- 1Institut Mondor de Recherche Biomédicale (IMRB)-INSERM U955, Ecole Doctorale Science de la Vie et de la Santé, Université Paris-Est Créteil, Paris, France
- 2Faculty of Medical Sciences, Neuroscience Research Center, Lebanese University, Hadath, Lebanon
- 3College of Health Sciences, Abu Dhabi University, Abu Dhabi, United Arab Emirates
- 4Institut National de Santé Publique, Epidémiologie Clinique et Toxicologie (INSPECT-LB), Beirut, Lebanon
- 5Faculty of Pharmacy, Lebanese University, Hadath, Lebanon
- 6University of Nicosia Medical School, Nicosia, Cyprus
- 7Hôpital Henri Mondor, AP-HP, Créteil, France
Background: To date, despite the application of secondary prevention worldwide, first-ever stroke survivors remain at imminent risk of stroke recurrence and death in the short and long term. The present study aimed to assess the cumulative risk rates and identify baseline differences and stroke characteristics of Lebanese survivors.
Methods: A prospective longitudinal study was conducted among survivors ≥18 years old who were followed-up for 15 months through a face-to-face interview. Kaplan–Meier method was used to calculate the cumulative rates of stroke mortality and recurrence. Cox-regression univariate and multivariable analyses were performed to identify the predictors of both outcomes.
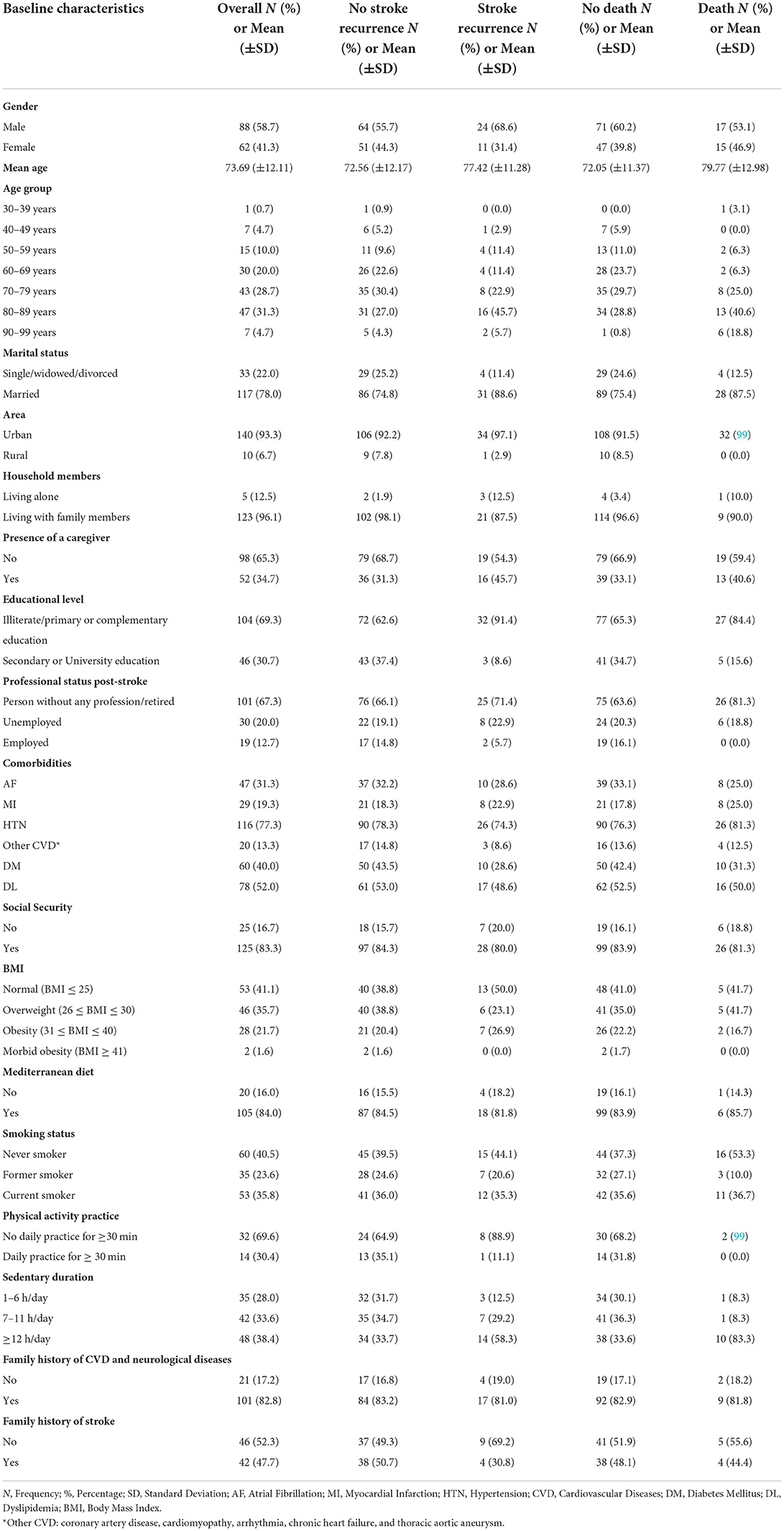
Results: Among 150 subjects (mean age 74 ± 12 years; 58.7% men vs. 44.3% women; 95.3% with ischemic stroke vs. 4.3% with intracerebral hemorrhage), high cumulative risk rates of stroke recurrence (25%) and death (21%) were highlighted, especially in the acute phase. Survival rates were lesser in patients with stroke recurrence compared to those without recurrence (Log rank test p < 0.001). Older age was the main predictor for both outcomes (p < 0.02). Large artery atherosclerosis was predominant in patients with stroke recurrence and death compared to small vessel occlusion (p < 0.02). Higher mental component summary scores of quality of life were inversely associated with stroke recurrence (p < 0.01). Lebanese survivors exhibited the highest percentages of depression and anxiety; elevated Hospital Anxiety and Depression Scale (HADS) scores were seen in those with stroke recurrence and those who died (≥80% with mean HADS scores ≥8). Lower Mini-Mental State Examination scores at the acute phase increased the risk of both outcomes by 10% (p < 0.03). Three out of 13 mortalities (23.1%) were presented with early epileptic seizures (p = 0.012). High educational level was the protective factor against stroke recurrence (p = 0.019). Administration of intravenous thrombolysis decreased the risk of both outcomes by 10% (p > 0.05).
Conclusion: Higher rates of stroke recurrence and death were observed in the first year following a stroke in Lebanon. Various factors were identified as significant determinants. Thus, health care providers and officials in Lebanon can use these findings to implement effective preventive strategies to best address the management of these factors to reduce the stroke burden and improve the short and long-term prognosis of stroke survivors.
Introduction
Stroke is a cerebrovascular disorder characterized by the sudden onset of symptoms and clinical signs caused by the disruption of blood supply to parts of the brain (1). It is a major health concern and is considered one of the most devastating neurological diseases worldwide (2). In Lebanon, as in several countries, stroke is one of the leading causes of death and morbidity (3, 4). Fifteen million people worldwide suffer from stroke annually, of which five million die, and another five million are left permanently disabled, placing a burden on the family and community (5). Normal life for the majority of stroke survivors is disrupted and they experience major disabilities affecting their physical and psychological wellbeing, resulting in long-term invalidity or death.
Stroke recurrence is highly prevalent in survivors and is one of the main functional outcomes in the short and long-term post-first-ever stroke (6). A recent global systematic review by Lin et al. including 37 studies conducted over the last 10 years, including 1,075,014 stroke patients, has shown an increasing pooled stroke recurrence rate ranging from 7.7% at 3 months, 9.5% at 6 months, 10.4% at 1 year to 39.7% at 12 years after the initial stroke (7). Neurological deficit caused by a recurrent stroke is more severe than the initial stroke, with a high percentage of prolonged disability and death. Hence, secondary prevention after the first stroke is crucial to reduce stroke recurrence and mortality (8).
The cause of stroke recurrence and mortality is multifactorial (8, 9). Various international papers have identified modifiable and non-modifiable risk factors, including age (10, 11), gender (12, 13), vascular events like hypertension (HTN) (14), hyperlipidemia and atherosclerotic cardiovascular diseases (15), diabetes mellitus (DM) (16), atrial fibrillation (AF) (17), previous history of cerebrovascular events and stroke subtypes (18), lifestyle factors like smoking habits (19), alcohol consumption (4), use of contraceptive pills, obesity and physical inactivity (18, 20, 21), and psychological complications post-first stroke (22, 23). It is imperative and necessary to identify the patients who are at high risk of stroke recurrence and mortality and who may benefit from a close and regular assessment and rapid implementation of preventive treatments (24).
Although the Global Burden of the Disease tends to provide regular worldwide data regarding stroke burden (25), there is still uncertainty in stroke estimates in low to middle-income countries without national-based health surveillance systems. In recent years, extensive data were published on stroke recurrence and fatality determinants worldwide but there is a scarcity of related studies from the Middle East and North Africa (MENA) region (26–30). However, the burden of stroke in low and middle-income countries, including Lebanon, is higher than in high-income countries and is still rising (31, 32). Stroke types, risk factors, knowledge, and adherence to medication were addressed in various Lebanese papers (2, 3, 33–35) but there are no research studies yet which investigate the recurrence of stroke and death after first-ever stroke. The purpose of this study was to measure the cumulative risk rates of stroke recurrence and death in a time-to-event survival analysis at 3, 6, and 12 months post-first-ever stroke and to identify their determinants among Lebanese first-ever stroke survivors.
Methods
We followed The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines for a proper reporting of this work (36).
Study design and population
An epidemiological observational multicenter prospective longitudinal study was conducted in five private and five public medical centers within two big governorates of Lebanon: Mount Lebanon and Beirut. The study period lasted 15 months, from February 2018 until May 2019. Approval of the protocol from ethics committees of all participating centers was granted before initiating any study procedure while abiding by the World Medical Association Declaration of Helsinki in 2013 (37).
The participants included first-ever ischemic or hemorrhagic stroke survivors who were admitted to the hospitals between February and May 2018. The inclusion criteria were as follows: (1) age ≥18 years, (2) Lebanese nationality, (3) having experienced first-ever stroke, well-identified by the following codes of the International Classification of Diseases-10 (ICD-10) (I60-I64) (38): cerebrovascular accident, stroke, ischemic stroke, hemorrhagic stroke, intracerebral hemorrhage or embolic/cerebral vascular thrombosis, and (4) a diagnosis confirmed clinically and through brain imaging. The exclusion criteria were the following: (1) admission for a recurrent stroke or transient ischemic accident or (2) a medical history of neurological and cognitive disorders. The participants (or their legal representatives) provided written informed consent to be enrolled in the study.
Sample size
Expected sample size was calculated using the Epi-Info 7 program estimating 116 participants, depending on the stroke prevalence of 3.9% obtained by Jurjus et al. (39). After accounting for missing data and lost follow-up data, a total of 150 subjects were included in the study.
Study procedures
Written consent from the eligible participants was gathered through an interview conducted by three well-trained investigators. Afterward, the participants were followed up for data collection at 3-, 6-, and 12-months post-stroke.
Clinical information was collected through a data collection form. It included the following: (1) age, gender, place of residence, marital status, number of kids, age of subject's custodian, level of education of the subject and his/her custodian, employment status, number of household members, number of rooms and type of health insurance, (2) lifestyle (eating habits, smoking, practice of physical activity, alcohol and other substances consumption, social support), (3) health indicators (anthropometric indices, family/medical/surgical history, comorbidities, treatment taken by subjects), (4) the disease and its severity (types/subtypes/location/symptoms, length of hospital stay, severity of disease, degree of disability, evaluation of the quality of life (QoL) and (5) the stroke consequences (neuropsychiatric disorders, cognitive disorders, hyperglycemia, fatigue, post-stroke pain, falls, pressure ulcers, pulmonary and urinary infections, deep vein thrombosis, pulmonary embolism, seizures, recurrence of stroke, and death).
Definitions
The initial Stroke or “Jalta Dimaghia,” the Arabic synonym, is the most familiar and most specific term for the disease in Lebanon. According to the World Health Organization, “it is a clinical syndrome consisting of rapidly developing clinical signs of focal (or global in case of coma) disturbance of cerebral function lasting more than 24 h or leading to death with no apparent cause other than that from a vascular origin” (40). Ischemic stroke was classified using the Trial of Org 10172 in the Acute Stroke Treatment (TOAST) criteria, which is divided into five subtypes: (1) large-artery atherosclerosis (LAA), (2) cardioembolism, (CE) (3) small-vessel occlusion (SVO), (4) stroke of other determined etiology (OE), and (5) stroke of undetermined etiology (UE) (41).
