- 1 Department of Neurology, Charite – University Medicine Berlin, Germany
- 2 Department of Neurology, Vivantes Klinikum im Friedrichshain Berlin, Germany
- 3 Department of Ophthalmology, Charite – University Medicine Berlin, Germany
- 4 Department of Radiology, Charite – University Medicine Berlin, Germany
- 5 Center for Stroke Research Berlin, Charite - Universitätsmedizin Berlin, Germany
- 6 Department of Neurology, Vivantes Auguste-Viktoria-Klinikum Berlin, Germany
Background: Microangiopathic brain lesions can be separated in diffuse lesions – leukoaraiosis – and focal lesions – lacunes. Leukoaraiosis and lacunes are caused by common cerebrovascular risk factors, but whether they represent a common entity is not sufficiently investigated. The present study aimed to determine the clinical profiles associated with the extent of leukoaraiosis and lacunes. Methods: Sixty-four consecutive patients with acute microangiopathic stroke were studied. Leukoaraiosis and lacunes were stratified according to their MRI-based extent. Standardized clinical assessment included clinical syndromes, cerebrovascular risk factors, cognitive performance, retinal imaging, ultrasonography, blood, and urine parameters. Results: Different clinical profiles for leukoaraiosis and lacunes were found. Regarding leukoaraiosis, the cognitive scores (SISCO, mini mental score examination, mental examination) and the presence of hyperlipidemia decreased as the severity of leukoaraiosis increased. Univariate and multivariate analysis revealed that these cognitive score values as well as the presence of hyperlipidemia correlated significantly with no or only mild leukoaraiosis. Regarding lacunes, the percentage of migraine, previous stroke events, hydrocephalus, left ventricular hypertrophy, and a higher National Institutes of Health Stroke Scale increased as the number of lacunar lesions increased. Statistical analysis revealed that these parameters correlated not significantly with the number of lacunes. Conclusions: The findings suggests that leukoaraiosis and lacunes are different microangiopathic entities potentially requiering different treatment concepts.
Introduction
Symptomatic microangiopathic brain lesions (MBL) constitute about one quarter of all ischemic strokes and are a leading cause of morbidity and mortality (Lammie, 2002). Morphologically, diffuse affection of cerebral white matter, i.e., – leukoaraiosis – and focal lesions – lacunes – are distinguished (Barkhof and Scheltens, 2002; Pantoni et al., 2002; Pantoni, 2010).
Previous population-based studies investigated a variety of clinical factors in the context of MBL. These were associated with arterial hypertension, diabetes mellitus, and hyperlipidemia (Hachinski et al., 1987; Sander et al., 2000; Wong et al., 2002). It is generally assumed that leukoaraiosis and lacunar lesions share common risk factors. However, few studies have conducted a clinical profile analysis of the two entities in a single study design (Kim et al., 2011).
The present study aimed to determine differences in the clinical profile between leukoaraiosis and lacunar lesions. In microangiopathic stroke patients, the associations and coincidence of specific clinical parameters regarding the extent of MBL were investigated.
Materials and Methods
Study Population
Consecutive patients with microangiopathic stroke who were admitted to our stroke unit during a 6-months recruiting period were prospectively enrolled. Inclusion criteria was a clinically defined lacunar syndrome according to published criteria (Fisher, 1965; Mohr and Marti-Vilalta, 1998). Exclusion criteria were cortical symptoms, hemorrhagic stroke, or large vessel stroke. The study was approved by the local ethics committee.
MRI Scanning
The MRI protocol comprised T2 weighted images, T2*-weighted images, diffusion-weighted images, and dark fluid sequences (Siemens 5; 1.5 T) in 6 mm slices from the vertex to the foramen magnum. Images were evaluated by two independent blinded observers (a senior neuroradiologist and an experienced stroke neurologist).
