- 1Departamento de Neurologia, Psicologia e Psiquiatria, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista (UNESP), Botucatu, Brazil
- 2Departamento de Doenças Tropicais e Diagnósticos por Imagem, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista (UNESP), Botucatu, Brazil
A 59-year-old man was admitted with respiratory tract infection, compromised conscience and generalized tonic–clonic seizures. His medical history included schizophrenia diagnosis, for which he had been being treated since he was 27 years old. EEG disclosed non-convulsive status epilepticus. A magnetic resonance image (MRI) acquired 3 days later showed increased left hippocampal volume with hyperintensity on T2-weighted and FLAIR sequences. After being treated with antibiotics and antiepileptic medications, the patient’s condition improved. A follow-up MRI showed reduction of the left hippocampus. The relationship between epilepsy and schizophrenia is not yet clear. This case illustrates this interaction. Hippocampal atrophy may have been caused by environmental aggression in the present patient with schizophrenia, perhaps in association with a predisposing genotype.
Background
Hippocampal atrophy (HA) is often seen with mesial temporal lobe epilepsy (MTLE), the most prevalent form of focal epilepsy in adults (1). Typically, patients with MTLE suffer from refractory seizures and may have a history of prolonged febrile seizures dating back to childhood (1). In adults, HA may be secondary to an acute insult. We report magnetic resonance image (MRI) findings disclosing hippocampal swelling associated with status epilepticus followed by HA 3 months later in an adult previously diagnosed with schizophrenia.
Introduction
A 59-year-old man was admitted to the emergency room with compromised consciousness for 1 day. Relatives reported he had been experiencing coughing, prostration, and fever during the week before admission and generalized tonic–clonic seizures 3 days earlier, after which he became unconscious. His medical history included schizophrenia diagnosis, for which he had been treated since he was 27 years old. He was taking risperidone 1 mg/day and biperiden 2 mg/day with satisfactory control of symptoms. Physical examination revealed that the patient had a tonic posture of the limbs and deviation of the head and eyes. Non-convulsive status epilepticus (NCSE) was suspected.
