- 1Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
- 2Department of Emergency Neurology and Stroke Unit, IRCCS Fondazione Mondino, Pavia, Italy
- 3Unit of Behavioral Neurology, Mondino Foundation, Pavia, Italy
- 4Neuroimmunology Unit, IRCCS Mondino Foundation, Pavia, Italy
- 5Department of Neuroradiology, IRCCS Mondino Foundation, Pavia, Italy
Introduction: Acute amnestic syndrome is an uncommon clinical presentation of neurological disease. Differential diagnosis encompasses several syndromes including Wernicke-Korsakoff and transient global amnesia (TGA). Structural lesions of the fornix account for a minority of cases of acute amnestic syndromes. Etiology varies from iatrogenic injury to ischemic, inflammatory, or neoplastic lesions. A prompt diagnosis of the underlying pathology is essential but challenging. The aim of this review is to systematically review the existing literature regarding cases of acute amnestic syndrome associated with non-iatrogenic lesions of the fornix.
Methods: We performed a systematic literature search on PubMed, Scopus, and Web of Science up to September 2023 to identify case reports and case series of patients with amnestic syndrome due to fornix lesions. The systematic review was conducted according to PRISMA guidelines. The research was limited to articles written in English. Cases of fornix damage directly ascribable to a surgical procedure were excluded.
Results: A total of 52 publications reporting 55 cases were included in the review. Focusing on acute/subacute onset, vascular etiology was highly prevalent, being responsible for 78% of cases, 40/55 (74%) of which were due to acute ischemic stroke. The amnestic syndrome was characterized by anterograde amnesia in all patients, associated with retrograde amnesia in 27% of cases. Amnesia was an isolated presentation in most cases. Up to two thirds of patients had persistent memory deficits of any severity at follow-up.
Discussion: Acute amnestic syndrome can be rarely caused by fornix lesions. In most cases of acute/subacute presentation, the etiology is ischemic stroke, mainly caused by strokes involving the subcallosal artery territory. The differential diagnosis is challenging and a distinction from common mimics is often difficult on a clinical basis. A high index of suspicion should be maintained to avoid misdiagnosis and provide adequate acute treatment to patients with time-dependent disease, also employing advanced neuroimaging. More research is needed to better understand the outcome and identify prognostic factors in patients with amnestic syndrome due to fornix lesions.
Introduction
Acute amnestic syndrome is an uncommon acute clinical presentation of neurological disease (1). Differential diagnosis encompasses many disorders such as transient global amnesia (TGA) and Wernicke-Korsakoff syndrome; moreover, the involvement of different anatomical structures can be responsible of this neurological syndrome (1).
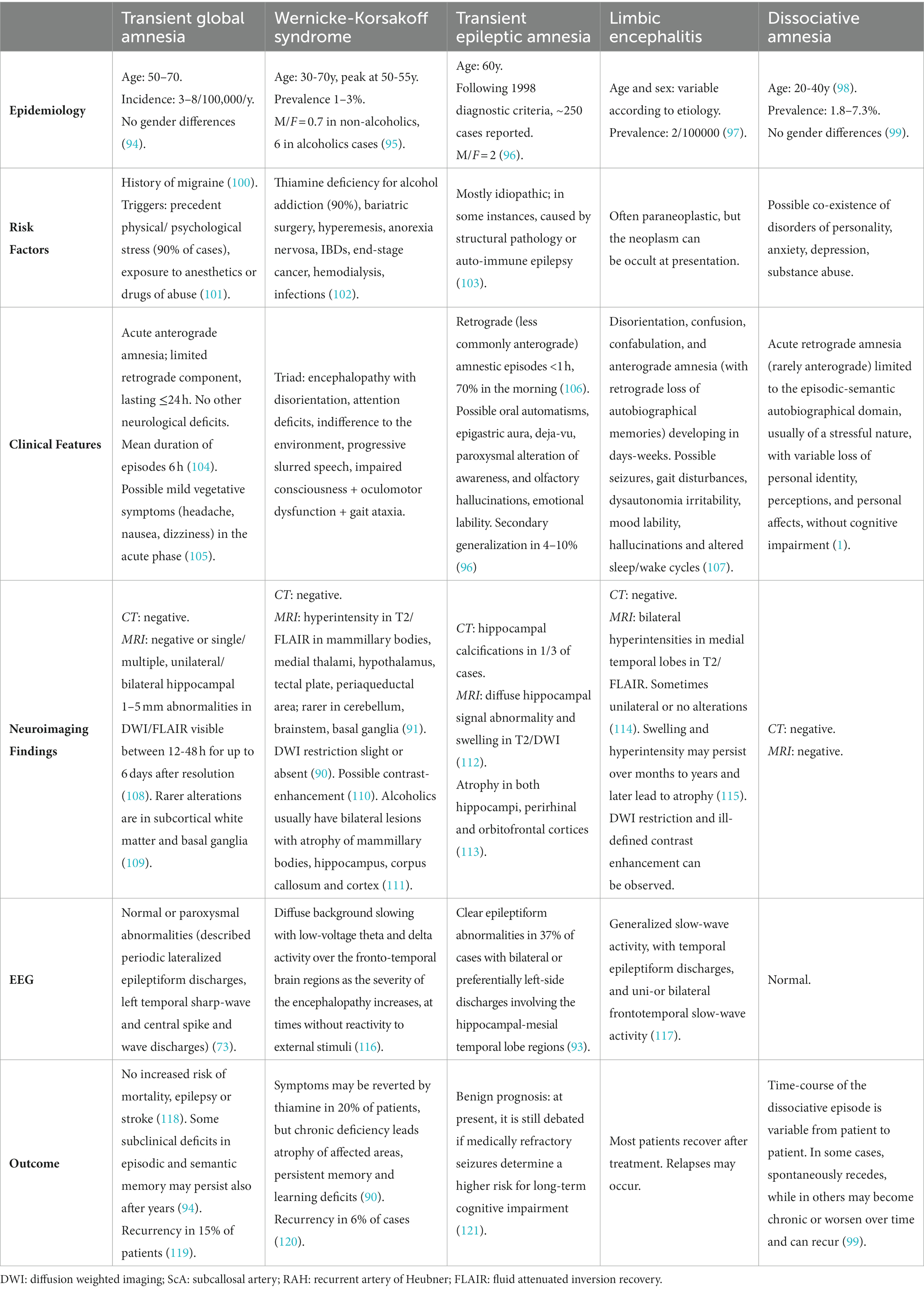
The fornix is both a commissure, a projection, and an association tract, mainly composed of fibers that represent the major hippocampal output. It has a key role within the Papez circuit (Figure 1), which is central in memory storage and is a part of the cholinergic, GABAergic and glutamatergic neurotransmission systems (4). The neural and neurochemical connections of the fornix with the nearby structures are reported in Figure 2. The fornices, as a part of this extended hippocampal-diencephalic system, are thought to contribute to the efficient encoding and normal recall of new episodic information (5). Specifically, considering amnesia as a symptom, the hippocampal formation, amygdala, paralimbic cortices, medial and anterior nuclei of the thalamus, mammillary bodies, basal forebrain and ventral striatum are all critical structures. Bilateral lesions to any of these components can cause significant episodic memory deficits, predominantly anterograde amnesia (6, 7); the severity of the memory deficit is related to the volume of damage of the affected structure (8).
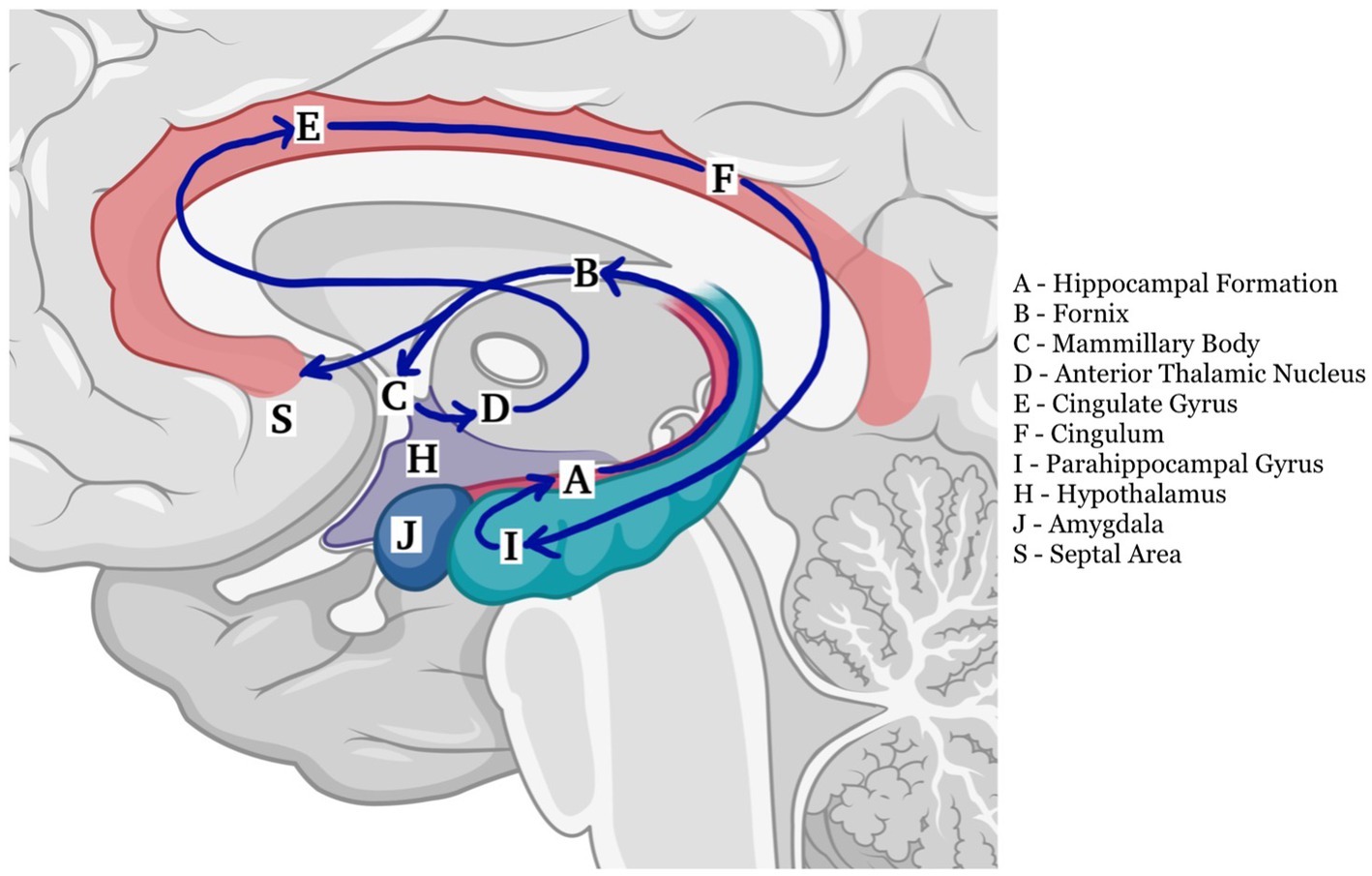
Figure 1. The Papez circuit plays a critical role in the processing and transfer of information for long storage (2). It begins in the hippocampus (A) and continues into the fornix (B) to reach the mamillary body (C), and then through the mammillothalamic tract continues to the anterior nucleus of the thalamus (D). From there it connects to the cingulate gyrus (E) by means of anterior thalamic radiations. The cingulum (F) courses around the corpus callosum to reach the entorhinal cortex (I), which projects to the hippocampus (A), closing the circuit (3) (created with BioRender.com).
