- 1Department of Neurosurgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 2School of Clinical Medicine, Guizhou Medical University, Guiyang, China
Stroke is a leading cause of death and long-term disability worldwide. Tissue plasminogen activator (tPA) is an effective treatment for ischemic stroke. However, only a small part of patients could benefit from it. Therefore, finding a new treatment is necessary. Bone marrow mesenchymal stromal cells (BMSCs) provide a novel strategy for stroke patients. Now, many patients take stem cells to treat stroke. However, the researches of the precise inflammatory mechanism of cell replacement treatment are still rare. In this review, we summarize the immune response of BMSCs treated to stroke and may provide a new perspective for stem cell therapy.
Introduction
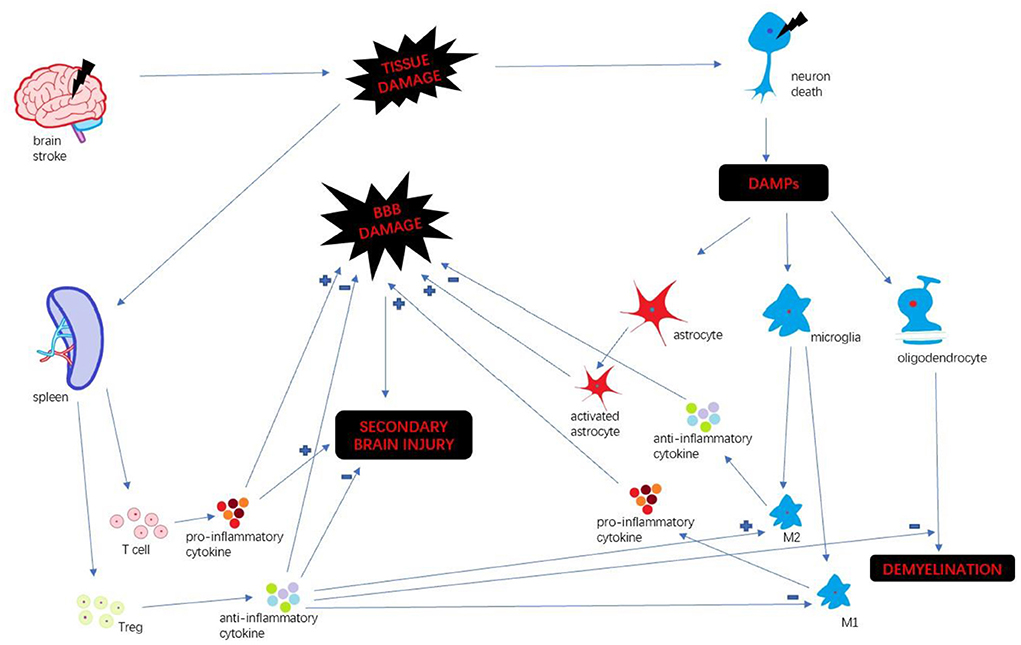
Stroke is a leading cause of death and long-term disability worldwide (1). Every year ~15 million people suffer from stroke in the world (2). Immune response plays a key factor in stroke progression. Neuroinflammation is an inflammatory response within the central nervous system (CNS), involving many different mediators such as cytokines, chemokines, reactive oxygen species and secondary messengers (3). Oxygen and glucose deprivation following brain tissue damage results in necrosis of neurons and released the different damage-associated molecular patterns (DAMP) which trigger neuroinflammation (4). DAMP include a wide variety of endogenous molecules released on tissue injury, which alter the blood-brain barrier (BBB) permeability, promote peripheral immune cell infiltration, and accelerate tissue edema and brain injury (5). Then microglia are activated and polarize M1 and M2 phenotypes. M1 microglia upregulate a variety of pro-inflammatory mediators which continually damage BBB integrity (6). In the periphery, spleen plays a pivotal role in humoral immunity. Following compromised BBB, spleen releases a mass of peripheral immune cells and inflammatory cytokines infiltrating brain insult. Those different pathways collectively exacerbate the secondary progression of ischemic brain injury (7). We summarize the inflammatory mechanism after stroke (Figure 1).
