- 1Shanxi Academy of Medical Sciences, Shanxi Bethune Hospital, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 2Shanxi Medical University, Taiyuan, China
Background: Stroke has become a major problem around the world, which is one of the main causes of long-term disability. Therefore, it is important to seek a biomarker to predict the prognosis of patients with stroke. This meta-analysis aims to clarify the relationship between the neutrophil-to-lymphocyte ratio (NLR) and the prognosis of stroke patients.
Methods: This study was pre-registered in PROSPERO (CRD42020186544). We performed systematic research in PubMed, Web of Science, and EMBASE databases for studies investigating the prognostic value of NLR. Based on the enrolled studies, patients were divided into the low-NLR cohort and the high-NLR cohort. Odds ratios (ORs) with 95% confidence intervals (CIs) were extracted and analyzed by the Review Manager 5.3 and Stata 12.0 software. Heterogeneity was estimated by using Cochran's Q test and I2 value. Sensitivity analyses and subgroup analyses were also performed to explore the potential sources of heterogeneity. Publication bias was assessed with funnel plots and assessed by Egger's tests.
Results: Forty-one studies with 27,124 patients were included. In the overall analysis, elevated NLR was associated with an increased mortality in acute ischemic stroke (AIS) patients (OR = 1.12, 95% CI = 1.07–1.16) and in acute hemorrhagic stroke (AHS) patients (OR = 1.23, 95% CI = 1.09–1.39), poorer outcomes in AIS patients (OR = 1.29, 95% CI = 1.16–1.44), and in AHS patients (OR = 1.11, 95% CI = 1.03–1.20). While in terms of hemorrhagic transformation (HT), elevated NLR was associated with an increased incidence of HT in AIS patients (OR = 1.15, 95% CI = 1.08–1.23).
Conclusions: This study demonstrated that elevated NLR was significantly associated with poor prognosis of stroke patients. High NLR is associated with a 1.1- to 1.3-fold increased risk of poor outcomes of AIS/AHS patients. NLR could be helpful as a potential prognostic biomarker to guide clinical decision making.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020186544.
Introduction
With almost 6 million deaths and more than 10% of all mortality every year, stroke has become one of the predominant threats to human health (1). There are two types of strokes, one is ischemic stroke, which accounts for 85% of all acute stroke, and the other is hemorrhagic stroke. According to previous reports, about 40% of all stroke deaths are attributable to hemorrhagic stroke (2). Currently, the major treatment for acute ischemic stroke is reperfusion therapy, which includes intravenous tissue plasminogen activator and endovascular therapy (EVT) (3). Exploring the key factors that affect the prognosis of stroke patients is crucial for clinicians to design appropriate treatments to improve the clinical efficacy and prognosis to stroke patients.
As we all know, there are two important pathophysiological mechanisms of stroke including oxidative stress and inflammation. After stroke, the inflammatory response is activated and plays a significant role in secondary brain injury (4). In recent years, the immunity has emerged as a new breakthrough target in the treatment strategy for acute stroke. Meanwhile, it is non-displaceable in predicting a poor prognosis (5). However, it is a complex process that can induce the activation and immunosuppression of a variety of inflammatory cells. Previous studies have found the different roles of neutrophils and lymphocytes in the progression and prognosis after stroke. Neutrophils could re-infiltrate the ischemic site in the first few hours after stroke, and then release chemical mediators related to increased tissue damage and poor neurological prognosis (6). At the same time, stroke could trigger a special immunosuppressive state (4), such as the activation of neutrophils, which leads to a decrease in lymphocytes (7), and certain types of lymphocytes are considered to be important brain protective immune regulators; the decrease of these lymphocytes may lead to deterioration of nerve function (8). Recently, the neutrophil-to-lymphocyte ratio (NLR) has become a powerful predictor of death in patients with cardiovascular disease or peripheral arterial occlusive disease. Previous studies reported a correlation between stroke severity and NLR determined at admission. Several studies suggested that the initial NLR was associated with mortality and infarct size in ischemic stroke patients.
However, the value of NLR in predicting the poor prognosis of stroke patients is still controversial. Some studies showed that NLR had no obvious effect on mortality (9, 10), while some studies demonstrated that a high NLR was an independent predictor of poor clinical outcomes in patients with stroke (11, 12). Thus, the aim of this study was to perform a meta-analysis to clear the relationship between NLR and the prognosis in patients with stroke.
Materials and Methods
Search Strategy
Registered in PROSPERO with the number CRD42020186544, this meta-analysis searched the databases, including PubMed, Web of Science, EMBASE, Scopus, and Google Scholar, which were papers published from the time of inception of the database to January 2021. We used the following search terms: “NLR or neutrophil-to-lymphocyte ratio or neutrophil-lymphocyte ratio” and “stroke or acute ischemic stroke or cerebrovascular accident or CVA or AIS or TIA or intra-cerebral hemorrhage or intracranial hemorrhage or AHS or subarachnoid hemorrhage” (Supplementary Table 1). Two investigators independently performed the literature search and resolved any disagreements via discussion. We screened retrieved articles in citation lists manually to ensure sensitivity of the search strategy.
Inclusion and Exclusion Criteria
The following eligibility criteria were utilized to reduce clinical heterogeneity: (a) patients were diagnosed with acute stroke, including ischemic stroke, and hemorrhagic stroke; (b) on or after admission, white blood cell counts and NLR were assessed or can be calculated; (c) odds ratios (ORs) or risk ratios (RRs) were provided with 95% confidence interval (CI) for survival outcomes or functional outcomes; and (d) prospective or retrospective cohort studies were considered eligible. Exclusion criteria were (a) the article was conference abstracts, letters, case reports, reviews, unrelated articles; (b) patients with systematic inflammatory disorders, such as recent myocardial infarction, liver or kidney failure, history of cancer; (c) the end point event of the study was not death, disabled, or hemorrhagic transformation; and (d) studies without enough data (refers to the absence of odds ratio or related data used to estimate odds ratio, lack of neutrophil–lymphocyte ratio or functional outcome after discharge). Disagreements were resolved by consensus between the two investigators.
Data Extraction
Relevant data were extracted by two independent investigators (WL and MH) from the eligible studies, including patients' characteristics, clinical data, and laboratory data such as first author, year of publication, patients area, sample size, study period, mean or median age, gender, National Institutes of Health Stroke Scale (NIHSS) or Glass Coma Scale (GCS), stroke type, time of onset, comorbid status, initial treatment, sampling time of the blood, research method, and cutoff value of NLR. We collected OR and 95% CI on the mortality (short term or long term), functional outcomes, and symptomatic intracranial hemorrhage, or parenchymal hematoma. We used multivariate regression analysis data, if the ORs of univariate and multivariate regression analyses were both available in the study. Any disagreement was settled via discussion with a third investigator.
Outcomes
The functional status was characterized by modified Rankin Scale (mRS) during clinical follow-up, with poor functional outcome as mRS ≥ 3, whereas the survival outcomes were measured by the occurrence of spontaneous intracerebral hemorrhage (sICH) or hemorrhagic transformation (HT), and mortality.
Quality Assessment
We applied the Newcastle–Ottawa Scale (NOS), which includes three factors: selection, comparability, and exposure to assess the quality of each enrolled study. The total score ranged from 0 to 9, and the score of 3 or less, 4–6, or 7 or more were considered to have low, intermediate, or high quality, respectively.
Statistical Analyses
The Review Manager version 5.3 software from Cochrane was applied in the analysis, and we utilized STATA 12.0 (STATA Corporation, College Station, TX, USA) to evaluate publication bias generated in the study. The prognostic value of NLR in stroke patients was estimated by forest and funnel plots. Based on the enrolled studies, patients were divided into the low-NLR cohort and the high-NLR cohort according to different cutoff values. Due to large sample size, we assumed that an OR is a good approximation to RR in our study; so we use the OR as the effect size of this meta-analysis. The log (OR) and its standard error were calculated by OR and 95% CI and used for aggregation. We merged the OR and 95% CI to analyze the implication of the NLR with poor endpoints. Furthermore, the heterogeneity was assessed comprehensively through subgroup analysis and sensitivity analysis. Heterogeneity between the studies was evaluated by Cochran's Q test and I2 statistic. A random-effect model was applied to calculate the pooled ORs and 95% CIs if there was significant heterogeneity among the enrolled studies (I2 > 50%, or p < 0.10); otherwise, the fixed-effect model was adopted (I2 <50% or p > 0.10). Publication bias was assessed by Egger's test. A p-value <0.05 was considered significant statistically.
Results
Literature Research
Figure 1 shows the research flow diagram. A total of about 935 potentially relevant records were selected after the initial literature research. After removing the duplications, a total of 287 studies were reviewed by titles and abstract. Of the remaining 287 articles, 204 papers were excluded due to meeting the exclusion criteria. Then, we inspected the remaining 83 articles with full texts, in which 42 studies lack enough data. Eventually, 41 articles with 27,124 patients were included in our analysis.