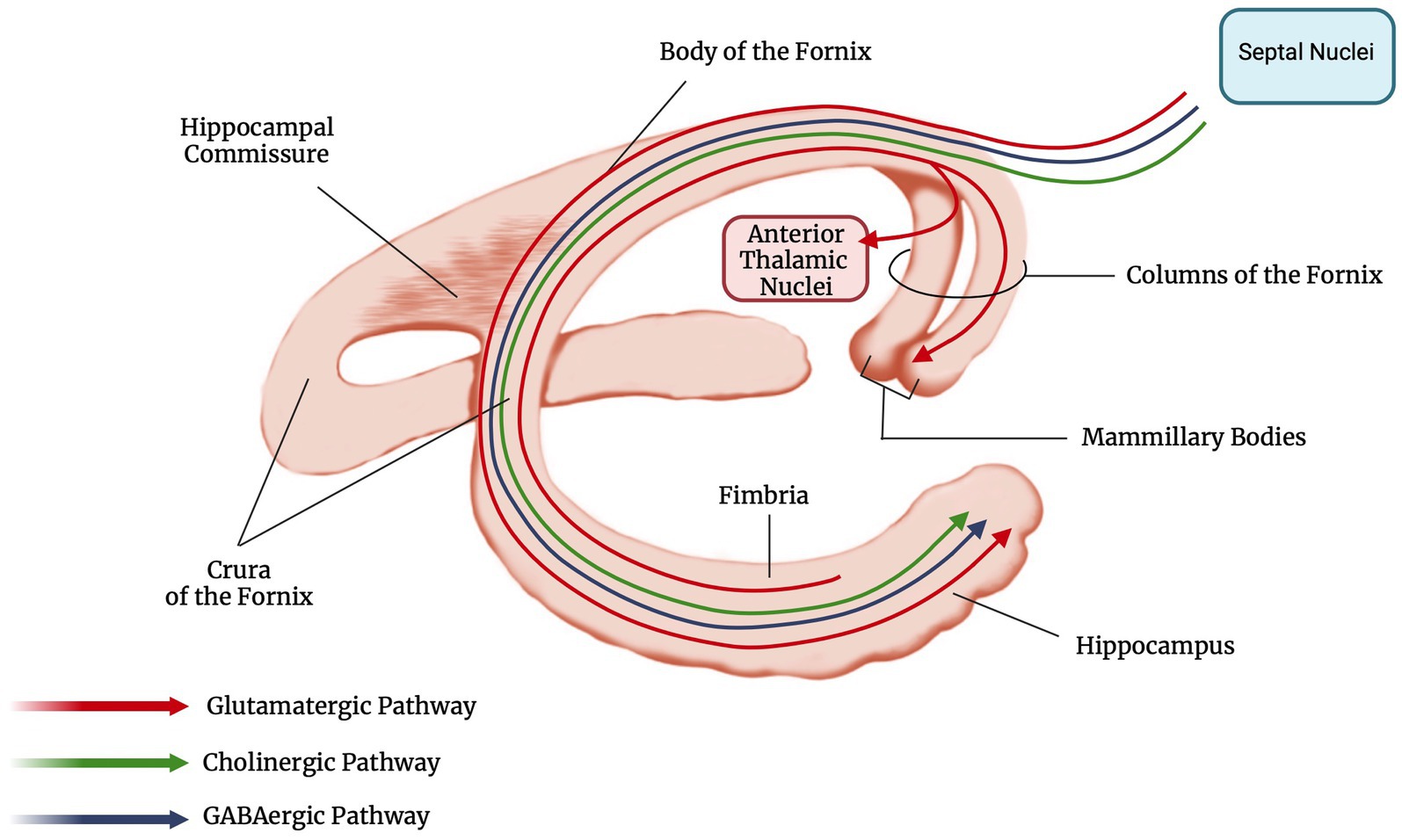
Figure 2. Anatomy and main neural connections of the fornix (created with BioRender.com).
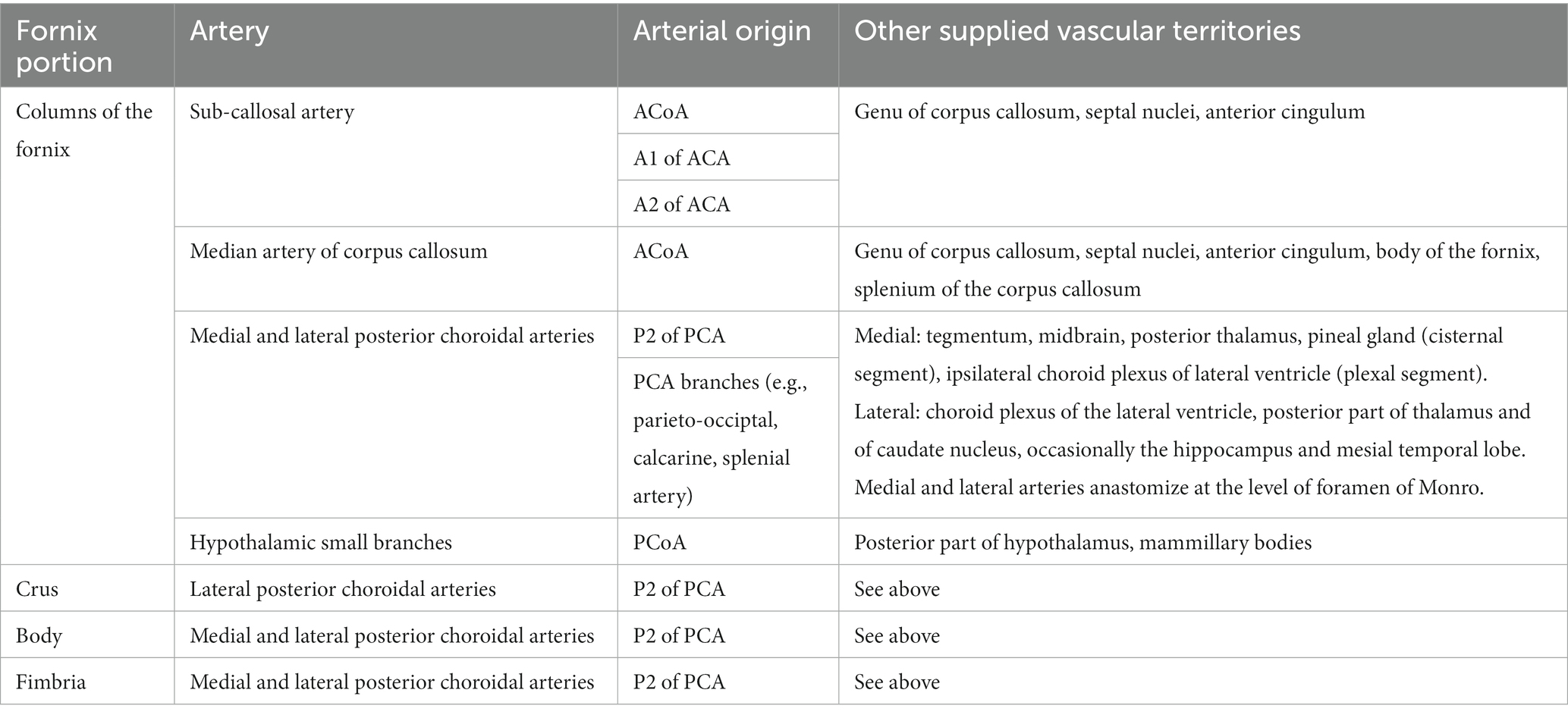
Fornix vascularization (Table 1) is mainly provided by the subcallosal artery (ScA), which vascularizes also the genu of the corpus callosum, the septal nuclei and anterior cingulum in some subjects (9). The medial or lateral posterior choroidal arteries arising from the posterior circulation can also be involved (10).
The consequences of forniceal injury in humans can be seen from post-operative cases involving forniceal transection or injury. The typical syndrome includes anterograde amnesia (11–26), that can be associated with a temporally graded retrograde amnesia (particularly in cases of fornix bilateral columns damage) (27). A lateralized fornix function has also been suggested by clinical reports, with the right fornix involved in visuospatial memory and the left fornix in verbal memory (20).
Overall, structural lesions in the fornix account for a minority of cases of anterograde amnesia, and several etiologies have been reported, including ischemic, inflammatory, neoplastic, and iatrogenic lesions (21, 28–30). Due to its rarity, acute amnestic syndrome due to fornix lesions has been mainly described in case reports and short case series.
The aim of this study is to systematically review the existing literature regarding cases of acute amnestic syndrome associated with fornix lesions, with a focus on clinical features, etiology, and differential diagnosis, to provide useful information to improve the diagnosis and treatment of these patients on clinical grounds.
Methods
This review is reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (31).
Data sources and searches
We performed a systematic literature search on PubMed, Scopus, and Web of Science up to September 2023 to identify case reports/case series of patients with acute amnestic syndrome due to lesions of the fornix. The research was limited to articles written in English. Search terms are provided in Supplementary Table S1.
Study selection
The screening of eligible publications was carried out independently by two raters. First, the titles and abstracts of all citations were reviewed. Next, the full text of potentially relevant citations was reviewed. Discrepancies were resolved by consensus.
Studies were included if they met all the following eligibility criteria: (1) acute/subacute amnestic syndrome (< 1 month); (2) amnesia was a prominent feature of the clinical picture at presentation; (3) fornix lesion confirmed by neuroimaging.
Studies were excluded if they met one or more of the following criteria: (1) fornix damage that could be directly attributed to a surgical or endovascular procedure; (2) presence of extensive damage to other limbic structures that could explain the memory deficit.
Reference lists of eligible studies were also reviewed To identify additional relevant reports. All studies were independently analyzed By two reviewers (F.M. and F.F.) and discrepancies were resolved By discussion and consensus.
