- 1Department of Otolaryngology, Ruian People’s Hospital, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Burns and Plastic Surgery, The Second Affiliated Hospital of Shantou University Medical College, Shantou, China
Background: A growing body of evidence suggests that immunological processes have a significant role in developing idiopathic sudden sensorineural hearing loss (SSHL). However, few studies have examined the association between immune cell phenotype and SSHL using Mendelian Randomization (MR).
Methods: The online genome-wide association studies (GWAS) database was used to compile data from GWAS covering 731 immunophenotypes and SSHL. Inverse variance weighted (IVW) analysis was primarily used for MR study, and single nucleotide polymorphisms (SNPs) associated with immunophenotypes served as dependent variables. A sensitivity study and the false discovery rate (FDR) correction were used to examine the MR hypothesis. In addition, the possibility of reverse causality between immunophenotype and SSHL was validated by reverse MR. Reverse MR was analyzed in a manner consistent with forward MR.
Results: After FDR correction and sensitivity analysis, we screened 7 immunophenotypes, including IgD+ CD38dim %lymphocyte (95% CI: 1.0019, 1.0742, p = 3.87 × 10−2, FDR = 1.15 × 10−2); Unsw mem AC (95% CI: 1.004, 1.2522, p = 4.23 × 10−2, FDR = 2.25 × 10−2); CD86+ myeloid DC AC (95% CI: 1.0083, 1.1147, p = 2.24 × 10−2, FDR = 4.27 × 10−2); CD33dim HLA DR− AC (95% CI: 1.0046, 1.0583, p = 2.12 × 10−2, FDR = 4.69 × 10−2); SSC-A on CD8br (95% CI: 1.0028, 1.1461, p = 4.12 × 10−2, FDR = 4.71 × 10−2); CD45RA− CD4+ %T cell (95% CI: 1.0036, 1.0503, p = 2.32 × 10−2, FDR = 4.82 × 10−2); DP (CD4+CD8+) AC (95% CI: 1.011, 1.2091, p = 2.78 × 10−2, FDR = 4.97 × 10−2). There was a strong causal relationship with SSHL onset, and the reliability of the results was verified. Furthermore, the immunological cell profile and SSHL did not appear to be closely associated, as shown by reverse MR analysis.
Conclusion: Our study provides more support for the current hypothesis that immunophenotypes and the pathophysiology of SSHL are closely associated. Further validation is needed to assess the role of these immunophenotypes in SSHL.
Introduction
The National Institute on Deafness and Other Communication Disorders defines idiopathic sudden sensorineural hearing loss (SSHL), as a condition distinguished by an abrupt and inexplicable decrease in hearing of at least 30 decibels at 3 subsequent sounds (less than 3 dB) without a discernible cause (1). The loss of hearing usually involves only one side of the ear, with less than 2% of cases involving both ears. It might appear suddenly or within a few hours (2). A German study estimated the prevalence rate to be 160 instances per 100,000 individuals annually (3).
While the incidence of SSHL increases with age, there are no significant gender differences (4). SSHL is considered a medical emergency, and its evolution is variable and multifactorial, with limited relevant studies available (5). However, the impact on the patient should not be underestimated. Individuals diagnosed with SSHL often develop acute auditory losses that worsen rapidly or unexpectedly, and there is often a delay in seeking medical attention (3). Since it is not feasible to biopsy and pathologically analyze the inner ear in vivo, studies on the etiology of SSHL can only be conducted by analyzing data obtained from peripheral blood or imaging studies. Despite the large number of studies on SSHL, research into its pathogenesis remains limited (6). Though many individuals lack an apparent cause SSHL, abrupt deafness can be linked to infections, vascular injuries, autoimmune diseases, injury, internal ear anomalies, and neurological diseases (7). An increasing amount of research indicates that immune-associated processes may play essential role in deafness as well as mechanisms involving autoimmune and autoinflammatory diseases may also affect hearing. For example, autoimmune inner ear disease (8), Meniere’s disease (9), Cogan’s syndrome (10), and NLRP3-related autoinflammatory diseases (11) have been found to be strongly associated with SSHL (12). Research findings indicate that elevated antibody levels may lead to the condition through indirect reactions with internal hearing antigens or stimulated T-cells. Autoantibodies against collagen types 2 and 9 and other internal ear antigens were also identified in SSHL patients (13) Regarding the treatment of SSHL, oral corticosteroids are widely recognized as playing a primary role in suppressing the immune reaction (14). Nevertheless, the pathology of immune-mediated sensorineural hearing loss remains unclear (15). Thus, the purpose of our study was to determine that immune cell morphologies and SSHL are directly associated. By employing a Mendelian Randomization (MR) approach, we can minimize confounders and eliminate reverse causality. In the present study, we utilized genetic variants strongly linked to immunocyte phenotypes and SSHL as instrumental variables. This is a significant advancement in the comprehension of the direct mechanistic link between immunological cells and SSHL. This study provides more concrete evidence that the pathogenesis of SSHL may be linked to immunity, offering new perspectives on the diagnosis, treatment, and research direction of SSHL.
Methods
Research design
The possible association of 731 immunophenotypes and SSHL has been assessed using single-nucleotide polymorphisms (SNPs), which are considered instrumental variables (IVs) derived from extensive GWAS studies. The study design employed a Bi-directional Mendelian randomization (MR) analysis method and the SNPs required three key assumptions, as shown in Figure 1. The MR method relies on three main hypotheses. Firstly, the independence assumption states that SNPs and confounders are mutually independent. Secondly, the association hypothesis suggests a strong association between SNPs and exposure factors. Finally, the exclusivity hypothesis states that SNPs can only influence outcomes through exposure factors. Participant’s written authorization was obtained by confirming consent forms, and the data utilized in this investigation had been authorized by the appropriate ethical evaluation boards.
