- 1Department of Neurology, Yonsei University College of Medicine, Seoul, South Korea
- 2Integrative Research Center for Cerebrovascular and Cardiovascular Diseases, Yonsei University College of Medicine, Seoul, South Korea
Introduction: Cardioembolic stroke (CE) has poor outcomes and high recurrence rates. A low ankle-brachial index (ABI <0.9) is associated with atrial fibrillation (AF) and poor stroke outcomes. We investigated whether a low ABI is associated with stroke recurrence, major adverse cardiovascular events (MACE), and mortality in patients with CE and whether this association is affected by AF.
Methods: We enrolled patients with CE who underwent ABI measurements during hospitalization. Recurrent stroke was defined based on newly developed neurologic symptoms with relevant lesions 7 days after the index stroke. MACE comprised stroke recurrence, myocardial infarction, or death.
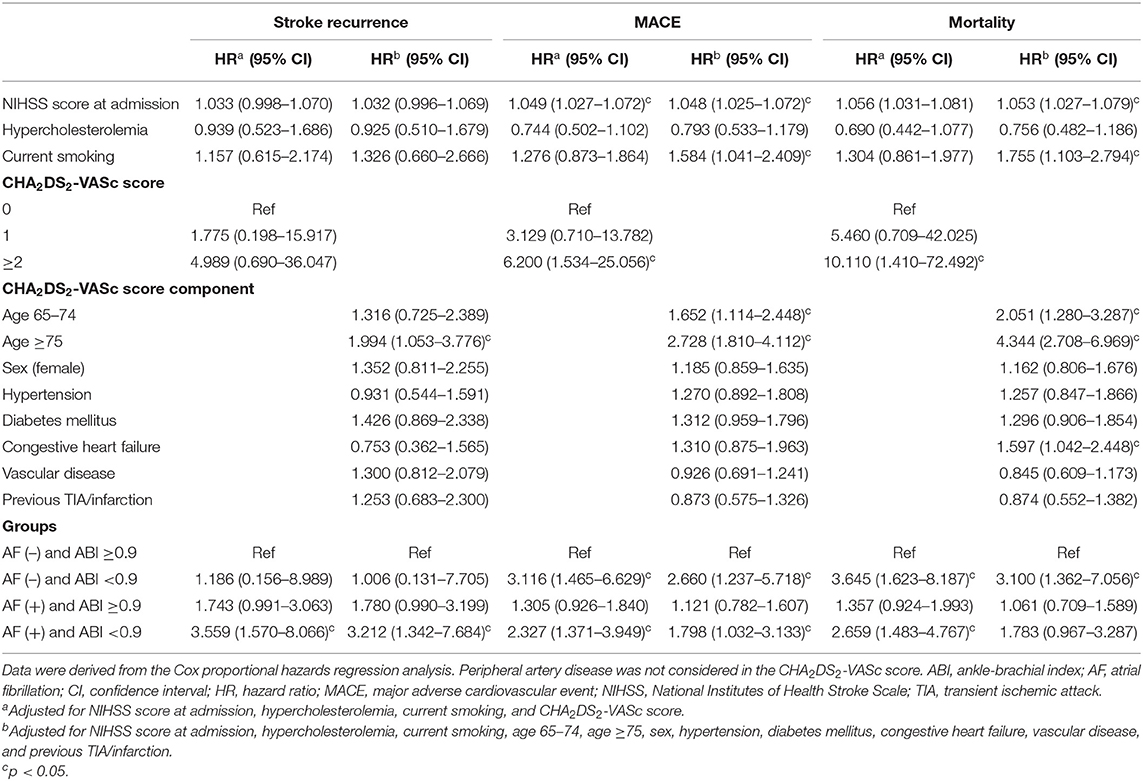
Results: Of 775 patients, 427 (55.1%) were AF patients and 348 (44.9%) were non-AF patients. Patients were followed up for a median of 33.6 (IQR, 18.0–51.6) months. In total, 194 (25.0%) patients experienced MACE, including 77 (9.9%) patients with stroke recurrence and 101 (13.0%) patients with mortality, during the study period. Multivariable Cox regression analysis showed that an ABI <0.9 was independently associated with MACE (AF patients: hazard ratio [HR] = 2.327, 95% confidence interval [CI] = 1.371–3.949, non-AF patients: HR = 3.116, 95% CI = 1.465–6.629) and mortality (AF patients: HR = 2.659, 95% CI = 1.483–4.767, non-AF patients: HR = 3.645, 95% CI = 1.623–8.187). Stroke recurrence was independently associated with an ABI <0.9 in AF patients (HR = 3.559, 95% CI = 1.570–8.066), but not in non-AF patients (HR = 1.186, 95% CI = 0.156–8.989).
Conclusions: We found that a low ABI is associated with stroke recurrence, MACE, and mortality in patients with CE. In particular, the association between ABI and recurrent stroke is only present in AF patients. A low ABI may be a useful prognostic marker in patients with CE, especially in AF patients.