Data extraction and quality assessment
Data was extracted by one reviewer and crosschecked by another. We extracted the following data: the first author’s last name, year of publication, patients’ demographic and clinical data, amnesic syndrome characteristics, associated neurologic deficits, neuroimaging data, etiology, and outcome; for patients with ischemic stroke, vascular risk factors and stroke etiology according to Trial of Org 10,172 in Acute Stroke Treatment classification (TOAST) classification (32) were recorded.
Quality of studies included in the systematic review was independently assessed by two authors (F.M. and F.F.) using the NIH Quality Assessment Tool for Case Series Studies (33).
Data synthesis and analysis
Data were summarized using descriptive statistics. Categorical variables were expressed as count (percentage). Continuous variables were summarized as mean (standard deviation, SD) or median (interquartile range, IQR) in case of normal and non-normal distribution, respectively.
Results
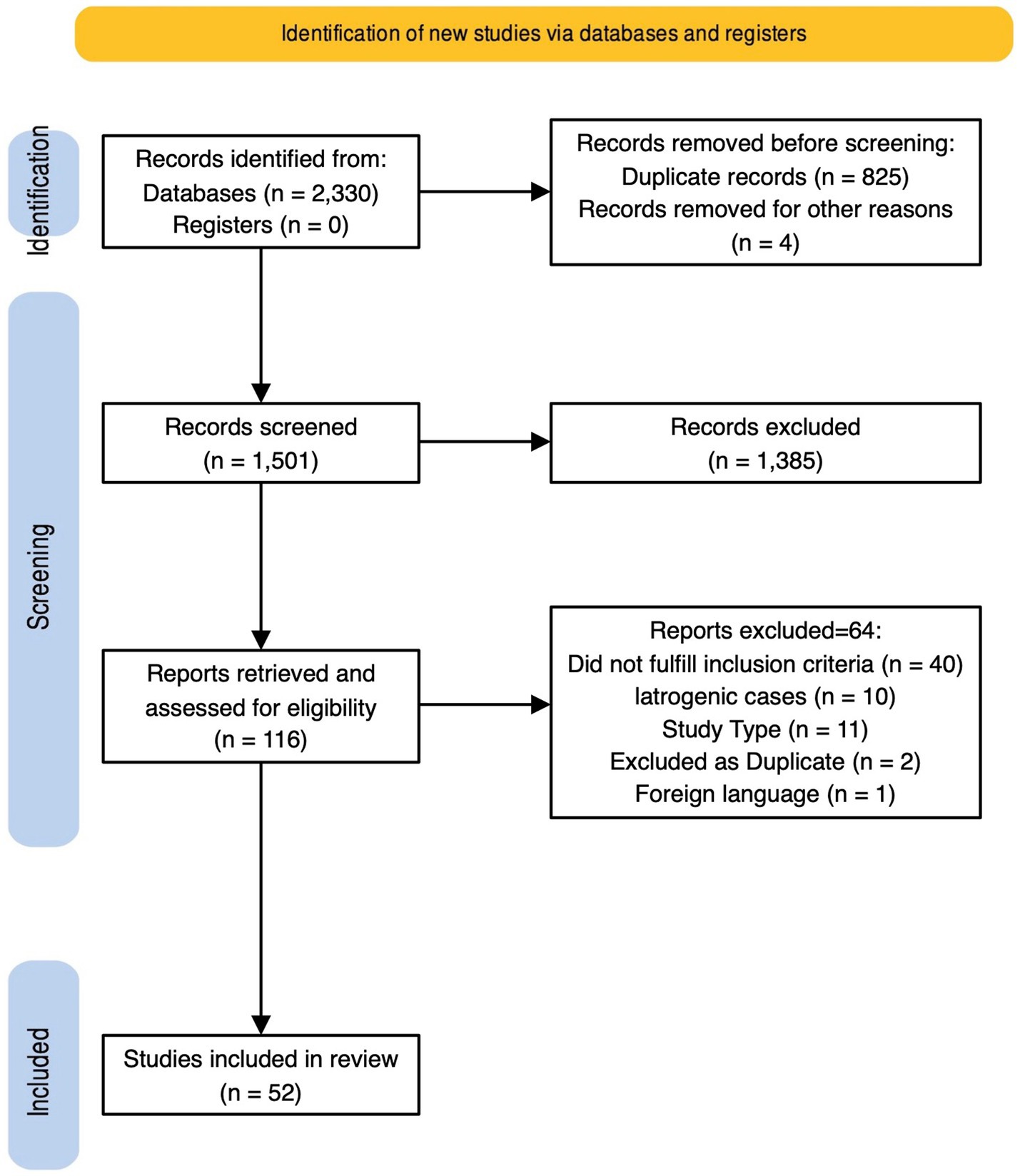
A total of 2,330 records were initially retrieved. After duplicates removal, 1,501 records were assessed for eligibility through abstract screening. 116 were included for full text review and 52 of them, reporting 55 cases, were finally included in the review. Study selection process is described in detail in Figure 3 (31, 34), and quality assessment in Supplementary Table S2. Details on included cases are provided in Supplementary Table S3.
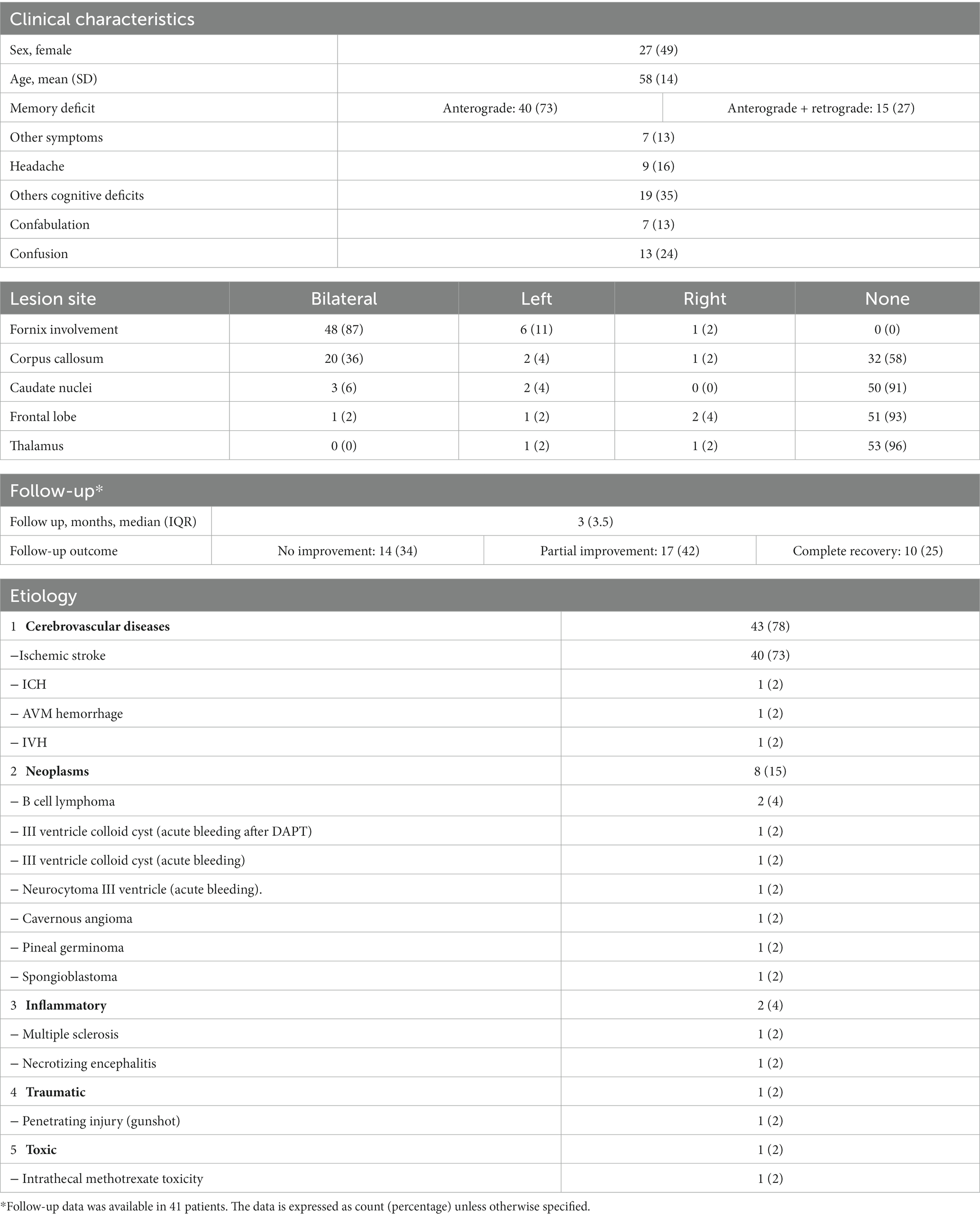
A total of 55 cases were included in the analysis. Twenty-seven patients (49%) were female with a mean (±SD) age of 58 (±14). As expected, focusing on acute/subacute onset, vascular etiology was highly prevalent, being responsible of 43/55 (78%) of cases, 40 (74%) of which were due to acute ischemic stroke (29, 35–71) and 3 (5%) to hemorrhagic stroke (72–75).
Neoplastic etiology was the second most common, being identified in 15% of patients. The associated amnestic syndrome was caused either by fornix infiltration [B cell lymphoma (28, 76), pineal germinoma (77), spongioblastoma (78)] or following an acute intratumoral hemorrhage with secondary forniceal damage (79, 80), facilitated by antithrombotic therapy (81). Less frequently inflammatory (30, 82), traumatic (83), or toxic (84) etiologies were described (Table 2).
Neuroimaging revealed bilateral forniceal involvement in most patients (48/55, 87%). Corpus callosum (23/55, 42%) and more rarely caudate nuclei (5/55, 9%) and thalamus (2/55, 4%) were also injured.
The amnestic syndrome was characterized by an anterograde amnesia in all patients, which was associated with retrograde amnesia in 15/55 (27%) of cases. The retrograde memory impairment was often limited to the recent past and had a significant temporal gradient, coherently with what previously discussed on the role of the fornix in memory function (85, 86).
Amnesia was often an isolated presentation. Other cognitive deficits were present in 37% of cases, predominantly an executive dysfunction.
Other neurological deficits were quite rare and affected only 14% of patients. Confusion and confabulation were reported, respectively, in 14 (25%) and 8 (14%) of patients. Confabulation was more frequent (60%) in patients with caudate nucleus involvement than in those without caudate nucleus lesions (8%). Confusion was also associated with corpus callosum (CC) involvement and was present in 43% of patients with CC lesions versus 9% of patients without CC damage.
Follow-up data were reported in 41/55 (75%) of patients. No standardized outcome reporting prevented a detailed analysis of the recovery trajectory of memory deficit after the acute phase. However, from available information, the outcome at last follow-up (median follow-up of 3 months) was: no improvement in 14/41 (34%), partial improvement in 17/41 (41%) and complete recovery in 10/41 (23%) patients. The characteristics of reported cases are summarized in Table 2.