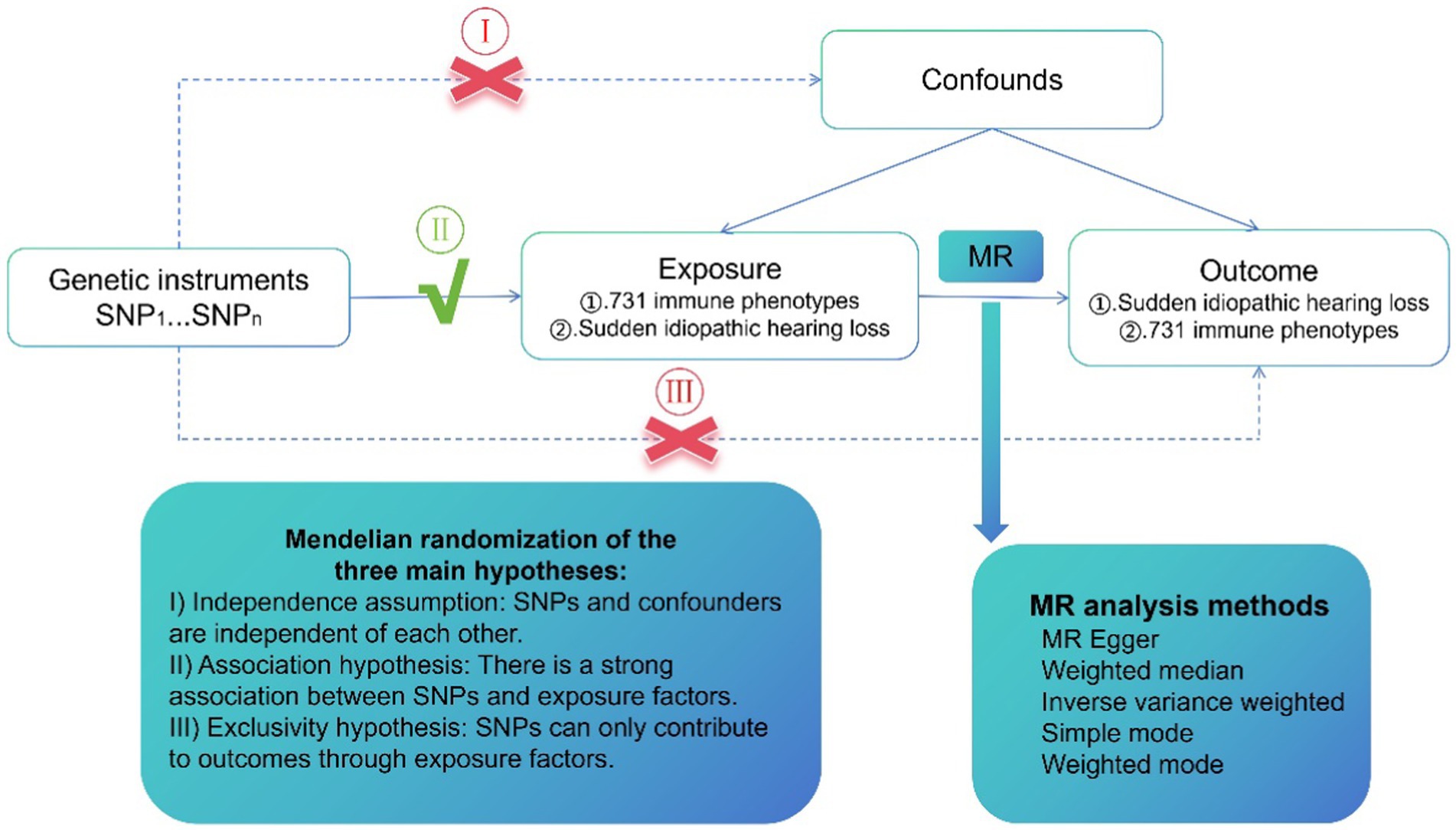
Figure 1. A design scheme for Mendelian randomization (MR). Forward MR: the exposure factor is the immune phenotypes and the outcome factor is Idiopathic sudden sensorineural hearing loss (SSHL). Reverse MR: the exposure factor is SSHL and the end factor is immune phenotypes. Single nucleotide polymorphisms (SNPs) stands for single nucleotide diversity.
Availability of SSHL genome-wide association study (GWAS) data
The GWAS ID: finn-b-H8_HL_IDIOP associated with SSHL obtained the summary-level data using the IEU Open GWAS program (https://gwas.mrcieu.ac.uk/datasets/finn-b-H8_HL_IDIOP/). Following evaluation and estimation, the dataset includes 196,592 instances and 1,491 controls with European ancestry, including 16,380,424 variations (16).
Collection of the GWAS data across immunity
The publically available GWAS database has been the source of all immunophenotypes information. We selected immunophenotypes-related data with numbers between GCST0001391 and GCST0002121 (17). The research included a broad spectrum of 731 immunophenotypes, including 118 relative cell counts (RC), 32 morphologic parameters (MP), 389 median fluorescence intensity (MFI) values indicating surface antigen levels, and 118 absolute cell counts (AC).
The immunophenotype dataset contained parameters such as MFI, AC, and RC that collected data on B-cells, Treg panels, cDCs, T-cell activation phase, monocytes, myeloid cell types, and TBNK (T-cells, B-cells, and natural killer cells). The TBNK and cDCs panels were included in the MP feature. There were 3,757 Europeans, that were involved in the initial GWAS for immune-mediated modeling, did not belong to any overlapped groups. Correlation analyses were run on the nearly 22,000,000 SNPs that were identified using high-density arrays whenever traits including gender, age, and age square were taken into consideration. Supplementary file 1 and the website at https://www.ebi.ac.uk/gwas/ provide access for the complete information.