Introduction
Globally, stroke occurs in over 12 million people each year and is the third leading cause of death and disability (1). The majority of strokes are ischemic strokes and are caused by atherosclerosis, small vessel occlusion, or cardioembolism. Among these causes, cardioembolic stroke (CE) accounts for 25–40% of cerebral infarctions (2). Furthermore, CE is associated with a 3-fold case fatality rate and relatively high recurrence rates of 22.0–35.5% compared to other types of ischemic stroke (2, 3). Atrial fibrillation (AF) is the most common cause of CE and the presence of AF has been reported to increase the risk of stroke recurrence by 54% (4). In addition, although stroke mortality has been reduced by 36% over the past 30 years, the disability-adjusted life-years were ranked second following ischemic heart disease in patients aged 50 years and older (1, 5). A high stroke burden in the elderly may be linked to an increased incidence of risk factors, including AF, and resulting cardiovascular events (6). Oral anticoagulant therapy with a vitamin K antagonist or a direct oral anticoagulant can reduce the relative risk of stroke recurrence by 60–70% (7, 8). However, many patients still experience recurrent stroke and poor cardiovascular outcomes despite taking oral anticoagulants (9). Therefore, it is crucial to identify high-risk populations among CE patients to reduce further the risk of stroke recurrence.
Peripheral artery disease (PAD) is characterized by abnormal narrowing of arteries that reduces blood flow to the limbs. PAD is typically diagnosed if the ankle-brachial index (ABI) is below 0.9 (10). We previously reported that an ABI <0.9 is a predictive factor of initial stroke severity (11) and an independent predictor of poor functional outcome at 3 months in patients with acute stroke (12). Large population-based studies showed that PAD is associated with the incidence of AF (13–15). Meta-analysis demonstrated that presence of PAD was associated with a 31% increase in risk for incident AF (16). However, outcome of acute ischemic stroke patients who have both PAD and AF is largely unknown.
Therefore, the purpose of this study is to reveal the impact of a low ABI on the outcome in acute ischemic stroke patients with CE. We sought to determine whether a low ABI is associated with stroke recurrence, major adverse cardiovascular events (MACE), and all-cause mortality in patients with CE. We also investigated whether the associations differ between patients with and without AF.
Materials and Methods
Study Sample
A single-center, hospital-based, retrospective observational study was conducted using prospectively collected stroke registry data. Between January 1, 2007, and June 30, 2013, 3,821 consecutive patients with acute cerebral infarction or transient ischemic attack (TIA) within 7 days of symptom onset were admitted to the study hospital and registered. During admission, all patients were thoroughly investigated for demographic data, medical history, vascular risk factors, and clinical manifestations. All patients underwent brain computed tomography (CT) and/or magnetic resonance imaging (MRI), 12-lead electrocardiography, and standard blood tests. Acute cerebral infarction was defined as the sudden onset of acute neurological deficits of presumed vascular etiology lasting 24 h or evidence of acute infarction on brain CT or MRI. TIA was diagnosed when a patient showed transient (<24 h) neurologic dysfunction of vascular origin and did not have acute lesions on CT or MRI. Patients were treated using standard treatment protocols based on the guidelines for acute ischemic stroke. Stroke classifications were determined during weekly conferences. Based on the consensus of three stroke neurologists, stroke subtypes were classified according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification (17). Briefly, large artery atherosclerosis is defined when there is significant (≥50%) stenosis of the large artery relevant to the acute infarction. Cardioembolism is defined when there is at least one potential cardiac source of embolism, which is defined in the TOAST classification. A patient with a small vessel occlusion should have one of the classic clinical lacunar syndromes and a relevant subcortical hemispheric or brain stem lesion with a diameter smaller than 1.5 cm. Stroke of other determined etiology includes patients with a rare cause of stroke, such as non-atherosclerotic vasculopathy, hypercoagulable state, and hematologic disorder. Stroke of undetermined etiology is defined when the mechanism of stroke cannot be determined and is further subdivided into undetermined etiology because of multiple causes identified, undetermined etiology attributable to negative evaluation despite extensive examinations, and undetermined etiology attributable to incomplete evaluation (18).
From the 3,821 patients, we excluded patients with stroke subtypes other than CE, including TIA (n = 52), large artery atherosclerosis (n = 762), small vessel occlusion (n = 329), stroke of other determined cause (n = 89), stroke of undetermined cause (n = 1,499; two or more causes [n = 682], negative evaluation [n = 806], and incomplete evaluation [n = 11]), and those in whom ABI measurements were not performed (n = 315). After exclusion, 775 patients with CE were finally enrolled in this study (Supplementary Table 1). Patients with CE were divided into two groups based on the presence of AF (Figure 1).
