- 1Division of Biomechanics and Research Development, Department of Biomechanics, and Center for Research in Human Movement Variability, University of Nebraska at Omaha, Omaha, NE, United States
- 2Graduate School of Engineering Science, Osaka University, Osaka, Japan
- 3National University of Ukraine on Physical Education and Sport, Scientific Research Institute, Kyiv, Ukraine
- 4Department of Movement Physiology, Bogomoletz Institute of Physiology, Kyiv, Ukraine
- 5Department of Electronic Engineering, Igor Sikorsky Kyiv Polytechnic Institute, Kyiv, Ukraine
- 6Faculty of Applied Sciences, Ukrainian Catholic University, Lviv, Ukraine
- 7Department of Psychology, State University of New York at New Paltz, New Paltz, NY, United States
Dexterous postural control subtly complements movement variability with sensory correlations at many scales. The expressive poise of gymnasts exemplifies this lyrical punctuation of release with constraint, from coarse grain to fine scales. Dexterous postural control upon a 2D support surface might collapse the variation of center of pressure (CoP) to a relatively 1D orientation—a direction often oriented towards the focal point of a visual task. Sensory corrections in dexterous postural control might manifest in temporal correlations, specifically as fractional Brownian motions whose differences are more and less correlated with fractional Gaussian noises (fGns) with progressively larger and smaller Hurst exponent H. Traditional empirical work examines this arrangement of lower-dimensional compression of CoP along two orthogonal axes, anteroposterior (AP) and mediolateral (ML). Eyes-open and face-forward orientations cultivate greater variability along AP than ML axes, and the orthogonal distribution of spatial variability has so far gone hand in hand with an orthogonal distribution of H, for example, larger in AP and lower in ML. However, perturbing the orientation of task focus might destabilize the postural synergy away from its 1D distribution and homogenize the temporal correlations across the 2D support surface, resulting in narrower angles between the directions of the largest and smallest H. We used oriented fractal scaling component analysis (OFSCA) to investigate whether sensory corrections in postural control might thus become suborthogonal. OFSCA models raw 2D CoP trajectory by decomposing it in all directions along the 2D support surface and fits the directions with the largest and smallest H. We studied a sample of gymnasts in eyes-open and face-forward quiet posture, and results from OFSCA confirm that such posture exhibits the classic orthogonal distribution of temporal correlations. Head-turning resulted in a simultaneous decrease in this angle Δθ, which promptly reversed once gymnasts reoriented their heads forward. However, when vision was absent, there was only a discernible negative trend in Δθ, indicating a shift in the angle’s direction but not a statistically significant one. Thus, the narrowing of Δθ may signify an adaptive strategy in postural control. The swift recovery of Δθ upon returning to a forward-facing posture suggests that the temporary reduction is specific to head-turning and does not impose a lasting burden on postural control. Turning the head reduced the angle between these two orientations, facilitating the release of postural degrees of freedom towards a more uniform spread of the CoP across both dimensions of the support surface. The innovative aspect of this work is that it shows how fractality might serve as a control parameter of adaptive mechanisms of dexterous postural control.
1 Introduction
Gymnastics training exerts a profound and transformative impact on postural performance (Asseman et al., 2004; Asseman et al., 2008; Hrysomallis, 2011; Paillard, 2017; Paillard, 2019), molding individuals into adept conductors of equilibrium and control. The intricate maneuvers and dynamic routines of gymnastics necessitate a heightened integration of proprioceptive acuity, spatial precision, and sophisticated neuromuscular coordination. The navigation through flips, twists, and landings compels continuous physiological adaptation, fostering the development of a finely tuned postural control system. Notably, while the scientific discourse explicitly recognizes the contribution of diverse muscle groups and the progressive enhancement of core strength to the observed postural stability, some remaining unanswered scientific questions center on how specific elements within gymnastics training selectively impact diverse facets of postural control, the enduring effects of such training on injury mitigation, and the intricate interplay between gymnastic-induced postural adaptations and the aging process. An essential question in the present manuscript is how simple perturbations from routine daily activities prompt the postural skills cultivated in gymnastics training.
Gymnastics training fosters the development of dexterity, supporting adult-like postural coordination and control even in children (Busquets et al., 2021). Notably, gymnastics training significantly affects response latency, particularly for upper body muscles situated along the frontal aspect and precisely along the anteroposterior (AP) axis (Debu and Woollacott, 1988; Calavalle et al., 2008; Zemková and Kováčiková, 2023). Even young gymnasts improve their ability to use proprioceptive information for postural stability (Garcia et al., 2011). Expert gymnasts have a distinctive capacity to reintegrate proprioceptive input with other modalities, particularly vision, which sets them apart from non-gymnast athletes (Vuillerme et al., 2001b). The attentional demands associated with regulating postural sway increase with task difficulty, yet gymnasts exhibit a more negligible effect during unipedal stances (Vuillerme and Nougier, 2004). This effect holds even without visual information, indicating adeptness at using alternative compensatory sensory modalities (Vuillerme et al., 2001a). Furthermore, gymnasts showcase increased instability in the postural center of pressure (CoP) compared to head movements compared to their non-gymnast counterparts (Gautier et al., 2008).
Understanding how gymnasts coordinate these exquisite postural feats may depend on understanding how they coordinate their quiet postural sway across time. Motor variations and sensory corrections proceed in a finely tuned coordination across time, and empirical indicators of correlations in sway can reveal the underlying control processes. Examining the 2D CoP trajectory of an individual reveals that CoP fluctuation at one timepoint might correlate with CoP fluctuations at other times (Duarte and Zatsiorsky, 2000; Blaszczyk and Klonowski, 2001; Błaszczyk et al., 2014). Postural sway might be correlated across time, with a stronger correlation likely between two points closer together. As we consider longer timescales, there is more room for the sway to vary. So, temporal correlations of CoP fluctuations might decay. However, an important question is how slowly those temporal correlations decay between successive CoP fluctuations. For instance, a gradual adjustment in muscle posture from fatigue may mean that CoP fluctuations might have more random fluctuations with weak temporal correlations as the body enacts faster changes in posture. This slow, gradual decay of correlations across time can leave long-range traces of this correlation-time “memory” within the time series. When this decay of correlation follows a single power law, we call it “monofractality.” The traditional examination of human postural dynamics often characterizes the monofractality of the 2D CoP trajectory as fractional Brownian motion (fBm), wherein sample-to-sample displacements resemble fractional Gaussian noise (fGn) (Burdet and Rougier, 2007; Kuznetsov et al., 2013). Detrended Fluctuation Analysis (DFA) offers a compelling analytical method to estimate these monofractal temporal correlations between CoP fluctuations that vary across longer separations in time Peng et al. (1994, 1995). It estimates a Hurst exponent HfGn, indicating the strength of this power-law decay. Specifically, HfGn relates with how the SD-like variations in CoP fluctuations grow across many timescales, encoding how the correlation among sequential fluctuations might decay slowly across longer separations in time. The HfGn reveals the strength of persistent correlations (0.5 < HfGn < 1.0; large values in the CoP fluctuation time series are typically followed by large values and vice versa) or anti-persistent correlations (0 < HfGn < 0.5; large values in the time series are typically followed by small values and vice versa) in CoP fluctuations over time (Hurst, 1951; Eke et al., 2002).
The fractal structure has offered a robust empirical connection to postural control strategies in non-gymnasts (Blaszczyk and Klonowski, 2001; Gilfriche et al., 2018). The fractal nature of postural CoP fluctuations in healthy adults is robust to sensory perturbations, such as alterations in visual input (Stambolieva, 2011). Learning to rely on non-visual proprioceptive inputs can be essential in the adaptability and flexibility of athletic postural control mechanisms (Coubard et al., 2014; Picot et al., 2022). Discerning the fractal architecture of CoP fluctuations may allow us to discern the reliance on proprioception. For instance, athletes exhibit postural control in sports demanding relatively swift throwing capabilities (e.g., handball and tennis), showing pronounced temporal correlations in CoP fluctuations (Caballero et al., 2020; Caballero et al., 2021). Athletes with elevated CoP fractal dimensions along the AP axis show greater flexibility in making more sparing use of vision while finding their execution of intricate posture-motor tasks more so on proprioception (Borzucka et al., 2020; Wyatt et al., 2021). In experimentation using galvanic vestibular stimulation (GVS) to destabilize posture, temporal correlations in CoP correlate inversely with the likelihood of post-GVS falls (Van den Hoorn et al., 2018); gymnastics practice counteracts postural control disruption caused by GVS (Hopper et al., 2014; Maitre and Paillard, 2016), although these adaptations tend to be very specific and the transfer effect is limited (Asseman et al., 2004; Asseman et al., 2008; Paillard, 2017; Paillard, 2019). Various non-gymnast models have shown that fractal fluctuations are an essential support in the coordination of postural adaptation, for instance, for allowing the body to respond to a destabilizing postural perturbation or engage in a secondary, more visual perceptual task which might also destabilize posture (Furmanek et al., 2021; Ihlen et al., 2013; Jacobson et al., 2021; Kelty-Stephen et al., 2021b; Kelty-Stephen et al., 2021a; Kelty-Stephen et al., 2023; Mangalam and Kelty-Stephen, 2021a; Mangalam et al., 2020a; Mangalam et al., 2020b; Mangalam et al., 2021; Morales and Kolaczyk, 2002; Palatinus et al., 2014; Shimizu et al., 2002). The present work aims to understand how gymnastic training might transfer to quiet standing and to examine the fractal temporal correlations in postural response to perturbation of quiet standing.
The implication of fractal temporal correlations in control provides an exciting window into the network connectivity pervading physiological systems. For instance, a prevailing understanding of postural control presumes that individual physiological mechanisms (e.g., visual, proprioceptive, and vestibular) might be so independent as to, for example, rank their relative prominence (Fitzpatrick and McCloskey, 1994; Peterka and Benolken, 1995; Ivanenko et al., 1999; Horlings et al., 2009; Wiesmeier et al., 2015). An incautious reading of the preceding paragraph might leave the incorrect view that fractal temporal correlations can be another curious intervention that can act upon various, separate processes. We see the value of a targeted intervention. However, the sometimes difficult-to-grasp fact is that fractality is not necessarily a localizable mechanism. Certainly, we can envision that the exogenous application of fractal stimulation, localized to any specific component or modality of the movement system, might yield some benefits (Nozaki et al., 1999b; Nozaki et al., 1999a; Raffalt et al., 2021; Raffalt et al., 2023; Rizzo et al., 2020; Soma et al., 2003). However tempting though such interpretations can be, fractality itself might reflect endogenous interactions spanning a wide range of scales bustling and, indeed, chaotically boiling over with interactions across scales (Wagman and Miller, 2003; Turvey and Sheya, 2017; Profeta and Turvey, 2018). Hence, fractality might reflect the unfolding of network relationships, widely presumed to be neural or neuronal (Plenz and Thiagarajan, 2007; Kello, 2013). It is also as likely to reflect networks of non-neural physiological media no less important for behavior and more important than previously thought to the function of any neural network (Ingber, 2006; Bashan et al., 2012; Bartsch et al., 2015; Ivanov et al., 2016; Bogdan, 2019; Ivanov, 2021). A wealth of intriguing theoretical research on networks and their fractal or multifractal implications suggests that networks featuring numerous connections spanning multiple scales of activity can occasionally yield a singular fractal dimension (Bak et al., 1987; Bak et al., 1988), sometimes many fractal dimensions (Bonachela and Munoz, 2009; Bonachela et al., 2010), and sometimes only “power law-like” processes that implicate fractal and multifractality dimensionalities under structural constraints (Stephen et al., 2009; Xiao et al., 2021). These network approaches appear limited by how best to understand the growth of new nodes (Gorochowski et al., 2018) to close the gap between the theoretical model and idiosyncratic constraints of the empirical model systems. We intend our work as an empirical branch to complement all of this theoretical work in which we can measure physiological time series and, much like Bogdan (2019) suggested, read the estimated sets of fractal dimensions as revealing the network dynamics—not localized in one tissue but spanning multiple scales of functional activity Turvey and Fonseca (2014). This relatively empirical use of fractal geometries allows network modeling across body parts (Carver et al., 2017; Mangalam et al., 2020a; b; Stephen et al., 2012b).
The 2D planarity of the support surface constitutes the widest possible constraints on postural control of the CoP trajectory. However, according to the tradition of movement science inspired by Bernstein (1967), coordination and control proceed by coalescing movement degrees of freedom into lower-dimensional solutions. So, the movement degrees of freedom may not always use the full dimensionality of the task constraints but may hover between collapsing and releasing degrees of freedom, fluidly reorganizing in response to unexpected perturbations. This point is as valid for postural control as for any other task (Latash et al., 2007; Latash, 2012). Models of postural variability make appeals to an intermittent controller sculpting distinctive sway patterns through a cyclical process, and by turns, this intermittent controller restrains CoP by collapsing its variability to a saddle-like stable manifold oriented along one “major” axis and, when it turns “off,” releases CoP into a spiraling circular expansion from the center point (Asai et al., 2009; Asai et al., 2013). That is, said another way, postural synergies compress variability from the two dimensions of the support surface to a relatively 1D axis of stability that avoids potentially less stable sway in the orthogonal direction. We often see this saddle of stability in a preponderance of temporal correlations in either the AP or ML axis but not both; for example, postural sway manifests augmented fractal scaling along the AP direction, accompanied by a diminished scaling exponent along the ML direction (Blaszczyk and Klonowski, 2001; Amoud et al., 2007; Kuznetsov et al., 2013), suggestive of more proactive and passive control strategies along the AP and ML axes, respectively (Figure 1A). Hence, in this traditional examination, the strategic allocation of temporal variability across the AP and ML axes by postural control mirrors its nuanced approach to more straightforward, task-oriented variability (Balasubramaniam et al., 2000). What we might expect, then, is that perturbations to posture might warrant the release of the ongoing postural synergy and consequent disengagement of saddle-type control, giving way to 2D spiral-type behavior (Figure 1B).
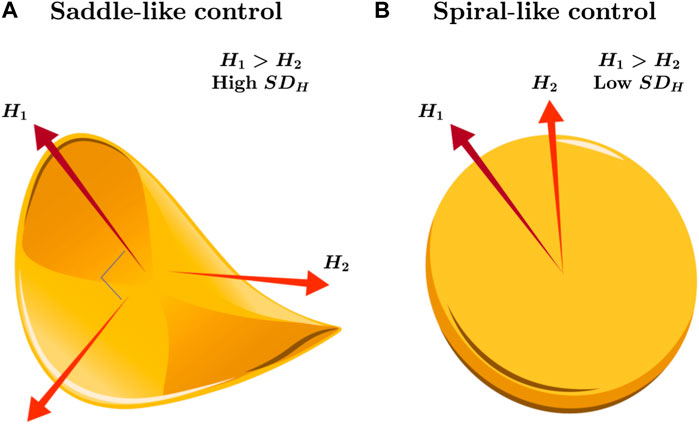
Figure 1. Schematic illustration of the saddle- and spiral-like strategies for controlling posture along the 2D support surface. (A) A stable equilibrium could emerge when examining temporal correlations predominantly in either the AP or ML axis but rarely in both, leading to a saddle-type control. For instance, heightened fractal scaling (i.e., large Hurst exponent H1) may indicate strong temporal correlations in postural sway along the AP direction, coupled with a reduced scaling exponent along the orthogonal ML direction (i.e., H2 < H1). This strategy could implicate more proactive control strategies along the AP axis and passive strategies along the ML axis with large spatial variability in temporal correlations (i.e., high SDH). (B) Alternatively, releasing the postural synergy constrained across the AP or ML axes in postural control might, for example, under perturbations or changes in orientation, disengage saddle-type control and pave the way for a transition to 2D spiral-type control, accompanied by relatively smaller spatial variability in temporal correlations (i.e., low SDH).
