- 1Department of Surgery, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Surgical Oncology, Fox Chase Cancer Center, Philadelphia, PA, United States
- 3Cardiology Department, Athens Naval Hospital, Athens, Greece
- 4Division of Vascular Surgery, Cardiovascular Center, Tufts Medical Center, Boston, MA, United States
- 5Institute for Life Sciences, University of Southampton, United Kingdom
- 6µ-VIS X-ray Imaging Centre, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, United Kingdom
- 7First Department of Pathology, National and Kapodistrian University of Athens, Athens, Greece
- 8Aristotle University of Thessaloniki, Thessaloniki, Greece
- 9Department of Surgery and Cancer, Imperial College, London, United Kingdom
- 10Department of Pathology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Background: There is a growing interest in exploring intraoperative methods for margin assessment of malignant breast specimens in breast-conserving surgeries (BCS). Micro-computed tomography (micro-CT) has already exhibited clinical value, yielding high-resolution three-dimensional (3D) volumetric images. Against this background, this study aimed to systematically evaluate the role of micro-CT in intraoperative margin assessment (IMA) in BCS.
Methods: A systematic literature review has been conducted in Scopus, EMBASE, and PubMed up to 10 December 2024. Studies reporting the diagnostic indices of micro-CT for IMA compared to histopathologic results were utilized for a diagnostic accuracy meta-analysis.
Results: Eight out of the initially retrieved 2,921 studies evaluated the role of micro-CT in IMA and were eligible for calculating the pooled diagnostic indices. In those studies, 988 specimens/margins were scanned, and the scanning time ranged from 4 to 30 min. The pooled diagnostic indices were: a sensitivity of 0.63 (95% CI: 0.45–0.79), a specificity of 0.78 (95% CI: 0.68–0.85), and an accuracy of 0.77 (95% CI: 0.71–0.84) for micro-CT based IMA compared to the gold-standard histopathological assessment.
Conclusion: This study demonstrates that micro-CT imaging is a promising IMA technique for BCS by providing high-resolution 3D images. These images can be acquired within a few minutes, allowing surgeons to assess margin status intra-operatively, and identify more than 70% of positive margins where reoperation rates are likely to decrease. Although these findings are encouraging, their clinical translation is still under investigation, and adequately empowered clinical trials are warranted to investigate the re-excision and local recurrence rates after micro-CT IMA assessment.
Systematic Review Registration: https://osf.io/342h8.
Introduction
Βreast cancer is the most common cancer in women worldwide, with an estimated 441,000 new cases in the United States in 2030 (Rosenberg et al., 2015). The long-term survival rate among women who undergo mastectomy is the same as that among women who undergo breast-conserving surgery (BCS) (Veronesi et al., 2002); therefore, the treatment of choice for most women with relatively small breast cancers is BCS, followed by radiation therapy (Veronesi et al., 2002; Smitt et al., 1995). Current American Society of Clinical Oncology guidelines for patients with invasive breast cancer undergoing BCS specify that a margin of no ink on tumor is adequate, and there is no benefit to obtaining wider margins (Moran et al., 2014a). Given the required specimen processing, it usually takes more than a day to determine the final margin status after BCS. A population-based study showed that after ‘‘no tumor on ink’’ consensus guidelines, re-excision rates after breast conservation were 14% with a 4% conversion to mastectomy (Moran et al., 2014a; Morrow et al., 2017; Moran et al., 2014b). Also, positive margin rates after BCS have been reported to range from 5% to 30% -depending on the histologic subtype-, which highlights the clinical importance of accurate intraoperative margin assessment (IMA) (Moran et al., 2014a; Morrow et al., 2017; Moran et al., 2014b). Re-excisions are linked with poorer outcomes and increased psychological and economic burden (Smitt et al., 1995).
Therefore, the predictive value of novel intraoperative methods for IMA is increasingly being investigated (Pradipta et al., 2020; Schnabel et al., 2014; Esbona et al., 2012). Specimen intraoperative radiographs have been shown to decrease re-excisions (McCormick et al., 2004; Bathla et al., 2011; Kaufman et al., 2007; Muttalib et al., 2004). In a single study of 93 patients, two-dimensional (2D) specimen radiographs decreased the re-excision rate from 12% to 5% (McCormick et al., 2004). In a recent study, the surgeon who used three-dimensional (3D) imaging by means of X-ray tomosynthesis achieved a reduction in re-excision rate from 9% to 5% (Partain et al., 2020). Several studies have investigated the diagnostic accuracy of intraoperative frozen section biopsy, imprint cytology, and other novel techniques for IMA during BCS including spatial frequency domain imaging (SFDI), targeted fluorescence imaging, 18F-fluorodeoxyglucose specimen-positron emission mammography, intraoperative magnetic resonance imaging (MRI), handheld optical imaging probe, and radiofrequency spectroscopy (MarginProbe) (Watanabe et al., 2018; Papa et al., 2016; Zysk et al., 2015; Thill et al., 2011; Maloney et al., 2018; Dowling et al., 2024; Manhoobi et al., 2022).
Additionally, micro-computed tomography (micro-CT) has emerged as a promising ex vivo imaging modality that provides high-resolution 3D volumetric images. Recent advances in micro-CT technology have led to significant improvements in both spatial and contrast resolution, enabling the acquisition of high-resolution images with voxel (i.e., the 3-D analog of pixel) sizes in the -μm range (Katsamenis et al., 2023). These technical advancements have expanded the potential clinical applications of micro-CT to various medical specialties, including surgical oncology. Therefore, micro-CT has the potential to be a valuable imaging tool for clinical research and diagnosis in the field of surgical oncology and other medical specialties (Cengiz et al., 2018; Hutchinson et al., 2017; Katsameni et al., 2019).
Breast cancer cells are about 10–20 microns in size (Milano et al., 2016). Pathology lab microscopes approach resolution down to about 0.2 μm, and current high-resolution lab-based micro-CT scanners can resolve sub-micrometer-sized features; however, this typically requires millimeter-sized specimens, and/or involves limitations in the field of view (Walton et al., 2015; Walsh et al., 2021). These constraints render such high magnification levels impractical for imaging larger specimens within the context of BCS; an exception may be technologies such as HiP-CT, which employ hierarchical magnification to achieve high resolution across larger volumes (Walton et al., 2015; Walsh et al., 2021).
Micro-CT scanners can be installed in the pathology lab or operating theater, allowing for real-time assessment of scanned specimens by pathologists, radiologists, and surgeons. This can provide valuable information for determining if additional excision is necessary to achieve negative margins. In the context of BCS, micro-CT has the potential to improve, expedite, and aid in determining the size and other volumetric characteristics of breast tumors in intact lumpectomy specimens (DiCorpo et al., 2020; Sarraj et al., 2015; Janssen et al., 2019).
In this study, we aimed to systematically review the clinical applications of micro-CT in breast cancer management and to perform an updated meta-analysis to evaluate the diagnostic accuracy of micro-CT-based IMA compared to conventional histopathologic evaluation.
Materials and methods
Literature search
A systematic literature review regarding micro-CT applications in breast cancer is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Page et al., 2021) (Supplementary Table S1). The protocol of this meta-analysis has been prospectively registered at the Open Science Framework registries (https://osf.io/342h8). Two primary reviewers (SMMY and KR) independently searched the literature in Scopus, EMBASE, and PubMed. All articles published before 10 December 2024, were screened for inclusion. The keywords used in our search query included: [(“micro-CT” OR “micro tomography” OR “micro-computed tomography”) AND (“breast cancer “OR “breast malignancy” OR “malignant breast disease”)]. Extensive hand-searching of the references was performed in the retrieved articles to identify other papers not previously detected.
Eligibility criteria and quality assessment
Peer-reviewed studies were included if they evaluated the role of micro-CT scanning for IMA in patients with breast cancer. The exclusion criteria were determined as (a) any phantom, animal, or cadaver studies and (b) any literature reviews, editorials, and conference abstracts. Two authors (SMMY and KR) independently screened all articles according to the eligibility criteria previously set. Any disagreement was resolved through discussion and with the consultation of a senior author (MV). Two reviewers (MV and ASP) independently assessed the methodological quality of the included studies according to the Quality Assessment of Diagnosis Accuracy Study (QUADAS-2) form (Whiting et al., 2011).
Data extraction and statistical analysis
A data extraction worksheet was created to provide a descriptive review of the reported results. Two reviewers (SMMY and ACL) extracted the numeric baseline characteristics of the studies, and another author (MV) rechecked them.
