- Department of Neurology, Yangpu Hospital, Tongji University School of Medicine, Shanghai, China
Background: Red blood cell distribution width (RDW) is considered to be related to coronary heart disease and heart failure and all-cause mortality, but its relationship with acute ischemic stroke is still unclear. In this study, we aimed to explore the relationship between RDW and the stroke severity and functional outcomes of ischemic stroke.
Methods: We retrospectively reviewed patients with acute ischemic stroke between September 2016 and January 2020. Demographic, clinical, stroke complications, laboratory data, and treatment were collected for all patients. Stroke severity and functional outcomes were evaluated by NIHSS score, modified Rankin Scale (mRS), and Barthel Index (BI) at 3 months. Furthermore, multiple logistic regression analysis was used to assess the relationship between RDW and stroke severity and functional outcomes.
Results: A total of 629 patients with acute ischemic stroke were included and were categorized into four groups according to the quartiles of RDW (< 12.4, 12.4–12.9, 13.0–13.4, > 13.4). After multivariable analysis, higher RDW was directly associated with moderate to severe stroke (OR 2.21, 95% CI, 1.30–3.75, P = 0.003), mRS score of 3–6 at 3 months (OR 1.86, 95% CI, 1.02–3.41, P = 0.044), and BI score below 85 at 3 months (OR 2.27, 95% CI, 1.25–4.12, P = 0.007) in patients with ischemic stroke.
Conclusion: Our results demonstrate that RDW is associated with stroke severity and unfavorable functional outcomes at 3 months in patients with ischemic stroke.
Introduction
Red cell distribution width (RDW) is an indicator reflecting the volume of red blood cells (RBCs) which is routinely calculated during automated cell counters (1). As a measurement method of circulating erythrocyte size variability, it is expressed by the erythrocyte size variation coefficient (2). In previous studies, RDW has been proven to be related to the prognosis of patients with cardiovascular diseases, such as coronary heart disease (3) and heart failure (4, 5), as well as the incidence of all-cause mortality (6, 7).
Acute ischemic stroke is the leading cause of disability and mortality around the globe (8). The annual death rate of stroke in China is about 157 per 100,000 people (9). Given the huge burden of stroke, it is increasingly important to find indicators to evaluate the clinical severity and prognosis of stroke. A large population-based prospective study found an independent relation between higher RDW and the risk of stroke in patients with coronary disease during a median follow-up of 5 years (10). Furthermore, several studies reported that higher RDW was a prognostic factor of poor functional outcome at 3 months (11, 12) and increased mortality (12, 13) in patients with ischemic stroke. In addition, recent studies have shown that in patients with ischemic stroke, higher RDW at baseline is more likely to have poor 1-year prognosis and mortality (14, 15).
There are few reports on the relationship between RDW and stroke severity and functional outcomes in Chinese patients with ischemic stroke. In this study, we aimed to evaluate the association between RDW and stroke severity and functional outcomes in patients with ischemic stroke based on the Chinese population.
Materials and methods
Population and study design
This was a retrospective cohort study consisting of patients admitted to the Department of Neurology at Yangpu Hospital Tongji University School of Medicine for acute ischemic stroke between September 2016 and January 2020. The patients who met the following inclusion criteria were included: (1) ≥18 years old; (2) diagnosis of acute ischemic stroke within 7 days of presentation of symptoms; and (3) independent prior to stroke. The exclusion criteria are as follows: (1) infection on admission, (2) a history of hematologic diseases, (3) immune system diseases or use of immunosuppressants, and (4) incomplete clinical and follow-up data. This study was approved by the ethics committee of Yangpu Hospital Tongji University School of Medicine.
Clinical data
We collected the patients' demographic data (age, gender), clinical characteristics (hypertension, diabetes, atrial fibrillation [AF], coronary heart disease, hyperlipidemia, smoking, and alcohol drinking), stroke complications (post-stroke pneumonia, symptomatic intracranial hemorrhage [sICH]), laboratory parameters, and treatment. Smoking was defined as current or former cigarette smoking. Alcohol consumption was defined as current alcohol intake > 80g/day. Any intracerebral hemorrhage that leads to the deterioration of neurological function is defined as sICH (16). Blood samples were obtained and evaluated in the hospital's biochemistry department within 24 h after admission.