Microangiopathic Brain Lesions
Leukoaraiosis was defined according to published criteria (Pantoni et al., 2002; Pantoni, 2010). Subjects with discrete leukoaraiosis were not considered to be affected (Hachinski et al., 1987; Barkhof and Scheltens, 2002; Pantoni et al., 2002). The extent of leukoaraiosis was divided into mild, moderate, and severe changes (Blennow et al., 1991; Pantoni et al., 2002). Lacunar lesions were defined as lesions with a diameter of 6–15 mm in typical locations. The number of lacunar lesions was classified into single, two to five and more than five lacunar lesions (Wahlund et al., 2001).
Cerebrovascular Risk Factors
Diagnostic work up included a standardized questionnaire evaluating history of cardiovascular diseases including myocardial infarction (MI), coronary artery disease (CAD), previous stroke, and family history. On admission, the leading clinical symptoms, the National Institutes of Health Stroke Scale (NIHSS), Rankin Scale (RS), and Barthel Index (BI) were documented. All other clinical data were recorded 7 days after the acute stroke event. Arterial hypertension was defined by either repeated elevated systolic blood pressure >160 and/or diastolic blood pressure >95 (Carter, 2004) or the previous use of antihypertensive drugs. Diabetes was defined by either HbA1c ≥6% or use of antidiabetics (Peters et al., 1996). Hyperlipidemia was defined either by total cholesterol ≥6.5 mmol/l, triglycerides ≥2.3, HDL cholesterol ≤1.0 mmol/l, or use of lipid-lowering drugs. Proteinuria was defined as urinary albumin >20 mg/dl. Renal insufficiency was defined as serum creatinine >120 μmol/l. Patients who did not currently smoke were divided in non-smokers and past smokers (≥5 years). Current smokers were evaluated in pack years (py). Alcohol consumption was stratified into g alcohol/month (Vriz et al., 1998). Body mass index (BMI, kg/m2) was calculated from height and weight measurements.
Cognitive Functions
Cognitive functions were assessed by the SIDAM score SISCO (Structured Interview for the Diagnosis of Dementia of the Alzheimer Type, multi-infarct dementia, and dementias of other etiology according to DSM-III-R and ICD-10) including the SIDAM Mini Mental Score Examination (MMSE) and mental examination (ME; Zaudig, 1992). In addition, the Beck Depression Inventory was performed (Beck et al., 1961).
Retinal Imaging
Retinal imaging to evaluate hypertensive retinopathy was performed after 5 min dark adaptation in mydriatic pupils. An ophthalmologist who was unaware of the clinical data evaluated the retinal imaging according to a standardized protocol. Hypertensive retinopathy was classified in mild, moderate, and severe retinopathy (Wong et al., 2002; Wong, 2004).
Vascular Ultrasonography
The pulsatility index (PI) of the MCA was recorded with a Doppler device (Multi-Dop X4, DWL) with the PI calculated automatically. Intima-media-thickness of the common carotid artery was defined by duplex sonography according to the method described by Simons et al. (1999).
Electrocardiography
Left ventricular hypertrophy (LVH) was identified by the gender specific Cornell voltage-duration product (RaVL + SVIII) × QRS> 2440 mm·ms in men and (RaVL + SVIII + 8mm) × QRS >2440 mm·ms in women. An additional acceptance criterion was based on the non-gender-specific Sokolow–Lyon voltage combination (SV1 + RV5 or RV6) > 38 mm (Dahlof et al., 1998). Atrial fibrillation was also defined by either conventional electrocardiogram or history of atrial fibrillation.
Data Analysis
Data were presented as mean ± SD for continuous variables and as proportions for categorical variables. Exploratory data analysis was performed to explore possible associations and to generate hypotheses. Due to the small number of patients, unadjusted p-values from Fisher’s exact tests are reported only to give a hint on possible associations. Due to the small sample size, variables leukoaraiosis and lacunar lesions were dichotomized to at least ensure useful group sizes for group comparisons: leukoaraiosis was dichotomized by either non or only mild changes on MRI, or moderate to severe leukoaraiosis and lacunes were dichotomized by either no or one lacunar lesion, or ≥2 lacunes.
