- Department of Neurology, Wayne State University, Detroit, MI, United States
A clear narrative of acute symptomatic seizures (ASyS) in older adults is lacking. Older adults (≥60 years) have the highest incidence of seizures of all age groups and necessitate a tailored approach. ASyS has a bimodal peak in infancy and old age (82.3–123.2/100,000/year after 65 years of age). ASyS can represent half of the new-onset seizures in older adults and can progress to acute symptomatic status epilepticus (ASySE) in 52–72% of the patients. Common etiologies for ASyS in older adults include acute stroke and metabolic disturbances. For ASySE, common etiologies are acute stroke and anoxic brain injury (ABI). Initial testing for ASyS should be consistent with the most common and urgent etiologies. A 20-min electroencephalogram (EEG) is less sensitive in older adults than in younger adults and might not help predict chronic epilepsy. The prolonged postictal phase is an additional challenge for acute management. Studies note that 30% of older adults with ASyS subsequently develop epilepsy. The risk of wrongly equating ASyS as the first seizure of epilepsy is higher in older adults due to the increased long-term challenges with chronic anti-seizure medication (ASM) treatment. Specific challenges to managing ASyS in older adults are related to their chronic comorbidities and polypharmacy. It is unclear if the prognosis of ASyS is dependent on the underlying etiology. Short-term mortality is 1.6 to 3.6 times higher than younger adults. ASySE has high short-term mortality, especially when it is secondary to acute stroke. An acute symptomatic etiology of ASySE had five times increased risk of short-term mortality compared to other types of etiology.
Introduction
Definition
The International League Against Epilepsy (ILAE) defines acute symptomatic seizures (ASyS) as seizures occurring in close temporal relationship with an acute central nervous system (CNS) insult of varying etiologies (1). The definition also includes seizures occurring in a preexisting background of epilepsy and fulfills other criteria for ASyS. Differentiating ASyS from unprovoked seizures is essential due to prognostic implications. ASyS differs from remote symptomatic seizures and progressive symptomatic seizures as the prognosis is different. For seizures to be considered ASyS, ILAE has proposed the following: seizure occurrence 1 week after stroke, head trauma, and anoxic brain injury (ABI), 1 day for metabolic causes, 7–48 h from the last alcoholic drink, and active intracranial infection or inflammation. Studies on optimal laboratory cutoff values to better delineate toxic–metabolic causes are lacking. Seizures due to anti-seizure medication (ASM) non-adherence are considered provoked seizures (1).
Older adults with epilepsy have the highest incidence of epilepsy of all age groups, with at least 25% of newly diagnosed seizures occurring after 60 years of age (2, 3). They constitute a separate treatment group compared to other adults due to the high incidence of comorbidities, associated polypharmacy, and age or disease-related changes in pharmacodynamics and pharmacokinetics (4).
There is a lack of consensus on who is considered an older adult in general and in those with seizures. We define older adults as ≥60 years of age for this review unless otherwise mentioned. Josephson et al. suggested using an age threshold of 65–70 years to define “elderly-onset epilepsy.” However, such a recommendation is lacking in ASyS or current older adults with “younger-onset epilepsy” (5).
Epidemiology
Studies note that 34% of all afebrile seizures are ASyS (incidence 39 persons/100,000/year) (6). Men are more susceptible than women (42 vs. 27/100,000/year). Mirroring the incidence of epilepsy, ASyS also has two peaks, i.e., infancy and >74 years (7, 8). The rates start increasing gradually after 45 years of age: 55/100,000/year for 55–65 years; 82.3/100,000/year for 65–75 years; and 123.2/100,000/year for 75+ years. Older male adults are 1.6 to 2.6 more likely to have ASyS than older female adults (8).
According to Holt-Seitz et al. (9) ASyS represented half of the new-onset seizures in older adults. Nearly half were due to cerebrovascular disease (CVD), and 20% were due to metabolic disturbances (8). The rising incidence of CVD and metabolic disturbances with age (renal and hepatic dysfunction and diabetes) is likely responsible for the increased incidence of ASyS.
Acute symptomatic seizures represent 52–72% of cases of status epilepticus [SE; acute symptomatic status epilepticus (ASySE)] (10). ILAE definition of SE does not differentiate between the etiology of epilepsy and that of SE. For example, in a patient with a remote symptomatic cause of epilepsy such as CVD, the new SE might be triggered by a metabolic insult. This lack of differentiation is significant in older adults due to the potential for long-term polypharmacy (11). However, we assume that the definition of ASyS that includes “seizures occurring in a preexisting background of epilepsy” can be extrapolated to SE.