By now tissue plasminogen activator (tPA) is a proven treatment for acute ischemic stroke (8). However, the use of tPA is restricted by the narrow time window of 4.5 h after ischemic stroke onset, which has limited its use to only a small minority of patients (9). Thrombectomy also is an available approval therapy, which has restricted therapeutic outcome (10). Hence finding a novel effective treatment that could ameliorate the secondary progression of ischemic stroke injury will benefit stroke patients who cannot use tPA (11).
Bone marrow mesenchymal stromal cells (BMSCs) offer an innovative strategy. Stem cell is a kind of special cell which could self-renew, proliferate, and differentiate into specialized cells for cell replacement treatment to stroke (12). Many researches showed that transplanted BMSCs home to sites of injury, which may depend on chemotactic signals (13). Zheng et al. observed that intravenously delivered BMSCs are entrapped in lung microvasculature and are cleared to the liver in 1 day (14). Other researches demonstrated that injected BMSCs preferentially migrate to spleen after stroke (11). Cells through intracerebral transplantation could directly migrate into the infract brain tissue, however, it is more invasive (15). BMSCs take effects through different pathways after stroke, including migrating into ischemic infarction (11), proliferating neuroblasts, replacing impaired cells (16), promoting angiogenesis and neurogenesis (17) and secret a great bunch of neurotrophins. However, BMSCs also cause thrombus and increased intracranial hypertension (15). From many recent researches, except the effects mentioned above, BMSCs could mediate neuroinflammation to accelerate neurofunctional recovery. Therefore, the present review teases out the immunomodulatory effects of BMSCs transplantation after stroke.
BMSC and central nervous system
With the release of DAMPs following stroke, the microglia become activated, polarizing M1 and M2 phenotypes (18). M1 microglia secrete pro-inflammatory mediators, such as IL-1, IL-6, IL-12, TNF-α, and aggravate brain damage. In contrast, M2 microglia secrete anti-inflammatory cytokines, such as TGF-β and IL-10, accelerating neural repair (19). Stromal derived factor-1 (SDF-1) is mainly produced in microglia/macrophage in a rat middle cerebral artery occlusion (MCAO) model. Shiota et al. found a mesenchymal stem cell (MSC) line (B10) transplantation increased SDF-1 mRNA level from an early time point that persisted until 14 days after MCAO (20). Some researchers found that transplanted BMSCs reduced microglia activation, conferring immunomodulatory effect (21, 22). A study by Nijboer et al. indicated that the number of M2-like (CD206+) microglia was highly increased through intranasal MSC administration (23). In another article, Yang et al. confirmed those findings that BMSCs transplantation promoted M2 phenotype polarization, and decreased the expression of M1 maker in vivo and in vitro (24). Those researches suggest that BMSCs transplantation could impact M2 polarization meditating inflammatory response.
Astrocytes maintain structure for neurons and contribute to keeping homeostasis of the extracellular environment (25). Also, activated astrocytes play a key participant in neuroinflammation by secreting a large number of inflammatory mediators. The activation of astrocytes could result in dense glial scars, exacerbating neurological deterioration and affecting long-term neuronal recovery (26). Shiota et al. also found B10 transplantation increased the differentiation of neuronal progenitor cells to astrocytes (20). A group of researchers found that BMSCs co-culture enhanced the resistance of astrocytes to hemin neurotoxicity. And they found that BMSCs transplantation promotes astrocytes vimentin expression, and enhance astrocytes antioxidation (26). Zhang et al. co-cultured BMSCs with neurons and astrocytes which exposed to oxygen-glucose deprivation, and found that BMSCs exerted neuroprotection through hindering the apoptosis of neurons and astrocytes (27). Those evidences showed that BMSCs diminished the apoptosis of astrocytes and enhanced its neuroprotection.
Oligodendrocyte precursor cells (OPCs) are immature forms of oligodendrocytes which are essential for repair of damaged white matter after ischemic injury (28). After brain ischemia, immature oligodendrocytes proliferate in the peri-infract areas. Then newly created oligodendrocytes establish contact with un-myelinated axons and form functional myelin sheaths around them (29). BMSCs could reduce the expression of IL-1β protein that could impede the recruitment of OPCs (30). It's reported that BMSCs treatment increased oligodendrogenesis after MCAO, and elevated the number of Nissl-stained neurons in the cortex. Hence, researchers indicated that BMSCs transplantation protects the myelin sheath and promotes axonal restoration (31). In the study by Zarriello et al., OPCs co-cultured with BMSCs increased myelination compared to control group (32). There are some reports that M2 phenotype microglia promoted OPCs differentiation (33). It suggested that BMSCs facilitated OPCs differentiation through promoting M2 phenotype polarization and improved myelination.