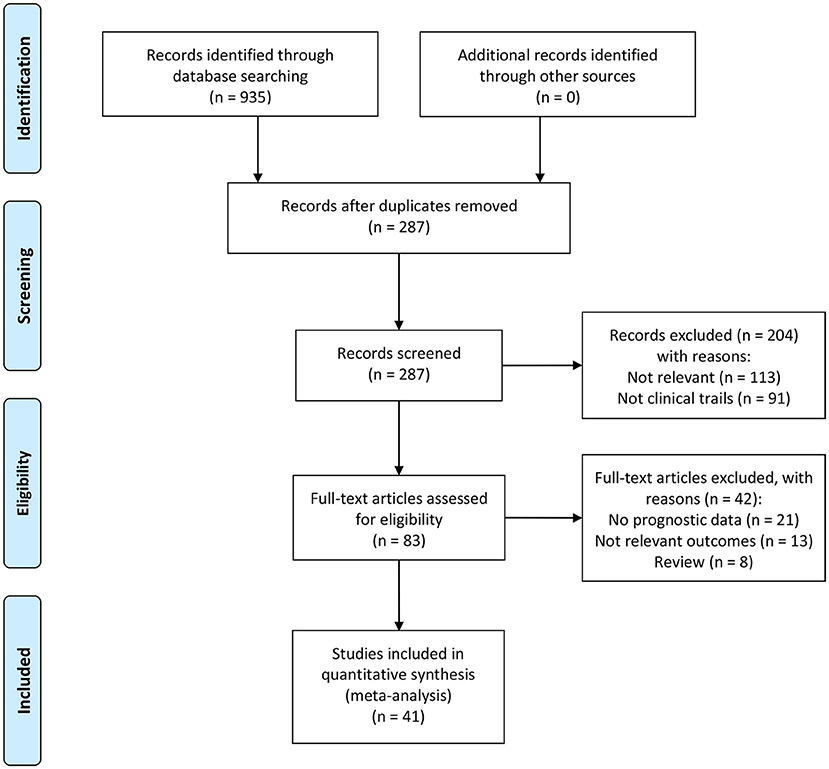
Figure 1. The flowchart of the selection process of studies according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).
Study Characteristics
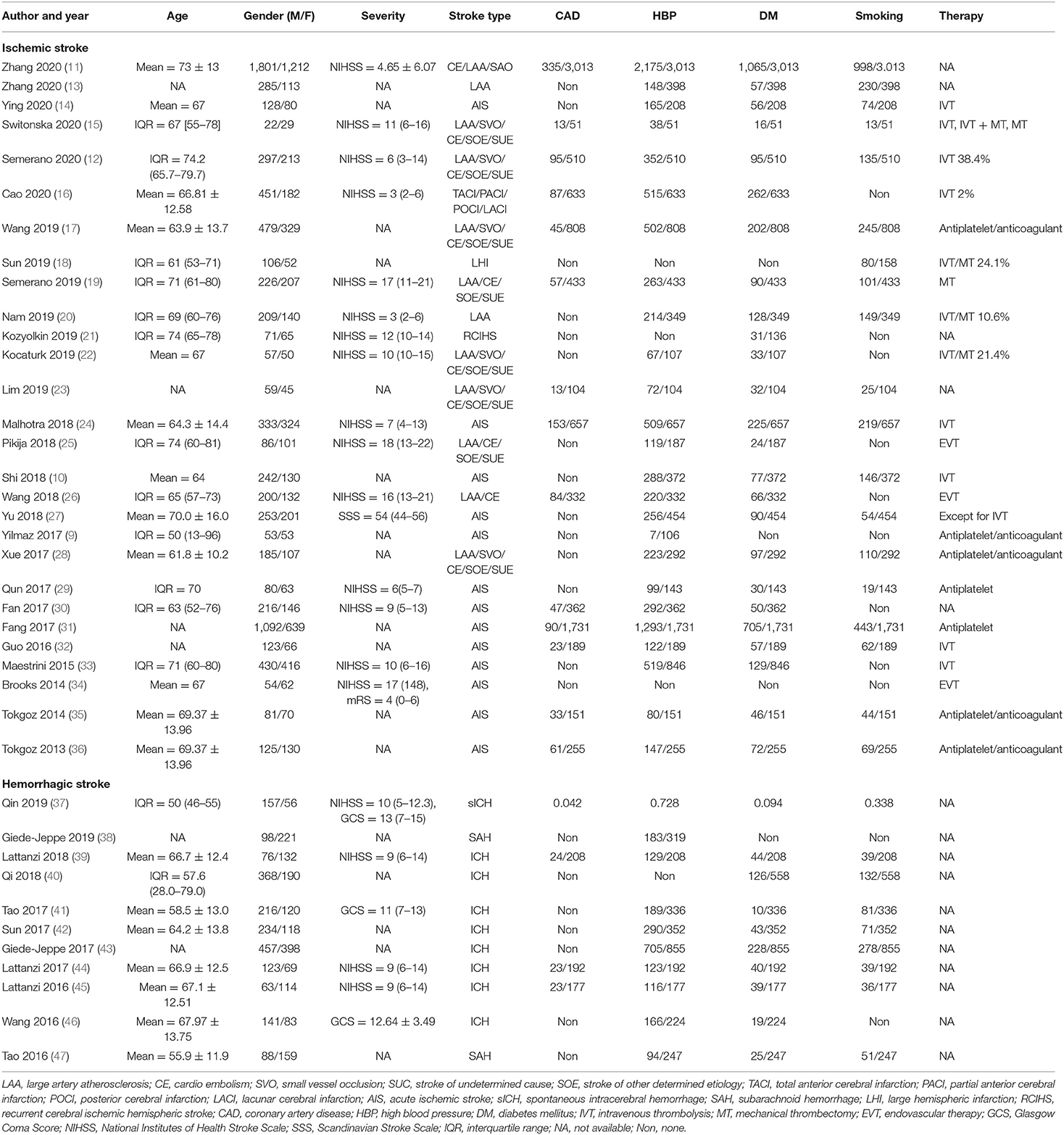
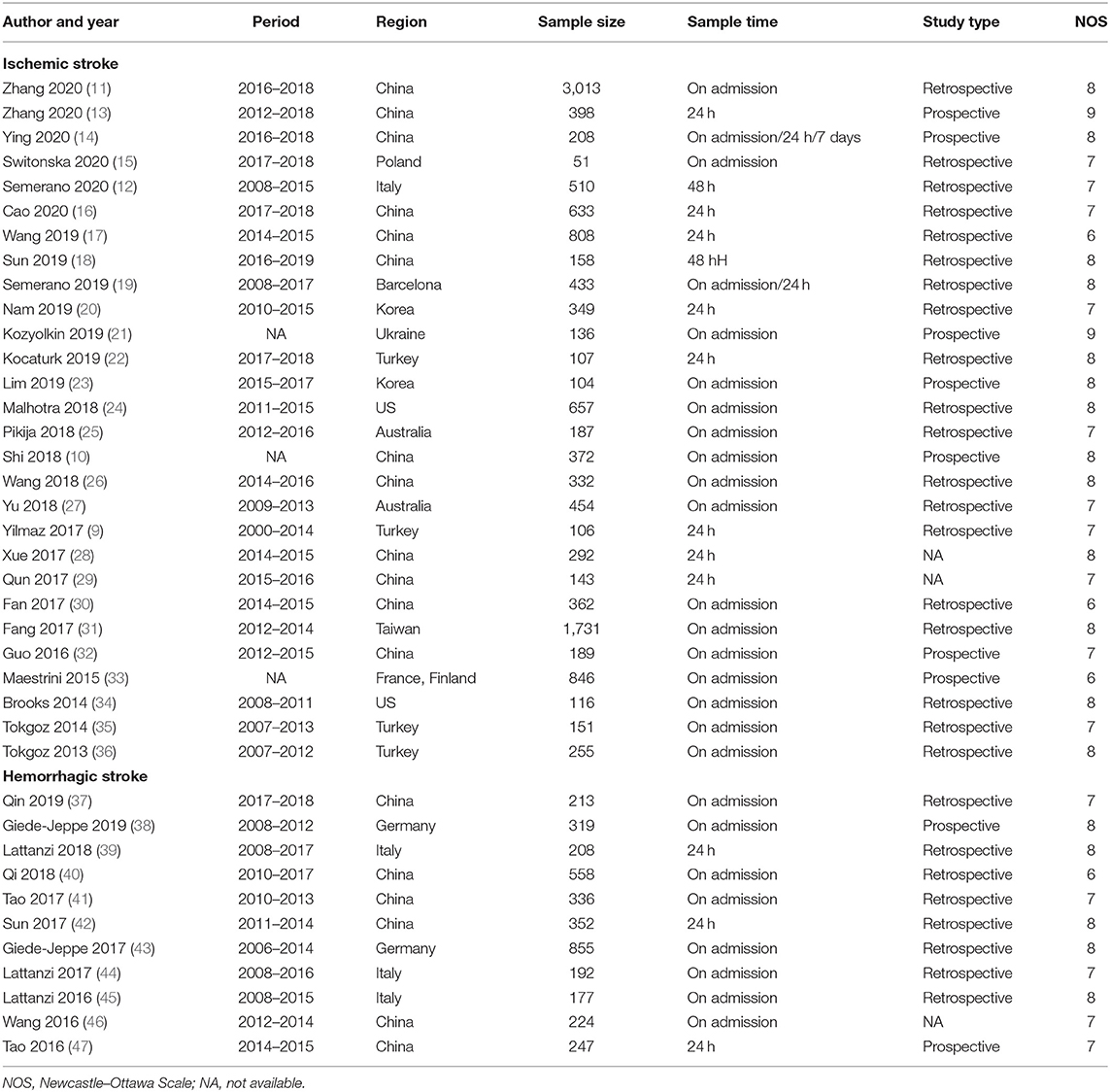
The clinical characteristics of patients described in the studies are shown in Table 1. Characteristics of the included studies are reflected in Table 2. Of the 41 studies, 30 reported ischemic stroke events (9–36, 48, 49), and 11 reported hemorrhagic stroke events (37–47). Nine studies were prospective in design, and 32 studies were retrospective. Among them, blood samples were taken out on admission, in 24 h after admission, in 48 h after admission, or in the first week after admission. For ischemic stroke, the sample size ranges from 51 to 3,013, and the research regions cover Asia, Europe, Australia, and North America. Most of the studies contain multiple stroke types, including large artery atherosclerosis (LAA) type, cardioembolism (CE) type, small vessel occlusion (SVO) type, stroke of other determined etiology (SOE) type, and stroke of undetermined etiology (SUE) type. In terms of hemorrhagic stroke, six of them were from China, and four were from Europe. The most frequently evaluated subtype of hemorrhagic stroke was ICH (n = 9) and subarachnoid hemorrhage (n = 2). The cutoff values of NLR varied between studies. Overall, all ORs and 95% CI are adjusted and obtained from the multiple regression analysis. The NOS scores ranged from 6 to 9, indicating a moderate to high quality of included studies.
