- 1State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences (CAS), Guiyang, China
- 2Chongqing Key Laboratory of Karst Environment and School of Geographical Sciences of Southwest University, Chongqing, China
- 3Chongqing Jinfo Mountain Karst Ecosystem National Observation and Research Station, School of Geographical Sciences, Southwest University, Chongqing, China
As an important link in the global carbon cycle, the carbon sink function of inland water bodies has attracted much attention in recent years. In particular, the autochthonous production (AP) associated with aquatic photosynthesis in karst surface waters converts dissolved inorganic carbon (DIC) into autochthonous organic carbon (the “carbon sink by carbonate weathering coupled with aquatic photosynthesis, CCW”), which is the key to the formation of a long-term stable carbonate weathering sink. After nearly 20 years of development, the “CCW” theory, as a nature-based solution, has been found to have a win-win mechanism of sink enhancement and water environment improvement. The specific mechanism is that dissolved aquatic CO2 (CO2(aq)) fertilization can effectively alleviate the carbon limitation of the water body, promote the productivity of the water body to achieve carbon sink enhancement, and achieve the inhibition of eutrophication through the modification of the biological structure and the co-precipitation of CaCO3 and phosphorus to enhance the efficiency of phosphorus removal. In conclusion, the carbon fertilization of AP effect in karst surface water bodies has a huge carbon storage capacity and water environment improvement capacity. This paper reviews the detailed process of AP effect in karst surface waters, especially about the possibility of carbon sink and eutrophication mitigation win-win by CO2 fertilization of water bodies and its mechanism of action. Finally, based on the current research gaps, we outline the future research priorities of AP in karst surface water bodies. This study will provide new theoretical basis and scientific support for the regulation of carbon sinks and water quality safety in karst surface waters.
Introduction
Global CO2 emissions have been on an upward trend since the industrial revolution. From the 1960s to the present, it has grown by an average of 2.2% per year (Friedlingstein et al., 2024). Data for 2024 suggests that global carbon emissions are still growing and that the 1.5°C target may no longer be achievable, but the 2°C target may still be (Deng et al., 2025). In order to respond to the threat posed by climate change to the survival and development of humankind, China has proposed a “dual-carbon” goal of reaching peak CO2 emissions by 2030 and achieving carbon neutrality by 2060. Currently, reducing carbon emissions and increasing carbon sequestration is one of the key research objectives of the scientific community. Nature-based Solutions (NbS) is gradually being widely recognized and accepted by the international community as an important concept and methodology that can synergistically address societal challenges and promote the harmonious coexistence of human beings and nature (Chang et al., 2024). Ecosystem carbon sinks, as a nature-based climate solution, are the greenest, most economical, and most scalable technological pathway to achieve carbon neutrality goals, and have attracted sustained attention from relevant researchers. In addition, based on modern carbon cycle research, it has been estimated that the amount of atmospheric carbon sources is larger than the amount of carbon sinks at the global scale, and there is an imbalance between the carbon sources and sinks, so the destination of this part of the “missing carbon sink” has become the focus of research in recent years (Houghton et al., 2018; Kirschbaum et al., 2019; Liu et al., 2018).
The global carbon cycle refers to the process of carbon transport, transformation and cyclic turnover among the various layers of the Earth (including the atmosphere, hydrosphere, biosphere, soil circle and lithosphere). Studies of the global carbon cycle have traditionally considered the vertical exchange of CO2 between two active compartments (land and oceans) and a third compartment (the atmosphere) (Middelburg, 2025). Inland waters, as an important link between land, oceans and the atmosphere, play a key role in the global cycling of elements that affect the biogeochemical balance between these systems (Webb et al., 2019). In addition, inland waters act as carbon sinks and sources by burying large amounts of carbon in reservoirs, lakes and flood plains and emitting large amounts of CO2 and methane (Cole et al., 2007). Thus, inland waters play an important role in the global carbon cycle. Additionally, previous studies of terrestrial carbon sinks have focused on soil and vegetation (Arneth et al., 2017; Pan et al., 2011), and the lithosphere, as the largest global carbon reservoir, has not received the attention it deserves (Liu et al., 2010; 2018; 2021). proposed and developed the theory of “the carbon sink by carbonate weathering coupled with aquatic photosynthesis, CCW” by integrating the processes of carbonate weathering, global water cycle and aquatic photosynthesis. The theory suggests that dissolved inorganic carbon (DIC) produced by carbonate weathering is not only an important inorganic carbon sink, but also can be converted into autochthonous organic carbon (AOC) by autochthonous production (AP) and buried in rivers, lakes and oceans, and ultimately enters the lithosphere to form a long-term stable carbon sink, which makes carbonate weathering have a role in controlling climate change on any time scale (Liu et al., 2018). The AP in inland waters refers to photosynthesis that is controlled by the concentration of nutrients (carbon, nitrogen, phosphorus, and trace elements, etc.), i.e., the process of biomass accumulation and the intensity of accumulation are influenced by the concentration of nutrients. The ability of carbonate weathering to form a lasting and stable carbon sink depends greatly on the efficiency of the AP, i.e., how much AOC is actually effectively preserved (Shao et al., 2025). Inland water bodies (rivers, lakes, reservoirs, etc.) are “active reactors” linking the two carbon pools of the Earth’s terrestrial and marine ecosystems and are actively involved in global carbon and nutrient cycling (Battin et al., 2023; Cole et al., 2007). Inland waters, as sites of AOC production and burial, have a disproportionate impact on the global carbon cycle compared to their size (Liu et al., 2025; Tranvik et al., 2009). Studies have shown that CO2 emission fluxes from some terrestrial waters have decreased globally (Finlay et al., 2015; Ran et al., 2021), and dissolved organic carbon (DOC) concentrations and organic carbon (OC) burial have increased over the past 60–150 years (Anderson et al., 2014). This change is mainly due to global warming and intensified human activities, with an increase in AOCs produced by photosynthesis in aquatic plants, i.e., caused by the enhanced AP effect (Liu et al., 2021). In the latest AOC estimates, carbon sinks from enhanced autochthonous production could range from 0.38 to 1.8 billion tons per year, and will become even more important in the context of future climate change and intensifying human activities (Liu et al., 2021).
The AP effect in karst surface waters not only acts as a carbon sequestration and stabilization process, but also is able to generate water environment effects through coupled physical-chemical-biological processes. This is mainly reflected in two aspects. On the one hand, the high pH characteristic of karst aquatic systems results in a low percentage of CO2 in the water in the DIC, and thus the AP efficiency may be CO2 limited, while CO2 is able to limit aquatic plant growth and influence community structure by regulating the carbon (C): nitrogen (N): phosphorus (P) stoichiometric ratio (Bao et al., 2020; Hammer et al., 2019; Shao et al., 2023a). It is important to emphasize that CO2 limitation (carbon limitation) here refers to the availability of free CO2 as a limiting factor for aquatic plant photosynthesis in karst water aquatic systems (see CO2 FERTILIZATION EFFECT AND ITS CARBON SINK for details). On the other hand, the high Ca2+ and DIC characteristic of karst surface water bodies makes AP produce more calcium carbonate deposits and contribute to co-precipitation with phosphate, forming a “self-purification mechanism” for the improvement of the water environment (Hamilton et al., 2009; He et al., 2025a; Sun et al., 2022). This mechanism may be important for water quality improvement (i.e., enhancement of the AP through DIC fertilization) in karst areas covering 15% of the global land surface. In conclusion, the effect of CO2 fertilization and its carbon sink mechanism based on carbon limitation in aquatic ecosystems and considering the mitigation of eutrophication by CO2 is the focus of future research on ecosystem services and management. This new knowledge will provide new scientific and technological support for surface waters to realize artificial carbon sinks and mitigate eutrophication.
This paper reviews the carbon fertilization of autochthonous production in karst surface waters and their roles in carbon reduction and eutrophication mitigation, with special emphasis on the win-win mechanism of carbon sink enhancement and water quality improvement where CO2 fertilization in karst surface aquatic ecosystems can effectively mitigate carbon limitation in the water body, promote productivity to achieve carbon sink enhancement in the water body, and achieve an inhibitory effect on eutrophication by changing the biotic structure and increasing the efficiency of phosphorus removal via co-precipitation of CaCO3 and phosphorus, and finally suggest future research priorities. These insights provide theoretical references for increasing carbon sinks and improving water quality.