Stroke recurrence was the main outcome, which was defined the same criteria as that of the initial stroke. Both ischemic and hemorrhagic stroke recurrences were recorded. Only recurrences that occurred 21 days after the initial event was considered (12). Mortality was defined as death from any cause within 12 months after the first-ever stroke onset. If a patient died within the year of follow-up, the cause of death was researched in the hospital or primary care medical records.
To determine the initial stroke characteristics, the Questionnaire for Verifying Stroke-Free Status (QVSFS) was used. This questionnaire was used to investigate if the subjects ever had the following stroke symptoms: sudden painless weakness on one side of the body, sudden numbness or a dead feeling on one side of the body, sudden painless loss of vision in one or both eyes, sudden loss of one-half of vision, sudden loss of the ability to understand what people are saying; and sudden loss of the ability to express ideas verbally or in writing (42). Stroke severity was measured by the National Institutes of Health Stroke Scale (NIHSS), which identifies the level of consciousness, vision (demonstrated by horizontal eye movements and visual field), facial palsy, motor function extremities, ataxia, sensations, speech dysarthria, or aphasia, and attention to multiple types of stimuli. The scale is divided into 2 levels: <21: non-severe stroke, ≥21: severe stroke; however, a NIHSS cutoff score ≤5 predicts a favorable outcome among survivors during the follow-up periods (43, 44). [Cronbach's alpha of (r) = 0.942]. We utilized the validated Arabic translation of NIHSS (45). Disability and dependence in Activities of Daily Living (ADL) were measured by the modified Rankin Scale (mRS), which is the most commonly used scale, with mild disability (independence) graded 0–2 and moderate to severe disability graded ≥3 (46) [Cronbach's alpha of (r) = 0.946]. The QoL was assessed by the short form (SF12), which consisted of 12 items including eight scales: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and perceived mental health (MH), and was divided into two summary scores [physical (PCS) and mental component summaries (MCS)]. They demonstrated the mental and physical functions and overall health-related QoL. PCS and MCS were computed through the scores 12 questions and ranged from 0 (lowest level of health) to 100 (highest level of health) with a cut-off of 50 for PCS and 42 for MCS. The scoring was calculated using the United States (US) norm-based scoring algorithm in the Statistical Package for the Social Sciences software (SPSS) (47, 48). The Arabic version of the SF-12 was used (49). A recent study by Haddad et al. was conducted for the validation of the Arabic version among Lebanese adults (50). The cognitive function was evaluated by the Mini-Mental State of Examination (MMSE), with a total score of 30 points where the cut-off point was set at 24, and a higher score defines a normal cognitive function (51). It has been classified into three levels: 24–30 = no cognitive impairment; 18–23 = mild cognitive impairment; and 0–17 = severe cognitive impairment (52) [Cronbach's alpha of (r) = 0.882]. Previous research has validated the use of the Arabic version of MMSE among the Lebanese population (53). The severity of psychological disorders, such as anxiety and depression, was assessed using the Hospital Anxiety and Depression Scale (HADS), which was divided into two scales of seven elements: a scale for depression and a scale for anxiety. Scores ranged from 0 to 7 = normal, 8 to 10 = borderline, 11 to 21 = abnormal (54), [Cronbach's alpha of (r) = 0.906], the Arabic validated version was utilized in this study (55). In addition, other scales and scores were utilized, such as the Social Support Rating Scale (SSRS) (56), the Fatigue Severity Scale (FSS) (57), the Modified Ashworth Scale (MAS)(58), “Douleur Neuropathique4” (DN4) questionnaire (59), and the Visual Analog Scale (VAS) (60).
Data processing and analysis
Continuous variables were presented as means ± Standard Deviation (SD) and categorical variables as numbers and percentages. A Survival (Time-to-Event) Analysis was utilized, and the Kaplan–Meier method was used to obtain the cumulative risk rates of stroke recurrence and any cause of death at 3-, 6-, and 12-month follow-up. Univariate and multivariable cox proportional hazards regressions were analyzed to determine the predictors of stroke recurrence and death depending on the time of the event occurrence. The explanatory variables were first tested individually against the dependent variable for the presence of a significant association. Variables for which no significant association was found were removed from the model. Regression analyses were then performed. In the multivariable logistic regression model, we included variables reported in the literature to be associated with 1-year stroke recurrence and death post-stroke considering them as potential confounders, such as age, gender, educational level, and stroke severity, in addition to the variables that showed a significant association at p ≤ 0.05 across any category in the univariate analysis. The logistic regression models were examined for the goodness of fit. Deviance values were calculated to analyze how well the model fitted each case. In all cases, it was concluded that the model fit was adequate, and the experimental removal of outliers did not violate the model. The strength of association was interpreted using the adjusted hazard ratio (AHR) with a 95% confidence interval (CI). Statistical significance was set at p ≤ 0.05. All these analyses were carried out using the SPSS software, version 25 (SPSSTM Inc., Chicago, IL USA).
Ethical considerations
The study protocol was reviewed and approved by the ethics committees and directors of the participating hospitals (NEUR-2018-001, HDF-1152). Ethical clearance was obtained through a formal letter granted in line with the World Medical Association Declaration of Helsinki in 2013 (37). Written consent was obtained from the subjects after explaining all details of the study. Participants were also informed that there will be no risks or direct benefits from their collaboration with this study. The participation was completely voluntary and enrolled subjects retained the right to withdraw at any time throughout the study. In addition, to maintain confidentiality, all data were coded in the questionnaire, and the materials will be discarded once the legal retention period expired.
Results
Baseline characteristics
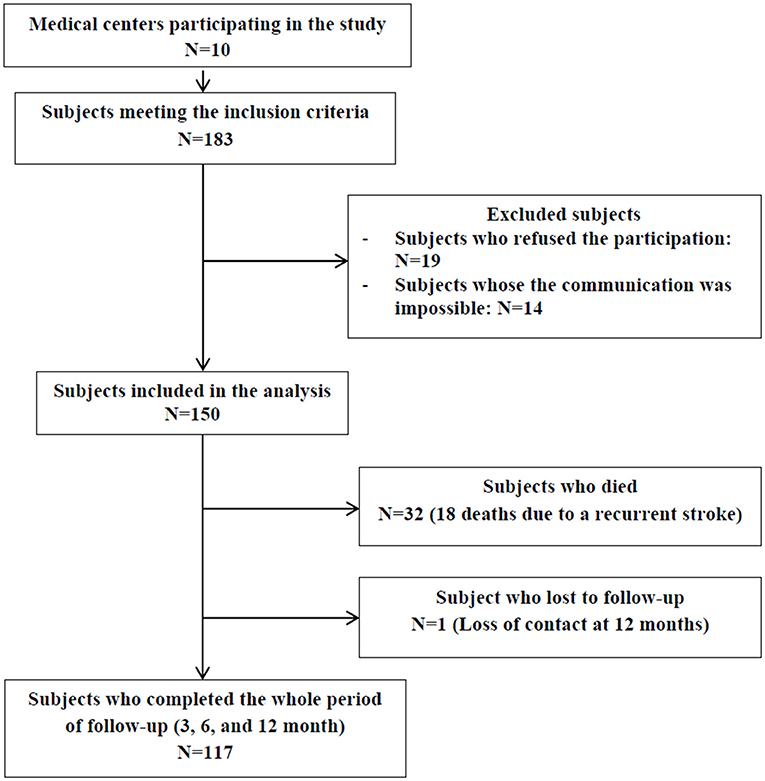
The study population consisted of 150 participants admitted to 10 medical centers in Mount Lebanon and Beirut between February and May 2018. A total of 117 subjects completed the whole follow-up period (3-, 6-, and 12-month), 32 died and one was lost to follow-up at 12 months post-stroke (Figure 1).
Baseline characteristics for all patients, no stroke recurrence/no death groups, and stroke recurrence/death groups are shown in Table 1. The participants had a mean age of 73.69 ± 12.11 years. The population included 88 men (58.7%) and 62 women (44.3%). Stroke recurrence and death were high in subjects with old age (51.4 and 59.4%, respectively), with low educational level (91.4 and 84.4%, respectively), with no employment post-stroke (94.3 and 100%, respectively), and in subjects with a sedentary duration of ≥12 h (58.3 and 83.3%, respectively).
Stroke characteristics and their severity
The median interval between the onset of stroke symptoms to admission was 2 h (ranging from 0 to 48 h) for all subjects (mean of 3.43 ± 5.94 h). In addition, the median duration of hospital stay was 7 days (ranging from 2 to 45 days) (mean of 9.69 ± 8.35 days) for all subjects.
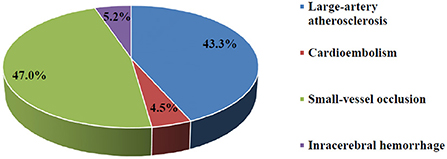
A total of 95.3% of subjects suffered from ischemic stroke compared to 4.7% who suffered from intracerebral hemorrhagic stroke (Figure 2). No subarachnoid hemorrhage was found in the present study. Ischemic stroke cases were categorized into 3 subtypes: LAA (58, 45.7%), CE (6, 4.7%), and SVO (63, 42%). A total of 46.7 and 40% of cases involved the left and right hemispheres, respectively. A majority (70.7%) of subjects were not able to express themselves verbally or in writing at the time of stroke and 70% experienced unilateral weakness.
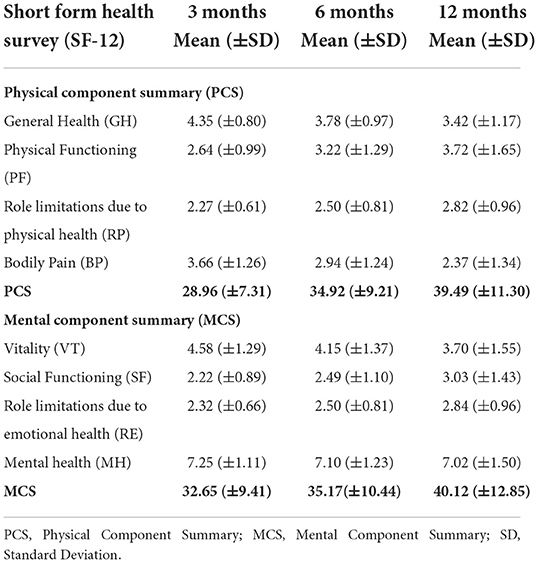
Stroke severity was estimated as a percentage of NIHSS categories at every follow-up. At 3-month post-stroke, 16.8% of subjects were found to have a severe stroke (NIHSS score ≥ 21). Regarding the degree of disability, a significant proportion of subjects (18%) died (mRS = 6) 3 months post-stroke, whereas, 16% were bedridden (mRS = 5). These percentages decreased from 9.8 and 9.3% (mRS = 5), to 1.6 and 0.8% (mRS = 6) respectively, in the 6- and 12-month follow-ups. The QoL scores are summed up in Table 2, showing decreased PCS and MCS components of QoL (means between 28 and 40) at 3-, 6-, and 12-month follow-up periods. These levels were less than the theoretical averages (cut-off of 50 for PCS and 42 for MCS). At index admission, 47 (31.3%) subjects were already on antiplatelet and anticoagulation agents. At index discharge, these drugs were prescribed to 141 (94%) subjects.
Risk rates of stroke recurrence and death
Figure 3 shows a high probability of stroke recurrence and death in the first 3 months post-stroke and a significant reduction in these consequences 6 to 12 months (p < 0.001) post-stroke. A total of 38 recurrent strokes occurred during the study period, 22 (14.7%) during 3 months post-stroke, nine (7.3%) from 3 to 6 months post-stroke, and seven (5.9%) from 6 to 12 months post-stroke. Additionally, a total of 32 mortalities were reported, 27 (18%) in the 3 months, three (2.4%) in the 3–6 months, and two (1.7%) in the 6–12 months following the first stroke. The reported causes of death were as follows: recurrent stroke (n = 18, 56.3%), brain herniation (n = 5, 15.6%), myocardial infarction (n = 3, 9.4%), complications post-stroke (n = 3, 9.4%), ARDS post-stroke (n = 1, 3.1%), and pulmonary embolism (n = 1, 3.1%).
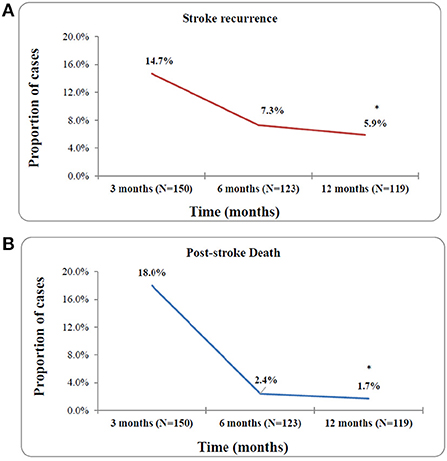
Figure 3. Risk rates of stroke recurrence (A) and any-cause of death (B) at 3, 6, and 12 month post-stroke. *p-value < 0.001.
