- 1Department of Microbiology, Mohanlal Sukhadia University, Udaipur, Rajasthan, India
- 2Department of Botany, University of Lucknow, Lucknow, Uttar Pradesh, India
- 3Department of Biochemistry, University of Lucknow, Lucknow, Uttar Pradesh, India
- 4Department of Biotechnology, Graphic Era (Deemed to be University), Dehradun, Uttarakhand, India
- 5Amity Institute of Environmental Toxicology Safety and Management, Amity University, Noida, India
- 6College of Life Science & Biotechnology, Korea University, Seoul, Republic of Korea
The rising global rates of metabolic disorders, such as obesity, type 2 diabetes, non-alcoholic fatty liver disease, and metabolic syndrome, call for new treatment methods beyond traditional drugs. The human gut microbiota, made up of trillions of microorganisms that plays a crucial role in maintaining metabolic balance through complex biochemical processes and interactions between hosts and microbes. Dysbiosis, which involves changes in microbial composition and a decrease in diversity, has become a major factor in metabolic problems. This disruption impacts the production of short-chain fatty acid, increase in permeability of intestine, and causes enduring low-grade inflammation. This review features into the potential of treatments based on microbiome for metabolic syndromes, focusing on probiotics, prebiotics, synbiotics, and postbiotics. It also encompasses innovative methods such as engineered microbial consortium, fecal microbiota transplantation (FMT), and vaginal microbiota transplantation (VMT). Probiotics show significant promise in improving blood sugar control and enhancing lipid levels. Prebiotics help bring about positive changes in microbial composition and the production of beneficial metabolites. Synbiotic combinations provide added benefits by helping good microbes thrive while supplying nutrients they can ferment. Postbiotics have recent research focus because they are safer, more stable, easier to store, and less likely to contribute to antibiotic resistance comparative to live probiotics. Even now there are substantial complications in translating microbiome research into standardized therapeutics despite of promising pre-clinical outcomes and some initial clinical data. These comprises individual variances, strain-specificity, dosage problems, regulation issues, and the necessity for personalised treatment strategies. Future success will depend upon personalized medicine, technological developments, and the incorporation of multi-omics strategy to generate metabolic health therapeutics depending on targeted microbiomes.
1 Introduction
The universal upsurge in the metabolic disorders which encompasses obesity, type 2 diabetes mellitus, non-alcoholic fatty liver disease, metabolic syndrome, and cardiovascular diseases, presents one of the major challenges in health care in the 21st century which substantially impacts the modern medication and public health policy. The number of adult persons suffering from diabetes globally has surpassed 800 million which is more than quadruple upsurge since 1990 (1). Furthermore, the prevalence of obesity among adults in the United States from August 2021 to August 2023 was 40.3% (2). This sharp intensification of the metabolic dysfunctions has sparked an urgent exploration for novel strategies of treatment beyond conventional drug therapies.
One of the greatest promising areas in this pursuit is the novel arena of treatments based on microbiome. This approach leverages the complex relationship between the gut microbiota and metabolic balance to create innovative treatment options. The human gut microbiota, made up of trillions of microorganisms in the gastrointestinal tract, is often referred to as a “forgotten organ.” When the microbiome is disturbed, it can greatly affect metabolism through various biochemical pathways and signalling networks (3). This microbial community contains about 100 times more genes than the human genome. It serves as a dynamic link between environmental factors, dietary components, and host physiology, managing vital metabolic processes. These include energy extraction, nutrient absorption, immune response, and hormone regulation.
The changes in gut microbiota composition and function, known as dysbiosis, are closely related to the development of metabolic disorders. This shift has changed our perspective and they are no longer seen only as issues within the host but as complex ecosystem imbalances involving interactions between hosts and microbes. The mechanisms behind these associations involve multiple interconnected pathways, such as altered short-chain fatty acid production, increased intestinal permeability causing metabolic endotoxemia, disrupted bile acid processing, changed incretin hormone release, and persistent low-grade inflammation (4).
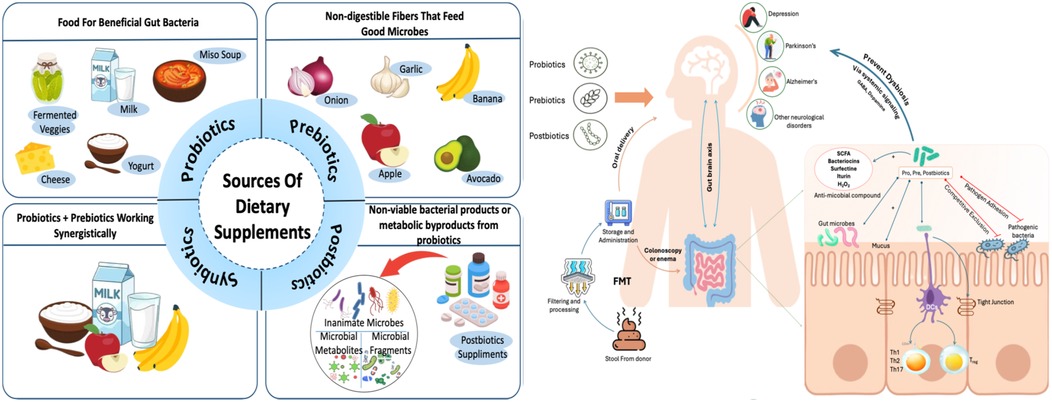
The range of microbiome-based treatments includes probiotics (live helpful microorganisms), prebiotics (substances that support beneficial bacteria growth), synbiotics (mixtures of probiotics and prebiotics), and postbiotics (active compounds produced by probiotics). Advanced microbiome therapies that use engineered microbes have come forth as innovative solutions that can be programmed to generate specific therapeutic molecules on-site while responding to conditions within the gut environment (5).
Microbiome-based treatments have the potential to make a substantial economic difference by preventing disease progression, lowering reliance on medications, and promoting long-term metabolic health. This review summarizes the current understanding of microbiome-based therapies for metabolic disorders. It explores the vital connections among the metabolic health and gut microbiome which evaluates the therapeutic potential of several microbial intrusions, scrutinizes clinical trial outcomes, and looks forward to future advancements in this speedily changing arena. By evaluating both successes and challenges, this review enlighten researchers, healthcare providers, and policymakers about the immense potential of microbiome-based strategies while recognizing the considerable work needed to achieve their full therapeutic benefits in addressing the global rise in metabolic disorders.
2 Gut microbiota and its relation with metabolic disorders
The gastrointestinal tract of a human accommodates an intricate and dynamic ecosystem of microbes that are collectively labelled as the gut microbiota subsequently work as a crucial regulator of metabolism and health of the host. This complicated microbial community, encompassing more than 100 trillion microbial cells and rendering more than 1,000 distinct microbial species, sustains a sophisticated interdependent association with the human host (6). The gut microbiota has progressed to become a vital constituent of human physiology, prompting several metabolic processes involving immune function, metabolism of lipid, regulation of glucose, and energy homeostasis (7). Recent developments in microbiota study have discovered that modifications in composition and function of gut microbiota which is termed as dysbiosis, is intimately connected to various metabolic disorders pathogenesis, including type 2 diabetes, metabolic syndrome, obesity, and cardiovascular disease (7, 8).