IVs selection
Suitable IVs from the different GWAS results were obtained separately for MR analysis. For immune cells, we screened for SNPs in the European 1,000 Genomes reference group using a p-value less than 1 × 10−5 as a statistical criterion and linking disequilibrium elimination (the screening condition was r2 < 0.001, within 10,000 kb). We selected F < 10 to represent the reliability of the weak instrument and then assessed the F-value after adjusting its significance level to 5 × 10−8.
MR analysis
To thoroughly evaluate the causal relationship between 731 immune phenotypes and SSHL, we conducted forward MR analysis with the 731 immune phenotypes as the exposure factors and reverse MR analysis with SSHL as the exposure factor to enhance the reliability of the causal inference and eliminate confounding factors. We employed the inverse-variance weighted (IVW) method (18), median-based weighted method (19), and mode-based weighted method (20), with the IVW model serving as our primary analysis approach as it is widely recognized as a robust method in MR studies. Subsequently, Cochran’s Q statistic (21) and the chosen IVs were examined for overall heterogeneity by a compatible p-value. Furthermore, we utilized the “leave-one-out” approach (22) to assess the robustness of the MR results and identify sources of heterogeneity. Finally, we used the powerful tool MR-PRESSO (23) to eliminate the consequences of pleiotropy on the horizon, remove outlier SNPs, estimate corrected results, and test for differences between pre- and post-corrected results.
Statistical analysis
The R 4.3.1 program was used to perform the statistical analysis (http://www.Rproject.org). The “TwoSampleMR” program (24) was the primary tool used to assess the probable association across 731 immunophenotypes and SSHL. The findings were considered trustworthy when an FDR modification (FDR < 0.05) had been applied to control for various comparisons and a threshold value of p < 0.05 was achieved.
Results
Forward MR analysis: immunophenotypes on SSHL
To find a possible link between susceptibility and 731 immune-mediated immunophenotypes, we conducted a bidirectional MR investigation in the present study. We identified seven immune phenotypes associated with SSHL using mainly the IVW method and using FDR correction (FDR < 0.05) as a criterion for correlation (Supplementary file 2: Table S1). Subsequently, we assessed the stability of the results by sensitivity analyses, which revealed that seven immunophenotypes were associated with SSHL (Figure 2; Supplementary file 2: Table S2). The screening program identified two panel for B cells (IgD+ CD38dim % lymphocyte and Unsw mem AC), one panel for conventional Dendritic Cells (cDCs) identified as CD86+ myeloid DC AC, one panel for myeloid cells (CD33dim HLA DR− AC), two panels for detecting TBNKs (T cells, B cells, and NK cells) which included SSC-A on CD8br and DP (CD4+CD8+) AC, and one panel is for the maturation stage of T cells (CD45RA− CD4+ %T cells). Utilizing the IVW method, we obtained an odds ratio (OR) for SSHL risk of 1.0374 (95% CI: 1.0019–1.0742, p = 3.87 × 10−2, FDR = 1.15 × 10−2) for IgD+ CD38dim % lymphocyte. Weighted mode and weighted median methods were also employed, resulting in odds ratios of 1.0366 (95% CI: 1.0015–1.0729, p = 4.89 × 10−2) and 1.0139 (95% CI: 0.9657–1.0644, p = 5.79 × 10−1). By the IVW method, we obtained an outcome Unsw mem AC odds ratio (OR) for SSHL risk of 1.1212 (95% CI: 1.004–1.2522, p = 4.23 × 10−2, FDR = 2.25 × 10−2). And it was obtained by weighted mode method (OR = 1.2363, 95% CI: 0.9835–1.5539, p = 8.68 × 10−2) and weighted median method (OR = 1.126, 95% CI: 0.9618–1.3181, p = 1.40 × 10−1). By the IVW method, we obtained an outcome CD86+ myeloid DC AC odds ratio (OR) for SSHL risk of 1.0602 (95% CI: 1.0083–1.1147, p = 2.24 × 10−2, FDR = 4.27 × 10−2). And it was obtained by weighted mode method (OR = 1.0476, 95% CI: 0.995–1.1029, p = 9.15 × 10−2) and weighted median method (OR = 1.0538, 95% CI: 0.9887–1.1231, p = 1.07 × 10−1). By the IVW method, we obtained an outcome CD33dim HLA DR− AC odds ratio (OR) for SSHL risk of 1.0311 (95% CI: 1.0046–1.0583, p = 2.12 × 10−2, FDR =4.69 × 10−2). And it was obtained by weighted mode method (OR = 1.0284, 95% CI: 0.9968–1.0611, p = 9.19 × 10−2) and weighted median method (OR = 1.026, 95% CI: 0.9917–1.0616, p = 1.39 × 10−1). By the IVW method, we obtained an outcome SSC-A on CD8br odds ratio (OR) for SSHL risk of 1.072 (95% CI: 1.0028–1.1461, p = 4.12 × 10−2, FDR =4.71 × 10−2). And it was obtained by weighted mode method (OR = 1.087, 95% CI: 1.0092–1.1708, p = 4.27 × 10−2) and weighted median method (OR = 1.0889, 95% CI: 1.0005–1.1851, p = 4.86 × 10−2). By the IVW method, we obtained an outcome CD45RA− CD4+ %T cell odds ratio (OR) for SSHL risk of 1.0267 (95% CI: 1.0036–1.0503, p = 2.32 × 10−2, FDR =4.82 × 10−2). And it was obtained by weighted mode method (OR = 1.0291, 95% CI: 1.0019–1.057, p = 4.64 × 10−2) and weighted median method (OR = 1.0202, 95% CI: 0.9892–1.0521, p = 2.04 × 10−1). Finally, by the IVW method, we obtained an outcome DP (CD4+CD8+) AC odds ratio (OR) for SSHL risk of 1.1056 (95% CI: 1.011–1.2091, p = 2.78 × 10−2, FDR =4.97 × 10−2). And it was obtained by weighted mode method (OR = 1.0879, 95% CI: 0.9498–1.2462, p = 2.41 × 10−1) and weighted median method (OR = 1.1003, 95% CI: 0.9732–1.2439, p = 1.27 × 10−1).