Results
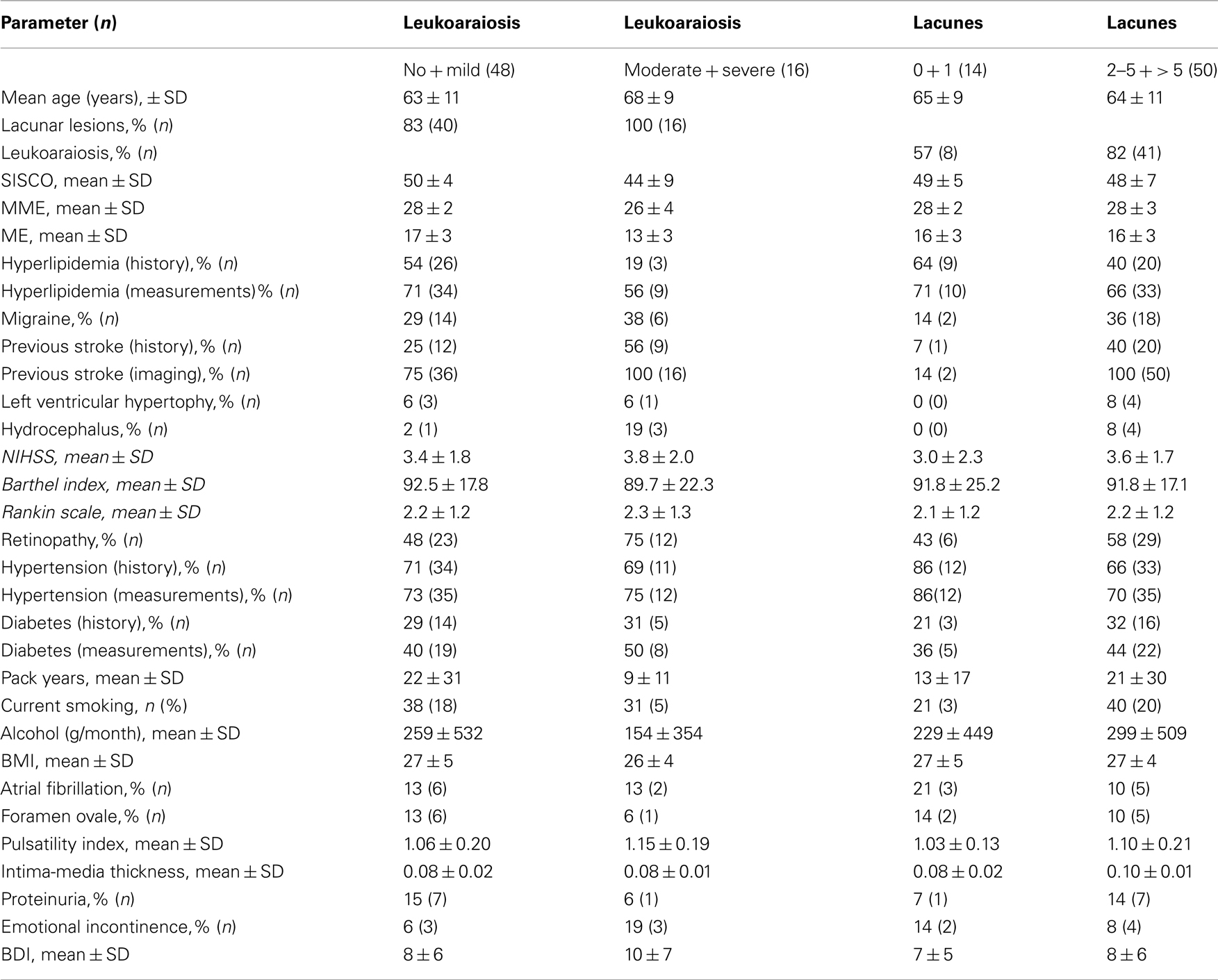
Sixty-four patients (25 female, 35 male, mean age of 65.5 ± 10.8 years) were investigated. Baseline characteristics of the study population are given in the Table 1.