Gut microbiota of the humans primarily comprises bacteria from four major phylum: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. Firmicutes and Bacteroidetes constitutes 70%–90% of the overall microbial population (9). The phylum Firmicutes comprises microbial genera for instance Lactobacillus, Clostridium, Ruminococcus, and Enterococcus which generates short-chain fatty acids (SCFAs) (10). Bacteroidetes, primarily characterized by Bacteroides species, are specialized in breaking down complex carbohydrates and plant fibers (11).
The Firmicutes to Bacteroidetes (F/B) ratio has acquired recognition as a probable marker for metabolic health. Greater F/B ratios are usually found in individuals with obesity and metabolic problems (12). Dysbiosis associated with obesity is characterized by diminished microbial diversity, reduced abundance of beneficial taxa for instance Bifidobacterium species and Akkermansia muciniphila, and development of potentially harmful microbes (13). Type 2 diabetes is linked with decreased diversity of microbes and reduced abundance of butyrate producing microbes such as Faecalibacterium prausnitzii, Eubacterium rectale, and Roseburia species (7).
2.2 Factors influencing gut microbiota
The gut microbiome's function and composition are affected by an intricate mix of interior and exterior aspects which act over dissimilar timeframes. Comprehending these features is significant for generating targeted treatments for metabolic syndromes and enhancing therapeutic methods (14).
The most substantial changeable factor impacting the composition and metabolic results of the gut microbiota is diet. Fluctuations in diet can rapidly alter microbial communities within few hours to days (15). Western foods that are high in refined sugars, processed foods, and saturated fats, promotes the progression of potentially harmful microorganisms while restraining beneficial forms related to metabolic strength (16). Whereas, diets which consist of an assortment of dietary fiber, plant-based foods, and fermented products encourage microbial diversity and beneficial metabolites production (9). Dietary fiber serves as a chief food source for microbial fermentation, leads to synthesis of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate (17). The mediterranean diet has unswervingly shown an association to superior microbial diversity and healthier metabolic consequences (7, 18).
Genetic factors of the host significantly impact the gut microbiota composition. Twin researches displays that around 10%–20% of microbial disparity comes from genetic factors (19, 20). The gut microbiome experiences momentous variations throughout an individual's life, with initial establishment at birth and variations through childhood, adulthood, and elderly phase (21). Environmental aspects such as geographical position, sanitation, climate, and exposure to contaminants impacts composition of gut microbiota (22). Choices of lifestyle, for instance stress levels, physical activity, sleep habits, and social networks also form microbial communities (23). Medicines particularly antibiotics have a robust influence on the gut microbiota 's composition and function, with effects that can sustain for months or even years (24).
2.3 Dysbiosis & metabolic disorders
Dysbiosis is an imbalance in the gut microbiome's diversity, composition, or metabolic action (25). This imbalance in microbiota is manifested by declined diversity, altered bacterial ratios, loss of advantageous bacteria, overgrowth of potentially damaging species, and reduced synthesis of helpful metabolites (26). The relationship among dysbiosis and metabolic dysfunction is a two-way connection; metabolic disorders might cause dysbiosis and on the other hand dysbiosis might worsen metabolic disorders (27).
Dysbiosis associated with obesity is characterized by diminished microbial diversity, augmented F/B ratio, reduced abundance of beneficial taxa for instance Bifidobacterium species and Akkermansia muciniphila, and development of potentially harmful microbes (13). A. muciniphila is a mucin degrading bacterium which constitutes 1%–5% of the gut microbiota in healthy persons, is consistently abridged in obesity and metabolic syndrome. Clinical researches have exhibited that abundance of A. muciniphila inversely associates with body mass index, inflammatory markers and resistance to insulin (28).
Type 2 diabetes is linked with distinctive variations in the composition and function of gut microbiome (29). Diabetic individuals show decreased diversity of microbes, changed representation of main bacterial taxa, and functional variations in metabolism of microbes (30). Specific changes encompass reduced abundance of butyrate producing microbes such as Faecalibacterium prausnitzii, Eubacterium rectale, and Roseburia species accompanied by augmented depiction of opportunistic pathogens and pro-inflammatory microbes (7).
Non-alcoholic fatty liver disease (NAFLD) is connected to dysbiosis of gut microbiota via the gut-liver axis (31). Alterations by dysbiosis accord to pathogenesis of NAFLD through several mechanisms involving upsurged permeability of intestines causing the portal circulation of microbial LPS and other inflammatory intermediaries, changed metabolism of bile acid, and dysregulated metabolite production (7). Novel pathways for therapeutic intercessions have been unlocked through understanding of the mechanisms of metabolic dysfunction allied with dysbiosis (27).
3 Role of probiotics, prebiotics, synbiotics and postbiotics in regulating gut microbiota and prevention of metabolic disorders
3.1 Probiotics, prebiotics, synbiotics and postbiotics
Kollath defined probiotics as active substances that perform critical functions for health (32). In 2001, the Food and Agriculture Organisation (FAO) and the World Health Organisation (WHO) given a definition, characterising probiotics as “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” (33, 34). Probiotic bacteria are often classified into two types: conventional and non-conventional. Lactobacillus, Streptococcus, Escherichia, and Bifidobacterium are examples of conventional strains, whereas non-conventional strains like Akkermansia, Faecalibacterium, Eubacterium, Roseburia, Christensenella, and Clostridium have recently received attention for their health-promoting potential (35).
Probiotics have a variety of beneficial effects, including competitive exclusion of pathogens, production of antimicrobial compounds, improvement in intestinal barrier functions, immunomodulation, and modulation of the gut-brain axis (36, 37, 38). They compete for nutrients and adhesion sites, secrete antimicrobial compounds such as SCFAs, organic acids, hydrogen peroxide (39), and bacteriocins (40), and strengthen gut integrity by upregulating mucin and tight junction proteins such as occludin and claudin-1 (41, 42). Probiotics modulate innate and adaptive immunity by modifying dendritic cells, macrophages, B and T lymphocytes, and boosting anti-inflammatory cytokines. While probiotics have long been acknowledged for their role in gut health, more emphasis is now being paid to prebiotics, which are dietary components that nourish and promote the activity of these beneficial microbes (43).
Gibson and Roberfroid created the notion of prebiotics in 1995 (44). Prebiotics are defined as “substrates which are selectively utilised by host microbes conferring a health benefit” (45). Prebiotics are non-digestible dietary components which helps the host by selectively augmenting the growth and/or activity of certain intestinal flora. Inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and lactulose are examples of common prebiotics (46, 47). Prebiotic fermentation synthesizes short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate. These SCFAs lower gut pH from ∼6.5 to ∼5.5, inhibiting pathogenic bacteria and promoting beneficial microbes (48, 49).
Individual gut microbiota differences, dosing challenges, gastrointestinal side effects, short shelf life, and safety concerns for immunocompromised individuals, particularly under processing conditions such as pasteurisation or baking, limit probiotic effectiveness (50, 51).