BMSC and peripheral immune system
Spleen is a critical organ in peripheral immune system. After brain damage, spleen could release immune cells and pro-inflammatory mediators which permeate BBB and exacerbate the secondary injuries of cerebral tissue (34). Chiu et al. found that spleen volume decreased over 48 h, then progressively increased following stroke (35). In the research studied by Yang et al., MCAO model rats received human multipotent adult progenitor cells derived from bone marrow. They found that the grafts restored spleen mass reduction (36). Acosta et al. showed that intravenous BMSCs transplantation preferentially migrated to spleen and mitigated inflammation after chronic stroke (11). Our previous study first demonstrated that intracerebral human BMSCs migrated from brain to spleen via lymphatic vessels, led by inflammatory signals (37). Those suggested that BMSCs perhaps exert an important role in peripheral immune response via spleen.
Following ischemic brain injury, T lymphocytes are activated, infiltrating into damaged brain tissue, and accumulating in the necrotic core (38). Then T cells release many pro-inflammatory cytokines, such as IL-1, IL-6, etc., which induce secondary injuries in the CNS (39). Some researchers demonstrated that T cells also had a detrimental effect on early stroke evolution (40). Oppositely, regulatory T cells (Treg), a special subset of T cells, exert a protective function in neural repair. Much evidence showed that Treg protected compromised BBB (41), intensified white matter repair (42) and promoted M2 microglia polarization to diminish neuroinflammation after stroke (43). Some investigators found that BMSCs with the population of Tregs conferred maximal neuroprotection. In their study, as the immune mediator, the existence of a minority Tregs population within the therapeutic BMSCs population exerted the immunomodulatory and neuroprotective function provided by BMSCs transplantation (44). In another article, Zarriello et al. reported that the native Treg population presented about 0.4% percent of BMSCs, which influenced macrophage polarization toward the more regenerative M2 phenotype. And they cultured oligodendrocyte progenitor cells (OPCs) with BMSCs containing their native Tregs. The result showed that Tregs conferred increased myelination by increasing myelin production (32). The exact molecular mechanisms of how BMSCs influence on Treg is still needed to be further studied.
BMSC and immunomodulatory molecules
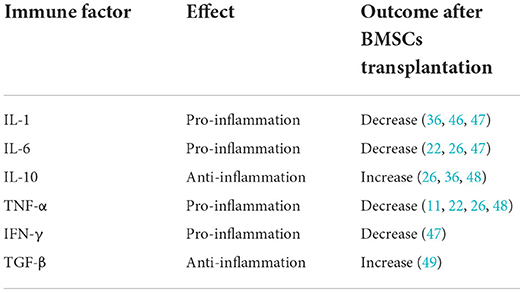
Both central neural cells and peripheral immune cells secret immune factors which play critical roles in central and peripheral system. Immune factors activate inflammatory cascades following cerebral damage (45). Cells transplantation changes the expression of inflammatory cytokines. Few articles systematically summarized the variations of immune factors after BMSCs therapy. Therefore, we reviewed the relevant literature for a summary (Table 1).
Interleukin-1 (IL-1) is a typical pro-inflammatory cytokine, first identified as the endogenous pyrogen. The main IL-1 family are IL-1α and IL-1β, which show high sequence homology despite being products of different genes. When brain injury occurred, the up-regulation of IL-1 level were observed (50). Many evidences showed that high levels of IL-1 exacerbated post-stroke damage, though mechanisms involved still unclear (51). Interleukin-6 (IL-6) is identified as a B-cell differentiation factor. IL-6 was observed to be significantly upregulated in Muridae and human patients after stroke (52). Tumor necrosis factor-α (TNF-α) is another important pro-inflammatory cytokine in neuroinflammation. The levels of TNF-α were improved in the damaged brain tissue after an ischemic insult. After brain damage, TNF-α penetrate impaired BBB (53). A number of articles reported the detrimental effects of TNF-α on both glia and neuronal functioning during ischemic stroke (54). As mentioned above, spleen plays a crucial role in neuroinflammation. Interferon gamma (IFN-γ) is associated with the splenic response, which enhances neural injury following middle cerebral artery occlusion (55). The evidences mentioned above unraveled that pro-inflammatory cytokines could lead further cerebral damage.