Acute ischemic stroke of the fornix
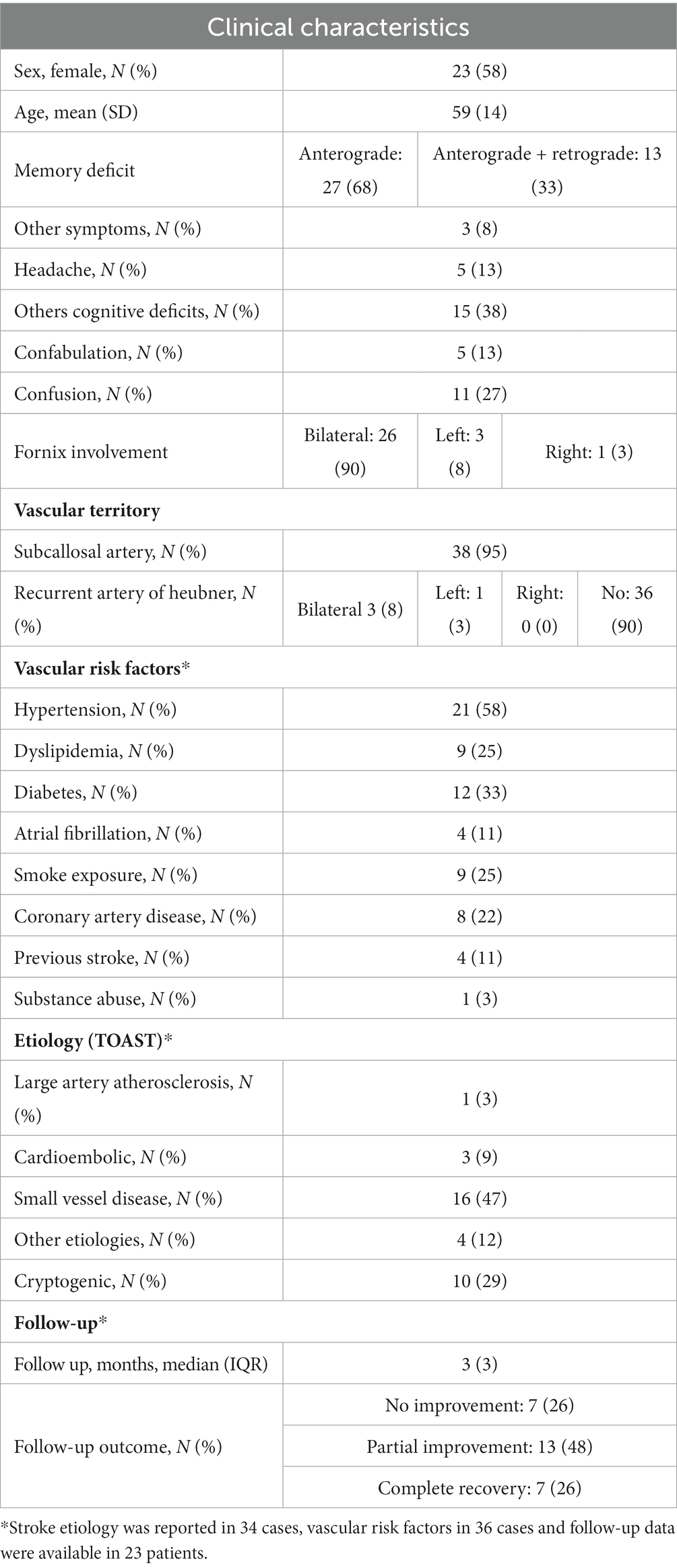
Considering that ischemic stroke involving the fornix accounted for about ¾ of the cases of acute amnestic syndrome due to fornix lesions, it deserves a deeper analysis. Clinical characteristics of ischemic stroke patients are summarized in Table 3.
The term “amnestic syndrome of the subcallosal artery” (ScA) was first proposed by Moussouttas et al. (29) to describe this clinical presentation of ischemic stroke, as ScA is often the culprit vessel.
We retrieved a total of 40 patients with acute ischemic stroke involving the fornix with acute anterograde amnesia that was not better explained by other lesions. Mean (±SD) age of stroke patients was 59 (±14) years and 23 (58%) were females.
MRI data were available in all patients and revealed an isolated fornix involvement in 22 (52%) cases, 90% of which were bilateral. The most frequently associated involved structure was the corpus callosum (18, 45%), as expected by the vascular territory of the ScA previously described. Along with ScA involvement, 4/40 (10%) of patients had a concomitant stroke in the territory of recurrent artery of Heuber (RAH). Examples of MRI findings in two cases of acute ischemic stroke of the fornix are reported in Figure 4.
Figure 4. MRI findings of two patients with acute ischemic stroke of the fornix. In the first patient DWI sequence revealed restricted diffusion involving the columns of the fornix, the corpus callosum and the right caudate nucleus (A); these structures are included in the vascular territory of the ScA (fornix, corpus callosum) and the right RAH (caudate head). FLAIR sequence obtained at 1-month shows gliotic lesions evolution (B). In the second patient, MRI acquired at symptom onset reveals acute ischemic stroke involving only forniceal columns both in DWI (C) and FLAIR (D) sequences. Forniceal columns appear swollen as well. DWI, diffusion weighted imaging; ScA, subcallosal artery; RAH, recurrent artery of Heubner; FLAIR, fluid attenuated inversion recovery.
Two cases of vertebrobasilar stroke with fornix involvement have been reported. One case of vertebral artery dissection with subsequent fornix ischemia was attributed to embolism involving the left lateral posterior choroidal artery (38). The presence of vertigo and right lateral homonymous hemianopsia (due to the concomitant embolic occlusion of left P2-PCA) helped in the diagnosis and localization (38). Another case, with right fornix and anteromedial right thalamus infarction was attributed to the occlusion of the tubero-thalamic artery due to its peculiar lesions distribution (56). The memory disturbance was indistinguishable from the other reported cases (38, 56).
Vascular risk factors were reported in 36/40 (90%) of case reports; patients’ medical history was not adequately reported in the remaining 4 cases. As expected, at least one vascular risk factor was reported in the majority of them (28/36, 78%), of which the most prevalent risk factor was arterial hypertension, affecting 21/36 (58%) of patients, followed by diabetes mellitus 12/36 (33%).
Regarding clinical characteristics at presentation, the memory deficit was only anterograde in 27/40 (68%) of patients, while 32% had also retrograde amnesia.
The presence of associated neurological symptoms is an important feature because it can be an essential clue to suspect an ischemic etiology. However, only 3 (8%) of patients had other neurological deficits and 5 (13%) reported headache. A total of 15 (38%) patient had other cognitive deficits, often executive dysfunction, that were usually revealed by subsequent formal neuropsychological testing.
Confusion 11/40 (28%) and confabulation 5/40 (13%) were not infrequently associated with amnesia.
As for the general study population, confabulation was more frequent in cases with RAH involvement which determines caudate nuclei lesion (6% vs. 75%), while confusion in case of reported corpus callosum ischemia (9% vs. 50%).
Stroke etiology was reported in 34/40 (85%) of cases. The predominant etiology according to TOAST classification (32) was small vessel disease (n = 16, 47%), followed by other known etiology (n = 4, 12%), cardioembolic (n = 3, 9%) and large artery atherosclerosis (n = 1, 3%); 10 (29%) were cryptogenic. The etiology reported for the 4 patients with other known etiologies were: vasospasm after subarachnoid hemorrhage, giant cell arteritis, vertebral artery dissection and one patient was diagnosed a pulmonary neoplasm during the diagnostic workup for cryptogenic stroke.
Regarding reperfusion therapies, none of the patients was treated with either intravenous thrombolysis or mechanical thrombectomy in the acute phase.
Outcome data were of limited quality and reported in 27/40 (68%) of cases; modified Rankin Scale was not reported in any case. From available information, 7 (25.9%) did not recover, 13 (48.1%) partially recovered, and 7 (25.9%) had complete resolution of symptoms.
Discussion
Acute amnestic syndrome with anterograde memory deficit is an uncommon clinical presentation and poses a significant diagnostic challenge. To our knowledge, we are reporting the largest and most comprehensive systematic review of case reports of acute amnestic syndrome due to non-iatrogenic lesions of the fornix.
Our review highlights that, apart from surgical cases where the diagnosis is straightforward, several different etiologies should be considered. However, when the symptom onset is acute, as for other neurological syndromes, the most relevant etiology is cerebrovascular disease: this rare stroke syndrome should be considered in these cases. In particular, our study confirm that the ScA is the main culprit vessel, and, in the majority of cases, the etiology of stroke is small vessel disease.
A first significant finding is that the clinical characteristics of the amnestic syndrome associated with fornix lesions seem to be non-specific, and often memory deficit occurs in isolation. As a consequence, the clinical picture widely overlaps with that of more frequent etiologies like Wernicke-Korsakoff syndrome and, especially, TGA. While additional neurological deficits are a strong element against a diagnosis of TGA, they are unfortunately quite rare, especially if fornix alone is involved. These results are in line with previous findings on ischemic amnesia (87).
Several neurological diseases should be considered in the differential diagnosis. TGA is the most common and typically presents with isolated anterograde amnesia with a limited retrograde component. Focal neurological deficits are absent, and the transient nature of the memory deficit is a key element for diagnosis, but of limited utility during the acute evaluation. MRI can demonstrate hippocampal punctate diffusion lesions (HPDL) (Figures 5A,B) or rarely extra-hippocampal punctate diffusion lesions (E-HPDL), but it can also be normal (88, 89).