To address the limits of standalone probiotics and prebiotics, synbiotics have emerged as a viable technique that mixes the two to provide greater health advantages. In 1955, Gibson and Roberfroid (44) proposed synbiotics, which are cooperative concoctions of probiotics and prebiotics envisioned to enhance survival, colonisation, and activity of the probiotic in the gut (52, 53). Synbiotics enhance implantation and function by specifically stimulating helpful bacteria, overcoming difficulties like as pH and oxidative stress, both of which restrict probiotic viability (54).
Postbiotics have arisen as a current research focus because they are safer, more stable, easier to store, and less likely to contribute to antibiotic resistance than live probiotics. As stated by the International Scientific Association for Probiotics and Prebiotics (ISAPP), postbiotics are “preparations of inanimate microorganisms and/or their components that confers a health benefit on the host” (50). Postbiotics improve health by inhibiting microorganisms, improving the intestinal barrier, and modifying immune responses via interactions with host receptors such as TLRs and NLRs (55).
While probiotics and prebiotics each provide significant health benefits, synbiotics combine their strengths to produce synergistic effects, and postbiotics provide a safer, more stable alternative with bioactive components that influence host health without the risks associated with live microorganisms (55).
3.2 Impact of diet & probiotics, prebiotics, synbiotics and postbiotics on Gut Microbiota
The gut microbiota, with roughly 3.8 × 10¹³ microorganisms, outnumbers human cells and plays a crucial role in supporting host health (56, 57). This intricate micro ecology which includes bacteria, yeasts, viruses, and parasites categorised into five primary phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia. Firmicutes and Bacteroidetes account for over 90% of the total microbial population (57, 58). Gut microbiota composition varies across individuals and is determined by factors such as age, genetics, birth mode, infant feeding practices, antibiotic usage, geography, and, most importantly, food (59). Diet has a bi-directional relationship with the microbiota, influencing nutrient absorption and metabolism (60).
Diet has a strong influence on the gut microbiome through macronutrients, micronutrients, and bioactive chemicals, influencing microbial composition, diversity, and function (61). Dietary fibre influences the gut microbiota, especially by boosting the abundance of SCFA-producing bacteria (62). Fibres like inulin, guar gum, resistant starch, galacto-oligosaccharides, fructo-oligosaccharides, and arabinoxylan oligosaccharides consistently promote beneficial microbes like Bifidobacterium, Faecalibacterium, Ruminococcus, Lactobacillus, Akkermansia, and Roseburia (63). Recent research indicates that high-protein diets increase gut microbial diversity and modify microbiota composition differently from normal-protein diets, enriching Akkermansia and Bifidobacterium while decreasing Prevotella (62, 58). High-fat diets, particularly those high in saturated fats, promote dysbiosis by boosting Firmicutes and Proteobacteria while lowering Bacteroidetes (63, 64, 57).
Probiotics regulate the synthesis of gastrointestinal hormones such as leptin, ghrelin, and GLP-1, which helps with hunger regulation and metabolic health (65). They create short-chain fatty acids from dietary fibre fermentation and produce organic acids and bacteriocins that inhibit infections (66). Bifidobacteria form acetate, which helps other gut microbes thrive. Bifidobacteria is one of the most profuse and substantially functional group of microbes in healthy individual's microbiome, primarily in newborns where they encompass approximately 90% of total microbiota (67). Prebiotics have a good effect on gut health by suppressing type 2 T helper immune responses and boosting calcium absorption by creating SCFAs through fermentation (46, 47). Synbiotics can assist regulate the gut microbiota during weight loss and improve blood glucose, lipid profiles, and body weight in T2DM patients (68, 69). Postbiotics boost gut and metabolic health by regulating immunity and improving glucose and insulin metabolism while avoiding the hazards associated with live bacteria (50).
3.3 Microbiome based metabolic treatment
The gut microbiome is defined as the collective genomes of all bacteria living in the gut. A change in gut microbiome homeostasis caused by changes in genetics, nutrition status, lifestyle, and other factors can lead to microbiome dysbiosis, which in turn results in chronic diseases including inflammatory bowel disease (IBD) (70), cardiovascular disease (71), neurological diseases such as autism and Parkinson's, metabolic conditions such as obesity and diabetes, and certain cancers type 2 diabetes (T2D), metabolic dysfunction-associated steatotic liver disease (MASLD), hypertension, and hyperlipidemia, all of which are increasingly prevalent worldwide (72). Several microbiome-based treatment techniques have emerged as viable approaches for treating metabolic and inflammatory illnesses. These include probiotics, prebiotics, synbiotics, and faecal microbiota transplantation (FMT) as depicted in Figure 1.
3.3.1 Probiotics, prebiotics, and synbiotics treatment technique
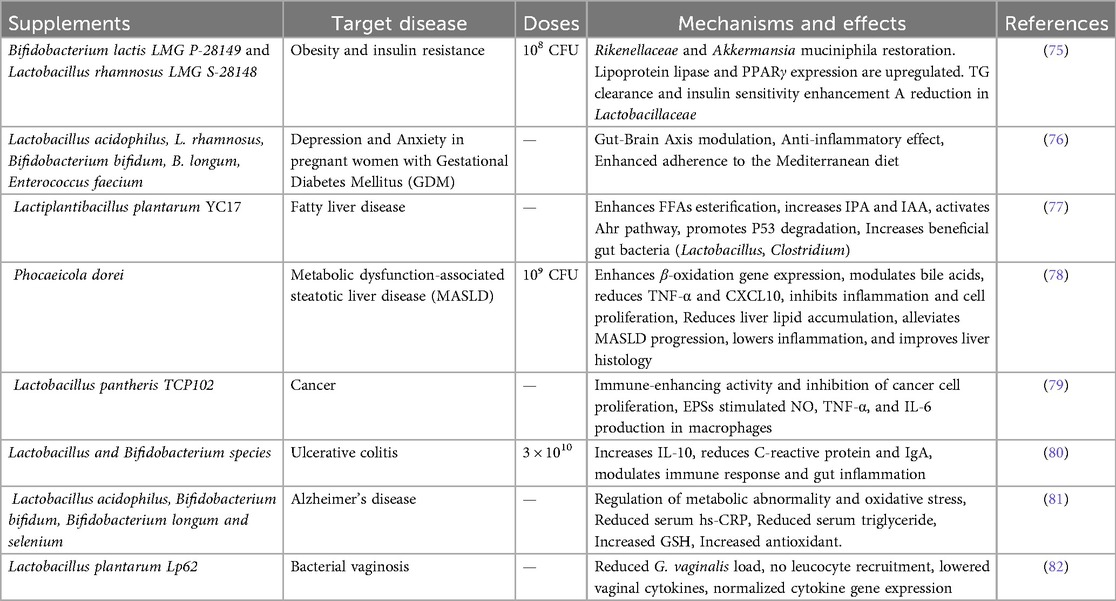
Prebiotics, probiotics, synbiotics (combinations of the two), and postbiotics are important microbiome-based treatments for treating metabolic diseases as shown in Table 1. While they may have potential benefits, such as enriching beneficial microorganisms, enhancing gut barrier function, and altering host metabolism, clinical evidence of their safety and efficacy is sparse. Probiotics frequently encounter colonisation resistance, exhibit strain and site-specific effects (73). Additionally, a study using an in vitro gut model with fecal samples from obese donors showed that synbiotic supplementation with Limosilactobacillus reuteri KUB-AC5 and Wolffia globosa powder increased beneficial bacteria, decreased Enterobacteriaceae, and enhanced levels of butyrate while lowering detrimental p-cresol (74). Postbiotics and synthetic microbes are developing alternatives with promising metabolic benefits, but they face challenges such as complex host-microbe interactions, unclear metabolic pathways, and tailored delivery.