Etiology
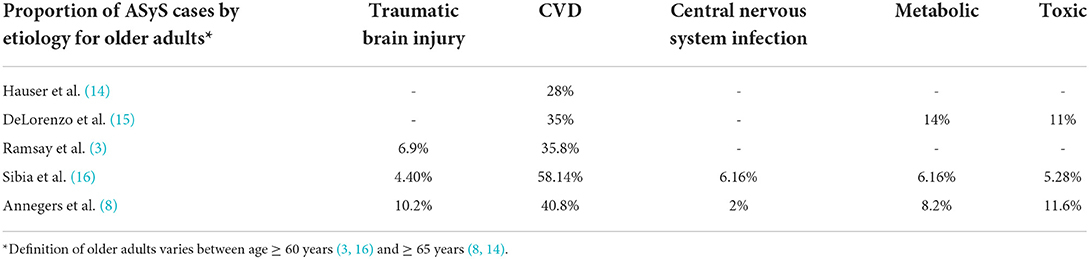
Cerebrovascular disease is the most common etiology of ASyS in older adults and accounts for 28–58% of ASyS (Table 1). ASyS are two times as likely with hemorrhagic CVD as compared to ischemic CVD (12). Cortical location predicts the risk of seizures after hemorrhagic and ischemic CVD (12). Regional ischemia leading to excitatory neurotransmitter release and local irritation due to acute mass effect from cerebral hemorrhage is the proposed pathophysiological mechanism underlying CVD-related AsyS (12). Other etiologies include metabolic (6–14%), traumatic brain injury (TBI) (7–10%), CNS infections (2–6%), and toxin-related (5–12%). Post-traumatic ASyS commonly occurs in the setting of acute subdural hematoma (13).
Acute stroke was responsible for 35% of ASySE in older adults (15). Other common etiologies in decreasing frequency were hypoxia (17%), metabolic disorders (14%), and alcohol-related (11%) (15).
Clinical presentations and diagnostic workup
Clinical presentations and differential diagnosis
Older adults can have atypical seizure presentations, such as epileptic aura, subtle confusion, aphasia, or prolonged postictal altered mental status. Non-motor manifestations, including somnolence and clumsiness, might be more common than convulsive seizures (17). Focal impaired awareness seizures originating from the frontal lobe are more common in the elderly than other types (18). Due to these atypical presentations, clinicians should consider seizures in the differential diagnosis of other common presentations in older adults, such as transient ischemic attacks, syncope, and falls.
Convulsive syncope is a seizure triggered by syncopal mechanisms caused by loss of vascular supply to the brain (19). The population incidence of convulsive syncope is unknown but is commonly seen in clinical practice. It can present as a transient myoclonic activity when the patient collapses with loss of consciousness. Surprisingly, head deviation, automatisms, and visual and auditory hallucinations, usually associated with focal onset seizures, are common (60–80%) in convulsive syncope (20) and can further lead to diagnostic dilemmas.
Diagnostic testing
The American Academy of Neurology has published practice guidelines for the management of the first unprovoked seizure (21). However, a similar guideline is not available for first or recurrent provoked or ASyS.
Neuroimaging
The initial testing for ASyS in older adults should be consistent with the most common and urgent etiologies. Focal deficits with and after a seizure should prompt evaluation for CVD, including CT head to rule out acute intracranial hemorrhage and MRI brain for acute ischemic stroke. Neuroimaging is also helpful to evaluate underlying neoplastic or infectious processes. One study focusing on older adults has found CT head with the new-onset seizures to show an acute pathology in 35% of patients (9).
Electrophysiologic studies
Initial electroencephalogram (EEG) was abnormal in 73% (61 out of 84) of older adults with new-onset seizures. Notably, 64% showed focal slowing but only 39% had epileptiform discharges. However, these included patients with all types of seizures and were not limited to AsyS (9). Routine EEG was less sensitive in older adults and might not help distinguish an ASyS with no clear etiology from an unprovoked seizure with possible epilepsy (22). Prolonged EEGs or serial EEGs should be considered if the long-term risk of seizures and diagnosis of epilepsy is to be ascertained (23). The yield of detecting interictal epileptiform discharges increased by 50% when ambulatory EEG was performed, as compared to routine EEG (3). Prolonged EEG can help detect non-convulsive SE in older adults who present with acute confusional states (24). However, Rossetti et al. demonstrated that prolonged EEG, despite increasing seizure detection, did not change the outcomes as compared to routine EEG in critically ill patients without recent seizures (25).
For patients with suspected convulsive syncope, capturing the episode on EEG or prolonged cardiac electrophysiological tests (event monitor, loop recorder) might be required for a definitive diagnosis. A simultaneous tilt-table and EEG might help confirm the diagnosis in a small number of patients (26). Clinicians should direct management toward finding and treating the cause of syncope.