Increasing autochthonous production in inland waters
The AP in inland waters refers to photosynthesis controlled by nutrient concentration, i.e., the process of biomass accumulation and the intensity of accumulation is influenced by the control of nutrient concentration. Especially for karst aquatic systems, the AP effect is affected by free CO2 limitation (e.g., He et al., 2025b). That is, the higher the free CO2 concentration, the stronger the carbon stabilization process and the higher the carbon sink formation. Specifically, aquatic plants convert DIC (HCO3− or CO2) to autochthonous organic carbon (AOC) through the biological pump effect, part of which is further buried as a long-term stable carbon sink, and part of which is transported to downstream water bodies and eventually into the ocean after transformation by complex physicochemical and biological processes (Liu et al., 2010; Liu et al., 2018).
Karst regions account for about 15% of the global land area, and karst surface waters are characterized by high pH, Ca2+ and DIC due to the high weathering rate of carbonates. Studies have shown that the DIC content in karst aquatic ecosystems is 6–10 times higher than that in non-karst areas (Wang et al., 2024; Yi et al., 2021). Studies have shown that a strong AP also exists in karst surface aquatic ecosystems, where aquatic photosynthetic organisms fix part of the DIC produced by carbonate weathering through their own photosynthesis to form AOC that is buried in the sediment, forming a long-term stable carbon sink (Liu et al., 2010; Liu et al., 2018; Liu et al., 2021) (Figure 1). Aquatic photosynthetic organisms (phytoplankton, submerged plants, and microorganisms) convert DIC to AOC during photosynthesis through the AP, a process known as carbon stabilization. The production and burial of AOC from the AP is a net carbon sink, which is an important indicator for global carbon cycle studies (Liu et al., 2018; Shao et al., 2025). In karst surface aquatic ecosystems, the higher the concentration of dissolved inorganic carbon in the water column, the higher the aquatic productivity, the more organic carbon produced, and the existence of a significant AP DIC(CO2) fertilization effect (Chen et al., 2017; Lai et al., 2022; Yang et al., 2016; Zeng et al., 2019). Experimental studies at the large-scale karst water-carbon cycle simulation experimental site in Shawan, Puding, Guizhou showed that different land uses control the concentrations and ratios of inorganic nutrients, such as DIC, N and P, in exposed surface waters, which in turn affects the structure and productivity of aquatic plants in surface waters, and the biomass of submerged plants, as an important participant in AP, changes in tandem with the concentration of DIC in the water (Bao et al., 2020; Bao et al., 2022), and the AOC fixed by autochthonous production in surface water bodies increased with increasing DIC concentration, with a range of 156–493 t C km-2a −1 (Chen et al., 2017). Yang et al. (2016) showed that phytoplankton biomass and TOC concentration increased with increasing water column DIC concentration in the Pearl River, a typical karst river, and the contribution of AOC to TOC was 65%. These studies suggest that the study of AP in inland water bodies will be an important indication of the role of karst aquatic ecosystems in carbon sequestration. In addition, carbonate mineral-rich areas are similarly characterized by high pH and high DIC concentrations in their surface water bodies due to similarly controlled carbonate weathering (Zhang et al., 2015). Our recent study found the same AP effect in surface waters of the Loess Plateau, which is rich in carbonate minerals (Shao et al., 2023a; 2023b; 2024). In addition, similar autochthonous production has been found in European lakes (Nõges et al., 2016) and in the Mississippi River Basin in the USA (Waterson and Canuel, 2008). In recent decades, this autochthonous production seems to be increasing, corresponding to decreasing CO2 emissions from surface waters (Finlay et al., 2009; Jia et al., 2022; Ran et al., 2021) and increasing dissolved organic carbon storage and organic carbon burial (Anderson et al., 2014; Gilarranz et al., 2022; He et al., 2020; Mendonça et al., 2017),a trend that may be closely related to enhanced AP. Based on global-scale estimates, the strengthening AP in inland waters could achieve sequestration of 0.38–1.8 billion tons of carbon equivalent per year (Liu et al., 2021).
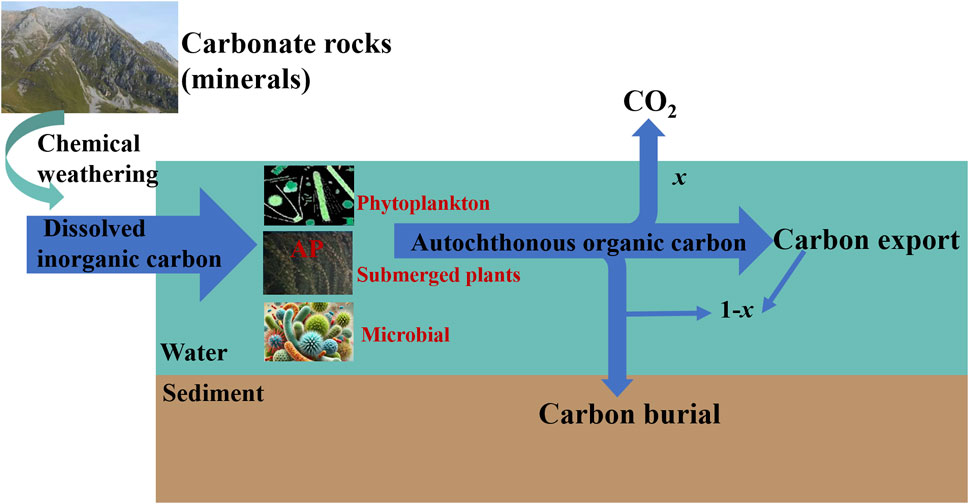
Figure 1. A conceptual model for carbon sink by carbonate weathering coupled with aquatic photosynthesis autochthonous production (AP) (CaCO3+H2O + CO2→Ca2++2HCO3−
CO2 fertilization effect and its carbon sink
Most studies of eutrophication in water bodies have focused on the limiting effects of N and P on the primary production of phytoplankton. Since the 1980s, evidence of consistent relationships between phosphorus in the water column and phytoplankton biomass in different study areas has supported the “phosphorus limitation paradigm” (Edmondson, 1970; Hecky and Kilham, 1988; Schindler and Fee, 1973). In contrast, C has long been generally not recognized as a limiting factor for aquatic primary production (Conley et al., 2009; Elser et al., 2009). This understanding is based on two common misconceptions. 1. CO2 in freshwater waters is sufficiently high to meet the carbon needs of aquatic plants for photosynthetic growth because many surface water bodies are often supersaturated with CO2 (Cole et al., 1994) and because atmospheric CO2 is continuously replenished. 2. Although CO2 accounts for only a small fraction of DIC in most cases, many photosynthetic organisms have a carbon concentrating mechanism (CCM) that uses HCO3− as a source of carbon in addition to CO2 directly, so CO2 does not limit aquatic primary productivity in water bodies. In fact, for the first view, even if pCO2 is high, it does not necessarily mean that the actual concentration of CO2 in water is high. Especially in high pH karst aquatic ecosystems, where the carbonate equilibrium system is dominated by HCO3− or CO32−, the CO2 concentration in the water is often less than 1% of DIC (Liu et al., 2018) (Figure 2). Coupled with intense photosynthesis can easily turn the water column into an unsaturated state (Visser et al., 2016) resulting in a high pH and carbon-limited aquatic environment. In addition, CO2 exchange between water and air is an extremely slow process, and the diffusion rate of CO2 in water is only one ten-thousandth of that in the atmosphere (Stumm and Morgan, 1983) and the rate of CO2 replenishment in water is much smaller than the rate of CO2 fixation by aquatic plants. For the second scenario, although CCM mechanisms are prevalent, the use of HCO3− requires additional energy and nutrients, which makes the affinity of many photosynthetic organisms for HCO3− significantly lower than for CO2. In addition, C-N-P concentrations and ratios in aquatic ecosystems are changing under the continuing influence of human activities (Elser et al., 2022; Elser et al., 2000). In particular, anthropogenic-mediated increases in N and P effectiveness in water bodies can make C limitation and CO2 fertilization effects more pronounced (Hammer et al., 2019; Zagarese et al., 2021). Studies have shown that in eutrophic freshwater systems, a doubling of atmospheric CO2 can increase productivity by more than 50%, and that aquatic photosynthetic carbon sinks may increase more than expected (Schippers et al., 2004). In addition, CO2 can contribute to the primary productivity of water bodies in supersaturated lakes. For example, Jansson et al. (2012) found that productivity in CO2 supersaturated lakes (even in the presence of P limitation) reached even 10 times the productivity of lakes in equilibrium with atmospheric CO2. In addition, in a study of hard-water lakes and pilot experiments in Denmark, it was found that phytoplankton biomass was significantly higher than in soft water, and that their growth was co-limited by C-P (Kragh and Sand-Jensen, 2018). In another study from an eutrophic lake with different alkalinity, significant CO2 limitations were found for phytoplankton photosynthesis, growth rate, maximum biomass and organic carbon production (Hammer et al., 2019). These results suggest that alkaline surface water bodies are more susceptible to carbon limitation than soft water environments, highlighting the advantages of karstic water bodies in enhancing carbon sinks. The CO2 fertilization effect of AP and its resulting AOC have received much attention for their important contribution to the global missing carbon sink, which is often overlooked in discussions of freshwater ecosystems’ contribution to the global carbon sink due to the traditional view that AOC are structurally simple and easily consumed by bacterioplankton. However, recent studies have revealed that recalcitrant DOC (RDOC) similar to that in the ocean exists in inland water ecosystems, and in particular, the unique water chemistry of karst regions (high Ca2+, DIC and pH) favors the formation and accumulation of RDOC (Shao et al., 2024; Xia et al., 2022).
