- 1Quanzhou First Hospital, Fujian Medical University, Fujian, China
- 2Department of Radiology, Affiliated Hospital of Zunyi Medical University, Guizhou, China
Background: Mobile stroke unit (MSU) is deployed to shorten the duration of ischemic stroke recognition to thrombolysis treatment, thus reducing disability, mortality after an acute stroke attack, and related economic burden. Therefore, we conducted a comprehensive systematic review of the clinical trial and economic literature focusing on various outcomes of MSU compared with conventional emergency medical services (EMS).
Methods: An electronic search was conducted in four databases (PubMed, OVID Medline, Embase, and the Cochrane Controlled Register of Trials) from 1990 to 2021. In these trials, patients with acute stroke were assigned to receive either MSU or EMS, with clinical and economic outcomes. First, we extracted interested data in the pooled population and conducted a subgroup analysis to examine related heterogeneity. We then implemented a descriptive analysis of economic outcomes. All analyses were performed with R 4.0.1 software.
Results: A total of 22,766 patients from 16 publications were included. In total 7,682 (n = 33.8%) were treated in the MSU and 15,084 (n = 66.2%) in the conventional EMS. Economic analysis were available in four studies, of which two were based on trial data and the others on model simulations. The pooled analysis of time metrics indicated a mean reduction of 32.64 min (95% confidence interval: 23.38–41.89, p < 0.01) and 28.26 minutes (95% CI: 16.11–40.41, p < 0.01) in the time-to-therapy and time-to-CT completion, respectively in the MSU. However, there was no significant difference on stroke-related neurological events (OR = 0.94, 95% CI: 0.70–1.27, p = 0.69) and in-hospital mortality (OR = 1.11, 95% CI: 0.83–1.50, p = 0.48) between the MSU and EMS. The proportion of patients with modified Ranking scale (mRS) of 0–2 at 90 days from onset was higher in the MSU than EMS (p < 0.05). MSU displayed favorable benefit-cost ratios (2.16–6.85) and incremental cost-effectiveness ratio ($31,911 /QALY and $38,731 per DALY) comparing to EMS in multiple economic publications. Total cost data based on 2014 USD showed that the MSU has the highest cost in Australia ($1,410,708) and the lowest cost in the USA ($783,463).
Conclusion: A comprehensive analysis of current research suggests that MUS, compared with conventional EMS, has a better performance in terms of time metrics, safety, long-term medical benefits, and cost-effectiveness.
Introduction
Acute stroke is a disease with high morbidity, disability, mortality, recurrence, and complications (1–5). The most effective treatment is revascularization within the time window (6). Advances in mobile stroke units can shorten the prehospital time due to the timeliness of thrombolytic therapy, thus improving the prognosis of acute stroke patients (7–10). Fatima et al. conducted a meta-analysis to assess the differences in time domain indexes and clinical outcomes between the MSU and regular care, discovering improved functional outcomes due to timely thrombolytic therapy (11). Therefore, scarce healthcare resources need to be effectively allocated based on more information about health economic assessments in this field. Considering the economic impact of stroke sequelae on different populations worldwide would help health authorities make reliable decisions regarding whether it deserves to implement a policy preferring MSUs.
To clarify related economic and social burden, the Global Burden of Disease study in 2016 did a careful survey that estimated the number of stroke cases worldwide to be 80.1 million, with 41.1 million women and 39 million men (12). Moreover, stroke is the second leading cause of death globally (5.5 million) after ischemic heart disease. Given the severity of the problem, the economic burden of stroke prevention and treatment represents a significant share of health care budgets in many countries (13–15). Furthermore, the cost of post-stroke care accounts for a considerable proportion of public expenditure (16). This review aims to assess various outcomes of MSUs, including time metrics, adverse events, functional results, economic estimations, compared to the conventional EMS for patients with acute stroke. We hypothesize that MSU has better clinical outcomes and is more cost-effective than EMS.
Methods
Search Strategy
We searched PubMed, OVID Medline, Embase, and the Cochrane Controlled Register of Trials (CENTRAL) from 1990 to 2021 for clinical trials and economic evaluations that compared MSUs with EMS for suspected patients with acute stroke. A comprehensive strategy of searching was developed by combining medical subject headings and keywords: “mobile stroke unit,” “stroke,” “ambulance,” “thrombolysis,” “functional evaluation,” and/or “cost-effectiveness” Articles published in English were included. The reference lists of the identified studies were manually searched for any missing clinical trials s or economic evaluations.
Inclusion and Exclusion Criteria
Type of Study
The review included clinical trials and economic analyses.
Participant
Patients with acute stroke receiving the MSU or EMS treatment were included.
Intervention
MSU consists of prehospital thrombolysis. The MSU vehicle is a specialized ambulance equipped with a CT scanner and point-of-care laboratory and staffed by a paramedic, radiology technician, and physician specializing in neurology and emergency medicine. This vehicle is sent in response when an acute stroke is alarmed by an emergency call. Depending on the clinical symptoms, such as disabling stroke symptoms, head CT scanning and blood tests are done at the site. After being recognized as Radiologists make CT scanning interpretations, and thrombolysis is started immediately within the MSU vehicle.
Comparator
EMS consists of in-hospital thrombolysis. The stroke patients are taken to the hospital through emergency medical services either in the specialized stroke centers or emergency department and given thrombolysis.