This saddle-type control of 2D CoP along AP and ML directions coincides with a conceptualization of postural control as an inverted pendulum fixed on two anatomical axes: the body executes rotations around one or more “hinges” aligned along the AP axis (Komura et al., 2005; Kuo, 2007; Kuo and Donelan, 2010). The saddle-type control is an apparent reason for finding temporal correlations that are strongest in one direction and weakest in the orthogonal direction, with stronger temporal correlations showing stronger signatures of coordination across time along one dimension than another. Despite the undeniable efficacy of measuring ground reaction forces along the AP and ML axes using force platforms, exclusive reliance on stability parameters within these orientations may inadvertently foster a misleading impression of their universal applicability across individuals (Granat et al., 1991; Sparto and Redfern, 2001). Indeed, effective postural control necessitates continuous integration of motor control spanning multiple directions (Peterka, 2002; Peterka and Loughlin, 2004; O’Connor and Kuo, 2009). In addition, the observed sway exhibits flexibility and fluidity, exceeding theoretical expectations from the inverted-pendulum model (Kelty-Stephen and Mangalam, 2023). Consequently, overfocus on orthogonal axes risks blinding us to spiral-type transients that might characterize the response to perturbation, allowing the formation of new saddle-type manifolds along novel task orientations.
Indeed, if the intermittent release of saddle-type control giving way to spiral-type control is adaptive, then the task-sensitive dissolution of orthogonality between the two postural-control directions could be a strategy for response to perturbation. Fortunately, Seleznov et al. (2020) have pioneered an analytical route that can help postural-control research to escape conventional AP-vs.-ML simplifications and explore how temporal correlations might transition between saddle and spiral orientations. This method, known as oriented fractal scaling component analysis (OFSCA), allows us to discern the directions, along the entire 2D support surface, of the strongest and weakest temporal correlations in sway. Although these directions can align with the anatomical AP and ML axes (Blaszczyk and Klonowski, 2001; Amoud et al., 2007; Kuznetsov et al., 2013), we know more about reorienting postural control from one direction to another (Balasubramaniam et al., 2000) and dramatically less about transient spiral-type states interspersed between postural adaptations. We might use OFSCA to analyze 2D CoP fluctuations to find saddle-type and spiral-type control signatures in the orthogonality or suborthogonality of the major and minor axes of temporal correlations in CoP.
The present study aims to clarify the foregoing possibilities. We seek here to call on the known postural dexterity of gymnasts to fill in empirical gaps and questions left from recent findings using the OFSCA. In recent comparisons of young healthy adults, older healthy adults, and adults with Parkinson’s disease (Mangalam et al., 2024b; Mangalam et al., 2024a), the healthy adults exhibited posture control with the strongest and weakest temporal correlations along two orthogonal directions, closely aligning with the anatomical AP and ML axes. Older adults show CoP with significantly narrower angles between the direction of the strongest and weakest temporal correlation, and adults with Parkinson’s disease showed dramatically more narrowing of this angle (Mangalam et al., 2024b). At first blush, this narrowing of directions of the strongest and weakest temporal correlations in CoP might look like the signature of a deficit. However, closer analysis suggests this narrowing might be an adaptive strategy: young healthy adults and older healthy adults showed significantly more narrowing of these two directions in experimental task settings that destabilized posture (e.g., closing eyes and standing on foam surface), and older adults with Parkinson’s disease showed no such change under the same task settings. Hence, healthy adults appear to exhibit brief narrowing of the axes in the CoP temporal correlations in response to perturbations. Meanwhile, individuals with Parkinson’s disease showed no flexibility in this narrowing (Mangalam et al., 2024b).
These OFSCA results may provide a novel empirical lens for testing the prior theoretical work [e.g., Asai et al. (2009, 2013)] emphasizing postural control vacillating intermittently between saddle- and spiral-type regimes. Younger adults show the clearest signature of this saddle-type control with their strongly orthogonal axes of the strongest and weakest temporal correlations, and older healthy adults may have less of this orthogonality in stable task settings. However, both show the narrowing of these directions that might correspond to greater 2D homogeneity in unstable conditions. The challenge for our interpretation is that individuals with Parkinson’s disease showed at once dramatically more narrowing and much less task-sensitive narrowing. A wider set of postural-control datasets of CoP, including healthy young and older adults alongside older adults with Parkinson’s disease, did show some task-sensitive narrowing of the two directions in response to unstable task settings (Mangalam et al., 2024a). But generally, it appeared that postural control in adults with Parkinson’s disease might spend disproportionately more time in the spiral-type mode, perhaps too much for a clear, unambiguous response to task manipulations. Is the loss of orthogonality a sign of disease? Or is it only a sign of disease when it shows weaker task sensitivity? So, we aimed to determine what orthogonality we might see in the 2D distribution of temporal correlations of gymnasts—under quiet standing with and without perturbations. Presumably, gymnasts show an adaptive response to the perturbation of quiet standing. So, we wish to use the gymnasts as a gold standard of quiet standing.
Our proposal to implicate the extremal axes of H in saddle-type and spiral-type regimes of control rests on traditions of control theory that are fundamentally topological. That is, invoking topological concerns “saddles” or “spirals” rests on an expectation of control strategies that are minimal and abstract and not directly invested in the anatomical constraints or details incidental to one or another model body or organism (Bernstein, 1967; Kelso, 1995; Holmes et al., 2006; Latash et al., 2007; Latash, 2012). Asai et al. (2009) did not invoke H as part of the control variables—they invoked the orthogonal dimensions of 1) the angle of the inverted pendulum sway and 2) the instantaneous change in the angle. Asai et al. (2009, 2013) did not even indicate whether they intended the angle to express rotation on the AP or the ML axis—AP and ML go entirely unmentioned. However, the actual observable of “angle” is not necessary to invoke this saddle/spiral control strategy, as it is replaceable with CoP or center of mass (CoM) (Nakamura et al., 2021). The angle of sway in any given axis is purely collinear with the movement of CoP in the same axis. As for the second axis, the convention of using instantaneous velocity reflects the convenient fact that the derivative of a pendular sine wave is the most straightforward algorithmic route to an orthogonal axis. A cosine wave is least correlated with the original sine wave. The proposed orthogonality of angular change from an angle matters more than the incidental fact of the pendulum, which is that the first derivative is orthogonal. If the angular position is collinear with AP or ML axes, we can replace angular change with the complementary axes (i.e., ML or AP, respectively). If angular change is orthogonal to angle, that is, a variable collinear with AP, then angular change must also be collinear with an axis orthogonal to AP—in this case, the topology on one (angular position vs. angular change) plane is the topology on the other (AP vs. ML). So, the details of which observables appear as labels are secondary to the topology, ideally a generic template for all anatomical/observable anchors.
We propose that the spatial distribution of sway variability presents a valuable perspective for discerning inter-individual variability in postural control, mainly influenced by gymnastics experience. We deliberately departed from conventional constraints: the strict adherence to AP versus ML directions and right angles. Instead, we sought a deeper understanding of postural control among trained gymnasts by examining task-specific alterations in the angles and directions between the smallest and largest fractal scaling patterns observed in 2D CoP trajectories. Our approach aimed to leverage the nuanced impact of gymnastics training on the robustness of postural control. We employed an innovative analytical technique known as oriented fractal scaling component analysis (OFSCA), as introduced by Seleznov et al. (2020) and subsequently used by Mangalam et al. (2024b), Mangalam et al. (2024a) to study postural deficits in older adults and individuals with Parkinson’s disease. This method decomposes the 2D CoP trajectories into directions corresponding to the most active and passive control mechanisms. This individualized portrayal of postural control was subsequently applied to the CoP 2D trajectories of a cohort of 17 trained gymnasts. Participants engaged in various conditions, including standing upright with eyes open versus closed and a perturbation condition involving rhythmic head rotation with both eyes open or closed. We intentionally selected this specific postural condition due to its inherent difficulty, anticipating it would yield the most pronounced angular anisotropy in postural sway. The chosen posture was anticipated to induce the most conspicuous directional variations in the individual’s swaying pattern. We posited that manipulating vision by closing the eyes and inducing vestibular perturbation through head rotation would each result in proprioceptive reintegration, evident in alterations to the spatial distribution of temporal correlations within CoP fluctuations.
Following the logic described above, we generated predictions with a twofold structure: predictions of effects for exogenous task manipulations (Hypothesis 1) and predictions of endogenous temporal correlations estimated by the OFSCA (Hypothesis 2). The OFSCA proceeds by estimating the angles θ1 and θ2 between the directions corresponding to the maximum and minimum values of the scaling exponent (i.e., indicated by H1 and H2 corresponding to the strongest and weakest temporal correlations, respectively) with respect to the force platform’s ML axis. It then returns the angle between these two axes as Δθ. First, we expected that perturbing quiet standing with an instructed head-turning would narrow the angle between the two directions of the strongest and weakest temporal correlations (i.e.,
2 Materials and methods
2.1 Participants
A total of 17 gymnasts (11 men and 6 women; age: 19.7 ± 2.2 years; height: 174.0 ± 11.3 cm; body mass: 66.9 ± 13.4 kg) participated in this study. All participants exhibited high proficiency in their respective sports, having undergone at least 5 years of dedicated professional training. None of the subjects reported any musculoskeletal or neurological disorders that could impede their involvement in the study. Before their active participation, each athlete provided verbal and written informed consent.
2.2 Experimental protocol
We examined the spatial distribution of temporal correlations in the 2D CoP trajectory across four postural conditions.
• upright stance, eyes open, before head rotation (EO-B-HR)
• upright stance, eyes open, after head rotation (EO-A-HR)
• upright stance, eyes closed (EC-B-HR)
• head rotation counterclockwise, eyes closed (EC-HR)
• upright stance, eyes close, after head rotation (EC-A-HR)
Head rotation in both eyes open and eyes closed conditions were executed counterclockwise, following a self-selected, comfortable rhythm at approximately once per second, corresponding to
2.3 Data acquisition
Participants’ ground reaction forces in the six postural conditions were captured through a stabilographic force platform featuring four high-precision force transducers. The recording and subsequent analysis of stabilographic oscillations across four channels were executed through a NI6070E interface (National Instruments, Inc., Austin, TX) software scripted in LabView. The signals were sampled at a frequency of 100 Hz, and before input, all signals underwent band-pass filtering within the range of 0.5–200 Hz.
2.4 Oriented fractal scaling component analysis (OFSCA)
Comprehensively characterizing a 2D CoP trajectory typically relies on the consideration of two independent fractional Brownian motion (fBm) sample paths corresponding to CoP along the AP and ML directions, denoted as (x(1)[i]) and (x(2)[i]), where i = 1, 2, …, N denotes the trajectory’s length. This assumption posits that these two components consistently maintain orthogonality, implying that the scaling property of each angular component, specifically the projection onto a rotated direction, remains uniform and robust to any rotational transformation. However, this presumed spatial “isotropy” may not universally apply; instead, it might be an exception rather than the norm, as there is no inherent justification to assume that all natural 2D CoP trajectories inherently exhibit isotropic behavior (Qian et al., 1998; Jin et al., 2017). We employed the oriented fractal scaling component analysis (Seleznov et al., 2020) to delve into the spatially anisotropic autocorrelation characteristics of 2D CoP planar trajectories. This analytical approach assesses the angle-dependent scaling properties of the trajectory by utilizing a higher-order directional detrending moving average (DDMA) (Tsujimoto et al., 2016) and dissects the observed 2D CoP trajectory into two distinct components, each characterized by varying orientations and scaling properties.
The OFSCA method characterizes the spatial distribution of temporal correlations within CoP fluctuations along the primary directions in which posture exhibits its extremal temporal correlations by analyzing the observed 2D CoP trajectories ϵ1 and ϵ2 at angles θ1 and θ2 relative to the horizontal reference direction (Figure 2A). It begins by estimating fractional Gaussian noise (fGn) within the observed 2D CoP trajectory, over all angles within the range 0 ≤ θ < π (Figure 2B). Subsequently, the DDMA analysis quantifies the strength of temporal correlations in these expanded trajectories at each angle. The original components indicate the directions θmax and θmin corresponding to angles between directions of the maximum and minimum values of these scaling exponents H1 and H2, respectively, from the force-plate ML axis (Figure 2C; these values consistently run orthogonal to the original orientations of the components, as shown in Figure 2D). Ultimately, the orientations of H1 and H2 allow reconstructing the actual 2D trajectory comprising ϵ1 and ϵ2, along with their corresponding directions (Figure 2E).
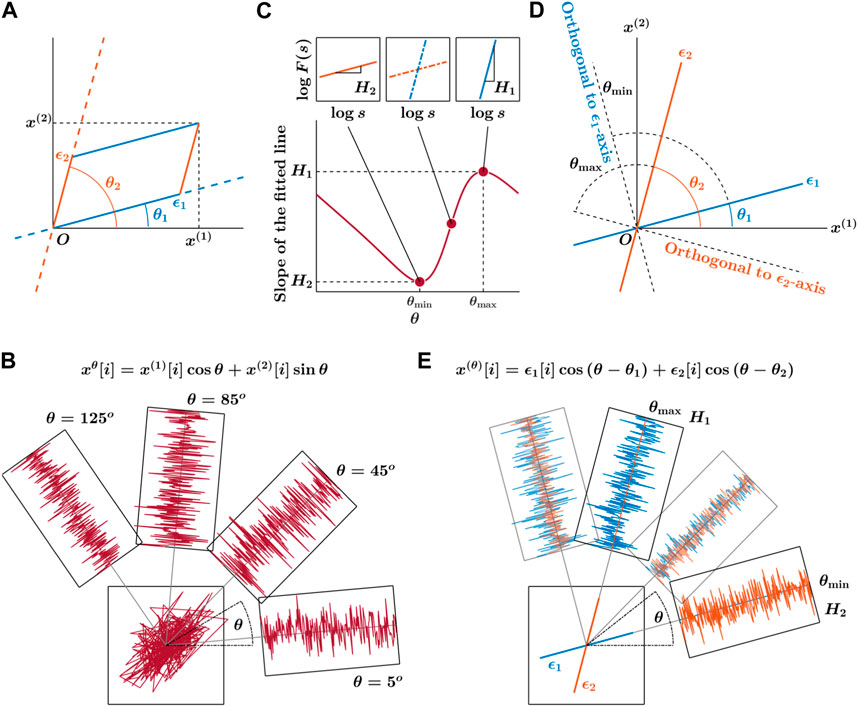
Figure 2. Primary depiction of the detection of angle-dependent temporally correlated components
To gain a comprehensive understanding of the OFSCA method, we recommend the original work by Seleznov et al. (2020). Readers seeking additional insights into applying this method in clinical populations can refer to our earlier studies on individuals with Parkinson’s disease (Mangalam et al., 2024b, a).