Figure 4 represents the Kaplan–Meier curves of cumulative risk rates over 1 year of follow-up. Cumulative recurrence risk rates among first-ever stroke survivors increased from 15% at the 3 months to 22% at 6 months and 25% at 12 months follow-up. A similar trend was observed for the cumulative any-cause of death risk, which increased from 18% at 3 months to 20% at 6 months to 21% at 12 months post-stroke. The difference between patients with and without 1-year stroke recurrence is shown in Figure 5. The survival rates decreased in patients with stroke recurrence compared to those without recurrence (log rank test p < 0.001).
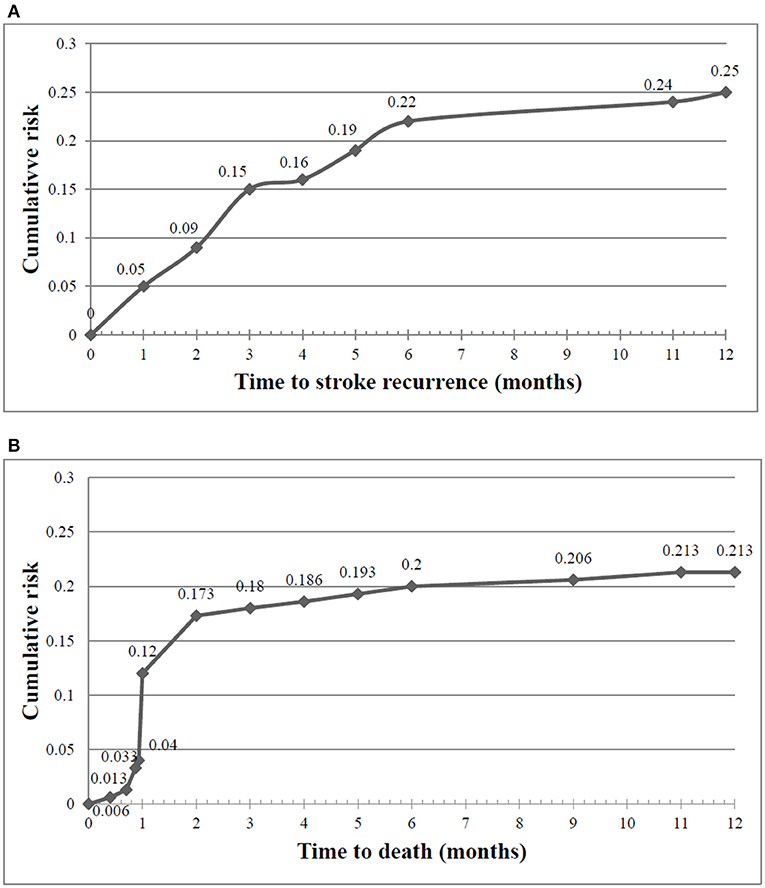
Figure 4. Cumulative risk rates of stroke recurrence (A) and any-cause of death (B) at 3, 6, and 12 month post-stroke.

Figure 5. Kaplan Meier estimates of 1-year probability of survival after a first-ever stroke among subjects with and without stroke recurrence. Log rank test P < 0.001.
Predictors of outcomes: Stroke recurrence and any-cause of death
Univariate and Multivariable analyses were performed using Cox proportional unadjusted (UHR) and adjusted hazard ratios (AHR).
One-year stroke recurrence predictors
Tables 3, 4 show the UHR of stroke recurrence according to the baseline characteristics, in-hospital course, and post-stroke consequences. Adjusted hazard risks of stroke recurrence are presented in Table 5.
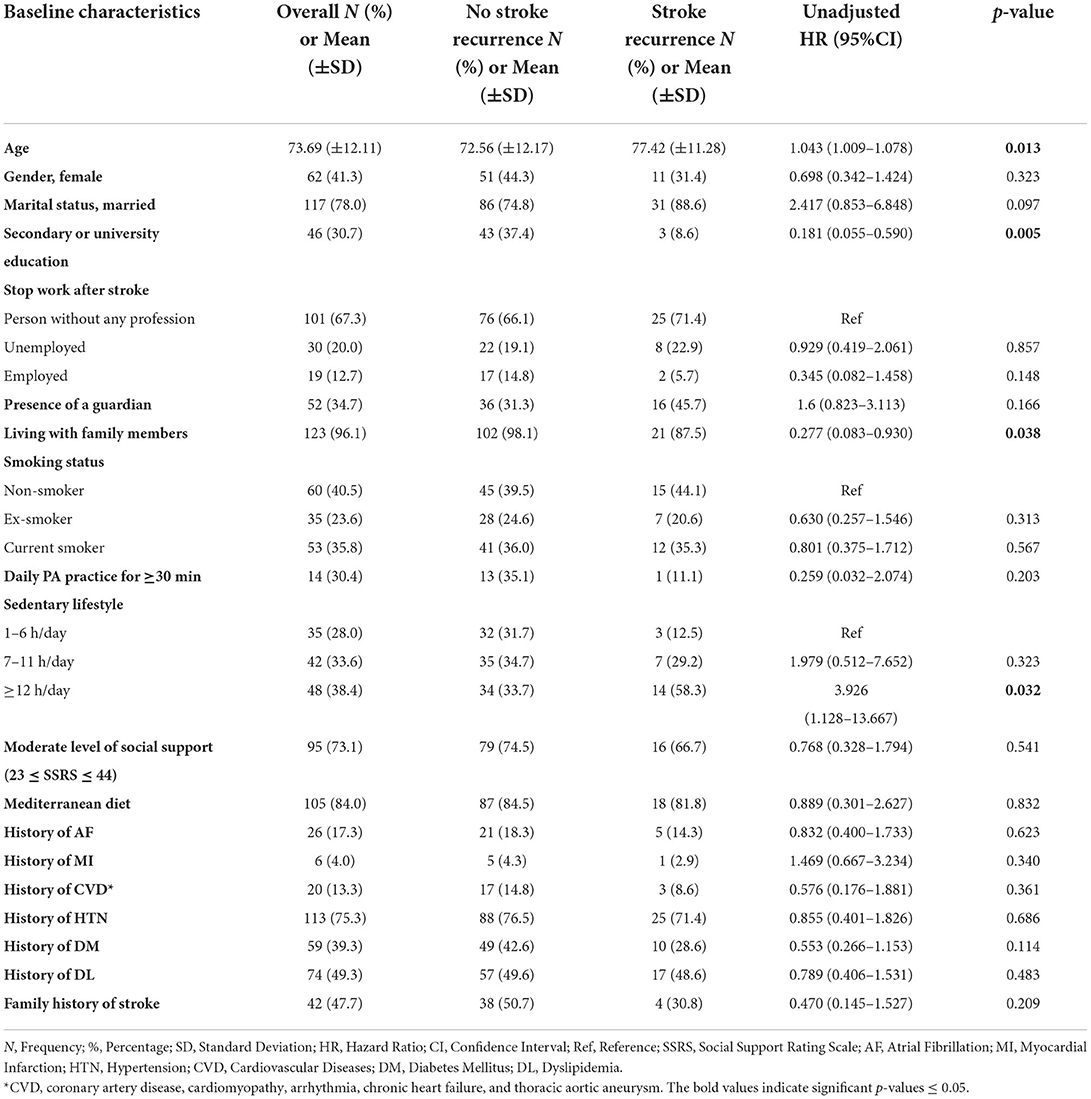
Table 3. The association of baseline characteristics with 1-year stroke recurrence using cox proportional hazard regression univariate analysis.
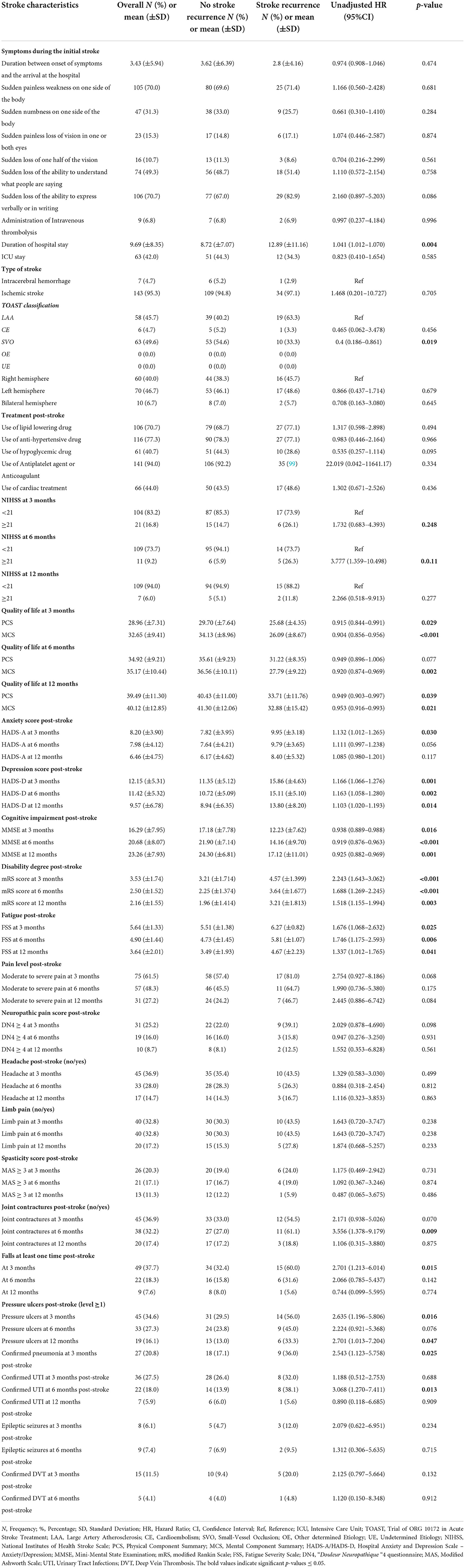
Table 4. The association of stroke in-hospital course and complications post-stroke with 1-year stroke recurrence using Cox Proportional Hazard regression univariate analysis.
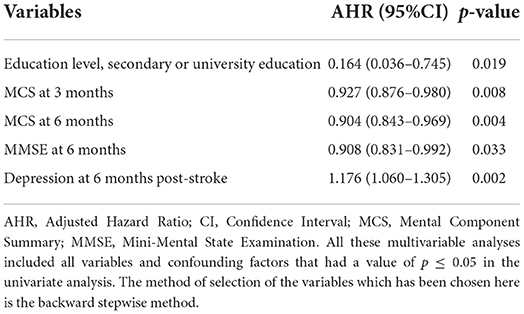
Table 5. Independent predictors of 1-year stroke recurrence using cox proportional hazard regression multivariable analysis.
The baseline factors associated positively with stroke recurrence were the older age with a mean of 77 ± 11 years [p = 0.016, UHR = 1.042, 95% CI (1.008–1.077)], and the sedentary duration of ≥12 h [p = 0.032, UHR = 3.926, 95% CI (1.128–13.667)]. Whereas, living with family members was negatively associated with stroke recurrence [p = 0.038, UHR = 0.277, 95% CI (0.083–0.930)], and a high educational level was the independent protective factor against stroke recurrence [p= 0.019, AHR = 0.164, 95% CI (0.036–0.745)].
Regarding hospital course, survivors with stroke recurrence had a longer duration of hospital stay than those without stroke recurrence (12.89 ± 11.16 days vs. 8.72 ± 7.07 days, respectively) [p = 0.004, UHR = 1.041, 95% CI (1.012–1.070)]. Moreover, subjects with SVO had 60% lower risk of stroke recurrence than those with LAA [p = 0.019, UHR = 0.4, 95% CI (0.186–0.861)].
Regarding post-stroke consequences, we studied the severity of the stroke, QoL, and functional, mental, neurological, and cognitive outcomes post-stroke. The stroke recurrence was positively associated with severe stroke (NIHSS ≥ 21) at 6-month post-stroke [p = 0.011, UHR = 3.777, 95% CI (1.359–10.498)]. Moreover, higher PCS and MCS scores of QoL at 3, 6, and 12 months post-stroke were inversely related to stroke recurrence; however, after adjusting for age and other explanatory factors, the mental dimensions and higher MCS scores at 3- and 6-month follow-up had strong independent opposite relations with stroke recurrence [p = 0.008, AHR = 0.927, 95% CI (0.876–0.980); p = 0.004, AHR = 0.904, 95% CI (0.843–0.969), respectively]. Similarly, elevated MMSE scores had a significant adjusted low risk of stroke recurrence [p = 0.033, AHR = 0.908, 95% CI (0.831–0.992)]. On the other hand, elevated HADS scores for anxiety and depression had a 1-fold increase of the stroke recurrence risk, especially, depression at 6 months post-stroke presented a significant adjusted higher risk [p = 0.002, AHR = 1.176, 95% CI (1.060–1.305)].