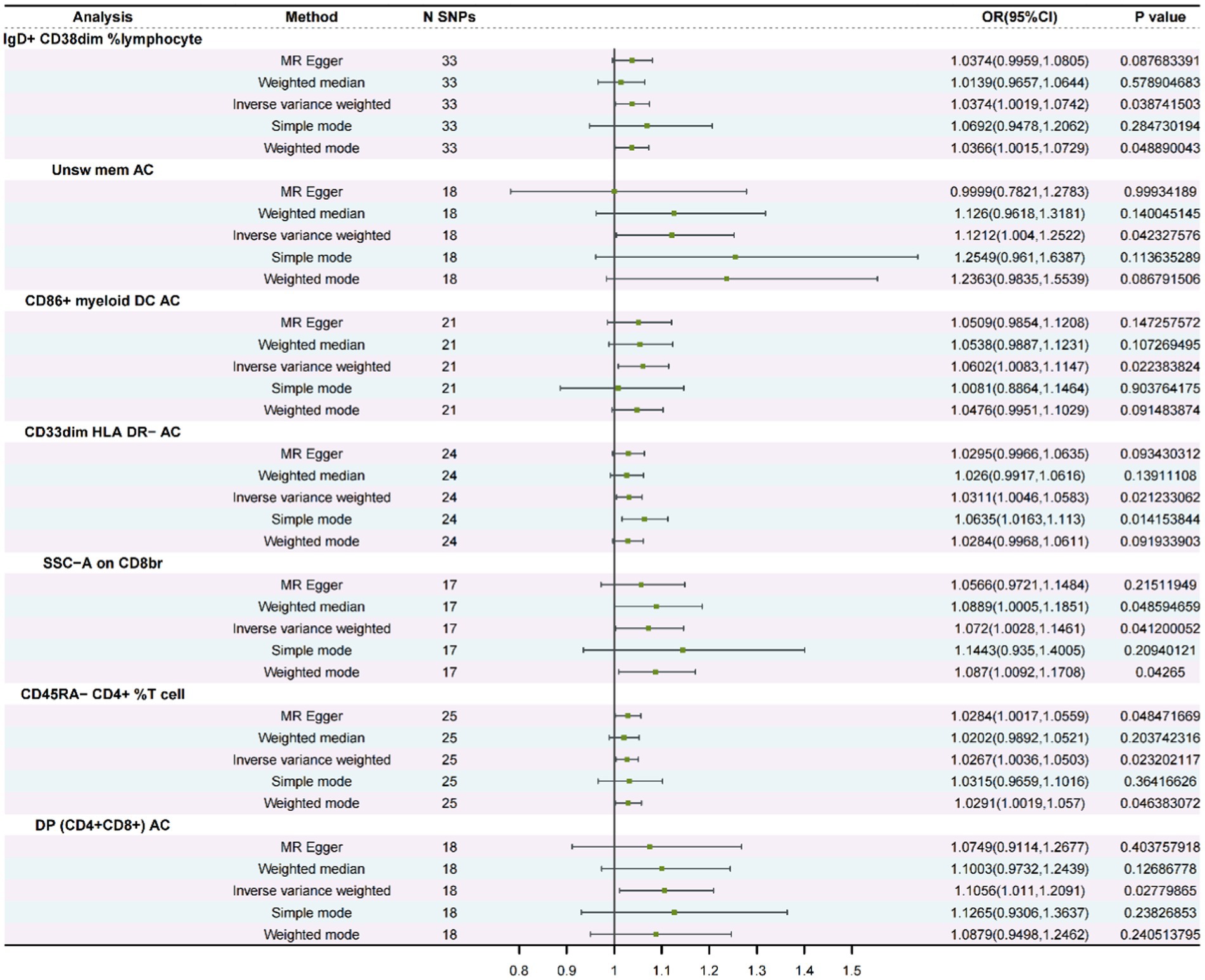
Figure 2. A forest diagram involving five approaches to analyze the association between immunological phenotypes and SSHL (Forward MR).