Stroke severity and function outcomes
The National Institutes of Health Stroke Scale (NIHSS) score was assessed for all patients by qualified neurologists at admission to evaluate stroke severity (17). Based on a previous study, patients were divided into two groups: moderate to severe stroke (≥ 5 points) and mild stroke (< 5 points) (18). We evaluated the prognosis of clinical function by modified Rankin Scale (mRS) and Barthel index (BI) at 3 months after stroke onset. Patients were followed-up by neurologists in our hospital outpatient department or through telephone interviews. Patients with an mRS score of 3–6 at 3 months or with an BI score below 85 at 3 months were considered to have unfavorable functional outcomes (19).
Statistical analysis
The study population was divided into four groups according to the distribution of RDW in quartiles (Q1–Q4). Continuous variables are expressed as mean ± standard deviation or median of the interquartile range. The Kolmogorov–Smirnov test was used to analyze the normality of distribution. The continuous univariate comparison was performed using the unpaired t-test, the Mann–Whitney U test, or the Kruskal–Wallis H test, when appropriate. Categorical variables are expressed as percentages and analyzed by the chi-square test. Multivariable logistic regression analysis including two models was used to analyze the relationship between RDW and stroke severity and unfavorable functional outcomes, separately. The adjusted variables of model 1 included age, gender, hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary heart disease; smoking, alcohol drinking, platelet, RBC, WBC, Hs-CRP, FBG, TC, TG, HDL, LDL, the use of antiplatelet, anticoagulation agents, and statin. In addition to the variables of model 1, model 2 added NIHSS score, intravenous thrombolysis, endovascular therapy, symptomatic intracranial hemorrhage, and post-stroke pneumonia as covariates. All statistical analyses with p-values < 0.05 were defined as statistically significant. SPSS Statistics 22.0 software (SPSS Inc., Chicago, IL) was performed for all statistical analyses.
Results
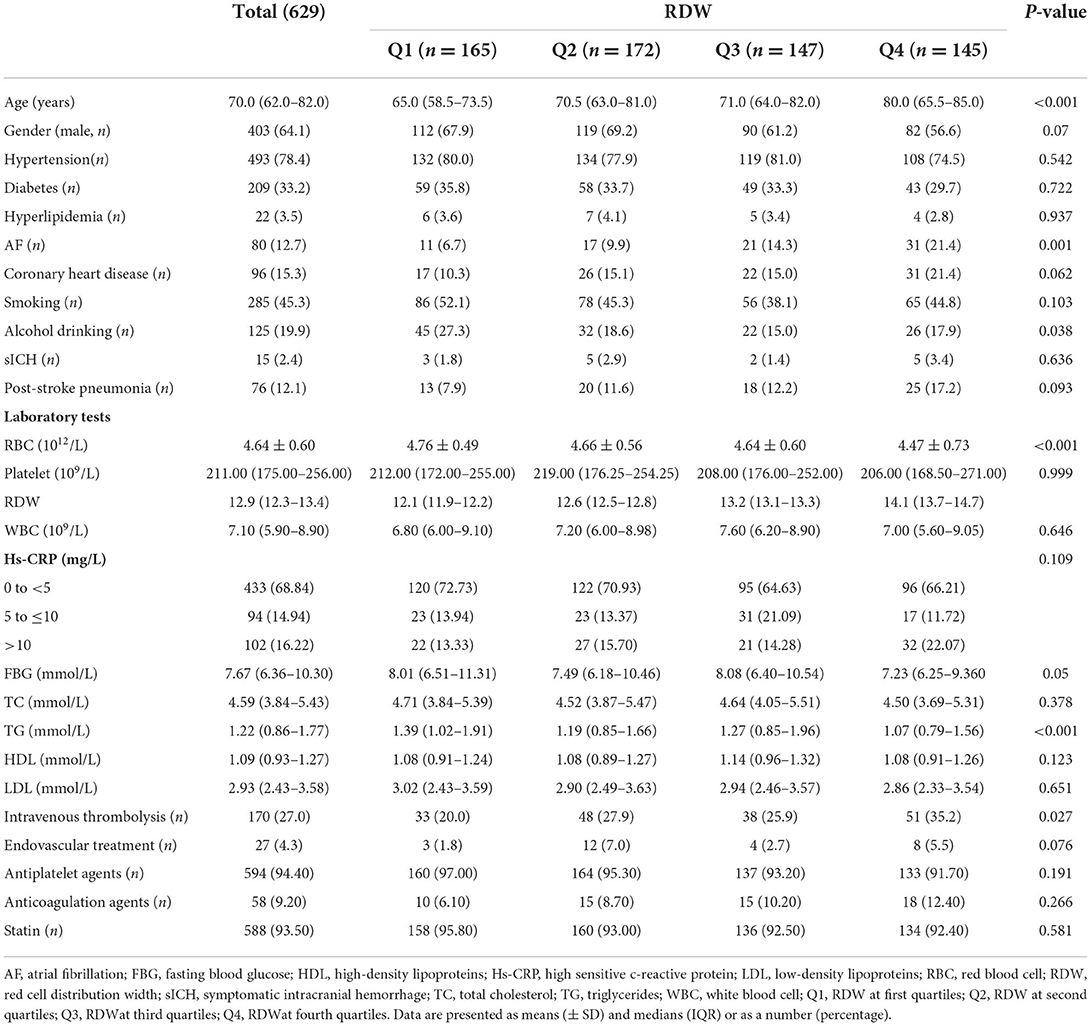
A total of 629 patients with acute ischemic stroke were included in the study. The median age of patients was 70.0 (58.5–73.5) years old, 64.1% of the patients were male subjects. Based on quartiles of RDW, 165 patients were in Q1 (RDW<12.4), 172 patients were in Q2 (RDW 12.4–12.9), 147 patients were in Q3 (RDW 13.0–13.4), and 145 patients were in Q4 (RDW >13.4). Table 1 shows the baseline characterization of demographics, clinical, stroke complications, laboratory parameters, and treatment according to RDW quartiles. Patients in higher RDW quartiles were older and had a higher prevalence of AF, whereas lower RBC counts were more frequently presented in higher RDW quartiles.
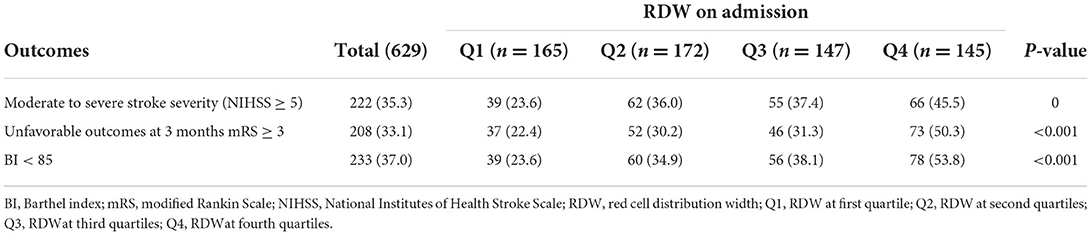
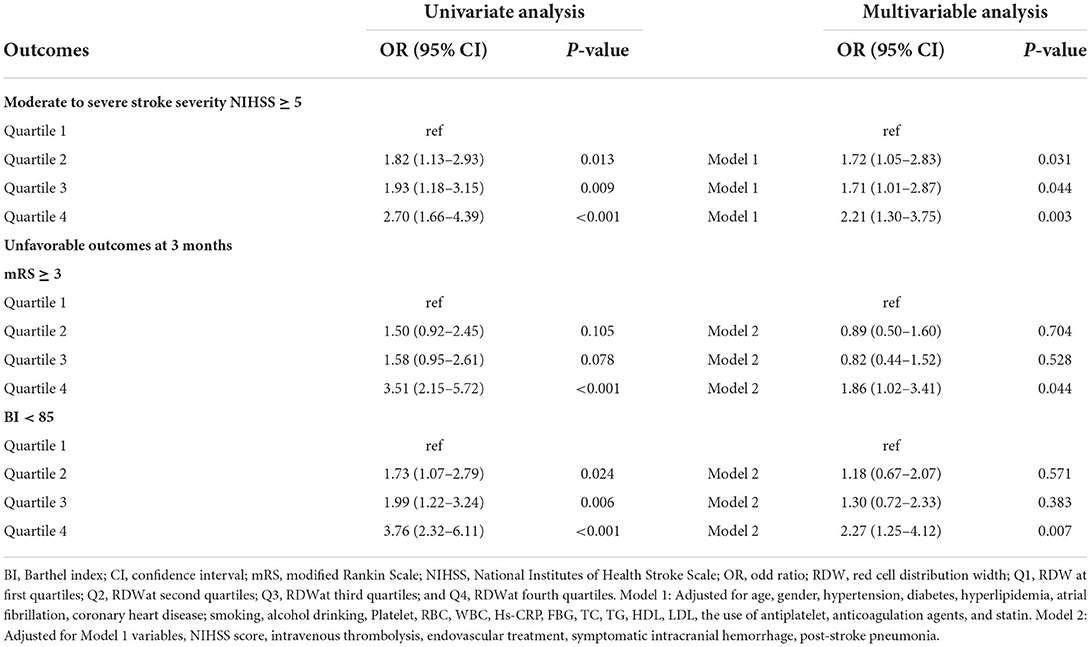