Outcome. Clinical outcomes: (i) alarm to therapy decision (intravenous thrombolysis or intra-arterial recanalization), (ii) alarm to end of CT, (iii) in-hospital mortality, (iv) stroke-related or neurological events, and (v) mRs at 90 days from onset. Stroke-related or neurological events include fatal ischaemic stroke, fatal reinfarction, fatal primary intracranial hemorrhage, and fatal secondary intracranial hemorrhage. Economic outcomes: (i) MSU cost, (ii) incremental cost (difference between mean costs of intervention and mean costs of the comparator), and (iii) cost-effectiveness analysis (CEA).
Any researches involving participants younger than 18 years, interventions other than MSU, or study type other than clinical trials or economic evaluation were excluded.
Data Extraction
The data were extracted by two authors using a structured template form. Any disagreement between the two authors was resolved by discussion.
Statistical Analysis
Data analysis was performed through founded dichotomous outcomes like in-hospital mortality. Odds ratio (OR) is an effect size with a 95% confidence interval (CI), and study weights were estimated from the random-effects analysis. Forest plots for interested data were demonstrated. The chi-square test was conducted to evaluate whether the observed differences were heterogeneous. The I2 test was used to access inconsistencies among included studies as the percentage of variation was measured where heterogeneity was classified as 0–30% (mild), 31–50% (moderate), 51–80% (substantial), and 81–100% (considerable). Subgroup analysis was conducted when substantial to severe heterogeneity occurred. All tests were two-tailed, and a p-value < 0.05 was considered statistically significant. We carried out the data synthesis using narrative demonstration, with a summary of the characteristics