Laboratory studies
One of the commonest etiologies is an acute metabolic disturbance. Relevant testing, including serum glucose, electrolytes, renal, and hepatic function tests, would guide appropriate diagnosis and treatment. The other common etiologies are infections, i.e., systemic or CNS (meningitis and encephalitis). Fever and non-reactive leukocytosis should lead to further workup, including urine analysis, chest X-ray, and lumbar puncture. Toxicology testing, including alcohol level, should be performed at presentation. Alcohol (induced or withdrawal) is a common cause of ASyS and ASySE. Elevated serum alcohol levels can diagnose alcohol-induced ASyS. However, for the diagnosis of alcohol-withdrawal seizures, reliable history of consistent alcohol use and recent abstinence and low serum alcohol level are needed. A clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) score can measure alcohol withdrawal symptoms and prompt appropriate prevention of alcohol-withdrawal seizures (27).
Outcomes and management
Outcomes
Short-term and long-term mortality of ASyS
The short-term risk of death with ASyS is high (around 20% in the first month post-ASyS). However, it is higher in older adults (28.4–40.5%) as compared to younger adults (11.2 vs. 17.7%). Thus, within the same population, the risk of death was 1.6 to 3.6 times for older adults (28). Similarly, short-term mortality due to ASySE is two times the rate in older adults compared to younger adults. This difference persists after excluding myoclonic SE due to ABI (29).
Despite increased short-term mortality of ASyS, studies show that long-term mortality is similar to ASyS and unprovoked seizures (30). ASyS does not predict functional outcomes at 6 months for patients with intracerebral hemorrhage in a prospective trial (31). However, these studies are not specific to older adults.
Mortality of ASyS by etiology
Cerebrovascular disease and ABI were the most common causes of ASyS in those with short-term mortality (28). Population-based studies fail to reveal if the increased risk of death is due to ASyS or the underlying etiology.
Similarly, ASySE in older adults has poor short-term outcomes (11). Acute symptomatic etiology was the commonest type of etiology for SE (52–58%) and also had a >6 times risk of poor outcome (death or new neurological impairment) as compared to other types of etiologies (11, 32). Two-thirds of patients with ASySE had a poor outcome, and 57% had inpatient mortality. CVD was the predominant acute symptomatic etiology (33). Similarly, Hui et al. (34) found that the acute symptomatic etiology of SE had five times increased risk of short-term mortality (49% mortality rate), with CVD the most common reason. In a study of convulsive SE in older adults, acute symptomatic etiology was the commonest cause, seen in 60% of the patients. Out of 33 patients, nine (27%) patients progressed to refractory SE. However, acute symptomatic etiology did not increase the likelihood of progressing to Refractory Status Epilepticus (RSE). CVD followed by metabolic disturbance was the most likely reason. None of the patients with CVD died. However, acute symptomatic etiology was associated with increased short-term mortality, seen in five out of six patients (35). Thus, in most studies, acute symptomatic etiology of ASySE and CVD as the specific etiology suggests poor outcomes in older adults.
Mortality in older adults with ASySE and acute ischemic CVD (39%) is much greater than in ischemic CVD alone (14%) or SE due to remote ischemic CVD (5%) (36). This finding demonstrates that the high mortality is due to “synergistic effects of SE and ischemic brain injury” (36). The increased mortality was not explained by the increased severity of CVD as measured by the size of the CVD. However, 63% of patients with acute ischemic CVD and 75% of patients with acute ischemic CVD + SE had negative CT or MRI imaging. This finding suggests that some of these patients possibly had a prolonged postictal focal deficit, which is commonly seen in older adults (37). It is unclear if the severity of ischemic CVD as measured by NIHSS is correlated with worse outcomes in concurrent SE (36).
There is conflicting evidence on whether acute stroke treatment with Tissue Plasminogen Activator (TPA) or thrombectomy increases the risk of ASyS. Two studies found that thrombolysis and thrombectomy increase the risk of poststroke seizures (38, 39) but were not replicated in a case–control study (40).
Risk of recurrence
The risk of subsequent unprovoked seizures after ASyS is about 30% and does not meet the criteria for a diagnosis of epilepsy (41, 42). Although ASyS has high short-term mortality, the risk of developing epilepsy is significantly lower than unprovoked or remote symptomatic seizures (43). A 10-year follow-up study found that the risk of future seizure recurrence is 3.3 times higher in ASySE as compared to ASyS. The risk is further modified by the underlying etiologies of ASyS, with ABI conferring the highest risk, followed by metabolic and structural causes (42). Thus, treatment with ASM in older adults with ASySE might be warranted, but not with ASyS.