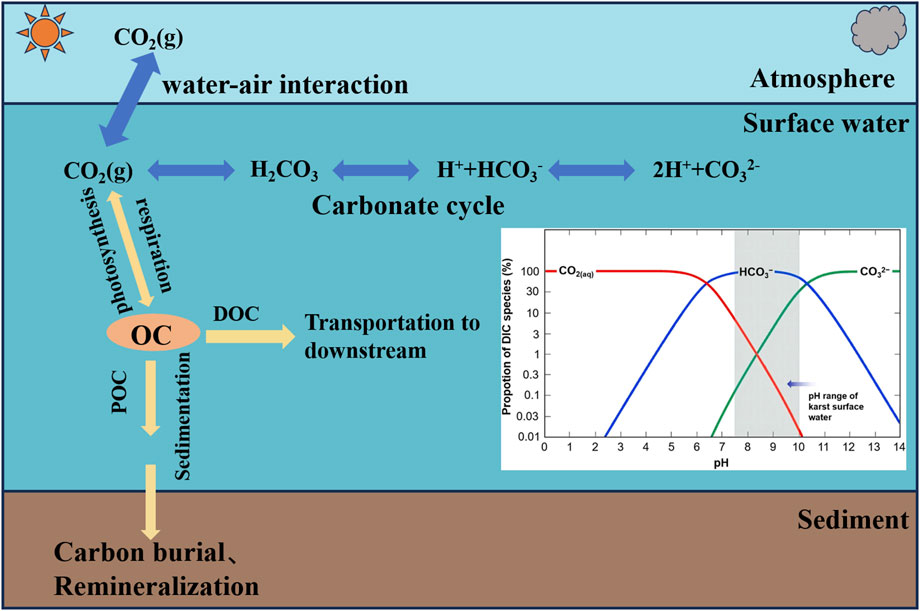
Figure 2. Schematic diagram of carbon cycling in inland waters (modified from Raymond and Hamilton, 2018), with inset plots of the proportion of dissolved inorganic carbon fractions (CO2(aq), HCO3−, and CO32−) as a function of pH (25°C, freshwater) (modified from Zeng et al., 2019). The process of water-air exchange in which surface water absorbs or releases CO2 from or to the atmosphere. When the CO2 concentration in surface water exceeds the equilibrium concentration of dissolved CO2 in water and gas, CO2 escapes from seawater and is discharged into the atmosphere. When the primary production of surface water is strong, the partial pressure of CO2 on the surface of surface water decreases, more atmospheric CO2 enters the seawater, and the inorganic carbon content of surface water increases until a new round of dissolution equilibrium is reached. Aquatic plants convert DIC (CO2) in water into autochthonous organic carbon (AOC) through the biological pump effect, part of which is further buried as a long-term stable carbon sink, and part of which is transported to downstream waterbodies and ultimately to the oceans after transformation through complex physicochemical and biological processes. The inset shows that in karst surface waters, carbonate equilibrium favors HCO3−, resulting in a lower concentration of dissolved CO2.
Mechanisms of mitigation of eutrophication of water bodies by autochthonous production
Impacts of carbon limitation from autochthonous production on aquatic plant community cpmposition and succession
Phytoplankton and submerged plants are the main primary producers in freshwater aquatic systems, and the stoichiometric ratios and growth of their organisms are regulated by environmental inorganic elements due to the different nutrient requirements and uptake of different algae and submerged plants (Iversen et al., 2019; Sardans et al., 2012). Previous studies have focused on the regulation of biological structures by N and P, with little consideration of the effects of C (King, 1970; Velthuis et al., 2022 showed that elevated CO2 increased phytoplankton C:N by 4% and C:P by 9% and growth rate by 6% under nutrient-sufficient conditions, while nutrient-limited conditions amplified the effects of CO2 on C:N and C:P, which increased by 27% and 17%, respectively. In addition, studies have shown that decreases in CO2 in the water column often correspond to algal bloom (Visser et al., 2016) and lead to the dominance of a few algal species, such as cyanobacteria (due to their having almost the most effective CCM). In fact, most phytoplankton have CCM (Giordano et al., 2005; Price et al., 2008; Shi et al., 2016), but there are differences in inorganic carbon utilization model between cyanobacteria and eukaryotic algae. Carbon acquisition pattern for CO2(aq) and HCO3− varied among the same phytoplankton species, and in descending order were: diatoms, coccolithophores, chlorophytas, and cyanobacteria (Low-Decarie et al., 2015; Low-Decarie et al., 2011). Compared with most planktonic algae, the more efficient CCM of cyanobacteria can convert more HCO3− to CO2 to maintain the carbon demand for photosynthesis and growth in C-limited waters, which greatly improves their photosynthetic performance and survival under CO2 limitation, and thus makes them easy to become dominant species (Visser et al., 2016). In addition, it was found that the CCM was inhibited when cells were P-limited, suggesting that P plays an irreplaceable role in maintaining the normal functioning of the CCM (Reinfelder, 2011), which gives cyanobacteria a more competitive advantage in eutrophic and C-limited water bodies and may further develop into cyanobacterial blooms. However, CCM is an active uptake energy-consuming process, and cyanobacterial HCO3− acquisition mechanisms include BicA, SbtA, and BCT1, all of which are highly energy-consuming (Shibata et al., 2001), and when the DIC of the water body is elevated, especially when CO2 is added to the water body, algae that originally had a weak CCM or did not have a CCM will gain a new competitive advantage, causing the proportion of cyanobacteria in the whole community to decrease, and the eutrophication hazard was reduced (Shi et al., 2016). In addition, in terms of the chemical composition of phytoplankton, more carbon is required for the growth of green algae relative to cyanobacteria, and thus differences in their chemical elemental compositions may also result in a competitive advantage for carbon (King, 1970; Rojo et al., 2020; Townsend et al., 2007). Recent work suggests that phytoplankton community composition may vary with CO2 concentration gradients, and that taxa with inefficient CCMs or their RuBisCO low affinity for CO2 are more dominant in high CO2 environments (Cabrerizo et al., 2020; Katkov et al., 2020). Over the years, our studies at the Shawan Karst Large-scale Analog Test Site have found that high C:N and C:P drive green algae and diatoms to have a competitive advantage over cyanobacteria (Bao et al., 2020; Bao et al., 2021). In addition, using a typical karst shallow lake, we found that the limiting threshold of CO2 concentration for planktonic algae was 15 μmol/L in the Erhai Sea of Yunnan (Lai et al., 2022),and 30 μmol/L in the Caohai lake of Guizhou, and that the cyanobacterials relative to the green algae when the water body C(CO2):N > 0.2 (molar ratio) and C(CO2):P > 10 and diatoms decreased (Zhang et al., 2023). It is hypothesized that high CO2 concentrations may raise the competitive advantage of algae other than cyanobacteria, thus helping to control cyanobacterial outbreaks in the early stages of eutrophication and mitigating eutrophication of water bodies.