Overall Prognostic Analysis
Association of Neutrophil-Lymphocyte Ratio and Mortality
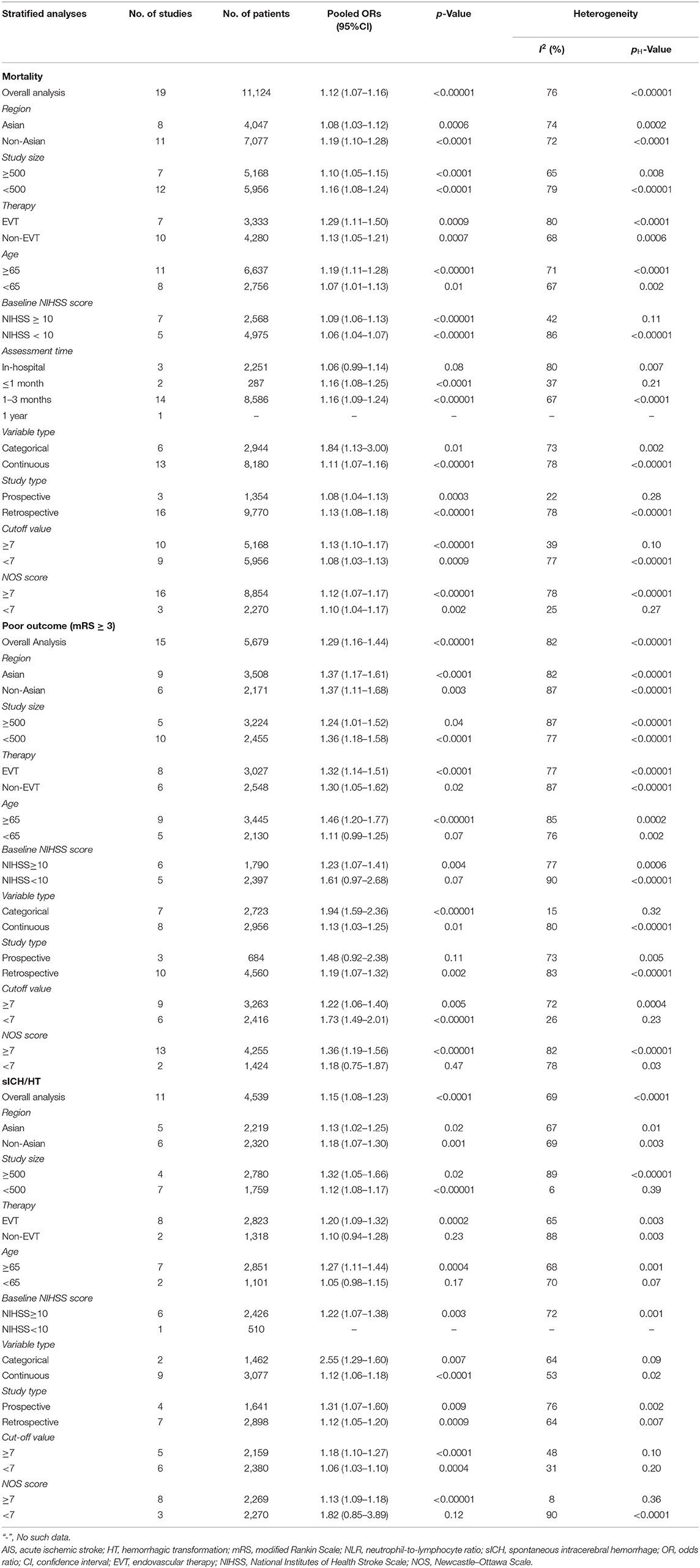
There were 19 studies with 11,124 patients that reported the association between acute ischemic stroke and mortality. After pooling the ORs, we found that the high NLR was correlated with an increased mortality of the AIS patients with an OR of 1.12 (95% CI, 1.07–1.16; p < 0.00001, Figure 2A) in a random-effect model, with evidence of moderate heterogeneity (τ2 <0.01; I2 = 76%; p < 0.00001).
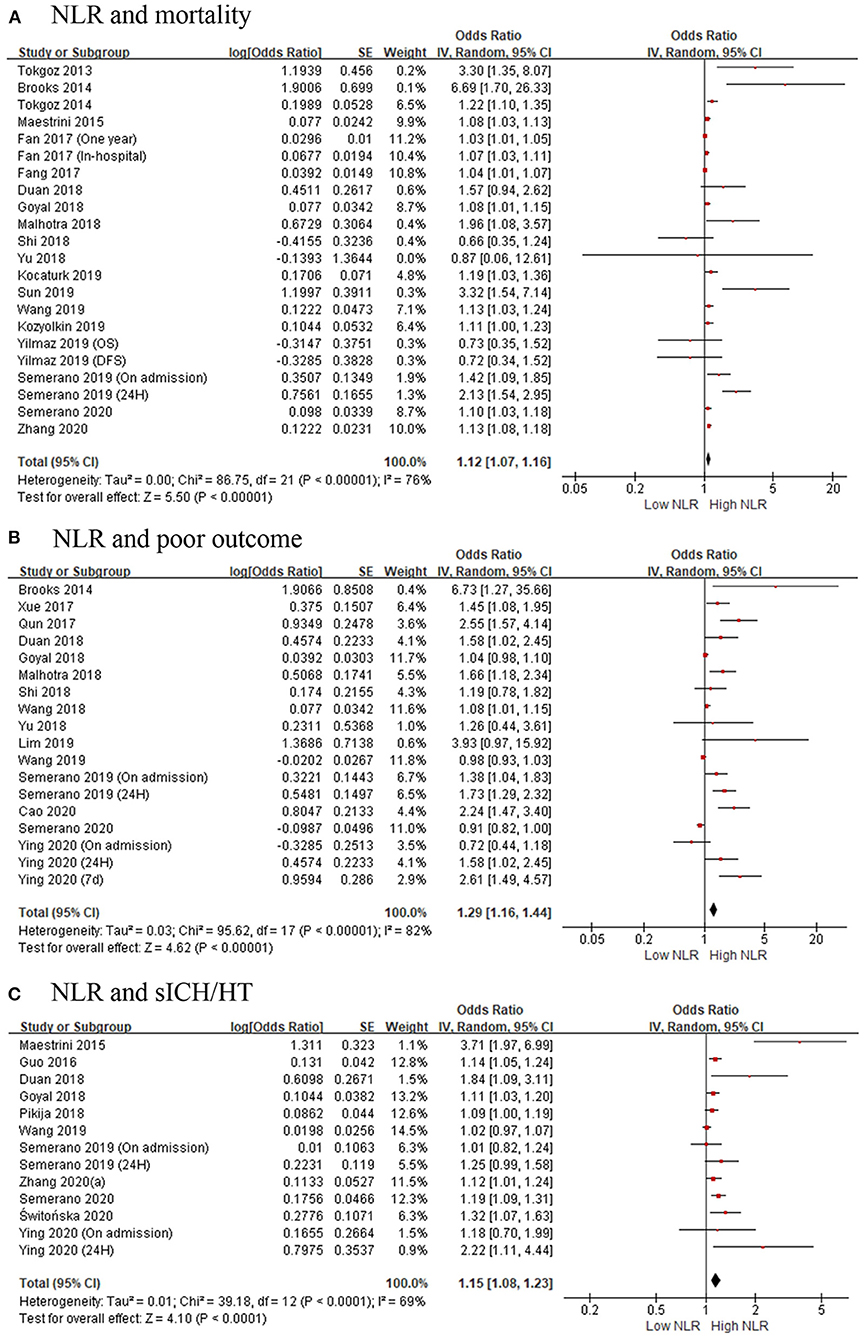
Figure 2. Forest plots of included studies evaluating the association in ischemic stroke patients between (A) neutrophil-to-lymphocyte ratio (NLR) and mortality, (B) NLR and poor outcome (mRS ≥ 3), and (C) NLR and the occurrence of sICH/HT. HT, hemorrhagic transformation; mRS, modified Rankin Scale; NLR, neutrophil-to-lymphocyte ratio; sICH, spontaneous intracerebral hemorrhage.
