- 1Department of Neurology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
- 2School of Medicine, Shanghai Jiao Tong University, Shanghai, China
The classical triad—ophthalmoplegia, cerebellar dysfunction, and altered mental state—in addition to bilateral symmetrical periventricular lesions are actually common to see, and clinicians tend to associate that with Wernicke's encephalopathy (WE). The diagnosis is strengthened with a likely deficiency of thiamine. We herein describe a malnourished patient with clinical triad and hyperintensities in the circumventricular regions, and she turned out to have neuromyelitis optica spectrum disorder (NMOSD) after many twists and turns. Despite totally different pathogenic mechanisms, NMOSD can mimic WE, sometimes even exhibiting radiological features similar to that of WE, thereby complicating the diagnosis. Our case highlights how similar these two diseases could be and the importance of differential diagnosis in clinical practice, which are so far rarely reported. Some clinical and radiological differences of these two diseases are summarized to help establish a prompt diagnosis.
Background
NMOSD is a relapsing autoimmune disorder of the central nervous system (CNS), predominately affecting the optic nerve and spinal cord (1). WE is a metabolic brain disease resulting from thiamine deficiency, characterized by the triad of ophthalmoplegia, altered mental state, and trunk ataxia (2). Despite totally different pathogenic mechanisms, these two diseases have some overlaps in clinical and imaging features. However, the differential diagnosis between NMOSD and WE is overlooked in clinical settings (3). Here, we report a case in which the clinical and radiological findings misled us into the diagnosis of non-alcoholic WE before the AQP4-Ab positive result was readily available.
Case Presentation
A 28-year-old woman was admitted to the hospital with progressive ocular symptoms as well as newly developed hemianesthesia and mental signs. Half a month before hospitalization, she complained of double eyelid droop and diplopia; the ocular weakness and fatigue was stable throughout the day and had nothing to do with physical activity. The local hospital suspected myasthenia gravis, a computed tomography scan of her brain revealed nothing, and her serum was collected to detect the acetylcholine antibody. While waiting for a hospital bed, she developed numbness in her right upper and lower extremities. At the same time, she presented with prolonged sleep duration and mental symptoms. She was then transferred to our institution. On admission, she was noted to have difficulty walking by herself due to truncal ataxia. There was no cold, fever, or diarrhea before her symptom onset.
She had been suffering from chronic anemia, and her family reported that she had dietary deficiencies due to poor appetite although she denied it herself. She was on no other medications, not pregnant, and did not use alcohol or illicit drugs.
On physical examination, the patient presented obviously unbalanced nutrition; she was 1.65 m in height yet only weighed 40 kg. She was conscious and oriented and scored 23 in the MMSE, mainly due to impaired memory and calculation performance. Eye signs were obvious; she had drooping eyelids (both 5 mm). The ophthalmoplegia was remarkable; she demonstrated bilateral abducens nerve palsies and adduction deficit of both eyes. Limitation and nystagmus on vertical gaze were also noted. There was slight dysmetria on both finger–nose and heel–shin testing. Limb dysdiadochokinesia was also observed. Bilateral pyramidal signs were positive. Sensory examination indicated hyperesthesia on the right side. Findings on muscle tone and strength tests were normal.
She had an abnormal Hb level of 92 g/L, accompanied by low levels of serum folate and ferritin; vitamin B12 level was normal. Laboratory tests also revealed elevated levels of CA199 and CA242; both were gastrointestinal tumor markers although an abdominal enhanced CT scan reported no findings. The CSF revealed an increased white blood cell count of 28/mL (dominantly lymphocytes); other routine analyses of the cerebrospinal fluid (CSF) were normal, including protein level and IgG index (0.49). Also, no oligoclonal bands were found. Additional laboratory workup—including rheumatology analysis—was negative.
