2021b). Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell. Res. Ther. 12 (1), 583–613. doi:10.1186/s13287-021-02650-w
Zhen, G., and Cao, X. (2014). Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol. Sci. 35 (5), 227–236. Epub 2014/04/22. doi:10.1016/j.tips.2014.03.005
Zhen, G., Wen, C., Jia, X., Li, Y., Crane, J. L., Mears, S. C., et al. (2013). Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19 (6), 704–712. Epub 2013/05/21. doi:10.1038/nm.3143
Zhu, G., Zhang, T., Chen, M., Yao, K., Huang, X., Zhang, B., et al. (2021). Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact. Mater. 6 (11), 4110–4140. doi:10.1016/j.bioactmat.2021.03.043
Zhu, H., Guo, Z. K., Jiang, X. X., Li, H., Wang, X. Y., Yao, H. Y., et al. (2010). A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 5 (3), 550–560. Epub 2010/03/06. doi:10.1038/nprot.2009.238
Keywords: microenvironmental, stem cell, arthritis, MSCs, interference, regeneration
Citation: Shao Z, Wang B, Gao H and Zhang S (2024) Microenvironmental interference with intra-articular stem cell regeneration influences the onset and progression of arthritis. Front. Genet. 15:1380696. doi: 10.3389/fgene.2024.1380696
Received: 02 February 2024; Accepted: 30 April 2024;
Published: 22 May 2024.
Edited by:
Chenyu Sun, AMITA Health, United StatesReviewed by:
Suyu Wang, Tongji University, ChinaHongye Peng, China Academy of Chinese Medical Sciences, China
Copyright © 2024 Shao, Wang, Gao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenqi Zhang, 19154571592@163.com
 Zhuce Shao
Zhuce Shao Benlong Wang
Benlong Wang