Leukoaraiosis
Using four groups (no, mild, moderate, and severe leukoaraiosis) for evaluation of mean values, we observed that the cognitive scores SISCO, ME, and MMSE as well as the percentage of patients with hyperlipidemia decreased as the severity of leukoaraiosis increased. Using two groups (non or mild leukoaraiosis versus moderate or severe leukoaraiosis) for statistical analysis, Fisher’s exact test revealed that the cognitive score values for ME, MME, and SISCO above median correlated significantly with no or mild leukoaraiosis. The same was found for the percentage of patients with hyperlipidemia.
Due to the small sample size using multivariable methods is a problematical issue. To inspect the joint influence of the risk factors detected by univariate analysis on the probability for at least moderate leukoaraiosis and to adjust for age, small logistic regression models were selected and fitted corresponding to the following criteria: as few as possible missing observations, significant exploratory variables, goodness of fit (calibration, Hosmer–Lemeshow-Test), usefulness at prediction of outcome (Nagelkerke R2), and ability for discrimination (AUCROC). Only models with age and two additional exploratory variables were incorporated. With the caveat of small sample size and therefore purely empirical selection of models there were two “best” selected models which roughly complied with the criteria above (Table 2).
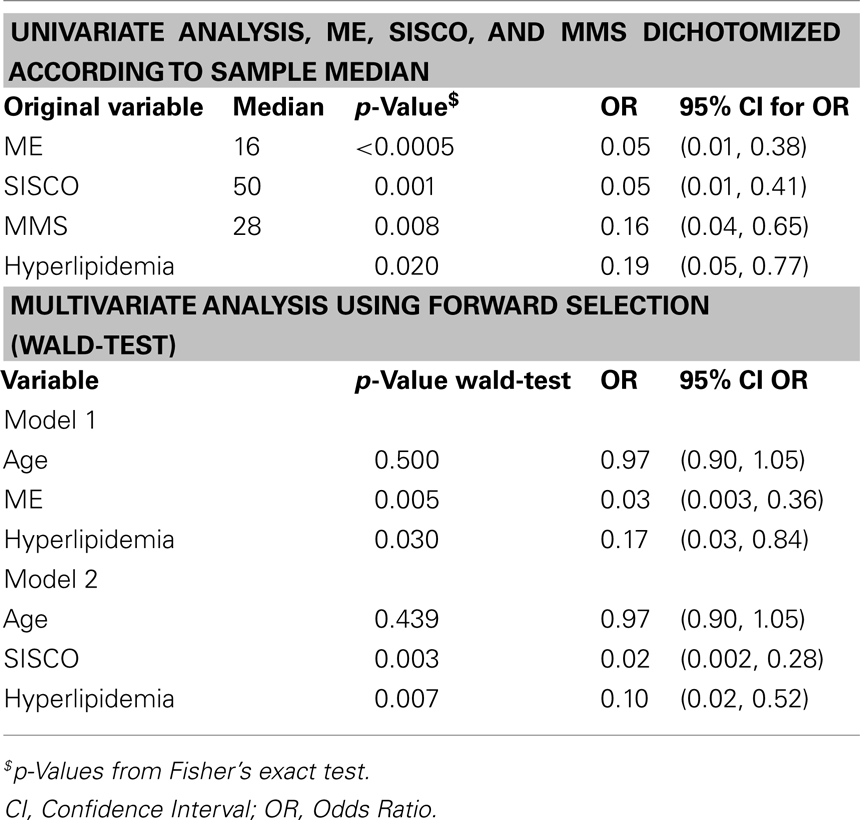
Table 2. Statistical analysis (dichotomization in no leukoaraiosis + mild leukoaraiosis versus moderate leukoaraiosis + severe leukoaraiosis).