Table 2 shows the comparison of stroke severity and functional outcomes according to quartiles of RDW. Among the 629 patients included, a total of 222 (35.3) had moderate to severe stroke at admission. Patients with higher quartiles had a higher proportion of moderate to severe stroke (P = 0.001). At 3 months, there were 208 (33.1) patients with an mRS score of 3 to 6 and 233 (37.0) patients with a BI score below 85. Meanwhile, patients in the Q4 subgroup had a higher frequency of mRS score of 3–6 and BI score below 85 (P < 0.001). The distribution of the NIHSS score, mRS score, and BI score according to quartiles of RDW is shown in Figure 1. Table 3 shows the odds ratios for NIHSS, mRS, and BI by RDW quartiles. Univariate analysis demonstrated that patients with RDW in the highest quartile had a higher risk of moderate to severe stroke on admission (OR 2.70, 95% CI, 1.66–4.39, P < 0.001) when compared with the first quartile. And patients with RDW in the fourth quartile had a higher relative risk of mRS score of 3 to 6 (OR 3.51, 95% CI, 2.15–5.72, P < 0.001) and BI score below 85 (OR 3.76, 95% CI, 2.32–6.11, P < 0.001) when compared to the first quartile. After multivariable analysis using model 1 variables, RDW remained associated with moderate to severe stroke (OR 2.21, 95% CI, 1.30–3.75, P = 0.003). Further analysis with multiple logistic regression using Model 2 revealed that RDW was associated with mRS score of 3 to 6 (OR 1.86, 95% CI, 1.02–3.41, P = 0.044) and BI scores below 85 (OR 2.27, 95% CI, 1.25–4.12, P = 0.007).