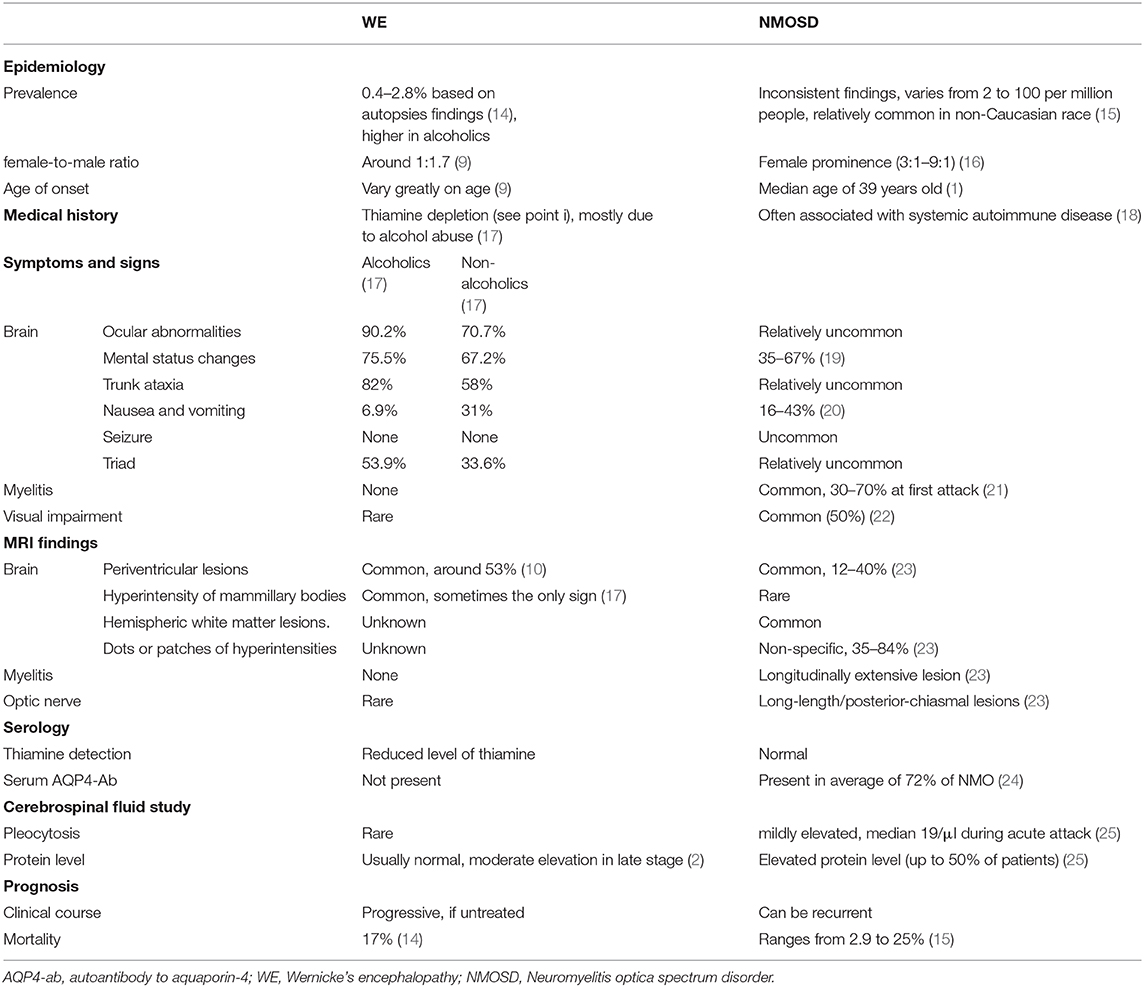
Magnetic resonance imaging of the brain (Figure 1) showed symmetric T2 hyperintensities predominantly located within periaqueductal gray matter and periventricular regions of the fourth ventricles. A diffusion-weighted sequence also showed a hyperintense signal along the floor of the fourth ventricle. The presence of a lateral ventricle lesion was also noted. All brain lesions did not show contrast enhancement. Nerve conduction studies and visual evoked potentials were normal.