2.5 Statistical analysis
We submitted each postural 2D CoP trajectory to the OFSCA and computed the angle Δθ between the major and minor directions θ1 and θ2, respectively, of postural control as
3 Results
3.1 Gymnasts control posture along suborthogonal directions that deviate from the anatomical AP and ML directions
We submitted all postural CoP trajectories to the OFSCA with fourth-order DDMA. The plots depicted in Figures 3–5 represent the orientation decomposition of the 2D CoP trajectory of a representative gymnast in an upright stance, eyes closed, before head rotation (EC-B-HR), head rotation counterclockwise, eyes closed (EC-HR), and upright stance, eyes closed, after head rotation (EC-A-HR). We evaluated the angle dependence of
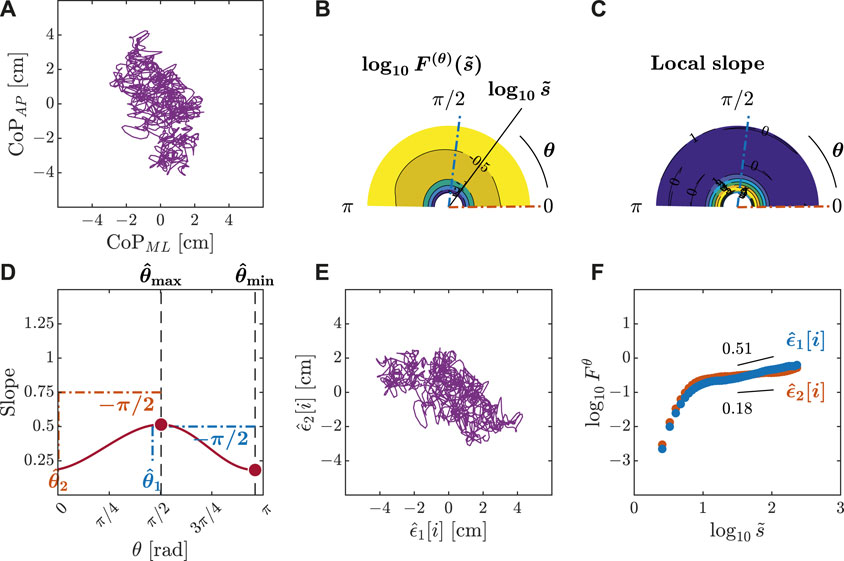
Figure 3. Orientation decomposition of the CoP trajectory of a representative gymnast with eyes closed before head rotation. (A) CoP along the anatomical AP and ML axes. (B) θ-dependent heterogeneity in CoP fluctuations, indicated by the angle dependence of
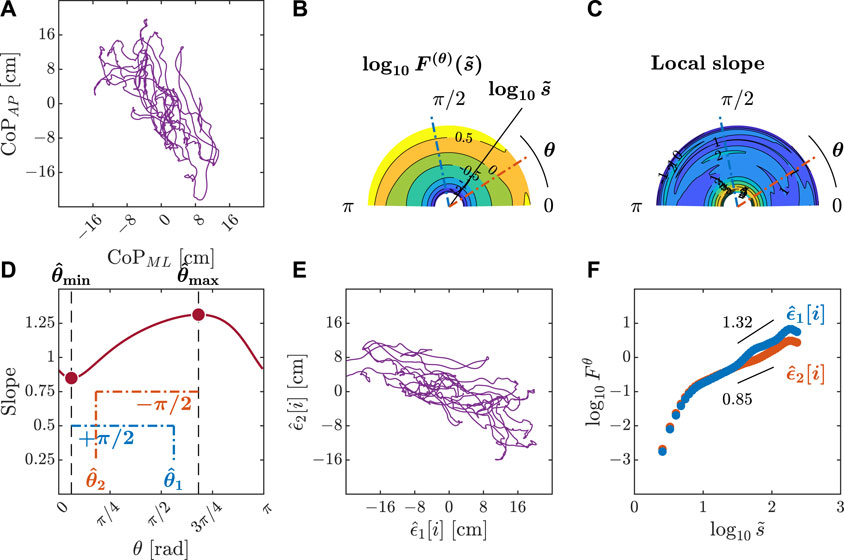
Figure 4. Orientation decomposition of the CoP trajectory of a representative gymnast with eyes closed during head rotation. (A) CoP along the anatomical AP and ML axes. (B) θ-dependent heterogeneity in CoP fluctuations, indicated by the angle dependence of
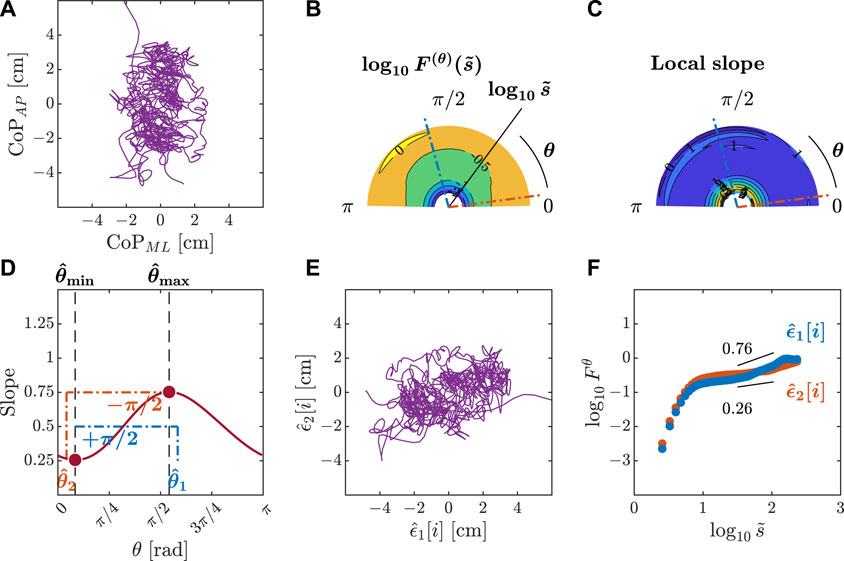
Figure 5. Orientation decomposition of the CoP trajectory of a representative gymnast with eyes closed after head rotation. (A) CoP along the anatomical AP and ML axes. (B) θ-dependent heterogeneity in CoP fluctuations, indicated by the angle dependence of
Contrary to the conventional belief attributing postural control to the anatomical AP and ML axes, the 17 gymnasts exhibited distinct maximum and minimum values of HfGn, H1 and H2, along divergent directions. In Figures 6, 7, we illustrate the major and minor directions of postural control—θ1 (purple traces) and θ2 (yellow traces)—corresponding to the maximum and minimum strengths of temporal correlations in postural CoP, H1 and H2, for each participant in each postural condition. Notably, these control directions deviate from the anatomical AP and ML axes, and the angle between them is often smaller than the 90° between the AP and ML axes. Moreover, we observed significant variability in both angles among participants and across the six postural conditions for each participant. In Figure 6, the angles θ1 and θ2 exhibit a notable alignment with the AP and ML axes before head rotation with eyes open. However, during head rotation under the same condition, these angles significantly deviate from the AP and ML axes, only to realign, albeit to a lesser extent, post-rotation with eyes open. This pattern of variability is consistently observed across all three conditions for participant P5. Examining the variability in these directional control of posture across all participants reveals a low degree of variability before head rotation with eyes open (scan the left-most column in Figure 6 from top to bottom). However, participant variability is substantially increased during head rotation, particularly evident in the deviation from the anatomical AP and ML axes (scan the second-from-the-left column in Figure 6 from top to bottom). Participant P3, for instance, demonstrates a notably high degree of obliqueness in this context. Thus, we discovered that trained gymnasts manage posture along directions divergent from the anatomical AP and ML axes, actively altering these directions based on the demands of different task contexts.
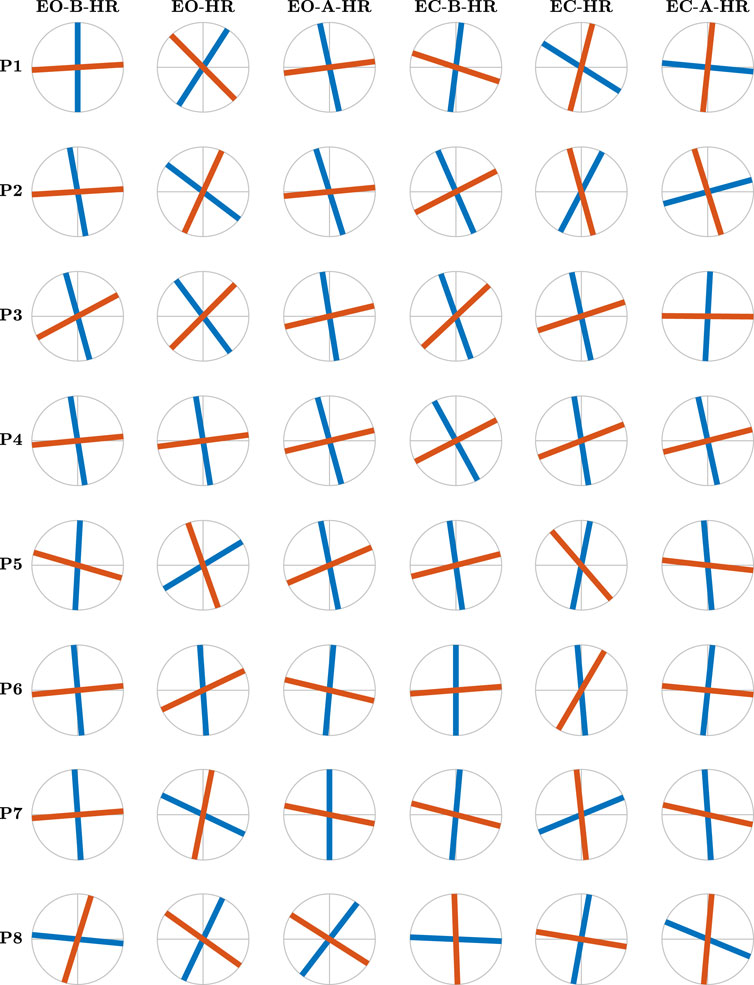
Figure 6. The major and minor directions of postural control, θ1 (blue traces) and θ2 (red traces), respectively, corresponding to the maximum and minimum strengths of temporal correlations in postural CoP, for participants 1–8 as revealed by the OFSCA. Notice that first, the two directions in which posture is controlled deviate from the anatomical AP and ML axes and that the angle between the two components is smaller than the 90o between the AP and ML axes. Continued in Figure 7.
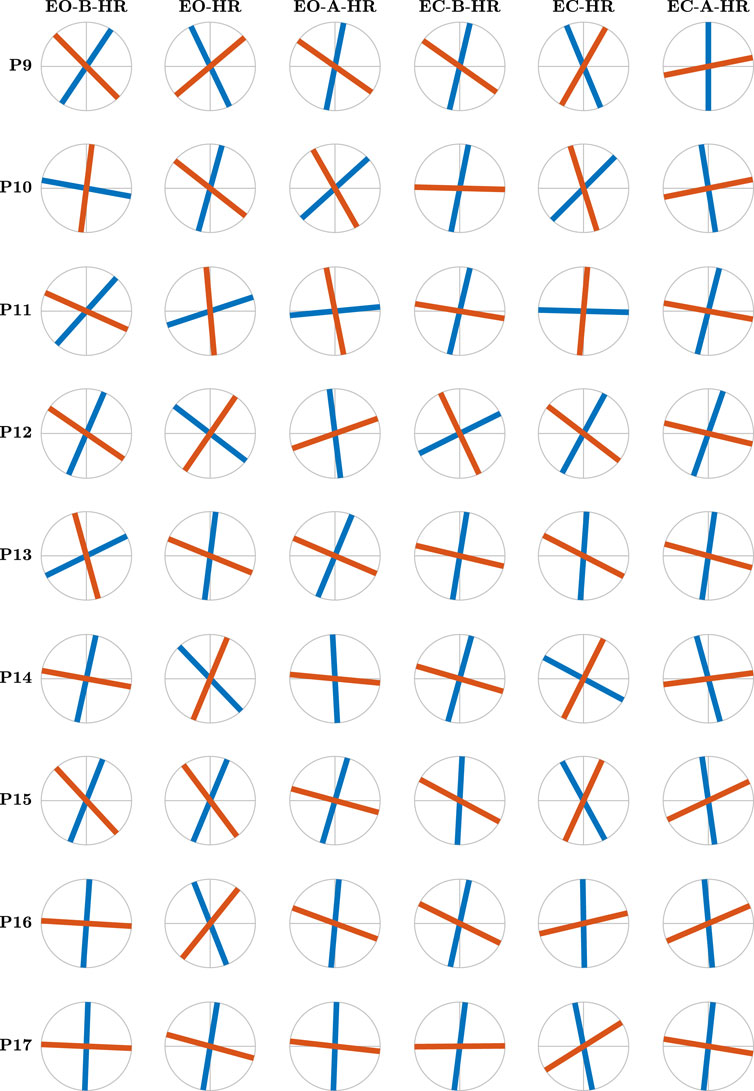
Figure 7. Figure 6 continues… The major and minor directions of postural control, θ1 (blue traces) and θ2 (red traces), respectively, corresponding to the maximum and minimum strengths of temporal correlations in postural CoP, for participants 9–17 as revealed by the OFSCA.
3.2 Vision and head rotation differently affected the directions in which posture is controlled
The linear mixed-effect modeling generated coefficients B for each covariate, signifying the average change in Δθ associated with group membership in class variable values or a unit increase in the corresponding continuous variable (Table 1). Each coefficient was accompanied by a standard error SE, representing the variation around the average change in Δθ. We ran two models, the first to model only the exogenous factors indicating experimental task setting, namely, two class variables Vision (i.e., equaling “EyesClosed” versus a baseline value of “EyesOpen”) and Postural Condition (i.e., equaling “Head rotation” or “After” both relative to “Before”) and their interaction. We omitted this factor because the interaction failed to improve model fit [χ2(2) = 2.045, p = 0.360]. Our second model added the endogenous factors SDH, H1, and H2 to test how the change from wider to narrower angle between the major and minor axes θ1 and θ2 correspond both to changes in spatial variability in axial temporal correlations (i.e., SDH) and to overall range (e.g., “max − min”) of temporal correlations. We present the estimated coefficients from the linear mixed-effect model in the format B ± SE, noting the corresponding t-statistic (equal to B/SE) and the p-value estimate based on Satterthwaite’s method.