EEG was performed with a 32-channel recorder (Nihon Kohden, Tokyo, Japan). Electrodes were positioned according to the 10–20 international system of electrode placement and with additional Silverman’s anterior temporal electrodes. EEG disclosed recurrent seizures, and the NCSE was terminated with midazolan and phenytoin (Figure 1).
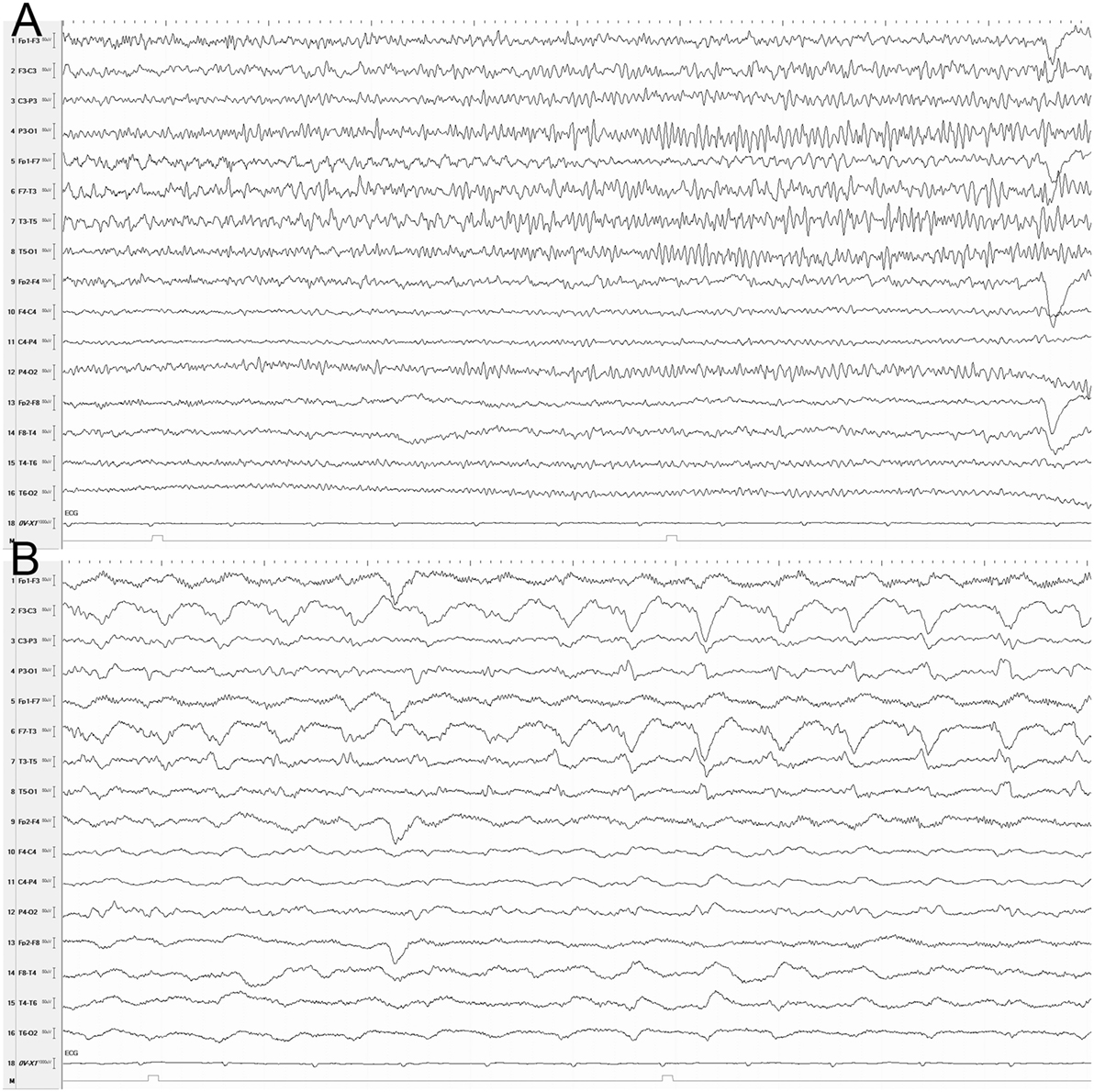
Figure 1. EEG (longitudinal montage) showing a seizure at onset (A) and 30 s later (B). (A) Rhythmic theta activity, mainly in the left temporal region. (B) Rhythmic delta and sharp waves in the left hemisphere. Sensitivity, 10 µV; time constant: 0.3 s; high filter, 30 Hz; Notch filter, 60 Hz; 10 s/page.
Blood samples showed elevated C-reactive protein (4.0 mg/L), leukocytosis (14,000 white blood cells/μL; 77% neutrophils, 12% lymphocytes, and 11% monocytes), and very elevated creatine phosphokinase (12,000 U/L); all other parameters were normal. Cerebrospinal fluid and chest X-rays were also normal. A computerized tomography scan of the brain on the day of admission showed no alterations.
An MRI was acquired using a 3 T scanner (Siemens, Verio, Erlangen, Germany) with a 12-channel head array coil 3 days later. Coronal slices with 3-mm thickness perpendicular to the long axis of the hippocampus were included to evaluate temporal lobe abnormalities. MRI showed increased left hippocampal volume with hyperintensity on T2-weighted and FLAIR sequences (Figure 2A). After being treated with ceftriaxone, the patient’s responsiveness and overall clinical condition improved. Corticosteroids and antiviral therapy were not used. A follow-up MRI 3 months after discharge showed reduction of the left hippocampus with enlargement of the left ventricle temporal horn (Figure 2B). Six months after discharge, the patient remains seizure-free using valproate 1,500 mg/day.