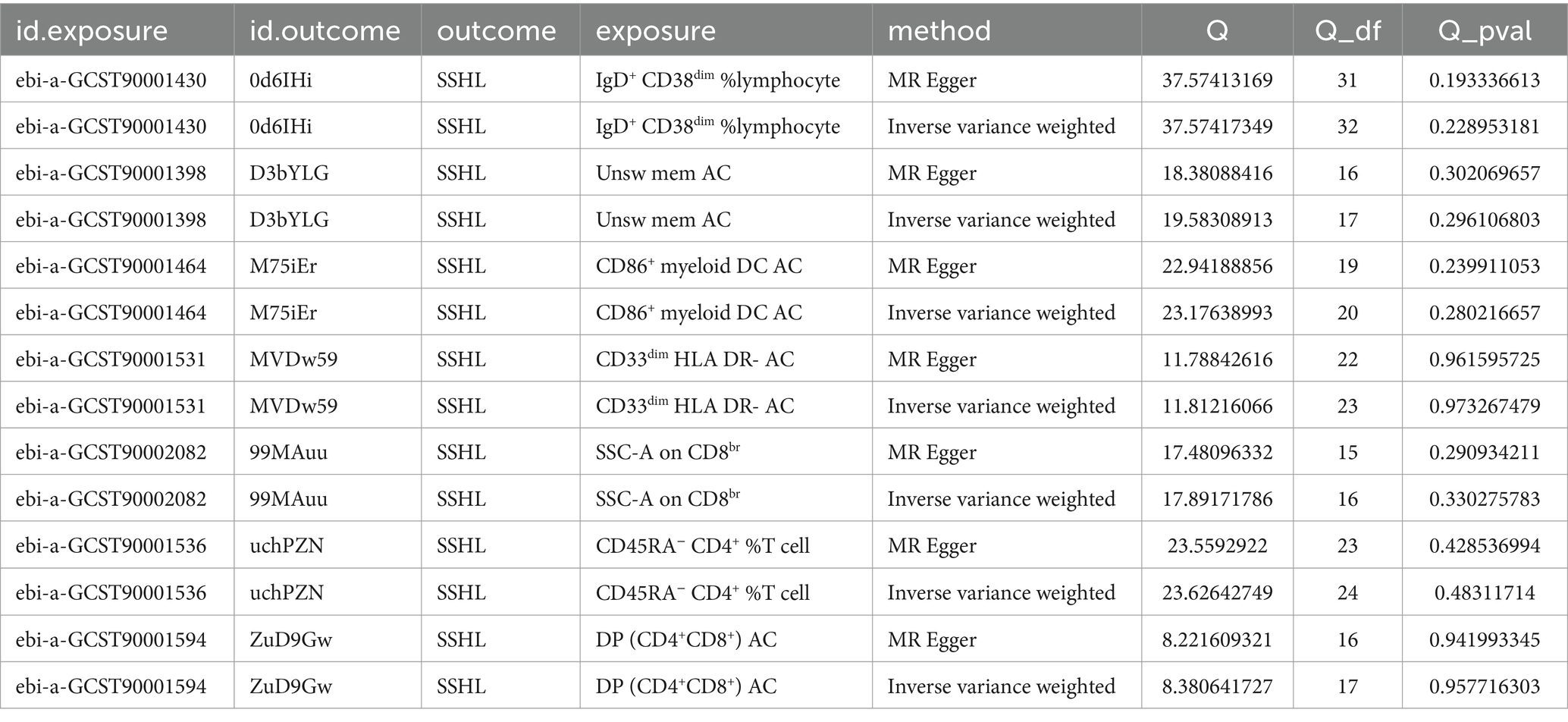
Furthermore, for the seven immunophenotypes examined, the MR-Egger’s intercepting analysis and the MR-PRESSO worldwide testing showed no horizontal pleiotropy (Supplementary file 2: Table S3). Subsequently, Cochran’s Q test and “leave-one-out” screening were performed, wherein none of the obtained results displayed any heterogeneity (Table 1 and Supplementary Figure S1). Scatter plots, funnel plots and forest plots serve to accentuate the rigor of the outcomes (Supplementary Figures S2–S4).
Reverse MR analysis: SSHL on immunophenotypes
We combined seven immunocyte phenotypes obtained from forward MR analyses: IgD+ CD38dim % lymphocytes and Unsw mem AC, CD86+ myeloid DC AC, CD33dim HLA DR− AC, SSC-A on CD8br and DP (CD4+CD8+) AC, and CD45RA− CD4+ % T cells, and used them as exposure factors. To investigate the potential direct association between the two, SSHL was employed as an outcome factor. Moreover, utilizing 1 × 10−5 as the threshold screening condition yielded a total of 23 SNPs. The reverse MR approach’s findings are displayed in Table 2, indicating that there was no causal association between SSHL and these seven immunophenotypes.