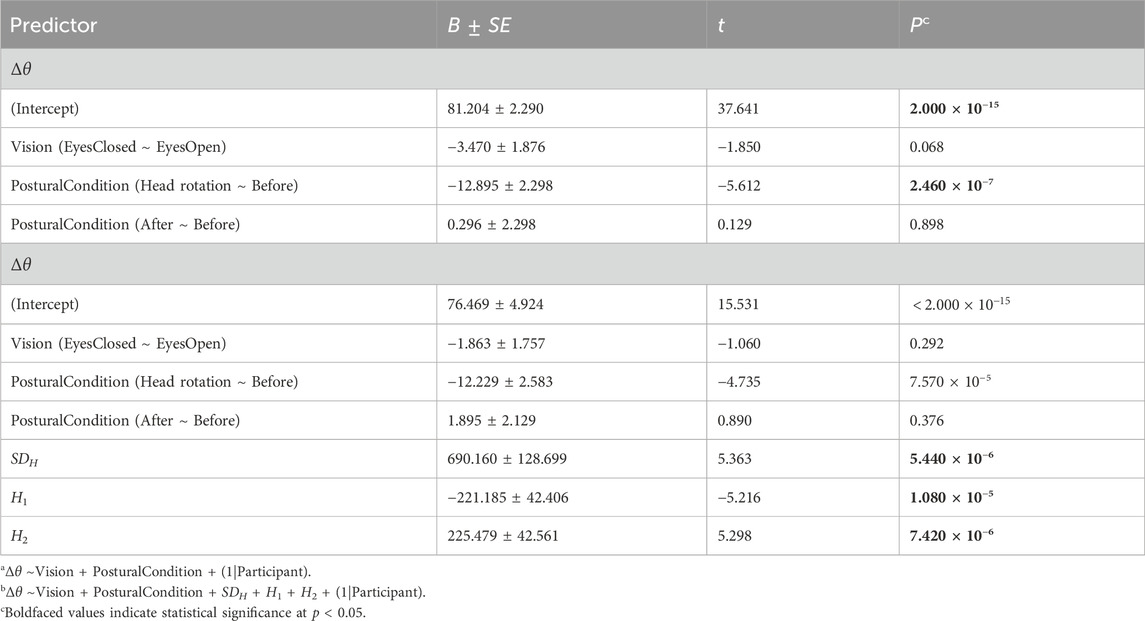
Table 1. Outcomes of the linear mixed-effects (LME) modelsa,b examining the influence of vision, postural conditions, and temporal correlations in CoP fluctuations on θ1, θ2, and Δθ.
The first model indicated that the head-turn perturbation had a much stronger effect of narrowing Δθ than closing eyes, suggesting a more substantial support for Hypothesis 1a than Hypothesis 1b. The first model returned only trends in reduction of Δθ by an average of 3.470 ± 1.876° (t = −1.850, p = 0.068; Figure 8). These values are consistent with previous findings in healthy young and older adults and individuals with Parkinson’s disease (Mangalam et al., 2024b; a). Head rotation during an upright stance showed considerably more reduction in Δθ by an average of 12.895 ± 2.298 (t = −5.612, p = 2.460 × 10−7) with respect to the baseline trial before the head turn. Nonetheless, head rotation during an upright stance did not induce any discernible adaptation in the trial following head rotation, as Δθ did not show any statistical difference with Δθ observed in the baseline condition (p > 0.05). Thus, the reduction of Δθ appears specific to the head turn and disappears as quickly as the gymnast returns to face-forward posture. This flexible rebound back to orthogonal axes thus makes the observed reduction of Δθ appear less like liability and more like an adaptive response of postural control with minimal liabilities to the orthogonality of temporal correlations in subsequent postural sway.
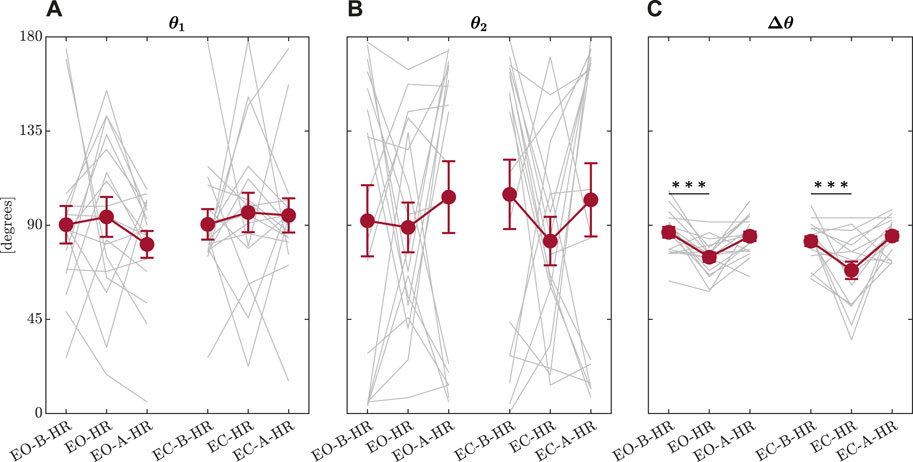
Figure 8. Summary of the output from the OFSCA. Mean values of θ1, the direction corresponding to the maximum value of temporal correlations, H1 (A), θ2, the direction corresponding to the minimum value of temporal correlations, H2 (B), and Δθ, the angle between these two directions along which the posture is controlled (C). Error bars indicate ± 1S.E.(N = 17). Light grey traces represent individual participants. *p < 0.05; ***p < 0.001.
The second model showed that incorporating information about endogenous temporal correlations dramatically improved our ability to predict the changes in Δθ [χ2(3) = 25.165, p = 1.426 × 10−5]. The effect of head-turning remained as strong as before, suggesting that the experimental manipulation was robust across many participants’ strategies of using temporal correlations to maintain postural control. Supporting the notion that orthogonality and generally greater Δθ went hand in hand with the greater variability of presumably saddle-type control (Hypothesis 2a), there was a strong positive effect of SDH on Δθ (B ± SE = 690.160 ± 128.699, t = 5.363, p = 5.440 × 10−6), suggesting that greater variability of Hurst exponent encoding the strength of temporal correlations across the angular space went hand in hand with greater Δθ. This finding helped confirm our intuition that greater angles between the major and minor axes closer to orthogonality might be associated with greater spatial variety as in saddle-type control (Hypothesis 2a). Conversely, this finding suggested that the narrower angles between the major and minor axes corresponded to more homogeneous values of H across all directions in the 2D plane. The second model indicated that the range between H1 and H2 might operate differently from the standard deviation. Specifically, although greater SDH was associated with greater Δθ, it appears that increasing H1 would decrease Δθ (B ± SE = −221.185 ± 42.406°, t = −5.216, p = 1.180 × 10−5) and increasing H2 would increase Δθ (B ± SE = 225.479 ± 42.561°, t = 5.298, p = 7.420 × 10−6). This evidence partially undermines our Hypothesis 2b that increasing the range of H would correspond to an increase of Δθ: independently increasing either H1 or H2 while keeping the other constant would increase or decrease the range, respectively, but co-occur with smaller or larger Δθ, also respectively. In short, although greater SDH co-occurs with larger Δθ, greater range co-occurs with smaller Δθ. So, the effect of the extremal temporal correlations appears to operate in the opposite direction from the SD of temporal correlations. If we imagined a bell-shaped curve describing the distribution of angular temporal correlations, greater Δθ corresponds to a fatter bell-shaped distribution with shorter tails. Conversely, smaller Δθ corresponds to a thinner bell-shaped distribution with longer tails. Postural control appears to balance its spatial distribution of fractal temporal correlations between extremes and average variability.
4 Discussion
The present study tested two hypotheses about the nuanced spatial distribution of temporal correlations in CoP fluctuations among trained gymnasts. First, we predicted in Hypothesis 1a and 1b that head-turning and closing eyes would narrow the angle between the axes of the strongest and weakest temporal correlations in 2D CoP. Second, we predicted that greater Δθ corresponding to the more orthogonal orientation of axes with the strongest and weakest temporal correlation would correspond to endogenous variability in temporal correlations—both in terms of the standard deviation of Hurst exponents H, encoded by SDH (Hypothesis 2a) and in terms of the range of H, encoded by H1 and H2 (Hypothesis 2b). Results supported Hypothesis 1a but not Hypothesis 1b, and they supported Hypothesis 2a but not 2b. Regarding the first hypothesis, head-turning led to a concurrent reduction of the Δθ that showed no signs of lingering once gymnasts returned their heads to a facing-forward position, but vision showed only a negative trend, indicating a change of Δθ in negative direction but not a statistically reliable one. Hence, narrowing Δθ might be an adaptive postural-control strategy that respects saddle-type postural synergy until a perturbation prompts the intermittent release of this synergy, giving way to a spiral-type mode. The rapid rebound of Δθ with a return to face-forward posture suggests that the temporary reduction is specific to head-turning and not an ongoing burden to postural control.
We propose that the postural system’s capacity for brief, task-sensitive reductions in Δθ may extend the body of fractal temporal-correlational evidence of intermittent postural control. In particular, we propose that the intermittent adoption of orthogonal and suborthogonal axes of extremal fractal temporal correlation might manifest the adoption of saddle-type and spiral-type CoP control, respectively. This understanding elaborates prior understandings that fractal temporal correlations reflect the coordination of corrections to sway across time (Blaszczyk and Klonowski, 2001; Amoud et al., 2007; Kuznetsov et al., 2013). Narrowing the angle between major and minor axes corresponds to fractal temporal correlations spread more homogeneously across angular directions. This 2D homogeneity reflects an opening of postural control to coordinate information across various potential task orientations. Conversely, increasing the angle between the two components of temporal correlations in CoP fluctuations might indicate a postural synergy collapsing the potential two-dimensionality of CoP into a 1D axis of relatively stronger temporal correlations. This may signify a more efficient use of sensorimotor integration for postural control canalized into a task-relevant orientation (e.g., Balasubramaniam et al., 2000). Subsequent investigations could test this hypothesis more explicitly using manipulations demanding releasing bodily degrees of freedom. Future work could also model the spatial distribution of temporal correlations from a holistic coordination perspective (Latash et al., 2007; Latash, 2012; Latash, 2024; Mangalam et al., 2020a; Mangalam et al., 2020b), considering the intricacies of individuals’ musculoskeletal features, training regimens, and even within the cohort of injured gymnasts.
The strength of the head-turning relative to closing eyes contrasts starkly with traditional attributions of minor role to vestibular rather than proprioceptive and visual information (Fitzpatrick and McCloskey, 1994; Peterka and Benolken, 1995; Ivanenko et al., 1999; Horlings et al., 2009; Wiesmeier et al., 2015). However, vestibular input plays a significant role in tonically activating anti-gravitative muscles through the lateral and medial vestibulospinal tracts, in conjunction with reticulospinal pathways (Fetter et al., 1991; Wilson, 2013). Unilateral suppression of vestibular processing by the postural syndrome observed in various animal species following unilateral vestibular system suppression (Lacour and Borel, 1993; Dieringer, 1995) and in humans as well (Curthoys and Halmagyi, 1995; Lacour et al., 1997; Redfern et al., 2004; Cousins et al., 2013). In the present case of gymnasts, angular nuances in CoP available from OFSCA might reflect sport-specific sensorimotor adaptations, encompassing lower extremity strength/power, proprioceptive acuity, and integrating vestibular information (e.g., Glass and Ross, 2021). The adequacy of sensory information crucial for maintaining balance and postural stability relies heavily on head stability during body movement and suprapostural tasks (Kelty-Stephen et al., 2021b; Mangalam et al., 2021). The so-called “Head-Stabilization Strategy (HSS)” is posited to mitigate potential ambiguities in interpreting sensory inputs for balance, primarily relying on a geocentric sensorimotor frame of reference (orientation to the vertical). However, incorporating egocentric (head orientation with respect to the body) or exocentric (orientation to environmental objects) frames of reference refines head stabilization (Lacour et al., 1997; Cullen, 2012). Individuals naturally leaning on the HSS-type geocentric frame of reference may diminish the angle between the two components due to uncertainties in interpreting vestibular information from head rotation. This entails proprioceptive reintegration by extending bodily degrees of freedom and leaning more into the 2D spiral-type distribution of more uniform temporal correlations across the 2D support surface.
Of course, within this topological question of saddle vs. spiral, the differences among angle or CoP or AP are less material than the implication of H as a control parameter on phase-plane axes. We intend that replacement explicitly as a substantive way to test the long-discussed proposal that fractal temporal correlations have something to do with control—with our term “something” intentionally vague to reflect the ambiguity of these proposals (Doyle et al., 2004; Pascolo et al., 2006; Amoud et al., 2007; Lin et al., 2008; Kirchner et al., 2012; Munafo et al., 2016; Ducharme and van Emmerik, 2018; Gilfriche et al., 2018; Quijoux et al., 2021). It is intriguing that Asai et al. (2009) offers control models to explain the proposed outcome of fractal temporal correlations in postural observables, assuming simplicity that fractal temporal correlations are sooner the product of the real underlying control parameters. However, just as topology is flexible regarding the specific labeling of axes, modeling is just a logical correspondence from premise to outcome. Without an experimental manipulation of the proposed outcome, the topological models generating fractal patterning from an angular position do not yet prohibit the converse possibility that fractal patterning has a non-zero causal status in influencing the angular change. We suspect that the fractal scaling variation may be a control parameter sooner. Hence, we intend the novel step of reversing the role of fractality from outcome to potential control parameter and, so, of testing the capacity of the saddle and spiral topologies to hold on a phase plane written in terms of H on the two dimensions of the force platform.
Given all of this interchangeability of parameters, it was for good reason that Weinberg coined his Third Law of Progress in Theoretical Physics: “You may use any degrees of freedom you like to describe a physical system, but if you use the wrong ones, you’ll be sorry” (Weinberg, 1983, p. 16). The reason for OFSCA is that the axes implicit in the observables forming the vector basis of the measurement could be wrong degrees of freedom, and flows within those bases might follow their intrinsic dimensionality. The power of the OFSCA method may be that it can take a fixed coordinate system like the 2D force platform, and it can then model the phase of anisotropic flow within that 2D coordinate system without using degrees of freedom with the wrong angular orientation to characterize that flow. H may be a suitable degree of freedom to embody control. Indeed, though we do not presume to manipulate H in this study, if H is a suitable candidate for postural control parameters, we should expect that H should embody the same abstract topological structures invoked in modern control theory. Any failure, at least, to find traces of known topology associated with intermittent control should hopefully be a strong point of evidence against rhetoric around fractal-themed control.
There is much yet to learn about the application of OFSCA to understanding postural control dynamics. At the very least, in the short term, we can say that the conventional premise, attributing postural control to the anatomical AP and ML axes, is incomplete. Unlike traditional stabilographic analysis, the OFSCA method unravels a potential disparity between this portrayed and actual control dynamics. In the longer term, we see a potential for OFSCA to inform theories of intermittent control using feedback and instability to reset postural synergies. Individuals adeptly tailor their posture control to align with task demands and anatomical constraints, exhibiting control in oblique directions to the AP and ML axes, complete with suborthogonal angles between the controlled axes. Furthermore, this spatial anisotropy in temporal correlations in CoP fluctuations diverges across individuals, potentially encapsulating variations in muscular strength, neurological control, and task-specific constraints. In contrast, the conventional orthogonal decomposition of the CoP trajectory adheres to a generic concept, lacking the discriminative power to distinguish among individuals in these critical dimensions. Closing the eyes diminished the angle between these two orientations, implying an amplified dependence on proprioception for postural control, effectively releasing bodily degrees of freedom across the 2D support surface. Subsequent head rotations accentuated this angular reduction, signifying a heightened liberation of bodily movement degrees of freedom. This process facilitates the integration of vestibular information with proprioceptive input, enhancing the overall control of posture. In contrast, head rotations failed to induce any discernible adaptation, with the posture swiftly reverting to its normal state within a single trial. This nuanced exploration provides valuable insights into the adaptive mechanisms gymnasts use to navigate sensory inputs during postural control.