Elevated CO2 in the water column not only affects planktonic community structure but also promotes the growth of submerged plants (Pagano and Titus, 2004). To alleviate CO2 limitation, many submersed plants already have the ability to utilize HCO3− (Maberly and Gontero, 2017). However, unlike phytoplankton algae, submerged plants are less capable of inorganic carbon utilization and light energy acquisition than phytoplankton, and they are more carbon limited and light limited in the water column (Allen and Spence, 1981). In general, the CO2 threshold at which phytoplankton begin to utilize HCO3− as a primary carbon source is 10–15 μm (Van Dam et al., 2018), whereas submersed plants such as Verticillium brasiliensis begin to utilize HCO3− as a carbon source at 50 μm (Bao et al., 2021), which puts submerged plants are often at a competitive disadvantage in eutrophic waters. Submerged plants have higher C:N and C:P ratios than phytoplankton in terms of their elemental chemical count ratios (e.g., C:N:P = 160:23:1 for cyanobacteria (King, 1970), and C:N:P = 582:82:1 for charophyte (Rojo et al., 2020)). Thus, submerged plants are better adapted to grow in C-rich environments (Iversen et al., 2019). Studies have shown that submerged plants can support higher productivity in high DIC environments than in low DIC environments when water body N:P increases (Kaijser et al., 2021). Therefore, the CO2 fertilization effect of AP may be more conducive to promoting the primary productivity and growth of submerged plants and solidifying their dominant position. Submerged plants are the main primary producers in shallow lakes and play an important role in water quality restoration and eutrophication management. Dense submerged plants can provide complex ecological niches for macrofauna, compete with algae for nutrients, promote sedimentation and reduce sediment re-suspension, improve the underwater light environment, and secrete chemosensory substances to inhibit algal growth, leading to more favorable water quality conditions (Scheffer et al., 2001). Taken together, carbon limitation of biological pumps may favor submerged plants to gain an advantage in competition with planktonic algae or enhance the competitive advantage of algae other than cyanobacteria (Shi et al., 2016), which is important for mitigating cyanobacterial-type eutrophication in water bodies.
Autochthonous production effectively promote the co-precipitation of CaCO3 with dissolved phosphate
The carbon fertilization of AP mitigates eutrophication of water bodies in addition to altering the structure of aquatic organisms. In addition, the AP forms AOC and promote carbonate deposition, of which carbonate deposition has a significant P removal function (Hamilton et al., 2009; Murphy et al., 1983). CaCO3 precipitation is regulated by coupled inorganic chemical and biological effects. However, most studies have focused on the effect of inorganic chemistry on CaCO3 precipitation, and the role of biology in it has not attracted enough attention. However, it has been found that biological effects can effectively induce CaCO3 precipitation. For example, indoor experimental studies showed that there was no calcite precipitation at the water-gas interface in the water column without organisms, despite the water column being supersaturated due to degassing, and that calcite precipitation did not occur at the bottom as it normally does in rivers, whereas calcite precipitation occurred in the presence of biofilm (Rogerson et al., 2008). In addition, laboratory studies have found that cyanobacteria are capable of inducing calcite deposition (Obst et al., 2009), photoheterotrophic bacteria are capable of inducing calcite precipitation and the rate of calcite precipitation is controlled by the growth rate of the bacteria, similar to that of cyanobacteria and anaerobic phototrophic bacteria (Bundeleva et al., 2012). Field studies have shown that photosynthesis in aquatic plants induces CaCO3 precipitation (De Montety et al., 2011; Nimick et al., 2011), especially in karstic surface waters with high DIC, Ca2+ and high pH, where aquatic photosynthetic organisms utilize the DIC produced by carbonate weathering to be converted to autochthonous organic carbon via the autochthonous production (AP) (AOC-organic carbon pump, AOCP) and accompanied by precipitation of secondary calcium carbonate (inorganic carbon pump) (CaCO3+H2O + CO2→Ca2++2HCO3−
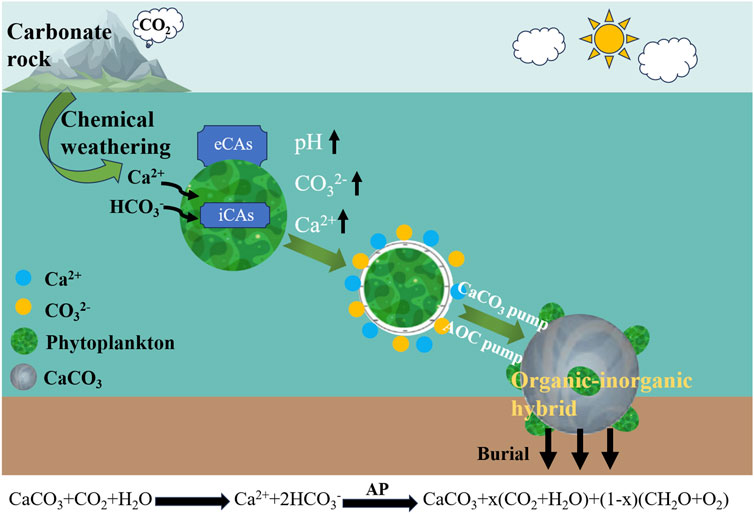
Figure 3. Sedimentation mechanism of aquatic photosynthetic organisms in karstic surface waters utilizing DIC produced by carbonate weathering converted to autochthonous organic carbon (AOC-Organic Carbon Pump, AOCP) accompanied by secondary calcium carbonate (Inorganic Carbon Pump) via the autochthonous production (AP). The double-pumping effect of organic carbon pump and inorganic carbon pump generates organic-inorganic hybrid that are further buried to form carbon storage.
The carbon fertilization of AP is not only a key mechanism for carbon sequestration and stabilization during carbonate weathering carbon sinks in terrestrial surface ecosystems, but also influences the cycling processes of other elements, including phosphorus (P), which has a controlling effect on eutrophication (Sun et al., 2022). The AP process can produce CaCO3 crystallization and precipitation. During calcium carbonate precipitation, dissolved phosphate co-precipitates with calcite, resulting in the conversion of soluble to insoluble phosphorus in water (Otsuki and Wetzel, 1972). Meanwhile, CaCO3 crystals have a large specific surface area and more surface adsorption sites, which can make soluble reactive phosphorus (SRP) adsorbed on the surface of calcium carbonate crystals through adsorption or coordination. Co-precipitation of SRP with calcium carbonate and adsorption on the surface of calcium carbonate crystals can remove SRP from the water body, thus alleviating the development of eutrophication, which is considered to be an important “self-purification mechanism” for eutrophic water bodies (Lin and Singer, 2006). Kamiya et al. (2004) and Liu et al. (2016) used X-ray diffraction (XRD) techniques to analyze the products of CaCO3 after immersion in (NH4)2HPO4 solution as well as foliar precipitates in water, respectively, and found that these products are the most thermodynamically stable hydroxyapatite (HAP)-containing carbonates.
It was found that aquatic organisms in karst surface water bodies further increased the pH value of water bodies under photosynthesis, which induced the co-precipitation of SRP and CaCO3 in the water, and more than 74% of the phosphorus co-precipitated with calcium carbonate when the pH value was between 9.5 and 10, and that the co-precipitation of calcium carbonate and phosphate in karst lakes due to the increase in pH value caused by enhanced AP could be used to control the lake’s eutrophication (Otsuki and Wetzel, 1972). This was confirmed by Dittrich and Koschel (2002) that the P removal by CaCO3-P co-precipitation is more pronounced when photosynthesis is strong in water. Similarly, the Sicilian reservoirs in Italy had contingency plans to stop cyanobacterial outbreaks by spreading lime powder to the reservoirs to remove P, which proved to be highly effective (Naselli-Flores et al., 2003). In addition, Hamilton et al. (2009) conducted a nutrient-enriched enclosure experiment on a nutrient-poor lake and showed that added P promoted increased productivity and exacerbated CaCO3 precipitation, but that much of the added P ended up being deposited back into the sediment as co-precipitation with CaCO3, creating a negative feedback mechanism on lake eutrophication (Corman et al., 2015; 2016). in river studies also found that co-precipitation of phosphate with calcium carbonate reduced P levels in the water column and increased the N:P ratio of the water column, making the primary productivity of the river P-limited, while the reduction in the P concentration in the water column may reduce biological N uptake. In summary, in karst aquatic systems, this carbon sink mechanism can synergize with the efficient carbonate pump (CP) to significantly contribute to the removal of phosphorus (P). Based on this concept, we propose the “AOCP -CP removal hypothesis”: Phosphorus uptake and utilization by organic processes will synergize with inorganic mechanisms (i.e., adsorption and co-precipitation of phosphorus by the carbonate pump) (He et al., 2025a) (Figure 4). In addition, for water containing a certain concentration of iron, high redox potential makes iron ions show high valence, high pH is conducive to the formation of iron hydroxide (Fe(OH)3) colloid, (Fe(OH)3) colloid has a large surface area, which can promote P adsorption on iron oxide/ferric hydroxide and co-precipitation occurs together. Moreover, Ca-P can be converted to Fe-P with lower activity, thus realizing the purpose of P removal (He et al., 2025b; Hoffman et al., 2013) (Figure 4). In summary, in addition to its role in carbon sequestration and carbon stabilization, the carbon fertilization of AP can play a key role in the restoration of ecosystems in eutrophic water bodies.