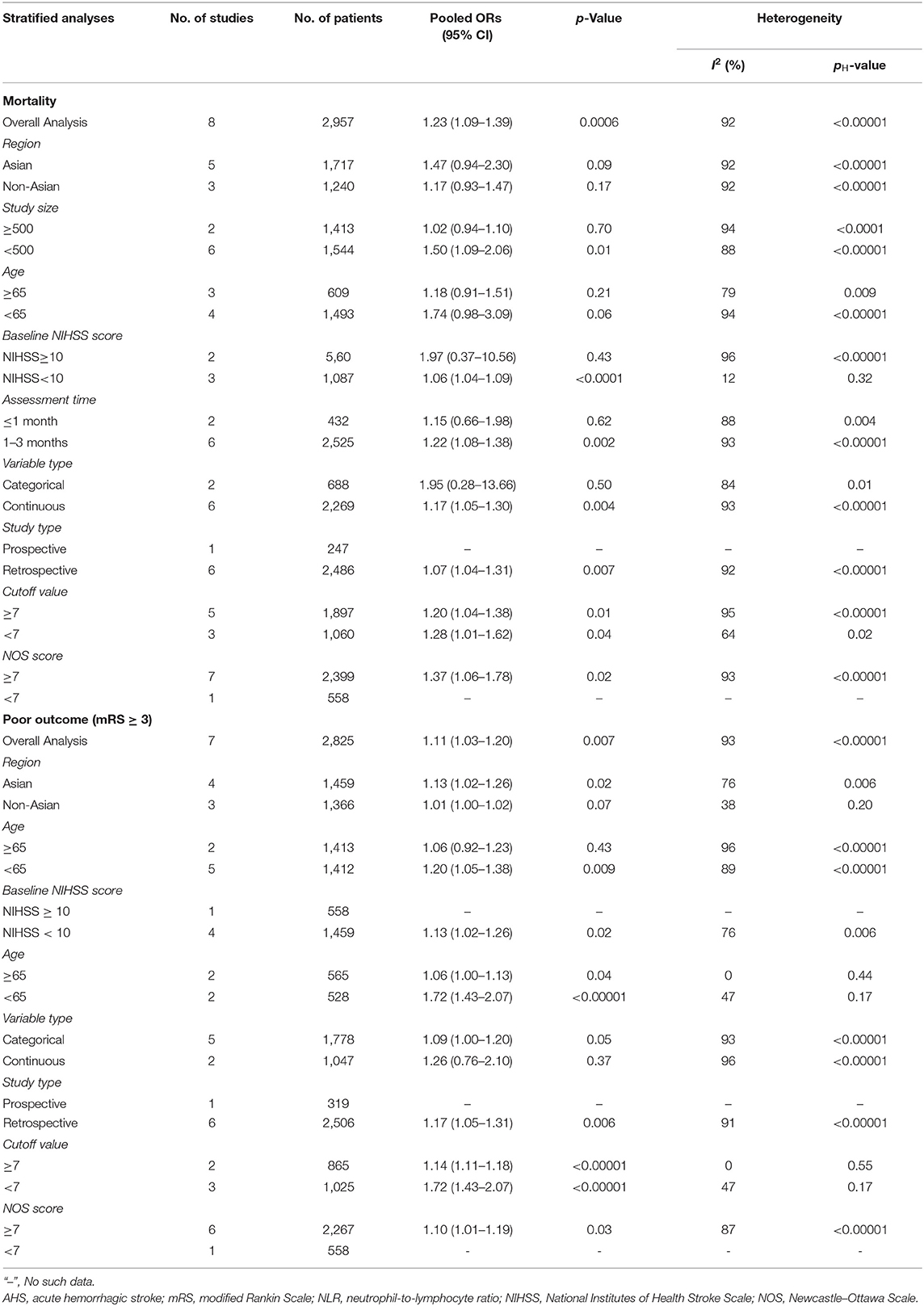
As for hemorrhagic stroke, a total of eight articles with 2,957 participants were included in this meta-analysis. The result showed that the higher risk of death was associated with high NLR, and the pooled OR was 1.23 (95% CI, 1.09–1.39; p = 0.0006, Figure 3A), using a random-effect model. Significant heterogeneity between the eight studies was observed (τ2= 0.02; I2 = 92%; p < 0.00001).
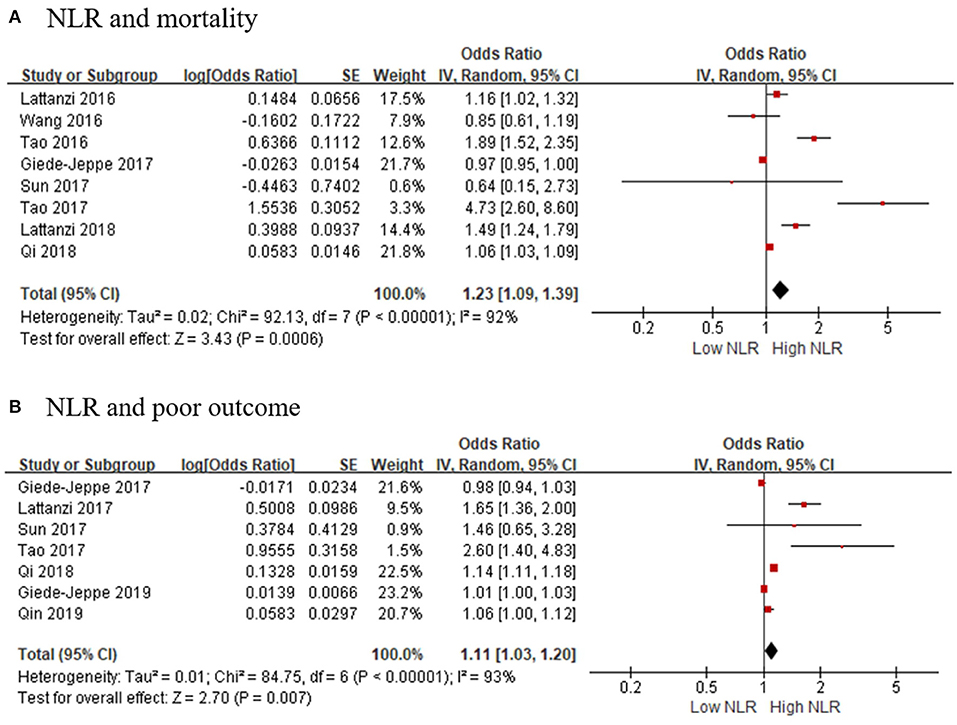
Figure 3. Forest plots of included studies evaluating the association in hemorrhagic stroke patients between (A) NLR and mortality, and (B) NLR and poor outcome (mRS ≥ 3). mRS, modified Rankin Scale; NLR, neutrophil-to-lymphocyte ratio.