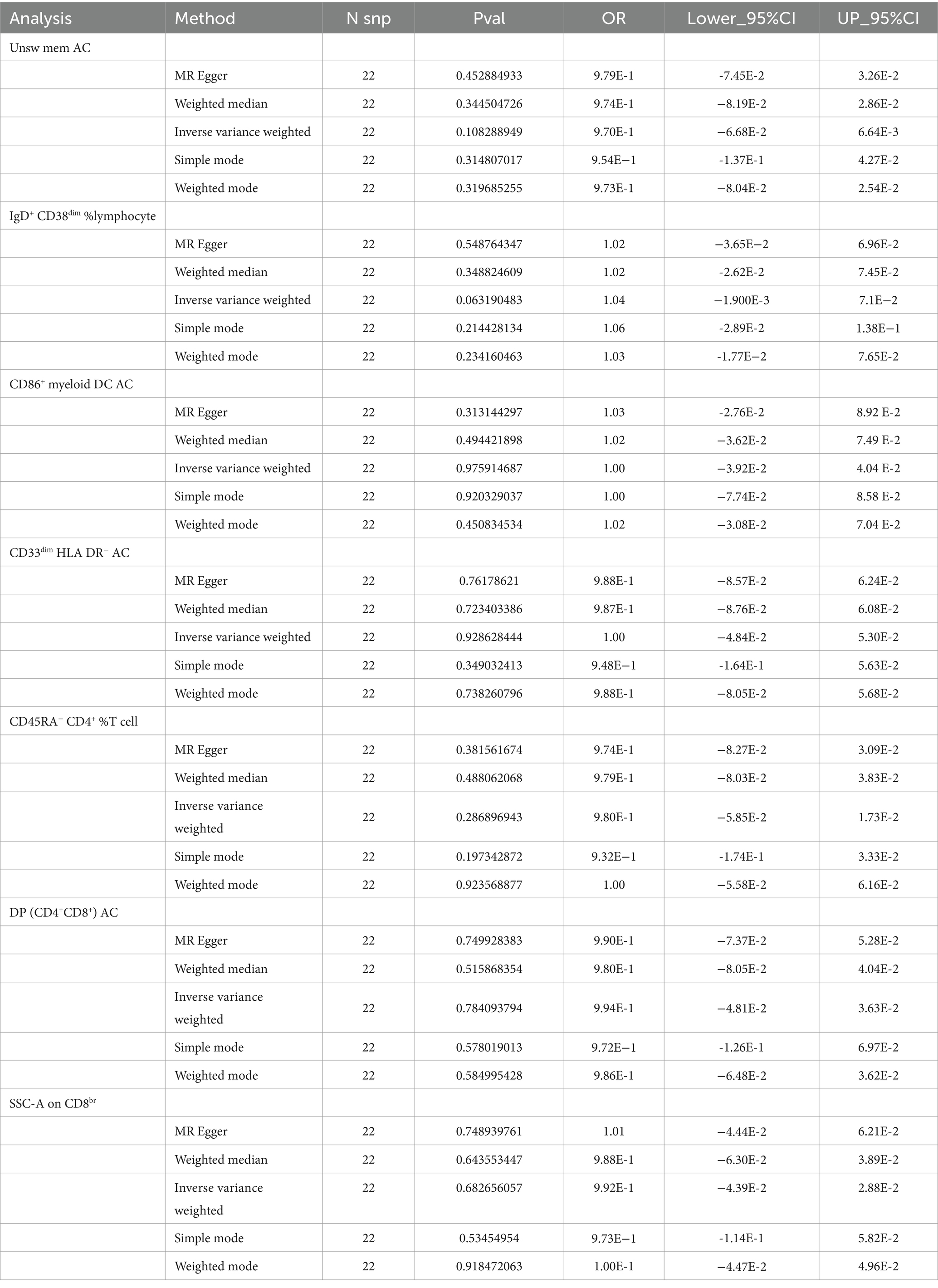
Table 2. The causal relationships between SSHL and seven immune cell phenotypes analyzed by five methods (Reverse MR).
Discussion
In the quest to unravel the complex web of immunocyte phenotypes contributing to Idiopathic sudden sensorineural hearing loss (SSHL), establishing causality remains a significant challenge. Traditional observational studies, while invaluable, often mired by confounding factors and reverse causation, limiting their ability to infer causal relationships. Mendelian Randomization (MR), leveraging gene variations as auxiliary factors, offers a robust alternative by capitalizing on the random assortment of alleles at conception to mimic randomized controlled trials. The Bidirectional MR method was used in the present study to examine the mechanistic interaction between SSHL and 731 immunocyte phenotypes, and results were validated by sensitivity analyses showing that 7 cell immunocyte phenotype as danger factors for SSHL. Additionally, we performed reverse MR with immunophenotype as the final phenotype and SSHL as a risk factor. The results demonstrated that there was no significant association between the two, which further supported the validity of the findings. The study revealed that the proportion of IgD+ CD38dim% lymphocytes raised the probability of developing SSHL. The role of IgD, though less elucidated compared to other immunoglobulins, is thought to influence B cell activity modulation. On the B cell surface, IgD participates in the primary immune response along with IgM as part of the B cell receptor (BCR), and is closely associated with immunity-related diseases. Additionally, the expression level of CD38 reflects the activation status and maturity of B cell. This result aligns with multiple current case reports where patients with B-cell abnormalities were diagnosed with SSHL. (25, 26) Among them, R-CHOP treatment led to remission in three cases, while two patients died during chemotherapy. One hypothesis suggests that SSHL in these patients may be related to a labyrinthine infarct caused by the lymphoma accumulation (27).
Based on MR data, SSC − A on CD8br and CD45RA− CD4+ %T cell were both linked to an increased probability of SSHL. There is growing evidence of the involvement of the immune system, particularly T-lymphocytes and specific autoimmune reactive antibodies (28). Humoral and cell-driven responses cause susceptible antigen-presenting cells (B and T-cells) to produce autoantibodies toward tissues involved in the auditory pathway when interleukin-17, interferon-gamma, and tumor necrosis factor are expressed (12). Ben-Sasson SZ et al. confirmed that the cytokine IL-1 can enhance the antigen-driven response of CD4 and CD8 T cells (29). Additionally, in the presence of IL-1, monocytes produce autoantibodies that damage hearing organs and trigger an acute inflammatory episode (30). Our Mendelian randomization (MR) results support this finding, indicating a genetic association between CD4 and CD8 T cells and SSHL. Several immunomodulators have been explored for their potential use in immune-mediated hearing loss. These include anakinra (IL-1 inhibitor), canakinumab (IL-1 inhibitor), tocilizumab (IL-6 inhibitor), infliximab (TNF-alpha inhibitor), and rituximab (B-cell inhibitor), all of which have displayed efficacy. Intriguingly, Zhou et al. presented a MR analysis (31) indicating that C-reactive protein is a risk factor for SSHL, while TNF-α and fibrinogen do not increase the risk for SSHL. Therefore, TNF-α, an inflammatory marker, exhibited elevated levels in SSHL, but without any relation to its progression.