Furthermore, concerning the functional outcome and post-stroke complications, the high disability degree at 3-, 6-, and 12-months post-stroke predicted a 1-year stroke recurrence, with the largest risk in the acute phase at 3 months. Higher mRS at 3 months post-stroke increased two times the stroke recurrence risk [p < 0.001, UHR = 2.243, 95% CI (1.643–3.062); p < 0.001]. The following factors affecting the functional outcome were all found as risk factors for 1-year stroke recurrence: fatigue at 3-, 6-, and 12-month post-stroke [p = 0.025, UHR = 1.676, 95% CI (1.068–2.632); p = 0.006, UHR = 1.746, 95% CI = (1.175–2.593); p = 0.041, UHR = 1.337, 95% CI (1.012–1.765), respectively], joint contractures at 6-month post-stroke [p = 0.009, UHR = 3.556, 95% CI (1.378–9.179)], falls at least one time at 3-month post-stroke [p = 0.015, UHR = 2.701, 95% CI (1.213–6.014)], pressure ulcers (level ≥ 1) at 3- and 12-month post-stroke [p = 0.016, UHR = 2.635, 95% CI (1.196–5.806); p = 0.047, UHR = 2.701, 95% CI (1.013–7.204), respectively], confirmed pneumonia at 3-month post-stroke [p = 0.025, UHR = 2.543, 95% CI (1.123–5.758)], and confirmed urinary tract infections at 6-month post-stroke [p = 0.013, UHR = 3.068, 95% CI (1.270–7.411)].
One-year any-cause of death predictors
The univariate analysis is tabulated in Tables 6, 7. Table 8 summarizes the multivariable analysis.
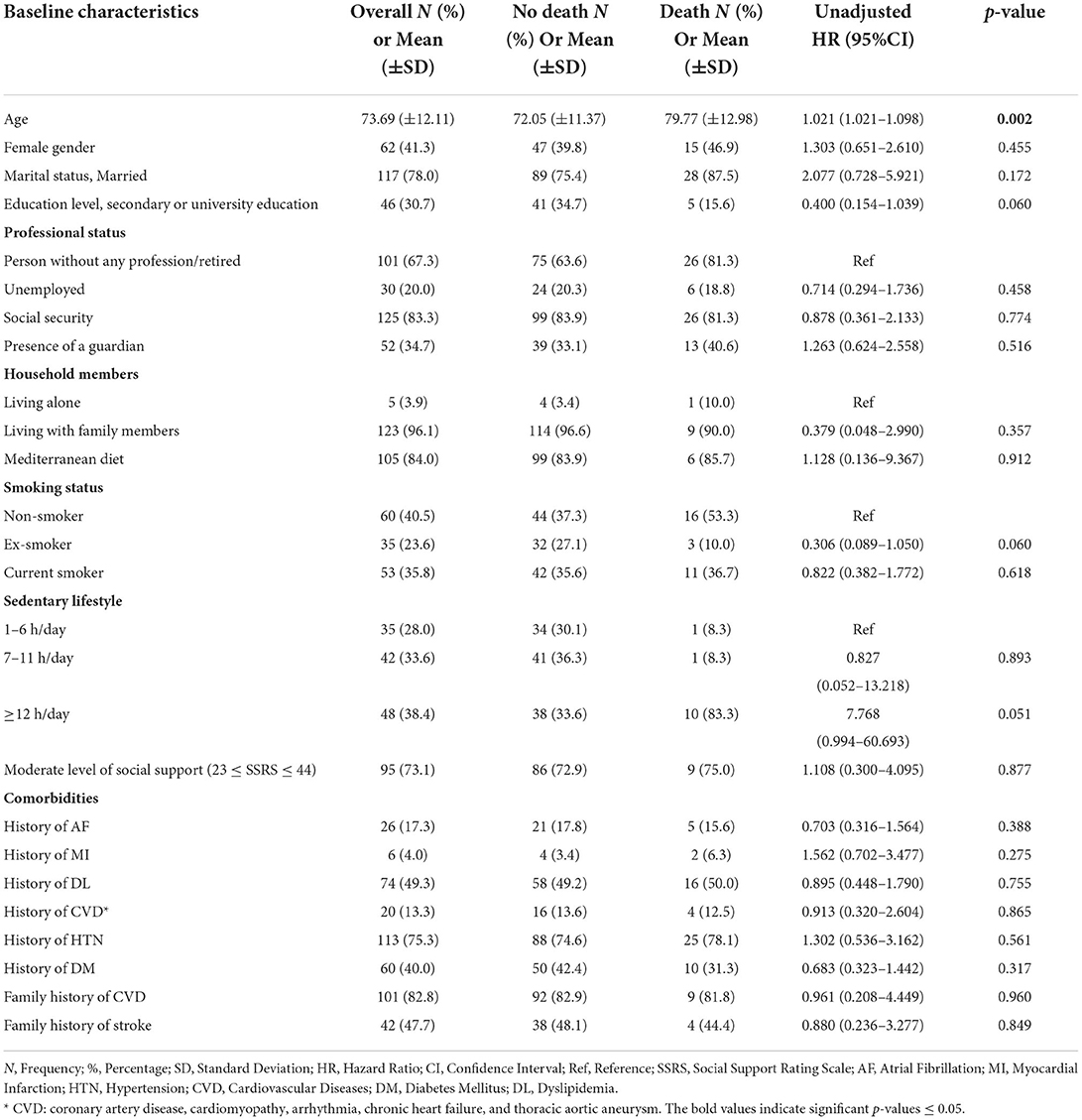
Table 6. The association of baseline characteristics with 1-year any-cause of death post-stroke using cox proportional hazard regression univariate analysis.
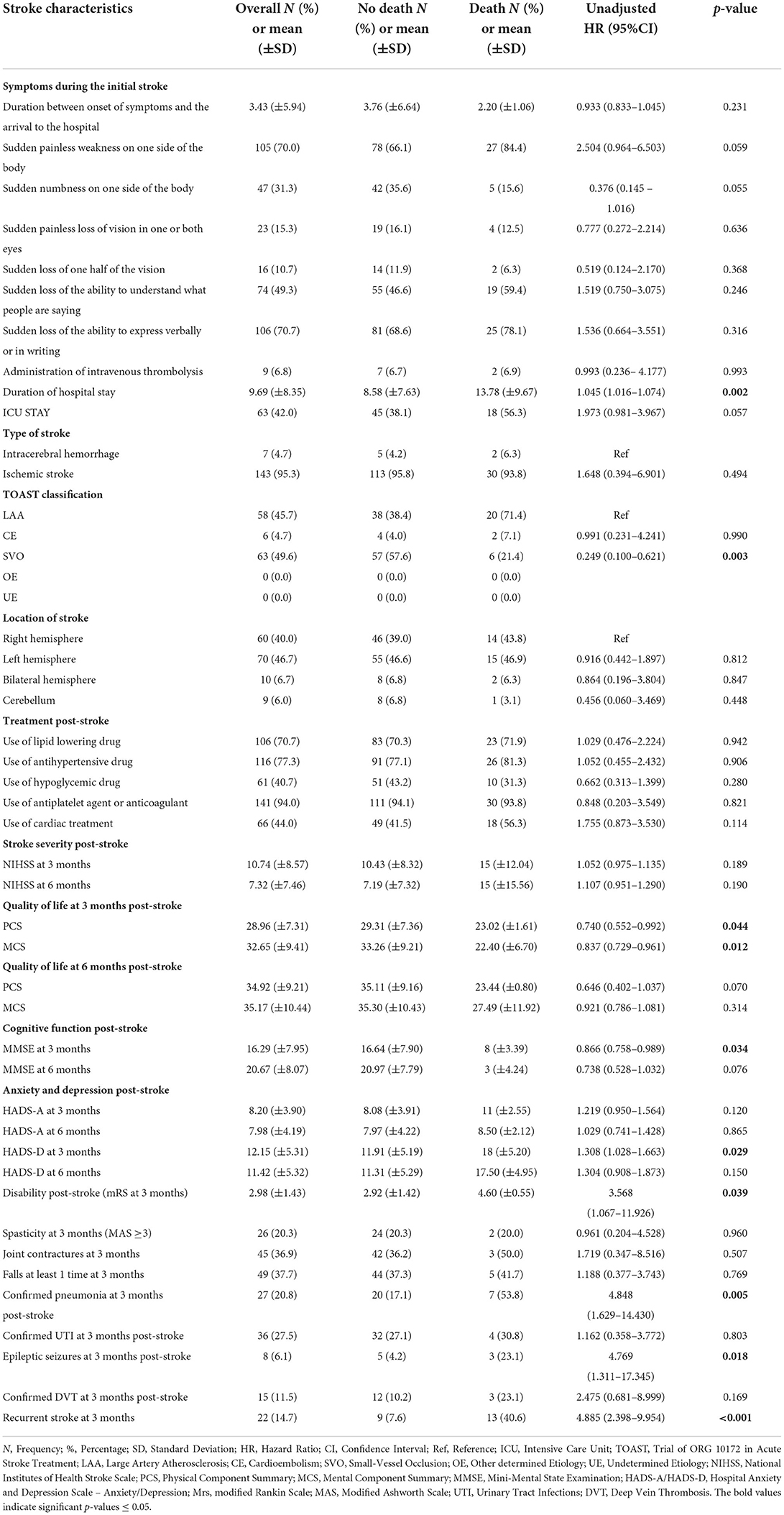
Table 7. The association of stroke in-hospital course and complications post-stroke with 1-year any-cause of death post-stroke using cox proportional hazard regression univariate analysis.
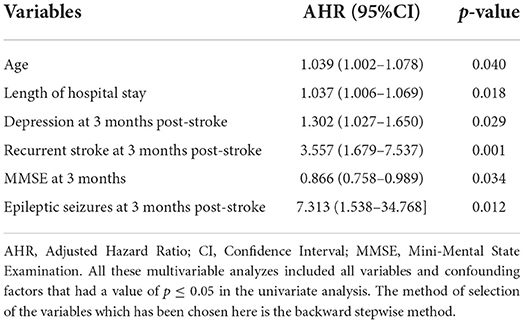
Table 8. Independent predictors of 1-year any-cause of death using cox proportional hazard regression multivariable analysis.
The death rate increased within the first year of stroke with the advanced age (mean of 80 ± 13 years) [p = 0.040, AHR = 1.039, 95% CI (1.002–1.078)]. Patients who died within the first year post-stroke had a longer duration of hospital stay at index stroke (mean of 13.78 ± 9.67) [p = 0.018, AHR = 1.037, 95% CI (1.006–1.069)], and 71.4% (20/28) were affected by LAA ischemic stroke vs. 21.4% with SVO [p = 0.003, UHR = 0.249, 95% CI (0.100–0.621)].
Regarding the post-stroke course, various factors were significant. Higher PCS and MCS of QoL scores were inversely associated with the 1-year mortality post-stroke [p = 0.044, UHR = 0.740, 95% CI (0.0552–0.992), p = 0.012, UHR = 0.837, 95% CI (0.729–0.961), respectively]. Furthermore, higher MMSE scores at 3 months post-stroke were negatively associated with the 1-year mortality [p = 0.034, AHR = 0.866, 95% CI = (0.758–0.989)], whereas elevated scores of mRS for disability and HADS for depression 3-month post-stroke were positively associated with mortality within the first year post-stroke. Death cumulative risk rate had a 3-fold increase among subjects with high disability than those without [p= 0.039, UHR = 3.568, 95% CI = (1.067–11.926)]. As for the depression that occurred 3 months post-stroke, a higher HADS score was independently associated with a higher risk of 1-year death post-stroke [p = 0.029, AHR = 1.302, 95% CI = (1.027–1.650)].
Subjects with confirmed pneumonia 3-month post-stroke significantly had an increased risk of death 1-year death post-stroke [p = 0.005, UHR = 4.848, 95% CI (1.629–14.430)].
Moreover, after adjusting for age and other explanatory factors, the risk of death in the first year following initial stroke was independently associated with epileptic seizures at 3-month post-stroke [p = 0.012, AHR = 7.313, 95% CI (1.538–34.768)] and with recurrent stroke at 3- month post-stroke [p = 0.001, AHR = 3.557, 95% CI (1.679–7.537)].