Salehi et al. found that BMSCs transplanted in rat middle cerebral artery occlusion, resulting down-regulation of IL-1 (46). Huang et al. demonstrated that treated intracerebral hemorrhage rats with BMSCs showed significantly abated expression of IL-1α, IL-6 and IFN-γ (47). In the study by Acosta et al., human BMSCs therapy to MCAO rats reduce TNF-α density (11). Tobin et al. also reported that microglia co-cultured with BMSCs reduced the secretion of IL-6, TNF-α (22). These reports suggested that BMSCs could alleviate inflammation via decreasing pro-inflammatory cytokine, such as IL-1, IL-6, TNF-α and IFN-γ.
In contrast, interleukin-10 (IL-10) is a key anti-inflammatory cytokine following ischemic stroke. In vitro and in vivo models of ischemic stroke showed the neuroprotection of IL-10. Expression of IL-10 in the cerebrum boost neuronal and glial cell survival and dampen of inflammatory responses though a range of signaling pathways (56). Current evidence demonstrated that IL-10 is increased in the brain after stroke (57). Transforming growth factor-β (TGF-β) is another classic anti-inflammatory mediator in brain injury. After stroke, TGF-β was observed in the ischemic brain lesions (58). Many evidence showed that TGF-β mediated microglial phenotype and facilitate neural repair after stroke (59). The finding by Islam et al. demonstrated that TGF-β in ischemic brain exerted sustained anti-inflammatory effects (60). Accordingly, anti-inflammatory cytokines could alleviate inflammatory reaction in the brain.
Liu et al. elucidated that BMSCs treated to MCAO rats increased the expression of IL-10 (48) and Yang et al. confirmed those results (36). In the article by Nakajima et al., BMSCs overexpressing IL-10 exert neuroprotection in acute ischemic stroke (61). Moisan et al. indicated the overexpression of TGF-β in human BMSCs treat MCAO rats (49). These articles supported that BMSCs therapy could ameliorate neuroinflammation though modulating anti-inflammatory cytokine, like IL-10 and TGF-β.
Except mediating immune factors, BMSCs promote angiogenesis and neurogenesis to reduce inflammation by secreting a multitude of growth factors or neurotrophins such as brain-derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) (62, 63). Many evidences showed that growth factors have the potential of immunomodulation (64–66). It was reported that HGF therapy could inhibit the disruption of BBB and exert anti-apoptotic and anti-inflammatory effects after cerebral ischemia (67). BDNF signals involved in regulating the production of inflammatory cytokines and oxidative stress (68). IGF-1 could facilitate anti-inflammatory phenotypes on both microglia and astrocytes (69) and decrease the inflammatory cascade (70). And VEGF binds to its receptor to activate downstream signals involved in endothelial activation and vascular inflammation (71). Some researcher found that BMSCs therapy could increase the expression of VEGF and HGF in MCAO model (72, 73). Similarly, Cho et al. observed that the proportions of VEGF-positive cells were higher in the therapy group (74). An article form Li et al. unraveled that concentrations of BDNF and IGF-1, which were mainly derived from transplanted BMSCs, were markedly higher than control group (75). Kim et al. observed similar results (76). Those researches indicated that BMSCs may regulate neuroinflammation through growth factor pathways. However, the exact mechanism still needs to be further investigated.
Conclusions
To date, growing proof shows the potential for cell replacement therapies to treat stroke. But still many difficulties must be overcome. The precise molecular mechanism of BMSCs treated to stroke is still elusive, which needs to be further studied. Even so, the current studies reported that BMSCs conduct neuroprotective effects after stroke and many patients benefit from it. Immune system is a crucial part to repair the injury. These wide variety of inflammatory pathways may provide new therapeutic targets, thereby giving stroke patients another chance.