3.3.2 Faecal microbiota transplantation technique
FMT includes introducing stool from a healthy donor into a patient's gastrointestinal tract to restore gut bacteria equilibrium. It is now a well-established treatment for recurrent Clostridioides difficile infection (CDI), with cure rates of up to 90%. Beyond CDI, FMT is being studied for its therapeutic potential in inflammatory bowel disease (IBD), with clinical studies indicating that it can reduce gut inflammation and microbial composition (83, 84). Le et al. found that FMT resulted in durable increases in gut microbial diversity and decreased pathogenic taxa in paediatric ulcerative colitis (85). Similarly, Shekar et al. found that FMT may assist Parkinson's patients by increasing the production of beneficial metabolites like short-chain fatty acids (SCFAs), promoting gut-brain axis activity, and potentially decreasing disease progression (86).
An important variable in mental health, the microbial-gut-brain (MGB) axis, is modulated by FMT, providing a novel treatment for depression. FMT restores a healthy microbial ecosystem by addressing dysbiosis in the gut microbiota milieu. This affects important targets such the NLRP3 inflammasome and Sig-1R, which are linked to neuroinflammatory and neurochemical pathways linked to depressive disorders. Furthermore, FMT can enhance the antidepressant potential by utilizing the medicinal qualities of advantageous herbs (87).
3.3.3 Vaginal microbiota transplantation
The vaginal microbiota has a somewhat lesser diversity of microorganisms compared to the intestinal tract. A wide variety of lactic acid bacteria largely control the vaginal environment in order to preserve homeostasis (88). In addition to fluctuating throughout pregnancy and menopause, the vaginal microbiota's composition can also alter dynamically over shorter timescales of days to months (89, 90).
The vaginal microbiota of pre-term birth (PTB) showed that the PTB group had a significantly higher proportion of harmful bacteria (such as Desulfovibrionaceae, Helicobacter, and Gardnerella) and a significantly lower proportion of beneficial bacteria (such as Lactobacillus, Ruminococcus, and Megamonas). This difference was closely linked to the blood's biochemical parameters (white blood cells, neutrophils, NLR, and SIRI (91).
Vaginal Microbiota Transplantation (VMT) aids in sustaining the vaginal acidity and hinder the pathogenic bacteria by restoring the central Lactobacillus species. Inhibiting the NF-κB signaling pathway is one of its main strategies, which lowers inflammatory cytokines like TNF-α, IL-1β, and IL-6. This promotes healing and lessens tissue inflammation. In addition to being more biocompatible than antibiotics, VMT can work better when combined with other therapies. VMT is a promising noninvasive approach to the treatment of endometritis, with safety and microbial benefits. With its safety and microbiological advantages, VMT is a viable noninvasive treatment for endometritis (92).
The safety and effectiveness of VMT in treating bacterial vaginosis, recurring yeast infections, and other vaginal disorders have been shown in numerous studies. Additionally, the technique has demonstrated encouraging outcomes in lowering pregnant women's risk of premature birth and sexually transmitted diseases. For women who have ongoing vaginal issues, VMT is a minimally invasive, safe, and effective therapy alternative (93).
Compared to babies born vaginally, babies born via cesarean section (C-section) frequently have a different gut flora and are more susceptible to atopic and immune-related disorders (94). Bifidobacterium, Bacteroides, and Parabacteroides are generally more abundant in vaginally born newborns than in C-section babies (95). Human milk oligosaccharide (HMO) breakdown is frequently accelerated by these first colonizers, leading to the generation of short-chain fatty acids (SCFA) and colonization resistance which shape the microbiome and immune system leading to a healthier life (96, 97). On the other hand, skin and hospital-associated bacteria such Staphylococcus, Enterococcus, Klebsiella, and Clostridium species (95, 98) frequently invade newborns born after cesarean section. These are more likely to have genes for antibiotic resistance and frequently lack the capacity to break down HMOs or generate SCFAs (99).
3.3.4 Artificial microbial consortia technique
Emerging personalized strategies such as engineered microbial consortia are gaining attention for targeted microbiome-based interventions in metabolic disorders. Artificial microbial consortia (AMCs) are precisely designed communities of microorganisms tailored to modulate the gut microbiota and address specific pathological states. These consortia could include naturally helpful microorganisms or genetically engineered strains with higher medicinal potential. AMCs involve the deliberate selection and assembly of microbial strains with specific metabolic, immunomodulatory, and ecological roles (100). A study found that co-administration of Bifidobacterium pseudocatenulatum JJ3 and the engineered strain BsS-RS06551 significantly reduced obesity and associated metabolic dysfunctions in high-fat diet-induced obese mice (101).
3.3.5 Precision dietary modulation
Precision dietary modulation uses personalised nutritional therapies to target the gut microbiota and takes into account individual-specific characteristics such as genetics, dietary patterns, lifestyle behaviours, and metabolomic signatures (102). This approach tailors dietary advice to prevent and manage metabolic and gastrointestinal illnesses (103). Despite its potential, the application of precision nutrition is hampered by hurdles such as the complexities of microbiome analysis and an imperfect understanding of causative microbiome-health interactions.
3.3.6 Clinical trials: success and failure
Clinical trials are critical in turning preclinical microbiome research into therapeutic applications, providing a controlled environment to assess safety, effectiveness, and mechanistic outcomes in human populations. However, the effects of microbiome-based therapies in metabolic illnesses have varied, indicating the complex and individualised nature of host-microbe interactions.
Faecal microbiota transplantation (FMT) has showed promise for improving metabolic parameters. Wu et al. found that both FMT alone and FMT combined with metformin significantly improved insulin resistance (HOMA-IR), body mass index (BMI), and glycaemic management in a randomised clinical trial comprising 31 newly diagnosed type 2 diabetic mellitus (T2DM) (104). These approaches resulted in successful donor microbiota colonisation, enhanced microbial diversity, and modification of more than 200 microbial species. Notably, Bifidobacterium adolescentis and Synechococcus sp. were adversely linked with HOMA-IR, which highlights the therapeutic potential of microbiota manipulation in T2DM therapy. Similarly, targeted probiotic and synbiotic formulations have shown moderate but significant improvements in metabolic health. Othman et al. conducted a clinical trial on obese people, comparing food alone to prebiotic (carob) or probiotic treatment in 45 participants. While all groups lost weight, the prebiotic and probiotic groups saw greater increases in fat mass loss, muscle strength, insulin sensitivity, sleep quality, and psychological well-being (105). In a randomized controlled trial, the FMT from healthy individual into a patient suffering from IBS with mild to modest depression and anxiety, exhibited alleviation in anxiety and depression after treatment along with IBS symptoms, leading to substantial improvement in the quality of life (106). Adult patients suffering from Major Depressive Disorder (MDD) on undergoing FMT from healthy donor showed significant augmentation in mean gastrointestinal symptom scores and demonstrated greater enhancements in quality of life measures (107).