Discussion
The current study is the only hospital-based study in Lebanon that provides data on long-term stroke recurrence and death rates over 1 year of follow-up post-first-ever stroke and to identify the associated risk factors. High rates of stroke recurrence (25%) and death (21.3%) in the first year post-stroke were highlighted in our population. Older age was the main predictor of both these outcomes. Subjects with stroke recurrence and death were more likely to have a poor QoL with low scores of MCS and PCS, moderate to severe disability and motor deficit, and severe cognitive impairment, associated with high levels of anxiety and depression. Early recurrent stroke and epileptic seizures were the main independent predictors of 1-year mortality following a stroke.
The cumulative risk rate of stroke recurrence over 1-year of follow-up was 25%, exceeding the 10–20% rates reported in previous studies in different countries, including Japan (61, 62), China (63), Spain (64, 65), U.S. (66–68), U.K. (12), Turkey (69), and Iran (27). This can be explained first by inappropriate re-education and poor knowledge in patients regarding post-stroke healthy habits for survival and improving overall lifestyle to ensure a better QoL and functional outcome. A recent study by Khalil H. et al., 2020 conducted a community-based survey targeting Lebanese adults aged 50 years and above to assess their stroke-related knowledge and concluded that there is a lack of adequate stroke-related knowledge among Lebanese older people (3). Higher levels of education were a significant predictor of better knowledge (3, 70); Almost 69.3% (104/150) of our study population had a low level of education, of whom 32 had experienced a stroke recurrence (32/35, 91.4%). Second, genetic makeup may be a possible reason for high stroke recurrence. Stroke prevalence in Lebanon may be higher than in other developing countries in the region (34) and population aging in Lebanon is higher than in any other Arab country (71, 72). Third, for the background behind this higher recurrence rate, increased vascular risk factors such as HTN, AF, DM, and DL (32, 73, 74) were remarkable in this study but were not statistically significant. The highest rate of recurrence found in this study was in the early stage, which is relatively comparable with the reported rates by previous literature (27, 75–78). Age and stroke severity at the time of the index stroke are important determinants of stroke recurrence and are associated with early and long term prognosis (27).
Regarding mortality post-stroke, the cumulative risk rate of all-cause of mortality was 21.3% at 1-year of follow-up, which was similar to the results of the study by Abdo et al. in Lebanon in 2019 (79). Various studies worldwide have assessed the long-term post-stroke mortality rate. A cumulative risk rate of death of 40.8% 1-year post-stroke was reported in East Africa (80), with rates of 34.5% in Iran (81), 26.9% in Saudi Arabia (82), 15% in China (76), 28% in Brazil (78), 16% in the US (77), 22% to 29% in the UK (83), and 29.4% in Czech Republic (84). Compared with these rates from different countries, the mortality rate over 1-year post-stroke in Lebanon was less than the rate obtained in East Africa, Iran, Saudi Arabia, Brazil, the U.K., and the Czech Republic, but a little greater than those obtained in China and the U.S. Among Middle Eastern countries, Lebanon represents the lowest 1-year fatality rate following a stroke, which might be because of the difference in patient characteristics or the health-care system.
Most of the 32 deaths observed in the follow-up period were caused by cardiac or neurovascular complications. However, recurrence of stroke was responsible for 56% of these deaths in our study, 41% in the 3 months, 9% during the 3 to 6 months, and 6% at 12 months post first-ever stroke, where the possible reason is older age (72% were ≥80 years old). Similar to other results (79, 85–88), stroke recurrence increases the risk of death four times among stroke survivors.
Several factors are known to influence short- and long-term stroke recurrence and mortality.
Age was found as the main predictor of recurrence and death (64, 89). In our population, we found a significantly higher risk of 1-year stroke recurrence and 1-year mortality with advanced age. Elderly individuals were more likely to have a more severe stroke and increased comorbidities, especially HTN, which was found to be higher in those with stroke recurrence but this difference was not statistically significant and could be attributable to the low sample size.
Men were more exposed to stroke recurrence than women (68.6 vs. 31.4%, respectively); however, this difference was statistically significant neither for stroke recurrence nor for death post-stroke. Several studies from U.S., Europe, and China showed similar outcomes for the sexes (68, 90–92).
Similarly, there was no significant association between comorbidities, such as HTN, DM, AF, and dyslipidemia, and stroke recurrence and death within the first year post-stroke. This may be due to the fact that the majority of the patients were on the lipid-lowering and antithrombotic drugs after the stroke; hence, the non-modifiable risk factors were controlled. Saade et al. in 2021, conducted a study to evaluate the adherence to medication in secondary prevention post-stroke and found that 83% of stroke patients were adherent to their medications (35).
The risk of stroke recurrence in subjects with prolonged sitting hours (≥12 h) was four times higher than in those with shorter sitting hours, thus indicating that physical inactivity increases the risk of stroke relapse (93, 94). Most stroke survivors are engaged in physical inactivity and sedentary behavior, due to many barriers including depression, low motivation, poor to moderate social support, and physical impairment (95). The American Heart Association and the American Stroke Association recommend the following: at least 30 min of moderate-intensity physical exercise (i.e., gait, upper extremity function, balance, muscle strength, motor skills, efficiency in self-care, occupational, and leisure-time activities), sufficient to break a sweat or raise heart rate, one to three times a week (93, 95, 96).
Inversely, a higher educational level was a strong independent protective factor against stroke recurrence. Individuals with a higher level of education were 18% less likely to have a secondary stroke over 1 year after the initial stroke. Previous findings suggested that educational level is an important predictor of long-term prognosis of stroke (97, 98). This group of participants understands and has the knowledge of stroke outcome and recurrence risk factors, as well as secondary preventive habits including practicing physical activity, and adopting a healthy lifestyle after stroke i.e., decreased consumption of alcohol and salt and increased consumption of fruits and vegetables, compliance to medications, and relevant rehabilitation process (97, 99).
Living with family members was found to have a significant negative association with 1-year stroke recurrence, which was consistent with previous studies (98, 100). The important step in the continuum of care for stroke survivors is receiving care from family members while living at home (101, 102).
Interestingly, our study highlighted the significant relationships between ischemic stroke subtypes and the cumulative risk rates of stroke recurrence and death, which showed a predominance of LAA stroke subtype in patients with 1-year stroke recurrence and 1-year all-cause of death compared to SVO. We found that almost 63% of patients with stroke recurrence and 71% of deceased patients were affected by LAA at index stroke. One-year mortality and 1-year stroke recurrence were the lowest for SVO stroke. This major difference was also reported by Kolmos et al. in a newly published systematic review comprising 26 studies conducted between 1997 and 2019 worldwide with similar inclusion criteria (103). Pre-existing conditions, specifically vascular risk factors, including HTN, DL, DM, and AF, in LAA patients with stroke recurrence and death were higher than those with SVO stroke in our study and previous studies (103, 104). A study in Egypt showed that SVO was significantly higher among patients with late recurrence (1 year after stroke or more), while LAA was significantly higher among those with early recurrence (within 1 year) of stroke (6).
Although we did not find any statistical significance in the effectiveness of intravenous thrombolysis as a first line treatment in the reduction of stroke recurrence and mortality as per the stroke index in our study, a higher survival rate and a lesser stroke relapse within 1 year were observed in patients who were treated with intravenous thrombolysis. Only nine LAA patients received intravenous thrombolysis, of whom 7 patients aged between 45 and 78 years survived for 1-year post-stroke and were free of stroke recurrence. This finding shed the light on the efficacy of intravenous thrombolysis on post-stroke prognosis (105, 106). Previous studies suggested that the main barrier against receiving intravenous thrombolysis in Lebanon and other developing countries was delayed in-hospital presentation to recombinant tissue plasminogen activator administration (107, 108). A standardized stroke protocol is lacking in Lebanese hospitals and should be implemented (109).
The patients with stroke recurrence or mortality within 1 year post-stroke had prolonged hospital stay at stroke index (initial stroke occurrence) more than those without stroke recurrence or mortality, which was statistically significant in our study (110). Ween et al., in 2000, studied the impact of early recovery rates after stroke on the functional outcome prediction among stroke survivors and found that the length of hospital stay was significantly prolonged in patients with a poor outcome, thus helping us to estimate the stroke prognosis and guide them for efficient rehabilitation programs (111).
Stroke survivors' health-related QoL is one of the important outcomes of rehabilitation. Stroke has a major impact on the QoL of survivors even among those who have no or minimal post-stroke disability (112). Although the outcomes of most patients with minor symptoms, defined by a low NIHSS score, are favorable, the incidence of permanent stroke-related sequelae, recurrent stroke, or medical complications of stroke is still possible (113). Most stroke survivors perceive their QoL as low compared to their pre-stroke status (114). Several factors such as functional status, ADL, anxiety, depression, neurological and cognitive functions, and environmental and other personal factors have been reported to predict the QoL in stroke survivors, which can worsen the long-term prognosis (115–120). In low resource countries (121, 122), such as Lebanon (108, 123, 124), additional factors like health costs, employment status, and emotional disorders have been reported to influence the stroke survivors' QoL.
The present study findings showed low scores of PCS and MCS components of QoL in all subject; however, the scores were lower in survivors with stroke recurrence and those who died over 1 year of follow-up, especially in the early stage.
The MCS of the QoL (SF, MH, RE, and VT) was found as an independent determinant of stroke recurrence. Hence, percentages of anxiety and depression post-stroke, measured by HADS, were 51.2, 48.3, 36.5%, and 77.2, 74.2, and 56.5% at 3-, 6-, and 12-month post-stroke, respectively. A systematic review conducted by Rafsten et al., in 2018, revealed an overall pooled prevalence of post-stroke anxiety disorders of 29.3% during the first year (125). While the present study has shown a high level of post-stroke anxiety among Lebanese survivors compared to the rate in the aforementioned review.
On the other hand, reviews by Ayerbe et al. and Hacket et al., revealed a cumulative rate of post-stroke depression of 33% (126, 127). Furthermore, a systematic review conducted in the MENA region by Kaadan and Larson, included 34 studies with the lowest rates reported in Saudi Arabia (17%), and Iran (18%), whereas, higher rates are reported in Algeria (56.1%), Jordan (64%), and Morocco (73.2%) (128). Lebanese survivors showed the highest rate of post-stroke depression, which is close to the rate in Moroccan people. This could be due to the general poor QoL following stroke among the Lebanese population, lack of proper care, rehabilitation services, and additional training for healthcare professionals on the symptoms of depression. Another possible explanation could be the use of different methods of assessment (123). There is evidence of a strong relationship between the common psychological disorders post-stroke, anxiety and depression, and the stroke recurrence and death over 1 year following stroke (22, 129–131). Elevated HADS scores for anxiety and depression (80–90% with mean HADS scores ≥8) were seen in subjects with stroke recurrence and death.
Other studies have shown that cognitive impairment after stroke increases the risk of long-term stroke recurrence and shortens long-time survival, especially in the acute phase (90, 132, 133). Almost half (53.7%) of the Lebanese stroke survivors complained of severe cognitive impairment (MMSE ≤ 17) in the early stage post-stroke (3 months post-stroke), 28.3% at 6 months, and 18.8% at 12 months post-stroke. Higher MMSE scores were inversely associated with stroke recurrence and death. Furthermore, after adjusting for age and other explanatory factors, Higher MMSE score found to strong protective factor predictor for both outcomes.
Subjects with stroke recurrence were positively associated with an occurrence of a severe stroke 6 months post-stroke and with moderate to severe disability and high mRS scores (85 and 100% with mean mRS scores >3, respectively), which are consistent with previous study results (80, 134, 135). Subjects with motor deficits, such as fatigue (mean FSS scores > 4), joint contractures (61.1%), falls (60%) and pressure ulcers (33–56%) had a greater risk of stroke recurrence as the risk increased by two to three times in them. The control of motor movement in executing ADL is the main problem after stroke and is one of the factors contributing to a low survival' QoL (136). A Swedish study, conducted in 2014 among 35,000 stroke patients (81% with first-ever stroke), followed up at 3 and 12 months, found a 16% decline among survivors, from a level of independence in ADL to a level of dependence in ADL (137). On the contrary, despite the motor deficits mentioned previously that were mainly in the acute phase, our findings reported a slight improvement of the motor function and level of independence from 3 to 12 months of follow-up. These findings are in agreement with the findings of a review by Wondergem et al. conducted in 2017 that included 28 studies (138), and those of Langhorne et al. conducted in 2011 (139).