Interestingly, the relationship between the standard deviation of temporal correlations (SDH) across the 2D support surface and the range of temporal correlations (Δθ) is inversely proportional: larger SDH coincides with larger Δθ, while greater range is associated with smaller Δθ. This suggests that extremal temporal correlations operate in the opposite direction from the overall standard deviation of temporal correlations. Visualizing this relationship as a bell-shaped curve, larger Δθ corresponds to a broader distribution with shorter tails, while lower Δθ corresponds to a narrower distribution with longer tails. Postural control maintains a balance in the spatial distribution of fractal temporal correlations, ranging between extremes and average variability. Future studies could delve deeper into this phenomenon, exploring its nuances in healthy adults, aging and diseased populations, and specifically trained groups such as gymnasts to provide a comprehensive understanding of the underlying mechanisms across diverse demographics.
The present treatment of gymnasts’ postural control dynamics offers a valuable gold standard for understanding what the OFSCA results might look like in one of the most dexterous populations. The targeted response of Δθ to the head-turn and the prompt rebound in trained gymnasts makes the spiral-type angular homogeneity seem like an adaptive strategy. This case helps to clarify an earlier ambiguity as to how we might extend this OFSCA modeling strategy to understand the pathologies of motor control. For instance, Parkinson’s disease contributes to deficits in postural control, potentially disrupting directional stability and heightening susceptibility to falls (Bloem et al., 2001; Grimbergen et al., 2004). In comparing healthy adults and adults with Parkinson’s disease, we found that task-settings-inducing instability prompted the narrowing of Δθ in healthy populations (Mangalam et al., 2024b). This finding aligns neatly with the present findings in trained gymnasts. We also found that even healthy older adults showed age-related and task-dependent narrowing. However, people with Parkinson’s showed narrowing of Δθ that was less sensitive to task setting and more sensitive to their diagnosis. The present work represents our attempt to understand how gymnasts’ task-sensitive narrowing of Δθ reflects known themes in the intermittent postural control strategy. This alignment of narrower and wider Δθ with spiral-type and saddle-type control strategies offers the novel understanding that postural control with Parkinson’s disease may use the spiral-type strategy in excess—just as our head-turn manipulation signifies the impact of vestibular perturbation, it may be that the narrowing of Δθ with age is a function of vestibular degeneration (Iwasaki and Yamasoba, 2015; Anson and Jeka, 2016) contributing to fall risk in the elderly (Liston et al., 2014). This minimal vestibular degeneration leaves intact the capacity for the postural control to use spiral-type release of postural degrees of freedom as a temporary response to perturbation. Adults with Parkinson’s may constitute an extreme case of spiral-type control, not necessarily from vestibular degeneration but from multifarious deficits in sensory integration rather than task-sensitive adaptation. Their more drastic and less task-sensitive reduction of Δθ may thus reflect postural control that has lost the capacity to collapse degrees of freedom into synergies aligning with saddle-type modes. Hence, OFSCA offers an unprecedented capacity to dissociate multiple forms of change in fractal scaling, at once vindicating but crucially elaborating upon more straightforward notions of fractal temporal correlations as somehow crucial to health (Goldberger et al., 2002; Lipsitz, 2002; Torre et al., 2019). OFSCA opens up the possibility that more nuanced modeling of physiological anisotropy might make it possible to dissociate task-related changes in fractal temporal correlations from deficits due to more longitudinal or clinical concerns.
That being said, the present manuscript is not without its limitations. We have yet to ascertain the consistency of OFSCA results for an individual across repeated trials and whether differences in control patterns persist upon repeated testing, considering that subtle changes in task constraints could induce variation in control strategies. Furthermore, it remains to be investigated whether spatial variability in fractal temporal correlations can predict critical aspects of postural control, such as the ability to respond to unpredictable perturbations or resilience to falls during more dynamic scenarios such as walking. Expanding the application of OFSCA results to predict clinical performance measures would offer a novel and valuable contribution to our understanding of spatial variation in these fractal patterns. Spatial variation in fractal temporal structure has previously been linked to intermittent control strategies that regulate posture in healthy adults. Changes in this intermittency have been shown to elucidate posture in healthy older adults and older adults with Parkinson’s disease (Mangalam et al., 2024b), suggesting the potential of these findings to inform interventions to enhance postural stability across diverse populations. Future research involving high-performance athletes on one end of the spectrum and clinical populations on the other might provide complementary insights to establish the OFSCA results on a firmer theoretical foundation and elucidate the causal significance of fractal temporal correlations as a control parameter in postural adaptations.
On less theoretical grounds, an inherent limitation of our study is using only one trial per condition to assess postural control. Typically, postural control data are gathered across multiple trials to ensure reliability and consistency and to mitigate the impact of outlier trials. By employing only one trial per condition, the variability within each condition may not be fully captured, potentially limiting the generalizability of our findings. Furthermore, the duration of each trial, set at 20 s, may be considered relatively brief in the context of quiet-standing research. Extended trial durations are often preferred to stabilize postural adjustments and better capture nuanced changes in postural control over time. The short duration of our trials may restrict the depth of insight into participants’ postural control abilities and could affect the robustness of our conclusions. Future research endeavors should consider employing multiple trials per condition and extending the duration of each trial to enhance the comprehensiveness and reliability of postural control assessments.
Effective postural control amid intricate gymnastic tasks necessitates precise sensory integration and somaesthetic reweighting, as a failure in these can significantly heighten the risk of injury. The present study illuminates an unrecognized facet of gymnasts’ postural performance—spatial anisotropy in temporal correlations in CoP fluctuations, signifying postural control extending beyond the conventional anatomical AP and ML axes (Janusz et al., 2016). Anisotropy escapes most traditional portrayals of postural control following practical considerations like the output from force platforms, anatomical constraints, and inverted-pendulum modeling (Rocchi et al., 2004). The innovative OFSCA method affords empirical traction on postural control mechanisms long discussed in the literature Asai et al. (2009) but beyond the scope of these traditional portrayals. The OFSCA departure into modeling anisotropy explicitly has substantial implications, particularly in the realm of proprioceptive reintegration—a crucial aspect of sensorimotor integration that distinguishes gymnasts from their non-gymnast counterparts (Debu and Woollacott, 1988; Vuillerme et al., 2001a; Vuillerme and Nougier, 2004; Calavalle et al., 2008; Gautier et al., 2008; Busquets et al., 2021; Zemková and Kováčiková, 2023).
Potential physiological explanations for these findings may involve localized tissues or specific subsystems. Notably, heightened activity within the sympathetic nervous system has been associated with increased covariation among diverse physiological systems (Garcia et al., 2011). The robust connections between the visual and vestibular systems (Abekawa et al., 2018; Rühl et al., 2018) may elucidate these observed effects. However, the apparent simplicity of these explanations is quickly complicated by qualifications, interactions, and contextual sensitivities. For instance, the interactions between the visual and vestibular systems must be qualified by factors such as aging (Lui et al., 2019), migraines (Bednarczuk et al., 2019), neuritis (Roberts et al., 2018), and their interactions with somatosensation (Moro and Harris, 2018). Then again, what is specific needs not necessarily only to be what is local. These findings may be no less specific to physiological tissues that spread globally through the body and whose function is to mediate among various components at various scales. The present results are consonant with and supportive of explanations of perception-action that rest on the multiscaled “tensegrity”-like dynamics of connective-tissue networks that span the entire body (Turvey and Fonseca, 2014; Levin et al., 2017; Scarr et al., 2024). Here, the physiological substrates afforded by the fascia embody a complex geometrical relationship that balances various muscular, skeletal, and neural tissues. This specific anatomical relationship leverages abstract geometrical balances between tension and compression that proceed downward into the finest scales of cytoskeletal dynamics supporting cell motility (Ingber, 2006; Ingber, 2008) and upward to the larger scales of intentional organism coordination of behavior (Mangalam et al., 2020a; Mangalam et al., 2020b; Profeta and Turvey, 2018; Turvey and Fonseca, 2014; Van Orden et al., 2012). The nesting of tension-compression balances proceeds symmetrically across scales deformed only by anatomical and metabolic constraints. The result is a multifractal geometry reflecting a rich family of scale-invariant processes reflecting a common ancestry in the cascading dynamics of nonlinear cross-scale interactions. Indeed, nonlinear cross-scale interactions have become a major highlight of modern explanations for how the nervous system can provide such adaptive, context-sensitive support (Morgane et al., 2005; Thayer, 2006; Suckling et al., 2008; Changizi and Destefano, 2010; Deco et al., 2011; Damm et al., 2020; Stylianou et al., 2021).
The vast reach of connective tissue networks thwarts the simple reduction of our phenomena to local components. However, the support for such context-sensitive behavior is yet physiological. Support for the role of tensegrity-like connective tissue in perception and action appears through “ultrafast” adaptive responses unfolding faster than neural transmission could support, appearing within cells belonging to multicellular organisms (Ingber, 2006; Ingber, 2008), single-celled organisms (Grebecka et al., 1999), small organisms just aiming to stay upright (Frantsevich and Gorb, 2002; Endlein and Federle, 2013), as well as humans standing (Marsden et al. 1983), locomoting (Moritz and Farley, 2005; Kiely and Collins, 2016), and using language (Kelso et al., 1984; Moreno et al., 2011). Complicating matters for the physiologist aiming to reduce function to anatomical components, tensegrity may be more generic than the idiosyncrasies of individual model-organism anatomy. Although we can point here to likely vestibular contributions, various previously named model systems exhibit sensitivity to mechanical rotation without having any vestibular system (Brunet, 1951; Svidersky and Plotnikova, 2002; Dilão and Hauser, 2013). And that is not to say that everything can be a tensegrity system. On the contrary, tensegrity-based approaches to physiology offer the specific guarantee that the connective tissues serve to regulate a peculiar sort of geometry called “multifractal” (Turvey and Fonseca, 2014). The tensegrity mechanism’s specificity increases in abstract geometrical properties sooner than in anatomical materials. The physiological diversity offers many “anchors” that can enact generic geometrically invariant “templates” (Holmes et al., 2006).
Thus, the present work informs what Turvey and Fonseca (2014) called the “multifractal tensegrity” (MFT) hypothesis. The MFT hypothesis articulated two crucial points in light of the foregoing observations. First, it retrospectively proposed that the widespread evidence of multifractal fluctuations in physiological systems have inevitably entailed cascading tensegrity-like dynamics; this point was not exclusive to the MFT hypothesis but followed upon prior proposals that multifractal physiological fluctuations reflected cascade dynamics (Ivanov et al., 2001; West, 2006). The more forward-looking second point of the MFT hypothesis was that the empirically estimable multifractal profile of bodily coordination might provide an empirical means for modeling the control of dexterous action. This latter point has received extensive support through the correlational study of postural, suprapostural, perceptual, and cognitive performance (Bell et al., 2019; Booth et al., 2018; Carver et al., 2017; Dixon and Kelty-Stephen, 2012; Hajnal et al., 2018; Kelty-Stephen and Dixon, 2014; Kelty-Stephen et al., 2021b; Mangalam et al., 2020a; Mangalam et al., 2020b; Mangalam and Kelty-Stephen, 2021a; Mangalam et al., 2021; Stephen et al., 2012a; Teng et al., 2016) as well as through experimental tests of multifractal stimulation on some of the same performance measures (Stephen and Dixon, 2011; Ward and Kelty-Stephen, 2018; Bloomfield et al., 2021; Kelty-Stephen et al., 2023).
This prior work has shown a systematic relationship between prior multifractal stimulation or fluctuation and subsequent performance measures. There has thus been a major intermediary blank in the modeling where we might have only hoped that multifractality bore upon the topology of control strategies. The present work aims to bolster support for this second part of the MFT hypothesis by filling in that blank. Specifically, this work suggests that multifractality is not merely an epiphenomenon of some fundamentally non-multifractal control strategies but could be part and parcel of control itself. The present work provides initial positive evidence about the plausibility that multifractal parameters could themselves serve as control parameters governing topologies for postural control. It may be particularly compelling that we have not presumed any novel topologies for postural control here. It only invoked pre-existing topologies that had once been proposed to produce a multifractal structure. Postural control might reflect the interplay of yet-unfamiliar multifractal flows through well-established topological constraints.
The present proposal is that multifractal tensegrity acts on known topologies of postural control, and it calls for new elaborations of theoretical discourse. On the one hand, these results give new force to a long history of promises that fractal and multifractal fluctuations might support or govern postural control. These prior promises rested on circumstantial evidence of differences in fractal or multifractal outcomes between diagnosis groups or tasks (Lipsitz et al., 1990; Lipsitz and Goldberger, 1992; Duarte and Zatsiorsky, 2000; Blaszczyk and Klonowski, 2001; Lipsitz, 2002; Amoud et al., 2007; Kuznetsov et al., 2013). Although these works appeal to a role for endogenous stochastic processes exerting scale invariant form to postural corrections, perhaps from finding no better alternative at hand, they found this role for control in the exogenous dimensional frame of the orthogonal AP and ML axes of the force plate. And for all this commitment to the exogenous force-plate dimensionality, these proposals never made clear contact with the topologies that contemporary models of control have founded in force-plated dimensions and used to reduce fractality to inverted-pendulum dynamics (Bottaro et al., 2005; 2008; Asai et al., 2009; Asai et al., 2013; Ahn and Hogan, 2012; Nomura et al., 2013; Nakamura et al., 2021). On the other hand, the present work substantiates the rhetoric about fractal-based control by offering a more concrete foundation in the same topologies the inverted-pendulum models invoke. The major changes we propose here are twofold: first, thanks to the OFSCA modeling, we have identified the control topologies in a dimensional frame endogenous to the body rather than in the exogenous force-plate dimensions, and second, multifractality (i.e., literally, the variability in fractality) is the control-parametric domain on which the topology is defined rather than an interesting by-product.