Association of Neutrophil-to-Lymphocyte Ratio and Poor Outcomes
Fifteen studies showed the relationship of NLR and poor outcomes in 5,679 AIS patients. As shown in Figure 2B, the gathered OR was 1.29 (95% CI, 1.16–1.44; p < 0.00001) in a random-effect model, which means the higher the NLR is, the poorer the outcomes are. The heterogeneity detected between the articles was τ2= 0.03; I2 = 82%; p < 0.00001.
We selected seven articles to explore the connection of high NLR and poor outcomes in patients with hemorrhagic stroke. The pooled OR was 1.11 (95% CI, 1.03–1.20; p = 0.007, Figure 3B), suggesting that poor outcomes in patients with AHS is associated with a higher NLR. Heterogeneity among the studies was τ2= 0.01; I2 = 93%; p < 0.00001.
Association of Neutrophil-to-Lymphocyte Ratio and Hemorrhagic Transformation or Spontaneous Intracerebral Hemorrhage
Eleven articles with 4,539 patients provided ORs and 95% CI for the risk factor for HT or sICH. Figure 2C shows a significant correlation between NLR and HT or sICH rates in patients with AIS, with a pooled OR of 1.15 (95% CI, 1.08–1.23; p < 0.0001). The heterogeneity discovered between the articles was τ2= 0.01; I2 = 69%; p < 0.0001.
Subgroup Prognostic Analyses
Subgroup Analysis of Mortality
Subgroup analysis of mortality in AIS patients is demonstrated in Table 3. We classified the mortality as follows: region, sample of sizes, treatment methods, cutoff value, median or mean age, stroke severity, follow-up period, data type, study type, and Newcastle–Ottawa Scale (NOS) quality scores. In general, elevated NLR value and higher risk of death in AIS patients were viewed constantly in all subgroups, except for in-hospital mortality. In the subgroup based on region, Asia and non-Asia groups were both observed to be associated with high NLR, with the pooled OR being 1.08 (95% CI, 1.03–1.12, p = 0.0006) and 1.19 (95% CI, 1.10–1.28, p < 0.0001), respectively. Cohorts with EVT were more likely to have a risk of death, with an OR of 1.29 (95% CI, 1.11–1.50, p = 0.0009). Furthermore, stroke severity, follow-up period, and study type could be the potential source of heterogeneity.
We further carried out another subgroup of mortality in AHS patients. The subgroup was stratified by the before-mentioned criterion. As shown in Table 4, we found that the region of study and age of patients with elevated NLR were not related to mortality in AHS patients. Generally speaking, stroke severity could be a potential source of heterogeneity.
Subgroup Analysis of Poor Outcomes
Subgroup analysis of poor outcomes in AIS patients revealed that elevated NLR was significantly associated with poor outcomes in studies performed by categorical variables. In addition, when stratified by the study data type, heterogeneity was evidently reduced in categorical variables, meaning the study data type may be the resource of heterogeneity (Table 3).
Table 4 shows the subgroup of the relationship between NLR and poor outcomes in AHS patients. Interestingly, we found that poor outcomes could be significantly associated with stroke severity in both the NIHSS score ≥ 10 and NIHSS score <10 subgroups, because the heterogeneity in the subgroups was both shrunk.
Subgroup Analysis of Hemorrhagic Transformation or Spontaneous Intracerebral Hemorrhage
Subgroup based on age revealed that the NLR had considerable effect on occurrence of HT in elderly individuals with an OR of 1.27 (95% CI, 1.11–1.44, p = 0.0004), whereas no significant association was observed in studies in non-elderly individuals. Interestingly, heterogeneity was obviously decreased after stratifying the cutoff value of NLR, and the results showed that NLR was closely related to HT in both the cutoff value of NLR > 7 and cutoff value ≤ 7 subgroups. In addition, in the small sample size (n < 500) subgroup, the heterogeneity was evidently reduced (I2 = 6, p = 0.39), meaning the sample size could be the hidden origin of heterogeneity (Table 3).
Publication Bias
Publication bias was assessed in studies that provided outcomes in both AIS and AHS patients. After performing funnel plots, we found significant bias in ischemic stroke because the funnel plots were asymmetric (Figure 4). Furthermore, Egger's tests indicated some degree of publication bias (both p < 0.05, Supplementary Figure 1). Then, the trim and fill method was applied to solve these problems. After the adjustment, the results showed that high NLR was associated with mortality, poor outcome (mRS ≥ 3), and the occurrence of sICH or HT with adjusted ORs of 1.14 (95% CI, 1.07–1.16, p < 0.0001), 1.10 (95% CI, 0.99–1.23, p = 0.088), and 1.10 (95% CI, 1.02–1.87, p = 0.012), respectively. As for bias in hemorrhagic stroke, although the funnel plots were not completely symmetrical (Figure 5), the Egger's test does not suggest significant bias (Supplementary Figure 2). Sensitivity analysis was performed by excluding the included studies one by one (Supplementary Tables 2, 3), and the results did not change significantly from before deletion, suggesting that the results of this study were relatively stable.
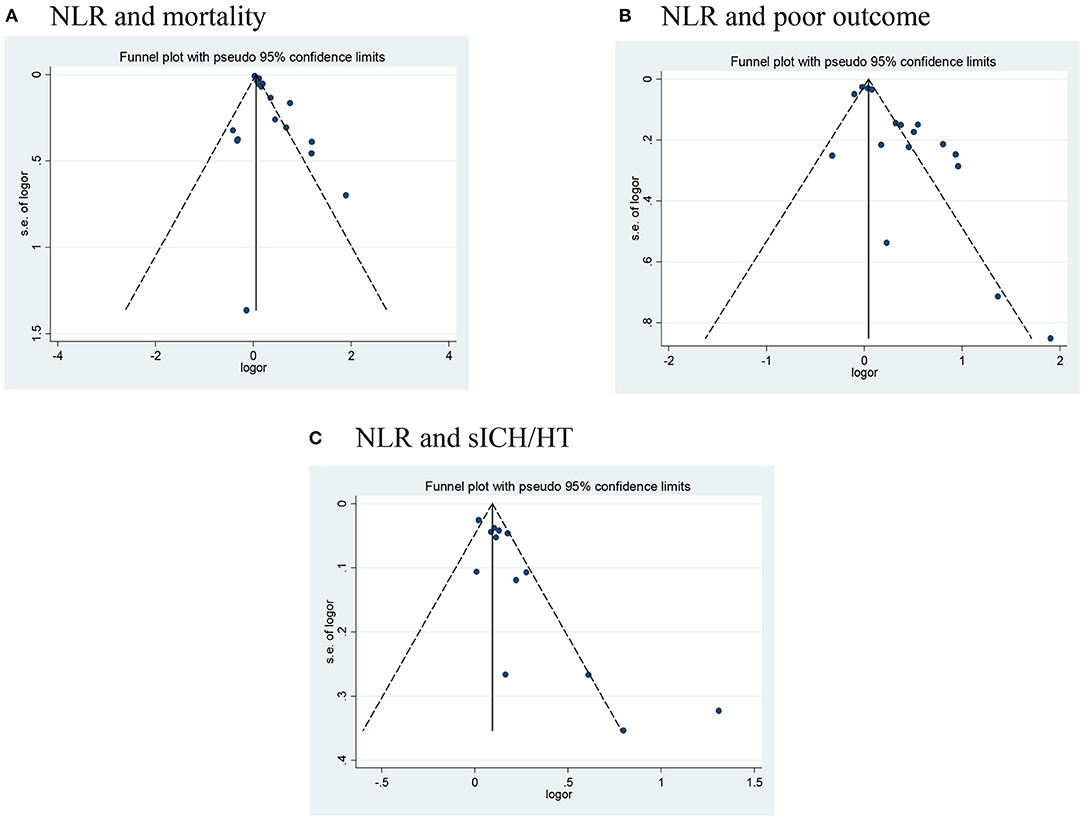
Figure 4. Funnel plots of (A) NLR and mortality, (B) NLR and poor outcome (mRS ≥ 3), and (C) NLR and the occurrence of sICH/HT in ischemic stroke. HT, hemorrhagic transformation; mRS, modified Rankin Scale; NLR, neutrophil-to-lymphocyte ratio; sICH, spontaneous intracerebral hemorrhage.