It is unclear if seizure recurrence risk after ASyS is more in older adults than in young adults or if there is risk stratification with different etiologies. More than three seizures at presentation and epileptiform activity on initial EEG were predictors of subsequent unprovoked seizures and epilepsy (9).
A meta-analysis noted that ASyS increases the risk of poststroke epilepsy. Although this finding is not specific to older adults, most CVDs occur in older adults, so the conclusion can potentially be extrapolated to the older adult cohort (44). ASyS due to CVD carries the highest weight in the SeLECT score that provides risk-stratification of postischemic stroke epilepsy (45). A large majority of participants in the SeLECT study were ≥60 years and provided ample evidence of seizure risk in this cohort. Similarly, ASyS is a risk factor for the development of epilepsy in posthemorrhagic CVD as measured by the CAVE score (46).
For all adults with TBI, the risk of recurrent seizures increases if seizures occur within 1 week of injury, with severe and penetrating injury, prolonged loss of consciousness, intracerebral hemorrhage, and subdural hemorrhage requiring surgical evacuation (49).
Physicians often encounter outpatient scenarios where patients are inappropriately started on ASMs due to ASyS in an acute setting. The abovementioned risk factors in addition to patient comfort and projected consequences of a seizure (even if low risk) such as injuries in job setting and loss of driving privileges should be considered to decide continuation vs. gradual weaning off of the ASM.
Management
Initial treatment of ASyS is directed toward the management of underlying etiologies. Patients with ischemic CVD presenting within a thrombolytic or endovascular window should receive cerebral revascularization treatment accordingly. In the 2019 American Heart Association guideline for the early management of ischemic CVD, IV Alteplase is reasonable in patients presenting with seizures at symptom onset if the residual deficits are attributed to CVD (50).
For patients with metabolic disturbances, correction of electrolyte and glucose disturbances is the most effective management for ASyS. Due to polypharmacy and multiple comorbidities, older adults may be prone to these disturbances. Investigating and treating underlying metabolic etiologies long-term is crucial to preventing future episodes. ASM is indicated to prevent recurrent seizures if there is an expected delay in correcting some metabolic derangement and hypoxia. Older adults are also prone to systemic and CNS infections due to immunosenescence (51, 52). New-onset seizures, especially in the setting of encephalopathy, should prompt early investigation and treatment of these infections. For patients with toxin ingestion or medication-induced (e.g., digoxin) ASyS, the culprit drug or toxin cessation and antidote administration (if available) are the most effective ways to treat seizures. Drug cessation and close monitoring of levels can help normalize epileptiform activity on EEG (53).
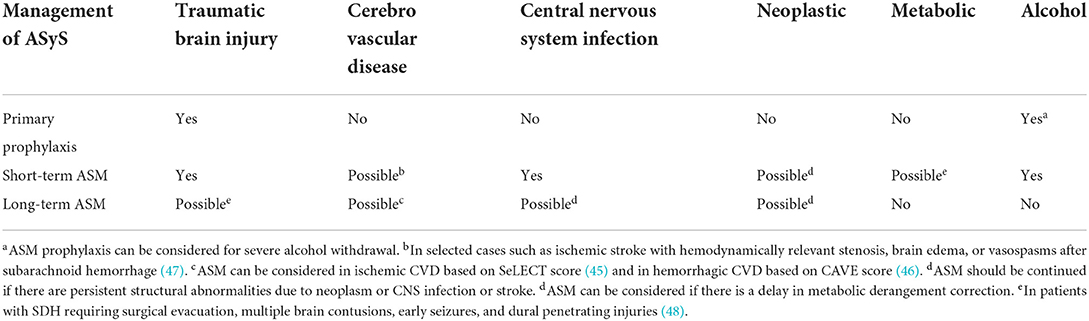
Anti-seizure medication is indicated for ASySE and might be necessary for treating ASyS that persist despite treatment of the underlying cause. Unfortunately, studies to guide the long-term management of ASM in the setting of ASyS are lacking. Patients who have interictal epileptiform discharges and persistent structural abnormalities or who present with SE are at a higher risk of developing epilepsy. The indication and duration of ASM in ASyS differ depending on the underlying etiology (Table 2) (43, 48, 50, 54–56). A 1–3 month duration has been proposed for short-term ASM use (43). For patients with ASyS due to posterior reversible encephalopathy syndrome (PRES), 3 months of ASM treatment have been proposed (57).
Primary seizure prophylaxis is not recommended for patients with acute ischemic CVD(50)or with intracranial neoplasm (54, 55). The Brain Trauma Foundation recommends 7-day anti-seizure medication prophylaxis after a TBI (56). Benzodiazepines or phenobarbital may be considered for seizure prophylaxis in severe alcohol withdrawal as determined by the CIWA-Ar score (43).