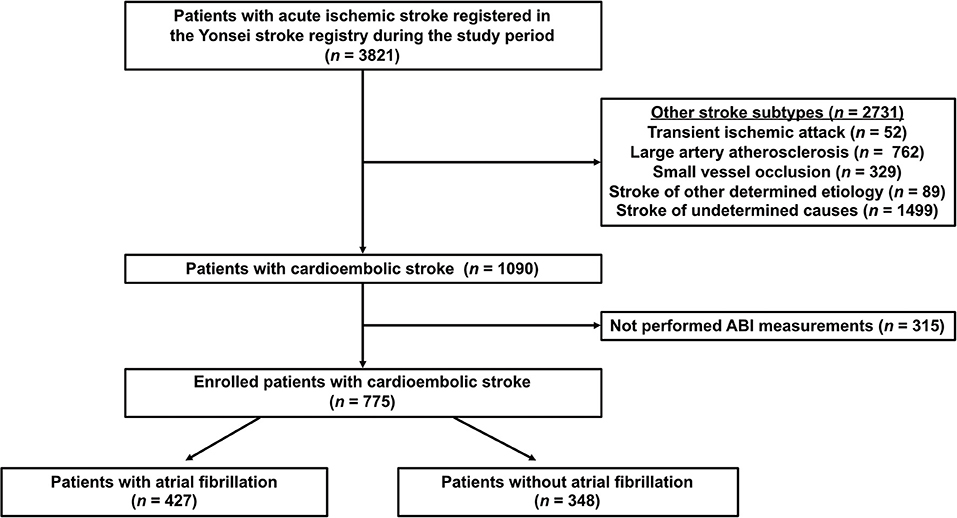
Figure 1. Flowchart of participants according to inclusion and exclusion criteria. ABI, ankle-brachial index.
This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System. The requirement for informed consent was waived because of the retrospective nature of the analysis (4-2019-1196).
Clinical Variables
Upon admission, we collected data on baseline characteristics, including sex, age, and neurological deficits [National Institutes of Health Stroke Scale (NIHSS) score], presence of risk factors, and laboratory data (glucose, low-density lipoprotein, and total cholesterol). Hypertension was defined as a resting systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg after repeated measurements during hospitalization or through the current use of antihypertensive medication. Diabetes mellitus was defined as a fasting plasma glucose level of ≥7 mmol/L or through the current use of an oral hypoglycemic agent or insulin. Hypercholesterolemia was diagnosed in patients taking lipid-lowering agents after a diagnosis of hypercholesterolemia or in those with low-density lipoprotein cholesterol levels ≥4.1 mmol/L or total cholesterol levels ≥6.2 mmol/L. Current smoking was defined as having smoked a cigarette within 1 year prior to admission. Congestive heart failure was determined based on history of heart failure diagnosis, treatment with loop diuretics, and ejection fraction of ≤35% on echocardiography. Coronary artery disease was diagnosed when a patient had a history of coronary artery disease (acute myocardial infarction, unstable angina, coronary artery bypass graft, or percutaneous coronary artery stent/angioplasty) or the presence of significant stenosis (≥50%) in any of the three main coronary arteries on multislice CT coronary angiography upon admission. The CHA2DS2-VASc scores were calculated for all patients, with one point assigned to patients with a history of congestive heart failure, hypertension, aged 65–74 years, diabetes mellitus, female sex, vascular diseases (coronary artery disease or atheroma in the aortic arch/ascending/descending aorta), and two points for patients aged ≥75 years and those with a history of stroke or TIA. The CHA2DS2-VASc scores were also divided into three groups (0: low risk, 1: intermediate risk, ≥2: high risk) based on a previous study (19).
ABI Measurement
The ABI was measured in the supine position using an automatic device (VP-1000; Colin Co., Ltd., Komaki, Japan), which has been validated previously (20). The device automatically and simultaneously measures four-limb blood pressure using the oscillometric method. The ABI was calculated as the ratio of the ankle systolic blood pressure divided by the higher systolic blood pressure of the arms. After obtaining bilateral ABI values, the lower value was used for analysis. A low ABI was defined as ABI <0.9. Patients with low ABI were defined as when the ABI <0.9 is present in one or more sides of the ankle (11, 12). The mean values of the systolic and diastolic blood pressures in both ankles were also used for analysis.
Follow-Up and Outcome Measures
After discharge, each patient was followed up regularly at 3 months, 1 year, and then yearly. At each follow-up visit, medical information including occurrence of any cardiovascular events, newly detected vascular risk factors, and re-admission to another hospital was obtained via face-to-face interviews with neurologists or through clinical research associates in the outpatient clinic. When the patients missed a scheduled visit, we obtained the information from the patients or their proxy through a telephone interview with a structured questionnaire. Short-term functional outcomes at 3 months were determined using a structured interview with the modified Rankin Scale (mRS). A poor short-term outcome was defined as an mRS ≥3. MACE were defined as any stroke recurrence, myocardial infarction occurrence, or death. Stroke recurrence was defined as newly developed neurologic symptoms with relevant lesions on brain CT and/or MRI 7 days after an index stroke or hospital discharge. Loss of follow-up occurred in 110 (14.2%) of 775 patients. When the patients were admitted to another hospital due to stroke recurrence or occurrence of myocardial infarction, we attempted to obtain medical records and imaging results. If this was not possible, stroke recurrence was determined by the investigators using telephone interview. Deaths among participants from January 1, 2007, to December 31, 2013, were confirmed by matching the information in the death records and identification numbers assigned to participants at birth (18). We obtained data for the date and cause of death from the death certificates from the Korean National Statistical Office.