In addition, MR results showed that Unsw mem AC, CD86+ myeloid DC AC, CD33dim HLA DR− AC and DP (CD4+CD8+) AC were linked to an increased probability of SSHL. Professional APC, such as dendritic cell, monocyte/macrophage and B-cell, detect foreign pathogens through specialized receptors known as pattern recognition receptors (PRRs), making them crucial components of the immune system. Despite the importance of APCs in immune responses, research on their role in SSHL is currently limited. However, Dichhoeck et al. (32) reported that 40 out of 100 patients had respiratory infections associated with SSHL. In addition, Seltzer and Mark (33) revealed the enhancement of magnetic resonance imaging (MRI) in the interior ear of SSHL sufferers indicates the existence of persistent inflammation. The results support the strong correlation between autoimmune and SSHL. However, several limitations must be considered when interpreting our findings. Firstly, although MR is more resistant to unmeasured confounders than traditional epidemiological methods, our results may still be affected by unobserved environmental and physiological factors. In addition, SSHL encompasses various types based on the frequency and degree of hearing loss, including high-frequency descending, low-frequency descending, flat descending, and total deafness. Since we used summary statistics rather than raw data, detailed analysis of these subgroups was not feasible. Furthermore, we were unable to obtain specific treatment information for each patient from the FinnGen database we used making our conclusions somewhat limited. Finally, it is challenging to generalize our findings to other ethnic populations because the study’s findings only cover Europeans.
Conclusion
We obtained a substantial number of study samples and utilized IVs as a tool to mitigate the influence of confounding factors. Our comprehensive Bidirectional MR analysis demonstrated a significant correlation between 7 immune phenotypes and SSHL. This scientifically rigorous approach minimizes the impact of reverse causality, and the findings may open new avenues for research and advancements in the diagnosis, treatment, and intervention of SSHL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Supervision. QZ: Conceptualization, Data curation, Formal analysis, Software, Validation, Writing – original draft, Writing – review & editing. LZ: Formal analysis, Writing – original draft, Data curation, Software, Validation. LC: Data curation, Writing – original draft, Formal analysis, Visualization. CZ: Data curation, Writing – original draft. ZD: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Investigation, Writing – original draft. SL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The grant from the Wenzhou Science and Technology Bureau (2022Y1497) contributed to partially fund this research.
Acknowledgments
The authors are thankful to Wenzhou Science and Technology Bureau for financial support and public GWAS databases for providing the online resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1368002/full#supplementary-material
References
1. Young, Y-H . Contemporary review of the causes and differential diagnosis of sudden sensorineural hearing loss. Int J Audiol. (2020) 59:243–53. doi: 10.1080/14992027.2019.1689432
2. Herrera, M, Berrocal, JRG, Arumí, AG, Lavilla, MJ, Plaza, G, and de la Comisión, GDT. Actualización del consenso sobre el diagnóstico y tratamiento de la sordera súbita idiopática. Acta Otorrinolaringol Esp. (2019) 70:290–300. doi: 10.1016/j.otorri.2018.04.010
3. Schreiber, BE, Agrup, C, Haskard, DO, and Luxon, LM. Sudden sensorineural hearing loss. Lancet. (2010) 375:1203–11. doi: 10.1016/S0140-6736(09)62071-7
4. Singh, A, and Kumar Irugu, DV. Sudden sensorineural hearing loss – a contemporary review of management issues. J Otol. (2020) 15:67–73. doi: 10.1016/j.joto.2019.07.001
5. Prince, ADP, and Stucken, EZ. Sudden sensorineural hearing loss: a diagnostic and therapeutic emergency. J Am Board Fam Med. (2021) 34:216–23. doi: 10.3122/jabfm.2021.01.200199
6. Teranishi, M, Katayama, N, Uchida, Y, Tominaga, M, and Nakashima, T. Thirty-year trends in sudden deafness from four nationwide epidemiological surveys in Japan. Acta Otolaryngol. (2007) 127:1259–65. doi: 10.1080/00016480701242410
7. Lin, JRJ, Atashband, S, Irvine, RA, and Westerberg, BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. (2010) 120:1011–21. doi: 10.1002/lary.20873
8. Ciorba, A, Corazzi, V, Bianchini, C, Aimoni, C, Pelucchi, S, Skarżyński, PH, et al. Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol. (2018) 32:2058738418808680. doi: 10.1177/2058738418808680
9. Ciccone, MM, Scicchitano, P, Gesualdo, M, Cortese, F, Zito, A, Manca, F, et al. Idiopathic sudden sensorineural hearing loss and ménière syndrome: the role of cerebral venous drainage. Clin Otolaryngol. (2018) 43:230–9. doi: 10.1111/coa.12947
10. Tirelli, G, Tomietto, P, Quatela, E, Perrino, F, Nicastro, L, Cattin, L, et al. Sudden hearing loss and Crohn disease: when Cogan syndrome must be suspected. Am J Otolaryngol. (2015) 36:590–7. doi: 10.1016/j.amjoto.2015.02.013