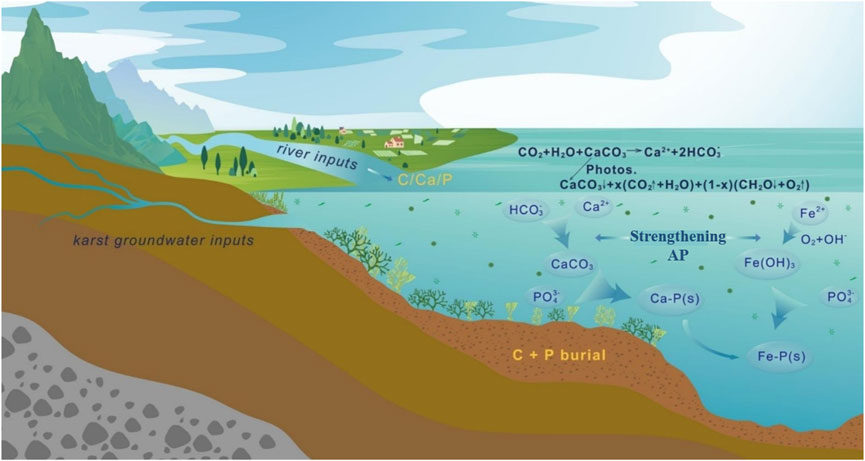
Figure 4. The strengthening autochthonous production (AP) on P scavenging in karst lake ecosystems (modified from He et al., 2025a).
Conclusion and future research directions
In karst surface water ecosystems, phytoplankton and submerged aquatic plants can fix part of the carbonate weathering carbon sink through photosynthesis, forming a stable carbon sink. The higher the concentration of dissolved inorganic carbon (DIC), the higher the aquatic productivity, and there is a “DIC (CO2) fertilization effect”. At the same time, AP in karst surface water has the effect of improving the water environment. The CO2 fertilization of water bodies based on the carbon limitation of biological pumps, as a natural land-based solution, can be the focus of future research on carbon sequestration and eutrophication mitigation in water bodies, which can provide a new research framework to realize the win-win situation of carbon sequestration and eutrophication mitigation. The breakthrough of this theoretical mechanism will provide a new theoretical support for future artificial carbon sinks and water quality control.
Although some progress has been made in AP’s pollution reduction and sink enhancement studies. However, there are still some limitations in this case study. First, most of the current studies have been carried out independently for a single region and a single period of time, and there is a lack of broad global studies and high-frequency, time-scaled monitoring. Secondly, most of the previous studies have focused on CO2, and quantitative studies on biogeochemical processes associated with emissions of other greenhouse gases (methane and nitrous oxide) need to be strengthened. Finally, the mechanisms of AP-related carbon sinks and eutrophication mitigation effects should be further elucidated. In summary. The study of CO2 fertilization and its carbon sink and eutrophication mitigation effects on AP in karst surface waters needs to be further strengthened, especially in the research direction, theory and methodology, which still need to be further improved and systematically elucidated.
Based on the review in this paper, we suggest that future research should focus on the following aspects.
Establishment of a network for momtoring carbon fluxes in karst surface waters
The processes of horizontal carbon exchange and vertical carbon burial are two key processes for carbon storage in karst surface waters. Accurate measurement of the fluxes of the above processes is the basis for assessing the AP-related carbon sinks in karst surface waters. Future studies should use hydrological modeling, radioisotopes, stable carbon isotopes, and machine learning to accurately quantify the lateral transport fluxes and vertical carbon burial fluxes in karst surface waters, to study their transport and transformation mechanisms, and to trace the sources of different carbon.
Enhance quantitative studies of biogeochemical processes associated wit mathane (CH4) and nirtrous qxide (N2O) emissions
As potentiated greenhouse gases (GHGs), CH4 and N2O are of great significance to the global carbon cycle. Previous studies related to AP have mainly focused on CO2 fluxes and their influence mechanisms. In order to more accurately assess the AP-related carbon sinks in karst surface waters, the emission fluxes of CH4 and N2O need to be comprehensively considered, especially the microbial contributions to the carbon cycle and their emission fluxes of methane and nitrous oxide. Future studies could quantify the biogeochemical processes associated with methane and nitrous oxide emissions through field investigations combined with remote sensing techniques.
Feedback mechanisms between dic fertilization in AP and its carbon sink and eutrophication mitigation effects with climate, land use and lithology
In order to systematically elucidate the DIC fertilization and its carbon sink and eutrophication mitigation effects on the productivity of karst surface waters at the global scale, we need to consider different climatic (temperature and precipitation differences), land use (N-P nutrient inputs), and lithological (carbonate-silicate rock pH and DIC differences) conditions in an integrated manner in the future. In particular, the following scientific questions need to be further investigated: (1) What are the mechanisms and conditions under which surface water body DIC contributes to AP fertilization? (2) What are the coupling relationships and mechanisms between C:N:P:Si and phytoplankton-submerged plant community structure in surface waters? (3) What are the carbon sink and eutrophication mitigation effects of AP under DIC fertilization in surface waters? The solution of the above questions is of great significance for the promotion of DIC (CO2) fertilization of AP and its carbon sink and eutrophication mitigation effects in a wider range of inland water bodies.
Study on the enhancement potential of sriticial interventions on carbon sinks and water environment effects
Enhanced rock weathering (ERW) has been rapidly developed in recent years as an important technology for anthropogenic capture of atmospheric CO2. Due to the rapid kinetic characteristics of carbonate dissolution, carbonate rock-based enhanced rock weathering (ECW) techniques has become an important development direction for ERW. We provide a preliminary quantitative assessment of the carbon sink potential, implementation cost, and carbon footprint generated by ECW, and conduct a comprehensive study on the sink enhancement efficiency (stability) of ECW implementation and its potential environmental impacts. On the basis of ECW, we propose that a new model of enhanced carbonate rock weathering (ECCW) coupled with photosynthesis of aquatic organisms should be developed, with particular emphasis on the important contribution of the carbon fertilization of AP in inland waters in stabilizing large-scale CO2 removal by ECW. In the future, sink enhancement efficiency should be a key concern for future ECW implementation, and the goal should be to convert a greater proportion of dissolved inorganic carbon (DIC) from enhanced rock weathering into endogenous organic carbon sequestration through synchronized regulation of aquatic photosynthesis to stabilize carbon sinks generated by ECW towards 100%.