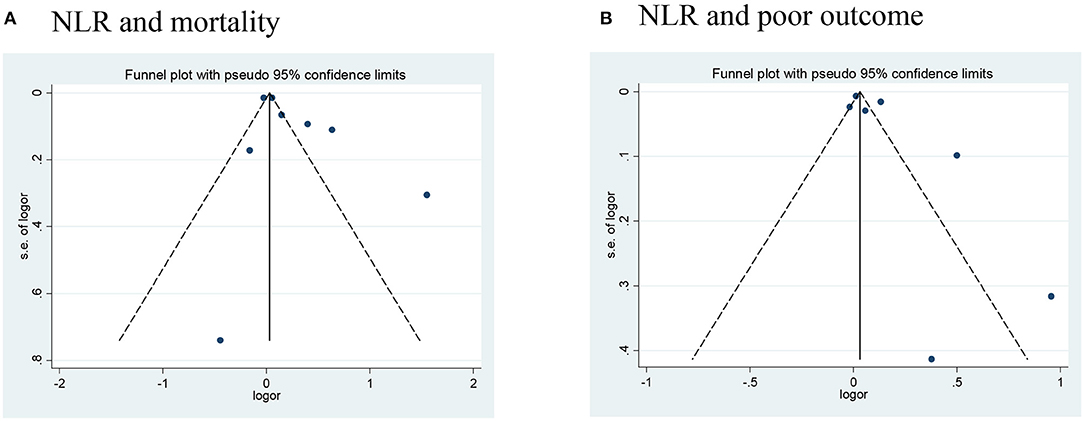
Figure 5. Funnel plots of (A) NLR and mortality, and (B) NLR and poor outcome (mRS ≥ 3) in hemorrhagic stroke. mRS, modified Rankin Scale; NLR, neutrophil-to-lymphocyte ratio.
Discussion
Stroke is one of the important diseases that seriously threaten human health, which has high mortality and morbidity (50). Despite its high incidence, there are few effective treatments to improve the quality of life of patients. Therefore, it is of great clinical significance to find simple and accurate serum biomarkers to assess the degree of early neurological damage and prognosis of stroke patients. The purpose of this meta-analysis was to evaluate the prognostic of NLR in patients with stroke. Inflammation is considered to be the secondary damage mechanism of stroke. We carried out the meta-analysis including 41 articles based on criterion with 27,124 individuals, and the result revealed that NLR was independently associated with prognosis in patients with stroke.
In recent years, inflammation has shown to have a strong relationship with the occurrence of stroke (51). Post-stroke inflammation has a harmful effect on brain injury, but it may play a protective role in tissue restoration and regeneration, and its role changes over time (52). As the marker of systemic inflammation, white blood cell counts were significantly increased after stroke (53) and related to the poor prognosis of stroke patients (54, 55). As we all know, different subtypes of WBC may have different effects on the inflammatory response of damaged tissues. Neutrophils are the major subtype of white blood cells that can respond earlier after stroke and show an active inflammatory response (52). Neutrophils first accumulate in the cerebral blood vessels within a few hours, which may cause the expansion of the infarction and block the microvessels (56). On the other hand, neutrophils increase the expression of matrix metalloprotein 9, which directly destroys the blood–brain barrier, causing secondary brain injury or hemorrhagic transformation (57, 58). Previous studies have confirmed that early leukocytosis and neutrophilia are related to the infarct volume assessed by DWI in the early stage of ischemic stroke (59). Neutrophils are recruited to the ischemic area of brain tissue and may release some proteolytic enzymes or free oxygen free radicals and other inflammatory mediators into the damaged area (5). It has recently been reported that when patients with acute cerebral infarction are admitted to the hospital, a higher total number of white blood cells and neutrophil counts are associated with the severity of stroke (60). Similar with cerebral infarction, some studies showed that elevated neutrophil level was related to hematoma volume and outcomes in ICH patients. Moreover, studies revealed that in a rat autologous blood model, neutrophils penetrated in and around the hematoma, which reached a peak at 2–3 days (61). Activated neutrophils can release a variety of proteolytic enzymes and pro-inflammatory proteases, which can damage the brain tissue directly.
Subpopulations of lymphocytes, especially T lymphocytes, may have regulatory functions in inflammation-induced neuroprotection (62). Lymphocytes in ischemic brain tissue rise later than neutrophils. Lymphocytes have been found to play an important role in healing or repairing inflammation (63). However, the role of lymphocytes in the pathogenesis of stroke remains controversial. It has been reported that other subtypes of T cells (pro-inflammatory lymphocytes) are the main source of cytotoxic substances and have a negative effect on ischemic brain tissue (64). Then, there is also experimental evidence that certain subtypes of lymphocytes (mainly regulatory T cells and B cells) have regulatory functions, and these cells are responsible for the reduction of ischemic tissue volume in ischemic stroke and the improvement of function after neurological deficit (65). Early studies indicated that a higher level of lymphocytes could upregulate the anti-inflammatory cytokine interleukin (IL)-10 and suppress inflammatory cytokines including tumor necrosis factor (TNF)-α and IL-6, which can play an anti-inflammatory effect (66). In addition, clinical evidence shows that lower lymphocyte count is associated with poor early neurological function improvement and poor long-term functional prognosis (67).
NLR stands for an easily available, non-invasive, and inexpensive marker that can be routinely used to evaluate systemic inflammation in clinical work. The mechanism behind the clinical significance of NLR in stroke is that inflammation plays a central role in all types of stroke, from the occurrence and development of injury to recovery (68). The underlying mechanism of elevated NLR and poor prognosis may be related to excessive activation of inflammation and immunosuppression (69). First, after an ischemic stroke, the damaged brain tissue will produce a strong inflammatory response and, consequently, produce inflammatory biomarkers (69). Although inflammation is necessary for early repair after stroke, excessive activation of inflammation can cause damage to the brain tissue, leading to deterioration of neurological function and brain edema (70, 71). Second, the theories about immunosuppression suggest that after a stroke, catecholamines are released into the blood through over-activated sympathetic nerves, which may reduce circulating lymphocyte level and increase the risk of infection (71). Recently, NLR has been proposed as an independent predictor of severity and mortality to predict coronary syndrome (72).
Application prognostic biomarkers may enhance risk stratification, help design individual treatment, and determine follow-up schedules. In the stage of customized treatment strategy, NLR may be a key sticking point in the risk stratification of acute stroke patients. Moreover, neurologists can develop more frequent and stricter follow-up strategies for patients who may have a poor prognosis. In short, in the different stages of diagnosis, treatment, and follow-up, the application of these preoperative lymphocyte-related systemic inflammation biomarkers may improve the accuracy of current prognostic models and help make clinical decisions.
This meta-analysis adopted strict inclusion and exclusion criteria, and covered 41 medium-to-high-quality studies, including retrospective studies and prospective studies, and successively explored the relationship between NLR and the prognosis of ischemic stroke and hemorrhagic stroke. Our meta-analysis found that high NLR has a predictive effect on the prognosis in stroke patients, mainly in terms of mortality, poor prognosis, and hemorrhagic transformation. Meanwhile, NLR has a stronger predictive effect on ischemic stroke than hemorrhagic stroke. Many studies also support that NLR is an independent risk factor for predicting short-term outcomes in both ischemic and hemorrhagic stroke. Zhang et al. (11) reported that NLR was the best independent predictor associated with mortality and poor outcome in AIS patients. Ying et al. (14) suggested that NLR could predict the HT and outcome in AIS patients with r-tPA treatment. Switonska et al. (15) indicated that NLR is an inexpensive tool that could identify the increased risk of early symptomatic hemorrhage after recanalization in AIS. Giede-Jeppe et al. (38) proved that in aSAH patients, NLR represents an independent parameter associated with unfavorable functional outcome. For NLR and AIS, the results of meta-analysis show that NLR has a significant correlation with the two. In the AHS patients, although the meta-analysis results suggest that patients with higher NLR have a poorer prognosis, the heterogeneity between the studies is high, and no single article that can significantly reduce the heterogeneity was found in the sensitivity analysis. In the subsequent subgroup analysis, the source of heterogeneity was deeply explored, and the stability of the results was further proved. Furthermore, we found that a high NLR is more closely related to the prognosis of AIS patients after endovascular treatment. Therefore, as a potential prognostic biomarker, NLR will help to more accurately determine the prognosis of stroke patients.
There are also some limitations in this study: First, the inflammatory process is relatively complex. We use a relatively simple ratio of neutrophils to lymphocytes to show the effect of inflammation on the prognosis of patients with stroke. It only reflects the general trend, not the full picture of the inflammatory process. Second, the results of some subgroup analysis suggest that there is a high degree of heterogeneity among the studies, and there may be potential selection bias or other confounding factors. Third, the ORs obtained by this meta-analysis are small; this requires clinicians to use it with caution and explain carefully. It is necessary to combine the patient's own situation to predict the prognosis of acute stroke patients. Moreover, there were few studies with negative results in this meta-analysis, which might lead to potential publication bias. Besides, only two subarachnoid hemorrhage cases were included in this meta-analysis that might result in an inaccuracy of the results. We look forward to more researches to deeply discuss this relationship. Finally, the optimal cutoff values of NLR remain undetermined. In this study, the NLR cutoff values of each article are different, and different NLR cutoff values may cause higher heterogeneity, which may interfere with the accuracy of our analysis results. Therefore, the establishment of a standard NLR cutoff value will promote in-depth research on its prognostic value.