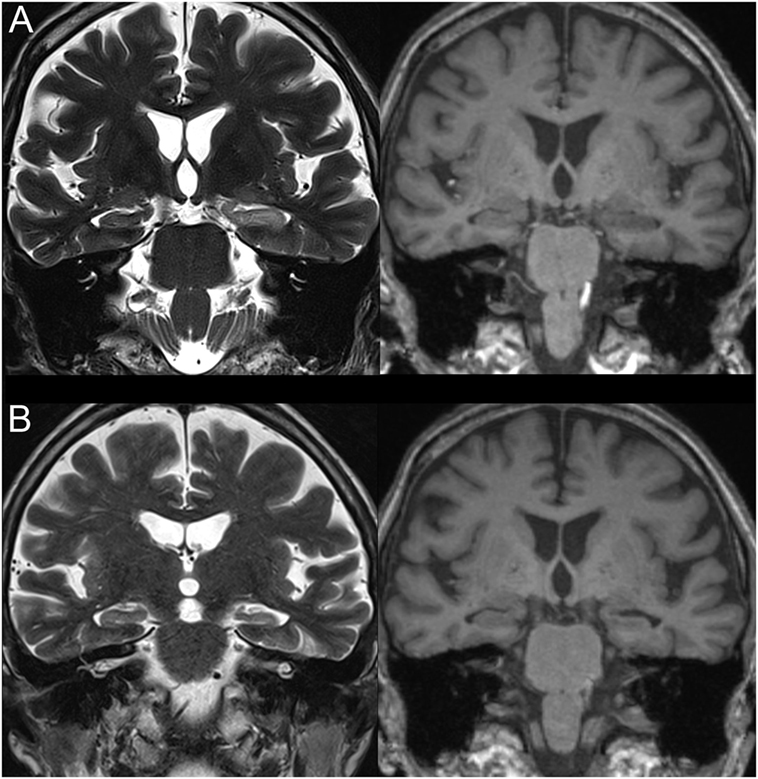
Figure 2. T1- (right) and T2- (left) weighted magnetic resonance imaging images (coronal slices). (A) Increased left hippocampal volume with increased T2 and decreased T1 signals. (B) Reduced left hippocampal volume without signal changes.
Discussion
Psychotic symptoms occur in 2–7% of patients with epilepsy (2). There is controversy regarding whether schizophrenia is a risk factor for epilepsy (3). Prevalence rates of psychosis in epilepsy may differ according to seizure-symptom onset interval. When the interval is short (post-ictal psychosis), estimates indicate that 2–11% of patients with epilepsy have comorbid psychosis. Interictal psychosis may be present in 3–9% of epilepsy cases (2).
The relationship between epilepsy and schizophrenia is not yet clear. Gelisse et al. (4) showed that the prevalence of epilepsy and acute symptomatic seizures is relatively low in patients with schizophrenia, compared to the general population. In a study comparing epilepsy patients receiving disability benefits with people with other somatic diseases, Stefansson et al. (5) found that 6.2% of the epilepsy group had a psychosis diagnosis, particularly schizophrenia and paranoid states, compared to only 2.3% of the control group, suggesting that the association between seizures and schizophrenia may involve common genetic or environmental causes (3). In a large 9-year Danish study (N = 558,958), an increased risk of schizophrenia (44%) was found in people with a history of febrile seizures. Both febrile seizures and epilepsy were associated with a more than doubled risk of schizophrenia (6). Likewise, MTLE has also been related strongly with febrile seizures (7).
Magnetic resonance image findings in patients with schizophrenia have included enlarged ventricles and whole-brain volume reduction, especially in the prefrontal cortex, superior temporal gyrus, hippocampus, and amygdala (3). Similar to MTLE, amygdalo–hippocampal circuitry might play an important role in the development of schizophrenia (8). Meanwhile, several neuroimaging studies have demonstrated acute swelling of the hippocampus after prolonged febrile seizures. These findings are related predominantly to prolonged febrile seizures in childhood (9, 10).
Genetic predisposition is an important causal factor of febrile seizures and HA (11). Several genes have been associated with susceptibility to schizophrenia (3). Abnormal neurodevelopmental hypothesis may explain the common occurrence of epilepsy and schizophrenia (3).
Subacute progressive decrease in the level of consciousness is the typical presentation of encephalitis. Infectious and autoimmune encephalitis are important differential diagnosis, which were considered in the current case. Most of infectious encephalitis are viral, and psychiatric symptoms are common early in the course of autoimmune encephalitis (12). Unfortunately, viral panel in the CSF and antibody testing were not performed. The hypotheses of viral and autoimmune encephalitis were weakened by the long duration of the psychiatric symptoms and the substantial response to antibiotics without the significant use of corticosteroids or antiviral therapy. Probably toxic-metabolic encephalopathy caused by pulmonary bacterial infection was the trigger to the status epilepticus.
In subjects with MTLE, the development of HA remains under investigation (13). The cause–consequence relationship between seizures and HA still have to be clarified. In adults, prolonged seizure and status epilepticus may lead to pathologically documented HA (14). In the present case, history of subtle or febrile seizures was negative, but they cannot be completely excluded. Therefore, in this setting, the interaction between genetical background with schizophrenia phenotype, age, and the acute infectious disease probably were decisive for the HA pathogenesis.
Concluding Remarks
Epilepsy and schizophrenia may share susceptibility. Epilepsy is associated with an increased risk of schizophrenia-like symptoms. This case illustrates this possible relationship. HA may have been caused by environmental aggression in the present patient with schizophrenia, perhaps in association with a predisposing genotype.