of each included study. An overview of the combined estimation related to the MSU effect was measured for quantitative synthesis. Finally, a descriptive analysis was performed on economic outcomes. We adjusted costs by US Consumer Price Inflation Rate (based on 2014). All calculations were done with R 4.0.1 software, The R Foundation for Statistical Computing, 2004 (http://www.r-project.org). The meta-package was used to perform the meta-analyses.
Risk of Bias
We included RCTs and non-RCTs in our study; however, no RCTs were designed as double-blind trials because of the nature of the intervention. The Revised Cochrane risk-of-bias tool (RoB 2.0) was used to appraise the methodological quality of the included studies by two reviewers independently.
Results
Characteristics of the Included Studies
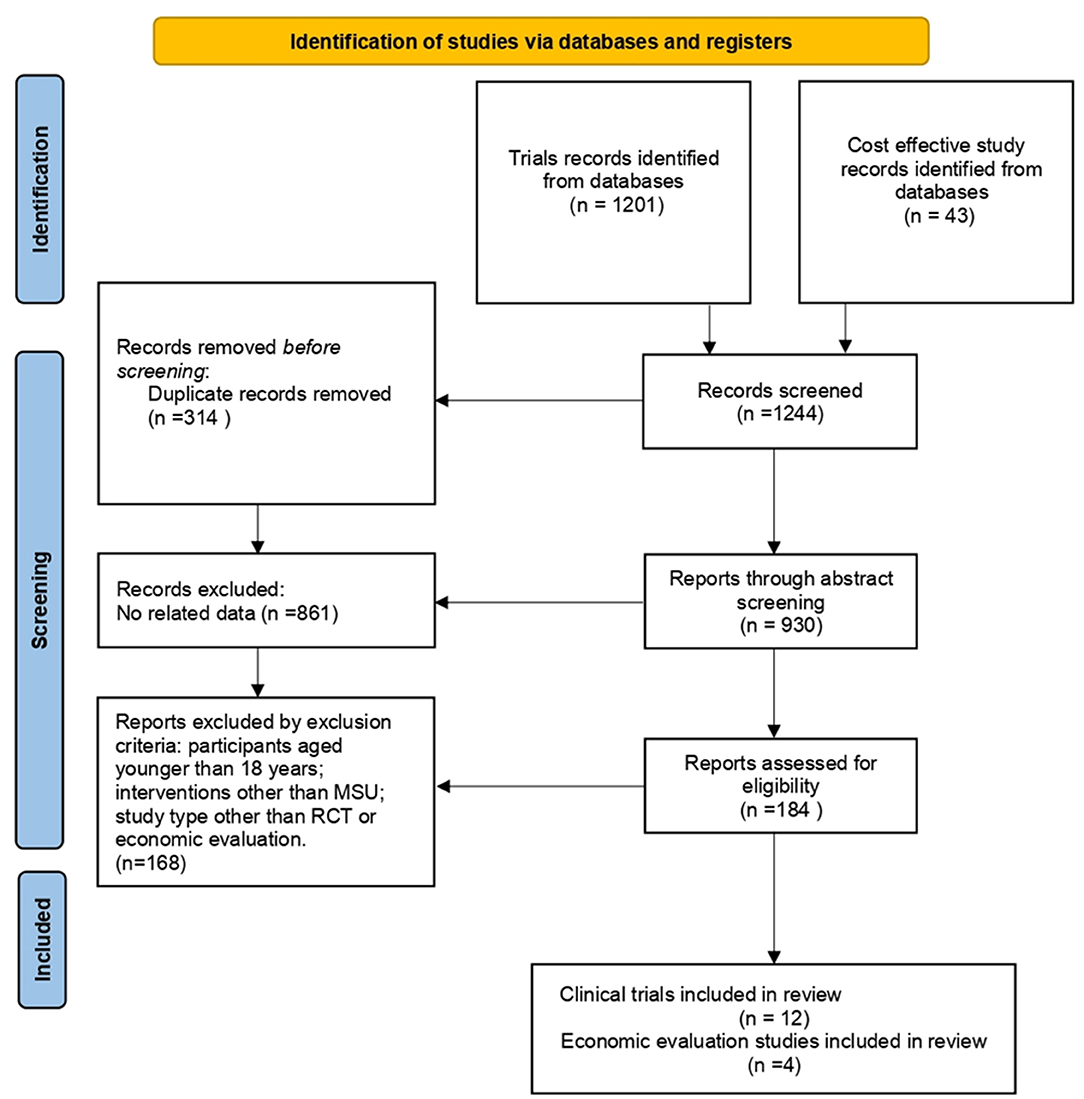
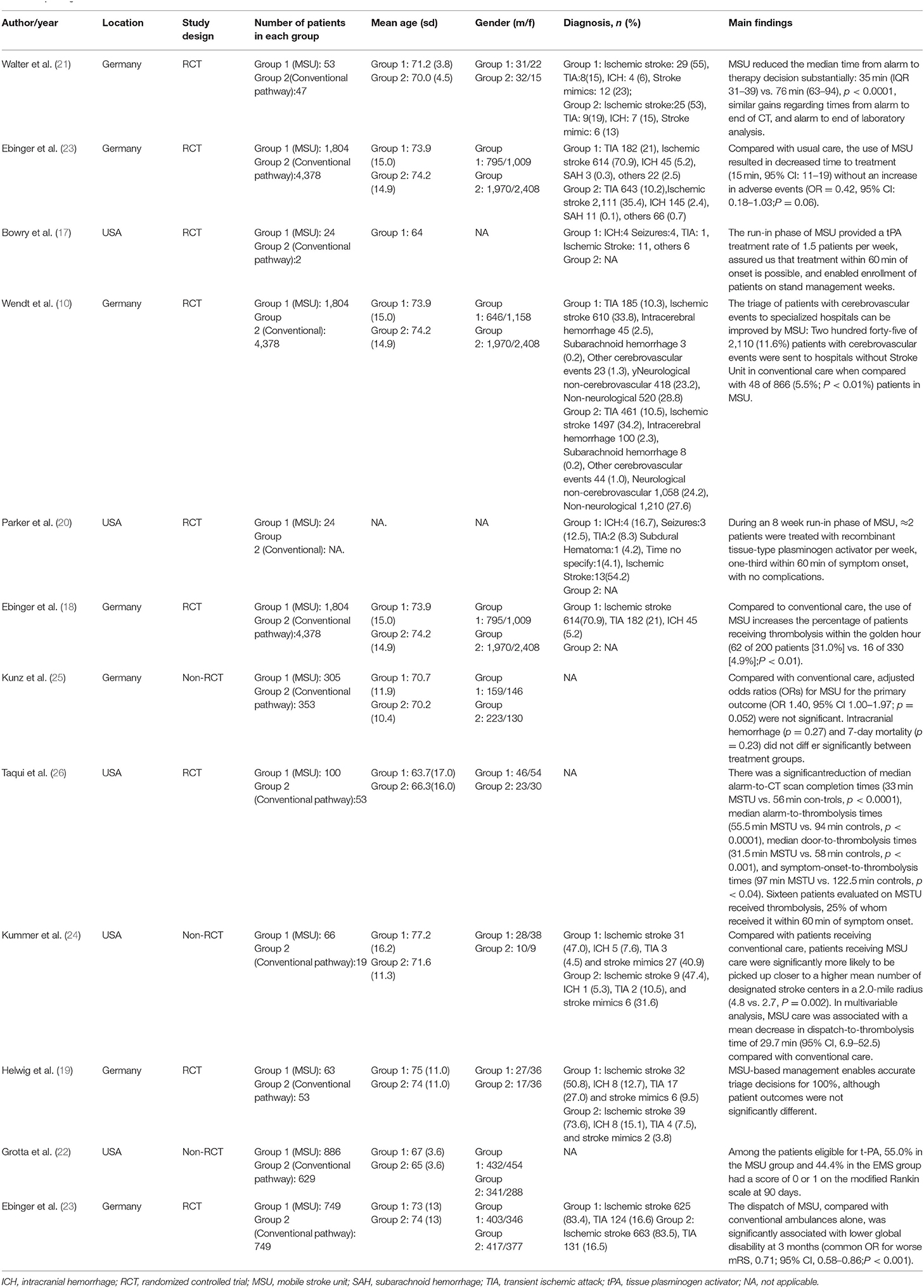
After screening 1,244 articles (Figure 1), a total of 16 articles were retrieved, including 12 clinical trials (9, 10, 17–26) and 4 economic studies (27–30). Characteristics of the included studies are described in Tables 1, 2. Most trials were done in Germany, except for five studies from the United States (Table 1). A combined total of 7,682 patients treated with MSUs were included in the trial group, and 15,084 patients treated by the conventional EMS were included in the control group (one clinical trial lacked a control group). The population's average age included in each clinical trial was around 70 years old, and more females than males. The diagnostic profile showed more patients with ischemic stroke than with hemorrhagic stroke. In addition, a complete of four economic studies were found, two based on trial data and the others on model simulations (Table 2). The earliest one was published in 2014, and the most recent one in 2021.