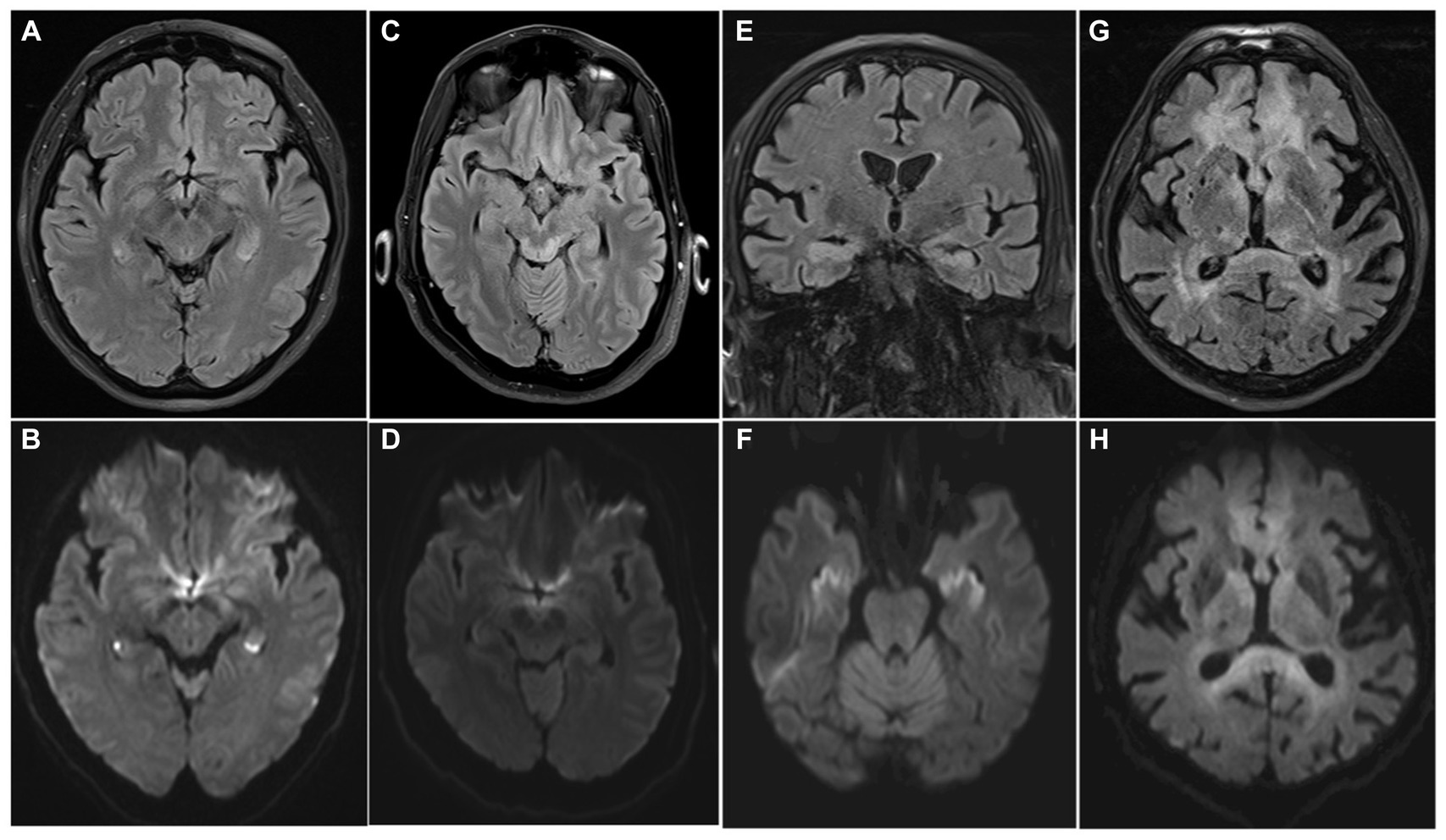
Figure 5. MRI differential diagnosis of acute amnestic syndrome. Transient global amnesia (A,B), Wernicke-Korsakoff syndrome (C,D), limbic encephalitis (E,F), and lymphoma (G,H) can present with acute anterograde amnesia, with or without other neurological symptoms. Bilateral HPDLs in a case of TGA are showed (A) (FLAIR) and (B) (DWI). Typical tectal plate lesions associated with alcoholic Wernicke-Korsakoff syndrome are showed in FLAIR and DWI sequences (C,D). MRI shows bilateral hyperintensity of the hippocampi on both FLAIR (E) and DWI (F) sequences in a case of paraneoplastic limbic encephalitis. A central nervous system lymphoma involving the fornix, but also frontal lobes and periventricular withe matter is showed in (G) (FLAIR) and (H) (DWI), appearing as a diffuse hyperintense alteration on both sequences.
Wernicke-Korsakoff syndrome is another mimic, but the medical history often reveals the cause of the thiamine deficit aiding in the diagnostic process; MRI can show hyperintensity in T2/FLAIR in mammillary bodies, medial thalami, hypothalamus, tectal plate, periaqueductal area; rarer in cerebellum, brainstem, basal ganglia (Figures 5C,D) (90, 91).
Transient epileptic amnesia (TEA) is a form of late-onset temporal lobe epilepsy that can present with the same syndrome (92). However, memory deficit is prevalently retrograde and of short duration (<1 h), episodes are often recurrent, and electroencephalography can show epileptiform abnormalities in the hippocampal-mesial temporal lobe regions (93).
Limbic encephalitis (Figures 5E,F), dissociative amnesia and neoplasms as lymphoma (Figures 5G,H) are other potential differential diagnosis but are usually more easily distinguishable from the clinical characteristics, and amnesia is rarely the only neurological symptom. The principal characteristics of the main pathologies included in the differential diagnosis are summarized in Table 4.
Our analysis has also shown that extraforniceal involvement (e.g., corpus callosum, caudate nuclei) seems to be associated with higher prevalence of additional clinical features, like confabulation and confusion. In cases of ischemic stroke, the involvement of RAH territory is often responsible of this clinical presentation. Confabulation in the context of caudate nuclei stroke has previously been reported and may explain these findings (122). Moreover, the concomitant ScA and RAH involvement has already been proposed as a variable that can aggravate the clinical picture in patients with fornix infarction following AcoA aneurysms treatment (123) and our study supports this finding. Other focal neurological deficits are rare and point to the involvement of other structures outside the limbic system, often in the context of embolism (38).
Another point that emerges is that, while this syndrome has been previously named “amnestic syndrome of the subcallosal artery” (29), it can also occur as a consequence of a posterior circulation stroke in exceedingly rare cases (38, 56). The presence of neurological deficits typical of posterior circulation involvement as ataxia or visual field deficits point to this uncommon circumstance (38).
A striking finding is that in the reported ischemic stroke cases, reperfusion therapy was never administered in the acute phase. This is probably due to a combination of delayed presentation to ED and delayed diagnosis. This is in line with previous literature on ischemic stroke presenting with amnesia, where Michel et al. reported that an ischemic etiology was considered in only 39% of cases (87). The main differential diagnosis was TGA, and many patients had an indistinguishable neurological presentation. Minor focal neurological signs, higher age, and more vascular risk factors were identified as potential clues to reach the correct diagnosis, but the discrimination based on the clinical syndrome was poor (87). Also in this cohort, ischemic patients had a high prevalence of vascular risk factors, but they were often quite young with a mean age of less than 60 years, further complicating the diagnosis. Considering that in most cases the etiology is small vessel disease, endovenous thrombolysis would be the only valid reperfusion therapy and the therapeutic window is narrow; therefore, waiting for the resolution of symptoms to confirm a TGA diagnosis is not possible.
Our study and previous ones on ischemic amnesia demonstrate that the use of MRI in the acute phase of the disease could be useful to assist clinician and would be especially valuable in this rare stroke presentation, where the decision based on the clinical data could be difficult.
As the need for a timely diagnosis clearly emerges from the literature, two useful radiological signs should be kept in mind to rapidly identify the peculiar appearance of acute ScA stroke in DWI sequence. The “goblet” sign (64) and the “watch out” sign (60) can be identified on axial images and are due to the fornix columns and the genu of the corpus callosum involvement, that are the main areas involved in ScA stroke (Figure 6). Beside these highly recognizable patterns, the use of 3D MRI sequences has been suggested to detect smaller infarcts (94).
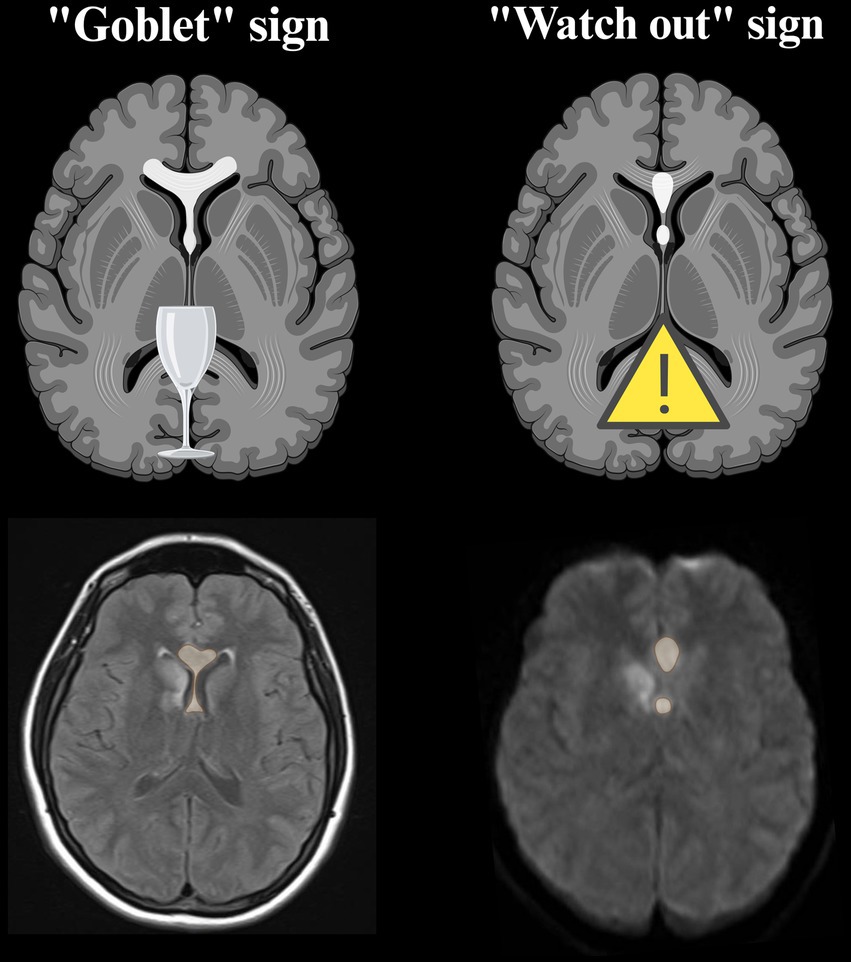
Figure 6. Neuroradiological signs proposed to rapidly recognize the lesional pattern of ScA stroke. They are best visualized on axial DWI sequence in the acute phase (60, 64). The possible concomitant involvement of RAH territory can occur in association with ScA stroke, as can be seen in the MRI images of our cases (created with BioRender.com). DWI, diffusion weighted imaging; ScA, subcallosal artery; RAH, recurrent artery of Heubner.
In our study, only 26% of patients had a complete recovery at last follow-up, with three quarter of them having persistent memory deficit. Moreover, a rapid resolution of symptoms was extremely uncommon, as only one patient with a vascular etiology in our cohort had transient symptoms and was a case of hemorrhage and not of fornix infarction (70). The persistence of the amnestic syndrome in our cases confirms the crucial role of the fornix in the Papez circuit. The quality follow-up data is limited, so no definitive conclusions can be drawn.
Overall, the reported cases and especially data pointing to a non-specific clinical presentation, potential worse outcome, and absent reperfusion therapies utilization, strongly suggest the need to maintain a high index of suspicion to rapidly identify and timely treat patients with this rare stroke syndrome. Patients with different etiologies from ischemic stroke can also benefit from treatment and at least a partially restored memory function has been reported (75, 95).