Author contributions
MS: Writing – review and editing. ZL: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review and editing, Formal Analysis. SZ: Writing – review and editing. HS: Writing – review and editing. HH: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (42130501, 42141008, 42307070, and 42407287), the Guizhou Provincial 2021 Science and Technology Subsidies (GZ2021SIG), and the Autonomous Strategy Project of the State Key Laboratory of Environmental Geochemistry (SKLEG2024106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allen, E., and Spence, D. (1981). The differential ability of aquatic plants to use the inorganic carbon supply in freshwaters. New Phytol. 87, 269–283. doi:10.1111/j.1469-8137.1981.tb03198.x
Anderson, N. J., Bennion, H., and Lotter, A. F. (2014). Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob. Change Biol. 20, 2741–2751. doi:10.1111/gcb.12584
Arneth, A., Sitch, S., Pongratz, J., Stocker, B., Ciais, P., Poulter, B., et al. (2017). Historical carbon dioxide emissions caused by land-use changes are possibly larger than assumed. Nat. Geosci. 10, 79–84. doi:10.1038/NGEO2882
Bao, Q., Liu, Z., Zhao, M., Hu, Y., Li, D., Han, C., et al. (2020). Primary productivity and seasonal dynamics of planktonic algae species composition in karst surface waters under different land uses. J. Hydrology 591, 125295. doi:10.1016/j.jhydrol.2020.125295
Bao, Q., Liu, Z., Zhao, M., Hu, Y., Li, D., Han, C., et al. (2022). Role of carbon and nutrient exports from different land uses in the aquatic carbon sequestration and Eutrophication process. Sci. Total Environ. 813, 151917. doi:10.1016/j.scitotenv.2021.151917
Battin, T., Lauerwald, R., Bernhardt, E., Bertuzzo, E., Gener, L. G., Hall, R., et al. (2023). River ecosystem metabolism and carbon biogeochemistry in a changing world. Nature 613, 449–459. doi:10.1038/s41586-022-05500-8
Bundeleva, I., Shirokova, L., Bénézeth, P., Pokrovsky, O., Kompantseva, E., and Balor, S. (2012). Calcium carbonate precipitation by anoxygenic phototrophic bacteria. Chem. Geol. 291, 116–131. doi:10.1016/j.chemgeo.2011.10.003
Cabrerizo, M., Álvarez-Manzaneda, M. I., Leon-Palmero, E., Guerrero-Jiménez, G., de Senerpont Domis, L., Teurlincx, S., et al. (2020). Warming and CO2 effects under oligotrophication on temperate phytoplankton communities. Water Res. 173, 115579. doi:10.1016/j.watres.2020.115579
Chang, C., Erbaugh, J., Fajardo, P., Lu, L., Molnár, I., Papp, D., et al. (2024). Global evidence of human well-being and biodiversity impacts of natural climate solutions. Nat. Sustain. 8, 75–85. doi:10.1038/s41893-024-01454-z
Chen, B., Yang, R., Liu, Z., Sun, H., Yan, H., Zeng, Q., et al. (2017). Coupled control of land uses and aquatic biological processes on the diurnal hydrochemical variations in the five ponds at the Shawan Karst Test Site, China: implications for the carbonate weathering-related carbon sink. Chem. Geol. 456, 58–71. doi:10.1016/j.chemgeo.2017.03.006
Cole, J., Caraco, N., Kling, G., and Kratz, T. (1994). Carbon dioxide supersaturation in the surface waters of lakes. Sci. (New York, N.Y.) 265, 1568–1570. doi:10.1126/science.265.5178.1568
Cole, J., Prairie, Y., Caraco, N., McDowell, W., Tranvik, L., Striegl, R., et al. (2007). Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185. doi:10.1007/s10021-006-9013-8
Conley, D., Paerl, H., Howarth, R., Boesch, D., Seitzinger, S., Havens, K., et al. (2009). Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015. doi:10.1126/science.1167755
Corman, J., Moody, E., and Elser, J. (2015). Stoichiometric impact of calcium carbonate deposition on nitrogen and phosphorus supplies in three montane streams. Biogeochemistry 126, 285–300. doi:10.1007/s10533-015-0156-6
Corman, J., Moody, E., and Elser, J. (2016). Calcium carbonate deposition drives nutrient cycling in a calcareous headwater stream. Ecol. Monogr. 86, 448–461. doi:10.1002/ecm.1229
De Montety, V., Martin, J., Cohen, M., Foster, C., and Kurz, M. (2011). Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem. Geol. 283, 31–43. doi:10.1016/j.chemgeo.2010.12.025
Deng, Z., Zhu, B., Davis, S., Ciais, P., Guan, D., Gong, P., et al. (2025). Global carbon emissions and decarbonization in 2024. Nat. Rev. Earth Environ. 6, 231–233. doi:10.1038/s43017-025-00658-x
Dittrich, M., and Koschel, R. (2002). Interactions between calcite precipitation (natural and artificial) and phosphorus cycle in the hardwater lake. Hydrobiologia 469, 49–57. doi:10.1023/A:1015571410442
Edmondson, W. (1970). Phosphorus, nitrogen, and algae in lake Washington after diversion of sewage. Science 169, 690–691. doi:10.1126/science.169.3946.690
Elser, J., Andersen, T., Baron, J., Bergström, A. K., Jansson, M., Kyle, M., et al. (2009). Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326, 835–837. doi:10.1126/science.1176199
Elser, J., Devlin, S., Yu, J., Baumann, A., Church, M., Dore, J., et al. (2022). Sustained stoichiometric imbalance and its ecological consequences in a large oligotrophic lake. Proc. Natl. Acad. Sci. U. S. A. 119, e2202268119. doi:10.1073/pnas.2202268119
Elser, J., Fagan, W., Denno, R., Dobberfuhl, D., Folarin, A., Huberty, A., et al. (2000). Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580. doi:10.1038/35046058
Finlay, K., Leavitt, P., Wissel, B., and Prairie, Y. (2009). Regulation of spatial and temporal variability of carbon flux in six hard-water lakes of the Northern Great Plains. Limnol. Oceanogr. 54, 2553–2564. doi:10.4319/lo.2009.54.6_part_2.2553
Finlay, K., Vogt, R., Bogard, M., Wissel, B., Tutolo, B., Simpson, G., et al. (2015). Decrease in CO2 efflux from northern hardwater lakes with increasing atmospheric warming. Nature 519, 215–218. doi:10.1038/nature14172
Friedlingstein, P., O’Sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Landschützer, P., et al. (2025). Global carbon budget 2024. Earth Syst. Sci. Data 17, 965–1039. doi:10.5194/essd-17-965-2025
Gilarranz, L., Narwani, A., Odermatt, D., Siber, R., and Dakos, V. (2022). Regime shifts, trends, and variability of lake productivity at a global scale. Proc. Natl. Acad. Sci. U. S. A. 119, e2116413119. doi:10.1073/pnas.2116413119
Giordano, M., Beardall, J., and Raven, J. (2005). CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 56, 99–131. doi:10.1146/annurev.arplant.56.032604.144052
Gomez, F., Mlewski, C., Boidi, F., Farías, M. E., and Gérard, E. (2018). Calcium carbonate precipitation in diatom-rich microbial mats: the laguna negra hypersaline lake, catamarca, Argentina. J. Sediment. Res. 88, 727–742. doi:10.2110/jsr.2018.37
Gromiec, M. J., and Morgan, J. (1983). Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. Ecol. Model. 19, 227–230. doi:10.1016/0304-3800(83)90061-3
Hamilton, S., Bruesewitz, D., Horst, G., Weed, D., and Sarnelle, O. (2009). Biogenic calcitephosphorus precipitation as a negative feedback to lake eutrophication. Can. J. Fish. Aquat. Sci. 66, 343–350. doi:10.1139/F09-003
Hammer, K., Kragh, T., and Kaj, S. (2019). Inorganic carbon promotes photosynthesis, growth, and maximum biomass of phytoplankton in eutrophic water bodies. Freshwat. Biol. 64, 1956–1970. doi:10.1111/fwb.13385
He, H., Liu, Z., Chen, C., Wei, Y., Bao, Q., Sun, H., et al. (2020). The sensitivity of the carbon sink by coupled carbonate weathering to climate and land-use changes: sediment records of the biological carbon pump effect in Fuxian Lake, Yunnan, China, during the past century. Sci. Total Environ. 720, 137539. doi:10.1016/j.scitotenv.2020.137539