Pulmonary infections at 3 months post-stroke were positively associated with stroke recurrence and death over 1 year of follow-up. Almost one-third of subjects with stroke recurrence and half of the subjects who died post-stroke presented with early pulmonary infections. In addition, urinary tract infections at 6 months post-stroke were significantly higher in subjects with stroke recurrence (38.1%). It was found that the majority of these subjects (70–80%) had a severe stroke (NIHSS ≥ 21) and increased disability with high mRS scores (mRS ≥ 3), which were similar to the results of previous studies (110, 140). Stroke may affect the immunological status and level of independence of survivors; thus, severe stroke patients are prone to infections leading to post-stroke readmissions because of recurrent aspiration pneumonia and urinary catheterizations. This condition causes an increase in disability, immobility, and elevated inflammatory markers that contribute to atherogenesis and thrombosis, leading to long-term sequelae, recurrent stroke, and subsequent death (110, 140–145).
Finally, epileptic seizures at 3 months post-stroke (8/131, 6.1%) were reported in one-fifth of subjects who died (3/13, 23.1%) over 1 year of follow-up, including two subjects who died after 4–5 months and one subject at 11 months post-stroke. Stroke recurrence was the primary cause of death among the three subjects. When adjusting for age, stroke severity, and recurrence of stroke, epileptic seizures remained associated with mortality post-stroke. The risk of mortality in the first year post-initial stroke was 7-fold higher in subjects with seizures than those without seizures at 3 months post-stroke. Seizures were linked with severe cognitive impairment and with moderate to severe disability post-stroke (60%). The AHR in the current study is higher than the reported HR in previous studies (146–148). This could be explained by the smaller number of patients with epileptic seizures in this study which could have negatively affected the precision of results. Further large cohort studies are needed to confirm our findings.
Strengths and limitations
This study has several limitations. First, the small sample size recruited following the previous study considering a low prevalence of stroke in Lebanon of 3.9% according to other countries (39). Second, participating hospitals were limited to the regions of Beirut and Mount Lebanon, even though subjects came from all governorates, they were not representative of the overall population of Lebanon. Third, other recurrence correlates, such as carotid artery sclerosis, imaging findings, and medication adherence may need to be studied to provide more insight into the process of recurrence and death. Therefore, this study could function as a preliminary study for stroke recurrence and death post-stroke and their predictive factors among Lebanese survivors.
However, the prospective multicenter longitudinal study design that was conducted may have decreased recall and selection bias. In addition, we used standardized validated reliable international measuring instruments, and the study was performed by highly qualified and well-trained investigators face-to-face with the subjects, which may have lowered the degree of bias usually resulting from self-completed questionnaires. Furthermore, we used the Arabic-validated version of the measuring instruments, which could have prevented information bias. Nevertheless, future studies with larger sample sizes are required to confirm the current study results.
Conclusion
Stroke recurrence and death were commonly found in the first year post-stroke, with the largest rates recorded in the acute phase. The risk of stroke recurrence in Lebanon is higher compared to those in western and other eastern countries. A large number of the patients died or had recurrent events due to poor functional, neurological, cognitive, and mental prognosis. Lower cognitive scores, and greater neuropsychological, disability, and severity scales were positively associated with both these outcomes among the Lebanese population. Therefore, the primary public goal is to reduce stroke complications. Implementing effective therapies for secondary prevention is necessary in the acute phase (stroke unit management, thrombolytic, and other reperfusion therapies), as well as rehabilitation and long-term follow-up efforts are needed in order to cope with the burden of stroke in people who have developed or survived a stroke.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to CB, celinaboutros@gmail.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committees and directors of the participating hospitals (NEUR-2018-001, HDF-1152). Ethical clearance was obtained through a formal letter granted in line with the World Medical Association Declaration of Helsinki in 2013. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HH, PS, and CB contributed to the conception and design of the study. CB, WK, and MT organized the database. CB performed the statistical analysis and wrote the first draft and the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The authors declare that this study received funding from Association Robert Debré pour la Recherche Médicale (ARDRM). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We would like to acknowledge Professor Youssef Fares for his support and cooperation in facilitating the access and the communication with some participating hospitals. We would also thank the patients and caregivers whose contribution made this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.973200/full#supplementary-material
Abbreviations
HTN, Hypertension; DM, Diabetes Mellitus; AF, Atrial Fibrillation; MENA, Middle East and North Africa; STROBE, Strengthening the Reporting of Observational studies in Epidemiology; ICD-10, International Classification of Diseases-10; QVSFS, Questionnaire for Verifying Stroke-Free Status; NIHSS, National Institutes of Health Stroke Scale; ADL, Activities of Daily Living; mRS, modified Rankin Scale; Quality of life, QoL, SF12, Short form Health Survey 12; PF, physical functioning; RP, role limitations due to physical problems; BP, bodily pain; GH, general health; VT, vitality; SF, Social Functioning; RE, role limitations due to emotional problems; MH, Mental health; PCS, Physical Component Summary; MCS, Mental Component Summary; US, United States; SPSS, Statistical Package for the Social Sciences software; MMSE, Mini-Mental State Examination; HADS, Hospital Anxiety and Depression Scale; SSRS, Social Support Rating Scale; FSS, Fatigue Severity Scale; MAS, Modified Ashworth Scale; DN4, Douleur Neuropathique4; VAS, Visual Analogue Scale; SD, Standard Deviation; AHR, Adjusted Hazard Ratio; CI, confidence interval; DL, Dyslipidemia; MI, Myocardial infarction; CVD, cardiovascular diseases; UHR, Unadjusted Hazard Ratio; UK, United Kingdom.
References
1. Rahayu LP, Sudrajat DA, Nurdina G, Agustina EN, Putri TARK. The risk factor of recurrence stroke among stroke and transient ischemic attack patients in Indonesia. KnE Life Sci. (2019) 87–97. doi: 10.18502/kls.v4i13.5229
2. Bou Ali I, Farah R, Zeidan RK, Chahine MN, Al Sayed G, Asmar R, et al. Stroke symptoms impact on mental and physical health: a Lebanese population based study. Rev Neurol. (2021) 177:124–31. doi: 10.1016/j.neurol.2020.03.026
3. Khalil HM, Lahoud N. Knowledge of stroke warning signs, risk factors, and response to stroke among Lebanese older adults in Beirut. J Stroke Cerebrovasc Dis. (2020) 29:104716. doi: 10.1016/j.jstrokecerebrovasdis.2020.104716
4. Han J, Mao W, Ni J, Wu Y, Liu J, Bai L, et al. Rate and determinants of recurrence at 1 year and 5 years after stroke in a low-income population in Rural China. Front Neurol. (2020) 11:2. doi: 10.3389/fneur.2020.00002
5. WHO. Stroke, Cerebrovascular Accident (2021). Available online at: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed February 25, 2021).
6. Elnady HM, Mohammed GF, Elhewag HK, Mohamed MK, Borai A. Risk factors for early and late recurrent ischemic strokes. Egypt J Neuro Psychiatry Neurosurg. (2020) 56:56. doi: 10.1186/s41983-020-00190-3
7. Lin B, Zhang Z, Mei Y, Wang C, Xu H, Liu L, et al. Cumulative risk of stroke recurrence over the last 10 years: a systematic review and meta-analysis. Neurol Sci. (2021) 42:61–71. doi: 10.1007/s10072-020-04797-5
8. Zhuo Y, Wu J, Qu Y, Yu H, Huang X, Zee B, et al. Clinical risk factors associated with recurrence of ischemic stroke within two years: a cohort study. Medicine. (2020) 99:1–6. doi: 10.1097/MD.0000000000020830
9. Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CDA. Cause of stroke recurrence is multifactorial. Stroke. (2003) 34:1457–63. doi: 10.1161/01.STR.0000072985.24967.7F
10. Khanevski AN, Bjerkreim AT, Novotny V, Naess H, Thomassen L, Logallo N, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta Neurol Scand. (2019) 140:3–8. doi: 10.1111/ane.13093
11. Xia X, Yue W, Chao B, Li M, Cao L, Wang L, et al. Prevalence and risk factors of stroke in the elderly in Northern China: data from the National Stroke Screening Survey. J Neurol. (2019) 266:1449–58. doi: 10.1007/s00415-019-09281-5
12. Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. (2020) 51:2435–44. doi: 10.1161/STROKEAHA.120.028992
13. Chen CY, Weng WC, Wu CL, Huang WY. Association between gender and stoke recurrence in ischemic stroke patients with high-grade carotid artery stenosis. J Clin Neurosci. (2019) 67:62–7. doi: 10.1016/j.jocn.2019.06.021
14. Zonneveld TP, Richard E, Vergouwen MD, Nederkoorn PJ, de Haan R, Roos YB, et al. Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev. (2018) 7:Cd007858. doi: 10.1002/14651858.CD007858.pub2
15. Yagita Y. Cholesterol and inflammation in stroke recurrence. J Atheroscler Thromb. (2019) 26:406–7. doi: 10.5551/jat.ED107
16. Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. (2019) 10:780–92. doi: 10.1111/jdi.12932
17. Tanaka K, Koga M, Lee KJ, Kim BJ, Park EL, Lee J, et al. Atrial fibrillation-associated ischemic stroke patients with prior anticoagulation have higher risk for recurrent stroke. Stroke. (2020) 51:1150–7. doi: 10.1161/STROKEAHA.119.027275
18. Chin YY, Sakinah H, Aryati A, Hassan BM. Prevalence, risk factors and secondary prevention of stroke recurrence in eight countries from south, east and southeast asia: a scoping review. Med J Malaysia. (2018) 73:90–9.
19. Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, et al. Impact of smoking status on stroke recurrence. J Am Heart Assoc. (2019) 8:e011696. doi: 10.1161/JAHA.118.011696
20. Oza R, Rundell K, Garcellano M. Recurrent ischemic stroke: strategies for prevention. Am Fam Phys. (2017) 96:436–40.
21. Kumral E, Erdogan CE, Ari A, Bayam FE, Saruhan G. Association of obesity with recurrent stroke and cardiovascular events. Rev Neurol. (2021) 177:414–21. doi: 10.1016/j.neurol.2020.06.019
22. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. (2019) 50:102–9. doi: 10.1016/j.arr.2019.01.013
23. McCurley JL, Funes CJ, Zale EL, Lin A, Jacobo M, Jacobs JM, et al. Preventing chronic emotional distress in stroke survivors and their informal caregivers. Neurocrit Care. (2019) 30:581–9. doi: 10.1007/s12028-018-0641-6
24. Arsava EM, Kim GM, Oliveira-Filho J, Gungor L, Noh HJ, Lordelo Mde J, et al. Prediction of early recurrence after acute ischemic stroke. JAMA Neurol. (2016) 73:396–401. doi: 10.1001/jamaneurol.2015.4949
25. GBD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
26. El-Gohary TM, Alshenqiti AM, Ibrahim SR, Khaled OA, Ali ARH, Ahmed MS. Risk factors and types of recurrent stroke: a Saudi hospital based study. J Phys Ther Sci. (2019) 31:743–6. doi: 10.1589/jpts.31.743
27. Salehi M, Amiri A, Thrift AG, Kapral MK, Sposato L, Behrouz R, et al. Five-year recurrence rate and the predictors following stroke in the mashhad stroke incidence study: a population-based cohort study of stroke in the middle east. Neuroepidemiology. (2018) 50:18–22. doi: 10.1159/000485509
28. Amiri A, Kapral MK, Thrift AG, Sposato LA, Saber H, Behrouz R, et al. The incidence and characteristics of stroke in urban-dwelling Iranian women. J Stroke Cerebrovasc Dis. (2018) 27:547–54. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.050
29. Kumral E, Gulluoglu H, Alakbarova N, Karaman B, Deveci EE, Bayramov A, et al. Association of leukoaraiosis with stroke recurrence within 5 years after initial stroke. J Stroke Cerebrovasc Dis. (2015) 24:573–82. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.002
30. Deleu D, Hamad AA, Kamram S, El Siddig A, Al Hail H, Hamdy SMK. Ethnic variations in risk factor profile, pattern and recurrence of non-cardioembolic ischemic stroke. Arch Med Res. (2006) 37:655–62. doi: 10.1016/j.arcmed.2006.01.001
31. Pandian JD, Kalkonde Y, Sebastian IA, Felix C, Urimubenshi G, Bosch J. Stroke systems of care in low-income and middle-income countries: challenges and opportunities. Lancet. (2020) 396:1443–51. doi: 10.1016/S0140-6736(20)31374-X
32. Farah R, Zeidan RK, Chahine MN, Asmar R, Chahine R, Salameh P, et al. Prevalence of stroke symptoms among stroke-free residents: first national data from Lebanon. Int J Stroke. (2015) 10(Suppl. A100):83–8. doi: 10.1111/ijs.12563
33. Salameh P, Farah R, Hallit S, Zeidan RK, Chahine MN, Asmar R, et al. Self-reported history of stroke and long-term living conditions near air pollution sources: results of a national epidemiological study in Lebanon. Environ Monit Assess. (2018) 190:153. doi: 10.1007/s10661-018-6545-2
34. Lahoud N, Salameh P, Saleh N, Hosseini H. Prevalence of Lebanese stroke survivors: a comparative pilot study. J Epidemiol Glob Health. (2016) 6:169–76. doi: 10.1016/j.jegh.2015.10.001
35. Saade S, Kobeissy R, Sandakli S, Malaeb D, Lahoud N, Hallit S, et al. Medication adherence for secondary stroke prevention and its barriers among lebanese survivors: a cross-sectional study. Clini Epidemiol Global Health. (2021) 9:338–46. doi: 10.1016/j.cegh.2020.10.007
36. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
37. WHO. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
38. WHO. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010. (2010). Available from: https://icd.who.int/browse10/2010/en#!/G46.3 (accessed September 22, 2017).