The strengths of our review include a comprehensive systematic literature search, with specific criteria for inclusion and quality appraisal. However, this study has several limitations. Our findings are limited by the quality of reported data; this is especially relevant for the description of unspecific symptoms as confabulation and confusion. Not uniform follow-up and outcome reporting is another significant limitation. Most importantly, case series and reports are uncontrolled, and while they can suggest hypotheses, they cannot establish definitive associations and they are not robust enough for statistical inference.
Conclusion
Acute amnestic syndrome can be rarely caused by fornix lesions. In most cases of acute/subacute presentation the etiology is vascular, mainly caused by acute ischemic stroke involving the ScA territory. The differential diagnosis is challenging and the distinction from common mimics as TGA is often impossible only on a clinical basis. A high index of suspicion should be maintained to avoid misdiagnosis of time-dependent diseases and to provide adequate acute treatment. A significant percentage of patients with amnestic syndrome due to fornix lesions seems to have persistent memory deficits at follow-up. However, due to the limited quality of available data, more studies are needed to better understand their outcome and identify prognostic factors in patients with amnestic syndrome due to fornix lesions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. FF: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AM: Visualization, Writing – review & editing, Conceptualization, Methodology. YM: Writing – review & editing, Data curation. MG: Visualization, Writing – review & editing, Conceptualization, Supervision. ACo: Supervision, Writing – review & editing. AP: Supervision, Validation, Writing – review & editing, Conceptualization, Data curation. ACa: Funding acquisition, Supervision, Validation, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Italian Ministry of Health “Ricerca Corrente 2022–2024” granted to IRCCS Mondino Foundation.
Conflict of interest
AP and ACo are Associate Editors of Frontiers in Neurology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1338291/full#supplementary-material
References
1. Alessandro, L, Ricciardi, M, Chaves, H, and Allegri, RF. Acute amnestic syndromes. J Neurol Sci. (2020) 413:116781. doi: 10.1016/J.JNS.2020.116781
2. Papez, JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. (1995) 7:103–12. doi: 10.1176/JNP.7.1.103
3. Shah, A, Jhawar, SS, and Goel, A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. (2012) 19:289–98. doi: 10.1016/J.JOCN.2011.04.039
4. Patestas, MAGLP. Limbic system In: A textbook of neuroanatomy. Oxford, England: Blackwell (2006). 344–59.
5. Thomas, AG, Koumellis, P, and Dineen, RA. The fornix in health and disease: an imaging review. Radiographics. (2011) 31:1107–21. doi: 10.1148/rg.314105729
6. Arena, JE, and Rabinstein, AA. Transient global amnesia. Mayo Clin Proc. (2015) 90:264–72. doi: 10.1016/J.MAYOCP.2014.12.001
7. Matthews, BR. Memory dysfunction. Continuum (Minneap Minn). (2015) 21:613–26. doi: 10.1212/01.CON.0000466656.59413.29
9. Marinković, S, Milisavljević, M, and Marinković, Z. Branches of the anterior communicating artery. Microsurgical anatomy. Acta Neurochir (Wien). (1990) 106:78–85. doi: 10.1007/BF01809337
10. Mugikura, S, and Takahashi, S. Fornix infarction due to involvement of posterior circulation. J Stroke Cerebrovasc Dis. (2015) 24:2883–5. doi: 10.1016/J.JSTROKECEREBROVASDIS.2015.07.009
11. Mazarakis, NK, Summers, F, Murray, AD, Waiter, GD, and Fouyas, IP. Partial recovery from amnesia following bilateral surgical fornix transection is correlated with cortical plasticity. Br J Neurosurg. (2011) 25:658–61. doi: 10.3109/02688697.2011.584982
12. Ibrahim, I, Young, CA, and Lamer, AJ. Fornix damage from solitary subependymal giant cell astrocytoma causing postoperative amnesic syndrome. Br J Hosp Med (Lond). (2013) 70:478–9. doi: 10.12968/HMED.2009.70.8.43545
13. Tsivilis, D, Vann, SD, Denby, C, Roberts, N, Mayes, AR, Montaldi, D, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. (2008) 11:834–42. doi: 10.1038/nn.2149
14. Ruggeri, M, and Sabatini, U. Recovery from amnesic confabulatory syndrome after right fornix lesion. Neurorehabil Neural Repair. (2008) 22:404–9. doi: 10.1177/1545968307313506
15. Iizuka, O, Suzuki, K, and Mori, E. Severe amnesic syndrome and collecting behavior after surgery for Craniopharyngioma. Cogn Behav Neurol. (2007) 20:126–30. doi: 10.1097/WNN.0b013e31804c6fb8
16. Poreh, A, Winocur, G, Moscovitch, M, Backon, M, Goshen, E, Ram, Z, et al. Anterograde and retrograde amnesia in a person with bilateral fornix lesions following removal of a colloid cyst. Neuropsychologia. (2006) 44:2241–8. doi: 10.1016/j.neuropsychologia.2006.05.020
17. Aggleton, JP, Mcmackin, D, Carpenter, K, Hornak, J, Kapur, N, Halpin, S, et al. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. (2000) 123:800–15. doi: 10.1093/brain/123.4.800
18. Yasuno, F, Hirata, M, Takimoto, H, Taniguchi, M, Nakagawa, Y, Ikejiri, Y, et al. Retrograde temporal order amnesia resulting from damage to the fornix. J Neurol Neurosurg Psychiatry. (1999) 67:102–5. doi: 10.1136/jnnp.67.1.102
19. Calabrese, P, Markowitsch, HJ, Harders, AG, Scholz, M, and Gehlen, W. Fornix damage and memory. A case report. Cortex. (1995) 31:555–64. doi: 10.1016/S0010-9452(13)80066-4
20. McMackin, D, Cockburn, J, Anslow, P, and Gaffan, D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir. (1995) 135:12–8. doi: 10.1007/BF02307408/METRICS
21. Hodges, JR, Carpenter, K, and Hodges, JR. Anterograde amnesia with fornix damage following removal of IIIrd ventricle colloid cyst. J Neurol Neurosurg Psychiatry. (1991) 54:633–8. doi: 10.1136/jnnp.54.7.633
22. Tucker, DM, Roeltgen, DP, Tully, R, Hartmann, J, and Boxell, C. Memory dysfunction following unilateral transection of the fornix: a hippocampal disconnection syndrome. Cortex. (1988) 24:465–72. doi: 10.1016/S0010-9452(88)80010-8
23. Sweet, W, Talland, G, and Ervin, F. Loss of recent memory following section of fornix. Trans Am Neurol Assoc. (1959) 84:76–82.
24. Vann, SD, Denby, C, Love, S, Montaldi, D, Renowden, S, and Coakham, HB. Memory loss resulting from fornix and septal damage: impaired supra-span recall but preserved recognition over a 24-hour delay. Neuropsychology. (2008) 22:658–68. doi: 10.1037/a0012542
25. Gaffan, D, and Gaffan, EA. Amnesia in man following transection of the fornix. A review. Brain. (1991) 114:2611–8. doi: 10.1093/BRAIN/114.6.2611
26. Baweja, R, Mensinkai, A, Reddy, K, and Sahlas, DJ. Fornix infarction after clipping of anterior communicating artery aneurysm. Can J Neurol Sci. (2015) 42:205–7. doi: 10.1017/CJN.2015.27
27. Fernandez-Romero, R, and Spica, DM. Memory dysfunction. Continuum (Minneap Minn). (2021) 27:1562–85. doi: 10.1212/CON.0000000000001020
28. Carota, A, Rizzo, E, Broways, P, and Calabrese, P. Pure anterograde memory deficit due to secondary lymphoma of the fornix. Eur Neurol. (2013) 70:242. doi: 10.1159/000351781
29. Moussouttas, M, Giacino, J, and Papamitsakis, N. Amnestic syndrome of the subcallosal artery: a novel infarct syndrome. Cerebrovasc Dis. (2005) 19:410–4. doi: 10.1159/000086104
30. Magnin, E, Bereau, M, Chamard, L, Jary, A, Moreau, T, and Berger, E. Isolated pure verbal amnesia as the disease onset of probable multiple sclerosis with a left forniceal black hole. Rev Neurol (Paris). (2016) 172:396–8. doi: 10.1016/J.NEUROL.2016.03.008
31. Moher, D, Liberati, A, Tetzlaff, J, Altman, DG, Antes, G, Atkins, D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/JOURNAL.PMED.1000097
32. Adams, HP, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
33. Study Quality Assessment Tools | NHLBI, NIH. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Accessed October 15, 2023]
34. Haddaway, NR, Page, MJ, Pritchard, CC, and McGuinness, LA. PRISMA2020: an R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. (2022) 18:e1230. doi: 10.1002/CL2.1230
35. Thapa, K, Setyapranata, S, Choi, PMC, and Clare, I. Bilateral fornix infarction as a cause of acute amnesia. Acta Neurol Belg. (2022) 123:1635–6. doi: 10.1007/s13760-022-02159-w
36. Miranda, MO, Suarez, EMA, Frutos, R, Vera, JE, Ferrer, FP, and Prado, JAL. Amnestic syndrome of the subcallosal artery with additional penetrating vessel involvement. J Neurol Sci. (2015) 359:438–9. doi: 10.1016/j.jns.2015.10.014
37. Turine, G, Gille, M, Druart, C, Rommel, D, and Rutgers, MP. Bilateral anterior fornix infarction: the “amnestic syndrome of the subcallosal artery”. Acta Neurol Belg. (2016) 116:371–3. doi: 10.1007/s13760-015-0553-6
38. Kurokawa, T, Baba, Y, Fujino, K, Kuroiwa, Y, Tomita, Y, Nakane, M, et al. Vertebral artery dissection leading to fornix infarction: a case report. J Stroke Cerebrovasc Dis. (2015) 24:e169–72. doi: 10.1016/J.JSTROKECEREBROVASDIS.2015.03.030
39. Gupta, M, Kantor, MA, Tung, CE, Zhang, N, and Albers, GW. Transient global amnesia associated with a unilateral infarction of the fornix: case report and review of the literature. Front Neurol. (2015) 5:291. doi: 10.3389/fneur.2014.00291
40. Meila, D, Saliou, G, and Krings, T. Subcallosal artery stroke: infarction of the fornix and the genu of the corpus callosum. The importance of the anterior communicating artery complex. Case series and review of the literature. Neuroradiology. (2015) 57:41–7. doi: 10.1007/s00234-014-1438-8
41. Rizek, P, Pasternak, S, Leung, A, and Jenkins, M. Acute-onset anterograde amnesia caused by isolated bilateral fornix infarction. Can J Neurol Sci. (2013) 40:738–9. doi: 10.1017/S0317167100015031
42. Murr, N, Thaisetthawatkul, P, Helvey, J, and Fayad, P. Selective infarction of the anterior genu fornices associated with giant cell arteritis. J Stroke Cerebrovasc Dis. (2012) 21:327–9. doi: 10.1016/j.jstrokecerebrovasdis.2010.08.006
43. Korematsu, K, Hori, T, Morioka, M, and Kuratsu, J. Memory impairment due to a small unilateral infarction of the fornix. Clin Neurol Neurosurg. (2010) 112:164–6. doi: 10.1016/j.clineuro.2009.10.016
44. Adamovich, BL, Gualberto, G, Roberts, T, Haut, MW, and Gutmann, L. Teaching neuro images: amnesia due to fornix infarction. Neurology. (2009) 73:e86:e86. doi: 10.1212/WNL.0b013e3181bd80af
45. Renou, P, Ducreux, D, Batouche, F, and Denier, C. Pure and acute Korsakoff syndrome due to a bilateral anterior fornix infarction: a diffusion tensor tractography study. Arch Neurol. (2008) 65:1252–3. doi: 10.1001/archneur.65.9.1252
46. Wang, J, Ke, J, Zhou, C, and Yin, C. Amnesia due to the injury of Papez circuit following isolated fornix column infarction. J Stroke Cerebrovasc Dis. (2018) 27:1431–3. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.040
47. Ren, C, Yuan, J, Tong, S, Xue, Y, Wu, H, Li, W, et al. Memory impairment due to a small acute infarction of the columns of the fornix. J Stroke Cerebrovasc Dis. (2018) 27:e138–43. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.039