He, H., Liu, Z., Chen, J., Li, D., Wang, Y., Han, Y., et al. (2025a). Enhanced biological pump and carbonate pump synergy: the primary pathway for phosphorus clearance in the century-long dynamics of a karst lake. Glob. Planet. Change 245, 104694. doi:10.1016/j.gloplacha.2025.104694
He, H., Liu, Z., Li, D., Liu, X., Han, Y., Sun, H., et al. (2024). Effects of carbon limitation and carbon fertilization on karst lake-reservoir productivity. Water Res. 261, 122036. doi:10.1016/j.watres.2024.122036
Hecky, R., and Kilham, P. (1988). Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33, 796–822. doi:10.4319/lo.1988.33.4part2.0796
Hoffman, A. R., Armstrong, D. E., and Lathrop, R. C. (2013). Influence of phosphorus scavenging by iron in contrasting dimictic lakes. Can. J. Fish. Aquat. Sci. 70, 941–952. doi:10.1139/cjfas-2012-0391
Houghton, R., Baccini, A., and Walker, W. (2018). Where is the residual terrestrial carbon sink? Glob. Change Biol. 24, 3277–3279. doi:10.1111/gcb.14313
Iversen, L. L., Winkel, A., Baastrup-Spohr, L., Hinke, A. B., Alahuhta, J., Baattrup-Pedersen, A., et al. (2019). Catchment properties and the photosynthetic trait composition of freshwater plant communities. Science 366, 878–881. doi:10.1126/science.aay5945
Jansson, M., Karlsson, J., and Jonsson, A. (2012). Carbon dioxide supersaturation promotes primary production in lakes. Ecol. Lett. 15, 527–532. doi:10.1111/j.1461-0248.2012.01762.x
Jia, J., Sun, K., Lü, S., Li, M., Wang, Y., Yu, G., et al. (2022). Determining whether Qinghai–Tibet Plateau waterbodies have acted like carbon sinks or sources over the past 20 years. Sci. Bull. 67, 2345–2357. doi:10.1016/j.scib.2022.10.023
Kaijser, W., Lorenz, A., Birk, S., and Hering, D. (2021). The interplay of nutrients, dissolved inorganic carbon and algae in determining macrophyte occurrences in rivers. Sci. Total Environ. 781, 146728. doi:10.1016/j.scitotenv.2021.146728
Kamiya, M., Hatta, J., Shimada, E., Ikuma, Y., Yoshimura, M., and Monma, H. (2004). AFM analysis of initial stage of reaction between calcite and phosphate. Mater. Sci. Eng. B 111, 226–231. doi:10.1016/S0921-5107(04)00210-7
Katkov, E., Low-Decarie, E., and Fussmann, G. (2020). Intra-annual variation of phytoplankton community responses to factorial N, P, and CO2 enrichment in a temperate mesotrophic lake. Freshwat. Biol. 65, 960–970. doi:10.1111/fwb.13482
Kirschbaum, M., Zeng, G., Ximenes, F., Giltrap, D., and Zeldis, J. (2019). Towards a more complete quantification of the global carbon cycle. Biogeosciences 16, 831–846. doi:10.5194/bg-16-831-2019
Kragh, T., and Sand-Jensen, K. (2018). Carbon limitation of lake productivity. Proc. R. Soc. B 285, 20181415. doi:10.1098/rspb.2018.1415
Lai, C., Ma, Z., Liu, Z., Sun, H., Yu, Q., Xia, F., et al. (2023). Alleviating eutrophication by reducing the abundance of Cyanophyta due to dissolved inorganic carbon fertilization: insights from Erhai Lake, China. J. Environ. Sci. 131, 68–83. doi:10.1016/j.jes.2022.10.030
Lin, Y.-P., and Singer, P. (2006). Inhibition of calcite precipitation by orthophosphate: speciation and thermodynamic considerations. Geochimica Cosmochimica Acta 70, 2530–2539. doi:10.1016/j.gca.2006.03.002
Liu, J., Luo, X., Zhang, N., and Wu, Y. (2016). Phosphorus released from sediment of Dianchi Lake and its effect on growth of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 23, 16321–16328. doi:10.1007/s11356-016-6816-9
Liu, S., Wang, J., Xu, W., Zhang, P., Zhang, S., Chen, X., et al. (2025). Human activities reshape greenhouse gas emissions from inland waters. Glob. Change Biol. 31, e70139. doi:10.1111/gcb.70139
Liu, Z., Dreybrodt, W., and Wang, H. (2010). A new direction in effective accounting for the atmospheric CO2 budget: considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Science Rev. 99, 162–172. doi:10.1016/j.earscirev.2010.03.001
Liu, Z., Macpherson, G., Groves, C., Martin, J., Yuan, D., and Zeng, S. (2018). Large and active CO2 uptake by coupled carbonate weathering. Earth-Science Rev. 182, 42–49. doi:10.1016/j.earscirev.2018.05.007
Liu, Z., Yan, H., and Zeng, S. (2021). Increasing autochthonous production in Inland waters as a contributor to the missing carbon sink. Front. Earth Sci. 9, 620513. doi:10.3389/feart.2021.620513
Low-Decarie, E., Bell, G., and Fussmann, G. (2015). CO2 alters community composition and response to nutrient enrichment of freshwater phytoplankton. Oecologia 177, 875–883. doi:10.1007/s00442-014-3153-x
Low-Decarie, E., Fussmann, G., and Bell, G. (2011). The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Glob. Change Biol. 17, 2525–2535. doi:10.1111/j.1365-2486.2011.02402.x
Maberly, S., and Gontero, B. (2017). Ecological imperatives for aquatic CO2-concentrating mechanisms. J. Exp. Bot. 68, 3797–3814. doi:10.1093/jxb/erx201
Mendonça, R., Müller, R. A., Clow, D., Verpoorter, C., Raymond, P., Tranvik, L., et al. (2017). Organic carbon burial in global lakes and reservoirs. Nat. Commun. 8, 1694. doi:10.1038/s41467-017-01789-6
Middelburg, J. J. (2024). Closing the inland water carbon cycle. Sci. Adv. 10, eadt3893. doi:10.1126/sciadv.adt3893
Müller, B., Meyer, J., and Gächter, R. (2016). Alkalinity regulation in calcium carbonate-buffered lakes. Limnol. Oceanogr. 61, 341–352. doi:10.1002/lno.10213
Murphy, T. P., Hall, K. J., and Yesaki, I. (1983). Coprecipitation of phosphate with calcite in a naturally eutrophic lake. Limnol. Oceanogr. 28, 58–69. doi:10.4319/lo.1983.28.1.0058
Naselli-Flores, L., Barone, R., and Mosello, R. (2003). Eutrophication control by lime addition: a preliminary approach in Sicilian reservoirs. Hydrobiologia 504, 297–303. doi:10.1023/B:HYDR.0000008529.57172.d6
Nimick, D., Gammons, C., and Parker, S. (2011). Diel biogeochemical processes and their effect on the aqueous chemistry of streams: a review. Chem. Geol. 283, 3–17. doi:10.1016/j.chemgeo.2010.08.017
Nõges, P., Cremona, F., Laas, A., Martma, T., Rõõm, E. I., Toming, K., et al. (2016). Role of a productive lake in carbon sequestration within a calcareous catchment. Sci. Total Environ. 550, 225–230. doi:10.1016/j.scitotenv.2016.01.088
Obst, M., Wehrli, B., and Dittrich, M. (2009). CaCO3 nucleation by cyanobacteria: laboratory evidence for a passive, surface-induced mechanism. Geobiology 7, 324–347. doi:10.1111/j.1472-4669.2009.00200.x
Otsuki, A., and Wetzel, R. (1972). Coprecipitation of phosphate with carbonates in a marl lake. Limnol. Oceanogr. 17, 763–767. doi:10.4319/lo.1972.17.5.0763
Pagano, A., and Titus, J. (2004). Submersed macrophyte growth at low pH: contrasting responses of three species to dissolved inorganic carbon enrichment and sediment type. Aquat. Bot. 79, 65–74. doi:10.1016/j.aquabot.2004.01.004
Pan, Y., Birdsey, R., Fang, J., Houghton, R., Kauppi, P., Kurz, W., et al. (2011). A large and persistent carbon sink in the world's forests. Science 333, 988–993. doi:10.1126/science.1201609
Price, G., Badger, M., Woodger, F., and Long, B. (2008). Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59, 1441–1461. doi:10.1093/jxb/erm112
Ran, L., Butman, D., Battin, T., Yang, X., Tian, M., Duvert, C., et al. (2021). Substantial decrease in CO2 emissions from Chinese inland waters due to global change. Nat. Commun. 12, 1730. doi:10.1038/s41467-021-21926-6
Raymond, P. A., and hamilton, S. K. (2018). Anthropogenic influences on riverine fluxes of dissolved inorganic carbon to the oceans. Limnol. Oceanogr. Lett. 3, 143–155. doi:10.1002/lol2.10069
Reinfelder, J. (2011). Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 3, 291–315. doi:10.1146/annurev-marine-120709-142720
Rogerson, M., Pedley, H., Wadhawan, J., and Middleton, R. (2008). New insights into biological influence on the geochemistry of freshwater carbonate deposits. Geochimica Cosmochimica Acta 72, 4976–4987. doi:10.1016/j.gca.2008.06.030