Author contributions
ZW and XW were responsible for drafting of the initial manuscript. YL and GC contributed to the initial draft. KX was the supervisor. All authors contributed to the critical revision of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81901173 and 82060231); Science and Technology Foundation of Guizhou Provincial Health Commission (gzwjkj2021-205).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; BMSCs, bone marrow mesenchymal stromal cells; CNS, central nervous system; DAMP, damage-associated molecular patterns; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IFN-γ, interferon gamma; IGF-1, insulin-like growth factor-1; IL-1, interleukin-1; IL-6, interleukin-6; IL-10, interleukin-10; MCAO, middle cerebral artery occlusion; MSC, mesenchymal stem cell; OPCs, oligodendrocyte precursor cells; SDF-1, stromal derived factor-1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; tPA, tissue plasminogen activator; Treg, regulatory T cells; VEGF, vascular endothelial growth factor.
References
1. Soufiany I, Hijrat KA, Soufiany S, Chen L. Bypass surgery for ischemic stroke caused by intracranial artery stenosis or occlusion. Brain Sci Adv. (2018) 4:49–60. doi: 10.26599/BSA.2018.9050003
2. Incontri Abraham D, Gonzales M, Ibarra A, Borlongan CV. Stand alone or join forces? Stem cell therapy for stroke. Expert Opin Biol Ther. (2019) 19:25–33. doi: 10.1080/14712598.2019.1551872
3. Levard D, Buendia I, Lanquetin A, Glavan M, Vivien D, Rubio M. Filling the gaps on stroke research: focus on inflammation and immunity. Brain Behav Immun. (2021) 91:649–67. doi: 10.1016/j.bbi.2020.09.025
4. Chaudhry SR, Hafez A, Rezai Jahromi B, Kinfe TM, Lamprecht A, Niemela M, et al. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int J Mol Sci. (2018) 19:19072035. doi: 10.3390/ijms19072035
5. Stanzione R, Forte M, Cotugno M, Bianchi F, Marchitti S, Rubattu S. Role of DAMPs and of leukocytes infiltration in ischemic stroke: insights from animal models and translation to the human disease. Cell Mol Neurobiol. (2020). doi: 10.1007/s10571-020-00966-4
6. Peng Y, Chen F, Li S, Liu X, Wang C, Yu C, et al. Tumor-associated macrophages as treatment targets in glioma. Brain Sci Adv. (2020) 6:306–23. doi: 10.26599/BSA.2020.9050015
7. Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. (2021) 335:113518. doi: 10.1016/j.expneurol.2020.113518
8. Bulmer T, Volders D, Kamal N. Analysis of thrombolysis process for acute ischemic stroke in urban and rural hospitals in Nova Scotia Canada. Front Neurol. (2021) 12:645228. doi: 10.3389/fneur.2021.645228
9. Zhu Z, Kalyan BS, Chen L. Therapeutic potential role of exosomes for ischemic stroke. Brain Sci Adv. (2019) 5:128–43. doi: 10.1177/2096595820902588
10. Chavda V, Madhwani K, Chaurasia B. Stroke and Immunotherapy: Potential Mechanisms and its implications as immune-therapeutics. Eur J Neurosci. (2021) 54:4338–57. doi: 10.1111/ejn.15224
11. Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. (2015) 46:2616–27. doi: 10.1161/STROKEAHA.115.009854
12. Zhu H, Tan Y, Gu Q, Han W, Li Z, Meyer JS, et al. Regulations in the United States for cell transplantation clinical trials in neurological diseases. Transl Neurosci Clin. (2015) 1:114–24. doi: 10.18679/CN11-6030_R.2015.015
13. Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. (2014) 5:148. doi: 10.3389/fimmu.2014.00148
14. Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T, et al. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics. (2016) 6:291–301. doi: 10.7150/thno.13728
15. Zhang HL, Xie XF, Xiong YQ, Liu SM, Hu GZ, Cao WF, et al. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. (2018) 1680:143–54. doi: 10.1016/j.brainres.2017.12.017
16. Zhang Y, Dong N, Hong H, Qi J, Zhang S, Wang J. Mesenchymal stem cells: therapeutic mechanisms for stroke. Int J Mol Sci. (2022) 23:23052550. doi: 10.3390/ijms23052550
17. Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. (2007) 27:1684–91. doi: 10.1038/sj.jcbfm.9600475