Quality Assessment
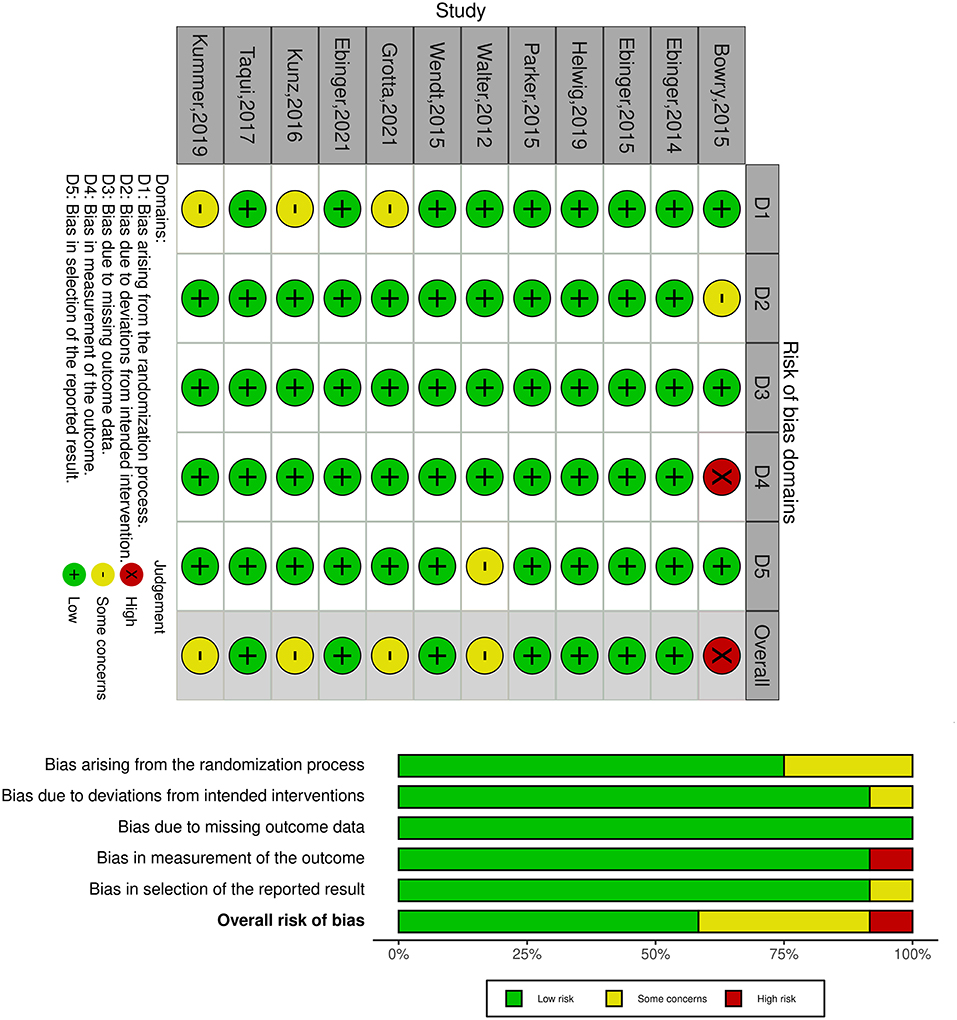
Selection, performance, detection, attrition, and reporting bias were assessed as high, low, or some concerns. As shown in Figure 2, all RCTs were considered high risk of bias. The trial dates ranged from 2014 to 2021, of which seven were assessed low risk of bias, with five moderate and one high.
Time Metrics
A pooled analysis of the time from alarm to therapy decision for all patients showed that the MSU group had a mean reduction of 32.64 min (95% confidence interval: 23.38–41.89, p < 0.01; one outlier was excluded (see Figure A1) compared with the control group. The pooled analysis of patient time from alarm to CT completion presented similar results, with a mean reduction of 28.26 min (95% confidence interval: 16.11–40.41, p < 0.01) for patients in the MSU group compared to the regular care group (Figure 3).
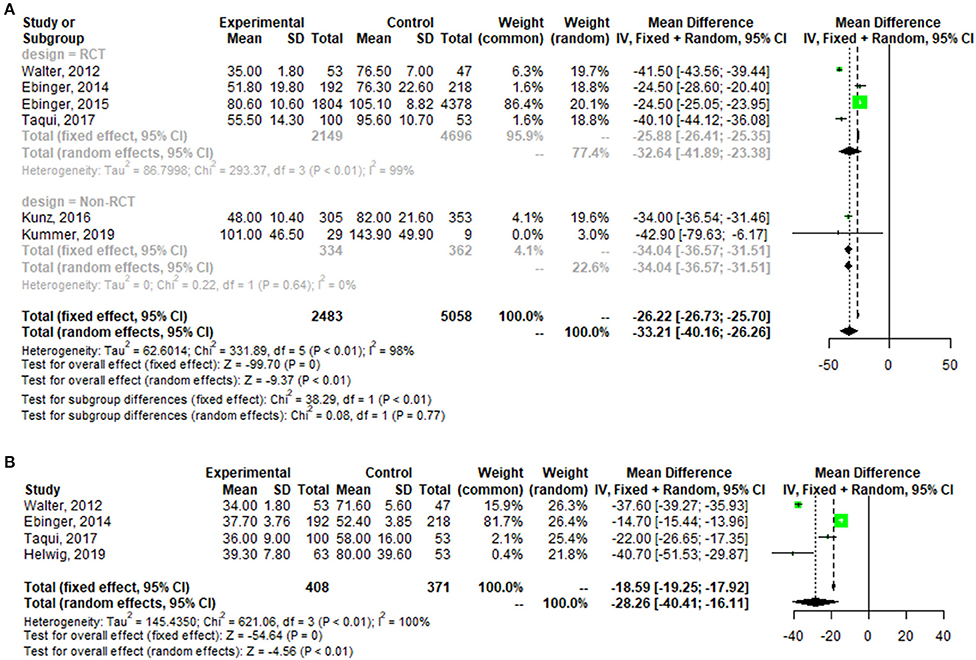
Figure 3. Forest plot for change in the time from alarm to therapy decision (A), and the time from alarm to CT completion (B).