Rojo, C., Sánchez-Carrillo, S., Rodrigo, M., Puche, E., Cirujano, S., and Álvarez-Cobelas, M. (2020). Charophyte stoichiometry in temperate waters. Aquat. Bot. 161, 103182. doi:10.1016/j.aquabot.2019.103182
Sardans, J., Rivas-Ubach, A., and Penuelas, J. (2012). The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect. Plant Ecol. Evol. Syst. 14, 33–47. doi:10.1016/j.ppees.2011.08.002
Scheffer, M., Carpenter, S., Foley, J. A., Folke, C., and Walker, B. (2001). Catastrophic shifts in ecosystems. Nature 413, 591–596. doi:10.1038/35098000
Schindler, D., and Fee, E. (1973). Diurnal variation of dissolved inorganic carbon and its use in estimating primary production and CO2 invasion in lake 227. J. Fish. Res. Board Can. 30, 1501–1510. doi:10.1139/f73-240
Schippers, P., Lurling, M., and Scheffer, M. (2004). Increase of atmospheric CO2 promotes phytoplankton productivity. Ecol. Lett. 7, 446–451. doi:10.1111/j.1461-0248.2004.00597.x
Shao, M., Liu, Z., Sun, H., He, H., Li, Q., Zeng, S., et al. (2024). Multi-tracer evidence of hydrology and primary production controls on dissolved organic matter composition and stability in the semi-arid aquatic continuum. Geochimica Cosmochimica Acta 384, 80–92. doi:10.1016/j.gca.2024.09.015
Shao, M., Liu, Z., Sun, H., Lai, C., Ma, Z., He, X., et al. (2023a). C-N-P driven changes to phytoplankton community structure and gross primary productivity in river-fed reservoir ecosystems on the Chinese Loess Plateau. J. Hydrology 616, 128781. doi:10.1016/j.jhydrol.2022.128781
Shao, M., Liu, Z., Sun, H., Ma, Z., Lai, C., He, H., et al. (2023b). Multi-tracer evidence for the presence of autochthonous organic carbon and the role of biological carbon pump in two river–reservoir ecosystems on the Chinese Loess Plateau. Chem. Geol. 635, 121608. doi:10.1016/j.chemgeo.2023.121608
Shao, M., Liu, Z., Zeng, S., Sun, H., He, H., Adnan, M., et al. (2025). Carbon sinks associated with biological carbon pump in karst surface waters: progress, challenges, and prospects. Environ. Res. 267, 120712. doi:10.1016/j.envres.2024.120712
Shi, X., Zhao, X., Zhang, M., Yang, Z., Xu, P., and Kong, F. (2016). The responses of phytoplankton communities to elevated CO2 show seasonal variations in the highly eutrophic Lake Taihu. Can. J. Fish. Aquat. Sci. 73, 727–736. doi:10.1139/cjfas-2015-0151
Shibata, M., Ohkawa, H., Kaneko, T., Fukuzawa, H., Tabata, S., Kaplan, A., et al. (2001). Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. U. S. A. 98, 11789–11794. doi:10.1073/pnas.191258298
Sun, H. C. H., Liu, Z., Wei, Y., Ma, S., Bao, Q., Zhang, Y., et al. (2022). Nutrient limitations on primary productivity and phosphorus removal by biological carbon pumps in dammed karst rivers: implications for eutrophication control. J. Hydrol. 607, 127480. doi:10.1016/j.jhydrol.2022.127480
Townsend, S., Schult, J., Douglas, M., and Skinner, S. (2007). Does the Redfield ratio infer nutrient limitation in the macroalga Spirogyra fluviatilis? Freshw. Biol. 53, 509–520. doi:10.1111/j.1365-2427.2007.01916.x
Tranvik, L., Downing, J., Cotner, J., Loiselle, S., Striegl, R., Ballatore, T., et al. (2009). Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 54, 2298–2314. doi:10.4319/lo.2009.54.6_part_2.2298
Van Dam, B., Tobias, C., Holbach, A., Paerl, H., and Zhu, G. (2018). CO2 limited conditions favor cyanobacteria in a hypereutrophic lake: an empirical and theoretical stable isotope study. Limnol. Oceanogr. 63, 1643–1659. doi:10.1002/lno.10798
Velthuis, M., Keuskamp, J., Bakker, E., Boersma, M., Sommer, U., van Donk, E., et al. (2022). Differential effects of elevated pCO2 and warming on marine phytoplankton stoichiometry. Limnol. Oceanogr. 67, 598–607. doi:10.1002/lno.12020
Visser, P., Verspagen, J., Sandrini, G., Stal, L., Matthijs, H., Davis, T., et al. (2016). How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 54, 145–159. doi:10.1016/j.hal.2015.12.006
Wang, W., Li, S., Zhong, J., Yi, Y., Yue, F.-J., Han, Z., et al. (2024). Unraveling the factors influencing CO2 emissions from hydroelectric reservoirs in Karst and Non-Karst regions: a comparative analysis. Water Res. 248, 120893. doi:10.1016/j.watres.2023.120893
Waterson, E., and Canuel, E. (2008). Sources of sedimentary organic matter in the Mississippi River and adjacent Gulf of Mexico as revealed by lipid biomarker and δ13CTOC analyses. Org. Geochem. 39, 422–439. doi:10.1016/j.orggeochem.2008.01.011
Webb, J. R., Santos, I. R., Maher, D. T., and Finlay, K. (2019). The importance of aquatic carbon fluxes in net ecosystem carbon budgets: a catchment-scale review. Ecosystems 22 (3), 508–527. doi:10.1007/s10021-018-0284-7
Xia, F., Liu, Z., Zhao, M., Li, Q., Li, D., Cao, W., et al. (2022). High stability of autochthonous dissolved organic matter in karst aquatic ecosystems: evidence from fluorescence. Water Res. 220, 118723. doi:10.1016/j.watres.2022.118723
Yang, M., Liu, Z., Sun, H., Yang, R., and Chen, B. (2016). Organic carbon source tracing and DIC fertilization effect in the Pearl River: insights from lipid biomarker and geochemical analysis. Appl. Geochem. 73, 132–141. doi:10.1016/j.apgeochem.2016.08.008
Yi, Y., Zhong, J., Bao, H., Mostofa, K., Xu, S., Xiao, H., et al. (2021). The impacts of reservoirs on the sources and transport of riverine organic carbon in the karst area: a multi-tracer study. Water Res. 194, 116933. doi:10.1016/j.watres.2021.116933
Zagarese, H., Sagrario, M., Wolf-Gladrow, D., Nõges, P., Nõges, T., Kangur, K., et al. (2021). Patterns of CO2 concentration and inorganic carbon limitation of phytoplankton biomass in agriculturally eutrophic lakes. Water Res. 190, 116715. doi:10.1016/j.watres.2020.116715
Zeng, S., Liu, H., Liu, Z., Kaufmann, G., Zeng, Q., and Chen, B. (2019). Seasonal and diurnal variations in DIC, NO3- and TOC concentrations in spring-pond ecosystems under different land-uses at the Shawan Karst Test Site, SW China: carbon limitation of aquatic photosynthesis. J. Hydrology 574, 811–821. doi:10.1016/j.jhydrol.2019.04.090
Zhang, F., Jin, Z., Yu, J., Zhou, Y., and Zhou, L. (2015). Hydrogeochemical processes between surface and groundwaters on the northeastern Chinese Loess Plateau: implications for water chemistry and environmental evolutions in semi-arid regions. J. Geochem. Explor. 159, 115–128. doi:10.1016/j.gexplo.2015.08.010
Zhang, Y., Liu, Z., Wu, Y., Ma, S., Cao, W., Lai, C., et al. (2023). Relationships between biomass of phytoplankton and submerged macrophytes and physicochemical variables of water in Lake Caohai, China: implication for mitigation of cyanobacteria blooms by CO2 fertilization. J. Hydrology 617, 129111. doi:10.1016/j.jhydrol.2023.129111
Keywords: inland waters, aquatic photosynthesis, autochthonous production, nature-based solution, emission reduction and sink enhancement
Citation: Shao M, Liu Z, Zeng S, Sun H and He H (2025) Carbon fertilization of autochthonous production in karst surface waters and its role in carbon reduction and eutrophication mitigation—a nature-based solution (NbS). Front. Geochem. 3:1622714. doi: 10.3389/fgeoc.2025.1622714
Received: 04 May 2025; Accepted: 01 July 2025;
Published: 11 July 2025.
Edited by:
Nadia Valentina Martínez-Villegas, Instituto Potosino de Investigación Científica y Tecnológica (IPICYT), MexicoReviewed by:
Wei-dong Zhai, Southern Marine Science and Engineering Guangdong Laboratory, ChinaQingguang Li, Guizhou University, China
Copyright © 2025 Shao, Liu, Zeng, Sun and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaihua Liu, bGl1emFpaHVhQHZpcC5neWlnLmFjLmNu
 Mingyu Shao1
Mingyu Shao1 Zaihua Liu
Zaihua Liu Hailong Sun
Hailong Sun