Statistical Analysis
SPSS (version 26, SPSS, Chicago, IL, USA) and SAS (version 9.4, SAS Inc., Cary, NC, USA) were used for statistical analysis. The statistical significance of intergroup differences was assessed using the χ2 or Fisher's exact test for categorical variables and the independent two-sample t-test or Mann-Whitney U-test for continuous variables. Data are expressed as mean ± standard deviation or median [interquartile ranges (IQRs)] for continuous variables, and numbers (%) for categorical variables. The correlation between an ABI <0.9 and CHA2DS2-VASc scores was determined using the Spearman rank test. Mediation analysis was performed to evaluate whether a low ABI mediates the association between AF and outcomes. Survival curves were generated according to the Kaplan-Meier method and compared using the log-rank test. Log-log plots were performed to verify the proportional hazards assumption of the strata (Supplementary Figure 1). Multivariable Cox proportional hazard regression was performed after adjusting for the NIHSS score at admission, CHA2DS2-VASc variables (age, sex, hypertension, diabetes mellitus, congestive heart failure, vascular disease, and previous TIA/infarction), and additional cardiovascular risk factors (hypercholesterolemia and smoking) to investigate the independent association of an ABI <0.9 with unfavorable outcomes. All p-values were two-tailed, and differences were considered significant at p <0.05.
Results
Patient Demographics and Clinical Characteristics
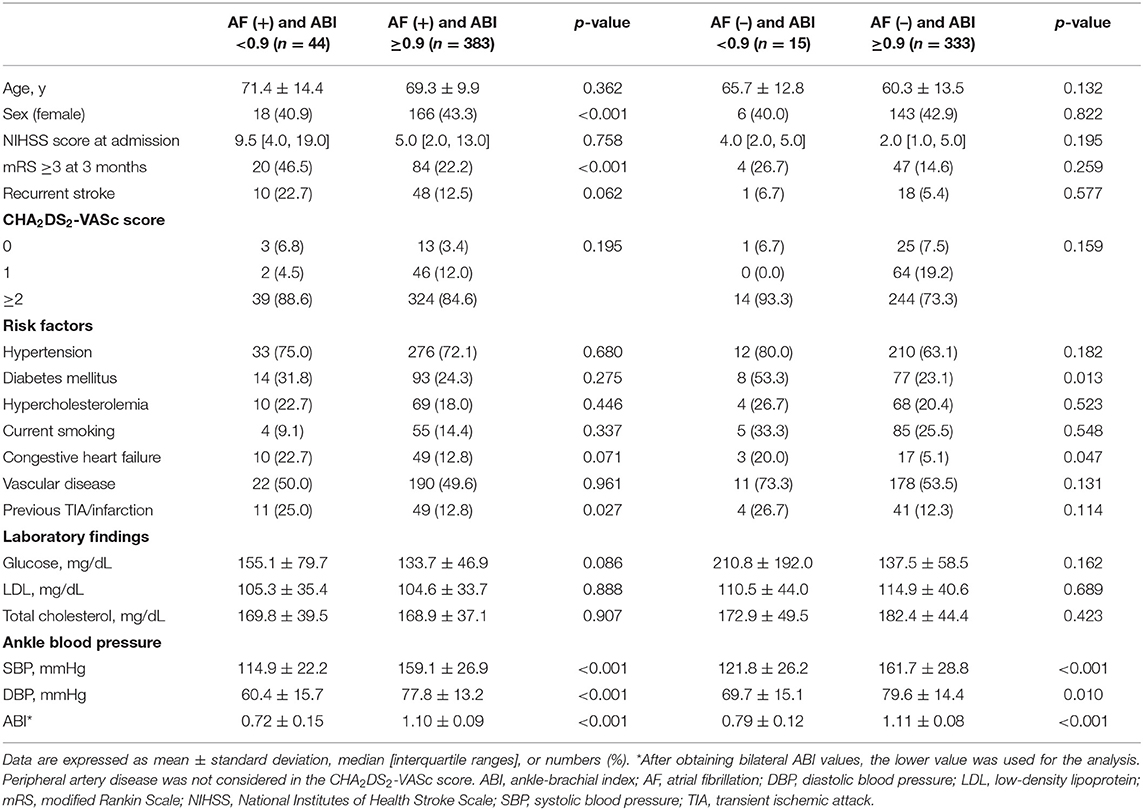
Of the 3,821 patients, 775 (20.3%) were eligible and classified as CE. The mean age of the study patients was 65.5 ± 12.7 years, and 333 (43.0%) were women. We divided CE patients into 427 (55.1%) patients with AF and 348 (44.9%) patients without AF. Compared to non-AF patients, AF patients were older, more likely to have hypertension and congestive heart failure, and less likely to be current smokers (all p < 0.05). Patients with a low ABI were also more frequent in the group of AF patients (10.3 vs. 4.3%, p = 0.002). The initial NIHSS score was higher in AF patients (5.0 [2.0, 13.0] vs. 2.0 [1.0, 5.0], p < 0.001). CHA2DS2-VASc scores were higher in AF patients than in non-AF patients (3.4 ± 1.7 vs. 2.7 ± 1.7, p < 0.001). A poor short-term outcome (mRS ≥3) at 3 months was more frequent in AF patients (24.7 vs. 15.2%, p = 0.001; Supplementary Table 2).