Despite these positive findings, many restrictions remain. A randomised trial by (NCT03125564) evaluating FMT in diarrhoea-predominant IBS patients found no significant improvement in overall IBS severity, but showed notable improvement in bloating symptoms (72% vs. 30%, p = 0.005), linked to reduced hydrogen sulphide-producing bacteria and microbial changes (e.g., ↓Ruminococcus gnavus, ↑Lawsonibacter) (108). Many clinical trials are unclear about probiotic strain specification, dosing regimen, and product formulation, with over 1,000 trials failing to publish standardised product information (109, 110).
4 Limitations, challenges and future outlook
The arena of microbiome-based therapeutics for metabolic disorders has observed remarkable advancement over the past decade, transitioning from conceptual bases to clinical realities with recent FDA approvals of live biotherapeutic products like Rebyota™ and Vowst™ (111). However, despite these substantial milestones, the translation of microbiome research into therapeutic interventions for metabolic ailments faces several complex challenges spanning scientific, technological, regulatory, and clinical domains.
One of the biggest challenges in microbiome-based treatments is the large differences in gut microbiome composition and function among individuals (112). The human gut microbiota shows significant diversity, which is affected by genetics, diet, lifestyle, environmental factors, and medical history (6, 113). This variation makes it hard to create standardized therapies that work well for all patients. Although many studies show links between changes in the microbiome and metabolic disorders, proving direct cause-and-effect relationships is tough (5). The lack of standard methods in microbiome research creates major hurdles for applying findings clinically, as differences in sample collection, processing, sequencing methods, and analysis can influence results and hinder reproducibility (111).
The rules around microbiome-based therapies are complex and change often, creating serious issues for developing and selling products. Engineering microbiome-based therapies is tricky, especially when it comes to keeping live products viable, stable, and consistent. Quality control must ensure the identity, purity, strength, and safety of microbes, while also maintaining consistency between batches (111). Safety assessments need to thoroughly evaluate both direct and indirect risks to the host, which includes concerns about microbial transfer, development of antibiotic resistance, and reactions from the immune system (114).
Forming clinical trials for treatments based on microbiome presents exclusive operational complications (5). Patient stratification strategies are important for identifying those who are most expected to benefit from definite treatments, but discovering dependable biomarkers is still a difficulty (115). Educating healthcare providers and gaining patient acceptance are additional hurdles for implementation.
The future of microbiome-based treatments will be influenced by several new technological advancements. Artificial intelligence and machine learning can help spot complex patterns and create predictive models for treatment response and customized therapy selection (115). Precision medicine based on individual microbiome profiles, genetic factors, and metabolic status will likely take center stage in future development. Multi-omics approaches that combine microbiome, metabolomic, proteomic, and genomic data may provide better targeting for therapies (116).
The future success of microbiome-based therapies for metabolic disorders will rely on ongoing scientific innovation, changes in regulation, and cooperation across academia, industry, and healthcare (115).
Author contributions
NA: Data curation, Formal analysis, Writing – original draft. VG: Data curation, Formal analysis, Writing – original draft. MK: Formal analysis, Writing – review & editing. AC: Supervision, Writing – review & editing. RC: Data curation, Writing – review & editing. PK: Conceptualization, Supervision, Writing – review & editing. NS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Urgent Action Needed as Global Diabetes Cases Increase Four-fold over Past Decades. Geneva: WHO (2024). Available online at: https://www.who.int/news/item/13-11-2024-urgent-action-needed-as-global-diabetes-cases-increase-four-fold-over-past-decades (Accessed November 13, 2024).
2. Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023. Hyattsville, MD: National Center for Health Statistics (2023).
3. Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. (2020) 9(3):179–92. doi: 10.1007/s13679-020-00379-w
4. Singh RK, Chang HW, Yan DI, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15(1):73. doi: 10.1186/s12967-017-1175-y
5. Vazquez-Uribe R, Hedin KA, Licht TR, Nieuwdorp M, Sommer MO. Advanced microbiome therapeutics as a novel modality for oral delivery of peptides to manage metabolic diseases. Trends Endocrinol Metab. (2025) 36(1):29–41. doi: 10.1016/j.tem.2024.04.021
6. Ezenabor EH, Adeyemi AA, Adeyemi OS. Gut microbiota and metabolic syndrome: relationships and opportunities for new therapeutic strategies. Scientifica (Cairo). (2024) 2024(1):4222083. doi: 10.1155/2024/4222083
7. Sasidharan Pillai S, Gagnon CA, Foster C, Ashraf AP. Exploring the gut microbiota: key insights into its role in obesity, metabolic syndrome, and type 2 diabetes. J Clin Endocrinol Metab. (2024) 109(11):2709–19. doi: 10.1210/clinem/dgae499
8. Sabico S, Al-Mashharawi A, Al-Daghri NM, Yakout S, Alnaami AM, Alokail MS, et al. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. J Transl Med. (2017) 15(1):249. doi: 10.1186/s12967-017-1354-x
9. Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity: a microbiome-wide analysis from population studies. JAMA Netw Open. (2021) 4(7):e2118811. doi: 10.1001/jamanetworkopen.2021.18811
10. Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. (2020) 8(11):1715. doi: 10.3390/microorganisms8111715
11. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19(1):55–71. doi: 10.1038/s41579-020-0433-9
12. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. (2020) 12(5):1474. doi: 10.3390/nu12051474
13. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. (2013) 498(7452):99–103. doi: 10.1038/nature12198
14. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. (2018) 555(7695):210–5. doi: 10.1038/nature25973
15. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505(7484):559–63. doi: 10.1038/nature12820
16. Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. (2017) 8:838. doi: 10.3389/fimmu.2017.00838
17. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165(6):1332–45. doi: 10.1016/j.cell.2016.05.041
18. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. (2020) 69(7):1218–28. doi: 10.1136/gutjnl-2019-319654
19. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. (2014) 159(4):789–99. doi: 10.1016/j.cell.2014.09.053
20. Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. (2016) 48(11):1413–7. doi: 10.1038/ng.3693
21. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562(7728):583–8. doi: 10.1038/s41586-018-0617-x
22. Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. (2018) 24(10):1526–31. doi: 10.1038/s41591-018-0160-1
23. Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, et al. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. (2019) 14(10):e0222394. doi: 10.1371/journal.pone.0222394
24. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. (2016) 34(3):260–8. doi: 10.1159/000443360
25. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. (2017) 17(4):219–32. doi: 10.1038/nri.2017.7
26. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. (2012) 148(6):1258–70. doi: 10.1016/j.cell.2012.01.035
27. Zhao MA, Chu J, Feng S, Guo C, Xue B, He K, et al. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother. (2023) 164:114985. doi: 10.1016/j.biopha.2023.114985
28. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. (2016) 65(3):426–36. doi: 10.1136/gutjnl-2014-308778
29. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. (2012) 490(7418):55–60. doi: 10.1038/nature11450
30. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. (2015) 528(7581):262–6. doi: 10.1038/nature15766
31. Albillos A, De Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. (2020) 72(3):558–77. doi: 10.1016/j.jhep.2019.10.003
32. Latif A, Shehzad A, Niazi S, Zahid A, Ashraf W, Iqbal MW, et al. Probiotics: mechanism of action, health benefits and their application in food industries. Front Microbiol. (2023) 14:1216674. doi: 10.3389/fmicb.2023.1216674
33. FDA. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. U.S. Food and Drug Administration Guidance for Industry. Silver Spring, MD: FDA (2024). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-clinical-trials-live-biotherapeutic-products-chemistry-manufacturing-and-control-information
34. Munir A, Javed GA, Javed S, Arshad N. Levilactobacillus brevis from carnivores can ameliorate hypercholesterolemia: in vitro and in vivo mechanistic evidence. J Appl Microbiol. (2022) 133(3):1725–42. doi: 10.1111/jam.15678
35. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
36. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. (2019) 10:S49–66. doi: 10.1093/advances/nmy063
37. Umair M, Jabbar S, Zhaoxin L, Jianhao Z, Abid M, Khan KUR, et al. Probiotic-based bacteriocin: immunity supplementation against viruses. An updated review. Front Microbiol. (2022) 13:876058. doi: 10.3389/fmicb.2022.876058
38. Peng X, Ed-Dra A, Song Y, Elbediwi M, Nambiar RB, Zhou X, et al. Lacticaseibacillus rhamnosus alleviates intestinal inflammation and promotes microbiota-mediated protection against Salmonella fatal infections. Front Immunol. (2022) 13:973224. doi: 10.3389/fimmu.2022.973224
39. Ahire JJ, Jakkamsetty C, Kashikar MS, Lakshmi SG, Madempudi RS. In vitro evaluation of probiotic properties of Lactobacillus plantarum UBLP40 isolated from traditional indigenous fermented food. Probiotics Antimicrob Proteins. (2021) 13(5):1413–24. doi: 10.1007/s12602-021-09775-7
40. Fantinato V, Camargo HR, Sousa ALOPD. Probiotics study with Streptococcus salivarius and its ability to produce bacteriocins and adherence to KB cells. Rev Odontol UNESP. (2019) 48:e20190029. doi: 10.1590/1807-2577.02919
41. Chang YH, Jeong CH, Cheng WN, Choi Y, Shin DM, Lee S, et al. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J Dairy Sci. (2021) 104(7):7415–25. doi: 10.3168/jds.2020-19820
42. Bu Y, Liu Y, Liu Y, Wang S, Liu Q, Hao H, et al. Screening and probiotic potential evaluation of bacteriocin-producing lactiplantibacillus plantarum in vitro. Foods. (2022) 11(11):1575. doi: 10.3390/foods11111575
43. Gupta V, Garg R. Probiotics. Indian J Med Microbiol. (2009) 27(3):202–9. doi: 10.4103/0255-0857.53201
44. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. (1995) 125(6):1401–12. doi: 10.1093/jn/125.6.1401
45. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14(8):491–502. doi: 10.1038/nrgastro.2017.75
46. Gu J, Thomas-Ahner JM, Riedl KM, Bailey MT, Vodovotz Y, Schwartz SJ, et al. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol Nutr Food Res. (2019) 63(8):1800636. doi: 10.1002/mnfr.201800636
47. You S, Ma Y, Yan B, Pei W, Wu Q, Ding C, et al. The promotion mechanism of prebiotics for probiotics: a review. Front Nutr. (2022) 9:1000517. doi: 10.3389/fnut.2022.1000517
48. Ney LM, Wipplinger M, Grossmann M, Engert N, Wegner VD, Mosig AS. Short chain fatty acids: key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. (2023) 13(3):230014. doi: 10.1098/rsob.230014
49. Marnpae M, Balmori V, Kamonsuwan K, Nungarlee U, Charoensiddhi S, Thilavech T, et al. Modulation of the gut microbiota and short-chain fatty acid production by gac fruit juice and its fermentation in in vitro colonic fermentation. Food Funct. (2024) 15(7):3640–52. doi: 10.1039/D3FO04318E
50. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EM, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18(9):649–67. doi: 10.1038/s41575-021-00440-6
51. Ma L, Tu H, Chen T. Postbiotics in human health: a narrative review. Nutrients. (2023) 15(2):291. doi: 10.3390/nu15020291
52. Zubair A. A commentary on mechanism of action and types of synbiotics. J Prob Health. (2022) 10:290. doi: 10.35248/2329-8901.22.10.290
53. Lee S, Choi SP, Choi HJ, Jeong H, Park YS. A comprehensive review of synbiotics: an emerging paradigm in health promotion and disease management. World J Microbiol Biotechnol. (2024) 40:280. doi: 10.1007/s11274-024-04085-w
54. Aggeletopoulou I, Konstantakis C, Assimakopoulos SF, Triantos C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb Pathog. (2019) 137:103774. doi: 10.1016/j.micpath.2019.103774
55. Jastrząb R, Graczyk D, Siedlecki P. Molecular and cellular mechanisms influenced by postbiotics. Int J Mol Sci. (2021) 22(24):13475. doi: 10.3390/ijms222413475
56. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. (2016) 14(8):e1002533. doi: 10.1371/journal.pbio.1002533
57. Zhang P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int J Mol Sci. (2022) 23(17):9588. doi: 10.3390/ijms23179588