Conclusion
In summary, our research refers that a high NLR value is closely related to the prognosis of stroke patients. High NLR is associated with a 1.1- to 1.3-fold increased risk of poor outcomes of AIS/AHS patients. Elevated NLR can predict the mortality, poor prognosis, and the occurrence of spontaneous cerebral hemorrhage in stroke patients, and our subgroup analysis suggests that a high NLR is more closely related to the prognosis of AIS patients after endovascular treatment. This low-cost and easy-to-obtain biomarker will play a greater and more profound role in clinical work in the future. Future studies need to combine with TOAST classification, OCSB classification, and other indicators in order to better predict the prognosis of stroke patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
WL and MH conceived the presented idea and designed the research. ZD and XL searched databases and performed data analysis and statistical analysis. WL, MH, YS, and XL were responsible for writing the manuscript. All authors critically revised the article for important intellectual content and approved the final manuscript.
Funding
This work was supported by the Key Research and Development Program, Science and Technology Department of Shanxi Province (grant number 201703D421018), Selected Program for Science and Technology Activities of Overseas Students, Science and Technology Department of Shanxi Province (grant number 2018-1059-13), Scientific Research Program for Returned Overseas Students, Science and Technology Department of Shanxi Province (grant number HGKY2019096), and Talent introduces research launches of Shanxi Bethune Hospital (grant number 2020RC007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the editors and reviewers for providing very useful and professional comments during the revision of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.686983/full#supplementary-material
Supplementary Figure 1. Publication bias assessment with Egger's tests for the outcomes in ischemic stroke, (a) NLR and mortality, (b) NLR and poor outcome (mRS ≥ 3), and (c) NLR and the occurrence of sICH/HT in ischemic stroke. HT, hemorrhagic transformation, mRS, modified Rankin Scale, NLR, neutrophil-to-lymphocyte ratio, sICH, spontaneous intracerebral hemorrhage.
Supplementary Figure 2. Publication bias assessment with Egger's tests for the outcomes in hemorrhagic stroke, (a) NLR and mortality, (b) NLR and poor outcome (mRS ≥ 3). mRS, modified Rankin Scale, NLR, neutrophil-to-lymphocyte ratio.
Supplementary Table 1. Search strategy of present systematic review and meta-analysis.
Supplementary Table 2. Sensitivity analyses for NLR in AIS patients. AIS, acute ischemic stroke, NLR, neutrophil-to-lymphocyte ratio, sICH, spontaneous intracerebral hemorrhage, OA, On admission, OR, Odds ratio, CI, confidence interval, DFS, disease-free survival, OS, overall survival.
Supplementary Table 3. Sensitivity analyses for NLR in AHS patients. AHS, acute hemorrhagic stroke, mRS, modifie.
Abbreviations
AIS, acute ischemic stroke; AHS, acute hemorrhagic stroke; CE, cardio embolism; CI, confidence interval; CAD, coronary artery disease; DM, diabetes mellitus; DFS, disease-free survival; EVT, endovascular therapy; GCS, Glasgow Coma Score; HT, hemorrhagic transformation; HBP, high blood pressure; IVT, intravenous thrombolysis; IQR, interquartile range; LAA, large artery atherosclerosis; LACI, lacunar cerebral infarction; LHI, large hemispheric infarction; mRS, modified Rankin Scale; MT, mechanical thrombectomy; NLR, neutrophil-to-lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; NA, not available; Non, none; NOS, Newcastle-Ottawa Scale; OR, Odds ratio; OA, On admission; OS, overall survival; PACI, partial anterior cerebral infarction; POCI, posterior cerebral infarction; RCIHS, recurrent cerebral ischemic hemispheric stroke; SVO, small vessel occlusion; SUC, stroke of undetermined cause; SOE, stroke of other determined etiology; sICH, spontaneous intracerebral hemorrhage; SAH, subarachnoid hemorrhage; SSS, Scandinavian Stroke Scale; TACI, total anterior cerebral infarction.
References
1. Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165260. doi: 10.1016/j.bbadis.2018.09.012
3. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. (2020) 48:1654–63. doi: 10.1097/CCM.0000000000004597
4. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/STROKEAHA.120.030429
5. Hermann DM, Kleinschnitz C, Gunzer M. Implications of polymorphonuclear neutrophils for ischemic stroke and intracerebral hemorrhage: predictive value, pathophysiological consequences and utility as therapeutic target. J Neuroimmunol. (2018) 321:138–43. doi: 10.1016/j.jneuroim.2018.04.015
6. Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. (2020) 11:108–21. doi: 10.1007/s12975-019-00694-y
7. Petrone AB, Eisenman RD, Steele KN, Mosmiller LT, Urhie O, Zdilla MJ. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurol Sci. (2019) 40:1877–85. doi: 10.1007/s10072-019-03919-y
8. Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. (2016) 26:26–32. doi: 10.1016/j.coph.2015.09.009
9. Yilmaz E, Bayram Kacar A, Bozpolat A, Zararsiz G, Gorkem BS, Karakukcu M, et al. The relationship between hematological parameters and prognosis of children with acute ischemic stroke. Childs Nerv Syst. (2017) 34:655–61. doi: 10.1007/s00381-017-3673-x
10. Shi J, Peng H, You S, Liu Y, Xu J, Xu Y, et al. Increase in neutrophils after recombinant tissue plasminogen activator thrombolysis predicts poor functional outcome of ischaemic stroke: a longitudinal study. Eur J Neurol. (2018) 25:687–45. doi: 10.1111/ene.13575
11. Zhang XG, Xue J, Yang WH, Xu XS, Sun HX, Hu L, et al. Inflammatory markers as independent predictors for stroke outcomes. Brain Behav. (2020) 11:e01922. doi: 10.1002/brb3.1922
12. Semerano A, Strambo D, Martino G, Comi G, Filippi M, Roveri L, et al. Leukocyte counts and ratios are predictive of stroke outcome and hemorrhagic complications independently of infections. Front Neurol. (2020) 11:201. doi: 10.3389/fneur.2020.00201
13. Zhang WB, Zeng YY, Wang F, Cheng L, Tang WJ, Wang XQ. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. (2020) 12:2428–39. doi: 10.18632/aging.102752
14. Ying A, Cheng Y, Lin Y, Yu J, Wu X, Lin Y. Dynamic increase in neutrophil levels predicts parenchymal hemorrhage and function outcome of ischemic stroke with r-tPA thrombolysis. Neurol Sci. (2020) 41:2215–23. doi: 10.1007/s10072-020-04324-6
15. Switońska M, Piekuś-Słomka N, Słomka A, Sokal P, Zekanowska E, Lattanzi S. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sci. (2020) 10:771. doi: 10.3390/brainsci10110771
16. Cao X, Zhu Q, Xia X, Yao B, Liang S, Chen Z, et al. The correlation between novel peripheral blood cell ratios and 90-day mortality in patients with acute ischemic stroke. PLoS ONE. (2020) 15:e0238312. doi: 10.1371/journal.pone.0238312
17. Wang L, Song Q, Wang C, Wu S, Deng L, Li Y, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. (2019) 406:116445. doi: 10.1016/j.jns.2019.116445
18. Sun W, Li G, Liu Z, Miao J, Yang Z, Zhou Q, et al. A nomogram for predicting the in-hospital mortality after large hemispheric infarction. BMC Neurol. (2019) 19:347. doi: 10.1186/s12883-019-1571-4
19. Semerano A, Laredo C, Zhao Y, Rudilosso S, Renú A, Llull L, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical Thrombectomy. Stroke. (2019) 50:3456–64. doi: 10.1161/STROKEAHA.119.026743
20. Nam KW, Kim TJ, Lee JS, Park SH, Jeong HB, Yoon BW, et al. Neutrophil-to-lymphocyte ratio predicts early worsening in stroke due to large vessel disease. PLoS ONE. (2019) 14:e0221597. doi: 10.1371/journal.pone.0221597
21. Kozyolkin O, Kuznietsov A, Novikova L. Prediction of the lethal outcome of acute recurrent cerebral ischemic hemispheric stroke. Medicina. (2019) 55:331. doi: 10.3390/medicina55060311
22. Kocaturk O, Besli F, Gungoren F, Kocaturk M, Tanriverdi Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. (2019) 40:139–46. doi: 10.1007/s10072-018-3604-y
23. Lim HH, Jeong IH, An GD, Woo KS, Kim KH, Kim JM, et al. Early prediction of severity in acute ischemic stroke and transient ischemic attack using platelet parameters and neutrophil-to-lymphocyte ratio. J Clin Lab Anal. (2019) 33:e22714. doi: 10.1002/jcla.22714