Comparison According to Presence of AF and Low ABI
Of the 427 patients with AF, the mean age was 69.5 ± 10.5 years, and 184 (43.1%) AF patients were female (Table 1). When compared with AF patients with an ABI ≥0.9, those with an ABI <0.9 were less likely to be female and more likely to have a previous TIA/infarction and lower ankle blood pressure (all p < 0.05). Poor short-term outcomes at 3 months were more frequent in AF patients with an ABI <0.9 than in those with an ABI ≥0.9 (46.5 vs. 22.2%, p < 0.001). Among AF patients, CHA2DS2-VASc scores were higher in patients with an ABI <0.9 than in those with an ABI ≥0.9 (Spearman rank test; p < 0.05; Figure 2A). The mean age of the 348 non-AF patients was 60.5 ± 13.5 years, and 149 (42.8%) of them were female. Compared to non-AF patients with an ABI ≥0.9, those with an ABI <0.9 were more likely to have diabetes mellitus, congestive heart failure, and lower ankle blood pressure (all p < 0.05). Among non-AF patients, CHA2DS2-VASc scores were higher in patients with an ABI <0.9 than in those with an ABI ≥0.9 (Spearman rank test; p < 0.05; Figure 2B).
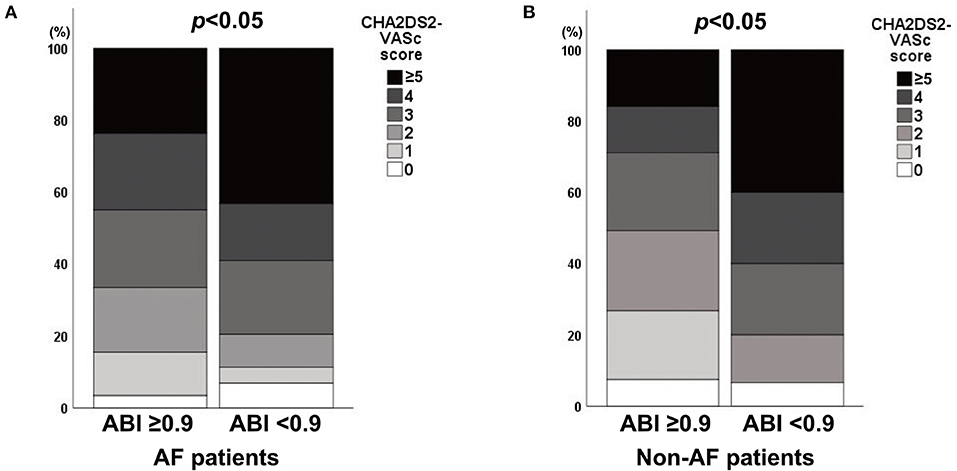
Figure 2. Association between the CHA2DS2-VASc score and an ABI <0.9 in (A) AF and (B) non-AF patients. ABI, ankle-brachial index; AF, atrial fibrillation.
Association Between Low ABI and Long-Term Outcomes
Patients were followed up for a median of 33.6 (IQR, 18.0–51.6) months. A total of 194 (25.0%) patients had MACE, including 77 (9.9%) with stroke recurrence and 101 (13.0%) with all-cause mortality, during the study period. Stroke recurrence rates were higher in patients with AF than in those without AF (13.6 vs. 5.5%, p < 0.001) and higher in patients with an ABI <0.9 than in those with an ABI ≥0.9 (18.6 vs. 9.2%, p = 0.02).
Kaplan-Meier curves showed that an ABI <0.9 was associated with stroke recurrence, MACE occurrence, and all-cause mortality in patients with CE (log-rank test, all p < 0.05; Supplementary Figure 2). In particular, an ABI <0.9 was associated with stroke recurrence in patients with AF (log-rank test, p = 0.015; Figure 3A). However, an ABI <0.9 was not associated with stroke recurrence in non-AF patients (log-rank test, p = 0.655; Figure 3B). MACE and all-cause mortality were associated with an ABI <0.9 in both AF and non-AF patients (log-rank test, all p < 0.05). Analysis of four groups according to the presence of AF and an ABI <0.9 showed that stroke recurrence was highest in patients who had both AF (+) and an ABI <0.9. Occurrence of MACE and mortality was higher in patients with AF (+) and ABI <0.9 and patients with AF (–) and an ABI <0.9 compared to those with AF (+) and an ABI ≥0.9 and those with AF (–) and an ABI ≥0.9 (log-rank test, all p < 0.001; Figure 4).
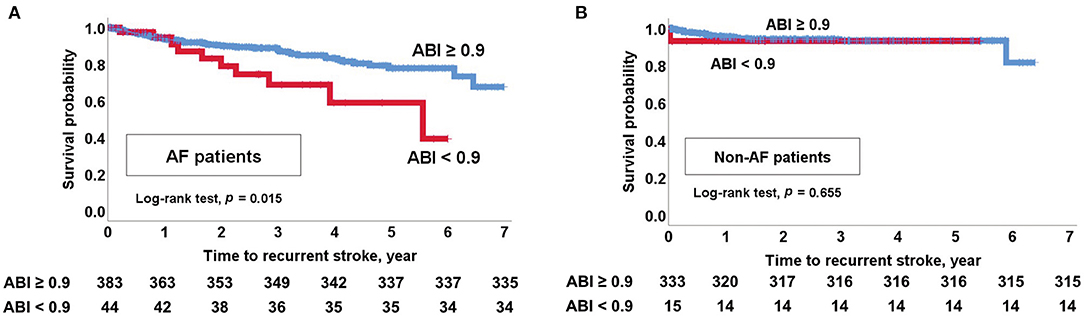
Figure 3. Kaplan-Meier survival analysis. (A) Recurrent stroke in AF patients with an ABI <0.9. (B) Recurrent stroke in non-AF patients with an ABI <0.9. ABI, ankle-brachial index; AF, atrial fibrillation.