39. Jurjus AR, Tohme RA, Ephrem G, Hussein IA, Jurjus R. Incidence and prevalence of circulatory diseases in Lebanon: a physician's inquiry. Ethn Dis. (2009) 19:1.
40. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
41. Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
42. Fitzpatrick AL, Ngo QV, Ly KA, Ton TG, Longstreth WT Jr, Vo TT, et al. Symptoms and risk factors for stroke in a community-based observational sample in Viet Nam. J Epidemiol Glob Health. (2012) 2:155–63. doi: 10.1016/j.jegh.2012.06.001
43. Alotaibi SST, Alzahrani AKJ, Al Nasserullah LZ, Nasser BAA, Mohammad N, Alhusayni LTA, et al. An overview on stroke diagnosis & management approach. Arch Pharm Pract. (2020) 11:61.
44. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. (1994) 25:2220–6. doi: 10.1161/01.STR.25.11.2220
45. Hussein HM, Abdel Moneim A, Emara T, Abd-Elhamid YA, Salem HH, Abd-Allah F, et al. Arabic cross cultural adaptation and validation of the National Institutes of Health Stroke Scale. J Neurol Sci. (2015) 357:152–6. doi: 10.1016/j.jns.2015.07.022
46. Lee SY, Kim DY, Sohn MK, Lee J, Lee S-G, Shin Y-I, et al. Determining the cut-off score for the Modified Barthel Index and the Modified Rankin Scale for assessment of functional independence and residual disability after stroke. PLoS ONE. (2020) 15:e0226324. doi: 10.1371/journal.pone.0226324
47. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34:220–33. doi: 10.1097/00005650-199603000-00003
48. Ware J, Kosinski M, Keller S. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA: The Health Institute, New England Medical Center (1998).
49. Al-Shehri AH, Taha AZ, Bahnassy AA, Salah M. Health-related quality of life in type 2 diabetic patients. Ann Saudi Med. (2008) 28:352–60. doi: 10.5144/0256-4947.2008.352
50. Haddad C, Sacre H, Obeid S, Salameh P, Hallit S. Validation of the Arabic version of the “12-item short-form health survey” (SF-12) in a sample of Lebanese adults. Arch Public Health. (2021) 79:56. doi: 10.1186/s13690-021-00579-3
51. Duan J, Lv Y-B, Gao X, Zhou J-H, Kraus VB, Zeng Y, et al. Association of cognitive impairment and elderly mortality: differences between two cohorts ascertained 6-years apart in China. BMC Geriatr. (2020) 20:29. doi: 10.1186/s12877-020-1424-4
52. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. (1992) 40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x
53. El-Hayeck R, Baddoura R, Wehbé A, Bassil N, Koussa S, Abou Khaled K, et al. An arabic version of the mini-mental state examination for the lebanese population: reliability, validity, and normative data. J Alzheimers Dis. (2019) 71:525–40. doi: 10.3233/JAD-181232
54. Christensen AV, Dixon JK, Juel K, Ekholm O, Rasmussen TB, Borregaard B, et al. Psychometric properties of the Danish Hospital Anxiety and Depression Scale in patients with cardiac disease: results from the DenHeart survey. Health Qual Life Outcomes. (2020) 18:9. doi: 10.1186/s12955-019-1264-0
55. Al Aseri ZA, Suriya MO, Hassan HA, Hasan M, Sheikh SA, Al Tamimi A, et al. Reliability and validity of the Hospital Anxiety and Depression Scale in an emergency department in Saudi Arabia: a cross-sectional observational study. BMC Emerg Med. (2015) 15:28. doi: 10.1186/s12873-015-0051-4
56. Xiao J, Huang B, Shen H, Liu X, Zhang J, Zhong Y, et al. Association between social support and health-related quality of life among Chinese seafarers: a cross-sectional study. PLoS ONE. (2017) 12:e0187275. doi: 10.1371/journal.pone.0187275
57. Rosti-Otajärvi E, Hämäläinen P, Wiksten A, Hakkarainen T, Ruutiainen J. Validity and reliability of the fatigue severity scale in finnish multiple sclerosis patients. Brain Behav. (2017) 7:e00743. doi: 10.1002/brb3.743
58. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206
59. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. (2005) 114:29–36. doi: 10.1016/j.pain.2004.12.010
60. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. (2008) 31:1601–7. doi: 10.1093/sleep/31.11.1601
61. Kono Y, Yamada S, Kamisaka K, Araki A, Fujioka Y, Yasui K, et al. Recurrence risk after noncardioembolic mild ischemic stroke in a Japanese population. Cerebrovasc Dis. (2011) 31:365–72. doi: 10.1159/000323233
62. Takashima N, Arima H, Kita Y, Fujii T. Two-year recurrence after first-ever stroke in a general population of 1.4 million Japanese Patients- The Shiga Stroke and Heart Attack Registry Study. Circ J. (2020) 84:943–8. doi: 10.1253/circj.CJ-20-0024
63. Wang G, Jing J, Pan Y, Meng X, Zhao X, Liu L, et al. Does all single infarction have lower risk of stroke recurrence than multiple infarctions in minor stroke? BMC Neurol. (2019) 19:7. doi: 10.1186/s12883-018-1215-0
64. Modrego PJ, Mainar R, Turull L. Recurrence and survival after first-ever stroke in the area of Bajo Aragon, Spain. A prospective cohort study. J Neurol Sci. (2004) 224:49–55. doi: 10.1016/j.jns.2004.06.002
65. Roquer J, Rodríguez-Campello A, Cuadrado-Godia E, Vivanco-Hidalgo RM, Jiménez-Conde J, Perich X, et al. Acute brain MRI–DWI patterns and stroke recurrence after mild-moderate stroke. J Neurol. (2010) 257:947–53. doi: 10.1007/s00415-009-5443-5
66. Sozener CB, Lisabeth LD, Shafie-Khorassani F, Kim S, Zahuranec DB, Brown DL, et al. Trends in stroke recurrence in mexican americans and non-hispanic whites. Stroke. (2020) 51:2428–34. doi: 10.1161/STROKEAHA.120.029376
67. Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, et al. Geographic variation in one-year recurrent ischemic stroke rates for elderly Medicare beneficiaries in the USA. Neuroepidemiology. (2010) 34:123–9. doi: 10.1159/000274804
68. Lambert C, Chaudhary D, Olulana O, Shahjouei S, Avula V, Li J, et al. Sex disparity in long-term stroke recurrence and mortality in a rural population in the United States. Ther Adv Neurol Disord. (2020) 13:1756286420971895. doi: 10.1177/1756286420971895
69. Kumral E, Evyapan D, Gökçay F, Karaman B, Orman M. Association of baseline dyslipidemia with stroke recurrence within five-years after ischemic stroke. Int J Stroke. (2014) 9(Suppl. A100):119–26. doi: 10.1111/ijs.12341
70. Soto-Cámara R, González-Bernal JJ, González-Santos J, Aguilar-Parra JM, Trigueros R, López-Liria R. Knowledge on Signs and Risk Factors in Stroke Patients. J Clin Med. (2020) 9:1–14. doi: 10.3390/jcm9082557
71. Abdulrahim S, Ajrouch KJ, Antonucci TC. Aging in Lebanon: challenges and opportunities. Gerontologist. (2015) 55:511–8. doi: 10.1093/geront/gnu095
73. Zablith N, Diaconu K, Naja F, El Koussa M, Loffreda G, Bou-Orm I, et al. Dynamics of non-communicable disease prevention, diagnosis and control in Lebanon, a fragile setting. Confl Health. (2021) 15:4. doi: 10.1186/s13031-020-00337-2
74. Mansour Z, Said R, Dbaibo H, Mrad P, Torossian L, Rady A, et al. Non-communicable diseases in Lebanon: results from World Health Organization STEPS survey 2017. Public Health. (2020) 187:120–6. doi: 10.1016/j.puhe.2020.08.014
75. Daneshfard B, Izadi S, Shariat A, Toudaji MA, Beyzavi Z, Niknam L. Epidemiology of stroke in Shiraz, Iran. Iran J Neurol. (2015) 14:158–63.