On the other hand, the present work raises new questions about the tuning of control. A question for future research may be how multifractality enters the control process. For instance, various theories of postural control currently debate the relatively continuous or intermittent form of control (Lipsitz et al., 1990; Lipsitz and Goldberger, 1992; Duarte and Zatsiorsky, 2000; Blaszczyk and Klonowski, 2001; Lipsitz, 2002; Amoud et al., 2007; Kuznetsov et al., 2013). However, the longstanding premise for many of these theories is that the nervous system uses some form of feedback to enact control relative to an internal model. For instance, the longstanding appeal to internal models in motor control has invoked a drama played out between a representation of current bodily position and “efference copies” that encode intended changes to that position (Mittelstaedt, 1998; Kuo, 2005; Maurer et al., 2006). This drama implies that the nervous system explicitly tunes control toward correcting the error, narrowing the distance between the current and the desired posture. An important future question in continuation of the present work is thus whether our implication of multifractality as a control parameter explicitly means that the central nervous system “sets,” “monitors” and “regulates” multifractality. We suspect that answer is “no” for at least two reasons: the ergodicity-breaking behavior of biological activity thwarts representativity of the entire behavior by any single or sequence of observations (Stephen and Dixon, 2011; Mangalam and Kelty-Stephen, 2021b; Kelty-Stephen and Mangalam, 2023; Mangalam et al., 2023), and the multifractality we find in human movement control reflects both responsivities at multiple time scales and nonlinear convolution of responsivities across those scales (Mandelbrot, 1982; Stephen and Dixon, 2011; Lovejoy and Schertzer, 2018; Kelty-Stephen and Mangalam, 2022), ensuring stochastic casual support rather than the simple deterministic cause. We expect that the multifractality appearing in postural control is the consequence of multiplicative cascades (Furmanek et al., 2021; Kelty-Stephen, 2018; Kelty-Stephen et al., 2021b; Kelty-Stephen et al., 2021a; Mangalam and Kelty-Stephen, 2021a; Mangalam et al., 2021). We also expect that control topologies are sooner the emergent compromise between endogenous multifractal-dimensional cascade dynamics and the exogenous dimensionality of the force plate’s task constraints (Jacobson et al., 2021). However, these points will require more explicit tests than current evidence provides.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB of the National University of Ukraine on Physical Education and Sport. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MM: Conceptualization, Formal Analysis, Software, Visualization, Writing–original draft, Writing–review and editing. IS: Conceptualization, Formal Analysis, Software, Validation, Writing–original draft, Writing–review and editing. EK: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. AP: Conceptualization, Validation, Writing–original draft, Writing–review and editing. DK-S: Formal Analysis, Writing–original draft, Writing–review and editing. KK: Conceptualization, Funding acquisition, Project administration, Software, Validation, Writing–original draft, Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. The Japan Society for the Promotion of Science (# 22F22376) supported IS. MM was supported by the Center of Research in Human Movement Variability of the University of Nebraska at Omaha and the National Institute of General Medical Sciences (# P20GM109090). KK was supported by the Japan Agency for Medical Research and Development (# 20hk0102071h0001) and the Japan Society for the Promotion of Science (# 17K09533, 21K08140, and 26461094).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abekawa, N., Ferrè, E. R., Gallagher, M., Gomi, H., and Haggard, P. (2018). Disentangling the visual, motor and representational effects of vestibular input. Cortex 104, 46–57. doi:10.1016/j.cortex.2018.04.003
Ahn, J., and Hogan, N. (2012). A simple state-determined model reproduces entrainment and phase-locking of human walking. PloS One 7, e47963. doi:10.1371/journal.pone.0047963
Amoud, H., Abadi, M., Hewson, D. J., Michel-Pellegrino, V., Doussot, M., and Duchêne, J. (2007). Fractal time series analysis of postural stability in elderly and control subjects. J. Neuroengineering Rehabilitation 4, 12. doi:10.1186/1743-0003-4-12
Anson, E., and Jeka, J. (2016). Perspectives on aging vestibular function. Front. Neurology 6, 269. doi:10.3389/fneur.2015.00269
Asai, Y., Tasaka, Y., Nomura, K., Nomura, T., Casadio, M., and Morasso, P. (2009). A model of postural control in quiet standing: robust compensation of delay-induced instability using intermittent activation of feedback control. PLoS One 4, e6169. doi:10.1371/journal.pone.0006169
Asai, Y., Tateyama, S., and Nomura, T. (2013). Learning an intermittent control strategy for postural balancing using an EMG-based human-computer interface. PLoS One 8, e62956. doi:10.1371/journal.pone.0062956
Asseman, F., Caron, O., and Crémieux, J. (2004). Is there a transfer of postural ability from specific to unspecific postures in elite gymnasts? Neurosci. Lett. 358, 83–86. doi:10.1016/j.neulet.2003.12.102
Asseman, F. B., Caron, O., and Crémieux, J. (2008). Are there specific conditions for which expertise in gymnastics could have an effect on postural control and performance? Gait Posture 27, 76–81. doi:10.1016/j.gaitpost.2007.01.004
Bak, P., Tang, C., and Wiesenfeld, K. (1987). Self-organized criticality: an explanation of the 1/f noise. Phys. Rev. Lett. 59, 381–384. doi:10.1103/PhysRevLett.59.381
Bak, P., Tang, C., and Wiesenfeld, K. (1988). Self-organized criticality. Phys. Rev. A 38, 364–374. doi:10.1103/PhysRevA.38.364
Balasubramaniam, R., Riley, M. A., and Turvey, M. (2000). Specificity of postural sway to the demands of a precision task. Gait Posture 11, 12–24. doi:10.1016/S0966-6362(99)00051-X
Bartsch, R. P., Liu, K. K., Bashan, A., and Ivanov, P. C. (2015). Network physiology: how organ systems dynamically interact. PloS One 10, e0142143. doi:10.1371/journal.pone.0142143
Bashan, A., Bartsch, R. P., Kantelhardt, J. W., Havlin, S., and Ivanov, P. C. (2012). Network physiology reveals relations between network topology and physiological function. Nat. Commun. 3, 702. doi:10.1038/ncomms1705
Bednarczuk, N. F., Bonsu, A., Ortega, M. C., Fluri, A.-S., Chan, J., Rust, H., et al. (2019). Abnormal visuo-vestibular interactions in vestibular migraine: a cross sectional study. Brain 142, 606–616. doi:10.1093/brain/awy355
Bell, C. A., Carver, N. S., Zbaracki, J. A., and Kelty-Stephen, D. G. (2019). Non-linear amplification of variability through interaction across scales supports greater accuracy in manual aiming: evidence from a multifractal analysis with comparisons to linear surrogates in the fitts task. Front. Physiology 10, 998. doi:10.3389/fphys.2019.00998
Błaszczyk, J., Beck, M., and Sadowska, D. (2014). Assessment of postural stability in young healthy subjects based on directional features of posturographic data: vision and gender effects. Acta Neurobiol. Exp. 74, 433–442. doi:10.55782/ane-2014-2006
Blaszczyk, J. W., and Klonowski, W. (2001). Postural stability and fractal dynamics. Acta Neurobiol. Exp. 61, 105–112. doi:10.55782/ane-2001-1390
Bloem, B. R., Grimbergen, Y. A., Cramer, M., Willemsen, M., and Zwinderman, A. H. (2001). Prospective assessment of falls in Parkinson’s disease. J. Neurology 248, 950–958. doi:10.1007/s004150170047
Bloomfield, L., Lane, E., Mangalam, M., and Kelty-Stephen, D. G. (2021). Perceiving and remembering speech depend on multifractal nonlinearity in movements producing and exploring speech. J. R. Soc. Interface 18, 20210272. doi:10.1098/rsif.2021.0272
Bogdan, P. (2019). Taming the unknown unknowns in complex systems: challenges and opportunities for modeling, analysis and control of complex (biological) collectives. Front. Physiology 10, 1452. doi:10.3389/fphys.2019.01452
Bonachela, J. A., De Franciscis, S., Torres, J. J., and Munoz, M. A. (2010). Self-organization without conservation: are neuronal avalanches generically critical? J. Stat. Mech. Theory Exp. 2010, P02015. doi:10.1088/1742-5468/2010/02/P02015
Bonachela, J. A., and Munoz, M. A. (2009). Self-organization without conservation: true or just apparent scale-invariance? J. Stat. Mech. Theory Exp. 2009, P09009. doi:10.1088/1742-5468/2009/09/P09009
Booth, C. R., Brown, H. L., Eason, E. G., Wallot, S., and Kelty-Stephen, D. G. (2018). Expectations on hierarchical scales of discourse: multifractality predicts both short-and long-range effects of violating gender expectations in text reading. Discourse Process. 55, 12–30. doi:10.1080/0163853X.2016.1197811
Borzucka, D., Krȩcisz, K., Rektor, Z., and Kuczyński, M. (2020). Differences in static postural control between top level male volleyball players and non-athletes. Sci. Rep. 10, 19334. doi:10.1038/s41598-020-76390-x
Bottaro, A., Casadio, M., Morasso, P. G., and Sanguineti, V. (2005). Body sway during quiet standing: is it the residual chattering of an intermittent stabilization process? Hum. Mov. Sci. 24, 588–615. doi:10.1016/j.humov.2005.07.006
Bottaro, A., Yasutake, Y., Nomura, T., Casadio, M., and Morasso, P. (2008). Bounded stability of the quiet standing posture: an intermittent control model. Hum. Mov. Sci. 27, 473–495. doi:10.1016/j.humov.2007.11.005
Brunet, P. (1951). The formation of the ootheca by Periplaneta americana: I. The micro-anatomy and histology of the posterior part of the abdomen. J. Cell Sci. 3, 113–127. doi:10.1242/jcs.s3-92.18.113
Burdet, C., and Rougier, P. (2007). Analysis of center-of-pressure data during unipedal and bipedal standing using fractional brownian motion modeling. J. Appl. Biomechanics 23, 63–69. doi:10.1123/jab.23.1.63
Busquets, A., Ferrer-Uris, B., Angulo-Barroso, R., and Federolf, P. (2021). Gymnastics experience enhances the development of bipedal-stance multi-segmental coordination and control during proprioceptive reweighting. Front. Psychol. 12, 661312. doi:10.3389/fpsyg.2021.661312
Caballero, C., Barbado, D., Hérnandez-Davó, H., Hernández-Davó, J. L., and Moreno, F. J. (2021). Balance dynamics are related to age and levels of expertise. Application in young and adult tennis players. PloS One 16, e0249941. doi:10.1371/journal.pone.0249941
Caballero, C., Barbado, D., Urbán, T., García-Herrero, J. A., and Moreno, F. J. (2020). Functional variability in team-handball players during balance is revealed by non-linear measures and is related to age and expertise level. Entropy 22, 822. doi:10.3390/e22080822
Calavalle, A., Sisti, D., Rocchi, M. B. L., Panebianco, R., Del Sal, M., and Stocchi, V. (2008). Postural trials: expertise in rhythmic gymnastics increases control in lateral directions. Eur. J. Appl. Physiology 104, 643–649. doi:10.1007/s00421-008-0815-6
Carver, N. S., Bojovic, D., and Kelty-Stephen, D. G. (2017). Multifractal foundations of visually-guided aiming and adaptation to prismatic perturbation. Hum. Mov. Sci. 55, 61–72. doi:10.1016/j.humov.2017.07.005
Changizi, M. A., and Destefano, M. (2010). Common scaling laws for city highway systems and the mammalian neocortex. Complexity 15, 11–18. doi:10.1002/cplx.20288
Coubard, O. A., Ferrufino, L., Nonaka, T., Zelada, O., Bril, B., and Dietrich, G. (2014). One month of contemporary dance modulates fractal posture in aging. Front. Aging Neurosci. 6, 17. doi:10.3389/fnagi.2014.00017
Cousins, S., Kaski, D., Cutfield, N., Seemungal, B., Golding, J. F., Gresty, M., et al. (2013). Vestibular perception following acute unilateral vestibular lesions. PLoS One 8, e61862. doi:10.1371/journal.pone.0061862
Cullen, K. E. (2012). The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 35, 185–196. doi:10.1016/j.tins.2011.12.001
Curthoys, I. S., and Halmagyi, G. M. (1995). Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J. Vestib. Res. 5, 67–107. doi:10.3233/VES-1995-5201
Damm, L., Varoqui, D., De Cock, V. C., Dalla Bella, S., and Bardy, B. (2020). Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neurosci. Biobehav. Rev. 112, 553–584. doi:10.1016/j.neubiorev.2019.12.024
Debu, B., and Woollacott, M. (1988). Effects of gymnastics training on postural responses to stance perturbations. J. Mot. Behav. 20, 273–300. doi:10.1080/00222895.1988.10735446
Deco, G., Jirsa, V. K., and McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56. doi:10.1038/nrn2961
Dieringer, N. (1995). ’vestibular compensation’: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog. Neurobiol. 46, 97–129. doi:10.1016/0301-0082(95)80009-W
Dilão, R., and Hauser, M. J. (2013). Chemotaxis with directional sensing during Dictyostelium aggregation. Comptes Rendus. Biol. 336, 565–571. doi:10.1016/j.crvi.2013.10.008
Dixon, J. A., and Kelty-Stephen, D. G. (2012). Multi-scale interactions in Dictyostelium discoideum aggregation. Phys. A Stat. Mech. its Appl. 391, 6470–6483. doi:10.1016/j.physa.2012.07.001
Doyle, T. L., Dugan, E. L., Humphries, B., and Newton, R. U. (2004). Discriminating between elderly and young using a fractal dimension analysis of centre of pressure. Int. J. Med. Sci. 1, 11–20. doi:10.7150/ijms.1.11
Duarte, M., and Zatsiorsky, V. M. (2000). On the fractal properties of natural human standing. Neurosci. Lett. 283, 173–176. doi:10.1016/S0304-3940(00)00960-5
Ducharme, S. W., and van Emmerik, R. E. (2018). Fractal dynamics, variability, and coordination in human locomotion. Kinesiol. Rev. 7, 26–35. doi:10.1123/kr.2017-0054
Eke, A., Herman, P., Kocsis, L., and Kozak, L. (2002). Fractal characterization of complexity in temporal physiological signals. Physiol. Meas. 23, R1–R38. doi:10.1088/0967-3334/23/1/201
Endlein, T., and Federle, W. (2013). Rapid preflexes in smooth adhesive pads of insects prevent sudden detachment. Proc. R. Soc. B Biol. Sci. 280, 20122868. doi:10.1098/rspb.2012.2868
Fetter, M., Diener, H.-C., and Dichgans, J. (1991). Recovery of postural control after an acute unilateral vestibular lesion in humans. J. Vestib. Res. 1, 373–383. doi:10.3233/VES-1991-1405
Fitzpatrick, R., and McCloskey, D. (1994). Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J. Physiology 478, 173–186. doi:10.1113/jphysiol.1994.sp020240
Frantsevich, L., and Gorb, S. (2002). Arcus as a tensegrity structure in the arolium of wasps (Hymenoptera: vespidae). Zoology 105, 225–237. doi:10.1078/0944-2006-00067
Furmanek, M. P., Mangalam, M., Kelty-Stephen, D. G., and Juras, G. (2021). Postural constraints recruit shorter-timescale processes into the non-Gaussian cascade processes. Neurosci. Lett. 741, 135508. doi:10.1016/j.neulet.2020.135508
Garcia, C., Barela, J. A., Viana, A. R., and Barela, A. M. F. (2011). Influence of gymnastics training on the development of postural control. Neurosci. Lett. 492, 29–32. doi:10.1016/j.neulet.2011.01.047
Gautier, G., Thouvarecq, R., and Vuillerme, N. (2008). Postural control and perceptive configuration: influence of expertise in gymnastics. Gait Posture 28, 46–51. doi:10.1016/j.gaitpost.2007.09.007
Gilfriche, P., Deschodt-Arsac, V., Blons, E., and Arsac, L. M. (2018). Frequency-specific fractal analysis of postural control accounts for control strategies. Front. Physiology 9, 293. doi:10.3389/fphys.2018.00293
Glass, S. M., and Ross, S. E. (2021). Direction-specific signatures of sport participation in center of pressure profiles of division I athletes. Int. J. Sports Phys. Ther. 16, 1260–1272. doi:10.26603/001c.28227
Goldberger, A. L., Amaral, L. A., Hausdorff, J. M., Ivanov, P. C., Peng, C.-K., and Stanley, H. E. (2002). Fractal dynamics in physiology: alterations with disease and aging. Proc. Natl. Acad. Sci. U. S. A. 99, 2466–2472. doi:10.1073/pnas.012579499
Gorochowski, T. E., Grierson, C. S., and Di Bernardo, M. (2018). Organization of feed-forward loop motifs reveals architectural principles in natural and engineered networks. Sci. Adv. 4, eaap9751. doi:10.1126/sciadv.aap9751
Granat, M., Barnett, R., Kirkwood, C., and Andrews, B. (1991). Technique for calculating the direction of postural sway. Med. Biol. Eng. Comput. 29, 599–601. doi:10.1007/BF02446092
Grebecka, L., Pomorski, P., Grebecki, A., and Lopatowska, A. (1999). Components of perinuclear and intranuclear cytoskeleton in the intact cells and in the isolated nuclei of Amoeba proteus. Acta Protozool. 38, 263–271.