11. Nakanishi, H, Kawashima, Y, Kurima, K, Chae, JJ, Ross, AM, Pinto-Patarroyo, G, et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc Natl Acad Sci USA. (2017) 114:E7766–75. doi: 10.1073/pnas.1702946114
12. Samaha, NL, Almasri, MM, Johns, JD, and Hoa, M. Hearing restoration and the stria vascularis: evidence for the role of the immune system in hearing restoration. Curr Opin Otolaryngol Head Neck Surg. (2021) 29:373–84. doi: 10.1097/MOO.0000000000000738
13. Greco, A, Fusconi, M, Gallo, A, Marinelli, C, Macri, GF, and De Vincentiis, M. Sudden sensorineural hearing loss: an autoimmune disease? Autoimmun Rev. (2011) 10:756–61. doi: 10.1016/j.autrev.2011.05.005
14. Kuhn, M, Heman-Ackah, SE, Shaikh, JA, and Roehm, PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. (2011) 15:91–105. doi: 10.1177/1084713811408349
15. Tripathi, P, and Deshmukh, P. Sudden sensorineural hearing loss: a review. Cureus. (2022) 14:e29458. doi: 10.7759/cureus.29458
16. Yang, Y, Ma, X, Pang, W, and Jiang, C. Causal associations of PM2.5 and GDM: a two-sample Mendelian randomization study. Toxics. (2023) 11:171. doi: 10.3390/toxics11020171
17. Wang, C, Zhu, D, Zhang, D, Zuo, X, Yao, L, Liu, T, et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry. (2023) 23:590. doi: 10.1186/s12888-023-05081-4
18. Xu, J, Zhang, S, Tian, Y, Si, H, Zeng, Y, Wu, Y, et al. Genetic causal association between Iron status and osteoarthritis: a two-sample Mendelian randomization. Nutrients. (2022) 14:3683. doi: 10.3390/nu14183683
19. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
20. Fu, Y, Xu, F, Jiang, L, Miao, Z, Liang, X, Yang, J, et al. Circulating vitamin C concentration and risk of cancers: a Mendelian randomization study. BMC Med. (2021) 19:171. doi: 10.1186/s12916-021-02041-1
21. Kulinskaya, E, and Dollinger, MB. An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med Res Methodol. (2015) 15:49. doi: 10.1186/s12874-015-0034-x
22. Cao, Z, Wu, Y, Li, Q, Li, Y, and Wu, J. A causal relationship between childhood obesity and risk of osteoarthritis: results from a two-sample Mendelian randomization analysis. Ann Med. (2022) 54:1636–45. doi: 10.1080/07853890.2022.2085883
23. Luo, J, Xu, Z, Noordam, R, van Heemst, D, and Li-Gao, R. Depression and inflammatory bowel disease: a bidirectional two-sample Mendelian randomization study. J Crohns Colitis. (2022) 16:633–42. doi: 10.1093/ecco-jcc/jjab191
24. Zhou, H, Zhang, Y, Liu, J, Yang, Y, Fang, W, Hong, S, et al. Education and lung cancer: a Mendelian randomization study. Int J Epidemiol. (2019) 48:743–50. doi: 10.1093/ije/dyz121
25. Berger, JR, Jones, R, and Wilson, D. Intravascular lymphomatosis presenting with sudden hearing loss. J Neurol Sci. (2005) 232:105–9. doi: 10.1016/j.jns.2005.01.001
26. Nageris, B, Or, R, Hardan, I, and Polliack, A. Sudden onset deafness as a presenting manifestation of chronic lymphocytic leukemia. Leuk Lymphoma. (1993) 9:269–71. doi: 10.3109/10428199309147381
27. Miyake, Z, Tomidokoro, Y, Tsurubuchi, T, Matsumura, A, Sakamoto, N, Noguchi, M, et al. Intravascular large B-cell lymphoma presenting with hearing loss and dizziness: a case report. Medicine (Baltimore). (2019) 98:e14470. doi: 10.1097/MD.0000000000014470
28. Brookes, GB . Immune complex-associated deafness: preliminary communication. J R Soc Med. (1985) 78:47–55. doi: 10.1177/014107688507800110
29. Mantovani, A, Dinarello, CA, Molgora, M, and Garlanda, C. IL-1 and related cytokines in innate and adaptive immunity in health and disease. Immunity. (2019) 50:778–95. doi: 10.1016/j.immuni.2019.03.012
30. Arakelyan, A, Nersisyan, L, Poghosyan, D, Khondkaryan, L, Hakobyan, A, Löffler-Wirth, H, et al. Autoimmunity and autoinflammation: a systems view on signaling pathway dysregulation profiles. PLoS One. (2017) 12:e0187572. doi: 10.1371/journal.pone.0187572
31. Zhou, T, Chen, M, Yuan, Z, Xia, Z, Zhang, S, Zhang, Z, et al. Inflammatory markers and the risk of idiopathic sudden sensorineural hearing loss: a Mendelian randomization study. Front Neurol. (2023) 14:1111255. doi: 10.3389/fneur.2023.1111255
32. Van Dishoeck, HA, and Bierman, TA. Sudden perceptive deafness and viral infection; report of the first one hundred patients. Ann Otol Rhinol Laryngol. (1957) 66:963–80. doi: 10.1177/000348945706600406
Keywords: idiopathic sudden sensorineural hearing loss, immunophenotypes, Mendelian randomization, causality, sensitivity analysis
Citation: Li W, Zhou Q, Zhou L, Cao L, Zhu C, Dai Z and Lin S (2024) Causal role of immune cell phenotypes in idiopathic sudden sensorineural hearing loss: a bi-directional Mendelian randomization study. Front. Neurol. 15:1368002. doi: 10.3389/fneur.2024.1368002
Edited by:
Agnieszka J. Szczepek, Charité University Medicine Berlin, GermanyReviewed by:
Marisa Flook Pereira, Granada Biosanitary Research Institute (ibs.GRANADA), SpainJakov Ajduk, Sisters of Charity Hospital, Croatia
Copyright © 2024 Li, Zhou, Zhou, Cao, Zhu, Dai and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijian Dai, wsjjzj@163.com; Sen Lin, Inns@wmu.edu.cn
†These authors have contributed equally to this work
 Wanqing Li1†
Wanqing Li1† Qiang Zhou
Qiang Zhou Longhe Cao
Longhe Cao Sen Lin
Sen Lin