To compare the diagnostic accuracy of micro-CT IMA to the final pathology, a pooled receiver operating characteristics (ROC) curve–also called hierarchical summary ROC (HSROC) – was created by the metandi package of Stata 17 (Stata Corp. LLC, US) based on logit transformed standard errors. Due to the expected heterogeneity, a mixed effects method was utilized in the HSROC analysis. The raw amounts of the diagnostic contingency tables, including true positive (TP), false positive (FP), true negative (TN), and false negative (FN), were collected or recalculated from the eligible studies. For studies that did not directly report all values required to construct 2 × 2 contingency tables (true positive, false positive, true negative, false negative), we recalculated missing values from available diagnostic indices (e.g., sensitivity, specificity, PPV) and sample sizes. In cases where 0 cells were present in the tables, continuity corrections were applied (Yates correction), and in one study, values were averaged from multiple evaluators. Studies with ambiguous or incomplete raw data were either excluded or approximated conservatively to avoid inflation of pooled estimates. To investigate the degree of heterogeneity of the diagnostic accuracy in the eligible studies for the meta-analysis, I2 based on normal standard errors (√pq/n) and random effect weighting for pooled results were calculated.
Furthermore, we performed subgroup and sensitivity analyses to validate the significance of our findings: (i) a subgroup analysis on industrial funding report could provide the diagnostic indices of micro-CT-based IMA according to data presented or not by industry sponsored studies, and (ii) sensitivity analyses addressing: a) large sample size effect, b) individual margin-level assay, c) different micro-CT diagnostic technique, and d) different margin assessment methods could indicate whether the initial diagnostic indices are substantially affected when omitting the relevant studies from the meta-analysis.
Our outcomes were assessed using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach (https://gdt.gradepro.org/app), which provides a systematic approach to making clinical practice recommendations.
Results
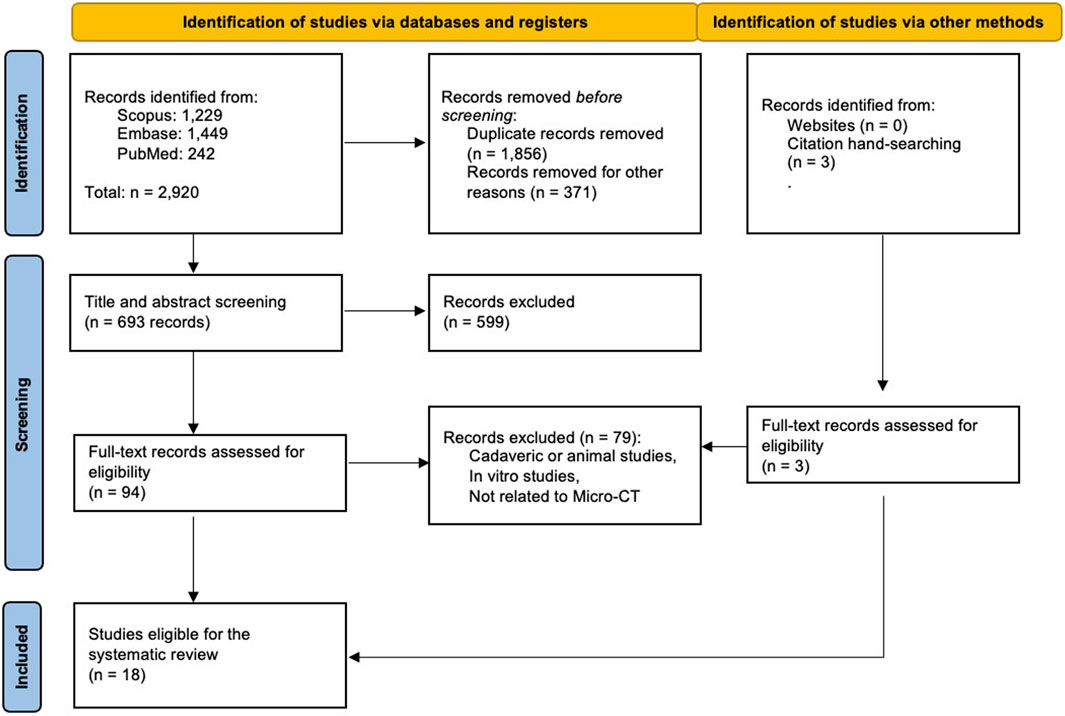
Our literature search initially yielded 2,921 articles (Figure 1). Of them, eight studies reported the accuracy of micro-CT-based IMA and were deemed eligible for this diagnostic accuracy meta-analysis (DiCorpo et al., 2020; Janssen et al., 2019; Tang et al., 2013a; McClatchy et al., 2018; Göker et al., 2020; Bourke and Abel, 2020; Streeter et al., 2023; Qiu et al., 2018). Eleven studies also evaluated the role of micro-CT in measuring the tumor size (DiCorpo et al., 2020; Sarraj et al., 2015; Tang et al., 2016), investigating the microcalcifications (Janssen et al., 2019; Tang et al., 2013a; McClatchy et al., 2018; Bourke and Abel, 2020; Qiu et al., 2018; Brahimetaj et al., 2022; Streeter et al., 2021; Chen et al., 2009; Gufler et al., 2011; Willekens et al., 2014; Kenkel et al., 2017; Tang et al., 2013b), and the differentiation between benign and malignant lymph nodes (Tang et al., 2013b). Due to the small sample sizes and heterogeneity of the reviewed studies, a meta-analysis of those outcomes was impossible, and they were not eligible for this meta-analysis.
Most studies were retrospective, but three eligible studies (Tang et al., 2013a; Bourke and Abel, 2020; Streeter et al., 2023), and one of the three phases of the study by Janssen et al. (Janssen et al., 2019) were prospectively designed. Most of the studies were conducted in the USA (4/8, 50%) and the Netherlands (2/8, 25%), followed by Belgium, and Australia (one study for each). The most commonly used micro-CT scanners were SkyScan models (1173, 1176, and 1275) (DiCorpo et al., 2020; Janssen et al., 2019; Bourke and Abel, 2020; Qiu et al., 2018; Tang et al., 2013b). The studies analyzed here use voxel sizes in the range of 4–200 µm (based on the reported data; Supplementary Table S2).
According to the QUADAS-2 checklist, most included studies had a low risk of bias. The “applicability concerns” part of this checklist was filled based on our main research question about the contribution of micro-CT scanning to the investigation of margin pathology (Supplementary Figure S1).