48. Chen, J, Zayas, V, and Gold, R. Anterograde amnesia and fornix infarction. Med Health R I. (2008) 91:258.
49. Den Heijer, T, Ruitenberg, A, Bakker, J, Hertzberger, L, and Kerkhoff, H. Bilateral caudate nucleus infarction associated with variant in circle of Willis. J Neurol Neurosurg Psychiatry. (2007) 78:1175–5. doi: 10.1136/JNNP.2006.112656
50. Saito, Y, Matsumura, K, and Shimizu, T. Anterograde amnesia associated with infarction of the anterior fornix and genu of the Corpus callosum. J Stroke Cerebrovasc Dis. (2006) 15:176–7. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.004
51. Qi-Huang, S, Barnaby, J, Mahmoudzadeh, S, Lev, S, and Patel, S. Acute amnesia caused by left fornix infarction: a case report of an unusual entity. Radiol Case Rep. (2022) 17:1626–30. doi: 10.1016/j.radcr.2022.02.038
52. Izgi, E, Ayasli, A, Ogul, Y, and Ogul, H. Unusual stroke cause: bilaterally fornix infarction in a patient with biotinidase deficiency. QJM. (2023) 116:944–6. doi: 10.1093/qjmed/hcad180
53. Zhang, H, Zhang, Y, Jiang, X, Lv, P, and Dong, Y. Bilateral fornix infarction presented with acute amnesia: case report. Neurocase. (2022) 28:63–5. doi: 10.1080/13554794.2021.2024857
54. Moudgil, SS, Azzouz, M, Al-Azzaz, A, Haut, M, and Gutmann, L. Amnesia due to fornix infarction. Stroke. (2000) 31:1418–9. doi: 10.1161/01.STR.31.6.1418
55. Park, SA, Hahn, JH, Kim, JI, Na, DL, and Huh, K. Memory deficits after bilateral anterior fornix infarction. Neurology. (2000) 54:1379–82. doi: 10.1212/WNL.54.6.1379
56. Ghannam, M, Alshaer, Q, Ukatu, H, Alkuwaiti, M, and Streib, C. Acute amnestic syndrome and ischemic stroke: a case series. Neurol Clin Pract. (2021) 11:263–7. doi: 10.1212/CPJ.0000000000000928
57. Jiang, J, Wang, X, Chen, Y, and Shang, X. What causes anterograde amnesia? Eur Neurol. (2021) 84:139–44. doi: 10.1159/000512408
58. Sidra, M, Hattingh, L, and Ramadan, H. Don’t forget the fornix. Pract Neurol. (2021) 21:336–7. doi: 10.1136/practneurol-2020-002802
59. Cho, MJ, Shin, D-I, Han, M-K, and Yum, KS. Acute amnesia during pregnancy due to bilateral fornix infarction: a case report. World J Clin Cases. (2020) 8:4494–8. doi: 10.12998/wjcc.v8.i19.4494
60. Holla, V, Pene, S, and Rakesh, SM. Acute amnestic syndrome – “watch out” for fornix infarct. Neurol India. (2020) 68:498–9. doi: 10.4103/0028-3886.280642
61. Karri, M, Ramasamy, B, and Perumal, S. Ischemic amnesia caused by bilateral fornix infarction: a rare entity. Ann Indian Acad Neurol. (2019) 22:333–4. doi: 10.4103/aian.AIAN_303_18
62. Boardman, J, and Zermansky, A. Ischaemic stroke of the fornix and genu of the corpus callosum presenting with a Korsakoff-like syndrome. BMJ Case Rep. (2019) 12:e228506. doi: 10.1136/bcr-2018-228506
63. Takano, Y, Tatewaki, Y, Mutoh, T, Ohara, Y, Yamamoto, S, and Taki, Y. Isolated fornix infarction with damage to the limbic system as a cause of persistent amnesia: a case report. Am J Case Rep. (2018) 19:1382–5. doi: 10.12659/AJCR.912508
64. Pardina-Vilella, L, Pinedo-Brochado, A, Vicente, I, Bocos-Portillo, J, Martínez-Arroyo, A, Ontañon, JM, et al. The goblet sign in the amnestic syndrome of the subcallosal artery infarct. Neurol Sci. (2018) 39:1463–5. doi: 10.1007/S10072-018-3425-Z/FIGURES/1
65. Zhu, QY, Zhu, HC, and Song, CR. Acute amnesia associated with damaged fiber tracts following anterior fornix infarction. Neurology. (2018) 90:706–7. doi: 10.1212/WNL.0000000000005306
66. Salvalaggio, A, Cagnin, A, Nardetto, L, Manara, R, and Briani, C. Acute amnestic syndrome in isolated bilateral fornix stroke. Eur J Neurol. (2018) 25:787–9. doi: 10.1111/ene.13592
67. Jang, SH, and Seo, YS. A new neural tract between injured fornix and brainstem cholinergic nucleus in a stroke patient. Am J Phys Med Rehabil. (2016) 95:e94–5. doi: 10.1097/PHM.0000000000000469
68. Kannath, SK, Malik, V, and Rajan, JE. Isolated subcallosal artery infarction secondary to localized cerebral vasospasm of anterior communicating artery complex following subarachnoid hemorrhage. World Neurosurg. (2017) 107:1043.e15–8. doi: 10.1016/j.wneu.2017.07.052
69. Kürşad Akpınar, Ç, and Sayılır, İ. Amnesia due to bilateral fornix infarction. Turk J Neurol. (2017) 23:77–8. doi: 10.4274/tnd.98958
70. Ji, Y, Xie, Y, Wang, T, Cao, D, Li, J, Han, J, et al. Four patients with infarction in key areas of the Papez circuit, with anterograde amnesia as the main manifestation. J Int Med Res. (2020) 48:030006052093936. doi: 10.1177/0300060520939369
71. Takahashi, M, Wakasugi, T, Hatakeyama, M, Sekiya, K, Shimbo, J, Sato, A, et al. Amnesia as a result of symmetrical infarction of the bilateral fornices. Neurol Clin Neurosci. (2016) 4:165–5. doi: 10.1111/NCN3.12065
72. Abu-Alya, AI, Halthore, V, Khosla, T, Chavda, D, and Veerappan, V. A case report of transient amnesia following spontaneous intracerebral hemorrhage of the fornix. Cureus. (2023) 15:e34519. doi: 10.7759/cureus.34519
73. Kwon, HG, and Jang, SH. Bilateral fornix injury due to cerebral infarct and traumatic intraventricular hemorrhage: a case study. Clin Neurol Neurosurg. (2013) 115:99–101. doi: 10.1016/j.clineuro.2012.04.004
74. Katoh, M, Sawamura, Y, Moriwaki, T, Yoshino, M, Aoki, T, Abumiya, T, et al. A case of cavernous angioma in the septum pellucidum. Clin Neurol Neurosurg. (2013) 115:1126–7. doi: 10.1016/J.CLINEURO.2012.09.010
75. Verfaellie, M, Bauer, RM, and Bowers, D. Autonomic and behavioral evidence of “implicit” memory in amnesia. Brain Cogn. (1991) 15:10–25. doi: 10.1016/0278-2626(91)90012-W
76. Yoon, SS, Na, DL, and Park, KC. Retrograde amnesia associated with fornix lymphoma. Eur Neurol. (2008) 60:155–8. doi: 10.1159/000145334
77. Yoshida, M, Hayashi, T, Fujii, K, Kawai, K, Tsuji, S, and Iwata, A. Recovered recall memory after decompression of the fornix by surgical removal of pineal tumor. Neurology. (2016) 86:790–1. doi: 10.1212/WNL.0000000000002394
78. Heilman, KM, and Sypert, GW. Korsakoff’s syndrome resulting from bilateral fornix lesions. Neurology. (1977) 27:490–13. doi: 10.1212/WNL.27.5.490
79. Sivakumaran, R, and Edwards, RJ. Amnesia due to spontaneous haemorrhage into a colloid cyst. Br J Neurosurg. (2015) 29:110–1. doi: 10.3109/02688697.2014.957649
80. Nishibayashi, H, Uematsu, Y, Terada, T, and Itakura, T. Neurocytoma manifesting as intraventricular hemorrhage-case report. Neurol Med Chir (Tokyo). (2006) 46:41–5. doi: 10.2176/nmc.46.41
81. Snyder, R, Lee, S, Heck, K, Mandel, JJ, Patel, AJ, and Jalali, A. Hemorrhagic cavum vergae colloid cyst: a presentation of anterograde amnesia without hydrocephalus. Surg Neurol Int. (2022) 13:148. doi: 10.25259/SNI_886_2021
82. Yamamoto, T, Kurobe, H, Kawamura, J, Hashimoto, S, and Nakamura, M. Subacute dementia with necrotising encephalitis selectively involving the fornix and splenium. Retrograde development of Alzheimer’s neurofibrillar tangles in the subiculum. J Neurol Sci. (1990) 96:159–72. doi: 10.1016/0022-510X(90)90129-B
83. D’esposito, M, Verfaellie, M, Alexander, MP, and Katz, DI. Amnesia following traumatic bilateral fornix transection. Neurology. (1995) 45:1546–50. doi: 10.1212/WNL.45.8.1546
84. Kwan, BYM, Krings, T, Bernstein, M, and Mandell, DM. A novel mechanism of toxic injury to the Papez circuit from chemotherapy. J Clin Neurosci. (2015) 22:760–2. doi: 10.1016/j.jocn.2014.10.008
85. Chan, D, Henley, SMD, Rossor, MN, and Warrington, EK. Extensive and temporally ungraded retrograde amnesia in encephalitis associated with antibodies to voltage-gated potassium channels. Arch Neurol. (2007) 64:404–10. doi: 10.1001/ARCHNEUR.64.3.404
86. Ribot, T. Diseases of memory: Essays in the positive psychology. Kegan Paul, Trench & Co. (1882). doi: 10.1037/12818-000
87. Michel, P, Beaud, V, Eskandari, A, Maeder, P, Demonet, JF, and Eskioglou, E. Ischemic amnesia: causes and outcome. Stroke. (2017) 48:2270–3. doi: 10.1161/STROKEAHA.117.017420/FORMAT/EPUB