Lacunar Lesions
Using four groups (no, single, two to five, and >5 lacunar lesion) for evaluation of mean values, the percentage of migraine, previous stroke events, hydrocephalus, LVH, and a higher NIHSS increased as the number of lacunar lesions increased. Using two groups (no or one lacunar lesion versus ≥2 lacunar lesions) for statistical analysis, univariate analysis (for continuous variables: Man–Whitney test; for categorical variables: Fisher’s exact test) revealed no significantly associated clinical parameters even at significance level 0.10. Therefore in view of only 14 patients with less then two lacunar lesions multivariate analysis was not performed.
Discussion
The pathogenesis of MBL is poorly understood. It remains unclear why some patients develop isolated lacunes while other patients also develop leukoaraiosis (Hassan et al., 2003). Hypertension is a major risk factor but fails to account for much of the risk (Boiten et al., 1993; Hassan et al., 2003). Imaging and autopsy-based studies propose two types of MBL. They separate patients with single lacunes without leukoaraiosis, a microatheromatous disease of larger perforating arteries, and the usual vascular risk factors versus patients with multiple lacunes and leukoaraiosis, diffuse arteriosclerosis of smaller perforating arteries, and a high frequency of hypertension (Fisher, 1969; Boiten et al., 1993; Khan et al., 2007).
Only few studies analyzed the two entities of MBL in a single study design. The present study investigated a variety of clinical parameters with regard to the extent of leukoaraiosis and lacunes. The study results demonstrated evidence for different clinical profiles in leukoaraiosis and lacunes, respectively.
The most reliable parameter regarding the severity of leukoaraiosis was the cognitive performance measured with the SIDAM including the SISCO, MMSE, and ME. The SIDAM test covers a broad range of cognitive functions for diagnosis of even mild cognitive impairment (Zaudig, 1992). Even the subitems MMSE and ME were sufficient to indicate at least moderate leukoaraiosis. Consistently, other MRI-based studies found that the extent of leukoaraiosis is strongly correlated with cognitive performance (Merino, 2008) and that the progression of leukoaraiosis is associated with progression in cognitive decline (Dijk et al., 2008). Interestingly, the presence of hyperlipidemia was a negative indicator of leukoaraiosis. Some authors suggest a protective role of hyperlipidemia against cerebral small-vessel disease (Warsch and Wright, 2010). Since 96% of our patients with known hyperlipidemia used statins, pharmacological effects have also to be considered.
Regarding the extent of lacunes, a higher NIHSS, previous stroke events, and LVH were more present in patients with multiple lacunes, but no statistical significance was found. Interestingly, the presence of migraine was higher in patients with multiple lacunes. Migraine is a leading clinical feature in some monogenetic small-vessel diseases such CADASIL or HERNS (Dichgans et al., 1998; Kruit et al., 2004). The etiology of MBL associated with migraine is unknown (Kruit et al., 2004). Spreading depolarizations are assumed to be the pathophysiological correlate of the migraine (Moskowitz, 2007). Small-vessel disease of the brain likely causes recurrent small perfusion deficits that might give rise to spreading depolarizations and migraine, consecutively (Dreier et al., 2002).
Arterial hypertension and older age were found in the vast majority of patients with leukoaraiosis and lacunes, as shown in other studies (de Leeuw et al., 2002). This validates arterial hypertension as an important risk factor for leukoaraiosis and lacunar lesions (Bryan et al., 1997; Lee et al., 2000). However, a correlation with the severity of MBL was not found. 25% of the study cohort had no hypertension, indicating that MBL are not synonymous with hypertensive microangiopathy.