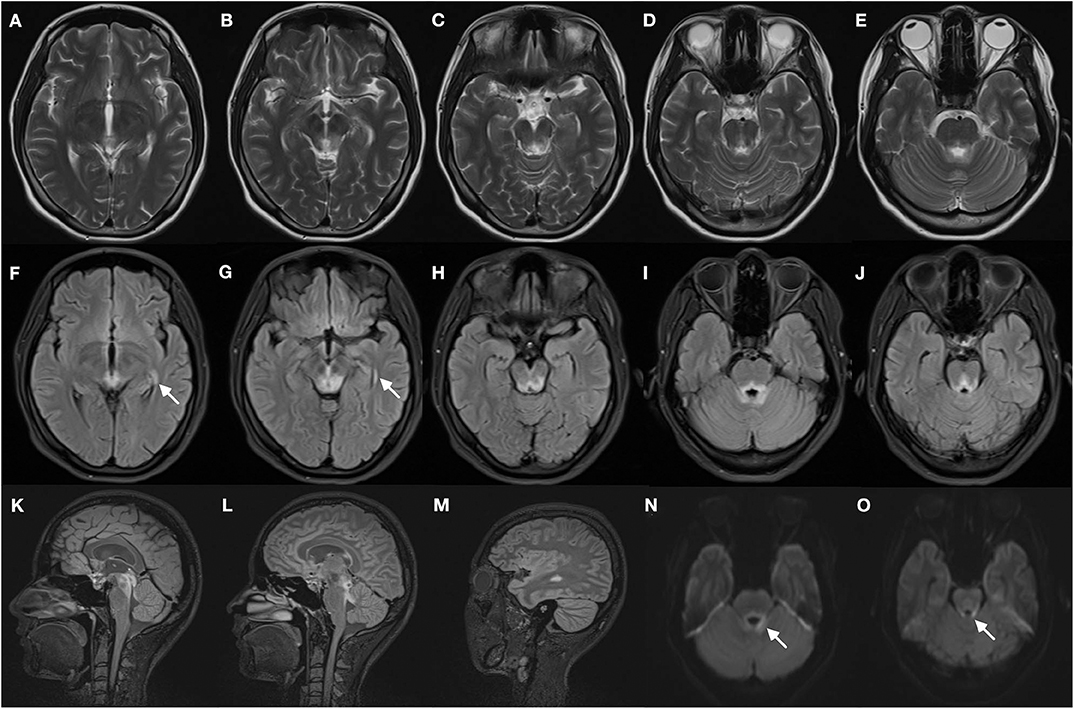
Figure 1. Brain magnetic resonance images of the patient. T2-weighted images (A–E) demonstrate bilateral symmetrical hyperintense lesions in periaqueductal regions and around the fourth ventricle, which include superior colliculus (A), ventral tegmental area (B), inferior colliculus (C), and superior cerebellar peduncle (D,E). The abnormal regions revealed more obvious hyperintensity signal on corresponding FLAIR images (F–J), increased signal in the lateral ventricles was found (F,G, arrows). The FLAIR sagittal slice showed apparent hyperintense signal in the periaqueductal gray region (K) and surrounding the fourth ventricle (L), and lesions in the lateral ventricles (M). DWI images also manifest restricted water diffusion adjacent to the fourth ventricle (N,O, arrows).
In view of the possible diagnosis of WE, thiamine was started intramuscularly (300 mg 12-h). However, poor clinical recovery was observed. Her mental status worsened and exhibited hallucination, and the ocular palsy also progressed.
Then came the crucial result that her serum and cerebral fluid tested positive for NMOSD-IgG (1:32 and 1:3.2, respectively). Thereafter, the spinal cord was evaluated, and MRI of the spine revealed a suspicious enhanced T2-weighted signal at the lower thoracic cord region (Figure 2).
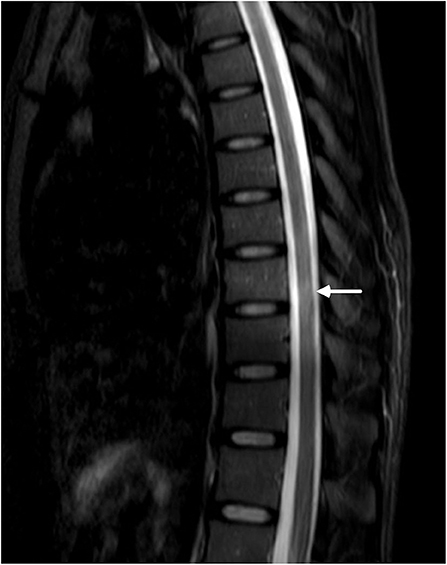
Figure 2. MRI of the lower thoracic spine showed T2 hyperintense lesions from T7 through T9 (arrow). However, the signal was blurry, and we considered it to be undetermined.
We corrected the diagnosis, and the patient started with treatment of high-dose intravenous steroids, but still little improvement was detected. The patient then experienced bilateral visual loss, but MRI results of the optic nerve reported no findings. After that, intravenous immunoglobulin treatment was given, followed by intravenous cyclophosphamide therapy. On discharge, she showed moderate improvement in eye movement and ataxia although her vision remained unchanged.