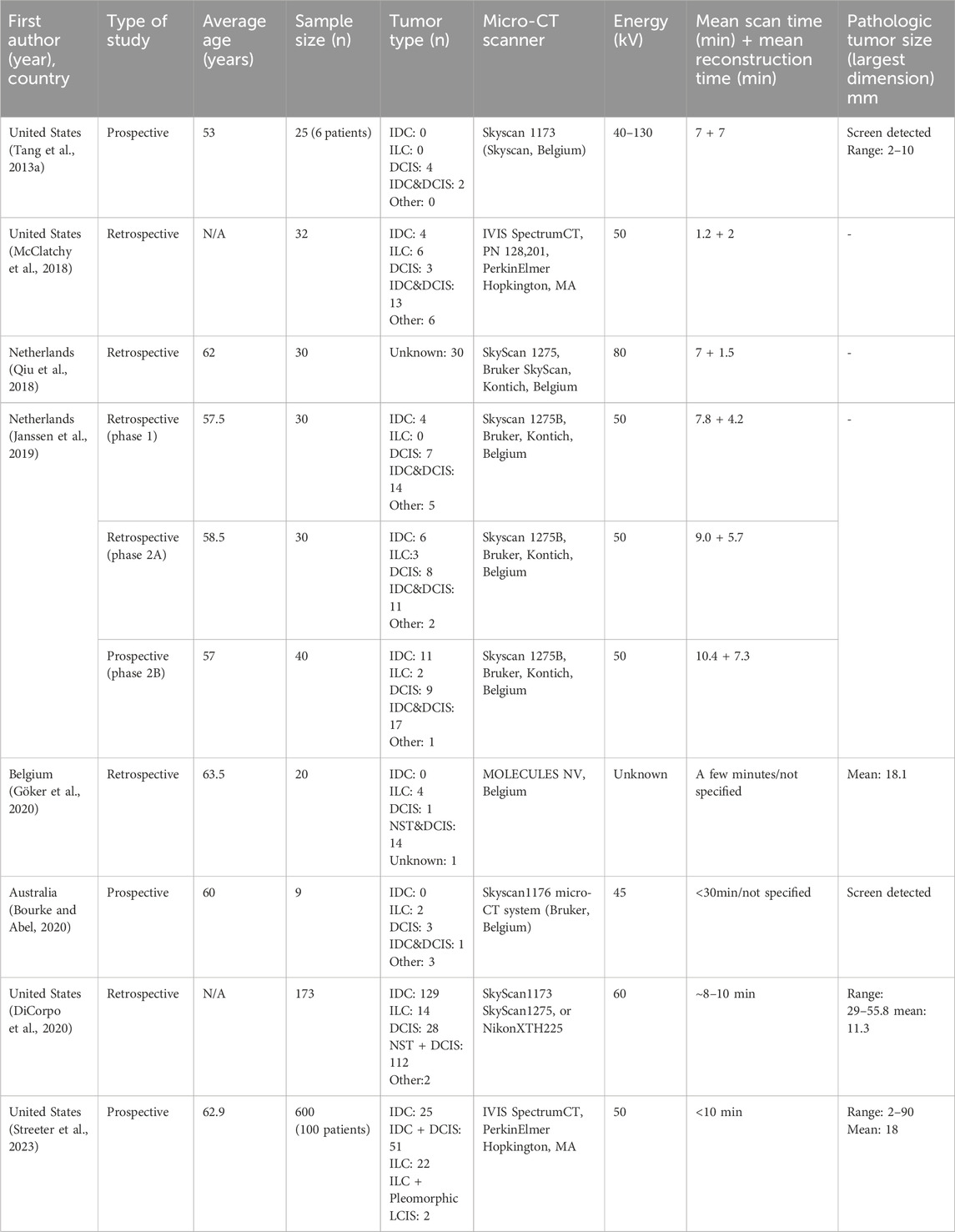
A total of 988 samples (specimens/margins) from eight studies were included (Table 1). The participants’ average ages ranged from 37 to 84 years. Moreover, the pathologic tumor size (largest diameter) was between 2 (Tang et al., 2013b) and 90 mm (DiCorpo et al., 2020). When reported, the scanning time ranged between 4 and 30 min. Two studies only evaluated screen-detected nonpalpable tumors (Tang et al., 2013a; Bourke and Abel, 2020). Invasive ductal carcinomas (IDC), invasive lobular carcinoma (ILC), and pure ductal carcinoma in situ (DCIS) constituted 40.7%, 13.4%, and 14.3% of the specimens. Two studies reported the focality of malignant lesions: 112/125 cases were considered unifocal, while 23/125 cases were deemed multifocal (DiCorpo et al., 2020; Tang et al., 2013a). In every eligible study, hematoxylin and eosin slides were evaluated under the microscope by the pathologist to determine the histopathological margin status. Resection margins were considered positive in case invasive cancer or DCIS reached into the inked border of the excision specimen. Only two studies (McCormick et al., 2004; Metcalfe et al., 2017) defined the positive margin for pure DCIS. Those studies considered the margin, positive if DCIS was within 2 mm of the inked margin’s surface.
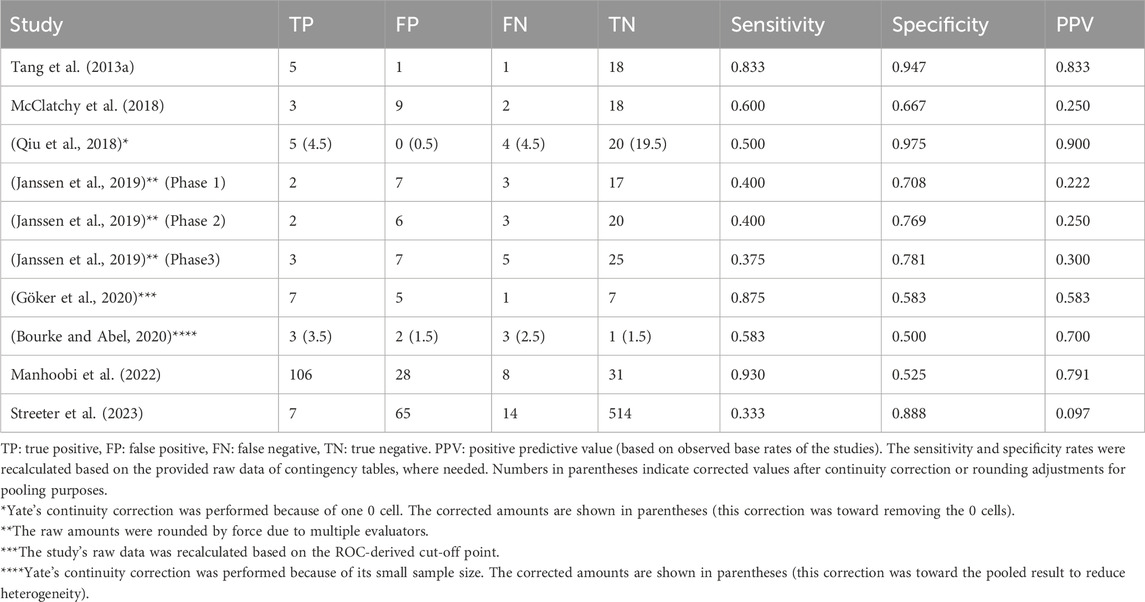
To generate pooled diagnostic indices for the micro-CT-based IMA, the existing raw data of diagnostic contingency tables for studies including TP, FP, FN, and TN were gathered or recalculated (Table 2) so that the pooled diagnostic indices could be accurately calculated (Table 3).
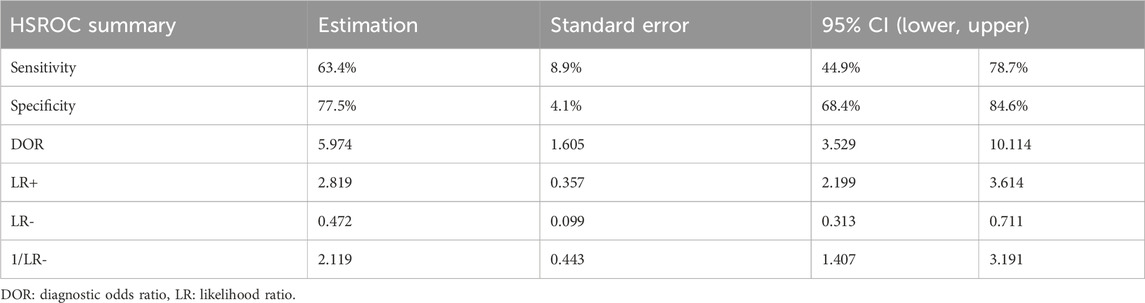
Table 3. Pooled ROC analysis on the diagnostic indices of micro-CT intraoperative margin assessment compared to the final pathology report.
A mixed effects logistic regression model (HSROC analysis) yielded the following pooled diagnostic indices: a sensitivity of 0.63 (95% CI: 0.45–0.79) and a specificity of 0.78 (95% CI: 0.68–0.85) (Table 3). The generated graph (Figure 2) did not have a symmetric distribution, and several studies were outside the predicted region.
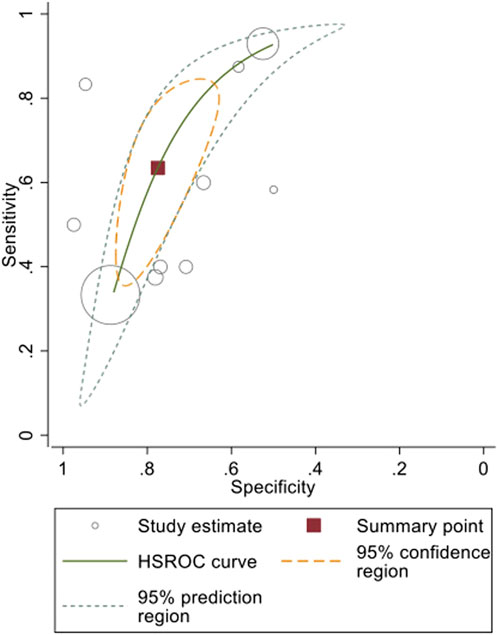
Figure 2. Pooled HSROC curve and diagnostic indices of micro-CT-based intraoperative margin assessment compared to final histopathological assessment.