Adverse Events
Pooled analysis of stroke-related or neurological events was not statistically different between the two groups (OR = 0.94, 95% CI: 0.70–1.27, p = 0.69), it was the same at in-hospital mortality (OR = 1.11, 95% CI: 0.83–1.50, p = 0.48) (Figure 4).
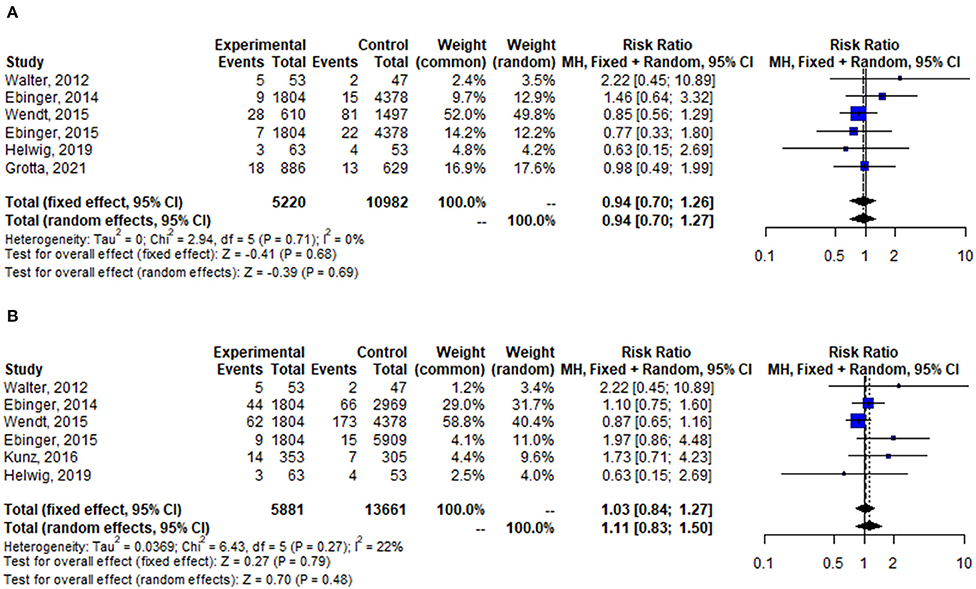
Figure 4. Forest plot for change in stroke-related or neurological events (A), and in-hospital mortality (B).
mRS
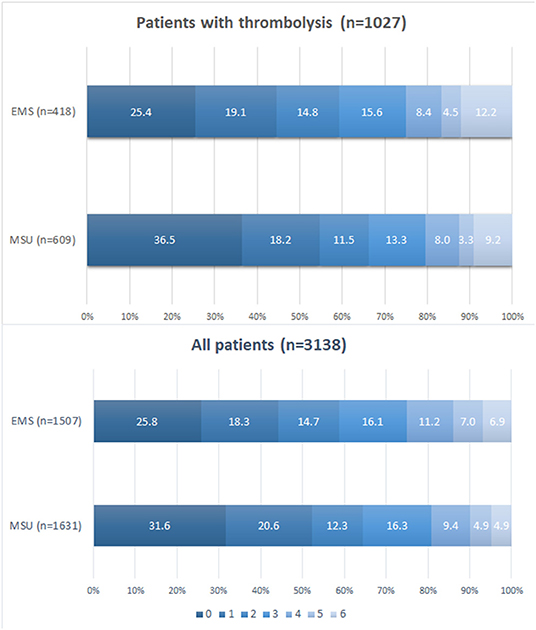
Four of the twelve clinical studies reported mRs at 90 days from onset. Figure 5 discovered the distribution of mRS by treated population at 90 days from onset. Pooled data showed that the proportion of patients with mRS 0–2 at 90 days from onset was higher in the MSU group than in the EMS group among the thrombolysis population and all populations (p < 0.05).