18. Deng W, Mandeville E, Terasaki Y, Li W, Holder J, Chuang AT, et al. Transcriptomic characterization of microglia activation in a rat model of ischemic stroke. J Cereb Blood Flow Metab. (2020) 40(1_suppl): S34–48. doi: 10.1177/0271678X20932870
19. Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. (2016) 142:23–44. doi: 10.1016/j.pneurobio.2016.05.001
20. Shiota Y, Nagai A, Sheikh AM, Mitaki S, Mishima S, Yano S, et al. Transplantation of a bone marrow mesenchymal stem cell line increases neuronal progenitor cell migration in a cerebral ischemia animal model. Sci Rep. (2018) 8:14951. doi: 10.1038/s41598-018-33030-9
21. Li Z, Ye H, Cai X, Sun W, He B, Yang Z, et al. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res Bull. (2019) 153:324–33. doi: 10.1016/j.brainresbull.2019.10.001
22. Tobin MK, Stephen TKL, Lopez KL, Pergande MR, Bartholomew AM, Cologna SM, et al. Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc. (2020) 9:e013583. doi: 10.1161/JAHA.119.013583
23. Nijboer CH, Kooijman E, van Velthoven CT, van Tilborg E, Tiebosch IA, Eijkelkamp N, et al. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. (2018) 27:313–25. doi: 10.1089/scd.2017.0148
24. Yang F, Li WB, Qu YW, Gao JX, Tang YS, Wang DJ, et al. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J Stem Cells. (2020) 12:633–58. doi: 10.4252/wjsc.v12.i7.633
25. Augusto-Oliveira M, Arrifano GP, Takeda PY, Lopes-Araujo A, Santos-Sacramento L, Anthony DC, et al. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci Biobehav Rev. (2020) 118:331–57. doi: 10.1016/j.neubiorev.2020.07.039
26. Chen X, Liang H, Xi Z, Yang Y, Shan H, Wang B, et al. BM-MSC Transplantation alleviates intracerebral hemorrhage-induced brain injury, promotes astrocytes vimentin expression, and enhances astrocytes antioxidation via the Cx43/Nrf2/HO-1 Axis. Front Cell Dev Biol. (2020) 8:302. doi: 10.3389/fcell.2020.00302
27. Zhang Y, Yu S, Tuazon JP, Lee JY, Corey S, Kvederis L, et al. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res. (2019) 14:597–604. doi: 10.4103/1673-5374.247464
28. Chen D, Huang Y, Shi Z, Li J, Zhang Y, Wang K, et al. Demyelinating processes in aging and stroke in the central nervous system and the prospect of treatment strategy. CNS Neurosci Ther. (2020) 26:1219–29. doi: 10.1111/cns.13497
29. Itoh K, Maki T, Lok J, Arai K. Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. (2015) 1623:135–49. doi: 10.1016/j.brainres.2015.04.039
30. Zhou Y, Zhang J, Wang L, Chen Y, Wan Y, He Y, et al. Interleukin-1beta impedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion. Brain Behav Immun. (2017) 60:93–105. doi: 10.1016/j.bbi.2016.09.024
31. Yu X, Wu H, Zhao Y, Guo Y, Chen Y, Dong P, et al. Bone marrow mesenchymal stromal cells alleviate brain white matter injury via the enhanced proliferation of oligodendrocyte progenitor cells in focal cerebral ischemic rats. Brain Res. (2018) 1680:127–36. doi: 10.1016/j.brainres.2017.12.019
32. Zarriello S, Neal EG, Kaneko Y, Borlongan CV. T-regulatory cells confer increased myelination and stem cell activity after stroke-induced white matter injury. J Clin Med. (2019) 8:8040537. doi: 10.3390/jcm8040537
33. Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. (2013) 16:1211–18. doi: 10.1038/nn.3469
34. Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR, et al. transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. (2012) 7:1017–24. doi: 10.1007/s11481-012-9406-8
35. Chiu NL, Kaiser B, Nguyen YV, Welbourne S, Lall C, Cramer SC. The volume of the spleen and its correlates after acute stroke. J Stroke Cerebrovasc Dis. (2016) 25:2958–61. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.012
36. Yang B, Hamilton JA, Valenzuela KS, Bogaerts A, Xi X, Aronowski J, et al. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cells. (2017) 35:1290–302. doi: 10.1002/stem.2600