The limiting factor of the present study is the small number of patients due to the intraindividually extraordinary expense for conducting a broad and general investigation. Follow up studies have to confirm the assumption of different microangiopathic entities. As a drawback of the MR protocol- the smallest lacunar lesions with a diameter of 5–6 mm might have been missed.
In conclusion, only few studies separately investigated leukoaraiosis and lacunar lesions. The present study found different clinical profiles of patients with leukoaraiosis and lacunes. This suggests that the pathophysiological process is likely to differ and different treatment concepts may be required (Khan et al., 2007).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was conducted in the setting of a government-sponsored stroke research project “Kompetenznetz Schlaganfall” (Bundesministerium für Bildung und Forschung, FK 01GI9903) and in the setting of the Center for Stroke Research Berlin.
References
Barkhof, F., and Scheltens, P. (2002). Imaging of white matter lesions. Cerebrovasc. Dis. 13, 21–30.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, N., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571.
Blennow, K., Wallin, A., Uhlemann, C., and Gottfries, C. G. (1991). White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol. Scand. 83, 187–193.
Boiten, J., Lodder, J., and Kessels, F. (1993). Two clinically distinct lacunar infarct entities? A hypothesis. Stroke 24, 652–656.
Bryan, R. N., Wells, S. W., and Miller, T. J. (1997). Infarctlike lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly – data from the cardiovascular health study. Radiology 202, 47–54.
Carter, B. L. (2004). Implementing the new guidelines for hypertension: JNC 7, ADA, WHO-ISH. J. Manag. Care Pharm. 10, 18–25.
Dahlof, B., Devereux, R. B., Julius, S., Kjeldsen, S. E., Beevers, G., de Faire, U., Fyhrquist, F., Hedner, T., Ibsen, H., Kristianson, K., Lederballe-Pedersen, O., Lindholm, L. H., Nieminen, M. S., Omvik, P., Oparil, S., and Wedel, H. (1998). Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan intervention for endpoint reduction in hypertension. Hypertension 32, 989–997.
de Leeuw, F. E., de Groot, J. C., Oudkerk, M., Witteman, J. C., Hofman, A., van Gijn, J., and Breteler, M. M. (2002). Hypertension, and cerebral white matter lesions in a prospective cohort study. Brain 125, 765–772.
Dichgans, M., Mayer, M., Uttner, I., Brüning, R., Müller-Höcker, J., Rungger, G., Ebke, M., Klockgether, T., and Gasser, T. (1998). The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann. Neurol. 44, 731–739.
Dijk, E. J., Prins, N. D., Vrooman, H. A., Hofman, A., Koudstaal, P. J., and Breteler, M. M. (2008). Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 39, 2712–2719.
Dreier, J. P., Kleeberg, J., Petzold, G., Priller, J., Windmüller, O., Orzechowski, H. D., Lindauer, U., Heinemann, U., Einhäupl, K. M., and Dirnagl, U. (2002). Endothelin-1 potently induces Leão’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain 125, 102–112.
Hassan, A., Hunt, B. J., O’Sullivan, M., Parmar, K., Bamford, J. M., Briley, D., Brown, M. M., Thomas, D. J., and Markus, H. S. (2003). Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 126, 424–432.
Khan, U., Porteous, L., Hassan, A., and Markus, H. S. (2007). Risk factor profile of cerebral small vessel disease and its subtypes. J. Neurol. Neurosurg. Psychiatr. 78, 702–706.
Kim, M. H., Moon, J. S., Park, S. Y., An, S. A., Kim, O. J., Kim, N. K., and Oh, S. H. (2011). Different risk factor profiles between silent brain infarction and symptomatic lacunar infarction. Eur. Neurol. 65, 250–256.
Kruit, M. C., van Buchem, M. A., Hofman, P. A., Bakkers, J. T., Terwindt, G. M., Ferrari, M. D., and Launer, L. J. (2004). Migraine as a risk factor for subclinical brain lesions. JAMA 291, 427–434.
Lammie, G. A. (2002). Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 12, 358–370.