Cost-Effectiveness
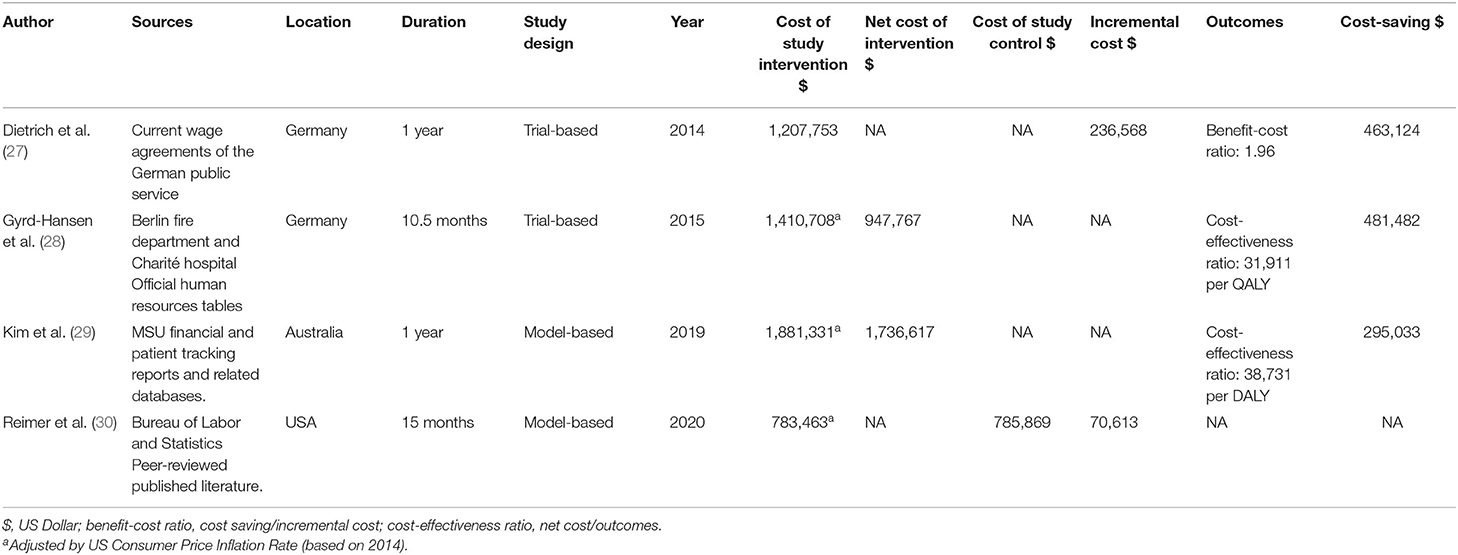
The cost-effectiveness outcomes of the included economic studies are shown in Table 2. The first cost analysis of MSU was from trial-based research in Germany, which showed a favorable benefit-cost ratio between 2.16–6.85 at an operating distance between 26.73 and 40.32 miles, depending on the staffing (27). Another estimation was from Phantom-S study in Berlin (28), Germany, a 21-month multicenter RCT involving 28 hospitals, which found that the annual net cost of MSU deployment ($947,767) was balanced by an expected health benefit of 29.7 quality-adjusted life years (QALYs) per year. This calculation produced the incremental cost-effectiveness ratio of $31,911/QALY (Based on 2014, using cpi to remove inflationary effects). Kim et al. (29) conducted a model-based economic evaluation of MSU, obtaining data from MSU missions in Melbourne, Australia. This calculation concluded that deployment of MSUs avoided 27.94 DALYs and cost an additional $38,731 per DALY avoided. The recent economic evaluation is based on data obtained from the US Bureau of Labor and Statistics and peer-reviewed published literature, focusing on analyzing the incremental costs of MSU during the transport of patients. The model output was that use of MSUs cost an additional $70,613 per year, which would avoid 76 secondary transfers and 76 ED encounters (30).
Discussion
The study assesses various outcomes of MSU in patients with acute stroke, explicitly focusing on the time metrics adverse events, long-term functional outcome, and cost-effectiveness. Twelve clinical studies reporting the previous MSU clinical trials and four economic research presenting related costs and benefits between 2012 and 2021 were included. Although pooled data from clinical studies demonstrated the significant time-saving effect of MSU, considerable heterogeneity exists between studies. It was probably a result of the different ranges of MSU deployment among studies [e.g., Walter et al. (21) reported the distance from a base station to the scene as 30 km as compared to approximately 10 km in Ebinger et al. (9) and 6.6 km in Helwig et al. (19)]. Furthermore, that stroke-related or neurological events and in-hospital mortality were not significantly improved is consistent with the previous meta-analysis (11). Next, the long-term outcome of mRs at 90 days from onset favored the MSU group, too. To consider the settings of RCTs the baseline mRs are similar between groups. In this way, the effect of MSU on reducing disability could be a reasonable extrapolation. Given that Prehospital time-saving may improve long-term outcomes, a couple of longitudinal researches are being prepared (31, 32). It should be informative to discuss the distance between potential stroke patients and the stroke unit. There are regional differences between countries, with stroke units sometimes very far away. MSU may be more effective regarding time to treatment and mortality in provincial cities and rural areas than in metropolises (33). However, the locations were primarily in urban instead of rural areas, except BEST MSU trial (22), thus raising many concerns about different outcomes for MSU in rural settings.
Four economic studies were conducted in three different countries. Despite inherent differences in health care systems, the MSU is a more expensive intervention than the EMS for acute stroke management in all selected countries. Therefore, the fundamental question is whether or not the MSU offers enough benefit to offset the additional costs incurred within the treatment. The present study's data show that despite the higher costs of the MSU, it creates upper QALYs and DALYs for patients compared with EMS. In the studies that present QALYs and DALYs indicators, MSU has generated higher values, inferring that the MSU program increased life expectancy, quality of life, and mRS in the long-term.
Study Limitations
Our study has several limitations. First, data acquisition may vary by caregiver, so we cannot exclude information bias. Second, except for Grotta et al., studies did not adequately document the time to alarm and arrival at the scene regarding stroke so we may have overestimated the time saved for patients treated in the MSU. Third, most studies reported clinical outcomes for a maximum of 90 days from onset, which lacks long-term data to warrant the conclusion. Furthermore, the benefits that MSU provides to patients are obvious, but it is unclear what the national health service is willing to pay. In the absence of clear indicators of value, it is questionable to interpret conclusions about cost-effectiveness from the perspective of medical decision-making.