Ethics Statement
Retrospective case report (exempted). Informed consent was signed for publication. Patient was not identified.
Author Contributions
NC, AB, and PC made substantial contributions to the collection, analysis and interpretation of the data, and revising the manuscript. SY made substantial contributions to the analysis and interpretation of the data and revising the manuscript. EF and LB made substantial contributions to the conception and design of the study, collection, analysis and interpretation of data, as well as to the preparation of the first draft and further revisions of the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest Statement
EF, NC, AB, PC, SY, and LB report no disclosures.
Funding
Supported by grant number 2011/02961-2, São Paulo Research Foundation (FAPESP).
References
1. Engel J Jr. Mesial temporal lobe epilepsy: what have we learned? Neuroscientist (2001) 7(4):340–52. doi:10.1177/107385840100700410
2. Araújo-Filho GM, Rosa VP, Yacubian ELT. Psychiatric disorders in epilepsy: a proposal for classification by the ILAE commission on neuropsychiatry. J Epilepsy Clin Neurophysiol (2008) 14(3):119–23.
3. Cascella NG, Schretlen DJ, Sawa A. Schizophrenia and epilepsy: is there a shared susceptibility? Neurosci Res (2009) 63(4):227–35. doi:10.1016/j.neures.2009.01.002
4. Gelisse P, Somalian JC, Genton P. Is schizophrenia a risk factor for epilepsy or acute symptomatic seizures? Epilepsia (1999) 40:1566–71. doi:10.1111/j.1528-1157.1999.tb02041.x
5. Stefansson SB, Olafsson E, Hauser WA. Psychiatric morbidity in epilepsy: a case controlled study of adults receiving disability benefits. J Neurol Neurosurg Psychiatry (1998) 64:238–41. doi:10.1136/jnnp.64.2.238
6. Vestergaard M, Pedersen CB, Christensen J, Madsen KM, Olsen J, Mortensen PB. Febrile seizures and risk of schizophrenia. Schizophr Res (2005) 73:343–9. doi:10.1016/j.schres.2004.07.004
7. Malmgren K, Thom M. Hippocampal sclerosis – origins and imaging. Epilepsia (2012) 53(Suppl 4):19–33. doi:10.1111/j.1528-1167.2012.03610.x
8. Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain (1999) 122(Pt 4):593–624. doi:10.1093/brain/122.4.593
9. VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol (1998) 43:413–26. doi:10.1002/ana.410430403
10. Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain (2002) 125:1951–9. doi:10.1093/brain/awf202
11. Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol (2004) 7(2):161–4. doi:10.1097/00019052-200404000-00013
12. Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol (2016) 12(1):1–13. doi:10.3988/jcn.2016.12.1.1
13. Kobayashi E, Li LM, Lopes-Cendes I, Cendes F. Magnetic resonance imaging evidence of hippocampal sclerosis in asymptomatic, first-degree relatives of patients with familial mesial temporal lobe epilepsy. Arch Neurol (2002) 59(12):1891–4. doi:10.1001/archneur.59.12.1891
Keywords: temporal lobe epilepsy, hippocampal sclerosis, neuroimaging, schizophrenia, EEG
Citation: Fujisao EK, Cristaldo NR, Braga AM, Cunha PR, Yamashita S and Betting LE (2017) Hippocampal Damage and Atrophy Secondary to Status Epilepticus in a Patient with Schizophrenia. Front. Neurol. 8:24. doi: 10.3389/fneur.2017.00024
Received: 24 September 2016; Accepted: 17 January 2017;
Published: 06 February 2017
Edited by:
Fernando Cendes, State University of Campinas, BrazilReviewed by:
Marino M. Bianchin, Universidade Federal do Rio Grande do Sul, BrazilMario Alonso, Instituto Nacional de Neurologia y Neurocirugía, Mexico
Copyright: © 2017 Fujisao, Cristaldo, Braga, Cunha, Yamashita and Betting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luiz Eduardo Betting, YmV0dGluZ0BmbWIudW5lc3AuYnI=
 Elaine Keiko Fujisao
Elaine Keiko Fujisao Nathalia Raquel Cristaldo
Nathalia Raquel Cristaldo Aline Marques da Silva Braga1
Aline Marques da Silva Braga1 Paulina Rodrigues Cunha
Paulina Rodrigues Cunha Luiz Eduardo Betting
Luiz Eduardo Betting