37. Xu K, Lee JY, Kaneko Y, Tuazon JP, Vale F, van Loveren H, et al. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica. (2019) 104:1062–73. doi: 10.3324/haematol.2018.206581
38. Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. (2012) 32:598–611. doi: 10.1038/jcbfm.2012.6
39. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. (2011) 17:796–808. doi: 10.1038/nm.2399
40. Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. (2010) 115:3835–42. doi: 10.1182/blood-2009-10-249078
41. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics−2014 update: a report from the American heart association. Circulation. (2014) 129:399–410. doi: 10.1161/01.cir.0000442015.53336.12
42. Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. (2021) 54:1527–42 e8. doi: 10.1016/j.immuni.2021.04.022
43. Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J Cereb Blood Flow Metab. (2017) 37:967–79. doi: 10.1177/0271678X16648712
44. Neal EG, Acosta SA, Kaneko Y, Ji X, Borlongan CV. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J Cereb Blood Flow Metab. (2019) 39:1750–58. doi: 10.1177/0271678x18766172
45. He J, Liu J, Huang Y, Tang X, Xiao H, Hu Z. Oxidative stress, inflammation, and autophagy: potential targets of mesenchymal stem cells-based therapies in ischemic stroke. Front Neurosci. (2021) 15:641157. doi: 10.3389/fnins.2021.641157
46. Salehi MS, Pandamooz S, Safari A, Jurek B, Tamadon A, Namavar MR, et al. Epidermal neural crest stem cell transplantation as a promising therapeutic strategy for ischemic stroke. CNS Neurosci Ther. (2020) 26:670–81. doi: 10.1111/cns.13370
47. Huang P, Freeman WD, Edenfield BH, Brott TG, Meschia JF, Zubair AC. Safety and efficacy of intraventricular delivery of bone marrow-derived mesenchymal stem cells in hemorrhagic stroke model. Sci Rep. (2019) 9:5674. doi: 10.1038/s41598-019-42182-1
48. Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. (2009) 6:207–13. doi: 10.1038/cmi.2009.28
49. Moisan A, Favre I, Rome C, De Fraipont F, Grillon E, Coquery N, et al. Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplant. (2016) 25:2157–71. doi: 10.3727/096368916X691132
50. Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech. (2012) 5:823–33. doi: 10.1242/dmm.008557
51. Murray KN, Parry-Jones AR, Allan SM. Interleukin-1 and acute brain injury. Front Cell Neurosci. (2015) 9:18. doi: 10.3389/fncel.2015.00018
52. Tso AR, Merino JG, Warach S. Interleukin-6 174G/C polymorphism and ischemic stroke: a systematic review. Stroke. (2007) 38:3070–5. doi: 10.1161/STROKEAHA.107.492231
53. Pan W, Kastin AJ. Tumor necrosis factor and stroke: role of the blood-brain barrier. Prog Neurobiol. (2007) 83:363–74. doi: 10.1016/j.pneurobio.2007.07.008
54. Watters O, O'Connor JJ. A role for tumor necrosis factor-alpha in ischemia and ischemic preconditioning. J Neuroinflammation. (2011) 8:87. doi: 10.1186/1742-2094-8-87
55. Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol. (2014) 9:679–89. doi: 10.1007/s11481-014-9560-2
56. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics−2014 update: a report from the American heart association. Circulation. (2014) 129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80
57. Chang LT, Yuen CM, Liou CW, Lu CH, Chang WN, Youssef AA, et al. Link between interleukin-10 level and outcome after ischemic stroke. Neuroimmunomodulation. (2010) 17:223–8. doi: 10.1159/000290038
58. Pal G, Vincze C, Renner E, Wappler EA, Nagy Z, Lovas G, et al. Time course, distribution and cell types of induction of transforming growth factor betas following middle cerebral artery occlusion in the rat brain. PLoS ONE. (2012) 7:e46731. doi: 10.1371/journal.pone.0046731
59. Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, et al. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest. (2017) 127:280–92. doi: 10.1172/JCI88647
60. Islam A, Choudhury ME, Kigami Y, Utsunomiya R, Matsumoto S, Watanabe H, et al. Sustained anti-inflammatory effects of TGF-beta1 on microglia/macrophages. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:721–34. doi: 10.1016/j.bbadis.2017.12.022
61. Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, et al. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev. (2017) 6:102–11. doi: 10.1016/j.omtm.2017.06.005
62. Brooks B, Ebedes D, Usmani A, Gonzales-Portillo JV, Gonzales-Portillo D, Borlongan CV. Mesenchymal stromal cells in ischemic brain injury. Cells. (2022) 11:11061013. doi: 10.3390/cells11061013
63. Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. (2020) 11:345. doi: 10.1186/s13287-020-01855-9
64. Wang HK, Chen JS, Hsu CY, Su YT, Sung TC, Liang CL, et al. A novel NGF receptor agonist B355252 ameliorates neuronal loss and inflammatory responses in a rat model of cerebral Ischemia. J Inflamm Res. (2021) 14:2363–76. doi: 10.2147/JIR.S303833
65. Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, et al. Growth factors in ischemic stroke. J Cell Mol Med. (2011) 15:1645–87. doi: 10.1111/j.1582-4934.2009.00987.x
66. Oliveira SL, Pillat MM, Cheffer A, Lameu C, Schwindt TT, Ulrich H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. (2013) 83:76–89. doi: 10.1002/cyto.a.22161
67. Sowa K, Nito C, Nakajima M, Suda S, Nishiyama Y, Sakamoto Y, et al. Impact of dental pulp stem cells overexpressing hepatocyte growth factor after cerebral ischemia/reperfusion in rats. Mol Ther Methods Clin Dev. (2018) 10:281–90. doi: 10.1016/j.omtm.2018.07.009
68. Teixeira FG, Salgado AJ. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regen Res. (2020) 15:75–7. doi: 10.4103/1673-5374.264455
69. Sohrabji F. Estrogen-IGF-1 interactions in neuroprotection: ischemic stroke as a case study. Front Neuroendocrinol. (2015) 36:1–14. doi: 10.1016/j.yfrne.2014.05.003
70. Motani A, Forster L, Tull S, Anggard EE, Ferns GA. Insulin-like growth factor-I modulates monocyte adhesion to EAhy 926 endothelial cells. Int J Exp Pathol. (1996) 77:31–5. doi: 10.1046/j.1365-2613.1996.960098.x
71. Wang L, Astone M, Alam SK, Zhu Z, Pei W, Frank DA, et al. Suppressing STAT3 activity protects the endothelial barrier from VEGF-mediated vascular permeability. Dis Model Mech. (2021) 14:049029. doi: 10.1242/dmm.049029
72. Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. (2011) 13:675–85. doi: 10.3109/14653249.2010.549122
73. Bi M, Wang J, Zhang Y, Li L, Wang L, Yao R, et al. Bone mesenchymal stem cells transplantation combined with mild hypothermia improves the prognosis of cerebral ischemia in rats. PLoS ONE. (2018) 13:e0197405. doi: 10.1371/journal.pone.0197405
74. Cho DY, Jeun SS. Combination therapy of human bone marrow-derived mesenchymal stem cells and minocycline improves neuronal function in a rat middle cerebral artery occlusion model. Stem Cell Res Ther. (2018) 9:309. doi: 10.1186/s13287-018-1011-1
75. Li X, Huang M, Zhao R, Zhao C, Liu Y, Zou H, et al. Intravenously delivered allogeneic mesenchymal stem cells bidirectionally regulate inflammation and induce neurotrophic effects in distal middle cerebral artery occlusion rats within the first 7 days after stroke. Cell Physiol Biochem. (2018) 46:1951–70. doi: 10.1159/000489384
Keywords: stroke, bone marrow mesenchymal stromal cell, stem cell therapy, inflammation, neuroprotection
Citation: Wang Z, Wang X, Liao Y, Chen G and Xu K (2022) Immune response treated with bone marrow mesenchymal stromal cells after stroke. Front. Neurol. 13:991379. doi: 10.3389/fneur.2022.991379
Received: 11 July 2022; Accepted: 29 August 2022;
Published: 20 September 2022.
Edited by:
Ge Li, Guangdong Academy of Medical Sciences, ChinaReviewed by:
Jingfei Shi, Capital Medical University, ChinaBipin Chaurasia, Neurosurgery Clinic, Nepal
Copyright © 2022 Wang, Wang, Liao, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaya Xu, xkaya@sina.com
†These authors have contributed equally to this work
 Zili Wang
Zili Wang Xudong Wang1,2†
Xudong Wang1,2† Guangtang Chen
Guangtang Chen Kaya Xu
Kaya Xu