Grimbergen, Y. A., Munneke, M., and Bloem, B. R. (2004). Falls in Parkinson’s disease. Curr. Opin. Neurology 17, 405–415. doi:10.1097/01.wco.0000137530.68867.93
Hajnal, A., Clark, J. D., Doyon, J. K., and Kelty-Stephen, D. G. (2018). Fractality of body movements predicts perception of affordances: evidence from stand-on-ability judgments about slopes. J. Exp. Psychol. Hum. Percept. Perform. 44, 836–841. doi:10.1037/xhp0000510
Holmes, P., Full, R. J., Koditschek, D., and Guckenheimer, J. (2006). The dynamics of legged locomotion: models, analyses, and challenges. SIAM Rev. 48, 207–304. doi:10.1137/S0036144504445133
Hopper, D. M., Grisbrook, T. L., Newnham, P. J., and Edwards, D. J. (2014). The effects of vestibular stimulation and fatigue on postural control in classical ballet dancers. J. Dance Med. Sci. 18, 67–73. doi:10.12678/1089-313X.18.2.67
Horlings, C., Küng, U., Honegger, F., Van Engelen, B., Van Alfen, N., Bloem, B., et al. (2009). Vestibular and proprioceptive influences on trunk movements during quiet standing. Neuroscience 161, 904–914. doi:10.1016/j.neuroscience.2009.04.005
Hrysomallis, C. (2011). Balance ability and athletic performance. Sports Med. 41, 221–232. doi:10.2165/11538560-000000000-00000
Hurst, H. E. (1951). Long-term storage capacity of reservoirs. Trans. Am. Soc. Civ. Eng. 116, 770–799. doi:10.1061/TACEAT.0006518
Ihlen, E. A., Skjæret, N., and Vereijken, B. (2013). The influence of center-of-mass movements on the variation in the structure of human postural sway. J. Biomechanics 46, 484–490. doi:10.1016/j.jbiomech.2012.10.016
Ingber, D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827. doi:10.1096/fj.05-5424rev
Ingber, D. E. (2008). Tensegrity-based mechanosensing from macro to micro. Prog. Biophysics Mol. Biol. 97, 163–179. doi:10.1016/j.pbiomolbio.2008.02.005
Ivanenko, Y. P., Grasso, R., and Lacquaniti, F. (1999). Effect of gaze on postural responses to neck proprioceptive and vestibular stimulation in humans. J. Physiology 519, 301–314. doi:10.1111/j.1469-7793.1999.0301o.x
Ivanov, P. C. (2021). The new field of network physiology: building the human physiolome. Front. Netw. Physiology 1, 711778. doi:10.3389/fnetp.2021.711778
Ivanov, P. C., Liu, K. K., and Bartsch, R. P. (2016). Focus on the emerging new fields of network physiology and network medicine. New J. Phys. 18, 100201. doi:10.1088/1367-2630/18/10/100201
Ivanov, P. C., Nunes Amaral, L. A., Goldberger, A. L., Havlin, S., Rosenblum, M. G., Stanley, H. E., et al. (2001). From 1/f noise to multifractal cascades in heartbeat dynamics. Chaos 11, 641–652. doi:10.1063/1.1395631
Iwasaki, S., and Yamasoba, T. (2015). Dizziness and imbalance in the elderly: age-related decline in the vestibular system. Aging Dis. 6, 38–47. doi:10.14336/AD.2014.0128
Jacobson, N., Berleman-Paul, Q., Mangalam, M., Kelty-Stephen, D. G., and Ralston, C. (2021). Multifractality in postural sway supports quiet eye training in aiming tasks: a study of golf putting. Hum. Mov. Sci. 76, 102752. doi:10.1016/j.humov.2020.102752
Janusz, B. W., Beck, M., Szczepańska, J., Sadowska, D., Bacik, B., Juras, G., et al. (2016). Directional measures of postural sway as predictors of balance instability and accidental falls. J. Hum. Kinet. 52, 75–83. doi:10.1515/hukin-2015-0195
Jin, Y., Wu, Y., Li, H., Zhao, M., and Pan, J. (2017). Definition of fractal topography to essential understanding of scale-invariance. Sci. Rep. 7, 46672. doi:10.1038/srep46672
Kello, C. T. (2013). Critical branching neural networks. Psychol. Rev. 120, 230–254. doi:10.1037/a0030970
Kelso, J. S. (1995). Dynamic patterns: the self-organization of brain and behavior. Cambridge, MA: MIT Press.
Kelso, J. S., Tuller, B., Vatikiotis-Bateson, E., and Fowler, C. A. (1984). Functionally specific articulatory cooperation following jaw perturbations during speech: evidence for coordinative structures. J. Exp. Psychol. Hum. Percept. Perform. 10, 812–832. doi:10.1037/0096-1523.10.6.812
Kelty-Stephen, D. G. (2018). Multifractal evidence of nonlinear interactions stabilizing posture for phasmids in windy conditions: a reanalysis of insect postural-sway data. PloS One 13, e0202367. doi:10.1371/journal.pone.0202367
Kelty-Stephen, D. G., and Dixon, J. A. (2014). Interwoven fluctuations during intermodal perception: fractality in head sway supports the use of visual feedback in haptic perceptual judgments by manual wielding. J. Exp. Psychol. Hum. Percept. Perform. 40, 2289–2309. doi:10.1037/a0038159
Kelty-Stephen, D. G., Furmanek, M. P., and Mangalam, M. (2021a). Multifractality distinguishes reactive from proactive cascades in postural control. Chaos, Solit. Fractals 142, 110471. doi:10.1016/j.chaos.2020.110471
Kelty-Stephen, D. G., Lee, I. C., Carver, N. S., Newell, K. M., and Mangalam, M. (2021b). Multifractal roots of suprapostural dexterity. Hum. Mov. Sci. 76, 102771. doi:10.1016/j.humov.2021.102771
Kelty-Stephen, D. G., Lee, J., Cole, K. R., Shields, R. K., and Mangalam, M. (2023). Multifractal nonlinearity moderates feedforward and feedback responses to suprapostural perturbations. Percept. Mot. Ski. 130, 622–657. doi:10.1177/00315125221149147
Kelty-Stephen, D. G., and Mangalam, M. (2022). Turing’s cascade instability supports the coordination of the mind, brain, and behavior. Neurosci. Biobehav. Rev. 141, 104810. doi:10.1016/j.neubiorev.2022.104810
Kelty-Stephen, D. G., and Mangalam, M. (2023). Multifractal descriptors ergodically characterize non-ergodic multiplicative cascade processes. Phys. A Stat. Mech. its Appl. 617, 128651. doi:10.1016/j.physa.2023.128651
Kiely, J., and Collins, D. J. (2016). Uniqueness of human running coordination: the integration of modern and ancient evolutionary innovations. Front. Psychol. 7, 262. doi:10.3389/fpsyg.2016.00262
Kirchner, M., Schubert, P., Schmidtbleicher, D., and Haas, C. (2012). Evaluation of the temporal structure of postural sway fluctuations based on a comprehensive set of analysis tools. Phys. A Stat. Mech. its Appl. 391, 4692–4703. doi:10.1016/j.physa.2012.05.034
Komura, T., Nagano, A., Leung, H., and Shinagawa, Y. (2005). Simulating pathological gait using the enhanced linear inverted pendulum model. IEEE Trans. Biomed. Eng. 52, 1502–1513. doi:10.1109/TBME.2005.851530
Kuo, A. D. (2005). An optimal state estimation model of sensory integration in human postural balance. J. Neural Eng. 2, S235–S249. doi:10.1088/1741-2560/2/3/S07
Kuo, A. D. (2007). The six determinants of gait and the inverted pendulum analogy: a dynamic walking perspective. Hum. Mov. Sci. 26, 617–656. doi:10.1016/j.humov.2007.04.003
Kuo, A. D., and Donelan, J. M. (2010). Dynamic principles of gait and their clinical implications. Phys. Ther. 90, 157–174. doi:10.2522/ptj.20090125
Kuznetsov, N., Bonnette, S., Gao, J., and Riley, M. A. (2013). Adaptive fractal analysis reveals limits to fractal scaling in center of pressure trajectories. Ann. Biomed. Eng. 41, 1646–1660. doi:10.1007/s10439-012-0646-9
Lacour, M., Barthelemy, J., Borel, L., Magnan, J., Xerri, C., Chays, A., et al. (1997). Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp. Brain Res. 115, 300–310. doi:10.1007/PL00005698
Lacour, M., and Borel, L. (1993). Vestibular control of posture and gait. Arch. Ital. Biol. 131, 81–104.
Latash, M. L. (2012). The bliss (not the problem) of motor abundance (not redundancy). Exp. Brain Res. 217, 1–5. doi:10.1007/s00221-012-3000-4
Latash, M. L. (2024). “Synergic control of movement: from single muscles to the whole body,” in Progress in motor control. Editors M. F. Levin, M. Petrarca, D. Piscitelli, and S. Summa (Cambridge, MA: Academic Press), 25–47. doi:10.1016/B978-0-443-23987-8.00010-9
Latash, M. L., Scholz, J. P., and Schöner, G. (2007). Toward a new theory of motor synergies. Mot. Control 11, 276–308. doi:10.1123/mcj.11.3.276
Levin, S., de Solórzano, S. L., and Scarr, G. (2017). The significance of closed kinematic chains to biological movement and dynamic stability. J. Bodyw. Mov. Ther. 21, 664–672. doi:10.1016/j.jbmt.2017.03.012
Lin, D., Seol, H., Nussbaum, M. A., and Madigan, M. L. (2008). Reliability of COP-based postural sway measures and age-related differences. Gait Posture 28, 337–342. doi:10.1016/j.gaitpost.2008.01.005
Lipsitz, L. A. (2002). Dynamics of stability: the physiologic basis of functional health and frailty. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 57, B115–B125. doi:10.1093/gerona/57.3.B115
Lipsitz, L. A., and Goldberger, A. L. (1992). Loss of ’complexity’ and aging: potential applications of fractals and chaos theory to senescence. JAMA 267, 1806–1809. doi:10.1001/jama.1992.03480130122036
Lipsitz, L. A., Mietus, J., Moody, G. B., and Goldberger, A. L. (1990). Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation 81, 1803–1810. doi:10.1161/01.CIR.81.6.1803
Liston, M. B., Bamiou, D.-E., Martin, F., Hopper, A., Koohi, N., Luxon, L., et al. (2014). Peripheral vestibular dysfunction is prevalent in older adults experiencing multiple non-syncopal falls versus age-matched non-fallers: a pilot study. Age Ageing 43, 38–43. doi:10.1093/ageing/aft129
Lovejoy, S., and Schertzer, D. (2018). The weather and climate: emergent laws and multifractal cascades. Cambridge, MA: Cambridge University Press.
Lui, K. Y., Hewston, P., and Deshpande, N. (2019). Visual–vestibular interaction for postural control during sit-to-stand: effects of aging. Mot. Control 23, 115–126. doi:10.1123/mc.2017-0045
Maitre, J., and Paillard, T. (2016). Postural effects of vestibular manipulation depend on the physical activity status. PLoS One 11, e0162966. doi:10.1371/journal.pone.0162966
Mangalam, M., Carver, N. S., and Kelty-Stephen, D. G. (2020a). Global broadcasting of local fractal fluctuations in a bodywide distributed system supports perception via effortful touch. Chaos, Solit. Fractals 135, 109740. doi:10.1016/j.chaos.2020.109740
Mangalam, M., Carver, N. S., and Kelty-Stephen, D. G. (2020b). Multifractal signatures of perceptual processing on anatomical sleeves of the human body. J. R. Soc. Interface 17, 20200328. doi:10.1098/rsif.2020.0328
Mangalam, M., and Kelty-Stephen, D. G. (2021a). Hypothetical control of postural sway. J. R. Soc. Interface 18, 20200951. doi:10.1098/rsif.2020.0951
Mangalam, M., and Kelty-Stephen, D. G. (2021b). Point estimates, Simpson’s paradox, and nonergodicity in biological sciences. Neurosci. Biobehav. Rev. 125, 98–107. doi:10.1016/j.neubiorev.2021.02.017
Mangalam, M., Kelty-Stephen, D. G., Kiyono, K., and Stergiou, N. (2024a). Spatial variability and directional shifts in postural control in Parkinson’s disease. Clin. Park. Relat. Disord. 10, 100249. doi:10.1016/j.prdoa.2024.100249
Mangalam, M., Kelty-Stephen, D. G., Seleznov, I., Popov, A., Likens, A. D., Kiyono, K., et al. (2024b). Older adults and individuals with Parkinson’s disease control posture along suborthogonal directions that deviate from the traditional anteroposterior and mediolateral directions. Sci. Rep. 14, 4117. doi:10.1038/s41598-024-54583-y
Mangalam, M., Kelty-Stephen, D. G., Sommerfeld, J. H., Stergiou, N., and Likens, A. D. (2023). Temporal organization of stride-to-stride variations contradicts predictive models for sensorimotor control of footfalls during walking. Plos One 18, e0290324. doi:10.1371/journal.pone.0290324
Mangalam, M., Lee, I.-C., Newell, K. M., and Kelty-Stephen, D. G. (2021). Visual effort moderates postural cascade dynamics. Neurosci. Lett. 742, 135511. doi:10.1016/j.neulet.2020.135511
Marsden, C., Merton, P., and Morton, H. (1983). Rapid postural reactions to mechanical displacement of the hand in man. Adv. Neurology 39, 645–659.