A pooled diagnostic accuracy of 0.77 (95% CI: 0.71–0.84; I2 = 72.1%) was obtained using the generic method and random effects model (Figure 3). The pooled sensitivity and specificity based on the generic method are also shown (Supplementary Figure S2, S3). Additionally, a diagnostic odds ratio of 5.97 (95% CI: 2.20–10.11) was generated, indicating that if a specimen had a pathologically positive margin, it was 6-fold more likely that intraoperative micro-CT scanning would provide a true positive outcome than a false positive one. These findings have been assessed using the GRADE approach (Supplementary Table S2), which yielded that the evidence derived from our meta-analysis is of low certainty and high importance. The inconsistency of the eligible studies, the potential small-study effect, and the heterogeneity observed contribute to the low certainty outcome.
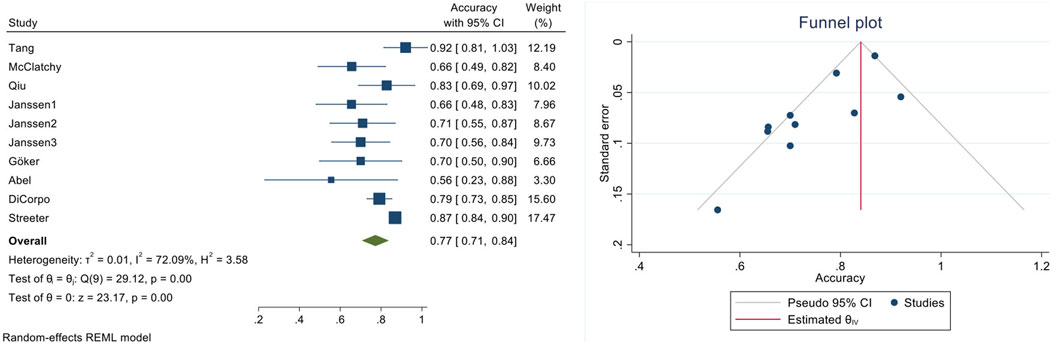
Figure 3. Quantitative diagnostic accuracy meta-analysis of micro-CT-based intra-operative margin assessment compared to final histopathological assessment (left: forest plot, right: funnel plot).
The subgroup and sensitivity analyses conducted for clinically relevant scenarios did not substantially affect the pooled diagnostic indices. More specifically:
1. The subgroup analysis on industrial funding (from micro-CT system manufacturers) led to the exclusion of two studies with declared industrial funding (by Janssen et al. (2019), Cengiz et al. (2018), DiCorpo et al. (2020), Manhoobi et al. (2022). Pooling the remaining data from non-industry sponsored studies yielded a pooled accuracy equal to 0.80 (0.70–0.89), which was not lower than the initial pooled accuracy [0.77 (0.71–0.84)] (Supplementary Figure S4).
2. Since most of the overall sample size was attributable to the study of Streeter et al. (2023), Tang et al. (2016), this study was excluded in the sensitivity analysis addressing the large sample size effect. In addition, the samples of the mentioned study were individual margin-level which was another reason for conducting this sensitivity analysis. Pooling the data from the remaining studies yielded a pooled accuracy equal to 0.75 (0.68–0.82), the 95% CIs of which overlapped with the corresponding ones of the initial pooled accuracy [0.77 (0.71–0.84)] (Supplementary Figure S5).
3. Since Goker et al. (2021), Janssen et al. (2019) used micro-PET CT for IMA, this study was excluded in the sensitivity analysis accounting for potential micro-CT differences within the eligible studies. Pooling the data from the remaining studies yielded a pooled accuracy equal to 0.78 (0.71–0.85), which was not lower than the initial pooled accuracy [0.77 (0.71–0.84)] (Supplementary Figure S6).
4. Since the study of DiCorpo et al. (2020), Manhoobi et al. (2022) assessed margins with the touch cytology method, this study was excluded in the sensitivity analysis accounting for potential IMA differences within the eligible studies. Pooling the data from the remaining studies yielded a pooled accuracy equal to 0.76 (0.69–0.84), the 95% CIs of which overlapped with the corresponding ones of the initial pooled accuracy [0.77 (0.71–0.84)] (Supplementary Figure S7).
5. Since the studies of Streeter et al. (2021), Tang et al. (2016) and Tang et al. (2022), Walsh et al. (2021) used shaved cavity margins, they were excluded in the final sensitivity analysis. Pooling the data from the remaining studies yielded a pooled accuracy equal to 0.74 (0.68–0.79), the 95% CIs of which overlapped with the corresponding ones of the initial pooled accuracy [0.77 (0.71–0.84)] (Supplementary Figure S8).
Discussion
Intraoperative margin assessment (IMA)
Our analysis encompassing eight relevant studies and 988 scanned specimens/margins, revealed a pooled specificity of 78% which is higher than the 69% reported in a previous meta-analysis from 4 studies and 260 samples (Manhoobi et al., 2022). Despite the relatively small number of eligible studies, this meta-analysis revealed that micro-CT-based IMA has reasonable diagnostic indices and may be a useful adjunct to pathologic margin assessment for patients undergoing BCS. The subgroup and sensitivity analyses performed increase the robustness of the observed outcomes since omitting different studies in each case had little or no effect on the pooled indices.
To the best of our knowledge, there is no agreement on the best method of IMA in BCS. Specimen mammography is used in many centers to document marker resection. Based on an intraoperative interpretation of the generated 2D images, surgeons may take an additional margin if the clip, seed, or wire is close to one margin (Butler-Henderson et al., 2014). High-resolution 2D specimen analysis of these lumpectomy walls can also ensure adequate removal of residual microcalcifications, often only detected by 2D imaging (Bathla et al., 2011). A recent meta-analysis reported a sensitivity and specificity of 0.55 (95% CI 0.47–0.63) and 0.85 (95% CI 0.78–0.90) for specimen mammography-based IMA compared to the final histopathologic report (Lin et al., 2022).
Given what we know about the pathologic differences between breast cancer histologies, questions arise about the accuracy of micro-CT-based IMA in different clinical situations. Specifically, the diffuse, less circumscribed growth pattern of ILCs may complicate the identification of a positive margin using radiographic images (Manhoobi et al., 2022). The available information in this study could not evaluate the diagnostic indices of micro-CT IMA in different histologies separately. Only the study by Dicorpo et al. evaluated the accuracy of the micro-CT for different histologies (Göker et al., 2020). In their study, the accuracy of micro-CT-based IMA (78.9%) did not increase significantly when IDCs were solely evaluated (78.9% vs 79.8%, respectively).
The sensitivity and specificity of intraoperative frozen section analysis is reported to be 86% (95% CI: 78–91); and 96% (95% CI: 92–98), respectively (St John et al., 2017). However, intraoperative frozen section analysis has substantial limitations, including time resource allocations, labor intensity, technical challenges, and cost considerations (Butler-Henderson et al., 2014; Rana et al., 2022; Jaafar, 2006). The mean time required for the frozen section is 27.8 min (range: 20–50 min) (St John et al., 2017), while micro-CT-based IMA currently takes only 4–10 min on average. It is also reported that the cost of micro-CT scanning is $130 per specimen scanned, which is cheaper than the cost of a frozen section ($327 per patient in the Netherlands) (Qiu et al., 2018).
Some surgeons perform cavity shave margins (CSM) to decrease the re-excision rate. CSM after a lumpectomy has been associated with a lower rate of positive margin (19%) and reoperation (10%), both lower than when no CSM is taken (34% and 21%, respectively) (Streeter et al., 2021). However, routine CSM may increase the volume of tissue excised, affecting cosmetic outcomes. Therefore, CSM can be avoided if there is a reliable way to assess the margins intra-operatively, allowing for targeted excision of margins where necessary.
This study revealed that micro-CT could accurately detect a positive margin in 77% of the scanned cases and potentially decrease re-operations in 77% of BCS requiring re-excision due to a positive margin on the final pathology report. Recent evidence suggests that rates of re-operation range from 5% to more than 30% in breast cancer patients undergoing BCS (Moran et al., 2014a; Morrow et al., 2017). Reoperations are estimated to increase hospital costs by $11,621 for each BCS and $26,276 for each mastectomy, with an average of $16,072 for each additional surgery (Metcalfe et al., 2017). Another study demonstrated that if the reoperation rate could be reduced to 10% in British Columbia, the average saving would be $1,055 per patient undergoing attempted BCS, translating into annual savings of $1.9 million in British Columbia (Pataky and Baliski, 2016). Of course, micro-CT scanning cannot compensate for suboptimal surgical planning. Still, the preliminary data gathered in our review support that micro-CT could complement the existing framework for BCS outcome assessment.