Figure 4. Kaplan-Meier survival analysis for (A) recurrent stroke, (B) major adverse cardiovascular events, and (C) all-cause mortality according to AF and an ABI <0.9. ABI, ankle-brachial index; AF, atrial fibrillation.
Multivariable Cox regression analysis after adjusting for the initial NIHSS score, smoking status, hypercholesterolemia, and CHA2DS2-VASc variables showed that an ABI <0.9 was independently associated with MACE occurrence [AF patients: hazard ratio (HR) 2.327, 95% confidence interval (CI) 1.371–3.949, non-AF patients: HR 3.116, 95% CI 1.465–6.629] and all-cause mortality (AF patients: HR 2.659, 95% CI 1.483–4.767, non-AF patients: HR 3.645, 95% CI 1.623–8.187). In particular, an ABI <0.9 was independently associated with stroke recurrence in AF patients (HR 3.559, 95% CI 1.570–8.066). However, an ABI <0.9 was not associated with stroke recurrence in non-AF patients (HR 1.186, 95% CI 0.156–8.989) (Table 2).
Discussion
We demonstrated that PAD, defined as an ABI <0.9, was independently associated with long-term MACE occurrence and all-cause mortality in patients with CE. In particular, in AF patients, an ABI <0.9 was independently associated with an increased risk of stroke recurrence, long-term MACE occurrence, and all-cause mortality after adjusting for CHA2DS2-VASc variables. Meanwhile, an ABI <0.9 was associated with MACE and all-cause mortality in non-AF patients but not with stroke recurrence. These findings suggest that PAD is associated with poor long-term prognosis in patients with CE and that PAD is only associated with recurrent stroke in patients with AF.
A low ABI is an established marker of systemic atherosclerosis, which is associated with increased mortality in the elderly population (21) and stroke patients (22). Other studies found that PAD, defined as an ABI <0.9, is related to increased severity of the initial stroke (11), a higher risk of recurrence (23), and poor outcomes in patients with all ischemic stroke (12). Consistent with these reports, our findings showed that after adjusting for CHA2DS2-VASc variables and cardiovascular risk factors, an ABI <0.9 is independently associated with a worse stroke prognosis.
Previous studies evaluated the prognostic effect of PAD in all stroke or non-cardioembolic stroke patients (24, 25). However, the prognostic value of PAD in CE is not fully understood. This may be associated with concerns regarding the accuracy of ABI measurements in patients with CE. To address these concerns, the reliability of ABI measurements during AF was studied by comparing ABI values measured during AF and sinus rhythm from the same patients. The study concluded that the ABI is accurate for the diagnosis of PAD also during AF (26). Another concern is the interaction between AF and PAD. Both are strong prognostic factors after index stroke (27–29). We found that an ABI <0.9 is more prevalent in AF patients than in non-AF patients and the presence of an ABI <0.9 is associated with a high CHA2DS2-VASc score. We also found that a low ABI mediates the association between AF and outcome (Supplementary Table 3). To overcome this interaction, multivariate analysis was conducted after adjusting for the CHA2DS2-VASc score. We demonstrated that a low ABI is an independent prognostic indicator for poor functional outcome, MACE, and mortality.
Among stroke subtypes, the stroke recurrence rate was higher in patients with CE [3]. AF is the most frequent cardiac condition associated with CE and is a major determinant of recurrent stroke (30). We showed that patients with AF are more likely to have stroke recurrence. Furthermore, our study demonstrated that patients with AF and an ABI <0.9 more frequently experienced recurrent stroke than patients with AF and an ABI ≥0.9. In contrast, an ABI <0.9 was not associated with stroke recurrence in non-AF patients. Therefore, the association between PAD and stroke recurrence is only valid in patients with AF, showing that PAD is differentially associated with stroke recurrence in patients with CE.
Several hypotheses can explain the effect of AF on stroke outcome in patients with PAD. First, the CHA2DS2-VASc score is known to be associated with systemic atherosclerosis, which increases the risk of stroke recurrence (23, 31–33). Therefore, high CHA2DS2-VASc scores in PAD patients with AF may lead to an increased risk of recurrence. Second, only in patients with both AF and PAD there was a significant association with stroke recurrence after adjusting for CHA2DS2-VASc variables. A previous study reported that a direct link and a dose-effect response relationship exist between PAD and AF (16). Therefore, the overlap between AF and PAD may have a synergistic effect and promote stroke recurrence (34, 35). Third, AF is a hemodynamic disorder, which causes endothelial damage by abnormal flow shear forces (36). We found that AF directly or indirectly affects poor functional outcome, MACE, and mortality through PAD in the mediation analysis. However, stroke recurrence was not mediated through PAD (defined by ABI <0.9) (Supplementary Table 3), while AF was associated with stroke recurrence through PAD (defined by continuous ABI) (Supplementary Table 4). These finding suggest that there may be a mediating effect of PAD between AF and stroke recurrence. Thus, atherosclerosis exacerbated by AF may increase the risk of adverse outcomes, including recurrent stroke (37). Finally, AF and PAD can exist as bystanders. Stroke recurrence rates may be higher because each is a significant risk factor for recurrent stroke (23, 27). However, further prospective studies are needed to assess the causal relationship between AF with PAD and stroke recurrence.