76. Chen Y, Wright N, Guo Y, Turnbull I, Kartsonaki C, Yang L, et al. Mortality and recurrent vascular events after first incident stroke: a 9-year community-based study of 0.5 million Chinese adults. Lancet Global Health. (2020) 8:e580–e90. doi: 10.1016/S2214-109X(20)30069-3
77. Hartmann A, Rundek T, Mast H, Paik MC, Boden–Albala B, Mohr JP, et al. Mortality and causes of death after first ischemic stroke. Neurology. (2001) 57:2000. doi: 10.1212/WNL.57.11.2000
78. Cabral NL, Muller M, Franco SC, Longo A, Moro C, Nagel V, et al. Three-year survival and recurrence after first-ever stroke: the Joinville stroke registry. BMC Neurol. (2015) 15:70. doi: 10.1186/s12883-015-0317-1
79. Abdo R, Abboud H, Salameh P, El Hajj T, Hosseini H. Mortality and predictors of death poststroke: data from a multicenter prospective cohort of Lebanese Stroke Patients. J Stroke Cerebrovasc Dis. (2019) 28:859–68. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.033
80. Tessua KK, Munseri P, Matuja SS. Outcomes within a year following first ever stroke in Tanzania. PLoS ONE. (2021) 16:e0246492. doi: 10.1371/journal.pone.0246492
81. Novbakht H, Shamshirgaran SM, Sarbakhsh P, Savadi-Oskouei D, Yazdchi MM, Ghorbani Z. Predictors of long-term mortality after first-ever stroke. J Educ Health Promot. (2020) 9:45. doi: 10.4103/jehp.jehp_8_19
82. Almekhlafi MA. Trends in one-year mortality for stroke in a tertiary academic center in Saudi Arabia: a 5-year retrospective analysis. Ann Saudi Med. (2016) 36:197–202. doi: 10.5144/0256-4947.2016.197
83. Gulliford MC, Charlton J, Rudd A, Wolfe CD, Toschke AM. Declining 1-year case-fatality of stroke and increasing coverage of vascular risk management: population-based cohort study. J Neurol Neurosurg Psychiatry. (2010) 81:416. doi: 10.1136/jnnp.2009.193136
84. Bryndziar T, Matyskova D, Sedova P, Belaskova S, Zvolsky M, Bednarik J, et al. Predictors of short- and long-term mortality in ischemic stroke: a community-based study in Brno, Czech Republic. Cerebrovasc Dis. (2021). doi: 10.1159/000519937
85. Lekoubou A, Nkoke C, Dzudie A, Kengne AP. Recurrent stroke and early mortality in an urban medical unit in cameroon. J Stroke Cerebrovasc Dis. (2017) 26:1689–94. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.031
86. Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. (2011) 42:1489–94. doi: 10.1161/STROKEAHA.110.602615
87. Wang T-A, Wu T-H, Pan S-L, Chen H-H, Chiu SY-H. Impacts of treatments on recurrence and 28-year survival of ischemic stroke patients. Sci Rep. (2021) 11:15258. doi: 10.1038/s41598-021-94757-6
88. Singh RJ, Chen S, Ganesh A, Hill MD. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke. (2018) 13:787–96. doi: 10.1177/1747493018798526
89. Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, Ois A, Rodríguez-Campello A, Cuadrado-Godia E, et al. Biological Age is a predictor of mortality in ischemic stroke. Sci Rep. (2018) 8:4148. doi: 10.1038/s41598-018-22579-0
90. Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke. Stroke. (2003) 34:122–6. doi: 10.1161/01.STR.0000047852.05842.3C
91. Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. (2003) 34:1114–9. doi: 10.1161/01.STR.0000068410.07397.D7
92. Yu C, An Z, Zhao W, Wang W, Gao C, Liu S, et al. Sex differences in stroke subtypes, severity, risk factors, and outcomes among elderly patients with acute ischemic stroke. Front Aging Neurosci. (2015) 7:174. doi: 10.3389/fnagi.2015.00174
93. Butler EN, Evenson KR. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Top Stroke Rehabil. (2014) 21:246–55. doi: 10.1310/tsr2103-246
94. Hou L, Li M, Wang J, Li Y, Zheng Q, Zhang L, et al. Association between physical exercise and stroke recurrence among first-ever ischemic stroke survivors. Sci Rep. (2021) 11:13372. doi: 10.1038/s41598-021-92736-5
95. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2532–53. doi: 10.1161/STR.0000000000000022
96. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke. (2011) 42:227–76. doi: 10.1161/STR.0b013e3181f7d043
97. Che B, Shen S, Zhu Z, Wang A, Xu T, Peng Y, et al. Education level and long-term mortality, recurrent stroke, and cardiovascular events in patients with ischemic stroke. J Am Heart Assoc. (2020) 9:e016671. doi: 10.1161/JAHA.120.016671
98. Pennlert J, Asplund K, Glader EL, Norrving B, Eriksson M. Socioeconomic status and the risk of stroke recurrence: persisting gaps observed in a Nationwide Swedish Study 2001 to 2012. Stroke. (2017) 48:1518–23. doi: 10.1161/STROKEAHA.116.015643
99. Hiraga A. Perception of recurrent stroke risk among stroke survivors. Neuroepidemiology. (2011) 37:88–9. doi: 10.1159/000330354
100. Tsivgoulis G, Safouris A, Kim D-E, Alexandrov AV. Recent advances in primary and secondary prevention of atherosclerotic stroke. J Stroke. (2018) 20:145–66. doi: 10.5853/jos.2018.00773
101. Kucukyazici B, Verter V, Nadeau L, Mayo NE. Improving post-stroke health outcomes: can facilitated care help? Health Policy. (2009) 93:180–7. doi: 10.1016/j.healthpol.2009.07.010
102. Intamas U, Rawiworrakul T, Amnatsatsue K, Nanthamongkolchai S, Palmer MH. Care of stroke survivors in community: a case study of rural Thai community. J Health Res. (2021) 35:77–87. doi: 10.1108/JHR-07-2019-0172
103. Kolmos M, Christoffersen L, Kruuse C. Recurrent ischemic stroke - a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2021) 30:835–43. doi: 10.1016/j.jstrokecerebrovasdis.2021.105935
104. Saber H, Thrift AG, Kapral MK, Shoamanesh A, Amiri A, Farzadfard MT, et al. Incidence, recurrence, and long-term survival of ischemic stroke subtypes: a population-based study in the Middle East. Int J Stroke. (2017) 12:835–43. doi: 10.1177/1747493016684843
105. Robinson T, Zaheer Z, Mistri AK. Thrombolysis in acute ischaemic stroke: an update. Ther Adv Chronic Dis. (2011) 2:119–31. doi: 10.1177/2040622310394032
106. Muruet W, Rudd A, Wolfe CDA, Douiri A. Long-term survival after intravenous thrombolysis for ischemic stroke: a propensity score-matched cohort with up to 10-year follow-up. Stroke. (2018) 49:607–13. doi: 10.1161/STROKEAHA.117.019889
107. El Sayed MJ, El Zahran T, Tamim H. Acute stroke care and thrombolytic therapy use in a tertiary care center in Lebanon. Emerg Med Int. (2014) 2014:438737. doi: 10.1155/2014/438737
108. Abdo R, Hosseini H, Salameh P, Abboud H. Acute ischemic stroke management in Lebanon: obstacles and solutions. Funct Neurol. (2019) 34:167–76.
109. Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's Target: Stroke initiative. Stroke. (2011) 42:2983–9. doi: 10.1161/STROKEAHA.111.621342
110. Abreu P, Magalhães R, Baptista D, Azevedo E, Silva MC, Correia M. Readmissions and mortality during the first year after stroke-data from a population-based incidence study. Front Neurol. (2020) 11:636. doi: 10.3389/fneur.2020.00636
111. Ween JE, Mernoff ST, Alexander MP. Recovery rates after stroke and their impact on outcome prediction. Neurorehabil Neural Repair. (2000) 14:229–35. doi: 10.1177/154596830001400309
112. Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. (2002) 33:1840–4. doi: 10.1161/01.STR.0000019289.15440.F2
113. Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With The Guidelines-Stroke. Stroke. (2011) 42:3110–5. doi: 10.1161/STROKEAHA.111.613208
114. Palmcrantz S, Widén Holmqvist L, Sommerfeld DK. Young individuals with stroke: a cross sectional study of long-term disability associated with self-rated global health. BMC Neurol. (2014) 14:20. doi: 10.1186/1471-2377-14-20
115. Kwon S, Park JH, Kim WS, Han K, Lee Y, Paik NJ. Health-related quality of life and related factors in stroke survivors: Data from Korea National Health and Nutrition Examination Survey (KNHANES) 2008 to 2014. PLoS ONE. (2018) 13:e0195713. doi: 10.1371/journal.pone.0195713
116. Abubakar SA, Isezuo SA. Health related quality of life of stroke survivors: experience of a stroke unit. Int J Biomed Sci. (2012) 8:183–7.
117. Zhu W, Jiang Y. Determinants of quality of life in patients with hemorrhagic stroke: a path analysis. Medicine. (2019) 98:e13928. doi: 10.1097/MD.0000000000013928
118. Chen CM, Tsai CC, Chung CY, Chen CL, Wu KP, Chen HC. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Outcomes. (2015) 13:118. doi: 10.1186/s12955-015-0314-5
119. Gurcay E, Bal A, Cakci A. Health-related quality of life in first-ever stroke patients. Ann Saudi Med. (2009) 29:36–40. doi: 10.4103/0256-4947.51814
120. Kim K, Kim YM, Kim EK. Correlation between the activities of daily living of stroke patients in a community setting and their quality of life. J Phys Ther Sci. (2014) 26:417–9. doi: 10.1589/jpts.26.417
121. Oni OD, Aina OF, Ojini FI, Olisah VO. Quality of life and associated factors among poststroke clinic attendees at a University Teaching Hospital in Nigeria. Niger Med J. (2016) 57:290–8. doi: 10.4103/0300-1652.190602
122. Khalid W, Rozi S, Ali TS, Azam I, Mullen MT, Illyas S, et al. Quality of life after stroke in Pakistan. BMC Neurol. (2016) 16:250. doi: 10.1186/s12883-016-0774-1
123. Khazaal W, Taliani M, Boutros C, Abou-Abbas L, Hosseini H, Salameh P, et al. Psychological complications at 3 months following stroke: prevalence and correlates among stroke survivors in Lebanon. Front Psychol. (2021) 12:663267. doi: 10.3389/fpsyg.2021.663267
124. Abdo RR, Abboud HM, Salameh PG, Jomaa NA, Rizk RG, Hosseini HH. Direct medical cost of hospitalization for acute stroke in Lebanon: a prospective incidence-based multicenter cost-of-illness study. Inquiry. (2018) 55:46958018792975. doi: 10.1177/0046958018792975
125. Rafsten L, Danielsson A, Sunnerhagen KS. Anxiety after stroke: a systematic review and meta-analysis. J Rehabil Med. (2018) 50:769–78. doi: 10.2340/16501977-2384
126. Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:14–21. doi: 10.1192/bjp.bp.111.107664
127. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. (2014) 9:1017–25. doi: 10.1111/ijs.12357
128. Kaadan MI, Larson MJ. Management of post-stroke depression in the Middle East and North Africa: too little is known. J Neurol Sci. (2017) 378:220–4. doi: 10.1016/j.jns.2017.05.026
129. Bartoli F, Di Brita C, Crocamo C, Clerici M, Carrà G. Early post-stroke depression and mortality: meta-analysis and meta-regression. Front Psychiatry. (2018) 9:530. doi: 10.3389/fpsyt.2018.00530
130. Zhang S, Xu M, Liu ZJ, Feng J, Ma Y. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. (2020) 10:125–38. doi: 10.5498/wjp.v10.i6.125
131. Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carrà G, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat. (2013) 2013:862978. doi: 10.1155/2013/862978
132. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. (2014) 2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05
133. Melkas S, Jokinen H, Hietanen M, Erkinjuntti T. Poststroke cognitive impairment and dementia: prevalence, diagnosis, and treatment. Degener Neurol Neuromuscul Dis. (2014) 4:21–7. doi: 10.2147/DNND.S37353
134. Mar J, Masjuan J, Oliva-Moreno J, Gonzalez-Rojas N, Becerra V, Casado MÁ, et al. Outcomes measured by mortality rates, quality of life and degree of autonomy in the first year in stroke units in Spain. Health Qual Life Outcomes. (2015) 13:36. doi: 10.1186/s12955-015-0230-8
135. Zhang J, Zhu P, Liu B, Yao Q, Yan K, Zheng Q, et al. Time to recurrence after first-ever ischaemic stroke within 3 years and its risk factors in Chinese population: a prospective cohort study. BMJ Open. (2019) 9:e032087. doi: 10.1136/bmjopen-2019-032087
136. Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. (2016) 10:442. doi: 10.3389/fnhum.2016.00442
137. Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. (2015) 46:389–94. doi: 10.1161/STROKEAHA.114.006538
138. Wondergem R, Pisters MF, Wouters EJ, Olthof N, de Bie RA, Visser-Meily JM, et al. The course of activities in daily living: who is at risk for decline after first ever stroke? Cerebrovasc Dis. (2017) 43:1–8. doi: 10.1159/000451034
139. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. (2011) 377:1693–702. doi: 10.1016/S0140-6736(11)60325-5
140. Bjerkreim AT, Thomassen L, Brøgger J, Waje-Andreassen U, Næss H. Causes and predictors for hospital readmission after ischemic stroke. J Stroke Cerebrovasc Dis. (2015) 24:2095–101. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.019
141. Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, et al. Stroke-induced immunodepression and post-stroke infections: Lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. (2009) 158:1184–93. doi: 10.1016/j.neuroscience.2008.07.044
142. Li SJ, Hu HQ, Wang XL, Cao BZ. Correlation between post-stroke pneumonia and outcome in patients with acute brain infarction. Zhonghua Yi Xue Za Zhi. (2016) 96:2796–801. doi: 10.3760/cma.j.issn.0376-2491.2016.35.007
143. Liu Z, Lin W, Lu Q, Wang J, Liu P, Lin X, et al. Risk factors affecting the 1-year outcomes of minor ischemic stroke: results from Xi'an stroke registry study of China. BMC Neurol. (2020) 20:379. doi: 10.1186/s12883-020-01954-3
144. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. (2010) 9:105–18. doi: 10.1016/S1474-4422(09)70266-2
145. Santos Samary C, Pelosi P, Leme Silva P, Rieken Macedo Rocco P. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Critical Care. (2016) 20:391. doi: 10.1186/s13054-016-1573-1
146. Reuck J. Seizures and epilepsy in stroke patients: an updated review. Int J Res Stud Med Health Sci. (2020) 5:39–45. doi: 10.22259/ijrsmhs.0512008
147. Burneo JG, Fang J, Saposnik G. Network ftIotRotCS. Impact of seizures on morbidity and mortality after stroke: a Canadian multi-centre cohort study. Eur J Neurol. (2010) 17:52–8. doi: 10.1111/j.1468-1331.2009.02739.x
Keywords: stroke, recurrence, death, cumulative risk rate, Lebanon, survivors, factors, burden
Citation: Boutros CF, Khazaal W, Taliani M, Said Sadier N, Salameh P and Hosseini H (2022) One-year recurrence of stroke and death in Lebanese survivors of first-ever stroke: Time-to-Event analysis. Front. Neurol. 13:973200. doi: 10.3389/fneur.2022.973200
Received: 19 June 2022; Accepted: 14 October 2022;
Published: 14 November 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Raffaele Ornello, University of L'Aquila, ItalyParneet Grewal, Medical University of South Carolina, United States
Copyright © 2022 Boutros, Khazaal, Taliani, Said Sadier, Salameh and Hosseini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celina F. Boutros, celinaboutros@gmail.com; celina.boutros@u-pec.fr
†These authors have contributed equally to this work
‡ORCID: Celina F. Boutros orcid.org/0000-0001-7789-8478
 Celina F. Boutros
Celina F. Boutros Walaa Khazaal
Walaa Khazaal Maram Taliani
Maram Taliani Najwane Said Sadier
Najwane Said Sadier Pascale Salameh
Pascale Salameh Hassan Hosseini1,4,7
Hassan Hosseini1,4,7