Maurer, C., Mergner, T., and Peterka, R. (2006). Multisensory control of human upright stance. Exp. Brain Res. 171, 231–250. doi:10.1007/s00221-005-0256-y
Mittelstaedt, H. (1998). Origin and processing of postural information. Neurosci. Biobehav. Rev. 22, 473–478. doi:10.1016/S0149-7634(97)00032-8
Morales, C. J., and Kolaczyk, E. D. (2002). Wavelet-based multifractal analysis of human balance. Ann. Biomed. Eng. 30, 588–597. doi:10.1114/1.1478082
Moreno, M. A., Stepp, N., and Turvey, M. (2011). Whole body lexical decision. Neurosci. Lett. 490, 126–129. doi:10.1016/j.neulet.2010.12.041
Morgane, P. J., Galler, J. R., and Mokler, D. J. (2005). A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 75, 143–160. doi:10.1016/j.pneurobio.2005.01.001
Moritz, C. T., and Farley, C. T. (2005). Human hopping on very soft elastic surfaces: implications for muscle pre-stretch and elastic energy storage in locomotion. J. Exp. Biol. 208, 939–949. doi:10.1242/jeb.01472
Moro, S. S., and Harris, L. R. (2018). Vestibular–somatosensory interactions affect the perceived timing of tactile stimuli. Exp. Brain Res. 236, 2877–2885. doi:10.1007/s00221-018-5346-8
Munafo, J., Curry, C., Wade, M. G., and Stoffregen, T. A. (2016). The distance of visual targets affects the spatial magnitude and multifractal scaling of standing body sway in younger and older adults. Exp. Brain Res. 234, 2721–2730. doi:10.1007/s00221-016-4676-7
Nakamura, A., Suzuki, Y., Milosevic, M., and Nomura, T. (2021). Long-lasting event-related beta synchronizations of electroencephalographic activity in response to support-surface perturbations during upright stance: a pilot study associating beta rebound and active monitoring in the intermittent postural control. Front. Syst. Neurosci. 15, 660434. doi:10.3389/fnsys.2021.660434
Nomura, T., Oshikawa, S., Suzuki, Y., Kiyono, K., and Morasso, P. (2013). Modeling human postural sway using an intermittent control and hemodynamic perturbations. Math. Biosci. 245, 86–95. doi:10.1016/j.mbs.2013.02.002
Nozaki, D., Collins, J. J., and Yamamoto, Y. (1999a). Mechanism of stochastic resonance enhancement in neuronal models driven by 1/f noise. Phys. Rev. E 60, 4637–4644. doi:10.1103/PhysRevE.60.4637
Nozaki, D., Mar, D. J., Grigg, P., and Collins, J. J. (1999b). Effects of colored noise on stochastic resonance in sensory neurons. Phys. Rev. Lett. 82, 2402–2405. doi:10.1103/PhysRevLett.82.2402
O’Connor, S. M., and Kuo, A. D. (2009). Direction-dependent control of balance during walking and standing. J. Neurophysiology 102, 1411–1419. doi:10.1152/jn.00131.2009
Paillard, T. (2017). Plasticity of the postural function to sport and/or motor experience. Neurosci. Biobehav. Rev. 72, 129–152. doi:10.1016/j.neubiorev.2016.11.015
Paillard, T. (2019). Relationship between sport expertise and postural skills. Front. Psychol. 10, 1428. doi:10.3389/fpsyg.2019.01428
Palatinus, Z., Kelty-Stephen, D. G., Kinsella-Shaw, J., Carello, C., and Turvey, M. T. (2014). Haptic perceptual intent in quiet standing affects multifractal scaling of postural fluctuations. J. Exp. Psychol. Hum. Percept. Perform. 40, 1808–1818. doi:10.1037/a0037247
Pascolo, P., Barazza, F., and Carniel, R. (2006). Considerations on the application of the chaos paradigm to describe the postural sway. Chaos, Solit. Fractals 27, 1339–1346. doi:10.1016/j.chaos.2005.04.111
Peng, C.-K., Buldyrev, S. V., Havlin, S., Simons, M., Stanley, H. E., and Goldberger, A. L. (1994). Mosaic organization of DNA nucleotides. Phys. Rev. E 49, 1685–1689. doi:10.1103/PhysRevE.49.1685
Peng, C.-K., Havlin, S., Stanley, H. E., and Goldberger, A. L. (1995). Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87. doi:10.1063/1.166141
Peterka, R. J. (2002). Sensorimotor integration in human postural control. J. Neurophysiology 88, 1097–1118. doi:10.1152/jn.2002.88.3.1097
Peterka, R. J., and Benolken, M. S. (1995). Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp. Brain Res. 105, 101–110. doi:10.1007/BF00242186
Peterka, R. J., and Loughlin, P. J. (2004). Dynamic regulation of sensorimotor integration in human postural control. J. Neurophysiology 91, 410–423. doi:10.1152/jn.00516.2003
Picot, B., Rémy-Neris, O., and Forestier, N. (2022). Proprioceptive postural control strategies differ among non-injured athletes. Neurosci. Lett. 769, 136366. doi:10.1016/j.neulet.2021.136366
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and Team, R. C. (2007). Linear and nonlinear mixed effects models. R. Package Version 3.1-137. Available at: https://cran.r-project.org/package=nlme.
Plenz, D., and Thiagarajan, T. C. (2007). The organizing principles of neuronal avalanches: cell assemblies in the cortex? Trends Neurosci. 30, 101–110. doi:10.1016/j.tins.2007.01.005
Profeta, V. L., and Turvey, M. T. (2018). Bernstein’s levels of movement construction: a contemporary perspective. Hum. Mov. Sci. 57, 111–133. doi:10.1016/j.humov.2017.11.013
Qian, H., Raymond, G. M., and Bassingthwaighte, J. B. (1998). On two-dimensional fractional Brownian motion and fractional Brownian random field. J. Phys. A Math. General 31, L527. doi:10.1088/0305-4470/31/28/002
Quijoux, F., Nicolaï, A., Chairi, I., Bargiotas, I., Ricard, D., Yelnik, A., et al. (2021). A review of center of pressure (COP) variables to quantify standing balance in elderly people: algorithms and open-access code. Physiol. Rep. 9, e15067. doi:10.14814/phy2.15067
Raffalt, P. C., Sommerfeld, J. H., Stergiou, N., and Likens, A. D. (2023). Stride-to-stride time intervals are independently affected by the temporal pattern and probability distribution of visual cues. Neurosci. Lett. 792, 136909. doi:10.1016/j.neulet.2022.136909
Raffalt, P. C., Stergiou, N., Sommerfeld, J. H., and Likens, A. D. (2021). The temporal pattern and the probability distribution of visual cueing can alter the structure of stride-to-stride variability. Neurosci. Lett. 763, 136193. doi:10.1016/j.neulet.2021.136193
R Core Team (2013). R: a language and environment for statistical computing. R Version 4.3.0. Available at: https://www.R-project.org/.
Redfern, M. S., Talkowski, M. E., Jennings, J. R., and Furman, J. M. (2004). Cognitive influences in postural control of patients with unilateral vestibular loss. Gait Posture 19, 105–114. doi:10.1016/S0966-6362(03)00032-8
Rizzo, R., Zhang, X., Wang, J. W., Lombardi, F., and Ivanov, P. C. (2020). Network physiology of cortico–muscular interactions. Front. Physiology 11, 558070. doi:10.3389/fphys.2020.558070
Roberts, R., Ahmad, H., Patel, M., Dima, D., Ibitoye, R., Sharif, M., et al. (2018). An fMRI study of visuo-vestibular interactions following vestibular neuritis. NeuroImage Clin. 20, 1010–1017. doi:10.1016/j.nicl.2018.10.007
Rocchi, L., Chiari, L., and Cappello, A. (2004). Feature selection of stabilometric parameters based on principal component analysis. Med. Biol. Eng. Comput. 42, 71–79. doi:10.1007/BF02351013
Rühl, R. M., Bauermann, T., Dieterich, M., and Zu Eulenburg, P. (2018). Functional correlate and delineated connectivity pattern of human motion aftereffect responses substantiate a subjacent visual-vestibular interaction. NeuroImage 174, 22–34. doi:10.1016/j.neuroimage.2018.02.057
Scarr, G., Blyum, L., Levin, S. M., and de Solórzano, S. L. (2024). Moving beyond Vesalius: why anatomy needs a mapping update. Med. Hypotheses 183, 111257. doi:10.1016/j.mehy.2023.111257
Seleznov, I., Popov, A., Kikuchi, K., Kolosova, E., Kolomiiets, B., Nakata, A., et al. (2020). Detection of oriented fractal scaling components in anisotropic two-dimensional trajectories. Sci. Rep. 10, 21892. doi:10.1038/s41598-020-78807-z
Shimizu, Y., Thurner, S., and Ehrenberger, K. (2002). Multifractal spectra as a measure of complexity in human posture. Fractals 10, 103–116. doi:10.1142/S0218348X02001130
Soma, R., Nozaki, D., Kwak, S., and Yamamoto, Y. (2003). 1/f noise outperforms white noise in sensitizing baroreflex function in the human brain. Phys. Rev. Lett. 91, 078101. doi:10.1103/PhysRevLett.91.078101
Sparto, P. J., and Redfern, M. S. (2001). Quantification of direction and magnitude of cyclical postural sway using ellipses. Biomed. Eng. Appl. Basis Commun. 13, 213–217. doi:10.4015/S1016237201000261
Stambolieva, K. (2011). Fractal properties of postural sway during quiet stance with changed visual and proprioceptive inputs. J. Physiological Sci. 61, 123–130. doi:10.1007/s12576-010-0129-4
Stephen, D. G., Anastas, J. R., and Dixon, J. A. (2012a). Scaling in cognitive performance reflects multiplicative multifractal cascade dynamics. Front. Physiology 3, 102. doi:10.3389/fphys.2012.00102
Stephen, D. G., and Dixon, J. A. (2011). Strong anticipation: multifractal cascade dynamics modulate scaling in synchronization behaviors. Chaos, Solit. Fractals 44, 160–168. doi:10.1016/j.chaos.2011.01.005
Stephen, D. G., Hsu, W.-H., Young, D., Saltzman, E. L., Holt, K. G., Newman, D. J., et al. (2012b). Multifractal fluctuations in joint angles during infant spontaneous kicking reveal multiplicativity-driven coordination. Chaos, Solit. Fractals 45, 1201–1219. doi:10.1016/j.chaos.2012.06.005
Stephen, D. G., Mirman, D., Magnuson, J. S., and Dixon, J. A. (2009). Lévy-like diffusion in eye movements during spoken-language comprehension. Phys. Rev. E 79, 056114. doi:10.1103/PhysRevE.79.056114
Stylianou, O., Racz, F. S., Kim, K., Kaposzta, Z., Czoch, A., Yabluchanskiy, A., et al. (2021). Multifractal functional connectivity analysis of electroencephalogram reveals reorganization of brain networks in a visual pattern recognition paradigm. Front. Hum. Neurosci. 15, 740225. doi:10.3389/fnhum.2021.740225
Suckling, J., Wink, A. M., Bernard, F. A., Barnes, A., and Bullmore, E. (2008). Endogenous multifractal brain dynamics are modulated by age, cholinergic blockade and cognitive performance. J. Neurosci. Methods 174, 292–300. doi:10.1016/j.jneumeth.2008.06.037
Svidersky, V., and Plotnikova, S. (2002). Insects and vertebrates: analogous structures in higher integrative centers of the brain. J. Evol. Biochem. Physiology 38, 627–639. doi:10.1023/A:1022073218825
Teng, D. W., Eddy, C. L., and Kelty-Stephen, D. G. (2016). Non-visually-guided distance perception depends on matching torso fluctuations between training and test. Atten. Percept. Psychophys. 78, 2320–2328. doi:10.3758/s13414-016-1213-5
Thayer, J. F. (2006). On the importance of inhibition: central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose-Response 4, 2–21. doi:10.2203/dose-response.004.01.002.Thayer
Torre, K., Vergotte, G., Viel, É., Perrey, S., and Dupeyron, A. (2019). Fractal properties in sensorimotor variability unveil internal adaptations of the organism before symptomatic functional decline. Sci. Rep. 9, 15736. doi:10.1038/s41598-019-52091-y
Tsujimoto, Y., Miki, Y., Shimatani, S., and Kiyono, K. (2016). Fast algorithm for scaling analysis with higher-order detrending moving average method. Phys. Rev. E 93, 053304. doi:10.1103/PhysRevE.93.053304
Turvey, M. T., and Fonseca, S. T. (2014). The medium of haptic perception: a tensegrity hypothesis. J. Mot. Behav. 46, 143–187. doi:10.1080/00222895.2013.798252
Turvey, M. T., and Sheya, A. (2017). Non-obvious influences on perception-action abilities. Psychonomic Bull. Rev. 24, 1597–1603. doi:10.3758/s13423-016-1223-2
Van den Hoorn, W., Kerr, G. K., Van Dieen, J. H., and Hodges, P. W. (2018). Center of pressure motion after calf vibration is more random in fallers than non-fallers: prospective study of older individuals. Front. Physiology 9, 273. doi:10.3389/fphys.2018.00273
Van Orden, G., Hollis, G., and Wallot, S. (2012). The blue-collar brain. Front. Physiology 3, 207. doi:10.3389/fphys.2012.00207
Vuillerme, N., Danion, F., Marin, L., Boyadjian, A., Prieur, J., Weise, I., et al. (2001a). The effect of expertise in gymnastics on postural control. Neurosci. Lett. 303, 83–86. doi:10.1016/S0304-3940(01)01722-0
Vuillerme, N., and Nougier, V. (2004). Attentional demand for regulating postural sway: the effect of expertise in gymnastics. Brain Res. Bull. 63, 161–165. doi:10.1016/j.brainresbull.2004.02.006
Vuillerme, N., Teasdale, N., and Nougier, V. (2001b). The effect of expertise in gymnastics on proprioceptive sensory integration in human subjects. Neurosci. Lett. 311, 73–76. doi:10.1016/S0304-3940(01)02147-4
Wagman, J. B., and Miller, D. B. (2003). Nested reciprocities: the organism–environment system in perception–action and development. Dev. Psychobiol. 42, 317–334. doi:10.1002/dev.10114
Ward, R. M., and Kelty-Stephen, D. G. (2018). Bringing the nonlinearity of the movement system to gestural theories of language use: multifractal structure of spoken English supports the compensation for coarticulation in human speech perception. Front. Physiology 9, 1152. doi:10.3389/fphys.2018.01152
Weinberg, S. (1983). “Why the renormalization group is a good thing,”. Dept of Physics in Asymptotic realms of Physics (Cambridge, MA: MIT Press), 1–19. Available at: https://cir.nii.ac.jp/crid/1573387449631142912.
West, B. J. (2006). Where medicine went wrong: rediscovering the path to complexity. Singapore: World Scientific.
Wiesmeier, I. K., Dalin, D., and Maurer, C. (2015). Elderly use proprioception rather than visual and vestibular cues for postural motor control. Front. Aging Neurosci. 7, 97. doi:10.3389/fnagi.2015.00097
Wilson, V. J. (2013). Mammalian vestibular physiology. New York, NY: Springer Science & Business Media.
Wyatt, H. E., Vicinanza, D., Newell, K. M., Irwin, G., and Williams, G. K. (2021). Bidirectional causal control in the dynamics of handstand balance. Sci. Rep. 11, 405. doi:10.1038/s41598-020-79730-z
Xiao, X., Chen, H., and Bogdan, P. (2021). Deciphering the generating rules and functionalities of complex networks. Sci. Rep. 11, 22964. doi:10.1038/s41598-021-02203-4
Keywords: fall, fractal, hurst exponent, motor disorder, multiscale, oriented fractal scaling component analysis, postural control
Citation: Mangalam M, Seleznov I, Kolosova E, Popov A, Kelty-Stephen DG and Kiyono K (2024) Postural control in gymnasts: anisotropic fractal scaling reveals proprioceptive reintegration in vestibular perturbation. Front. Netw. Physiol. 4:1393171. doi: 10.3389/fnetp.2024.1393171
Received: 28 February 2024; Accepted: 05 April 2024;
Published: 18 April 2024.
Edited by:
Robert Hristovski, Saints Cyril and Methodius University of Skopje, North MacedoniaReviewed by:
Natàlia Balagué, University of Barcelona, SpainAli Foroutannia, University of Canberra, Australia
Adam W. Kiefer, University of North Carolina at Chapel Hill, United States
Copyright © 2024 Mangalam, Seleznov, Kolosova, Popov, Kelty-Stephen and Kiyono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhur Mangalam, bW1hbmdhbGFtQHVub21haGEuZWR1; Ken Kiyono, a2l5b25vLmtlbi5lc0Bvc2FrYS11LmFjLmpw
 Madhur Mangalam
Madhur Mangalam Ivan Seleznov
Ivan Seleznov Elena Kolosova3,4
Elena Kolosova3,4 Anton Popov
Anton Popov