This study has several limitations. First, the definition of PAD was not based on medical history and radiological study findings. It is known that medical histories obtained from PAD patients are often inaccurate, and subclinical PAD is prevalent. In this study, radiological examinations to detect atherosclerosis in the extremities were not routinely conducted. However, the ABI is recommended for use in clinical practice and research according to guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) (10). Second, this study was a retrospective cohort study, and there was a follow-up loss of 14.2%. This may have influenced the statistical analyses. Third, our findings may not be generalizable to other populations or ethnicities because our study population was limited to Korean patients.
Conclusion
This study showed that PAD is associated with poor long-term outcomes, including stroke recurrence, MACE, and all-cause mortality in patients with CE. In particular, the association between PAD and recurrent stroke is only observed in patients with AF. Therefore, an ABI <0.9 may be a useful prognostic marker in patients with CE, especially in those with AF.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Severance Hospital, Yonsei University Health System. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MH and HN: conceptualization, methodology, investigation, writing—original draft preparation, and writing—review and editing. MH: formal analysis. MH, MB, JC, KS, and EP: data curation. YK and JH: supervision. HN: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Numbers: HI19C0481 and HC19C0028).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.705904/full#supplementary-material
References
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Kolominsky Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. (2001) 32:2735–40. doi: 10.1161/hs1201.100209
3. Saber H, Thrift AG, Kapral MK, Shoamanesh A, Amiri A, Farzadfard MT, et al. Incidence, recurrence, and long-term survival of ischemic stroke subtypes: a population-based study in the Middle East. Int J Stroke. (2017) 12:835–43. doi: 10.1177/1747493016684843
4. Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. (2020) 51:2435–44. doi: 10.1161/STROKEAHA.120.02899
5. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
6. Yiin GS, Howard DP, Paul NL, Li L, Luengo Fernandez R, Bull LM, et al. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation. (2014) 130:1236–44. doi: 10.1161/CIRCULATIONAHA.114.010942
7. Diener H, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. (2012) 11:225–31. doi: 10.1016/S1474-4422(12)70017-0
8. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
9. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. (2020) 87:677–87. doi: 10.1002/ana.25700
10. Gerhard Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. (2017) 22:NP1–43. doi: 10.1177/1358863X17701592
11. Lee DH, Kim J, Lee HS, Cha MJ, Kim YD, Nam HS, et al. Low ankle-brachial index is a predictive factor for initial severity of acute ischaemic stroke. Eur J Neurol. (2012) 19:892–8. doi: 10.1111/j.1468-1331.2011.03652.x
12. Kim J, Lee DH, Cha M, Song T, Park JH, Lee HS, et al. Low ankle-brachial index is an independent predictor of poor functional outcome in acute cerebral infarction. Atherosclerosis. (2012) 224:113–7. doi: 10.1016/j.atherosclerosis.2012.06.058
13. O'Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Peripheral arterial disease and risk of atrial fibrillation and stroke: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. (2014) 3:e001270. doi: 10.1161/JAHA.114.001270
14. Griffin WF, Salahuddin T, O'Neal WT, Soliman EZ. Peripheral arterial disease is associated with an increased risk of atrial fibrillation in the elderly. Europace. (2016) 18:794–8. doi: 10.1093/europace/euv369
15. Bekwelem W, Norby FL, Agarwal SK, Matsushita K, Coresh J, Alonso A, et al. Association of peripheral artery disease with incident atrial fibrillation: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc. (2018) 7: e007452. doi: 10.1161/JAHA.117.007452
16. Proietti M, Farcomeni A. Association between peripheral artery disease and incident risk of atrial fibrillation: strong evidence coming from population-based cohort studies. J Am Heart Assoc. (2018) 7:e009126. doi: 10.1161/JAHA.118.009126
17. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41.