Few observational studies have found preventive benefits against ASyS with statins (58, 59). However, prospective, randomized studies are lacking to strongly recommend statin use at this time. The management of ASyS is summarized in Table 2.
Choice of ASM
Choosing an ASM in the setting of ASyS in older adults is challenging. Physicians need to consider underlying etiologies of ASyS, concurrent medications, comorbidities, and altered drug metabolism.
Strong hepatic enzyme inducers such as carbamazepine, phenytoin, and phenobarbital and enzyme inhibitor such as valproic acid should be used with caution due to potential interaction with multiple drugs used in older adults (60). Similarly, these ASMs should be avoided in patients with neoplasms due to drug–drug interaction with chemotherapeutic agents (61). They are also known to be atherogenic due to the potential of increasing serum cholesterol and, hence, are a suboptimal choice for poststroke seizures (61), a common reason for ASyS in older adults. Levetiracetam can improve cognitive outcomes after hemorrhagic CVD (62). It is comparable with phenytoin for the efficacy of primary and secondary seizure prophylaxis in TBI (43).
Patients with metabolic derangement secondary to renal or hepatic dysfunction require further consideration. Hepatically and renally metabolized or cleared ASM should be avoided or dose adjusted accordingly. Older adults can have physiologically decreased creatinine clearance (CLCR). Specifically, for levetiracetam, dose adjustment is required for CLCR < 80 ml/min/1.73 m2 (63). Similarly, a maximum dose of 300 mg/day is recommended for lacosamide for severe renal impairment (CLCR < 30 ml/min) (64). An additional dose of up to 50% once a day on baseline is recommended for levetiracetam and lacosamide in end-stage renal disease after hemodialysis on dialysis days (63, 64).
Randomized trials on ASMs targeting older adults are limited. However, two systematic reviews and meta-analyses suggest that lamotrigine, levetiracetam, and lacosamide are first-choice ASMs based on their high efficacy and reasonable tolerance in older adults (65, 66). Overall, older adults require lower doses to be seizure-free. For example, a median lamotrigine daily dose of 100 mg was sufficient for seizure freedom in many patients (67). A reasonable strategy for ASMs in older adults is “Start low, go slow, and stay low.”
Special considerations in the management of ASyS in the older adults
Differentiating between ASyS and unprovoked seizures can be challenging. However, the diagnostic dilemma has worse outcomes in older adults. The risk outcome of diagnosing a seizure as “unprovoked” when it was ASyS is more with older adults as they might be started on unnecessary chronic ASMs. This action can add to the medication burden in patients with polypharmacy (68). ASMs are frequently associated with adverse effects in older adults, including falls, cognitive impairment, and long-term complications like osteoporosis (69). In addition, they increase the risk of hospitalization (70).
Older adults can have prolonged postictal symptoms, including altered mental status, lasting hours to days (37). Thus, even if the etiology is known to be acute symptomatic, this prolonged postictal state could be confused with non-convulsive SE and could lead to needless treatment with benzodiazepines, ASMs, and anesthetic medications. This intervention further leads to an increased risk of intubation and increased length of stay which are known to have worse short-term and long-term outcomes (11). Obtaining an emergent EEG is of paramount importance in these patients to distinguish the postictal state from non-convulsive SE. Even in patients with established epilepsy, failure to distinguish between ASyS and unprovoked seizures will lead to an unnecessary increase in the dose of baseline ASMs.
Conclusion
Diagnosis and management of ASyS is an emerging field with many unanswered questions in older adults, including patient selection and risk factors for primary prophylaxis, short-term and long-term outcomes, and ASM management. Some novel clinical endeavors such as post-ASyS clinics and the multicenter Post-Acute Symptomatic Seizure Investigation and Outcomes Network (PASSION) project will help to further clarify many unknowns in this field (71).
Author contributions
WK and RM were responsible for the conception and design of the paper, drafting, critical revision, and final approval of the article to be published.
Conflict of interest
RM has received funding for investigator-initiated trial from Eisai Co.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. (2010) 51:671–5. doi: 10.1111/j.1528-1167.2009.02285.x
2. Olafsson E, Hauser WA, Ludvigsson P, Gudmundsson G. Incidence of epilepsy in rural Iceland: a population-based study. Epilepsia. (1996) 37:951–5. doi: 10.1111/j.1528-1157.1996.tb00532.x
3. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. (2004) 62:S24–29. doi: 10.1212/WNL.62.5_suppl_2.S24
4. Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol. (2009) 8:1019–30. doi: 10.1016/S1474-4422(09)70240-6
5. Josephson CB, Engbers JDT, Sajobi TT, Jette N, Agha-Khani Y, Federico P, et al. Towards a clinically informed, data-driven definition of elderly onset epilepsy. Epilepsia. (2016) 57:298–305. doi: 10.1111/epi.13266
6. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. (1996) 71:576–86. doi: 10.4065/71.6.576
7. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. (2008) 49:8–12. doi: 10.1111/j.1528-1167.2008.01443.x
8. Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia. (1995) 36:327–33. doi: 10.1111/j.1528-1157.1995.tb01005.x
9. Holt-Seitz A, Wirrell EC, Sundaram MB. Seizures in the elderly: etiology and prognosis. Can J Neurol Sci J Can Sci Neurol. (1999) 26:110–4.