Conclusion
In conclusion, a comprehensive analysis of current research on MSU indicates that this acute stroke management strategy is a better choice in various economic and social settings in terms of time metrics, safety, long-term medical benefits, and cost-effectiveness.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JC: methodology, formal analysis, and writing—original draft. XL and GZ: methodology and writing—review and editing. RH: writing—review and editing. YC: conceptualization, resources, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ahmadi M, Laumeier I, Ihl T, Steinicke M, Ferse C, Endres M, et al. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:49–60. doi: 10.1016/S1474-4422(19)30369-2
2. Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1804–18. doi: 10.1016/j.jacc.2019.12.072
3. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
4. Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. (2013) 309:1136–44. doi: 10.1001/jama.2013.842
5. Wilcock AD, Zachrison KS, Schwamm LH, Uscher-Pines L, Zubizarreta JR, Mehrotra A. Trends among rural and urban medicare beneficiaries in care delivery and outcomes for acute stroke and transient ischemic attacks, 2008-2017. JAMA Neurol. (2020) 77:863–71. doi: 10.1001/jamaneurol.2020.0770
6. Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door-to-imaging time and imaging-to-needle time. JAMA Neurol. (2014) 71:1155–61. doi: 10.1001/jamaneurol.2014.1528
7. Itrat A, Taqui A, Cerejo R, Briggs F, Cho SM, Organek N, et al. Telemedicine in prehospital stroke evaluation and thrombolysis: taking stroke treatment to the doorstep. JAMA Neurol. (2016) 73:162–8. doi: 10.1001/jamaneurol.2015.3849
8. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. (2020) 368:l6983. doi: 10.1136/bmj.l6983
9. Ebinger M, Winter B, Wendt M, Weber JE, Waldschmidt C, Rozanski M, et al. Effect of the Use of Ambulance-Based Thrombolysis on Time to Thrombolysis in Acute Ischemic Stroke. JAMA. (2014) 311:1622. doi: 10.1001/jama.2014.2850
10. Wendt M, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, Weber JE, et al. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the prehospital acute neurological therapy and optimization of medical care in stroke study. Stroke. (2015) 46:740–5. doi: 10.1161/STROKEAHA.114.008159
11. Fatima N, Saqqur M, Hussain MS, Shuaib A. Mobile stroke unit versus standard medical care in the management of patients with acute stroke: a systematic review and meta-analysis. Int J Stroke. (2020) 15:595–608. doi: 10.1177/1747493020929964
12. Global regional and and national burden of stroke 1990-2016: 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:439–58. doi: 10.1016/S1474-4422(19)30034-1
13. Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. (2007) 370:1929–38. doi: 10.1016/S0140-6736(07)61696-1
14. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. (2014) 383:245–54. doi: 10.1016/S0140-6736(13)61953-4
15. Guy GP, Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic burden of chronic conditions among survivors of cancer in the United States. J Clin Oncol. (2017) 35:2053–61. doi: 10.1200/JCO.2016.71.9716
16. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
17. Bowry R, Parker S, Rajan SS, Yamal JM, Wu TC, Richardson L, et al. Benefits of Stroke Treatment Using a Mobile Stroke Unit Compared With Standard Management: The BEST-MSU Study Run-In Phase. Stroke. (2015) 46:3370–4. doi: 10.1161/STROKEAHA.115.011093
18. Ebinger M, Kunz A, Wendt M, Rozanski M, Winter B, Waldschmidt C, et al. Effects of Golden Hour Thrombolysis. JAMA Neurol. (2015) 72:25. doi: 10.1001/jamaneurol.2014.3188
19. Helwig SA, Ragoschke-Schumm A, Schwindling L, Kettner M, Roumia S, Kulikovski J, et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol. (2019) 76:1484–92. doi: 10.1001/jamaneurol.2019.2829
20. Parker SA, Bowry R, Wu T-C, Noser EA, Jackson K, Richardson L, et al. Establishing the first mobile stroke unit in the United States. Stroke. (2015) 46:1384–91. doi: 10.1161/STROKEAHA.114.007993
21. Walter S, Kostopoulos P, Haass A, Keller I, Lesmeister M, Schlechtriemen T, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. The Lancet Neurology. (2012) 11:397–404. doi: 10.1016/S1474-4422(12)70057-1
22. Grotta JC, Yamal JM, Parker SA, Rajan SS, Gonzales NR, Jones WJ, et al. Prospective, multicenter, controlled trial of mobile stroke units. N Engl J Med. (2021) 385:971–81. doi: 10.1056/NEJMoa2103879
23. Ebinger M, Siegerink B, Kunz A, Wendt M, Weber JE, Schwabauer E, et al. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. (2021) 325:454–66. doi: 10.1001/jama.2020.26345
24. Kummer BR, Lerario MP, Hunter MD, Wu X, Efraim ES, Salehi Omran S, et al. Geographic analysis of mobile stroke unit treatment in a dense urban area: the New York City METRONOME registry. J Am Heart Assoc. (2019) 8:e013529. doi: 10.1161/JAHA.119.013529
25. Kunz A, Ebinger M, Geisler F, Rozanski M, Waldschmidt C, Weber JE, et al. Functional outcomes of prehospital thrombolysis in a mobile stroke treatment unit compared with conventional care: an observational registry study. Lancet Neurol. (2016) 15:1035–43. doi: 10.1016/S1474-4422(16)30129-6
26. Taqui A, Cerejo R, Itrat A, Briggs FB, Reimer AP, Winners S, et al. Reduction in time to treatment in prehospital telemedicine evaluation and thrombolysis. Neurology. (2017) 88:1305–12. doi: 10.1212/WNL.0000000000003786
27. Dietrich M, Walter S, Ragoschke-Schumm A, Helwig S, Levine S, Balucani C, et al. Is prehospital treatment of acute stroke too expensive? An economic evaluation based on the first trial. Cerebrovasc Dis. (2014) 38:457–63. doi: 10.1159/000371427
28. Gyrd-Hansen D, Olsen KR, Bollweg K, Kronborg C, Ebinger M, Audebert HJ. Cost-effectiveness estimate of prehospital thrombolysis: results of the PHANTOM-S study. Neurology. (2015) 84:1090–7. doi: 10.1212/WNL.0000000000001366
29. Kim J, Easton D, Zhao H, Coote S, Sookram G, Smith K, et al. Economic evaluation of the melbourne mobile stroke unit. Int J Stroke. (2020) 16:466–75. doi: 10.1177/1747493020929944
30. Reimer AP, Zafar A, Hustey FM, Kralovic D, Russman AN, Uchino K, et al. Cost-consequence analysis of mobile stroke units vs. standard prehospital care and transport. Front Neurol. (2019) 10:1422. doi: 10.3389/fneur.2019.01422
31. Yamal JM, Rajan SS, Parker SA, Jacob AP, Gonzalez MO, Gonzales NR, et al. Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. (2018) 13:321–7. doi: 10.1177/1747493017711950
32. STOP-MSU: Stopping Haemorrhage With Tranexamic Acid for Hyperacute Onset Presentation Including Mobile Stroke Units. Available online at: https://ClinicalTrials.gov/show/NCT03385928.