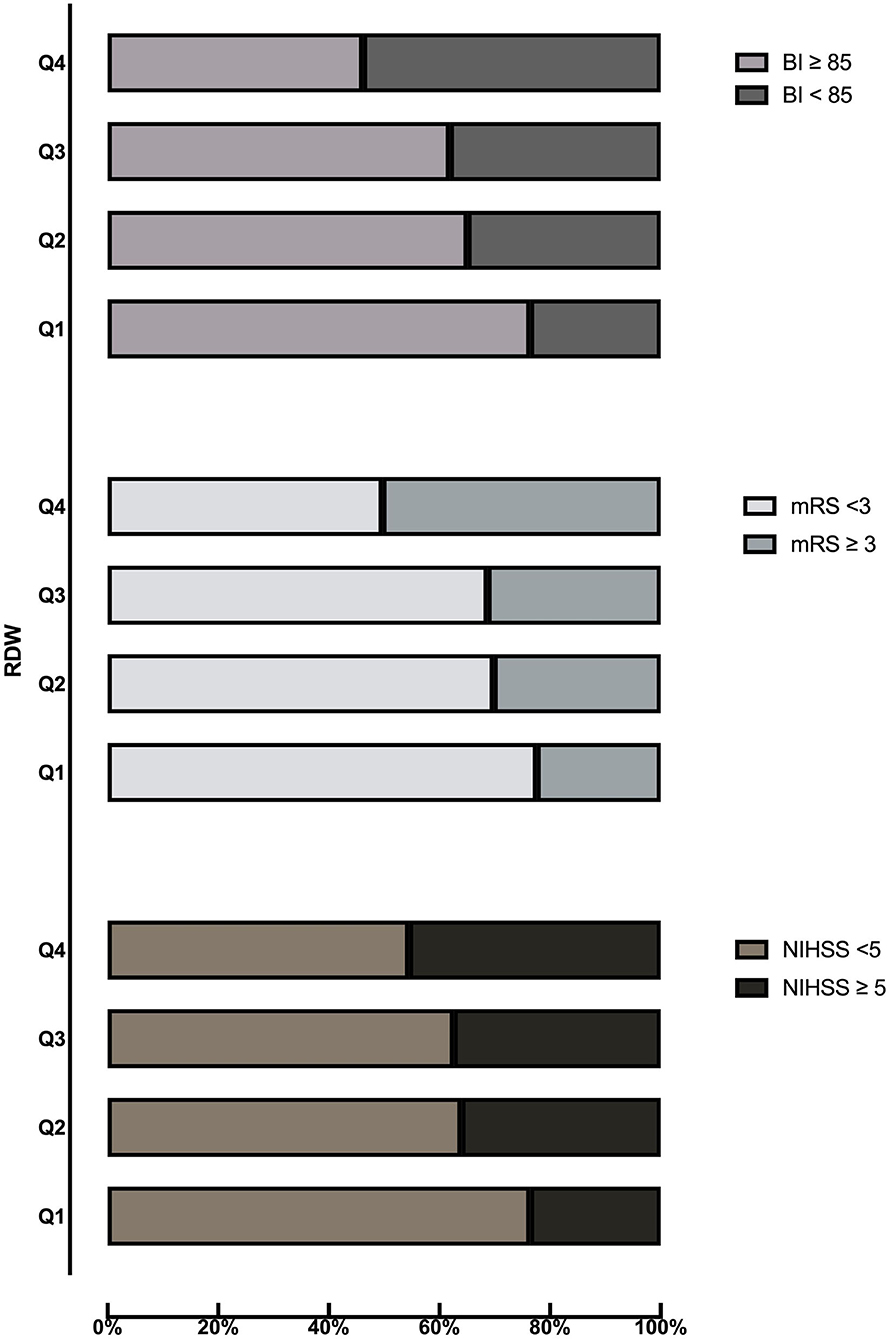
Figure 1. Distribution of NIHSS score, mRS score, and BI score according to quartiles of RDW. BI, Barthel index; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; RDW, red cell distribution width.
Discussion
To our best knowledge, this is the first study to evaluate the association between RDW and stroke severity and functional outcomes in patients with ischemic stroke based on the Chinese population. And we found that RDW was associated with stroke severity and unfavorable functional outcomes at 3 months.
A number of studies have assessed the relationship between RDW and the high risk of stroke in patients with heart diseases. An independent relationship was discovered between elevated RDW and stroke in a study of coronary heart disease patients with a median follow-up of 5 years (10). In another study, increased basal RDW was observed in patients with heart failure having stroke when compared with those without stroke after follow-up for 1 year (20). Moreover, Saliba et al. (21) found that RDW was directly related to stroke risk and improved the prediction accuracy of stroke in patients with AF regardless of anemia status.