Discussion
In our case, the patient presented the classical triad of ocular signs, mental status changes, and unsteadiness of gait; even the MRI lesion patterns perfectly corresponded to the diagnosis of non-alcoholic WE. Underlying digestive tract cancer (clued by her chronic anemia and elevated tumor markers) was suspected to be responsible for the malabsorption of vitamin B1, making the diagnosis fairly reassuring. But there were some discoveries that did not match the diagnosis. First, the pleocytosis in CSF was incompatible with a WE diagnosis. Second, the patient didn't achieve the desired therapeutic effect. WE patients are reported to have good and even dramatic responses to thiamine treatment, and in our case, very little recovery occurred despite adequate supplementation with thiamine.
Interestingly, WE and NMOSD both feature periependymal lesions surrounding the ventricular system, thus sharing some clinical and imaging features. Contrasting different pathogenic processes of these two diseases may help us to better understand such unique brain abnormalities. For NMOSD seropositive for anti-AQP4 antibody, the pathogenesis is initiated by the specific autoantibody AQP4-IgG targeting the autoantigen, aquaporin-4, thus particularly demonstrating abnormalities in brain regions with high AQP4 expression, that is, pial and ependymal surfaces in contact with the CSF (4). The entry of AQP4–IgG into the CNS and its binding inhibits regional AQP4 water permeability, causing astrocyte swelling (5). The exact mechanism that accounts for the typical lesion pattern of WE remains unclear. One possible hypothesis is that thiamine deficiency results in insufficient production of thiamine pyrophosphate (6), which is an important coenzyme to process carbohydrate metabolism in the brain (7). As a result of energy metabolism dysfunction, lactic acid starts to accumulate in the focal regions of brain. And it is reported that lactic acidosis may mediate AQP-4 overexpression in astrocytes, leading to cytotoxic edema (8). Once again, AQP-4 is particularly concentrated on circumventricular areas (4), which are also selective histological lesion locations for WE.
At present, the diagnosis of WE is primarily clinical as thiamine measurement is often unavailable in many hospitals, and no specific abnormalities have been identified in other laboratory tests (9). MRI also functions as a supportive diagnostic adjunct. Although it is reported that MRI has a high specificity of 93% for the diagnosis of WE (10), we should not forget that such brain lesions are also typical patterns of NMOSD. NMOSD has variable clinical presentations; involvement of brainstem and diencephalon could cause the clinical triad of ophthalmoplegia, ataxia, and confusion, all of which can be seen in WE. And we particularly have to point out that visual loss is not exclusive for NMOSD, WE can also manifest as visual deterioration, which is often resulted from optic disk swelling (11) but in relatively low incidence.
The differential diagnosis between WE and NMO tends to be overlooked and possibly underreported, which may lead to an incorrect or delayed diagnosis. In a case reported by Nyo et al. (12), a young woman with SLE developed the clinical triad of mild confusion, ataxia, and ophthalmoplegia; hyperintensity in the thalami and cerebellar of MRI was noticed, and then she was diagnosed as WE secondary to a likely gastrointestinal SLE. After treating with intravenous methylprednisolone and oral prednisone, the patient had a full neurological recovery. However, in our opinion, the possibility of NMOSD should be considered for the following reasons: (1) She was a young woman, (2) she had a medical history of SLE, (3) the symptoms of pancerebellar ataxia and dysarthria were unusual for WE, (4) she had raised protein concentrations in CSF, (5) she had indefinite thiamine deficiency, and (6) effective treatment of cortisol was combined with thiamine. Long-term follow-up is highly recommended for this person, and AQP4-Ab should be detected if possible.
Its extremely important to differentiate WE from NMOSD for they are distinct in treatment. NMOSD requires immunosuppressive therapy, and glucocorticoid is effective at reducing inflammation (13). Although WE requires emergent thiamine supplementation, the management of glucocorticoid should be cautious because exact effects of steroid treatment in WE are unclear.
We summarize features that differ between NMOSD and WE in Table 1; meanwhile, some important points are highlighted below.