33. Shuaib A, Jeerakathil T. The mobile stroke unit and management of acute stroke in rural settings. CMAJ. (2018) 190:E855–e8. doi: 10.1503/cmaj.170999
Appendix
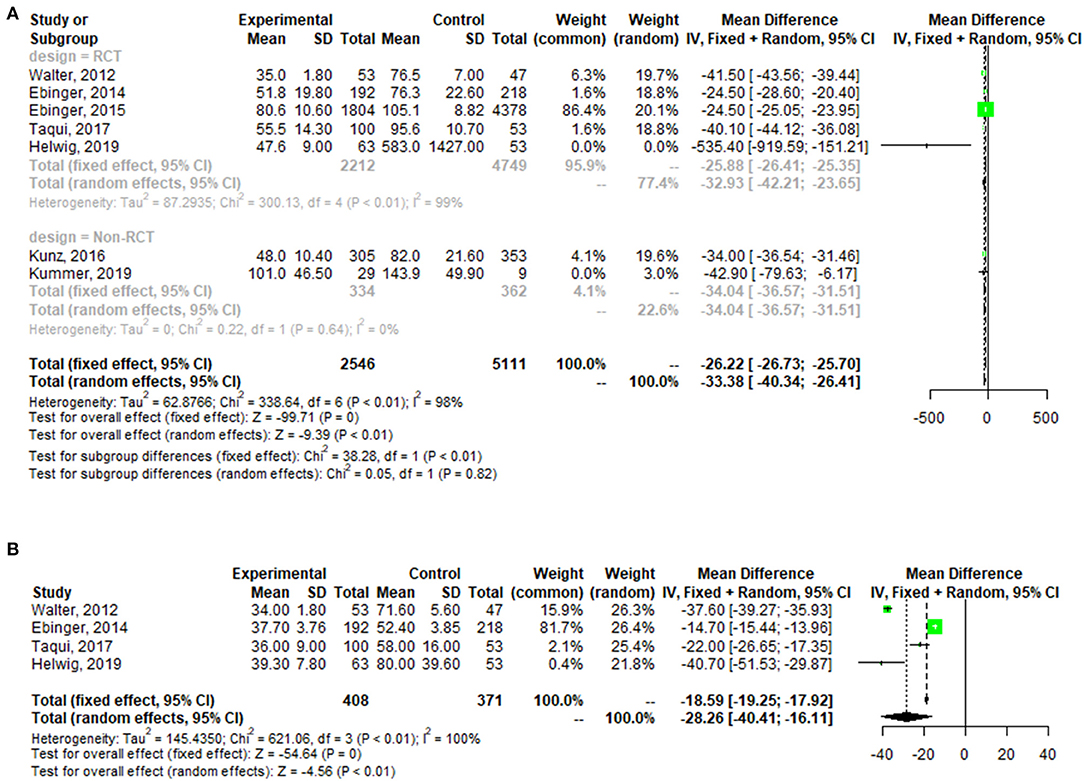
Figure A1. Forest plot for change in the time from alarm to therapy decision [adding Helwig (19)] (A), and the time from alarm to CT completion (B).
Keywords: mobile stroke unit, emergency care, meta-analysis, systematic review, cost-effectiveness
Citation: Chen J, Lin X, Cai Y, Huang R, Yang S and Zhang G (2022) A Systematic Review of Mobile Stroke Unit Among Acute Stroke Patients: Time Metrics, Adverse Events, Functional Result and Cost-Effectiveness. Front. Neurol. 13:803162. doi: 10.3389/fneur.2022.803162
Received: 28 October 2021; Accepted: 28 January 2022;
Published: 09 March 2022.
Edited by:
Nishant K. Mishra, Yale University, United StatesReviewed by:
Francisco Moniche, Virgen del Rocío University Hospital, SpainAravind Ganesh, University of Calgary, Canada
Copyright © 2022 Chen, Lin, Cai, Huang, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieyun Chen, chenjieyunfj@126.com
 Jieyun Chen
Jieyun Chen Xiaoying Lin1
Xiaoying Lin1 Gaofeng Zhang
Gaofeng Zhang