24. Malhotra K, Goyal N, Chang JJ, Broce M, Pandhi A, Kerro A, et al. Differential leukocyte counts on admission predict outcomes in patients with acute ischaemic stroke treated with intravenous thrombolysis. Eur J Neurol. (2018) 25:1417–24. doi: 10.1111/ene.13741
25. Pikija S, Sztriha LK, Killer-Oberpfalzer M, Weymayr F, Hecker C, Ramesmayer C, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflamm. (2018) 15:319. doi: 10.1186/s12974-018-1359-2
26. Wang H, Zhang M, Hao Y, Zi W, Yang D, Zhou Z, et al. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg. (2018) 115:e312–21. doi: 10.1016/j.wneu.2018.04.042
27. Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. (2018) 387:115–8. doi: 10.1016/j.jns.2018.02.002
28. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
29. Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotoxic Res. (2017) 31:444–52. doi: 10.1007/s12640-017-9707-z
30. Fan L, Gui L, Chai E-Q, Wei C-J. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J Clin Lab Anal. (2017) 32:e22244. doi: 10.1002/jcla.22244
31. Fang YN, Tong MS, Sung PH, Chen YL, Chen CH, Tsai NW, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed J. (2017) 40:154–62. doi: 10.1016/j.bj.2017.03.002
32. Guo Z, Yu S, Xiao L, Chen X, Ye R, Zheng P, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflamm. (2016) 13:199. doi: 10.1186/s12974-016-0680-x
33. Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. (2015) 85:1408–16. doi: 10.1212/WNL.0000000000002029
34. Brooks SD, Spears C, Cummings C, VanGilder RL, Stinehart KR, Gutmann L, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointervent Surg. (2014) 6:578–83. doi: 10.1136/neurintsurg-2013-010780
35. Tokgoz S, Keskin S, Kayrak M, Seyithanoglu A, Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. (2014) 23:2163–8. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007
36. Tokgoz S, Kayrak M, Akpinar Z, Seyithanoglu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. (2013) 22:1169–74. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011
37. Qin J, Li Z, Gong G, Li H, Chen L, Song B, et al. Early increased neutrophil-to-lymphocyte ratio is associated with poor 3-month outcomes in spontaneous intracerebral hemorrhage. PLoS ONE. (2019) 14:e0211833. doi: 10.1371/journal.pone.0211833
38. Giede-Jeppe A, Reichl J, Sprügel MI, Lücking H, Hoelter P, Eyüpoglu IY, et al. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. (2019) 132:400–7. doi: 10.3171/2018.9.JNS181975
39. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. (2018) 387:98–102. doi: 10.1016/j.jns.2018.01.038
40. Qi H, Wang D, Deng X, Pang X. Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-day mortality in spontaneous intracerebral hemorrhage. Med Sci Monitor. (2018) 24:9282–91. doi: 10.12659/MSM.911645
41. Tao C, Hu X, Wang J, Ma J, Li H, You C. Admission neutrophil count and neutrophil to lymphocyte ratio predict 90-day outcome in intracerebral hemorrhage. Biomarkers Med. (2017) 11:33–42. doi: 10.2217/bmm-2016-0187
42. Sun Y, You S, Zhong C, Huang Z, Hu L, Zhang X, et al. Neutrophil to lymphocyte ratio and the hematoma volume and stroke severity in acute intracerebral hemorrhage patients. Am J Emerg Med. (2017) 35:429–33. doi: 10.1016/j.ajem.2016.11.037
43. Giede-Jeppe A, Bobinger T, Gerner ST, Sembill JA, Sprügel MI, Beuscher VD, et al. Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis. (2017) 44:26–34. doi: 10.1159/000468996
44. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini MJO. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotargrt. (2017) 8:57489–94. doi: 10.18632/oncotarget.15423
45. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. (2016) 47:1654–7. doi: 10.1161/STROKEAHA.116.013627
46. Wang F, Hu S, Ding Y, Ju X, Wang L, Lu Q, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2016) 25:182–7. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.013
47. Tao C, Wang J, Hu X, Ma J, Li H, You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2016) 26:393–401. doi: 10.1007/s12028-016-0332-0
48. Duan Z, Wang H, Wang Z, Hao Y, Zi W, Yang D, et al. Neutrophil-lymphocyte ratio predicts functional and safety outcomes after endovascular treatment for acute ischemic stroke. Cerebrovasc Dise. (2018) 45:221–7. doi: 10.1159/000489401
49. Goyal N, Tsivgoulis G, Chang JJ, Malhotra K, Pandhi A, Ishfaq MF, et al. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. (2018) 49:1985–7. doi: 10.1161/STROKEAHA.118.021477
50. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:3754–832. doi: 10.1161/STR.0000000000000046
51. Zhang SR, Phan TG, Sobey CG. Targeting the immune system for ischemic stroke. Trends Pharmacol Sci. (2021) 42:96–105. doi: 10.1016/j.tips.2020.11.010
52. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
53. Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. (2003) 139:93–101. doi: 10.1016/S0165-5728(03)00134-6
54. Furlan JC, Vergouwen MD, Fang J, Silver FL. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol. (2014) 21:215–22. doi: 10.1111/ene.12233
55. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. (1987) 257:2318–24. doi: 10.1001/jama.257.17.2318
56. Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. (2009) 22:294–301. doi: 10.1097/WCO.0b013e32832b4db3
57. McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. (2007) 27:4403–12. doi: 10.1523/JNEUROSCI.5376-06.2007
58. McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. (2008) 28:9451–62. doi: 10.1523/JNEUROSCI.2674-08.2008
59. Cui LL, Zhang Y, Chen ZY, Su YY, Liu Y, Boltze J. Early neutrophil count relates to infarct size and fatal outcome after large hemispheric infarction. CNS Neurosci Ther. (2020) 26:829–36. doi: 10.1111/cns.13381
60. Tarkanyi G, Karadi ZN, Szabo Z, Szegedi I, Csiba L, Szapary L. Relationship between leukocyte counts and large vessel occlusion in acute ischemic stroke. BMC Neurol. (2020) 20:440. doi: 10.1186/s12883-020-02017-3
61. Babcock AA, Toft-Hansen H, Owens T. Signaling through MyD88 regulates leukocyte recruitment after brain injury. J Immunol. (2008) 181:6481–90. doi: 10.4049/jimmunol.181.9.6481
62. Chamorro Á, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. (2012) 8:401–10. doi: 10.1038/nrneurol.2012.98
63. Lei TY, Ye YZ, Zhu XQ, Smerin D, Gu LJ, Xiong XX, et al. The immune response of T cells and therapeutic targets related to regulating the levels of T helper cells after ischaemic stroke. J Neuroinflamm. (2021) 18:25. doi: 10.1186/s12974-020-02057-z
64. Liesz A, Hu X, Kleinschnitz C, Offner H. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke. (2015) 46:1422–30. doi: 10.1161/STROKEAHA.114.008608
65. Liesz A, Zhou W, Na SY, Hämmerling GJ, Garbi N, Karcher S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. (2013) 33:17350–62. doi: 10.1523/JNEUROSCI.4901-12.2013
66. Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. (2020) 130:2777–88. doi: 10.1172/JCI135530
67. Kim J, Song TJ, Park JH, Lee HS, Nam CM, Nam HS, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
68. Lasek-Bal A, Jedrzejowska-Szypulka H, Student S, Warsz-Wianecka A, Zareba K, Puz P, et al. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J physiol Pharmacol. (2019) 70:209–17. doi: 10.1101/503953
69. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
70. Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, et al. Inflammation following stroke. J Clin Neurosci. (2006) 13:1–8. doi: 10.1016/j.jocn.2005.07.005
71. Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. (2018) 114:42–54. doi: 10.1016/j.neuint.2018.01.002
Keywords: inflammation, ischemic stroke, hemorrhagic stroke, meta-analysis, neutrophil-to-lymphocyte ratio, prognosis, systematic review
Citation: Li W, Hou M, Ding Z, Liu X, Shao Y and Li X (2021) Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 12:686983. doi: 10.3389/fneur.2021.686983
Received: 28 March 2021; Accepted: 23 August 2021;
Published: 24 September 2021.
Edited by:
Nishant K. Mishra, Yale University, United StatesReviewed by:
Surasak Saokaew, University of Phayao, ThailandPallab Bhattacharya, National Institute of Pharmaceutical Education and Research, Ahmedabad, India
Copyright © 2021 Li, Hou, Ding, Liu, Shao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyi Li, xinyili2003@163.com
†These authors have contributed equally to this work and share first authorship
 Wenxia Li
Wenxia Li Miaomiao Hou1†
Miaomiao Hou1† Xinyi Li
Xinyi Li