Thiamine Deficiency
A history of thiamine depletion is necessary before reaching a diagnosis of WE, and it has been emphasized as the fourth sign apart from the triad in the revised criteria (17). If commercially available, measurement of blood thiamine or its phosphate ester concentrations should be performed to confirm the thiamine deficiency. Although alcohol abuse accounts for most cases, non-alcoholic WE has also been frequently reported, and according to Galvin et al. (17), the most common conditions are tumors, gastrointestinal disease, and surgery as well as hyperemesis gravidarum. Other causes reported include starvation, dieting, and unbalanced diets. Symptoms such as wet beriberi or polyneuropathy also hint at thiamine insufficiency (26). However, we sometimes overestimate the unbalanced nutrition or a potential malignant disorder as shown in our case. Still, any patient suspected of having WE should be treated with a high dose of thiamine supplementation immediately, considering the significant mortality and neurological disabilities. Also, recovery of the neurological state after administration of thiamine is valuable for diagnosis (9).
CSF Assay
Lumbar puncture should be done on every suspected WE. CSF testing is normal in most WE patients although increased protein levels in late stages were occasionally reported (2). Moderate pleocytosis in the CSF is often a feature of NMOSD (25), which is rare to see in WE.
Brain MRI Differences
Distinguishing WE from NMOSD can be tough when they manifest a similar altered signal in lesions around the third ventricles, adjacent to the fourth ventricle, or surrounding the lateral ventricles. Thalamus, hypothalamus, and brainstem are also susceptible in both diseases. But, generally speaking, brain lesions in the mammillary bodies favor the diagnosis of WE (27), whereas NMOSD frequently features abnormalities in hemispheric white matter and optic nerve lesions (28).
Spinal Cord Involvement
To our knowledge, WE selectively involves cerebral, and there is no spinal cord engagement. Therefore, thorough investigations in search of an underlying sensory level is warranted when making differential diagnoses. Also, in patients suspected to have NMOSD, spinal cord MRI should be performed.
AQP4-IgG
The AQP4-Ab assay should be tested for suspected NMOSD, and sometimes, the presence of this specific marker can play a determinant role in distinguishing these diseases. However, the absence of AQP4-IgG doesn't exclude the possibility of NMOSD (29).
Conclusion
The overlaps in phenotypes between WE and NMOSD might obscure the correct diagnosis. Our case highlights that, for those presenting with typical triad and circumventricular lesions, NMOSD should not be neglected as an important differential diagnosis, especially for young women. Some clinical and neuroimaging evidence can help distinguish NMOSD from WE. Prompt recognition and proper treatment can prevent neurological deterioration and improve the outcome. Still, patients suspected to have WE should be empirically treated with thiamine promptly.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Shanghai sixth hospital ethics committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LY drafted the manuscript. ZX, JD, and JY critically reviewed the manuscript.
Funding
This work was supported by Western Medicine Guidance Project of Shanghai Science and Technology Commission (Grant No. 19411971400).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to our colleagues in the Department of Neurology and Radiology at Shanghai Jiao Tong University Affiliated Sixth People's Hospital who assisted us in our work. We thank Zhijuan Miao for her help in case information management.
References
1. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. (2007) 6:805–15. doi: 10.1016/S1474-4422(07)70216-8
2. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke's encephalopathy. N Engl J Med. (1985) 312:1035–9. doi: 10.1056/NEJM198504183121606
3. Shan F, Zhong R, Wu L, Fan Y, Long Y, Gao C. Neuromyelitis optica spectrum disorders may be misdiagnosed as Wernicke's encephalopathy. Int J Neurosci. (2016) 126:922–7. doi: 10.3109/00207454.2015.1084619
4. Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays ofastrocyte and ependymocyte plasma membranes in rat brain and spinalcord. Proc Natl Acad Sci USA. (1998) 95:11981–6. doi: 10.1073/pnas.95.20.11981
5. Papadopoulos MC, Verkman A. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. (2012) 11:535–44. doi: 10.1016/S1474-4422(12)70133-3
6. Schenker S, Henderson GI, Hoyumpa A Jr, Mccandless DW. Hepatic and Wernicke's encephalopathies: current concepts of pathogenesis. Am J Clin Nutr. (1980) 33:2719–26. doi: 10.1093/ajcn/33.12.2719