10. Costello DJ, Cole AJ. Treatment of acute seizures and status epilepticus. J Intensive Care Med. (2007) 22:319–47. doi: 10.1177/0885066607307506
11. Sadeghi M, Eshraghi M, Akers KG, Hadidchi S, Kakara M, Nasseri M, et al. Outcomes of status epilepticus and their predictors in the elderly-a systematic review. Seizure. (2020) 81:210–21. doi: 10.1016/j.seizure.2020.08.021
12. Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. (2000) 57:1617–22. doi: 10.1001/archneur.57.11.1617
13. Won SY, Konczalla J, Dubinski D, Cattani A, Cuca C, Seifert V, et al. A systematic review of epileptic seizures in adults with subdural haematomas. Seizure. (2017) 45:28–35. doi: 10.1016/j.seizure.2016.11.017
14. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. (1993) 34:453–8. doi: 10.1111/j.1528-1157.1993.tb02586.x
15. DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. (1995) 12:316–25. doi: 10.1097/00004691-199512040-00003
16. Sibia RS, Kumar S A, Sharma H. Seizure in later life: an ode to the elderly. Int J Res Med Sci. (2017) 2:1393–5. doi: 10.5455/2320-6012.ijrms20141128
17. Ghosh S, Jehi LE. New-onset epilepsy in the elderly: challenges for the internist. Cleve Clin J Med. (2014) 81:490–8. doi: 10.3949/ccjm.81a.13148
18. Waterhouse E, Towne A. Seizures in the elderly: nuances in presentation and treatment. Cleve Clin J Med. (2005) 72:S26. doi: 10.3949/ccjm.72.Suppl_3.S26
19. Leppik IE. Epilepsy in the elderly: scope of the problem. Int Rev Neurobiol. (2007) 81:1–14. doi: 10.1016/S0074-7742(06)81001-9
20. Lempert T, Bauer M, Schmidt D. Syncope: a videometric analysis of 56 episodes of transient cerebral hypoxia. Ann Neurol. (1994) 36:233–7. doi: 10.1002/ana.410360217
21. Evidence-based guideline: Management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Available online at: https://pubmed.ncbi.nlm.nih.gov/25901057/ (accessed May 25, 2022).
22. Acharya JN, Acharya VJ. Epilepsy in the elderly: special considerations and challenges. Ann Indian Acad Neurol. (2014) 17:S18–26. doi: 10.4103/0972-2327.128645
23. Van Cott AC. Epilepsy and EEG in the tElderly. Epilepsia. (2002) 43:94–102. doi: 10.1046/j.1528-1157.43.s.3.10.x
24. Manfredonia F, Saturno E, Lawley A, Gasverde S, Cavanna AE. The role of electroencephalography in the early diagnosis of non-convulsive status epilepticus in elderly patients with acute confusional state: two possible strategies? Seizure. (2019) 73:39–42. doi: 10.1016/j.seizure.2019.11.002
25. Rossetti AO, Schindler K, Sutter R, Rüegg S, Zubler F, Novy J, et al. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: a multicenter randomized clinical trial. JAMA Neurol. (2020) 77:1225–32. doi: 10.1001/jamaneurol.2020.2264
26. LaRoche S, Taylor D, Walter P. Tilt table testing with video EEG monitoring in the evaluation of patients with unexplained loss of consciousness. Clin EEG Neurosci. (2011) 42:202–5. doi: 10.1177/155005941104200311
27. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. (1989) 84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x
28. Hesdorffer DC, D'Amelio M. Mortality in the first 30 days following incident acute symptomatic seizures. Epilepsia. (2005) 46:43–5. doi: 10.1111/j.1528-1167.2005.00408.x
29. Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Time trends in incidence, mortality, and case-fatality after first episode of status epilepticus. Epilepsia. (2001) 42:1031–5. doi: 10.1046/j.1528-1157.2001.0420081031.x
30. Hesdorffer DC, Benn EKT, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? mortality and risk for recurrent seizure. Epilepsia. (2009) 50:1102–8. doi: 10.1111/j.1528-1167.2008.01945.x
31. De Herdt V, Dumont F, Hénon H, Derambure P, Vonck K, Leys D, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. (2011) 77:1794–800. doi: 10.1212/WNL.0b013e31823648a6
32. Sinha S, Satishchandra P, Kalband BR, Thennarasu K. New-onset status epilepticus and cluster seizures in the elderly. J Clin Neurosci. (2013) 20:423–8. doi: 10.1016/j.jocn.2012.02.050
33. Vilella L, González Cuevas M, Quintana Luque M, Toledo M, Sueiras Gil M, Guzmán L, et al. Prognosis of status epilepticus in elderly patients. Acta Neurol Scand. (2018) 137:321–8. doi: 10.1111/ane.12867
34. Hui ACF, Lam AK, Wong A, Chow KM, Chan ELY, Choi SL, et al. Generalized tonic-clonic status epilepticus in the elderly in China. Epileptic Disord Int Epilepsy J Videotape. (2005) 7:27–31.