Lee, S. C., Park, S. J., Ki, H. K., Gwon, H. C., Chung, C. S., Byun, H. S., Shin, K. J., Shin, M. H., and Lee, W. R. (2000). Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension 36, 73–77.
Mohr, J. P., and Marti-Vilalta, J.-L. (1998). “Lacunes,” in Stroke: Pathophysiology, diagnosis, and management, eds H. J. M. Barnett, J. P. Mohr, B. M. Stein, and F. M. Yatsu (Philadelphia, PA: Churchill Livingstone), 599–622.
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701.
Pantoni, L., Simoni, M., Pracucci, G., Schmidt, R., Barkhof, F., and Inzitari, D. (2002). Visual rating scales for age-related white matter changes (leukoaraiosis): can the heterogeneity be reduced? Stroke 33, 2827–2833.
Peters, A. L., Davidson, M. B., Schriger, D. L., and Hasselblad, V. (1996). A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. Meta-analysis research group on the diagnosis of diabetes using glycated hemoglobin levels. JAMA 276, 1246–1252.
Sander, D., Winbeck, K., Klingelhöfer, J., and Conrad, B. (2000). Extent of cerebral white matter lesions is related to changes of circadian blood pressure rhythmicity. Arch. Neurol. 57, 1302–1307.
Simons, P. C., Algra, A., Bots, M. L., Grobbee, D. E., and van der Graaf, Y. (1999). Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART study (second manifestations of ARTerial disease). Circulation 100, 951–957.
Vriz, O., Piccolo, D., Cozzutti, E., Milani, L., Gelisio, R., Pegoraro, F., Garavelli, G., D’Este, D., and Palatini, P. (1998). The effects of alcohol consumption on ambulatory blood pressure and target organs in subjects with borderline to mild hypertension. HARVEST study group. Am. J. Hypertens 11, 230–234.
Wahlund, L. O., Barkhof, F., Fazekas, F., Bronge, L., Augustin, M., Sjogren, M., Wallin, A., Ader, H., Leys, D., Pantoni, L., Pasquier, F., Erkinjuntti, T., Scheltens, P., and European Task Force on Age-Related White Matter Changes. (2001). A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32, 1318–1322.
Warsch, J. R. L., and Wright, C. B. (2010). Stroke: hyperlipidemia and cerebral small-vessel disease. Nat. Rev. Neurol. 6, 307–308.
Wong, T. Y. (2004). Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 3, 179–183.
Wong, T. Y., Klein, R., Sharrett, A. R., Couper, D. J., Klein, B. E., Liao, D. P., Hubbard, L. D., Mosley, T. H., and ARIC Investigators. (2002). Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 288, 67–74.
Keywords: microangiopathic brain lesions, leukoaraiosis, lacunar lesion, cardiovascular risk factor
Citation: Leistner S, Koennecke H-C, Dreier JP, Strempel A-K, Kathke M, Nikolova A, Heuschman P, Malzahn U, Audebert HJ and Mackert B-M (2011) Clinical characterization of symptomatic microangiopathic brain lesions. Front. Neur. 2:61. doi: 10.3389/fneur.2011.00061
Received: 19 April 2011; Accepted: 04 September 2011;
Published online: 22 September 2011.
Edited by:
Bruce Coull, University of Arizona, USAReviewed by:
Ashfaq Shuaib, University of Alberta, CanadaMichael Brainin, Donau-Universität Krems, Austria
Marc Malkoff, University of New Mexico, USA
Copyright: © 2011 Leistner, Koennecke, Dreier, Strempel, Kathke, Nikolova, Heuschman, Malzahn, Audebert and Mackert. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Stefanie Leistner, Department of Neurology, Charite – University Medicine Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany. e-mail:c3RlZmFuaWUubGVpc3RuZXJAY2hhcml0ZS5kZQ==

 Hans-Christian Koennecke2
Hans-Christian Koennecke2