In addition, some studies have evaluated the association between RDW and stroke based on the general population. In a case-control study of 224 patients with first ischemic stroke, Ramírez-Moreno et al. (22) found that patients in the fourth quartile of RDW had a significantly higher risk of stroke compared with those in the lower quartile. And during a mean follow-up period of 15.2 years, a large general population study including 26,879 participants reported that RDW in the highest quartile was associated with increased incidence of total stroke and ischemic stroke (23). However, Chen et al. (6) reported that high RDW was independently associated with an increased risk of all-cause mortality, but not the development of stroke in a community population during a median follow-up period of 15.9 years. Furthermore, a series of previous studies have confirmed that RDW is an independent risk factor for increasing poor clinical outcomes at 3 months (11, 12) and all-cause mortality (12, 13, 24, 25) after ischemic stroke. Recently, Ye et al. (26) and Pinho et al. (15) found that baseline RDW is a potential predictor of 1-year survival in patients with ischemic stroke treated with intravenous thrombolysis. Moreover, a retrospective observational single-center study showed that RDW is an independent predictor of 1-year mortality and prognosis in patients with acute anterior circulation stroke after endovascular therapy (14).
Furthermore, there were a few reports on the relationship between RDW and stroke severity, but the conclusions varied. Kara et al. (27) supported that increased RDW values were significantly associated with increased stroke severity in acute ischemic stroke, whereas Ntaios et al. (28) reported that RDW does not predict severity or functional outcome in patients with acute ischemic stroke after multivariable analysis. Our study indicated that the RDW is not only associated with moderate to severe stroke but also associated with unfavorable functional outcomes at 3 months.
Although many studies have shown the relationship between RDW and stroke, its pathophysiological mechanism is not clear. It is widely known that inflammation plays an important role in the pathophysiological processes of ischemic stroke (29, 30). High RDW suggests an increase in extensive RBC size heterogeneity and may reflect a potential inflammatory state, which is associated with adverse clinical outcomes and leads to impaired erythrocyte maturation (10). Previous studies have proved that inflammatory cytokines can inhibit erythropoietin-induced erythrocyte maturation by inhibiting bone marrow, which is reflected by the increase of RDW (31). Inflammatory cytokines and markers such as CRP, interleukin-6, and tumor necrosis factor-alpha have been confirmed to be related to RDW (32–34). Furthermore, high oxidative stress may lead to the increase of RDW by prolonging the survival time of red blood cells (27). And it is reported that the increase in RDW is related to high oxidative stress and low antioxidant level (35, 36). In this study, the relationship between RDW and stroke severity and unfavorable functional outcomes remained significant even after the adjustment of CRP and WBC. Additionally, chronic inflammation and oxidative stress may contribute to the development of atherosclerosis, and the increase of RDW may be an indicator of the development of atherosclerosis (27). Several cohort studies have found a close relationship between high RDW and increased intimal-medial thickness, a well-known risk factor for ischemic stroke (23, 37). Finally, it should be noted that RDW is also related to other medical conditions, including old age, anemia, liver disease, and lung disease, which may affect the clinical prognosis (12).
Several limitations of this study should be acknowledged as follows. First, this was a retrospective observational study with a single center and small sample size. And a few patients were not included in the final analysis due to the lack of clinical or laboratory data, so it might produce selection bias. Second, RDW was only measured once for all participants, so we could not evaluate the RDW dynamically, which is not conducive to a better understanding of the association between RDW and the prognosis of stroke. Finally, we adjusted for possible risk factors in multivariable analysis. However, there may be some residual confounding factors that affect the severity and clinical prognosis of stroke and cannot be completely excluded.
In conclusion, our study demonstrated that RDW is associated with stroke severity and unfavorable functional outcomes at 3 months in patients with ischemic stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Yangpu Hospital Tongji University School of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-HY conceived and designed the experiments. X-QZ and X-SX collected the data. DZ and X-GZ analyzed the data. JX wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by Shanghai Sailing Program (20YF1445000) and the Shanghai Municipal Planning Commission of Science and Research Fund (20204Y0123).