7. Manzo L, Locatelli C, Candura SM, Costa LG. Nutrition and alcohol neurotoxicity. Neurotoxicology. (1994) 15:555–65.
8. Morishima T, Aoyama M, Iida Y, Yamamoto N, Hirate H, Arima H, et al. Lactic acid increases aquaporin 4 expression on the cell membrane of cultured rat astrocytes. Neurosci Res. (2008) 61:18–26. doi: 10.1016/j.neures.2008.01.005
9. Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. (2007) 6:442–55. doi: 10.1016/S1474-4422(07)70104-7
10. Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandezsola J, Urbanomarquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. Am J Roentgenol. (1998) 171:1131–7. doi: 10.2214/ajr.171.4.9763009
11. Cooke CA, Hicks E, Page AB, Mc Kinstry S. An atypical presentation of Wernicke's encephalopathy in an 11-year-old child. Eye. (2006) 20:1418–20. doi: 10.1038/sj.eye.6702275
12. Nyo MTL, Magazi D, Ally MMTM. Systemic lupus erythematosus: a possible cause of non-alcoholic Wernicke's encephalopathy. S Afr Med J. (2017) 107:299. doi: 10.7196/SAMJ.2017.v107i4.12053
13. Torres J, Pruitt A, Balcer L, Galetta S, Markowitz C, Dahodwala N. Analysis of the treatment of neuromyelitis optica. J Neurol Sci. (2015) 351:31–5. doi: 10.1016/j.jns.2015.02.012
14. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. (1970) 7:1–206.
15. Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite M, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler J. (2015) 21:845–53. doi: 10.1177/1352458515572406
16. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Clinical spectrum of CNS aquaporin-4 autoimmunity. Neurology. (2012) 78:1179–85. doi: 10.1212/WNL.0b013e31824f8069
17. Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy: EFNS guidelines for Wernicke encephalopathy. Eur J Neurol. (2010) 17:1408–18. doi: 10.1111/j.1468-1331.2010.03153.x
18. Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non-organ-specific autoimmunity. Arch Neurol. (2008) 65:78–83. doi: 10.1001/archneurol.2007.17
19. Eizaguirre MB, Alonso R, Vanotti S, Garcea O. Cognitive impairment in neuromyelitis optica spectrum disorders: what do we know? Mult Scler Relat Dis. (2017) 18:225–9. doi: 10.1016/j.msard.2017.10.003
20. Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Dig World Core Med J. (2006) 65:1479–82. doi: 10.1212/01.wnl.0000183151.19351.82
21. Wingerchuk DM. Neuromyelitis optica: current concepts. Front Biosci. (2004) 9:834–40. doi: 10.2741/1267
22. Merle H, Olindo S, Bonnan M, Donnio A, Richer R, Smadja D, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. (2007) 114:810–5.e2. doi: 10.1016/j.ophtha.2006.06.060
23. Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. (2015) 84:1165–73. doi: 10.1212/WNL.0000000000001367
24. Asgari N, Skejoe HPB, Lillevang ST, Steenstrup T, Stenager E, Kyvik KO. Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): a population-based, descriptive study. BMC Neurol. (2013) 13:33. doi: 10.1186/1471-2377-13-33
25. Jarius S, Paul F, Franciotta D, Ruprecht K, Ringelstein M, Bergamaschi R, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. (2011) 306:82–90. doi: 10.1016/j.jns.2011.03.038
26. Chaves LCL, Faintuch J, Kahwage S, Alencar FDA. A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg. (2002) 12:328–34. doi: 10.1381/096089202321088093
27. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. Am J Roentgenol. (2009) 192:501–8. doi: 10.2214/AJR.07.3959
28. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. (2006) 63:7. doi: 10.1001/archneur.63.7.964
Keywords: neuromyelitis optica, Wernicke's encephalopathy, triad, magnetic resonance imaging, periventricular lesions, differential diagnosis
Citation: Ye L, Xu Z, Deng J and Yang J (2020) Classical Triad and Periventricular Lesions Do Not Necessarily Indicate Wernicke's Encephalopathy: A Case Report and Review of the Literature. Front. Neurol. 11:451. doi: 10.3389/fneur.2020.00451
Received: 16 May 2019; Accepted: 28 April 2020;
Published: 10 June 2020.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Jorge Tolivia, University of Oviedo, SpainMichael Levy, Massachusetts General Hospital, United States
Copyright © 2020 Ye, Xu, Deng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajun Yang, c2RfeWFuZ2pqQHN1bWhzLmVkdS5jbg==
 Lisha Ye
Lisha Ye Zhouwei Xu1,2
Zhouwei Xu1,2