18. Nam HS, Kim HC, Kim YD, Lee HS, Kim J, Lee DH, et al. Long-term mortality in patients with stroke of undetermined etiology. Stroke. (2012) 43:2948–56. doi: 10.1161/STROKEAHA.112.661074
19. Ntaios G, Lip GY, Makaritsis K, Papavasileiou V, Vemmou A, Koroboki E, et al. CHADS2, CHA2S2DS2-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology. (2013) 80:1009–17. doi: 10.1212/WNL.0b013e318287281b
20. Han M, Kim YD, Choi JK, Ha J, Park E, Kim J, et al. Predicting stroke outcomes using ankle-brachial index and inter-ankle blood pressure difference. J Clin Med. (2020) 9:1125. doi: 10.3390/jcm9041125
21. Sheng C, Liu M, Zeng W, Huang Q, Li Y, Wang J. Four-limb blood pressure as predictors of mortality in elderly Chinese. Hypertension. (2013) 61:1155–60. doi: 10.1161/HYPERTENSIONAHA.111.00969
22. Milionis H, Vemmou A, Ntaios G, Makaritsis K, Koroboki E, Papavasileiou V, et al. Ankle-brachial index long-term outcome after first-ever ischaemic stroke. Eur J Neurol. (2013) 20:1471–8. doi: 10.1111/ene.12208
23. Hong JB, Leonards CO, Endres M, Siegerink B, Liman TG. Ankle-brachial index and recurrent stroke risk: meta-analysis. Stroke. (2016) 47:317–22. doi: 10.1161/STROKEAHA.115.011321
24. Abboud H, Monteiro Tavares L, Labreuche J, Arauz A, Bryer A, Lavados PM, et al. Impact of low ankle-brachial index on the risk of recurrent vascular events. Stroke. (2019) 50:853–8. doi: 10.1161/strokeaha.118.022180
25. Matsushima H, Hosomi N, Hara N, Yoshimoto T, Neshige S, Kono R, et al. Ability of the ankle brachial index and brachial-ankle pulse wave velocity to predict the 3-month outcome in patients with non-cardioembolic stroke. J Atheroscler Thromb. (2017) 24:1167–73. doi: 10.5551/jat.38901
26. Dabrowski M, Lewandowski J, Abramczyk P, Łoń I, Gaciong Z, Siński M. Atrial fibrillation does not affect ankle-brachial index measured using the Doppler method. Hypertens Res. (2018) 41:60–5. doi: 10.1038/hr.2017.89
27. Penado S, Cano M, Acha O, Hernández JL, Riancho JA. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med. (2003) 114:206–10. doi: 10.1016/s0002-9343(02)01479-1
28. Tsivgoulis G, Bogiatzi C, Heliopoulos I, Vadikolias K, Boutati E, Tsakaldimi S, et al. Low ankle-brachial index predicts early risk of recurrent stroke in patients with acute cerebral ischemia. Atherosclerosis. (2012) 220:407–12. doi: 10.1016/j.atherosclerosis.2011.11.009
29. Cha MJ, Kim YD, Nam HS, Kim J, Lee DH, Heo JH. Stroke mechanism in patients with non-valvular atrial fibrillation according to the CHADS2 and CHA2 DS2-VASc scores. Eur J Neurol. (2012) 19:473–9. doi: 10.1111/j.1468-1331.2011.03547.x
30. Kamel H, Healey JS. Cardioembolic stroke. Circ Res. (2017) 120:514–26. doi: 10.1161/circresaha.116.308407
31. Kim YD, Cha MJ, Kim J, Lee DH, Lee HS, Nam CM, et al. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. (2011) 42:930–4. doi: 10.1161/strokeaha.110.602987
32. Hsu PC, Lee WH, Lee HC, Tsai WC, Chu CY, Chen YC, et al. Association between modified CHA(2)DS(2)-VASc Score with Ankle-Brachial index <0.9. Sci Rep. (2018) 8:1175. doi: 10.1038/s41598-018-19243-y
33. Hsu PC, Lin TH, Lee WH, Chu CY, Chiu CA, Lee HH, et al. Association between the CHADS2 score and an ankle-brachial index of <0.9 in patients without atrial fibrillation. J Atheroscler Thromb. (2014) 21:322–8. doi: 10.5551/jat.21212
34. Winkel TA, Hoeks SE, Schouten O, Zeymer U, Limbourg T, Baumgartner I, et al. Prognosis of atrial fibrillation in patients with symptomatic peripheral arterial disease: data from the REduction of Atherothrombosis for Continued Health (REACH) registry. Eur J Vasc Endovasc Surg. (2010) 40:9–16. doi: 10.1016/j.ejvs.2010.03.003
35. Violi F, Daví G, Hiatt W, Lip GY, Corazza GR, Perticone F, et al. Prevalence of peripheral artery disease by abnormal ankle-brachial index in atrial fibrillation: implications for risk and therapy. J Am Coll Cardiol. (2013) 62:2255–6. doi: 10.1016/j.jacc.2013.07.035
36. Wang Z, Korantzopoulos P, Liu T. Carotid atherosclerosis in patients with atrial fibrillation. Curr Atheroscler Rep. (2019) 21:55. doi: 10.1007/s11883-019-0808-4
Keywords: ankle-brachial index, atrial fibrillation, outcome, peripheral artery disease, stroke
Citation: Han M, Baik M, Kim YD, Choi J, Seo K, Park E, Heo JH and Nam HS (2021) Low Ankle-Brachial Index Is Associated With Stroke Recurrence in Ischemic Stroke Patients With Atrial Fibrillation. Front. Neurol. 12:705904. doi: 10.3389/fneur.2021.705904
Received: 06 May 2021; Accepted: 23 September 2021;
Published: 20 October 2021.
Edited by:
Vincent Thijs, University of Melbourne, AustraliaReviewed by:
Andreas Kastrup, Klinikum Bremen Mitte, GermanyJanine Gronewold, Essen University Hospital, Germany
Copyright © 2021 Han, Baik, Kim, Choi, Seo, Park, Heo and Nam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyo Suk Nam, hsnam@yuhs.ac
 Minho Han
Minho Han Minyoul Baik1
Minyoul Baik1 Young Dae Kim
Young Dae Kim Ji Hoe Heo
Ji Hoe Heo Hyo Suk Nam
Hyo Suk Nam