Acknowledgments
We thank Chun-Chun Hu for her help in revising the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. (2017) 2017:7089493. doi: 10.1155/2017/7089493
2. Clarke K, Sagunarthy R, Kansal S, RDW as an additional marker in inflammatory bowel disease/undifferentiated colitis. Dig Dis Sci. (2008) 53:2521–3. doi: 10.1007/s10620-007-0176-8
3. Veeranna V, Zalawadiya SK, Panaich S, Patel KV, Afonso L. Comparative analysis of red cell distribution width and high sensitivity C-reactive protein for coronary heart disease mortality prediction in multi-ethnic population: findings from the 1999-2004 NHANES. Int J Cardiol. (2013) 168:5156–61. doi: 10.1016/j.ijcard.2013.07.109
4. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the duke databank. J Am Coll Cardiol. (2007) 50:40–7. doi: 10.1016/j.jacc.2007.02.067
5. Nakashima K, Ohgami E, Kato K, Yoshitomi S, Maruyama T, Harada M. Prognostic significance of red cell distribution width in hospitalized older patients with heart failure or infection. Geriatr Gerontol Int. (2019) 19:988–92. doi: 10.1111/ggi.13755
6. Chen PC, Sung FC, Chien KL, Hsu HC, Su TC, Lee YT. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol. (2010) 171:214–20. doi: 10.1093/aje/kwp360
7. Zhang Q, Hu M, Sun J, Ma S. The combination of neutrophil-to-lymphocyte ratio and platelet correlation parameters in predicting the no-reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Scand Cardiovasc J. (2020) 54:352–7. doi: 10.1080/14017431.2020.1783457
8. Mortality GBD, Causes Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1
9. Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. (2011) 42:3651–4. doi: 10.1161/STROKEAHA.111.635755
10. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. (2008) 117:163–8. doi: 10.1161/CIRCULATIONAHA.107.727545
11. Turcato G, Cervellin G, Cappellari M, Bonora A, Zannoni M, Bovi P, et al. Early function decline after ischemic stroke can be predicted by a nomogram based on age, use of thrombolysis, RDW and NIHSS score at admission. J Thromb Thrombolysis. (2017) 43:394–400. doi: 10.1007/s11239-016-1456-y
12. Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost. (2012) 108:349–56. doi: 10.1160/TH12-03-0165
13. Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. (2009) 277:103–8. doi: 10.1016/j.jns.2008.10.024
14. Wang Z, Liu Y. Red cell distribution width as a predictor of one-year prognosis and mortality of endovascular therapy for acute anterior circulation ischemic stroke. J Stroke Cerebrovasc Dis. (2022) 31:106243. doi: 10.1016/j.jstrokecerebrovasdis.2021.106243
15. Pinho J, Marques SA, Freitas E, Araujo J, Taveira M, Alves JN, et al. Red cell distribution width as a predictor of 1-year survival in ischemic stroke patients treated with intravenous thrombolysis. Thromb Res. (2018) 164:4–8. doi: 10.1016/j.thromres.2018.02.002
16. Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. (2006) 37:556–61. doi: 10.1161/01.STR.0000196942.84707.71
17. Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
18. Tziomalos K, Sofogianni A, Angelopoulou SM, Christou K, Kostaki S, Papagianni M, et al. Left ventricular hypertrophy assessed by electrocardiogram is associated with more severe stroke and with higher in-hospital mortality in patients with acute ischemic stroke. Atherosclerosis. (2018) 274:206–11. doi: 10.1016/j.atherosclerosis.2018.05.030
19. Xue J, Zhang XG, Jiang HY, Cui XK, Zhang D, Yao ZW, et al. An increase in neutrophil-to-lymphocyte ratio predicts poor functional outcomes in older patients with acute ischemic stroke: a retrospective study. J Integr Neurosci. (2021) 20:399–404. doi: 10.31083/j.jin2002040