For successful integration of micro-CT into surgical workflows, real-time coordination between radiology, pathology, and surgical teams is essential. Scanning times reported in current studies range from 4to 10 min, which makes intraoperative use feasible if interpretation can be synchronized with the natural surgical pause after excision and before closure. This compares favorably with current practice, where intraoperative margin assessment typically relies on snap -frozen section analysis. While widely used, frozen sectioning is resource-intensive, requiring skilled personnel, specialized equipment, and up to an hour of surgical downtime, thus significantly impacting operating theatre efficiency and healthcare costs (Omidifar et al., 2022). Moreover, frozen sections provide only 2D slices, sampling a small proportion of the tissue, and are prone to artifacts, especially in fatty breast tissue.
X-ray micro-CT offers an attractive alternative, capable of rapid, non-destructive, 3D imaging of the entire excised specimen and can be incorporated into clinical workflows with minimal disruption. However, conventional X-ray attenuation -based imaging lacks sufficient soft tissue contrast in fresh, hydrated specimens, due to water’s attenuation properties. This challenge may be addressed using advanced techniques such as X-ray phase-contrast μCT (XPCμCT), which has shown promising results in enhancing soft tissue visualization without staining or dehydration (Massimi et al., 2021).
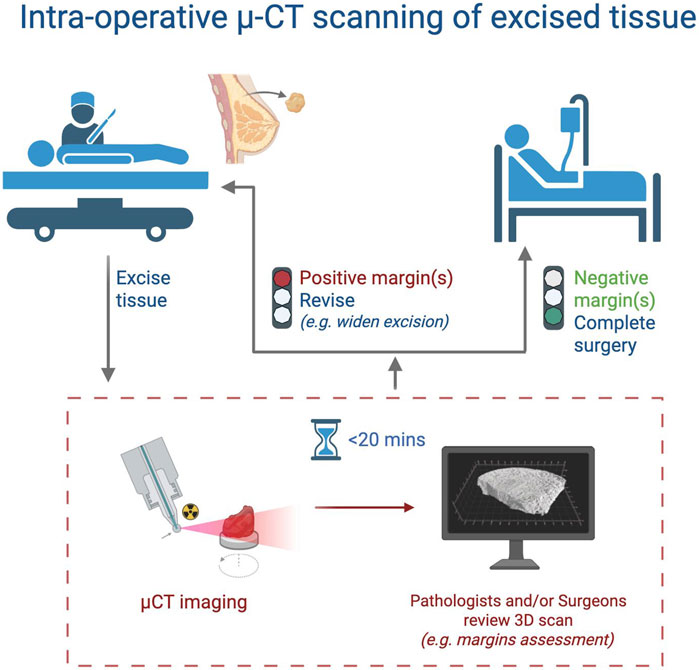
One pragmatic model for intraoperative deployment (Figure 4) involves locating the scanner in an adjacent suite and notifying the imaging team immediately following specimen excision. Using predefined templates and interpretation protocols (e.g., Janssen et al., 2019) radiologists or trained surgeons can rapidly assess margins and alert the surgical team if wider excision is required, thereby avoiding repeat procedures (Janssen et al., 2019). This approach could significantly reduce revision rates, healthcare costs, and patient morbidity but further clinical studies are needed to optimize and validate these workflow models.
Other potential uses for micro-CT in breast cancer management
The number and characteristics of microcalcifications are only sometimes apparent on mammograms (Imamura et al., 2008) and specimen radiographs (Liberman et al., 1994); yet, micro-CT could visualize microcalcification structures in high resolution with a high discriminating power to distinguish benign from malignant appearance (Gufler et al., 2011). The benign microcalcifications are on average bigger and rounder than the malignant ones, which are smaller and more elongated. The surface area to volume ratio of benign microcalcifications also appears to be lower than that of malignant ones in micro-CT-derived images (Willekens et al., 2014).
Another potential micro-CT use might be tumor size measurement. Micro-CT measurements have the highest correlation coefficient, followed by MRI, compared to the largest pathologic tumor dimension (DiCorpo et al., 2020; Sarraj et al., 2015), and there is a statistically significant agreement between micro-CT and standard pathology for T-stage classification, mainly in invasive ductal carcinomas (IDCs) (Göker et al., 2020). Moreover, micro-CT can also identify and discriminate different tumor growth patterns; i) IDCs are viewed as expanding spheres where the width of the gross margin is based on the lengths of speculation extensions, ii) ILCs grow as multiple individual tongues of tumor extending irregularly (Merrill et al., 2017), and iii) DCIS has a radial growth pattern around the branch duct system as visualized in 3D images provided by micro-CT (Merrill et al., 2017).
Finally, micro-CT also creates 3D images of the excised axillary lymph node internal structure and vasculature. Benign lymph nodes have a regular oval contour, and the interior appearance is usually homogenous. In contrast, malignant lymph nodes have irregular shapes and a more heterogeneous internal structure, sometimes having calcifications. Although more studies are required, preliminary data have shown that micro-CT can also be an effective intraoperative method for evaluating sentinel lymph nodes in the upfront and post-neoadjuvant setting to determine whether lymph nodes contain tumor cells (Tang et al., 2013b).
Existing challenges for micro-CT utilization in breast cancer management
Conventional micro-CT imaging involves capturing X-ray attenuation differences between various tissue components by taking radiographs at numerous projection angles (100s–1000s). This is achieved either by rotating the specimen around a fixed axis or by rotating the X-ray source and detector around a stationary specimen, depending on the system configuration. The resulting information is then fed into specialized algorithms to reconstruct the sample’s internal structure. These algorithms use mathematical tools to map microscopic density variations in the sample and reconstruct them into a 3D rectangular grid, expressed in grayscale (with brighter voxels indicating higher attenuation). However, this approach can sometimes misidentify fibrous tissues as tumors and lead to tumor size overestimation (particularly in patients receiving neoadjuvant treatment) or potentially increased risk of missing positive margins (Janssen et al., 2019). This issue stems from the similarity in X-ray attenuation between fibroglandular tissue and tumor, which makes reliable distinction difficult. While micro-CT offers excellent contrast between adipose tissue and denser regions, advanced methods such as phase-contrast microtomography, photon-counting CT, dual-energy imaging, or the use of contrast agents, can help address this limitation. Use of the technology in tandem with more specific imaging modalities such as conventional histology or immunohistochemistry enhances tissue discrimination, although this reduces its utility in intraoperative settings. The over/underestimation of invasive tumor size by micro-CT could also be related to tissue processing and tissue fixation in formalin that may expand or shrink the tissue and affect the pathologic tumor size and stage (DiCorpo et al., 2020; Sarraj et al., 2015). Micro-CT could also overestimate the tumor size due to lumpectomy procedure-related factors such as bleeding, edema, inflammatory reactions, or fibrosis following a recent tissue core biopsy (Sarraj et al., 2015).
Another significant limitation of this technique is the need for more relative training to read specimen micro-CT imaging. A radiological atlas and training dataset, such as the consensus guideline by Janssen et al., could improve reader performance by surgeons, pathologists, and/or radiologists (Janssen et al., 2019; McClatchy et al., 2018). Their three-phase study illustrated increased positive predictive value and sensitivity when introducing relevant implementation guidelines. It indicates that more training leads to better micro-CT reporting (Janssen et al., 2019). The most effective utilization of micro-CT technology would involve collaboration between radiologists and pathologists trained in interpreting micro-CT data, also known as 3D X-ray Histology datasets. This approach leverages the expertise of radiology specialists, who have extensive experience in working with X-ray volumetric data interpretation and analysis, along with pathology experts, who can interpret high-resolution images generated by micro-CT. Currently, most radiology experts need to become more familiar with the histology-level resolution images produced by micro-CT. In contrast, pathologists with experience in interpreting these high-resolution images have limited exposure to volumetric data. Therefore, collaboration between these two specialties is crucial for maximizing the potential benefits of micro-CT in breast cancer management and surgical oncology in general. To date, radiologists and pathologists can both reimburse micro-CT scanning in the United States despite being classified as a radiological procedure. Adding experimental CPT codes could further contribute to the financial sustainability of micro-CT as a routine clinical partner for pathologists and radiologists that can prevent the re-excision of the margin (Papazoglou et al., 2022).