58. Sălcudean A, Cîmpian DM, Popovici RA, Forna N, Corodan-Comiati DM, Sasu AB, et al. Dietary habits and their influence on the microbiome and mental health in adolescents. Nutrients. (2025) 17(9):1496. doi: 10.3390/nu17091496
59. Rinninella E, Tohumcu E, Raoul P, Fiorani M, Cintoni M, Mele MC, et al. The role of diet in shaping human gut microbiota. Best Pract Res Clin Gastroenterol. (2023) 62–63:101828. doi: 10.1016/j.bpg.2023.101828
60. Ramos S, Martín MÁ. Impact of diet on gut microbiota. Curr Opin Food Sci. (2021) 37:83–90. doi: 10.1016/j.cofs.2020.09.006
61. Mora-Flores LP, Moreno-Terrazas Casildo R, Fuentes-Cabrera J, Pérez-Vicente HA, de Anda-Jáuregui G, Neri-Torres EE. The role of carbohydrate intake on the gut microbiome: a weight of evidence systematic review. Microorganisms. (2023) 11(7):1728. doi: 10.3390/microorganisms11071728
62. Li T, Lu X, Yang X. Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans. Food Funct. (2017) 8(1):262–9. doi: 10.1039/C6FO01290F
63. Fu J, Zheng Y, Gao Y, Xu W. Dietary fiber intake and gut Microbiota in human health. Microorganisms. (2022) 10(12):2507. doi: 10.3390/microorganisms10122507
64. Soveid N, Barkhidarian B, Samadi M, Hatami M, Gholami F, Yekaninejad MS, et al. Animal and plant protein intake association with mental health, tryptophan metabolites pathways, and gut microbiota in healthy women: a cross-sectional study. BMC Microbiol. (2024) 24(1):390. doi: 10.1186/s12866-024-03534-8
65. van Son J, Koekkoek LL, La Fleur SE, Serlie MJ, Nieuwdorp M. The role of the gut microbiota in the gut–brain axis in obesity: mechanisms and future implications. Int J Mol Sci. (2021) 22(6):2993. doi: 10.3390/ijms22062993
66. Hegarty JW, Guinane CM, Ross RP, Hill C, Cotter PD. Bacteriocin production: a relatively unharnessed probiotic trait? F1000Res. (2016) 5:2587. doi: 10.12688/f1000research.9615.1
67. Jena R, Singh NA, Ahmed N, Choudhury PK. Bifidobacteria in antibiotic-associated dysbiosis: restoring balance in the gut microbiome. World J Microbiol Biotechnol. (2025) 41(8):297. doi: 10.1007/s11274-025-04517-1
68. Sergeev IN, Aljutaily T, Walton G, Huarte E. Effects of synbiotic supplement on human gut Microbiota, body composition and weight loss in obesity. Nutrients. (2020) 12(1):222. doi: 10.3390/nu12010222
69. Jiang H, Cai M, Shen B, Wang Q, Zhang T, Zhou X. Synbiotics and gut Microbiota: new perspectives in the treatment of type 2 diabetes Mellitus. Foods. (2022) 11(16):2438. doi: 10.3390/foods11162438
70. Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. (2014) 20(5):1192. doi: 10.3748/wjg.v20.i5.1192
71. Hansen TH, Gøbel RJ, Hansen T, Pedersen O. The gut microbiome in cardio-metabolic health. Genome Med. (2015) 7(1):33. doi: 10.1186/s13073-015-0157-z
72. Zhang X, Cai X, Zheng X. Gut microbiome-oriented therapy for metabolic diseases: challenges and opportunities towards clinical translation. Trends Pharmacol Sci. (2021) 42(12):984–7. doi: 10.1016/j.tips.2021.09.003
73. Mkilima T. Synthetic biology approaches for restoring gut microbial balance and engineering disease-specific microbiome therapeutics. Microb Pathog. (2025) 207:107931. doi: 10.1016/j.micpath.2025.107931
74. Mok K, Tomtong P, Ogawa T, Nagai K, Torrungruang P, Charoensiddhi S, et al. Synbiotic-driven modulation of the gut microbiota and metabolic functions related to obesity: insights from a human gastrointestinal model. BMC Microbiol. (2025) 25:250. doi: 10.1186/s12866-025-03953-1
75. Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham AL, et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. (2016) 18(5):1484–97. doi: 10.1111/1462-2920.13181
76. Bozdoğan FB K, Kabaran S, Tazeoğlu A. Effect of probiotic supplementation on maternal depression, anxiety and attachment in gestational diabetes by improving Mediterranean diet quality: a randomized controlled trial. Clin Exp Obstet Gynecol. (2024) 51(11):237. doi: 10.31083/j.ceog5111237
77. Ding FF, Zhou NN, Mao YJ, Yang J, Limbu SM, Galindo-Villegas J, et al. Lactiplantibacillus plantarum attenuate gossypol-induced hepatic lipotoxicity by altering intestinal microbiota for enriching microbial tryptophan metabolites in Nile tilapia (oreochromis niloticus). Microbiome. (2025) 13:180. doi: 10.1186/s40168-025-02172-0
78. Choi J, Choi YR, Jeong MK, Song HH, Yu JS, Song SH, et al. Phocaeicola dorei ameliorates progression of steatotic liver disease by regulating bile acid, lipid, inflammation and proliferation. Gut Microbes. (2025) 17(1):2539448. doi: 10.1080/19490976.2025.2539448
79. Sheng S, Fu Y, Pan N, Zhang H, Xiu L, Liang Y, et al. Novel exopolysaccharide derived from probiotic Lactobacillus pantheris TCP102 strain with immune-enhancing and anticancer activities. Front Microbiol. (2022) 13:1015270. doi: 10.3389/fmicb.2022.1015270
80. Agraib LM, Yamani MI, Tayyem R, Abu-Sneineh AT, Rayyan YM. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: a pilot, randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN. (2022) 51:83–91. doi: 10.1016/j.clnesp.2022.08.020
81. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. (2019) 38(6):2569–75. doi: 10.1016/j.clnu.2018.11.034
82. Selis NN, Oliveira HBM, Souza CLS, Almeida JB, Andrade YMFS, Silva LSC, et al. Lactobacillus plantarum Lp62 exerts probiotic effects against Gardnerella vaginalis ATCC 49154 in bacterial vaginosis. Lett Appl Microbiol. (2021) 73(5):579–89. doi: 10.1111/lam.13547
83. Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. (2017) 389(10075):1218–28. doi: 10.1016/S0140-6736(17)30182-4
84. Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. (2019) 118(Suppl 1):S23–31. doi: 10.1016/j.jfma.2018.08.011
85. Le J, Hakimjavadi H, Parsana R, Chamala S, Michail S. Fecal microbiota transplantation induces sustained gut microbiome changes in pediatric ulcerative colitis: a combined randomized and open-label study. Gastro Hep Adv. (2025) 4:100741. doi: 10.1016/j.gastha.2025.100741
86. Shekar S, Venkatachalapathy R, Jayaraman A, Siddhu NSS. Fecal microbiota transplantation for Parkinson’s disease: a systematic review of clinical evidence. Med Microecol. (2025) 25:100128. doi: 10.1016/j.medmic.2025.100128
87. Zhang Q, Bi Y, Zhang B, Jiang Q, Mou CK, Lei L, et al. Current landscape of fecal microbiota transplantation in treating depression. Front Immunol. (2024) 15:1416961. doi: 10.3389/fimmu.2024.1416961
88. Brown SE, Tuddenham S, Shardell MD, Klebanoff MA, Ghanem KG, Brotman RM. Bacterial vaginosis and spontaneous clearance of Chlamydia trachomatis in the longitudinal study of vaginal Flora. J Infect Dis. (2023) 228(6):783–91. doi: 10.1093/infdis/jiad142