20. Kaya A, Isik T, Kaya Y, Enginyurt O, Gunaydin ZY, Iscanli MD, et al. Relationship between red cell distribution width and stroke in patients with stable chronic heart failure: a propensity score matching analysis. Clin Appl Thromb Hemost. (2015) 21:160–5. doi: 10.1177/1076029613493658
21. Saliba W, Barnett-Griness O, Elias M, Rennert G. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. (2015) 128:e11-8. doi: 10.1016/j.amjmed.2014.09.020
22. Ramirez-Moreno JM, Gonzalez-Gomez M, Ollero-Ortiz A, Roa-Montero AM, Gomez-Baquero MJ, Constantino-Silva AB. Relation between red blood cell distribution width and ischemic stroke: a case-control study. Int J Stroke. (2013) 8:E36. doi: 10.1111/ijs.12091
23. Soderholm M, Borne Y, Hedblad B, Persson M, Engstrom G. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. PLoS One. (2015) 10:e0124957. doi: 10.1371/journal.pone.0124957
24. Zhao H, Zhao Y, Wu Z, Cheng Y, Zhao N. Red cell distribution width is associated with all-cause mortality in patients with acute stroke: a retrospective analysis of a large clinical database. J Int Med Res. (2021) 49:300060520980587. doi: 10.1177/0300060520980587
25. Wang L, Wang C, Wu S, Li Y, Guo W, Liu M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: a cohort study and systematic review. Ann Transl Med. (2020) 8:81. doi: 10.21037/atm.2019.12.142
26. Ye WY Li J, Li X, Yang XZ, Weng YY, Xiang WW, et al. Predicting the one-year prognosis and mortality of patients with acute ischemic stroke using red blood cell distribution width before intravenous thrombolysis. Clin Interv Aging. (2020) 15:255–63. doi: 10.2147/CIA.S233701
27. Kara H, Degirmenci S, Bayir A, Ak A, Akinci M, Dogru A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat. (2015) 11:733–9. doi: 10.2147/NDT.S81525
28. Ntaios G, Gurer O, Faouzi M, Aubert C, Michel P. Red cell distribution width does not predict stroke severity or functional outcome. Int J Stroke. (2012) 7:2–6. doi: 10.1111/j.1747-4949.2011.00609.x
29. Semple JW, Italiano JE. Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. (2011) 11:264–74. doi: 10.1038/nri2956
30. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
31. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. (2005) 20:83–90. doi: 10.1191/0267659105pf793oa
32. Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. (2009) 158:659–66. doi: 10.1016/j.ahj.2009.07.024
33. Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. (2010) 16:230–8. doi: 10.1016/j.cardfail.2009.11.003
34. Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. (2011) 107:1241–5. doi: 10.1016/j.amjcard.2010.12.023
35. Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women's health and aging study I. Clin Nutr. (2010) 29:600–4. doi: 10.1016/j.clnu.2010.03.001
36. Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A. Influence of vitamin C diet supplementation on endogenous antioxidant defences during exhaustive exercise. Pflugers Arch. (2003) 446:658–64. doi: 10.1007/s00424-003-1112-1
Keywords: red blood cell distribution width (RDW), ischemic stroke, stroke severity, function outcomes, predictor
Citation: Xue J, Zhang D, Zhang X-G, Zhu X-Q, Xu X-S and Yue Y-h (2022) Red cell distribution width is associated with stroke severity and unfavorable functional outcomes in ischemic stroke. Front. Neurol. 13:938515. doi: 10.3389/fneur.2022.938515
Received: 09 May 2022; Accepted: 20 October 2022;
Published: 09 November 2022.
Edited by:
Piotr Religa, Karolinska Institutet (KI), SwedenReviewed by:
Askiel Bruno, Augusta University, United StatesLekhjung Thapa, National Neuro Center (NNC), Nepal
Antonio Bonora, University Hospital, Italy
Copyright © 2022 Xue, Zhang, Zhang, Zhu, Xu and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-hua Yue, yunhua.yue@tongji.edu.cn
 Jie Xue
Jie Xue Dong Zhang
Dong Zhang Xiao-Guang Zhang
Xiao-Guang Zhang Xiao-Qiong Zhu
Xiao-Qiong Zhu Yun-hua Yue
Yun-hua Yue