Another limitation of the micro-CT is that imaging artifacts may be generated because of metal markers or wires (both standard localizing techniques for non-palpable tumors). Highly attenuative metallic components can interfere with imaging, resulting in “starvation,” “hardening,” and “scattering” artifacts, which are all well documented in clinical CT imaging literature. Tang et al. mitigated this issue by performing two separate acquisitions (Tang et al., 2016). The first specimen scan was performed with the marker or wire inside the specimen, and the full micro-CT images were obtained when the marker or wire was removed. Other techniques, such as using appropriate beam filtration, imaging with a narrower X-ray spectrum, or deploying dual-energy acquisition protocols, should also be considered in the future. Implementing post-processing algorithms, such as the metal artifact reduction algorithm, could be the most direct solution to this limitation.
Advancements and future directions
Future trials will shed light on the applicability of novel X-ray-based techniques in IMA, such as novel dedicated spiral breast CT equipped with a photon-counting detector, X-ray phase contrast micro-CT imaging, and photoacoustic breast imaging techniques. In the future, micro-PET-CT may emerge as the first-line intraoperative imaging modality, as it has yielded substantially higher sensitivity (90%) compared to the pooled sensitivity of micro-CT-based IMA (63%). Although micro-PET/CT is a promising modality for IMA, the 18F-FDG presence interferes with gamma probe sentinel lymph node detection. To overcome this limitation, the surgeons can use additional patent blue. The patient will be injected with 99mTc on the morning of the surgery to enhance the 99mTc signal and increase the 99mTc signal-to-background ratio (Göker et al., 2020). Further clinical studies, based on micro-PET-CT scanning of larger specimen samples, are warranted to shed additional light on the cost-feasibility balance. Moreover, coupling micro-CT with optical scatter imaging or spatial frequency-domain imaging may be helpful in differentiating highly scattering, collagen-rich fibrous tissues at the margins of BCS specimens (Streeter et al., 2021; Tank et al., 2022). Additionally, future trials investigating the use of contrast agents or the utility of combined radioactive seed localization might help increase the tumor-to-background contrast and localize tumor regions within the resected specimen (Goudreau et al., 2015).
Strengths and limitations of the study
This study provides a comprehensive evaluation of the existing evidence regarding micro-CT applications in breast cancer and can serve as a primer for the execution of further clinically oriented breast cancer trials assessing the utility of micro-CT as a routine clinical partner. However, it has some limitations that should be taken into consideration. The restricted number of included studies, the rather heterogeneous definition of negative margins, differences in imaging protocols and scanner types, and the relatively small number of scanned specimens may limit the generalizability of the findings. Additionally, the high degree of observed heterogeneity and the low certainty of evidence as characterized by the GRADE approach highlight the need for larger, more standardized studies. Nonetheless, the sensitivity analyses performed aim to compensate for the observed degree of heterogeneity. According to them, the pooled diagnostic accuracy decreased only in two scenarios: (a) after the elimination of the study having the largest sample size, and (b) after the elimination of the study examining the shave cavity margin. In both cases, however, the derived 95% CIs of the pooled accuracy overlapped with the initial ones, which makes less possible the overestimation of the micro-CT diagnostic accuracy initially. Future prospective studies with standardized definitions and uniform diagnostic criteria are needed to improve the reliability of pooled meta-analytic estimates. Finally, although we identified no clear asymmetry in the funnel plot, the limited number of included studies and small sample sizes limit the power of formal statistical tests for publication bias. In addition, some studies were sponsored by micro-CT system manufacturers, potentially introducing selective reporting or favorable results. Our sensitivity analysis (excluding industry-sponsored studies) did not significantly alter pooled outcomes, suggesting minimal effect, but the risk cannot be fully excluded. Finally, another limitation could be the possibility of selection bias among the individual studies which could be resolved only through a randomized diagnostic trial setting.
Conclusion
This study demonstrates that micro-CT imaging is a promising complementary IMA technique for BCS by providing high-resolution 3-D images. These images can be acquired within a few minutes, allowing surgeons to assess margin status intra-operatively, and identify more than 70% of positive margins where reoperation rates are likely to decrease. Although these findings seem to hold promise for IMA improvement in BCS, their clinical translation and applicability remains to be evaluated in clinical trials adequately empowered to investigate the re-excision and local recurrence rates after micro-CT IMA assessment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
SM: Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review and editing. MV: Investigation, Supervision, Writing – original draft, Writing – review and editing. AP: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review and editing. AL: Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review and editing. KR: Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review and editing. AW: Data curation, Investigation, Writing – original draft, Writing – review and editing. RB: Investigation, Writing – original draft, Writing – review and editing. OK: Investigation, Writing – original draft, Writing – review and editing. MP: Writing – original draft, Writing – review and editing. CC: Investigation, Writing – original draft, Writing – review and editing. ST: Supervision, Writing – original draft, Writing – review and editing. FT: Writing – original draft, Writing – review and editing, Investigation. DL: Project administration, Supervision, Writing – original draft, Writing – review and editing. JM: Investigation, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmede.2025.1571528/full#supplementary-material
References
Bathla, L., Harris, A., Davey, M., Sharma, P., and Silva, E. (2011). High resolution intra-operative two-dimensional specimen mammography and its impact on second operation for re-excision of positive margins at final pathology after breast conservation surgery. Am. J. Surg. 202, 387–394. doi:10.1016/j.amjsurg.2010.09.031
Bourke, A., and Abel, T. (2020). Can micro-computed tomography imaging improve interpretation of macroscopic margin assessment of specimen radiography in excised breast specimens? J. Cancer Res. Ther. 16, 1366. doi:10.4103/jcrt.jcrt_949_19
Brahimetaj, R., Willekens, I., Massart, A., Forsyth, R., Cornelis, J., Mey, J. D., et al. (2022). Improved automated early detection of breast cancer based on high resolution 3D micro-CT microcalcification images. BMC cancer 22, 162–13. doi:10.1186/s12885-021-09133-4
Butler-Henderson, K., Lee, A. H., Price, R. I., and Waring, K. (2014). Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast 23, 112–119. doi:10.1016/j.breast.2014.01.002
Cengiz, I. F., Oliveira, J. M., and Reis, R. L. (2018). Micro-CT–a digital 3D microstructural voyage into scaffolds: a systematic review of the reported methods and results. Biomaterials Res. 22, 26–11. doi:10.1186/s40824-018-0136-8
Chen, C. H., Cho, S. H., Tsai, F., Erten, A., and Lo, Y. H. (2009). Microfluidic cell sorter with integrated piezoelectric actuator. Biomed. microdevices 11, 1223–1231. doi:10.1007/s10544-009-9341-5
DiCorpo, D., Tiwari, A., Tang, R., Griffin, M., Aftreth, O., Bautista, P., et al. (2020). The role of Micro-CT in imaging breast cancer specimens. Breast cancer Res. Treat. 180, 343–357. doi:10.1007/s10549-020-05547-z
Dowling, G. P., Hehir, C. M., Daly, G. R., Hembrecht, S., Keelan, S., Giblin, K., et al. (2024). Diagnostic accuracy of intraoperative methods for margin assessment in breast cancer surgery: a systematic review and meta-analysis. Breastedinbg. Scotl. 76, 103749. doi:10.1016/j.breast.2024.103749
Esbona, K., Li, Z., and Wilke, L. G. (2012). Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann. Surg. Oncol. 19, 3236–3245. doi:10.1245/s10434-012-2492-2
Garcia, M. T., Mota, B. S., Cardoso, N., Martimbianco, A. L. C., Ricci, M. D., Carvalho, F. M., et al. (2021). Accuracy of frozen section in intraoperative margin assessment for breast-conserving surgery: a systematic review and meta-analysis. PloS one 16, e0248768. doi:10.1371/journal.pone.0248768
Göker, M., Marcinkowski, R., Van Bockstal, M., Keereman, V., Van Holen, R., Van Dorpe, J., et al. (2020). 18F-FDG micro-PET/CT for intra-operative margin assessment during breast-conserving surgery. Acta Chir. Belg. 120, 366–374. doi:10.1080/00015458.2020.1774163
Goudreau, S. H., Joseph, J. P., and Seiler, S. J. (2015). Preoperative radioactive seed localization for nonpalpable breast lesions: technique, pitfalls, and solutions. Radiographics 35, 1319–1334. doi:10.1148/rg.2015140293
Gufler, H., Wagner, S., and Franke, F. E. (2011). The interior structure of breast microcalcifications assessed with micro computed tomography. Acta Radiol. 52, 592–596. doi:10.1258/ar.2011.100489
Hutchinson, J. C., Shelmerdine, S. C., Simcock, I. C., Sebire, N. J., and Arthurs, O. J. (2017). Early clinical applications for imaging at microscopic detail: Microfocus computed tomography (micro-CT). Br. J. radiology 90, 20170113. doi:10.1259/bjr.20170113
Imamura, K., Ehara, N., Inada, Y., Kanemaki, Y., Okamoto, J., Maeda, I., et al. (2008). Microcalcifications of breast tissue: appearance on synchrotron radiation imaging with 6-μm resolution. Am. J. Roentgenol. 190, W234–W236. doi:10.2214/ajr.07.2610
Jaafar, H. (2006). Intra-operative frozen section consultation: concepts, applications and limitations. Malays. J. Med. Sci. MJMS 13, 4–12. Available online at: https://pubmed.ncbi.nlm.nih.gov/22589584/.