89. Lewis FM, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. (2017) 129(4):643–54. doi: 10.1097/AOG.0000000000001932
90. Tomas M, Palmeira-de-Oliveira A, Simoes S, Martinez-de-Oliveira J, Palmeira-de-Oliveira R. Bacterial vaginosis: standard treatments and alternative strategies. Int J Pharm. (2020) 587:119659. doi: 10.1016/j.ijpharm.2020.119659
91. Zhang J, Li L, Zhang M, Fang J, Xu Z, Zheng Y, et al. Distinct vaginal microbiome and metabolome profiles in women with preterm delivery following cervical cerclage. Front Cell Infect Microbiol. (2025) 15:1444028. doi: 10.3389/fcimb.2025.1444028
92. Lahouty M, Abdi M, Fadaee M, Nezhadi J. Potential of vaginal Microbiota transplantation (VMT) in endometritis management. Am J Reprod Immunol. (2025) 93(6):e70104. doi: 10.1111/aji.70104
93. Meng Y, Sun J, Zhang G. Vaginal microbiota transplantation is a truly opulent and promising edge: fully grasp its potential. Front Cell Infect Microbiol. (2024) 14:1280636. doi: 10.3389/fcimb.2024.1280636
94. Nieto PA, Nakama C, Trachsel J, Goad D, Soderborg TK, Tan DS, et al. Improving immune-related health outcomes post-cesarean birth with a gut microbiome-based program: a randomized controlled trial. Pediatr Allergy Immunol. (2025) 36(9):e70182. doi: 10.1111/pai.70182
95. Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574(7776):117–21. doi: 10.1038/s41586-019-1560-1
96. Kiely LJ, Busca K, Lane JA, van Sinderen D, Hickey RM. Molecular strategies for the utilisation of human milk oligosaccharides by infant gut-associated bacteria. FEMS Microbiol Rev. (2023) 47(6):fuad056. doi: 10.1093/femsre/fuad056
97. Kijner S, Cher A, Yassour M. The infant gut commensal Bacteroides dorei presents a generalized transcriptional response to various human milk oligosaccharides. Front Cell Infect Microbiol. (2022) 12:854122. doi: 10.3389/fcimb.2022.854122
98. Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon MC, et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe. (2021) 29(5):765–76. doi: 10.1016/j.chom.2021.02.021
99. Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome. (2017) 5(1):4. doi: 10.1186/s40168-016-0213-y
100. Mkilima T. Engineering artificial microbial consortia for personalized gut microbiome modulation and disease treatment. Ann N Y Acad Sci. (2025) 1548:29–55. doi: 10.1111/nyas.15352
101. Chen X, Gao M, Wang L, Qiang G, Wu Y, Huang H, et al. A synthetic microbial consortium protects against obesity by regulating vitamin B6 metabolism. Gut Microbes. (2024) 16(1):2304901. doi: 10.1080/19490976.2024.2304901
102. Mills S, Stanton C, Lane JA, Smith GJ, Ross RP. Precision nutrition and the microbiome, part I: current state of the science. Nutrients. (2019) 11(4):923. doi: 10.3390/nu11040923
103. De Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. (2017) 9(8):913. doi: 10.3390/nu9080913
104. Wu Z, Zhang B, Chen F, Xia R, Zhu D, Chen B, et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: a randomized, controlled, prospective study. Front Cell Infect Microbiol. (2023) 12:1089991. doi: 10.3389/fcimb.2022.1089991
105. Ben Othman R, Ben Amor N, Mahjoub F, Berriche O, El Ghali C, Gamoudi A, et al. A clinical trial about effects of prebiotic and probiotic supplementation on weight loss, psychological profile and metabolic parameters in obese subjects. Endocrinol Diabetes Metab. (2023) 6(2):e402. doi: 10.1002/edm2.402
106. Lin H, Guo Q, Wen Z, Tan S, Chen J, Lin L, et al. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb Cell Fact. (2021) 20(1):233. doi: 10.1186/s12934-021-01720-1
107. Green JE, Berk M, Mohebbi M, Loughman A, McGuinness AJ, Castle D, et al. Feasibility, acceptability, and safety of faecal microbiota transplantation in the treatment of major depressive disorder: a pilot randomized controlled trial. Can J Psychiatry. (2023) 68(5):315–26. doi: 10.1177/07067437221150508
108. Yau YK, Su Q, Xu Z, Tang W, Ching JYL, Mak JWY, et al. Randomised clinical trial: faecal microbiota transplantation for irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. (2023) 58(8):795–804. doi: 10.1111/apt.17703
109. Van den Nieuwboer M, Van De Burgwal LHM, Claassen E. A quantitative key-opinion-leader analysis of innovation barriers in probiotic research and development: valorisation and improving the tech transfer cycle. PharmaNutrition. (2016) 4(1):9–18. doi: 10.1016/j.phanu.2015.09.003
110. Van de Burgwal LHM, Van der Waal MB, Claassen E. Accelerating microbiota product development: the societal impact value cycle as a conceptual model to shape and improve public-private valorization processes. PharmaNutrition. (2018) 6(4):157–68. doi: 10.1016/j.phanu.2018.07.002
111. Microbiome Therapeutics Innovation Group, Barberio D. Navigating regulatory and analytical challenges in live biotherapeutic product development and manufacturing. Front Microbiomes. (2024) 3:1441290. doi: 10.3389/frmbi.2024.1441290
112. Zhou P, Chen C, Patil S, Dong S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front Nutr. (2024) 11:1355542. doi: 10.3389/fnut.2024.1355542
113. Yaqub MO, Jain A, Joseph CE, Edison LK. Microbiome-driven therapeutics: from gut health to precision medicine. Gastrointest Disord. (2025) 7(1):7. doi: 10.3390/gidisord7010007
114. Rouanet A, Bolca S, Bru A, Claes I, Cvejic H, Girgis H, et al. Live biotherapeutic products, a road map for safety assessment. Front Med. (2020) 7:237. doi: 10.3389/fmed.2020.00237
115. Nazir A, Hussain FHN, Raza A. Advancing microbiota therapeutics: the role of synthetic biology in engineering microbial communities for precision medicine. Front Bioeng Biotechnol. (2024) 12:1511149. doi: 10.3389/fbioe.2024.1511149
Keywords: gut micobiota, dysbiosis, metabolic disorder, diabetes, FMT, vaginal microbiota
Citation: Ahmed N, Gaur V, Kamle M, Chauhan A, Chauhan R, Kumar P and Singh NA (2025) Microbiome-based therapeutics for metabolic disorders: harnessing microbial intrusions for treatment. Front. Med. Technol. 7:1695329. doi: 10.3389/fmedt.2025.1695329
Received: 29 August 2025; Accepted: 8 October 2025;
Published: 30 October 2025.
Edited by:
Abhinav Saurabh, National Institutes of Health (NIH), United StatesReviewed by:
Satya P. Singh, Saurashtra University, IndiaCopyright: © 2025 Ahmed, Gaur, Kamle, Chauhan, Chauhan, Kumar and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep Kumar, cGtiaW90ZWNoQGdtYWlsLmNvbQ==; Namita Ashish Singh, bmFtaXRhLnNpbmdoQG1sc3UuYWMuaW4=
†These authors have contributed equally to this work
 Nafees Ahmed
Nafees Ahmed Vishwas Gaur
Vishwas Gaur Madhu Kamle
Madhu Kamle Abhishek Chauhan
Abhishek Chauhan Ritu Chauhan
Ritu Chauhan Pradeep Kumar
Pradeep Kumar Namita Ashish Singh
Namita Ashish Singh