35. Jayalakshmi S, Vooturi S, Chepuru R, Sahu S, Surath M. Aetiology and outcome of generalized convulsive status epilepticus in elderly. Seizure. (2015) 29:104–8. doi: 10.1016/j.seizure.2015.03.011
36. Waterhouse EJ, Vaughan JK, Barnes TY, Boggs JG, Towne AR, Kopec-Garnett L, et al. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res. (1998) 29:175–83. doi: 10.1016/S0920-1211(97)00071-5
37. Stefan H, May TW, Pfäfflin M, Brandt C, Füratsch N, Schmitz B, et al. Epilepsy in the elderly: comparing clinical characteristics with younger patients. Acta Neurol Scand. (2014) 129:283–93. doi: 10.1111/ane.12218
38. Alvarez V, Rossetti AO, Papavasileiou V, Michel P. Acute seizures in acute ischemic stroke: does thrombolysis have a role to play? J Neurol. (2013) 260:55–61. doi: 10.1007/s00415-012-6583-6
39. Naylor J, Thevathasan A, Churilov L, Guo R, Xiong Y, Koome M, et al. Association between different acute stroke therapies and development of post stroke seizures. BMC Neurol. (2018) 18:61. doi: 10.1186/s12883-018-1064-x
40. Zöllner JP, Misselwitz B, Mauroschat T, Roth C, Steinmetz H, Rosenow F, et al. Intravenous thrombolysis or mechanical thrombectomy do not increase risk of acute symptomatic seizures in patients with ischemic stroke. Sci Rep. (2020) 10:21083. doi: 10.1038/s41598-020-78012-y
41. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55:475–82. doi: 10.1111/epi.12550
42. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. (1998) 44:908–12. doi: 10.1002/ana.410440609
43. Gunawardane N, Fields M. Acute symptomatic seizures and provoked seizures: to treat or not to treat? Curr Treat Options Neurol. (2018) 20:41. doi: 10.1007/s11940-018-0525-2
44. Ferlazzo E, Gasparini S, Beghi E, Sueri C, Russo E, Leo A, et al. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. (2016) 57:1205–14. doi: 10.1111/epi.13448
45. Galovic M, Döhler N, Erdélyi-Canavese B, Felbecker A, Siebel P, Conrad J, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. (2018) 17:143–52. doi: 10.1016/S1474-4422(17)30404-0
46. The CAVE Score for Predicting Late Seizures After Intracerebral Hemorrhage Stroke. Available online at: https://www.ahajournals.org/doi/10.1161/strokeaha.114.004686 (accessed May 21, 2022).
47. Galovic M, Ferreira-Atuesta C, Abraira L, Döhler N, Sinka L, Brigo F, et al. Seizures and epilepsy after stroke: epidemiology, biomarkers and management. Drugs Aging. (2021) 38:285–99. doi: 10.1007/s40266-021-00837-7
48. Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. (2006) 108:433–9. doi: 10.1016/j.clineuro.2005.09.001
49. Risk of Post-Traumatic Epilepsy After Severe Head Injury in Patients With at Least One Seizure. Available online at: https://pubmed.ncbi.nlm.nih.gov/28919762/ (accessed May 25, 2022).
50. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
51. Saltzman RL, Peterson PK. Immunodeficiency of the elderly. Rev Infect Dis. (1987) 9:1127–39. doi: 10.1093/clinids/9.6.1127
52. Choi C. Bacterial meningitis in aging adults. Clin Infect Dis. (2001) 33:1380–5. doi: 10.1086/322688
53. Kerr DJ, Elliott HL, Hillis WS. Epileptiform seizures and electroencephalographic abnormalities as manifestations of digoxin toxicity. Br Med J Clin Res Ed. (1982) 284:162–3. doi: 10.1136/bmj.284.6310.162
54. Chang SM, Messersmith H, Vogelbaum MA. Anticonvulsant prophylaxis and steroid use in adults with metastatic brain tumors: ASCO/SNO joint endorsement summary of college of neurologic surgeons guidelines. J Oncol Pract. (2019) 15:395–7. doi: 10.1200/JOP.19.00058
55. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. (2004) 79:1489–94. doi: 10.4065/79.12.1489
56. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
57. Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what's certain, what's new? Pract Neurol. (2011) 11:136–44. doi: 10.1136/practneurol-2011-000010
58. Li Y, Zhang B, Zhang L, Xie D, Li Y. Efficacy of Statin therapy in post-stroke seizure prophylaxis: clues from an observational study of routine secondary prevention treatment. Seizure. (2019) 71:185–9. doi: 10.1016/j.seizure.2019.07.010
59. Guo J, Guo J, Li J, Zhou M, Qin F, Zhang S, et al. Statin treatment reduces the risk of poststroke seizures. Neurology. (2015) 85:701–7. doi: 10.1212/WNL.0000000000001814
60. French JA, Staley BA. AED treatment through different ages: as our brains change, should our drug choices also? Epilepsy Curr. (2012) 12:22–7. doi: 10.5698/1535-7511-12.4s.22
61. Hanaya R, Arita K. The new antiepileptic drugs: their neuropharmacology and clinical indications. Neurol Med Chir. (2016) 56:205–20. doi: 10.2176/nmc.ra.2015-0344
62. Taylor S, Heinrichs RJ, Janzen JM, Ehtisham A. Levetiracetam is associated with improved cognitive outcome for patients with intracranial hemorrhage. Neurocrit Care. (2011) 15:80–4. doi: 10.1007/s12028-010-9341-6
63. French J. Use of levetiracetam in special populations. Epilepsia. (2001) 42:40–3. doi: 10.1046/j.1528-1157.2001.0420s4040.x
64. Cawello W, Fuhr U, Hering U, Maatouk H, Halabi A. Impact of impaired renal function on the pharmacokinetics of the antiepileptic drug lacosamide. Clin Pharmacokinet. (2013) 52:897–906. doi: 10.1007/s40262-013-0080-7
65. Lezaic N, Gore G, Josephson CB, Wiebe S, Jetté N, Keezer MR. The medical treatment of epilepsy in the elderly: a systematic review and meta-analysis. Epilepsia. (2019) 60:1325–40. doi: 10.1111/epi.16068
66. Lattanzi S, Trinka E, Del Giovane C, Nardone R, Silvestrini M, Brigo F. Antiepileptic drug monotherapy for epilepsy in the elderly: a systematic review and network meta-analysis. Epilepsia. (2019) 60:2245–54. doi: 10.1111/epi.16366
67. Leppik IE, Brodie MJ, Saetre ER, Rowan AJ, Ramsay RE, Macias F, et al. Outcomes research: clinical trials in the elderly. Epilepsy Res. (2006) 68 Suppl 1:S71–76. doi: 10.1016/j.eplepsyres.2005.07.019
68. Baftiu A, Feet SA, Larsson PG, Burns ML, Henning O, Sætre E, et al. Utilisation and polypharmacy aspects of antiepileptic drugs in elderly versus younger patients with epilepsy: a pharmacoepidemiological study of CNS-active drugs in Norway, 2004-2015. Epilepsy Res. (2018) 139:35–42. doi: 10.1016/j.eplepsyres.2017.11.001
69. Poza JJ. Management of epilepsy in the elderly. Neuropsychiatr Dis Treat. (2007) 3:723–8. doi: 10.2147/NDT.S1026
70. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older americans. N Engl J Med. (2011) 365:2002–12. doi: 10.1056/NEJMsa1103053
71. Post-Acute Symptomatic Seizure (PASS) Clinic: A Continuity of Care Model for Patients Impacted by Continuous EEG Monitoring. Available online at: https://pubmed.ncbi.nlm.nih.gov/32524051/ (accessed May 23, 2022).
Keywords: acute symptomatic seizure, acute symptomatic status epilepticus, older adults, management, outcomes
Citation: Kong WY and Marawar R (2022) Acute symptomatic seizures and status epilepticus in older adults: A narrative review focusing on management and outcomes. Front. Neurol. 13:954986. doi: 10.3389/fneur.2022.954986
Received: 27 May 2022; Accepted: 01 August 2022;
Published: 26 August 2022.
Edited by:
Vineet Punia, Cleveland Clinic, United StatesReviewed by:
Isabelle Beuchat, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2022 Kong and Marawar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohit Marawar, gg8831@wayne.edu
 Wan Yee Kong
Wan Yee Kong Rohit Marawar
Rohit Marawar