Janssen, N. N., van Seijen, M., Loo, C. E., Vrancken Peeters, M. J. T., Hankel, T., Sonke, J. J., et al. (2019). Feasibility of micro–computed tomography imaging for direct assessment of surgical resection margins during breast-conserving surgery. J. Surg. Res. 241, 160–169. doi:10.1016/j.jss.2019.03.029
Katsamenis, O. L., Olding, M., Warner, J. A., Chatelet, D. S., Jones, M. G., Sgalla, G., et al. (2019). X-ray micro-computed tomography for nondestructive three-dimensional (3D) X-ray histology. Am. J. pathology 189, 1608–1620. doi:10.1016/j.ajpath.2019.05.004
Katsamenis, O. L., Basford, P. J., Robinson, S. K., Boardman, R. P., Konstantinopoulou, E., Lackie, P. M., et al. (2023). A high-throughput 3D X-ray histology facility for biomedical research and preclinical applications. Wellcome Open Res. 8, 366. doi:10.12688/wellcomeopenres.19666.1
Kaufman, C. S., Jacobson, L., Bachman, B. A., Kaufman, L. B., Mahon, C., Gambrell, L. J., et al. (2007). Intraoperative digital specimen mammography: rapid, accurate results expedite surgery. Ann. Surg. Oncol. 14, 1478–1485. doi:10.1245/s10434-006-9126-5
Kenkel, D., Varga, Z., Heuer, H., Dedes, K. J., Berger, N., Filli, L., et al. (2017). A micro ct study in patients with breast microcalcifications using a mathematical algorithm to assess 3d structure. Plos one 12, e0169349. doi:10.1371/journal.pone.0169349
Liberman, L., Evans, W., Dershaw, D. D., Hann, L. E., Deutch, B. M., Abramson, A. F., et al. (1994). Radiography of microcalcifications in stereotaxic mammary core biopsy specimens. Radiology 190, 223–225. doi:10.1148/radiology.190.1.8259409
Lin, C., Wang, K., Chen, H., Xu, Y., Pan, T., and Chen, Y. (2022). Specimen mammography for intraoperative margin assessment in breast conserving surgery: a meta-analysis. Sci. Rep. 12, 18440. doi:10.1038/s41598-022-23234-5
Maloney, B. W., McClatchy, D. M., Pogue, B. W., Paulsen, K. D., Wells, W. A., and Barth, R. J. (2018). Review of methods for intraoperative margin detection for breast conserving surgery. J. Biomed. Opt. 23, 100901. doi:10.1117/1.jbo.23.10.100901
Manhoobi, I. P., Bodilsen, A., Nijkamp, J., Pareek, A., Tramm, T., Redsted, S., et al. (2022). Diagnostic accuracy of radiography, digital breast tomosynthesis, micro-CT and ultrasound for margin assessment during breast surgery: a systematic review and meta-analysis. Acad. Radiol. 29, 1560–1572. doi:10.1016/j.acra.2021.12.006
Massimi, L., Suaris, T., Hagen, C. K., Endrizzi, M., Munro, P. R. T., Havariyoun, G., et al. (2021). Detection of involved margins in breast specimens with X-ray phase-contrast computed tomography. Sci. Rep. 11, 3663. doi:10.1038/s41598-021-83330-w
McClatchy, D. M., Zuurbier, R. A., Wells, W. A., Paulsen, K. D., and Pogue, B. W. (2018). Micro-computed tomography enables rapid surgical margin assessment during breast conserving surgery (BCS): correlation of whole BCS micro-CT readings to final histopathology. Breast Cancer Res. Treat. 172, 587–595. doi:10.1007/s10549-018-4951-3
McCormick, J. T., Keleher, A. J., Tikhomirov, V. B., Budway, R. J., and Caushaj, P. F. (2004). Analysis of the use of specimen mammography in breast conservation therapy. Am. J. Surg. 188, 433–436. doi:10.1016/j.amjsurg.2004.06.030
Merrill, A. L., Buckley, J., Tang, R., Brachtel, E., Rai, U., Michaelson, J., et al. (2017). A study of the growth patterns of breast carcinoma using 3D reconstruction: a pilot study. breast J. 23, 83–89. doi:10.1111/tbj.12688
Metcalfe, L. N., Zysk, A. M., Yemul, K. S., Jacobs, L. K., Oker, E. E., Underwood, H. R., et al. (2017). Beyond the Margins—economic costs and complications associated with repeated breast-conserving surgeries. JAMA Surg. 152, 1084–1086. doi:10.1001/jamasurg.2017.2661
Milano, D. F., Ngai, N. A., Muthuswamy, S. K., and Asthagiri, A. R. (2016). Regulators of metastasis modulate the migratory response to cell contact under spatial confinement. Biophysical J. 110, 1886–1895. doi:10.1016/j.bpj.2016.02.040
Moran, M. S., Schnitt, S. J., Giuliano, A. E., Harris, J. R., Khan, S. A., Horton, J., et al. (2014a). Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncology* Biology* Phys. 88, 553–564. doi:10.1016/j.ijrobp.2013.11.012
Moran, M. S., Schnitt, S. J., Giuliano, A. E., Harris, J. R., Khan, S. A., Horton, J., et al. (2014b). Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J. Clin. Oncol. 32, 1507–1515. doi:10.1200/jco.2013.53.3935
Morrow, M., Abrahamse, P., Hofer, T. P., Ward, K. C., Hamilton, A. S., Kurian, A. W., et al. (2017). Trends in reoperation after initial lumpectomy for breast cancer: addressing overtreatment in surgical management. JAMA Oncol. 3, 1352–1357. doi:10.1001/jamaoncol.2017.0774
Muttalib, M., Tisdall, M., Scawn, R., Shousha, S., Cummins, R., and Sinnett, H. (2004). Intra-operative specimen analysis using faxitron microradiography for excision of mammographically suspicious, non-palpable breast lesions. Breast 13, 307–315. doi:10.1016/j.breast.2004.02.005
Omidifar, N., Chogani, E., Zangouri, V., Keshavarz, K., and Talei, A. (2022). Cost-effectiveness analysis of intraoperative frozen section in women with breast cancer: evidence from south of Iran. Iran. J. Med. Sci. 47, 143–151. doi:10.30476/IJMS.2021.88887.1960
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin. Res. ed. 372, n71–11. doi:10.1136/bmj.n71
Papa, M., Allweis, T., Karni, T., Sandbank, J., Konichezky, M., Diment, J., et al. (2016). An intraoperative MRI system for margin assessment in breast conserving surgery: initial results from a novel technique. J. Surg. Oncol. 114, 22–26. doi:10.1002/jso.24246
Papazoglou, A. S., Karagiannidis, E., Liatsos, A., Bompoti, A., Moysidis, D. V., Arvanitidis, C., et al. (2022). Volumetric tissue imaging of surgical tissue specimens using micro–computed tomography: an emerging digital pathology modality for nondestructive, slide-free Microscopy—Clinical applications of digital pathology in 3 dimensions. Am. J. Clin. Pathology 159, 242–254. doi:10.1093/ajcp/aqac143
Partain, N., Calvo, C., Mokdad, A., Colton, A., Pouns, K., Clifford, E., et al. (2020). Differences in re-excision rates for breast-conserving surgery using intraoperative 2D versus 3D tomosynthesis specimen radiograph. Ann. Surg. Oncol. 27, 4767–4776. doi:10.1245/s10434-020-08877-w
Pataky, R., and Baliski, C. (2016). Reoperation costs in attempted breast-conserving surgery: a decision analysis. Curr. Oncol. 23, 314–321. doi:10.3747/co.23.2989
Pradipta, A. R., Tanei, T., Morimoto, K., Shimazu, K., Noguchi, S., and Tanaka, K. (2020). Emerging technologies for real-time intraoperative margin assessment in future breast-conserving surgery. Adv. Sci. 7, 1901519. doi:10.1002/advs.201901519