88. Piffer, S, Nannoni, S, Maulucci, F, Beaud, V, Rouaud, O, Cereda, CW, et al. Acute neurological disease as a trigger or co-occurrence of transient global amnesia: a case series and systematic review. Neurol Sci. (2022) 43:5959–67. doi: 10.1007/S10072-022-06259-6/TABLES/2
89. Bartsch, T, and Butler, C. Transient amnesic syndromes. Nat Rev Neurol. (2013) 9:86–97. doi: 10.1038/NRNEUROL.2012.264
90. Zuccoli, G, and Pipitone, N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. Am J Roentgenol. (2009) 192:501–8. doi: 10.2214/AJR.07.3959/ASSET/IMAGES/02_07_3959_05B.JPEG
91. Manzo, G, De Gennaro, A, Cozzolino, A, Serino, A, Fenza, G, and Manto, A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. Biomed Res Int. (2014) 2014:1–12. doi: 10.1155/2014/503596
92. Zeman, AZJ, Boniface, SJ, and Hodges, JR. Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. J Neurol Neurosurg Psychiatry. (1998) 64:435–43. doi: 10.1136/JNNP.64.4.435
93. Asadi-Pooya, AA. Transient epileptic amnesia: a concise review. Epilepsy Behav. (2014) 31:243–5. doi: 10.1016/J.YEBEH.2013.10.021
94. Guillery-Girard, B, Quinette, P, Desgranges, B, Piolino, P, Viader, F, De La Sayette, V, et al. Long-term memory following transient global amnesia: an investigation of episodic and semantic memory. Acta Neurol Scand. (2006) 114:329–33. doi: 10.1111/J.1600-0404.2006.00625.X
95. Chamorro, AJ, Rosón-Hernández, B, Medina-García, JA, Muga-Bustamante, R, Fernández-Solá, J, Martín-González, MC, et al. Differences between alcoholic and nonalcoholic patients with Wernicke encephalopathy: a multicenter observational study. Mayo Clin Proc. (2017) 92:899–907. doi: 10.1016/J.MAYOCP.2017.02.019
96. Mosbah, A, Tramoni, E, Guedj, E, Aubert, S, Daquin, G, Ceccaldi, M, et al. Clinical, neuropsychological, and metabolic characteristics of transient epileptic amnesia syndrome. Epilepsia. (2014) 55:699–706. doi: 10.1111/EPI.12565
97. Dubey, D, Pittock, SJ, Kelly, CR, McKeon, A, Lopez-Chiriboga, AS, Lennon, VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. (2018) 83:166–77. doi: 10.1002/ANA.25131
98. Spiegel, D, Loewenstein, RJ, Lewis-Fernández, R, Sar, V, Simeon, D, Vermetten, E, et al. Dissociative disorders in DSM-5. Depress Anxiety. (2011) 28:824–52. doi: 10.1002/DA.20874
99. Staniloiu, A, and Markowitsch, HJ. Dissociative amnesia. Lancet Psych. (2014) 1:226–41. doi: 10.1016/S2215-0366(14)70279-2
100. Liampas, I, Siouras, AS, Siokas, V, Tsouris, Z, Rikos, D, Brotis, A, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. (2022) 269:184–196. doi: 10.1007/s00415-020-10363-y
101. Werner, R, and Woehrle, JC. Prevalence of mimics and severe comorbidity in patients with clinically suspected transient global amnesia. Cerebrovasc Dis. (2021) 50:171–177. doi: 10.1159/000512602
102. Wijnia, JW, Oudman, E, van Gool, WA, Wierdsma, AI, Bresser, EL, Bakker, J, et al. Severe infections are common in thiamine deficiency and may be related to cognitive outcomes: A cohort study of 68 patients with wernicke-korsakoff syndrome. Psychosomatics. (2016) 57:624–633. doi: 10.1016/j.psym.2016.06.004
103. Baker, J, Savage, S, Milton, F, Butler, C, Kapur, N, Hodges, J, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. (2021) 3:fcab038. doi: 10.1093/BRAINCOMMS/FCAB038
104. Miller, JW, Petersen, RC, Metter, EJ, Millikan, CH, and Yanagihara, T. Transient global amnesia: clinical characteristics and prognosis. Neurology. (1987) 37:733–7. doi: 10.1212/WNL.37.5.733
105. Bartsch, T, and Deuschl, G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. (2010) 9:205–214. doi: 10.1016/S1474-4422(09)70344-8
106. Butler, CR, Graham, KS, Hodges, JR, Kapur, N, Wardlaw, JM, and Zeman, AZJ. The syndrome of transient epileptic amnesia. Ann Neurol. (2007) 61:587–98. doi: 10.1002/ANA.21111
107. Budhram, A, Leung, A, Nicolle, MW, and Burneo, JG. Diagnosing autoimmune limbic encephalitis. CMAJ. (2019) 191:E529–34. doi: 10.1503/CMAJ.181548
108. Sander, K, and Sander, D. New insights into transient global amnesia: recent imaging and clinical findings. Lancet Neurol. (2005) 4:437–444. doi: 10.1016/S1474-4422(05)70121-6
109. Piffer, S, Nannoni, S, Maulucci, F, Beaud, V, Rouaud, O, Förster, A, et al. Transient global amnesia with unexpected clinical and radiological findings: a case series and systematic review. J Neurol Sci. (2022) 441:120349. doi: 10.1016/J.JNS.2022.120349
110. Zuccoli, G, Gallucci, M, Capellades, J, Regnicolo, L, Tumiati, B, Giadás, TC, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. (2007) 28:1328–1331. doi: 10.3174/ajnr.A0544
111. Zuccoli, G, Cruz, DS, Bertolini, M, Rovira, A, Gallucci, M, Carollo, C, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. (2009) 30:171–176. doi: 10.3174/ajnr.A1280
112. Sparaco, M, Pascarella, R, Muccio, CF, and Zedde, M. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. (2022) 11:3940. doi: 10.3390/JCM11143940
113. Butler, C, van Erp, W, Bhaduri, A, Hammers, A, Heckemann, R, and Zeman, A. Magnetic resonance volumetry reveals focal brain atrophy in transient epileptic amnesia. Epilepsy Behav. (2013) 28:363–9. doi: 10.1016/j.yebeh.2013.05.018
114. Graus, F, Titulaer, MJ, Balu, R, Benseler, S, Bien, CG, Cellucci, T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
115. Urbach, H, Soeder, BM, Jeub, M, Klockgether, T, Meyer, B, and Bien, CG. Serial MRI of limbic encephalitis. Neuroradiology. (2006) 48:380–6. doi: 10.1007/S00234-006-0069-0/FIGURES/4
116. Faigle, R, Sutter, R, and Kaplan, PW. Electroencephalography of encephalopathy in patients with endocrine and metabolic disorders. J Clin Neurophysiol. (2013) 30:505–16. doi: 10.1097/WNP.0B013E3182A73DB9
117. Van Vliet, J, Mulleners, W, and Meulstee, J. EEG leading to the diagnosis of limbic encephalitis. Clin EEG Neurosci. (2012) 43:161–4. doi: 10.1177/1550059411433612
118. Arena, JE, Brown, RD, Mandrekar, J, and Rabinstein, AA. Long-term outcome in patients with transient global amnesia: A population-based study. Mayo Clin Proc. (2017) 92:399–405. doi: 10.1016/j.mayocp.2016.11.015
119. Ropper, AH. Transient global amnesia. N Engl J Med. (2023) 388:635–640. doi: 10.1056/NEJMra2213867
120. Sanvisens, A, Zuluaga, P, Fuster, D, Rivas, I, Tor, J, Marcos, M, et al. Long-term mortality of patients with an alcohol-related wernicke-korsakoff syndrome. Alcohol Alcohol. (2017) 52:466–471. doi: 10.1093/alcalc/agx013
121. Savage, SA, Butler, CR, Hodges, JR, and Zeman, AZ. Transient epileptic amnesia over twenty years: long-term follow-up of a case series with three detailed reports. Seizure. (2016) 43:48–55. doi: 10.1016/J.SEIZURE.2016.10.022
122. Carota, A, and Calabrese, P. Confabulations after bilateral consecutive strokes of the Lenticulostriate arteries. Case Rep Neurol. (2012) 4:61–7. doi: 10.1159/000337221
123. Mugikura, S, Kikuchi, H, Fujimura, M, Mori, E, Takahashi, S, and Takase, K. Subcallosal and Heubner artery infarcts following surgical repair of an anterior communicating artery aneurysm: a causal relationship with postoperative amnesia and long-term outcome. JPN J Radiol. (2018) 36:81–9. doi: 10.1007/S11604-017-0703-2/FIGURES/6
Keywords: amnesia, fornix, stroke, review, limbic system
Citation: Mazzacane F, Ferrari F, Malvaso A, Mottese Y, Gastaldi M, Costa A, Pichiecchio A and Cavallini A (2024) Acute amnestic syndrome in fornix lesions: a systematic review of reported cases with a focus on differential diagnosis. Front. Neurol. 15:1338291. doi: 10.3389/fneur.2024.1338291
Edited by:
Mark Gorman, Central Maine Medical Center, United StatesReviewed by:
Zeina Chemali, Massachusetts General Hospital and Harvard Medical School, United StatesHsueh-Sheng Chiang, University of Texas Southwestern Medical Center, United States
Copyright © 2024 Mazzacane, Ferrari, Malvaso, Mottese, Gastaldi, Costa, Pichiecchio and Cavallini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Pichiecchio, anna.pichiecchio@unipv.it
 F. Mazzacane
F. Mazzacane F. Ferrari
F. Ferrari A. Malvaso
A. Malvaso Y. Mottese5
Y. Mottese5 M. Gastaldi
M. Gastaldi A. Costa
A. Costa A. Pichiecchio
A. Pichiecchio A. Cavallini
A. Cavallini