Qiu, S.-Q., Dorrius, M. D., de Jongh, S. J., Jansen, L., de Vries, J., Schröder, C. P., et al. (2018). Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: a feasibility study in breast conserving surgery. Eur. J. Surg. Oncol. 44, 1708–1713. doi:10.1016/j.ejso.2018.06.022
Rana, M. K., Rana, A. P. S., Sharma, U., Barwal, T. S., and Jain, A. (2022). Evolution of frozen section in carcinoma breast: systematic review. Int. J. Breast Cancer 2022, 1–7. doi:10.1155/2022/4958580
Rosenberg, P. S., Barker, K. A., and Anderson, W. F. (2015). Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl. Cancer Inst. 107, djv159. doi:10.1093/jnci/djv159
Sarraj, W. M., Tang, R., Najjar, A. L., Griffin, M., Bui, A. H., Zambeli-Ljepovic, A., et al. (2015). Prediction of primary breast cancer size and T-stage using micro-computed tomography in lumpectomy specimens. J. pathology Inf. 6, 60. doi:10.4103/2153-3539.170647
Schnabel, F., Boolbol, S. K., Gittleman, M., Karni, T., Tafra, L., Feldman, S., et al. (2014). A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann. Surg. Oncol. 21, 1589–1595. doi:10.1245/s10434-014-3602-0
Smitt, M. C., Nowels, K. W., Zdeblick, M. J., Jeffrey, S., Carlson, R. W., Stockdale, F. E., et al. (1995). The importance of the lumpectomy surgical margin status in long term results of breast conservation. Cancer 76, 259–267. doi:10.1002/1097-0142(19950715)76:2<259::aid-cncr2820760216>3.0.co;2-2
St John, E. R., Al-Khudairi, R., Ashrafian, H., Athanasiou, T., Takats, Z., Hadjiminas, D. J., et al. (2017). Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery. Ann. Surg. 265, 300–310. doi:10.1097/sla.0000000000001897
Streeter, S. S., Maloney, B. W., Zuurbier, R. A., Wells, W. A., Barth, R. J., Paulsen, K. D., et al. (2021). Optical scatter imaging of resected breast tumor structures matches the patterns of micro-computed tomography. Phys. Med. and Biol. 66, 115021. doi:10.1088/1361-6560/ac01f1
Streeter, S. S., Zuurbier, R. A., diFlorio-Alexander, R. M., Hansberry, M. T., Maloney, B. W., Pogue, B. W., et al. (2023). Breast-Conserving surgery Margin guidance using micro-computed tomography: challenges when imaging radiodense resection specimens. Ann. Surg. Oncol. 30, 4097–4108. doi:10.1245/s10434-023-13364-z
Tang, R., Coopey, S. B., Buckley, J. M., Aftreth, O. P., Fernandez, L. J., Brachtel, E. F., et al. (2013a). A pilot Study evaluating shaved cavity margins with micro-computed tomography: a novel method for predicting lumpectomy Margin status intraoperatively. breast J. 19, 485–489. doi:10.1111/tbj.12146
Tang, R., Buckley, J. M., Fernandez, L., Coopey, S., Aftreth, O., Michaelson, J., et al. (2013b). Micro-computed tomography (Micro-CT): a novel approach for intraoperative breast cancer specimen imaging. Breast cancer Res. Treat. 139, 311–316. doi:10.1007/s10549-013-2554-6
Tang, R., Saksena, M., Coopey, S. B., Fernandez, L., Buckley, J. M., Lei, L., et al. (2016). Intraoperative micro-computed tomography (micro-CT): a novel method for determination of primary tumour dimensions in breast cancer specimens. Br. J. radiology 89, 20150581. doi:10.1259/bjr.20150581
Tank, A., Vergato, C., Waxman, D. J., and Roblyer, D. (2022). Spatial frequency domain imaging for monitoring immune-mediated chemotherapy treatment response and resistance in a murine breast cancer model. Sci. Rep. 12, 5864–5869. doi:10.1038/s41598-022-09671-2
Thill, M., Röder, K., Diedrich, K., and Dittmer, C. (2011). Intraoperative assessment of surgical margins during breast conserving surgery of ductal carcinoma in situ by use of radiofrequency spectroscopy. Breast 20, 579–580. doi:10.1016/j.breast.2011.08.134
Veronesi, U., Cascinelli, N., Mariani, L., Greco, M., Saccozzi, R., Luini, A., et al. (2002). Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 347, 1227–1232. doi:10.1056/nejmoa020989
Walsh, C. L., Tafforeau, P., Wagner, W., Jafree, D. J., Bellier, A., Werlein, C., et al. (2021). Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat. methods 18, 1532–1541. doi:10.1038/s41592-021-01317-x
Walton, L. A., Bradley, R. S., Withers, P. J., Newton, V. L., Watson, R. E. B., Austin, C., et al. (2015). Morphological characterisation of unstained and intact tissue micro-architecture by X-ray computed micro-and nano-tomography. Sci. Rep. 5, 10074. doi:10.1038/srep10074
Watanabe, G., Itoh, M., Duan, X., Watabe, H., Mori, N., Tada, H., et al. (2018). 18 F-fluorodeoxyglucose specimen-positron emission mammography delineates tumour extension in breast-conserving surgery: preliminary results. Eur. Radiol. 28, 1929–1937. doi:10.1007/s00330-017-5170-8
Whiting, P. F., Rutjes, A. W. S., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., et al. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536. doi:10.7326/0003-4819-155-8-201110180-00009
Willekens, I., Van de Casteele, E., Buls, N., Temmermans, F., Jansen, B., Deklerck, R., et al. (2014). High-resolution 3D micro-CT imaging of breast microcalcifications: a preliminary analysis. BMC cancer 14, 9–10. doi:10.1186/1471-2407-14-9
Zysk, A. M., Chen, K., Gabrielson, E., Tafra, L., May Gonzalez, E. A., Canner, J. K., et al. (2015). Intraoperative assessment of final margins with a handheld optical imaging probe during breast-conserving surgery may reduce the reoperation rate: results of a multicenter study. Ann. Surg. Oncol. 22, 3356–3362. doi:10.1245/s10434-015-4665-2
Keywords: micro-computed tomography, intraoperative margin assessment, breast-conserving surgery, breast cancer, 3D imaging
Citation: Meshkati Yazd SM, Vasigh M, Papazoglou AS, Liatsos AC, Ranjbar K, Williams AD, Bleicher RJ, Katsamenis OL, Pierotti ML, Cruz Pico CX, Theocharis S, Tsolaki F, Leff DR and Michaelson JS (2025) The role of micro-CT in breast cancer management: a systematic review on the clinical applications of micro-CT in breast cancer and a diagnostic accuracy meta-analysis on intraoperative margin assessment. Front. Med. Eng. 3:1571528. doi: 10.3389/fmede.2025.1571528
Received: 07 February 2025; Accepted: 02 September 2025;
Published: 25 September 2025.
Edited by:
Haiyan Li, The Sixth Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Shubo Wang, University of Delaware, United StatesSamuel Streeter, Dartmouth University, United States
Copyright © 2025 Meshkati Yazd, Vasigh, Papazoglou, Liatsos, Ranjbar, Williams, Bleicher, Katsamenis, Pierotti, Cruz Pico, Theocharis, Tsolaki, Leff and Michaelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas S. Papazoglou, YW5wYXBhem9nbG91QHlhaG9vLmNvbQ==; Mahtab Vasigh, bWFodGFidmFzaWdoQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Seyed Mostafa Meshkati Yazd1†
Seyed Mostafa Meshkati Yazd1† Mahtab Vasigh
Mahtab Vasigh Andreas S. Papazoglou
Andreas S. Papazoglou Alexandros C. Liatsos
Alexandros C. Liatsos Keivan Ranjbar
Keivan Ranjbar Stamatios Theocharis
Stamatios Theocharis Fani Tsolaki
Fani Tsolaki Daniel R. Leff
Daniel R. Leff James S. Michaelson
James S. Michaelson